Abstract
Objective
Breast cancer and breast cancer-directed radiation therapy (RT) may increase the risk of late effects, such as hypothyroidism. We conducted a systematic review and meta-analysis to investigate the association between breast cancer, RT, and risk of hypothyroidism in breast cancer survivors.
Methods
Through February 2022, we searched PubMed, EMBASE, and references of relevant articles, to identify papers on breast cancer and breast cancer-directed RT and subsequent risk of hypothyroidism. Articles were screened by title and abstract and reviewed for eligibility. We used a pre-formed data extraction sheet and identified key design elements that could potentially introduce bias. The main outcome was the confounder-adjusted relative risk (RR) of hypothyroidism in breast cancer survivors versus women without breast cancer, and in breast cancer survivors according to the receipt of RT to the supraclavicular lymph nodes. We used a random-effects model to calculate pooled RRs and associated 95% confidence intervals (95% CI).
Results
From 951 papers screened by title and abstract, 34 full-text papers were reviewed for eligibility. We included 20 studies published between 1985 and 2021–19 were cohort studies. Compared with women without breast cancer, the pooled RR of hypothyroidism in breast cancer survivors was 1.48 (95% CI: 1.17, 1.87), with highest risk associated with RT to the supraclavicular region (RR = 1.69, 95% CI: 1.16, 2.46). The most important limitations of the studies were small sample size yielding estimates with low precision, and lack of data on potential confounders.
Conclusion
Breast cancer and radiation therapy to the supraclavicular lymph nodes is associated with an increased risk of hypothyroidism.
Keywords: Breast cancer, Radiation therapy, Hypothyroidism, Treatment late effects, Meta-analysis
Highlights
-
•
Breast cancer survivors have an increased risk of hypothyroidism.
-
•
Radiation therapy to the supraclavicular lymph nodes further increases this risk.
-
•
The thyroid gland may be an organ at risk in breast cancer patients.
-
•
Using volume-based delineation may spare the thyroid gland from radiation damage.
1. Introduction
The 10-year life expectancy for breast cancer patients in high-income countries is over 75% [1]. Such long life expectancy highlights the need to identify harmful late effects of cancer treatments—one of these potential late effects being hypothyroidism.
Hypothyroidism is characterized by low levels of the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Synthesis and secretion of T3 and T4 is regulated through the release of thyroid-stimulating hormone (TSH). Circulating thyroid hormones exert feedback on TSH secretion. Reference values for TSH are in the range of 0.3–4.0 × 10−3 IU/L, and 12.0–21.0 pmol/L for free T4 (fT4), dependent on the assay used. Subclinical hypothyroidism is defined as increased TSH with normal fT4 values. Overt hypothyroidism is defined as increased TSH with sub-reference levels of fT4. Hypothyroidism can be associated with non-specific symptoms like fatigue, weight gain, increased sensitivity to cold, and muscle weakness, among others [2]. In Europe, the estimated prevalence of overt hypothyroidism is between 0.2% and 5.3% [3]. The incidence is higher in women and increases with age [3]. Although there is no cure for hypothyroidism, symptoms can be controlled via life-long thyroxine substitution.
Hypothyroidism is an established late effect of radiation therapy for head and neck cancer, manifesting months to years after treatment [4,5]. The thyroid gland is not routinely considered an organ-at-risk during radiation therapy in breast cancer patients [6]. However, several studies have suggested an association between breast cancer-directed radiation therapy and increased risk of hypothyroidism, though results are conflicting. To our knowledge, only one systematic review by Darvish et al. published in 2018, investigated the association between breast cancer-directed radiation therapy and hypothyroidism [7]. The review included 14 articles, with five studies included in the meta-analyses. The authors reported increased TSH levels after radiation therapy, indicating subclinical or overt hypothyroidism. Yet, the study provided no overall risk estimates of hypothyroidism after breast cancer radiation therapy.
Since the publication by Darvish and colleagues, several large observational studies have added to the evidence of an increased risk of hypothyroidism in breast cancer survivors [[8], [9], [10]]. These advocate for surveillance for hypothyroidism in breast cancer survivors to expedite diagnosis, disease control, and enhance quality of life. We therefore conducted a systematic review and meta-analysis to collate the evidence on the association of breast cancer, and breast cancer-directed radiation therapy, and subsequent risk of hypothyroidism in breast cancer survivors.
2. Methods and materials
2.1. Study protocol
This study was conducted according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11].
A study protocol was formulated in accordance with PRISMA for protocols and is available upon request [12].
2.2. Search strategy and eligibility criteria
Information sources for this review included the electronic databases PubMed and EMBASE. Through February 2022, we performed a comprehensive, systematic literature search to identify studies on the association between breast cancer or breast cancer treatment and subsequent risk of hypothyroidism. We reviewed reference lists of eligible papers to identify further potentially relevant studies. A full overview of the search strings and results is included in Supplementary Tables 1a and 1b
Studies were eligible for inclusion if they met the following criteria (1): the exposure was breast cancer and/or radiation therapy for breast cancer (2); the outcome was hypothyroidism, both subclinical and overt (3); the full-text paper was published in either English, Danish, Swedish, or Norwegian. We imposed no restrictions on study design or publication year. Only peer-reviewed, published articles were eligible. Meeting abstracts, letters, case descriptions and studies published as abstracts were ineligible for inclusion. Reasons for exclusion were recorded and are presented in Supplementary Table 2.
2.3. Study selection and data extraction
One reviewer (ES) performed the initial screening of titles and abstracts to remove irrelevant studies and retrieved full-text reports of potentially eligible studies. For overlapping studies, the most updated report was included. Subsequently, two investigators (ES, DCF) independently assessed eligibility, data extraction and risk of bias using a pre-specified data abstraction form (see Supplementary Table 3). Where effect estimates and corresponding 95% confidence intervals (95% CI) were not reported, we extracted raw data.
We defined hypothyroidism as diagnostic code of hypothyroidism, with or without prescriptions for thyroxine substitution, and/or biochemical measurements of TSH and/or T3 and T4.
2.4. Assessment of study quality and risk of bias
Due to the disagreement regarding the utility of composite scales for assessing risk of bias in non-randomized studies [13], we assessed the risk of bias by identifying key design elements of the studies’ internal validity a priori using the Guidance on Conducting Systematic reviews and Meta-analyses of Observational Studies of Etiology (COSMOS-E) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines as templates [14,15]. The qualitative review evaluated the potential for selection bias in the study population, information bias with regard to how the exposure, outcome and confounders were ascertained, and the extent of confounder control.
2.5. Statistical analyses
We tabulated the characteristics of the included studies. The main outcome of interest was the confounder-adjusted effect estimate for hypothyroidism risk, comparing breast cancer survivors to the general population, and comparing breast cancer survivors who received radiation therapy to the supraclavicular region with those who received radiation therapy to the breast/chest wall only. Measures of the relative risk (risk ratios, odds ratios, incidence rate ratios, hazard ratios) were considered equivalent given the rare outcome. Where adjusted effect estimates were not provided, raw data were used to calculate the crude estimates and 95% confidence intervals (CI) [8,[16], [17], [18], [19], [20], [21]]. Raw data calculations are available upon request. Using the restricted estimation maximum likelihood random-effects model, we pooled effect estimates displayed in forest plots.
To investigate the impact of different radiation regimes on the risk of hypothyroidism, we pooled results from studies comparing breast cancer survivors who had received radiation therapy to the supraclavicular region with those irradiated to the breast/chest wall only.
To investigate the robustness of the association, we performed two pre-planned sensitivity analyses. First, we restricted to studies with more than 500 breast cancer patients. Second, we restricted our meta-analysis to studies that defined hypothyroidism based on biochemical measures from blood samples, which were likely to capture subclinical hypothyroidism and thereby yield a higher incidence of hypothyroidism. In a separate meta-analysis, we restricted to studies that defined hypothyroidism based on registry data (diagnostic codes or prescription data).
We examined statistical heterogeneity using the I2 statistic. We evaluated the possibility of small study effects by visual inspection of asymmetry in the funnel plots. Funnel plots were constructed by plotting the effect estimate in each study by the inverse of its standard error. All statistical analyses were performed using R Statistical Software (version 4.1.3 (2022-03-10); The R Foundation for Statistical Computing).
3. Results
3.1. Literature search
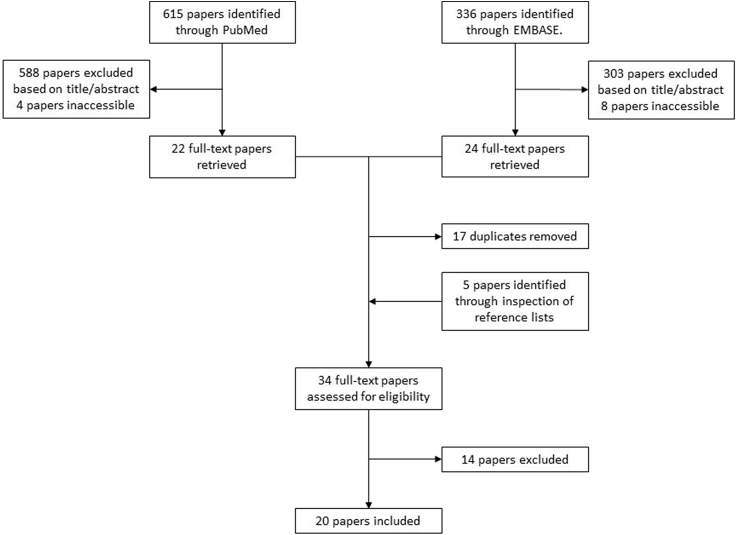
Our literature search identified 615 articles in PubMed and 336 articles in EMBASE. After screening by title and abstract, we retrieved 22 and 24 full text articles from PubMed and EMBASE, respectively; 17 papers were duplicates. We identified five additional papers through inspection of reference lists of the retrieved articles. In total, 34 full text papers were assessed for eligibility. After excluding 14 papers [7,[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]], 20 studies were included in the systematic review [[8], [9], [10],[16], [17], [18], [19], [20], [21],[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]]. Of these, 12 were included in one or several of the meta-analyses. The full study selection process is depicted in Fig. 1
Fig. 1.
Study selection process.
3.2. Study characteristics and quality assessment
An overview of the characteristics of the included studies is provided in Table 1. Nineteen studies were cohort studies [[8], [9], [10],[16], [17], [18], [19], [20],[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] and one was a randomized trial where patients were randomized to chest wall/breast radiation with or without supraclavicular radiation therapy [21]. The studies were conducted in 15 different countries and published between 1985 and 2021; most studies were published after 2010 (15 studies). The studies included 117 935 breast cancer patients in total—range 28 to 44 574 [8,35]. Ten studies included less than 200 study subjects at baseline, limiting precision and increasing in-study variability.
Table 1.
Characteristics of the included studies.
| First author, year, country | Study design | Participants | Age | Comparators | Outcome |
|---|---|---|---|---|---|
| Akyurek, 2014, Turkey | Cohort study | 28 breast cancer patients receiving supraclavicular irradiation between Oct 2010 and Dec 2012 | 50b (range 32–75) | No comparator group | Incidence of hypothyroidism by blood samples |
| Bruning, 1985, Netherlands | Cohort study | 250 female breast cancer patients: | Group: | Breast cancer vs. breast cancer-free | Thyroid profile by blood samples |
| 100 irradiated at the ipsilateral internal mammary artery lymph nodes (I) | I: 57.3a (SD 13.4) | ||||
| 100 mastectomy patients irradiated at the chest wall and ipsilateral internal mammary, infraclavicular, supraclavicular lymph nodes (II) | II: 62.3a (SD 12.2) | Irradiated breast cancer vs. not irradiated breast cancer | |||
| 50 patients admitted for primary breast cancer surgery without irradiation (III) | III: 73.7a (SD 9.0) | ||||
| IV: 69.3a (SD 6.9) | Irradiated to the supraclavicular region vs. irradiated to breast/chest wall only | ||||
| 100 non-breast cancer controls: | V: 68.0a (SD 8.3) | ||||
| 50 women admitted for endometrial carcinoma (IV) 50 women admitted for colorectal carcinoma (V) Patients were treated from Dec 1964 to Jan 1984. |
|||||
| Choi, 2021, Korea | Cohort study | 4073 breast cancer patients receiving radiation therapy after breast cancer surgery from 2007 to 2016. | 50.2b (range 17.9–84.4) | Breast cancer patients irradiated to the supraclavicular region vs. breast cancer patients irradiated to breast/chest wall only | Incidence of hypothyroidism by blood samples |
| Dorri, 2016, Iran | Cohort study | 30 breast cancer patients treated with radiation therapy. | Range 25-38 | No comparator group | Incidence of hypothyroidism by blood samples |
| Treatment period not stated. | |||||
| Falstie-Jensen, 2020, Denmark | Cohort study | 44 574 breast cancer survivors diagnosed 1996–2009 | 61a | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by diagnostic and/or prescription codes |
| 203 306 controls matched on year of birth, municipality of residence | Irradiated breast cancer vs. not irradiated breast cancer | ||||
| Irradiated to the supraclavicular region vs. irradiated to breast/chest wall only | |||||
| Huang, 2021, USA | Cohort study | 192 breast cancer patients treated with radiation therapy to the supraclavicular field from 2007 to 2019 | 54c (range 29–94) | No comparator group | Incidence of hypothyroidism by blood samples or treatment with levothyroxine or another thyroid supplementation |
| Jha, 2020, India | Cohort study | 191 breast cancer patients diagnosed 2015–2017 | Breast cancer patients: 47.6a (SD 10.4) | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by blood samples |
| Healthy controls: 45.8a (SD 9.5) | |||||
| 166 healthy controls | Malignant controls: 53.1a (SD 7.7) | ||||
| 87 malignant controls | |||||
| Joensuu, 1986, Finland | Cohort | 80 breast cancer patients irradiated 1975–1979 | At examination | No comparator group | Incidence of hypothyroidism by blood samples |
| 54.9a (SD 8.2) | |||||
| Kanyilmaz, 2017, Turkey | Cohort study | 243 breast cancer patients treated with radiation therapy 2009–2015. | 53b (range 28–82) | Irradiated to the supraclavicular region vs. irradiated to breast/chest wall only. | Incidence of hypothyroidism by blood samples. |
| 163 irradiated to the breast/chest wall and supraclavicular area. | |||||
| 80 irradiated to the breast/chest wall only. | |||||
| Khan, 2011, Great Britain | Cohort study | 16 938 breast cancer patients. | 66.9a (SD 12.3) | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by READ or OXMIS codes |
| 67 649 controls matched on age, gender, and primary care practice | |||||
| Treatment period not stated. | |||||
| Kikawa, 2017, Japan | Cohort study | 42 breast cancer patients receiving supraclavicular irradiation between 2007 and 2016 | High TSH group: 61.3a (SD 11.7) | No comparator group | Incidence of hypothyroidism by blood samples |
| Normal TSH group: 58.1a (SD 11.4) | |||||
| Kumar, 2018, USA | Cohort study | 261 women with operable, stage I-IIIB breast cancer | Cases 51a (SD 11) | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by blood samples |
| 261 controls matched on age | Controls 50a (SD 10) | ||||
| Treatment period not stated. | |||||
| Laway, 2012, India | Cohort study | 30 breast cancer patients | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by blood samples | |
| 59 healthy controls | |||||
| Treatment period not stated. | |||||
| Ng, 2019, Canada | Cohort study | 12 127 breast cancer patients diagnosed 2005–2009 | 80% aged≥50 years | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by diagnostic and prescription codes based on insurance claims |
| 20 150 controls matched on birth year and index date | |||||
| Pillai, 2019, India | Cohort study | 152 breast cancer patients. | 47b (range 27–72) | No comparator group | Prevalence and incidence of hypothyroidism by blood samples |
| Treatment period not stated. | |||||
| Reinertsen, 2009, Norway | Cohort study | 403 breast cancer patients treated 1998–2002. | At survey | Breast cancer vs. breast cancer-free | Data on hypothyroidism mainly based on self-report, supplemented with thyroid function tests |
| Cases 58b (range 32–78) | |||||
| 2015 age-matched controls | Controls 57b (range 33–79) | ||||
| Saraiva, 2005, Brazil | Cohort study | 26 breast cancer patients submitted to surgery | Range 30-85 | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by blood samples |
| 22 women with normal mammograms | |||||
| Treatment period not stated. | |||||
| Smith, 2008, USA | Cohort study | 38 255 breast cancer patients diagnosed 1992–2002 | 75a (SD 6) | Breast cancer vs. breast cancer-free | Incidence of hypothyroidism by diagnostic codes |
| 111 944 healthy controls | Irradiated breast cancer vs. not irradiated breast cancer | ||||
| Irradiated to the supraclavicular region vs. irradiated to breast/chest wall only | |||||
| Tunio, 2015, Saudia-Arabia | Randomized prospective trial | 20 patients with stage IIA-IIIC, operable breast cancer treated with ipsilateral supraclavicular radiation therapy. | SC group: 47a (range 23–76) | Irradiated to the supraclavicular region vs. irradiated to breast/chest wall only | Incidence of hypothyroidism by blood samples |
| 20 patients with stage IIA-IIIC breast cancer treated with radiation therapy to the breast and chest wall | Control group: 46a (range 23–67) | ||||
| Treatment period not stated. | |||||
| Wolny-Rokicka, 2016, Poland | Cohort study | 38 breast cancer patients irradiated to the breast after breast conserving surgery or mastectomy (BCT) | BCT: 58b | Irradiated to the supraclavicular region vs. irradiated to the breast/chest wall only | Incidence of hypothyroidism by blood samples |
| 32 breast cancer patients irradiated to the breast and supraclavicular lymph nodes (SC-RT) | SC-RT 55.5b | ||||
| Patients were treated between April 2012 and May 2015. |
Mean age.
Median age.
Does not state if mean or median.
The definition and way of measuring hypothyroidism varied considerably (see Supplementary Table 4). Sixteen studies evaluated thyroid function using biochemical methods such as serum values of TSH, fT4 and free T3 (fT3) [10,[16], [17], [18], [19], [20], [21],[35], [36], [37], [38], [39],[41], [42], [43],45]. Of these, eleven studies used serum concentrations of TSH, fT4 and/or fT3 to define a diagnosis of hypothyroidism, while five studies used elevated TSH levels only. The reference ranges for TSH, fT3 and fT4 varied slightly between studies, depending on national guidelines and assays used. Five studies used diagnostic codes and/or prescription data from databases to define hypothyroidism [8,9,37,40,44]. Huang et al. assessed thyroid function by both biochemical tests and registry data [37]. In the studies by Choi et al. and Huang et al. patients were not routinely screened for hypothyroidism. Instead, the decision to evaluate serum thyroid hormone levels depended on the treating physician [10,37]. Accordingly, some cases of hypothyroidism may have been missed, leading to misclassification.
The follow-up time in the cohort studies varied substantially. Falstie-Jensen et al. had the longest follow-up, with a median follow-up time of 8.4 years in the breast cancer group [8]. The study by Khan et al. was restricted to patients who had survived for 5 years or more after breast cancer diagnosis [40]. In comparison, the maximum follow-up was 1 year in the study by Jha et al. [38]. Two studies reported a median time to onset of hypothyroidism of more than 1.5 years [10,20]. As such, the studies with short follow-up may have had insufficient time for hypothyroidism development.
Eight studies did not include a comparison group or did not provide enough information to calculate risk estimates from raw data [[35], [36], [37], [38], [39],41,42,45]. None of these were included in the meta-analyses. Nine studies [8,9,[16], [17], [18], [19],40,43,44] focused on the risk of hypothyroidism in breast cancer survivors versus the general population; three reported on the risk of hypothyroidism after radiation therapy compared with no radiation therapy [8,16,44]. Six studies reported the risk of hypothyroidism according to radiation therapy modality (i.e., radiation to breast/chest wall only vs. radiation to supraclavicular lymph nodes) [8,10,16,20,21,44]. Two studies used hospital controls, which may have introduced selection bias, as the prevalence of hypothyroidism in the hospital controls may not reflect that in the general population [16,38].
Most studies standardized results by age, via matching or adjustment. Several studies also adjusted for other potential confounders, such as comorbidities, tumor stage and grade, estrogen receptor status, number of physician visits and socioeconomic factors, among others. In the papers by Dorri et al. Akyurek et al. and Pillai et al. no information was provided on whether adjustments were made; these studies were judged at high risk of bias [35,36,42].
General limitations of the studies included small sample size, lack of data on potentially important risk factors for hypothyroidism such as lifestyle factors and comorbidities, and selection bias.
3.3. Meta-analyses
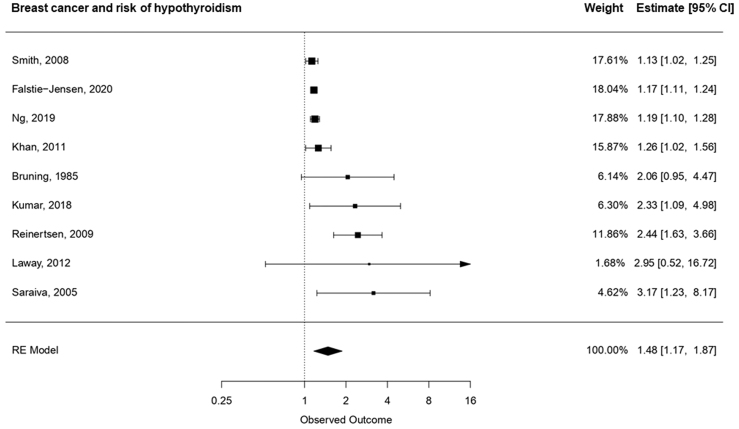
We observed considerable statistical heterogeneity, as indicated by an overall I2 statistic of 93.8% (95% CI: 69.3%, 99.0%). To investigate the risk of hypothyroidism in breast cancer survivors, we pooled results from nine studies comparing the incidence of hypothyroidism in breast cancer survivors versus healthy controls [8,9,[16], [17], [18], [19],40,43,44]. As summarized in Fig. 2a, breast cancer survivors had increased risk of hypothyroidism, with a pooled relative risk of 1.48 (95% CI: 1.17, 1.87). The results did not vary by publication year.
Fig. 2a.
Pooled relative risk and associated 95% CIs for the association between breast cancer and hypothyroidism, ordered according to the magnitude of the relative risk.
Due to only three eligible studies, we did not generate a pooled estimate for studies that investigated the risk of hypothyroidism among breast cancer survivors who received radiation therapy compared with those who did not [8,16,44].
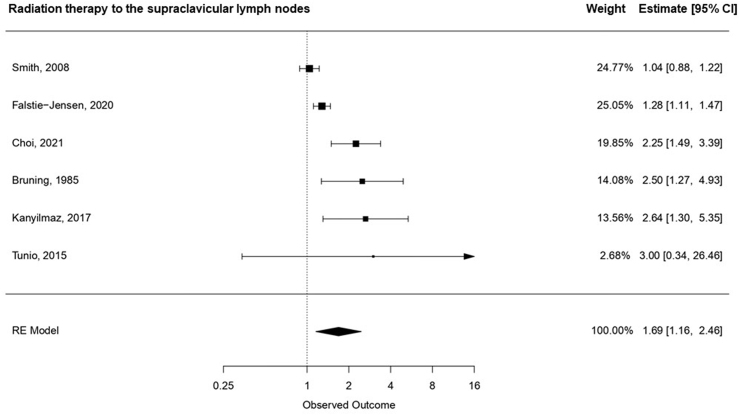
Among the six studies that investigated the effect of supraclavicular radiation therapy on the risk of hypothyroidism [8,10,16,20,21,44], radiation therapy to the supraclavicular field was associated with a 69% increased risk of hypothyroidism (95% CI: 1.16, 2.46) (Fig. 2b) compared with radiation to the breast and chest wall only.
Fig. 2b.
Pooled relative risk and associated 95% CIs for the association between radiation therapy to the supraclavicular lymph nodes and hypothyroidism, ordered according to the magnitude of the relative risk.
When we restricted to studies with large sample size (>500 breast cancer patients), the pooled estimate was 1.17 (95% CI: 1.13, 1.22). The risk estimates were higher when restricting to a biochemistry-based diagnosis of hypothyroidism (pooled estimate 2.44 (95% CI 1.80, 3.30)) compared with a register-based diagnosis (pooled estimate 1.17 (95% CI 1.13, 1.22).
Visual inspection of the funnel plots indicated some asymmetry indicating publication bias or small study effects.
4. Discussion
Our systematic review and meta-analyses suggested that breast cancer survivors have an increased risk of hypothyroidism compared with women without a history of breast cancer. Radiation therapy, particularly when directed to the supraclavicular field, was associated with increased risk of hypothyroidism.
Several factors warrant consideration when interpreting our findings. Our findings may be attributable to detection bias—breast cancer patients may be more likely to be diagnosed with hypothyroidism due to more frequent contact with the healthcare system. Smith et al. found that hypothyroidism was associated with an increased number of physician visits, supporting such bias [44]. Nevertheless, after adjusting for the number of physician visits, the association between breast cancer and elevated risk of hypothyroidism remained in their study. Confounding factors such as differences in iodide intake due to socioeconomic position might vary across studies, and only five studies adjusted for confounding by socioeconomic position [8,9,40,43,44]. Low iodide intake is associated with hypothyroidism, and low socioeconomic position [46].
There may be a true causal association between breast cancer and/or breast cancer-directed radiation therapy and hypothyroidism. Breast cancer is associated with increased concentrations of cytokines, such as tumor necrosis factor. Breast cancer-associated alterations in cytokine signaling may inhibit the hypothalamus-pituitary-thyroid axis as well as the thyrocytes, mediating a causal effect between breast cancer and hypothyroidism [47,48]. Still, radiation therapy for head and neck cancers and lymphoma is associated with increased risk of hypothyroidism, suggesting that radiation therapy may be the underlying cause [4,5]. Radiation therapy may damage small thyroid vessels and the thyroid capsule, as well as radiation-induced atherosclerosis and direct thyroid cell injury, resulting in thyroid dysfunction and hypothyroidism [49]. Nonetheless, the design of the studies included in our systematic review and meta-analysis did not enable us to distinguish whether it is breast cancer itself, breast cancer treatment, or perhaps the two combined that contributed to the excess risk of hypothyroidism in breast cancer survivors.
Other factors may contribute to an elevated risk of hypothyroidism in breast cancer survivors. These include the dose of radiation received by the thyroid gland. Higher mean radiation dose to the thyroid gland has been documented in patients who received radiation therapy including the supraclavicular field compared with radiation therapy to the breast or chest wall only, with/without the regional axillary lymph nodes [10]. The mean radiation dose received by the thyroid gland was higher in patients who developed hypothyroidism than in the euthyroid patients [10]. A study by Reinertsen et al. found that CT-guided radiation therapy was associated with increased risk of post-breast cancer hypothyroidism compared with standardized field radiation therapy [43]. The authors hypothesized that this was due to higher radiation dose to the thyroid associated with the CT-based technique where an unconventional cranial oblique field resulted in an exit dose of radiation hitting the neck region.
The volume of an individual patient's thyroid gland may also influence the risk of hypothyroidism after radiation therapy. Smaller thyroid volume has also been associated with increased risk of hypothyroidism [21]. A case-control study found that breast cancer patients with post-radiation therapy hypothyroidism had significantly smaller thyroid gland volume compared with their age-matched euthyroid controls [50]. These findings are reflected in the study by Huang et al. who found that patients with larger thyroid volumes spared from irradiation had lower risk of hypothyroidism [37]. A smaller thyroid gland and a higher mean radiation dose has also been associated with increased risk of hypothyroidism in studies investigating radiation-induced hypothyroidism in patients with head and neck cancers [51]. Together, these findings suggest that radiation therapy damaging the thyroid gland may lead to hypothyroidism, though further evidence is needed incorporating detailed information on the radiation dose to the thyroid and evaluating a dose-response association.
Hypothyroidism may manifest as a delayed effect of radiation therapy [20]. Kanyilmaz et al. reported a cumulative incidence of hypothyroidism increasing from 8% three years after radiation therapy to 35% at five years [20]. Among patients who received radiation therapy to the supraclavicular field, the cumulative incidence increased from 10% to 40% at years three and five, respectively. Kanyilmaz et al. and Choi et al. found that the association between lymph node irradiation and hypothyroidism was more prominent in younger breast cancer patients [10,20]. If younger patients are more susceptible to radiation-induced sequelae, such as hypothyroidism, this may have contributed to the results in the afore-mentioned paper by Smith et al. [44]. Their study population included women eligible for Medicare—therefore aged over 65 years. As the risk of hypothyroidism increases with age, the excess risk in breast cancer survivors may be less pronounced compared with aged-matched women without breast cancer (HR = 1.13, 95% CI 1.10, 1.35). Still, there was little difference in the risk of hypothyroidism in patients treated with radiation therapy to the supraclavicular lymph nodes compared with patients who did not receive radiation therapy (HR = 1.04, 95% CI 0.89, 1.23).
Radiation therapy techniques have evolved over time. Over the past decade, volume-based radiation therapy has replaced the more traditional field-based techniques. In 2015, the European Society for Radiotherapy and Oncology (ESTRO) published guidelines for target volume contouring [52,53]. One of the major modifications in the ESTRO guidelines compared to previous guidelines was the shift from the “supraclavicular” area towards what is now referred to as clinical target volume lymph node level 4 (CTVn-L4) [52]. For most patients, the thyroid gland is excluded from the radiation field, and the radiation dose to the thyroid gland is reduced [54]. However, most of the studies included in the current systematic review and meta-analysis, included patients who were treated with radiation therapy before 2015. Our results therefore reflect how radiation therapy may have impacted thyroid function before the ESTRO guidelines were implemented. It is likely that dissemination of the guidelines into clinical practice will reduce the risk of radiation damage to the thyroid gland. We therefore anticipate that our observed excess risk of hypothyroidism in breast cancer survivors will diminish in contemporary patients.
Our study has some additional limitations. First, we note that only two of the studies included men [18,35]. Hypothyroidism is more prevalent in women, which could explain some of the observed excess risk [3]. However, the comparator group in the studies were also women, so the baseline prevalence should be comparable between the two groups. As such, it seems unlikely that the observed excess risk of hypothyroidism in breast cancer survivors is attributable to sex. Furthermore, studies in head and neck cancer patients, which include both men and women, have also shown increased risk of hypothyroidism after radiation therapy directed to the supraclavicular region, supporting our findings [5]. Second, the quality of the systematic review is directly related to the quality of the included studies. Thus, their limitations must be considered when interpreting our findings. We note that several studies in our review had small sample size, leading to risk estimates with low precision. Still, when we restricted to studies with more than 500 breast cancer patients, the excess risk of hypothyroidism remained.
The definition and method of measuring hypothyroidism varied across studies. Most studies measured levels of TSH and/or T3 and/or T4 in blood samples; however, some used increased TSH levels only, while others used increased TSH in combination with lowered T3 and/or T4 to identify hypothyroidism. Subclinical hypothyroidism does not have an ICD code and was not included in the register-based studies; possibly leading to misclassification. We expect such misclassification to be non-differential in breast cancer survivors and their non-cancer counterparts. For overt hypothyroidism, we anticipate some differential misclassification due to more frequent healthcare contact in breast cancer survivors compared with individuals without cancer; this is supported by our sensitivity analyses. Finally, several studies were difficult to interpret due to a lack of transparency in their methods section; some failed to describe the selection of the study population [35,36].
Although we used a broad study search and a systematic design, we may have missed relevant studies if the investigated relationship was not explicitly mentioned in the title, abstract or key words of the papers. Furthermore, limiting by language may have biased our review and meta-analysis towards the inclusion of studies with statistically significant results, as authors might be more likely to publish results in an English language journal if the results are statistically significant [55].
Compared with the systematic review by Darvish et al. the current study has several advantages. First, we included more articles and more recent evidence on the association. The meta-analysis by Darvish and colleagues included 478 participants as compared with over 500 000 study subjects included in one of the three meta-analyses performed in this study. Darvish et al. used cases as their own controls (i.e., before/after radiation therapy) thereby precluding comparison with the background population. Nevertheless, Darvish and colleagues concluded that the thyroid gland is an organ-at-risk in breast cancer patients receiving radiation therapy, corroborating our findings.
Some of the included studies also investigated the relationship between chemotherapy and endocrine therapy and risk of hypothyroidism, either alone or when combined with radiation therapy [8,10,17,20,21,38,44], which was not an objective of the current review. Radiation therapy to the supraclavicular lymph nodes is administered to high-risk patients. It is therefore likely that these patients were treated with systemic therapy such as chemotherapy and/or endocrine therapy in addition to radiation therapy. Systemic therapy may affect thyroid function [56,57]. A recently published systematic review suggested slightly increased risk of thyroid dysfunction in patients treated with tamoxifen, but not with the aromatase inhibitor letrozole [56]. Cytotoxic effects of chemotherapy may also impact thyroid function, and may sensitize the thyroid gland to radiation therapy [57]. Taken together, these findings suggest that the interplay and potential synergistic effects of systemic therapy and radiation therapy on thyroid dysfunction should be clarified in future studies.
In conclusion, this systematic review suggests that breast cancer survivors, especially those treated with radiation therapy directed to the supraclavicular region, have increased risk of hypothyroidism. These findings indicate that the thyroid gland may be an organ-at-risk in breast cancer patients receiving radiation therapy. Nonetheless, further dissemination of the ESTRO 2015 guidelines, which recommended volume-based delineation, are likely to mitigate damage to the thyroid gland and decrease any potential excess risk of hypothyroidism in contemporary breast cancer patients.
Funding
This work was supported by grants to DCF from The Independent Research Fund Denmark, Medicine (DFF-4183-00359) and the Eva and Henry Frænkels Foundation, Denmark. Elisabeth Solmunde is supported by a grant from The Independent Research Fund Denmark, Medicine (1149-0013 B) to DCF and a grant from the Danish Cancer Society (R320-A18464-B5768) to ES.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.02.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro L.E., Surks M.I. In: Principles and practice of endocrinology and metabolism. third ed. Becker K.L., editor. LIPPINCOTT WILLIAMS & WILKINS; Philadelphia, PA 19106 USA: 2001. Hypothyroidism; pp. 445–453. [Google Scholar]
- 3.Chiovato L., Magri F., Carlé A. Hypothyroidism in context: where we've been and where we're going. Adv Ther. 2019;36(Suppl 2):47–58. doi: 10.1007/s12325-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alterio D., Jereczek-Fossa B.A., Franchi B., D'Onofrio A., Piazzi V., Rondi E., et al. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: a retrospective analysis of seventy-three patients. Int J Radiat Oncol Biol Phys. 2007;67(1):144–150. doi: 10.1016/j.ijrobp.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 5.Feen Rønjom M. Radiation-induced hypothyroidism after treatment of head and neck cancer. Dan Med J. 2016;63(3) [PubMed] [Google Scholar]
- 6.Sciacca F. Organs at risk. Reference article 2020 [Available from: https://radiopaedia.org/articles/80650.
- 7.Darvish L., Ghorbani M., Teshnizi S.H., Roozbeh N., Seif F., Bayatiani M.R., et al. Evaluation of thyroid gland as an organ at risk after breast cancer radiotherapy: a systematic review and meta-analysis. Clin Transl Oncol. 2018;20(11):1430–1438. doi: 10.1007/s12094-018-1875-7. [DOI] [PubMed] [Google Scholar]
- 8.Falstie-Jensen A.M., Esen B., Kjærsgaard A., Lorenzen E.L., Jensen J.D., Reinertsen K.V., et al. Incidence of hypothyroidism after treatment for breast cancer-a Danish matched cohort study. Breast Cancer Res. 2020;22(1):106. doi: 10.1186/s13058-020-01337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng H.S., Vitry A., Koczwara B., Roder D., McBride M.L. Patterns of comorbidities in women with breast cancer: a Canadian population-based study. Cancer Causes Control. 2019;30(9):931–941. doi: 10.1007/s10552-019-01203-0. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.H., Chang J.S., Byun H.K., Son N.H., Hong C.S., Hong N., et al. Risk of hypothyroidism in women after radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2021;110(2):462–472. doi: 10.1016/j.ijrobp.2020.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase S.C. Systematic reviews and meta-analysis. Plast Reconstr Surg. 2011;127(2):955–966. doi: 10.1097/PRS.0b013e318200afa9. [DOI] [PubMed] [Google Scholar]
- 14.Dekkers O.M., Vandenbroucke J.P., Cevallos M., Renehan A.G., Altman D.G., Egger M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16(2) doi: 10.1371/journal.pmed.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., et al. Strengthening the reporting of observational studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Bruning P., Bonfrer J., De Jong-Bakker M. Primary hypothyroidism in breast cancer patients with irradiated supraclavicular lymph nodes. Br J Cancer. 1985;51(5):659–663. doi: 10.1038/bjc.1985.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N.B., Fink A., Levis S., Xu P., Tamura R., Krischer J. Thyroid function in the etiology of fatigue in breast cancer. Oncotarget. 2018;9(39):25723–25737. doi: 10.18632/oncotarget.25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laway B.A., Shafi K.M., Majid S., Lone M.M., Afroz F., Khan S., et al. Incidence of primary hypothyroidism in patients exposed to therapeutic external beam radiation, where radiation portals include a part or whole of the thyroid gland. Indian J Endocrinol Metab. 2012;16(Suppl 2):S329–S331. doi: 10.4103/2230-8210.104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraiva P.P., Figueiredo N.B., Padovani C.R., Brentani M.M., Nogueira C.R. Profile of thyroid hormones in breast cancer patients. Braz J Med Biol Res. 2005;38(5):761–765. doi: 10.1590/s0100-879x2005000500014. [DOI] [PubMed] [Google Scholar]
- 20.Kanyilmaz G., Aktan M., Koc M., Demir H., Demir L.S. Radiation-induced hypothyroidism in patients with breast cancer: a retrospective analysis of 243 cases. Med Dosim. 2017;42(3):190–196. doi: 10.1016/j.meddos.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Tunio M.A., Al Asiri M., Bayoumi Y., Stanciu L.G., Al Johani N., Al Saeed E.F. Is thyroid gland an organ at risk in breast cancer patients treated with locoregional radiotherapy? Results of a pilot study. J Cancer Res Therapeut. 2015;11(4):684–689. doi: 10.4103/0973-1482.167613. [DOI] [PubMed] [Google Scholar]
- 22.Cella L., Liuzzi R., Conson M., D'Avino V., Salvatore M., Pacelli R. Development of multivariate NTCP models for radiation-induced hypothyroidism: a comparative analysis. Radiat Oncol. 2012;7(1) doi: 10.1186/1748-717X-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim N., Chang J.S., Shah C., Shin H., Keum K.C., Suh C.O., et al. Hypofractionated volumetric-modulated arc therapy for breast cancer: a propensity-score-weighted comparison of radiation-related toxicity. Int J Cancer. 2021;149(1):149–157. doi: 10.1002/ijc.33525. [DOI] [PubMed] [Google Scholar]
- 24.Mirsadraei M., Seilanian Toosi M., Mohebbi S., Khalili-Hezarjaribi H. Evaluating dose to thyroid gland in women with breast cancer during radiotherapy with different radiation energies at supraclavicular fossa region. J Radiother Pract. 2018;17(4):436–440. [Google Scholar]
- 25.Namdar A.M., Mohammadzadeh M., Okutan M., Mesbahi A. A review on the dosimetrical and radiobiological prediction of radiation-induced hypothyroidism in radiation therapy of head-and-neck cancer, breast cancer, and Hodgkin's lymphoma survivors. Pol J Med Phys Eng. 2018;24(2):137–148. [Google Scholar]
- 26.Pulickal S.G., Sebastian N., Bhaskaran R., Aparna P. Effect of change in neck position on thyroid dose and volume in supraclavicular irradiation for breast cancer using conformal technique. J Radiother Pract. 2022; 21(2):234-238 [Google Scholar]
- 27.Torino F., Barnabei A., Paragliola R., Baldelli R., Appetecchia M., Corsello S.M. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23(11):1345–1366. doi: 10.1089/thy.2013.0241. [DOI] [PubMed] [Google Scholar]
- 28.Mireștean C.C., Iancu R.I., Iancu D.P.T. An underestimated toxicity radiation-induced hypothyroidism in patients multimodally treated for breast cancer. J Clin Med. 2021;10(23) doi: 10.3390/jcm10235503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyrop K.A., Damone E.M., Deal A.M., Carey L.A., Lorentsen M., Shachar S.S., et al. Obesity, comorbidities, and treatment selection in Black and White women with early breast cancer. Cancer. 2021;127(6):922–930. doi: 10.1002/cncr.33288. [DOI] [PubMed] [Google Scholar]
- 30.Gul A., Faaruq S., Abbasi N.Z., Siddique T., Ali A., Shehzadi N.N., et al. Estimation of absorbed dose to thyroid in patients treated with radiotherapy for various cancers. Radiat Protect Dosim. 2013;156(1):37–41. doi: 10.1093/rpd/nct043. [DOI] [PubMed] [Google Scholar]
- 31.Schottenfeld D. The relationship of breast cancer to thyroid disease. J Chron Dis. 1968;21(5):303–313. doi: 10.1016/0021-9681(68)90039-8. [DOI] [PubMed] [Google Scholar]
- 32.Moossa A.R., Evans D.A., Brewer A.C. Thyroid status and breast cancer. Reappraisal of an old relationship. Ann R Coll Surg Engl. 1973;53(3):178–188. [PMC free article] [PubMed] [Google Scholar]
- 33.Falstie-Jensen A.M., Kjærsgaard A., Lorenzen E.L., Jensen J.D., Reinertsen K.V., Dekkers O.M., et al. Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality - a Danish population-based study. Breast Cancer Res. 2019;21(1):44. doi: 10.1186/s13058-019-1122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlan L.C., Klabunde C.N., Ambs A.H., Gibson T., Bernstein L., McTiernan A., et al. Comorbidities, therapy, and newly diagnosed conditions for women with early stage breast cancer. J Cancer Surviv. 2009;3(2):89–98. doi: 10.1007/s11764-009-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akyurek S., Babalioglu I., Kose K., Gokce S.C. Thyroid dysfunction following supraclavicular irradiation in the management of carcinoma of the breast. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi. 2014;24(2):139–144. [Google Scholar]
- 36.Dorri Giv M., Bahreini Toosi M.H., Aghamiri S.M., Akbari F., Taeb S. Calculation of thyroid dose with planner system and evaluation of thyroid function after radiotherapy for patients with breast cancer. J Biomed Phys Eng. 2016;6(4):220–234. [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H., Roberson J., Hou W., Mani K., Valentine E., Ryu S., et al. NTCP model for hypothyroidism after supraclavicular-directed radiation therapy for breast cancer. Radiother Oncol. 2021;154:87–92. doi: 10.1016/j.radonc.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Jha C.K., Mishra A., Yadav S.B., Agarwal G., Singh S., Chand G., et al. Thyroid dysfunctions and autoimmunity in breast cancer patients: a prospective case-control study. Arch Endocrinol Metab. 2021;64(6):743–750. doi: 10.20945/2359-3997000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joensuu H., Viikari J. Thyroid function after postoperative radiation therapy in patients with breast cancer. Acta Radiol Oncol. 1986;25(3):167–170. doi: 10.3109/02841868609136397. [DOI] [PubMed] [Google Scholar]
- 40.Khan N.F., Mant D., Carpenter L., Forman D., Rose P.W. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105(Suppl 1):S29. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikawa Y., Kosaka Y., Hashimoto K., Hohokabe E., Takebe S., Narukami R., et al. Prevalence of hypothyroidism among patients with breast cancer treated with radiation to the supraclavicular field: a single-centre survey. ESMO Open. 2017;2(1) doi: 10.1136/esmoopen-2017-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai U.S., Kayal S., Cyriac S., Nisha Y., Dharanipragada K., Kamalanathan S.K., et al. Late effects of breast cancer treatment and outcome after corrective interventions. Asian Pac J Cancer Prev APJCP. 2019;20(9):2673–2679. doi: 10.31557/APJCP.2019.20.9.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinertsen K.V., Cvancarova M., Wist E., Bjøro T., Dahl A.A., Danielsen T., et al. Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol Biol Phys. 2009;75(3):764–770. doi: 10.1016/j.ijrobp.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 44.Smith G.L., Smith B.D., Giordano S.H., Shih Y.C., Woodward W.A., Strom E.A., et al. Risk of hypothyroidism in older breast cancer patients treated with radiation. Cancer. 2008;112(6):1371–1379. doi: 10.1002/cncr.23307. [DOI] [PubMed] [Google Scholar]
- 45.Wolny-Rokicka E., Tukiendorf A., Wydmański J., Roszkowska D., Staniul B.S., Zembroń-Łacny A. Thyroid function after postoperative radiation therapy in patients with breast cancer. Asian Pac J Cancer Prev APJCP. 2016;17(10):4577–4581. doi: 10.22034/APJCP.2016.17.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen N., Bülow I., Laurberg P., Ovesen L., Perrild H., Jørgensen T. Low socio-economic status and familial occurrence of goitre are associated with a high prevalence of goitre. Eur J Epidemiol. 2003;18(2):175–181. doi: 10.1023/a:1023001400945. [DOI] [PubMed] [Google Scholar]
- 47.Pang X.P., Yoshimura M., Hershman J.M. Suppression of rat thyrotroph and thyroid cell function by tumor necrosis factor-alpha. Thyroid. 1993;3(4):325–330. doi: 10.1089/thy.1993.3.325. [DOI] [PubMed] [Google Scholar]
- 48.Poth M., Tseng Y.C., Wartofsky L. Inhibition of TSH activation of human cultured thyroid cells by tumor necrosis factor: an explanation for decreased thyroid function in systemic illness? Thyroid. 1991;1(3):235–240. doi: 10.1089/thy.1991.1.235. [DOI] [PubMed] [Google Scholar]
- 49.Jereczek-Fossa B.A., Alterio D., Jassem J., Gibelli B., Tradati N., Orecchia R. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30(4):369–384. doi: 10.1016/j.ctrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Johansen S., Reinertsen K.V., Knutstad K., Olsen D.R., Fosså S.D. Dose distribution in the thyroid gland following radiation therapy of breast cancer--a retrospective study. Radiat Oncol. 2011;6:68. doi: 10.1186/1748-717X-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rønjom M.F., Brink C., Bentzen S.M., Hegedüs L., Overgaard J., Petersen J.B., et al. External validation of a normal tissue complication probability model for radiation-induced hypothyroidism in an independent cohort. Acta Oncol (Stockh) 2015;54(9):1301–1309. doi: 10.3109/0284186X.2015.1064160. [DOI] [PubMed] [Google Scholar]
- 52.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Biete Sola A., et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 53.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Sola A.B., et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118(1):205–208. doi: 10.1016/j.radonc.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Kaidar-Person O., Offersen B.V., Boersma L., Meattini I., Dodwell D., Wyld L., et al. Tricks and tips for target volume definition and delineation in breast cancer: lessons learned from ESTRO breast courses. Radiother Oncol. 2021;162:185–194. doi: 10.1016/j.radonc.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 55.Egger M., Zellweger-Zähner T., Schneider M., Junker C., Lengeler C., Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350(9074):326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]
- 56.Marina D., Rasmussen Å K., Buch-Larsen K., Gillberg L., Andersson M., Schwarz P. Influence of the anti-oestrogens tamoxifen and letrozole on thyroid function in women with early and advanced breast cancer: a systematic review. Cancer Med. 2023;12(2):967-982 doi: 10.1002/cam4.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hancock S.L., Cox R.S., McDougall I.R. Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med. 1991;325(9):599–605. doi: 10.1056/NEJM199108293250902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.