Abstract
In late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered the global coronavirus disease 2019 (COVID-19) pandemic. Although most infections cause a self-limited syndrome comparable to other upper respiratory viral pathogens, a portion of individuals develop severe illness leading to substantial morbidity and mortality. Furthermore, an estimated 10%–20% of SARS-CoV-2 infections are followed by post-acute sequelae of COVID-19 (PASC), or long COVID. Long COVID is associated with a wide variety of clinical manifestations including cardiopulmonary complications, persistent fatigue, and neurocognitive dysfunction. Severe acute COVID-19 is associated with hyperactivation and increased inflammation, which may be an underlying cause of long COVID in a subset of individuals. However, the immunologic mechanisms driving long COVID development are still under investigation. Early in the pandemic, our group and others observed immune dysregulation persisted into convalescence after acute COVID-19. We subsequently observed persistent immune dysregulation in a cohort of individuals experiencing long COVID. We demonstrated increased SARS-CoV-2-specific CD4+ and CD8+ T-cell responses and antibody affinity in patients experiencing long COVID symptoms. These data suggest a portion of long COVID symptoms may be due to chronic immune activation and the presence of persistent SARS-CoV-2 antigen. This review summarizes the COVID-19 literature to date detailing acute COVID-19 and convalescence and how these observations relate to the development of long COVID. In addition, we discuss recent findings in support of persistent antigen and the evidence that this phenomenon contributes to local and systemic inflammation and the heterogeneous nature of clinical manifestations seen in long COVID.
Keywords: COVID-19, inflammation
Summary
WHAT IS ALREADY KNOWN ON THIS TOPIC:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes severe clinical impact and death.
Many patients with severe SARS-CoV-2 infection exhibit extreme immune perturbations.
WHAT ARE THE NEW FINDINGS:
Immune dysregulation persists beyond the acute stages of coronavirus disease 2019 (COVID-19).
Many patients with long COVID exhibit a immunological signature that includes sustained S-protein specific T-cell activation.
HOW MIGHT THESE FINDINGS IMPACT CLINICAL PRACTICE?
Further investigation into the mechanisms behind long COVID may provide information about immunological targets for symptom resolution or long COVID prevention.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has killed over 6 million people globally to date. 1 While most people infected with SARS-CoV-2 have self-limited disease and recover within 1–2 weeks, approximately 20% developed more severe illness in the initial waves of COVID-19, with respiratory involvement leading to hospitalization, respiratory failure, and increased mortality.2–9 While immunity acquired through natural infection and vaccination has decreased the frequency and presentation of severe disease, COVID-19 continues to be associated with hospitalizations and death. In addition to the acute disease process, a portion of people develop post-acute sequelae of COVID-19 (PASC), also referred to as long COVID.10,11 The phenomenon of post-acute sequelae is not unique to SARS-CoV-2, as post-viral syndromes have been previously recognized in other viruses such as SARS-CoV-1, H1N1 influenza, Epstein-Barr virus (EBV), and others. 12 Many post-viral syndromes are associated with neurocognitive dysfunction and rheumatologic symptoms, which could suggest a common underlying etiology driving chronic symptoms. However, the extraordinary number of global infections over a short period of time seen during the COVID-19 pandemic has led to a high burden of individuals experiencing persistent and sometimes debilitating syndromes.
SARS-CoV-2 is an enveloped, positive-sense RNA virus belonging to the Coronaviridae family. Notably, coronaviruses have caused two recent outbreaks: SARS-CoV-1 in 2003 and Middle East respiratory syndrome (MERS-CoV) in 2012. SARS-CoV-1 binds host cells via the angiotensin-converting enzyme 2 (ACE2) receptor, whereas MERS-CoV utilizes dipeptidylpeptidase 4 (DPP4).13,14 Similar to SARS-CoV-1, SARS-CoV-2 utilizes host cell receptor ACE2.15,16 ACE2 plays a homeostatic role in the renin–angiotensin–aldosterone system by regulating angiotensin II levels. 17 Notably, this receptor is found on various cell types throughout the body, contributing to the heterogeneous nature of clinical manifestations seen in both acute COVID-19 and long COVID.18–25 The SARS-CoV-1 epidemic infected approximately 8000 people with a reported case fatality rate of ~10% across 30 different countries before it was ultimately contained within 1 year. 26 MERS-CoV has infected approximately 2500 people across 28 countries and appears to be the most lethal of the three coronaviruses with an estimated case-fatality rate of ∼34%. 27 SARS-CoV-2, while having a lower case fatality rate than SARS-CoV-1 and MERS-CoV, is much more transmissible, contributing to its lack of containment and subsequent global spread. 28 Notably, both SARS-CoV-1 and MERS-CoV were associated with prolonged symptom duration and post-viral syndromes.29,30
While the clinical features and underlying immunology for severe acute COVID-19 have been extensively studied since its recognition in late 2019, the mechanisms and immunology driving long COVID have yet to be fully elucidated. Earlier in the pandemic, our group and others found that immune dysregulation persisted into convalescence after acute SARS-CoV-2 infection. 31 In a subsequent study, we observed similar findings in individuals with long COVID. 32 We proposed that a portion of this persistent dysregulation was likely driven by viral reservoirs leading to chronic immune activation and inflammation. Recently, other groups have found evidence supporting the presence of persistent antigen after acute COVID-19, and some have tied this finding to the development of long COVID.33–42 In this review, we provide a summary of findings during acute COVID-19 infection and convalescence and how these observations relate to the potential role of persistent antigen contributing to long COVID.
Acute COVID-19
Clinical presentation and risk factors
COVID-19 symptoms typically begin around 3- to 6-day post-infection and can involve most organ systems (e.g., respiratory, gastrointestinal, cardiovascular, neurocognitive).4,5,7,8,43 Most commonly reported symptoms include cough, myalgias, headache, and nasal congestion.44–47 Self-limited COVID-19 illness often resolves in 7–10 days5,48,49; however, lower respiratory tract involvement can progress to acute respiratory distress syndrome, leading to the need for mechanical ventilation and dramatic increases in morbidity and mortality.5,48,50 Early in the pandemic, reports emerged that over half of cases progressed to severe disease.5,49,51 As the true prevalence became clearer, severity incidence settled around 15%–20%.2–4,6–9 Risk factors for severe disease include older age, male sex, and a variety of comorbid conditions (e.g., immunocompromised states, metabolic disorders).2–5,8,9,52,53 Since its origins in late 2019, SARS-CoV-2 has continued to mutate, leading to various strains with enhanced transmission. Each wave is associated with sequential dominant variants, but timing of surges varied by geographic location. To date, there is limited data comparing the immunologic differences in COVID-19 based on the dominant variant at the time, especially with more recent variants such as Omicron.54–63 Over time, SARS-CoV-2 has appeared to become less virulent while transmissibility has increased, although this is confounded by accumulated population immunity.44,58,59,61,63–68 Even though SARS-CoV-2 can have a longer incubation period than some other respiratory viruses, viremia appears to peak at time of symptom onset. Furthermore, viral shedding has been noted in asymptomatic COVID-19 patients, contributing to increased transmission.36,69–73
T-cell immune responses during acute illness
The immunopathology behind severe COVID-19 has been intensively studied since the beginning of the pandemic (Figure 1). Investigations have shown that severe illness is associated with delays in mounting an effective adaptive immune response, caused by prominent lymphopenia with concurrent hyperactivation and inflammation promoting tissue damage that leads to hospitalization and potential mortality.74–82 Initial reports on the differences in clinical outcomes with SARS-CoV-2 showed elevations in inflammatory cytokine levels such as interleukin (IL)-6, inducible protein 10, IL-10, and tumor necrosis factor alpha in patients with severe illness. In addition, prominent lymphopenia was associated with increased severity.49,83–93 One of the first larger-scale analyses, including over 500 hospitalized patients, observed that severe disease was associated with depletion of total, CD4+, and CD8+ T cells, as well as increases in various inflammatory cytokines compared to milder illness. 94 They also found that more severe disease was associated with increased expression of programmed cell death protein 1 (PD1), a regulatory molecule that can be a sign of cellular activation and exhaustion. Mathew et al. 95 analyzed similar metrics in acute COVID-19 patients and compared them to patients who recovered from COVID-19 and uninfected controls. While they noted similar findings of lymphopenia and increased inflammation, they also analyzed various T-cell populations and found a decrease in naïve CD4+ T cells, an increase in activated and terminally differentiated CD4+ and CD8+ T cells, and an increase in circulating T-follicular helper (cTfh) cells. Subsequent studies analyzed more extensive panels of cell surface markers and noted functional exhaustion in CD4+ and CD8+ T cells, increased expression of regulatory markers (e.g., PD1, TIGIT, TIM3), and increased expression of activation markers (e.g., HLA-DR) in severe disease.48,94–101
Figure 1.
Immune dysregulation of non-hospitalized vs hospitalized individuals during acute SARS-CoV-2 infection.
(Blue) Mild illness is associated with a timely immune response, indicated by appropriate activation of the adaptive immune response. This is evident by an increase in cytokine production and upregulation of activation markers on the surface of T cells (non-hospitalized visit 1). While mild illness can lead to a return to baseline function (blue dashed line), we observed that non-hospitalized patients had increased expression of activation and exhaustion markers 1-month post-infection (non-hospitalized visit 2, blue dotted line), indicating persistent immune dysregulation. (Orange/Red) Delayed viral clearance contributes to hyperactivation and worsening of symptoms, leading to hospitalization and mortality. This hyperactivation is associated with upregulation of several activation and exhaustion markers on the surface of T cells, as well as an increase in inflammatory cytokine production (hospitalized/mortality). Furthermore, persistent hyperactivation can lead to eventual exhaustion (hypoactivation) and either recovery (orange dashed line) or mortality (red dashed line).
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
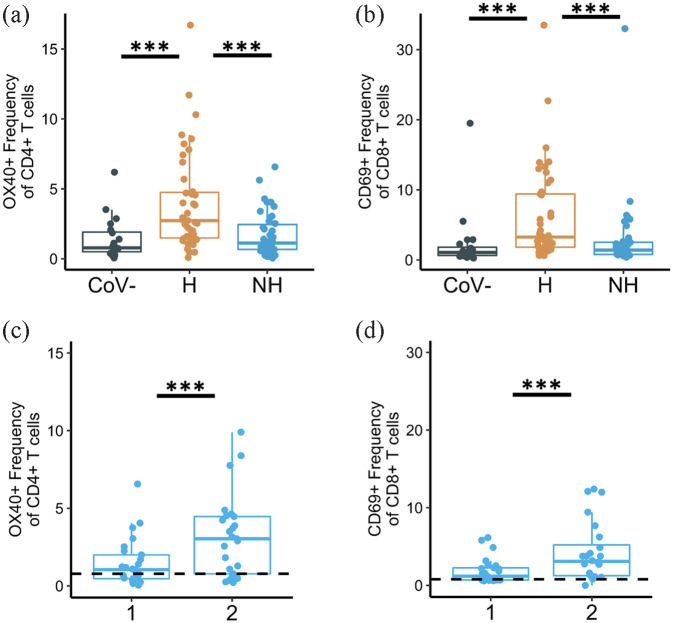
While these early reports focused on severe presentations of COVID-19, we sought to explore differences in the immune response between hospitalized and non-hospitalized patients during acute infection. To do this, we obtained peripheral blood samples from 85 COVID-19 infected donors (46 hospitalized and 39 non-hospitalized) within the early months of the pandemic. For detailed protocol, please see Files et al. 31 In brief, peripheral blood mononuclear cells (PBMCs) were isolated and washed from each sample. 100K PBMCs from each sample were then stained with immunological markers, including CD3, CD4, CD8, OX40, and CD69, and analyzed on a BD FACS Symphony flow cytometer. All data collected were in accordance with the University of Alabama at Birmingham’s Institutional Review Board. We observed that hospitalized patients had increased expression of activation markers OX40 and CD69 on CD4+ and CD8+ T cells, respectively, compared to both non-hospitalized patients and uninfected controls (Figure 2(a) and (b)). Hospitalized patients had increased expression of several other activation markers on T cells, including HLA-DR, CD38, and CD-154. We also found increased expression of classical exhaustion markers including PD1 and TIM3 in hospitalized patients, while TIGIT was increased on T cells from COVID-19 patients regardless of hospitalization status. 31 Around the same time, other groups observed that non-hospitalized patients had increased expression of activation markers compared to uninfected controls albeit lower than hospitalized patients.102–105 Carsetti et al. 102 observed increases in HLA-DR expression on CD4+ T cells from both mild and severe COVID-19 patients. Oja et al. 104 noted similar increases in PD1 in severe COVID-19 patients.
Figure 2.
Expression of activation markers on CD4+ and CD8+ T cells during acute COVID-19 infection. (a) OX40 expression on CD4+ T cells and (b) CD69 expression on CD8+ T cells from healthy, pre-pandemic controls (“CoV-”), hospitalized COVID-19-infected samples (“H”), and non-hospitalized COVID-19-infected samples (“NH”). All samples collected during acute infection and early convalescent time points. (c) OX40 expression on CD4+ T cells and (d) CD69 expression on CD8+ T cells from longitudinal non-hospitalized patient samples.
Visit number is shown on the x-axis and is represented by “1” and “2” for “Visit 1” and “Visit 2,” respectively. All data modified from Files et al. 31
***p < 0.001; significance determined by unpaired Wilcoxon tests (a and b) or paired Wilcoxon tests (c and d).
COVID-19, corona virus 2019.
B-cell and antibody immune responses during acute illness
We also explored differences in the B-cell and humoral responses between hospitalized and non-hospitalized patients. Notably, we found increased expression of activation markers CD69 and CD95 on B cells from hospitalized patients compared to both non-hospitalized patients and uninfected controls. 31 Other groups noted similar findings of increased expression of activation markers, as well as decreases in total B cells.95,102,106–108 Mathew et al. 95 observed an increase in CD27+CD38+ plasmablasts, comprising >30% of circulating B cells in some patients. Similarly, Carsetti et al. 102 observed an increase in plasmablasts in relation to more severe illness. They also noted that symptomatic patients, regardless of severity, had increased antibody production, and that this production seemed to persist into early convalescence. 102 This increase in antibody production and expansion of plasmablasts aligns with the hyperactivation seen in severe illness. This may be in part due to an initial delay in effective adaptive immune responses, leading to immune hyperactivation and perhaps contributing to viral persistence.
While these data evaluated the humoral responses of COVID-19-infected donors obtained prior to COVID-19 vaccination efforts, more recent studies have investigated SARS-CoV-2-specific humoral responses in vaccinated and unvaccinated individuals. Vaccination leads to an overall lower rate of infection,109–112 although breakthrough infection is associated with a weaker humoral response against variants such as Delta and Omicron.113–117 In addition, symptomatic breakthrough infection was associated with higher antibody levels than asymptomatic infection with mainly the Delta variant. 118 However, increased antibody responses were also found in vaccinated individuals with no infection vs unvaccinated individuals with Delta variant infection. 119 These data indicate that humoral immunity should continue to be assessed against the various SARS-CoV-2 variants, as well as how newer vaccination booster products impact the immune response to circulating and future strains.
Prolonged immune responses during convalescence
Another area of active inquiry has been the longitudinal impact of SARS-CoV-2 infection on T-cell activation following initial infection. Using the same protocol as described above and in Files et al., 31 we observed that many non-hospitalized patients exhibited sustained activation into the convalescent period. In longitudinal samples collected from two visit timepoints in non-hospitalized patients, we measured activation and exhaustion markers into early convalescence. Notably, all samples were collected within the first 40-day post-symptom onset. We found evidence that immune dysregulation persisted regardless of initial symptom severity as shown by the increased OX40 expression on CD4+ T cells and increased CD69 expression on CD8+ T cells at the later visit 2 timepoint (Figure 2(c) and (d)). 31 We observed a similar phenomenon with the activation marker HLA-DR and exhaustion markers TIM3 and TIGIT expression on T cells. Other groups have reported persistent adaptive immune system dysregulation into convalescence, notably changes in cell populations toward a more inflammatory, reactive state with increases in pro-inflammatory cytokines from various cells, and decreases in naïve T-cell and B-cell populations.120–127 Multiple hypotheses could explain these findings including the inadequate dampening of the acute inflammatory response to SARS-CoV-2 infection, or a failure to eliminate viral antigen. This continual shift of T-cell populations toward activation states could suggest continual exposure to antigen due to failure to clear the virus. Persistent exposure to antigen leads to the immune system continuously activating various immune cells in response to active infection, thus leading to an increase in activated cell populations. Other viruses associated with persistent antigen such as cytomegalovirus (CMV) and HIV also demonstrate a shift toward effector and terminally differentiated cell populations. CMV is associated with a long-term increase in both memory and effector-memory cells which has been linked to immunosenescence.128–130 HIV is associated with an increase in effector cells during acute infection, and an increase in effector and exhausted cells in chronic infection, with and without antiretroviral therapy.131–138
There are reports of persistent SARS-CoV-2-specific humoral and cellular responses over 8-month post-infection, regardless of initial severity, demonstrating that the body sustains longitudinal T- and B-cell immunity to SARS-CoV-2.120,121,124 Specifically, one group observed elevated SARS-CoV-2-specific CD4+ T-cell responses in COVID-19 patients, regardless of acute disease severity. 104 Our group and others found SARS-CoV-2-specific cTfh responses correlate with antibody neutralization.139–142 cTfh are a specific subset of CD4+ T cells that support humoral responses such as the development of neutralizing antibodies.143–150 Previous work on memory responses to seasonal and pandemic coronaviruses demonstrated durable memory T-cell responses to SARS-CoV-1 lasting as long as 17-year post-infection and to SARS-CoV-2 over 1-year post-infection. 151 In addition, other studies have shown cross-reactive antibodies to SARS-CoV-1 and seasonal coronaviruses in SARS-CoV-2 convalescent patients, as well as in SARS-CoV-2-uninfected controls, demonstrating conserved epitopes between coronaviruses and further suggesting long-lasting immunity.152,153 One potential mechanism for antigen persistence is cross-reactive responses from prior coronavirus infections due to a lack of naïve lymphocytes, resulting in suboptimal adaptive responses to SARS-CoV-2. Based on these observations, we hypothesized that viral reservoirs may contribute to the persistent immune activation and dysregulation observed in early convalescence and could provide the basis for a subset of long COVID presentations.
An early observation from the COVID-19 pandemic was the phenomenon of persistently positive PCR-based testing, particularly when sampling the lower airway. Although this was often a consequence residual viral proteins and high-sensitivity PCR-based testing platforms, recovery of replication competent virus was observed, particularly in immunosuppressed hosts. 154 Several groups have observed prolonged viral shedding in patients who recovered from SARS-CoV-2 infection with resolution of symptoms.155–159 Persistent SARS-CoV-2 RNAemia has been associated with severe illness.160,161 Similarly, Cai et al. 83 noted that slower viral clearance was associated with progression to critical illness, suggesting persistent virus contributes to prolonged immune activation, inflammation, and ultimately tissue damage. The recognition of post-acute sequelae syndromes raises the possibility that delays in eliminating viral proteins may contribute to persistent symptoms.
Long COVID
Clinical presentation and risk factors
While many individuals recover fully from acute SARS-CoV-2 infection, a portion of individuals develop persistent symptoms that can last for weeks or months now referred to as long COVID or PASC. Risk factors for long COVID development include severe acute illness, older age, female sex, lack of vaccination, and various comorbidities.162–167 Long COVID has a wide variety of manifestations including cardiopulmonary complications, persistent fatigue, and neurocognitive dysfunction, among a diverse array of persistent syndromes.164–166,168–171 Many early characterizations of long COVID reported varying symptom durations following initial diagnosis, ranging from 4 weeks to 4 months. The heterogeneous nature of long COVID and inconsistencies in defining symptom duration for diagnosis have complicated initial efforts to estimate overall prevalence. Prior to a broadly accepted definition, early reports stated long COVID prevalence as low as 9% and as high as 81%. 172 The World Health Organization has since defined long COVID as new or persistent symptoms lasting for at least 2 months and occurring at least 3-month post-infection. 10 Recent studies have reported long COVID prevalence to be 10%–20%.10,11 These data were largely published using cohorts from early variants. Thus, the frequency of severe disease, a long COVID risk factor, was greater than what is being observed in subsequent waves.44,63–68,173 Similarly, population immunity was minimal during early phases of COVID-19, but through a patchwork of vaccination and repeated natural infection, that is no longer the case. More recent reports observed that, despite SARS-CoV-2 overall causing less severe disease, presentations consistent with long COVID continue to arise, albeit at lower frequencies than prior variants.63,64,174 Data regarding long COVID risk after vaccination are underexplored, though a recent report observed that fully vaccinated patients with breakthrough infection had only a slightly reduced risk compared to unvaccinated individuals (HR = 0.85 (95% CI 0.82, 0.89)). 162 Although the massive burden of long COVID is now appreciated, the mechanisms and associated immune responses driving the clinical sequelae of long COVID have been less characterized.
Adaptive immunity during long COVID
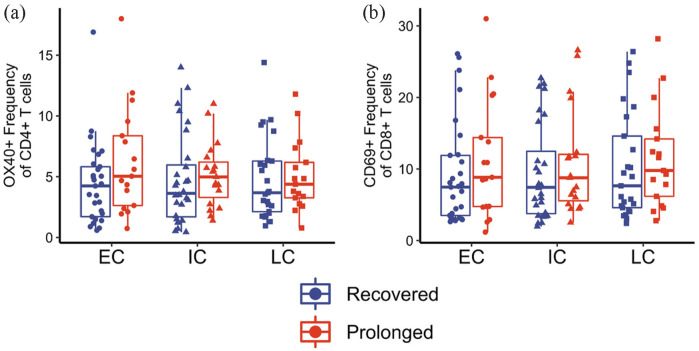
To explore potential immunologic signatures of long COVID, our group evaluated immune responses in a cohort of 50 patients infected with COVID-19 during the year 2020, meaning all samples were obtained prior to vaccination. A detailed overview of the protocols and the cohort used is given in Files et al. 31 In brief, longitudinal samples were collected from patients at three differing timepoints: (1) early convalescence (75 or less days from initial symptom onset), (2) intermediate convalescence (76–150 days from symptom onset), and (3) late convalescence (151+ days from symptom onset). Of note, 20 of these patients had persistent symptoms for at least 4-week post-infection, thus meeting the criteria for long COVID. The remaining 30 individuals experienced symptom resolution within 14 days of initial symptom onset. 32 All data collected were in accordance with the University of Alabama at Birmingham’s Institutional Review Board. We hypothesized that individuals with long COVID would exhibit persistent immune dysregulation that our group and others had observed in convalescent COVID-19 samples. However, our findings showed minimal systemic differences between recovered and long COVID groups in overall immune dysregulation between early, intermediate, or late convalescent timepoints. Using assays similar to our previous findings, isolated PMBCs from each timepoint were stained with various immune markers and analyzed on a BD FACS Symphony flow cytometer. Representative data showing OX40 expression on CD4+ T cells and CD69 expression on CD8+ T cells between the two groups are shown in Figure 3(a) and (b), respectively. No significant differences were identified between patients with prolonged symptoms (long COVID) or recovered infection. Additional T-cell activation and exhaustion markers were also investigated, including HLA-DR, CD38, CD154, PD1, TIGIT, and TIM3, as well as other immune cell populations including B cells and monocytes, but no statistically significant differences were observed.
Figure 3.
Expression of activation markers on CD4+ and CD8+ T cells during long COVID. (a) OX40 expression on CD4+ T cells and (b) CD69 expression on CD8+ T cells from patients with prolonged symptoms (long COVID; red) and recovered COVID-19 samples (dark blue).
Samples collected at early convalescent (“EC”), intermediate convalescent (“IC”), or late convalescent (“LC”) timepoints. All data modified from Files et al. 31
***p < 0.001; significance determined by unpaired Wilcoxon tests.
In this study, our group also investigated SARS-CoV-2-specific T-cell and antibody responses to the spike protein. More detailed protocol can be obtained in Files et al. 31 In brief, prepared peptides covering the whole length of the SARS-CoV-2 spike protein were prepared. PBMCs from each sample at each timepoint were stimulated with spike protein peptides for 18 h. Samples were then stained with various immunological markers including OX40, PDL1, CD69, and CD137.175,176 Samples were analyzed on a BD FACS Symphony flow cytometer. In agreement with numerous other studies at the time, we observed persistent SARS-CoV-2-specific T-cell activation throughout our cohort as far as 6 month post-infection.41,121,177–179 Many of these studies, including Dan et al., 121 demonstrated a decay in the T-cell response magnitude over time. We observed a similar decrease in CD4+ T-cell response magnitude in individuals from our recovered group (Figure 4(a) and (b); shown in blue). In contrast, those experiencing long COVID demonstrated a sustained SARS-CoV-2-specific CD4+ T-cell response magnitude over time (Figure 4(a) and (b); shown in red). The differences in antigen-specific T-cell responses were statistically significant for response magnitudes at the late convalescent timepoint. Overall, these observations suggest patients with long COVID have increased SARS-CoV-2-specific CD4+ and CD8+ T-cell magnitude responses during late convalescence as compared to those who recovered quickly and without persistent symptoms. Several studies have subsequently shown similar findings in patients with long COVID at various timepoints.35,41,121,177,179–188 Galan et al. 180 observed increased levels of functional memory CD8+ T cells, increased CD4+ regulatory T cells, and increased expression of PD1 on T cells in long COVID patients 7-month post-infection. Similarly, Peluso et al. 177 noted increased SARS-CoV-2-specific adaptive immune responses in long COVID patients, regardless of their initial disease severity, as far as 9-month post-infection. In addition, Phetsouphanh et al. 183 noted similar findings of SARS-CoV-2-specific adaptive immune responses as well as a lack a naïve T and B cells in long COVID patients at 8-month post-infection. Taken together, these data demonstrate that antigen-specific responses are sustained in long COVID patients at levels significantly higher than individuals with prompt resolution of symptoms. This observation raises the possibility that persistent viral reservoirs are maintained and drive the persistence of SARS-CoV-2 specific responses seen in long COVID patients.
Figure 4.
Antigen-specific CD4+ T-cell responses are sustained into late convalescence in long COVID-19 patients. (a) OX40+PDL1+ expression on CD4+ T cells following stimulation with S-protein peptide pool from patients with prolonged symptoms (long COVID; red) and recovered COVID-19 samples (dark blue). Samples collected at early convalescent (“EC”), intermediate convalescent (“IC”), or late convalescent (“LC”) timepoints. (b) OX40+PDL1+ expression on CD4+ T cells following stimulation with S-protein peptide pool shown as days post-symptom onset.
All data modified from Files et al. 31
**p < 0.01 and *p < 0.05; significance determined by unpaired Wilcoxon tests (a) and by linear mixed effects model (b).
Localized immune responses during long COVID
One limitation of our study was that it exclusively investigated immune signatures from peripheral blood samples. Other groups have defined tissue-specific immunopathology and shown similar findings of persistent immune activation and inflammation.105,178,182,183,189–193 Several have now observed persistent immune activation and elevation of proteins associated with apoptosis, tissue repair, and epithelial injury in the lungs,105,193–202 while others have observed persistent immune activation and inflammation in the central nervous system (CNS) of patients with persistent neurocognitive symptoms.203–206 Apple et al. 206 proposed that persistent immune activation and inflammation in the CNS may contribute to neurocognitive manifestations of long COVID after observing elevated inflammatory markers in the CSF from patients with long COVID neurocognitive symptoms. In line with these organ-specific findings, SARS-CoV-2 antigen has been recovered from multiple body sites including the gastrointestinal tract, hepatic tissues, the brain, lymphoid tissues, and in circulation up to 7-month post-infection34,40,207,208 Some groups proposed that, much like other chronic infections, persistent antigen exposure leads to chronic immune activation and subsequent systemic and compartmentalized inflammation, contributing to the wide array of clinical manifestations seen in long COVID.105,165,177,178,181,183,189–193,209–212
Post-acute sequalae due to antigen persistence
Antigen persistence and subsequent clinical sequelae post-infection are not novel to SARS-CoV-2 as infectious causes of chronic autoimmune and inflammatory disorders have been explored for decades.12,213–217 Chronic infections such as HIV, CMV, and EBV are associated with viral latency and persistence, leading to chronic exposure to viral antigens and subsequent immune activation and inflammation, contributing to the various sequelae of these infections. For example, HIV is known to cause accelerated aging due to chronic immune activation leading to early senescence and exhaustion of various immune cell populations.136–138,218–220 This persistent inflammation contributes to earlier development of a variety of comorbidities such as cardiometabolic disorders and neurocognitive dysfunction.136,219,221,222 EBV and CMV are two other latent viruses known to become reactivated in immunocompromised hosts and contribute to continued damage and inflammation throughout the body. 223 Latent viral infections are associated with continual, low-level viremia that can lead to reactivation and subsequent symptoms. This low-level, often compartmentalized viremia can continuously activate the immune system, contributing to negative effects later in life such as increased risk of comorbidities and early senescence and exhaustion of various immune cell populations. Other viruses such as influenza, Ebola, Coxsackie, and now SARS-CoV-2 have been associated with post-acute sequelae, and some have demonstrated antigen persistence.12,224–232
For example, prolonged viral shedding has been observed after Ebola and West Nile virus infection for several years after acute infection.224–227 Similarly, several papers have provided support for the presence of viral reservoirs for SARS-CoV-2.33,34,36–42,207,208 Recent reports have observed persistent RNAemia and circulating SARS-CoV-2 spike in individuals with long COVID.38,40 Others have observed tissue- and organ-specific SARS-CoV-2 RNA and antigen persistence. Chertow et al. 207 performed autopsies on COVID-19 patients and reported persistent SARS-CoV-2 RNA in a wide array of anatomical locations including the respiratory tract, gastrointestinal tract, cardiovascular tissue, reproductive tissue, peripheral nerves, and several brain regions, almost 8-month post-infection, although this study is biased toward more severe acute disease. In addition, others have demonstrated associations between persistent antigenemia and long COVID manifestations, including in the olfactory bulb perhaps explaining persistent anosmia.233,234 Zollner et al. 235 reported SARS-CoV-2 antigen in the gastrointestinal tract of patients who were later diagnosed with irritable bowel disease post-SARS-CoV-2 infection.
Long COVID mechanisms and current evidence
Collectively, these findings strongly suggest that persistent SARS-CoV-2 antigen is a common feature in individuals recovering from COVID-19 infection and plays a role in the pathogenesis of long COVID. As suggested in Figure 5, this persistent viral antigen could explain the ongoing symptoms, persistent inflammation, and the sustained T-cell response magnitude observed in long COVID patients. However, this observation does not exclude a variety of other mechanisms that may also contribute to long COVID symptomatology. While our study focused on immune cell populations, other groups measured various plasma inflammatory markers in patients experiencing long COVID and found marked elevations that could contribute to systemic symptoms such as fever and fatigue.105,182,183,190,209,210,212,236 As discussed earlier, acute disease severity strongly correlates with likelihood and burden of long COVID symptoms.162–167 Symptoms such as dyspnea, anxiety, and frailty commonly occur during severe disease and are at least partially due to end organ damage and fibrosis that commonly occurs during various critical illnesses.237–244 A few groups have shown evidence of EBV reactivation in patients with long COVID35,39,245; this could explain persistent fatigue and other long COVID symptoms. Others have shown support for gut dysbiosis, showing perturbations in the microbiome over 6-month post-infection, which may also explain a variety of gastrointestinal-related symptoms.246–249 Also, a variety of reports have identified signatures of autoimmune responses from patients experiencing long COVID including the evidence of autoantibodies in long COVID patients, whereas others have not.35,37,39,250,251 The above processes are not mutually exclusive, resulting in a variety of complex overlapping symptomatic presentations all falling under the umbrella of long COVID. In summary, these findings lend credence to the numerous proposed mechanisms behind long COVID including persistent viral reservoirs, systemic and tissue-specific inflammation, latent virus reactivation, gut dysbiosis, and autoimmunity.33,35,37,164,189,250–253 While our data and others have shown support for persistent viral antigen and sustained immune activation and inflammation, there is also support for other proposed mechanisms. The wide array of clinical manifestations of long COVID suggests that there are likely multiple mechanisms driving pathogenesis, which contributes to the complexities of determining the biological basis of the condition. Future investigation is needed to further identify and describe these pathologic mechanisms of long COVID to identify novel therapeutics that may be beneficial in treating patients with prolonged symptoms.
Figure 5.
Proposed mechanism for how viral reservoirs and dysregulated immune responses contribute to the development of long COVID.
Persistent antigen contributes to cell population changes such as a decrease in naïve T and B cells and an increase in effector cells, as well as increased expression of activation and exhaustion markers as the immune system attempts to regulate this chronic activation state. We propose that viral reservoirs contribute to this dysregulation and lead to both localized and systemic inflammation depending on where antigen is located. Several groups have demonstrated antigen persistence in a variety of tissues (e.g., brain, lungs, heart, adipose, gastrointestinal tract) in SARS-CoV-2-infected patients both acutely and chronically. In addition, several groups have demonstrated tissue-specific immune responses and inflammation in relation to corresponding organ-specific symptoms.
COVID, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Conclusions
Since the beginning of the COVID-19 pandemic, a large numbers of studies have been performed investigating the immunopathology following infection with SARS-CoV-2. During acute SARS-CoV-2 infection, delayed viral clearance contributes to hyperactivation and worsening of symptoms, leading to hospitalization and mortality. Indeed, our group and others observed increased expression of activation and exhaustion markers on various immune cell populations and increased systemic inflammatory cytokines that persists into convalescence, suggesting sustained immune activation (Figure 1). We also observed sustained SARS-CoV-2-specific T-cell response magnitudes in long COVID patients into late convalescence. Other groups showed similar findings of SARS-CoV-2-specific lymphocytes over 1-year post-infection. We proposed that persistent viral reservoirs could contribute to the immune dysregulation seen in early convalescence and explain the persistent SARS-CoV-2 specific immune responses observed in long COVID patients during late convalescence. This persistent activation could contribute to the localized and systemic inflammation observed in long COVID which could manifest as various sequelae depending on where the persistent antigen is located. Indeed, several groups have demonstrated antigen persistence in a variety of tissues in SARS-CoV-2-infected patients (Figure 5). Further research on determining if certain immunologic or inflammatory signatures correlate with specific long COVID manifestations should be performed, as well as how various prevention and treatment options such as vaccination and monoclonal antibodies influence the development of long COVID.
Footnotes
Author contributions: SO primarily wrote the paper, receiving assistance from JKF and NE on manuscript text, and assistance from NE and TF on figure conceptualization and design. SO drafted Figures 1 and 5. JKF drafted Figures 2–4. All authors provided edits and feedback on the initial draft. SO, JKF, and NE provided final edits on the manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Author NE—intellectual property (IP), “Human neutralizing antibodies against SARS-CoV-2/COVID-19” licensed to the Plantform Corp.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Author NE has received grant support from the National Institutes of Health (K08AI129705).
References
- 1.Dong E, Du H, Gardner L.An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20: 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrenfeldt LJ, Nielsen CR, Möller S, et al. Burden and prevalence of risk factors for severe COVID-19 disease in the ageing European population–A SHARE-based analysis. Z Gesundh Wiss 2022; 30: 2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anesi GL, Halpern SD, Delgado MK.Covid-19 related hospital admissions in the United States: needs and outcomes. BMJ 2020; 369: m2082. [DOI] [PubMed] [Google Scholar]
- 4.Bohn MK, Hall A, Sepiashvili L, et al. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda) 2020; 35: 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaeuffer C, Le Hyaric C, Fabacher T, et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill 2020; 25: 2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPadden J, Warner F, Young HP, et al. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. PLoS One 2021; 16: e0243291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendiola-Pastrana IR, Lopez-Ortiz E, Rio de la Loza-Zamora JG, et al. SARS-CoV-2 variants and clinical outcomes: a systematic review. Life (Basel) 2022; 12: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Coronavirus disease (covid-19): Post covid-19 condition, 2021. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed 22 September 2022).
- 11.Centers for Disease Control and Prevention. Nearly one in five American adults who have had COVID-19 still have “long covid”, 2022. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm (accessed 22 September 2022).
- 12.Choutka J, Jansari V, Hornig M, et al. Unexplained post-acute infection syndromes. Nat Med 2022; 28: 911–923. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Li W, Farzan M, et al. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005; 309: 1864–1868. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Shi X, Jiang L, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res 2013; 23: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000; 87: E1–E9. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004; 203: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MY, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020; 24: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salamanna F, Maglio M, Landini MP, et al. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med (Lausanne) 2020; 7: 594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000; 275: 33238–33243. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Li HB, Lyu JR, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis 2020; 96: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020; 14: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiris JS, Yuen KY, Osterhaus AD, et al. The severe acute respiratory syndrome. N Engl J Med 2003; 349: 2431–2441. [DOI] [PubMed] [Google Scholar]
- 27.Zumla A, Hui DS, Perlman S.Middle east respiratory syndrome. Lancet 2015; 386: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis 2020; 20: e238–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and middle east respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52: jrm00063. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Shin HS, Park HY, et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors. Psychiatry Investig 2019; 16: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Files JK, Boppana S, Perez MD, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest 2021; 131: e140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Files JK, Sarkar S, Fram TR, et al. Duration of post-COVID-19 symptoms is associated with sustained SARS-CoV-2-specific immune responses. JCI Insight 2021; 6: e151544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022; 54: 1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung CCL, Goh D, Lim X, et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022; 71: 226–229. [DOI] [PubMed] [Google Scholar]
- 35.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SK, Lee CW, Park DI, et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin Gastroenterol Hepatol 2021; 19: 1387.e2–1394.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proal AD, VanElzakker MB.Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021; 12: 698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ram-Mohan N, Kim D, Rogers AJ, et al. Association between SARS-CoV-2 RNAemia and post-acute sequelae of COVID-19. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022; 185: 881.e20–895.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis 2023; 76: e487–e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 2021; 64: 103230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson BK, Francisco EB, Yogendra R, et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol 2021; 12: 746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merad M, Blish CA, Sallusto F, et al. The immunology and immunopathology of COVID-19. Science 2022; 375: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 44.Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022; 399: 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenforde MW, Billig Rose E, Lindsell CJ, et al. Characteristics of adult outpatients and inpatients with COVID-19 - 11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu C, Lei Q, Li W, et al. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China: a single-center experience. J Infect Public Health 2020; 13: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andeweg SP, Vennema H, Veldhuijzen I, et al. Elevated risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals. Sci Transl Med 2023; 15: eabn4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blanca D, Nicolosi S, Bandera A, et al. Comparison between the first and second COVID-19 waves in internal medicine wards in Milan, Italy: a retrospective observational study. Intern Emerg Med 2022; 17: 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutta A.COVID-19 waves: variant dynamics and control. Sci Rep 2022; 12: 9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Shabasy RM, Nayel MA, Taher MM, et al. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol 2022; 204: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol 2022; 7: eabo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu T, Zhang M, Deng A, et al. Comparison of omicron and delta variant infection COVID-19 cases - guangdong province, China, 2022. China CDC Wkly 2022; 4: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klaser K, Molteni E, Graham M, et al. COVID-19 due to the B.1.617.2 (Delta) variant compared to B.1.1.7 (Alpha) variant of SARS-CoV-2: a prospective observational cohort study. Sci Rep 2022; 12: 10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mileto D, Micheli V, Fenizia C, et al. Reduced neutralization of SARS-CoV-2 omicron variant by BNT162b2 vaccinees’ sera: a preliminary evaluation. Emerg Microbes Infect 2022; 11: 790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siddiqui S, Alhamdi HWS, Alghamdi HA.Recent chronology of COVID-19 pandemic. Front Public Health 2022; 10: 778037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vo GV, Bagyinszky E, An SSA. COVID-19 genetic variants and their potential impact in vaccine development. Microorganisms 2022; 10: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022; 399: 2263–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bager P, Wohlfahrt J, Bhatt S, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis 2022; 22: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid-19 severity with omicron variant in South Africa. N Engl J Med 2022; 386: 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022; 399: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glenet M, Lebreil AL, Heng L, et al. Asymptomatic COVID-19 adult outpatients identified as significant viable SARS-CoV-2 shedders. Sci Rep 2021; 11: 20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the republic of Korea. JAMA Intern Med 2020; 180: 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyamae Y, Hayashi T, Yonezawa H, et al. Duration of viral shedding in asymptomatic or mild cases of novel coronavirus disease 2019 (COVID-19) from a cruise ship: a single-hospital experience in Tokyo, Japan. Int J Infect Dis 2020; 97: 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murata T, Sakurai A, Suzuki M, et al. Shedding of viable virus in asymptomatic SARS-CoV-2 carriers. mSphere 2021; 6: e00019–e00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saurabh S, Kumar R, Gupta MK, et al. Prolonged persistence of SARS-CoV-2 in the upper respiratory tract of asymptomatic infected individuals. QJM 2020; 113: 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen YT, Shao SC, Hsu CK, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care 2020; 24: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long B, Brady WJ, Koyfman A, et al. Cardiovascular complications in COVID-19. Am J Emerg Med 2020; 38: 1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi K, Zeng W, Ye M, et al. Clinical, laboratory, and imaging features of pediatric COVID-19: a systematic review and meta-analysis. Medicine (Baltimore) 2021; 100: e25230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036.e9–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021; 184: 4953.e16–4968.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilk AJ, Rustagi A, Zhao NQ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020; 26: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020; 75: 1742–1752. [DOI] [PubMed] [Google Scholar]
- 84.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020; 92: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146: 128.e4–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8: 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020; 55: 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med 2020; 12: e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan M, Liu Y, Zhou R, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020; 160: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol 2020; 146: 119.e4–127.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci 2020; 7: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020; 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020; 369: eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al Balushi A, AlShekaili J, Al Kindi M, et al. Immunological predictors of disease severity in patients with COVID-19. Int J Infect Dis 2021; 110: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song JW, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 2020; 11: 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei LL, Wang WJ, Chen DX, et al. Dysregulation of the immune response affects the outcome of critical COVID-19 patients. J Med Virol 2020; 92: 2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Youngs J, Provine NM, Lim N, et al. Identification of immune correlates of fatal outcomes in critically ill COVID-19 patients. PLoS Pathog 2021; 17: e1009804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 2020; 17: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17: 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carsetti R, Zaffina S, Piano Mortari E, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol 2020; 11: 610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzoni A, Maggi L, Capone M, et al. Cell-mediated and humoral adaptive immune responses to SARS-CoV-2 are lower in asymptomatic than symptomatic COVID-19 patients. Eur J Immunol 2020; 50: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 104.Oja AE, Saris A, Ghandour CA, et al. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur J Immunol 2020; 50: 1998–2012. [DOI] [PubMed] [Google Scholar]
- 105.Grau-Exposito J, Sanchez-Gaona N, Massana N, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun 2021; 12: 3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oliviero B, Varchetta S, Mele D, et al. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol Immunol 2020; 17: 1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pusnik J, Richter E, Schulte B, et al. Memory B cells targeting SARS-CoV-2 spike protein and their dependence on CD4(+) T cell help. Cell Rep 2021; 35: 109320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shuwa HA, Shaw TN, Knight SB, et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y) 2021; 2: 720.e4–735.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med 2022; 386: 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med 2022; 386: 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harris RJ, Hall JA, Zaidi A, et al. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med 2021; 385: 759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med 2021; 385: 1718–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calcoen B, Callewaert N, Vandenbulcke A, et al. High incidence of SARS-CoV-2 variant of concern breakthrough infections despite residual humoral and cellular immunity induced by BNT162b2 vaccination in healthcare workers: a long-term follow-up study in Belgium. Viruses 2022; 14: 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Favresse J, Dogne JM, Douxfils J.Assessment of the humoral response in Omicron breakthrough cases in healthcare workers who received the BNT162b2 booster. Clin Chem Lab Med 2022; 60: e153–e156. [DOI] [PubMed] [Google Scholar]
- 115.Mohlendick B, Ciuciulkaite I, Elsner C, et al. Individuals with weaker antibody responses after booster immunization are prone to omicron breakthrough infections. Front Immunol 2022; 13: 907343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Staerke NB, Reekie J, Nielsen H, et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat Commun 2022; 13: 4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tay MZ, Rouers A, Fong SW, et al. Decreased memory B cell frequencies in COVID-19 delta variant vaccine breakthrough infection. EMBO Mol Med 2022; 14: e15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang SL, Mat Ripen A, Leong CT, et al. COVID-19 breakthrough infections and humoral immune response among BNT162b2 vaccinated healthcare workers in Malaysia. Emerg Microbes Infect 2022; 11: 1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fernandez-Rivas G, Barallat J, Quirant-Sanchez B, et al. Follow up of the humoral response in healthcare workers after the administration of two dose of the anti SARS-CoV-2 vaccines-effectiveness in delta variant breakthrough infections. Viruses 2022; 14: 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breton G, Mendoza P, Hagglof T, et al. Persistent cellular immunity to SARS-CoV-2 Infection. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371: eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mudd PA, Remy KE.Prolonged adaptive immune activation in COVID-19: implications for maintenance of long-term immunity? J Clin Invest 2021; 131: e143928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orologas-Stavrou N, Politou M, Rousakis P, et al. Peripheral blood immune profiling of convalescent plasma donors reveals alterations in specific immune subpopulations even at 2 months post SARS-CoV-2 infection. Viruses 2020; 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sherina N, Piralla A, Du L, et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021; 2: 281.e4–295.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 2020; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun 2021; 12: 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Townsend L, Dyer AH, Naughton A, et al. Longitudinal analysis of COVID-19 patients shows age-associated T cell changes independent of ongoing Ill-health. Front Immunol 2021; 12: 676932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pardieck IN, Beyrend G, Redeker A, et al. Cytomegalovirus infection and progressive differentiation of effector-memory T cells. F1000Res 2018; 7: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pitard V, Roumanes D, Lafarge X, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008; 112: 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pourgheysari B, Khan N, Best D, et al. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007; 81: 7759–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aandahl EM, Quigley MF, Moretto WJ, et al. Expansion of CD7(low) and CD7(negative) CD8 T-cell effector subsets in HIV-1 infection: correlation with antigenic load and reversion by antiretroviral treatment. Blood 2004; 104: 3672–3678. [DOI] [PubMed] [Google Scholar]
- 132.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001; 410: 106–111. [DOI] [PubMed] [Google Scholar]
- 133.Paiardini M, Cervasi B, Albrecht H, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol 2005; 174: 2900–2909. [DOI] [PubMed] [Google Scholar]
- 134.Pastor L, Urrea V, Carrillo J, et al. Dynamics of CD4 and CD8 T-Cell subsets and inflammatory biomarkers during early and chronic HIV infection in mozambican adults. Front Immunol 2017; 8: 1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takata H, Kakazu JC, Mitchell JL, et al. Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8(+) T cells. EBioMedicine 2022; 84: 104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deeks SG.HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deeks SG, Verdin E, McCune JM.Immunosenescence and HIV. Curr Opin Immunol 2012; 24: 501–506. [DOI] [PubMed] [Google Scholar]
- 138.Grosso TM, Alcami J, Arribas JR, et al. HIV and aging, biological mechanisms, and therapies: what do we know? AIDS Rev 2022; 25: 79–86. [DOI] [PubMed] [Google Scholar]
- 139.Boppana S, Qin K, Files JK, et al. SARS-CoV-2-specific circulating T follicular helper cells correlate with neutralizing antibodies and increase during early convalescence. PLoS Pathog 2021; 17: e1009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gong F, Dai Y, Zheng T, et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J Clin Invest 2020; 130: 6588–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Juno JA, Tan HX, Lee WS, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med 2020; 26: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 142.Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 2020; 52: 971.e3–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013; 5: 176ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol 2011; 186: 5556–5568. [DOI] [PubMed] [Google Scholar]
- 145.Haltaufderhyde K, Srikiatkhachorn A, Green S, et al. Activation of peripheral T follicular helper cells during acute dengue virus infection. J Infect Dis 2018; 218: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013; 39: 770–781. [DOI] [PubMed] [Google Scholar]
- 147.Heit A, Schmitz F, Gerdts S, et al. Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J Exp Med 2017; 214: 2139–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013; 39: 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mikell I, Sather DN, Kalams SA, et al. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 2011; 7: e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sterrett S, Peng BJ, Burton RL, et al. Peripheral CD4 T follicular cells induced by a conjugated pneumococcal vaccine correlate with enhanced opsonophagocytic antibody responses in younger individuals. Vaccine 2020; 38: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
- 152.Grobben M, van der Straten K, Brouwer PJ, et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. Elife 2021; 10: e70330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 2020; 130: 5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383: 2586–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fotouhi F, Salehi-Vaziri M, Farahmand B, et al. Prolonged viral shedding and antibody persistence in patients with COVID-19. Microbes Infect 2021; 23: 104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Long H, Zhao J, Zeng HL, et al. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: a prospective study. BMC Infect Dis 2021; 21: 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou B, She J, Wang Y, et al. Duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis 2020; 71: 2240–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li N, Wang X, Lv T.Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol 2020; 92: 2286–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yan D, Zhang X, Chen C, et al. Characteristics of viral shedding time in SARS-CoV-2 Infections: a systematic review and meta-analysis. Front Public Health 2021; 9: 652842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically Ill patients with coronavirus disease 2019. Clin Infect Dis 2020; 71: 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bae S, Kim JY, Lim SY, et al. Dynamics of viral shedding and symptoms in patients with asymptomatic or mild COVID-19. Viruses 2021; 13: 2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Al-Aly Z, Bowe B, Xie Y.Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022; 28: 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Asadi-Pooya AA, Akbari A, Emami A, et al. Risk factors associated with long COVID syndrome: a retrospective study. Iran J Med Sci 2021; 46: 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Desai AD, Lavelle M, Boursiquot BC, et al. Long-term complications of COVID-19. Am J Physiol Cell Physiol 2022; 322: C1–C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Montani D, Savale L, Noel N, et al. Post-acute COVID-19 syndrome. Eur Respir Rev 2022; 31: 210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sneller MC, Liang CJ, Marques AR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med 2022; 175: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One 2021; 16: e0260568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324: 1723–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–1032. [DOI] [PubMed] [Google Scholar]
- 170.Mehandru S, Merad M.Pathological sequelae of long-haul COVID. Nat Immunol 2022; 23: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med 2020; 20: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022; 226: 1593–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jassat W, Abdool Karim SS, Mudara C, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health 2022; 10: e961–e969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bauer L, Rissmann M, Benavides FFW, et al. In vitro and in vivo differences in neurovirulence between D614G, delta and omicron BA.1 SARS-CoV-2 variants. Acta Neuropathol Commun 2022; 10: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dan JM, Lindestam Arlehamn CS, Weiskopf D, et al. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol 2016; 197: 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reiss S, Baxter AE, Cirelli KM, et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One 2017; 12: e0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 2021; 36: 109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poon MML, Rybkina K, Kato Y, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol 2021; 6: eabl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Visvabharathy L, Hanson B, Orban Z, et al. Neuro-COVID long-haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv. 2021. [Google Scholar]
- 180.Galan M, Vigon L, Fuertes D, et al. Persistent overactive cytotoxic immune response in a spanish cohort of individuals with long-COVID: identification of diagnostic biomarkers. Front Immunol 2022; 13: 848886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Newell KL, Waickman AT.Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection Immunity and inflammation in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol 2022; 77: 102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 224: 1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23: 210–216. [DOI] [PubMed] [Google Scholar]
- 184.Ryan FJ, Hope CM, Masavuli MG, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med 2022; 20: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020; 369: 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Filbin MR, Mehta A, Schneider AM, et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med 2021; 2: 100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gisby J, Clarke CL, Medjeral-Thomas N, et al. Longitudinal proteomic profiling of dialysis patients with COVID-19 reveals markers of severity and predictors of death. Elife 2021; 10: e64827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol 2021; 6: eabg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martinez-Colon GJ, Ratnasiri K, Chen H, et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci Transl Med 2022: 14: eabm9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cheon IS, Li C, Son YM, et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci Immunol 2021; 6: eabk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Montes-Ibarra M, Oliveira CLP, Orsso CE, et al. The impact of long COVID-19 on muscle health. Clin Geriatr Med 2022; 38: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vanderheiden A, Klein RS.Neuroinflammation and COVID-19. Curr Opin Neurobiol 2022; 76: 102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vijayakumar B, Boustani K, Ogger PP, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity 2022; 55: 542.e5–556 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chopra V, Flanders SA, O’Malley M, et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med 2021; 174: 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mendez R, Latorre A, Gonzalez-Jimenez P, et al. Reduced diffusion capacity in COVID-19 survivors. Ann Am Thorac Soc 2021; 18: 1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 2020; 55: 2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 2020; 80: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Heneka MT, Golenbock D, Latz E, et al. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther 2020; 12: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou L, Zhang M, Wang J, et al. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis 2020; 36: 101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Apple AC, Oddi A, Peluso MJ, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol 2022; 9: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Daniel C, Stein S, Ramelli S, et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Res Sq. Epub ahead of print 20 December 2021. DOI: 10.21203/rs.3.rs-1139035/v1. [DOI] [Google Scholar]
- 208.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schultheiss C, Willscher E, Paschold L, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med 2022; 3: 100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Busatto GF, de Araujo AL, Castaldelli-Maia JM, et al. Post-acute sequelae of SARS-CoV-2 infection: relationship of central nervous system manifestations with physical disability and systemic inflammation. Psychol Med 2022; 52: 2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chiang KC, Imig JD, Kalantar-Zadeh K, et al. Kidney in the net of acute and long-haul coronavirus disease 2019: a potential role for lipid mediators in causing renal injury and fibrosis. Curr Opin Nephrol Hypertens 2022; 31: 36–46. [DOI] [PubMed] [Google Scholar]
- 212.Maamar M, Artime A, Pariente E, et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin 2022; 38: 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Francis L, Perl A.Infection in systemic lupus erythematosus: friend or foe? Int J Clin Rheumtol 2010; 5: 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kivity S, Agmon-Levin N, Blank M, et al. Infections and autoimmunity–friends or foes? Trends Immunol 2009; 30: 409–414. [DOI] [PubMed] [Google Scholar]
- 215.Mawanda F, Wallace R.Can infections cause Alzheimer’s disease? Epidemiol Rev 2013; 35: 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Piekut T, Hurla M, Banaszek N, et al. Infectious agents and Alzheimer’s disease. J Integr Neurosci 2022; 21: 73. [DOI] [PubMed] [Google Scholar]
- 217.Soldan SS, Lieberman PM.Epstein-barr virus and multiple sclerosis. Nat Rev Microbiol 2022; 21: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nou E, Lo J, Grinspoon SK.Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30: 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Paiardini M, Muller-Trutwin M.HIV-associated chronic immune activation. Immunol Rev 2013; 254: 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Srinivasa S, Fitch KV, Petrow E, et al. Soluble CD163 is associated with shortened telomere length in HIV-infected patients. J Acquir Immune Defic Syndr 2014; 67: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maciel RA, Kluck HM, Durand M, et al. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis 2018; 70: 30–35. [DOI] [PubMed] [Google Scholar]
- 222.Peng X, Ouyang J, Isnard S, et al. Sharing CD4+ T cell loss: when COVID-19 and HIV collide on immune system. Front Immunol 2020; 11: 596631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.de Melo Silva J, Pinheiro-Silva R, Dhyani A, et al. Cytomegalovirus and epstein-barr infections: prevalence and impact on patients with hematological diseases. Biomed Res Int 2020; 2020: 1627824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fischer WA, Brown J, Wohl DA, et al. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute ebola virus infection. Open Forum Infect Dis 2017; 4: ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Keita AK, Vidal N, Toure A, et al. A 40-month follow-up of Ebola virus disease survivors in guinea (PostEbogui) reveals long-term detection of Ebola viral ribonucleic acid in semen and breast milk. Open Forum Infect Dis 2019; 6: ofz482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Murray K, Walker C, Herrington E, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis 2010; 201: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Group PIS, Sneller MC, Reilly C, et al. A longitudinal study of Ebola sequelae in liberia. N Engl J Med 2019; 380: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Carod-Artal FJ.Post-Ebolavirus disease syndrome: what do we know? Expert Rev Anti Infect Ther 2015; 13: 1185–1187. [DOI] [PubMed] [Google Scholar]
- 229.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006; 333: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Magnus P, Gunnes N, Tveito K, et al. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 2015; 33: 6173–6177. [DOI] [PubMed] [Google Scholar]
- 231.Morroy G, Keijmel SP, Delsing CE, et al. Fatigue following acute Q-fever: a systematic literature review. PLoS One 2016; 11: e0155884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rojas M, Monsalve DM, Pacheco Y, et al. Ebola virus disease: an emerging and re-emerging viral threat. J Autoimmun 2020; 106: 102375. [DOI] [PubMed] [Google Scholar]
- 233.de Melo GD, Lazarini F, Levallois S, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 2021; 13: eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kandemirli SG, Altundag A, Yildirim D, et al. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol 2021; 28: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zollner A, Koch R, Jukic A, et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology 2022; 163: 495.e8–506.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Group P-CC. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022; 10: 761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bevilacqua Filho CT, Schmidt AP, Felix EA, et al. Risk factors for postoperative pulmonary complications and prolonged hospital stay in pulmonary resection patients: a retrospective study. Braz J Anesthesiol 2021; 71: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bhattacharya B, Maung A, Barre K, et al. Postoperative delirium is associated with increased intensive care unit and hospital length of stays after liver transplantation. J Surg Res 2017; 207: 223–228. [DOI] [PubMed] [Google Scholar]
- 239.Elfstrom KM, Hatefi D, Kilgo PD, et al. What happens after discharge? An analysis of long-term survival in cardiac surgical patients requiring prolonged intensive care. J Card Surg 2012; 27: 13–19. [DOI] [PubMed] [Google Scholar]
- 240.Lai CC, Ho CH, Chen CM, et al. Long-term risk of dementia after acute respiratory failure requiring intensive care unit admission. PLoS One 2017; 12: e0180914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Livingston DH, Tripp T, Biggs C, et al. A fate worse than death? Long-term outcome of trauma patients admitted to the surgical intensive care unit. J Trauma 2009; 67: 341–348; discussion 348–349. [DOI] [PubMed] [Google Scholar]
- 242.Mahesh B, Choong CK, Goldsmith K, et al. Prolonged stay in intensive care unit is a powerful predictor of adverse outcomes after cardiac operations. Ann Thorac Surg 2012; 94: 109–116. [DOI] [PubMed] [Google Scholar]
- 243.Unda SR, Labagnara K, Birnbaum J, et al. Impact of hospital-acquired complications in long-term clinical outcomes after subarachnoid hemorrhage. Clin Neurol Neurosurg 2020; 194: 105945. [DOI] [PubMed] [Google Scholar]
- 244.Williams MR, Wellner RB, Hartnett EA, et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg 2002; 73: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 245.Gold JE, Okyay RA, Licht WE, et al. Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation. Pathogens 2021; 10: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alharbi KS, Singh Y, Hassan Almalki W, et al. Gut microbiota disruption in COVID-19 or post-COVID illness association with severity biomarkers: a possible role of pre / pro-biotics in manipulating microflora. Chem Biol Interact 2022; 358: 109898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022; 71: 544–552. [DOI] [PubMed] [Google Scholar]
- 248.Schult D, Reitmeier S, Koyumdzhieva P, et al. Gut bacterial dysbiosis and instability is associated with the onset of complications and mortality in COVID-19. Gut Microbes 2022; 14: 2031840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhou Y, Zhang J, Zhang D, et al. Linking the gut microbiota to persistent symptoms in survivors of COVID-19 after discharge. J Microbiol 2021; 59: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ 2021; 374: n1648. [DOI] [PubMed] [Google Scholar]
- 251.Yong SJ.Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021; 53: 737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Salmon-Ceron D, Slama D, De Broucker T, et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect 2021; 82: e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yeoh YK, Zuo T, Lui GC, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021; 70: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]