Abstract
The present study aimed to examine the clinical and pathogenic characteristics, diagnosis and treatment of primary canaliculitis to provide further guidance for its clinical management. The present prospective study enrolled 50 patients (50 eyes) diagnosed with primary canaliculitis between May 2018 and April 2021 at Department of Ophthalmology, Affiliated Wuxi Clinical College of Nantong University, Wuxi, China. The patients' general clinicopathological information, clinical characteristics, microbiological profiles and treatment outcomes were analyzed and summarized. All the patients presented with persistent red eyes and eye discharge. Examination of discharge smears revealed that 96% of patients tested positive for Actinomyces and all smears were negative for fungi. Microbial cultures indicated that 82% of cases were positive for bacteria. A total of 51 bacterial strains were cultured; of these, 27.5% were aerobes, 35.3% were anaerobes and 37.2% were facultative anaerobes. A total of 56.9% of strains were gram-positive and 43.1% were gram-negative. The three most common bacteria, including Streptococcus spp., Capnocytophaga spp. and Propionibacterium, were analyzed. Only 3 cases (6%) of microbial cultures were positive for Actinomyces and all cases were negative for fungi in microbial cultures. Among the 50 cases, 45 were cured with conservative treatment [intracanalicular ointment infiltration (IOI)]. Five patients responded poorly to conservative treatment; however, they were cured with surgical treatment. In the current study, the majority of canaliculitis cases were caused by mixed infections, predominantly Actinomyces. The results revealed that the culture positivity rate of Actinomyces was low; however, the smear staining positivity rate was high. Fungus was smear- and culture-negative in all cases. In conclusion, patients with canaliculitis had a good prognosis after timely diagnosis and treatment.
Keywords: primary canaliculitis, Actinomyces, anaerobic bacteria, gram-positive bacteria, intracanalicular ointment infiltration
Introduction
Lacrimal canaliculitis is an infection of the lacrimal canaliculus, which is the proximal part of the tear drainage system of the eye. It is a relatively rare condition and typically occurs in people aged >40. Although it is most commonly caused by a bacterial pathogen, it can also be caused by a fungal or viral infection (1,2). The infection may lead to the formation of small dacryoliths. Multiple dacryoliths can be present and obstruct the lacrimal outflow system with time; finally, the infection and obstruction of lacrimal canaliculus are combined to form a vicious circle. Lacrimal canaliculitis can be divided into primary canaliculitis and secondary canaliculitis according to the cause of the disease (3). Secondary canaliculitis is most commonly associated with punctal or intracanalicular plug placement, while there is often no history of surgery related to the lacrimal canaliculus in primary canaliculitis.
Primary canaliculitis is an uncommon disease, comprising 2% of all patients with lacrimal disease worldwide (4), typically manifesting as pouting erythematous punctum, punctal or canalicular swelling, and expressible punctal discharge. It is caused by various microorganisms, with Actinomyces infection reported to be the most common pathogen (5). However, owing to its rarity and lack of typical presentation in the clinics, primary canaliculitis is often misdiagnosed as chronic conjunctivitis, chronic dacryocystitis, chalazion or hordeolum (4,6-8), which causes a delay in effective treatment. Therefore, there is often a prolonged symptomatic period until diagnosis; this can have serious impacts on work and life and even lead to adverse psychological effects.
The present study aimed to summarize the pathogenic characteristics, diagnosis and treatment of patients with primary canaliculitis admitted to Affiliated Wuxi Clinical College of Nantong University (Wuxi, China) between May 2018 and April 2021. This information may serve as a basis for developing guidelines for the clinical diagnosis and treatment of canaliculitis. To the best of our knowledge, the current study is the first to report this information for the specific region analyzed.
Materials and methods
Participants
Patients diagnosed with primary canaliculitis according to the clinical manifestations and examinations (such as pouting erythematous punctum, punctal or canalicular swelling, as well as expressible punctal discharge) at the Department of Ophthalmology, Affiliated Wuxi Clinical College of Nantong University (Wuxi, China), between May 2018 and April 2021, were enrolled in the present prospective study (Table I). Patients with canaliculitis secondary to trauma or punctal and canalicular plugs were excluded. All patients underwent slit-lamp examination and lacrimal duct irrigation and exploration. Ultrasound biomicroscopy (UBM) (Quantel Medical, Cournon d'Auvergne Cedex, France) was performed with a 50 MHz probe as an ancillary examination only in patients with severe lacrimal duct dilatation. The protocol of the current prospective study was approved by the Institutional Review Board of the Affiliated Wuxi Clinical College of Nantong University (approval no. 2022-Y-98). Prior to inclusion, written informed consent was obtained from all patients. The present study adhered to the tenets of The Declaration of Helsinki.
Table I.
Patient clinicopathological characteristics (n=50).
| Characteristic | Number of patients, n (%) |
|---|---|
| Sex | |
| Male | 11(22) |
| Female | 39(78) |
| Age, years | |
| 38-63 | 27(54) |
| 63-87 | 23(46) |
| Lacrimal duct position | |
| Superior | 17(34) |
| Inferior | 29(58) |
| Superior + inferior | 4(8) |
| Eye position | |
| Left eye | 30(60) |
| Right eye | 20(40) |
Specimen collection and smear staining examination
Care was taken in specimen collection since this can directly impact the results of smears and cultures. Following the application of one drop of topical anesthesia (Oxybuprocaine Hydrochloride Eye Drops; Santen Pharmaceutical), the eyes were cleaned and disinfected. The affected lacrimal punctum was dilated. Thereafter, the mucopurulent discharge and concretions were manually expressed from the punctum using two sterile cotton buds to compress the affected canaliculus. Possibly contaminated discharge was removed from the anterior segment. A sterile spatula was subsequently used to obtain a discharge from the middle or posterior segment. The dacryoliths from discharge were crushed evenly and then smears were immediately prepared for Gram staining according to the manufacturer's protocols and microscopic examination under a fluorescence microscope (Olympus BX53; Olympus Corporation).
Bacterial culture and identification
The collected specimens (both mucopurulent discharge and dacryoliths) were used for bacterial, fungal and anaerobic cultures. Specimens for aerobic culture were inoculated onto Columbia blood agar plates, nutrient broth and chocolate agar plates without antibiotics (All of the above plates were purchased from Shanghai Comagal Microbial Technology Co., Ltd.). Plates were incubated for 24-48 h at 35˚C with 5% CO2. Subsequently, bacteria from culture-positive specimens were isolated to obtain single colonies. Specimens for anaerobic culture were plated into CDC blood agar plates (Qingdao Hi-Tech Industrial Park Hope Bio-Technology Co., Ltd.). The plates were placed in an AnaeroPack system (bioMérieux, Inc.) at 35˚C for 2-7 days. Subsequently, bacteria from culture-positive specimens were streaked for isolation on the same medium.
The VITEK® MS automated rapid mass spectrometry microbial identification system (bioMérieux, Inc.) was used to identify the isolated colonies. The analysis was performed according to the manufacturer's instructions.
Treatments
Initially, an intracanalicular ointment infiltration (IOI) treatment with an ophthalmic corticosteroid/antibiotic combination (tobramycin and dexamethasone eye ointment; TobraDex; Alcon) was adopted for all patients, which was a safe and non-invasive approach that was initially introduced by Xu et al (9). Specifically, the discharges and concretions were expressed thoroughly, followed by lacrimal duct irrigation with physiological saline. Subsequently, ~0.2 ml antibiotic/corticosteroid eye ointment was injected into the lacrimal duct weekly for 2-8 weeks and topical antibiotic eye drops were administered four times a day.
A total of five patients who responded poorly to IOI underwent routine surgical treatment. First, a silicone Crawford tube (Shandong Bausch & Lomb Freda Pharmaceutical Co., Ltd.,) was intubated into the lacrimal passage through the upper and lower lacrimal puncta under topical anesthesia. Using probe guidance, an incision was made on the canaliculus, parallel to the eyelid margin and at a distance of 2 mm from the punctum. The canalicular lumen was exposed to remove intracanalicular dacryoliths and hyperplastic granulation tissue. After that, the canalicular incision was closed with an 8-0 absorbable thread. Subsequently, the lacrimal duct was irrigated and injected with 0.2 ml antibiotic/corticosteroid eye ointment. Irrigation and injection with eye ointment were performed weekly for 2-8 weeks after surgery. In addition, the patients were treated with topical antibiotic eye drops four times daily. The silicone drainage tube remained in place for 3-6 months.
Follow-up
Follow-up was performed monthly for 3-12 months after the end of conservative treatment or for 3-12 months after silicone drainage tube removal in patients who underwent surgical treatment. During follow-up, conjunctival hyperemia, discharge, epiphora, canalicular swelling and lacrimal duct irrigation were observed.
Data analysis
Graphs were plotted using GraphPad Prism version 9 (GraphPad Software; Dotmatics). Count data are presented as frequency (%).
Results
Patient characteristics
A total of 50 patients diagnosed with primary canaliculitis were recruited to the present study. The age of the participants ranged from 38-87 years (average age, 63 years), and there were 11 males (22%) and 39 females (78%) (Table I). There were 17 cases (34%) of superior canaliculitis, 29 cases (58%) of inferior canaliculitis and 4 cases (8%) of superior and inferior canaliculitis. A total of 30 cases (60%) were affected on the left eye, whereas 20 cases (40%) were affected on the right eye. All patients had a history of chronic red eye and increased discharge, occasionally accompanied by epiphora (10%).
Clinical manifestations
Slit-lamp examination revealed conjunctival hyperemia. The inflamed lacrimal canaliculus was hyperemic and edematous. A yellowish-white mucopurulent discharge attached to the punctum was observed (Fig. 1). Upon lacrimal duct irrigation the discharges always regurgitated from the affected punctum; however, the inflamed lacrimal canaliculus was patent in most patients.
Figure 1.
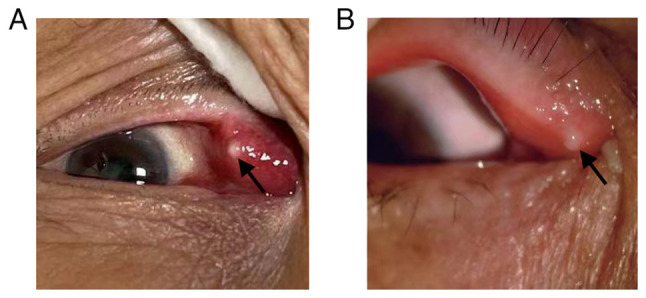
Slit-lamp examination of a patient with primary canaliculitis. (A) Lacrimal canaliculus was hyperemic and hypertrophic. (B) A yellowish-white mucopurulent discharge attached to the punctum was observed. Black arrows pointed to mucopurulent discharge on the lacrimal punctum.
Lacrimal duct exploration revealed a dilated canalicular lumen that was not smooth, narrowed or obstructed, with a palpable gritty feel on the canalicular wall. Canalicular compression produced a sulfurous, cottage cheese-like discharge, dacryoliths, which varied in color (Fig. 2). UBM revealed canalicular dilation in patients with several dacryoliths, with uneven, moderate-to-high intensity signals within the lumen (Fig. 3).
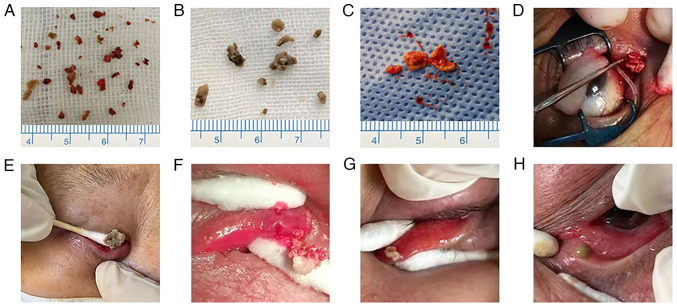
Figure 2.
Dacryoliths and mucopurulent discharges from 8 representative cases with primary canaliculitis. Various dacryoliths presents different shapes and colors (A-G). Panel A exhibits concretions and debris-like dacryoliths, panels B-E exhibit sulfurous-like dacryoliths, panel F exhibits sediment-like dacryoliths, and panel G exhibits cheese-like dacryoliths. Panel H exhibits mucopurulent secretions, which contains no dacryoliths.
Figure 3.
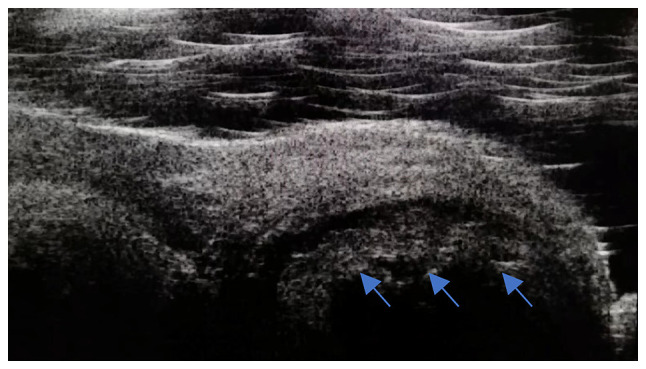
Ultrasound biomicroscopy showing canalicular dilation. The dacryoliths (blue arrows) exhibited uneven, moderate-to-high signals within the lumen.
Discharge smears
Among the 50 cases with canaliculitis, 48 showed dacryoliths in their discharge specimens. The dacryoliths were crushed to prepare smears for Gram staining and microscopic examination; all smears of dacryoliths revealed the presence of Actinomyces species (spp.) (Fig. 4). The discharge in the two cases without dacryoliths was negative for Actinomyces. All smears were also positive for cocci or bacilli, with more cases testing positive for cocci. None of the smears was positive for fungi.
Figure 4.
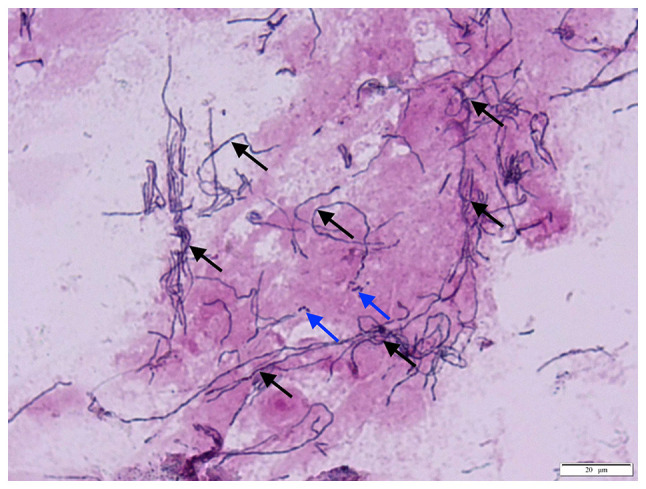
Smears revealing scattered Actinomyces species (black arrows) and cocci (blue arrows) in dacryoliths, after being crushed and smeared for Gram staining and microscopic examination (magnification, x1,000).
Culture
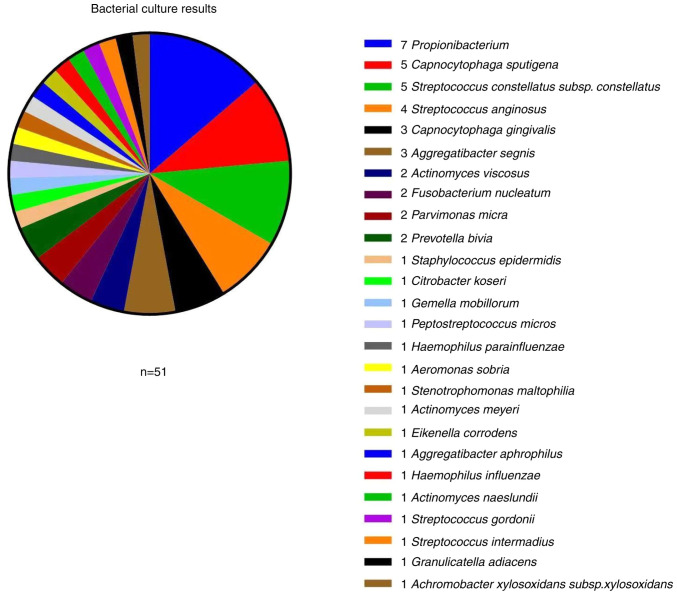
Bacterial, fungal and anaerobic cultures were prepared. Among the 50 cases with canaliculitis, 41 were positive for bacteria, with a culture positivity rate of 82%. A total of 51 relatively heterogeneous bacterial strains were identified in these cultures. The top three most frequently identified bacteria were Streptococcus spp. (11 strains), Capnocytophaga spp. (eight strains) and Propionibacterium (seven strains; (Fig. 5). Aerobes accounted for 27.5% (14/51), anaerobes for 35.3% (18/51) and facultative anaerobes for 37.2% (19/51) of the identified strains. Among the 51 strains, 56.9% (29/51) were gram-positive and 43.1% (22/51) were gram-negative (Table II). All patient cultures were negative for fungal infection.
Figure 5.
Bacterial culture results identify 51 relatively heterogeneous bacterial strains in eye mucopurulent discharge and dacryoths cultures. The top three most frequently identified bacteria were Streptococcus spp. (11 strains), Capnocytophaga spp. (eight strains) and Propionibacterium (seven strains). Spp., species.
Table II.
Distribution of the 51 strains isolated from bacterial cultures.
| A, Aerobes (n=14) | |
|---|---|
| Strain | Total, n (%) |
| Gram-positive bacteria | |
| Streptococcus anginosus | 4 (7.84) |
| Staphylococcus epidermidis | 1 (1.96) |
| Streptococcus constellatus subsp. constellatus | 5 (9.80) |
| Streptococcus gordonii | 1 (1.96) |
| Streptococcus intermedius | 1 (1.96) |
| Gram-negative bacteria | |
| Stenotrophomonas maltophilia | 1 (1.96) |
| Achromobacter xylosoxidans subsp. xylosoxidans | 1 (1.96) |
| B, Obligate anaerobes (n=18) | |
| Strain | Total, n (%) |
| Gram-positive bacteria | |
| Propionibacterium | 7 (13.73) |
| Actinomyces viscosus | 2 (3.92) |
| Peptostreptococcus micros | 1 (1.96) |
| Actinomyces meyeri | 1 (1.96) |
| Parvimonas micra | 2 (3.92) |
| Actinomyces naeslundii | 1 (1.96) |
| Gram-negative bacteria | |
| Fusobacterium nucleatum | 2 (3.92) |
| Prevotella bivia | 2 (3.92) |
| C, Facultative anaerobes (n=19) | |
| Strain | Total, n (%) |
| Gram-positive bacteria | |
| Citrobacter koseri | 1 (1.96) |
| Gemella morbillorum | 1 (1.96) |
| Granulicatella adiacens | 1 (1.96) |
| Gram-negative bacteria | |
| Capnocytophaga sputigena | 5 (9.80) |
| Capnocytophaga gingivalis | 3 (5.88) |
| Haemophilus parainfluenzae | 1 (1.96) |
| Aeromonas sobria | 1 (1.96) |
| Aggregatibacter segnis | 3 (5.88) |
| Eikenella corrodens | 1 (1.96) |
| Aggregatibacter aphrophilus | 1 (1.96) |
| Haemophilus influenzae | 1 (1.96) |
Subsp., subspecies.
Treatment outcomes
Among the 50 cases with canaliculitis, 45 showed improvements in eye redness, discharge, epiphora and canalicular oedema following IOI treatment. Clinically meaningful improvements were generally achieved 3-4 days after treatment initiation, and most patients were cured within 2-8 weeks. No relapse occurred at the follow-up appointments, and the success rate was 90%. A total of 5 patients who responded poorly to IOI treatment or experienced relapse underwent surgical treatment. Canalicular dacryoliths and hyperplastic granulation tissues were completely removed during surgery, and bicanalicular lacrimal drainage tube placement was performed. Ocular symptoms were alleviated in all cases after surgery, without complications (such as canalicular luminal narrowing or scarring, lacrimal pump dysfunction or canalicular fistula formation) during follow-up. All patients with canaliculitis achieved satisfactory treatment outcomes. The total cure rate was 100%.
Discussion
The results of the present study are similar to previous reports, stating that middle-aged and older women were more commonly affected by primary canaliculitis and that the lower eyelid was involved more often than the upper eyelid (6-8). There were also similarities in the clinical presentations and the prolonged symptomatic period until diagnosis, including pouting erythematous punctum, punctal or canalicular swelling, and expressible punctal discharge (7-14). UBM and dacryoendoscopy may be used as diagnostic tools for canaliculitis (13-15); however, these require special equipment and techniques. In most cases, a thorough clinical examination is sufficient for making a correct diagnosis (8,12).
To the best of our knowledge, there are few detailed descriptions of laboratory findings in canaliculitis in the literature. The present study comprised 50 cases of canaliculitis, of which 48 had canalicular dacryolith. Discharge smear staining examination revealed a 96% (48/50) positivity rate of Actinomyces, which is similar to previous reports (10,16,17). Most smears were also positive for cocci or bacilli, with more positive smears for cocci. Canaliculitis is typically associated with mixed bacterial growth (5,16).
The culture positivity rate of canaliculitis reportedly ranges from 11.1-91.0% (7,8,10,14,17,18). The present study found a bacterial culture positivity rate of 82%, which was moderately high. The anaerobic culture may have increased the bacterial detection rate. The bacterial strains were relatively heterogeneous (mostly anaerobes or facultative anaerobes and gram-positive bacteria). The three commonest strains identified through culture were Streptococcus spp., Capnocytophaga spp. and Propionibacterium. In previous studies (8-10,12,13,17,18), Streptococcus and Staphylococcus were isolated as the commonest causative agents. This difference could have occurred as a result of specimen contamination and differences in culture conditions or due to changes in the microbiological profile of canaliculitis. Specimens should be collected aseptically to reduce contamination and increase culture accuracy. In the current study, anaerobic culture was also performed to increase the detection rate for anaerobic bacteria. Only three cases (6%) were positive for Actinomyces, which is similar to the culture results of other studies (7,10,13,17). The low positivity rate for Actinomyces found in the current study may be related to the small sample size, difficulty in isolating Actinomyces and stringent culture requirements. Perumal et al (16) reported that the success of bacterial culture could be variable but that smear examination analysis can assist in determining the etiology. Conducting both smear and culture examinations will help improve the pathogen detection rate in canaliculitis and guide clinical treatment. All smears and cultures in the present study were negative for fungal infection, which is consistent with the findings of a previous report (17).
Various treatments have been described for canaliculitis. Conservative measures include topical antibiotics and intracanalicular antibiotic irrigation, punctal dilatation and canalicular expression (8,9,12). Surgical measures include punctoplasty and canalicular curettage, canaliculotomy with canalicular curettage, or canaliculostomy (4,11,13,14,18). Conservative management not only has a high failure rate of 65-100%, but also a high recurrence rate of 33% (3). Failure of antibiotic therapy is attributed to the inability of antibiotics to penetrate canalicular concretions. The concretions and inflamed tissue interfere with tear flow, causing a cycle of canalicular stasis and infection (19). Surgical treatment has proven to be effective (4,7,11,14,18); however, it could lead to canalicular luminal narrowing or scarring, lacrimal pump dysfunction and canalicular fistula formation (6,7,9,11,14).
IOI was described in 2015 by Xu et al (9) as a minimally invasive technique. The technique is particularly effective when the ointment is retained for a longer duration inside the canaliculus, thereby increasing its bioavailability around the focus of infection. The resolution rate of canaliculitis after intracanalicular ointment single injection was 72.7% in the study by Xu et al (9). One patient had a canalicular laceration during ointment infiltration, but no canalicular block was noted at the 2-month follow-up. In 2021, Alam et al (20) used the same technique; however, their protocol involved multiple sessions of ointment loading, which achieved a complete cure in all cases. Three patients developed a canalicular block, but the authors could not determine if this resulted from the primary disease or a cannula-related injury. The same treatment was selected for canaliculitis in the present study, where canalicular dacryoliths were thoroughly removed through the lacrimal punctum followed by intracanalicular injection of tobramycin/dexamethasone eye ointment. The treatment was performed weekly for 2-8 weeks, resulting in a cure rate of 90%. Five patients relapsed at subsequent follow-up sessions. These patients underwent canaliculotomy with canalicular curettage. Canalicular dacryoliths and hyperplastic granulation tissues were completely removed during surgery. The present study preserved the integrity of the punctum and performed bicanalicular lacrimal drainage placement. All five cases were cured without any complications. In the present study, all patients with canaliculitis achieved satisfactory treatment outcomes, and the resolution rate was 100%. During surgery, residual dacryoliths are observed in the lacrimal tubule (11,18). It is difficult to completely remove dacryoliths through the lacrimal punctal without canaliculotomy and residual dacryoliths may lead to relapse (5,19). Placement of the lacrimal drainage tube is beneficial for the drainage of tears through the inflamed canalicular system and for preventing lacrimal duct adhesion, narrowing or obstruction (21). According to a previous study, the ointment can incite a foreign body reaction or lead to lipogranuloma formation (22). No paracanalicular lipogranulomata were noted in the present study. If penetration of the canalicular walls occurs creating a false passage, the ointment injected may be deposited in the eyelid tissue, inducing lipogranulomatous inflammation. Therefore, the ointment administration should be performed carefully and gently. Eye ointment should only be injected by an experienced specialist.
The present study was limited by its relatively small sample size; thus, the present findings require further verification to be considered representative.
In conclusion, although primary canaliculitis is an uncommon clinical condition and may have no specific features, it is not difficult to be diagnosed using detailed smear staining examinations and laboratory testing. Surgery has a high resolution rate, and for a number of patients, it is the treatment of choice. By contrast, IOI is an effective and less invasive procedure with fewer complications and could be used as the gold standard for canaliculitis treatment. Thorough removal of the dacryoliths is the key to successful treatment and prevention of relapse. In the future, the present authors will endeavor to continue collecting data on this condition, hoping that clinical research results based on a larger sample will provide more evidence regarding the etiology of canaliculitis, which can further guide its treatment and diagnosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by The Fund of Top Talent Support Program for Young and Middle-aged People of Wuxi Health Committee (grant no. HB2020030), The Research Project of Wuxi Commission of Health (grant no. M202131), The Elderly Health Research Project of Jiangsu Province (grant no. 2022043) and The Social Development Project of Jiangsu Provincial Department of Science and Technology (grant no. BE2022699).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QW, SS and ZZ conceived the study. QW, SL, RH, HS and YG collected the clinical information of the patients. QW, SS, SL, RH, HS and ZZ analyzed and interpreted the clinical data. QW and SL wrote the draft of the manuscript. SS, YG and ZZ reviewed and edited the manuscript. ZZ was responsible for study supervision. QW and ZZ confirm the authenticity of all the raw data. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The protocol of the present prospective study was approved by the Institutional Review Board of the Affiliated Wuxi Clinical College of Nantong University (Wuxi, China; approval no. 2022-Y-98). Prior to inclusion, written informed consent was obtained from all patients. The present study adhered to the tenets of the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the patient whose images are presented.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Anand AR, Harinee R, Jeyalatha MV, Poonam NS, Therese KL, Rajeshwari H, Narasimhan L, Gopinath R. Microbiological profile of canaliculitis and their antibiotic susceptibility patterns: A 11-year review at a referral eye care centre. Indian J Med Microbiol. 2022;40:378–383. doi: 10.1016/j.ijmmb.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Ali MJ. Metagenomics of infective canaliculitis: The Lacriome paper 3. Eur J Ophthalmol. 2022;32:3346–3352. doi: 10.1177/11206721221091646. [DOI] [PubMed] [Google Scholar]
- 3.Freedman JR, Markert MS, Cohen AJ. Primary and secondary lacrimal canaliculitis: A review of literature. Surv Ophthalmol. 2011;56:336–347. doi: 10.1016/j.survophthal.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Baldursdóttir E, Sigurdsson H, Jónasson L, Gottfredsson M. Actinomycotic canaliculitis: Resolution following surgery and short topical antibiotic treatment. Acta Ophthalmol. 2010;88:367–370. doi: 10.1111/j.1755-3768.2008.01367.x. [DOI] [PubMed] [Google Scholar]
- 5.Hussain I, Bonshek RE, Loudon K, Armstrong M, Tullo AB. Canalicular infection caused by Actinomyces. Eye (Lond) 1993;7 (Pt 4):542–544. doi: 10.1038/eye.1993.118. [DOI] [PubMed] [Google Scholar]
- 6.Fulmer NL, Neal JG, Bussard GM, Edlich RF. Lacrimal canaliculitis. Am J Emerg Med. 1999;17:385–386. doi: 10.1016/s0735-6757(99)90093-1. [DOI] [PubMed] [Google Scholar]
- 7.Anand S, Hollingworth K, Kumar V, Sandramouli S. Canaliculitis: The incidence of long-term epiphora following canaliculotomy. Orbit. 2004;23:19–26. doi: 10.1076/orbi.23.1.19.28985. [DOI] [PubMed] [Google Scholar]
- 8.Kaliki S, Ali MJ, Honavar SG, Chandrasekhar G, Naik MN. Primary canaliculitis: Clinical features, microbiological profile, and management outcome. Ophthal Plast Reconstr Surg. 2012;28:355–360. doi: 10.1097/IOP.0b013e31825fb0cd. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Liu Z, Mashaghi A, Sun X, Lu Y, Li Y, Wu D, Yang Y, Wei A, Zhao Y, et al. Novel therapy for primary canaliculitis: A pilot study of intracanalicular ophthalmic corticosteroid/antibiotic combination ointment infiltration. Medicine (Baltimore) 2015;94(e1611) doi: 10.1097/MD.0000000000001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaldívar RA, Bradley EA. Primary canaliculitis. Ophthal Plast Reconstr Surg. 2009;25:481–484. doi: 10.1097/IOP.0b013e3181b8c185. [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, Choung HK, Kim NJ, Khwarg SI. One-snip punctoplasty and canalicular curettage through the punctum: A minimally invasive surgical procedure for primary canaliculitis. Ophthalmology. 2009;116:2027–2030.e2. doi: 10.1016/j.ophtha.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Gogandy M, Al-Sheikh O, Chaudhry I. Clinical features and bacteriology of lacrimal canaliculitis in patients presenting to a tertiary eye care center in the Middle East. Saudi J Ophthalmol. 2014;28:31–35. doi: 10.1016/j.sjopt.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang S, Lin B, Pan Q, Zheng M, Qin X, Wang Y, Zhang Z. Clinical features and surgical outcomes of primary canaliculitis with concretions. Medicine (Baltimore) 2017;96(e6188) doi: 10.1097/MD.0000000000006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Y, Zhang L, Li L, Fan X, Xiao C. Surgical procedure of canaliculoplasty in the treatment of primary canaliculitis associated with canalicular dilatation. BMC Ophthalmol. 2020;20(245) doi: 10.1186/s12886-020-01503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo B, Qi X. Utility of 80-MHz ultrasound biomicroscopy and lacrimal endoscopy in chronic lacrimal canaliculitis. J Ultrasound Med. 2021;40:2513–2520. doi: 10.1002/jum.15625. [DOI] [PubMed] [Google Scholar]
- 16.Perumal B, Carlson JA, Meyer DR. A pathological analysis of canaliculitis concretions: More than just Actinomyces. Scientifica (Cairo) 2016;2016(6313070) doi: 10.1155/2016/6313070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Deng SJ, Wang ZQ, Ding JW, Sun XG. Etiological and drug sensitivity analysis of lacrimal canaliculitis. Zhonghua Yan Ke Za Zhi. 2018;54:111–114. doi: 10.3760/cma.j.issn.0412-4081.2018.02.008. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 18.Kim UR, Wadwekar B, Prajna L. Primary canaliculitis: The incidence, clinical features, outcome and long-term epiphora after snip-punctoplasty and curettage. Saudi J Ophthalmol. 2015;29:274–277. doi: 10.1016/j.sjopt.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavilack MA, Frueh BR. Through curettage in the treatment of chronic canaliculitis. Arch Ophthalmol. 1992;110:200–202. doi: 10.1001/archopht.1992.01080140056026. [DOI] [PubMed] [Google Scholar]
- 20.Alam MS, Poonam NS, Koka K, Vijay V, Ganesh S. Intracanalicular antibiotic ointment loading as a management option for canaliculitis. Orbit. 2021;40:295–300. doi: 10.1080/01676830.2020.1801763. [DOI] [PubMed] [Google Scholar]
- 21.Jin X, Zhao Y, Tong N, Xu W. Use of crawford tube for chronic suppurative lacrimal canaliculitis. Ophthal Plast Reconstr Surg. 2014;30:229–232. doi: 10.1097/IOP.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Xiao C, Bi X, Zhou H, Ge S, Fan X. Palpebral lipogranuloma caused by transcanalicular ointment injection after laser canaliculoplasty. Ophthal Plast Reconstr Surg. 2011;27:333–337. doi: 10.1097/IOP.0b013e31821b6d92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.