Abstract
Background
There is a lack of evidence from nationwide samples on the disparity of initiating immune checkpoint inhibitors (ICIs) after metastatic lung cancer diagnosis.
Methods
We identified metastatic lung cancer patients diagnosed between 2015 and 2020 from a large, nationwide commercial claims database. We analyzed the time from metastatic lung cancer diagnosis to ICI therapy using Cox proportional hazard models. Independent variables included county-level measures (quintiles of percentage of racialized population, quintiles of percentage of population below poverty, urbanity, and density of medical oncologists) and patient characteristics (age, sex, Charlson comorbidity index, Medicare Advantage, and year of diagnosis). All tests were 2-sided.
Results
A total of 17 022 patients were included. Counties with a larger proportion of racialized population appeared to be more urban, have a greater percentage of its residents in poverty, and have a higher density of medical oncologists. In Cox analysis, the adjusted hazard ratio of the second, third, fourth, and highest quintile of percentage of racialized population were 0.89 (95% confidence interval [CI] = 0.82 to 0.98), 0.85 (95% CI = 0.78 to 0.93), 0.78 (95% CI = 0.71 to 0.86), and 0.71 (95% CI = 0.62 to 0.81), respectively, compared with counties in the lowest quintile. The slower ICI therapy initiation was driven by counties with the highest percentage of Hispanic population and other non-Black racialized groups.
Conclusions
Commercially insured patients with metastatic lung cancer who lived in counties with greater percentage of racialized population had slower initiation of ICI therapy after lung cancer diagnosis, despite greater density of oncologists in their neighborhood.
There have been major improvements in cancer treatment in recent years, particularly with the advent of immune checkpoint inhibitors (ICIs). These novel immunotherapies have improved the treatment and survival outcomes of many cancers, including lung cancer (1-5). The major improvements in survival have fueled the rapid adoption of these novel therapies. A recent study found that within 4 months after the US Food and Drug Administration approval of nivolumab, more than 60% of eligible lung cancer patients had received the drug (6). By the end of 2018, 55% of first-line treatments for newly diagnosed metastatic lung cancer and 59% of later-line treatments were using one of the approved immunotherapies (7).
Despite the tremendous potential and excitement, there are concerns with access to recently launched immunotherapies, particularly in disadvantaged populations. In this paper, we explore the disparity in utilization of checkpoint inhibitors among patients with metastatic lung cancer. Lung cancer is the leading cause of cancer deaths among men and women in the United States, making up an estimated 22% of all cancer deaths in 2021 (8). Nearly 60% of patients with lung cancer were first diagnosed at distant stage, with a 5-year survival of 6% for those diagnosed between 2010 and 2016 (8).
As ICIs are a relatively new class of drugs for lung cancer, research on the disparity in their use is scarce, and findings are mixed. Two recent studies of stage IV non-small cell lung cancer using the National Cancer Database, a hospital-based cancer registry in the United States, found that Black patients experienced delayed initiation and lower utilization of immunotherapy as the first course of treatment compared with White patients (9,10). Two studies using regional electronic health records, however, found no racial disparity in immunotherapy use (6,11). The difference in findings on racial disparity among existing studies is possibly because of the difference in their study populations, the fact that the National Cancer Database registry only documents the first course of treatment, and a lack of attention to contextual factors such as neighborhood disadvantage.
Individuals from marginalized communities are more likely to experience implicit interpersonal bias while interacting with the health-care workforce (12). Such racism and implicit bias were found to be associated with poorer treatment in domains such as patient centeredness, contextual knowledge of the patient, patient-provider communication, and treatment recommendations (12). In addition to characteristics of the individual patients, characteristics of the geographic location where patients reside could also influence the use of health-care services (13-15). Indeed, researchers who have decomposed racial disparity found that individual factors do not fully account for the observed differences, but location characteristics such as residential segregation are also possible sources of disparity (16,17). Racial residential segregation creates a platform for disinvestment in marginalized communities, leading to fewer employment opportunities, greater poverty, worse physical environments, and fewer public resources—all could impede access to care (16,18-21).
Since the first ICI’s approval in 2015 for metastatic lung cancer, there has been limited evidence on the disparity in their use. In particular, there is a lack of knowledge on how neighborhood disadvantage affected timely access to ICIs after metastatic lung cancer diagnosis. The aim of this work was to examine the impact of neighborhood disparity on access to immunotherapy in a nationwide sample of commercially insured population. We hypothesized that patients who lived in disadvantaged communities (ie, communities with a greater percentage of residents from racialized groups) had a slower initiation of checkpoint inhibitors after their lung cancer diagnosis. We followed the American Medical Association’s recommendation and used “racialized group” rather than “racial and ethnic minority” throughout this paper (22). The purpose of using this inclusive language is to avoid reinforcing the existing discriminatory narratives of the marginalized communities.
Methods
Data and study population
We conducted a retrospective cohort study using the de-identified Optum Clinformatics Data Mart. Optum is a large, adjudicated claims database that covers working-age adults and their dependents and older adults with Medicare Advantage, with a total annual enrollment between 15 and 20 million (23). Patients in Optum have medical and pharmacy benefits, allowing the analysis of overall utilization of cancer drugs.
We identified male and female patients aged 18 years and older with newly diagnosed metastatic lung cancer between January 2015 and December 2020 using the International Classification of Diseases versions 9 and 10 codes (Supplementary Table 1, available online). We used a published, validated algorithm for identifying metastatic lung cancer (both de novo and recurrent) from Optum data (24). Specifically, patients were required to have at least 1 inpatient claim with a diagnosis in any field of lung cancer or at least 2 outpatient claims with a diagnosis in any field of lung cancer that occurred at least 30 days apart, between January 2008 and December 2019 (identification period). Their first diagnosis of lung cancer in the identification period was defined as the index date. To identify metastatic cancer, patients were required to have at least 2 medical claims with a diagnosis in any field of secondary metastasis on separate dates, within 30 days before or any time after the index date. Patients were excluded if they had diagnosis of other cancers in the year prior to the index date. Finally, patients were required to have continuous enrollment in Optum 12 months before and at least 3 months after their metastatic diagnosis.
ICI therapy initiation
We examined all ICIs approved for lung cancer as of 2020. These drugs were ipilimumab, pembrolizumab, nivolumab, tezolizumab, and durvalumab. Claims of immunotherapies were identified using the Healthcare Common Procedure Coding System codes (Supplementary Table 2, available online). The service date on the first immunotherapy claim after metastatic cancer diagnosis was defined as the date of treatment initiation.
Area-level and patient-level characteristics
Using information from the Census Bureau (25), we divided all counties into 5 quintiles based on the percentage of population that are racialized groups and 5 quintiles based on the percentage of population who live in poverty. A higher quintile indicates a higher percentage of racialized population and a higher percentage of population below poverty line in the county, respectively. Using information from the rural-urban continuum codes (26), we grouped areas into metro, urban, and rural areas. Finally, we extracted density of medical oncologists in a county from a previous study, where physicians self-identified as providing oncologic care to patients were identified from the health-care provider taxonomy code in the National Provider Identifier data and aggregated at the county level (27). Patient-level characteristics include age, sex, Charlson comorbidity index (28), Medicare Advantage vs non-Medicare commercial insurance, and diagnosis year. Information on patient demographics such as age and sex were self-reported in Optum.
Statistical analysis
We first examined the uptake of ICIs in our study sample. To account for differential follow-up time and censoring, we estimated the restricted mean time from diagnosis to checkpoint inhibitor initiation (event of interest) and plotted Kaplan–Meier curves, with steeper curves indicating faster initiation. To illustrate geographic variation in checkpoint inhibitor adoption across the nation, we created maps of uptake by county.
Time from metastatic lung cancer diagnosis to initiating checkpoint inhibitors was then analyzed using Cox proportional hazard models. We aimed to construct the most parsimonious model that includes all variables that may affect checkpoint inhibitor initiation. To do so, we first examined the correlation between each neighborhood and patient characteristic and checkpoint inhibitor initiation. In the univariate analyses, percentage of racialized population, urbanity, and density of medical oncologists, as well as all patient-level characteristics, were statistically significantly correlated with initiation (P from log-rank test < 0.2). We then constructed 3 models. In the first model, to estimate the crude hazard ratios (HRs), we only included quintiles of percentage of racialized population in a county. In the second model, we adjusted for patient characteristics (age, sex, Charlson comorbidity index, Medicare Advantage, and year of diagnosis) in addition to county-level racialized population. In the third model, we further adjusted for urbanity and density of oncologists in a county in addition to the variables included in the second model. In a secondary analysis, we broke down the composition of racialized population into Black, Hispanic, and other racialized groups and included quintiles of these measures in the Cox model. In a sensitivity analysis, we restricted our study sample to those with at least 12 months of continuous enrollment period post metastatic cancer diagnosis. To examine the proportional hazards assumption, we tested the Schoenfeld residuals after fitting a Cox model (29). The P value for the main predictor of interest—racialized population—was 0.171, suggesting that the proportional hazards assumption was valid. In all regressions, we clustered standard errors at the county level. A 2-sided test with a P value of less than .05 is considered statistically significant. We used Stata 15.1 (College Station, TX) for all statistical analyses.
Results
Between 2015 and 2020, a total of 17 022 metastatic lung cancer patients in our data were diagnosed and met our inclusion criteria (Table 1). The average age of our study sample was 71.5 (9.3) years, and 52.7% were female. Approximately 20% of our study sample had 0 Charlson comorbidity, 24% had 1 or 2 comorbidities, and more than half (54%) had 3 or more. Approximately 81.4% of our sample had Medicare Advantage (as opposed to employer-based commercial insurance). Approximately 13% of our study population lived in poverty, and 86% lived in metro areas. On average, there were 4.5 medical oncologists per 100 000 population in the county. Over the entire follow-up period, approximately 41% of the study population used checkpoint inhibitors, whereas the remaining 59% were censored. The average length of enrollment in our dataset post metastatic cancer diagnosis was 15.6 months.
Table 1.
Characteristics of patient population by quintiles of racialized population in a county
| Characteristics | All patients (N = 17 022) | By racialized population quintiles |
||||
|---|---|---|---|---|---|---|
| 1 (lowest percentage) | 2 | 3 | 4 | 5 (highest percentage) | ||
| Age, mean (SD), y | 71.5 (9.3) | 70.9 (9.2) | 71.0 (9.1) | 71.7 (9.4) | 71.7 (9.2) | 72.1 (9.4) |
| Female, No. (%) | 8967 (52.7) | 1753 (51.3) | 1781 (52.4) | 1867 (54.4) | 1802 (53.2) | 1764 (52.2) |
| Percentage of population living below poverty line, mean (SD) | 13.1 (4.6) | 11.7 (4.6) | 11.7 (3.7) | 11.9 (4.0) | 14.5 (4.1) | 15.7 (4.9) |
| Urbanity of county, No (%) | ||||||
| Metro | 14 632 (85.9) | 2025 (59.2) | 3088 (90.7) | 3183 (92.6) | 3133 (92.5) | 3203 (94.7) |
| Urban | 2049 (12.0) | 1170 (34.2) | 276 (8.1) | 219 (6.4) | 223 (6.6) | 161 (4.8) |
| Rural | 351 (2.1) | 224 (6.6) | 41 (1.2) | 34 (1.0) | 32 (0.9) | 20 (0.6) |
| Number of medical oncologists per 100 000 in the county, mean (SD) | 4.5 (4.5) | 2.5 (3.5) | 3.6 (3.3) | 5.1 (4.0) | 6.4 (5.5) | 5.4 (4.9) |
| Charlson comorbidity index, No. (%) | ||||||
| 0 | 3333 (19.6) | 641 (18.9) | 671 (19.7) | 688 (20.0) | 681 (20.1) | 652 (19.3) |
| 1-2 | 4046 (23.8) | 896 (26.2) | 869 (25.5) | 798 (23.2) | 785 (23.2) | 698 (20.6) |
| ≥3 | 9124 (53.6) | 1797 (52.6) | 1758 (51.6) | 1836 (53.4) | 1822 (53.8) | 1911 (56.5) |
| Medicare Advantage, No. (%) | 13 861 (81.4) | 2731 (79.9) | 2754 (80.9) | 2781 (80.9) | 2789 (82.3) | 2806 (82.9) |
| Checkpoint inhibitor initiation, No. (%) | ||||||
| Yes | 6988 (41.0) | 1599 (46.8) | 1447 (42.5) | 1422 (41.4) | 1301 (38.4) | 1219 (36.0) |
| Censored | 10 044 (59.0) | 1820 (53.2) | 1958 (57.5) | 2014 (58.6) | 2087 (61.6) | 2165 (64.0) |
| Length of continuous enrollment after diagnosis, mo | 15.6 (14.2) | 13.8 (12.2) | 15.0 (13.6) | 15.4 (14.0) | 17.3 (15.5) | 16.7 (15.2) |
The racial composition of a county appeared to be correlated with poverty, urbanity, and density of medical oncologists (Table 1; Supplementary Tables 3-5, available online). We observed greater percentage of population living in poverty and in metro areas and greater density of medical oncologists in counties with greater percentage of its residents from racialized groups. Furthermore, patients residing in counties with a larger racialized population were less likely to use checkpoint inhibitors and had longer time between diagnosis and initiation. Lastly, the average length of enrollment after metastatic cancer diagnosis appeared to be slightly longer in patients residing in areas with a larger racialized population.
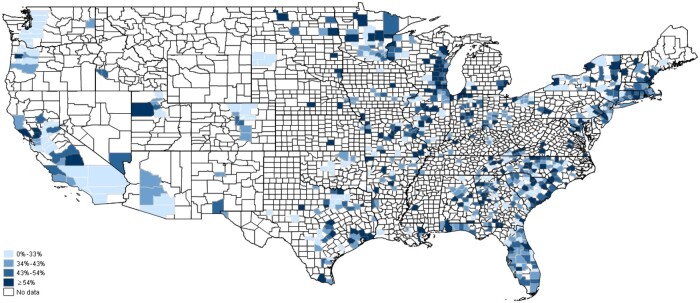
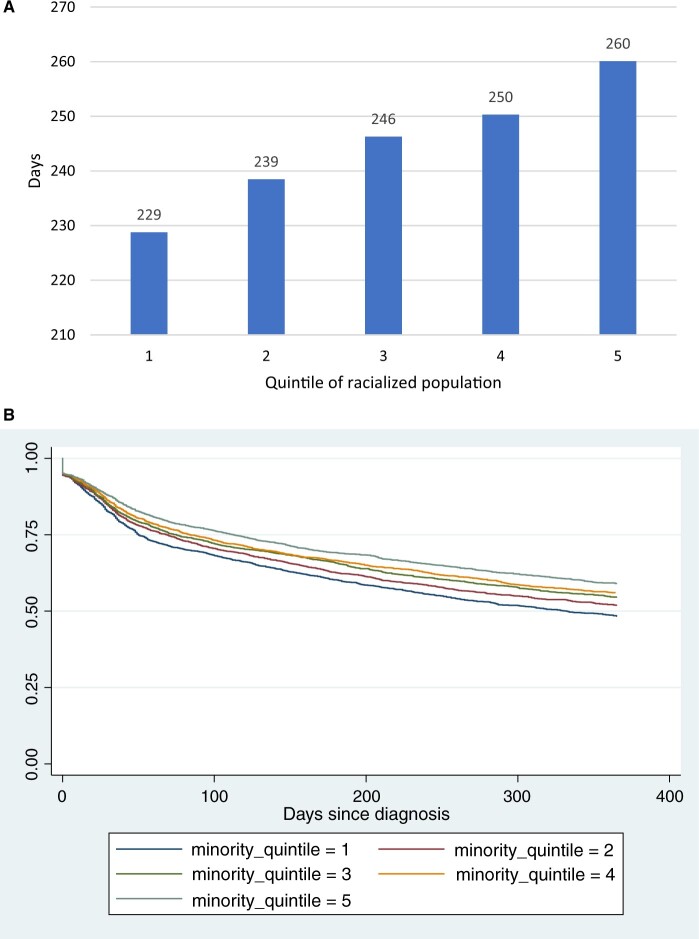
Across the nation, there was a large amount of geographic variation in checkpoint inhibitor adoption (Figure 1). In univariate analyses and spatial examinations, a larger percentage of racialized population, more urban, lower density of medical oncologists, as well as older age, female, more comorbidities, and enrollment in Medicare Advantage were statistically significantly correlated with slower initiation of checkpoint inhibitors (Figure 2; Supplementary Figures 1-12, available online). In particular, the restricted mean time from diagnosis to initiation was 229, 239, 246, 250, and 260 days for racialized population quintiles 1 through 5, and the differences between quintiles 2-5 and quintile 1 were all statistically significant (Figure 2). County-level poverty was not correlated with initiation in univariate analysis. Moreover, patient cohorts diagnosed in earlier years had slower initiation: the mean time from diagnosis to initiation of checkpoint inhibitors decreased from 325 days in the cohort diagnosed in 2015 to 180 days in the cohort diagnosed in 2020 (Supplementary Figure 1, available online).
Figure 1.
Geographic variation in uptake of immune checkpoint inhibitors between 2015 and 2020 by metastatic lung cancer patients with employer-sponsored commercial insurance or Medicare Advantage. Counties with at least 5 metastatic lung cancer patients diagnosed in our study period were included in this map.
Figure 2.
Mean days (restricted means) from metastatic cancer diagnosis to initiation of checkpoint inhibitors (A) and Kaplan-Meier curves (B) by quintiles of percentage of racialized population, both truncated at 1 year (length of follow-up for those diagnosed in 2020, the latest year). Higher quintiles indicate greater percentage of population from racialized groups. The differences in restricted mean time between quintiles 2-5 and quintile 1 were 10, 17, 22, and 31, respectively (all statistically significant).
In multivariate Cox regression analyses, greater percentage of racialized population in a county was associated with slower initiation of checkpoint inhibitors, with and without adjusting for other county and patient characteristics (Table 2, models 1-3). In the full model (model 3), the adjusted hazard ratios of the second, third, fourth, and highest quintile of racialized population in a county were 0.89 (95% confidence interval [CI] = 0.82 to 0.98), 0.85 (95% CI = 0.78 to 0.93), 0.78 (95% CI = 0.71 to 0.86), and 0.71 (95% CI = 0.62 to 0.81), respectively, compared with the lowest quintile of racialized population. In models 1 and 2, where fewer covariates were adjusted for, the hazard ratios of racialized population quintiles were almost the same as those from model 3.
Table 2.
Cox proportional hazard regression output with racialized population quintiles
| Variable | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Percentage of racialized population in a county quintile; referent: quintile 1, lowest percentage) | ||||||
| 2 | 0.90 (0.83 to 0.98) | .01 | 0.89 (0.81 to 0.96) | .005 | 0.89 (0.82 to 0.98) | .01 |
| 3 | 0.86 (0.78 to 0.95) | .002 | 0.85 (0.77 to 0.93) | .001 | 0.85 (0.78 to 0.93) | .001 |
| 4 | 0.80 (0.73 to 0.88) | <.001 | 0.79 (0.72 to 0.87) | <.001 | 0.78 (0.71 to 0.86) | <.001 |
| 5 (highest percentage) | 0.72 (0.62 to 0.83) | <.001 | 0.70 (0.61 to 0.81) | <.001 | 0.71 (0.62 to 0.81) | <.001 |
| Age group (referent: younger than 55), y | ||||||
| 55 to younger than 65 | — | — | 1.06 (0.90 to 1.26) | .47 | 1.06 (0.90 to 1.26) | .47 |
| 65 to younger than 75 | — | — | 1.03 (0.87 to 1.23) | .70 | 1.03 (0.87 to 1.22) | .72 |
| 75 and older | — | — | 0.80 (0.67 to 0.96) | .02 | 0.80 (0.67 to 0.96) | .01 |
| Female (referent: male) | — | — | 0.83 (0.80 to 0.87) | <.001 | 0.83 (0.79 to 0.87) | <.001 |
| Charlson comorbidities (referent: 0) | ||||||
| 1-2 | — | — | 1.02 (0.95 to 1.10) | .54 | 1.02 (0.95 to 1.10) | .55 |
| ≥3 | — | — | 0.93 (0.87 to 0.99) | .02 | 0.93 (0.87 to 0.99) | .02 |
| Medicare Advantage | — | — | 0.84 (0.78 to 0.91) | <.001 | 0.84 (0.78 to 0.92) | <.001 |
| Urbanity (referent: metro) | ||||||
| Urban | — | — | — | — | 1.03 (0.95 to 1.12) | .44 |
| Rural | — | — | — | — | 1.15 (0.98 to 1.36) | .09 |
| No. medical oncologists per 100 000 | — | — | — | — | 1.01 (0.99 to 1.01) | .14 |
| Diagnosis year (referent: 2015) | ||||||
| 2016 | — | — | 1.35 (1.21 to 1.50) | <.001 | 1.35 (1.21 to 1.50) | <.001 |
| 2017 | — | — | 2.01 (1.83 to 2.21) | <.001 | 2.01 (1.83 to 2.21) | <.001 |
| 2018 | — | — | 2.77 (2.53 to 3.02) | <.001 | 2.76 (2.53 to 3.02) | <.001 |
| 2019 | — | — | 4.06 (3.71 to 4.44) | <.001 | 4.05 (3.70 to 4.44) | <.001 |
| 2020 | — | — | 4.91 (4.44 to 5.42) | <.001 | 4.90 (4.43 to 5.41) | <.001 |
Model 1 only included quintiles of percentage of racialized population, to estimate crude hazard ratios. CI = confidence interval; HR = hazard ratio.
Model 2 adjusted for patient characteristics (age, sex, Charlson comorbidity index, Medicare Advantage, and year of diagnosis) in addition to percentage of racialized population.
Model 3 further adjusted for urbanity and density of oncologists in addition to the variables included in model 2.
In the secondary analysis, when the percentage of racialized population in a county was broken down into Black, Hispanic, and other racialized groups, the slower initiation in counties with a larger racialized population was driven by counties with the highest percentage of Hispanic population and other racialized groups (Table 3). The adjusted hazard ratio of the highest quintile of Hispanic population was 0.75 (95% CI = 0.67 to 0.83) compared with the lowest quintile (Table 3, model 3). The adjusted hazard ratio of the highest quintile of other racialized population was 0.79 (95% CI = 0.70 to 0.90) compared with the lowest quintile. In models 1 and 2, where fewer covariates were adjusted for, the hazard ratios of quintiles of Hispanic population and other racialized groups were almost the same as those from model 3.
Table 3.
Cox proportional hazard regression output with quintiles of Black, Hispanic, and other racialized populations
| Variable | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Percentage of Black population in a county quintile; referent: quintile 1 (lowest percentage) | ||||||
| 2 | 0.97 (0.89 to 1.06) | .48 | 0.95 (0.87 to 1.04) | .30 | 0.96 (0.88 to 1.05) | .33 |
| 3 | 0.93 (0.84 to 1.04) | .20 | 0.92 (0.83 to 1.02) | .10 | 0.92 (0.84 to 1.02) | .12 |
| 4 | 1.00 (0.91 to 1.09) | .91 | 0.97 (0.90 to 1.06) | .56 | 0.98 (0.90 to 1.06) | .57 |
| 5 (highest percentage) | 0.99 (0.92 to 1.08) | .89 | 0.99 (0.91 to 1.08) | .84 | 0.99 (0.90 to 1.08) | .79 |
| Percentage of Hispanic population in a county quintile; referent: quintile 1 (lowest percentage) | ||||||
| 2 | 1.01 (0.92 to 1.10) | .90 | 1.02 (0.94 to 1.10) | .69 | 1.02 (0.94 to 1.11) | .61 |
| 3 | 0.91 (0.83 to 1.00) | .04 | 0.91 (0.84 to 1.00) | .05 | 0.92 (0.84 to 1.01) | .07 |
| 4 | 0.94 (0.86 to 1.03) | .19 | 0.95 (0.86 to 1.04) | .24 | 0.95 (0.87 to 1.04) | .30 |
| 5 (highest percentage) | 0.73 (0.66 to 0.81) | <.001 | 0.74 (0.67 to 0.83) | <.001 | 0.75 (0.67 to 0.83) | <.001 |
| Percentage of other racialized population in a county quintile; referent: quintile 1 (lowest percentage) | ||||||
| 2 | 1.01 (0.92 to 1.10) | .17 | 1.06 (0.98 to 1.15) | .15 | 1.07 (0.98 to 1.17) | .14 |
| 3 | 1.03 (0.83 to 1.00) | .51 | 1.04 (0.96 to 1.13) | .34 | 1.05 (0.95 to 1.15) | .34 |
| 4 | 1.04 (0.86 to 1.03) | .49 | 1.01 (0.92 to 1.11) | .85 | 1.01 (0.92 to 1.12) | .80 |
| 5 (highest percentage) | 0.79 (0.66 to 0.81) | <.001 | 0.79 (0.70 to 0.89) | <.001 | 0.79 (0.70 to 0.90) | <.001 |
| Age group (referent: <55), y | ||||||
| 55 to younger than 65 | — | — | 1.05 (0.89 to 1.25) | .53 | 1.06 (0.89 to 1.25) | .53 |
| 65 to younger than 75 | — | — | 1.05 (0.89 to 1.25) | .57 | 1.05 (0.89 to 1.24) | .58 |
| 75 and older | — | — | 0.83 (0.69 to 0.99) | .04 | 0.83 (0.69 to 0.99) | .04 |
| Female (referent: male) | — | — | 0.84 (0.80 to 0.87) | <.001 | 0.84 (0.80 to 0.87) | <.001 |
| Charlson comorbidities (referent: 0) | ||||||
| 1-2 | — | — | 1.03 (0.96 to 1.10) | .43 | 1.03 (0.96 to 1.10) | .44 |
| ≥3 | — | — | 0.93 (0.87 to 0.99) | .02 | 0.93 (0.87 to 0.99) | .02 |
| Medicare Advantage | — | — | 0.83 (0.77 to 0.90) | <.001 | 0.83 (0.77 to 0.90) | <.001 |
| Urbanity (referent: metro) | ||||||
| Urban | — | — | — | — | 1.01 (0.93 to 1.11) | .76 |
| Rural | — | — | — | — | 1.14 (0.96 to 1.35) | .13 |
| No. medical oncologists per 100 000 | — | — | — | — | 1.00 (0.11 to 1.01) | .51 |
| Diagnosis year (referent: 2015) | ||||||
| 2016 | — | — | 1.35 (1.22 to 1.50) | <.001 | 1.35 (1.21 to 1.50) | <.001 |
| 2017 | — | — | 2.01 (1.83 to 2.21) | <.001 | 2.01 (1.83 to 2.21) | <.001 |
| 2018 | — | — | 2.78 (2.54 to 3.03) | <.001 | 2.77 (2.54 to 3.03) | <.001 |
| 2019 | — | — | 4.08 (3.73 to 4.47) | <.001 | 4.07 (3.72 to 4.46) | <.001 |
| 2020 | — | — | 4.93 (4.46 to 5.46) | <.001 | 4.92 (4.45 to 5.45) | <.001 |
Model 1 only included quintiles of percentage of racialized population, to estimate crude hazard ratios. CI = confidence interval; HR = hazard ratio.
Model 2 adjusted for patient characteristics (age, sex, Charlson comorbidity index, Medicare Advantage, and year of diagnosis) in addition to percentage of racialized population.
Model 3 further adjusted for urbanity and density of oncologists in addition to the variables included in model 2.
In the sensitivity analysis, when the study sample was limited to those with at least 12 months of continuous enrollment after metastatic cancer diagnosis, the results were robust (Supplementary Tables 6 and 7, available online).
Discussion
We analyzed the utilization of ICIs among adult patients with employer-based insurance or Medicare Advantage diagnosed with metastatic lung cancer between 2015 and 2020 and found large disparity in the timeliness of access. Patients residing in counties with a larger percentage of racialized population had statistically significantly slower initiation of checkpoint inhibitors following their metastatic cancer diagnosis. This association was driven by areas with the highest density of Hispanic population and other racialized groups.
In our analysis, we found that areas with a greater racialized population were also urban areas with greater density of medical oncologists. However, the greater availability of oncologists in these areas did not translate into a more rapid access to checkpoint inhibitors. Prior evidence suggested that racialized patients and White patients were to a large extent treated by different physicians. One study found that most visits made by racialized patients were with a small group of physicians who provided only a small percentage of care to White patients, and those physicians were less likely to be board certified and more likely to report that they were unable to provide high-quality care to their patients (30). In our case, though areas with high racialized population have more medical oncologists per population, it is possible that only a small percentage of oncologists in those areas actually served the large racialized population living in these territories, because of racial segregation in hospitals, lack of diversity and low cultural competency of health-care professionals, and inability of the health system to demonstrate trustworthiness, and this fraction of physicians caring for the racialized population may also be less well trained and may have less access to important clinical resources.
This implies that policies that aim to improve access to cancer care in racialized communities by increasing availability of medical oncologists in their county of residence may not achieve the intended goal if the characteristics of physicians who serve primarily racialized communities are not well understood. Prior evidence suggests that racialized physicians provide a disproportionate share of care to underserved populations—racialized groups, low income, Medicaid enrollees, uninsured, and non-English home language speakers (31). Our finding that increasing availability of medical oncologists may not improve access to quality care even among commercially insured individuals highlights the importance of improving the racial and ethnic diversity of the physician workforce, the cultural competency of physicians failing to treat racialized groups, and catchment area concordance as key to meeting national goals of eradicating health disparities.
The slower initiation of checkpoint inhibitors in counties with a larger racialized population observed in our analysis was driven by slower initiation in counties with the highest density of Hispanics and other non-Black racialized population. This could be due to language barriers. Prior research found that Spanish-speaking Americans received about one-third less health care (measured in spending) as compared with patients whose primary language is English (32). Furthermore, Americans with limited English proficiency are less likely to have a usual care provider (33). These findings highlighted the need for improving the cultural competency and language training of physicians to meet the needs of diverse patients.
In our sample, we did not observe any difference in time to initiation of checkpoint inhibitors among counties with different levels of poverty. This could be because our sample consisted of commercially insured (including Medicare Advantage) patients, and most plans after January 2016 had an annual out-of-pocket maximum (OOPM) on medical expenses (34). Checkpoint inhibitors are mostly covered under medical benefits, and because of the high costs associated with cancer diagnosis and ICIs, many patients might have reached their annual OOPM of medical expense by the time or shortly after they initiated immunotherapy. Once they reach annual OOPM, there is no cost sharing for the rest of the year.
There are several limitations that are worth noting. First, even though we used a validated algorithm to identify metastatic lung cancer, there were likely misclassifications. The validation study showed that the sensitivity and specificity of the algorithm was 55% and 85%, respectively (24). Second, information on severity of disease, such as stage and tumor size, was not available in claims data. This may introduce selection bias in our retrospective, observational design. Third, the average length of enrollment appeared to be slightly longer in counties with a larger racialized population. This may have biased our study results in a more conservative fashion as patients living in highly racialized areas had slower initiation and lower probability of initiating checkpoint inhibitors despite longer enrollment in our data. Fourth, information on the socioeconomic characteristics of individual patients was not available in our data. As a result, we were not able to examine how individual-level race influenced timeliness of initiating ICIs after lung cancer diagnosis. Finally, our study population consisted of commercially insured population from a large national insurer. The findings may not generalize to the traditional Medicare, Medicaid, or uninsured populations.
Metastatic lung cancer patients with employer-based insurance or Medicare Advantage who lived in counties with greater percentage of racialized population had slower initiation of checkpoint inhibitors after their cancer diagnosis, despite greater density of medical oncologists in their neighborhood. More research is needed on the provider, patient, and health-care system characteristics that contributed to slower access to care in areas with higher proportion of racialized population.
Supplementary Material
Contributor Information
Meng Li, Section of Cancer Economics and Policy, Department of Health Services Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Kaiping Liao, Section of Cancer Economics and Policy, Department of Health Services Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Alice J Chen, Sol Price School of Public Policy, University of Southern California, Los Angeles, CA, USA.
Tina Cascone, Department of Thoracic/Head and Neck Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Yu Shen, Department of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Qian Lu, Department of Health Disparities Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ya-Chen Tina Shih, Section of Cancer Economics and Policy, Department of Health Services Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Funding
This study is funded in part by the National Cancer Institute (R01CA207216 and R01CA225647, PI: Shih).
Notes
Role of the funder: The funder has no role in the design, analysis, interpretation, writing, or the decision to submit the manuscript for publication.
Disclosures: TC reports speaker fees/honoraria from The Society for Immunotherapy of Cancer, Bristol Myers Squibb, Roche, Medscape, and PeerView; travel, food and beverages expenses from Dava Oncology and Bristol Myers Squibb; advisory role/consulting fees from MedImmune/AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck & Co, Genentech, and Arrowhead Pharmaceuticals; and institutional research funding from MedImmune/AstraZeneca, Bristol Myers Squibb, Boehringer-Ingelheim and EMD Serono, outside of the submitted work. Other authors have no conflict of interest to disclose. YTS, a JNCI Associate Editor and coauthor of this article, was not involved in the editorial review or decision to publish this article.
Author contributions: Conceptualization: ML, AJC, TS. Data curation: ML. Formal analysis: KL. Investigation: ML, KL, AJC, YS, QL, TS. Methodology: ML, AJC, TS. Project administration: ML. Resources: ML. Software: ML. Supervision: ML, TS. Validation: ML, AJC, YS, QL, TS. Visualization: KL. Writing—original draft: ML. Writing—review & editing: ML, AJC, TC, YS, QL, TS.
Prior presentations: This study was presented as a poster at the ASCO 2022 conference on June 3, 2022.
Data availability
The raw/processed data required to reproduce the above findings cannot be shared under the data use agreement between University of Texas MD Anderson Cancer Center and OPTUM Insight. The Optum data can be requested, with licensing fee, by contacting connected@optum.com. More information can be found here: https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf.
References
- 1. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537-546. doi: 10.1200/JClinOncol.18.00149. [DOI] [PubMed] [Google Scholar]
- 4. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; for the KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 6. O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. doi: 10.1001/jamaoncol.2018.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The IQVIA Institute. Global Oncology Trends 2019. 2019. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019. Accessed September 1, 2022.
- 8. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 9. Deng W, Wang Y, Yang M, Liu X, Yang Z, Jiang W.. Racial disparities in time to treatment initiation for stage IV non-small cell lung cancer patients receiving immunotherapy. Int J Radiat Oncol Biol Phys. 2020;108(3):e154. doi: 10.1016/j.ijrobp.2020.07.1332. [DOI] [Google Scholar]
- 10. Verma V, Haque W, Cushman TR, et al. Racial and insurance-related disparities in delivery of immunotherapy-type compounds in the United States. J Immunother. 2019;42(2):55-64. doi: 10.1097/CJI.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 11. Brawley OW, Luhn P, Reese-White D, et al. Disparities in tumor mutational burden, immunotherapy use, and outcomes based on genomic ancestry in non-small-cell lung cancer. J Clin Oncol Glob Oncol. 2021;7:1537-1546. doi: 10.1200/GO.21.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60-e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirby JB, Lau DT.. Community and individual race/ethnicity and home health care use among elderly persons in the United States. Health Serv Res. 2010;45(5 pt 1):1251-1267. doi: 10.1111/j.1475-6773.2010.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golestaneh L, Cavanaugh KL, Lo Y, et al. Community racial composition and hospitalization among patients receiving in-center hemodialysis. Am J Kidney Dis off Dis. 2020;76(6):754-764. doi: 10.1053/j.ajkd.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golestaneh L, Karaboyas A, Cavanaugh K, et al. The role of place in disparities affecting Black men receiving hemodialysis. Kidney Int Rep. 2021;6(2):357-365. doi: 10.1016/j.ekir.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hargraves JL, Hadley J.. The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Serv Res. 2003;38(3):809-829. doi: 10.1111/1475-6773.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinick RM, Zuvekas SH, Cohen JW.. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev. 2000;57(suppl 1):36-54. doi: 10.1177/1077558700057001S03. [DOI] [PubMed] [Google Scholar]
- 18. Do DP, Frank R, Iceland J.. Black-White metropolitan segregation and self-rated health: investigating the role of neighborhood poverty. Soc Sci Med. 2017;187:85-92. doi: 10.1016/j.socscimed.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 19. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT.. Structural racism and health inequities in the USA: evidence and interventions. Lancet (London, England). 2017;389(10077):1453-1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 20. Gaskin DJ, Dinwiddie GY, Chan KS, McCleary R.. Residential segregation and disparities in health care services utilization. Med Care Res Rev. 2012;69(2):158-175. doi: 10.1177/1077558711420263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reschovsky JD, O’Malley AS.. Do primary care physicians treating minority patients report problems delivering high-quality care? Health Aff (Millwood)). 2008;27(3):w222-w231. doi: 10.1377/hlthaff.27.3.w222. [DOI] [PubMed] [Google Scholar]
- 22.American Medical Association. Advancing health equity: a guide to language, narrative and concepts glossary. https://www.ama-assn.org/about/ama-center-health-equity/advancing-health-equity-guide-language-narrative-and-concepts. Accessed September 8, 2022.
- 23.Optum. Optum claims data. 2021. https://www.optum.com/business/solutions/life-sciences/real-world-data/claims-data.html. Accessed May 24, 2021.
- 24. Whyte JL, Engel-Nitz NM, Teitelbaum A, Gomez Rey G, Kallich JD.. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;53(7):e49-e57. doi: 10.1097/MLR.0b013e318289c3fb. [DOI] [PubMed] [Google Scholar]
- 25. United States Census Bureau. Small Area Income and Poverty Estimates (SAIPE). 2019. https://www.census.gov/data-tools/demo/saipe/#/?map_geoSelector=aa_c. Accessed May 23, 2021.
- 26. U.S. Department of Agriculture Economic Research Service. Rural-urban continuum codes. 2020. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed May 23, 2021
- 27. Shih Y-CT, Kim B, Halpern MT.. State of physician and pharmacist oncology workforce in the United States in 2019. J Clin Oncol Pract. 2021;17(1):e1-e10. doi: 10.1200/OP.20.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klabunde CN, Potosky AL, Legler JM, Warren JL.. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 29. Stata.com. stcox PH-assumption tests. https://www.stata.com/manuals/ststcoxph-assumptiontests.pdf.
- 30. Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL.. Primary care physicians who treat Blacks and Whites. N Engl J Med. 2004;351(6):575-584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 31. Marrast LM, Zallman L, Woolhandler S, Bor DH, McCormick D.. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174(2):289-291. doi: 10.1001/jamainternmed.2013.12756. [DOI] [PubMed] [Google Scholar]
- 32. Himmelstein J, Himmelstein DU, Woolhandler S, et al. Health care spending and use among Hispanic adults with and without limited English proficiency, 1999-2018. Health Aff (Millwood). 2021;40(7):1126-1134. doi: 10.1377/hlthaff.2020.02510. [DOI] [PubMed] [Google Scholar]
- 33. Foiles Sifuentes AM, Robledo Cornejo M, Li NC, Castaneda-Avila MA, Tjia J, Lapane KL.. The role of limited English proficiency and access to health insurance and health care in the affordable care act era. Health Equity. 2020;4(1):509-517. doi: 10.1089/heq.2020.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UnitedHealthcare. Out-of-pocket maximum. 2022. https://www.uhc.com/united-for-reform/health-reform-provisions/out-of-pocket-maximum.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce the above findings cannot be shared under the data use agreement between University of Texas MD Anderson Cancer Center and OPTUM Insight. The Optum data can be requested, with licensing fee, by contacting connected@optum.com. More information can be found here: https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf.