Abstract
CDK9 paired with cyclin T1 forms the human P-TEFb complex and stimulates productive transcription through phosphorylation of the RNA polymerase II C-terminal domain. Here we report that CDK9 is ubiquitinated and degraded by the proteasome whereas cyclin T1 is stable. SCFSKP2 was recruited to CDK9/cyclin T1 via cyclin T1 in an interaction requiring its PEST domain. CDK9 ubiquitination was modulated by cyclin T1 and p45SKP2. CDK9 accumulated in p45SKP2−/− cells, and its expression during the cell cycle was periodic. The transcriptional activity of CDK9/cyclin T1 on the class II major histocompatibility complex promoter could be regulated by CDK9 degradation in vivo. We propose a novel mechanism whereby recruitment of SCFSKP2 is mediated by cyclin T1 while ubiquitination occurs exclusively on CDK9.
Transcriptional elongation is regulated by both positive and negative transcription elongation factors and is recognized as an important target for transcriptional regulation (37). The human positive transcription elongation factor b (P-TEFb) is composed of a 43-kDa catalytic subunit, CDK9 (previously known as PITALRE) (13), and an 87-kDa regulatory subunit, cyclin T1 (33, 46). Cyclin T1 is the predominant cyclin associated with CDK9 in HeLa nuclear extracts, although CDK9 is also present in complexes with cyclins T2 and K (9, 33). Cyclin T1 is most closely related to the C-type cyclins, which, paired with their associated CDKs, function in transcriptional regulation by phosphorylating the carboxy-terminal domain (CTD) of RNA polymerase II (RNAPII) (6).
P-TEFb was originally identified by its ability to stimulate RNAPII transcriptional elongation in vitro (29, 30). The CTD of RNAPII present in preinitiation complexes and early elongation complexes is hypophosphorylated but becomes hyperphosphorylated during productive elongation (25). P-TEFb is proposed to facilitate the transition from abortive to productive elongation by hyperphosphorylating the RNAPII CTD. Removal of the CTD in early elongation complexes abolished P-TEFb function, suggesting that the CTD is the target of P-TEFb function (28). CDK9 has been shown to phosphorylate the RNAPII CTD in vitro and is sensitive to 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole (DRB), which is a known inhibitor of transcriptional elongation (28, 49).
Ubiquitin-dependent proteolysis plays an essential role in a number of cellular processes, including cell cycle progression, transcription, and signal transduction (reviewed in reference 5). Proteins destined for degradation by the proteasome are recognized and ubiquitinated in a process that requires a conserved cascade of enzymatic reactions (reviewed in reference 21). The ubiquitin-activating enzyme E1 and an E2 ubiquitin-conjugating enzyme function with E3 ubiquitin-protein ligases to covalently attach ubiquitin to lysine residues in substrate proteins. A polyubiquitin chain is synthesized by transfer of additional ubiquitin molecules to the assembling ubiquitin chain. Polyubiquinated substrates are targeted by the 26S proteasome for degradation.
The SCF E3 ubiquitin ligase system mediates the ubiquitination of many cellular proteins. SCF is named for three of its core components, p19SKP1, CDC53/cullin, and an F-box containing protein. p19SKP1 and F-box proteins interact through the F-box motif (1), while CDC53 bridges this complex to an E2 enzyme, CDC34 (47). An additional component, Rbx1/Roc1, enhances the recruitment of CDC34 (38). Substrates targeted for ubiquitination are recognized by different E3 ligases via specific motifs. One such motif is the PEST (rich in proline, glutamate, serine, and threonine) sequence (35), which is found in many proteins whose abundance is regulated by proteolysis, including cyclin D1, IκBα, fos, jun, myc, and p53 (reviewed in reference 34). F-box proteins are responsible for substrate recognition by different SCF E3 ligases.
Here, we report that CDK9 is a novel target for SCFSKP2-dependent ubiquitination and degradation by the proteasome. CDK9 ubiquitination represents a unique example in which the SCF complex is recruited by the regulatory subunit, cyclin T1, while ubiquitination proceeds on its partner protein, CDK9. Our results have important implications for the regulation of P-TEFb activity in vivo.
MATERIALS AND METHODS
Chemicals, reagents, and plasmid constructions.
N-acetyl-l-leucyl-l-leucyl-l-norleucinol (LLnL) (Sigma; 20 mg of stock/ml stored in dimethyl sulfoxide [DMSO]) was used at 250 μM. Lactacystin (Calbiochem; 10 mM stock stored in DMSO) was used at 10 μM. MG132 (Calbiochem; 50 mM stock stored in ethanol) was used at 50 μM. E64 (Calbiochem; 50 mM stock stored in sterile water) was used at 50 μM. Cycloheximide (Sigma; 10 mg of stock/ml) was used at 30 μg/ml. The following antibodies were used: anti-HA (12CA5; Boehringer Mannheim); anti-Flag (M2; Sigma); anti-CDK9 and anti-cyclin T1 (33); anti-p19SKP1 and anti-CDC34 (obtained from M. Dorée); anti-p45SKP2 (26); anti-h-βTrCP (27); anti-CDK2, anti-CDK4, anti-CDK5, and anti-CDK7 (Santa Cruz); anti-ubiquitin and anti-tubulin (Sigma); and anti-CIITA (obtained from P. Louis-Plence). The following cDNAs have been previously described: HA-CDK9 (13); Flag-CDK9 (12); hemagglutinin (HA)-cyclin T1ΔPEST (46); Flag-cyclin T1 1-726 (17); HA-cyclin T2A (33); HA-Ub and His6-Ub (43); HA-CDC34, MT-p45SKP2, and MT-p45SKP2AxA (26); HA-cul-1 (14); and DMB-luc (42). To generate an N-terminal epitope-tagged cyclin T1 construct, we used PCR amplification with a forward primer containing the sequence for the HA peptide. The following primers were used to amplify cyclin T1: forward, 5′-CCTCTAGATG TACCCATACGACG TCCCAGAC TACGC TGAGGGAGAGAGGAAGAACAAC-3′; reverse, 5′-CCGGATCCTTACTTAGGAAGGGG TGGAAG-3′ (restriction sites are underlined, and the sequence coding for the HA epitope is in italics). The PCR product was digested with XbaI and BamHI and inserted into the NheI/BamHI sites of the pcDNA 3.1(+) vector (Invitrogen). To construct Flag-cyclin T1ΔNΔP, a PstI/BamHI fragment from HA-cyclin T1ΔPEST was cloned into PstI/BamHI-restricted Flag-cyclin T1 (positions 203 to 726) (17).
Cell culture, transfection, and immunochemistry.
Primary mouse embryonic fibroblasts (MEFs) were prepared and infected with recombinant adenovirus as described previously (32). The MEFs were lysed in TNT buffer (300 mM NaCl, 50 mM Tris [pH 7.5], and 0.5% Triton X-100). Samples were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE), and the proteins were transferred to polyvinylidene difluoride membranes by semidry electroblotting (Millipore, Bedford, Mass.). The membranes were incubated with the primary antibody for 1 h, washed, and incubated with the appropriate secondary antibody (Amersham) for 1 h. The proteins were visualized by chemiluminescence (Amersham) according to the manufacturer's protocol. HeLa and 293 cells were propagated in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and transiently transfected using calcium phosphate. For cell cycle analysis, HeLa cells were incubated in medium containing nocodazole (0.05 μg/ml) for 16 h. Mitotic cells harvested by shake-off were placed in fresh medium without the drug. Samples were harvested at various time points, washed in phosphate-buffered saline (PBS), and resuspended in cell lysis buffer (250 mM NaCl, 50 mM Tris [pH 7.4], 1 mM EDTA, 0.1% Nonidet P-40, 2 mM dithiothreitol [DTT], and complete protease inhibitors [Boehringer Mannheim]). Samples were analyzed by SDS-PAGE and immunoblotting as described above. For pulse-chase analysis, HeLa cells were incubated for 30 min in methionine- and cysteine-free RPMI 1640, pulse-labeled for 30 min with 1 mCi of [35S]methionine/cysteine (35S-Trans-label; ICN Biochemical)/ml, washed twice in cold PBS, and resuspended in complete medium. At the indicated time points, the cells were washed twice in PBS and lysed in TNT buffer. The lysates were precleared, and protein was immunoprecipitated with the appropriate antibody, followed by five washes in lysis buffer. Samples were resolved by SDS–10% PAGE and visualized by autoradiography. Cycloheximide T1/2 experiments were performed as follows. Transfected HeLa cultures were treated with cycloheximide (30 μg/ml) for various times. Some cultures were pretreated for 1 h with proteasome or lysosome inhibitors. The cells were washed twice in PBS and resuspended in cell lysis buffer. Samples were analyzed by SDS-PAGE and immunoblotting as described above. Coimmunoprecipitation analysis was performed as follows. HeLa or 293 cells transfected with the plasmids were treated for 2 h with 250 μM LLnL prior to being harvested. The cells were washed twice in PBS and resuspended in 1 ml of lysis buffer. The proteins were immunoprecipitated with the indicated antibodies, followed by SDS-PAGE and immunoblotting. Ubiquitinated conjugates were analyzed as follows. 293 cells transfected with various plasmids were treated for 2 h with 250 μM LLnL prior to being harvested. The cells were washed twice in PBS and lysed as described previously (31). Briefly, cell pellets were resuspended in 100 μl of denaturing lysis buffer (50 mM Tris [pH 7.5], 0.5 mM EDTA, 1% SDS, and 1 mM DTT) and boiled for 10 min before the addition of 1 ml of TNN buffer (50 mM Tris [pH 7.5], 250 mM NaCl, 5 mM EDTA [pH 8], 0.5% Nonidet P-40, 1 mM DTT, and complete protease inhibitors [Boehringer Mannheim]). The proteins were immunoprecipitated with the appropriate antibodies, followed by SDS-PAGE and immunoblotting. For transactivation experiments, transfected HeLa or 293 cells were lysed and assayed for luciferase activity 48 h posttransfection. DMB luciferase activity was normalized to pRL-TK (Promega), which encodes the Renilla luciferase from the TK promoter, as an internal control.
Fusion protein affinity chromatography.
CDK9 and cyclin T1 were expressed as glutathione S-transferase (GST) fusion proteins in BL21 (Pharmacia), and GST fusion protein purification was performed as described previously (2).
In vitro binding studies.
HA-CDK9, HA-cyclin T1, HA-cyclin T1ΔPEST, and myc-p45SKP2 were translated in vitro in a coupled transcription-translation rabbit reticulocyte lysate system (Promega) in the presence of [35S]methionine according to the manufacturer's protocol. For immunoprecipitation analysis, translated proteins in 0.5 ml of TNN buffer were incubated for 2 h at 4°C with the appropriate antibody prebound to protein A beads. The beads were then washed five times in TNN buffer and resuspended in loading buffer. The proteins were resolved by SDS–10% PAGE and visualized by autoradiography.
RESULTS
CDK9, but not cyclin T1, is degraded by the proteasome.
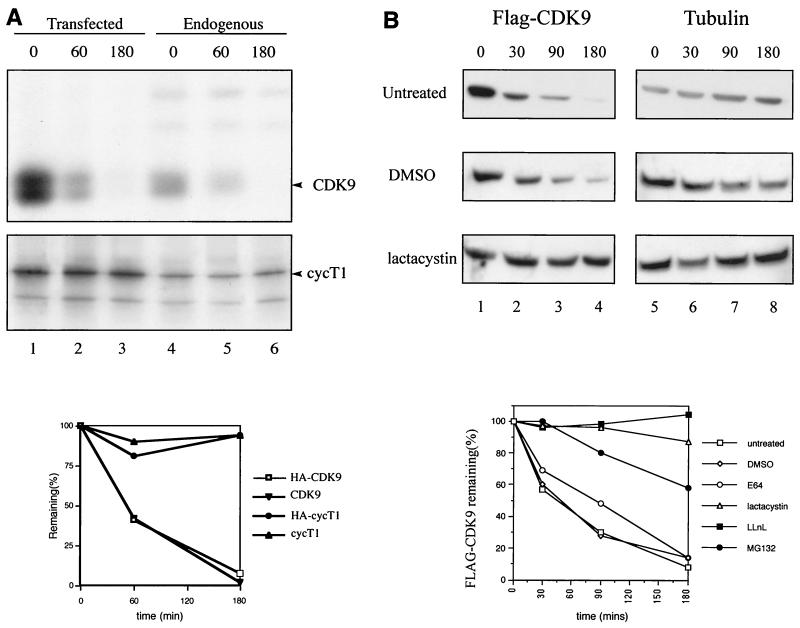
Among the targets of the ubiquitination pathway are several proteins involved in transcription, including Gcn4, c-Fos, c-Jun, and RNAPII following exposure to DNA-damaging agents (reviewed in reference 5). We examined the stability of CDK9 and cyclin T1, which form human P-TEFb. Both endogenous CDK9 and transfected HA-CDK9 were rapidly degraded (half-life [T1/2] = approximately 50 min), while endogenous or transfected cyclin T1 was stable (Fig. 1A). CDK9 degradation observed by pulse-chase analysis was confirmed using cycloheximide T1/2 experiments. We verified that CDK2, CDK4, CDK5, and CDK7 present in the same extracts were stable (data not shown). To determine whether CDK9 degradation occurred via the proteasome, the T1/2 of CDK9 was analyzed in HeLa cells treated with various proteasome inhibitors. The lysosome inhibitor E64 had no significant effect on CDK9 stability, while the proteasome inhibitors lactacystin, LLnL, and MG132 significantly stabilized Flag-CDK9 (Fig. 1B). None of the treatments affected the stability of cyclin T1 or tubulin (data not shown). These results indicate that CDK9 is degraded in vivo via the proteasomal pathway.
FIG. 1.
(A) CDK9, but not cyclin T1 (cycT1), is a short-lived protein in vivo. HeLa cells were either nontransfected (lanes 4 to 6) or transfected with 10 μg each of plasmids expressing HA-CDK9 (lanes 1 to 3, top) or HA-cyclin T1 (lanes 1 to 3, bottom). The cells were labeled with 35S-Trans-label and subjected to pulse-chase analysis. Endogenous CDK9 and endogenous cyclin T1 were immunoprecipitated using the appropriate antibodies, and transfected HA-CDK9 and HA-cyclin T1 were immunoprecipitated using anti-HA. Below is a graphical representation of the levels of CDK9 and cyclin T1. (B) Proteasome inhibitors stabilize the T1/2 of CDK9. HeLa cells were transfected with 2 μg of plasmid expressing Flag-CDK9. The stability of CDK9 was analyzed by cycloheximide T1/2 experiments and immunoblotting of lysates with either anti-Flag or anti-tubulin antibodies. The cells were pretreated for 1 h with the proteasome inhibitor lactacystin (10 μM), LLnL (250 μM), or MG132 (50 μM) or the lysozome inhibitor E64 (50 μM) or were untreated or mock treated with DMSO as indicated. The results are shown as a graphical representation of the levels of Flag-CDK9 for each treatment after normalization to tubulin levels in the same extracts. Representative immunoblots are shown. Time (in minutes) is shown above the blots.
CDK9 interacts with components of the SCF-type E3 ubiquitin ligase SCFSKP2 and the E2 enzyme CDC34 in vitro and in vivo.
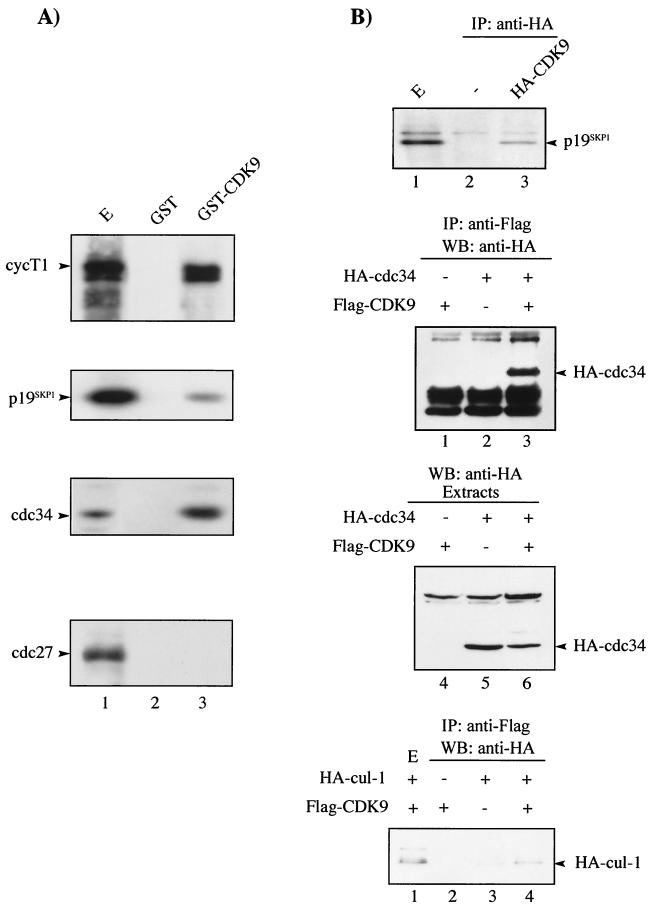
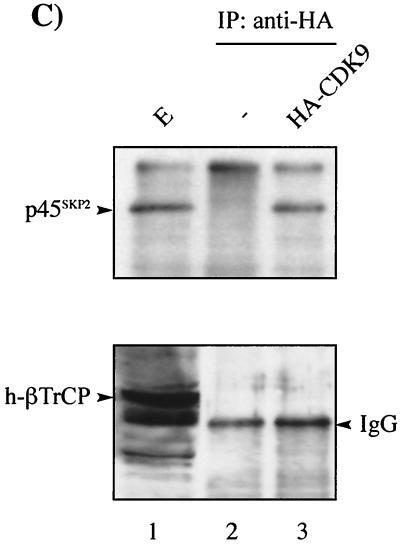
We investigated whether CDK9 interacted with components of the SCF or anaphase-promoting complex pathway by in vitro binding studies using GST-CDK9 fusion protein. GST-CDK9 interacted with p19SKP1, a component of the SCF complex, and CDC34, as well as cyclin T1, but failed to interact with CDC27, a component of the anaphase-promoting complex pathway (Fig. 2A). These results were confirmed in vivo by coimmunoprecipitation analysis. CDK9 immunoprecipitated from transfected cells was found to interact specifically with endogenous p19SKP1, CDC34, and cul-1 (Fig. 2B). While p19SKP1 and cul-1/CDC53 are core components of the SCF complex, the F-box protein is variable and is believed to determine the substrate specificity of the SCF complex. We tested whether CDK9 could interact with known human F-box proteins in vivo. HA-CDK9 interacted specifically with p45SKP2 but failed to interact with h-βTrCP (Fig. 2C). Taken together, these data demonstrate that CDK9 interacts, either directly or indirectly, with the SCF-type E3 ligase SCFSKP2.
FIG. 2.
CDK9 interacts with components of the SCF complex in vitro and in vivo. (A) GST or GST-CDK9 was equilibrated with HeLa extract and washed extensively. Extract (E) (lane 1), GST (lane 2), and GST-CDK9-bound materials (lane 3) were resolved by SDS-PAGE followed by immunoblotting using antibody to cyclin T1 (cycT1), p19SKP1, CDC34, and CDC27 as indicated. (B) CDK9 interacts with the SCF complex as shown by coimmunoprecipitation analysis. (Top) HeLa cells were mock transfected (−) (lane 2) or transfected with 2 μg of HA-CDK9 (lanes 1 and 3). Transfected CDK9 was immunoprecipitated using anti-HA antibody, followed by SDS-PAGE and immunoblotting using antibody against p19SKP1 (lanes 2 and 3). An aliquot of cell extract from HA-CDK9-transfected cells (E) was subjected to direct immunoblotting using antibody against p19SKP1 (lane 1). (Middle and bottom) 293 cells were transfected with Flag-CDK9 (2 μg) or HA-CDC34 (2 μg) (middle) or HA-cul-1 (10 μg) (bottom) either alone or in combination as indicated. Transfected CDK9 was immunoprecipitated using anti-Flag antibody, followed by SDS-PAGE and immunoblotting using anti-HA antibody. An aliquot of cell extract (E) was subjected to direct immunoblotting using anti-HA antibody. (C) CDK9 interacts with endogenous p45SKP2 in vivo. HeLa cells were transfected with HA-CDK9 (2 μg). Transfected CDK9 was immunoprecipitated using anti-HA antibody, followed by SDS-PAGE and immunoblotting using antibodies against p45SKP2 or h-βTrCP as indicated (lanes 2 and 3). An aliquot of cell extract from HA-CDK9-transfected cells (E) was subjected to direct immunoblotting using antibody against p45SKP2 or h-βTrCP as indicated (lane 1). +, present; −, absent. IgG, immunoglobulin G.
The interaction between CDK9 and SCFSKP2 occurs via cyclin T1 and requires its PEST domain.
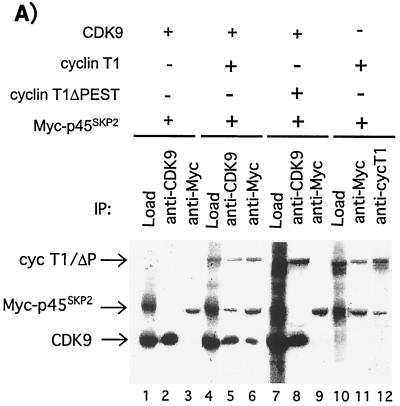
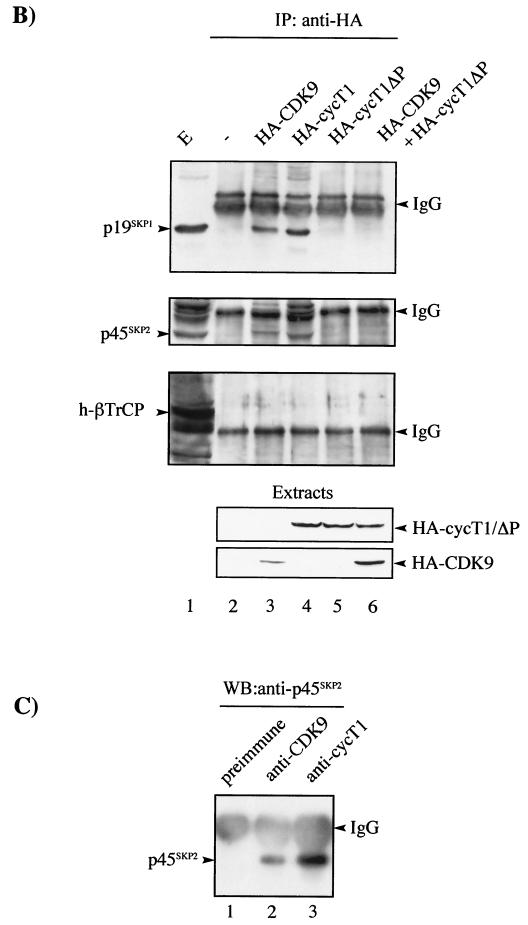
While in vitro and in vivo binding studies demonstrated that CDK9 interacted with SCFSKP2, parallel analysis revealed that cyclin T1 was also involved (data not shown). Both CDK9 and cyclin T1 are ubiquitously expressed in human tissues (13, 46). Therefore, to investigate the role of cyclin T1 in the interaction between CDK9 and SCFSKP2, in vitro-transcribed and -translated p45SKP2, CDK9, and cyclin T1 were used in coimmunoprecipitation analysis (Fig. 3A). No direct interaction was observed between CDK9 and p45SKP2 (lanes 1 to 3). However, in the presence of cyclin T1, a trimolecular complex, CDK9/cyclin T1/p45SKP2, could be immunoprecipitated (lanes 4 to 6). In contrast to CDK9, cyclin T1 interacted directly with p45SKP2 (lanes 10 to 12). Cyclin T1 contains a C-terminal PEST sequence from residues 709 to 726 (46). Since PEST sequences have been implicated as recognition motifs for F-box proteins (34), we investigated whether the cyclin T1 PEST domain was involved in the recruitment of SCFSKP2 to CDK9/cyclin T1. In contrast to full-length cyclin T1, cyclin T1ΔP did not interact significantly with p45SKP2 (Fig. 3A, compare lanes 5 to 6 with 8 to 9) but could interact with CDK9 (lane 8).
FIG. 3.
The interaction between CDK9 and SCFSKP2 occurs via cyclin T1 and requires the PEST domain. (A) CDK9, cyclin T1 (cycT1), PEST-minus cyclin T1 (cycT1ΔP), and myc-tagged p45SKP2 (myc-p45SKP2) were in vitro transcribed and translated. Reaction mixtures containing 1.25 μl of each of the indicated translated proteins were subjected to immunoprecipitation using the indicated antibodies or were analyzed directly (Load). Samples were resolved by SDS-PAGE followed by autoradiography. +, present; −, absent. (B) 293 cells were mock transfected (−) (lane 2); transfected with 2 μg of either HA-CDK9 (lane 3), HA-cyclin T1 (lane 4), or HA-cyclin T1ΔP (lane 5); or cotransfected with 2 μg each of HA-CDK9 and HA-cyclin T1ΔP (lane 6). The transfected proteins immunoprecipitated with anti-HA antibody or an aliquot of cell extract from mock-transfected cells (E) were analyzed by SDS-PAGE and by immunoblotting using antibody against p19SKP1, p45SKP2, or h-βTrCP as indicated. Cell extracts were also subjected to direct immunoblot analysis using anti-HA (Extracts). (C) Extracts from 293 cells were subjected to immunoprecipitation with antibody against CDK9 (lane 2), cycT1 (lane 3), or normal rabbit serum (preimmune) (lane 1). The immunoprecipitates were subjected to immunoblotting using antibody against p45SKP2. IgG, immunoglobulin G.
These results were confirmed in vivo by coimmunoprecipitation analysis with the endogenous SCFSKP2 complex. Lysates of cells transfected with HA-CDK9, HA-cyclin T1, or HA-cyclin T1ΔP or cotransfected with HA-CDK9 and HA-cyclin T1ΔP were immunoprecipitated with anti-HA antibodies. Western blotting of immunoprecipitates was performed using antibodies against p19SKP1, p45SKP2, and h-βTrCP (Fig. 3B). Both HA-CDK9 and HA-cycT1 interacted with p19SKP1 and p45SKP2, while no interaction was observed between HA-cycT1ΔP and p19SKP1, p45SKP2, or h-βTrCP. HA-CDK9 and HA-cycT1 failed to interact with h-βTrCP. The interaction observed between HA-CDK9 and the SCF complex is most likely mediated by endogenous cyclin T1. Indeed, overexpression of HA-cyclin T1ΔP inhibited HA-CDK9/p19SKP1 and HA-CDK9/p45SKP2 interactions (Fig. 3B, lane 6). Comparable amounts of transfected proteins were present in immunoprecipitates (Fig. 3B). Taken together, these data suggest either that the E3 ligase, SCFSKP2, binds directly to the PEST domain of cyclin T1 or that the presence of the PEST domain confers an appropriate structural conformation on cyclin T1 necessary for its interaction with SCFSKP2. In any case, these data strongly suggest that cyclin T1 recruits SCFSKP2 to CDK9/cyclin T1 in an interaction requiring its PEST domain. Finally, to verify that the interactions between CDK9/cyclin T1 and SCFSKP2 also occur with the endogenous proteins in vivo, coimmunoprecipitations were performed using 293 cell extract as a source of protein. Endogenous p45SKP2 was immunoprecipitated using either anti-CDK9 or anti-cyclin T1 antibodies but not by normal rabbit serum (Fig. 3C).
CDK9 expression is enhanced in p45SKP2−/− cells and shows periodicity during the cell cycle.
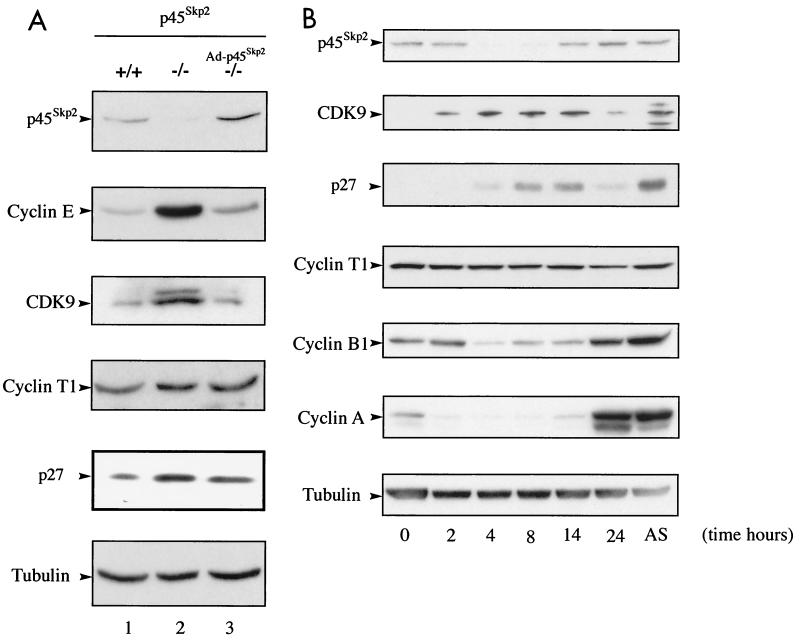
To confirm the role of p45SKP2 in CDK9 degradation, we examined its expression in embryonic fibroblasts (MEFs) from p45SKP2+/+ and p45SKP2−/− mice (32). The abundances of CDK9 and other reported SCFSKP2 substrates, cyclin E, and p27Kip1, were increased in p45SKP2−/− MEFs, whereas expression of cyclin T1 and tubulin was unchanged (Fig. 4A). Accumulation of CDK9, cyclin E, and p27Kip1 could be reversed by infection of p45SKP2−/− MEFs with a recombinant adenovirus encoding p45SKP2 (Fig. 4A).
FIG. 4.
(A) Accumulation of CDK9 in p45SKP2−/− cells. Immunoblot analysis of p45SKP2, CDK9, cyclin T1, and various cell cycle regulators in MEFs from p45SKP2+/+ and p45SKP2−/− mice and p45SKP2−/− MEFs that had been infected with a recombinant adenoviral vector encoding p45SKP2 (Ad-p45SKP2). (B) CDK9 expression shows periodicity during the cell cycle. HeLa cells were incubated in the presence of nocodazole for 16 h. Mitotic cells resumed the cell cycle after being placed in fresh medium. The cells were collected at the indicated time points. AS, asynchronous cells. Expression of p45SKP2, CDK9, cyclin T1, and other cell cycle regulators was analyzed by immunoblotting.
Since expression of p45SKP2 is regulated during the cell cycle (26, 52), we wished to determine whether CDK9 expression shows periodicity during the cell cycle. HeLa cells were blocked in mitosis by treatment with nocodazole, and mitotic cells were allowed to resume the cell cycle by being placed in fresh medium without the drug. Extracts of cells collected at regular intervals were analyzed for expression of p45SKP2, CDK9, cyclin T1, and other cell cycle regulators (Fig. 4B). CDK9 expression was regulated during the cell cycle. It peaked in G1 phase by comparison with expression of the S-phase marker, cyclin A, and the G2/M marker, cyclin B1. Its expression correlated inversely with that of p45SKP2, which was expressed during S/G2 and mitosis, and mirrored that of p27Kip1. Cyclin T1 expression was stable throughout the cell cycle. These results support a role for p45SKP2 in CDK9 degradation.
CDK9 but not cyclin T1 is ubiquitinated in vivo.
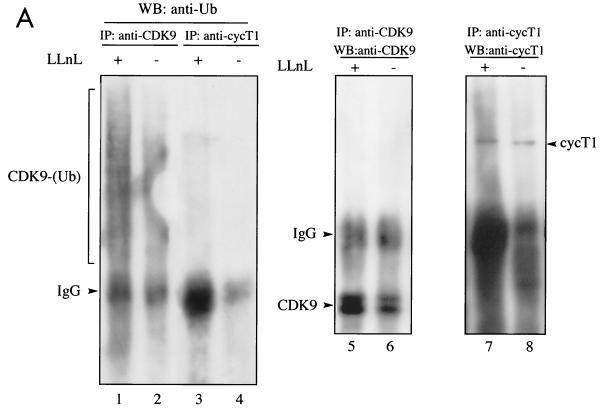
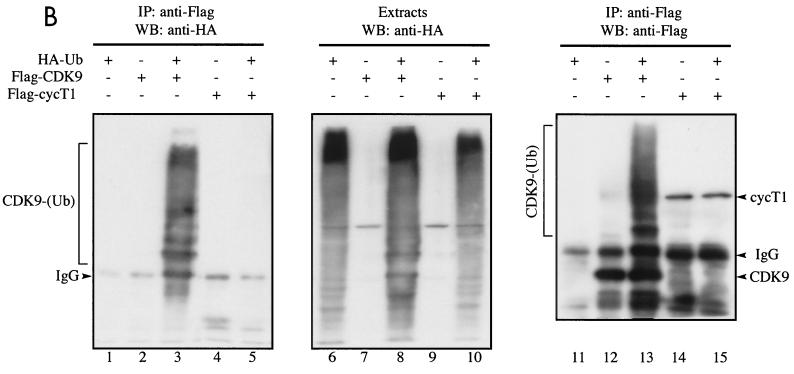
Our interpretation of the data presented above is that the degradation of CDK9 represents a unique example in which cyclin T1 is used to recruit SCFSKP2 (Fig. 3) but is not itself a target for degradation (Fig. 1). Rather, its partner protein, CDK9, is targeted for proteolytic destruction. To further explore this possibility, we investigated the ubiquitination of CDK9 and cyclin T1 in vivo. First, we analyzed ubiquitination of endogenous CDK9 and cyclin T1. Cells were mock treated or treated with proteasome inhibitor and then lysed under highly denaturing conditions which retain covalently attached ubiquitin but dissociate noncovalent interactions. Anti-ubiquitin immunoblotting revealed slower-migrating species in extracts immunoprecipitated with anti-CDK9 but not anti-cyclin T1 (Fig. 5A). Treatment with proteasome inhibitor significantly increased the amount of ubiquitinated CDK9 detected. Next, we analyzed the ubiquitination of transfected CDK9 and cyclin T1. Cells transfected with Flag-CDK9 or Flag-cyclin T1 together with a plasmid expressing HA-ubiquitin (43) were lysed under highly denaturing conditions. Transfected CDK9 or cyclin T1 was immunoprecipitated using anti-Flag, and ubiquitinated conjugates were detected by anti-HA immunoblotting. Ubiquitinated conjugates of Flag-CDK9 but not Flag-cyclin T1 were readily detected (Fig. 5B). Expression levels of HA-ubiquitin were comparable in all samples (lanes 6 to 10). Under the denaturing conditions used, cyclin T1 could not be detected in Flag-CDK9 immunoprecipitates, nor could CDK9 be detected in Flag-cyclin T1 immunoprecipitates (data not shown). Slower-migrating species of Flag-CDK9, which likely represent ubiquitinated CDK9 conjugates, were revealed by anti-Flag immunoblotting of immunoprecipitates (lanes 12 to 13). Coexpression of Flag-CDK9 and HA-ubiquitin greatly increased the efficiency of Flag-CDK9 ubiquitination (compare lane 12 with lane 13). This may be due to the formation of multiubiquitinated chains containing N-terminally tagged ubiquitin which are resistant to proteasomal degradation (7). These experiments show that CDK9 and not cyclin T1 is targeted for ubiquitination in vivo.
FIG. 5.
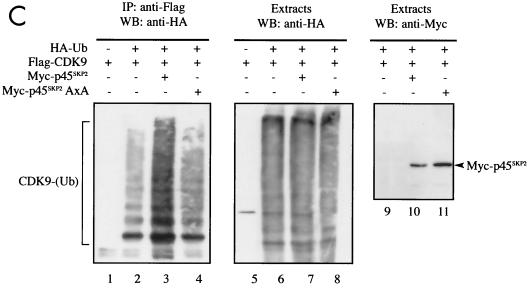
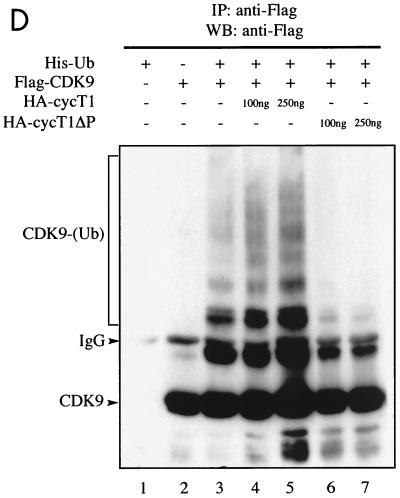
CDK9 but not cyclin T1 is ubiquitinated in vivo. (A) Denatured extracts of mock-treated or LLnL-treated 293 cells were subjected to immunoprecipitation (IP) using anti-CDK9 or anti-cyclin T1 (cycT1) antibodies as indicated, followed by immunoblotting using anti-ubiquitin (Ub) (lanes 1 to 4), anti-CDK9 (lanes 5 and 6), or anti-cyclin T1 (lanes 7 and 8) antibodies. (B) 293 cells were transfected with a plasmid expressing HA-ubiquitin (5 μg) either alone or together with plasmids expressing Flag-CDK9 (2 μg) or Flag-cyclin T1 (10 μg) as indicated. Denatured cell extracts of LLnL-treated cells were immunoprecipitated using anti-Flag antibody, followed by immunoblotting using anti-HA (lanes 1 to 5) or anti-Flag (lanes 11 to 15) antibodies. The cell extracts were subjected to direct immunoblot analysis using anti-HA (lanes 6 to 10). (C) Overexpression of p45SKP2 augments HA-CDK9 ubiquitination in vivo. 293 cells were transfected with a plasmid expressing Flag-CDK9 (2 μg) either alone or together with plasmids expressing myc-p45SKP2 (5 μg) or myc-p45SKP2AxA (5 μg) and with HA-ubiquitin (5 μg) as indicated. Denatured cell extracts of LLnL-treated cells were immunoprecipitated using anti-Flag antibody, and ubiquitinated conjugates were revealed by immunoblot analysis using anti-HA antibody (lanes 1 to 4). Other aliquots were subjected to direct immunoblot analysis using anti-HA (lanes 5 to 8) and anti-Myc (lanes 9 to 11) antibodies. (D) Overexpression of full-length cyclin T1 or cyclin T1ΔPEST modulates HA-CDK9 ubiquitination in vivo. 293 cells were transfected with a plasmid expressing Flag-CDK9 (2 μg) either alone or together with various amounts of plasmids expressing full-length cyclin T1 or cyclin T1ΔPEST and with His6-tagged ubiquitin (5 μg) as indicated. Denatured cell extracts of LLnL-treated cells were immunoprecipitated using anti-Flag antibody, and slower-migrating ubiquitinated conjugates were revealed by immunoblotting using anti-Flag antibody. +, present; −, absent. IgG, immunoglobulin G.
Ubiquitination and degradation of CDK9 in vivo is modulated by overexpression of p45SKP2, cyclin T1, and cyclin T1ΔPEST.
To further investigate the importance of p45SKP2 in the ubiquitination of CDK9, lysates of cells coexpressing Flag-CDK9, either wild-type myc-tagged p45SKP2 or a mutant of p45SKP2 that cannot interact with cyclin A-cdk2 (myc-p45SKP2AxA) (26) and HA-ubiquitin, were assayed for the presence of ubiquitinated CDK9 conjugates. CDK9 ubiquitination was augmented by overexpression of wild-type p45SKP2 but not by mutant p45SKP2AxA (Fig. 5C). Expression levels of HA-ubiquitin and wild-type and mutant forms of p45SKP2 were comparable. Coimmunoprecipitation analysis confirmed that p45SKP2AxA was able to interact with cyclin T1 (data not shown). Overexpression of FWD-1, a mouse homologue of h-βTrCP (22), had no effect on CDK9 ubiquitination (data not shown).
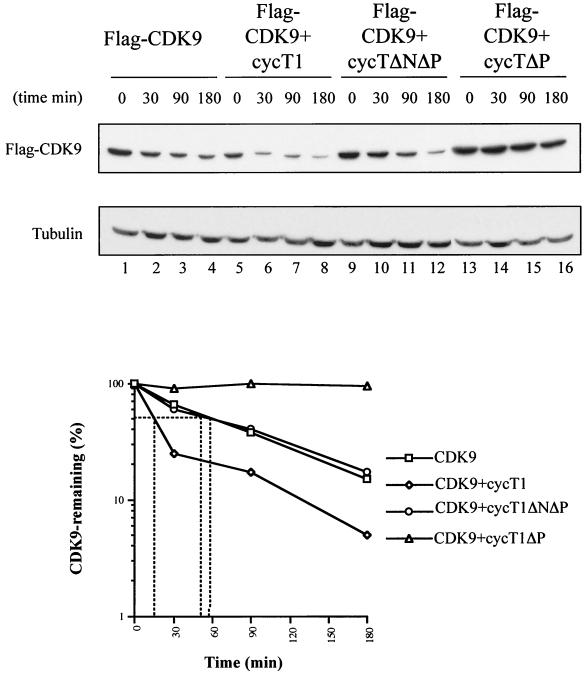
The interaction between CDK9 and SCFSKP2 is mediated by cyclin T1 and requires its C-terminal PEST domain (Fig. 3). We investigated whether CDK9 ubiquitination was affected by overexpression of cyclin T1 or cyclin T1ΔPEST. Overexpression of cyclin T1 augmented the level of CDK9 ubiquitination observed in vivo, while overexpression of HA-cyclin T1ΔPEST strongly inhibited CDK9 ubiquitination (Fig. 5D). We next investigated whether overexpression of cyclin T1 might also result in changes in CDK9 stability in vivo. The stability of Flag-CDK9 was significantly reduced by overexpression of cyclin T1 and increased by overexpression of cyclin T1ΔPEST (Fig. 6). Overexpression of a cyclin T1ΔPEST mutant containing an N-terminal deletion (cycT1ΔNΔP) that removes the CDK9 binding domain (10, 17) did not alter Flag-CDK9 stability, showing that its modulation depends on the interaction between CDK9 and cyclin T1. These results support the hypothesis that cyclin T1 is required for the recruitment of SCFSKP2 via an interaction requiring its PEST domain which ultimately results in ubiquitination and degradation of CDK9.
FIG. 6.
Modulation of CDK9 stability in vivo by overexpression of full-length and PEST-minus forms of cyclin (cyc) T1. HeLa cells were transfected with Flag-CDK9 (2 μg) alone or together with either cyclin T1 (2 μg), cyclin T1ΔP (2 μg), or cyclin T1ΔNΔP (2 μg). The cells were lysed after the addition of cycloheximide for the indicated times, and Flag-CDK9 was detected by immunoblotting using anti-Flag antibody. The results were quantified and expressed relative to tubulin stability.
Transcriptional activity of CDK9/cyclin T1 is regulated by CDK9 ubiquitination.
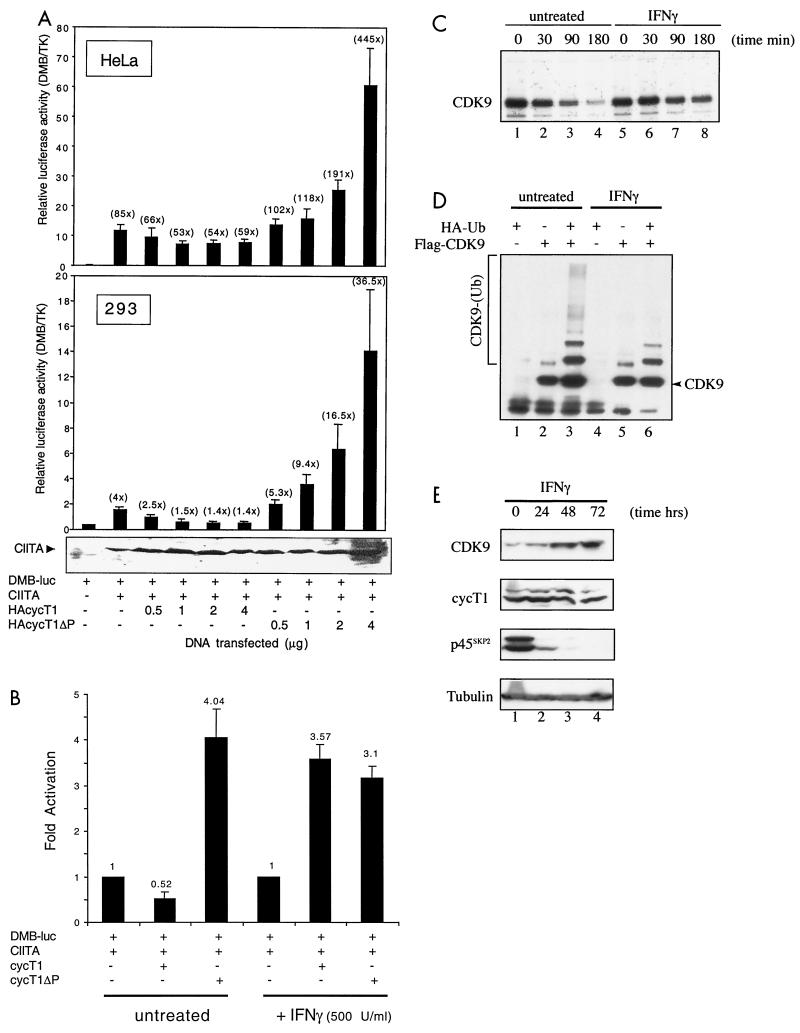
The role of cyclin T1 and CDK9 in transcriptional elongation raises the possibility that ubiquitination of CDK9 provides a mechanism to regulate transcription from cellular promoters. It was recently demonstrated that a dominant-negative mutant of CDK9 repressed activation of the DRA promoter, suggesting that transcription from the class II major histocompatibility complex (MHCII) promoter is P-TEFb dependent (19). The MHCII promoter is regulated by the class II transactivator (CIITA [20, 42]) and is strongly induced in vivo following treatment of cells with gamma interferon (IFN-γ) (reviewed in reference 39). We initially analyzed the effects of full-length and PEST-minus cyclin T1 on transactivation from the MHCII-HLA-DMB promoter. Overexpression of cyclin T1ΔPEST increased transactivation from the promoter in a dose-dependent manner, up to fivefold in HeLa cells and ninefold in 293 cells over that observed for CIITA alone (Fig. 7A). In contrast, overexpression of cyclin T1 caused promoter repression. The difference in transactivation potential between cyclin T1 and cyclin T1ΔPEST ranged from 1.5- to 7.5-fold in HeLa cells and from 2-to 26-fold in 293 cells, depending on the amount of cyclin T1 or cyclin T1ΔPEST DNA transfected. Expression of CIITA was not affected by overexpression of cyclin T1 or cyclin T1ΔPEST (Fig. 7A). Basal transcriptional activity in the absence of CIITA was not significantly affected by overexpression of cyclin T1 or cyclin T1ΔPEST (data not shown).
FIG. 7.
Ubiquitination of CDK9 is regulated following stimulation of cells with IFN-γ. (A) Overexpression of full-length and PEST-minus cyclin T1 modulates transactivation from the MHCII DMB promoter. HeLa cells were cotransfected with pDMB-luc (1 μg), pCIITA (500 ng), together with pRL-TK (100 ng) and increasing amounts of pHA-cyclin T1 (HAcycT1) or pHA-cyclin T1ΔP as indicated. Relative luciferase activity was calculated following normalization for Renilla luciferase activity expressed from the TK promoter present in the pTK-RL internal control plasmid. Shown are the mean relative luciferase activities (plus standard errors) obtained from at least three independent experiments. Fold transactivation relative to pDMB-luc alone is shown above the bars in parentheses. Below the graph is shown the expression level of CIITA in samples of 293 cells determined by Western blotting of extracts with anti-CIITA antibody. +, present; −, absent. (B) Transient transfections were performed as described for panel A except that after transfections the cells were mock treated or treated with IFN-γ at 500 U/ml for 24 h. The results are presented as fold activation relative to CIITA transactivation of pDMB-luc. (C) HA-CDK9-transfected 293 cells were mock treated or treated with IFN-γ for 24 h, labeled with 35S-Trans-label, and subjected to pulse-chase analysis. (D) IFN-γ modulates HA-CDK9 ubiquitination in vivo. 293 cells transfected with a plasmid expressing Flag-CDK9 (2 μg) either alone or together with a plasmid expressing HA-ubiquitin (Ub) (5 μg) as indicated were mock treated or treated with IFN-γ (500 U/ml) for 24 h. Denatured cell extracts of LLnL-treated cells were immunoprecipitated using anti-Flag antibody, and slower-migrating ubiquitinated conjugates were revealed by immunoblotting using anti-Flag antibody. (E) IFN-γ modulates the expression of CDK9 and p45SKP2. Extracts of 293 cells were prepared at 0, 24, 48, and 72 h after treatment with IFN-γ (500 U/ml). The expression of CDK9, cyclin T1, p45SKP2, and tubulin was analyzed by immunoblotting using specific antibodies.
Since the MHCII promoter is induced by IFN-γ, we next examined its effect on transactivation from the DMB promoter following overexpression of full-length or PEST-minus cyclin T1. In the absence of IFN-γ, cyclin T1 repressed transactivation while cyclinT1ΔPEST increased transactivation (Fig. 7B). In striking contrast, following treatment of cells with IFN-γ, cyclin T1 no longer produced a repressive effect but instead increased transactivation from the promoter to a level equivalent to that induced by cyclinT1ΔPEST. In the absence of IFN-γ, a sevenfold difference in transactivation potential was observed between cyclin T1 and cyclinT1ΔPEST, whereas following IFN-γ treatment, the transactivation potentials of these proteins were equivalent. Transactivation by cyclinT1ΔPEST was not significantly affected by IFN-γ treatment. Higher levels of basal promoter activity were observed following IFN-γ treatment (3.5-fold), presumably due to induction of endogenous CIITA expression. Thus, the increase in transactivation by transfected CIITA was not as high in IFN-treated samples, although the relative luciferase activities were equivalent in treated and untreated samples.
To further investigate IFN-γ-induced modulation of cyclin T1 transactivation potential, the effect of IFN-γ on CDK9 stability was examined. In IFN-γ-treated cells, CDK9 was significantly more stable (Fig. 7C) and CDK9 ubiquitination was reduced (Fig. 7D). To investigate the mechanism by which IFN-γ treatment regulates CDK9 ubiquitination, we analyzed the expression of CDK9 and p45SKP2 following IFN-γ treatment. CDK9 expression was increased dramatically, consistent with its increased stability and decreased ubiquitination, while the expression of p45SKP2, initially high in untreated cells, was almost completely shut down (Fig. 7E). Cyclin T1 expression was unaffected. The steady-state expression of both CDK9 and p45SKP2 in untreated cells did not fluctuate significantly over 72 h (data not shown). Taken together, these data strongly suggest that treatment with IFN-γ inhibits expression of p45SKP2, leading to reduced ubiquitination of CDK9. The consequent increase in CDK9 stability results in increased transactivation from cellular promoters that are regulated by CDK9/cyclin T1.
DISCUSSION
In this report, we show that the catalytic subunit of human P-TEFb, CDK9, is a novel substrate of SCFSKP2. CDK9 can be ubiquitinated and degraded by the proteasome, whereas cyclin T1 is stable. We show that SCFSKP2 is recruited to CDK9/cyclin T1 via cyclin T1 in an interaction requiring its PEST domain. CDK9 ubiquitination can be modulated by both cyclin T1 and p45SKP2. The expression of CDK9 was periodic during the cell cycle and correlated inversely with that of p45SKP2. Furthermore, CDK9 accumulated in p45SKP2−/− MEFs. While we cannot exclude the possibility that the cell cycle-regulated degradation of CDK9 and its accumulation in p45SKP2−/− cells may be due to the effect of p45SKP2 on the cell cycle by promoting S-phase progression (52), the body of experiments presented strongly suggest that CDK9 is indeed a bona fide substrate of SCFSKP2 and that its regulation during the cell cycle is due to the periodic expression of p45SKP2. Finally, the transcriptional activity of CDK9/cyclin T1 on the MHCII promoter could be regulated by CDK9 degradation in vivo.
SCFSKP2 has been implicated in the ubiquitination of several proteins, including E2F-1, p27Kip1, and cyclin E, that are important for cell cycle progression (4, 31, 32, 40, 44). The proteolytic degradation of CDK9 is unique among the family of CDK proteins. While other CDKs play a central role in regulated proteolysis both at the G1-S transition and in mitosis through phosphorylation of their cyclin partners, the CDK subunits are not targets for ubiquitination. Instead, the abundance of the cyclin subunits is regulated by proteolysis and provides a mechanism for cell cycle progression (reviewed in reference 23).
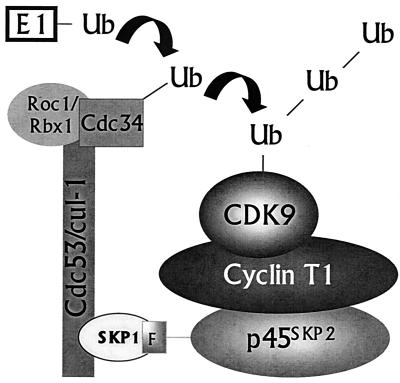
Figure 8 shows a schematic representation of a proposed model for CDK9 ubiquitination. A unique feature of this model is that recruitment of the SCF complex occurs via cyclin T1 while CDK9 serves as the target for ubiquitination. The possibility that an oligomeric protein may contain both short-lived and long-lived subunits has been hypothesized previously (18). In a study using X-β-galactosidase mutants that contain either a destabilizing amino-terminal residue X or a ubiquitin acceptor lysine residue, it was observed that mixed tetramers recruited ubiquitination activity, although only the moiety bearing a wild-type lysine was degraded. It was suggested that two determinants for degradation could be located on different subunits of an oligomer and target the lysine-bearing component for destruction in a process termed trans-recognition. Somewhat analogous examples are provided by two viral proteins: the Vpu protein of human immunodeficiency virus type 1, which targets the cellular protein CD4 for h-βTrCP-mediated ubiquitination and degradation (27), and the human papilloma virus E6 oncoprotein, which targets p53 for rapid ubiquitin-dependent proteolysis through its interaction with E6-AP (16, 36). Like cyclin T1, neither Vpu nor E6 is a target for degradation itself. Rather, these viral proteins serve simply to connect their respective targets to the proteolytic pathway. Cyclin T1 is the first cellular protein shown to act as a connector protein able to target its partner for ubiquitination without being degraded itself in the reaction. It also provides evidence for the concept of trans-recognition, in which an otherwise long-lived protein is targeted for destruction in trans, and suggests that this mechanism has a functional role in ubiquitin-mediated degradation of a wild-type cellular protein.
FIG. 8.
Proposed model of CDK9 ubiquitination mediated by an interaction between cyclin T1 and SCFSKP2. See the text for details. Ub, ubiquitin.
CDK9/cyclin T1 is an important factor involved in transcriptional elongation. While CDK9 activity has been shown to be increased upon activation of peripheral blood lymphocytes or differentiation of promonocytic cell lines (11, 15, 50), our data provide the first indications that CDK9 is regulated in a cell cycle-dependent manner by the ubiquitin pathway. This has important implications for the regulation of cellular transcription. While P-TEFb is considered to be a general transcriptional elongation factor, recent evidence suggests that its function may be more specific (8). Our finding that CDK9 is differentially expressed during the cell cycle suggests that its activity maybe more specifically targeted to genes that are expressed in G1/S phase. The regulated destruction of a transcriptional elongation factor by SCFSKP2 could provide a mechanism for linking efficient gene expression with cell cycle position. In this regard, a CDK9 mutant that cannot be ubiquitinated might be expected to alter cell cycle progression, and analysis of cells expressing such a mutant may lead to the identification of genes whose expression is regulated by P-TEFb.
We have shown that regulation of CDK9 expression can modulate transactivation from a specific P-TEFb-dependent promoter (Fig. 7). Importantly, these data show that ubiquitination of CDK9 is regulated in response to physiological stimuli and results in increased promoter transactivation. Stimulation of cells with IFN-γ inhibited expression of p45SKP2, leading to reduced ubiquitination of CDK9. While the mechanism of p45SKP2 shutdown in IFN-γ-treated cells is unclear, we observed that IFN-γ-treated cells accumulated in G1 phase of the cell cycle (data not shown), in agreement with previous reports (41, 51). Thus, IFN-γ treatment may inhibit the cell cycle-dependent expression of p45SKP2 by blocking cells in G0/G1, although it is possible that other mechanisms of p45SKP2 inhibition exist in IFN-γ-treated cells. In any case, lowered expression of p45SKP2 leads to reduced ubiquitination of CDK9 and a consequent increase in CDK9 stability. The importance of regulation of CDK9 ubiquitination in vivo is revealed by the subsequent increase in transactivation from the MHCII promoter. Demonstration that a cell-activating signal received at the plasma membrane can ultimately affect CDK9 ubiquitination and P-TEFb transactivation potential suggests that a complex, highly regulated network of interacting pathways combine to control transcriptional activity in vivo.
Although in vitro reconstitution of the ubiquitination pathway has been achieved for only a few substrates, these clearly do not require connector proteins in the role played by cyclin T1. While the purpose served by the requirement for cyclin T1 in the degradation of CDK9 is still unclear, the data shown in Fig. 7A suggest that cyclin T1 may act as a genuine regulator of P-TEFb-mediated transactivation by controlling CDK9 degradation. Under conditions that do not support CDK9 degradation, mimicked by overexpression of cyclin T1ΔPEST, cyclin T1 clearly acts as a positive regulator of transcription. In contrast, conditions under which CDK9 ubiquitination and degradation are observed lead to promoter repression. In previous reports describing promoter transactivation by cyclin T1, cyclin T1ΔPEST has been used in the experiments (3, 10, 46). Thus, cyclin T1 can be considered a molecular switch controlling CDK9 activity. Cell cycle-regulated expression of p45SKP2 would likely be an important factor governing the cyclin T1-mediated transcriptional switching mechanism. Other factors, such as phosphorylation events, may also play a role. While additional partners for cyclin T1 have not been reported, it is possible that the timely degradation of CDK9 would allow cyclin T1 to interact with other cellular partners. From the other perspective, CDK9 is known to interact predominantly with cyclin T1 but also with cyclins T2 and K (9, 33). Inspection of the sequence of these additional CDK9 partners revealed that they do not contain an obvious PEST domain. Importantly, the binding of CDK9 with cyclin T1 or T2 is mutually exclusive. Therefore, this unusual requirement for cyclin T1 in the degradation of CDK9 could provide a further mechanism to fine tune the level of expression of CDK9. For example, the CDK9/cyclin T1 complex would be active in transcriptional elongation except when the expression of p45SKP2 results in the degradation of the majority of CDK9 in the cell. However, the remaining CDK9 associated with cyclins T2 and K would be protected from proteolytic degradation. In support of this hypothesis, low-level expression of CDK9 was observed at 2 and 24 h following mitosis (Fig. 4B). This low level of CDK9 may be important in a homeostatic function to promote low-level expression at many genes until the periodic disappearance of p45SKP2 allows high-level expression of the active CDK9/cyclin T1 CTD kinase in G1/S phase (4 to 14 h following mitosis) (Fig. 4B). Alternatively, it is possible that constitutive, low-level expression of CDK9/cyclin T is required at a distinct subset of cellular promoters. Therefore, the presence of multiple CDK9 partners that interact differently with the ubiquitination machinery could provide a useful mechanism for regulating not only the timing but also the magnitude of CDK9 degradation. This additional level of regulation would not be expected for targets involved in cell cycle progression or pathway inhibitors such as IκB. In these cases, destruction of all of the target molecules would be optimally required. CDK9, in contrast, is involved in transcriptional elongation at a great many cellular promoters which may have different requirements for expression at different times in the cell cycle.
Transcriptional elongation at RNAPII-dependent genes is likely regulated by a complex interplay between positive regulators, such as P-TEFb, and negative regulators, DRB-sensitivity inducing factor (DSIF) and negative elongation factor (49). DSIF has been shown to repress RNAPII elongation in vitro, using HeLa nuclear extracts that have been immunodepleted of P-TEFb, or in the presence of DRB (45, 48). In the presence of P-TEFb, the RNAPII CTD becomes hyperphosphorylated, presumptively by CDK9. Since DSIF is unable to interact with the hyperphosphorylated form of RNAPII CTD, DSIF repression is relieved and transcriptional elongation can proceed (45). However, it is unclear how DSIF-mediated repression may occur in vivo when P-TEFb is present. Ubiquitination and degradation of CDK9 could allow transient inactivation of P-TEFb and thus provide a mechanism by which the effects of positive and negative regulators of transcriptional elongation are finely balanced to allow efficient gene expression.
In addition to CDK9, described in this study, other proteins involved in transcription, such as transcription factors and RNAPII following exposure to DNA-damaging agents, are regulated by the ubiquitin pathway (reviewed in references 21 and 24). Thus, the formation and activity of transcriptional complexes may be tightly regulated in normal cells to allow an appropriate program of gene expression. Misregulation of these processes could have severe consequences and may be involved in cellular transformation.
ACKNOWLEDGMENTS
We thank the members of the M. Benkirane, P. Corbeau, and M. Dorée laboratories; G. Cavalli, K.-T. Jeang, C. Neuveut, and D. Price for critical reading of the manuscript; P. Atger for art work; and J. Demaille for his support. We thank D. Price for anti-CDK9 and anti-cyclin T1 antibodies, R. Benarous for anti-h-βTrCP antibody, M. Dorée for anti-p19SKP1 and anti-CDC34 antibodies, W. Krek for anti-p45SKP2 antibody, and P. Louis-Plence for anti-CIITA antibody. cDNAs were obtained from the following individuals: X. Grana (HA-CDK9), A. Rice (Flag-CDK9), K. Jones (HA-cyc T1ΔP), R. Gaynor [Flag-cyclin T1 (1–726) and Flag-cyclin T1 (203–726)], W. Krek (HA-CDC34, MT-p45SKP2, and MT-p45SKP2AxA), and P. Louis-Plence (DMB-luc).
This work was supported by grants from the HFSP and ANRS to M.B and S.E, ARC No. 9387 to S.E., and Ministère de la Recherche No. 5343 to M.B. R.E.K. was supported by an ANRS fellowship.
REFERENCES
- 1.Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper J W, Elledge S J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 2.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 3.Bieniasz P D, Grdina T A, Bogerd H P, Cullen B R. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc Natl Acad Sci USA. 1999;96:7791–7796. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrano A C, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper K F, Strich R. Functional analysis of the Ume3p/Srb11p-RNA polymerase II holoenzyme interaction. Gene Expr. 1999;8:43–57. [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison M J, Hochstrasser M. Epitope-tagged ubiquitin. A new probe for analyzing ubiquitin function. J Biol Chem. 1991;266:21150–21157. [PubMed] [Google Scholar]
- 8.Foskett S M, Ghose R, Tang D N, Lewis D E, Rice A P. Antiapoptotic function of Cdk9 (TAK/P-TEFb) in U937 promonocytic cells. J Virol. 2001;75:1220–1228. doi: 10.1128/JVI.75.3.1220-1228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu T J, Peng J, Lee G, Price D H, Flores O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J Biol Chem. 1999;274:34527–34530. doi: 10.1074/jbc.274.49.34527. [DOI] [PubMed] [Google Scholar]
- 10.Garber M E, Wei P, KewalRamani V N, Mayall T P, Herrmann C H, Rice A P, Littman D R, Jones K A. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998;12:3512–3527. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garriga J, Peng J, Parreno M, Price D H, Henderson E E, Grana X. Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene. 1998;17:3093–3102. doi: 10.1038/sj.onc.1202548. [DOI] [PubMed] [Google Scholar]
- 12.Gold M O, Yang X, Herrmann C H, Rice A P. PITALRE, the catalytic subunit of TAK, is required for human immunodeficiency virus Tat transactivation in vivo. J Virol. 1998;72:4448–4453. doi: 10.1128/jvi.72.5.4448-4453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grana X, De Luca A, Sang N, Fu Y, Claudio P P, Rosenblatt J, Morgan D O, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatakeyama S, Kitagawa M, Nakayama K, Shirane M, Matsumoto M, Hattori K, Higashi H, Nakano H, Okumura K, Onoe K, Good R A, Nakayama K. Ubiquitin-dependent degradation of IκBα is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci USA. 1999;96:3859–3863. doi: 10.1073/pnas.96.7.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann C H, Carroll R G, Wei P, Jones K A, Rice A P. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J Virol. 1998;72:9881–9888. doi: 10.1128/jvi.72.12.9881-9888.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov D, Kwak Y T, Nee E, Guo J, Garcia-Martinez L F, Gaynor R B. Cyclin T1 domains involved in complex formation with Tat and TAR RNA are critical for tat-activation. J Mol Biol. 1999;288:41–56. doi: 10.1006/jmbi.1999.2663. [DOI] [PubMed] [Google Scholar]
- 18.Johnson E S, Gonda D K, Varshavsky A. cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature. 1990;346:287–291. doi: 10.1038/346287a0. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa S, Okamoto T, Peterlin B M. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12:61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 20.Kern I, Steimle V, Siegrist C A, Mach B. The two novel MHC class II transactivators RFX5 and CIITA both control expression of HLA-DM genes. Int Immunol. 1995;7:1295–1299. doi: 10.1093/intimm/7.8.1295. [DOI] [PubMed] [Google Scholar]
- 21.King R W, Deshaies R J, Peters J M, Kirschner M W. How proteolysis drives the cell cycle. Science. 1999;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koepp D M, Harper J W, Elledge S J. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell. 1999;97:431–434. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- 24.Krek W. Proteolysis and the G1-S transition: the SCF connection. Curr Opin Genet Dev. 1998;8:36–42. doi: 10.1016/s0959-437x(98)80059-2. [DOI] [PubMed] [Google Scholar]
- 25.Laybourn P J, Dahmus M E. Transcription-dependent structural changes in the C-terminal domain of mammalian RNA polymerase subunit IIa/o. J Biol Chem. 1989;264:6693–6698. [PubMed] [Google Scholar]
- 26.Lisztwan J, Marti A, Sutterluty H, Gstaiger M, Wirbelauer C, Krek W. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margottin F, Bour S P, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 28.Marshall N F, Peng J, Xie Z, Price D H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 29.Marshall N F, Price D H. Control of formation of two distinct classes of RNA polymerase II elongation complexes. Mol Cell Biol. 1992;12:2078–2090. doi: 10.1128/mcb.12.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall N F, Price D H. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J Biol Chem. 1995;270:12335–12338. doi: 10.1074/jbc.270.21.12335. [DOI] [PubMed] [Google Scholar]
- 31.Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama K, Nagahama H, Minamishima Y A, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Nakayama K, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 35.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 36.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 37.Shilatifard A. Factors regulating the transcriptional elongation activity of RNA polymerase II. FASEB J. 1998;12:1437–1446. doi: 10.1096/fasebj.12.14.1437. [DOI] [PubMed] [Google Scholar]
- 38.Skowyra D, Koepp D M, Kamura T, Conrad M N, Conaway R C, Conaway J W, Elledge S J, Harper J W. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science. 1999;284:662–665. doi: 10.1126/science.284.5414.662. [DOI] [PubMed] [Google Scholar]
- 39.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 40.Sutterlüty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 41.Tiefenbrun N, Melamed D, Levy N, Resnitzky D, Hoffman I, Reed S I, Kimchi A. Alpha interferon suppresses the cyclin D3 and cdc25A genes, leading to a reversible G0-like arrest. Mol Cell Biol. 1996;16:3934–3944. doi: 10.1128/mcb.16.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ting J P, Wright K L, Chin K C, Brickey W J, Li G. The DMB promoter: delineation, in vivo footprint, trans-activation, and trans-dominant suppression. J Immunol. 1997;159:5457–5462. [PubMed] [Google Scholar]
- 43.Treier M, Staszewski L M, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 44.Tsvetkov L M, Yeh K H, Lee S J, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 45.Wada T, Takagi T, Yamaguchi Y, Watanabe D, Handa H. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 1998;17:7395–7403. doi: 10.1093/emboj/17.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 47.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Mathias N, Kobayashi R, Wittenberg C, Tyers M. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi Y, Wada T, Handa H. Interplay between positive and negative elongation factors: drawing a new view of DRB. Genes Cells. 1998;3:9–15. doi: 10.1046/j.1365-2443.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yarden A, Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986;234:1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]