Abstract
Epithelial ovarian cancer (EOC) is the type of OC with the highest mortality rate. Due to the asymptomatic nature of the disease and few available diagnostic tests, it is mostly diagnosed at the advanced stage. Therefore, the present study aimed to discover predictive and/or early diagnostic novel circulating microRNAs (miRNAs or miRs) for EOC. Firstly, microarray analysis of miRNA expression levels was performed on 32 samples of female individuals: Eight plasma samples from patients with pathologically confirmed EOC (mean age, 45 (30–54) years), eight plasma samples from matched healthy individuals (HIs) (mean age, 44 (30–65) years), eight EOC tissue samples (mean age, 45 (30–54) years) and eight benign ovarian (mean age, 35 (17–70) years) neoplastic tissue samples A total of 31 significantly dysregulated miRNAs in serum and three miRNAs in tissue were identified by microarray. The results were validated using reverse transcription-quantitative PCR on samples from 10 patients with pathologically confirmed EOC (mean age, 47(30–54) years), 10 matched His (mean age, 40(26–65) years], 10 EOC tissue samples (mean age, 47(30–54) years) and 10 benign ovarian neoplastic tissue samples (mean age, 40(17–70) years). The ‘Kyoto Encyclopedia of Genes and Genomes’ (KEGG) database was used for target gene and pathway analysis. A total of three miRNAs from EOC serum (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p) and one microRNA from tissue samples (hsa-miR-200c-3p) were validated as significant to distinguish patients with EOC from HIs. KEGG pathway enrichment analysis showed seven significant pathways, which included ‘prion diseases’, ‘proteoglycans in cancer’, ‘oxytocin signaling pathway’, ‘hippo signaling pathway’, ‘adrenergic signaling in cardiomyocytes’, ‘oocyte meiosis’ and ‘thyroid hormone signaling pathway’, in which the validated miRNAs served a role. This supports the hypothesis that four validated miRNAs, have the potential to be a biomarker of EOC diagnosis and target for treatment.
Keywords: biomarkers, DIANA-miRPath, KEGG, liquid biopsy, miRNA, EOC
Introduction
Epithelial ovarian cancer (EOC) is the most common and lethal type of ovarian malignancy. Early detection of EOC is key in the clinic. A total of ~70% of EOC cases are diagnosed at the advanced stage (International Federation of Gynecology and Obstetrics stages IIB to IV) and <30% of these patients survive for >5 years (1). By contrast, the 5-year survival rate of patients diagnosed at stage I is 90% and the 5-year survival rate of patients diagnosed at stage II is up to 70% (2). EOC is an aggressive disease characterized by multiple metastases driven by dysregulated genes (3). Detection of tumor particles (cells, cell-free nucleic acids and exosomes) in body fluids (liquid biopsy) in the early stages of cancer would enable preclinical diagnosis and improve survival rate. Liquid biopsy is used for the detection of serum biomarkers and to prove that these markers are specific to the disease (4).
MicroRNAs (miRNAs or miRs) belong to the class of small (18–22 nucleotides) non-coding RNAs and are involved in post-transcriptional gene regulation and inhibition of gene expression. miRNAs regulate up to 60% of all human genes and are involved in development, differentiation, metabolism, proliferation, cell cycle, immune system, inflammation and carcinogenesis (5).
Bioinformatic analysis is a powerful approach to support the laboratory results in biomarker studies for early diagnosis of cancer; in miRNA studies, target genes and pathways should be investigated using bioinformatics tools. Therefore, the aim of the present study was to detect significant dysregulated serum and tissue miRNAs by comparing their expression levels in patients and healthy individuals (HIs), as well as in patients with EOC and simple ovarian cysts (SOCs). To the best of our knowledge, the present study is the first to compare fresh EOC tissue and serum.
Material and methods
Study outline
The present study involved three steps: i) Serum and tissue miRNA profiling by microarray; ii) dysregulated miRNA validation by reverse transcription-quantitative PCR (RT-qPCR) and iii) bioinformatics analysis (Fig. 1). The sample collection and analysis process were approved by the Istanbul University Faculty of Medicine Clinical Researches Ethics Committee (Istanbul, Turkey; approval no. 2014/1175). All experiments were performed in accordance with the approved guidelines and regulations indicating in World Medical Association Declaration of Helsinki (6).
Figure 1.
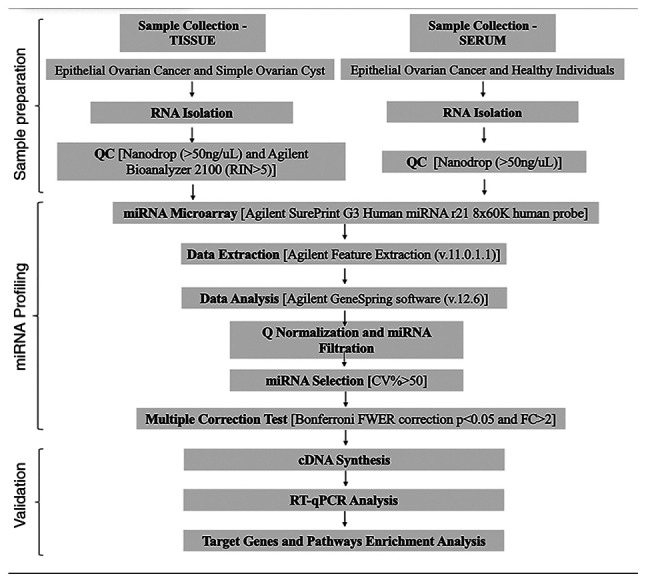
Workflow for expression analysis of miRNAs. CV, coefficient of variation; FWER, family-wise error-rate; miRNA, microRNA; Q, quantile; QC, quality control; RIN, RNA integrity number; RT-q, reverse transcription-quantitative.
Sample collection
A total of 40 samples were collected from four groups of females: Ten plasma samples from patients with pathologically confirmed EOC (mean age, 47 (30–54) years), ten plasma samples from matched healthy individuals (HIs) (mean age, 40 (26–65) years) eight EOC tissue samples (mean age, 47 (30–54) years) and eight benign ovarian (mean age, 40 (17–70) years) neoplastic tissue samples. No patients had been administered neoadjuvant chemotherapy and all patients had undergone primary cytoreductive surgery. All cases of OC were histologically diagnosed and classified in accordance with the World Health Organization criteria (7). For microarray analysis, the first eight samples with best qualified were used in each group; RNA samples extracted from tissues with RIN values >5 were chosen for microarray analysis and the total RNAs extracted from all serum samples were used. 10 samples per group were used for the validation step by RT-qPCR (Table I). The sample collection was performed at Istanbul University Medical Faculty Department of Obstetrics and Gynecology (Istanbul, Turkey) between January 1, 2015 and March 31, 2016. Peripheral blood was collected into EDTA tubes (5 ml) and immediately centrifuged at 3,500 × g for 15 min at 4°C. The serum supernatant was transferred to RNase-free tubes. Sample serum fractions were collected and stored at −80°C until needed. For the collection of tissue, after the routine surgical operation, ~500 mg tissue was deposited in a sterile centrifuge tube containing RNAlater™ stabilization and storage solution (Ambion; Thermo Fisher Scientific, Inc.), stored at 4°C overnight and then stored at −80°C until needed.
Table I.
Demographic and clinical characteristic of samples used in microarray and validation steps.
| Microarray | Validation | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Healthy individuals | Patients with EOC | Patients with SOC | Healthy individuals | Patients with EOC | Patients with SOC |
| Number of samples (source) | 8 (serum) | 8 (serum and tissue) | 8 (tissue) | 10 (serum) | 10 (serum and tissue) | 10 (tissue) |
| Median age (range), years | 44 (30–65) | 45 (30–54) | 35 (17–70) | 40 (26–65) | 47 (30–54) | 40 (17–70) |
| CA-125, U/ml | - | 1348.5 | 88.9 | - | 1520.2 | 83.2 |
| Alcohol consumption | N | N | N | N | N | N |
| Cigarette consumption | N | N | N | N | N | N |
| Treatment | N | N | N | N | N | N |
| Histological type | - | Three cases of stage 3C grade 3 serous cancer, two cases of stage 3B grade 3 serous cancer, one case each of stage 2B grade 1 serous cancer, stage 2A endometrioid and stage 1A grade 3 serous cancer | Five cases of endometrioid cyst, one case of dermoid cyst, two cases of serous cystadenoma | - | Five cases of stage 3C grade 3 serous cancer, two cases of stage 3B grade 3 serous cancer, one case each of stage 2B grade 1 serous cancer, stage 2A endometrioid and stage 1A grade 3 serous cancer | Four cases of endometrioid cyst, two cases each of dermoid cyst, mucinous cystadenoma and serous cystadenoma |
| Metastasis, n | - | 8 | - | - | 9 | - |
CA-125, cancer antijen-125; EOC, epithelial ovarian cancer; N, no; SOC, simple ovarian cyst; -, not available.
Total RNA isolation
To extract total RNA, including miRNA, from serum, a mirVana™ PARIS™ RNA and Native Protein Purification kit (Ambion; Thermo Fisher Scientific, Inc.) was used according to the manufacturer's protocol. To test purification quality and normalize variation, cel-miR-39 (Table SI; Qiagen GmbH), synthetic Caenorhabditis elegans miRNA (working solution of 1.6×10−8 copies/ml), was spiked in each serum sample before the extraction protocol. Synthetic cel-miR-39 was selected as the spike-in due to the absence of homologous sequences in Homo sapiens. Total RNA was eluted with 35 µl mirVana elution solution. The purity and concentration of RNA were determined by NanoDrop IMPLEN P-Class (Thermo Fisher Scientific, Inc.).
Total RNA was isolated from tissue samples for microarray and RT-qPCR analysis using a RNeasy® Plus Mini kit (Qiagen GmbH), according to the manufacturer's protocol. The quantity of obtained total RNA was measured by NanoDrop IMPLEN P-Class (Thermo Fisher Scientific, Inc.) and quality controls were conducted by an Agilent RNA 6000 Nano kit on an Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc.). RNA extract was evaluated with the bioanalyzer; the eight samples with the highest quality [RNA integrity number (RIN) value >5] were selected for microarray analysis; all serum samples were selected.
MiRNA microarray analysis
miRNA expression analysis was performed using miRNA microarray chips (SurePrint G3 Human miRNA r21 8×60K; Agilent Technologies, Inc.) and miRNA Labelling and Hybridization kit (Agilent Technologies, Inc.) according to the manufacturer's instructions. Total RNA extracted from both serum and tissue was labelled with cyanine 3-cytidine bisphosphate. The labelled miRNAs were hybridized for 24 h at 56°C on Human miRNA Microarray Version 16 slides (Agilent Technologies, Inc.), which include 1,368 miRNAs encoded by genes located across all chromosomes. The hybridized slides were scanned using an Agilent SureScan Microarray Scanner (Model G2600D; Agilent Technologies, Inc.) and the images were analyzed by Agilent Feature Extraction (v.11.0.1.1) software (Agilent Technologies, Inc.). Also, divisive hierarchical clustering method was followed for top to down clustering analysis. Microarray results were submitted to the Gene Expression Omnibus database (accession no. GSE216150).
RT-qPCR
The candidate miRNAs [P<0.05 and fold change (FC)>2] obtained from microarray bioinformatics analysis were validated by stem-loop RT-qPCR. miRNAs were quantified using TaqMan® MicroRNA Assays (Thermo Fisher Scientific, Inc.; Table SI). RT was performed with the SureCycler 8800 Thermal Cycler (Agilent Technologies, Inc.) using a TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Inc.). The samples were normalized using TaqMan MicroRNA Assays (Thermo Fisher Scientific, Inc.) spiked with cel-miR-39 for serum and U6 small nuclear RNA (snRNA) for tissue.
According to the manufacturer's protocol of TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Inc.) for cDNA synthesis, 7 µl reaction mix contained 0.15 µl 100 mM dNTP mix in 1.5 µl 10X RT Buffer, supplemented with 1 µl RT enzyme, 0.19 µl RNase Inhibitor and 4.16 µl Invitrogen™ UltraPure™ DNase/RNase-Free Distilled Water (Thermo Fisher Scientific, Inc.). cDNA synthesis was performed using 5 µl samples containing 4 ng/µl total RNA for serum and 10 ng/µl total RNA for tissue. The reaction mix and 3 µl 5X TaqMan MicroRNA Assays were reverse-transcribed for 30 min at 16°C, 30 min at 42°C, 5 min at 85°C and stopped at 4°C. RT-qPCR was performed using a Stratagene Mx3005P RT-qPCR system (Agilent Technologies, Inc.). For each 20 µl PCR reaction, 10 µl TaqMan miRNA RT-qPCR Assay (Applied Biosystems; Thermo Fisher Scientific, Inc.) and 20X TaqMan® MicroRNA Assays containing PCR primers and probes (5′-carboxyfluorescein and 3′-tetramethylrhodamine), 2.5 µl cDNA from serum and 6.5 µl Invitrogen™ UltraPure DNase/RNase-Free Distilled Water were mixed. For tissue, 1.33 µl cDNA and 7.67 µl Invitrogen UltraPure™ DNase/RNase-Free Distilled Water were added into each reaction. Every batch of amplifications included two water blanks and primers as no template negative controls for each of the cDNA products and RT-qPCR steps. The reaction was performed at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Data were normalized to cel-miR-39 for serum samples and U6 snRNA for tissue samples and analyzed using a Stratagene Mx3005P RT-qPCR system (Agilent Technologies, Inc.) with the automatic Cq setting for adapting baselines and thresholds for Cq determination. The 2−ΔΔCq method calculates relative fold changes using Cq values (8).
Statistical analysis of microarray results
Statistical analysis of raw microarray data extracted from Agilent Feature Extraction (v.11.0.1.1) software was conducted using GeneSpring v.12.6 software (Agilent Technologies, Inc.). Raw data were normalized by quantile normalization and probes <50% coefficient of variation were filtered. Samples with high variation and low quality (RIN value <5) were eliminated in the quality control step. Obtained probes involved thousands of signal results, requiring correction of these probes results. The most effective method to control family-wise error rate (FWER) is Bonferroni's test which is the exact value and the underlying P-values are validated for all dependency structures (9). Unpaired student's t test with Bonferroni's FWER correction was performed. P<0.05 and FC>2 were considered to indicate as a statistically significant difference; probes with these values in the gene expression analysis were identified as significantly dysregulated miRNAs.
Statistical analysis of RT-qPCR results
GraphPad Prism (v.7.04; Dotmatics) was used to perform statistical analysis for validation of RT-qPCR data. Mann-Whitney U test was used to analyze the difference in serum miRNA expression levels between EOC and HI and in tissue miRNA expression between EOC and SOC. SD of two replicates, confidence intervals (CIs) and P-values were calculated using Cq values obtained from RT-qPCR results. Receiver operating characteristic (ROC) curves were generated and areas under the ROC curves (AUC) were calculated to obtain sensitivity and specificity. The best threshold or cut-off value for the distinction between control and patient outcomes was set at 0.5. P<0.05 was considered to indicate a statistically significant difference.
Target gene and pathway enrichment analysis
Targets of 34 significantly differentially expressed miRNAs resulted from the microarray study (31 serum and three tissue miRNAs) were predicted using the DIANA-microT-CDS algorithm with a threshold of 0.8 and DIANA-miRPath v3.0 (10) was used to identify Kyoto Encyclopedia of Genes and Genomes' (KEGG) pathways (11) with P-value and false discovery rate <0.05.
Results
Patient characteristics
The demographic and clinical characteristics of patients are shown in Table I. There were three groups: HI, EOC and SOC; control groups were HIs for EOC in serum and SOC for EOC in tissue. In the EOC group, 8 patients were still alive at time of writing whereas 2 were not (died after 23 and 32 months of treatment).
Microarray results
In the serum samples, 31 miRNAs were significantly downregulated (Table II) in EOC samples compared to HI serum samples. However, three miRNAs were significantly upregulated in tissue samples in EOC tissues compared to SOC samples.
Table II.
Significantly differentially expressed miRs in EOC compared with HI serum and SOC tissue samples by microarray.
| miR | miRBase accession number | Corrected P-value | Regulation | Log fold-change | Sample source |
|---|---|---|---|---|---|
| Hsa-miR-1909-5p | MIMAT0004484 | 2.84×10−9 | Down | −4.88594 | Serum |
| Hsa-miR-6775-3p | MIMAT0027451 | 1.93×10−5 | Down | −4.66636 | Serum |
| Hsa-miR-3935 | MI0016591 | 1.11×10−7 | Down | −4.27686 | Serum |
| Hsa-miR-6511a-3p | MIMAT0025479 | 3.25×10−8 | Down | −4.11005 | Serum |
| Hsa-miR-6756-3p | MIMAT0027413 | 6.04×10−4 | Down | −4.06512 | Serum |
| Hsa-miR-885-5p | MIMAT0004947 | 5.21×10−7 | Down | −4.06315 | Serum |
| Hsa-miR-6794-3p | MIMAT0027489 | 1.32×10−9 | Down | −4.01289 | Serum |
| Hsa-miR-3646 | MI0016046 | 3.56×10−9 | Down | −3.98497 | Serum |
| Hsa-miR-6743-3p | MIMAT0027388 | 3.54×10−3 | Down | −3.94265 | Serum |
| Hsa-miR-548am-5p | MIMAT0022740 | 4.92×10−7 | Down | −3.84752 | Serum |
| Hsa-miR-7111-3p | MIMAT0028120 | 4.9×10−3 | Down | −3.81715 | Serum |
| Hsa-miR-4728-3p | MIMAT0019850 | 7.51×10−4 | Down | −3.79012 | Serum |
| Hsa-miR-6784-3p | MIMAT0027469 | 2.33×10−6 | Down | −3.78141 | Serum |
| Hsa-miR-6804-3p | MIMAT0027509 | 7.80×10−6 | Down | −3.67437 | Serum |
| Hsa-miR-1306-3p | MIMAT0004947 | 5.66×10−7 | Down | −3.59189 | Serum |
| Hsa-miR-6824-3p | MIMAT0027549 | 2.59×10−7 | Down | −3.57616 | Serum |
| Hsa-miR-6761-3p | MIMAT0027423 | 7.93×10−6 | Down | −3.45328 | Serum |
| Hsa-miR-449c-3p | MIMAT0013771 | 4.39×10−5 | Down | −3.40556 | Serum |
| Hsa-miR-195-3p | MIMAT0004615 | 6.82×10−5 | Down | −3.21695 | Serum |
| Hsa-miR-6847-3p | MIMAT0027595 | 9.39×10−5 | Down | −3.03194 | Serum |
| Hsa-miR-6730-3p | MIMAT0027362 | 9.03×10−5 | Down | −2.95574 | Serum |
| Hsa-miR-1296-5p | MIMAT0022740 | 6.10×10−5 | Down | −2.90261 | Serum |
| Hsa-miR-4695-3p | MIMAT0019789 | 4.30×10−5 | Down | −2.82112 | Serum |
| Hsa-miR-4763-5p | MIMAT0019912 | 3.10×10−5 | Down | −2.79861 | Serum |
| Hsa-miR-615-3p | MIMAT0003283 | 1.08×10−4 | Down | −2.73022 | Serum |
| Hur_2 | SERUM REFERENCE | 1.18×10−2 | Down | −2.55936 | Serum |
| Hsa-let-7d-3p | MIMAT0007882 | 7.22×10−3 | Down | −2.07235 | Serum |
| Hsa-miR-6776-3p | MIMAT0027453 | 9.53×10−6 | Down | −3.3754 | Serum |
| Hsa-miR-3184-3p | MIMAT0022731 | 3.69×10−6 | Down | −3.2145 | Serum |
| Hsa-miR-6759-3p | MIMAT0027419 | 1.58×10−5 | Down | −3.1554 | Serum |
| Hsa-miR-6894-3p | MIMAT0027689 | 5.72×10−5 | Down | −2.9809 | Serum |
| Hsa-miR-937-3p | MIMAT0004980 | 3.21×10−5 | Down | −2.7339 | Serum |
| Hsa-miR-6737-3p | MIMAT0027376 | 6.02×10−5 | Up | 3.851476 | Tissue |
| Hsa-miR-4665-3p | MIMAT0019740 | 1.1×10−7 | Up | 4.859524 | Tissue |
| Hsa-miR-200c-3p | MIMAT0000617 | 9.65×10−5 | Up | 7.873392 | Tissue |
miR, microRNA.
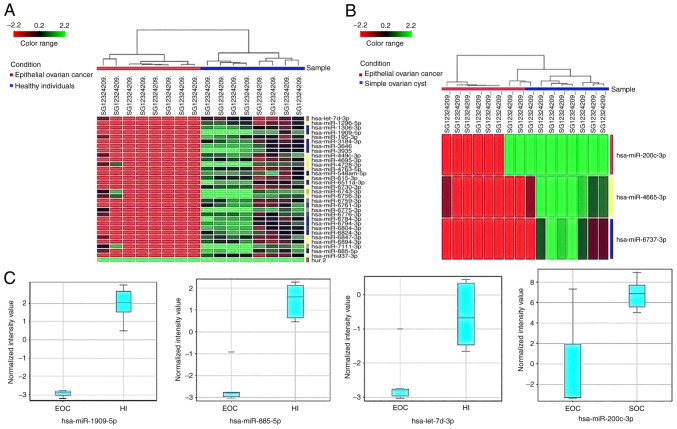
Significant changes in expression levels of the miRNA are shown in Fig. 2, determined using hierarchical clustering analysis. The hierarchical clustering of dysregulated miRNAs in serum and tissue demonstrated that the subgroups were well-differentiated from the unified set of differentially expressed miRNAs (Fig. 2A and B). According to serum results of the HIs and patients with EOC, 31 downregulated miRNAs were clearly separated (Fig. 2A). Additionally, in SOC and EOC tissues, three upregulated miRNAs (hsa-miR-200c-3p, hsa-miR-4665-3p and hsa-miR-6737-3p) were clearly separated (Fig. 2B). Expression levels for each significant (P<0.05 and fold change >2) miRNA in serum and tissue are presented as box-whisker plots in Fig. 2C.
Figure 2.
Hierarchical clustering and box-whisker plots of differentially expressed miRNAs in both serum and tissue samples. (A) Hierarchical clustering of differentially expressed miRs in serum EOC and HI samples. EOC serum samples are distributed under the red line and the HI samples under the blue line. Red rectangles indicate expression lower than the mean intensity and the green rectangles indicate expression higher than the mean intensity. (B) EOC and SOC tissue samples with P<0.05 determined by microarray analysis. EOC serum samples are distributed under the blue line and the SOC samples under the red line. Vertical codes represent different samples. (C) Box-whisker plots of serum miRs (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p) in both EOC and HI and the tissue miR (hsa-miR-200c-3p) in both SOC and EOC. EOC, epithelial ovarian cancer; HI, healthy individual; miR, microRNA; SOC, simple ovarian cyst.
RT-qPCR results
According to FC values, the first 10 miRNAs that had the highest logarithmic FC of the total 31 miRNAs were determined. Among all miRNAs after validation, three miRNAs (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p) were validated.
To confirm the miRNA microarray results, samples were analyzed for miRNA expression by RT-qPCR. The expression levels of serum miRNAs were compared in 10 EOC and HI serum samples. According to the RT-qPCR results, expression levels of hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p were significantly downregulated (Table III). For tissue miRNA validation, 10 EOC and 10 SOC tissue samples were used. RT-qPCR analysis indicated that hsa-miR-200c 3p expression was significantly upregulated in EOC tissues compared with SOC samples (Table III).
Table III.
Significantly dysregulated and validated miRs in serum and tissue.
| Sample Stages and miRNA Levels | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| miR | P-value | 95% CI | Standard deviation | Log fold-change | Regulation | Sample source | Up-Regulated | Down-Regulated |
| Hsa-miR-1909-5p | 0.002 | 1-1 | 0.0000 | −1.35 | Down | Serum | 3C (n=1) | 2B (n=1), 3B |
| (n=2), 2A | ||||||||
| (n=1), 3C | ||||||||
| (n=4), 1A | ||||||||
| (n=1) | ||||||||
| Hsa-miR-885-5p | 0.0195 | 0.563-0.997 | 0.1107 | −2.64 | Down | Serum | 1A (n=1) | 2B (n=1), 3B |
| (n=2), 2A | ||||||||
| (n=1), 3C | ||||||||
| (n=5) | ||||||||
| Hsa-let-7d-3p | 0.0488 | 0.460-0.941 | 0.1227 | −2.35 | Down | Serum | 1A (n=1) | 2B (n=1), 3B |
| (n=2), 2A | ||||||||
| (n=1), 3C | ||||||||
| (n=5) | ||||||||
| Hsa-miR-200c-3p | 0.0273 | 0.623-1.037 | 0.1056 | 2.87 | Up | Tissue | 2B (n=1), 3B | 1A (n=1) |
| (n=2), 2A | ||||||||
| (n=1), 3C | ||||||||
| (n=5) | ||||||||
CI, confidence interval; miR, microRNA.
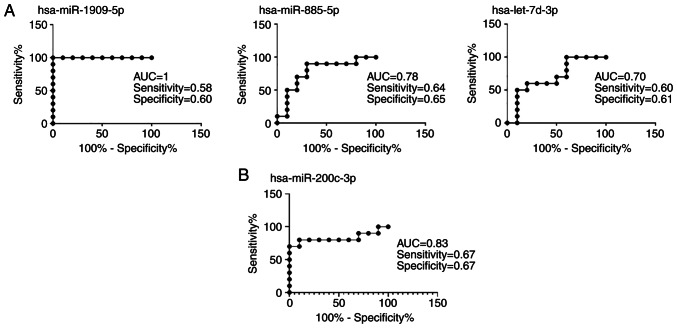
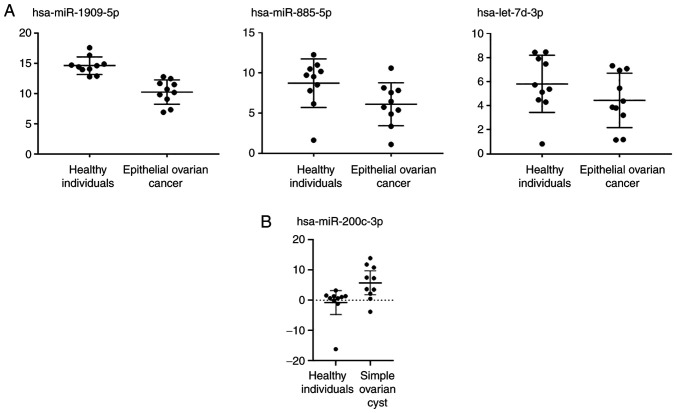
ROC curve analysis was performed to determine the discriminability of serum and tissue miRNAs detected (Fig. 3). The AUC was >0.50 for all candidate miRNAs. Therefore, they appear to be successful in distinguishing patients with EOC from HIs and patients with SOC. The relative expression levels of the miRNAs are shown in Fig. 4 for patients with EOC and the HIs. Relative expression levels of hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p were down regulated in EOC serum samples (Fig. 4A). Relative expression of hsa-miR-200c 3p was upregulated in EOC tissues compared to SOC samples (Fig. 4B).
Figure 3.
Receiver operating characteristic parameters for (A) serum and (B) tissue miRNA. AUC, area under the curve; miR, microRNA.
Figure 4.
Relative expression analysis of differentially expressed serum and tissue miRNAs (A) Relative expression of serum miRs was significantly lower in patients with EOC than healthy individuals. A total of three validated miRNAs were downregulated in patients with EOC. (B) Relative expression of validated tissue miR was significantly higher in patients with EOC than simple ovarian cyst (controls). EOC, epithelial ovarian cancer; miR, microRNA.
Target gene and pathway enrichment analysis of miRNAs
Following microarray analysis, 34 dysregulated miRNAs (31 serum and three tissue miRNAs) were determined. Using these significantly dysregulated miRNAs, target genes and pathways were identified by enrichment analysis. A total of 47 enriched pathways were found and the most significant pathways were selected according to P-value (P<0.0009). The top seven pathways (‘prion diseases’, ‘proteoglycans in cancer’, ‘oxytocin signaling pathway’, ‘hippo signaling pathway’, ‘adrenergic signaling in cardiomyocytes’, ‘oocyte meiosis’ and ‘thyroid hormone signaling pathway’) were taken into consideration because of their relation with ovarian cancer (Table IV). At least one validated miRNAs was involved in each of these pathways. When these pathways were evaluated, all except ‘oocyte meiosis’ had an interaction with OC.
Table IV.
Target gene and pathway enrichment analysis of dysregulated miRs identified from microarray results.
| KEGG pathway | P-value | Number of miRs | Number of genes | Associated miR | Associated gene |
|---|---|---|---|---|---|
| Prion diseases | 1.28×10−9 | 9 | 14 | miR-6775-3p, miR-548am-5p, miR-6824-3p, miR-195-3p, miR-7111-3p, miR-3184-3p, miR-200c-3pa, miR-4695-3p, miR-6737-3p | EGR1, STIP1, IL1B, BAX, HSPA5, NCAM1, NCAM2, NOTCH1, PRKACG, PRNP, PRKX, C6, MAPK1, ELK1 |
| Proteoglycans in cancer | 2.18×10−7 | 26 | 90 | miR-7111-3p, miR-3646, miR-200c-3pa, miR-6824-3p, miR-6759-3p, miR-6794-3p, miR-548am-5p, miR-4728-3p, miR-3184-3p, miR-195-3p, miR-4695-3p, miR-885-5pa, miR-449c-3p, miR-6894-3p, miR-6775-3p, miR-6847-3p, miR-6737-3p, miR-6730-3p, miR-3935, miR-6804-3p, miR-4763-5p, miR-6776-3p, miR-1296-5p, miR-6756-3p, miR-6784-3p, miR-6511a-3p | FZD7, ESR1, PPP1CA, BRAF, PRKCA, STAT3, WNT16, WNT7A, SMAD2, CBL, CAMK2G, NRAS, PTCH1, CAV1, WNT5A, PPP1CC, PXN, ROCK2, FRS2, FZD6, RDX, IQGAP1, ITGA5, RAF1, WNT2B, WNT4, TIAM1, IGF1R, EGFR, TLR4, RHOA, PPP1R12B, CAV2, KRAS, FZD3, RRAS2, MSN, ITGB5, MAPK13, ANK2, RPS6KB2, COL21A1, HPSE, CAMK2A, PTK2, ITGAV, ANK3, FZD4, PPP1R12A, DCN, PLCG1, CASP3, PIK3R3, CCND1, CTNNB1, HIF1A, FLNB, PRKACG, FLNA, ITGA2, SOS1, PIK3CG, PTPN11, PRKX, DDX5, SRC, FGF2, LUM, PRKCB, IGF1, GAB1, AKT3, PLCE1, PIK3CA, SDC2, FN1, CDKN1A, PDPK1, VMP1, WNT9B, TWIST1, FGFR1, MAPK1, ITPR2, KDR, ELK1, WNT9A, ERBB4, RPS6KB1, CD44 |
| Oxytocin signaling pathway | 7.45×10−6 | 26 | 79 | miR-3646, miR-6894-3p, miR-548am-5p, miR-6847-3p, miR-200c-3pa, miR-6775-3p, miR-3935, miR-4728-3p, miR-4695-3p, miR-6737-3p, miR-1909-5pa, miR-6794-3p, miR-3184-3p, miR-6824-3p, miR-1296-5p, miR-7111-3p, miR-6730-3p, miR-6756-3p, miR-195-3p, miR-6759-3p, miR-6804-3p, miR-885-5pa, miR-6776-3p, miR-6784-3p, -miR-449c-3p, -miR-6511a-3p | PPP1CA, FOS, PRKCA, CACNG8, EEF2K, GUCY1B3, MYLK4, ADCY5, CAMK2G, CAMK4, NRAS, PRKAA2, CD38, ADCY2, RCAN1, CALM1, GUCY1A3, CACNB4, PPP1CC, ROCK2, MAP2K5, PPP3R1, RAF1, KCNJ14, EGFR, GNAI3, RHOA, PPP1R12B, KRAS, CALM2, CAMKK2, NFATC4, NPR1, CAMK2A, PPP3CA, RYR1, PLCB1, PPP1R12A, JUN, PIK3, R3CCND1, PPP3CB, PRKACG, CACNB1, PTGS2, PIK3CG, PRKX, OXTR, SRC, PRKAA1, CAMK1, KCNJ6, CACNA1, CPRKCB, PRKAB2, KCNJ2, GNAQ, PRKAG2, GUCY1A2, PIK3CA, CDKN1A, CACNG3, CACNA2D1, CACNB2, MEF2C, CAMK1D, NFATC3, MAPK1, ITPR2, KCNJ5, KCNJ3, ELK1, PLCB4, MYLK, PLA2G4C, GNAI1, PPP3R2, CACNB3, ADCY6 |
| Hippo signaling pathway | 4.56×10−5 | 26 | 63 | miR-449c-3p, miR-200c-3, pmiR-6759-3p, miR-6894-3p, miR-6743-3p, miR-3935, miR-6784-3p, miR-6804-3p, miR-548am-5p, miR-7111-3p, miR-4728-3p, miR-195-3p, miR-4763-5p, miR-4695-3p, miR-3646, miR-1909-5pa, miR-6847-3p, miR-885-5pa, miR-6775-3p, miR-6776-3p, miR-6794-3p, miR-6824-3p, miR-6737-3p, miR-6756-3p, miR-3184-3p, miR-6730-3p | FZD7, PPP1CA, GSK3B, WNT16, PARD6G, WNT7A, YAP1, SMAD2, YWHAE, PPP2R2C, PPP2CA, NF2, WNT5A, BMPR1B, YWHAG, PPP1CC, GLI2, FZD6, WNT2B, WNT4, MOB1, BYWHAB, TEAD3, AMOT, WWTR1, TP53BP2, FZD3, BMP8B, YWHAQ, LLGL2, FZD4, CCND1, SMAD4, CTNNB1, TP73, PPP2R2A, PPP2R1A, LLGL1, DLG3, FRMD6, SAV1, TEAD1, YWHAZ, GDF6, CSNK1E, BMPR1A, CTNNA3, LEF1, RASSF1, WNT9B, MOB1A, CTNNA2, LATS1, BMP7, FGF1, FBXW11, PPP2R1B, PARD6B, INADL, TGFBR2, WNT9A, BMPR2, TGFB3 |
| Adrenergic signaling in cardiomyocytes | 1.04×10−4 | 25 | 62 | miR-548am-5p, miR-6824-3p, miR-4695-3p, miR-3646, miR-4728-3p, miR-6730-3p, miR-6776-3p, miR-885-5pa, miR-6756-3p, miR-6759-3p, miR-6847-3p, miR-6894-3p, miR-3184-3p, miR-7111-3p, miR-195-3p, miR-6737-3p, miR-200c-3pa, miR-6794-3p, miR-6784-3p, miR-6511a-3p, miR-4763-5p, miR-449c-3p, miR-1909-5pa, miR-6775-3p, miR-3935 | PPP1CA, RAPGEF3, SCN7A, PRKCACA, CNG8, PPP2R5E, ATP1B2, ADCY5, TPM1, CAMK2G, ATP2B2, PPP2R3A, ADCY2, CALM1, AGTR1, PPP2R2C, PPP2CA, CREB5, CACNB4, PPP1CC, BCL2, PPP2R5D, GNAI3, PPP1R1A, CALM2, MAPK13, PPP2R5A, RAPGEF4, CREB1, CAMK2A, SCN5A, PLCB1, PPP2R5B, ACTC1, PIK3R3, PLN, PPP2R2A, PPP2R1A, PRKACG, CACNB1, SLC8A1, ATP1A4, PIK3CG, PRKX, PPP2R3C, CACNA1C, GNAQ, AKT3, PIK3CA, AGTR2, ATP2B4, CACNG3, CACNA2D1, ATP2A2, CACNB2, MAPK1, PPP2R1B, PLCB4, GNAI1, CACNB3, KCNE1, ADCY6 |
| Oocyte meiosis | 3.3×10−4 | 21 | 55 | miR-3646, miR-6756-3p, miR-6759-3p, miR-885-5pa, miR-200c-3pa, miR-6847-3p, miR-548am-5p, miR-7111-3p, miR-6824-3p, miR-6784-3p, miR-449c-3p, miR-6775-3p, miR-3184-3p, miR-6894-3p, miR-195-3p, miR-4728-3p, miR-4695-3p, miR-6776-3p, miR-6737-3p, miR-6794-3p, miR-6730-3p | SLKE, SPL1, PPP1CA, PPP2R5E, SMC1A, ADCY5, CCNB1, PGR, CAMK2G, YWHAE, ADCY2, CALM1, PPP2CA, CPEB4, YWHAG, PPP1CC, CDK2, BUB1, PP3R1, CPEB1, CUL1, PPP2R5D, YWHAB, IGF1R, PLCZ1, ANAPC1, CALM2, YWHAQ, PPP2R5A, CAMK2A, PPP3CA, PPP2R5B, AR, PPP3CB, CCNE2, PPP2R1A, PRKACG, CPEB2, YWHAZ, ANAPC7, PRKX, MAD2L1, ANAPC5, RPS6KA3, IGF1, CDC27, RBX1, CPEB3, MAPK1, ITPR2, FBXW11, PPP2R1B, FBXO43, PPP3R2, ADCY6 |
| Thyroid hormone signaling pathway | 5.3×10−4 | 24 | 56 | miR-4695-3p, miR-6776-3p, miR-548am-5p, miR-3646miR-6759-3p, miR-6784-3p, miR-4763-5p, miR-6894-3p, miR-200c-3pa, miR-3184-3p, miR-7111-3p, miR-885-5pa, miR-6824-3p, miR-1296-5p, miR-4728-3p, miR-6737-3p, miR-6756-3p, miR-6511a-3p, miR-195-3p, miR-6794-3p, miR-6775-3p, miR-6847-3p, miR-449c-3p, miR-6730-3p | ESR1, GSK3B, PRKCA, ATP1B2, MED13L, NRAS, RCAN1, RXRG, DIO2, RAF1, MED13, SLC16A10, WNT4, THRA, PLCZ1, NOTCH2, RCAN2, KRAS, NCOA3, GATA4, MED1, ITGAV, PLCB1, TBC1D4, NOTCH1, PLCG1, PIK3R3, CCND1, CTNNB1, HIF1APLN, PRKACG, NCOR1, ATP1A4, NCOA2, PIK3CG, HDAC2, PRKX, KAT2A, SRC, PRKCB, KAT2B, EP300, AKT3, SLC16A2, PLCE1, PIK3CA, PDPK1, PFKFB2, ATP2A2, STAT1, RXRB, MAPK1, PLCB4, NCOA1, SIN3A |
Validated miR. KEGG, Kyoto Encyclopedia of Genes and Genomes; miR, microRNA.
Discussion
The physiological and pathological roles of altered miRNAs have been demonstrated in many tumor types such as breast, colon, pancreatic cancers (12) and may serve a key role in the diagnosis, prognosis and treatment of cancer (13). The cell-free forms of circulating miRNAs in peripheral blood are highly stable as they are protected from endogenous RNase activity (5) and are therefore potential candidates as biomarkers.
In the present study, three downregulated miRNAs (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p) in serum of patients with EOC and one upregulated miRNA (hsa-miR-200c-3p) in EOC tissue samples were validated by comparison with HIs and patients with SOC, respectively. After validation of the miRNAs, target genes and involved pathways were analyzed. After validation step, significantly dysregulated miRNAs (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p I serum; hsa-miR-200c 3p in tissue) were analyzed using KEGG analysis in terms of OC pathogenesis.
Prion diseases are fatal neurodegenerative diseases that occur in humans and some animals (14). These diseases are directly associated with misfolded prion proteins derived from normal prion proteins expressed by the prion protein gene (PRNP) (15). This conversion is caused by factors such as prion infection, mutations or unknown factors. Prion disease-associated pathways cause neuronal death and comprise ‘oxidative stress’, ‘regulated activation of complement’, ‘ubiquitin-proteasome and endosomal-lysosomal systems’, ‘synaptic alterations and dendritic atrophy’, ‘corticosteroid response’ and ‘endoplasmic reticulum stress’ (14). Kim et al (15) found that prion disease-associated somatic mutations of the PRNP gene are caused by aberrant expression of oncogenes and a stability gene(s) causing genetic variants and metastatic properties. Somatic mutations of the PRNP gene can be observed in patients with OC at a rate of 5.3% (15–20). In the present study, one validated miRNAs, hsa-miR-200c-3p, was involved in prion diseases pathway, according to its target genes. Hsa-miR-200c-3p is involved in regulating cellular transformation, such as epithelial-mesenchymal transition (EMT), metastasis, cell proliferation and differentiation, inflammation, angiogenesis, cellular transformation, apoptosis, chemoresistance and tissue turnover (21–25).
Proteoglycans (PGs) are extracellular matrix components and play a key role in cell signaling and structural organization, which controls normal and pathological processes (26). Altered expression and structural variable caused by different types of covalently linked glycosaminoglycan chains PGs accumulate in remodeled tumor stroma in malignancy (26–28). They affect tumor growth via formation of a permissive provisional matrix that regulates tissue organization, cell-cell and cell-matrix interactions and tumor cell signaling (26). Studies indicate a role of PGs in OC. Davies et al (29) found a larger variety of heparan sulfate PGs in normal ovaries compared with ovarian tumors. It was also suggested that stromal induction of one type (syndecan-1) of heparan sulfate PG contributes to the pathogenesis of ovarian malignancy (29). Furthermore, PGs have been suggested as therapy targets for several cancer types, such as melanoma, breast cancer, squamous cell carcinoma, mesothelioma and neuroblastoma (30,31). Certain PGs are also considered as biomarkers of cancer. For example, syndecan-1 and glypican-1 have been recorded as biomarkers for OC and their upregulation is an indicator of poor prognosis (32). Therefore, PGs are one of the most important proteins in several cancers and also OC (28). In the present study, when analyzing the miRNAs involved in ‘proteoglycan in cancer’, only one validated tissue miRNA, hsa-miR-200c-3p, was found to be involved in PG biosynthesis. Therefore, upregulation of hsa-miR-200c-3p results in aberrant expression patterns of proteoglycans and regulate OC progression via PGs.
Oxytocin is a key hormone in the female reproductive system, especially during pregnancy and lactation. Notably, there is an association between decreased risk of OC and oxytocin, which is released during breastfeeding (33,34). Although the underlying molecular mechanism is not clear, there have been some studies to illuminate the role of oxytocin in OC. Therefore, the present study may bring a new perspective to OC pathogenesis. Similarly to the present study, Zhang et al (35) identified ‘oxytocin signaling pathway’ in KEGG enrichment pathways in EOC cases via next generation sequencing (NGS). Ji et al (34) showed that increased levels of oxytocin receptors in ovarian tumors are associated with decreased metastasis-free survival time. Therefore, it was hypothesized that the oxytocin signaling pathway serves a role in OC metastasis. Additionally, it was demonstrated that oxytocin antagonizes OC via the VEGF pathway (34). Morita et al (36) and Ji et al (34) demonstrated the inhibitory role of oxytocin on OC cell proliferation, migration and invasion in SKOV3 and A2780 cell lines. In another study, oxytocin has a protective effect via controlling anti-inflammatory mechanisms by interaction with lower levels of systemic and tumor-associated IL-6 (37). Therefore, the oxytocin signaling pathway may have a key role in OC migration, invasion, proliferation and metastasis and molecular regulators involved in this pathway have the potential to determine OC pathogenesis. Considering this, it was hypothesized that three dysregulated and validated miRNAs, hsa-miR-200c-3p, hsa-miR-1909-5p and hsa-miR-885-5p, identified in the present study, were involved in OC development via their effect on the oxytocin signaling pathway. Hsa-miR-1909-5p regulates the growth of epithelial ovarian tumors, endothelial cell function and angiogenesis of serous ovarian tumors (38). Hsa-miR-885-5p serves a regulatory role in OC and downregulation of hsa-miR-885-5p may drive tumor growth, invasion, metastasis, EMT and chemotherapy resistance (39–43). The aforementioned studies support the suggestion that the miRNAs validated in the present study have the potential as a diagnostic and therapeutic target for OC. However, underlying mechanisms should be validated with further studies.
The Hippo signaling pathway is a highly conserved pathway in Drosophila and mammals and has the role in controlling organ size and tumor growth (44–47). It limits cell number by regulating proliferation and apoptosis (47,48). The primary components of the Hippo pathway induce tumorigenesis by inducing repair and regeneration of tumor stem cells and proliferation (47–50). Previous studies have found that deregulation of the Hippo pathway is associated with tumor progression, which occurs extensively in gynecological malignancies, including endometrial, ovarian and cervical cancer (47–50). Yes1-associated transcriptional regulator (YAP1) is a key downstream oncogene of the Hippo signaling pathway, which plays a role in promoting tumorigenesis in human OC by promoting cell proliferation and apoptosis resistance (47,51). Ye et al (52) demonstrated that YAP expression serves an important role in regulating proliferation and differentiation of ovarian germline stem cells and ovarian function. The transcription of the Hippo pathway is actuated by the neuron-restrictive silencer factor to promote proliferation of OC cells, which increases dephosphorylation of YAP, macrophage stimulating 1 and large tumor suppressor kinase 1 (48). Xu et al (53) showed that miRNA-149-5p directly targets the core kinase components of the Hippo signaling pathway and promotes chemotherapeutic resistance in OC. Similarly to the oxytocin signaling pathway, three dysregulated miRNAs validated in the present study, hsa-miR-200c-3p, hsa-miR-1909-5p and hsa-miR-885-5p, serve a role in the hippo signaling pathway. Altered expression of these miRNAs may cause OC due to the hippo signaling pathway. These findings supports the significance of our validated miRNAs on OC in terms of diagnosis, treatment or therapy response.
The β-adrenergic signaling pathway mediates sympathetic nervous system-induced fight-or-flight stress responses that contribute to the initiation and progression of cancer (54). There are several β-adrenergic mediating mechanisms such as increased expression of pro-inflammatory cytokines, like IL-6 and IL-8, by tumor cells (55,56), matrix metalloproteinase-associated increases in tissue invasion (57,58), VEGF-mediated increases in angiogenesis (59) and focal adhesion kinase-mediated resistance to anoikis/apoptosis (60). Zhang et al (35) analyzed differential gene expression between EOC and healthy samples by NGS and obtained 117 dysregulated genes that are related to important pathways, including ‘adrenergic signaling in cardiomyocytes’. Another study also showed that β2-adrenergic signaling is the underlying biological pathway through which stress influences OC development (61). A study also found a feed-forward loop that is the underlying mechanism by which sustained adrenergic signaling increases tumor innervation, resulting in increased norepinephrine accumulation and enhanced tumor growth (62). In adrenergic signaling pathway, as in oxytocin and hippo signaling pathways, three dysregulated miRNAs validated in the present study, hsa-miR-200c-3p, hsa-miR-1909-5p and hsa-miR-885-5p, were found to have a role via their target genes. Depending on the effect of adrenergic signaling pathway by miRNA's target genes, these validated miRNAs may serve a crucial role in OC development. Because β-adrenergic signaling pathway promotes tumor growth and accelerate metastasis in OC.
Thyroid hormones serve major roles in cell proliferation, development and metabolism (63). Thyroid hormone receptors control tumor cell proliferation and cancer cell defense pathways under physiological conditions (64,65). Previous studies have reported that thyroid hormones not only serve a key role in the promotion of cancer cell proliferation, but also inhibit apoptosis (65). In epidemiological analyses, risk of OC development was shown to be almost double in ovarian cancer patients with a history of hyperthyroidism compared to hypothyroidism OC patients (64). Hyperthyroidism is caused by thyroid hormones and triiodothyronine hormone (T3) is one of these hormones. T3 is shown having direct inflammatory effects on ovarian surface epithelial (OSE) cells function in vitro. OSE cell responses to T3 include an increased expression of estrogen receptor alpha (ERα) mRNA, which encodes the ER isoform most strongly associated with OC (66). Thyroid hormone binding to αvβ3 integrin promotes cell proliferation in OC and natural thyroid hormone derivatives may antagonize these actions (67). A study focused on the potential association between miRNAs associated with oxidative stress and dysregulation of thyroid hormone in cancer progression (63). They suggested that thyroid hormone regulates the expression of different miRNAs either directly or indirectly to effect oxidative stress. In the present study, hsa-miR-200c-3p and hsa-miR-885-5p interacted with thyroid hormone signaling pathways via target genes. Therefore, it may be suggested that our validated miRNAs have an increasing value for understanding the OC pathogenesis.
To the best of our knowledge, hsa-let-7d-3p has previously not been detected as associated with enriched pathways. According to the DIANA-microT-CDS algorithm, target genes of hsa-let-7d-3p were SIGLEC6, AKAP6, ZIC1 and ASXL2 but none of these genes were found in enriched pathways with other miRNAs. It was hypothesized that this miRNA alone affects mechanisms that play a role in cancer development. When RT-qPCR results for hsa-let-7d-3p were analyzed, expression of this miRNA differed in each patient in terms of disease stage. In 10 serum samples from patients, hsa-let-7d-3p was downregulated in 9 patients who were staged at IIA, IIB, IIIB and IIIC. Only in the sample from a stage IA patient was hsa-let-7d-3p upregulated (Table III). These findings indicated that this miRNA was downregulated in advanced stages of the disease. However, studies have suggested that it plays a role in the formation of several cancer types, including OC (68–73). According to the function of its target genes, hsa-let-7d-3p is involved in cell differentiation, cell cycle, tumor, cell proliferation, apoptotic resistance, drug resistance and metastasis (68,74–84).
The present miRNAs identified as biomarkers are candidates for OC prediction. The aim of the present study was to find novel miRNA candidates and simultaneously study tumor tissue and serum of the same patients. This was to question whether changes in miRNA expression profiles in the serum and in the tissue can be identified. miRNA expression that changed significantly in serum did not change significantly in tissue. According to the results obtained by liquid biopsy, serum biomarker studies did not fully reflect tissue changes because different miRNAs were found in liquid biopsy samples compared to tissue samples of same patient. This makes it important to identify biomarkers specific to liquid biopsy for the diagnosis, treatment and monitoring of the disease. Novel serum miRNAs (hsa-miR-1909-5p, hsa-miR-885-5p and hsa-let-7d-3p) were identified and validated, which showed that they may contribute to the emergence of EOC. In addition, among tissue miRNAs, hsa-miR-200c-3p was a promising candidate for a prognostic and diagnostic biomarker for EOC. Hence, these identified miRNAs may serve as both prognostic and diagnostic biomarkers in EOC. Depending on enriched pathways, the validated miRNAs were demonstrated to serve crucial roles in OC pathogenesis. Therefore, they need to be further studied as diagnostic, prognostic and therapeutic serum biomarkers of OC. Although the results demonstrated OC pathogenesis, the small sample size is a limitation for biomarker research. Thus, it is suggested that further studies are conducted with a large-scale analysis. Additionally, the present study only included validation of four serum and three tissue miRNAs; other differently expressed serum miRNAs determined by microarray need to be quantitatively validated and assessed for diagnostic and prognostic implications in EOC.
Supplementary Material
Acknowledgements
The authors would like to thank Ms Kathleen M Sullivan (Translating and Interpreting Department, Istanbul Yeni Yuzyil University, Istanbul, Turkey) for language editing.
Glossary
Abbreviations
- EOC
epithelial ovarian cancer
- HI
healthy individual
- SOC
simple ovarian cyst
- ROC
receiver operating characteristic
- EMT
epithelial-mesenchymal transition
- miRNA
microRNA
- KEGG
Kyoto Encyclopedia of Genes and Genomes
Funding Statement
The present study was supported by the Istanbul University Scientific Research Projects Department (grant nos. 27339 and 47803). Genera Era Diagnostic covered the publishing fee for this article.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus repository, (ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216150) with accession number GSE216150. All data generated or analyzed during this study are included in this published article.
Authors' contributions
TG, EGA, MKH, ST and KA designed the study. KA and ST collected samples. EGA and MKH performed and analyzed the microarray. BD and EET performed RT-qPCR. NC analyzed RT-qPCR data. BG and US performed target gene and pathway enrichment analysis. TG, EGA, MKH, BD, EET and NC wrote the manuscript. All authors have read and approved the final manuscript. TG, EGA, MKH, BD, BG and US confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Whole sample collection and analysis was approved by Istanbul University Faculty of Medicine Clinical Researches Ethics Committee (Istanbul, Turkey; approval no. 2014/1175) on August 8, 2014. All patients and volunteers provided written informed consent. For patients aged <18 years, informed consent was signed by their parent/guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stewart C, Ralyea C, Lockwood S. Ovarian cancer: An integrated review. Semin Oncol Nurs. 2019;35:151–156. doi: 10.1016/j.soncn.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Retamales-Ortega R, Oróstica L, Vera C, Cuevas P, Hernández A, Hurtado I, Vega M, Romero C. Role of nerve growth factor (NGF) and miRNAs in epithelial ovarian cancer. Int J Mol Sci. 2017;18:507. doi: 10.3390/ijms18030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Li H, Xu Y, Lv X. Identification of candidate biomarkers for epithelial ovarian cancer metastasis using microarray data. Oncol Lett. 2017;14:3967–3974. doi: 10.3892/ol.2017.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumusoglu E, Gunel T. The role of circulating biomarkers in the early diagnosis of ovarian cancer. Ovarian Cancer. 2018 [Google Scholar]
- 5.Lan H, Lu H, Wang X, Jin H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. Biomed Res Int. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Medical Association, corp-author. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 7.Barber HR, Sommers SC, Synder R, Kwon TH. Histologic and nuclear grading and stromal reactions as indices for prognosis in ovarian cancer. Am J Obstet Gynecol. 1975;121:795–807. [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71:353–360. doi: 10.4097/kja.d.18.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460–W466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40((Database issue)):D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, Zhang X. Circulating MicroRNAs in cancer: Potential and challenge. Front Genet. 2019;10:626. doi: 10.3389/fgene.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan WN, Zhang YQ, Wang XM, Liu YJ, Zhang YX, Que YH, Zhao WJ, Li P. Down-regulated miR-22 as predictive biomarkers for prognosis of epithelial ovarian cancer. Diagn Pathol. 2014;9:178. doi: 10.1186/s13000-014-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.KEGG, corp-author. Kyoto Encyclopedia of Genes and Genomes. 2021 [Google Scholar]
- 15.Kim YC, Won SY, Jeong BH. Identification of prion disease-related somatic mutations in the prion protein gene (PRNP) in cancer patients. Cells. 2020;9:1480. doi: 10.3390/cells9061480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishimaru D, Andrade LR, Teixeira LS, Quesado PA, Maiolino LM, Lopez PM, Cordeiro Y, Costa LT, Heckl WM, Weissmüller G, et al. Fibrillar aggregates of the tumor suppressor p53 core domain. Biochemistry. 2003;42:9022–9027. doi: 10.1021/bi034218k. [DOI] [PubMed] [Google Scholar]
- 17.Silva JL, Vieira TC, Gomes MP, Bom AP, Lima LM, Freitas MS, Ishimaru D, Cordeiro Y, Foguel D. Ligand binding and hydration in protein misfolding: Insights from studies of prion and p53 tumor suppressor proteins. Acc Chem Res. 2010;43:271–279. doi: 10.1021/ar900179t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva JL, De Moura Gallo CV, Costa DC, Rangel LP. Prion-like aggregation of mutant p53 in cancer. Trends Biochem Sci. 2014;39:260–267. doi: 10.1016/j.tibs.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Ano Bom AP, Rangel LP, Costa DC, de Oliveira GA, Sanches D, Braga CA, Gava LM, Ramos CH, Cepeda AO, Stumbo AC, et al. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: Implications for cancer. J Biol Chem. 2012;287:28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcken R, Wang G, Boeckler FM, Fersht AR. Kinetic mechanism of p53 oncogenic mutant aggregation and its inhibition. Proc Natl Acad Sci USA. 2012;109:13584–13589. doi: 10.1073/pnas.1211550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilming Elgaaen B, Olstad OK, Haug KB, Brusletto B, Sandvik L, Staff AC, Gautvik KM, Davidson B. Global miRNA expression analysis of serous and clear cell ovarian carcinomas identifies differentially expressed miRNAs including miR-200c-3p as a prognostic marker. BMC Cancer. 2014;14:80. doi: 10.1186/1471-2407-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panda H, Pelakh L, Chuang TD, Luo X, Bukulmez O, Chegini N. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod Sci. 2012;19:786–796. doi: 10.1177/1933719112438448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokol E, Kedzierska H, Czubaty A, Rybicka B, Rodzik K, Tański Z, Bogusławska J, Piekiełko-Witkowska A. microRNA-mediated regulation of splicing factors SRSF1, SRSF2 and hnRNP A1 in context of their alternatively spliced 3′UTRs. Exp Cell Res. 2018;363:208–217. doi: 10.1016/j.yexcr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Fischer DC, Noack K, Runnebaum IB, Watermann DO, Kieback DG, Stamm S, Stickeler E. Expression of splicing factors in human ovarian cancer. Oncol Rep. 2004;11:1085–1090. [PubMed] [Google Scholar]
- 25.Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: A marker of aggressiveness and chemoresistance in female reproductive cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theocharis AD, Karamanos NK. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019:75–76. 220–259. doi: 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: Regulation of inflammation and autophagy in cancer biology. FEBS J. 2017;284:10–26. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 29.Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, Slade RJ, Martin-Hirsch P, Gallagher JT, Jayson GC. Distribution and clinical significance of heparan sulfate proteoglycans in ovariasn cancer. Clin Cancer Res. 2004;10:5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 30.Nikitovic D, Berdiaki A, Spyridaki I, Krasanakis T, Tsatsakis A, Tzanakakis GN. Proteoglycans-Biomarkers and targets in cancer therapy. Front Endocrinol (Lausanne) 2018;9:69. doi: 10.3389/fendo.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilieva KM, Cheung A, Mele S, Chiaruttini G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE, et al. Chondroitin sulfate proteoglycan 4 and its potential as an antibody immunotherapy target across different tumor types. Front Immunol. 2017;8:1911. doi: 10.3389/fimmu.2017.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneda A, Lendorf ME, Couchman JR, Multhaupt HA. Breast and ovarian cancers: A survey and possible roles for the cell surface heparan sulfate proteoglycans. J Histochem Cytochem. 2012;60:9–21. doi: 10.1369/0022155411428469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turck D, Comité de nutrition de la Société française de pédiatrie Breast feeding: Health benefits for child and mother. Arch Pediatr. 2005;12((Suppl 3)):S145–S165. doi: 10.1016/j.arcped.2005.10.006. (In French) [DOI] [PubMed] [Google Scholar]
- 34.Ji H, Liu N, Yin Y, Wang X, Chen X, Li J, Li J. Oxytocin inhibits ovarian cancer metastasis by repressing the expression of MMP-2 and VEGF. J Cancer. 2018;9:1379–1384. doi: 10.7150/jca.23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Luo M, Yang H, Zhu S, Cheng X, Qing C. Next-generation sequencing-based genomic profiling analysis reveals novel mutations for clinical diagnosis in Chinese primary epithelial ovarian cancer patients. J Ovarian Res. 2019;12:19. doi: 10.1186/s13048-019-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004;109:525–532. doi: 10.1002/ijc.20017. [DOI] [PubMed] [Google Scholar]
- 37.Cuneo MG, Szeto A, Schrepf A, Kinner EM, Schachner BI, Ahmed R, Thaker PH, Goodheart M, Bender D, Cole SW, et al. Oxytocin in the tumor microenvironment is associated with lower inflammation and longer survival in advanced epithelial ovarian cancer patients. Psychoneuroendocrinology. 2019;106:244–251. doi: 10.1016/j.psyneuen.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groeneweg JW, Foster R, Growdon WB, Verheijen RH, Rueda BR. Notch signaling in serous ovarian cancer. J Ovarian Res. 2014;7:95. doi: 10.1186/s13048-014-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marone M, Scambia G, Giannitelli C, Ferrandina G, Masciullo V, Bellacosa A, Benedetti-Panici P, Mancuso S. Analysis of cyclin E and CDK2 in ovarian cancer: Gene amplification and RNA overexpression. Int J Cancer. 1998;75:34–39. doi: 10.1002/(SICI)1097-0215(19980105)75:1<34::AID-IJC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Gakiopoulou H, Korkolopoulou P, Levidou G, Thymara I, Saetta A, Piperi C, Givalos N, Vassilopoulos I, Ventouri K, Tsenga A, et al. Minichromosome maintenance proteins 2 and 5 in non-benign epithelial ovarian tumours: Relationship with cell cycle regulators and prognostic implications. Br J Cancer. 2007;97:1124–1134. doi: 10.1038/sj.bjc.6603992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussein NA, Kholy ZA, Anwar MM, Ahmad MA, Ahmad SM. Plasma miR-22-3p, miR-642b-3p and miR-885-5p as diagnostic biomarkers for pancreatic cancer. J Cancer Res Clin Oncol. 2017;143:83–93. doi: 10.1007/s00432-016-2248-7. [DOI] [PubMed] [Google Scholar]
- 42.Afanasyeva EA, Mestdagh P, Kumps C, Vandesompele J, Ehemann V, Theissen J, Fischer M, Zapatka M, Brors B, Savelyeva L, et al. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011;18:974–984. doi: 10.1038/cdd.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet. 2012;286:1537–1543. doi: 10.1007/s00404-012-2456-6. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, He J, Dong J, Meyer TF, Xu T. The HIPPO pathway in gynecological malignancies. Am J Cancer Res. 2020;10:610–629. [PMC free article] [PubMed] [Google Scholar]
- 45.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz-Galvan S, Felipe-Abrio B, Verdugo-Sivianes EM, Perez M, Jiménez-García MP, Suarez-Martinez E, Estevez-Garcia P, Carnero A. Downregulation of MYPT1 increases tumor resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness. Mol Cancer. 2020;19:7. doi: 10.1186/s12943-020-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin X, Feng D, Li P, Lv Y. LncRNA LINC00857 regulates the progression and glycolysis in ovarian cancer by modulating the Hippo signaling pathway. Cancer Med. 2020;9:8122–8132. doi: 10.1002/cam4.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng P, Zuo Y, Feng S, Li Z, Chen W, Li H, Wang X. Knockdown of NRSF inhibits cell proliferation of ovarian cancer via activating Hippo pathway. Life Sci. 2018;215:73–79. doi: 10.1016/j.lfs.2018.10.070. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, AOCS Study group. Bowtell DD, Harvey KF. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 50.He C, Lv X, Hua G, Lele SM, Remmenga S, Dong J, Davis JS, Wang C. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene. 2015;34:6040–6054. doi: 10.1038/onc.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, Hilsenbeck SG, Orsulic S, Goode S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye H, Li X, Zheng T, Hu C, Pan Z, Huang J, Li J, Li W, Zheng Y. The Hippo signaling pathway regulates ovarian function via the proliferation of ovarian germline stem cells. Cell Physiol Biochem. 2017;41:1051–1062. doi: 10.1159/000464113. [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Xiao J, Chen M, Yuan L, Li J, Shen H, Yao S. miR1495p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway. Int J Oncol. 2018;52:815–827. doi: 10.3892/ijo.2018.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, Jennings N, Arevalo J, Lutgendorf SK, Gallick GE, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 56.Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 59.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 60.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, Allen JK, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang T, Tworoger SS, Hecht JL, Rice MS, Sood AK, Kubzansky LD, Poole EM. Association of ovarian tumor β2-adrenergic receptor status with ovarian cancer risk factors and survival. Cancer Epidemiol Biomarkers Prev. 2016;25:1587–1594. doi: 10.1158/1055-9965.EPI-16-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen JK, Armaiz-Pena GN, Nagaraja AS, Sadaoui NC, Ortiz T, Dood R, Ozcan M, Herder DM, Haemmerle M, Gharpure KM, et al. Sustained adrenergic signaling promotes Intratumoral innervation through BDNF Induction. Cancer Res. 2018;78:3233–3242. doi: 10.1158/0008-5472.CAN-16-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang PS, Wang CS, Yeh CT, Lin KH. Roles of thyroid hormone-associated microRNAs affecting oxidative stress in human hepatocellular carcinoma. Int J Mol Sci. 2019;20:5220. doi: 10.3390/ijms20205220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YC, Yeh CT, Lin KH. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int J Mol Sci. 2019;20:4986. doi: 10.3390/ijms20204986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis PJ, Glinsky GV, Lin HY, Leith JT, Hercbergs A, Tang HY, Ashur-Fabian O, Incerpi S, Mousa SA. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne) 2014;5:240. doi: 10.3389/fendo.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rae MT, Gubbay O, Kostogiannou A, Price D, Critchley HO, Hillier SG. Thyroid hormone signaling in human ovarian surface epithelial cells. J Clin Endocrinol Metab. 2007;92:322–327. doi: 10.1210/jc.2006-1522. [DOI] [PubMed] [Google Scholar]
- 67.Shinderman-Maman E, Cohen K, Moskovich D, Hercbergs A, Werner H, Davis PJ, Ellis M, Ashur-Fabian O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci Rep. 2017;7:16475. doi: 10.1038/s41598-017-16593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang CJ, Hsu CC, Chang CH, Tsai LL, Chang YC, Lu SW, Yu CH, Huang HS, Wang JJ, Tsai CH, et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011;26:1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 69.Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103:1252–1260. doi: 10.1016/j.fertnstert.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F, Guo Z, Zhang X, Liu J, Shen Q, et al. Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 2014;13:206. doi: 10.1186/1476-4598-13-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyerinas B, Park SM, Murmann AE, Gwin K, Montag AG, Zillhardt M, Hua YJ, Lengyel E, Peter ME. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int J Cancer. 2012;130:1787–1797. doi: 10.1002/ijc.26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakurai M, Miki Y, Masuda M, Hata S, Shibahara Y, Hirakawa H, Suzuki T, Sasano H. LIN28: A regulator of tumor-suppressing activity of let-7 microRNA in human breast cancer. J Steroid Biochem Mol Biol. 2012;131:101–106. doi: 10.1016/j.jsbmb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Ramberg H, Alshbib A, Berge V, Svindland A, Tasken KA. Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer. 2011;10:50. doi: 10.1186/1476-4598-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ning YX, Luo X, Xu M, Feng X, Wang J. Let-7d increases ovarian cancer cell sensitivity to a genistein analog by targeting c-Myc. Oncotarget. 2017;8:74836–74845. doi: 10.18632/oncotarget.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun H, Ding C, Zhang H, Gao J. Let7 miRNAs sensitize breast cancer stem cells to radiation-induced repression through inhibition of the cyclin D1/Akt1/Wnt1 signaling pathway. Mol Med Rep. 2016;14:3285–3292. doi: 10.3892/mmr.2016.5656. [DOI] [PubMed] [Google Scholar]
- 76.Prahm KP, Novotny GW, Hogdall C, Hogdall E. Current status on microRNAs as biomarkers for ovarian cancer. APMIS. 2016;124:337–355. doi: 10.1111/apm.12514. [DOI] [PubMed] [Google Scholar]
- 77.Huang YC, Hung WC, Chen WT, Jiang WH, Yu HS, Chai CY. Effects of MEK and DNMT inhibitors on arsenic-treated human uroepithelial cells in relation to Cyclin-D1 and p16. Toxicol Lett. 2011;200:59–66. doi: 10.1016/j.toxlet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Choi JH, Choi KC, Auersperg N, Leung PC. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocr Metab. 2004;89:5508–5516. doi: 10.1210/jc.2004-0044. [DOI] [PubMed] [Google Scholar]
- 79.Choo KB, Soon YL, Nguyen PN, Hiew MS, Huang CJ. MicroRNA-5p and −3p co-expression and cross-targeting in colon cancer cells. J Biomed Sci. 2014;21:95. doi: 10.1186/s12929-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Liu X, Datta A, Govindarajan K, Tam WL, Han J, George J, Wong C, Ramnarayanan K, Phua TY, et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J Clin Invest. 2009;119:2171–2183. doi: 10.1172/JCI37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan Z, Wang F, Zhao Z, Zhao X, Qiu J, Nie C, Wei Y. BIM-mediated AKT phosphorylation is a key modulator of arsenic trioxide-induced apoptosis in cisplatin-sensitive and -resistant ovarian cancer cells. PLoS One. 2011;6:e20586. doi: 10.1371/journal.pone.0020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K, Hoppe-Seyler F. Dependence of Intracellular and Exosomal microRNAs on Viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11:e1004712. doi: 10.1371/journal.ppat.1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krug LM, Miller VA, Filippa DA, Venkatraman E, Ng KK, Kris MG. Bcl-2 and bax expression in advanced non-small cell lung cancer: Lack of correlation with chemotherapy response or survival in patients treated with docetaxel plus vinorelbine. Lung Cancer. 2003;39:139–143. doi: 10.1016/S0169-5002(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Jia Q, Zhang Q, Wan Y. Rab25 upregulation correlates with the proliferation, migration, and invasion of renal cell carcinoma. Biochem Biophys Res Commun. 2015;458:745–750. doi: 10.1016/j.bbrc.2015.01.144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus repository, (ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216150) with accession number GSE216150. All data generated or analyzed during this study are included in this published article.