Abstract
Background
Lower heart rates (HRs) prolong diastole, which increases filling pressures and wall stress. As a result, lower HRs may be associated with higher brain natriuretic peptide (BNP) levels and incident atrial fibrillation (AF). Beta-blockers may increase the risk for AF due to suppression of resting HRs.
Objective
Examine the relationships of HR, BNP, beta-blockers and new-onset AF in the REVEAL-AF and SPRINT cohort of subjects at risk for developing AF.
Methods
In REVEAL-AF, 383 subjects without a history of AF and a mean CHA2DS2VASC score of 4.4 ± 1.3 received an insertable cardiac monitor and were followed up to 30 months. In SPRINT, 7595 patients without prior history of AF and a mean CHA2DS2VASC score of 2.3 ± 1.2 were followed up to 60 months.
Results
The median daytime HR in the REVEAL-AF cohort was 75bpm [IQR 68–83]. Subjects with below-median HRs had 2.4-fold higher BNP levels compared to subjects with above-median HRs (median BNP [IQR]: 62 pg/dl [37−112] vs. 26 pg/dl [13–53], p < 0.001). HRs <75bpm were associated with a higher incidence of AF: 37% vs. 27%, p < 0.05. This was validated in the SPRINT cohort after adjusting for AF risk factors. Both a HR < 75bpm and beta-blocker use were associated with a higher rate of AF: 1.9 vs 0.7% (p < 0.001) and 2.5% vs. 0.6% (p < 0.001), respectively. The hazard ratio for patients on beta-blockers to develop AF was 3.72 [CI 2.32, 5.96], p < 0.001.
Conclusions
Lower HRs are associated with higher BNP levels and incident AF, mimicking the hemodynamic effects of diastolic dysfunction. Suppression of resting HR by beta-blockers could explain their association with incident AF.
Keywords: Arrhythmia, Diastolic dysfunction, HFpEF, Insertable cardiac monitor, Natriuretic peptide
Abbreviations
- ACEi
Angiotensin converting enzyme inhibitor
- ACS
Acute coronary syndrome
- AF
Atrial fibrillation
- AMI
Acute myocardial infarction
- ARB
Angiotensin receptor blocker
- BB
Beta-blocker
- BMI
Body mass index
- BNP
Brain natriuretic peptide
- BP
Blood pressure
- Ca2+
Calcium
- CAD
Coronary artery disease
- CI
Confidence Interval
- CVA
Cerebrovascular accident
- HFpEF
Heart failure with preserved ejection fraction
- HR
Heart rate
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- Na+
Sodium
- SD
Standard deviation
- TIA
Transient ischemic attack
1. Introduction
Atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF) frequently manifest together [1]. Multiple shared risk factors - including older age, diabetes, hypertension, and obesity - predispose to increased myocardial stiffness and higher filling pressures, which in turn causes congestive heart failure, atrial myopathy and AF. Not surprisingly, increased brain natriuretic peptide (BNP) levels - a marker of elevated filling pressures - predict the development of HFpEF and AF [2].
Lower resting heart rates (HRs) are a newly-appreciated factor contributing to both HFpEF exacerbation and AF incidence [[3], [4], [5]]. Low resting heart rates are associated with a high level of cardiovascular fitness [6], and pharmacological HR lowering with beta-blockers improves outcomes in patients with reduced systolic function. Hence, it may seem counterintuitive that lower HRs increase filling pressure to an extent that becomes pathological in the setting of an impaired diastolic function [7,8]. The detrimental effects of lower HRs on atrial and ventricular load result from the combination of higher filling pressures, increased wall stress, slowing of myocardial relaxation [3] and reflected peripheral pressure waves being superimposed onto systole that increase central blood pressure [8,9].
Several trials enrolling patients with preserved ejection fraction and hypertension point to an association of unfavorable clinical outcomes with drug-induced suppression of resting HRs [3,[10], [11], [12], [13], [14]]. The LIFE hypertension trial [11] compared antihypertensive treatment with atenolol and losartan: The atenolol group, which had a 6 bpm lower average HR than the losartan group, had a 13% higher rate of cardiovascular endpoints despite the same blood pressure reduction in both groups. In the SIGNIFY trial of 19,102 patients with coronary artery disease (CAD) and preserved ejection fraction, selective HR lowering by 10 bpm with ivabradine increased the relative risk for heart failure and AF by 20% and 40%, respectively [12]. Consistent with these findings, we recently reported that beta-blocker use in subjects with HFpEF is associated with elevated BNP levels and more heart failure hospitalizations [13,14].
Based on the above, we hypothesized that low-normal HRs would be independently associated with higher BNP levels and incident AF in preserved EF populations. First, we tested this in the REVEAL-AF study, which enrolled patients who did not have a history of AF, had a high CHA2DS2VASC score, and received insertable cardiac monitors [15]. In this cohort we sought to 1) evaluate whether below-median HRs are associated with higher BNP levels, 2) determine whether lower HRs are associated with incident AF and 3) determine if beta-blocker use impacts BNP levels and AF incidence. Secondly, we analyzed the SPRINT cohort of hypertensive subjects at risk for AF to confirm the association of lower HRs and beta-blocker use on incident AF [16].
2. Methods
2.1. REVEAL-AF study
The REVEAL-AF study was supported by Medtronic and conducted in compliance with applicable local laws and regulation of each participating country. The REVEAL-AF study and its design have been previously reported [15,17]. In brief, REVEAL-AF is a prospective, single-arm, open-label, multicenter clinical study of insertable cardiac monitors to establish the incidence of AF in patients without prior AF history but deemed to be at risk for developing AF. Patients were included with a CHADS2 score of ≥3 or alternatively a CHADS2 score of 2 with an additional AF risk factor (CAD, renal impairment, sleep apnea, or COPD). While enrollment was based on CHADS2 score, gathered data allowed for calculation of CHA2DS2VASC score. Patients with a creatinine clearance of ≤30 ml/min, and/or receiving hemodialysis, or taking antiarrhythmic medications were excluded. All patients underwent a minimum of 24-h Holter monitoring prior to insertion of an internal cardiac monitor and patients with any AF were excluded. Follow up of enrolled patients continued for at least 18 months and up to 30 months.
Analyzed Population: We used the REVEAL-AF database to investigate the relationship between HR and BNP levels in patients at risk for AF. HRs were averaged between 8AM and 8PM from day two to eight post-implantation. Six patients developed AF during the initial week of HR monitoring and were excluded from the HR analysis. Adjudicated AF (symptomatic or asymptomatic) lasting ≥6 min was defined as new-onset AF; this cut-off was derived from the ASSERT Trial [18].
2.2. SPRINT study
The SPRINT trial was sponsored by the NHLBI and approved by the institutional review board at each participating study site. The trial results and its design have been previously reported [16,19]. In brief, 9361 patients were randomly assigned to intensive vs. standard blood pressure control. Participants needed to be at least 50 years of age with hypertension and an increased risk of cardiovascular events – defined by one or more of the following: cardiovascular disease, chronic kidney disease with an estimated glomerular filtration rate ≥20 ml/min/m2, a Framingham risk score ≥15%, or age ≥75 years. Patients with diabetes or prior stroke were excluded.
Analyzed Population: Of the 9361 participants of the SPRINT trial, patients with missing ECG data or history of AF were excluded from the secondary analysis. 7595 participants were included in the analysis investigating the relationship between HR, beta-blocker use and incident AF. Baseline average HR was derived from three seated measurements taken at the initial clinical visit. 12-lead ECG at baseline, 2 years, 4 years and close-out visit were used to determine presence of AF. Based on longitudinal medication inventories beta-blocker use was categorized into ‘on beta-blocker’ at any point during the trial vs. ‘never on beta-blocker’ for the duration of the trial. An additional sensitivity analysis compared patients who were on beta-blockers throughout the entire trial to patients who never received beta-blockers.
2.3. Statistical analysis
Data are reported using descriptive statistics, mean ± standard deviation or median with interquartile range (IQR). Parametric testing for normally distributed data was performed using Student's t-test. Non-parametric testing was performed using the Mann-Whitney U Test. Standard log-transformed BNP levels were used in the regression analysis. The chi-squared test was used for the analysis of categorical data and the survival analysis. Multivariable Cox-proportional hazard ratios models were fit to estimate the contribution of HR and beta-blocker use on the incidence of AF during follow-up. In the REVEAL-AF cohort, the model was corrected for age, body mass index (BMI), sex, coronary artery disease, beta-blocker use and HR as a binary variable (above or below the median HR of the cohort of 75bpm). In the SPRINT cohort, the model was corrected for age, sex, HR as a continuous variable, BMI, history of acute cardiovascular events, chronic kidney disease, beta-blocker use and intensive blood pressure treatment. Proportionality of hazards was verified using Schoenfeld's residuals. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Study populations
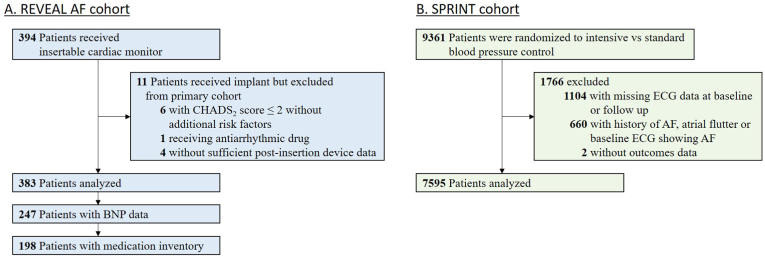
Among the 394 REVEAL-AF participants who underwent insertable cardiac monitor placement, 383 patients were analyzed (Fig. 1A). The mean age for the study population was 71.5 ± 9.8 years, 52% of participants were male, and the mean CHA2DS2VASC score was 4.4 ± 1.3. The median HR was 75bpm [IQR 68–83] and 127 patients (33%) developed AF. Baseline characteristics for the 376 patients who were in sinus rhythm during the first week stratified by median HR are summarized in Table 1. Age, BMI, CAD, and beta-blocker use were different between the groups. Baseline BNP data was available for 247 of 376 patients. The relationship of HR, BNP and incident AF was examined in those 247 patients while the remaining patients were omitted from this aspect of the analysis.
Fig. 1.
Flow diagram of the REVEAL-AF and SPRINT cohort. A) 11 out of 394 REVEAL-AF patients were excluded from the primary cohort. B) 1766 out of 9361 patients in the SPRINT trial were excluded from the secondary analysis.
Table 1.
REVEAL AF patients’ baseline characteristics.
| HR < 75bpm (n = 190) | HR ≥ 75bpm (n = 186) | p-value | |||
|---|---|---|---|---|---|
| Mean Age in years (SD) | 74.2 | (8.1) | 68.8 | (10.7) | <0.001 |
| Male sex | 96 | (51%) | 101 | (54%) | 0.46 |
| Mean Body mass index in kg/m2 (SD) | 30.4 | (5.9) | 32.0 | (6.8) | 0.013 |
| CHA2DS2-VASc score | |||||
| Mean (SD) | 4.6 | (1.3) | 4.2 | (1.3) | 0.006 |
| 2 | 5 | (3%) | 18 | (10%) | |
| 3 | 40 | (21%) | 36 | (19%) | |
| 4 | 50 | (26%) | 54 | (29%) | |
| 5 | 47 | (25%) | 51 | (27%) | |
| 6 | 32 | (17%) | 19 | (10%) | |
| 7 | 11 | (6%) | 7 | (4%) | |
| 8 | 5 | (3%) | 1 | (1%) | |
| Mean LVEF in % (SD) | 58.6 | (8.6) | 59.0 | (7.5) | 0.67 |
| Heart Failure | 46 | (24%) | 32 | (17%) | 0.09 |
| Hypertension | 178 | (94%) | 179 | (93%) | 0.79 |
| Coronary artery disease | 126 | (66%) | 100 | (54%) | 0.013 |
| Diabetes | 115 | (61%) | 126 | (68%) | 0.14 |
| Sleep Apnea | 50 | (26%) | 52 | (28%) | 0.72 |
| TIA/CVA | 68 | (36%) | 70 | (38%) | 0.71 |
| Renal Failure | 28 | (15%) | 34 | (18%) | 0.35 |
| Beta-blockers | 132 | (69%) | 83 | (45%) | <0.001 |
| Diltiazem/Verapamil | 18 | (9%) | 15 | (8%) | 0.63 |
Parametric testing used for comparison. Abbreviations: ACEi angiotensin converting enzyme inhibitor; ARB angiotensin receptor blocker; CVA cerebrovascular accident; LVEF left ventricular ejection fraction; SD standard deviation; TIA transient ischemic attack.
7595 of the 9361 SPRINT participants were included in the analyses of HR, beta-blocker use and incident AF (Fig. 1B). The mean age for this study population was 67.5 ± 9.2 years, 64% were male, and the mean CHA2DS2VASC score was 2.3 ± 1.2. Baseline patient characteristics stratified by HR are summarized in Table 2.
Table 2.
Baseline characteristics of the SPRINT cohort.
| HR < 75bpm (n = 5533) | HR ≥ 75bpm (n = 2062) | p-value | |||
|---|---|---|---|---|---|
| Mean Age in years (SD) | 68.4 | (9.1) | 65.1 | (9.0) | <0.001 |
| Male sex | 3623 | (65%) | 1260 | (61%) | <0.001 |
| Mean Body mass index in kg/m2 (SD) | 29.6 | (5.6) | 30.6 | (6.2) | <0.001 |
| History of ACS, AMI or coronary revascularization | 781 | (14%) | 162 | (8%) | <0.001 |
| Intensive Blood Pressure Treatment | 2786 | (50%) | 1000 | (48%) | 0.158 |
| Mean systolic blood pressure (SD) | 139.9 | (15.4) | 138.8 | (15.4) | 0.006 |
| Mean diastolic blood pressure (SD) | 76.9 | (11.6) | 82.0 | (11.4) | <0.001 |
| Chronic kidney disease | 1564 | (28%) | 476 | (23%) | <0.001 |
| Mean 10-year Framingham risk in % (SD) | 20.1 | (10.8) | 19.3 | (11.0) | <0.001 |
| Active Tobacco use | 589 | (11%) | 422 | (20%) | <0.001 |
| Statin use | 2454 | (45%) | 779 | (38%) | <0.001 |
Parametric testing used for comparison. Abbreviations: ACS acute coronary syndrome; AMI acute myocardial infarction; SD standard deviation.
3.2. REVEAL-AF
3.2.1. Heart rate and BNP
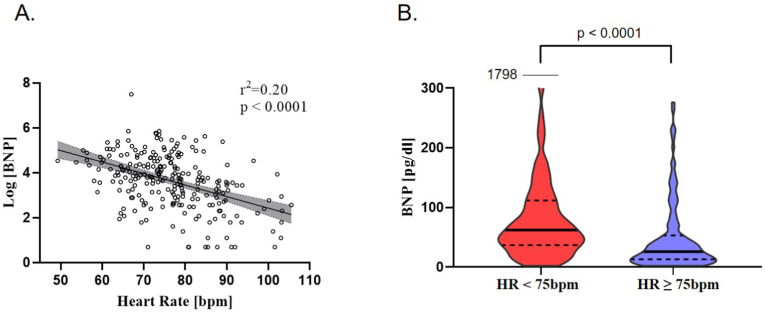
Below-median HRs (median 75bpm, IQR 68–83) were associated with higher BNP levels, as illustrated in Fig. 2A. Patients with a HR less than 75bpm had a 2.4-fold higher BNP level compared with patients with HRs of ≥75bpm: median BNP 62 pg/dl [interquartile range, IQR 37–112] vs. 26 pg/dl [IQR 13–53], p < 0.001, Fig. 2B.
Fig. 2.
Relationship of heart rate and BNP level in the REVEAL-AF cohort. A) Lower HRs are associated with higher BNP levels. B) Violin plot of BNP levels demonstrates that HRs below the median (75bpm) are associated with higher BNP levels compared to patients with above-median HRs. Solid line indicates median, dashed line indicates first and third quartile.
3.2.2. Heart rate and new-onset atrial fibrillation
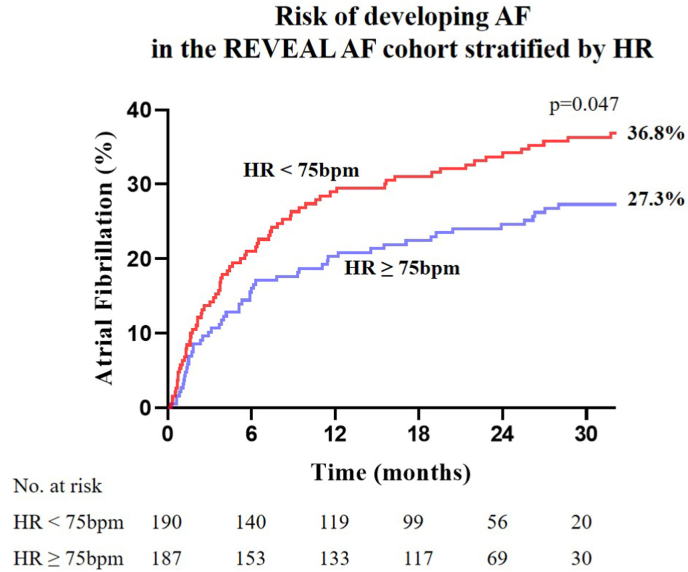
AF was detected in 121 (32.2%) of the 376 REVEAL-AF patients included in this analysis over the duration of follow up. The median resting HR during sinus rhythm for those who developed AF was 73bpm [IQR 66–79] compared with 77bpm [IQR 68–83] among those who did not develop AF (p = 0.01). Median follow-up was 24 months, with 294 patients (78%) completing 18 months of follow-up. Stratified by median HR, patients with HRs <75bpm had a significantly higher AF incidence over the course of the study (36.8% vs. 27.3%, hazard ratio 1.447 [95% Confidence Interval (CI) 1.013, 2.067], p = 0.047) as shown in Fig. 3. The hazard ratio by multivariable Cox-proportional analysis for patients to develop AF when HR is < 75bpm, and correcting for age, sex, BMI, history of CAD, and beta-blocker use was 1.208 [CI 0.825, 1.767], p = 0.331 (Supplemental Table 1). Although the association of HR < 75bpm with new-onset AF was not statistically significant in the multivariable analysis, we retested the HR-AF association in the SPRINT trial – a larger cohort that provided greater statistical power.
Fig. 3.
Risk of developing AF in the REVEAL-AF cohort stratified by heart rate. Kaplan-Meier plot of cumulative AF incidence over 30 months of follow up stratified by the median HR. Patients with HR < 75bpm had a significantly higher risk for developing AF over the course of the REVEAL-AF study.
3.2.3. New-onset atrial fibrillation and BNP
In patients with BNP data, 79 patients who developed AF had BNP levels almost twice as high compared with the 168 patients who did not develop AF: median BNP 65 pg/dl [IQR 26–141] vs. 38 pg/dl [IQR 16–72], p = 0.0008.
3.2.4. Beta-blocker and BNP
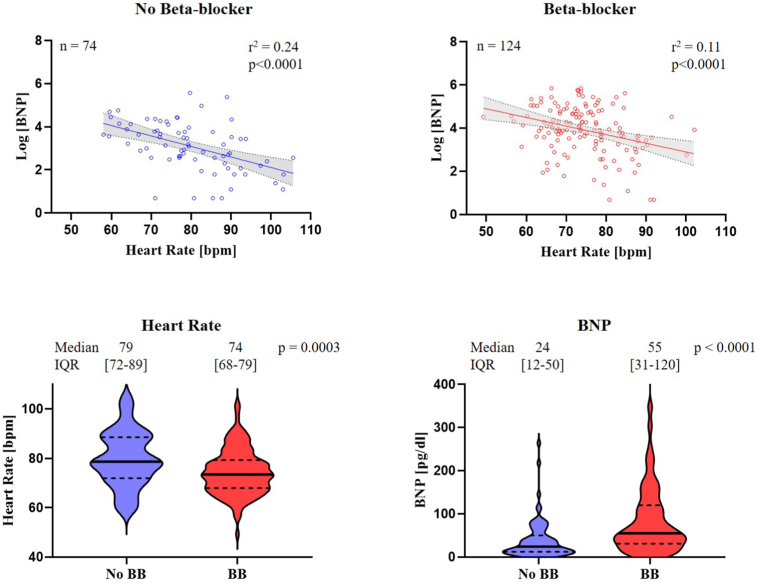
Medication inventories and BNP data were available for 198 of the 383 participants. 124 subjects received beta-blockers and their median HR was 5 bpm lower compared to 74 patients without beta-blocker use, suggesting that moderate doses of beta-blockers were prescribed. Patients on beta-blockers had markedly increased BNP levels (median 55 pg/dl [IQR 31–120]) compared with patients who did not receive beta-blockers (median 24 pg/dl [IQR 12–50], p < 0.001), Fig. 4.
Fig. 4.
Relationship of beta-blocker use on heart rate and BNP level in the REVEAL-AF cohort. Beta-blocker use is associated with higher BNP levels. Top left: Association between BNP level and HR for patients without beta-blockers. Top right: Association between BNP level and HR for patients with beta-blockers. Bottom left: Violin plot shows the effect of beta-blocker use on the distribution of HRs as compared to no beta-blocker use. Solid line indicates median, dashed line indicates first and third quartile. Bottom right: Violin plot of BNP levels demonstrates that pharmacological HR lowering with beta-blocker is associated with higher BNP levels compared to patients without beta-blockers.
3.2.5. Beta-blocker and new-onset atrial fibrillation
The likelihood of developing AF on beta-blockers over the course of the study was 34.1% vs 27.4% in participants without beta-blockers, although this did not reach statistical significance in this smaller subset (hazard ratio 1.342 [CI 0.842, 2.137], p = 0.221).
3.3. SPRINT
3.3.1. Heart rate and new-onset atrial fibrillation
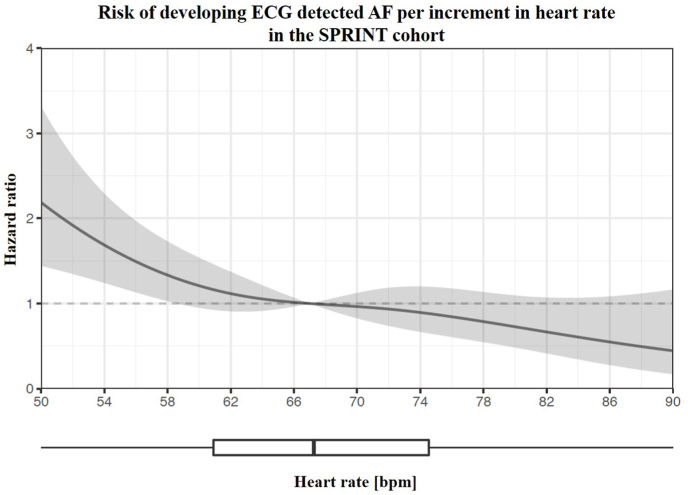
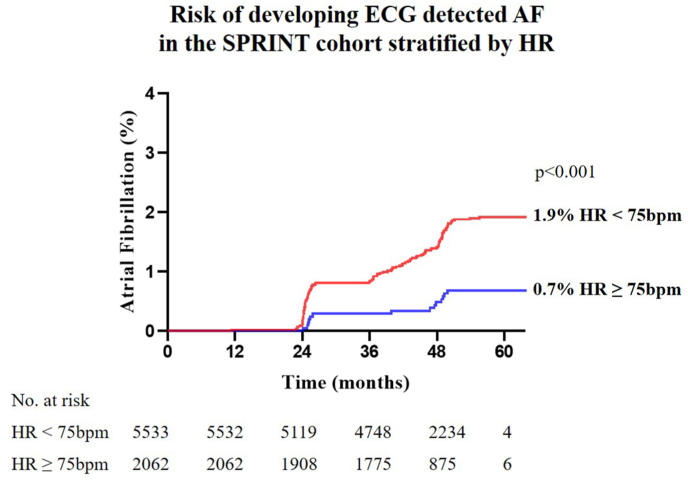
AF was detected in 120 participants (1.6%) of the SPRINT cohort during follow up. The median resting HR during sinus rhythm for those who developed AF was 61 bpm [IQR 55–69] compared with 67 bpm [IQR 60–76] among those who did not develop AF (p < 0.001). Restricted cubic splines further detail the non-linear relationship of HR and incident AF (Fig. 5). Analogous to the REVEAL-AF cohort, patients with resting HRs <75 bpm (5533 patients, 73%) were significantly more likely to develop AF as compared to patients with HR ≥ 75 bpm (2062 patients, 27%): hazard ratio by multivariable Cox-proportional analysis 2.233, CI [1.270, 3.926], p = 0.005. This relationship was also present when using the SPRINT median HR of 67 bpm (Table 3), hazard ratio 1.907 [1.287, 2.825], p = 0.001. Cumulative AF incidence over the course of the SPRINT trial is illustrated in Fig. 6: HR < 75 bpm compared to HR ≥ 75 bpm was associated with a higher incidence of AF 1.92% vs. 0.68% (hazard ratio 2.841, CI [1.901, 4.245], p < 0.001).
Fig. 5.
Restricted cubic splines showing the continuous non-linear association between heart rate and adjusted hazard of incident AF in the SPRINT cohort. Binary logistic regression models with restricted cubic splines (4 knots) were adjusted in a nested fashion for age, sex, BMI, history of CAD, intensive blood pressure treatment and chronic kidney disease. The median HR of the study (67 bpm) was chosen as reference. Shaded areas represent the upper and lower 95% confidence interval. Dashed line corresponds to a hazard ratio of 1. Box plot shows the HR distribution.
Table 3.
Multivariable cox-proportional hazard ratio of new-onset atrial fibrillation among all SPRINT trial patients with HR as binary variable. Left: above and below the median HR of the SPRINT cohort of 67bpm. Right:above and below the median HR of the REVEAL-AF cohort of 75bpm for comparison.
| Hazard ratio for HR < 67 bpm | p-value | Hazard ratio for HR < 75 bpm | p-value | |
|---|---|---|---|---|
| Heart rate as binary variable | 1.907 [1.287, 2.825] | 0.001 | 2.233 [1.270, 3.926] | 0.005 |
| Age (continuous variable) | 1.084 [1.060, 1.108] | <0.001 | 1.083 [1.059, 1.108] | <0.001 |
| Female sex | 0.772 [0.519, 1.149] | 0.202 | 0.757 [0.509, 1.125] | 0.168 |
| Body mass index (continuous variable) | 1.061 [1.030, 1.093] | <0.001 | 1.061 [1.030, 1.093] | <0.001 |
| History of ACS, AMI or coronary revascularization | 1.684 [1.085, 2.614] | 0.020 | 1.721 [1.110, 2.668] | 0.015 |
| Chronic kidney disease | 1.108 [0.753, 1.630] | 0.603 | 1.129 [0.768, 1.662] | 0.536 |
| Intensive BP treatment | 0.775 [0.539, 1.114] | 0.169 | 0.776 [0.540, 1.116] | 0.171 |
Multivariable model corrected for all variables listed in the table. Abbreviations: ACS acute coronary syndrome; AMI acute myocardial infarction; BP blood pressure.
Fig. 6.
Risk of developing ECG detected AF in the SPRINT cohort stratified by heart rate. Kaplan-Meier plot of cumulative AF incidence stratified by HR. Patients with HR < 75bpm had a significantly higher risk for developing AF over the course of the SPRINT trial.
3.3.2. Beta-blocker use and new-onset atrial fibrillation
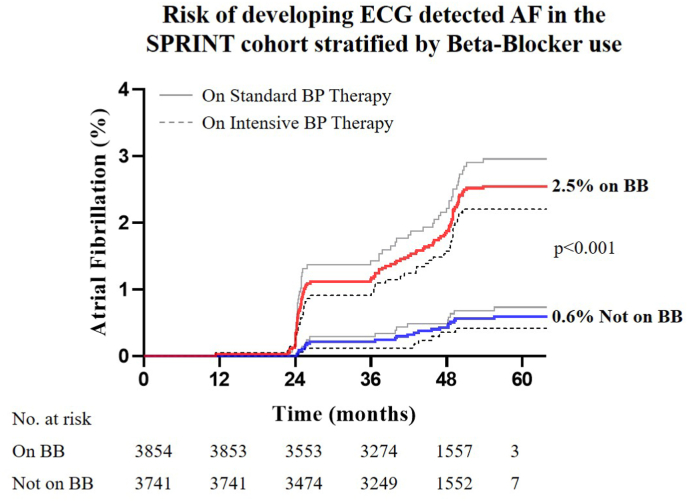
3854 patients (51%) received beta-blockers during SPRINT, while 3741 did not (Supplemental Table 2 lists types and doses of beta-blockers). Beta-blocker use was associated with a higher rate of AF: 2.5% vs 0.6%, hazard ratio 4.369 [CI 3.055, 6.249], p < 0.001 (Fig. 7). The hazard ratio by multivariable Cox-proportional analysis for patients on beta-blocker therapy to develop AF when correcting for age, HR, sex, BMI, history of CAD, intensive vs standard blood pressure management and chronic kidney disease was 3.716 [CI 2.317, 5.958], p < 0.001. In a multivariable sensitivity analysis, this association was confirmed in the subset of patients that never changed beta-blocker status throughout the trial (Table 4, Supplemental Figure 1). A second sensitivity analysis accounting for death as a competing risk showed that beta-blockers still significantly increased the risk of AF when adjusting for confounding variables (Supplemental Table 3). There was no significant interaction between continuous heart rate and the use of beta-blockers, suggesting that patients on beta-blockers are at higher risk for AF independent of their baseline HR.
Fig. 7.
Risk of developing ECG detected AF in the SPRINT cohort stratified by beta-blocker use. Kaplan-Meier plot of cumulative AF incidence stratified by beta-blocker use. Patients on beta-blocker therapy at any point of the trial were significantly more likely to develop AF (2.5%) compared with patients not on beta-blockers (0.6%), hazard ratio 4.369 [CI 3.055, 6.249], p < 0.001. Intensive (dotted grey line) or standard (solid grey line) did not significantly alter the outcomes of beta-blocker therapy on incident AF.
Table 4.
Multivariable cox-proportional hazard ratio of new onset atrial fibrillation for the subset of SPRINT trial patients who did not change beta-blocker status during the entire duration of the SPRINT trial. Beta-blocker use is a binary variable: on beta-blocker therapy during the entire duration of the SPRINT trial versus never on beta-blocker therapy.
| Hazard ratio for new onset AF | p-value | |
|---|---|---|
| On beta-blocker | 3.607 [2.244, 5.800] | <0.001 |
| Heart rate (continuous variable) | 0.958 [0.951, 0.985] | <0.001 |
| Age | 1.080 [1.055, 1.105] | <0.001 |
| Female sex | 0.742 [0.499, 1.105] | 0.142 |
| Body mass index | 1.056 [1.024, 1.089] | 0.001 |
| History of AMI, ACS or coronary revascularization | 1.235 [0.792, 1.928] | 0.352 |
| Chronic kidney disease | 1.001 [0.680, 1.474] | 0.995 |
| Intensive BP treatment | 0.705 [0.489, 1.014] | 0.060 |
Multivariable model corrected for all variables listed in the table. Abbreviations: ACS acute coronary syndrome; AMI acute myocardial infarction; BP blood pressure.
4. Discussion
Over the last two decades, basic and clinical research has advanced our understanding of AF initiation and maintenance, as well as the risk factors associated with AF development [20]. Different stimuli, such as oxidative stress with aging, inflammation and hemodynamic stress can result in atrial myopathy, which is the substrate for the development of AF [21] and systemic thromboembolism [22]. Atrial stretch in the setting of increased atrial afterload (heart failure, hypertension, left ventricular hypertrophy) [23], as well as increased atrial preload with obstructive sleep apnea, are known to promote atrial remodeling [24,25]. Clinical outcomes in studies enrolling patients with preserved ejection fraction point to potential adverse effects from pharmacological HR lowering. We hypothesized that in patients with preserved ejection fraction, HR would be inversely related to AF incidence and that beta-blocker use may exacerbate that risk. We examined the relationship between HR, natriuretic peptide levels, and incident AF in the REVEAL-AF cohort of 383 patients and the SPRINT cohort of 7595 patients. In the REVEAL-AF analysis we found that 1) below-median HRs and beta-blocker use were associated with increased BNP levels 2) lower HRs were associated with an increased risk for AF and 3) beta-blocker use was a significant confounding variable in the low HR group, which had a higher incidence of new-onset AF. In the secondary analysis of the SPRINT hypertension trial we also found that low HRs were associated with incident AF. Additionally, there was a strong association between beta-blocker use and incident AF even after correcting for HR, suggesting an additional HR-independent mechanism. This is concordant with an analysis of the LIFE hypertension trial showing that atenolol was associated with more than 30% increase in incident AF as compared to losartan [26].
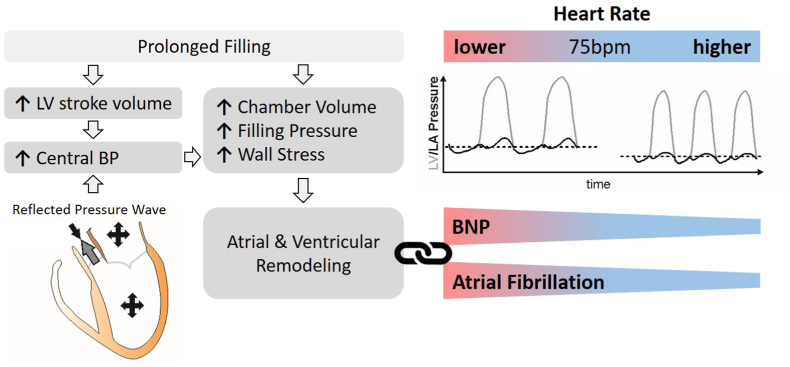
The finding herein that HRs on the lower end of “normal” contribute to incident AF is novel and likely explained by pressure-mediated pathogenic atrial remodeling (Fig. 8). As HR slows, prolonged left ventricular filling time increases myocardial load and stroke volume via the Frank-Starling mechanism, leading to higher filling pressures and wall stress in all chambers [7,8]. The mechanical properties of atrial and ventricular myocardium are comparable [[27], [28], [29], [30]] and an increase in wall stress can trigger remodeling in both chambers. A recent imaging analysis of hypertensive patients found a significant association between beta-blocker use and reduced atrial function and the development of atrial myopathy [31]. It is therefore plausible that low HRs and beta-blocker use exacerbate both diastolic and systolic atrial dysfunction, predisposing patients to the development of AF [32,33]. In summary, our post-hoc analyses of REVEAL-AF and SPRINT support the hypothesis of a mechanistic relationship between low HR and AF. Viewed through this lens, “vagally-mediated” AF observed in endurance athletes - typically ascribed to heterogeneity in atrial myocyte refractoriness - may also be consequent to or exacerbated by the hemodynamic effects of a low resting HR.
Fig. 8.
Mechanisms and impact of heart rate on natriuretic peptide (BNP) levels and incident atrial fibrillation. Left: Prolonged chamber filling at higher filling pressures increase chamber volumes and wall stress. Right: Below the median HRs are associated with higher BNP levels and incident AF.
4.1. Heart rate-dependent cellular mechanisms that promote atrial remodeling
Cellular calcium handling is a key component of myocardial contractile and electrical performance. Low HRs and adrenergic blockade slow intracellular calcium handling resulting in negative lusitropy and dromotropy that can contribute to atrial and ventricular diastolic dysfunction [34]. In isolated myocardium from patients with HFpEF we have previously reported increased diastolic calcium levels relative to normal myocardium, leaving the myofilaments activated in diastole [33]. An imbalance in cytosolic calcium also alters cellular electrophysiology: Removal of excess calcium from the cytosol via the Na+/Ca2+ exchanger (three Na + for one Ca2+) leads to a net depolarizing current, which can cause delayed afterdepolarizations that are proarrhythmic. Downregulation of L-type calcium channels in response to cytosolic Ca2+-overload shortens action potential duration and also promotes AF [35]. In addition, low HRs and beta-adrenergic blockade may decrease titin phosphorylation, resulting in increased myocardial stiffness and impaired diastolic recoil [36]. The aforementioned factors contribute to a decrease in atrial systolic and diastolic function further exacerbated by the concomitant increase in LV stiffness, which increases atrial afterload. Fibroblast proliferation in response to increased atrial pressure and resultant atrial fibrosis furthers the vicious cycle of atrial myopathy [37].
4.2. Natriuretic peptides as markers of atrial and ventricular remodeling
Natriuretic peptides (ANP and BNP) are secreted in response to atrial and ventricular distention [38,39] and correlate with markers of inflammation (IL-6) and fibrosis (TGF-beta 1, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1) [40]. An inflammatory milieu and hemodynamic stress are important precursors to the development of AF [21,41]. Our analysis reveals that patients who developed AF had baseline BNP levels almost twice as high as patients who did not develop AF, supporting the notion that elevated BNP is a relevant biomarker for atrial myopathy and incident AF. While the median BNP level of 65 pg/dl in the AF group may not seem overtly abnormal from a clinical standpoint, it is markedly higher than in a cohort of healthy adults (median BNP in the 6th decade of life 17.7 pg/dl and median BNP in the 7th and 8th decade of life 25.8 pg/dl) [42]. A recent analysis of the LOOP study by Diederichsen et al. also showed that natriuretic peptide levels are associated with increased odds of AF detection and higher AF burden. Importantly, this analysis also confirmed that age, BMI and HR are important predictors of new-onset AF [43].
4.3. Heart rate modifications and outcomes in atrial fibrillation and HFpEF
The unequivocal benefits of beta-blockers in heart failure with reduced ejection fraction have not been reproduced in HFpEF clinical trials. On the contrary, the use of beta-blockers in randomized studies of patients with normal ejection fractions has been associated with worsening heart failure [[11], [12], [13], [14]]. Furthermore, selective HR lowering with ivabradine in a small randomized HFpEF trial did not improve any clinical outcomes [44] and in patients with CAD, who did not have heart failure at baseline, resulted in more heart failure and AF [12]. Both ivabradine trials indicate that heart rate suppression alone - in the absence of pleiotropic effects of adrenergic blockade – can explain the observed increase in heart failure and AF.
The lack of benefit from pharmacological HR lowering in HFpEF patients in normal sinus rhythm coupled with the findings of this study conversely raise the possibility that an increased average HR might be a therapeutic target in both paroxysmal AF and HFpEF [45]. We previously published two small cohort studies of patients with pacemakers and concomitant diastolic dysfunction and/or overt HFpEF [46,47]. These studies revealed improvements in heart failure symptoms and functional status accompanied by lower natriuretic peptide levels with higher rate physiologic pacing. The recently published myPACE study [48] provides further evidence for improved quality of life, lower natriuretic peptide levels and lower AF burden with a higher pacemaker HR in patients with HFpEF. The PACE-HFpEF study (NCT04546555) is an additional prospective clinical trial that evaluates the effects of higher HRs in HFpEF and AF.
4.4. Limitations
These are secondary analyses of 1) the REVEAL-AF trial, which prospectively evaluated subjects at risk for incident AF using insertable cardiac monitors and 2) participants of the SPRINT hypertension trial who did not have a prior history of AF. While both trials provided reliable HR assessments, the BNP data and medication inventories were incomplete (duration and intensity of exposure in REVEAL-AF). Although we recently confirmed the inverse relationship between HR and left atrial pressures [7], neither trial included hemodynamic or volumetric chamber assessments and BNP data from the SPRINT trial is not publically available to date. The SPRINT trial adjudicated AF based on ECG detection at 2 years, 4 years and the close out visit, likely underestimating the overall incidence of AF.
Despite correcting for the established AF risk factors, residual confounders cannot be excluded. This secondary analysis should therefore only be viewed as hypothesis generating and will need to be explored more definitively in randomized controlled trials.
5. Conclusion
Lower HRs are associated with higher BNP levels and incident AF, supporting the hypothesis that lower HRs mimic and/or exacerbate the hemodynamic effects of diastolic dysfunction and promote atrial myopathy.
Pharmacological HR lowering with beta-blockers in patients with baseline sinus rhythm was associated with increased BNP levels and incident AF. The effects of beta-blockers on clinical outcomes in patient populations outside the context of heart failure with reduced ejection fraction will need to be re-evaluated.
Credit author statement
All authors have substantially contributed to the work presented in this manuscript. Individual contributions are detailed below. Nicole Habel MD PhD: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing; Jeanne de Lavallaz MD: Data curation, Formal analysis, Writing – review & editing; Margaret Infeld MD MS: Conceptualization, Writing – review & editing; Jodi L. Koehler MS: Data curation, Formal analysis; Paul D. Ziegler MS: Data curation, Resources; Daniel L. Lustgarten MD PhD: Writing – review & editing; Markus Meyer MD PhD: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding/support
This research was supported by grant R01 HL-122744 from the National Institutes of Health (Dr. Meyer).
Conflict of interest disclosures
Dr. Meyer reports having licensed patents on the use of pacemakers to prevent and treat heart failure with preserved ejection fraction; the relationship is modest. Jodi Koehler and Paul Ziegler are employees and shareholders of Medtronic. The remaining authors have no disclosure.
Handling Editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2023.200182.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Reddy Y.N.V., Obokata M., Verbrugge F.H., Lin G., Borlaug B.A. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J. Am. Coll. Cardiol. 2020;76:1051–1064. doi: 10.1016/j.jacc.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T.J., Larson M.G., Levy D., et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 3.Meyer M., LeWinter M.M. Heart rate and heart failure with preserved ejection fraction: time to slow beta-blocker use? Circ. Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohm M., Schumacher H., Linz D., et al. Low resting heart rates are associated with new-onset atrial fibrillation in patients with vascular disease: results of the ONTARGET/TRANSCEND studies. J. Intern. Med. 2015;278:303–312. doi: 10.1111/joim.12373. [DOI] [PubMed] [Google Scholar]
- 5.Grundvold I., Skretteberg P.T., Liestol K., et al. Low heart rates predict incident atrial fibrillation in healthy middle-aged men. Circ. Arrhythm. Electrophysiol. 2013;6:726–731. doi: 10.1161/CIRCEP.113.000267. [DOI] [PubMed] [Google Scholar]
- 6.Saxena A., Minton D., Lee D.C., et al. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin. Proc. 2013;88:1420–1426. doi: 10.1016/j.mayocp.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman D.N., Rambod M., Lustgarten D.L., Lobel R., LeWinter M.M., Meyer M. Heart rate-induced myocardial Ca(2+) retention and left ventricular volume loss in patients with heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B., Lacy P.S., Thom S.M., et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 9.Rimoldi S.F., Messerli F.H., Cerny D., et al. Selective heart rate reduction with ivabradine increases central blood pressure in stable coronary artery disease. Hypertension. 2016;67:1205–1210. doi: 10.1161/HYPERTENSIONAHA.116.07250. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder I.C., Groenveld H.F., Crijns H.J., et al. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 11.Dahlof B., Devereux R.B., Kjeldsen S.E., et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 12.Fox K., Ford I., Steg P.G., et al. Ivabradine in stable coronary artery disease without clinical heart failure. N. Engl. J. Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 13.Nambiar L., Silverman D., Vanburen P., LeWinter M., Meyer M. Beta-blocker cessation in stable outpatients with heart failure with a preserved ejection fraction. J. Card. Fail. 2020;26:281–282. doi: 10.1016/j.cardfail.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Silverman D.N., Plante T.B., Infeld M., et al. Association of beta-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiffel J.A., Verma A., Kowey P.R., et al. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol. 2017;2:1120–1127. doi: 10.1001/jamacardio.2017.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group S.R., Wright J.T., Jr., Williamson J.D., et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiffel J., Verma A., Halperin J.L., et al. Rationale and design of REVEAL AF: a prospective study of previously undiagnosed atrial fibrillation as documented by an insertable cardiac monitor in high-risk patients. Am. Heart J. 2014;167:22–27. doi: 10.1016/j.ahj.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Healey J.S., Connolly S.J., Gold M.R., et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosius W.T., Sink K.M., Foy C.G., et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin. Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellman J., Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr. Physiol. 2015;5:649–665. doi: 10.1002/cphy.c140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J. Cardiovasc. Electrophysiol. 2012;23:797–799. doi: 10.1111/j.1540-8167.2012.02341.x. [DOI] [PubMed] [Google Scholar]
- 22.Brambatti M., Connolly S.J., Gold M.R., et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 23.Medi C., Kalman J.M., Spence S.J., et al. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011;22:1317–1324. doi: 10.1111/j.1540-8167.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- 24.Otto M.E., Belohlavek M., Romero-Corral A., et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am. J. Cardiol. 2007;99:1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y.K., Shi Y., Benito B., et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;9:1409–14016 e1. doi: 10.1016/j.hrthm.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Wachtell K., Lehto M., Gerdts E., et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) study. J. Am. Coll. Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Pagel P.S., Kehl F., Gare M., Hettrick D.A., Kersten J.R., Warltier D.C. Mechanical function of the left atrium: new insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiology. 2003;98:975–994. doi: 10.1097/00000542-200304000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Hondo T., Okamoto M., Kawagoe T., et al. Effects of heart rate on left atrial contractile performance and left ventricular filling during atrial systole in dogs. Jpn. Heart J. 1995;36:367–375. doi: 10.1536/ihj.36.367. [DOI] [PubMed] [Google Scholar]
- 29.Stone H.L. Effect of heart rate on left atrial systolic shortening in the dog. J. Appl. Physiol. 1975;38:1110–1116. doi: 10.1152/jappl.1975.38.6.1110. [DOI] [PubMed] [Google Scholar]
- 30.Williams J.F., Jr., Sonnenblick E.H., Braunwald E. Determinants of atrial contractile force in the intact heart. Am. J. Physiol. 1965;209:1061–1068. doi: 10.1152/ajplegacy.1965.209.6.1061. [DOI] [PubMed] [Google Scholar]
- 31.Sardana M., Syed A.A., Hashmath Z., et al. Beta-blocker use is associated with impaired left atrial function in hypertension. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadakia H.H., Channah S., Heywood J.T., et al. Left atrial diastolic dysfunction, an underappreciated cause of pulmonary venous hypertension and decompensated heart failure. J. Card. Fail. 2011;17:S10. [Google Scholar]
- 33.Selby D.E., Palmer B.M., LeWinter M.M., Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J. Am. Coll. Cardiol. 2011;58:147–154. doi: 10.1016/j.jacc.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runte K.E., Bell S.P., Selby D.E., et al. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ. Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nattel S., Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 36.Hamdani N., Herwig M., Linke W.A. Tampering with springs: phosphorylation of titin affecting the mechanical function of cardiomyocytes. Biophys. Rev. 2017;9:225–237. doi: 10.1007/s12551-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rucker-Martin C., Pecker F., Godreau D., Hatem S.N. Dedifferentiation of atrial myocytes during atrial fibrillation: role of fibroblast proliferation in vitro. Cardiovasc. Res. 2002;55:38–52. doi: 10.1016/s0008-6363(02)00338-3. [DOI] [PubMed] [Google Scholar]
- 38.Goetze J.P., Friis-Hansen L., Rehfeld J.F., Nilsson B., Svendsen J.H. Atrial secretion of B-type natriuretic peptide. Eur. Heart J. 2006;27:1648–1650. doi: 10.1093/eurheartj/ehl109. [DOI] [PubMed] [Google Scholar]
- 39.Goetze J.P., Bruneau B.G., Ramos H.R., Ogawa T., de Bold M.K., de Bold A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020;17:698–717. doi: 10.1038/s41569-020-0381-0. [DOI] [PubMed] [Google Scholar]
- 40.Stanciu A.E., Vatasescu R.G., Stanciu M.M., Serdarevic N., Dorobantu M. The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine. 2018;103:63–68. doi: 10.1016/j.cyto.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Harada M., Van Wagoner D.R., Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida Y., Nakanishi K., Daimon M., et al. Alteration of cardiac performance and serum B-type natriuretic peptide level in healthy aging. J. Am. Coll. Cardiol. 2019;74:1789–1800. doi: 10.1016/j.jacc.2019.07.080. [DOI] [PubMed] [Google Scholar]
- 43.Diederichsen S.Z., Haugan K.J., Brandes A., et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J. Am. Coll. Cardiol. 2019;74:2771–2781. doi: 10.1016/j.jacc.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 44.Komajda M., Isnard R., Cohen-Solal A., et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur. J. Heart Fail. 2017;19:1495–1503. doi: 10.1002/ejhf.876. [DOI] [PubMed] [Google Scholar]
- 45.Meyer M., LeWinter M.M., Zile M.R. A targeted treatment opportunity for HFpEF: taking advantage of diastolic tone. Circulation. 2021;144:1269–1271. doi: 10.1161/CIRCULATIONAHA.121.056412. [DOI] [PubMed] [Google Scholar]
- 46.Wahlberg K., Arnold M.E., Lustgarten D., Meyer M. Effects of a higher heart rate on quality of life and functional capacity in patients with left ventricular diastolic dysfunction. Am. J. Cardiol. 2019;124:1069–1075. doi: 10.1016/j.amjcard.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeshwant S.C., Zile M.R., Lewis M.R., Lewinter M., Meyer M. Safety and feasibility of a nocturnal heart rate elevation-exploration of a novel treatment concept. J. Card. Fail. 2019;25:67–71. doi: 10.1016/j.cardfail.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Infeld M., Wahlberg K., Cicero J. Effect of personalized accelerated pacing on quality of life, physical activity, and atrial fibrillation in patients with preclinical and overt heart failure with preserved ejection fraction: the myPACE randomized clinical trial. JAMA Cardiol. 2023 doi: 10.1001/jamacardio.2022.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.