Abstract
H1 linker histones are involved in facilitating the folding of chromatin into a 30-nm fiber. Mice contain eight H1 subtypes that differ in amino acid sequence and expression during development. Previous work showed that mice lacking H10, the most divergent subtype, develop normally. Examination of chromatin in H10−/− mice showed that other H1s, especially H1c, H1d, and H1e, compensate for the loss of H10 to maintain a normal H1-to-nucleosome stoichiometry, even in tissues that normally contain abundant amounts of H10 (A. M. Sirotkin et al., Proc. Natl. Acad. Sci. USA 92:6434–6438, 1995). To further investigate the in vivo role of individual mammalian H1s in development, we generated mice lacking H1c, H1d, or H1e by homologous recombination in mouse embryonic stem cells. Mice lacking any one of these H1 subtypes grew and reproduced normally and did not exhibit any obvious phenotype. To determine whether one of these H1s, in particular, was responsible for the compensation present in H10−/− mice, each of the three H1 knockout mouse lines was bred with H10 knockout mice to generate H1c/H10, H1d/H10, or H1e/H10 double-knockout mice. Each of these doubly H1-deficient mice also was fertile and exhibited no anatomic or histological abnormalities. Chromatin from the three double-knockout strains showed no significant change in the ratio of total H1 to nucleosomes. These results suggest that any individual H1 subtype is dispensable for mouse development and that loss of even two subtypes is tolerated if a normal H1-to-nucleosome stoichiometry is maintained. Multiple compound H1 knockouts will probably be needed to disrupt the compensation within this multigene family.
DNA in the nuclei of all eukaryotic cells is packaged into repeating units of nucleosomes that form the basic unit of chromatin. Each nucleosome consists of an octamer core containing two molecules of each of the core histones, H2a, H2b, H3, and H4. H1 linker histones bind to the nucleosome core particle and the linker DNA between nucleosomes to facilitate further compaction of chromatin into a 30-nm fiber. Recent studies have shown that the chromatin complex, especially the nucleosome and its modifications, can have a profound influence on transcription (reviewed in references 9 and 26).
Although histones are highly conserved proteins, multicellular organisms contain a variety of subtypes exhibiting significant sequence divergence. Among the histone classes, the H1 linker histones are the most divergent group. In mammals, there are at least eight H1 subtypes, including the somatic H1s, H1a to H1e, germ cell-specific H1t and H1oo, and replacement linker histone H10 (11, 24). These subtypes exhibit distinct patterns of expression during differentiation and development (12, 24). The significance of the diversity present within the H1 family is not understood. The genes for H1a through H1e and H1t are tightly linked on mouse chromosome 13 (25). The H10 gene is located on mouse chromosome 15 (2). H10 is the smallest and most divergent member of the H1 family (27). H10 accumulates in quiescent cells and during terminal differentiation and terminal cell division, reaching levels as high as 30% of the total H1 in certain tissues, such as adult liver. Despite the unique properties and developmental regulation of H10, previous studies in our laboratory showed that mice develop normally without H10 (23). Analysis of chromatin from H10-null mice indicated that the level of the somatic H1s, especially H1c, H1d, and H1e, was increased so as to maintain a normal ratio of H1 to nucleosomes in H10-deficient chromatin. In certain tissues, such as adult liver, H1c, H1d, and H1e accounted for 95% of the remaining H1, suggesting that these subtypes are responsible for compensating for loss of H10.
The present study was undertaken with the following two experimental objectives: first, to determine whether or not any one of several H1 subtypes is essential for mouse development; second, to determine whether H1c, H1d, or H1e is responsible for compensating for the loss of H10 in H10−/− mice. To achieve the first goal, we generated null mutations in each of the three somatic H1 genes by homologous recombination in embryonic stem (ES) cells and then produced mice lacking each of these individual subtypes. To achieve the second goal, we bred each of these three H1 knockout mice to H10 null mice and ultimately produced H1c/H10, H1d/H10, and H1e/H10 double-knockout mice.
MATERIALS AND METHODS
Disruption of the H1c gene in ES cells and generation of chimeric mice.
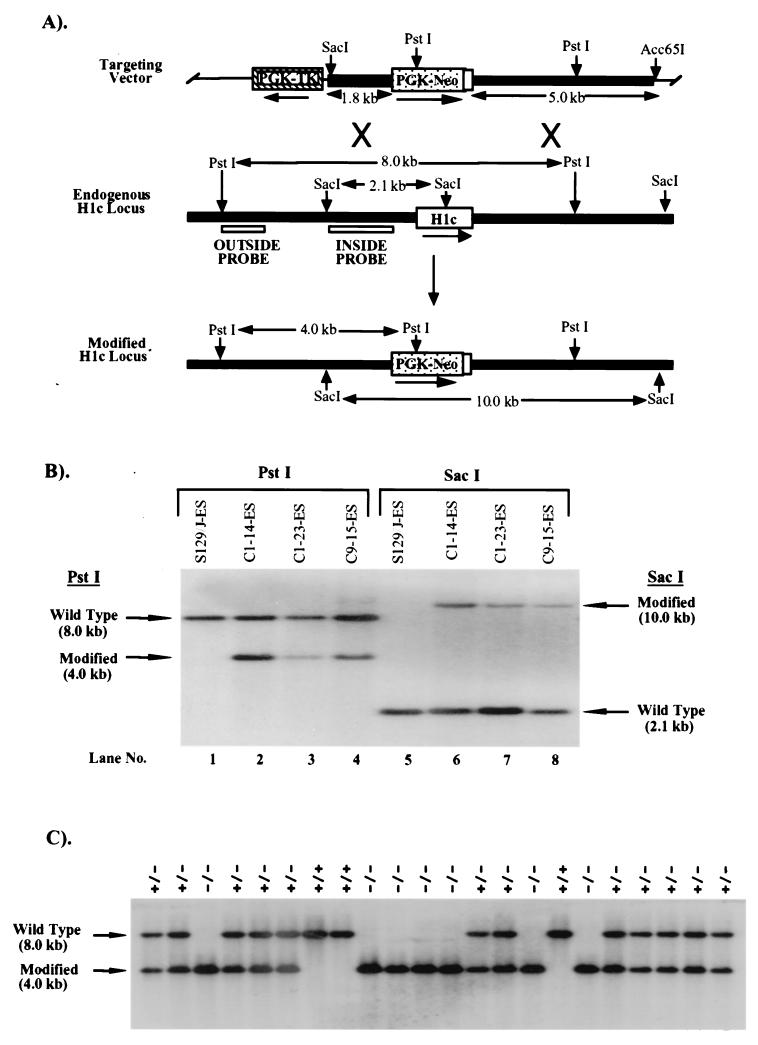
All of the genomic DNA clones used in construction of targeting vectors were isolated by screening a strain 129J/sv mouse genomic DNA library with gene-specific probes described previously (4, 25). Twenty-five and 50 μg of Acc65I-linearized H1c targeting vector (Fig. 1A) were electroporated separately into 2 × 107 or 4 × 107 E14-1 ES cells (7) at 400 V and 250 μF using a Bio-Rad Gene Pulser, and cells resistant to active G418 (Genticin; GIBCO-BRL) at 200 μg/ml and 2 μM ganciclovir (Syntex) were selected. Genomic DNA from 500 individual G418/ganciclovir-resistant colonies was prepared as previously described (8), pooled (two colonies per pool), digested with PstI, and screened for homologous recombination events by Southern blot analysis with a 0.9-kb 5′-flanking region probe (outside probe, Fig. 1A). Three of 240 clones gave a 4-kb PstI hybridizing fragment expected from the modified allele (Fig. 1B). To confirm that these three clones had undergone homologous recombination, Southern blot analysis of SacI-digested genomic DNA was also performed using a probe lying within the targeting construct (inside probe; Fig. 1A). The three positive clones showed the expected 10-kb band from the modified locus (Fig. 1B).
FIG. 1.
Targeted disruption of the H1c gene in mouse ES cells and mice. (A) Homologous-recombination strategy in ES cells. The H1c targeting vector (top) was constructed by removing a 566-bp ApaI/MscI segment from a 6.4-kb EcoRI genomic fragment cloned in pGEM-3Z (Promega), containing the H1c coding region (open box), and inserting by blunt-end ligation a 1.8-kb ApaI/HindIII fragment (stippled box) from PGK-NEO. The 7.2-kb H1c/PGK-NEO insert was subsequently released by SalI digestion and inserted into the XhoI site of pPGK-TK (19). To increase the length of homology within the short arm (5′ of PGK-NEO), the 0.8-kb ClaI-XhoI short-arm fragment was removed and replaced with a ClaI-XhoI 1.8-kb homologous H1c 5′ region fragment from H1c plasmid subclone HS7 (22). The transcriptional orientations of the genes are indicated by arrows. A homologous recombination event (X's) between the targeting vector and the endogenous H1c locus (middle) results in production of a modified H1c locus (bottom) in which a segment from 58 bp 5′ of the translation initiation codon to codon 170 was replaced with PGK-NEO. (B) Identification of ES cell clones containing the modified H1c allele. After an initial screening of pools of two ES cell clones, ES cell DNA (10 μg) from individual clones was digested with PstI (lanes 1 to 4) and Southern blot hybridized with the outside probe (A). Correct targeting was confirmed (lanes 5 to 8) by SacI digestion of ES cell DNA and blot hybridization with the inside probe (A). Results obtained with DNA from untransfected ES cells are shown in lanes 1 and 5. The expected positions of the hybridizing fragments from the unmodified (wild-type) and modified H1c loci and their respective sizes are indicated. (C) Genotype analysis of offspring from parents heterozygous for the modified H1c allele. Siblings that were heterozygous for the modified H1c allele were bred, and 15 μg of tail DNA from offspring was digested with PstI and blot hybridized with the inside probe (Fig. 1A). The deduced genotype of each animal is indicated above each lane. The expected positions of the hybridizing fragment from the wild-type and modified loci and their corresponding sizes are indicated.
The three ES cell clones (C1-14-ES, C1-25-ES, and C9-15-ES) containing the modified H1c locus were injected into C57BL/6 blastocysts, and the blastocysts were transferred to CD-1 pseudopregnant females to generate chimeric mice as described previously (8). The three cell lines generated chimeras ranging in chimerism between 30 and 70% based on coat color. Male and female chimeras from each line were then mated with C57BL/6 mice. One female chimera transmitted the agouti coat color marker and the modified H1c allele. An H1c heterozygous mutant mouse was bred to C57BL/6 females. Mouse tail DNA was prepared as described previously (8).
Disruption of the H1d gene in ES cells and generation of chimeric mice.
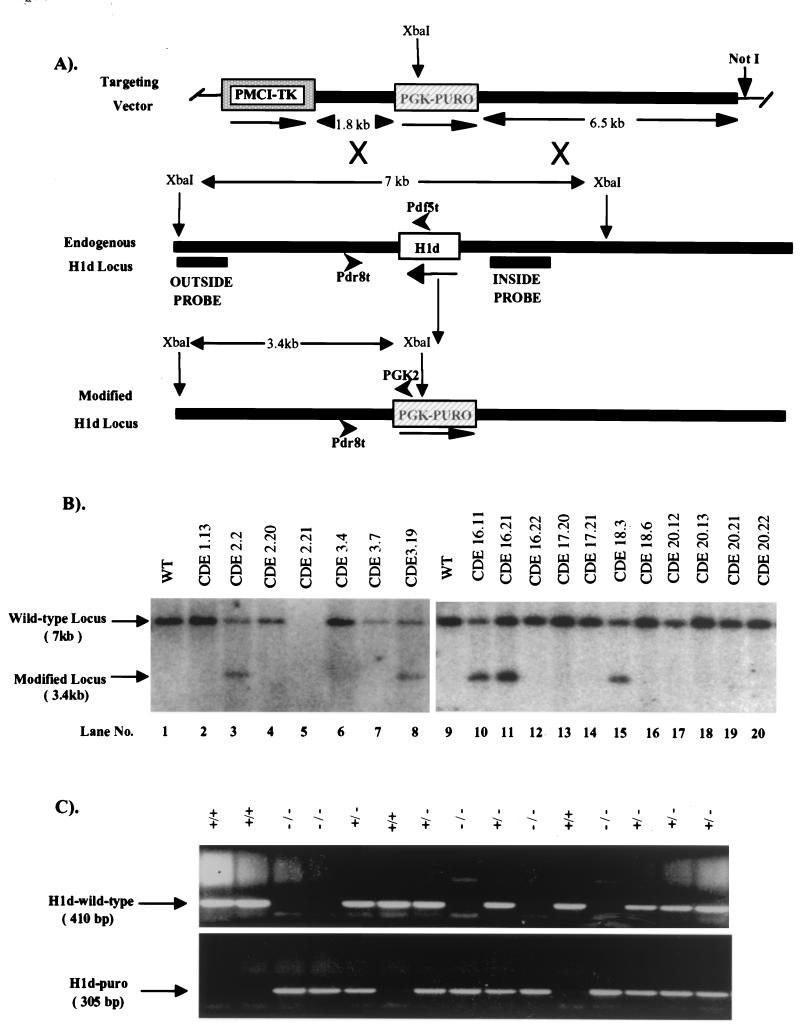
A 50-μg sample of the H1d targeting vector (Fig. 2A) was linearized with NotI and electroporated into 4 × 107 cells of a WW6 ES cell clone (WEC9.6 previously targeted at the H1c and H1e loci). Colonies resistant to puromycin (Sigma) at 2 μg/ml and 2 μM ganciclovir (Syntex) were isolated and expanded. Genomic DNA from 678 puromycin/ganciclovir-resistant colonies was digested with XbaI and screened for homologous recombination events by Southern blot hybridization with a 0.55-kb 3′-flanking region probe (outside probe, Fig. 2A). Nine clones gave a 3.4-kb XbaI hybridizing fragment expected from the modified allele (Fig. 2B). Seven positive clones were injected into C57BL/6 recipient blastocysts, and chimeric mice were derived as described above. All cell lines generated chimeras ranging in chimerism between 80 and 99% based on coat color. Male and female chimeras from each line were then mated with C57BL/6 mice. Seven lines transmitted the modified allele. Three lines derived from ES cell clones (CDE7.3, CDE16.11, and CDE24.4) in which H1d gene targeting had occurred in trans to the previous H1c and H1e gene targetings were used for the analyses described here.
FIG. 2.
Targeted disruption of the H1d gene in mouse ES cells and mice. (A) Homologous recombination strategy in ES cells. To generate the H1d targeting vector (top), the Scramble plasmid system (Lexicon) was used. The positive and negative selectable marker genes PGK-PURO (striped box) and PMCI-TK cassettes (shaded box) were inserted into vector 901 at the AscI and RsrII sites, respectively. The H1d targeting vector (top) was then constructed by inserting a 6.5-kb H1d 5′ ClaI/EcoRI fragment (in which the EcoRI site lies 616 bp 5′ of the H1d ATG codon) and a 1.8-kb PCR-amplified H1d 3′ fragment (beginning 120 bp 3′ of the stop codon) into the SmaI/NotI and KpnI sites, respectively, in modified vector 901. A homologous recombination event (X's) between the targeting vector and the endogenous H1d locus (middle) results in production of a modified H1d locus (bottom), in which a 1.4-kb fragment, including the entire H1d coding sequence (open box), 616 bp of the 5′ noncoding sequence, and 120 bp of the 3′ noncoding sequence, is removed. (B) Identification of ES cell clones containing the modified H1d allele. ES cell DNA (10 μg) was digested with XbaI and blot hybridized with the outside probe (A). The expected positions of the hybridizing fragments from the unmodified (wild-type) and modified H1d loci and their respective sizes are indicated. Lanes 1 and 9 contained DNA from wild-type ES cells. Clones analyzed in lanes 3, 8, 10, 11, and 15 underwent a homologous recombination event. (C) Genotype analysis of offspring from parents heterozygous for the modified H1d allele. Siblings that were heterozygous for the modified H1d allele were bred, and 1 μg of tail DNA from offspring was used for PCR analysis with the following primers shown in panel A: H1d wild-type allele, the H1d 5′ sequence-specific primers (Pdf5t [5′ AAGCCTAAAGCTTCTAAGCCG 3′] and Pdr8t [5′ CTAGAGAACCCCCCTAATGC 3′]; predicted band size, 410 bp); H1d null allele (H1d-Puro): the PGK-PURO gene-specific primer (PGK2 [5′ GCTGCTAAAGCGCATGCTCCA 3′]) and the H1d 5′ sequence-specific primer (Pdr8t [5′ CTAGAGAACCCCCCTAATGC 3′]; predicted band size, 305 bp). The deduced genotype of each animal is indicated above each lane. The migration of PCR products from the wild-type and modified loci and their corresponding sizes are indicated.
Disruption of the H1e gene in ES cells and generation of chimeric mice.
The same protocol was utilized for disruption of the H1e gene as described above for H1c gene, except that the H1e targeting vector (Fig. 3A) was linearized with SalI. Genomic DNA was extracted from 240 G418/ganciclovir-resistant colonies and digested with EcoRI, and Southern blot hybridization was performed with a 0.9-kb 5′-flanking region probe (outside probe, Fig. 3A). Eleven clones gave a 4.5-kb EcoRI hybridizing fragment expected from the modified allele (Fig. 3B).
FIG. 3.
Targeted disruption of the H1e gene in mouse ES cells and mice. (A) Homologous recombination strategy in ES cells. The H1e targeting vector (top) was constructed by removing a 720-bp MscI fragment from a 6.5-kb EcoRI H1e genomic DNA fragment cloned in pGEM-3Z (Promega), containing the H1e coding region (open box), and inserting by blunt-end ligation a 1.8-kb ApaI/HindIII fragment (shaded box) from pPGK-NEO. A 1.8-kb SalI/XhoI fragment from pMCI-TK was inserted into the SalI site at the 5′ end of the gene. A homologous recombination event (X's) between the targeting vector and the endogenous H1e locus (middle) results in production of a modified H1e locus (bottom), in which a 720-bp fragment, including the entire H1e coding sequence, along with 49 bp of the 5′ noncoding sequence and 11 bp of the 3′ noncoding sequence, is removed. (B) Identification and confirmation of ES cell clones containing the modified H1e allele. ES cell DNA (10 μg) was digested with EcoRI, followed by Southern blot analysis using the outside probe shown in panel A. The expected positions of the hybridizing fragments from the unmodified (wild-type) and modified H1c loci and their respective sizes are indicated. Clones analyzed in lanes 1, 4, 5, 8, 9, 11, 13, 15, 17, 18, and 23 underwent a homologous recombination events. (C) Genotype analysis of offspring from parents heterozygous for the modified H1e allele. Siblings that were heterozygous for the modified H1e allele were bred, and 15 μg of tail DNA from offspring was digested with EcoRI, blotted, and hybridized with the inside probe shown in panel A. The deduced genotype of each animal is indicated above each lane. The wild-type and modified loci and their corresponding sizes are indicated.
Four clones containing the modified H1e locus were injected into C57BL/6 recipient blastocysts, and chimeric mice were derived as described above. Two cell lines (E2-5 and E22-3) generated chimeras ranging in chimerism between 70 and 100% based on coat color. Male chimeras from these two cell lines were mated with C57BL/6 mice. Both cell lines successfully transmitted the modified H1e allele through the germ line.
Preparation and analysis of histones.
Mice were sacrificed by cervical dislocation, and tissues were immediately rinsed with ice-cold phosphate-buffered saline. Histone proteins were prepared by 0.2 N sulfuric acid extraction and fractionated by high-performance liquid chromatography (HPLC) as described previously (14, 23). The effluent from the HPLC column was monitored at 214 nm, and the peaks were recorded using a Hewlett-Packard 1090 system. Peak areas were determined with a Hewlett-Packard peak integrator program. Time-of-flight mass spectrometry TOF-MS analysis was performed as described previously (25).
Histological analysis of mouse tissues.
Blocks of mouse organs were fixed in neutral buffered 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy.
RESULTS
Production and characterization of mice lacking one of three somatic H1 subtypes.
The mouse H1c, H1d, and H1e genes encode proteins of 212, 221, and 219 amino acids, respectively (25). None of these genes contains introns. H1c, H1d, and H1e genomic clones for each of these subtypes were isolated from a mouse strain 129J/sv genomic library with gene-specific flanking region probes.
To generate targeting vectors for each of these genes, vectors containing both positive and negative selectable marker genes for transfection in ES cells were constructed. Either the entire H1 coding region (for H1e and H1d) or most of the coding region (for H1c) was removed from the respective genomic clone and replaced with a positive selectable marker gene, either PGK-neo (for H1c and H1e targeting vectors) or PGK-puro (for the H1d targeting vector). A tk gene was inserted either 5′ (H1c and H1e) or 3′ (H1d) of the modified gene for negative selection (Fig. 1A, 2A, and 3A). After transfection and selection of resistant ES cell clones, Southern blot analyses were used to identify clones containing a modified allele (Fig. 1B, 2B, and 3B) and to verify the transmission of the modified allele through the germ line of chimeric mice produced from correctly targeted ES cell clones (data not shown). Mice heterozygous for any of these three H1 gene mutations were phenotypically normal. To determine whether any of the three H1 genes (H1c, H1d, and H1e) is essential for mouse development, mice heterozygous for the modified H1 allele were interbred to generate H1c−/−; H1e−/−; or H1d−/− mice. Southern blot analyses (for H1c and H1e) or PCR assays (for H1d) were used to genotype F2 progeny (Fig. 1C, 2C, and 3C). Of 38 F2 animals from interbreeding H1c heterozygotes, 8 (21%) carried only the wild-type allele, 18 (47%) carried one copy of the modified allele, and 12 (32%) carried two copies of the modified allele. Of 98 F2 animals from interbreeding H1d heterozygotes, 27 (28%) carried only the wild-type allele, 50 (51%) carried one copy of the modified allele, and 21 (21%) carried two copies of the modified allele. Of 48 F2 animals from interbreeding H1e heterozygotes, 10 (21%) carried only the wild-type allele, 24 (50%) carried one copy of the modified allele, and 14 (29%) carried two copies of the modified allele. The ratio of the three classes of animals, in each case, is not significantly different from the expected values for Mendelian transmission of the two alleles.
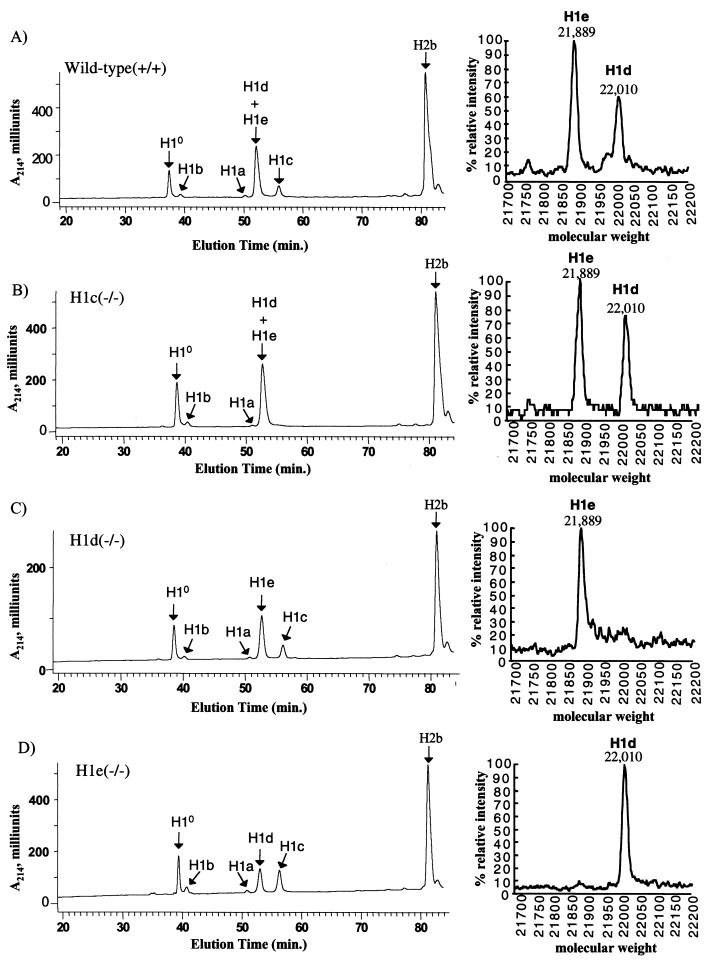
Since each gene modification resulted in either removal of the complete open reading frame (H1d and H1e) or removal of the translation initiation codon and nearly 80% of the coding region (H1c), it was very unlikely that mice homozygous for the modified allele could produce that H1 subtype. To confirm this expectation, H1 histone extracts from livers were analyzed by reverse-phase HPLC analysis. As described previously (23) and as shown in Fig. 4A, this method resolves H10 and the five somatic H1s. H1d and H1e elute as a single peak in the chromatogram, but by collecting this peak and subjecting it to TOF-MS analysis, they can be separated. Whereas H1c, H1d, and H1e were readily detected in extracts of wild-type animals (Fig. 4A), in each case, the subtype corresponding to the modified gene was not detectable in extracts from homozygous mutant animals (Fig. 4B, C, and D). We conclude that the modification introduced into each of the three H1 genes indeed resulted in a null mutation. As described previously (23) and discussed below, the HPLC analysis also allows calculation of the H1-to-nucleosome ratio in tissues of the knockout mice. Calculation of this ratio from experiments like that shown in Fig. 4 showed that the ratio was not affected in liver chromatin from any of the three single-knockout mice (data not shown).
FIG. 4.
Reverse-phase HPLC analysis of histones from wild-type and H1c, H1d, and H1e homozygous mutant mice. (Left side) Approximately 100 μg of total histone extract of chromatin from livers of 20-week-old mice was fractionated by reverse phase HPLC as previously described (23). Panels: A, wild-type; B, homozygous H1c mutant; C, homozygous H1d mutant; D, homozygous H1e mutant. The identity of the histone subtype(s) in each peak has been reported previously (3, 15, 25). (Right side) Fractions eluting between 52 and 54 min (corresponding to the peak marked H1d+H1e) were collected and subjected to TOF-MS analysis. The identity of the two H1 subtypes detected in this analysis was demonstrated in reference 25.
All of the three types of H1-null mice exhibited normal birth weight and size and were indistinguishable from heterozygous and wild-type littermates. We also determined whether homozygous null mutant mice were fertile by mating −/− male and female animals of each strain. These matings produced litters of normal size, and the progeny appeared normal. In addition, hematoxylin-and-eosin-stained, paraffin-embedded sections from over 30 tissues of mutant and control littermates were examined for pathological or abnormal histological features. No consistent differences between wild-type and homozygous mutant animals were detected, even in tissues, such as those of the brain, liver, pancreas, kidney, and lung, that normally exhibit high levels of H1c, H1d, and H1e. All of these results indicate that the loss of any of these three somatic H1 subtypes does not impair normal development or reproductive capacity.
Generation and characterization of three types of doubly H1-null mice deficient for H10 and one of three somatic H1 subtypes.
As mentioned, previous work from our laboratory showed that mice lacking H10, the most divergent H1 subtype, also develop normally and exhibit no apparent abnormalities (23). Analysis of chromatin from tissues of these mice showed that the amounts of H1c, H1d, and H1e were all increased, resulting in maintenance of a normal stoichiometry of total H1 to nucleosomes, even in tissues, such as that of the liver, in which H10 normally constitutes 30% of the total H1 (22, 23). To determine whether one of these increased H1 subtypes is responsible for compensating for loss of H10 in H10-null mice, we combined each of the three null somatic H1 alleles with the H10-null allele. First, doubly heterozygous mutant mice were produced by breeding H10−/− mice with each of the mice null for one of the three somatic subtypes. Double-mutant heterozygotes of each specific type were then interbred to produce double-null mice, H1c/H10, H1d/H10, and H1e/H10 homozygous mutants. All three types of doubly H1-null mice appeared phenotypically normal. To determine their fertility, double-null male and female mice were bred. Such breedings showed that each of the double-knockout mouse strains was fertile and reproduced normally. In addition, for each double-knockout strain, examination of hematoxlin-and-eosin-stained sections of over 40 tissues from six double-knockout animals failed to reveal any pathology or abnormal histology.
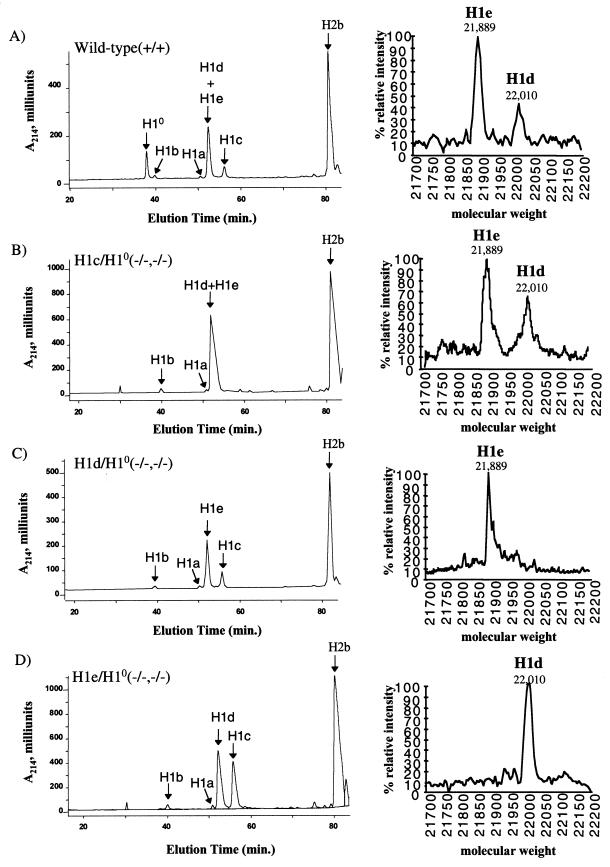
The absence of any phenotypic abnormality in the double mutants suggested that the normal stoichiometry of H1 to nucleosomes might be maintained, even in the absence of two H1 subtypes, by the remaining H1 subtypes. To investigate this possibility, we compared both the H1 subtype composition and the stoichiometry of linker histones and nucleosomes in liver chromatin of the three types of double mutants and wild-type controls. Adult liver was chosen for this analysis because it normally contains the highest levels of H10 of any tissue, nearly 30% of the total H1 (12, 23; Table 1), and because the three somatic H1 subtypes, H1c, H1d, and H1e, constitute almost all of the other linker histones. Therefore, the liver would be expected to exhibit the largest perturbation in these parameters resulting from a deficiency in H10 and one of the other three subtypes. Total chromatin-bound histones were extracted from liver nuclei by solubilization in sulfuric acid and fractionated by reverse-phase HPLC (Fig. 5). As shown in Fig. 4 and 5, in addition to resolving H10 and the five somatic H1s, this method also separates the H1s from the nucleosomal core histones, allowing us to estimate the linker histone-to-nucleosome ratio by measuring the total amount of H1s relative to one of the core histones, e.g., H2b. As described above and shown in Fig. 5 (right panels), the relative amounts of H1d and H1e in the combined H1d/H1e peak were obtained by collecting this peak and subjecting it to TOF-MS analysis. The results of this analysis (Table 1) confirm the complete absence of H10 and the expected somatic H1 subtype in each type of doubly homozygous mutant animal. Quantitative measurements from these analyses carried out on four double-knockout mice and four wild-type control animals showed that the linker histone-to-nucleosome ratio was the same in liver chromatin of all of the animals (Table 1), indicating that the stoichiometry between H1 and nucleosomes was not altered by the loss of H10 and any one of the three somatic H1 subtypes. The observed ratio of about 0.7 H1 molecule per nucleosome is in good agreement with previous measurements made on mouse liver chromatin by other methods (1) and with our earlier determinations (23). These results demonstrate that there is sufficient excess capacity for synthesis of linker histones to maintain normal chromatin stoichiometry even when the genes for two abundant subtypes are inactivated.
TABLE 1.
H1 subtype composition of liver chromatin from wild-type and double-mutant micea
| Mice | % of total H1b
|
Total H1/nucleosome ratioc | |||||
|---|---|---|---|---|---|---|---|
| H10 | H1a | H1b | H1c | H1d | H1e | ||
| Wild type | 27.4 ± 0.6 | 0.9 ± 0.1 | 1.9 ± 0.1 | 11.6 ± 0.4 | 16.1 ± 0.1 | 42.1 ± 0.2 | 0.73 ± 0.02 |
| H10/H1c (−/−, −/−) | 0 | 1.5 ± 0.2 | 2.7 ± 0.7 | 0 | 27.1 ± 0.3 | 68.6 ± 0.4 | 0.71 ± 0.01 |
| H10/H1d (−/−, −/−) | 0 | 1.8 ± 0.5 | 5.1 ± 3.1 | 22.7 ± 3.9 | 0 | 70.5 ± 4.5 | 0.71 ± 0.04 |
| H10/H1e (−/−, −/−) | 0 | 1.8 ± 0.1 | 3.8 ± 0.7 | 44.6 ± 1.7 | 49.7 ± 2.1 | 0 | 0.76 ± 0.03 |
Data were from HPLC analyses of wild-type and three H1 double-mutant strains. Values are means ± standard deviations of individual determinations made on four 5-month-old mice of each of the indicated genotypes.
Determined by the ratio of the A214 of the indicated H1 peak to the total A214 of all H1 peaks. The A214 values of the individual H1 peaks were adjusted to account for the differences in the number of peptide bonds in each H1 subtype.
Determined by the ratio of the total A214 of all H1 peaks to half of the A214 of the H2b peak. The A214 values of the H1 and H2b peaks were adjusted to account for the differences in the number of peptide bonds in each H1 subtype and H2b.
FIG. 5.
Reverse-phase HPLC analysis of histones from wild-type mice and three types of doubly homozygous mutant mice. Approximately 100 μg of total histone extract of chromatin from livers of 20-week-old mice were analyzed as described in the legend to Fig. 4. Panels: A, wild type; B, H1c/H10 homozygous double mutant; C, H1d/H10 homozygous double mutant; D, H1e/H10 homozygous double mutant.
DISCUSSION
In this study, mice carrying a null mutation in one of three different somatic H1 linker histone genes were generated by homologous recombination in ES cells. We are certain that the mutations we introduced are null mutations for two reasons. First, the homologous recombination removed all (H1d and H1e) or most (H1c) of the coding region of the respective gene. Second, examination of chromatin extracts by HPLC and TOF-MS showed that the respective H1 histone proteins were completely lacking in homozygous mutant animals. Despite the absence of the specific H1 subtype, homozygous mutant mice were viable and fertile. They also appeared phenotypically normal and did not exhibit any anatomic or histologic abnormality.
The absence of any obvious abnormality in the three types of somatic H1 mutants is not completely surprising because earlier work from our laboratory and others showed that mice lacking either of the highly divergent H1 subtypes, “replacement” subtype H10 (23) or testis-specific subtype H1t (5, 6, 14), develop normally. Mice lacking H1a, a somatic subtype that is highly enriched in developing spermatocytes, also appear normal and do not appear to exhibit any abnormality in spermatogenesis (13, 18). However, H10 accumulates to significant levels in only a very limited number of cell types during embryogenesis and such cells are in the final stages of differentiation. And while H10 is widely expressed after birth, it accumulates only in quiescent or terminally differentiated cells (12). Therefore, its role may be related to maintenance of terminally differentiated states (reviewed in reference 27). Likewise, H1t and H1a accumulate at specific stages of spermatogenesis, whereupon they are rapidly replaced by other basic proteins (10, 16, 17). In contrast, H1c, H1d, and H1e are widely expressed in both dividing and nondividing cell types, both during embryogenesis and in postnatal life. Nevertheless, mice lacking any one of these three somatic H1 subtypes appear normal.
The most likely explanation for the absence of a phentoype in the three single-knockout strains described here is that other H1 subtypes can compensate for the deficiency created in these animals. We showed previously in H10-null mice that chromatin from tissues, such as that of the liver, that normally contain abundant H10, contain increased levels of H1c, H1d, and H1e. The levels of these somatic H1s rose sufficiently to maintain a normal H1-to-nucleosome stoichiometry in these animals (23). We made similar observations in H1t-deficient germ cells (14). Likewise, in the present study, we found that in each of the three types of singly H1-null animals, H10 and the remaining somatic H1s, especially the remaining members of the H1c-H1d-H1e set, increased in chromatin to maintain the H1-to-nucleosome ratio at a normal value. In light of the differences in the regulation of the replacement linker histone H10 gene and the three replication-dependent H1 genes (H1c, H1d, and H1e), it is interesting that both H10 and the remaining replication-dependent H1s are increased in each of the three types of singly H1-null animals. It is also interesting that the two H1s that are least abundant in adult liver, H1a and H1b, do not contribute significantly to maintenance of the H1-to-nucleosome ratio at a constant value in these knockout mice. These observations suggest that the deposition of H1 histones at the vacated sites in chromatin is primarily regulated by the ongoing synthesis of H1s in that tissue rather than calling upon underexpressed members of the H1 gene family.
The existence of compensation at the level of chromatin stoichiometry seen both in our earlier work and in the present study prompted us to attempt to disrupt the compensation by generating compound knockout strains by breeding each of the three H1 knockout lines described here with the H10-null animals we reported upon previously (23). These combinations are of interest both because H10 is increased in liver chromatin in each of the three types of singly H1-null animals and because H1c, H1d, and H1e are all increased in chromatin in H10-null mice. Nevertheless, we found that each of the doubly H1-deficient strains (H1c/H10; H1d/H10; and H1e/H10) were normal and that chromatin from these animals has a normal ratio of total H1 to nucleosomes. This compensation occurred even in H1e/H10-null animals, in which nearly 70% of the H1 normally present in liver chromatin was eliminated. Apparently, there is considerable additional capacity in the system that regulates the amounts of H1 subtypes that can be deposited in chromatin. For example, the number of molecules of H1c and H1d present in liver chromatin of H1e/H10 null mice is three- to fourfold higher than in wild-type mice (Table 1). We do not know whether this is due to increased rates of synthesis of these subtypes in the knockout animals or increased deposition from a pool of excess molecules.
Although we did not observe a phenotype even in double-null mutants, the ES cell lines and mice described here should prove to be invaluable for future studies aimed at producing animals in which the H1-to-nucleosome stoichiometry has been altered. The compensation observed in double-null mutants suggests that producing mice with H1-depleted chromatin will require combining three or more null mutations in a single strain. Unfortunately, this task is made especially difficult by the tight linkage of the somatic H1 genes (H1a through H1e) and H1t on MMU13. We attempted to obtain a recombinant chromosome carrying the null alleles of H1c and H1e by interbreeding H1c-H1e doubly heterozygous mutants, but we failed to observe recombination among the 1,024 meioses analyzed. Thus, further reductions in the H1 content of mice will require sequential gene targeting in ES cells. Since the H10 gene is located on MMU15, as described here, such mice can be bred with H10-null mice to reduce the H1 content still further.
Despite the absence of a phenotype, the mice described here also should prove to be very valuable for studies aimed at determining whether regulation of gene expression is altered due to changes in the composition of H1 subtypes in chromatin. It is quite possible that expression of certain genes is changed in the knockout mice without a phenotypic effect. The advent of DNA microarray technology (20, 21) allows measurements on the expression of many genes simultaneously in single and compound H1 knockout strains. Such experiments should lead to identification of specific genes in which expression is affected by changes in H1 subtype composition or overall H1-to-nucleosome stoichiometry.
ACKNOWLEDGMENTS
This work was supported by NIH grant CA 79057 (A.I.S).
We thank the AECOM Cancer Center Gene Targeting Facility, the Laboratory of Macromolecular Analysis, and the Comparative Pathology facility. We thank Qingcong Lin for helpful discussion and Hui Xu for technical assistance.
REFERENCES
- 1.Bates D L, Thomas J O. Histones H1 and H5: one or two molecules per nucleosome? Nucleic Acids Res. 1981;9:5883–5894. doi: 10.1093/nar/9.22.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brannan C I, Gilbert D J, Ceci J D, Matsuda Y, Chapman V M, Mercer J A, Eisen H, Johnston L A, Copeland N G, Jenkins N A. An interspecific linkage map of mouse chromosome 15 positioned with respect to the centromere. Genomics. 1992;13:1075–1081. doi: 10.1016/0888-7543(92)90021-j. [DOI] [PubMed] [Google Scholar]
- 3.Brown D T, Sittman D B. Identification through overexpression and tagging of the variant type of the mouse H1e and H1c genes. J Biol Chem. 1993;268:713–718. [PubMed] [Google Scholar]
- 4.Dong Y, Sirotkin A M, Yang Y S, Brown D T, Sittman D B, Skoultchi A I. Isolation and characterization of two replication-dependent mouse H1 histone genes. Nucleic Acids Res. 1994;22:1421–1428. doi: 10.1093/nar/22.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drabent B, Saftig P, Bode C, Doenecke D. Spermatogenesis proceeds normally in mice without linker histone H1t. Histochem Cell Biol. 2000;113:433–442. doi: 10.1007/s004180000146. [DOI] [PubMed] [Google Scholar]
- 6.Fantz D A, Hatfield W R, Horvath G, Kistler M K, Kistler W S. Mice with a targeted disruption of the H1t gene are fertile and undergo normal changes in structural chromosomal proteins during spermiogenesis. Biol Reprod. 2001;64:425–431. doi: 10.1095/biolreprod64.2.425. [DOI] [PubMed] [Google Scholar]
- 7.Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan P M, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 9.Kornberg R D, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 10.Lennox R W, Cohen L H. The alterations in H1 histone complement during mouse spermatogenesis and their significance for H1 subtype function. Dev Biol. 1984;103:80–84. doi: 10.1016/0012-1606(84)90009-5. [DOI] [PubMed] [Google Scholar]
- 11.Lennox R W, Cohen L H. The H1 subtypes of mammals: metabolic characteristics and tissue distribution. In: Stein G S, Stein J L, Marzluff W F, editors. Histone genes: structure, organization and regulation. New York, NY: John Wiley & Sons; 1984. pp. 373–396. [Google Scholar]
- 12.Lennox R W, Cohen L H. The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem. 1983;258:262–268. [PubMed] [Google Scholar]
- 13.Lin Q. Ph.D. thesis. A genetic approach to the functions of specific H1 linker histones in mammalian spermatogenesis. Bronx, New York: Albert Einstein College of Medicine of Yeshiva University; 1998. [Google Scholar]
- 14.Lin Q, Sirotkin A, Skoultchi A I. Normal spermatogenesis in mice lacking the testis-specific linker histone H1t. Mol Cell Biol. 2000;20:2122–2128. doi: 10.1128/mcb.20.6.2122-2128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindner H, Helliger W, Dirschlmayer A, Jaquemar M, Puschendorf B. High-performance capillary electrophoresis of core histones and their acetylated modified derivatives. Biochem J. 1992;283:467–471. doi: 10.1042/bj2830467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meistrich M L, Bucci L R, Trostle-Weige P K, Brock W A. Histone variants in rat spermatogonia and primary spermatocytes. Dev Biol. 1985;112:230–240. doi: 10.1016/0012-1606(85)90137-x. [DOI] [PubMed] [Google Scholar]
- 17.Oko R J, Jando V, Wagner C L, Kistler W S, Hermo L S. Chromatin reorganization in rat spermatids during the disappearance of testis-specific histone, H1t, and the appearance of transition proteins TP1 and TP2. Biol Reprod. 1996;54:1141–1157. doi: 10.1095/biolreprod54.5.1141. [DOI] [PubMed] [Google Scholar]
- 18.Rabini S, Franke K, Saftig P, Bode C, Doenecke D, Drabent B. Spermatogenesis in mice is not affected by histone H1.1 deficiency. Exp Cell Res. 2000;255:114–124. doi: 10.1006/excr.1999.4767. [DOI] [PubMed] [Google Scholar]
- 19.Rudnicki M A, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- 20.Schena M, Shalon D, Davis R W, Brown P O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 21.Shalon D, Smith S J, Brown P O. A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res. 1996;6:639–645. doi: 10.1101/gr.6.7.639. [DOI] [PubMed] [Google Scholar]
- 22.Sirotkin A M. Ph.D. thesis. A genetic approach to H1 histone function. Bronx, New York: Albert Einstein College of Medicine of Yeshiva University; 1996. [Google Scholar]
- 23.Sirotkin A M, Edelmann W, Cheng G, Klein-Szanto A, Kucherlapati R, Skoultchi A I. Mice develop normally without the H10 linker histone. Proc Natl Acad Sci USA. 1995;92:6434–6438. doi: 10.1073/pnas.92.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M, Hennebold J D, Macfarlane J, Adashi E Y. A mammalian oocyte-specific linker histone gene H1oo: homology with the genes for the oocyte-specific cleavage stage histone (cs-H1) of sea urchin and the B4/H1M histone of the frog. Development. 2001;128:655–664. doi: 10.1242/dev.128.5.655. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z F, Sirotkin A M, Buchold G M, Skoultchi A I, Marzluff W F. The mouse histone H1 genes: gene organization and differential regulation. J Mol Biol. 1997;271:124–138. doi: 10.1006/jmbi.1997.1166. [DOI] [PubMed] [Google Scholar]
- 26.Wolffe A P. Chromatin: structure and function. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 27.Zlatanova J, Doenecke D. Histone H1 zero: a major player in cell differentiation? FASEB J. 1994;8:1260–1268. doi: 10.1096/fasebj.8.15.8001738. [DOI] [PubMed] [Google Scholar]