Abstract
Cytoplasmic stresses, including heat shock, osmotic stress, and oxidative stress, cause rapid inhibition of protein synthesis in cells through phosphorylation of eukaryotic initiation factor 2α (eIF2α) by eIF2α kinases. We have investigated the role of heme-regulated inhibitor (HRI), a heme-regulated eIF2α kinase, in stress responses of erythroid cells. We have demonstrated that HRI in reticulocytes and fetal liver nucleated erythroid progenitors is activated by oxidative stress induced by arsenite, heat shock, and osmotic stress but not by endoplasmic reticulum stress or nutrient starvation. While autophosphorylation is essential for the activation of HRI, the phosphorylation status of HRI activated by different stresses is different. The contributions of HRI in various stress responses were assessed with the aid of HRI-null reticulocytes and fetal liver erythroid cells. HRI is the only eIF2α kinase activated by arsenite in erythroid cells, since HRI-null cells do not induce eIF2α phosphorylation upon arsenite treatment. HRI is also the major eIF2α kinase responsible for the increased eIF2α phosphorylation upon heat shock in erythroid cells. Activation of HRI by these stresses is independent of heme and requires the presence of intact cells. Both hsp90 and hsc70 are necessary for all stress-induced HRI activation. However, reactive oxygen species are involved only in HRI activation by arsenite. Our results provide evidence for a novel function of HRI in stress responses other than heme deficiency.
Protein synthesis in intact reticulocytes and their lysates is dependent on the availability of heme. In heme deficiency, protein synthesis is inhibited, with a marked decrease in the formation of 40S–eukaryotic initiation factor 2 (eIF2)–Met-tRNAf GTP (43S preinitiation complex) and with increased phosphorylation of the α subunit of eIF2. eIF2 is an initiation factor which binds GTP and Met-tRNAf and is required for the formation of the 43S initiation complex. The phosphorylation of eIF2α in heme deficiency is the result of the activation of heme-regulated inhibitor (HRI), which is a heme-regulated eIF2α kinase (8, 9). The mechanism of inhibition of translation initiation by the phosphorylation of eIF2α has been studied extensively (18, 19). In brief, the recycling of eIF2 in initiation requires the exchange of bound GDP for GTP. Under physiological conditions, eIF2 has a 400-fold-greater affinity for GDP than for GTP. The exchange of tightly bound GDP for GTP requires eIF2B, which is rate limiting and is present at 15 to 25% of the amount of eIF2. When eIF2α is phosphorylated, the binding of eIF2(αP)-GDP to the regulatory subcomplex of eIF2B is much tighter than the binding of eIF2-GDP to eIF2B (25). This tighter interaction of phosphorylated eIF2 with eIF2B prevents the GDP-GTP exchange activity of eIF2B. In this manner, once the amount of phosphorylated eIF2 exceeds the amount of eIF2B, the shutoff of protein synthesis occurs.
Phosphorylation of eIF2α was later found to be a common regulatory mechanism of protein translation under various stress conditions and is carried out by the family of eIF2α kinases. In addition to HRI, there are three other known eIF2α kinases at present. They are the interferon-induced double-stranded RNA (dsRNA)-dependent eIF2α kinase (PKR), the GCN2 protein kinase, and the recently identified mammalian endoplasmic reticulum (ER) resident kinase (PERK), which is identical to PEK that is highly expressed in the pancreas (17, 38). These eIF2α kinases share extensive homology in their kinase catalytic domains and phosphorylate eIF2α at the serine 51 residue (reviewed in reference 23). However, the regulatory domains and the regulatory mechanism of each of these eIF2α kinases are very different. PKR is regulated by dsRNA through two N-terminal dsRNA-binding domains and is activated by dsRNA upon viral infection (reviewed in reference 33). GCN2 is activated under the condition of amino acid starvation through the C-terminal domain, which contains a His-tRNA synthase-like sequence (3, 20, 40). PERK is essential for ER stress and is activated by ER stress through its luminal domain, which is similar to the sensor domain of the ER-stress kinase Irel (16, 17, 38). HRI is regulated by heme through the two heme-binding domains in the N terminus and kinase insert domain (6, 34).
In addition to the stress conditions described above, there are other environmental stresses that are known to induce rapid inhibition of translation in the cell through eIF2α phosphorylation. These include heat shock, arsenite treatment, osmotic stress, and nutrient starvation (4). It remains to be determined which eIF2α kinase(s) is activated under these stresses. Recently, the availabilities of cells lacking PKR or PERK have been useful in addressing this question. It has been shown that PERK is essential for ER stress but not for cytoplasmic stresses such as arsenite or heat shock (16, 17). PKR is dispensable for either essential amino acid starvation or ER stress (17, 24).
The production of HRI knockout mice in our laboratory (14) enables us to determine the role of HRI in these stress responses. This work was performed using mouse reticulocytes and nucleated erythroid progenitor cells from fetal livers. Since HRI is most abundant in erythroid cells, these cells are most suitable for studying the functions of HRI. Furthermore, reticulocytes have been used classically as a model system for studying translation, since they have no nuclei and therefore no transcriptional regulation, which can also affect protein output.
We report here, for the first time, the presence of HRI in nucleated erythroid progenitor cells. In addition, in both reticulocytes and fetal liver erythroid progenitor cells, HRI is activated by various cytoplasmic stresses, including arsenite treatment, heat shock, and osmotic shock, but not by ER stress or nutrition starvation. Most importantly, our results demonstrate that HRI is the only eIF2α kinase that is activated by arsenite treatment of erythroid cells, as HRI−/− cells fail to increase eIF2α phosphorylation upon arsenite treatment. These results underscore the importance of HRI in stresses other than heme deficiency, particularly the oxidative stress induced by arsenite.
MATERIALS AND METHODS
Materials.
[35S]methionine (3,000 Ci/mmol) and [γ-32P]ATP (3,000 Ci/mmol) were purchased from New England Technologies Inc. Sodium arsenite, N-acetyl-l-cysteine (NAC), and clofibric acid (CIA) were from Sigma, and geldanamycin (GA) was from Calbiochem. The anti-PKR antibody was from Santa Cruz, and the anti-phosphorylated-eIF2α antibody was from Biosource International Inc.
Preparation of mouse reticulocytes.
Wild-type, HRI knockout, and PKR knockout mice were injected with phenylhydrazine at 40 mg/kg on days 0, 1, and 3 to induce hemolysis, subsequent erythropoiesis, and release of reticulocytes into the bloodstream. Blood samples were collected by heart puncture at day 7, and the percentages of reticulocytes in the peripheral blood were determined by new methylene blue staining. The percentages of reticulocytes were between 85 and 95%.
Stress challenge of reticulocytes.
The blood samples were washed twice with ice-cold phosphate-buffered saline supplemented with 5 mM glucose and resuspended in Dulbecco modified Eagle medium with 2% dialyzed fetal bovine serum at a concentration of 108 reticulocytes/ml. The cells were plated onto six-well plates (108/well) and preincubated for 30 min at 37°C in a tissue culture incubator for recovery. The cells were then subjected to different stresses: (i) heat shock at 42°C for 30 min, (ii) sodium arsenite at various concentrations and time periods as indicated in the figures, (iii) osmotic shock with 0.5 M NaCl for 1 h, and (iv) Thapsigargin (Tg) (1 to 20 μM) for ER stress for 1 h. For nutrient starvation, the reticulocytes were washed three times with either serum-free or methionine-free medium and maintained in these media for 1 h for serum or amino acid starvation. In examining the chaperone antagonists and reducing reagent, the cells were pretreated with GA (4 to 40 μg/ml), CIA (5 to 40 mM), and NAC (5 to 20 mM) for 30 min before the stress challenges. For protein synthesis, cells were pulse-labeled with 100 μCi of [35S]methionine in low-methionine medium (containing 1/10 of the methionine concentration of the complete medium) for 30 min after the stress challenge.
Preparation and stress challenge of mouse fetal liver cells.
Embryonic livers at day 14.5 from both wild-type and HRI−/− mice were excised and dispersed by being passed through a 21-gauge needle in Dulbecco modified Eagle medium with 2% fetal bovine serum. A single-cell suspension was preincubated in this culture medium for recovery for at least 45 min. The cells were used for stress response study as described above for reticulocytes.
Lysate preparation and Western blot analysis.
For Western blotting, the cells were lysed in sodium dodecyl sulfate (SDS) sample buffer by adding 1/3 volume of 4× SDS sample buffer directly into cell suspensions. The samples were then boiled for 3 min and loaded onto SDS-polyacrylamide gels (7% for HRI and 12% for eIF2αP and total eIF2α). Western blot analyses for HRI and eIF2α were carried out as described previously (2).
Immunoprecipitation (IP) and kinase assays.
Cells (108) were lysed in 500 μl of cell lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, 1 μM aprotinin, 1 μM leupeptin, 5 μM pepstatin A, and 1 mM phenylmethylsulfonyl fluoride) at 4°C for 15 min. Cell debris were removed by centrifugation at 10,000 × g for 5 min at 4°C. Cell lysates from equal numbers of cells (107) were mixed with anti-mouse HRI antiserum (14) or anti-PKR polyclonal antibody and incubated at 4°C for 2 h. Protein A-Sepharose beads (50 μl) were then added, and the samples were incubated at 4°C overnight with rocking. The Sepharose immunocomplex was washed five times with 1 ml of wash solution (50 mM Tris-HCl [pH 7.4], 50 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, and protease inhibitors). After the last wash, the Sepharose beads were resuspended in 20 μl of 2× kinase assay buffer (40 mM Tris-HCl [pH 8.0], 4 mM MgCl2, and 0.2 mM EDTA). eIF2α kinase assays were then performed by the addition of 3.5 μl of labeling mix (0.5 μl of 10 mM ATP, 5 μCi of [γ-32P]ATP, and 200 ng of the recombinant yeast eIF2α) at 30°C for 5 min for HRI or 20 min for PKR. The reactions were terminated by the addition of 10 μl of 3× SDS sample buffer. The extent of eIF2α phosphorylation was visualized by autoradiography after SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Tetracycline-inducible expression of HRI in CHO cells.
The cDNAs of both the wild type and an inactive mutant of HRI (K196R HRI) were put into the pCDNA4/TO vector with the tetracycline-inducible promoter (Invitrogen). The plasmid DNAs were transfected into the Chinese hamster ovary (CHO)-TRX cell line (Invitrogen), and stable cell lines were established by selection of the cells with both Zeocin and Blasticidin. Expression of HRI and its mutant was induced with 1 μg of tetracycline per ml for 24 h. Cells were then treated with arsenite at 100 μM for 1 h. Cells were washed once with cold phosphate-buffered saline and lysed in SDS sample buffer. The expression and activation of HRI were detected by Western blot analysis as described above.
RESULTS
Activation of HRI by arsenite treatment.
To characterize the role of HRI in the inhibition of translation in response to arsenite treatment, reticulocytes were produced by treating mice with phenylhydrazine as described in Materials and Methods. Cells were washed, incubated in culture medium, and then treated with various concentrations of sodium arsenite for 60 min or with 200 μM arsenite for different time periods. Protein synthesis was measured by the incorporation of [35S]methionine into the globin chains. Arsenite treatment shut off globin synthesis completely in these cells, since no detectable [35S]methionine incorporation into globins was observed in the arsenite-treated cells (Fig. 1A). Arsenite has been shown to induce eIF2α phosphorylation in NIH 3T3 cells (30). The extent of eIF2α phosphorylation in arsenite-treated reticulocytes was examined by Western blot analysis using anti-phospho-eIF2α antibody. As shown in Fig. 1A, there was a pronounced increase in eIF2α phosphorylation in the reticulocytes upon arsenite treatment, while the amount of total eIF2α remained constant.
FIG. 1.
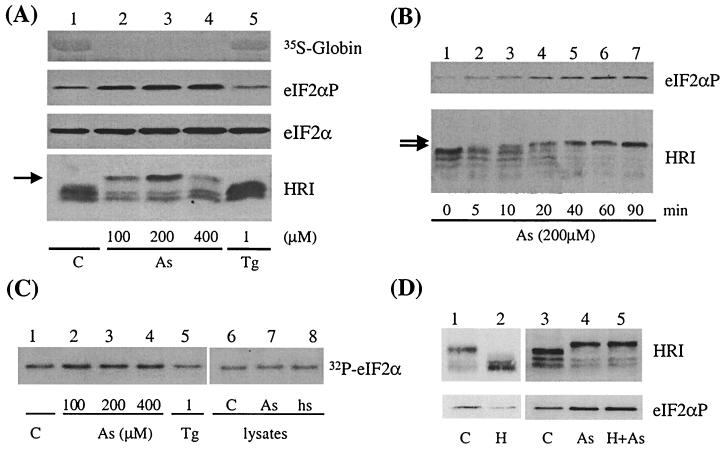
Activation of HRI by arsenite in mouse reticulocytes. (A) Effect of arsenite concentration on activation of HRI. Mouse reticulocytes were isolated as described in Materials and Methods. After recovery, the cells were treated with 100, 200, or 400 μM arsenite (As) or 1 μM Tg for 30 min and then labeled with [35S]methionine for an additional 30 min. Total cellular proteins were then separated by SDS-PAGE. The incorporation of [35S]methionine into globins was detected by exposing the nitrocellulose membrane to X-ray film. The extents of HRI activation and eIF2α phosphorylation were detected with anti-mouse HRI antibody and anti-eIF2αP antibody. Total eIF2α was determined with anti-eIF2α monoclonal antibody. The upshifted species of HRI are indicated by an arrow. C, control. (B) Time course of HRI activation by arsenite. Mouse reticulocytes were treated with 200 μM arsenite for different time periods as indicated. (C) Kinase activity of HRI. HRI in reticulocytes was immunoprecipitated from lysates of arsenite-treated, Tg-treated, or control reticulocytes with anti-HRI antibody, and the kinase activities were assayed in vitro by using recombinant yeast eIF2α as a substrate. The incorporation of 32P into eIF2α was detected by autoradiography. In lanes 7 and 8, the reticulocyte lysates from the control cells were either treated in vitro with 50 μM arsenite or heat shocked (hs) at 42°C for 30 min prior to immunoprecipitation. (D) Heme-independent activation of HRI by arsenite. Reticulocytes were treated with or without hemin (H, 40 μM) for 1 h prior to arsenite treatment (200 μM, 1 h).
We further determined whether there was corresponding activation of HRI in cells under arsenite treatment. The results in Fig. 1A and B showed that HRI was activated by the arsenite treatment. This activation of HRI was seen by its upshift in SDS-PAGE in a concentration-dependent and time-dependent manner. At 200 μM arsenite, upshift of HRI was seen at as early as 10 min. When separated in SDS–7% polyacrylamide gels, HRI from reticulocytes migrated as multiple bands (Fig. 1A and B) due to its autophosphorylation (see Fig. 2D and E). This was also observed when recombinant HRI was expressed in Escherichia coli. Multiple autophosphorylation is necessary for eIF2α kinase activity and also for the proper conformation for HRI to remain in a soluble state (2). The hyperphosphorylation of HRI upon arsenite treatment was accompanied by an increase of its eIF2α kinase activity as demonstrated by in vitro kinase assays of immunoprecipitated HRI (Fig. 1C, lanes 2 to 4). In addition, treatment of reticulocyte lysates in vitro with arsenite or heat shock (Fig. 1C, lanes 7 and 8) failed to activate HRI. These results indicate that the activation of HRI by arsenite and heat shock requires the presence of the intact reticulocytes. The activation of HRI by arsenite suggests that there is an additional function of HRI in cytoplasmic stress responses besides heme deficiency. Additionally, as shown in Fig. 1D, the activation of HRI by arsenite was not affected by pretreatment of the cells with hemin (40 μM), which prevented the activation of HRI in control cells. This result indicates that the mechanism of HRI activation by arsenite is different from that of heme deficiency.
FIG. 2.
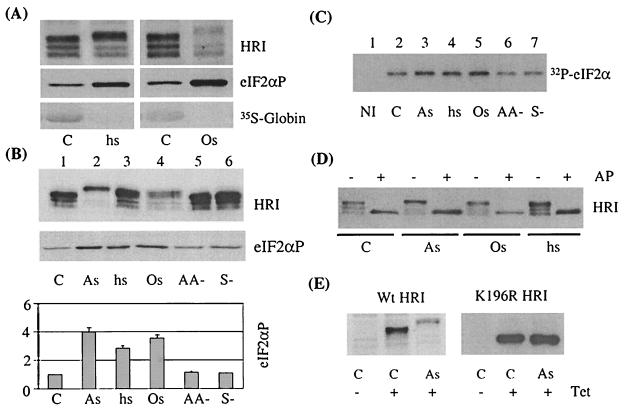
Differential activation of HRI by different stresses in reticulocytes. (A) Activation of HRI by osmotic stress and heat shock. Mouse reticulocytes were subjected to osmotic stress (Os) and heat shock (hs) for 30 min and then labeled with [35S]methionine for an additional 30 min. The extents of HRI activation and eIF2α phosphorylation and the incorporation of [35S]methionine into globins were detected as described in the legend to Fig. 1. C, control. (B) Effects of cytoplasmic stresses on HRI activation. Mouse reticulocytes were subjected to stresses as described in Materials and Methods. The extents of HRI activation and eIF2α phosphorylation were examined by Western blot analysis. The amounts of eIF2αP under various stresses were quantitated by densitometry scans of Western blots from three separate experiments and are shown here. As, arsenite; AA−, amino acid starvation; S−, serum starvation. (C) eIF2α kinase activity of HRI. The activity of HRI in either stressed or nonstressed reticulocytes, as indicated, was assayed by IP-coupled protein kinase assays as described in Materials and Methods and the legend to Fig. 1C. The incorporation of 32P into eIF2α was detected by autoradiography. NI, nonimmune serum control. (D) Effect of alkaline phosphatase (AP) on the electrophoretic mobility of HRI. Cell lysates from either stressed or nonstressed reticulocytes were treated with or without 2 U of calf intestine alkaline phosphatase at 37°C for 30 min. The phosphorylation status of HRI was analyzed by SDS-PAGE and Western blot analysis. (E) Autophosphorylation is required for the slower electrophoretic mobility of the HRI activated by arsenite treatment. Wild-type (Wt) HRI and its inactive mutant K196R HRI were expressed in CHO-TRX cells by induction with tetracycline (Tet) overnight as described in Materials and Methods. Cells were treated with arsenite for 1 h. The expression and activation of HRI and its mutant in CHO cells were examined as described above.
Tg is known to induce ER stress and specifically activates PERK to phosphorylate eIF2α (17). As expected, HRI was not activated by Tg (Fig. 1A and C, lanes 5). This is consistent with the fact that PERK is the eIF2α kinase responsible for the ER stress response (15, 16). Most interestingly, there was neither increased eIF2α phosphorylation nor inhibition of globin synthesis in the Tg-treated reticulocytes. The same results were obtained when higher concentrations of Tg (up to 20 μM) were used. These concentrations of Tg were sufficient to induce eIF2α phosphorylation in both NIH 3T3 and mouse embryonic fibroblast (MEF) cells (data not shown). Thus, there is no ER stress response in the reticulocytes, which is consistent with the fact that reticulocytes do not produce a significant amount of secretory proteins.
Activation of HRI by various cytoplasmic stresses in reticulocytes and nucleated erythroid progenitor cells.
Since HRI is activated by arsenite but not by Tg, the question whether HRI can also be activated by other cytoplasmic stresses was examined. Reticulocytes and fetal liver erythroid cells were subjected to different stress conditions as described in Materials and Methods. Activation of HRI in reticulocytes detected by electrophoretic mobility shift was observed under heat shock and osmotic stress, with corresponding increases of eIF2α phosphorylation and inhibition of globin synthesis under these conditions (Fig. 2A and B). However, HRI was not activated under amino acid starvation or serum starvation (Fig. 2B and C). The increases in eIF2αP in vivo were quantitated from Western blot signals from three separate experiments; there was a three- to fourfold-higher eIF2αP level in reticulocytes under these stress conditions (Fig. 2B). Although this method of detection does not allow estimation of the percentage of eIF2α phosphorylated, it is useful and adequate for determining the relative extents of eIF2α phosphorylation under different conditions.
The activation of HRI by various stresses was also confirmed by in vitro eIF2α kinase assays of immunoprecipitated HRI, as shown in Fig. 2C. The eIF2α kinase assays were performed in the linear range of the time course and HRI concentrations (data not shown). The increase in eIF2αP observed using the immunoprecipitated HRI was more modest than that observed in vivo (Fig. 2B). The reason for this is likely due to the activation of latent HRI by the removal of the heme during IP. Nonetheless, the results of IP-coupled eIF2α kinase assays did show the activation of HRI by arsenite, heat shock, and osmotic shock, in contrast to the lack of activation of HRI in nutrient starvation. These results indicated that HRI is a stress-activated kinase in response to several cytoplasmic stress conditions other than heme deficiency, which has been well characterized.
Interestingly, although HRI is activated by many stresses, the mechanisms of activation may be different, since activated HRI migrated differently in the SDS-PAGE. It is believed that arsenite is able to mimic most of the effects of heat shock, including inhibition of protein synthesis, induction of heat shock proteins, and activation of stress-activated kinases. However, we found that the upshift of HRI in SDS-PAGE caused by arsenite treatment was greater than that produced by heat shock (Fig. 2B). This observation suggests that arsenite induces a more extensive phosphorylation per HRI molecule and implies that mechanisms of activation of HRI by these two stress conditions may differ. However, definitive proof for different mechanisms of activation by different stress responses will require the actual identification of the phosphorylation sites. Osmotic shock-activated HRI migrated similarly to heat shock-activated HRI.
There are several different modifications that may alter the migration of a protein in SDS-PAGE, including proteolysis, deamidation, glycosylation, or phosphorylation. In order to demonstrate that the mobility shift of HRI upon activation is due to different extents of phosphorylation, the control and stressed reticulocyte lysates were treated with alkaline phosphatase. As shown in Fig. 2D, alkaline phosphatase treatment of the lysates converted all of the upshifted active HRI back to the fastest-migrating form. Thus, these upshifted species of HRI are the result of multiple phosphorylation of HRI. This conclusion is confirmed by our observation using the K196R mutant HRI, which undergoes autophosphorylation extremely poorly (2, 7). As shown in Fig. 2E, both wild-type and K196R mutant HRI were expressed in CHO cells upon induction with tetracycline. In response to arsenite treatment, wild-type HRI expressed in CHO cells was upshifted as in reticulocytes and fetal liver cells. In contrast, the K196R mutant HRI did not upshift upon arsenite treatment. Thus, autophosphorylation is required for the formation of the upshifted species of HRI upon stress.
The same stress experiments were carried out in mouse fetal liver nucleated erythroid cells. As shown in Fig. 3, HRI is also present in fetal liver erythroid progenitor cells and displayed multiple phosphorylated species as in reticulocytes. Similar to that in reticulocytes, HRI was activated by arsenite treatment (Fig. 3A) and by heat shock and osmotic stress (Fig. 3B) but not by ER stress, amino acid starvation, or serum starvation (Fig. 3B and C). One difference between reticulocytes and fetal liver cells is that there was increased eIF2α phosphorylation under amino acid or serum starvation in fetal liver cells even though HRI was not activated. The reason for this difference might be due to the absence of GCN2 in highly differentiated reticulocytes.
FIG. 3.
Activation of HRI by various cytoplasmic stresses in fetal liver erythroid progenitors. (A) Effect of arsenite. Fetal liver cells from both wild-type and HRI−/− mice were isolated as described in Materials and Methods. Cells were treated with different concentrations of arsenite (As) for 1 h. C, control. (B) Effect of osmotic stress (Os), heat shock (hs), and Tg. Fetal liver cells were treated with different cytoplasmic stresses: osmotic stress by NaCl (0.5 M, 1 h), heat shock (42°C, 30 min), and ER stress by Tg (1 μM, 1 h). (C) Effect of amino acid starvation and serum starvation. Starvation of cells was performed by incubation in either methionine (Met)-free or serum-free medium for 1 h. (D) Effects of arsenite treatment on protein synthesis in wild-type and HRI−/− fetal liver cells. Fetal liver cells were subjected to arsenite treatment for 90 min. [35S]methionine was added at time zero. Cells were harvested at different time periods as indicated. The total cellular proteins were separated by SDS-PAGE. The incorporation of [35S]methionine into globins was detected by autoradiography and was quantitated by scintillation counting of nitrocellulose strips containing globin chains.
HRI is the only eIF2α kinase activated by arsenite in erythroid cells.
As described above, HRI can be activated by cytoplasmic stresses such as arsenite treatment, heat shock, and osmotic stress. It remains to be determined how important HRI is in these stress responses. Is HRI the only eIF2α kinase responsible for these stresses? Other eIF2α kinase, especially PKR, are known to be present in reticulocytes and may also be involved in these stress responses. PKR−/− reticulocytes and HRI−/− reticulocytes and fetal liver cells were used to address this question. Arsenite treatment with either different concentrations (Fig. 3A and 4A) or different time periods (data not shown) failed to increase eIF2α phosphorylation in HRI−/− cells. Furthermore, arsenite treatment resulted in the inhibition of protein synthesis in HRI+/+, but not HRI−/−, fetal liver cells (Fig. 3D). This result demonstrates that HRI is the only eIF2α kinase activated in erythroid cells upon arsenite treatment. In contrast, increased eIF2α phosphorylation was still observed in HRI−/− reticulocytes and fetal liver cells under other stresses, such as heat shock and osmotic stress (Fig. 3B and 4A). Thus, even though HRI is activated by these stresses, it is not the only eIF2α kinase responsible for increased eIF2α phosphorylation under stress. Other eIF2α kinases may be activated and are also involved in heat shock and osmotic stress responses. However, HRI is the predominant eIF2α kinase activated in heat shock, since the extent of eIF2α phosphorylation was significantly reduced in HRI−/− cells compared to in wild-type cells. In osmotic stress, other eIF2α kinase members or phosphatases contribute more significantly, since no significant difference in eIF2α phosphorylation was observed between wild-type and HRI−/− cells.
FIG. 4.
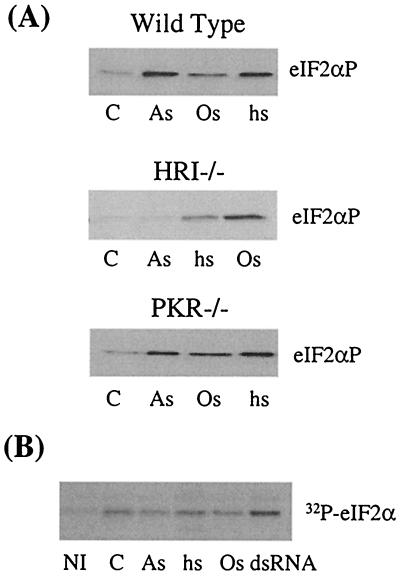
Cytoplasmic stresses of wild-type, HRI−/−, and PKR−/− reticulocytes. (A) eIF2α phosphorylation of wild-type, HRI−/−, and PKR−/− and reticulocytes upon different stresses. Reticulocytes from wild-type, HRI−/−, or PKR−/− mice were subjected to different stress conditions as described in the legend to Fig. 2A. As, arsenite; Os, osmotic stress; hs, heat shock; C, control. (B) eIF2α kinase activity of PKR. PKR from either stressed or nonstressed reticulocytes was immunoprecipitated with anti-PKR antibody. The kinase activity of PKR was determined by in vitro kinase assays as described in Materials and Methods. The incorporation of 32P into eIF2α was detected by autoradiography. NI, nonimmune serum control.
In contrast to the results obtained from HRI−/− reticulocytes, the eIF2α phosphorylation pattern of the stressed PKR−/− reticulocytes was nearly identical to that of wild-type cells (Fig. 4A). The activity of PKR was also measured by IP-coupled protein kinase assays. There was no detectable PKR activation upon introduction of these cytoplasmic stresses, while PKR was activated by dsRNA as expected (Fig. 4B). Thus, PKR is not essential for stress responses induced by arsenite, heat shock, or osmotic stress in reticulocytes. Collectively, these results demonstrate that HRI is the eIF2α kinase responsible for arsenite-induced eIF2α phosphorylation and is also the main one for the heat shock response in reticulocytes and fetal liver cells.
Role of ROS in stress-induced activation of HRI.
Stress-activated signaling cascades may be mediated by an altered redox potential in the cell during oxidative stress. The key contributors to altered redox potential are reactive oxygen species (ROS), which are formed in most cases by extracellular stress conditions. Arsenite is thought to be an oxidative stress inducer and can cause increases of intracellular ROS (10). Arsenite activation of HRI was found to occur only in intact reticulocytes and not in reticulocyte lysates (Fig. 1C). Thus, arsenite does not act directly on HRI.
The possibility of ROS involvement in HRI activation under different stresses was examined by using the reducing reagent NAC. NAC is a thiol-containing antioxidant capable of directly inactivating ROS as well as inducing glutathione production and thus blocks ROS-mediated signal transduction. Reticulocytes were pretreated with NAC for 30 min before being subjected to different stresses. The phosphorylation of eIF2α and the activation of HRI in these cells were then analyzed. Our results showed that pretreatment with NAC (20 mM) prevented the activation of HRI by arsenite with a concomitant decrease in eIF2α phosphorylation (Fig. 5). NAC had no effect on the activation of HRI by heat shock or osmotic stress (Fig. 5). NAC by itself also had no significant effect on the activity of HRI in untreated cells (Fig. 5). These results indicate the involvement of ROS in HRI activation upon arsenite treatment but not in heat shock or osmotic stress. We can also conclude that HRI can be activated through pathways other than ROS, as in the cases of heat shock and osmotic shock.
FIG. 5.
Role of ROS in HRI activation. Mouse reticulocytes were pretreated with different concentrations of the reducing reagent NAC as indicated for 30 min before being subjected to different stresses: no stress treatment (C), arsenite treatment (200 μM) for 1 h (As), heat shock at 42°C for 30 min, and osmotic stress with 0.5 M NaCl for 1 h. The activation of HRI and eIF2aP was examined.
Role of heat shock protein chaperones in HRI activation under stress conditions.
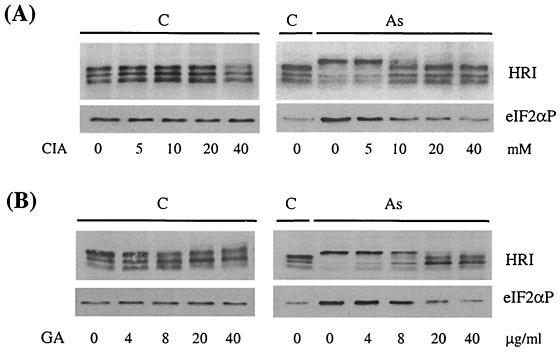
Both hsp90 and hsc70 have been shown to interact and regulate HRI activity in vitro (41, 42). We have examined the possible involvement of these two chaperones in the activation of HRI in intact reticulocytes upon stress. Reticulocytes were pretreated with GA, a specific inhibitor of hsp90 (29), or with CIA, an hsc70 antagonist (42), for 30 min prior to arsenite treatment. Our results demonstrate that the activation of HRI by arsenite is prevented by GA or CIA in a dose-dependent manner with a concomitant decrease of eIF2α phosphorylation (Fig. 6). CIA or GA alone had no significant effect on HRI activation or eIF2αP. Similar results were observed under heat shock and osmotic stress. Thus, these results strongly suggest that hsp90 and hsc70 are required for the activation of HRI by cytoplasmic stresses in intact reticulocytes.
FIG. 6.
Role of chaperones in activation of HRI by arsenite. Reticulocytes were pretreated with different concentrations of GA (4, 8, 20, and 40 μg/ml) (B) and CIA (5, 10, 20, and 40 mM) (A) for 30 min and then subjected to a 60-min incubation with (As) or without (C) arsenite treatment at 200 μM. The activation of HRI and eIF2αP was examined.
DISCUSSION
It has been well established that HRI is activated in heme deficiency of reticulocytes (reviewed in reference 8). Our recent study of HRI−/− mice demonstrates the physiological function of HRI in balancing globin synthesis with the availability of heme (14). However, it remains to be determined whether HRI also plays a role in regulating protein synthesis under other stress conditions. Here, we report a novel function of HRI in response to cytoplasmic stresses other than heme deficiency in both reticulocytes and immature nucleated erythroid cells. By using HRI−/− reticulocytes and fetal liver erythroid progenitor cells, we demonstrate that HRI is the only eIF2α kinase activated by arsenite in erythroid cells and is responsible for the increased eIF2α phosphorylation caused by arsenite treatment in these cells.
It has been speculated for some time about which eIF2α kinase family member is responsible for the increased eIF2α phosphorylation and the subsequent inhibition of protein synthesis caused by cytoplasmic stresses such as arsenite treatment, heat shock, and osmotic stress (4). Among the four known eIF2α kinases, PERK is not activated by either arsenite or heat shock (17). GCN2 was originally discovered in yeast and is activated during amino acid starvation (20). It was later also found in Drosophila, mouse, and human (3, 40). It has been reported that GCN2 can also be activated by glucose starvation (43) or serum starvation (3). To date, the contribution of GCN2 to arsenite treatment and other cytoplasmic stresses is still unknown. Our present study indicates that GCN2 is not likely to be activated by arsenite. As shown in Fig. 3, both wild-type and HRI−/− fetal liver cells responded to amino acid and serum starvation, indicating the presence of functional GCN2. However, HRI−/− fetal liver cells failed to respond to arsenite despite the response to amino acid and serum starvation.
In nonerythroid cells, PKR is likely the eIF2α kinase activated by arsenite, since little or no HRI is expressed in nonerythroid cells (13). It has been reported that PKR is activated by arsenite in murine interleukin-3-dependent NFS/N1.H7 cells (21) and NIH 3T3 and MEF cells (30). In addition PKR-null MEF cells are more resistant to arsenite-induced apoptosis (30). Recently, a cellular protein activator of PKR was cloned from human (PACT) (31) and mouse (RAX) (21). PACT/RAX is a dsRNA-binding protein, forms a heterodimer with PKR through its dsRNA-binding motifs, and activates PKR in vitro in the absence of dsRNA (21, 31). Arsenite treatment induces rapid phosphorylation of PACT/RAX and the association with PKR (30). However, we found here that PKR is not essential for arsenite-induced eIF2α phosphorylation in reticulocytes (Fig. 4A) and is not activated by arsenite in mouse erythroid cells as examined by IP-coupled protein kinase assays (Fig. 4B). One reason for this is that the activator of PKR, PACT/RAX, may be lacking or unable to be phosphorylated in erythroid cells upon stress. Therefore, PKR cannot be activated by arsenite in erythroid cells.
Since both PKR and HRI can be activated by various stresses, the involvement of these two eIF2α kinases seems to depend on the tissue-specific expression of these kinases. In erythroid cells, HRI is the most predominant eIF2α kinase and is responsible for the phosphorylation of eIF2α by arsenite and most other cytoplasmic stresses. In nonerythroid cells like NIH 3T3 and MEF cells, it is likely that PKR is activated by these stresses, since there is little to no HRI in these cells. Our results also indicate that ER stress and amino acid starvation are unique to PERK and GCN2, respectively. These stresses do not activate HRI (Fig. 1, 2, and 3). Similarly, it has been shown recently that PKR is dispensable for ER stress and amino acid starvation (24). The possible role of GCN2 in cytoplasmic stress remains to be investigated when GCN2−/− cells are available.
In the case of high-salt osmotic stress, the response is different from that to either arsenite or heat shock. There is no significant reduction of eIF2αP in either HRI−/− or PKR−/− reticulocytes. It is possible that GCN2 or PERK may be another eIF2α kinase responding to osmotic stress.
Unlike the regulation of HRI by heme, which binds directly to HRI and inhibits its activity, activation of HRI by arsenite treatment or heat shock is independent of heme. This conclusion is supported by two different experimental approaches. One of them is heme reversibility. Activation of HRI in heme deficiency in intact reticulocytes can be prevented by adding heme to the cells (14) (Fig. 1D), while arsenite activation of HRI cannot be suppressed by addition of hemin (Fig. 1D). This result indicates that the mechanisms of HRI activation by heme deficiency and arsenite treatment differ. The other supporting evidence is provided by the requirement of the intact living cells for the activation of HRI by arsenite and heat shock but not by heme deficiency. Once reticulocytes were lysed, HRI could not be activated by treatment with arsenite or heat shock (Fig. 1C). Thus, activation of HRI by arsenite is indirect and requires other components of the living cell. Furthermore, the difference in mechanisms of HRI activation by arsenite and heme deficiency is also indicated by the different phosphorylation statuses of HRI under these conditions. The upshift of HRI in SDS-PAGE caused by arsenite treatment, heat shock, and osmotic stress is higher than that caused by heme deficiency (data not shown).
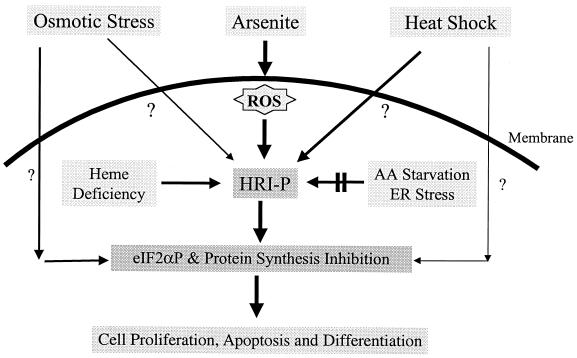
A model summarizing stress activation of eIF2α kinases in erythroid cells is shown in Fig. 7. Under stress conditions, HRI serves a major role in regulating protein synthesis in erythroid cells. It can be activated by several different stresses other than heme deficiency. Activation of HRI by heme deficiency occurs intracellularly, while activation by other stresses requires the presence of the intact cells. Arsenite-induced eIF2α phosphorylation is mediated exclusively through the activation of HRI, while increased eIF2α phosphorylation under osmotic stress or heat shock is mediated through activation of both HRI and other eIF2α kinases or through the inhibition of phosphatases. HRI contributes more significantly under heat shock than under osmotic stress. The mechanism of the activation of HRI by arsenite is different from that of activation by heat shock or osmotic shock. ROS are involved in the signaling of arsenite activation of HRI only. Furthermore, HRI activated by arsenite is hyperphosphorylated compared to HRI activated by heat shock or osmotic shock. HRI is not activated by nutrient starvation or by ER stress in either reticulocytes or fetal liver erythroid progenitor cells.
FIG. 7.
Model of the role of HRI in the stress response of erythroid cells. HRI is a major stress-activated eIF2α kinase in erythroid cells and can be activated by several different stresses in addition to heme deficiency. HRI is the only eIF2α kinase activated by arsenite. It is also the main eIF2α kinase activated by heat shock. The activation of HRI contributes to the regulation of cell proliferation, survival, and/or differentiation under stress challenges. AA, amino acid.
Both arsenite treatment and heat shock are known to activate the mitogen-activated protein (MAP) kinases, including ERKs, p38, and JNKs (32). The MAP kinase pathway is also important for activation of many signaling targets, which lead to modification of transcriptional factors and translational regulators (27). We have found that ERKs, JNKs, and p38 were expressed and were activated by arsenite in fetal liver cells (data not shown). However, we found that the ERK inhibitor PD98059 (27) and the p38 inhibitor SB203580 (36) had no significant effect on HRI activation and eIF2α phosphorylation in fetal liver cells or reticulocytes (data not shown). Similarly, we found that staurosporine, which is known to inhibit all MAP kinases but not eIF2α kinases (26), had no significant effect on HRI activation either (data not shown). Thus, it appears that the activation of HRI by stresses does not require the activation of MAP kinases.
Stress-activated signaling cascades can be affected by altered redox potential in the cell. ROS are a key contributor to altered redox potential (reviewed in reference 35). Arsenite can cause the formation of ROS and is thus thought to be an oxidative stress inducer (10). ROS are considered to be the primary intracellular change that serves as an important cellular component linking external stimuli with signal transduction. ROS can modulate signaling molecules, including both kinases and transcription factors. One of the most well-known targets of ROS is the stress-activated kinase (1). In this study, we also found that HRI activation by arsenite depends on ROS formation in the cell. ROS can modulate signaling molecules through two different means (37). One is to oxidize the cysteine of the target proteins directly and alter conformation and activity. The other is through cysteine-rich, redox-sensitive proteins such as thioredoxin and glutathione. It remains to be determined how ROS regulate the activity of HRI. However, it seems likely that glutathione acts as a mediator. The translation rate in rabbit reticulocyte lysates can be inhibited by GSSG (the oxidized form of glutathione) through the activation of HRI (11, 22).
The chaperone hsp90 is known to be a kinase chaperone, as it binds to various kinases and regulates their activities (5). Its role in the folding and maturation of newly synthesized HRI has been demonstrated in rabbit reticulocyte lysates (41). We show here that hsp90 is also essential for HRI activation under stress conditions. The mechanism by which hsp90 works in this process is still not clear. Both the nonactivated form and stress-activated hyperphosphorylated forms of HRI can be coimmunoprecipitated with hsp90 (data not shown).
The role of hsc70 in HRI regulation seems to be more complicated. A dual role of hsc70 in HRI regulation has been suggested by Uma et al. (42). Their in vitro study using rabbit reticulocyte lysates indicates that hsc70 is required for the folding and transformation of HRI into an active kinase and is subsequently required to negatively attenuate the activation of transformed HRI. Disruption of Hsc70 function by CIA in heme-deficient reticulocyte lysates resulted in the hyperactivation of HRI. However, this is not the case in intact reticulocytes. Treatment of reticulocytes with CIA alone did not cause the activation of HRI. Under cytoplasmic stress induced by arsenite, heat shock, and osmotic stress, CIA inhibits the stress-induced activation of HRI. Thus, in living cells, hsc70 is essential for HRI activation by stresses.
Contamination of drinking water with arsenic compounds poses an important health hazard. Long-term arsenic exposure can lead to many kinds of cancers, such as lung, bladder, and skin cancers, as well as to defects in erythropoiesis (28, 39). We have shown that HRI is involved in the regulation of erythroid cell proliferation (12). Our recent work on HRI knockout mice also indicates an essential role of HRI in stress-induced erythropoiesis (14). The unique role of HRI in the arsenite response and the availability of the HRI knockout mice generated in our laboratory provide us important tools to elucidate the molecular mechanisms of how arsenite affects erythropoiesis.
In summary, we have discovered a new function of HRI in erythroid cells under cytoplasmic stress responses other than heme deficiency. We also demonstrated the essential role of HRI in arsenite-induced eIF2α phosphorylation and inhibition of protein synthesis in erythroid cells.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant DK-16272 to J.-J.C. from the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Adler V, Yin Z, Tew K D, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, B. N., M. Rafie-Kolpin, L. Lu, A. Han, and J.-J. Chen. Multiple autophosphorylation is essential for the formation of the active and stable homodimer of heme-regulated eIF-2α kinase. Biochemistry, in press. [DOI] [PubMed]
- 3.Berlanga J J, Santoyo J, DeHaro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem. 1999;265:754–762. doi: 10.1046/j.1432-1327.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 4.Brostrom C O, Brostrom M A. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A J. Hsp90's secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 1999;9:262–268. doi: 10.1016/s0962-8924(99)01580-9. [DOI] [PubMed] [Google Scholar]
- 6.Chefalo P, Oh J, Rafie-Kolpin M, Chen J-J. Heme-regulated eIF-2α kinase purifies as a hemoprotein. Eur J Biochem. 1998;258:820–830. doi: 10.1046/j.1432-1327.1998.2580820.x. [DOI] [PubMed] [Google Scholar]
- 7.Chefalo P J, Yang J M, Ramaiah K V A, Gehrke L, Chen J-J. Inhibition of protein synthesis in insect cells by baculovirus-expressed heme-regulated eIF-2α kinase. J Biol Chem. 1994;269:25788–25794. [PubMed] [Google Scholar]
- 8.Chen J-J. Heme-regulated eIF-2α kinase. In: Sonenberg N, Hershey J W B, Mathews M B, editors. Translational control of gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. pp. 529–546. [Google Scholar]
- 9.Chen J-J, London I M. Regulation of protein synthesis by heme-regulated eIF-2α kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y C, Lin-Shiau S Y, Lin J K. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Clemens M J, Safer B, Merrick W C, Anderson W F, London I M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci USA. 1975;72:1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosby J S, Chefalo P J, Yeh I, Ying S, London I M, Leboulch P, Chen J-J. Regulation of hemoglobin synthesis and proliferation of differentiating erythroid cells by heme-regulated eIF-2α kinase. Blood. 2000;96:3241–3247. [PubMed] [Google Scholar]
- 13.Crosby J S, Lee K, London I M, Chen J-J. Erythroid expression of the heme-regulated eIF-2α kinase. Mol Cell Biol. 1994;14:3906–3914. doi: 10.1128/mcb.14.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, A., C. Yu, L. Lu, Y. Fujiwara, C. Browne, G. Chin, M. Fleming, P. Leboulch, S. H. Orkin, and J.-J. Chen. HRI is required for translational regulation and survival of erythroid precursors in iron-deficiency. EMBO J., in press. [DOI] [PMC free article] [PubMed]
- 15.Harding H P, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini D D, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 16.Harding H P, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 17.Harding H P, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 18.Hershey J W, Merrick W C. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, Hershey J W, Matthews M, editors. Translational control of gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. pp. 33–88. [Google Scholar]
- 19.Hershey J W B. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch A G. Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 199–244. [Google Scholar]
- 21.Ito T, Yang M, May W S. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 22.Kan B, London I M, Levin D H. Role of reversing factor in the inhibition of protein synthesis initiation by oxidized glutathione. J Biol Chem. 1988;263:15652–15656. [PubMed] [Google Scholar]
- 23.Kaufman R J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 24.Kimball S R, Clemens M J, Tilleray V J, Wek R C, Horetsky R L, Jefferson L S. The double-stranded RNA-activated protein kinase PKR is dispensable for regulation of translation initiation in response to either calcium mobilization from the endoplasmic reticulum or essential amino acid starvation. Biochem Biophys Res Commun. 2001;280:293–300. doi: 10.1006/bbrc.2000.4103. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamoorthy T, Pavitt G D, Zhang F, Dever T E, Hinnebusch A G. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21:5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin de la Vega C, Garcia A, Martin M E, Alcazar A, Marin O, Quevedo C, Salinas M. Resistance of initiation factor 2. (eIF-2alpha) kinases to staurosporine: an approach for assaying the kinases in crude extracts. Cell Signal. 1999;11:399–404. doi: 10.1016/s0898-6568(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 27.Morley S J, McKendrick L. Involvement of stress-activated protein kinase and p38/ERK mitogen-activated protein kinase signaling pathways in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J Biol Chem. 1997;272:17887–17893. doi: 10.1074/jbc.272.28.17887. [DOI] [PubMed] [Google Scholar]
- 28.Morse B S, Conlan M, Giuliani D G, Nussbaum M. Mechanism of arsenic-induced inhibition of erythropoiesis in mice. Am J Hematol. 1980;8:273–280. doi: 10.1002/ajh.2830080305. [DOI] [PubMed] [Google Scholar]
- 29.Neckers L, Schulte T W, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 30.Patel C V, Handy I, Goldsmith T, Patel R C. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 31.Patel R C, Sen G C. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter A C, Fanger G R, Vaillancourt R R. Signal transduction pathways regulated by arsenate and arsenite. Oncogene. 1999;18:794–802. doi: 10.1038/sj.onc.1203214. [DOI] [PubMed] [Google Scholar]
- 33.Proud C G. PKR: a new name and new roles. Trends Biochem Sci. 1995;20:241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- 34.Rafie-Kolpin M, Chefalo P J, Hussain Z, Hahn J, Ma S, Matts R L, Chen J-J. Two heme-binding domains of heme-regulated eukaryotic initiation factor-2alpha kinase. N terminus and kinase insertion. J Biol Chem. 2000;275:5171–5178. doi: 10.1074/jbc.275.7.5171. [DOI] [PubMed] [Google Scholar]
- 35.Ronai Z. Deciphering the mammalian stress response—a stressful task. Oncogene. 1999;18:6084–6086. doi: 10.1038/sj.onc.1203175. [DOI] [PubMed] [Google Scholar]
- 36.Saklatvala J, Rawlinson L, Waller R J, Sarsfield S, Lee J C, Morton L F, Barnes M J, Farndale R W. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 37.Sen C. Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol. 1998;55:1747–1758. doi: 10.1016/s0006-2952(97)00672-2. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Vattem K M, Sood R, An J, Liang J, Stramm L, Wek R C. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith A H, Lingas E O, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull W H O. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 40.Sood R, Porter A C, Olsen D, Cavener D R, Wek R C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uma S, Hartson S D, Chen J-J, Matts R L. Hsp90 is obligatory for the heme-regulated eIF-2α kinase to acquire and maintain an activatable conformation. J Biol Chem. 1997;272:11648–11656. doi: 10.1074/jbc.272.17.11648. [DOI] [PubMed] [Google Scholar]
- 42.Uma S, Thulasiraman V, Matts R L. Dual role for Hsc70 in the biogenesis and regulation of the heme-regulated kinase of the α subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 1999;19:5861–5871. doi: 10.1128/mcb.19.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang R, Wek S A, Wek R C. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol Cell Biol. 2000;20:2706–2717. doi: 10.1128/mcb.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]