Abstract
Background
Racial and ethnic differences in survival after a first cancer are well established but have not been examined after a second primary cancer (SPC) despite the increasing incidence among survivors.
Methods
We examined 39 029 female breast cancer survivors who developed an SPC between 2000 and 2014 in the Surveillance, Epidemiology, and End Results 18 database. Multivariable Cox proportional hazards regression for competing risks data was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for cancer and cardiovascular disease mortality after SPCs comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black survivors with Non-Hispanic White survivors. Models were adjusted for sociodemographics, tumor characteristics, and treatments of the first and second cancer. Analyses were stratified by SPC type.
Results
During 17 years of follow-up, there were 15 117 deaths after SPCs. The risk of cancer death was 12% higher among Non-Hispanic Black survivors (HR = 1.12, 95% CI = 1.05 to 1.19) and 8% higher among Hispanic survivors (HR = 1.08, 95% CI = 1.00 to 1.16) compared with Non-Hispanic White survivors. In subgroup analyses, the strongest associations were observed among Non-Hispanic Black survivors with a second breast or uterine cancer and among Hispanic survivors with a second breast cancer. Non-Hispanic Black survivors also experienced a 44% higher risk of cardiovascular disease death after SPC diagnosis than Non-Hispanic White survivors (HR = 1.44, 95% CI = 1.20 to 1.74).
Conclusions
Higher cancer mortality among Non-Hispanic Black and Hispanic survivors and higher cardiovascular mortality among Non-Hispanic Black survivors exist among women who survive a first breast cancer to develop an SPC. Studies focused on identifying the contributors to these disparities are needed to enable implementation of effective mitigation strategies.
In 2030, the expected number of breast cancer survivors in the United States will be as high as 5 million (1). Second primary cancers (SPCs) can be a major comorbidity for breast cancer survivors and are not uncommon, with 20% of survivors predicted to develop an SPC at 35 years (2-4). We previously observed that breast cancer survivors who developed an SPC experienced elevated mortality compared with women with a first cancer of the same type (5).
Racial and ethnic disparities in mortality after an SPC diagnosis has not been reported but is likely to be present given differences in mortality after a first cancer (6-8). There are many factors that can differ across race and ethnicity among survivors and affect survival disparities after SPCs. Patients who develop SPCs have survived their first cancer and thus overcome many existing challenges and barriers while gaining valuable knowledge about cancer treatment and early detection (9,10). At the same time, survivors may have endured high financial costs related to their care, leaving them unable to access high-quality health care when they developed SPCs, long-term adverse effects from treatments that could affect treatment options, and psychosocial stressors as they now face the challenge of dealing with multiple cancers (11,12). Some survivors may experience more adverse effects during treatment of their SPCs, which may lead to greater nonadherence (13). Providers may also be influenced by a patient’s prior experiences when considering treatment options. From a biological perspective, some SPCs may require more aggressive treatments due to their molecular profile (14).
Survivors who develop an SPC may also have higher mortality from cardiovascular disease (CVD) given shared risk factors, adverse effects of cancer treatment, and aging (15,16). Observational studies suggest that Non-Hispanic Black women have a higher prevalence of cardiac risk factors and are more likely to die from CVD than White women after a first breast cancer diagnosis (11,17). However, whether CVD mortality after a SPC differs by race and ethnicity is largely unknown.
In this study, we used data from the National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER) to examine whether racial and ethnic differences in cancer and CVD mortality exist after a second cancer diagnosis among breast cancer survivors. A workshop led by the National Cancer Institute identified understanding and addressing disparities among cancer survivors as an area where more knowledge is needed (18).
Methods
Study population and design
The study population was identified from the SEER 18 (2000-2016) database (19), which covers 18 US cancer registries and represents 27.8% of the population. Women aged 18 years and older diagnosed with incident invasive breast cancer and a subsequent SPC between January 1, 2000, and December 31, 2014, were included. SPC was defined as any type of invasive cancer diagnosed at least 6 months after the first breast cancer. They were followed until December 31, 2016, at least 2 years after the SPC diagnosis. Women who developed more than 2 cancers during the study period were excluded.
Race and ethnicity
SEER provided variables on racial and ethnic categories based on information abstracted from medical and administrative records. We categorized women into the following groups: Hispanic, Non-Hispanic American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic Black, and Non-Hispanic White. Non-Hispanic American Indian or Alaska Native (n = 173) and Pacific Islanders (n = 241) were excluded due to small sample size.
Ascertainment of cancer and tumor characteristics
Information on cancer type and number of cancers was provided by cancer registries. SEER identified multiple primary cancers based on a series of rigorous rules that considered the cancer site (International Classification of Disease for Oncology, 3rd Edition) topography codes), time since initial cancer diagnosis, tumor histology, tumor behavior, and laterality (20). Tumor characteristics were available for both cancers.
To account for prognostic differences by cancer types, a new variable was created in which SPCs were categorized into 4 broad categories based on the 5-year relative survival rate (S) between 2010 and 2016 of the different cancer types published by the American Cancer Society: good survival group (S ≥ 90%), moderate survival group (70% ≤ S < 90%), fair survival group (50% ≤ S < 70%), and poor survival group (S < 50%) (6,21) (see Supplementary Methods, available online for details).
Other covariates
Demographic variables, including age at diagnosis, year of diagnosis, region of cancer registry, marital status, insurance status (available since 2007), and initial treatments were extracted from medical records. For treatment variables, the category “no/unknown” was used by SEER instead of “no” to reflect the fact that based on medical records it is not possible to be certain that some patients in which there was no record of treatment did not receive treatments.
Ascertainment of county-level variables
Linkage to the 2000 US Census and American Community Survey (2007-2011, 2012-2016, 2014-2018) enabled us to incorporate median household income, percent of residents with less than ninth-grade education, percent of residents below federal poverty, and rural or urban status of the county.
Vital status and cause of death
Vital status and cause of death based on International Classification of Disease codes were provided by the National Center for Health Statistics (22). Death from CVD included death from “diseases of heart,” “hypertension without heart disease,” “cerebrovascular diseases,” “atherosclerosis,” “aortic aneurysm and dissection,” and “other diseases of arteries, arterioles, capillaries.”
Statistical analysis
The demographic and tumor characteristics of SPCs and the first breast cancer were described by racial and ethnic groups. We calculated proportions for categorical variables and means and standard deviations (SD) or medians (10th-90th percentile) for continuous variables.
Time-to-event analysis was conducted to compare the mortality after SPCs between racial and ethnic groups. Individuals contributed person-months from time at SPC diagnosis to time of death from cancer, from CVD, and from other conditions and could be censored at the time of last contact or December 31, 2016, whichever came first. One-half month of follow-up time was added to women with survival time of 0 months. Cumulative incidence function comparing cancer mortality between racial and ethnic groups was graphed considering competing risk of death from noncancer conditions. When estimating the cumulative incidence function, the racial and ethnic groups were propensity score weighted by age at SPC diagnosis. Hazard ratios (HRs) with 95% confidence intervals (CIs) for cancer mortality comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black to Non-Hispanic White survivors were estimated from multivariable Cox proportional hazards regression. The proportional hazard assumption was verified by graphing and testing the Schoenfeld residuals. We conducted 3 models that included potential confounders and risk factors. Model 1 adjusted for age at diagnosis of SPCs (continuous), year of diagnosis (2000-2004, 2005-2009, 2010-2014), and prognostic groups (previously described). Model 2 further adjusted for tumor stage and initial treatments of SPCs (surgery, chemotherapy, and radiation therapy), marital status (married vs single), region of cancer registry (West, South, Midwest, Northeast), county-level median household income (continuous), and percent of residents with less than ninth-grade education (continuous). Model 3, in addition, adjusted for characteristics of the initial breast cancer, including age at diagnosis, tumor stage, estrogen receptor (ER) status, and initial treatments (surgery, chemotherapy, and radiation therapy). Subgroup analyses were conducted among survivors who developed the top 4 most common SPCs, including second breast cancers (further adjusted for ER status of the second cancer in model 2 and 3), lung cancer, colorectal cancer, and uterine cancer separately. For second breast cancer, analyses were further stratified by hormone receptor status of the first and second cancer and by molecular subtype (luminal A, HER2-positive, triple negative) of the second cancer. For non-breast SPCs, to distinguish between mortality from first and second cancer, we quantified the hazard ratio for death from common SPC type and death from the first breast cancer. The association of race and ethnicity with cancer mortality among breast cancer survivors who did not develop a SPC was also assessed.
Details on the methods used to evaluate the association of race and ethnicity with CVD mortality and all-cause mortality after SPCs, stratified analyses by age categories, and sensitivity analyses are reported in Supplementary Methods (available online). All analyses were performed in software R (version 4.1.2). Two-sided P values less than .05 were considered statistically significant in hypothesis testing.
Results
Demographic and clinical characteristics
In this SEER study, there were 39 029 SPCs among women diagnosed with breast cancers between 2000 and 2014. Table 1 describes characteristics of the study population stratified by race and ethnicity. SPCs occurred at an earlier age in Hispanic (mean age = 62.2 years), Non-Hispanic Asian (mean age = 63.4 years), and Non-Hispanic Black (mean age = 63.5 years) survivors compared with Non-Hispanic White survivors (mean age = 68.8 years). Breast cancer was the most common SPC in all racial and ethnic groups. SPCs in Non-Hispanic Black women were less likely to be local stage compared with other groups. Hispanic and Non-Hispanic Black survivors were more likely to receive chemotherapy twice. Characteristics of the first breast cancer differed by race and ethnicity (Supplementary Table 1, available online). Only 59% of Hispanic and Non-Hispanic Black survivors had local-stage first breast cancer compared with more than 66% of Non-Hispanic Asian and Non-Hispanic White survivors. Almost 30% of first breast cancers in Non-Hispanic Black women were ER-negative.
Table 1.
Demographic and clinical characteristics of the breast cancer survivors diagnosed with an SPC by racial and ethnic groups
| Characteristic | Hispanic (n = 3197) | Non-Hispanic Asian (n = 2146) | Non-Hispanic Black (n = 4227) | Non-Hispanic White (n = 29 459) |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Age at diagnosis, mean (SD), y | 62.2 (13.6) | 63.4 (13.1) | 63.5 (13.4) | 68.8 (12.7) |
| ≤70 | 2229 (69.7) | 1481 (69.0) | 2869 (67.9) | 15 421 (52.3) |
| >70 | 968 (30.3) | 665 (31.0) | 1358 (32.1) | 14 038 (47.7) |
| Year of diagnosis | ||||
| 2000-2004 | 288 (9.0) | 206 (9.6) | 446 (10.6) | 3241 (11.0) |
| 2005-2009 | 1023 (32.0) | 699 (32.6) | 1359 (32.2) | 9972 (33.9) |
| 2010-2014 | 1886 (59.0) | 1241 (57.8) | 2422 (57.3) | 16 246 (55.1) |
| SPC cancer group | ||||
| Good survival (Sa ≥ 90%) | 1621 (50.7) | 1018 (47.4) | 2080 (49.2) | 12 186 (41.4) |
| Moderate survival (70% ≤ S < 90%) | 426 (13.3) | 320 (14.9) | 540 (12.8) | 4889 (16.6) |
| Fair survival (50% ≤ S < 70%) | 457 (14.3) | 335 (15.6) | 720 (17.0) | 5107 (17.3) |
| Poor survival (S < 50%) | 693 (21.7) | 473 (22.0) | 887 (21.0) | 7277 (24.7) |
| Top 4 SPC type | ||||
| Breast | 1420 (44.4) | 879 (41.0) | 1965 (46.5) | 9895 (33.6) |
| Lung | 259 (8.1) | 187 (8.7) | 451 (10.7) | 4179 (14.2) |
| Colorectum | 206 (6.4) | 187 (8.7) | 391 (9.3) | 2670 (9.1) |
| Uterus | 167 (5.2) | 166 (7.7) | 249 (5.9) | 1890 (6.4) |
| Tumor stage | ||||
| Local | 1516 (47.4) | 1119 (52.1) | 1907 (45.1) | 14 894 (50.6) |
| Regional | 811 (25.4) | 494 (23.0) | 1077 (25.5) | 6388 (21.7) |
| Distant | 697 (21.8) | 424 (19.8) | 1028 (24.3) | 6505 (22.1) |
| Missing | 173 (5.4) | 109 (5.1) | 215 (5.1) | 1672 (5.7) |
| Surgery | ||||
| Yes | 2180 (68.2) | 1579 (73.6) | 2794 (66.1) | 20 251 (68.7) |
| No/unknown | 1017 (31.8) | 567 (26.4) | 1433 (33.9) | 9208 (31.3) |
| Chemotherapy | ||||
| Yes | 1127 (35.3) | 715 (33.3) | 1577 (37.3) | 8674 (29.4) |
| No/unknown | 2070 (64.7) | 1431 (66.7) | 2650 (62.7) | 20 785 (70.6) |
| Frequency of chemotherapy | ||||
| Never | 1177 (36.8) | 901 (42.0) | 1548 (36.6) | 14 460 (49.1) |
| Once | 1373 (42.9) | 889 (41.4) | 1783 (42.2) | 11 244 (38.2) |
| Twice | 647 (20.2) | 356 (16.6) | 896 (21.2) | 3755 (12.7) |
| Radiation | ||||
| Yes | 656 (20.5) | 475 (22.1) | 904 (21.4) | 6544 (22.2) |
| No/unknown | 2541 (79.5) | 1671 (77.9) | 3323 (78.6) | 22 915 (77.8) |
| Marital status | ||||
| Singleb | 1373 (42.9) | 752 (35.0) | 2609 (61.7) | 13 087 (44.4) |
| Married/unmarried or domestic partner | 1592 (49.8) | 1251 (58.3) | 1295 (30.6) | 14 196 (48.2) |
| Missing | 232 (7.3) | 143 (6.7) | 323 (7.6) | 2176 (7.4) |
| Insurance statusc | ||||
| Insured | 1853 (71.0) | 1390 (80.0) | 2511 (74.7) | 20 037 (87.2) |
| Medicaid | 579 (22.2) | 250 (14.4) | 651 (19.4) | 1599 (7.0) |
| Uninsured | 65 (2.5) | 19 (1.1) | 57 (1.7) | 175 (0.8) |
| Missing | 114 (4.4) | 78 (4.5) | 141 (4.2) | 1179 (5.1) |
| County-level median household income, median [10th, 90th], $ | 56 200 [42 100, 77 400] | 63 800 [47 100, 85 800] | 51 400 [36 600, 72 800] | 56 300 [39 400, 78 600] |
| County-level <9th grade, median [10th, 90th], % | 9.85 [4.55, 15.8] | 7.34 [3.70, 13.8] | 5.93 [3.35, 13.3] | 5.86 [2.79, 13.3] |
| County-level below poverty, median [10th, 90th], % | 16.3 [9.32, 21.4] | 12.4 [8.35, 18.2] | 16.4 [9.51, 25.0] | 12.9 [7.34, 20.8] |
| Rural/urban status of county | ||||
| Rural | 2 (0.1) | 0 (0) | 26 (0.6) | 479 (1.6) |
| Urban | 3194 (99.9) | 2146 (100) | 4201 (99.4) | 28 978 (98.4) |
| Region of cancer registry | ||||
| West | 2630 (82.3) | 1896 (88.4) | 1201 (28.4) | 14 359 (48.7) |
| South | 113 (3.5) | 64 (3.0) | 1821 (43.1) | 6325 (21.5) |
| Midwest | 43 (1.3) | 21 (1.0) | 507 (12.0) | 3181 (10.8) |
| Northeast | 411 (12.9) | 165 (7.7) | 698 (16.5) | 5594 (19.0) |
| Time to censoring, median [10th, 90th], mo | 36 [3, 100] | 42 [5, 114] | 33 [2, 100] | 39 [3, 111] |
| Time since first breast cancer, median [10th, 90th], mo | 52 [13, 120] | 52 [13, 119] | 53 [14, 119] | 55 [13, 122] |
S =5-year relative survival. SPC = second primary cancer.
Single included never married, separated, divorced, and widowed.
Insurance status is available in year 2007 and onwards.
Cancer mortality
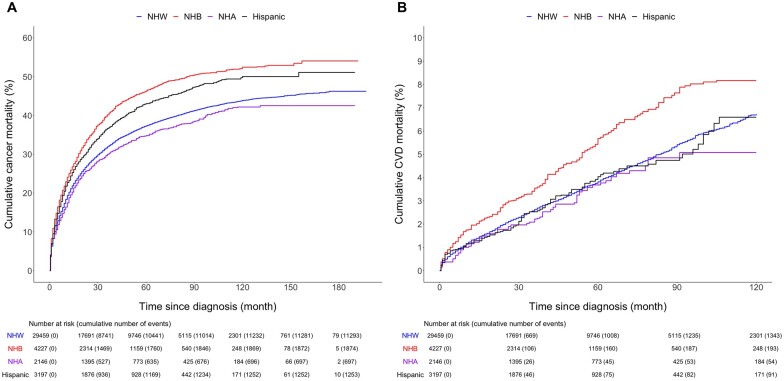
Non-Hispanic Black survivors had the highest cumulative cancer mortality during entire follow-up, followed by Hispanic, Non-Hispanic White, and Non-Hispanic Asian survivors (Figure 1A). The hazard ratio of death from cancer comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black survivors to Non-Hispanic White survivors are presented in Table 2. In model 1, the risk of death from cancer was elevated among Non-Hispanic Black (HR = 1.46, 95% CI = 1.39 to 1.53) and Hispanic (HR = 1.24, 95% CI = 1.17 to 1.31) survivors compared with Non-Hispanic White survivors, while a decreased risk was observed for Non-Hispanic Asian women (HR = 0.89, 95% CI = 0.83 to 0.96). In model 2, which further adjusted for tumor stage, treatments, marital status, and county-level factors, the hazard ratio of cancer death for Non-Hispanic Black (HR = 1.19, 95% CI = 1.12 to 1.25) and Hispanic (HR = 1.14, 95% CI = 1.06 to 1.21) survivors decreased respectively compared with model 1. In model 3, the fully adjusted model, the risk of cancer death increased by 12% (HR = 1.12, 95% CI = 1.05 to 1.19) among Non-Hispanic Black survivors and by 8% (HR = 1.08, 95% CI = 1.00 to 1.16) among Hispanic survivors compared with Non-Hispanic White survivors. Further adjustment of percent of residents below poverty, rural or urban status of the county, and individual health insurance status yielded similar results (Supplementary Table 2, available online). The hazard ratio of cancer death among Non-Hispanic Black breast cancer survivors who developed an SPC was increased to a slightly lesser degree compared with Non-Hispanic Black survivors who did not develop an SPC (Supplementary Table 3, available online).
Figure 1.
Cumulative cancer (A) and cardiovascular disease (CVD) (B) mortality for Hispanic, Non-Hispanic Asian (NHA), Non-Hispanic Black (NHB), and Non-Hispanic White (NHW) survivors diagnosed with second primary cancers (SPCs). The racial or ethnic groups were propensity-score weighted by age at SPC diagnosis. Weighted mean (SD) age was 69 (13) years across groups. Follow-up started from the diagnosis of the SPC and ended at December 31, 2016 (end of the study) for cancer mortality and at 120 months (10 years) for CVD mortality.
Table 2.
Hazard ratios (95% confidence intervals) of cancer death comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black survivors with Non-Hispanic White survivors overall and by type of SPCs
| SPC, race and ethnicity | No. of SPC | Person months | No. of deaths | Model 1a | Model 2a | Model 3a |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| All SPCs | ||||||
| Hispanic | 3197 | 144 973 | 1253 | 1.24 (1.17 to 1.31) | 1.14 (1.06 to 1.21) | 1.08 (1.00 to 1.16) |
| Non-Hispanic Asian | 2146 | 113 402.5 | 697 | 0.89 (0.83 to 0.96) | 0.93 (0.85 to 1.01) | 0.92 (0.85 to 1.01) |
| Non-Hispanic Black | 4227 | 182 490.5 | 1874 | 1.46 (1.39 to 1.53) | 1.19 (1.12 to 1.25) | 1.12 (1.05 to 1.19) |
| Non-Hispanic White | 29 459 | 1 437 088.5 | 11 293 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second breast cancerb | ||||||
| Hispanic | 1420 | 75 866 | 424 | 1.54 (1.39 to 1.72) | 1.24 (1.09 to 1.41) | 1.17 (1.01 to 1.35) |
| Non-Hispanic Asian | 879 | 54 737 | 172 | 0.89 (0.76 to 1.04) | 0.90 (0.75 to 1.08) | 0.85 (0.71 to 1.03) |
| Non-Hispanic Black | 1965 | 101 607 | 656 | 1.77 (1.62 to 1.94) | 1.27 (1.14 to 1.42) | 1.28 (1.14 to 1.44) |
| Non-Hispanic White | 9895 | 601 328.5 | 2087 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second lung cancer | ||||||
| Hispanic | 259 | 5914 | 177 | 1.03 (0.89 to 1.20) | 0.92 (0.78 to 1.08) | 0.86 (0.71 to 1.03) |
| Non-Hispanic Asian | 187 | 6181.5 | 112 | 0.76 (0.63 to 0.91) | 0.68 (0.55 to 0.84) | 0.68 (0.54 to 0.85) |
| Non-Hispanic Black | 451 | 10 569.5 | 318 | 1.07 (0.95 to 1.20) | 0.84 (0.74 to 0.95) | 0.81 (0.71 to 0.93) |
| Non-Hispanic White | 4179 | 101 526 | 2928 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second colorectal cancer | ||||||
| Hispanic | 206 | 9725.5 | 74 | 1.14 (0.90 to 1.44) | 1.17 (0.91 to 1.51) | 1.15 (0.88 to 1.52) |
| Non-Hispanic Asian | 187 | 10 696 | 52 | 0.85 (0.64 to 1.12) | 0.95 (0.70 to 1.28) | 0.99 (0.73 to 1.34) |
| Non-Hispanic Black | 391 | 17 925.5 | 155 | 1.40 (1.18 to 1.67) | 1.14 (0.95 to 1.38) | 1.07 (0.87 to 1.31) |
| Non-Hispanic White | 2670 | 137 148 | 957 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second uterine cancer | ||||||
| Hispanic | 167 | 8899 | 46 | 1.28 (0.94 to 1.73) | 1.27 (0.90 to 1.79) | 0.98 (0.66 to 1.44) |
| Non-Hispanic Asian | 166 | 9365 | 42 | 1.07 (0.78 to 1.47) | 1.13 (0.80 to 1.60) | 0.91 (0.61 to 1.36) |
| Non-Hispanic Black | 249 | 11 375.5 | 98 | 1.83 (1.48 to 2.28) | 1.31 (1.02 to 1.68) | 1.25 (0.95 to 1.65) |
| Non-Hispanic White | 1890 | 116 947.5 | 534 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
Model 1 adjusted for age at diagnosis of SPC, year of diagnosis of SPC, and type of SPC (4 cancer groups based on 5-year relative survival rate. This is only for all SPC model); Model 2: Model 1 + tumor stage of SPC, treatment of SPC (surgery, chemotherapy, and radiation therapy), marital status, median household income, percent less than ninth grade, and region of registry at SPC diagnosis; Model 3: Model 2 + age at diagnosis of first breast cancer, tumor stage of first breast cancer, estrogen receptor status of first breast cancer, treatment of first breast cancer (surgery, chemotherapy, and radiation therapy). CI = confidence interval; HR = hazard ratio; SPC = second primary cancer.
Model 2 and 3 further adjusted for estrogen receptor status of the second breast cancer.
In subgroup analyses by second cancer type, we observed an increase in risk of cancer death for Non-Hispanic Black (HR = 1.28, 95% CI = 1.14 to 1.44) and Hispanic (HR = 1.17, 95% CI = 1.01 to 1.35) survivors for breast cancer compared with Non-Hispanic White survivors (Table 2). These results remained in analysis restricted to women diagnosed with a breast cancer 1 year and more after diagnosis and by laterality (Supplementary Table 4, available online). An increased risk of cancer death among Non-Hispanic Black survivors was consistently observed among various molecular-based subgroups of the first and second breast cancers, except among women diagnosed with 2 hormone receptor-negative cancers (Supplementary Table 5, available online).
A statistically significant increase in mortality among Non-Hispanic Black survivors was observed for second colorectal and uterine cancer in model 1 but not in the fully adjusted model (Table 2). Hispanic survivors had a non-statistically significant increase in the risk of cancer death after second colorectal cancer. A 32% lower risk of cancer death was observed among Non-Hispanic Asian survivors after a second lung cancer. Based on our analysis of second lung, colorectal, and uterine cancer, it appears that both the SPC and first breast cancer contributed to the increased mortality among Non-Hispanic Black survivors after a second colorectal and uterine cancer, whereas this was not the case for lung cancer (Supplementary Table 6, available online).
Stratified analysis for the fully adjusted model did not show statistically significant interaction between race and ethnicity and age (70 years and younger and older than 70 years) at SPC diagnosis (Poverall interaction = .12). However, we observed a higher hazard ratio of death from cancer comparing Non-Hispanic Black survivors with Non-Hispanic White survivors among women 70 years or younger compared with women older than 70 years (HR≤70yrs = 1.17, 95% CI = 1.08 to 1.26; HR>70yrs = 1.07, 95% CI = 0.97 to 1.17; Pnon-Hispanic Black*age70 = .02) (Table 4).
Table 4.
Hazard ratios (95% confidence intervals) of death from cancer and cardiovascular disease comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black to Non-Hispanic White survivors among women diagnosed with SPCs age 70 years and younger, and older than 70 years of age
| Age, race and ethnicity | No. of SPC | Person-months | Death from cancer |
Death from cardiovascular disease |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of deaths | Model 1a | Model 2a | Model 3a | No. of deaths | Model 1a | Model 2a | Model 3a | |||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| ≤70 y | ||||||||||
| Hispanic | 2229 | 110 560.5 | 779 | 1.27 (1.18 to 1.37) | 1.10 (1.01 to 1.20) | 1.04 (0.94 to 1.14) | 28 | 1.17 (0.79 to 1.73) | 1.14 (0.76 to 1.73) | 1.03 (0.64 to 1.66) |
| Non-Hispanic Asian | 1481 | 86 278.5 | 420 | 0.88 (0.79 to 0.97) | 0.94 (0.84 to 1.04) | 0.91 (0.81 to 1.02) | 19 | 0.96 (0.60 to 1.52) | 1.08 (0.66 to 1.77) | 1.08 (0.64 to 1.83) |
| Non-Hispanic Black | 2869 | 137 919 | 1190 | 1.56 (1.46 to 1.66) | 1.24 (1.15 to 1.33) | 1.17 (1.08 to 1.26) | 80 | 2.60 (2.02 to 3.35) | 1.85 (1.39 to 2.46) | 1.70 (1.23 to 2.33) |
| Non-Hispanic White | 15 421 | 890 655.5 | 4990 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 246 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| >70 y | ||||||||||
| Hispanic | 968 | 34 412.5 | 474 | 1.17 (1.07 to 1.29) | 1.15 (1.04 to 1.28) | 1.08 (0.97 to 1.21) | 69 | 1.12 (0.88 to 1.43) | 0.99 (0.76 to 1.30) | 1.01 (0.75 to 1.35) |
| Non-Hispanic Asian | 665 | 27 124 | 277 | 0.90 (0.80 to 1.01) | 0.89 (0.78 to 1.02) | 0.92 (0.80 to 1.06) | 42 | 0.79 (0.58 to 1.08) | 0.84 (0.60 to 1.17) | 0.76 (0.52 to 1.10) |
| Non-Hispanic Black | 1358 | 44 571.5 | 684 | 1.29 (1.19 to 1.40) | 1.12 (1.02 to 1.22) | 1.07 (0.97 to 1.17) | 116 | 1.35 (1.12 to 1.64) | 1.28 (1.04 to 1.58) | 1.31 (1.04 to 1.64) |
| Non-Hispanic White | 14 038 | 546 433 | 6303 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1176 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| P interaction | ||||||||||
| Poverall interactionb | .12 | .07 | ||||||||
| PHispanic*age70c | .51 | .97 | ||||||||
| PNon-Hispanic Asian*age70c | .59 | .46 | ||||||||
| PNon-Hispanic Black*age70c | .02 | .009 | ||||||||
Model 1 adjusted for age at diagnosis of SPC, year of diagnosis of SPC, and type of SPC (4 cancer groups based on 5-year relative survival rate); Model 2: Model 1 + tumor stage of SPC, treatment of SPC (surgery, chemotherapy, and radiation therapy), marital status, median household income, precent ninth grade, and region of registry at SPC diagnosis; Model 3: Model 2 + age at diagnosis of first breast cancer, tumor stage of first breast cancer, estrogen receptor status of first breast cancer, treatment of first breast cancer (surgery, chemotherapy, and radiation therapy). CI = confidence interval; HR = hazard ratio; SPC = second primary cancer.
P value was from the likelihood ratio test comparing Model 3 with interaction between race and ethnicity and age category (70 years and younger vs older than 70 years) with Model 3 without interaction.
P value was for the interaction term between each race and ethnicity and age category (70 years and younger vs older than 70 years) using Model 3.
CVD mortality
Cumulative CVD mortality among Non-Hispanic Black survivors was higher than other groups during the first 10 years since diagnosis (Figure 1B). Non-Hispanic Black survivors with SPCs also had a greater risk of dying from CVD than Non-Hispanic White survivors (HR = 1.44, 95% CI = 1.20 to 1.74), whereas Hispanic and Non-Hispanic Asian survivors did not (Table 3). An increased risk from CVD death in Non-Hispanic Black survivors was observed among women with a second breast cancer (HR = 1.74, 95% CI = 1.30 to 2.32) and colorectal cancer (HR = 1.64, 95% CI = 1.02 to 2.64) but not lung cancer (HR = 1.08, 95% CI = 0.61 to 1.92). Hispanic survivors had a statistically significant increase in the risk of CVD death after a second colorectal cancer in model 1 but not in the fully adjusted model. The sample size for uterine cancer was too small to yield precise estimates.
Table 3.
Hazard ratios (95% confidence intervals) of death from cardiovascular disease comparing Hispanic, Non-Hispanic Asian, and Non-Hispanic Black to Non-Hispanic White survivors overall and by type of SPCs
| SPC, race and ethnicity | No. of deaths | Model 1a | Model 2a | Model 3a |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| All SPCs | ||||
| Hispanic | 97 | 1.12 (0.91 to 1.38) | 1.03 (0.82 to 1.29) | 1.01 (0.78 to 1.29) |
| Non-Hispanic Asian | 61 | 0.83 (0.64 to 1.07) | 0.89 (0.67 to 1.17) | 0.83 (0.61 to 1.13) |
| Non-Hispanic Black | 196 | 1.67 (1.44 to 1.94) | 1.47 (1.25 to 1.74) | 1.44 (1.20 to 1.74) |
| Non-Hispanic White | 1422 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second breast cancerb | ||||
| Hispanic | 36 | 1.04 (0.74 to 1.47) | 0.86 (0.57 to 1.31) | 0.91 (0.58 to 1.42) |
| Non-Hispanic Asian | 19 | 0.64 (0.41 to 1.02) | 0.75 (0.45 to 1.23) | 0.62 (0.35 to 1.12) |
| Non-Hispanic Black | 91 | 1.94 (1.55 to 2.43) | 1.62 (1.25 to 2.09) | 1.74 (1.30 to 2.32) |
| Non-Hispanic White | 479 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second lung cancer | ||||
| Hispanic | 7 | 0.97 (0.45 to 2.06) | 0.99 (0.45 to 2.15) | 0.81 (0.32 to 2.02) |
| Non-Hispanic Asian | 10 | 1.62 (0.85 to 3.08) | 1.80 (0.92 to 3.53) | 1.86 (0.87 to 3.96) |
| Non-Hispanic Black | 24 | 1.80 (1.17 to 2.78) | 1.31 (0.81 to 2.13) | 1.08 (0.61 to 1.92) |
| Non-Hispanic White | 148 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second colorectal cancer | ||||
| Hispanic | 18 | 1.76 (1.08 to 2.86) | 1.60 (0.96 to 2.67) | 1.54 (0.86 to 2.76) |
| Non-Hispanic Asian | 2 | 0.21 (0.05 to 0.86) | 0.20 (0.05 to 0.83) | 0.21 (0.05 to 0.86) |
| Non-Hispanic Black | 27 | 1.52 (1.01 to 2.28) | 1.64 (1.07 to 2.50) | 1.64 (1.02 to 2.64) |
| Non-Hispanic White | 203 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Second uterine cancer | ||||
| Hispanic | 4 | 1.02 (0.37 to 2.78) | 1.16 (0.41 to 3.25) | 0.89 (0.27 to 2.92) |
| Non-Hispanic Asian | 6 | 1.21 (0.53 to 2.78) | 1.33 (0.57 to 3.13) | 0.79 (0.24 to 2.59) |
| Non-Hispanic Black | 3 | 0.43 (0.14 to 1.37) | 0.49 (0.15 to 1.60) | 0.56 (0.17 to 1.83) |
| Non-Hispanic White | 98 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
Model 1 adjusted for age at diagnosis of SPC, year of diagnosis of SPC, and type of SPC (4 cancer groups based on 5-year relative survival rate. This is only for all SPC model); Model 2: Model 1 + tumor stage of SPC, treatment of SPC (surgery, chemotherapy, and radiation therapy), marital status, median household income, percent less than ninth grade, and region of registry at SPC diagnosis; Model 3: Model 2 + age at diagnosis of first breast cancer, tumor stage of first breast cancer, estrogen receptor status of first breast cancer, treatment of first breast cancer (surgery, chemotherapy, and radiation therapy). CI = confidence interval; HR = hazard ratio; SPC = second primary cancer.
Model 2 and 3 further adjusted for estrogen receptor status of the second breast cancer.
Stratified analysis overall did not show statistically significant interaction between race and ethnicity and age 70 years at SPC diagnosis (Poverall interaction =.07). However, the hazard ratio of death from CVD in Non-Hispanic Black women compared with Non-Hispanic White among women 70 years and younger was larger than among women older than 70 years (HR≤70yrs = 1.70, 95% CI = 1.23 to 2.33; HR>70yrs = 1.31, 95% CI = 1.04 to 1.64, Pnon-Hispanic Black*age 70 = .009) (Table 4).
All-cause mortality
Similar to cancer mortality, an increased risk of overall death among Hispanic and Non-Hispanic Black survivors but a decreased risk among Non-Hispanic Asian survivors compared with Non-Hispanic White survivors was observed (Supplementary Table 7, available online).
In sensitivity analyses, the Fine and Gray competing risk models showed comparable results with the Cox model for evaluating the risk of death from cancer and CVD (Supplementary Table 8, available online). Using age as time scale yielded similar results (Supplementary Table 9, available online). Similar racial and ethnic differences were observed among women diagnosed with a local-stage SPC (Supplementary Table 10, available online). The Non-Hispanic White–Non-Hispanic Black disparity in cancer mortality persisted during the entire study period while the Non-Hispanic White–Hispanic disparity appeared after 2005 (Supplementary Table 11, available online). When stratified by age 50 years (a surrogate for menopausal status) for cancer mortality, the hazard ratios among survivors 50 years and younger were similar to those who were older than 50 years (Supplementary Table 12, available online).
Discussion
To our knowledge, this is the first study to comprehensively examine the racial and ethnic disparities in survival outcomes after a second cancer. We observed that Hispanic and Non-Hispanic Black breast cancer survivors after a second cancer had higher cancer mortality than their Non-Hispanic White counterparts, whereas Non-Hispanic Asian survivors had lower mortality, even after adjusting for demographics, tumor characteristics, and county-level factors. Survivors of racial and ethnic minorities were diagnosed with SPCs up to 6 years younger than Non-Hispanic White survivors and within a shorter time from their first cancer. Increased cancer mortality was observed among Non-Hispanic Black survivors with second breast, colorectal, and uterine cancer and among Hispanic survivors with second breast cancer. We demonstrated that in breast cancer survivors who developed an SPC, mortality can be due to either their first cancer or their SPC. Non-Hispanic Black survivors also had increased risk of death from CVD, particularly when diagnosed with second breast and colorectal cancer. Larger differences in both cancer and CVD mortality were observed between Non-Hispanic Black and Non-Hispanic White survivors diagnosed with SPCs 70 years of age and younger, although the interaction between race and ethnicity and age categories was not statistically significant.
In this study, later stage at presentation and more aggressive tumor characteristics contributed to increased cancer mortality among Hispanic and Non-Hispanic Black survivors after SPCs, as did marital status, county-level education, and household income at diagnosis. There is no prior study to directly compare our findings with, but 2 large US studies reported that tumor characteristics, neighborhood socioeconomic status, insurance type, and marital status contributed to a statistically significant proportion (approximately 50%) of the racial and ethnic survival disparity among women diagnosed with a primary cancer (23,24). Racial and ethnic differences in screening uptake and adherence may also be contributors (25,26). Doses and toxicities from previous cancer treatments, particularly chemotherapy, can preclude SPC survivors from being prescribed the standard treatment. We observed more than 20% of Non-Hispanic Black survivors diagnosed with SPC received chemotherapy twice compared with 13% in Non-Hispanic White survivors. Providers may be less likely to prescribe aggressive treatment the second time around based on how patients tolerated it. Obesity is another factor that can affect mortality, and its prevalence is higher among Hispanic and Non-Hispanic Black cancer survivors (24,27). Preclinical data suggest that obesity may reduce the effectiveness of therapies such as tamoxifen through acquired resistance (28). Comorbidities, such as diabetes, can complicate treatment options and increase mortality (29).
Interestingly, Non-Hispanic Asian survivors in this study had lower mortality than Non-Hispanic White survivors for lung cancer. A meta-analysis showed that Asian patients with a first lung cancer experienced 14% lower mortality than White patients (30). The lower mortality could be due to more favorable behavior such as smoking, where Asian survivors are more likely to be never smokers and less likely to be former or current smokers (31). Asian patients are also more likely than White patients to have epidermal growth factor receptor-positive lung cancers, which can have a more favorable short-term outcomes when treated with targeted therapies involving tyrosine kinase inhibitor (32).
Non-Hispanic Black patients not only had higher risk of death from cancer than Non-Hispanic White patients, but they are also more likely to die from CVD, particularly women diagnosed at age 70 years and younger. Previous studies have shown that Black cancer survivors had a higher prevalence of CVD-related risk factors, CVD incidence, and mortality (17,31). In addition, Non-Hispanic Black women were more likely to receive chemotherapy due to the specific subtype or more aggressive disease. Agents such as anthracyclines can cause cardiotoxicity and an elevated risk of dying from CVD (33).
This study has several limitations. We were not able to investigate factors such as provider recommendation, receipt of appropriate treatment, treatment adherence, access to health care, patient behaviors, and health status, including smoking, increased weight, and comorbidities, all of which can play a role in the racial and ethnic disparities. There could be misclassification on cause of death from death certificates for survivors with SPCs (34). However, this should be nondifferential across racial and ethnic groups, resulting in an underestimation of the observed associations. The strengths of this study include the large sample size of SPC survivors representative of multiple racial and ethnic groups across the United States, the long-term follow-up, the high-quality data on cancer diagnosis, tumor characteristics, mortality, and the rigorous ascertainment of SPCs. The approach used in this article can be applied to other cancers.
In summary, this large population-based study observed racial and ethnic disparities in mortality among breast cancer survivors who developed an SPC. The findings are extremely concerning given the increasing prevalence of second cancer at a young age among women diagnosed with breast cancer. In our study, Hispanic and Non-Hispanic Black survivors had higher risk of death from cancer, and Non-Hispanic Black survivors had higher risk of death from CVD. A multilevel approach is needed to identify patient, provider, and systems level contributors to survival outcomes among breast cancer survivors.
Supplementary Material
Contributor Information
Zhengyi Deng, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Miranda R Jones, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Oncology, Kimmel Cancer Center, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Mei-Cheng Wang, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Antonio C Wolff, Department of Oncology, Kimmel Cancer Center, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Kala Visvanathan, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Oncology, Kimmel Cancer Center, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Funding
This work was supported by the Breast Cancer Research Foundation. MJ, AW and KV are supported by P30CA006973.
Notes
Role of funder: The funding source has no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author disclosures: K. Visvanathan reports funding from Cepheid and non-financial support from Optra Health Inc. K. Visvanathan also has a patent to C11625 issued. A.C. Wolf reports a patent to C12014 and its use licensed (license terminated by Cepheid 2020). Z. Deng, MR. Jones and MC. Wang have no disclosures.
KV, a JNCI Associate Editor and coauthor of this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: ZD: Conceptualization; Data curation; Methodology; Formal analysis; Writing-original draft; Writing-review and editing. MRJ: Writing-review and editing. M-CW: Writing-review and editing. ACW: Writing-review and editing. KV: Conceptualization; Funding acquisition; Methodology; Supervision; Writing-original draft; Writing-review and editing.
Prior presentation: 2021 virtual AACR San Antonio Breast Cancer Symposium, December 7-10, 2021. Abstract P3-14-05: Racial/ethnic disparities in cancer mortality after a second breast cancer. Cancer Research (2022) 82 (4_Supplement): P3-14-05.
Data availability
The data underlying this article are available in National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, at https://seer.cancer.gov/.
References
- 1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2. Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute, NIH Publ. No. 05-5302; 2006. [Google Scholar]
- 3. Molina-Montes E, Requena M, Sánchez-Cantalejo E, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;136(1):158-171. doi: 10.1016/j.ygyno.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 4. Nsouli-Maktabi HH, Henson DE, Younes N, Young HA, Cleary SD.. Second primary breast, endometrial, and ovarian cancers in Black and White breast cancer survivors over a 35-year time span: effect of age. Breast Cancer Res Treat. 2011;129(3):963-969. doi: 10.1007/s10549-011-1560-9. [DOI] [PubMed] [Google Scholar]
- 5. Deng Z, Jones MR, Wang M-C, Visvanathan K.. Mortality after second malignancy in breast cancer survivors compared to a first primary cancer: a nationwide longitudinal cohort study. NPJ Breast Cancer. 2022;8(1):1-10. doi: 10.1038/s41523-022-00447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/CAAC.21654/FORMAT/PDF/OEBPS/PAGES/1.PAGE.XHTML. [DOI] [PubMed] [Google Scholar]
- 7. Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532-1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 8. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheng JY, Visvanathan K, Thorner E, Wolff AC.. Breast cancer survivorship care beyond local and systemic therapy. Breast. 2019;48:S103-S109. doi: 10.1016/S0960-9776(19)31135-X. [DOI] [PubMed] [Google Scholar]
- 10. Earle CC, Burstein HJ, Winer EP, Weeks JC.. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21(8):1447-1451. doi: 10.1200/JClinOncol.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 11. Connor AE, Schmaltz CL, Jackson‐Thompson J, Visvanathan K.. Comorbidities and the risk of cardiovascular disease mortality among racially diverse patients with breast cancer. Cancer. 2021;127(15):2614-2622. doi: 10.1002/cncr.33530. [DOI] [PubMed] [Google Scholar]
- 12. Connor AE, Kaur M, Dibble KE, Visvanathan K, Dean LT, Hayes JH.. Racialized economic segregation and breast cancer mortality among women in Maryland. Cancer Epidemiol Biomarkers Prev. 2022;31(2):413-421. doi: 10.1158/1055-9965.EPI-21-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529-537. doi: 10.1007/S10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Z, Jones MR, Visvanathan K. Comparison of tumor profiles of second cancers among breast cancer survivors to first cancers in the SEER registries: a nationwide study [abstract]. Presented at: Proceedings of the American Association for Cancer Research Annual Meeting 2021; Apr 10-15 to May 17-21, 2021. Philadelphia: AACR. Cancer Res 2021; 81(suppl 13):Abstract nr 896. [Google Scholar]
- 15. Colzani E, Liljegren A, Johansson ALV, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29(30):4014-4021. doi: 10.1200/JClinOncol.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 16. Schairer C, Mink PJ, Carroll L, Devesa SS.. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311-1321. doi: 10.1093/JNCI/DJH253. [DOI] [PubMed] [Google Scholar]
- 17. Troeschel AN, Liu Y, Collin LJ, et al. Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer. Int J Epidemiol. 2019;48(6):1897-1905. doi: 10.1093/ije/dyz108. [DOI] [PubMed] [Google Scholar]
- 18. Gallicchio L, Tonorezos E, de Moor JS, et al. Evidence gaps in cancer survivorship care: a report from the 2019 National Cancer Institute cancer survivorship workshop. J Natl Cancer Inst. 2021;113(9):1136-1142. doi: 10.1093/JNCI/DJAB049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEERStat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975-2016 varying) - Linked To County Attributes - Total U.S.; 1969.
- 20. SEER. 2007 Multiple Primary and Histology Coding Rules. https://seer.cancer.gov/tools/mphrules/. Accessed January 4, 2022.
- 21.American Cancer Society, Inc. Cancer A-Z. https://www.cancer.org/cancer.html. Published 2021. Accessed September 20, 2021.
- 22. SEER. U.S. Mortality Data, 1969-2018. https://seer.cancer.gov/mortality/. Accessed January 4, 2022.
- 23. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL.. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25-33. doi: 10.1200/JClinOncol.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254-2261. doi: 10.1200/JClinOncol.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed AT, Welch BT, Brinjikji W, et al. Racial disparities in screening mammography in the United States: a systematic review and meta-analysis. J Am Coll Radiol. 2017;14(2):157-165.e9. doi: 10.1016/J.JACR.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 26. Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Van Ballegooijen M, Zauber AG, Jemal A.. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev. 2012;21(5):728-736. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY.. Trends in obesity prevalence in adults with a history of cancer: results from the US national health interview survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133-3140. doi: 10.1200/JClinOncol.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagalingam A, Siddharth S, Parida S, et al. Hyperleptinemia in obese state renders luminal breast cancers refractory to tamoxifen by coordinating a crosstalk between Med1, miR205 and ErbB. NPJ Breast Cancer. 2021;7(1):1-13. doi: 10.1038/s41523-021-00314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D.. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765-1772. doi: 10.1001/JAMA.294.14.1765 [DOI] [PubMed] [Google Scholar]
- 30. Klugman M, Xue X, Hosgood HD.. Race/ethnicity and lung cancer survival in the United States: a meta-analysis. Cancer Causes Control. 2019;30(11):1231-1241. doi: 10.1007/s10552-019-01229-4. [DOI] [PubMed] [Google Scholar]
- 31. Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253-261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Prim. 2015;1:1-16. doi: 10.1038/nrdp.2015.9. [DOI] [PubMed] [Google Scholar]
- 33. Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10(1):337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lund JL, Harlan LC, Yabroff KR, Warren JL.. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest. 2010;28(7):758-764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, at https://seer.cancer.gov/.