Abstract
OBJECTIVE
The aim of this study is to determine the incidence and characteristics of newborns with subconjunctival hemorrhage (SCH).
METHODS
In our study, patient files of term infants referred to the study hospital’s ophthalmology clinic in 2018–19 were analyzed. Demographic data of infants including gestational week, birth weight, gender, and head circumference were all recorded. The frequency of SCH detection was evaluated depending on delivery type. Demographic data of infants with and without retinal hemorrhage (RH) were compared.
RESULTS
A total of 172 eyes of 86 infants were included in study. Forty-two (48.8%) of 86 neonates were male, and 44 (51.2%) were female. Mean gestational week was 38.62±1.1. SCH was detected in 31.4% (27) in the right eye, 36% (31) in the left eye, and 32.6% (28) in both eyes. The diagnosis was made at the mean of 3.74 days (range 1–20). Mean birth weight was found as 3621.1±453.3 g, head circumference as 35.4±1.3 cm, height as 50.7±2 cm, and chest circumference as 33.6±1.4 cm. Mean Apgar score in 1st min was 7.1±0.4; 5th min was 9. About 11.6% (10) of the mothers were nulliparous, and 88.4% (76) were multiparous. It was found that 79 of the deliveries were vaginal and seven with cesarean section. RH was not detected in any of the infants born with cesarean section.
CONCLUSION
SCH and RH were more common in infants born vaginally. If SCH is detected, a fundus examination should be performed to not miss possible RH.
Keywords: Newborn, retinal hemorrhage, subconjunctival hemorrhage
Highlight key points
Bilateral SCH cases should be evaluated under the increased risk for coincidence of RH.
Infants with bilateral SCH should be directed to ophthalmologists for detailed ophthalmic examination.
Infants with RH accompanying to SCH should be followed up for a longer period of time in terms of vision disorders that may develop in the future.
Subconjunctival hemorrhage (SCH) is a common cause of acute, painless red eye encountered in ophthalmology clinics, and emergency services. SCH is defined histologically as bleeding between the conjunctiva and episclera [1]. SCH can occur secondary to systemic diseases, local effects, or trauma [2]. Although SCH is seen more frequently with advancing age, it can be met in all age groups, including newborns [3]. SCH may occur due to the rupture of thin vessels in the conjunctiva due to the sudden increase in pressure in the thorax [4]. Retinal hemorrhage (RH) may accompany SCH in infants. The incidence of RH in neonates has been reported between 2.6% and 50% depending on the examination time and the difference in examination techniques [5]. In general, birth-related RH does not affect the development of visual function and resorbs spontaneously in 2–8 weeks, but delayed resorption of macular hemorrhage may cause amblyopia [5–7]. The frequency of RH in infants varies depending on the type of delivery. RH was reported in newborns at a ratio/incidence of 75% of births with vacuum extraction, 33% of spontaneous vaginal deliveries, and 6.7% of cesarean section (C/S) [6]. Too short or too long and traumatic delivery is a risk factor for RH development [8]. Birth-related hemorrhage in infants can also be localized or diffuse at the intraretinal level [5].
Therefore, this study aimed to determine the congenital characteristics of infants with SCH and define the incidence of co-occurrence of SCH with RH.
MATERIALS AND METHODS
A total of 172 eyes of 86 infants born at 37 weeks and above, who were consulted for SCH in Kanuni Sultan Suleyman Training and Research Hospital in the ophthalmology clinic 2018–2019, and who had a detailed anterior segment and fundus examination were included in this retrospective study. Neonates with congenital anomalies and systemic diseases and those with any kind of bleeding disorder, or a history of congenital bleeding disorders, additional congenital ocular, or systemic pathologies were not included in the study. The study was conducted as per the Helsinki Declaration and by obtaining the Kanuni Sultan Suleyman Training and Research Hospital Clinical Research Ethics Committee approval with the number of KAEK/2020.06.101 in June 29, 2020. A statement of consent to publish these findings and images were gathered from the patients included.
Maternal, obstetric, and neonatal data such as gender, gestational week, birth weight, head circumference, chest circumference, height, 1st and 5th min Apgar scores, delivery type (vaginal delivery, c/s, intervened delivery), and complications related to delivery were all recorded. The side of the eye with SCH, the presence of RH in the eye with SCH, the grade of RH, and resolution time of the RH were recorded.
RH classification was made according to the criteria specified by Egge et al. [9] Grade 1 was classified as thin hemorrhage limited around the optic disc, grade 2 as patchy irregular bleeding not exceeding the optic disc’s diameter, and grade 3 as flame-like bleeding larger than an optic disk head or hemorrhage in the macula.
Statistical Analysis
SPSS (SPSS Inc, PASW Statistics for Windows, Version 18, Chicago, USA) program was used for statistical analysis. Whether the data comply with the normal distribution was evaluated using the Kolmogorov–Smirnov test for the 30 patients and above group, and the Shapiro–Wilk test for 30 patients and below group. Parameters not conforming to a normal distribution were evaluated using the Mann–Whitney U-test. Categorical variables were compared using the Chi-square test. P<0.05 was considered statistically significant.
RESULTS
A total of 86 infants were included in the study. Of whom 42 infants were male, and 44 were female. The mean gestational week of infants was 38.62±1.074 weeks. SCH was detected in 27 (31.4%) of the infants in the right eye, 31 (36%) in the left eye, and 28 (32.6%) in both eyes. The SCH diagnosis was made in a mean of postpartum 3.74 days (range 1–20). Demographic characteristics of infants with SCH are presented in Table 1. RH was detected in 16 infants (18.6%). It was found that 77 (89.5%) of the infants were born from multiparous mothers, and 9 (10.5%) were from nulliparous mothers. It was observed that 79 (91.9%) of the deliveries were vaginal, and 7 (8.1%) were with cesarean section.
TABLE 1.
Demographic characteristics of infants with subconjunctival hemorrhage (n=86)
| Birth weight (g) | 3621.05±453.37 |
| Height (cm) | 50.73±1.96 |
| Head circumference (cm) | 35.35±1.28 |
| Chest circumference (cm) | 33.55±1.44 |
| Gestational week | 38.62±1.07 |
| Apgar score 1st min. | 7.08±0.35 range (6–8) median 7 |
| Apgar score 5th min. | 9±0 range (9–9) median 9 |
| Rate of nulliparity | 10.5% |
| Maternal age | 27.64±3.26 |
| Delivery mode (%) | |
| Vaginal delivery n=79 | 91.27 |
| 10 maternal dystocia | 11.62 |
| 1 vacuum delivery | 1.16 |
| 1 delivery with meconium-stained amniotic fluid | 1.16 |
| 1 vaginal septic delivery at home | 1.16 |
| 7 others | 8.13 |
| Cesarean section n=7 | 8.13 |
An infant was delivered by vaginal delivery with intervention, one was diagnosed as perinatal asphyxia stage 1, one had meconium stained amniotic fluid, and one was delivered by vaginal septic delivery at home. Apart from these, seven of them had a history of maternal dystocia, one of these infants, who was born in 36th gestational week, had a history of hospitalization in an incubator for 20 days. Adrenal hematoma occurred in one patient, and clavicle fracture occurred in five patients due to dystocia. RH also developed in one infant with a history of interfered delivery and one infant with a history of dystocia and developed clavicle fracture.
RH was also detected in 16 (18.6%) of 86 infants with SCH. About 56.2% of RHs were grade 3, 25% were grade 2, and 18.8% were grade 1. The mean time for the complete resolution of RHs was 18.69 days (range 7–48).
The comparison of the congenital characteristics of the group with SCH and the group with SCH accompanied by RH is presented in Table 2. It was observed that 63 of 70 infants who did not develop RH were delivered by vaginal delivery and seven by cesarean section. It was found that all 16 infants who developed RH were born vaginally. Sixty-two (88.57%) of the mothers of infants who did not develop RH and 14 (87.5%) of the infants who developed RH were multiparous mothers. The group with SCH only and the group with RH did not differ in terms of delivery type and multiparity (p=0.223, p=0.470, respectively).
TABLE 2.
Comparison of the characteristics between the group with only subconjunctival hemorrhage and the group with accompanying retinal hemorrhage
| Subconjunctival hemorrhage (n=70) Median | Subconjunctival hemorrhage with retinal hemorrhage (n=16) Median | p | |
|---|---|---|---|
| Birth weight (g) | 3621.50 | 3619.06 | 0.881m |
| Height (cm) | 50.64 | 51.13 | 0.21m |
| Head circumference (cm) | 35.31 | 35.5 | 0.612m |
| Chest circumference (cm) | 33.50 | 33.75 | 0.253m |
| Gestational week | 38.7 | 38.31 | 0.152m |
| Apgar score 1st min. | 7.11 | 6.94 | 0.081m |
| Apgar score 5th min. | 9 | 9 | 1m |
| Rate of nulliparity (%) | 11.4 | 6.2 | 0.542c |
| Rate of vaginal delivery (%) | 90 | 100 | 0.187c |
m: Mann–Whitney U-Test; c: Chi-square test.
DISCUSSION
SCH related to delivery is a common ophthalmological complication in infants (Fig. 1). Pressure changes in the birth canal or dystocia are the most common etiologic factors for SCH [3]. The prevalence of SCH is reported to be 1.7–46.3% for infants delivered by vaginal delivery [10, 11], SCH spontaneously regresses and does not affect vision. Conjunctival hemorrhage was known to be associated with delivery mode, multiparity, rapid 2nd stage of delivery, black race, high birth weight, head circumference, and gestational week [12]. In accordance wtih the literature, in this study, 88.4% of infants with SCH had multiparous mothers, their mean birth weight was over 3500 g, their mean height was above 50 cm, and their mean Apgar score was high, and 91.9% of the patients were delivered vaginally. Besides, most of the mothers whose infants had either SCH or RH were multiparous (Table 2).
FIGURE 1.
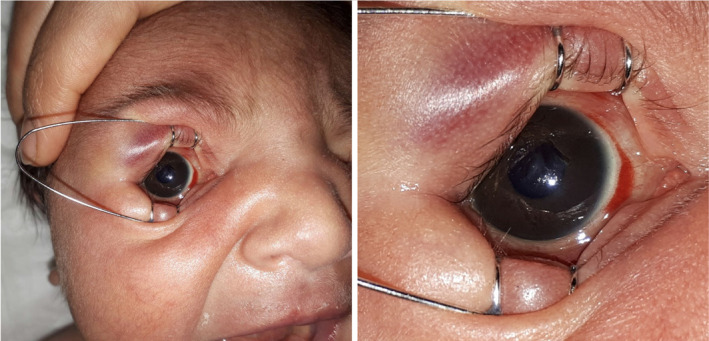
Subconjunctival hemorrhage in a newborn infant at postnatal 1st day.
RH is another ocular injury, which can accompany with SCH in neonates, especially whose had a history of dystocia and asphyxia. The incidence of RH in infants varies between 2.6% and 50% [6]. The present study revealed that RH was detected in 18.6% of the infants. Perinatal asphyxia is defined as the main cause of RH [13]. In this study, only a patients had perinatal asphyxia and he developed either SCH and RH. This can be explained by the limited number of participants and also the study center had an overall low rate of perinatal asphyxia. The incidence of RH is also related to the mode of delivery. It is mostly seen in vaginal delivery, especially in vacuum-assisted vaginal delivery. The reason of RH is thought to be mechanical trauma in the retinal vessels due to direct compression of the head [14]. While spontaneous vaginal delivery is pointed as a risk factor for RH, C/S is thought to be protective [15]. In this study, all patients who developed RH were born vaginally. There is conflicting data regarding the effect of the duration of 2nd stage of delivery on RH. Although Emerson et al. found that the duration of the 2nd stage of delivery did not affect the risk of developing RH, Williams et al. showed that the shortness of the 2nd stage (<30 min) of delivery increased the risk for RH [14, 16]. Probably, in case of 2nd stage of delivery to be shorter than expected, the sudden decrease in intracranial pressure of the fetus causes ruptures of the retinal vessels. Duration of the delivery stages were not included in this study, but 87.5% of our patients who developed RH were born from multiparous mothers, and the delivery of multiparous mothers is known to be faster than the nullipars’, because their birth canals are wider. RHs in infants of study resolved quickly without any visual loss. Figure 2 demonstrates RH of the infant and the complete resolution after 14 days (Fig. 2A, B). Almost all RHs are recorded to be resolved within 2 weeks, and none exceeded 4 weeks [5, 14]. In our study, the mean resolution of RH time is 18.69 day. Whether RH does not resolve or in case of delay in the resolution, this may result in abnormalities of visual functions [9, 16]. In particular, grade 3 RHs may take a long time to resolve [17]. A total of 56.3% of patients had grade 3 RH in the present study. Only a single one of them involved the macula. Complete resolution for the only RH involving macula took 48 days. Different incidence of RH grades was defined in the literature, and this may be associated with the timing of first fundus examination time. In this study, mean time to first fundus examination was quite early, 3.7 days following birth. RH is often seen bilaterally [7]. In this study, we also detected bilateral RH at a rate of 56.25%. Conjunctival and RH can be caused by traumatic reasons and can be seen together. A comprehensive newborn examination performed by a pediatrician includes an ocular examination. However, many significant ocular pathologies in the infant can be overlooked by pediatrician, and a detailed ocular examination is required to avoid it. RH which can accompany with SCH may have a negative effect on visual function. Hence, detailed eye examination including funduscopy should be performed for infants with SCH in order not to miss a possible accompanying RH.
FIGURE 2.
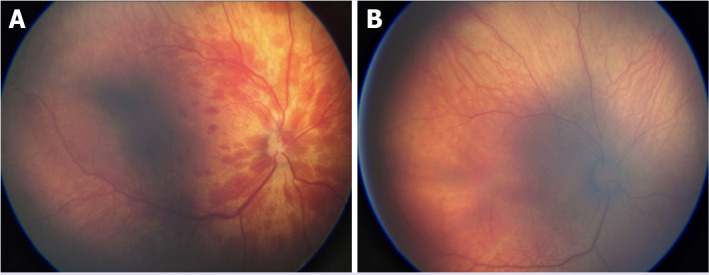
(A) Retinal hemorrhage in a newborn infant at postnatal 1st day and (B) completely resorbed retinal hemorrhage in the previous newborn infant at postnatal 14th day.
Asphyxia and traumatic delivery history are essential in the etiology of both subconjunctival and RH. The first examination of infant is performed by pediatricians, and SCH can be easily diagnosed. However, the presence of RH requires a detailed fundus examination by ophthalmologists to be visualized.
The present study had some limitations. First, authors could not give long-term outcome of patients due to the short follow-up time. Second, this study included a limited number of infants with SCH instead of all infants who were born vaginally in the study hospital. Hence, future studies including more infants with a long follow-up time are required to better understand the etiology and outcome of SCH and/or RH.
Conclusion
A considerable percentage of infants with SCH may also have a RH, which can lead visual abnormalities in follow-up. Therefore, authors suggest that infants with SCH should be directed to ophthalmologists for detailed ophthalmic examination. Especially, for bilateral SCH cases, physicians should pay more attention due to the increased risk for coincidence of RH. Besides, those infants should be followed up for a longer period in terms of vision disorders that may develop in the future.
Footnotes
Cite this article as: Karademir Z, Yilmaz Semerci S, Ozdemir FE, Bayramoglu SE. Subconjunctival hemorrhage in the newborn: Experience of a tertiary care hospital. North Clin Istanb 2023;10(1):74–78.
Ethics Committee Approval
The Kanuni Sultan Suleyman Training and Research Hospital Clinical Research Ethics Committee granted approval for this study (date: 29.06.2020, number: 2020.06.101).
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
The authors declared that this study has received no financial support.
Authorship Contributions
Concept – ZK, SYS, FEO, SEB; Design – ZK, SYS, FEO, SEB; Supervision – ZK, SYS, FEO, SEB; Fundings – ZK, SYS, FEO, SEB; Data collection and/or processing – ZK, SYS, FEO, SEB; Analysis and/or interpretation – ZK, SYS, FEO, SEB; Literature review – ZK, SYS, FEO, SEB; Writing – SYS; Critical review – ZK, SYS, FEO, SEB.
References
- 1.Leibowitz HM. The red eye. N Engl J Med. 2000;343:345–51. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- 2.Duke-Elder S. Conjunctival diseases. In: Duke-Elder S, editor. System of Ophthalmology, Volume VIII: Diseases of the Outer Eye, Part 1 Diseases of the Conjunctiva and the Associated Diseases of the Corneal Epithelium. 1st ed. London: Henry Kimpton; 1965. pp. 34–9. [Google Scholar]
- 3.Fukuyama J, Hayasaka S, Yamada K, Setogawa T. Causes of subconjunctival hemorrhage. Ophthalmologica. 1990;200:63–7. doi: 10.1159/000310079. [DOI] [PubMed] [Google Scholar]
- 4.Levin AV, Morad Y. Ocular manifestations of child abuse. In: Reece RM, Christian CW, editors. Child Abuse: Medical Diagnosis and Management. 3rd ed. Elk Grove Village, IL: American Academy of Pediatrics; 2008. pp. 211–26. [Google Scholar]
- 5.Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102–6. doi: 10.1016/j.jaapos.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Vinekar A, Govindaraj I, Jayadev C, Kumar AK, Sharma P, Mangalesh S, et al. Universal ocular screening of 1021 term infants using wide-field digital imaging in a single public hospital in India-a pilot study. Acta Ophthalmol. 2015;93:e372–6. doi: 10.1111/aos.12685. [DOI] [PubMed] [Google Scholar]
- 7.Watts P, Maguire S, Kwok T, Talabani B, Mann M, Wiener J, et al. Newborn retinal hemorrhages: a systematic review. J AAPOS. 2013;17:70–8. doi: 10.1016/j.jaapos.2012.07.012. Erratum J AAPOS 2013;17:341. [DOI] [PubMed] [Google Scholar]
- 8.Sezen F. Retinal haemorrhages in newborn infants. Br J Ophthalmol. 1971;55:248–53. doi: 10.1136/bjo.55.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egge K, Lyng G, Maltau JM. Retinal haemorrhages in the newborn. Acta Ophthalmol (Copenh) 1980;58:231–6. doi: 10.1111/j.1755-3768.1980.tb05715.x. [DOI] [PubMed] [Google Scholar]
- 10.Jain IS, Singh YP, Grupta SL, Gupta A. Ocular hazards during birth. J Pediatr Ophthalmol Strabismus. 1980;17:14–6. doi: 10.3928/0191-3913-19800101-04. [DOI] [PubMed] [Google Scholar]
- 11.Castro XSH, Quintero OH, Ferrer LG, Gorte PR. factores de riesgo en las affecciones oftalmologicas neonatales. [Article in Spanish] Rev Cubana Med Gen Integr. 2001;17:356–9. [Google Scholar]
- 12.Baum JD, Bulpitt CJ. Retinal and conjunctival haemorrhage in the newborn. Arch Dis Child. 1970;45:344–9. doi: 10.1136/adc.45.241.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parameĭ OV, Sidorenko EI. Relationship between perinatal pathology and refractogenesis, incidence and type of ocular diseases in children. [Article in Russian] Vestn Oftalmol. 1999;115:32–4. [PubMed] [Google Scholar]
- 14.Emerson MV, Pieramici DJ, Stoessel KM, Berreen JP, Gariano RF. Incidence and rate of disappearance of retinal hemorrhage in newborns. Ophthalmology. 2001;108:36–9. doi: 10.1016/s0161-6420(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q, Zhang Y, Yang Y, Li Z, Lin Y, Liu R, et al. Birth-related retinal hemorrhages in healthy full-term newborns and their relationship to maternal, obstetric, and neonatal risk factors. Graefes Arch Clin Exp Ophthalmol. 2015;253:1021–5. doi: 10.1007/s00417-015-3052-9. [DOI] [PubMed] [Google Scholar]
- 16.Williams MC, Knuppel RA, O’Brien WF, Weiss A, Spellacy WN, Pietrantoni M. Obstetric correlates of neonatal retinal hemorrhage. Obstet Gynecol. 1993;81:688–94. [PubMed] [Google Scholar]
- 17.Egge K, Lyng G, Maltau JM. Effect of instrumental delivery on the frequency and severity of retinal hemorrhages in the newborn. Acta Obstet Gynecol Scand. 1981;60:153–5. [PubMed] [Google Scholar]


