Abstract
Introduction:
Cannabis use rates are increasing in the United States. Patients with cancer use cannabis for many reasons, even without high-quality supporting data. This study sought to characterize cannabis use among patients seen in radiation oncology in a state that has legalized adult non-medical use cannabis and to identify key cannabis-related educational topics.
Methods:
Cannabis history was documented by providers using a structured template at patient visits in an academic radiation oncology practice 10/2020–11/2021. Cannabis use data, including recency/frequency of use, reason, and mode of administration, were summarized, and logistic regression was used to explore associations between patient and disease characteristics and recent cannabis use. A multivariable model employed stepwise variable selection using the Akaike Information Criterion (AIC).
Results:
Of 3143 patients total, 91 (2.9%) declined to answer cannabis use questions, while 343 (10.9%) endorsed recent use (≤1 month ago), 235 (7.5%) noted non-recent use (>1 month ago), and 2474 (78.7%) denied history of cannabis use. In multivariable analyses, those ≥50 years old [OR 0.409 (95%CI 0.294, 0.568), p<0.001] or with history of prior courses of radiation [OR 0.748 (95%CI: 0.572, 0.979), p=0.034] were less likely, while those with a mental health diagnosis not related to substance use [OR 1.533 (95%CI: 1.171, 2.005), p=0.002] or who smoked tobacco [OR 3.003 (95%CI 2.098,4.299) p<0.001] were more likely to endorse recent cannabis use. Patients reported pain, insomnia, and anxiety as the most common reasons for use. Smoking was the most common mode of administration.
Conclusions:
Patients are willing to discuss cannabis use with providers and reported recent cannabis use for a variety of reasons. Younger patients new to oncologic care and those with a history of mental illness or tobacco smoking may benefit most from discussions about cannabis given higher rates of cannabis use in these groups.
Introduction
The United States has seen increasing cannabis use in recent years, as changes in state-level legislation have made cannabis easier to obtain1–3. Cancer is a universal qualifying condition for medical cannabis, and many patients with cancer use cannabis1, 4. There is limited evidence suggesting that the local regulatory status of cannabis may impact cannabis use among those with cancer disproportionately5. Generally, populations of patients with cancer have noted both symptom-based reasoning for cannabis use as well as the use of cannabis to treat their cancer6. There is limited high-quality scientific data to support many uses of cannabis by those with cancer, and there is no clinical evidence to support the use of cannabis to treat cancer.
Providers often feel insufficiently informed on the topic of cannabis use in patients with cancer7, 8. Efforts to meet educational needs of providers so that they can better advise their patients have been stymied by limitations in available data driven by a host of policy decisions stretching back decades9. Some have tried to provide guidance to the community in the form of cannabis practice descriptions to assist other providers, and a host of reviews of the limited data have been prepared10–13. Fortunately, there is now an effort to develop needed data on patients with cancer through funding made available through the National Cancer Institute (NCI) in recent years. Understanding patient cannabis use patterns and providing education based upon the best available data are important parts of quality care provision even in the face of limited data to guide cannabis recommendations in oncology in general or radiation oncology14.
We sought to: 1) assess the extent to which patients would answer questions about cannabis, 2) determine the proportion of patients seen in a radiation oncology practice who are using cannabis, 3) characterize patient-reported reasons for cannabis use, mode of administration, and frequency of cannabis use, 4) examine relationships between patient and disease factors and recent cannabis use, and 5) identify important areas for provider intervention in the context of our present understanding of the risks and benefits of cannabis.
Materials and Methods
Cannabis use history collection
Cannabis use history collection was instituted in a single academic radiation oncology department in Michigan, a state that legalized medical cannabis use in 2008 and adult non-medical cannabis use in 12/2019. Cannabis use history was documented in physician and advanced practice provider notes using the SmartList function of the MiChart (EPIC, Verona, WI) electronic medical record (EMR). The SmartList items served as both prompts for use during the patient interview and as a mechanism for recording patient responses in such a way as to allow the data to later be captured from the medical record. Data were collected from charts for patients seen between 10/2020 to 11/2021 (from 10–23 months after legalization of adult non-medical use cannabis).
SmartList structure and application
There were four components to the EMR tool that were used to capture the data, one for each of the key components that was collected to include the following 1) history of cannabis use – Options: “Prefer not to answer”, “Never,” “Not in last month,” and “Used in last month”; 2) reason for cannabis use – Options: “Pain”, “Nausea”, “Anxiety”, “Depression”, “Insomnia”, “Poor appetite”, “Recreational (for the high)”, “To fight cancer”, and “Other” with a prompt for free text; 3) mode of administration – Options: “Smoke (inhalation)”, “Vape (inhalation)”, “Eat/edibles (oral)”, “Drink (oral)”, “Put under the tongue (sublingual)”, “Put on the skin (topical)”, “Rectal”, and “Other” with a prompt for free text.), and 4) frequency of use – Options: “More than once per day”, “Daily”, “Weekly”, or “Less than weekly”. Providers were given instructions as to the use of these forms prior to cannabis use history collection initialization. Providers were allowed to select multiple reasons for cannabis use and multiple modes of administration. Questions were asked of patients at follow-up and consultation visits.
Data collection
The Michigan Radiation Oncology Analytics Resource (M-ROAR)] was used to capture data from the medical record retrospectively under an IRB-approved protocol. Data captured included patient gender, age, race, ethnicity, treatment goal (curative vs. palliative/metastasis-directed therapy), radiation history, malignant vs. benign disease, tobacco smoking history, and mental health diagnosis history in addition to cannabis history data noted above.
Statistical analysis
Analysis largely focused on the subset of adult patients who agreed to answer questions related to cannabis use (n=3,052). Summary statistics (n, %) were computed for cannabis use history (Never, Not in last month, Used in last month), gender, age, race, ethnicity, prior radiation history, malignant disease, mental health diagnoses, and tobacco smoking history. For race, Asian and Other races were combined into a single category. Curative or palliative treatment was summarized only among those patients with malignant disease.
Univariate logistic regression models for recent cannabis use (Yes vs. No), the decision to answer the cannabis use question (Yes vs. No), and the frequency of cannabis administration (Daily or more vs. Less than daily use) were fit. Additionally, univariate logistic regression models for each mode of cannabis administration (Smoking, Vaping, Smoking or vaping, and Edible) were built separately. Age, gender, race, ethnicity, malignant disease, curative intent, prior radiation, mental health diagnoses, and tobacco smoking history were considered as predictors in each model. Whether patients used cannabis for pain was also included as a predictor in models for the frequency and mode of cannabis administration. Stepwise variable selection using the Akaike Information Criterion (AIC) was used to develop multivariable models, with age, gender, race, malignant disease, curative intent, prior radiation, and any mental health diagnosis as potential factors that could be included in the final model. Ethnicity was excluded as a predictor due to the low frequency of reported cannabis use among Hispanic patients (<10).
The alpha level for statistical significance was set at 0.05. R version 4.1.0 was used for analysis.
Results
History and patterns of cannabis use
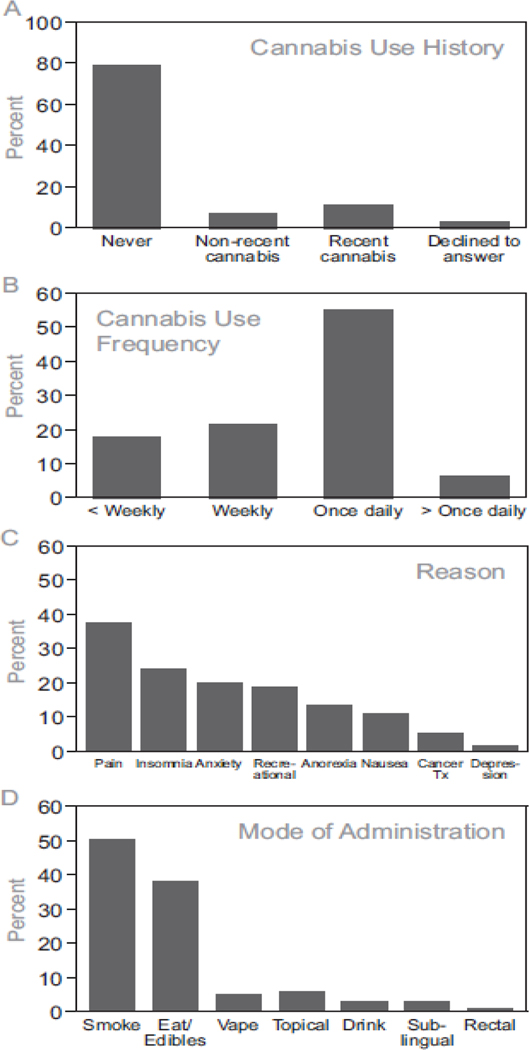
Clinicians used EMR tools for cannabis history documentation for 3143 adult patients seen for initial consultation or follow-up between 10/2020 and 11/2021 in an academic radiation oncology department (Table 1). A total of 6,651 unique patients were seen in this department during this time period. Therefore, cannabis history was documented for 47.3% of patients seen. Recent cannabis use was endorsed by 343 (10.9%) of patients, while 2474 (78.7%) said that they had never used cannabis and 235 (7.5%) reported non-recent cannabis use (Figure 1A). Only 91 (2.9%) patients declined to answer. Among those endorsing recent cannabis use, 207 (61.1%) used cannabis at least daily. The breakdown of use frequency included 60 (17.7%) less than weekly, 72 (21.2%) at least weekly but not daily, 186 (54.9%) daily, and 21 (6.2%) multiple times per day (Figure 1B). Patient reasons for cannabis use included pain (127 patients; 37.0%), insomnia (82 patients; 23.9%), anxiety (68 patients; 19.8%), recreational (63 patients; 18.4%), anorexia (46 patients; 13.4%), nausea (36 patients; 10.5%), cancer treatment (17 patients; 5.0%), and depression (4 patients; 1%; Figure 1C). Mode of administration selected by patients included smoking (173 patients; 50.4%), eating (e.g., edibles, 131 patients; 38.2%), vaping (17 patients; 5.0%), topical (20 patients; 5.8%), drinking (10 patients; 2.9%), sublingual (9 patients; 2.6%), and rectal (2 patients; 0.6%; Figure 1D).
Table 1.
Summary of patient characteristics by cannabis use.
| Variable | Recent cannabis [n (%)] | Non-recent cannabis [n (%)] | Never cannabis [n (%)] |
|---|---|---|---|
| Total | 343 (11.2) | 235 (7.7) | 2474 (81.1) |
|
| |||
| Age | — | — | — |
| 18–34 | 19 (21.1) | 7 (7.8) | 64 (71.1) |
| 35–49 | 64 (20.8) | 20 (6.5) | 223 (72.6) |
| 50 or greater | 260 (9.8) | 208 (7.8) | 2187 (82.4) |
|
| |||
| Gender | — | — | — |
| Male | 185 (11.7) | 145 (9.1) | 1256 (79.2) |
| Female | 158 (10.8) | 90 (6.1) | 1218 (83.1) |
|
| |||
| Race | — | — | — |
| White | 295 (11.3) | 205 (7.8) | 2122 (80.9) |
| Black | 30 (15.3) | 20 (10.2) | 146 (74.5) |
| All others | 18 (7.7) | 10 (4.3) | 206 (88.0) |
|
| |||
| Ethnicity | — | — | — |
| Non-Hispanic | 325 (11.2) | 221 (7.6) | 2349 (81.1) |
| Hispanic | 7 (12.1) | 4 (6.9) | 47 (81.0) |
| Unknown | 11 (11.1) | 10 (10.1) | 78 (78.8) |
|
| |||
| Disease type | — | — | — |
| Benign | 19 (11.2) | 12 (7.1) | 139 (81.8) |
| Malignant | 324 (11.2) | 223 (7.7) | 2335 (81.0) |
|
| |||
| Treatment goal * | — | — | — |
| Palliative | 121 (12.5) | 67 (6.9) | 782 (80.6) |
| Curative | 203 (10.6) | 156 (8.2) | 1553 (81.2) |
|
| |||
| Radiation history | — | — | — |
| No prior radiation | 219 (12.0) | 160 (8.8) | 1444 (79.2) |
| Prior radiation | 124 (10.1) | 75 (6.1) | 1030 (83.8) |
|
| |||
| Mental health (MH) | — | — | — |
| Any MH diagnosis | 145 (14.6) | 80 (8.0) | 770 (77.4) |
| Anxiety | 91 (15.0) | 48 (7.9) | 468 (77.1) |
| Depression | 68 (15.9) | 36 (8.4) | 323 (75.6) |
| MH diagnosis related to alcohol use | 17 (21.2) | 10 (12.5) | 53 (66.2) |
| MH diagnosis related to substance other than alcohol or tobacco | 12 (20.3) | 5 (8.5) | 42 (71.2) |
| MH diagnosis not related to substance use | 122 (13.8) | 68 (7.7) | 693 (78.5) |
|
| |||
| Tobacco smoking history | — | — | — |
| Never | 74 (6.7) | 51 (4.6) | 986 (88.7) |
| Former | 113 (12.5) | 82 (9.1) | 707 (78.4) |
| Current | 73 (18.9) | 46 (11.9) | 267 (69.2) |
Assessed among those with malignant disease
Definitions: Recent cannabis – cannabis use in the last month; Non-Recent cannabis – cannabis use more than one month ago
Abbreviations: n – number; OR – Odds Ratio; CI – Confidence Interval; ref. – reference for statistical testing; MH – mental health
Figure 1.
Patterns of cannabis use among patients with cancer. (A) Cannabis use history among adult patients (n=3143) seen in an academic radiation oncology practice. (B) Cannabis use frequency among those who had used cannabis in the last month (n=343). (C) Reasons for cannabis use among those who had used cannabis in the last month. (D) Cannabis mode of administration among those who had used cannabis in the last month.
Patient and disease characteristics and recent cannabis use
After characterizing cannabis use, we sought to assess patient and disease factors that were associated with higher rates of recent cannabis use. The younger two age groups were condensed due to small numbers in the 18–34 age group. In univariable models (Table 2), there was a significant effect of age. Patients over 50 were less likely to use cannabis than those under 50 [OR 0.411 (95% CI 0.312, 0.540) p<0.001]. No significant effect of race, ethnicity, benign vs. malignant disease, palliative vs. curative intent of radiation, or prior radiation treatment was detected. However, patients with a history of a mental health diagnoses were more likely to use cannabis than those without a mental health diagnosis. Specifically, patients with mental health diagnosis related to alcohol [OR 2.296 (95% CI 1.323, 3.987) p=0.003], a mental health diagnosis related to a substance other than alcohol or tobacco [OR 2.146 (95% CI 1.123, 4.099) p=0.021], or a mental health diagnosis not related to substance use [OR 1.588 (95% CI 1.227, 2.056) p<0.001] were more likely to use cannabis. Additionally, individuals who had formerly smoked [OR 2.007 (95% CI 1.476, 2.729) p<0.001] or who were currently smoking [OR 3.268 (95% CI 2.309, 4.625) p<0.001] tobacco were more likely to endorse cannabis use. In a multivariable analysis (Table 3), a significant effect of age was again observed with reduced likelihood of recent cannabis use among those 50 and over [OR 0.409 (95% CI 0.294, 0.568) p<0.001]. Patients with prior radiation were less likely to endorse recent cannabis use [OR 0.748 (95% CI 0.572, 0.979) p=0.034]. Again, patients with a mental health diagnosis not related to substance use [OR 1.533 (95%CI: 1.171, 2.005), p=0.002] or who smoked tobacco currently [OR 3.003 (95%CI 2.098,4.299) p<0.001] or in the past [2.143 (95%CI 1.569, 2.926) p<0.001] were more likely to endorse recent cannabis use.
Table 2.
Univariate models for recent cannabis use.
| Model | Predictor | OR (95% CI) | p-value |
|---|---|---|---|
| 1 | Age 18–49 (ref) | — | — |
| Age 50+ | 0.411 (0.312, 0.540) | <0.001 | |
|
| |||
| 2 | Male (ref) | — | — |
| Female | 0.915 (0.730, 1.146) | 0.438 | |
|
| |||
| 3 | White (ref) | — | — |
| Black | 1.426 (0.949, 2.142) | 0.088 | |
| All others | 0.657 (0.400, 1.079) | 0.097 | |
|
| |||
| 4 | Non-Hispanic (ref) | — | — |
| Hispanic | 1.085 (0.488, 2.412) | 0.841 | |
| Unknown | 0.988 (0.523, 1.870) | 0.972 | |
|
| |||
| 5 | Benign (ref) | — | — |
| Malignant | 1.007 (0.616, 1.645) | 0.979 | |
|
| |||
| 6 | Palliative (ref) | — | — |
| Curative | 0.840 (0.665, 1.061) | 0.143 | |
|
| |||
| 7 | No prior radiation (ref) | — | — |
| Prior radiation | 0.822 (0.651, 1.038) | 0.099 | |
|
| |||
| 8 | No MH diagnosis (ref) | — | — |
| Any MH diagnosis | 1.602 (1.274, 2.014) | <0.001 | |
|
| |||
| 9 | No MH diagnosis related to alcohol use (ref) | — | — |
| MH diagnosis related to alcohol use | 2.296 (1.323, 3.987) | 0.003 | |
|
| |||
| 10 | No MH diagnosis related to substance other than alcohol or tobacco (ref) | — | — |
| MH diagnosis related to substance other than alcohol or tobacco | 2.146 (1.123, 4.099) | 0.021 | |
|
| |||
| 11 | No MH diagnosis not related to substance use (ref) | — | — |
| MH diagnosis not related to substance use | 1.588 (1.227, 2.056) | <0.001 | |
|
| |||
| 12 | Never tobacco smoker (ref) | — | — |
| Former tobacco smoker | 2.007 (1.476, 2.729) | <0.001 | |
| Current tobacco smoker | 3.268 (2.309, 4.625) | <0.001 | |
Abbreviations: n – number; OR – Odds Ratio; CI – Confidence Interval; ref. – reference for statistical testing; MH – mental health
Table 3.
Multivariable model for recent cannabis use (outcome). Components selected by stepwise variable selection with AIC.
| Predictor | OR (95% CI) | p-value |
|---|---|---|
| Age 18–49 (ref) | — | — |
| Age 50+ | 0.409 (0.294, 0.568) | <0.001 |
|
| ||
| Benign (ref) | — | — |
| Malignant | 1.758 (0.787, 3.924) | 0.169 |
|
| ||
| No prior radiation (ref) | — | — |
| Prior radiation | 0.748 (0.572, 0.979) | 0.034 |
|
| ||
| No MH diagnosis related to alcohol use (ref) | — | — |
| MH diagnosis related to alcohol use | 1.731 (0.964, 3.108) | 0.066 |
|
| ||
| No MH diagnosis not related to substance use (ref) | — | — |
| MH diagnosis not related to substance use | 1.533 (1.171, 2.005) | 0.002 |
|
| ||
| Never tobacco smoker (ref) | — | — |
| Former tobacco smoker | 2.143 (1.569, 2.926) | <0.001 |
| Current tobacco smoker | 3.003 (2.098, 4.299) | <0.001 |
Abbreviations: n – number; OR – Odds Ratio; CI – Confidence Interval; ref. – reference for statistical testing; MH – mental health
Patient and disease characteristics associated with declining to answer questions about cannabis
After noting patterns of recent cannabis use, we sought to assess factors related to selecting the option of “prefer not to answer” to questions about cannabis. Of the 3143 patients assessed, only 91 (2.9%) declined to answer questions. In a univariate model, patients over 50 were less likely to select “prefer not to answer” vs. younger patients [OR 0.433 (95% CI 0.272, 0.718) p=0.001], those treated with curative intent were less likely to decline to answer than those treated with palliative intent [OR 0.580 (95% CI 0.382, 0.883) p=0.011; Table S1]. Patients who had previously received radiation were more likely to decline to answer than those without radiation history [OR 2.165 (1.416, 3.310) p<0.001]. In a multivariable model using AIC, age, curative vs. palliative intent, and prior radiation vs. no prior radiation remained significant (Table S2).
Patient and disease characteristics related to cannabis use characteristics.
We next assessed relationships between patient and disease characteristics and both cannabis use frequency and cannabis mode of administration. None of the factors assessed were related to the frequency of cannabis product administration (Table S3). Female patients were less likely to smoke cannabis vs. other modes of administration in a univariate model[OR 0.431 (95% CI 0.279, 0.665) p<0.001; Table S4)]. Patients with a mental health diagnosis related to alcohol use [OR 3.735 (95% CI 1.184, 11.776) p=0.025] or who formerly [OR 2.649 (95% CI 1.398, 5.019) p=0.003] or currently [OR 7.671 (95% CI 3.687, 15.958) p<0.001] smoked tobacco were more likely to endorse smoking cannabis (Table S4). Gender and tobacco smoking history were the only variables that remained significant at the 0.05 level in the multivariable model. Patients undergoing curative intent treatment were more likely to vape cannabis [OR 9.950 (95% CI 1.304, 75.954), p=0.027] in univariate models (Table S5). In a multivariable model with AIC, female patients were less likely to vape than males [OR 0.298 (95% CI 0.094, 0.945) p=0.040], and those undergoing curative intent radiation were more likely to vape [OR 11.114 (95% CI 1.448, 85.286) p=0.021; Table S6]. Female patients were more likely to use edible cannabis [OR 2.191 (95% CI 1.406, 3.414) p=0.001] while those currently smoking tobacco were less likely to use edible cannabis [OR 0.409 (95% CI 0.208, 0.804) p=0.010; Table S7]. Gender and current tobacco smoking remained significant in a multivariable model.
Discussion
In patients seen in an academic radiation oncology practice less than 2 years after legalization of recreational cannabis, the vast majority (97.1%) of patients were willing to answer questions about cannabis use, and 10.9% of those who answered these questions reported recently using cannabis. Therefore, cannabis use history collection appears to be feasible and acceptable using the approach described in our methods, and cannabis use rates are sufficient to justify such history collection. Upon review of the characteristics and patterns of cannabis use, there are important areas to target with provider and patient education including lack of evidence supporting cannabis as a cure for cancer, risks of smoking/inhalation of cannabis, practices for safe use of edible cannabis, and cannabis use in setting of mental health conditions. Provider and patient education should focus on key areas identified above and reviewed in the following paragraphs.
Recent cannabis use
Recent cannabis use was common (10.9%) among patients seen in our radiation oncology clinic. This has also been the case in other studies of patients with cancer, though rates vary broadly (10–40%)4, 6, 15. It is important to note that the definition of “recent cannabis use” has varied from study to study, with many defining recent cannabis use as cannabis use in the last year, while we defined recent cannabis use as use within the last month4. There has also been great variation among studies in how patients using cannabis are identified; approaches have included identification at time of a visit with a specific small group of providers, when seen by any provider at a cancer center, via online survey, or via national survey4, 6, 15. Patients who endorsed recent cannabis use in our study also appeared to use it frequently, at least once per day for most (61%).
Several patient and disease features appeared to be related to recent cannabis use, including age, mental health diagnoses, smoking history, and radiation history. Younger patients were more likely to be using cannabis, as has been shown in other studies4. Younger patients perceive lower risk associated with cannabis use and feel that cannabis is more accessible than their older counterparts4. Cannabis use rates were higher in those with a history of a mental health diagnosis, including those diagnoses related to substance use. Others have shown that patients with a mental health diagnosis may use cannabis more frequently and preferentially select more potent products for use, though the evidence regarding the impact of cannabis use on mental health conditions is limited and may vary among mental health diagnoses16–18. Patients with a history of radiation were less likely to endorse recent cannabis use. This seems to be counterintuitive, as a provider might anticipate greater use among those who had been more heavily treated; however, greater exposure to treatment could just be a marker of more severe disease which could impact the ability to access and/or consume cannabis, lead to more time with providers with unfavorable opinions of cannabis, more time to find therapies with stronger evidence, or more time to try cannabis and decide not to use it due to side effects or lack of effectiveness. Cannabis use was more common in those who smoked tobacco. Others have shown that co-use of cannabis and tobacco is common outside of the cancer space19, 20. Future studies should explore factors that are related to recent cannabis use in further detail. These findings can also serve to guide providers to patients most likely to benefit from discussions about cannabis.
The rate of response to questions about cannabis was higher than anticipated, with over 97% of patients choosing to directly answer the question about past month use. Age, treatment intent, and prior history of radiation were related to the decision not to answer questions about cannabis but mental health diagnoses, including those related to substance use, and tobacco smoking were not. Older individuals and those receiving curative treatment were more likely to answer questions while those with prior radiation history were less likely. This suggests that the already significant effect of age on recent cannabis use could be understated if younger patients are declining to discuss cannabis. Additionally, the finding that those with prior radiation history were less likely to answer questions about cannabis suggests that these individuals may be seeking to avoid conversations about cannabis in the patient/provider visit setting, perhaps due to past interactions with providers. The impact of stigma among cannabis providers has been documented in previous reports21. The percentage of patients who declined to answer cannabis questions was small, so it is difficult to infer broadly from this likely highly selected group. Patient comfort answering questions about cannabis is an important topic to explore both at the level of individual clinical encounters and at the level of large surveys.
Cannabis mode of administration
Regarding mode of administration, inhalation via smoking was most common in our sample followed by edible consumption via the oral route. Much less common were topical, inhalation via vaping, oral intake via drinking, sublingual, and rectal administrations. The American Lung Association recommends against smoking or vaping of cannabis due to concerns about negative impacts of these practices on lung health22. Though no link between cannabis use and lung cancer has been demonstrated to date, many of the same chemicals found in cigarette smoke are also found in combusted cannabis22, 23. In the interests of preserving lung health, we discourage inhalational cannabis use in our practice. When a rapid onset cannabis product is sought, the oral mucosal mode of administration (sublingual) is preferred. Male patients in our study population were more likely to smoke and therefore may be more likely to benefit from advice regarding cannabis smoking cessation. Patients who smoke tobacco were also more likely to smoke cannabis, potentially providing an opportunity to link smoking-related counseling for tobacco and cannabis in the same visit.
The second most common mode of administration indicated was oral intake of edibles. Edible cannabis use increases the risk of acute toxicity from excessive cannabis dosing due to protracted time to maximal plasma concentrations (1–2 hours) of cannabis taken orally compared to time to effect of seconds to minutes when cannabis is inhaled24. Patients should be encouraged to begin at a low dose and increase slowly, particularly when using edible cannabis to avoid ill effects from excessive dose. Females may be somewhat more likely to benefit from provision of this information, given that they are more likely to use edible cannabis in our sample. It has generally been our practice to encourage edibles over other forms of cannabis due to increased ability to control dose while avoiding potential short- and long-term lung toxicity from inhaled forms of cannabis. Mode of administration likely impacts both risk and benefit profiles of cannabis and is therefore an important consideration for providers25.
Reason for cannabis use
A diversity of reasons were endorsed for cannabis use in our patient population, including pain, insomnia, anxiety, recreational, anorexia, nausea, cancer treatment, and depression, similar to findings in the US and abroad in patients with cancer and without cancer though with distributions varying among populations 6, 26, 27. The best evidence for use of cannabis in patients with cancer is for treatment refractory chemotherapy-induced nausea, which is supported by randomized studies9, 28. It was therefore somewhat surprising to see nausea as the 6th most common reason for cannabis use in the present study. Cannabis for radiation-induced nausea has not been studied in a randomized fashion to the knowledge of the authors. There is limited and conflicting data regarding the use of cannabis for many of the reasons reported by patients in our study, though the percentages were similar to other studies in the literature; more data are needed to guide use of cannabis for these symptoms6, 28, 29. It has generally been our practice to pursue therapies with strong evidence and reserve cannabis or cannabinoids for scenarios where these therapies are not valid options for the patient or are ineffective. In cases where cannabis is used, we would begin with a low dose (1–2.5 mg total cannabinoids) balanced THC:CBD edible preparation and have the patient titrate for desired effect with minimal or tolerable side effects.
In our patient population, 5% of individuals using cannabis were doing so in order to treat their cancer. This is lower than numbers found in a dedicated medical cannabis clinic and in a more general survey of patients seen at a cancer center (29% and 26%, respectively) 6, 27. The popular literature is littered with anecdotal claims regarding cannabis; however, to date, there have been no high-quality studies that demonstrate a cancer outcome benefit from the use of cannabis. There is preclinical evidence for cannabis compound activity against cancer cells in the in vitro setting30, 31. Providers can provide valuable context for patients by explaining the key differences between in vitro and clinical studies. It is critical for providers to inform their patients that cannabis is not a treatment for cancer. Cannabis should not take the place of standard of care cancer treatments; this belief could lead to sub-standard care if patients delay or avoid presentation to oncology physicians based on this belief.
Cancer, cannabis, and mental health
Individuals with mental health diagnoses were more likely to endorse recent cannabis use in the present study. Available data give cause for concern. The effects of cannabis on the course of mental health likely varies from condition to condition16–18. Some mental health conditions are exacerbated by the use of cannabis. Regarding depression specifically, one study found that patients with cancer and depression demonstrated higher risk of suicide when they were also using cannabis32. This suggests that additional monitoring for decompensation of mental health conditions and referral to mental health providers are reasonable considerations in patients with cancer using cannabis in the context of mental health conditions. It is clear in our data and in reports of others that there are associations between the use of alcohol, tobacco, and other substances and disorders related to these substances and cannabis use. The relationships among these substances, mental health conditions, cancer, and cannabis should be the topic of future study and should guide providers as they work to optimize management of mental health conditions in patients with cancer.
Patient and provider education
Prior to cannabis use history collection in our clinic, we created a patient information sheet that is freely available through a clearinghouse at our institution (https://www.med.umich.edu/1libr/RadOnc/CannabisAndCancer.pdf). This document has been useful in our clinic, and other providers may find such sheets helpful in their own practices. We would welcome use and adaptation of this document at other centers. This is a living document upon which the authors welcome feedback as the literature grows in quantity and quality.
Limitations and Strengths
This is a retrospective study and may be subject to unforeseen biases or selection pressures unknown to the study team. The methodology used in this study has not been validated against an anonymous survey to determine its accuracy as a research tool. However, this approach can serve as an example of a real-world ready approach that uses electronic medical record-based prompts to remind providers to initiate cannabis use discussions with patients. Data are self-reported and may reflect recall or social desirability biases; however, the most obvious direction of such bias would be for patients to under-report use of cannabis, which only serves to underscore our primary conclusion that cannabis use is frequent enough in this population to merit attention. We did not collect information regarding the chemical composition of the cannabis product utilized by patients, which might influence the interpretation of the data. The data are from a single academic radiation oncology department between 10 and 23 months after legalization of recreational cannabis, and findings may not accurately represent those at community practices or in areas with longer or shorter exposure to legal adult non-medical use cannabis. We were also unable to accurately characterize systemic therapy history in our dataset, limiting our ability to assess chemotherapy impacts on cannabis use. The dataset was not restricted to a single disease site in radiation oncology, limiting site-specific interpretation but allowing for drawing of general conclusions. A summary of disease sites for patients treated with curative intent in the study is included in the supplement (Table S8). In the future, it is our hope to conduct site-specific examinations of cannabis use. The present analyses focused on cross-sectional analyses of reported cannabis use and associated patient characteristics. In the future, longitudinal studies will be possible from this dataset. This study is the largest examination of cannabis use in patients seen in radiation oncology or oncology in general to date to the knowledge of the authors.
Conclusion
Cannabis use history collection is possible and worthwhile given the frequency with which patients use cannabis and their willingness to answer questions related to cannabis use. We have described a mechanism for systematic collection of cannabis use history and guidance on key teaching points for providers and patients with the goal of improving the care of patients with cancer in the age of cannabis legalization.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge the contribution of additional members of the faculty in the Department of Radiation Oncology at the University of Michigan who contributed to this work, including: Kyle Cuneo, MD, Robert Dess, MD, Jason Hearn, MD, Will Jackson, MD, Shruti Jolly, MD, Michelle Kim, MD, Theodore S Lawrence, MD, PhD, Michelle Mierzwa, MD, Lori Pierce, MD, Caitlin Schonewolf, MD, Corey Speers, MD, PhD, and Daniel Wahl, MD, PhD. The authors would also like to acknowledge Steven Kronenberg for his assistance with figure creation.
Funding statement:
This work was supported by funding from the National Institutes of Health (4R01DA033397 to MI) and the Department of Veterans Affairs Health Services Research Development Research (RCS 19-333 to MI). The funding agencies had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest statement:
RJ has stock options as compensation for her advisory board role in Equity Quotient, a company that evaluates culture in health care companies; she has received personal fees from the Greenwall Foundation, Doris Duke Foundation, and the National Institutes of Health and grants or contracts for unrelated work from the National Institutes of Health, the Doris Duke Foundation, the Greenwall Foundation, the Komen Foundation, Genentech, and Blue Cross Blue Shield of Michigan for the Michigan Radiation Oncology Quality Consortium. She has served as an expert witness for Sherinian and Hasso, Dressman Benzinger LaVelle, and Kleinbard, LLC. MI has received grants for unrelated work from the National Institutes of Health, the Department of Veterans Affairs, and the Department of Defense. MI owns shares of Arborsense, a company developing graphene-based sensors for alcohol and drugs.
JAH receives salary support through grant funding from Blue Cross Blue Shield of Michigan via the Michigan Radiation Oncology Quality Consortium (MROQC). MMC, CM, TD, MD, SR, SM, SGA, AKB, KMR, DJH, DME, JT, SB, NE, JE, DE, CH, AML, MM, AFD, JS, and LNC have nothing to disclose.
Footnotes
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Government.
Data sharing statement: Data are available from the Data Office for Clinical and Translational Research at the University of Michigan (contact via email: DataOffice@umich.edu) for researchers who meet the criteria for access to sensitive data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Conference of State Legislatures. State Medical Marijuana Laws (URL: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx). Vol 20212021.
- 2.Chawla D, Yang YC, Desrosiers TA, Westreich DJ, Olshan AF, Daniels JL. Past-month cannabis use among U.S. individuals from 2002–2015: An age-period-cohort analysis. Drug Alcohol Depend. 2018;193:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Vol HHS Publication No. PEP20–07-01–001, NSDUH Series H-55. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2020. [Google Scholar]
- 4.Cousins MM, Jannausch ML, Coughlin LN, Jagsi R, Ilgen MA. Prevalence of cannabis use among individuals with a history of cancer in the United States. Cancer. 2021;127:3437–3444. [DOI] [PubMed] [Google Scholar]
- 5.Cousins MM, Jannausch M, Jagsi R, Ilgen M. Differences between cancer patients and others who use medicinal Cannabis. PLoS One. 2021;16:e0248227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pergam SA, Woodfield MC, Lee CM, et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123:4488–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak J, Liu J, Zou X, et al. Radiation oncologist perceptions of therapeutic cannabis use among cancer patients. Support Care Cancer. 2021;29:5991–5997. [DOI] [PubMed] [Google Scholar]
- 8.Braun IM, Wright A, Peteet J, et al. Medical Oncologists’ Beliefs, Practices, and Knowledge Regarding Marijuana Used Therapeutically: A Nationally Representative Survey Study. Journal of Clinical Oncology. 2018;36:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 10.Neben-Wittich MA. Medical Cannabis to Treat Symptoms From Head and Neck Radiation Therapy. Practical radiation oncology. 2021;11:313–316. [DOI] [PubMed] [Google Scholar]
- 11.Wilkie G, Sakr B, Rizack T. Medical Marijuana Use in Oncology: A Review. JAMA Oncology. 2016;2:670–675. [DOI] [PubMed] [Google Scholar]
- 12.Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Annals of Palliative Medicine. 2017:S215–S222. [DOI] [PubMed]
- 13.Boland EG, Bennett MI, Allgar V, Boland JW. Cannabinoids for adult cancer-related pain: systematic review and meta-analysis. BMJ supportive & palliative care. 2020;10:14–24. [DOI] [PubMed] [Google Scholar]
- 14.Rosewall T, Feuz C, Bayley A. Cannabis and Radiation Therapy: A Scoping Review of Human Clinical Trials. Journal of medical imaging and radiation sciences. 2020;51:342349. [DOI] [PubMed] [Google Scholar]
- 15.Weiss MC, Hibbs JE, Buckley ME, et al. A Coala-T-Cannabis Survey Study of breast cancer patients’ use of cannabis before, during, and after treatment. Cancer. 2022;128:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rup J, Freeman TP, Perlman C, Hammond D. Cannabis and mental health: Prevalence of use and modes of cannabis administration by mental health status. Addictive behaviors. 2021;121:106991. [DOI] [PubMed] [Google Scholar]
- 17.Lowe DJE, Sasiadek JD, Coles AS, George TP. Cannabis and mental illness: a review. Eur Arch Psychiatry Clin Neurosci. 2019;269:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botsford SL, Yang S, George TP. Cannabis and Cannabinoids in Mood and Anxiety Disorders: Impact on Illness Onset and Course, and Assessment of Therapeutic Potential. The American journal on addictions. 2020;29:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbar SA, Tomko RL, Salazar CA, Squeglia LM, McClure EA. Tobacco and cannabis co-use and interrelatedness among adults. Addictive behaviors. 2019;90:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindocha C, Brose LS, Walsh H, Cheeseman H. Cannabis use and co-use in tobacco smokers and non-smokers: prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in Great Britain, 2020. Addiction. 2021;116:2209–2219. [DOI] [PubMed] [Google Scholar]
- 21.Melnikov S, Aboav A, Shalom E, Phriedman S, Khalaila K. The effect of attitudes, subjective norms and stigma on health-care providers’ intention to recommend medicinal cannabis to patients. International Journal of Nursing Practice. 2021;27:e12836. [DOI] [PubMed] [Google Scholar]
- 22.(ALA) ALA. Marijuana and Lung Health. Vol 2021: American Lung Association; 2020. [Google Scholar]
- 23.Graves BM, Johnson TJ, Nishida RT, et al. Comprehensive characterization of mainstream marijuana and tobacco smoke. Scientific Reports. 2020;10:7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical pharmacokinetics. 2003;42:327–360. [DOI] [PubMed] [Google Scholar]
- 25.Vinette B, Côté J, El-Akhras A, Mrad H, Chicoine G, Bilodeau K. Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: a scoping review. BMC Cancer. 2022;22:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvamme SL, Pedersen MM, Alagem-Iversen S, Thylstrup B. Beyond the high: Mapping patterns of use and motives for use of cannabis as medicine. Nordisk Alkohol Nark. 2021;38:270–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghunathan NJ, Brens J, Vemuri S, Li QS, Mao JJ, Korenstein D. In the weeds: a retrospective study of patient interest in and experience with cannabis at a cancer center. Support Care Cancer. 2022;30:7491–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko GD, Bober SL, Mindra S, Moreau JM. Medical cannabis - the Canadian perspective. Journal of pain research. 2016;9:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caputo MP, Rodriguez CS, Padhya TA, Mifsud MJ. Medical Cannabis as Adjunctive Therapy for Head and Neck Cancer Patients. Cureus. 2021;13:e18396-e18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Molecular Cancer Therapeutics. 2011;10:1161–1172. [DOI] [PubMed] [Google Scholar]
- 31.Salazar M, Carracedo A, Salanueva IJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. The Journal of clinical investigation. 2009;119:1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Rahman O. Cannabis use among Canadian adults with cancer (2007–2016): results from a national survey. Expert Review of Pharmacoeconomics & Outcomes Research. 2020:1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.