Abstract
Osteoarthritis (OA) is the most prevalent musculoskeletal disease characterized by multiple joint structure damages, including articular cartilage, subchondral bone and synovium, resulting in disability and economic burden. Bone marrow lesions (BMLs) are common and important magnetic resonance imaging (MRI) features in OA patients. Basic and clinical research on subchondral BMLs in the pathogenesis of OA has been a hotspot. New evidence shows that subchondral bone degeneration, including BML and angiogenesis, occurs not only at or after cartilage degeneration, but even earlier than cartilage degeneration. Although BMLs are recognized as important biomarkers for OA, their exact roles in the pathogenesis of OA are still unclear, and disputes about the clinical impact and treatment of BMLs remain. This review summarizes the current basic and clinical research progress of BMLs. We particularly focus on molecular pathways, cellular abnormalities and microenvironmental changes of subchondral bone that contributed to the formation of BMLs, and emphasize the crosstalk between subchondral bone and cartilage in OA development. Finally, potential therapeutic strategies targeting BMLs in OA are discussed, which provides novel strategies for OA treatment.
Keywords: Osteoarthritis, Subchondral bone, Bone marrow lesions, Microenvironment, Crosstalk, Treatment
1. Introduction
OA is the most prevalent joint disease globally, affecting about 10 % of men and 18 % of women over the age of 60 (Woolf and Pfleger, 2003). It can affect almost any joints of the human body, and the most common symptoms include pain and dysfunction (Hwang and Kim, 2015). In recent years, emerging evidence demonstrates that subchondral bone plays an important role in the progression of OA (Hoshi et al., 2017). Subchondral bone abnormalities refer to microstructure changes of OA articular subchondral bone, including histopathological changes such as subchondral bone cyst, bone marrow lesions (BMLs), angiogenesis and osteophyte formation (Li et al., 2013).
BMLs mainly refer to MRI features of bone marrow abnormalities (Fig. 1), which are frequently detected in the affected joints of OA patients (Roemer et al., 2009). BMLs can be subdivided into two subtypes based on with or without cyst-like component (Perry et al., 2020). BMLs can be divided into traumatic or non-traumatic according to the etiology, and reversible or irreversible according to the progress of the disease (Kon et al., 2016). It was reported that almost 80 % of OA patients with knee pain had BMLs while only 30 % of patients with BMLs did not have knee pain (Felson et al., 2001). Culvenor et al. conducted a meta-analysis and reported that 4089 knees from 3255 asymptomatic uninjured adults processed BMLs with a pooled prevalence of up to 18 % (95%CI: 12 % to 24 %) (Culvenor et al., 2019). The MRI findings of BMLs are thought to be unspecific and characterized by regional decreased subchondral bone marrow signal intensity on T1-weighted images with a corresponding area of increased signal intensity on T2-weighted images in the painful joints (Roemer et al., 2009). The abnormal changes in the bone marrow signal intensity accompanied by pain symptoms were first reported and named “bone marrow edema (BME) syndrome” by Wilson et al. in 1988, which was referred to as increased water content in the focal bone marrow (Wilson et al., 1988). However, the abnormality described was only a transient change with no recurrences after conservative symptomatic treatments. A study to correlate ill-defined MRI signal changes with histologic findings by Zanettti et al. suggested that several histological abnormalities were found in subchondral bone including bone marrow necrosis, abnormal trabeculae, bone marrow fibrosis and bone marrow bleeding (Zanetti et al., 2000). Thus, the term “bone marrow lesions” has become generally used, especially in the OA research community (Roemer et al., 2009; Xu et al., 2012). Although most studies of BMLs regarding OA have been reported in the knee or hip joints, BMLs could also be found in joints of hand, ankle, and spine (Valderrabano et al., 2009; Rahme and Moussa, 2008; Haugen et al., 2011).
Fig. 1.
Sagittal MRI of knee joint BMLs in patients with OA (indicated by white arrow). a. BMLs without cyst-like component. b. BMLs with cyst-like component.
This review focuses on subchondral BMLs and summarizes the current research progress of BMLs, including the development prospect of MRI combined with artificial intelligence (AI) in the diagnosis and prognosis of OA. In addition, this review also focused on the changes in the microenvironment of the subchondral bone matrix, which may be caused by the regulation of osteoblasts, osteoclasts and immune cells (Horwood, 2013). Finally, we suggest that BMLs may have a promising role in future research and can be a candidate new target for OA treatment.
2. Epidemiology of BMLs
2.1. Role of BMLs in OA
The pathophysiology of OA has long been focused on articular cartilage deterioration which was considered the hallmark of OA (Peng et al., 2021). However, OA is now widely accepted as a whole-joint disease that several tissues including subchondral BMLs, synovium, menisci, ligaments, infrapatellar fat pad and tendons, play crucial roles in the process of joint degeneration (Loeser et al., 2012). BMLs are of particular interest due to their intimate relationships with pain symptom among OA patients (Zhang et al., 2011; Felson et al., 2007; Lo et al., 2009; Zhu et al., 2016a). Zhu et al. investigated the sensory innervation and osteoarthritic pain in animal model (Zhu et al., 2019). Then Zhou et al. found the related change in BMLs was an independent risk factor for joint pain by using clinical data (Zhou et al., 2022). The evolution of BMLs in OA changes in size or even regress over time, accompanied by the fluctuation or resolution of pain symptom. It should be noted that pain in OA is multifactorial in etiology (Lo et al., 2009), and the pathogenesis of why BMLs may cause pain is still unknown.
BMLs may be an early marker for disease progression. They are constantly involved in the progression of OA and are also reckoned as the potential risk factor for structural degeneration (Felson et al., 2003). Galateia et al. reported that thicker trabeculae with abnormal tissue composition located in the BMLs regions beneath cartilage deterioration, while extensive marrow changes represented active bone remodeling activity (Kazakia et al., 2013). Several longitudinal studies also found that the severity of BMLs was positively associated with cartilage defect, cartilage volume loss (Fan et al., 2021; Li et al., 2022b; Zhu et al., 2016a), joint space narrowing and joint replacement (Tanamas et al., 2010). It is of great importance to identify OA patients who are most likely to deteriorate by detecting BMLs (Klement and Sharkey, 2019). Since BMLs is closely related to pain and structural changes, many clinical trials of OA have taken BMLs as an outcome (Cai et al., 2020). A study and analysis pointed out that in patients with knee OA, cartilage lesions were not necessarily accompanied by BMLs, but BMLs were rare in tibiofemoral subregions without cartilage lesions. In addition, the presence of BMLs at baseline was associated with the worsening of cartilage integrity in the same tibiofemoral subregion over the next two years (Kothari et al., 2010). Similarly, effusion-synovitis was proved to be associated with BMLs in multiple cross-sectional and longitudinal studies (Wang et al., 2017). However, the whole picture of the interaction between BMLs and other structural abnormalities in the affected joint is still not fully understood (Kazakia et al., 2013).
2.2. Potential risk factors for BML formation
Considering that BMLs may be the potential target for the prevention and treatment of OA, it is crucial to explore the risk factors in the process of BMLs development. Weight loss in OA patients could relieve knee pain and improve function (Christensen et al., 2007). Yuan et al. conducted a systematic review and found moderate evidence supporting the significant association between obesity and the incidence of BMLs (Lim et al., 2014). Beckwée et al. reported that body weight increased the incidence of BMLs to a lesser extent, while significant associations were also found with higher body mass index (BMI) and increased body fat mass (Beckwee et al., 2015). It is widely accepted that weight gain and obesity act as biomechanical factors which are detrimental to the joint structures and may lead to the development of BMLs (Lim et al., 2014).
Dore et al. reported that cartilage defects of the affected joints are associated with site-specific BMLs, which may suggest that increased loading on and adjacent to the defects could accelerate the progress of BMLs (Dore et al., 2010; Ahedi et al., 2016). It is also possible that BMLs and cartilage defects may drive one another or co-occur in the pathway toward disease progression (Dore et al., 2010). In addition, synovial invasion, bone contusion and angiogenesis may also relate to BML formation (Munsch et al., 2020; Shabestari et al., 2016).
BMLs could be affected by systemic factors including inflammatory factors (C-reactive protein and interleukins) (Zhu et al., 2016b; Zhu et al., 2017) and serum lipids (serum cholesterol and triglycerides) (Lim et al., 2014). Previous studies indicated that low-grade inflammatory reaction could accelerate BML formation and serum lipids were significantly associated with subchondral bone atherosclerosis. It is worth noting that cardiovascular diseases and OA share some common risk factors and molecular pathways (Fernandes and Valdes, 2015). Excess accumulation of cholesterol may also contribute to the pathogenesis of BMLs (Song et al., 2021). Although OA is an age-related degenerative joint disease, no association between age and BMLs was found (Lim et al., 2014).
2.3. Predictive value of BMLs for osteoarthritic outcomes
MRI is a widely used technique in the diagnosis and prognosis of musculoskeletal diseases, with the advantages of non-invasive operation and direct visualization of various musculoskeletal tissues (Pelletier et al., 2013). As one of the most frequent MRI findings of OA, BMLs process the predictive value for OA progression and even joint replacement, which is thought to be the ultimate outcome of OA. Stephanie et al. reported that the severity of BMLs was negatively associated with cartilage loss over 2 years and positively associated with the risk of knee replacement over 4 years (Tanamas et al., 2010). A systematic review also confirmed the finding that the presence of BMLs may predict knee replacement independently (Pelletier et al., 2013). The results were in line with the findings of Dawn et al., which suggested that BMLs were site-specifically associated with cartilage defects (Dore et al., 2010). The Multicenter Osteoarthritis (MOST) Study reported that the presence of BMLs at baseline predicted subchondral bone attrition in the matching region over 30 months (Roemer et al., 2010). Another report on hand OA found that MRI-defined BMLs predicted radiographic joint space narrowing and erosion (Haugen et al., 2016). This evidence suggests that there may be potential value of BMLs in predicting OA progression and even end-stage of OA, although their value in clinical practice is not fully established. The cartilage defects and cartilage loss over time are closely related to BMLs detected by MRI in the same subregions of the tibial and femoral chambers, which indicates that the progress of OA structural abnormalities could have site-specific association with BMLs (Crema et al., 2013).
With the development of technology, artificial intelligence (AI) has been widely used in OA imaging. AI has made progress in shortening MRI acquisition time, data post-processing, disease diagnosis and prognosis (Calivà et al., 2022). A recent study pointed out that MRI-based three-dimensional texture of the infrapatellar fat pad was associated with future development of knee osteoarthritis (Li et al., 2022a). Although there is no report on MRI-based BMLs to predict OA. It is reported that AI's reading accuracy rate of BMLs in MRI is over 70 % (Tibrewala et al., 2020), which is thought to improve with the continuous innovation of technology in the future. More importantly, the clinical application value of BMLs detected by the AI approach will continue to be mined and confirmed.
3. Biological and cellular aspects
3.1. Subchondral bone microenvironment
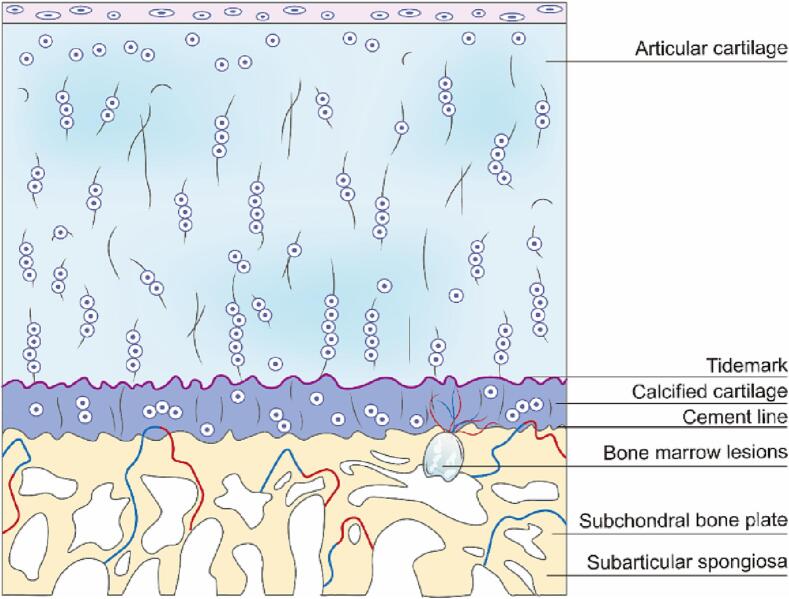
Subchondral bone refers to the bony layer beneath the articular cartilage and cement line that consists of the subchondral bone plate (SBP) and the subarticular spongiosa (Fig. 2). SBP is composed of cortical bone, which is a dense porous calcified plate crisscrossed by multiple blood vessels and nerve fibers. The subarticular sponge is located below SBP and is composed of trabecular bone (Hu et al., 2021; Dawn Doré, 2011). One of the primary functions of subchondral bone is to provide support to the overlying articular cartilage (Dawn Doré, 2011). Subchondral bone absorbs most of the mechanical force transmitted through joints and its architecture can adapt during mechanical stress to reduce the wear of cartilage. Subchondral bone also supplies nutrition to cartilage (Hu et al., 2020).
Fig. 2.
Schematic diagram of different layers of cartilage and subchondral bone.
In the local microenvironment of normal subchondral bone, mesenchymal stromal cells (MSCs), osteoblasts, osteoclasts, endothelial cells and immune cells are carefully coordinated by various biological and mechanical factors (Hu et al., 2021; Yan Hu et al., 2022). In OA joints, activated transforming growth factor β (TGF-β) is released from the bone matrix due to abnormal load and other factors, resulting in increased osteoblasts and enhanced osteogenic activity. The cells are relatively hypoxic during osteogenesis. A hypoxic environment leads to increased vascular endothelial growth factor (VEGF) expression and promotes angiogenesis (Abed et al., 2017; Corrado et al., 2013). A specific subtype of vessels, termed H-type vessels and defined by high costaining for CD31 and endomucin (CD31hiEmcnhi), has been identified to couple angiogenesis and osteogenesis (Xie et al., 2014; Kusumbe et al., 2014). In a single-cell sequencing study, it was found that the expression content of immune cells in the subchondral bone microenvironment of OA group was different from that of the normal group (Yan Hu et al., 2022). Abnormal subchondral bone microenvironment aggravates the subchondral bone injury, including BMLs, osteophyte, osteosclerosis and angiogenesis.
In this abnormal environment, osteocytes are stimulated to start the repair process by recruiting bone resorption cells and bone forming cells to maintain bone homeostasis, which is called subchondral bone remodeling (Zhu et al., 2020).
3.2. Subchondral bone microenvironment
With the change in subchondral bone structure, the microenvironment of subchondral BMLs is also changing. At present, there are emerging reports regarding the subchondral bone microenvironment in OA, but few studies have examined the longitudinal changes in the BMLs microenvironment. BMLs are characteristic imaging manifestations of subchondral bone lesions. The formation of BMLs is closely related to the changes in the subchondral bone microenvironment in OA. Therefore, investigating the role of the BMLs microenvironment in the development of subchondral bone degeneration and OA progression is of significant importance for understanding the etiology of OA.
Studies have shown that the microstructure of subchondral bone varies in different stages of OA. Increased bone remodeling and subchondral bone degradation occur in the early stages of OA and are considered determinants of OA progression (Bettica et al., 2002). Specifically, the subchondral bone plate becomes thinner and the porosity increases. Vascular infiltration has also been reported in the early stage (Koushesh et al., 2022). Subchondral sclerosis occurs only in the advanced stages of OA (Intema et al., 2010). In the late stage of OA, the main feature is subchondral bone sclerosis, whose microarchitectural characteristics are elevated apparent density, increased bone volume, thickening of the subchondral bone plate, increased trabecular thickness, a decrease of trabecular separation and bone marrow spacing, and transformation of trabeculae from rod-like into plate-like (Ding, 2010). The tidemark of OA joint is thickening, which contributes to articular cartilage thinning and deterioration (Goldring, 2009; Li et al., 2013). Although the bone density of the so-called “sclerotic” subchondral bone increases, its mineralization decreases, which is lower than that of normal joints or even osteoporotic joints. Therefore, the material stiffness of OA subchondral bone is decreased, which may exaggerate cartilage degeneration upon joint loading (Li and Aspden, 1997).
In a transcriptome analysis, significant differences in gene expression were found between BMLs and normal subchondral bone tissues. The up-regulated genes include stathmin 2 (STMN2), ATP binding cassette protein, thrombospondin 4 (THBS4), matrix metalloproteinase 13 (MMP-13) and chromosome 21 open reading frame, which are involved in a variety of functions, including bone remodeling, pain sensitivity and matrix conversion (Kuttapitiya et al., 2017). STMN2 is a phosphoprotein involved in the regulation of microtubule function, responsiveness to nerve growth factor (NGF), neuronal growth and osteogenesis. The upregulation of STMN2 in BMLs may lead to new neuronal structure in OA and expansion of BMLs, resulting in pain progression (Jin et al., 2004; Liu et al., 2011).
The most downregulated genes included hemoglobin, S100 calcium-binding protein A12, proplatelet basic protein ((chemokine C-X-C) motif ligand 7) and delta amino levulinate synthase 2 (Kuttapitiya et al., 2017). The decrease in hemoglobin was also consistent with the results of another study, which showed that the erythroid cells of hip joint were lower than those of normal femoral bone marrow (Trivanovic et al., 2022). These significantly altered gene expressions will cause changes in the subchondral bone microenvironment, which may eventually lead to the formation of BMLs. Therefore, there are still many places worth studying for the microenvironment of subchondral BMLs.
3.3. Pathology and formation process of BMLs
Although BMLs have been well described by using MRI, insufficient understanding of the pathology and formation process of BMLs remains. In a research analysis, compared with normal bone marrow tissue, the bone volume fraction was starkly reduced in BMLs areas, with marrow replaced by new blood vessels, dense fibrous connective tissue, hyaline cartilage and fibrocartilage (Kuttapitiya et al., 2017). But at the same time, some literatures also pointed out that patients with BML showed higher bone volume fraction than those without BMLs (Driban et al., 2012). In addition, subchondral cysts, thickening of bone trabeculae, inflammatory cell infiltration and tidal marks between cartilage and bone interface are also considered to be pathological changes of BMLs (Koushesh et al., 2022). The proposed pathological features, including cyst, subchondral fibrosis, vascular proliferation, abnormal cartilage formation, bone trabecular thickening, loss of tidal integrity and inflammatory cell infiltration, can be used to distinguish between BMLs and non-BMLs subchondral bone, and even OA and non-OA cases (Koushesh et al., 2022). However, the mechanism of pathological formation of BML is still not clear, but it may be caused by repeated microinjury and chronic or dysfunctional subchondral healing, specifically manifested as synovial invasion, bone remodeling and angiogenesis (Munsch et al., 2020; Shabestari et al., 2016).
Synovial invasion theory suggests that the formation of BMLs is due to synovial fluid entering the subchondral bone in the joint contact area by destroying the osteochondral connection (Li et al., 2013). Synovial fluid dispersion in the subchondral bone will naturally form BMLs, which can be confirmed by the detection of similar fluid in joint space and BMLs tissue (Koushesh et al., 2022).
Bone remodeling theory believes that the presence of mechanical stress in the subchondral bone will lead to microfracture and bone resorption (Li et al., 2013). Abnormal bone remodeling will form abnormal subchondral bone lesions, which is the beginning of BML formation. Bone remodeling is defined as the removal of mature bone tissue from the bone (bone resorption) and the formation of new bone tissue (bone formation). Bone remodeling not only can renew the bones of normal people, but also control the remodeling or replacement of bone after an injury. Subchondral bone in patients with OA will also undergo this process, including increased bone resorption and bone formation (Li et al., 2014). The formation of BMLs is associated with enhanced bone remodeling, which is thought to be a reversal. Further, it has been suggested that elevated bone remodeling and its associated stimulated vascularity are indispensable for the progression of OA (Burr and Gallant, 2012). Osteoclasts, mononuclear cells, osteoblasts, and osteocytes are the major cell types that participate in bone remodeling (Sims et al., 2016).
Although some studies point out that the reversal stage in bone remodeling may be the key treatment window period for regulating subchondral bone degeneration in OA (Li et al., 2013), more high-quality studies are needed as little is known about this reversal stage of OA. Angiogenesis is also considered to be a trigger of BML development. The increase in VEGF expression and other factors promote angiogenesis (Abed et al., 2017; Corrado et al., 2013). At present, we have only a vague understanding of the formation of BMLs, and its deeper mechanism needs to be explored.
4. Crosstalk of subchondral bone with cartilage
According to the previous description, OA subchondral bone lesions were deemed to occur earlier than cartilage degeneration and affect the process of cartilage degeneration. Meanwhile, cartilage degeneration may also react to subchondral bone remodeling. However, the signal pathways through which they interact need to be further clarified.
4.1. Feedback loop of “osteoclast-chondrocyte crosstalk”
The “osteoclast–chondrocyte crosstalk” can be accomplished in various pathways. First, microcracks or neovascularization provide a link between articular cartilage and subchondral bone, allowing osteoclasts and chondrocyte secreted mediators to diffuse and transport through microcracks or new vessels (Lajeunesse and Reboul, 2003). Second, osteoclast precursors can invade the hypertrophic cartilage region through neovascularization and interact with its cells to participate in cartilage matrix absorption and ossification (Tonna et al., 2016). Consistent with this, bone marrow-derived osteoclast precursors invade the neovascular-bone interface through blood circulation Fand differentiate into osteoclasts that contribute to articular cartilage erosion (Hasegawa et al., 2019). Furthermore, osteoclasts can degrade the osteochondral junction and resorb cartilage and calcified cartilage in MMP-mediated and cysteine protease-mediated ways (Löfvall et al., 2018), making it possible for mature osteoclasts in the subchondral bone to interact directly with chondrocytes in the cartilage layer. Finally, mechanical stress also plays a role in osteoclast–chondrocyte crosstalk. Abnormal mechanical loading may induce horizontal fractures and cartilage erosion at the osteochondral interface, leading to reduced capacity of the cartilage layer to bear mechanical stress and resulting in subchondral bone overload (Chen et al., 2020). In addition, in the early stages of OA, the increased turnover caused by mechanical overloads, such as increased bone remodeling and subchondral bone loss, will cause the shear force to concentrate on the cartilage layer and cause sustained damage (Hu et al., 2021; Yuan et al., 2014).
4.2. Regulation of osteoclasts by chondrocytes
Chondrocytes can regulate osteoclasts through a variety of pathways which may promote subchondral bone resorption. First, the effect of abnormal mechanical stress can induce the up-regulation of interleukin-1 (IL-1) expression in chondrocytes (Fujisawa et al., 1999). IL-1, IL-6 and prostaglandin E2 (PGE2) can enhance the expression of RANKL in osteoblasts, thereby stimulating the nucleation of osteoclast precursors and bone resorption (Cao et al., 2016). In addition, chondrocytes can also synthesize RANKL to induce osteoclastogenesis and subchondral osteoclast recruitment, contributing to subchondral bone loss and calcified cartilage microcracks (Martínez-Calatrava et al., 2012; Bertuglia et al., 2016). Second, other cytokines such as IL-6, IL-11 and tumor necrosis factor α (TNF-α) can also be produced by chondrocytes (Pearson et al., 2017; Maier et al., 1993). Briefly, inflammatory cytokines IL-6, IL-11 and TNF-α may induce osteoclast formation through a “remedial” pathway that has no role in normal bone (Kudo et al., 2003). Third, high mobility group box 1 protein (HMGB1) expression is upregulated in chondrocytes during the progression of OA (Aulin et al., 2020). HMGB1 released by hypertrophic chondrocytes can recruit osteoclasts that could invade growth plates and regulate bone development (Taniguchi et al., 2007). Forth, abnormal mechanical stress can also induce the expression of VEGF in chondrocytes, especially in hypertrophic chondrocytes, and VEGF can induce the recruitment of osteoclasts below the hypertrophic cartilage layer (Tanaka et al., 2005; Engsig et al., 2000).
4.3. Regulation of chondrocytes by osteoclasts
Similarly, osteoclasts can also influence chondrocytes through different pathways, which may contribute to cartilage degeneration. First, mechanical stress-induced TGF-β1 overexpression in osteoclasts promotes chondrocyte apoptosis and cartilage degeneration in OA (Buscemi et al., 2011; Zhang et al., 2018). Second, osteoclast activation and subchondral bone remodeling during early stage of OA are accompanied by increased calcium (Ca), phosphate (Pi), TGF-β1 and insulin-like growth factor (IGF-1) levels in the subchondral environment. The Ca-Pi complex up-regulates the expression of matrix metalloproteinase 3 (MMP-3) and MMP-13 in hypertrophic chondrocytes and is involved in cartilage degeneration in the early stage of OA (Jung et al., 2018). Third, recent studies have shown that exosomes also play a role in osteoclast-chondrocyte crosstalk. Osteoclast-derived exosomes let-7a-5p participate in the regulation of hypertrophic differentiation of chondrocytes by targeting Smad2, thus promoting endochondral ossification (Dai et al., 2020). Finally, the study of Cherifi et al. (Cherifi et al., 2021) showed that osteoclast-derived sphingosine 1-phosphate (S1P) regulated chondrocytes to secrete MMP through the S1P2 signaling pathway, thus promoting the decomposition of chondrocyte extracellular matrix and cartilage damage (Fig. 3).
Fig. 3.
Schematic diagram of cell interaction between cartilage and subchondral bone. The media secreted by osteoclasts and chondrocytes diffuse are transport through microcracks or new blood vessels. Osteoclast precursors can invade the hypertrophic cartilage region through neovascularization, interact with its cells and participate in cartilage matrix absorption and ossification. Chondrocytes regulate osteoclasts by secreting IL-1, IL-6, IL-11, TNF, HMGB1 and VEGF. Osteoclasts promote the expression of MMP in chondrocytes by secreting TGF-β, IGF-1 and Ca-Pi, which leads to the degradation of cartilage in the early stage of OA. (The mechanism diagram is drawn from reference (Hu et al., 2020)).
5. OA therapies targeting BMLs
At present, there is no effective treatment to alleviate OA symptoms and prevent OA progression except for joint replacement surgery at the end stage of disease (Carr et al., 2012; Bijlsma et al., 2011). Therapeutic attempts targeting articular cartilage have been disappointing. On the other hand, treatments of regulating subchondral bone have made significant progress, and the specific treatment of BMLs is worthy of in-depth investigation. Therefore, we will discuss several potential therapeutic strategies targeting BMLs in OA.
5.1. Regulating cells in BMLs
There have been several investigational drugs aiming to inhibit bone resorption and regulate bone formation to restore abnormal subchondral bone remodeling. For example, bisphosphonates (BPs), which induce osteoclast apoptosis and inhibit osteoclast activity, are classical drugs that inhibit bone resorption (Rogers et al., 2011). A 2-year clinical trial involving 2483 patients with medial compartment OA of the knee found that risedronate reduced biochemical markers of cartilage degradation but did not improve symptoms or slow radiological progress (Bingham et al., 2006). Another recent meta-analysis of randomized controlled trials involving 3013 patients showed that BPs did not significantly improve pain, structure and function compared with placebo (Vaysbrot et al., 2018). In addition, patients with symptomatic knee OA who received zoledronic acid intravenously each year had no significant reduction in cartilage volume loss, BMLs size, or pain score over 24 months compared with the placebo group (Aitken et al., 2018). In contrast, a recent report showed that BPs have an early preventive effect on subsets of OA patients (Hayes et al., 2020). And a meta-analysis of 3832 OA subjects also showed that BPs were effective in the treatment of OA pain compared with placebo (Davis et al., 2013). The efficacy of BPs is thereby inconclusive across studies, which may be due to various dosages and duration of intervention.
Similarly, calcitonin also showed the capacity to prevent subchondral trabecular bone loss and protect cartilage in animal OA models (Wen et al., 2016). However, a recent phase III clinical trial concluded that calcitonin did not improve joint space width or relieve pain (Karsdal et al., 2015). Estrogen is well-known to be very important for bone development and health. It regulates the balance of the subchondral bone microenvironment by inhibiting osteoclast activation and inducing osteoclast apoptosis. Although estrogen has not been specifically used in randomized clinical trials for OA, some promising results suggest that estrogen may regulate OA related subchondral bone remodeling (Zhu et al., 2020). One cross-sectional clinical study reported that elderly women with estrogen administration had a lower prevalence of knee OA–related subchondral bone attrition and subchondral BMLs than those without estrogen administration (Carbone et al., 2004). Raloxifene (RAL) is a commonly used drug for the treatment of postmenopausal osteoporosis. It can inhibit subchondral bone absorption, improve subchondral bone microstructure, and delay the progression of patellofemoral OA in ovariectomized rats (Bei et al., 2020).
In addition to inhibiting the activity of osteoclasts, regulating the function of osteoblasts is also a strategy to reverse subchondral bone reconstruction. High levels of TGF-β1 activation were found in human and mouse OA subchondral bone, which led to subchondral bone abnormalities, including increased osteoblast number, enhanced subchondral bone sclerosis and angiogenesis (Zhu et al., 2020). The enhancement of osteoclast activity is an important reason for the over-activation of TGF-β1 signal in the subchondral bone. Animal experiments have shown that inhibiting TGF-β1 pathway by blocking TGF-β1 receptor (sb-505,124) or neutralizing TGF-β1 in subchondral bone with neutralizing antibody (1d11) can reduce the progression of OA and rescue articular cartilage degeneration and subchondral osteosclerosis (Zhen et al., 2013). Canonical signaling via β-catenin is also involved in subchondral bone remodeling (Huang et al., 2018). Funck et al. found the activation of Wnt/β catenin signal in OA subchondral bone, inhibited Wnt in osteoblasts and reduced OA phenotype through the overexpression of its natural antagonist DKK1, which provides a new potential pharmacological target for OA subchondral bone remodeling (Funck-Brentano et al., 2014). These drugs have played an excellent role in the treatment of subchondral bone lesions. However, there are very few reports of effective drugs specifically targeting BMLs for OA treatment.
5.2. Cell therapies: bone marrow derived mesenchymal stem cells
MSCs are pluripotent adult cells that can be isolated from various adult or neonatal tissues, such as bone marrow, adipose tissue, placenta and umbilical cord. MSCs isolated from bone marrow (BMSCs) have been widely used in animal models and clinical practice to prove their chondrogenic potential, but the BMSC donors are lacking. Adipose-derived mesenchymal stem cells (AMSC) are a more readily available source of stem cells for OA treatment (Zhang et al., 2019). Due to their paracrine and immunosuppressive properties, MSCs-based treatment has shown reasonable differentiation ability in OA joints. Therefore, intra-articular injection of MSCs suspension for nutritional activity, or implantation of MSCs in combination with biodegradable materials for tissue engineering applications are possible treatment strategies for OA (Maumus et al., 2018).
At present, about 35 clinical studies on the treatment of OA BMLs with MSCs have been registered on the ClinicalTrails.gov website. 20 years ago, Wakitani et al. (Wakitani et al., 2002) reported for the first time that the transplanted BMSCs successfully repaired the isolated full-thickness cartilage defect of OA patients. The study consisted of 24 patients with knee OA who underwent high tibial osteotomy. The adherent cells in bone marrow aspirate were expanded into collagen gel and transplanted into articular cartilage defects of the medial femoral condyle, and then covered with autogenous periosteum during the 12 high tibial incisions. The other 12 subjects served as acellular controls. In the cell transplantation group, as early as 6.3 weeks after transplantation, the defect was covered by white to soft pink tissue, some of which were heterochromatic 42 weeks after transplantation. The cartilage defect was covered by soft white tissue, in which heterochromaticity was observed in almost all areas of the sampled tissue, and hyaline cartilage like tissue was partially observed. Although there was no significant difference in clinical improvement, the arthroscopic and histological grading scores of the cell transplantation group were better than those of the cell-free control group, indicating the therapeutic potential of BMSCs in OA cartilage repair and regeneration (Wakitani et al., 2002). Thus far, a large number of studies have reported that intra-articular injection of MSCs can improve OA cartilage volume and relieve pain. For example, in OA rat model, injection of BMSC-derived exosomes can alleviate knee OA pain (He et al., 2020). In addition, a phase II clinical trial showed that intra-articular injection of human AMSC could significantly improve joint function, pain, quality of life and cartilage regeneration in patients with OA (Lu et al., 2019). However, the reported favorable results mainly focus on pain relief and functional improvement, scarce tissue regeneration or subchondral bone reconstruction effects were reported. It is worth noting that Hernigou et al. (Hernigou et al., 2021) published a randomized controlled trial comparing BMSCs injection with contralateral joint replacement. The results not only showed that BMSCs therapy achieved a clinical score similar to that of total knee arthroplasty (TKA) in short-term and long-term follow-up, but also suggested that BMSCs therapy can effectively reduce the size of BMLs in patients with OA.
5.3. Blocking subchondral type H vessels/restoring abnormal subchondral bone remodeling
With the progress of OA, the subchondral bone microenvironment changes significantly, leading to braided bone and angiogenesis. There have been articles reported that increased osteoclast bone resorption leads to TGF- β1 over activation and will decouple bone resorption and bone formation, resulting in the sclerotic phenotype of subchondral bone in OA animal model (Cui et al., 2016; Tang et al., 2009). At present, the treatment of OA mainly focuses on inhibiting inflammation and subchondral bone reconstruction, while the therapeutic strategies for subchondral angiogenesis are limited. Blocking H-type angiogenesis in OA animal models has shown the potential to reduce cartilage destruction and subchondral bone loss (Hu et al., 2020; Nagai et al., 2010). As mentioned earlier, secreted factors such as TGF-β1and VEGF from osteoclasts or osteoblast line cells in OA subchondral bone microenvironment could promote subchondral angiogenesis. Therefore, these molecules may serve as potential drug targets for the treatment of OA. For example, the small molecule compound halofuginone can inhibit Smad2/3-dependent TGF-β1 signal to restore the coupling of subchondral bone reconstruction, alleviate H-type angiogenesis, and reduce cartilage degradation in rodent ACLT joints (Cui et al., 2016).
6. Summary
As a characteristic pathological structure of subchondral bone in OA, BMLs are of great significance to the occurrence and development of OA. BMLs are thought to occur earlier than cartilage degeneration and can be detected in the early stage of OA. Therefore, BMLs could serve as a biomarker for the early diagnosis of OA. Although MRI is the most superior clinical imaging for the evaluation of OA patients at present, its application is still limited, because it demands no movement for prolonged time which is not practical for elderly patients and most importantly it is very expensive while in same time MRI clinical findings are still not validated with purpose to treatment. However, with the development of AI technology, BMLs can be accurately distinguished from MRI (Tibrewala et al., 2020), and the diagnosis and prognosis of OA can be achieved with the help of AI in the future.
Second, through the pathological detection of BMLs in different stages, we can infer the different development stages of OA. Some articles pointed out that the size of BMLs is also related to OA pain, and the regression of BMLs can reduce joint pain (Driban et al., 2013). Clinical trials have taken the change of BMLs size for OA essential outcome evaluation. However, whether the change of BMLs will affect the changes of other related symptoms of OA remains to be studied. The role of BMLs investigation is not to provide a final diagnosis of OA but rather to serve as an additional input for decision-making.
Last, an increasing number of studies on BMLs targeted therapies for OA are undertaking, and the treatment of subchondral BMLs has also shown promising results. The role of BMLs in OA has become increasingly prominent as more in-depth research on BMLs emerges. Existing studies have proved that BMLs are closely related to OA cartilage degeneration, pain symptoms and so on, while more in-depth, broader, and higher-level research are needed to explore the applicable value of BMLs in the diagnosis and treatment of OA.
Funding
The present study was supported by the National Natural Science Foundation of China (32000925), Guangzhou Science and Technology Program (202002030481), Guangdong Basic and Applied Basic Research Foundation (2019A1515111169) and Wu Jieping Medical Foundation Program (3206750.2020-03-12).
CRediT authorship contribution statement
Article design: ZZ.
Literature collection: SX, FX, MY.
Writing - original draft: SX, FX, MY, DQ.
Writing - review & editing: SX, MY, WZ, XS, CH, ZZ, DC.
Approve the submission: All authors.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
The author thanks all the co-authors and participants who provided help in the process of writing this article, especially those who provided help in writing this article. We also thank Yumei Cao from Southern Medical University, Weizhong Qi from Zhujiang Hospital, Tingting Zhu from Anhui Medical University for commenting on the manuscript.
Data availability
The authors do not have permission to share data.
References
- Abed É., Delalandre A., Lajeunesse D. Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts. Arthritis Res. Ther. 2017;19:151. doi: 10.1186/s13075-017-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahedi H.G., Aitken D.A., Blizzard L.C., Ding C.H., Cicuttini F.M., Jones G. Correlates of hip cartilage defects: a cross-sectional study in older adults. J. Rheumatol. 2016;43:1406–1412. doi: 10.3899/jrheum.151001. [DOI] [PubMed] [Google Scholar]
- Aitken D., Laslett L.L., Cai G., Hill C., March L., Wluka A.E., Wang Y., Blizzard L., Cicuttini F., Jones G. A protocol for a multicentre, randomised, double-blind, placebo-controlled trial to compare the effect of annual infusions of zoledronic acid to placebo on knee structural change and knee pain over 24 months in knee osteoarthritis patients - ZAP2. BMC Musculoskelet. Disord. 2018;19:217. doi: 10.1186/s12891-018-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulin C., Lassacher T., Palmblad K., Erlandsson Harris H. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthr. Cartil. 2020;28:698–707. doi: 10.1016/j.joca.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Beckwee D., Vaes P., Shahabpour M., Muyldermans R., Rommers N., Bautmans I. The influence of joint loading on bone marrow lesions in the knee: a systematic review with meta-analysis. Am. J. Sports Med. 2015;43:3093–3107. doi: 10.1177/0363546514565092. [DOI] [PubMed] [Google Scholar]
- Bei M.J., Tian F.M., Xiao Y.P., Cao X.H., Liu N., Zheng Z.Y., Dai M.W., Wang W.Y., Song H.P., Zhang L. Raloxifene retards cartilage degradation and improves subchondral bone micro-architecture in ovariectomized rats with patella Baja-induced - patellofemoral joint osteoarthritis. Osteoarthr. Cartil. 2020;28:344–355. doi: 10.1016/j.joca.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Bertuglia A., Lacourt M., Girard C., Beauchamp G., Richard H., Laverty S. Osteoclasts are recruited to the subchondral bone in naturally occurring post-traumatic equine carpal osteoarthritis and may contribute to cartilage degradation. Osteoarthr. Cartil. 2016;24:555–566. doi: 10.1016/j.joca.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Bettica P., Cline G., Hart D.J., Meyer J., Spector T.D. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the chingford study. Arthritis Rheum. 2002;46:3178–3184. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- Bingham C.O., III, Buckland-Wright J.C., III, Garnero P., III, Cohen S.B., III, Dougados M., III, Adami S., III, Clauw D.J., III, Spector T.D., III, Pelletier J.P., III, Raynauld J.P., III, Strand V., III, Simon L.S., III, Meyer J.M., III, Cline G.A., III, Beary J.F., III Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- Burr D.B., Gallant M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J.J., Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr. Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Cai G., Aitken D., Laslett L.L., Pelletier J.P., Martel-Pelletier J., Hill C., March L., Wluka A.E., Wang Y., Antony B., Blizzard L., Winzenberg T., Cicuttini F., Jones G. Effect of intravenous zoledronic acid on tibiofemoral cartilage volume among patients with knee osteoarthritis with bone marrow lesions: a randomized clinical trial. JAMA. 2020;323:1456–1466. doi: 10.1001/jama.2020.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calivà F., Namiri N.K., Dubreuil M., Pedoia V., Ozhinsky E., Majumdar S. Studying osteoarthritis with artificial intelligence applied to magnetic resonance imaging. Nat. Rev. Rheumatol. 2022;18:112–121. doi: 10.1038/s41584-021-00719-7. [DOI] [PubMed] [Google Scholar]
- Cao Y., Jansen I.D., Sprangers S., Stap J., Leenen P.J., Everts V., de Vries T.J. IL-1β differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets. J. Leukoc. Biol. 2016;100:513–523. doi: 10.1189/jlb.1A1215-543R. [DOI] [PubMed] [Google Scholar]
- Carbone L.D., Nevitt M.C., Wildy K., Barrow K.D., Harris F., Felson D., Peterfy C., Visser M., Harris T.B., Wang B.W., Kritchevsky S.B. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;50:3516–3525. doi: 10.1002/art.20627. [DOI] [PubMed] [Google Scholar]
- Carr A.J., Robertsson O., Graves S., Price A.J., Arden N.K., Judge A., Beard D.J. Knee replacement. Lancet. 2012;379:1331–1340. doi: 10.1016/S0140-6736(11)60752-6. [DOI] [PubMed] [Google Scholar]
- Chen L., Yao F., Wang T., Li G., Chen P., Bulsara M., Zheng J.J.Y., Landao-Bassonga E., Firth M., Vasantharao P., Huang Y., Lorimer M., Graves S., Gao J., Carey-Smith R., Papadimitriou J., Zhang C., Wood D., Jones C., Zheng M. Horizontal fissuring at the osteochondral interface: a novel and unique pathological feature in patients with obesity-related osteoarthritis. Ann. Rheum. Dis. 2020;79:811–818. doi: 10.1136/annrheumdis-2020-216942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherifi C., Latourte A., Vettorazzi S., Tuckermann J., Provot S., Ea H.K., Ledoux A., Casas J., Cuvillier O., Richette P., Ostertag A., Hay E., Cohen-Solal M. Inhibition of sphingosine 1-phosphate protects mice against chondrocyte catabolism and osteoarthritis. Osteoarthr. Cartil. 2021;29:1335–1345. doi: 10.1016/j.joca.2021.06.001. [DOI] [PubMed] [Google Scholar]
- Christensen R., Bartels E.M., Astrup A., Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 2007;66:433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado A., Neve A., Cantatore F.P. Expression of vascular endothelial growth factor in normal, osteoarthritic and osteoporotic osteoblasts. Clin. Exp. Med. 2013;13:81–84. doi: 10.1007/s10238-011-0170-5. [DOI] [PubMed] [Google Scholar]
- Crema M.D., Felson D.T., Roemer F.W., Wang K., Marra M.D., Nevitt M.C., Lynch J.A., Torner J., Lewis C.E., Guermazi A. Prevalent cartilage damage and cartilage loss over time are associated with incident bone marrow lesions in the tibiofemoral compartments: the MOST study. Osteoarthr. Cartil. 2013;21:306–313. doi: 10.1016/j.joca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Crane J., Xie H., Jin X., Zhen G., Li C., Xie L., Wang L., Bian Q., Qiu T., Wan M., Xie M., Ding S., Yu B., Cao X. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann. Rheum. Dis. 2016;75:1714–1721. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor A.G., Oiestad B.E., Hart H.F., Stefanik J.J., Guermazi A., Crossley K.M. Prevalence of knee osteoarthritis features on magnetic resonance imaging in asymptomatic uninjured adults: a systematic review and meta-analysis. Br. J. Sports Med. 2019;53:1268–1278. doi: 10.1136/bjsports-2018-099257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Dong R., Han X., Li J., Gong X., Bai Y., Kang F., Liang M., Zeng F., Hou Z., Dong S. Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes. Am. J. Physiol. Cell Physiol. 2020 doi: 10.1152/ajpcell.00039.2020. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Davis A.J., Smith T.O., Hing C.B., Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? A meta-analysis and systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn Doré B.H.K. University of Tasmania; 2011. Bbiotech(Hons). The Role of Subchondral Bone in Osteoarthritis. Doctor of Philosophy. [Google Scholar]
- Ding M. Microarchitectural adaptations in aging and osteoarthrotic subchondral bone issues. Acta Orthop. Suppl. 2010;81:1–53. doi: 10.3109/17453671003619037. [DOI] [PubMed] [Google Scholar]
- Dore D., Martens A., Quinn S., Ding C., Winzenberg T., Zhai G., Pelletier J.P., Martel-Pelletier J., Abram F., Cicuttini F., Jones G. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res. Ther. 2010;12 doi: 10.1186/ar3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J.B., Tassinari A., Lo G.H., Price L.L., Schneider E., Lynch J.A., Eaton C.B., McAlindon T.E. Bone marrow lesions are associated with altered trabecular morphometry. Osteoarthr. Cartil. 2012;20:1519–1526. doi: 10.1016/j.joca.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J.B., Price L., Lo G.H., Pang J., Hunter D.J., Miller E., Ward R.J., Eaton C.B., Lynch J.A., McAlindon T.E. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker–longitudinal relationships with pain and structural changes: data from the osteoarthritis initiative. Arthritis Res. Ther. 2013;15 doi: 10.1186/ar4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engsig M.T., Chen Q.J., Vu T.H., Pedersen A.C., Therkidsen B., Lund L.R., Henriksen K., Lenhard T., Foged N.T., Werb Z., Delaissé J.M. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T., Ruan G., Antony B., Cao P., Li J., Han W., Li Y., Yung S.N., Wluka A.E., Winzenberg T., Cicuttini F., Ding C., Zhu Z. The interactions between MRI-detected osteophytes and bone marrow lesions or effusion-synovitis on knee symptom progression: an exploratory study. Osteoarthr. Cartil. 2021;29:1296–1305. doi: 10.1016/j.joca.2021.06.008. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Chaisson C.E., Hill C.L., Totterman S.M., Gale M.E., Skinner K.M., Kazis L., Gale D.R. The association of bone marrow lesions with pain in knee osteoarthritis. Ann. Intern. Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- Felson D.T., McLaughlin S., Goggins J., Lavalley M.P., Gale M.E., Totterman S., Li W., Hill C., Gale D. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann. Intern. Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Niu J., Guermazi A., Roemer F., Aliabadi P., Clancy M., Torner J., Lewis C.E., Nevitt M.C. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- Fernandes G.S., Valdes A.M. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur. J. Clin. Investig. 2015;45:405–414. doi: 10.1111/eci.12413. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Hattori T., Takahashi K., Kuboki T., Yamashita A., Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J. Biochem. 1999;125:966–975. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]
- Funck-Brentano T., Bouaziz W., Marty C., Geoffroy V., Hay E., Cohen-Solal M. Dkk-1-mediated inhibition of wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014;66:3028–3039. doi: 10.1002/art.38799. [DOI] [PubMed] [Google Scholar]
- Goldring S.R. Role of bone in osteoarthritis pathogenesis. Med. Clin. N. Am. 2009;93(25–35):xv. doi: 10.1016/j.mcna.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Kikuta J., Sudo T., Matsuura Y., Matsui T., Simmons S., Ebina K., Hirao M., Okuzaki D., Yoshida Y., Hirao A., Kalinichenko V.V., Yamaoka K., Takeuchi T., Ishii M. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat. Immunol. 2019;20:1631–1643. doi: 10.1038/s41590-019-0526-7. [DOI] [PubMed] [Google Scholar]
- Haugen I.K., Lillegraven S., Slatkowsky-Christensen B., Haavardsholm E.A., Sesseng S., Kvien T.K., van der Heijde D., Boyesen P. Hand osteoarthritis and MRI: development and first validation step of the proposed Oslo hand osteoarthritis MRI score. Ann. Rheum. Dis. 2011;70:1033–1038. doi: 10.1136/ard.2010.144527. [DOI] [PubMed] [Google Scholar]
- Haugen I.K., Slatkowsky-Christensen B., Boyesen P., Sesseng S., van der Heijde D., Kvien T.K. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann. Rheum. Dis. 2016;75:117–123. doi: 10.1136/annrheumdis-2014-205949. [DOI] [PubMed] [Google Scholar]
- Hayes K.N., Giannakeas V., Wong A.K.O. Bisphosphonate use is protective of radiographic knee osteoarthritis progression among those with low disease severity and being non-overweight: data from the osteoarthritis initiative. J. Bone Miner. Res. 2020;35:2318–2326. doi: 10.1002/jbmr.4133. [DOI] [PubMed] [Google Scholar]
- He L., He T., Xing J., Zhou Q., Fan L., Liu C., Chen Y., Wu D., Tian Z., Liu B., Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276. doi: 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernigou P., Delambre J., Quiennec S., Poignard A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int. Orthop. 2021;45:365–373. doi: 10.1007/s00264-020-04571-4. [DOI] [PubMed] [Google Scholar]
- Horwood N.J. Immune cells and bone: coupling goes both ways. Immunol. Investig. 2013;42:532–543. doi: 10.3109/08820139.2013.822762. [DOI] [PubMed] [Google Scholar]
- Hoshi H., Akagi R., Yamaguchi S., Muramatsu Y., Akatsu Y., Yamamoto Y., Sasaki T., Takahashi K., Sasho T. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368:379–387. doi: 10.1007/s00441-016-2563-y. [DOI] [PubMed] [Google Scholar]
- Hu W., Chen Y., Dou C., Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2020;80:413–422. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Chen X., Wang S., Jing Y., Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhao L., Chen D. Growth factor signalling in osteoarthritis. Growth Factors. 2018;36:187–195. doi: 10.1080/08977194.2018.1548444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H.S., Kim H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015;16:26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intema F., Hazewinkel H.A., Gouwens D., Bijlsma J.W., Weinans H., Lafeber F.P., Mastbergen S.C. In early OA, thinning of the subchondral plate is directly related to cartilage damage: results from a canine ACLT-meniscectomy model. Osteoarthr. Cartil. 2010;18:691–698. doi: 10.1016/j.joca.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Jin K., Mao X.O., Cottrell B., Schilling B., Xie L., Row R.H., Sun Y., Peel A., Childs J., Gendeh G., Gibson B.W., Greenberg D.A. Proteomic and immunochemical characterization of a role for stathmin in adult neurogenesis. FASEB J. 2004;18:287–299. doi: 10.1096/fj.03-0973com. [DOI] [PubMed] [Google Scholar]
- Jung Y.K., Han M.S., Park H.R., Lee E.J., Jang J.A., Kim G.W., Lee S.Y., Moon D., Han S. Calcium-phosphate complex increased during subchondral bone remodeling affects earlystage osteoarthritis. Sci. Rep. 2018;8:487. doi: 10.1038/s41598-017-18946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Byrjalsen I., Alexandersen P., Bihlet A., Andersen J.R., Riis B.J., Bay-Jensen A.C., Christiansen C. Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials. Osteoarthr. Cartil. 2015;23:532–543. doi: 10.1016/j.joca.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Kazakia G.J., Kuo D., Schooler J., Siddiqui S., Shanbhag S., Bernstein G., Horvai A., Majumdar S., Ries M., Li X. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthr. Cartil. 2013;21:94–101. doi: 10.1016/j.joca.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement M.R., Sharkey P.F. The significance of osteoarthritis-associated bone marrow lesions in the knee. J. Am. Acad. Orthop. Surg. 2019;27:752–759. doi: 10.5435/JAAOS-D-18-00267. [DOI] [PubMed] [Google Scholar]
- Kon E., Ronga M., Filardo G., Farr J., Madry H., Milano G., Andriolo L., Shabshin N. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1797–1814. doi: 10.1007/s00167-016-4113-2. [DOI] [PubMed] [Google Scholar]
- Kothari A., Guermazi A., Chmiel J.S., Dunlop D., Song J., Almagor O., Marshall M., Cahue S., Prasad P., Sharma L. Within-subregion relationship between bone marrow lesions and subsequent cartilage loss in knee osteoarthritis. Arthritis Care Res. (Hoboken) 2010;62:198–203. doi: 10.1002/acr.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushesh S., Shahtaheri S.M., McWilliams D.F., Walsh D.A., Sheppard M.N., Westaby J., Haybatollahi S.M., Howe F.A., Sofat N. The osteoarthritis bone score (OABS): a new histological scoring system for the characterisation of bone marrow lesions in osteoarthritis. Osteoarthr. Cartil. 2022;30:746–755. doi: 10.1016/j.joca.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo O., Sabokbar A., Pocock A., Itonaga I., Fujikawa Y., Athanasou N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttapitiya A., Assi L., Laing K., Hing C., Mitchell P., Whitley G., Harrison A., Howe F.A., Ejindu V., Heron C., Sofat N. Microarray analysis of bone marrow lesions in osteoarthritis demonstrates upregulation of genes implicated in osteochondral turnover, neurogenesis and inflammation. Ann. Rheum. Dis. 2017;76:1764–1773. doi: 10.1136/annrheumdis-2017-211396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse D., Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr. Opin. Rheumatol. 2003;15:628–633. doi: 10.1097/00002281-200309000-00018. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R.M. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J. Bone Miner. Res. 1997;12:641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C., Zheng M.H. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Ma Y., Cheng T.S., Landao-Bassonga E., Qin A., Pavlos N.J., Zhang C., Zheng Q., Zheng M.H. Identical subchondral bone microarchitecture pattern with increased bone resorption in rheumatoid arthritis as compared to osteoarthritis. Osteoarthr. Cartil. 2014;22:2083–2092. doi: 10.1016/j.joca.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Li J., Fu S., Gong Z., Zhu Z., Zeng D., Cao P., Lin T., Chen T., Wang X., Lartey R., Kwoh C.K., Guermazi A., Roemer F.W., Hunter D.J., Ma J., Ding C. MRI-based texture analysis of infrapatellar fat pad to predict knee osteoarthritis incidence. Radiology. 2022;304:611–621. doi: 10.1148/radiol.212009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li J., Zhu Z., Cao P., Han W., Ruan G., Fan T., Hunter D.J., Ding C. Signal intensity alteration and maximal area of pericruciate fat pad are associated with incident radiographic osteoarthritis: data from the osteoarthritis initiative. Eur. Radiol. 2022;32:489–496. doi: 10.1007/s00330-021-08193-1. [DOI] [PubMed] [Google Scholar]
- Lim Y.Z., Wang Y., Wluka A.E., Davies-Tuck M.L., Hanna F., Urquhart D.M., Cicuttini F.M. Association of obesity and systemic factors with bone marrow lesions at the knee: a systematic review. Semin. Arthritis Rheum. 2014;43:600–612. doi: 10.1016/j.semarthrit.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang R., Ko S.Y., Oyajobi B.O., Papasian C.J., Deng H.W., Zhang S., Zhao M. Microtubule assembly affects bone mass by regulating both osteoblast and osteoclast functions: stathmin deficiency produces an osteopenic phenotype in mice. J. Bone Miner. Res. 2011;26:2052–2067. doi: 10.1002/jbmr.419. [DOI] [PubMed] [Google Scholar]
- Lo G.H., Mcalindon T.E., Niu J., Zhang Y., Beals C., Dabrowski C., Le Graverand M.P., Hunter D.J., Group O.A.I.I. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr. Cartil. 2009;17:1562–1569. doi: 10.1016/j.joca.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfvall H., Newbould H., Karsdal M.A., Dziegiel M.H., Richter J., Henriksen K., Thudium C.S. Osteoclasts degrade bone and cartilage knee joint compartments through different resorption processes. Arthritis Res. Ther. 2018;20:67. doi: 10.1186/s13075-018-1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Dai C., Zhang Z., Du H., Li S., Ye P., Fu Q., Zhang L., Wu X., Dong Y., Song Y., Zhao D., Pang Y., Bao C. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10:143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R., Ganu V., Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J. Biol. Chem. 1993;268:21527–21532. [PubMed] [Google Scholar]
- Martínez-Calatrava M.J., Prieto-Potín I., Roman-Blas J.A., Tardio L., Largo R., Herrero-Beaumont G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res. Ther. 2012;14 doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maumus M., Pers Y.M., Ruiz M., Jorgensen C., Noël D. Mesenchymal stem cells and regenerative medicine: future perspectives in osteoarthritis. Med. Sci. (Paris) 2018;34:1092–1099. doi: 10.1051/medsci/2018294. [DOI] [PubMed] [Google Scholar]
- Munsch M.A., Safran M.R., Mai M.C., Vasileff W.K. Bone marrow lesions: etiology and pathogenesis at the hip. J. Hip Preserv. Surg. 2020;7:401–409. doi: 10.1093/jhps/hnaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Sato M., Kutsuna T., Kokubo M., Ebihara G., Ohta N., Mochida J. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis Res. Ther. 2010;12 doi: 10.1186/ar3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M.J., Herndler-Brandstetter D., Tariq M.A., Nicholson T.A., Philp A.M., Smith H.L., Davis E.T., Jones S.W., Lord J.M. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 2017;7:3451. doi: 10.1038/s41598-017-03759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.P., Cooper C., Peterfy C., Reginster J.Y., Brandi M.L., Bruyere O., Chapurlat R., Cicuttini F., Conaghan P.G., Doherty M., Genant H., Giacovelli G., Hochberg M.C., Hunter D.J., Kanis J.A., Kloppenburg M., Laredo J.D., McAlindon T., Nevitt M., Raynauld J.P., Rizzoli R., Zilkens C., Roemer F.W., Martel-Pelletier J., Guermazi A. What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann. Rheum. Dis. 2013;72:1594–1604. doi: 10.1136/annrheumdis-2013-203631. [DOI] [PubMed] [Google Scholar]
- Peng Z., Sun H., Bunpetch V., Koh Y., Wen Y., Wu D., Ouyang H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120555. [DOI] [PubMed] [Google Scholar]
- Perry T.A., Parkes M.J., Hodgson R.J., Felson D.T., Arden N.K., O'Neill T.W. Association between bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2020;28:316–323. doi: 10.1016/j.joca.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme R., Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am. J. Neuroradiol. 2008;29:838–842. doi: 10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer F.W., Frobell R., Hunter D.J., Crema M.D., Fischer W., Bohndorf K., Guermazi A. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthr. Cartil. 2009;17:1115–1131. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Roemer F.W., Neogi T., Nevitt M.C., Felson D.T., Zhu Y., Zhang Y., Lynch J.A., Javaid M.K., Crema M.D., Torner J., Lewis C.E., Guermazi A. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthr. Cartil. 2010;18:47–53. doi: 10.1016/j.joca.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M.J., Crockett J.C., Coxon F.P., Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Shabestari M., Vik J., Reseland J.E., Eriksen E.F. Bone marrow lesions in hip osteoarthritis are characterized by increased bone turnover and enhanced angiogenesis. Osteoarthr. Cartil. 2016;24:1745–1752. doi: 10.1016/j.joca.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Sims N.A., Martin T.J., Quinn J.M.W. Coupling: the influences of immune and bone cells. Osteoimmunology. 2016:169–185. [Google Scholar]
- Song Y., Liu J., Zhao K., Gao L., Zhao J. Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33:1911–1925. doi: 10.1016/j.cmet.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Aoyama J., Miyauchi M., Takata T., Hanaoka K., Iwabe T., Tanne K. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem. Cell Biol. 2005;123:275–281. doi: 10.1007/s00418-005-0773-6. [DOI] [PubMed] [Google Scholar]
- Tanamas S.K., Wluka A.E., Pelletier J.P., Pelletier J.M., Abram F., Berry P.A., Wang Y., Jones G., Cicuttini F.M. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford) 2010;49:2413–2419. doi: 10.1093/rheumatology/keq286. [DOI] [PubMed] [Google Scholar]
- Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., Feng X., van Hul W., Wan M., Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Yoshida K., Ito T., Tsuda M., Mishima Y., Furumatsu T., Ronfani L., Abeyama K., Kawahara K., Komiya S., Maruyama I., Lotz M., Bianchi M.E., Asahara H. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol. Cell. Biol. 2007;27:5650–5663. doi: 10.1128/MCB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibrewala R., Ozhinsky E., Shah R., Flament I., Crossley K., Srinivasan R., Souza R., Link T.M., Pedoia V., Majumdar S. Computer-aided detection AI reduces interreader variability in grading hip abnormalities with MRI. J. Magn. Reson. Imaging. 2020;52:1163–1172. doi: 10.1002/jmri.27164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonna S., Poulton I.J., Taykar F., Ho P.W., Tonkin B., Crimeen-Irwin B., Tatarczuch L., McGregor N.E., Mackie E.J., Martin T.J., Sims N.A. Chondrocytic ephrin B2 promotes cartilage destruction by osteoclasts in endochondral ossification. Development. 2016;143:648–657. doi: 10.1242/dev.125625. [DOI] [PubMed] [Google Scholar]
- Trivanovic D., Harder J., Leucht M., Kreuzahler T., Schlierf B., Holzapfel B.M., Rudert M., Jakob F., Herrmann M. Immune and stem cell compartments of acetabular and femoral bone marrow in hip osteoarthritis patients. Osteoarthr. Cartil. 2022;30:1116–1129. doi: 10.1016/j.joca.2022.05.001. [DOI] [PubMed] [Google Scholar]
- Valderrabano V., Horisberger M., Russell I., Dougall H., Hintermann B. Etiology of ankle osteoarthritis. Clin. Orthop. Relat. Res. 2009;467:1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaysbrot E.E., Osani M.C., Musetti M.C., McAlindon T.E., Bannuru R.R. Are bisphosphonates efficacious in knee osteoarthritis? A meta-analysis of randomized controlled trials. Osteoarthr. Cartil. 2018;26:154–164. doi: 10.1016/j.joca.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- Wang X., Jin X., Blizzard L., Antony B., Han W., Zhu Z., Cicuttini F., Wluka A.E., Winzenberg T., Jones G., Ding C. Associations between knee effusion-synovitis and joint structural changes in patients with knee osteoarthritis. J. Rheumatol. 2017;44:1644–1651. doi: 10.3899/jrheum.161596. [DOI] [PubMed] [Google Scholar]
- Wen Z.H., Tang C.C., Chang Y.C., Huang S.Y., Lin Y.Y., Hsieh S.P., Lee H.P., Lin S.C., Chen W.F., Jean Y.H. Calcitonin attenuates cartilage degeneration and nociception in an experimental rat model of osteoarthritis: role of TGF-β in chondrocytes. Sci. Rep. 2016;6:28862. doi: 10.1038/srep28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.J., Murphy W.A., Hardy D.C., Totty W.G. Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167:757–760. doi: 10.1148/radiology.167.3.3363136. [DOI] [PubMed] [Google Scholar]
- Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., Li C., Xie L., Crane J., Wan M., Zhen G., Bian Q., Yu B., Chang W., Qiu T., Pickarski M., Duong L.T., Windle J.J., Luo X., Liao E., Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Hayashi D., Roemer F.W., Felson D.T., Guermazi A. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin. Arthritis Rheum. 2012;42:105–118. doi: 10.1016/j.semarthrit.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Hu J.C., Liu Han, Wang Sicheng, Zhou Qirong, Zhang Hao, Guo Jiawei, Cao Liehu, Chen Xiao, Xu Ke, Su Jiacan. bioRxiv; 2022. Single-cell RNA-sequencing Analysis Reveals the Molecular Mechanism of Subchondral Bone Cell Heterogeneity in the Development of Osteoarthritis. [Google Scholar]
- Yuan X.L., Meng H.Y., Wang Y.C., Peng J., Guo Q.Y., Wang A.Y., Lu S.B. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014;22:1077–1089. doi: 10.1016/j.joca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Bruder E., Romero J., Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Nevitt M., Niu J., Lewis C., Torner J., Guermazi A., Roemer F., McCulloch C., Felson D.T. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–699. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.K., Li G.W., Zeng C., Lin C.X., Huang L.S., Huang G.X., Zhao C., Feng S.Y., Fang H. Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1) Bone Joint Res. 2018;7:587–594. doi: 10.1302/2046-3758.711.BJR-2018-0057.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Ma J., Han J., Zhang W., Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019;11:6275–6289. [PMC free article] [PubMed] [Google Scholar]
- Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., Askin F.B., Frassica F.J., Chang W., Yao J., Carrino J.A., Cosgarea A., Artemov D., Chen Q., Zhao Z., Zhou X., Riley L., Sponseller P., Wan M., Lu W.W., Cao X. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Han X., Wang L., Zhang W., Cui J., He Z., Xie K., Jiang X., Du J., Ai S., Sun Q., Wu H., Yu Z., Yan M. Associations of osteoclastogenesis and nerve growth in subchondral bone marrow lesions with clinical symptoms in knee osteoarthritis. J Orthop Translat. 2022;32:69–76. doi: 10.1016/j.jot.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Ding C., Jin X., Antony B., Han W., Laslett L.L., Cicuttini F., Jones G. Patellofemoral bone marrow lesions: natural history and associations with pain and structure. Arthritis Care Res. (Hoboken) 2016;68:1647–1654. doi: 10.1002/acr.22871. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Jin X., Wang B., Wluka A., Antony B., Laslett L.L., Winzenberg T., Cicuttini F., Jones G., Ding C. Cross-sectional and longitudinal associations between serum levels of high-sensitivity C-reactive protein, knee bone marrow lesions, and knee pain in patients with knee osteoarthritis. Arthritis Care Res. (Hoboken) 2016;68:1471–1477. doi: 10.1002/acr.22834. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Otahal P., Wang B., Jin X., Laslett L.L., Wluka A.E., Antony B., Han W., Wang X., Winzenberg T., Cicuttini F., Jones G., Ding C. Cross-sectional and longitudinal associations between serum inflammatory cytokines and knee bone marrow lesions in patients with knee osteoarthritis. Osteoarthr. Cartil. 2017;25:499–505. doi: 10.1016/j.joca.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Zhu S., Zhu J., Zhen G., Hu Y., An S., Li Y., Zheng Q., Chen Z., Yang Y., Wan M., Skolasky R.L., Cao Y., Wu T., Gao B., Yang M., Gao M., Kuliwaba J., Ni S., Wang L., Wu C., Findlay D., Eltzschig H.K., Ouyang H.W., Crane J., Zhou F.Q., Guan Y., Dong X., Cao X. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019;129:1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Chan Y.T., Yung P.S.H., Tuan R.S., Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.607764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.