Abstract
Background
Although unopposed estrogen exposure is considered a major driver of endometrial carcinogenesis, chronic inflammation and insulin resistance and hyperinsulinemia are also major endometrial cancer risk factors. However, it is unclear whether diets with inflammatory or insulinemic potential are associated with risk of endometrial cancer.
Methods
We followed 48 330 women from the Nurses’ Health Study (1984-2016) and 85 426 women from the Nurses’ Health Study II (1989-2017). Using food frequency questionnaires, we calculated repeated measures of empirical dietary inflammatory pattern (EDIP) and empirical dietary index for hyperinsulinemia (EDIH) scores, which characterize the potential of the whole diet to modulate circulating biomarkers of inflammation or C-peptide, respectively. We used multivariable-adjusted Cox regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for type I endometrial cancer risk.
Results
We documented 1462 type I endometrial cancer cases over 2 823 221 person-years of follow-up. In the pooled multivariable-adjusted analyses, women in the highest compared with lowest quintiles were at higher risk of type I endometrial cancer (EDIP HRQ5vsQ1 = 1.46, 95% CI = 1.24 to 1.73; Ptrend < .001; EDIH HRQ5vsQ1 = 1.58, 95% CI = 1.34 to 1.87; Ptrend < .001). Additional adjustment for body mass index attenuated the associations (EDIP HR = 1.03, 95% CI = 0.87 to 1.22; EDIH HR = 1.01, 95% CI = 0.85 to 1.21), and mediation analyses showed that body mass index may explain 60.4% (95% CI = 37.4% to 79.6%; P < .001) and 71.8% (95% CI = 41.0% to 90.4%; P < .001) of the association of endometrial cancer with EDIP and EDIH, respectively.
Conclusions
In this large cohort study, higher dietary inflammatory and insulinemic potential were each associated with increased endometrial cancer incidence, and this association may be almost entirely mediated by adiposity.
Endometrial cancer is the most common cancer of the female reproductive organs (1), and its incidence is projected to surpass colorectal cancer incidence to become the third leading type of cancer in US women by 2030 (2). This increase is driven by 2 key factors: an increase in the prevalence of obesity and an aging population (3). Established risk factors for endometrial cancer include unopposed estrogens, including those produced endogenously and menopausal hormone therapy. Other hormone-related risk factors include nulliparity or low parity and early menarche and/or late menopause, reflecting a larger lifetime cumulative number of ovulatory cycles (4).
Although the hormonal etiology of endometrial cancer is known, more than 70% of endometrial cancer cases may be attributable to modifiable factors (ie, diet, physical activity, or obesity) (5). Epidemiological and clinical data implicate insulin resistance and hyperinsulinemia [independent of estradiol levels (6)] as being key risk factors in endometrial cancer development (7), as insulin can stimulate cell proliferation (8-10). The molecular mechanisms underlying the relationship of insulin resistance with endometrial cancer are still under investigation (11). Chronic inflammation is also postulated to contribute to endometrial carcinogenesis. Proinflammatory cytokines (C-reactive protein, interleukin-6 [IL-6], IL-1 receptor antagonist, and tumor necrosis factor alpha [TNF-α]) have been associated with endometrial cancer risk (12-15). In obese women, adipose tissue secretes less adiponectin and more proinflammatory cytokines (TNF-α and IL-6) (16).
Excess body weight is most strongly associated with endometrial cancer compared with other obesity-related cancers (17). Yet, dietary risk factors have been implicated in endometrial cancer etiology independent of adiposity as well, particularly dietary glycemic load (GL) and coffee consumption (18). In 2018, the World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR) concluded there was probable evidence that coffee intake decreases endometrial cancer risk (18), which is consistent with the results from a recent meta-analysis (19). The WCRF–AICR also reported that GL, commonly used to assess the influence of carbohydrate-containing foods on postprandial blood glucose, was a probable endometrial cancer risk factor (20). Nevertheless, GL and the glycemic index have limited capacity to account for noncarbohydrate factors (ie, proteins and fats), which may also influence levels of circulating inflammatory and insulin-resistance biomarkers. They do not account for overall composition or frequency of food intake, which may affect insulin response, and they did not predict C-peptide, which was associated with endometrial cancer in a meta-analysis (8). To our knowledge, studies have not examined the association of dietary insulinemic or inflammatory potential with endometrial cancer incidence. Therefore, to capture the biologically relevant aspects of these diets for endometrial cancer, we used an empirical dietary inflammatory pattern (EDIP) (21) and empirical index for hyperinsulinemia (EDIH) (22) to assess long-term inflammatory and insulinemic potential of diet, respectively, in 2 prospective cohort studies.
Methods
Study population
The Nurses’ Health Study (NHS) consists of 121 701 female nurses aged 30-55 years at enrollment in 1976 (23) and the Nurses’ Health Study II (NHSII), established in 1989, consists of 116 429 female nurses aged 25-42 years (24). In both cohorts, information about medical history, lifestyle, and health conditions has been collected by self-administered questionnaires every 2 years since baseline. The cumulative follow-up rates in each cohort are approximately 90%.
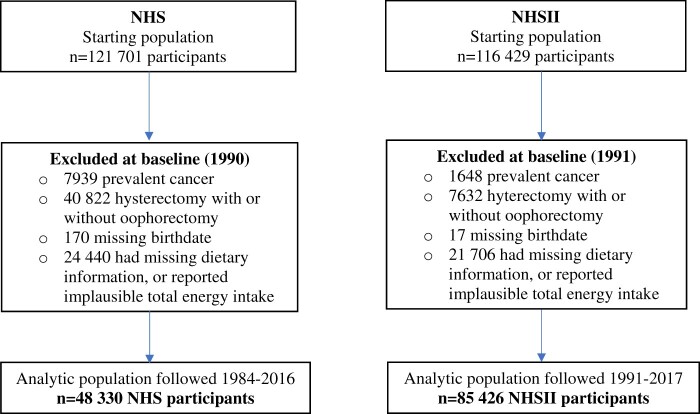
At the start of follow-up for NHS and NHSII in 1984 and 1991, respectively, we excluded nurses who had died or reported previous cancers except nonmelanoma skin cancer (NHS, n = 8109; NHSII, n = 1665), reported a hysterectomy or surgical menopause (NHS, n = 40 822; NHSII, n = 7632), or were missing the exposure (NHS, n= 24 440; NHSII, n = 21 706). At each subsequent follow-up cycle, we censored deaths or cancer diagnoses, as well as women reporting hysterectomy or surgical menopause. The final study population comprised 48 330 participants in NHS and 85 426 in NHSII with intact uterus (see Figure 1). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required.
Figure 1.
Flowchart of study population. NHS = Nurses’ Health Study; NHSII = Nurses’ Health Study II.
Dietary assessment
Diet was assessed using a semiquantitative food-frequency questionnaire (FFQ) administered every 4 years (starting from 1984 in NHS and 1991 in NHSII). The number of FFQ items was 116 items in 1984 and 1986 in NHS, and at least 130 items thereafter; in the NHSII, the FFQ from 1991 had more than 130 items. Participants were asked to report how often on average they consumed a specified portion size of each food during the previous year using 9 response categories (from “never or less than once per month” to “≥6 times/day”). The reproducibility and validity of the FFQ has been demonstrated using 24-hour dietary recalls and multiweek weighted dietary records as reference measurements of diet (25-28). The EDIH and EDIP were developed and validated to capture the potential of an overall dietary pattern to elicit blood inflammatory and insulinemic responses, respectively (21,22). EDIH was derived based on 39 predefined food groups from FFQs using stepwise regression models to identify a dietary pattern most predictive of fasting plasma C-peptide. EDIP was derived in a separate study based on 39 predefined food groups from FFQs using reduced-rank regression followed by stepwise linear regression models to identify a dietary pattern most predictive of 3 plasma inflammatory biomarkers (IL-6, C-reactive protein, and TNF-α receptor 2). EDIP and EDIH are weighted sums of 18 food groups (9 overlapping), with higher scores (more positive) indicating higher insulinemic or inflammatory potential and lower scores (more negative) suggesting lower insulinemic or inflammatory potential of the diet (Supplementary Table 1, available online). In independent datasets, EDIP and EDIH were validated using biomarkers of inflammation and insulin response (21,22,29). For each participant, we calculated EDIP and EDIH scores using updated FFQ data every 4 years.
Assessment of covariates
Information on lifestyle and other potential risk factors were collected at baseline and updated biennially during follow-up through self-administered questionnaires. Diet- and/or cancer-associated covariates were selected a priori and included age in months, body mass index (BMI), physical activity, age at menopause, menopausal status, postmenopausal hormone therapy use, parity, oral contraceptive use, and smoking status—all biennially updated. Covariates not updated included age at menarche and family history of endometrial cancer. BMI was carried forward 1 questionnaire cycle if missing.
Ascertainment of endometrial cancer
Participants reported disease diagnoses on biennial questionnaires. When a woman reported a cancer diagnosis, we sought permission to obtain the relevant medical records and pathology reports, and study physicians blinded to all questionnaire data reviewed the documents to verify diagnosis and establish an exact date of diagnosis. Histological subtype, grade, and stage at diagnosis were obtained from pathology records. Information on deceased women were obtained from the National Death Index, the US Postal Service, or next of kin; at least 98% of deaths were ascertained (30). As less than 10% of NHS and NHSII endometrial cancer cases were diagnosed with serous, clear cell, or other rare histologic types, we restricted our analysis to cases with type I endometrial cancer. Women diagnosed with nonepithelial tumors, types of epithelial tumors other than adenocarcinoma (eg, squamous cell), or noninvasive tumors (endometrial intraepithelial neoplasia, atypical hyperplasia, or adenocarcinoma in situ) were censored during follow-up. Furthermore, given the preponderance of type I endometrial cancer cases, we kept known (n = 1188) and unknown (n = 280) histological cases, assuming the majority of unknowns are type I (31).
Statistical analyses
Person-years were calculated from baseline when EDIP and EDIH scores were first available (1984 for NHS, 1991 for NHSII) to the date of type I endometrial or other cancer diagnosis (except nonmelanoma skin neoplasms), death, or end of study follow-up (NHS, June 2016; NHSII, June 2017), whichever occurred first. We used cumulative average of EDIP and EDIH calculated from repeated measure of FFQs to capture habitual long-term dietary intake and reduce within-person variation. Furthermore, we used baseline or recently updated EDIP and EDIH scores to evaluate the influence of dietary intake in the distant past or in the recent past, respectively. The scores were adjusted for total energy intake using the residual method (32). We used Cox proportional hazards regression models with time-varying covariates to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of endometrial cancer. For the test for trend, we assigned the quintile median to all participants in the quintile and modeled it as a continuous variable. Because the dietary pattern scores did not have intuitive a priori categories, we also used each dietary pattern as a continuous variable and modeled risk per 1-standard deviation increase. We used age in months as the underlying timescale and stratified by calendar year and cohort. In the multivariable-adjusted model, we adjusted for smoking status, physical activity, family history of endometrial cancer, age at menarche, age at menopause and menopausal status, parity, oral contraceptive use history, and menopausal hormone therapy. Additionally, we ran a multivariable-adjusted model further adjusting for BMI, a possible intermediate in the association of dietary inflammatory and insulinemic potential and endometrial cancer risk. We pooled data across the 2 cohorts for the main analysis and conducted sensitivity analyses within each cohort.
As diet and adiposity may interact to increase endometrial cancer risk, we further evaluated potential differences in the associations of diet scores with endometrial cancer risk by BMI categories. We tested for interaction between the empirical dietary indices (continuous) and stratification variables by including then excluding an interaction term in the model and evaluating its statistical significance with the Wald test. We also examined whether the association between EDIP or EDIH and endometrial cancer differed by other potential effect modifiers (physical activity, personal history of diabetes, and smoking status) and also adjusted for BMI within strata of the potential effect modifier (eg, adjusting for continuous BMI among normal weight participants).
Mediation analyses were performed to assess the extent to which associations may be statistically accounted for by BMI and estimated the mediation proportion (33-35). To do so, we calculated the mediation proportion and its 95% confidence interval (34) using the publicly available %Mediate macro designed by our group (https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/08/mediate.pdf). Briefly, this method uses a data duplication algorithm and reports point and interval estimates for the mediation proportion and the results for the mediation test using the difference method.
We performed several sensitivity analyses to test the robustness of our results. First, we ran the multivariable model while mutually adjusting for EDIP and EDIH. Second, we examined joint associations of the scores based on the median to evaluate their combined influence and between each score and BMI with endometrial cancer risk. Third, we restricted to confirmed type I endometrial cancer. Lastly, we evaluated a well-established index of diet quality, the Alternate Healthy Eating Index 2010 (AHEI-2010), in relation to endometrial cancer risk. All statistical analyses were performed using SAS 9.4 with 2-sided tests, and a P value less than .05 was considered statistically significant.
Results
Among 133 756 women and 2 823 221 person-years of follow-up, 1565 endometrial cancer cases were diagnosed of which 1462 were type I endometrial cancer cases (Supplementary Table 2, available online). Compared with women with lower EDIP or EDIH scores, those in the highest quintiles of each score tended to have higher BMI, greater weight change since age 18 years, and lower physical activity levels and were more likely to be current smokers and have a personal history of diabetes and a family history of endometrial cancer (Table 1). They also reported less consumption of coffee and alcohol.
Table 1.
Age-adjusted baseline characteristics by quintiles of empirical dietary inflammatory pattern (EDIP) and empirical dietary index for hyperinsulinemia (EDIH) in the Nurses’ Health Study (NHS, 1984) and Nurses’ Health Study II (NHSII, 1991)
| Characteristic | Quintiles of dietary pattern |
|||||
|---|---|---|---|---|---|---|
| NHS |
NHSII |
|||||
| Q1 (n = 9523) | Q3 (n = 9524) | Q5 (n = 9524) | Q1 (n = 16 690) | Q3 (n = 16 991) | Q5 (n = 16 990) | |
| EDIP, median (range)a | −1.1 (−1.6 to −0.9) | 0 (−0.1 to 0.1) | 1 (0.8 to 1.4) | −1.2 (−1.6 to −0.9) | 0 (−0.1 to 0.1) | 1.2 (0.9 to 1.7) |
| Age, mean (SD), y | 52.4 (7) | 52.4 (7.3) | 51.3 (7.3) | 39.4 (4.4) | 38.3 (4.6) | 37.6 (4.7) |
| BMI, mean (SD), kg/m2 | 23.9 (3.8) | 25 (4.4) | 27.1 (5.9) | 24.3 (4.9) | 24.7 (5.3) | 26.7 (6.7) |
| Weight change, mean (SD), kg | 7.2 (9.8) | 9.8 (10.6) | 14.5 (13.5) | 8.8 (11.1) | 10 (11.6) | 13.7 (14.1) |
| Lost ≥2 kg, % | 13.8 | 9.5 | 6.6 | 10.7 | 8.9 | 7.0 |
| Stable | 14.0 | 10.9 | 6.8 | 13.0 | 11.8 | 8.2 |
| Gained >2 kg, % | 72.2 | 79.6 | 86.6 | 76.4 | 79.3 | 84.8 |
| Physical activity, mean (SD), MET h/wk | 16.7 (24.9) | 13.8 (19.4) | 11.5 (17.3) | 24.4 (31.2) | 20.5 (25.3) | 18.5 (25.3) |
| Age at menarche, younger than 12 y, % | 21.2 | 21.5 | 23.1 | 24.4 | 22.7 | 25.5 |
| Ever use of OCs, % | 52.1 | 48.8 | 50.1 | 83.7 | 83.4 | 81.8 |
| Parous, % | 92.8 | 93.2 | 92.3 | 74.5 | 77.6 | 75.8 |
| Number of children, mean (SD) | 3.1 (1.4) | 3.1 (1.5) | 3.1 (1.6) | 2.1 (0.9) | 2.2 (0.9) | 2.2 (0.9) |
| Postmenopausal women, % | 51.4 | 51.9 | 52.4 | 1.9 | 1.9 | 2.0 |
| Postmenopausal hormone use, current use, %b | 15.4 | 12.0 | 8.8 | 76.5 | 77.9 | 76.7 |
| Former smoker, % | 40.0 | 33.2 | 29.9 | 30.0 | 22.7 | 18.0 |
| Current smoker, % | 27.0 | 22.0 | 22.0 | 14.6 | 9.7 | 10.0 |
| Personal history of diabetes, % | 1.6 | 2.7 | 7.0 | 0.6 | 1.0 | 2.2 |
| Family history of endometrial cancer, % | 2.6 | 3.0 | 3.1 | 2.3 | 2.3 | 2.5 |
| Alcohol intake, mean (SD), grams/day | 13.5 (14.5) | 5.4 (8.6) | 3.8 (8.3) | 6.3 (9.6) | 2.5 (4.6) | 1.5 (3.6) |
| Coffee intake, mean (SD), grams/day | 3.7 (1.8) | 2.4 (1.5) | 1.4 (1.3) | 2.7 (2) | 1.3 (1.4) | 1.5 (3.6) |
| EDIH, median (range)a | −1.1 (−1.5 to −0.9) | 0 (−0.1 to 0.1) | 1.1 (0.8 to 1.4) | −1.2 (−1.6 to −0.9) | 0 (−0.1 to 0.1) | 1.2 (0.9 to 1.7) |
| Age, mean (SD), y | 53.6 (7) | 52.4 (7.2) | 50.3 (7.1) | 39.1 (4.5) | 38.3 (4.6) | 37.9 (4.7) |
| BMI, mean (SD), kg/m2 | 23.7 (3.7) | 25.1 (4.4) | 27.1 (5.9) | 23.5 (4.4) | 24.8 (5.2) | 27.2 (6.8) |
| Weight change, mean (SD), kg | 6.7 (9.4) | 9.9 (10.8) | 14.7 (13.4) | 7.2 (10.1) | 10.2 (11.5) | 14.8 (14.3) |
| Lost ≥2 kg, % | 14.6 | 9.5 | 6.3 | 12.3 | 8.3 | 6.0 |
| Stable | 15.1 | 10.2 | 6.6 | 15.5 | 11.2 | 7.3 |
| Gained >2 kg, % | 70.3 | 80.3 | 87.1 | 72.2 | 80.5 | 86.8 |
| Physical activity, mean (SD), MET h/wk | 17.9 (25.3) | 13.4 (19.6) | 10.8 (15.7) | 28 (33.8) | 19.4 (24.4) | 17.1 (23.4) |
| Age at menarche (younger than 12 y), % | 20.5 | 22.0 | 22.7 | 23.7 | 23.7 | 25.5 |
| Ever use of OCs, % | 50.3 | 49.6 | 51.8 | 82.0 | 83.2 | 83.9 |
| Parous, % | 92.3 | 93.4 | 92.6 | 71.6 | 78.4 | 77.1 |
| Number of children, mean (SD) | 3.1 (1.5) | 3.1 (1.5) | 3.2 (1.5) | 2.1 (0.9) | 2.2 (0.9) | 2.2 (0.9) |
| Postmenopausal women, % | 51.7 | 52.5 | 51.3 | 1.8 | 1.9 | 2.1 |
| Postmenopausal hormone use, current use, %b | 15.2 | 12.1 | 9.3 | 76.0 | 72.3 | 76.9 |
| Former smoker, % | 39.1 | 33.2 | 30.9 | 29.5 | 22.0 | 18.9 |
| Current smoker, % | 22.5 | 22.4 | 24.7 | 10.9 | 10.6 | 12.2 |
| Personal history of diabetes, % | 1.5 | 2.5 | 7.2 | 0.6 | 0.9 | 2.2 |
| Family history of endometrial cancer, % | 2.9 | 2.9 | 3.0 | 2.3 | 2.4 | 2.7 |
| Alcohol intake, mean (SD), grams/day | 11.6 (13.8) | 5.8 (9.3) | 5.1 (9.6) | 5.3 (8.6) | 2.6 (4.9) | 2.3 (4.7) |
| Coffee intake, mean (SD), grams/day) | 3.2 (1.8) | 2.4 (1.6) | 1.8 (1.6) | 2.4 (1.9) | 1.4 (1.5) | 0.9 (1.3) |
Energy-adjusted values. Values are means (SD) or percentages and are standardized to the age distribution of the study population. BMI = body mass index; MET = metabolic equivalent task; OC = oral contraceptives; Q = quintile.
Among postmenopausal women.
Age-adjusted and multivariable-adjusted analyses showed consistent positive associations between EDIP and EDIH and endometrial cancer risk (Table 2), also apparent within each cohort (Supplementary Table 3, available online). In the multivariable-adjusted analysis, relative to those who consumed the least, women who consumed the most inflammatory diet had higher endometrial cancer risk (HRQ5vsQ1 = 1.46, 95% CI = 1.24 to 1.73; Ptrend < .001). Each 1-standard deviation increase of EDIP was associated with a 17% higher endometrial cancer risk (HR = 1.17, 95% CI = 1.11 to 1.24). However, the association was no longer statistically significant after adjusting for BMI (HRQ5vsQ1 = 1.03, 95% CI = 0.87 to 1.22; Ptrend = .31).
Table 2.
Hazard ratio (95% CI) of type 1 endometrial cancer according to quintiles of empirical dietary inflammatory pattern (EDIP)a and empirical dietary index for hyperinsulinemia (EDIH)a in the Nurses’ Health Study (NHS, 1984-2016) and Nurses’ Health Study II (NHSII, 1991-2017)b
| Dietary pattern | Quintiles of empirical hypothesis-oriented dietary indices |
P trend c | Per 1-SD increase | ||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| EDIPa | |||||||
| Cases/person-years | 255/501 249 | 248/501 349 | 281/501 494 | 321/501 814 | 357/502 070 | ||
| Age adjusted | 1 (referent) | 1.01 (0.85 to 1.21) | 1.16 (0.98 to 1.38) | 1.36 (1.16 to 1.61) | 1.54 (1.31 to 1.81) | <.001 | 1.19 (1.12 to 1.25) |
| Multivariable adjusted | 1 (referent) | 0.98 (0.82 to 1.16) | 1.11 (0.93 to 1.32) | 1.30 (1.10 to 1.53) | 1.46 (1.24 to 1.73) | <.001 | 1.17 (1.11 to 1.24) |
| Multivariable adjusted + BMI | 1 (referent) | 0.91 (0.77 to 1.09) | 0.99 (0.83 to 1.17) | 1.07 (0.91 to 1.27) | 1.03 (0.87 to 1.22) | .31 | 1.03 (0.97 to 1.09) |
| Multivariable adjusted + EDIHc | 1 (referent) | 0.91 (0.76 to 1.09) | 1.00 (0.83 to 1.20) | 1.13 (0.93 to 1.36) | 1.18 (0.95 to 1.46) | .04 | 1.08 (0.99 to 1.17) |
| Multivariable adjusted + EDIHc+ BMI | 1 (referent) | 0.93 (0.77 to 1.11) | 1.01 (0.84 to 1.21) | 1.11 (0.92 to 1.34) | 1.09 (0.87 to 1.35) | .20 | 1.05 (0.97 to 1.14) |
| EDIHa | |||||||
| Cases/person-years | 247/501 094 | 258/501 492 | 317/501 472 | 285/501 891 | 355/502 028 | ||
| Age adjusted | 1 (referent) | 1.08 (0.90 to 1.28) | 1.34 (1.13 to 1.58) | 1.25 (1.05 to 1.48) | 1.57 (1.34 to 1.86) | <.001 | 1.17 (1.11 to 1.23) |
| Multivariable adjusted | 1 (referent) | 1.06 (0.89 to 1.26) | 1.32 (1.12 to 1.57) | 1.24 (1.04 to 1.47) | 1.58 (1.34 to 1.87) | <.001 | 1.18 (1.12 to 1.25) |
| Multivariable adjusted + BMI | 1 (referent) | 0.95 (0.80 to 1.14) | 1.11 (0.94 to 1.32) | 0.95 (0.79 to 1.13) | 1.01 (0.85 to 1.21) | .92 | 1.00 (0.95 to 1.06) |
| Multivariable adjusted + EDIPc | 1 (referent) | 1.00 (0.83 to 1.20) | 1.22 (1.01 to 1.46) | 1.10 (0.90 to 1.34) | 1.33 (1.07 to 1.66) | .007 | 1.12 (1.03 to 1.21) |
| Multivariable adjusted + EDIPc + BMI | 1 (referent) | 0.93 (0.77 to 1.11) | 1.07 (0.89 to 1.29) | 0.90 (0.73 to 1.09) | 0.94 (0.74 to 1.17) | .52 | 0.97 (0.89 to 1.05) |
Energy-adjusted dietary pattern. CI = confidence interval; BMI = body mass index; MET = metabolic equivalent task.
Cox model was stratified for age in months, cohort study and year of questionnaire return with further adjustment for smoking status (never, past, current smoking), family history of endometrial cancer (no, yes), age at menarche (younger than 12, 12, 13, 14 years, 14 years and older), oral contraceptives (never, ever), parity (1, 2, ≥3 children), age at menopause (continuous), menopausal status (premenopausal, postmenopausal, dubious or missing), postmenopausal hormone use (never user, past user, current user—estrogen only for <5 years, current user—estrogen only for ≥5 years, current estrogen with progestin user for <5 years, current estrogen with progestin user for ≥5 years, current user of other types), and physical activity (linear MET hours per week). The multivariable-adjusted plus BMI models adjusted for all covariates in the multivariable-adjusted model and additionally for BMI (kg/m2, continuous).
P trend was calculated using continuous variables of dietary pattern in the model; model further mutually adjusting for EDIH or EDIP.
Similarly, higher EDIH was associated with increased endometrial cancer risk (HRQ5vsQ1 = 1.58, 95% CI = 1.34 to 1.87; Ptrend <.001). Each 1-standard deviation increase of EDIH was associated with an 18% increased risk of endometrial cancer (HR = 1.18, 95% CI = 1.12 to 1.25). Additional adjustment for BMI attenuated the association (EDIH HRQ5vsQ1 = 1.01, 95% CI = 0.85 to 1.21; Ptrend = .92). Results did not change when we considered all endometrial cancer cases (type I and type II, n = 1565) (Supplementary Table 4, available online). In a model including EDIP and EDIH (Table 2), we observed positive associations for EDIP (HR = 1.18, 95% CI = 0.95 to 1.46; Ptrend = .04) and EDIH (HR = 1.33, 95% CI = 1.07 to 1.66; Ptrend = .007). However, further controlling for BMI attenuated these associations. Results were somewhat stronger though materially unchanged when we included only confirmed type I cases (Supplementary Table 5, available online) or when we used dietary intake assessed at baseline only or with recently assessed diet (data not shown).
Given the attenuation with adjustment for BMI, we evaluated the extent to which the direct association of EDIP or EDIH with endometrial cancer may be statistically accounted for by BMI. Higher BMI statistically explained 60.4% (95% CI = 37.4% to 79.6%; P < .001) and 71.8% (95% CI = 41.0% to 90.4%; P < .001) of the direct association with EDIP and EDIH, respectively.
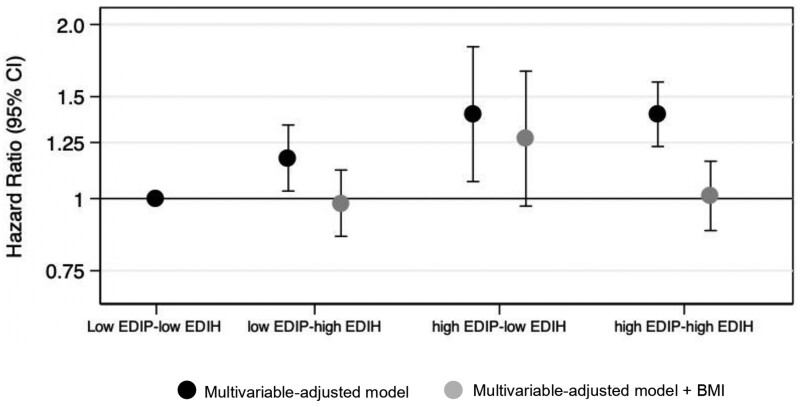
In the joint analysis of EDIP and EDIH (Figure 2), women in the highest categories for both EDIP and EDIH (high-high) had a 40% elevated risk of endometrial cancer (HRhigh EDIP/high EDIH = 1.40, 95% CI = 1.23 to 1.59) compared with those in the lowest category for both scores (low-low). Additional adjustment for BMI attenuated the association (HRhigh-high = 1.01, 95% CI = 0.88 to 1.16).
Figure 2.
Joint association of the EDIP and EDIH with risk of type 1 endometrial cancer in the Nurses’ Health Study (1984-2016) and Nurses’ Health Study II (1991-2017). EDIP and EDIH scores were dichotomized at the median resulting in 4 mutually exclusive groups (ie, low-low, low-high, high-low, and high-high). Low-low, the reference category, represents participants who persistently consumed low EDIP score diet or EDIH (below the median). Cox model was stratified for age in months, cohort study and year of questionnaire return with further adjustment for smoking status (never, past, current smoking), family history of endometrial cancer (no, yes), age at menarche (younger than 12 years, 12, 13, 14 years, 14 years and older), oral contraceptives (never, ever), parity (1, 2, ≥3 children), age at menopause (continuous), menopausal status (premenopausal, postmenopausal, dubious or missing), postmenopausal hormone use (never user, past user, current user—estrogen only for <5 years, current user—estrogen only for ≥5 years, current estrogen with progestin user for <5 years, current estrogen with progestin user for ≥5 years, current user of other types), and physical activity (linear MET hours per week). Multivariable-adjusted model further adjusted for BMI (kg/m2, continuous). BMI = body mass index; CI = confidence interval; EDIH = Empirical Dietary Index for Hyperinsulinemia; EDIP = Empirical Dietary Inflammatory Pattern; MET = metabolic equivalent.
A higher adherence to the AHEI-2010 a priori dietary pattern was associated with lower endometrial cancer risk, but the magnitude was less than for EDIP and EDIH (HRQ5vsQ1 = 0.82, 95% CI = 0.70 to 0.98; Ptrend = .01) (Supplementary Table 6, available online). However, additional adjustment for BMI also attenuated the association.
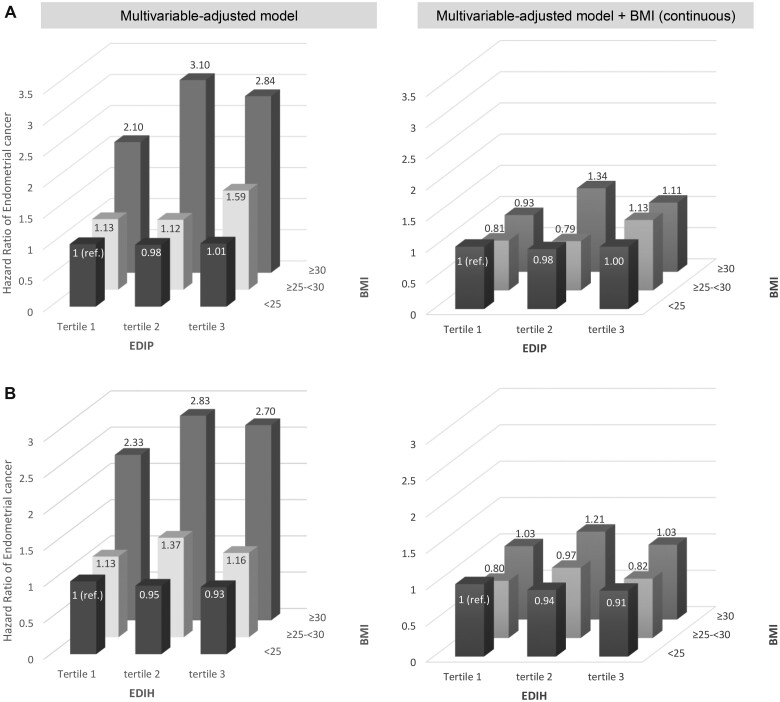
We observed an interaction between EDIP and BMI (Pinteraction = 0.04) (Table 3) but not with EDIH (Table 4). In the multivariable-adjusted model, among overweight women, the hazard ratio for Q5 vs Q1 was 1.21 (95% CI = 0.88 to 1.66; Ptrend = .08), which contrasted with the findings in other groups that tended toward a null association. Further adjusting for continuous BMI did not change the results (HRQ5vsQ1 = 1.19, 95% CI = 0.87 to 1.64). When we used other cut points to categorize BMI, we consistently found an interaction between BMI and EDIP (Supplementary Table 7, available online). The trend was clearer in the joint associations of EDIP and BMI. Compared with normal weight women consuming low insulinemic (Figure 3, B) or anti-inflammatory diets (Figure 3, A) (tertile 1), those who were obese and consuming proinflammatory diets had 2.84 times higher risk of endometrial cancer (95% CI = 2.18 to 3.70) (Figure 3, A).
Table 3.
Hazard ratio (95% CI) of type 1 endometrial cancer according to quintiles of empirical dietary inflammatory pattern (EDIP) stratified by body mass index, physical activity, diabetes history, and smoking status (pooled results from the Nurses’ Health Study and NHSII cohorts)a
| Stratification variables | Empirical dietary inflammatory pattern |
||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend b | P interaction c | |
| BMI | Multivariable-adjusted model | ||||||
| <25 kg/m2 | 1 (referent) | 0.97 (0.73 to 1.30) | 1.05 (0.78 to 1.41) | 0.94 (0.69 to 1.29) | 1.04 (0.74 to 1.47) | .92 | .04 |
| ≥25 to <30 kg/m2 | 1 (referent) | 0.95 (0.69 to 1.32) | 0.80 (0.57 to 1.12) | 1.25 (0.92 to 1.70) | 1.21 (0.88 to 1.66) | .08 | |
| ≥30 kg/m2 | 1 (referent) | 0.82 (0.59 to 1.13) | 1.11 (0.83 to 1.50) | 1.11 (0.83 to 1.48) | 1.12 (0.85 to 1.47) | .10 | |
| Multivariable-adjusted model + BMI | |||||||
| <25 kg/m2 | 1 (referent) | 0.96 (0.72 to 1.29) | 1.04 (0.78 to 1.39) | 0.93 (0.68 to 1.28) | 1.03 (0.73 to 1.44) | .99 | .66 |
| ≥25 to <30 kg/m2 | 1 (referent) | 0.94 (0.68 to 1.30) | 0.80 (0.57 to 1.11) | 1.23 (0.90 to 1.67) | 1.19 (0.87 to 1.64) | .09 | |
| ≥30 kg/m2 | 1 (referent) | 0.80 (0.58 to 1.11) | 1.06 (0.79 to 1.43) | 1.04 (0.78 to 1.38) | 0.95 (0.72 to 1.25) | .83 | |
| Physical activityd | Multivariable-adjusted model | ||||||
| < median | 1 (referent) | 1.03 (0.80 to 1.32) | 1.23 (0.97 to 1.57) | 1.34 (1.06 to 1.69) | 1.55 (1.23 to 1.94) | <.001 | .57 |
| ≥ median | 1 (referent) | 0.96 (0.75 to 1.23) | 1.01 (0.78 to 1.29) | 1.33 (1.04 to 1.70) | 1.42 (1.09 to 1.83) | .001 | |
| Multivariable-adjusted model + BMI | |||||||
| < median | 1 (referent) | 0.96 (0.74 to 1.23) | 1.07 (0.84 to 1.36) | 1.10 (0.86 to 1.39) | 1.07 (0.85 to 1.35) | .37 | .88 |
| ≥ median | 1 (referent) | 0.89 (0.69 to 1.14) | 0.89 (0.69 to 1.15) | 1.09 (0.85 to 1.39) | 1.04 (0.79 to 1.35) | .48 | |
| Personal history of diabetes | Multivariable-adjusted model | ||||||
| No | 1 (referent) | 1.00 (0.84 to 1.20) | 1.14 (0.96 to 1.36) | 1.28 (1.07 to 1.51) | 1.39 (1.17 to 1.65) | <.001 | .88 |
| Yes | 1 (referent) | 1.09 (0.53 to 2.26) | 1.22 (0.62 to 2.42) | 1.40 (0.73 to 2.67) | 1.35 (0.72 to 2.54) | .24 | |
| Multivariable-adjusted model + BMI | |||||||
| No | 1 (referent) | 0.94 (0.79 to 1.12) | 1.03 (0.86 to 1.2) | 1.08 (0.91 to 1.29) | 1.03 (0.86 to 1.23) | .41 | .85 |
| Yes | 1 (referent) | 1.20 (0.57 to 2.52) | 1.25 (0.62 to 2.52) | 1.38 (0.71 to 2.67) | 1.21 (0.63 to 2.32) | .67 | |
| Smoking status | |||||||
| Multivariable-adjusted model | |||||||
| Never | 1 (referent) | 1.15 (0.90 to 1.47) | 1.30 (1.02 to 1.65) | 1.32 (1.04 to 1.67) | 1.67 (1.32 to 2.11) | <.001 | .84 |
| Past | 1 (referent) | 0.97 (0.73 to 1.28) | 1.41 (1.08 to 1.82) | 1.07 (0.80 to 1.42) | 1.48 (1.13 to 1.95) | .003 | |
| Current | 1 (referent) | 0.88 (0.39 to 1.97) | 1.25 (0.59 to 2.66) | 1.51 (0.75 to 3.04) | 1.37 (0.68 to 2.78) | .21 | |
| Multivariable-adjusted model + BMI | |||||||
| Never | 1 (referent) | 0.99 (0.77 to 1.26) | 1.01 (0.79 to 1.28) | 1.23 (0.97 to 1.55) | 1.44 (1.14 to 1.80) | <.001 | .65 |
| Past | 1 (referent) | 1.00 (0.76 to 1.33) | 1.35 (1.03 to 1.76) | 1.46 (1.12 to 1.91) | 1.53 (1.16 to 2.01) | <.001 | |
| Current | 1 (referent) | 1.14 (0.58 to 2.25) | 0.72 (0.32 to 1.59) | 1.48 (0.75 to 2.93) | 1.43 (0.73 to 2.82) | .25 | |
Energy-adjusted dietary pattern. Cox model was stratified for age in months, cohort study and year of questionnaire return with further adjustment for smoking status (never, past, current smoking), family history of endometrial cancer (no, yes), age at menarche (younger than 12 years, 12, 13, and 14 years, 14 years and older), oral contraceptives (never, ever), parity (1, 2, ≥3 children), age at menopause (continuous), menopausal status (premenopausal, postmenopausal, dubious or missing), postmenopausal hormone use (never user, past user, current user—estrogen only for <5 years, current user—estrogen only for ≥5 years, current estrogen with progestin user for <5 years, current estrogen with progestin user for ≥5 years, current user of other types), and physical activity (linear MET hours per week). The multivariable-adjusted and BMI models adjusted for all covariates in the multivariable-adjusted model and additionally for BMI (kg/m2, continuous). BMI = body mass index; CI = confidence interval; MET = metabolic equivalent task; Q = quintiles.
P trend was calculated using continuous variables of dietary pattern in the model.
P interaction was calculated using the Wald test by including interaction terms [median EDIP value across quintiles (continuous variable) x effect modifier].
Cohort-specific median values for physical activity (NHS: 10.4 MET h/wk; NHS II: 12.9 MET h/wk).
Table 4.
Hazard ratio (95% CI) of type 1 endometrial cancer according to quintiles of empirical dietary index for hyperinsulinemia (EDIH) stratified by body mass index, physical activity, diabetes history, and smoking status (pooled results from the Nurses’ Health Study and NHSII cohorts)a
| Stratification variables | Empirical dietary index for hyperinsulinemia |
||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend b | P interaction c | |
| BMI | Multivariable-adjusted model | ||||||
| <25 kg/m2 | 1 (referent) | 0.98 (0.74 to 1.29) | 1.08 (0.81 to 1.44) | 0.88 (0.64 to 1.23) | 1.04 (0.73 to 1.48) | .94 | .03 |
| ≥25 to <30 kg/m2 | 1 (referent) | 1.17 (0.84 to 1.63) | 1.25 (0.91 to 1.73) | 1.11 (0.79 to 1.55) | 1.22 (0.87 to 1.71) | .35 | |
| ≥30 kg/m2 | 1 (referent) | 0.85 (0.61 to 1.20) | 1.14 (0.83 to 1.56) | 0.98 (0.72 to 1.33) | 1.10 (0.82 to 1.48) | .23 | |
| Multivariable-adjusted model + BMI | |||||||
| <25 kg/m2 | 1 (referent) | 0.97 (0.73 to 1.28) | 1.06 (0.80 to 1.41) | 0.87 (0.62 to 1.21) | 1.02 (0.71 to 1.45) | .83 | .69 |
| ≥25 to <30 kg/m2 | 1 (referent) | 1.16 (0.83 to 1.61) | 1.24 (0.90 to 1.72) | 1.09 (0.78 to 1.53) | 1.19 (0.85 to 1.67) | .42 | |
| ≥30 kg/m2 | 1 (referent) | 0.82 (0.58 to 1.16) | 1.07 (0.78 to 1.47) | 0.89 (0.65 to 1.21) | 0.90 (0.67 to 1.22) | .62 | |
| Physical activityd | Multivariable-adjusted model | ||||||
| < median | 1 (referent) | 1.08 (0.83 to 1.40) | 1.34 (1.05 to 1.70) | 1.27 (0.99 to 1.61) | 1.51 (1.19 to 1.90) | <.001 | .70 |
| ≥ median | 1 (referent) | 1.08 (0.84 to 1.37) | 1.36 (1.07 to 1.73) | 1.23 (0.95 to 1.60) | 1.69 (1.31 to 2.19) | <.001 | |
| Multivariable-adjusted model + BMI | |||||||
| < median | 1 (referent) | 0.97 (0.75 to 1.26) | 1.12 (0.87 to 1.43) | 0.96 (0.75 to 1.22) | 0.96 (0.75 to 1.22) | .58 | .50 |
| ≥ median | 1 (referent) | 0.96 (0.75 to 1.23) | 1.12 (0.88 to 1.43) | 0.94 (0.72 to 1.23) | 1.12 (0.85 to 1.47) | .46 | |
| Personal history of diabetes | Multivariable-adjusted model | ||||||
| No | 1 (referent) | 1.07 (0.89 to 1.27) | 1.27 (1.07 to 1.51) | 1.25 (1.05 to 1.49) | 1.49 (1.25 to 1.77) | <.001 | .93 |
| Yes | 1 (referent) | 1.38 (0.64 to 2.97) | 1.41 (0.69 to 2.88) | 1.13 (0.55 to 2.31) | 1.40 (0.70 to 2.79) | .54 | |
| Multivariable-adjusted model + BMI | |||||||
| No | 1 (referent) | 0.97 (0.81 to 1.15) | 1.08 (0.91 to 1.28) | 0.98 (0.82 to 1.17) | 1.00 (0.83 to 1.20) | .95 | .73 |
| Yes | 1 (referent) | 1.33 (0.61 to 2.90) | 1.27 (0.61 to 2.64) | 0.98 (0.47 to 2.03) | 1.11 (0.55 to 2.25) | .73 | |
| Smoking | |||||||
| Multivariable-adjusted model | |||||||
| Never | 1 (referent) | 1.04 (0.81 to 1.33) | 1.07 (0.84 to 1.36) | 0.98 (0.77 to 1.25) | 1.01 (0.79 to 1.29) | .89 | .56 |
| Past | 1 (referent) | 0.86 (0.65 to 1.13) | 1.17 (0.90 to 1.52) | 0.81 (0.61 to 1.09) | 0.96 (0.72 to 1.28) | .75 | |
| Current | 1 (referent) | 0.75 (0.32 to 1.72) | 1.11 (0.51 to 2.38) | 1.16 (0.57 to 2.39) | 1.03 (0.49 to 2.14) | .69 | |
| Multivariable-adjusted model + BMI | |||||||
| Never | 1 (referent) | 0.92 (0.72 to 1.18) | 0.89 (0.70 to 1.14) | 1.00 (0.79 to 1.26) | 0.98 (0.78 to 1.24) | .81 | .99 |
| Past | 1 (referent) | 0.93 (0.70 to 1.23) | 1.18 (0.90 to 1.55) | 1.19 (0.91 to 1.56) | 1.07 (0.81 to 1.42) | .28 | |
| Current | 1 (referent) | 1.12 (0.56 to 2.24) | 0.64 (0.28 to 1.45) | 1.30 (0.64 to 2.63) | 1.21 (0.60 to 2.42) | .56 | |
Energy-adjusted dietary pattern. Cox model was stratified for age in months, cohort study and year of questionnaire return with further adjustment for smoking status (never, past, current smoking), family history of endometrial cancer (no, yes), age at menarche (younger than 12 years, 12, 13, 14 years, 14 years and older), oral contraceptives (never, ever), parity (1, 2, ≥3 children), age at menopause (continuous), menopausal status (premenopausal, postmenopausal, dubious or missing), postmenopausal hormone use (never user, past user, current user—estrogen only for <5 years, current user—estrogen only for ≥5 years, current estrogen with progestin user for <5 years, current estrogen with progestin user for ≥5 years, current user of other types), and physical activity (linear MET hours per week). The multivariable-adjusted and BMI models adjusted for all covariates in the multivariable-adjusted model and additionally for BMI (kg/m2, continuous). BMI = body mass index; CI = confidence interval; MET = metabolic equivalent task; Q = quintiles
P trend was calculated using continuous variables of dietary pattern in the model.
Pinteraction was calculated using the Wald test by including interaction terms [median EDIH value across quintiles (continuous variable) x effect modifier].
Cohort-specific median values for physical activity (NHS: 10.4 MET h/wk; NHS II: 12.9 MET h/w).
Figure 3.
Joint association of each dietary pattern and BMI with type 1 endometrial cancer risk. A) EDIP. B) EDIH. Multivariable-adjusted model was stratified for age in months, cohort study and year of questionnaire return with further adjustment for smoking status (never, past, current smoking), family history of endometrial cancer (no, yes), age at menarche (younger than 12 years, 12, 13, 14 years, 14 years and older), oral contraceptives (never, ever), parity (1, 2, ≥3 children), age at menopause (continuous), menopausal status (premenopausal, postmenopausal, dubious or missing), postmenopausal hormone use (never user, past user, current user—estrogen only for <5 years, current user—estrogen only for ≥5 years, current estrogen with progestin user for <5 years, current estrogen with progestin user for ≥5 years, current user of other types), and physical activity (linear MET hours per week). Multivariable-adjusted model further adjusted for BMI (kg/m2, continuous). BMI = body mass index; EDIP = Empirical Dietary Inflammatory Pattern; EDIH = Empirical Dietary Index for Hyperinsulinemia; MET = metabolic equivalents.
Discussion
In these 2 large, prospective US cohorts, we investigated the associations of 2 dietary pattern scores reflecting the potential of habitual diets to contribute to chronic inflammation and hyperinsulinemia with type I endometrial cancer risk. Associations between EDIH or EDIP and endometrial cancer attenuated after adjusting for BMI, which suggest adiposity is likely to be an important mediator.
Adiposity is closely related to diet and can in part mediate its health effect. Higher EDIP and EDIH scores were associated with substantial long-term weight gain in previous studies (36); therefore, the models adjusting for BMI may highlight the adiposity-mediated association of the scores on endometrial cancer risk. Not surprisingly, our observation that BMI explained 60%-72% of the direct association with EDIP and EDIH adherence suggests the positive associations between EDIH or EDIP and endometrial cancer may not be independent of BMI. These dietary patterns are strongly associated with obesity (36), which, in turn, is associated with endometrial cancer (37). Therefore, preventing obesity through these diets could prevent a large proportion of cases (38). In addition, diet may interact with adiposity to modulate endometrial cancer risk. Our findings from the joint analysis confirms that the combined influence of the dietary scores and adiposity can be substantial.
Prior epidemiologic studies of the association of dietary inflammatory potential and risk of developing endometrial cancer have relied on the dietary inflammatory index (DII), and results have been mixed. A recent umbrella review of meta-analyses showed limited evidence for an association between DII and endometrial cancer risk (39). It is, however, difficult to directly compare these findings with ours, as the DII is mainly nutrient based and driven by dietary supplement use, whereas the EDIP is based exclusively on intakes of whole foods and food groups and is strongly correlated with systemic inflammatory biomarkers (29).
We observed that BMI modified the effect of the association between EDIP and endometrial cancer risk. Particularly, higher EDIP scores were suggestively associated with higher endometrial cancer risk among overweight women, which could be a chance finding given it was not observed in the obese category. Although none of the previous studies reported significant effect modification by BMI, the association was slightly stronger in women with a BMI of at least 25 kg/m2 in 2 studies (40,41). The underlying mechanism by which a proinflammatory diet might contribute to endometrial cancer risk among overweight women remains unknown, however, it may involve a complex interaction between several factors.
Proinflammatory diets are rich in red and processed meat and sugar-sweetened beverages but low in coffee and green and yellow vegetables. These components are in accordance with the inverse associations with endometrial cancer observed for coffee (42-44), monounsaturated fatty acid, and fiber, as well as compounds such as proanthocyanidins and β-carotene and the positive associations observed for sugar-sweetened beverages and red and processed meat (45-47). According to the 2018 WCRF–AICR summary report, high dietary GL (but not glycemic index) is a probable cause of endometrial cancer (18). A direct association between the GL and endometrial cancer risk was reported by several studies (48,49), but not others (50-52). Consumption of red and processed meat and butter have been associated with increased systemic inflammation and insulin resistance (7,53,54), which in turn could stimulate cell proliferation and inhibit apoptosis (7). Strengths of our study include the use of novel empirical hypothesis-oriented dietary patterns in 2 prospective cohorts, with large sample sizes and comprehensively collected data, which minimizes the potential for residual confounding. Additionally, we repeatedly measured diet over time, which reduces within-person variation. EDIH and EDIP, extensively applied in previous studies (36,55-57), comprise food combinations that maximally predict concentrations of C-peptide and inflammatory markers (21,22). Nonetheless, our study has limitations, including that self-reported dietary intake data may be subject to measurement errors. However, the FFQs have been validated against diet and biomarkers (58). Residual and/or unmeasured confounding cannot be completely ruled out; however, we controlled for potential confounders. Participants were all nurses and mostly White, which might limit generalizability.
In conclusion, the association between higher dietary inflammatory or insulinemic potentials and increased endometrial cancer incidence may be mediated by adiposity. Furthermore, among overweight women, higher EDIP was suggestively associated with endometrial cancer risk. Dietary recommendations emphasizing the importance of avoiding high-inflammatory dietary patterns could be considered for the prevention of endometrial cancer to prevent excess adiposity and ameliorate systemic inflammation and/or chronic insulin hypersecretion among overweight women.
Supplementary Material
Contributor Information
Andrea Romanos-Nanclares, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Fred K Tabung, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; The Ohio State University Comprehensive Cancer Center—Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, OH, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Jennifer A Sinnott, Department of Statistics, The Ohio State University, Columbus, OH, USA; Huntsman Cancer Institute, The University of Utah, Salt Lake City, UT, USA.
Britton Trabert, Huntsman Cancer Institute, The University of Utah, Salt Lake City, UT, USA; Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Immaculata De Vivo, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Mary C Playdon, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, and Huntsman Cancer Institute, Salt Lake City, UT, USA.
A Heather Eliassen, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Funding
This project was supported by the National Institutes of Health (U01 CA176726, UM1 CA186107, R00 CA218694) and Ramon Areces Foundation.
Notes
Role of the funder: The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author disclosures: The authors have no conflicts of interest to disclose.
Author contributions: Conceptualization: ARN, FKT, MCP, AHE. Formal analysis: ARN. Funding acquisition: MCP, AHE. Investigation: ARN, FKT, MCP, AHE. Methodology: ARN, FKT, JAS, BT, IDV, MCP, AHE. Resources/Data-curation: ARN, FKT, MCP, AHE. Writing—original draft: ARN, AHE. Writing—review & editing: ARN, FKT, JAS, BT, IDV, MCP, AHE.
Acknowledgements: The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Prior presentations: Presented at the 2022 American Association for Cancer Research (AACR) Annual Meeting, Poster Showcase, April 8-13, 2022.
Data availability
The data described in the article, code book, and analytic code will be made available upon application and approval. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).
References
- 1.American Cancer Society. Key statistics for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html. Published 2021. Accessed August 12, 2021.
- 2. Sheikh MA, Althouse AD, Freese KE, et al. USA endometrial cancer projections to 2030: should we be concerned? Future Oncol. 2014;10(16):2561-2568. doi: 10.2217/fon.14.192. [DOI] [PubMed] [Google Scholar]
- 3. Yasin HK, Taylor AH, Ayakannu T.. A narrative review of the role of diet and lifestyle factors in the development and prevention of endometrial cancer. Cancers. 2021;13(9):2149. doi: 10.3390/cancers13092149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Devivo I, Persson I, Adami HO.. Endometrial cancer. In: Adami HO, Hunter D, Trichopoulos D, eds. Textbook of Cancer Epidemiology. New York: Oxford University Press; 2008:468-493. doi: 10.1093/acprof:oso/9780195311174.003.0018. [DOI] [Google Scholar]
- 5. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States: potentially preventable cancers in US. CA Cancer J Clin. 2018;68(1):31-54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 6. Gunter MJ, Hoover DR, Yu H, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):921-929. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaaks R, Lukanova A, Kurzer MS.. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomark Prev. 2002;11(12):1531-1543. [PubMed] [Google Scholar]
- 8. Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR.. Insulin resistance and endometrial cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2015;51(18):2747-2758. doi: 10.1016/j.ejca.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 9. Hu Y, Zhang X, Ma Y, et al. Incident type 2 diabetes duration and cancer risk: a prospective study in two US cohorts. J Natl Cancer Inst. 2021;113(4):381-389. doi: 10.1093/jnci/djaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dashti SG, Simpson JA, Viallon V, et al. Adiposity and breast, endometrial, and colorectal cancer risk in postmenopausal women: quantification of the mediating effects of leptin, C‐reactive protein, fasting insulin, and estradiol. Cancer Med. 2022;11(4):1145-1159. doi: 10.1002/cam4.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sidorkiewicz I, Jóźwik M, Niemira M, Krętowski A.. Insulin resistance and endometrial cancer: emerging role for microRNA. Cancers. 2020;12(9):2559. doi: 10.3390/cancers12092559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trabert B, Eldridge RC, Pfeiffer RM, et al. Prediagnostic circulating inflammation markers and endometrial cancer risk in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial: prediagnostic inflammation markers and endometrial cancer. Int J Cancer. 2017;140(3):600-610. doi: 10.1002/ijc.30478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang T, Rohan TE, Gunter MJ, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011;20(5):971-977. doi: 10.1158/1055-9965.EPI-10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedenreich CM, Langley AR, Speidel TP, et al. Case–control study of inflammatory markers and the risk of endometrial cancer. Eur J Cancer Prev. 2013;22(4):374-379. doi: 10.1097/CEJ.0b013e32835b3813. [DOI] [PubMed] [Google Scholar]
- 15. Dossus L, Rinaldi S, Becker S, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case–control study. Endocr Relat Cancer. 2010;17(4):1007-1019. doi: 10.1677/ERC-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Renehan AG, Zwahlen M, Egger M.. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484-498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 17. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M.. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569-578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 18. World Cancer Research Fund/American Institute for Cancer Research. Continuous update project expert report 2018. Diet, nutrition, physical activity and endometrial cancer. https://www.wcrf.org/wp-content/uploads/2021/02/Endometrial-cancer-report.pdf. Accessed September 1, 2022.
- 19. Gao Y, Zhai P, Jiang F, Zhou F, Wang X.. Association between coffee drinking and endometrial cancer risk: a meta‐analysis. J Obstet Gynaecol Res. 2022;48(3):774-795. doi: 10.1111/jog.15139. [DOI] [PubMed] [Google Scholar]
- 20. Norat T, Aune D, Navarro Rosenblatt D, Vingeliene S, Abar L. WCRF/AICR Systematic Literature Review Continuous Update Project report. The Associations Between Food, Nutrition and Physical Activity and the Risk of Endometrial Cancer. https://www.wcrf.org/wp-content/uploads/2021/02/endometrial-cancer-slr.pdf. Published 2012. Accessed March 29, 2022.
- 21. Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560-1570. doi: 10.3945/jn.115.228718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tabung FK, Wang W, Fung TT, et al. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr. 2016;116(10):1787-1798. doi: 10.1017/S0007114516003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colditz GA, Manson JE, Hankinson SE.. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49-62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 24. Ley SH, Ardisson Korat AV, Sun Q, et al. Contribution of the Nurses’ Health Studies to uncovering risk factors for type 2 diabetes: diet, lifestyle, biomarkers, and genetics. Am J Public Health. 2016;106(9):1624-1630. doi: 10.2105/AJPH.2016.303314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC.. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 27. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243-249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 29. Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147(8):1567-1577. doi: 10.3945/jn.117.248377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rich-Edwards JW, Corsano KA, Stampfer MJ.. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016-1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 31. Burchardt NA, Shafrir AL, Kaaks R, Tworoger SS, Fortner RT.. Oral contraceptive use by formulation and endometrial cancer risk among women born in 1947-1964: the Nurses’ Health Study II, a prospective cohort study. Eur J Epidemiol. 2021;36(8):827-839. doi: 10.1007/s10654-020-00705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willett WC, Howe GR, Kushi LH.. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(suppl 4):1220S-1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 33. Greenland S, Rothman KJ, Lash TL. Measures of effect and association. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins;1998:47-64. [Google Scholar]
- 34. Lin DY, Fleming TR, De Gruttola V.. Estimating the proportion of treatment effect explained by a surrogate marker. Statist Med. 1997;16(13):1515-1527. doi:. [DOI] [PubMed] [Google Scholar]
- 35. Jun HJ, Austin SB, Wylie SA, et al. The mediating effect of childhood abuse in sexual orientation disparities in tobacco and alcohol use during adolescence: results from the Nurses’ Health Study II. Cancer Causes Control. 2010;21(11):1817-1828. doi: 10.1007/s10552-010-9609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL.. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J Nutr. 2019;149(5):804-815. doi: 10.1093/jn/nxy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaw E, Farris M, McNeil J, Friedenreich C.. Obesity and endometrial cancer. In: Pischon T, Nimptsch K, eds. Obesity and Cancer. Vol. 208. Recent Results in Cancer Research. Cham, Switzerland: Springer International Publishing; 2016:107-136. doi: 10.1007/978-3-319-42542-9_7. [DOI] [PubMed] [Google Scholar]
- 38. Onstad MA, Schmandt RE, Lu KH.. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34(35):4225-4230. doi: 10.1200/JClinOncol.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marx W, Veronese N, Kelly JT, et al. The dietary inflammatory index and human health: an umbrella review of meta-analyses of observational studies. Adv Nutr. 2021;12(5):1681-1690. doi: 10.1093/advances/nmab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shivappa N, Hébert JR, Zucchetto A, et al. Dietary inflammatory index and endometrial cancer risk in an Italian case–control study. Br J Nutr. 2016;115(1):138-146. doi: 10.1017/S0007114515004171 [DOI] [PubMed] [Google Scholar]
- 41. Nagle CM, Ibiebele T, Shivappa N, et al. ; for the Australian National Endometrial Cancer Study Group. Dietary inflammatory index, risk and survival among women with endometrial cancer. Cancer Causes Control. 2020;31(2):203-207. doi: 10.1007/s10552-019-01257-0. [DOI] [PubMed] [Google Scholar]
- 42. Zhao LG, Li ZY, Feng GS, et al. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer. 2020;20(1):101. doi: 10.1186/s12885-020-6561-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Merritt MA, Tzoulaki I, Tworoger SS, et al. Investigation of dietary factors and endometrial cancer risk using a Nutrient-wide Association Study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer Epidemiol Biomarkers Prev. 2015;24(2):466-471. doi: 10.1158/1055-9965.EPI-14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di Maso M, Boffetta P, Negri E, La Vecchia C, Bravi F.. Caffeinated coffee consumption and health outcomes in the US population: a dose–response meta-analysis and estimation of disease cases and deaths avoided. Adv Nutr. 2021;12(4):1160-1176. doi: 10.1093/advances/nmaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raglan O, Kalliala I, Markozannes G, et al. Risk factors for endometrial cancer: an umbrella review of the literature: risk factors for endometrial cancer. Int J Cancer. 2019;145(7):1719-1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 46. Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML.. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control. 2007;18(9):967-988. doi: 10.1007/s10552-007-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inoue-Choi M, Robien K, Mariani A, Cerhan JR, Anderson KE.. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2384-2394. doi: 10.1158/1055-9965.EPI-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turati F, Galeone C, Augustin LSA, La Vecchia C.. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients. 2019;11(10):2342. doi: 10.3390/nu11102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hatami Marbini M, Amiri F, Sajadi Hezaveh Z.. Dietary glycemic index, glycemic load, insulin index, insulin load and risk of diabetes-related cancers: a systematic review of cohort studies. Clin Nutr Espen. 2021;42:22-31. doi: 10.1016/j.clnesp.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 50. Brenner DR, Speidel T, Csizmadi I, et al. Glycemic load and endometrial cancer risk in a case-control study of Canadian women. Cancer Epidemiol. 2015;39(2):170-173. doi: 10.1016/j.canep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 51. Hartman TJ, McCullough ML, Hodge JM, Gaudet MM, Wang Y, Gapstur SM.. Dietary energy density, glycemic load, glycemic index, and risk for endometrial cancer in the CPS-II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2018;27(1):113-115. doi: 10.1158/1055-9965.EPI-17-0964. [DOI] [PubMed] [Google Scholar]
- 52. Sadeghi A, Sadeghian M, Nasiri M, et al. Carbohydrate quantity and quality affect the risk of endometrial cancer: a systematic review and dose-response meta-analysis. Clin Nutr. 2020;39(6):1681-1691. doi: 10.1016/j.clnu.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 53. Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM.. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102(1):42-47. doi: 10.1161/01.CIR.102.1.42. [DOI] [PubMed] [Google Scholar]
- 54. Lee Y, Kang D, Lee SA.. Effect of dietary patterns on serum C-reactive protein level. Nutr Metab Cardiovasc Dis. 2014;24(9):1004-1011. doi: 10.1016/j.numecd.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 55. Ma W, Jovani M, Nguyen LH, et al. Association between inflammatory diets, circulating markers of inflammation, and risk of diverticulitis. Clin Gastroenterol Hepatol. 2020;18(10):2279-2286.e3. doi: 10.1016/j.cgh.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tabung FK, Wang W, Fung TT, et al. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108(2):363-370. doi: 10.1093/ajcn/nqy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee DH, Li J, Li Y, et al. Dietary inflammatory and insulinemic potential and risk of type 2 diabetes: results from three prospective U.S. cohort studies. Diabetes Care. 2020;43(11):2675-2683. doi: 10.2337/dc20-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051-1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the article, code book, and analytic code will be made available upon application and approval. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).