Abstract
DNA polymerase μ (Polμ) is a newly identified member of the polymerase X family. The biological function of Polμ is not known, although it has been speculated that human Polμ may be a somatic hypermutation polymerase. To help understand the in vivo function of human Polμ, we have performed in vitro biochemical analyses of the purified polymerase. Unlike any other DNA polymerases studied thus far, human Polμ catalyzed frameshift DNA synthesis with an unprecedentedly high frequency. In the sequence contexts examined, −1 deletion occurred as the predominant DNA synthesis mechanism opposite the single-nucleotide repeat sequences AA, GG, TT, and CC in the template. Thus, the fidelity of DNA synthesis by human Polμ was largely dictated by the sequence context. Human Polμ was able to efficiently extend mismatched bases mainly by a frameshift synthesis mechanism. With the primer ends, containing up to four mismatches, examined, human Polμ effectively realigned the primer to achieve annealing with a microhomology region in the template several nucleotides downstream. As a result, human Polμ promoted microhomology search and microhomology pairing between the primer and the template strands of DNA. These results show that human Polμ is much more prone to cause frameshift mutations than base substitutions. The biochemical properties of human Polμ suggest a function in nonhomologous end joining and V(D)J recombination through its microhomology searching and pairing activities but do not support a function in somatic hypermutation.
Many cellular processes require a DNA polymerase (Pol), including DNA replication, DNA repair, recombination, translesion DNA synthesis, and somatic hypermutation. Polα, Polδ, Polɛ, and Polγ are replicative DNA polymerases in eukaryotes (16, 29). Polζ is a major polymerase required for DNA damage-induced mutagenesis (21, 22). Polθ is likely involved in repair of DNA interstrand cross-links (27). Polη, Polι, and Polκ belong to the Y (UmuC) family of DNA polymerases and are involved in error-free and error-prone translesion synthesis opposite various DNA lesions (9, 20, 24, 31).
Polβ is a major repair synthesis polymerase during base excision repair in higher eukaryotes (17, 33, 36). Polβ and terminal deoxynucleotidyltransferase (TdT) are members of the DNA polymerase X family (13). TdT catalyzes nucleotide addition to DNA in a template-independent manner (3, 5). This enzyme is restricted to lymphoid tissues and functions during V(D)J recombination of the immunoglobulin genes and T-cell receptor genes (3, 5, 32). Most recently, the two newest members of the DNA polymerase X family, designated Polλ and Polμ, have been identified in humans (1, 8, 10). According to protein sequence comparisons, Polλ is more closely related to Polβ while Polμ is phylogenetically closer to TdT (1, 8). The biological functions of Polλ and Polμ remain to be defined. It has been speculated that Polλ may play a role in meiosis (10) and that Polμ may be a somatic hypermutation polymerase (8).
V(D)J recombination and somatic hypermutation are two essential mechanisms for generating antibody diversity during immunoglobulin development. V(D)J recombination requires DNA strand cleavage by the lymphoid-specific RAG1 and RAG2 proteins, and the resulting double-strand breaks are repaired by a nonhomologous end joining (NHEJ) mechanism similar to that employed by other tissues to repair the broken ends of DNA. Proteins involved in NHEJ include Ku70, Ku80, DNA-PKcs, XRCC4, and DNA ligase IV (25). More proteins are likely needed during NHEJ, such as a specific factor that promotes microhomology search and microhomology pairing. Somatic hypermutation introduces mainly point mutations into the V region of immunoglobulin genes at a rate of 10−3 to 10−4/base pair/generation, which is ≈106-fold higher than the spontaneous mutation rate in the rest of the genome (30). Thus, somatic hypermutation probably requires a low-fidelity DNA polymerase that possesses extraordinarily high error rates of misincorporations opposite undamaged template bases (4). However, this hypothetical hypermutation polymerase has eluded extensive studies thus far.
To help understand the biological function of human Polμ, we have extensively analyzed its biochemical properties. Surprisingly, we found that human Polμ catalyzes frameshift DNA synthesis with an unprecedentedly high frequency. Furthermore, when the primer 3′ end contains base mismatches, human Polμ efficiently realigns the primer strand to form new base pairings further downstream with the template bases. These remarkable biochemical properties do not support a role for human Polμ in somatic hypermutation and suggest that human Polμ may function in NHEJ and V(D)J recombination by promoting microhomology search and microhomology pairing.
MATERIALS AND METHODS
Materials.
A mouse monoclonal antibody against the His6 tag was purchased from Qiagen (Valencia, Calif.). Alkaline phosphatase-conjugated anti-mouse immunoglobulin G was from Sigma Chemical Co. (St. Louis, Mo.). Oligonucleotides were synthesized by Operon (Alameda, Calif.). The yeast rad30 deletion mutant strain BY4741rad30Δ (MATα his3 leu2 met15 ura3 rad30Δ) was purchased from Research Genetics (Huntsville, Ala.). The Klenow fragment of Escherichia coli DNA polymerase I was purchased from Gibco BRL (Bethesda, Md.), Pfu DNA polymerase was obtained from Stratagene (La Jolla, Calif.), and restriction endonucleases were from New England Biolabs (Beverly, Mass.). Human Polβ was purified to apparent homogeneity as previously described (38).
Gene constructions.
Human Polμ is encoded by the POLM gene (1, 8). The POLM cDNA was obtained by PCR amplification from human pancreas cDNAs using Pfu DNA polymerase and two primers, 5′-GCTCTAGAGTCGACATGCTCCCCAAACGGCGG (PolMF primer) and 5′-ACATGCATGCAGGCCCCACCACAGC. The resulting 1.8-kb PCR product was then cloned into the SalI and SphI sites of the vector pEGUh6, yielding pEGUh6-POLM. The POLM gene was verified by DNA sequencing. This expression construct contains the 2μm origin for multicopy plasmid replication, the URA3 gene for plasmid selection, the inducible GAL1/GAL10 promoter, and six histidine codons preceding the ATG initiator codon of the human POLM gene. To construct the mutant polm gene, the pEGUh6-POLM plasmid was amplified by PCR with the PolMF primer and the primer 5′-CCCAAGCTTAGGATGGGCAGGGCCTCG. The resulting 1.2-kb DNA fragment was then cloned into the SalI and HindIII sites of the vector pEGUh6, yielding pEGUh6-polmΔC83. The mutant gene was verified by DNA sequencing. Expression of pEGUh6-polmΔC83 in yeast cells produces the mutant protein PolμΔC83 missing the C-terminal 83 amino acids of human Polμ.
Purification of human Polμ.
Yeast BY4741rad30Δ cells harboring pEGUh6-POLM were grown in minimum medium containing 2% sucrose for 2 days. Expression of Polμ was induced by diluting the culture 10-fold in 16 liters of YPG (2% Bacto Peptone, 1% yeast extract, 2% galactose) medium supplemented with 0.5% sucrose and incubation for 15 h at 30°C with shaking. The collected cells (≈100 g) were homogenized with zirconium beads in a Bead-Beater (Biospec Products, Bartlesville, Okla.) in an extraction buffer containing 50 mM Tris-HCl, pH 7.5, 600 mM KCl, 5 mM β-mercaptoethanol, 10% sucrose, and protease inhibitors (37). The clarified extract (≈120 ml) was loaded onto two connected HiTrap chelating columns (5 ml each) charged with NiSO4 (Amersham Pharmacia Biotech, Piscataway, N.J.), followed by washing the column sequentially with 100 ml of Ni buffer A (20 mM KH2PO4, pH 7.4, 0.5 M NaCl, 10% glycerol, 5 mM β-mercaptoethanol, and protease inhibitors) containing 10 mM imidazole and 100 ml of Ni buffer A containing 35 mM imidazole. Bound proteins were eluted with a linear gradient of 35 to 108 mM imidazole. The His6-tagged human Polμ was identified by Western blot analyses using a mouse monoclonal antibody specific to the His6 tag. The pooled sample (≈150 ml) was concentrated by polyethylene glycol 10,000 and desalted through 5 connected Sephadex G-25 columns (5 ml each) (Amersham Pharmacia Biotech) in fast-protein liquid chromatography (FPLC) buffer A (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 10% glycerol, and 5 mM β-mercaptoethanol) containing 80 mM KCl. The resulting sample (≈50 ml) was loaded onto an FPLC Mono S HR5/5 column (Amersham Pharmacia Biotech) and eluted with a 30-ml linear gradient of 80 to 600 mM KCl in FPLC buffer A. Human Polμ was eluted at ≈250 mM KCl. The Mono S fractions of human Polμ were concentrated to 250 μl by polyethylene glycol 10,000 and loaded onto an FPLC Superdex 200 gel filtration column that had been equilibrated in FPLC buffer A containing 150 mM KCl. Human Polμ was eluted at the ≈60-kDa position.
DNA polymerase assays.
A standard DNA polymerase reaction mixture (10 μl) contained 25 mM KH2PO4 (pH 7.0), 5 mM MgCl2, 5 mM dithiothreitol, 100 μg of bovine serum albumin/ml, 10% glycerol, 50 μM deoxynucleoside triphosphates (dNTPs) (dATP, dCTP, dTTP, and dGTP individually or together as indicated), 50 fmol of a DNA substrate containing a 32P-labeled primer, and purified DNA polymerase as indicated. After incubation at 30°C for 10 min or as otherwise indicated, reactions were terminated with 7 μl of a stop solution (20 mM EDTA, 95% formamide, 0.05% bromophenol blue, and 0.05% xylene cyanol). The reaction products were separated by electrophoresis on a 20% denaturing polyacrylamide gel and visualized by autoradiography.
Kinetic analysis of human Polμ.
Kinetic analysis of human Polμ was performed as previously described (6, 38). Briefly, the assays were performed using 50 fmol of a DNA substrate containing a 5′ 32P-labeled primer, 0.75 ng (14 fmol) of purified Polμ, and increasing concentrations of each dNTP (dATP, dCTP, dTTP, or dGTP). Incubations were for 10 min at 30°C under standard DNA polymerase assay conditions. Longer incubations of up to 120 min were required to detect some misincorporations by human Polμ. The reaction products were separated by electrophoresis on a 20% denaturing polyacrylamide gel and quantitated by scanning densitometry. The observed enzyme velocity (v) was plotted as a function of dNTP concentration. The plotted data was fitted by a nonlinear regression curve to the Michaelis-Menton equation, v = (Vmax × [dNTP])/(Km + [dNTP]), using the SigmaPlot software. Vmax and Km values for the incorporation of the correct and the incorrect nucleotides were obtained from the fitted curves. The relative error rate (finc) of nucleotide incorporation was calculated from the equation: finc = (Vmax/Km)incorrect/(Vmax/Km)correct.
RESULTS
Purification of human Polμ.
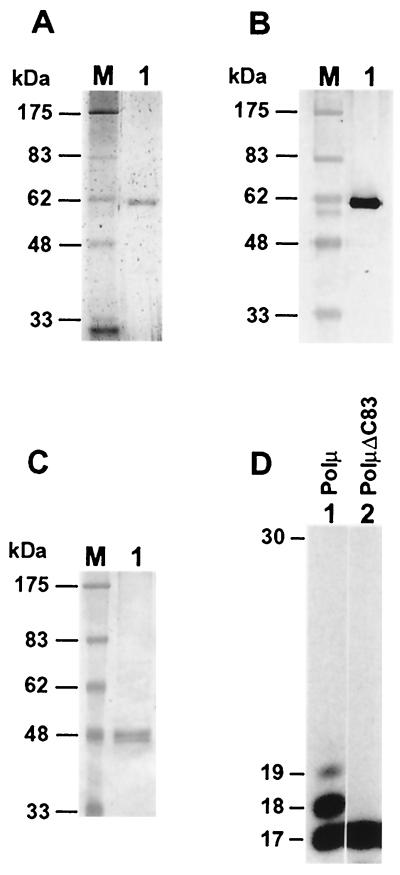
Following its expression in yeast cells, we have purified human Polμ to near homogeneity (Fig. 1A). The identity of human Polμ was confirmed by Western blot analysis using a mouse monoclonal antibody specific to the His6 tag at its N terminus (Fig. 1B). The purified human Polμ migrated as a 60-kDa protein on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel (Fig. 1A and B), consistent with its calculated molecular mass of 55 kDa. Separately, we deleted the C-terminal 83 amino acids of human Polμ by gene deletion. Since the mutant protein (PolμΔC83) lacks several conserved amino acid residues that are known to be critical for Polβ activity (2, 8), PolμΔC83 is expected to lose the polymerase activity. Using the same vector and under conditions identical to those with the wild-type human Polμ, the His6-tagged PolμΔC83 mutant protein was expressed in yeast cells and partially purified by an affinity Ni column. As indicated by Western blot analysis using the monoclonal antibody against the His6 tag, the PolμΔC83 protein migrated as a 48-kDa protein on an SDS–10% polyacrylamide gel (Fig. 1C), consistent with its calculated molecular mass of 45 kDa. Using similar amounts (as estimated from a Western blot analysis) of the Ni column fractions of Polμ and PolμΔC83, a DNA polymerase activity was readily detected with the wild-type Polμ (Fig. 1D, lane 1) but was undetectable with the PolμΔC8 mutant (Fig. 1D, lane 2). These results show that the DNA polymerase activity being studied is intrinsic to the purified human Polμ rather than a contaminant DNA polymerase from yeast.
FIG. 1.
Analysis of purified human Polμ. (A) Purified human Polμ (300 ng) was analyzed by electrophoresis on an SDS–10% polyacrylamide gel and visualized by silver staining. Protein mass markers (lane M) are indicated on the left. (B) Purified human Polμ (300 ng) was analyzed by Western blotting using a mouse monoclonal antibody against the N-terminal His6 tag. (C) The mutant human Polμ (PolμΔC83) was partially purified on a Ni column, and the sample (700 ng) was analyzed by Western blotting using the mouse monoclonal antibody against the N-terminal His6 tag. (D) The Ni column fractions containing similar amounts of human Polμ and PolμΔC83 as determined by a Western blot analysis were assayed for DNA polymerase activity, using the template 5′-GGATGGACTGCAGGATCCGGAGGCCGCGCG annealed with the 5′ 32P-labeled primer 5′-CGCGCGGCCTCCGGATC. The PolμΔC83 sample contained 70 ng of total proteins in the polymerase assay. DNA size markers in nucleotides are indicated on the left.
Human Polμ is a distributive polymerase that lacks a 3′ → 5′ proofreading exonuclease activity.
In a standard DNA polymerase assay, purified human Polμ extended the 32P-labeled 16-mer primer by 1 nucleotide in 2 min. With increasing reaction time, longer DNA strands were synthesized (Fig. 2A). However, DNA synthesis largely stopped after polymerizing only 6 nucleotides in 60 min (Fig. 2A, lane 6). When human Polμ was increased by 20-fold (10-fold molar excess over the template), only 9 nucleotides were polymerized in 10 min (data not shown). Thus, human Polμ is a distributive polymerase and is capable of only short-stretch DNA synthesis. To examine the 3′ → 5′ proofreading exonuclease activity, we incubated human Polμ with two DNA templates containing either a matched or a mismatched base pair at the primer 3′ end (Fig. 2B). While the proofreading exonuclease activity of the Klenow fragment of E. coli DNA polymerase I was readily detected (Fig. 2B, lanes 2 and 5), human Polμ did not degrade either the matched or the mismatched primers (Fig. 2B, lanes 3 and 6). These results show that human Polμ does not possess a 3′ → 5′ proofreading exonuclease activity.
FIG. 2.
Assays for distributive DNA synthesis and proofreading exonuclease of human Polμ. (A) DNA polymerase assays were performed with 1.5 ng (27 fmol) of human Polμ at 30°C for various times as indicated, using a 40-mer DNA template containing a 16-mer 5′ 32P-labeled (asterisk) primer as shown on the right. (B) DNA substrates (50 fmol) containing a T-A (template-primer) pair (lanes 1 to 3) or a T-T mismatch (lanes 4 to 6) (sequences shown on the right) at the primer 3′ end were incubated with purified human Polμ (5 ng; 90 fmol) for 10 min at 37°C in the DNA polymerase assay buffer without dNTPs. Similar assays were performed with the purified Klenow fragment (1 U) of E. coli DNA polymerase I, except that the incubation time was reduced to 2 min. The reaction products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. Lanes 1 and 4, no DNA polymerase. DNA size markers in nucleotides are indicated on the sides.
DNA synthesis fidelity of human Polμ.
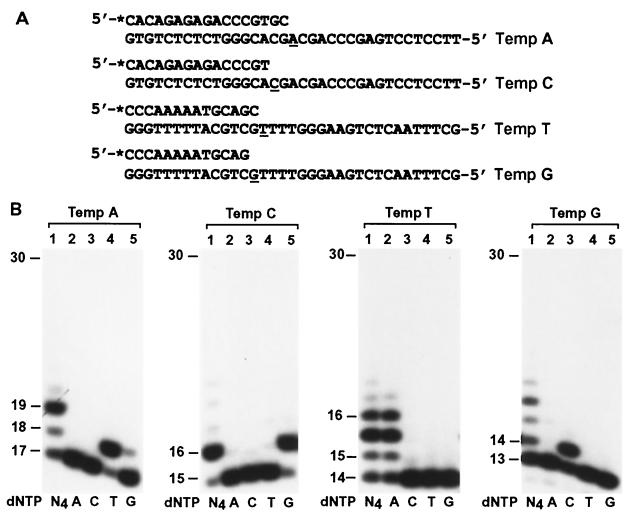
To determine whether the biochemical properties of human Polμ are consistent with a role in somatic hypermutation as speculated recently (8), we examined the DNA synthesis fidelity of this polymerase. Sequences from the JH4-JH5 intron of the rearranged human JH gene were chosen for analyzing Polμ fidelity such that the results could be compared with the reported hypermutation spectrum (18). DNA polymerase assays with purified human Polμ were performed in the presence of only one dNTP, using templates A, C, T, and G (Fig. 3A). Except for some G incorporation with the template A substrate (Fig. 3B, Temp A, lane 5), misincorporation by human Polμ was not detectable in these sequence contexts (Fig. 3B).
FIG. 3.
Fidelity of human Polμ. (A) Sequences from the JH4-JH5 intron of the rearranged human JH gene were used as DNA templates for polymerase assays; the analyzed template bases are underlined. Each primer was labeled at its 5′ end with 32P as indicated by an asterisk. (B) Polymerase assays were performed with 50 fmol of DNA and 1.5 ng (27 fmol) of human Polμ in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. DNA size markers in nucleotides are indicated on the left.
To quantitatively measure DNA synthesis fidelity, we performed steady-state kinetic analyses of nucleotide incorporation by human Polμ, using a method described by Creighton et al. (6). DNA polymerase assays were performed with increasing concentrations of a single dNTP using 14 fmol of purified human Polμ and 50 fmol of the primed templates A, C, T, and G (Fig. 3A), respectively. Four kinetic parameters were obtained: Vmax, Km, Vmax/Km, and finc (Table 1). As indicated by the Vmax/Km values, human Polμ activity is most efficient opposite template C and much less efficient opposite the other three template bases (Table 1). The fidelity of nucleotide incorporation is indicated by the (Vmax/Km)incorrect/(Vmax/Km)correct values (finc) (6). Except for G incorporation with the template A substrate, the misincorporation error rates (finc) of human Polμ were not exceptionally high (Table 1) compared to those of human DNA polymerases η, ι, and κ (14, 15, 19, 23, 28, 38, 39). Furthermore, the Polμ specificity of misincorporations at the examined A, C, T, and G sites were generally inconsistent with the reported hypermutation specificity at the corresponding sites of the human JH4-JH5 intron (18).
TABLE 1.
Kinetic measurement of nucleotide incorporation by human Polμa
| dNTP | Vmax (fmol/min; mean ± SD) | Km (μM; mean ± SD) | Vmax/Km | fincb |
|---|---|---|---|---|
| Template A | ||||
| dATP | 0.036 ± 0.0004 | 351.4 ± 183.3 | 0.00010 | 5.4 × 10−5 |
| dCTP | NDc | ND | 0.00038 | 2.1 × 10−4 |
| dTTP | 3.48 ± 0.17 | 1.89 ± 0.43 | 1.84 | 1 |
| dGTP | 2.13 ± 0.05 | 32.4 ± 2.55 | 0.066 | 3.6 × 10−2 |
| Template C | ||||
| dATP | 1.73 ± 0.24 | 140.4 ± 62.1 | 0.012 | 1.9 × 10−3 |
| dCTP | 2.83 ± 0.24 | 224.8 ± 53.9 | 0.013 | 2.0 × 10−3 |
| dTTP | 2.24 ± 0.30 | 174.6 ± 69.6 | 0.013 | 2.0 × 10−3 |
| dGTP | 3.5 ± 0.16 | 0.55 ± 0.12 | 6.36 | 1 |
| Template T | ||||
| dATP | 3.25 ± 0.03 | 7.76 ± 0.23 | 0.42 | 1 |
| dCTP | ND | ND | 0.00030 | 7.1 × 10−4 |
| dTTP | 0.20 ± 0.05 | 685.9 ± 309.3 | 0.00029 | 6.9 × 10−4 |
| dGTP | 0.033 ± 0.004 | 74.6 ± 25.0 | 0.00044 | 1.0 × 10−3 |
| Template G | ||||
| dATP | 0.11 ± 0.03 | 567.5 ± 457.7 | 0.00019 | 1.5 × 10−3 |
| dCTP | 1.90 ± 0.17 | 14.8 ± 4.02 | 0.13 | 1 |
| dTTP | ND | ND | 0.000038 | 2.9 × 10−4 |
| dGTP | ND | ND | 0.00027 | 2.1 × 10−3 |
DNA templates shown in Fig. 3A were used for kinetic analyses.
finc = (Vmax/Km)incorrect/(Vmax/Km)correct.
ND, not detected. The reaction velocity (v) remained linear throughout the entire dNTP concentration range used (0 to 3,000 μM). Therefore, individual Vmax and Km values could not be determined based on the v-versus-[dNTP] plot. The Vmax/Km value was determined by the slope of the initial velocity.
To further examine whether human Polμ is indeed prone to G misincorporation opposite template A, we performed the analysis again with a different template, 3′-GCCGGAGGCCAATCATACAAGCTTAC-5′ (the analyzed template A is underlined). In this sequence context, G, A, or C incorporations were not detected (data not shown). This and other experiments (see below) led us to infer that the G incorporation with the template A substrate shown in Fig. 3B is probably a result of −1 frameshift DNA synthesis opposite template C 2 nucleotides downstream rather than misincorporation opposite template A.
Frequent frameshift DNA synthesis by human Polμ.
We consistently observed that the DNA synthesis fidelity and nucleotide incorporation specificity of human Polμ were strongly influenced by the sequence context, with the single-nucleotide repeat sequences having the most dramatic effect. These observations led us to suspect that human Polμ may be especially prone to frameshift DNA synthesis in many sequence contexts. To directly examine this possibility, we analyzed DNA synthesis by human Polμ from template GG, TT, AA, and CC sequences.
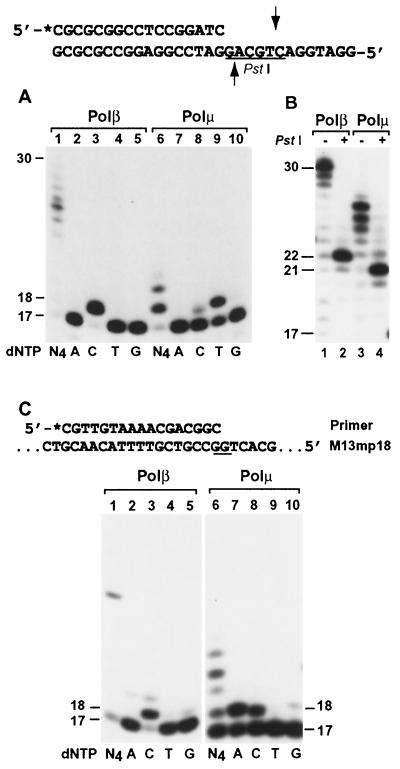
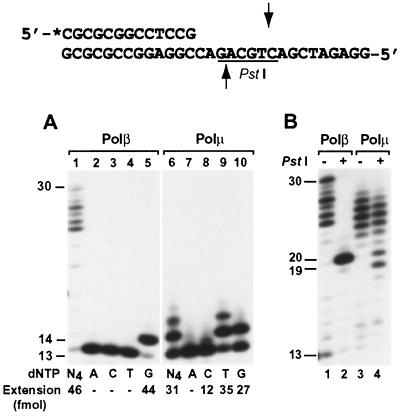
A labeled 17-mer primer was annealed to the template GG, in which the primer 3′ end was paired to the 3′ G of the GG sequence (Fig. 4A and B). Normal DNA synthesis would lead to C incorporation, as observed with Polβ-catalyzed DNA synthesis (Fig. 4A, lanes 1 to 5). Misaligning the primer 3′ C to the next template G would result in T incorporation and −1 frameshift DNA synthesis. As shown in Fig. 4A (lanes 6 to 10), human Polμ predominantly incorporated T. With higher Polμ concentration and extended incubation time, longer DNA strands were synthesized by human Polμ (Fig. 4B, lane 3), allowing us to examine the synthesis products by PstI restriction digestion. Normal DNA synthesis would yield a 22-mer 32P-labeled DNA fragment after the PstI cleavage, as was observed with human Polβ-catalyzed DNA synthesis (Fig. 4B, lane 2). With Polμ-catalyzed DNA synthesis, the PstI cleavage yielded a major 21-mer DNA fragment (Fig. 4B, lane 4). These results demonstrate that DNA synthesis by human Polμ at the examined template GG sequence is mediated predominantly by a −1 frameshift mechanism.
FIG. 4.
Frameshift DNA synthesis at the template GG sequence by human Polμ. (A) Using the indicated DNA substrate, standard DNA polymerase assays were performed with human Polβ (23 ng; 605 fmol) or human Polμ (1.5 ng; 27 fmol) in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. (B) Polymerase assays were performed at 37°C for 30 min, using human Polβ (23 ng; 605 fmol) or human Polμ (30 ng; 540 fmol) as indicated. After the polymerase reaction, 5 μl of the reaction products was mixed with 2 μl of H2O, 1 μl of the 10× PstI buffer (500 mM Tris-HCl, pH 8.0, 100 mM MgCl2, and 500 mM NaCl), and 2 μl of PstI (20 U). After incubation at 37°C for 4 h, the digested products were separated by electrophoresis on a 20% denaturing polyacrylamide gel and visualized by autoradiography. Samples without (−) or with (+) PstI treatment are indicated. Underline, PstI recognition sequence; arrows, PstI cleavage sites. (C) Using single-stranded M13mp18 containing a 17-mer 5′ 32P-labeled (asterisk) primer, DNA polymerase assays were performed with human Polβ (23 ng; 605 fmol) or human Polμ (7.5 ng; 135 fmol) at 30°C for 10 min in the presence of a single dNTP or all four dNTPs as indicated. The analyzed template GG sequence is underlined. DNA size markers in nucleotides are indicated on the sides.
To determine whether this surprising result reflects an artificially short DNA template, which may be structurally more flexible, or reflects an intrinsic biochemical property of human Polμ, we performed DNA synthesis at the GG sequence using single-stranded M13mp18 circular DNA (7,249 bases) as the DNA template (Fig. 4C). As expected, human Polβ incorporated a C opposite the 5′ G of the GG sequence (Fig. 4C, lanes 1 to 5). In contrast, −1 frameshift DNA synthesis would incorporate an A opposite the template T 5′ to the GG sequence (Fig. 4C). Again, human Polμ most frequently incorporated an A (53% primer extension) and less frequently incorporated the correct C (39% primer extension) (Fig. 4C, lanes 6 to 10), indicating that DNA synthesis at the template GG sequence was mediated mainly by a −1 frameshift mechanism. In the presence of all four dNTPs (Fig. 4C, lane 6), nucleotide sequence synthesized by human Polμ is consistent with 5′-AGTG (the −1 deletion product), based on the migration pattern of the DNA bands (mobility from fastest to slowest, C>A>T>G). Therefore, we conclude that the unprecedentedly high frequency of −1 frameshift DNA synthesis at the template GG sequence is an intrinsic property of human Polμ.
At the template TT sequence (Fig. 5), human Polβ incorporated an A opposite the 5′ T, as expected for normal DNA synthesis (Fig. 5A, lanes 1 to 5). The −1 frameshift DNA synthesis would lead to T incorporation opposite the template A 5′ to the TT sequence (Fig. 5). As shown in Fig. 5A (lanes 6 to 10), human Polμ predominantly incorporated T. Remarkably, the correct A incorporation by human Polμ had become barely detectable (Fig. 5A, lane 7). Cleavage of the synthesized DNA products by SphI restriction endonuclease would yield a 32P-labeled 24-mer DNA fragment, as was observed with human Polβ-catalyzed DNA synthesis (Fig. 5B, lane 2). With Polμ-catalyzed DNA synthesis, the SphI cleavage yielded a major 23-mer DNA fragment (Fig. 5B, lane 4). Polμ-synthesized products were less efficiently cleaved by SphI (Fig. 5B, compare lanes 2 and 4), probably due to shorter DNA strands and/or the 1-nucleotide loop on the template strand. These results show that DNA synthesis by human Polμ at the examined template TT sequence is mediated predominantly by a −1 frameshift mechanism.
FIG. 5.
Frameshift DNA synthesis at the template TT sequence by human Polμ. (A) Using the indicated DNA substrate, standard DNA polymerase assays were performed with human Polβ (23 ng; 605 fmol) or human Polμ (1.5 ng; 27 fmol) in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. (B) Polymerase assays were performed at 37°C for 30 min, using human Polβ (23 ng; 605 fmol) or human Polμ (30 ng; 540 fmol) as indicated. After the polymerase reaction, 5 μl of the reaction products was mixed with 2 μl of H2O, 1 μl of the 10× SphI buffer (100 mM Tris-HCl, pH 7.9, 100 mM MgCl2, 500 mM NaCl, and 10 mM dithiothreitol), and 2 μl of SphI (10 U). After incubation at 37°C for 4 h, the digested products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. Samples without (−) or with (+) SphI treatment are indicated. DNA size markers in nucleotides are indicated on the left. Asterisk, 32P label. Underline, SphI recognition sequence; arrows, SphI cleavage sites.
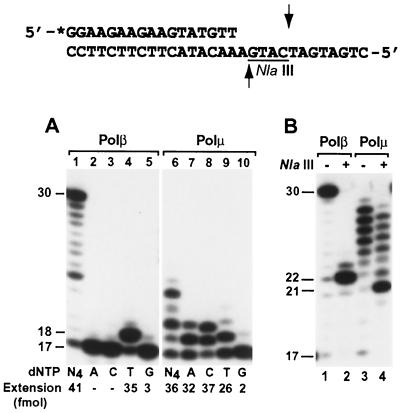
At the template AA sequence (Fig. 6), human Polβ incorporated a T opposite the 5′ A, as expected for normal DNA synthesis (Fig. 6A, lanes 1 to 5). The −1 frameshift DNA synthesis would lead to C incorporation opposite the template G 5′ to the AA sequence (Fig. 6). As shown in Fig. 6A (lanes 6 to 10), human Polμ most frequently incorporated C. Less frequently, A could also be incorporated by human Polμ (Fig. 6B, lane 7), which most likely resulted from −2 frameshift DNA synthesis by using the template T 3 nucleotides downstream of the primer 3′ end. Cleavage of the Polβ-synthesized products with NlaIII restriction endonuclease yielded a major 22-mer DNA band (Fig. 6B, lane 2), as expected for normal DNA synthesis. In contrast, identical treatment of the Polμ-synthesized products with NlaIII yielded a major 21-mer DNA band (Fig. 6B, lane 4). The less efficient cleavage of Polμ-synthesized products by NlaIII (Fig. 6B, compare lanes 2 and 4) was probably due to shorter DNA strands; the 1-nucleotide loop on the template strand; −2 frameshift DNA synthesis, which would destroy the NlaIII recognition site; or combinations of these factors. These results show that DNA synthesis by human Polμ at the examined template AA sequence is mediated predominantly by a −1 frameshift mechanism.
FIG. 6.
Frameshift DNA synthesis at the template AA sequence by human Polμ. (A) Using the indicated DNA substrate, standard DNA polymerase assays were performed with human Polβ (23 ng; 605 fmol) or human Polμ (1.5 ng; 27 fmol) in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. Quantitation of extended primers is shown below the gels. (B) Polymerase assays were performed at 37°C for 30 min, using human Polβ (23 ng; 605 fmol) or human Polμ (30 ng; 540 fmol) as indicated. After the polymerase reaction, 5 μl of the reaction products was mixed with 2 μl of H2O, 1 μl of the 10× NlaIII buffer (200 mM Tris-acetate, pH 8.0, 100 mM MgCl2, 500 mM potassium acetate, and 10 mM dithiothreitol), and 2 μl of NlaIII (20 U). After incubation at 37°C for 4 h, the digested products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. Samples without (−) or with (+) NlaIII treatment are indicated. DNA size markers in nucleotides are indicated on the left. Asterisk, 32P label; underline, NlaIII recognition sequence; arrows, NlaIII cleavage sites.
At the template CC sequence (Fig. 7), human Polβ incorporated a G opposite the 5′ C, as expected for normal DNA synthesis (Fig. 7A, lanes 1 to 5). The −1 frameshift DNA synthesis would lead to T incorporation opposite the template A 5′ to the CC sequence (Fig. 7). As shown in Fig. 7A (lanes 6 to 10), human Polμ favored T incorporation over the correct G incorporation. Minor C incorporation was also observed (Fig. 7A, lane 8), which most likely resulted from −2 frameshift DNA synthesis by using the template G 3 nucleotides downstream of the primer 3′ end. Cleavage of the Polβ-synthesized products with the PstI restriction endonuclease yielded a major 20-mer DNA band (Fig. 7B, lane 2), as expected for normal DNA synthesis. In contrast, following PstI cleavage of the Polμ-synthesized products, 1.3-fold more 19-mer DNA band than 20-mer DNA band was formed (Fig. 7B, lane 4). PstI cleavage of Polμ-synthesized products was significantly less efficient than that of Polβ-synthesized products (Fig. 7B, compare lanes 2 and 4). The precise cause of this difference is not known. Possible factors include shorter DNA strands, the 1-nucleotide loop on the template strand, some −2 frameshift DNA synthesis, or combinations of these. These results show that human Polμ prefers −1 frameshift DNA synthesis to normal DNA synthesis at the examined template CC sequence.
FIG. 7.
Frameshift DNA synthesis at the template TT sequence by human Polμ. (A) Using the indicated DNA substrate, standard DNA polymerase assays were performed with human Polβ (23 ng; 605 fmol) or human Polμ (1.5 ng; 27 fmol) in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. Quantitation of extended primers is shown below the gels. (B) Polymerase assays were performed at 37°C for 30 min, using human Polβ (23 ng; 605 fmol) or human Polμ (30 ng; 540 fmol) as indicated. After the polymerase reaction, 5 μl of the reaction products was treated with 20 U of PstI as for Fig. 4B. The digested products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. Samples without (−) or with (+) PstI treatment are indicated. DNA size markers in nucleotides are indicated on the left. Asterisk, 32P label; underlne, PstI recognition sequence; arrows, PstI cleavage sites.
As indicated by the steady-state kinetic values (Table 2), the rate of −1 frameshift synthesis by human Polμ was 20-, 28-, 4.7-, and 2.4-fold higher than normal DNA synthesis at the GG, TT, AA, and CC sequences, respectively, in the sequence contexts examined. Together, these results show that human Polμ catalyzes highly frequent frameshift DNA synthesis, which can predominate as the major DNA synthesis mechanism in some sequence contexts.
TABLE 2.
Kinetic measurement of −1 frameshift DNA synthesis by human Polμa
| dNTP | Vmax (fmol/min; mean ± SD) | Km (μM; mean ± SD) | Vmax/Km | Relative rateb |
|---|---|---|---|---|
| Template GG | ||||
| dCTP | 1.44 ± 0.13 | 135.3 ± 39.4 | 0.011 | 1 |
| dTTP | 4.76 ± 0.18 | 21.8 ± 2.37 | 0.22 | 20 |
| Template TT | ||||
| dATP | 1.96 ± 0.02 | 42.6 ± 1.87 | 0.046 | 1 |
| dTTP | 3.12 ± 0.08 | 2.46 ± 0.28 | 1.27 | 28 |
| Template AA | ||||
| dTTP | 2.86 ± 0.08 | 58.3 ± 6.37 | 0.049 | 1 |
| dCTP | 2.91 ± 0.09 | 12.7 ± 1.22 | 0.23 | 4.7 |
| Template CC | ||||
| dGTP | 4.36 ± 0.14 | 13.7 ± 1.8 | 0.32 | 1 |
| dTTP | 4.72 ± 0.09 | 6.10 ± 0.46 | 0.77 | 2.4 |
DNA templates GG, TT, AA, and CC (Fig. 4A, 5, 6, and 7, respectively) were used for kinetic analyses.
For each template, incorporation of the correct nucleotide reflects normal DNA synthesis, whereas incorporation of the other nucleotide reflects −1 frameshift DNA synthesis. Relative rate = (Vmax/Km)−1frameshift/(Vmax/Km)correct.
Mismatch extension by human Polμ.
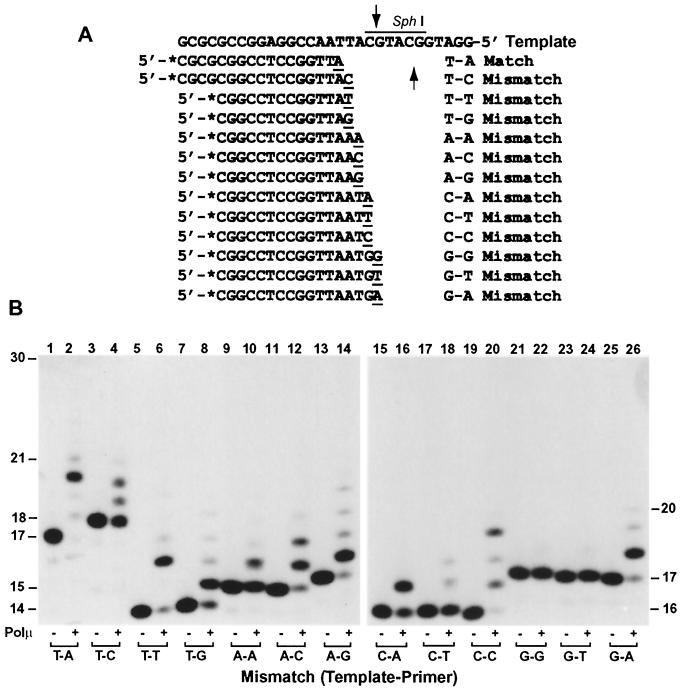
Without a 3′ → 5′ proofreading exonuclease activity, human Polμ cannot remove mismatched nucleotides at the primer 3′ end. To examine mismatch extension activity of human Polμ, we performed primer extension assays using the 12 possible base pair mismatches (Fig. 8A). As shown in Fig. 8B, except for G-G, and G-T (template-primer), all other mismatches were extended by human Polμ. T-T, A-G, C-C, and G-A mismatches were extended most efficiently (Fig. 8B, lanes 6, 14, 20, and 26).
FIG. 8.
Mismatch extensions by human Polμ. (A) Various primers labeled at their 5′ ends with 32P (asterisks) were annealed to the indicated template, generating 12 possible mismatches at the primer 3′ ends. One normal T-A-matched substrate was used as the control. The SphI recognition sequence is overlined, and the mismatched primer 3′ ends are underlined. Arrows, SphI cleavage sites. (B) Matched and mismatched substrates were incubated with (+) or without (−) human Polμ (3 ng; 54 fmol) under standard polymerase assay conditions. DNA size markers in nucleotides are indicated on the sides.
To identify nucleotides incorporated during mismatch extension, we performed the extension assays again in the presence of only one dNTP. Human Polμ incorporated G with the T-T mismatch (Fig. 9, lanes 1 to 5), C with the A-G mismatch (Fig. 9, lanes 16 to 20), and T with the G-A mismatch (Fig. 9, lanes 31 to 35). These incorporations are precisely predicted by a −1 frameshift synthesis mechanism involving misaligning the primer 3′ end with the next complementary template base prior to DNA synthesis. Human Polμ incorporated C with the T-G mismatch (Fig. 9, lanes 6 to 10) and T with the C-A mismatch (Fig. 9, lanes 21 to 25), which are predicted by a −2 frameshift synthesis mechanism involving misaligning the primer 3′ end with the complementary template base 2 nucleotides downstream prior to DNA synthesis. With the A-C mismatch, human Polμ preferentially incorporated A (Fig. 9, lane 12) and less frequently C (Fig. 9, lane 13), which was consistent with −2 frameshift synthesis by misaligning the primer 3′ C 2 nucleotides downstream with the template G and −1 frameshift synthesis using the template G 2 nucleotides downstream, respectively. With the C-C mismatch, human Polμ slightly preferred A incorporation over T incorporation, consistent with misaligning the primer 3′ C with the next template G as the preferred event prior to DNA synthesis (−1 frameshift) (Fig. 9, lanes 26 to 30). T incorporation (Fig. 9, lane 29) was consistent with −2 frameshift DNA synthesis using the template A 3 nucleotides downstream.
FIG. 9.
Nucleotide incorporation during mismatch extension. Using the mismatched DNA substrates as indicated (sequences shown in Fig. 8A), mismatch extension was performed with 3 ng (54 fmol) of human Polμ under standard polymerase assay conditions. The reactions were carried out using a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. DNA size markers in nucleotides are indicated on the sides.
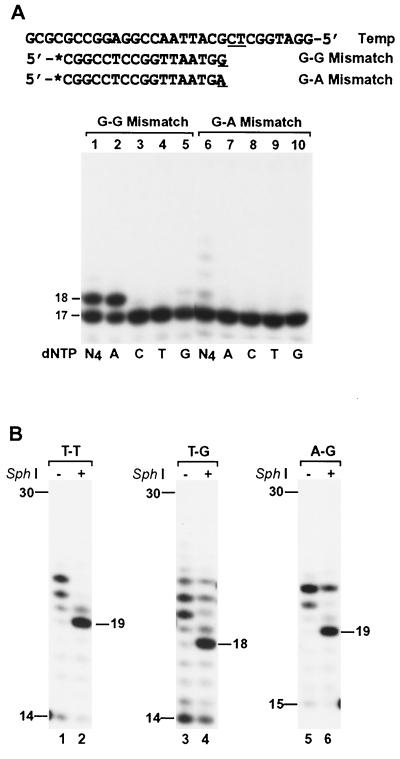
To test the notion that mismatch extension by human Polμ is mediated mainly by frameshift DNA synthesis, we slightly modified the DNA template and analyzed extensions from G-G and G-A mismatches. The template 3′-TA-5′ sequence immediately downstream of the mismatch was replaced by 3′-CT-5′ (Fig. 10A). Frameshift extension predicts that human Polμ should then be able to extend the G-G mismatch that was refractory to extension in the original sequence context (Fig. 8), since the primer 3′ G can pair with the next template C. Furthermore, A incorporation is predicted. As shown in Fig. 10A (lanes 1 to 5), the G-G mismatch was indeed effectively extended by human Polμ, and A was incorporated. In contrast, the G-A mismatch extension by human Polμ was drastically reduced in the new sequence context (Fig. 10A, lanes 6 to 10).
FIG. 10.
Evidence that mismatch extension by human Polμ is mainly mediated by frameshift DNA synthesis. (A) The template sequence of Fig. 8A was slightly modified such that the template 3′-TA-5′ immediately downstream of the G-G and G-A mismatches was replaced by 3′-CT-5′ (underlined). These mismatched DNA substrates were analyzed for extension by 3 ng (54 fmol) of human Polμ using a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs (N4) as indicated. The mismatched primer 3′ and A are underlined. Asterisk, 32P label. (B) Polymerase assays were performed with human Polμ (30 ng; 540 fmol) at 37°C for 30 min. After the polymerase reaction, 5 μl of the reaction products was treated with 10 U of SphI as for Fig. 5B. The digested products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. Samples without (−) or with (+) SphI treatment are indicated. DNA size markers in nucleotides are indicated on the sides. Mismatched DNA sequences are shown in Fig. 8A.
Similar to the paired T-A extension (Fig. 8B, lane 2), human Polμ extended the mismatched primers by only 1 or a few nucleotides under the reaction conditions of low enzyme concentration and short incubation time (Fig. 8B). With higher Polμ concentration and extended incubation time, longer DNA strands were synthesized from a mismatched primer 3′ end (Fig. 10B, lanes 1, 3, and 5), which allowed us to analyze the Polμ-synthesized products by SphI restriction digestion. After SphI cleavage, a 20-mer 32P-labeled DNA fragment is expected for extension without deletion. As shown in Fig. 10B (lanes 2 and 6), SphI cleavage of the Polμ-catalyzed T-T and A-G extension products yielded a major 19-mer DNA fragment, demonstrating −1 deletion as the major DNA product. SphI cleavage of the Polμ-catalyzed T-G extension products yielded a major 18-mer DNA fragment (Fig. 10B, lane 4), demonstrating −2 deletion as the major DNA product. Together, these results show that human Polμ can efficiently extend base mismatches by frameshift DNA synthesis.
Human Polμ promotes microhomology search and microhomology pairing in DNA.
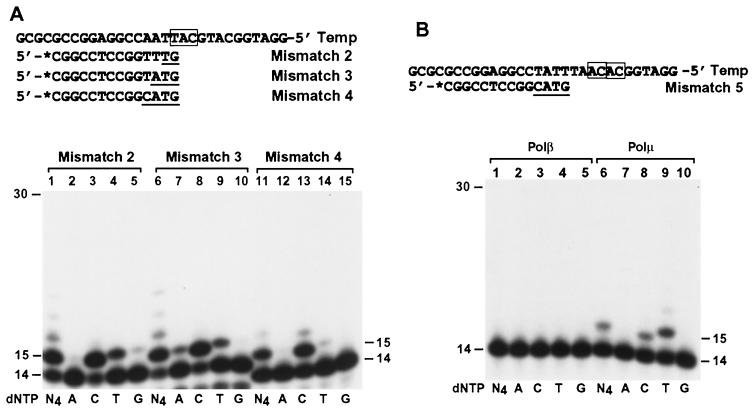
The unprecedented ability of human Polμ to perform frameshift DNA synthesis led us to suspect that this polymerase may be capable of promoting microhomology search by realigning two strands of DNA. To test this hypothesis, we prepared a DNA template to which three 32P-labeled primers were separately annealed. The resulting DNA substrates contained 2-, 3-, and 4-base mismatches, respectively, at the primer 3′ end (Fig. 11A). Up to 3-nucleotide microhomology was incorporated in the template DNA 2 nucleotides downstream from the primer 3′ end. Human Polμ was then incubated with the DNA substrates under polymerase reaction conditions. If human Polμ is able to promote homology search by realigning the template and the primer strands of DNA, DNA synthesis is expected and C incorporation is predicted. Indeed, DNA synthesis was observed and C was predominantly incorporated in every case (Fig. 11A, lanes 3, 8, and 13). Minor T incorporation was also observed with substrates containing two or three mismatches (Fig. 11A, lanes 4 and 9), probably as a result of mismatch extension without frameshift. Increasing mismatched bases from two to four at the primer 3′ end greatly decreased T incorporation by human Polμ (Fig. 11A, lanes 11 to 15), suggesting more efficient microhomology pairing with increasing mismatches at the primer 3′ end.
FIG. 11.
Microhomology search and microhomology pairing promoted by human Polμ. (A) A DNA template was separately annealed to three 32P-labeled (asterisks) 14-mer primers as shown, forming two, three, and four mismatches (underlined), respectively, at the primer ends. A sequence of 2 or 3 nucleotides (boxed) that could pair with the last 2 or 3 nucleotides of the primers was contained in the template 2 nucleotides downstream. (B) A 32P-labeled 14-mer primer was annealed to a template as shown, forming 4-nucleotide mismatches at the primer 3′ end (underlined). Two 3′-AC-5′ sequences located 4 and 6 nucleotides, respectively, downstream in the template (boxed) were complementary to the last 2 nucleotides of the primer. These DNA substrates were incubated with human Polβ (23 ng; 605 fmol) or human Polμ (3 ng; 54 fmol) under standard DNA polymerase assay conditions in the presence of a single dNTP (dATP [A], dCTP [C], dTTP [T], or dGTP [G]) or all four dNTPs as indicated. DNA size markers in nucleotides are indicated on the sides.
To examine whether the mismatched primer end can pair with a microhomologous region further downstream in the template, we modified the template sequence such that the last 2 nucleotides (5′-TG-3′) of the primer were complementary to two template 3′-AC-5′ sequences located 4 and 6 nucleotides, respectively, downstream (Fig. 11B). If microhomology pairing had occurred at the first template 3′-AC-5′ sequence 4 nucleotides away, human Polμ would incorporate a T. If microhomology pairing had occurred at the second template 3′-AC-5′ sequence 6 nucleotides away, human Polμ would incorporate a C. As predicted by such microhomology pairings, T incorporation by human Polμ was observed (Fig. 11B, lane 9), and less frequently, C incorporation was also observed (Fig. 11B, lane 8). A incorporation that would have resulted from primer extension without frameshift was not detected (Fig. 11B, lane 7). In contrast, human Polβ (even in greatly excessive amounts) was completely unresponsive to this DNA substrate (Fig. 11, lanes 1 to 5). These results show that human Polμ is capable of promoting microhomology search and subsequent microhomology pairing in DNA.
DISCUSSION
To facilitate understanding of the biological function of human DNA Polμ, we have purified this polymerase and analyzed its biochemical properties. Surprisingly, human Polμ catalyzes frameshift DNA synthesis with an unprecedentedly high frequency. The rate of frameshift DNA synthesis is greatly dependent on the sequence context of the template base to be copied. When the primer 3′ end is complementary to the next template base (template AA, TT, GG, and CC sequences), human Polμ most efficiently misaligns the primer end to the next template base prior to DNA synthesis, resulting in −1 deletion products. Remarkably, at the template AA, TT, GG, and CC sequences examined in this study, −1 frameshift synthesis has become the predominant mechanism of DNA synthesis by human Polμ, with rates ranging from 2.4- to 28-fold higher than the normal DNA synthesis. When the primer 3′ end is complementary to the template base 2 nucleotides downstream, human Polμ can often efficiently catalyze −2 primer-template misalignment prior to DNA synthesis, leading to −2 deletion (data not shown). Compared to template AA, TT, and GG sequences, frameshift DNA synthesis opposite template CC is less efficient, which seems to be inversely correlated to the relatively higher catalytic efficiency of human Polμ opposite template C. We have additionally examined DNA templates in which the primer end could be misaligned backward to “loop out” the primer. DNA synthesis based on such a mechanism would produce insertion products. However, under the conditions used in this study, there was no evidence supporting such an insertion frameshift DNA synthesis by human Polμ (data not shown).
Recently, Dominguez et al. (8) proposed that human Polμ might function as a DNA mutator polymerase in somatic hypermutation of immunoglobulin genes. Extensive analyses of immunoglobulin gene mutations have indicated that hypermutation mainly results in base substitutions (point mutations) (4, 12). Thus, a major hypermutation DNA polymerase must satisfy at least two biochemical requirements: (i) be highly error prone and (ii) have higher base substitution rates than frameshift DNA synthesis rates. Using DNA sequences derived from the JH4-JH5 intron of the rearranged human JH gene, the measured error rates of human Polμ are not exceptionally high (Table 1). Except for G incorporation with template A, which may result from −1 frameshift DNA synthesis, all other error rates most likely reflect the base substitution rates of human Polμ. These error rates suggest that human Polμ is significantly more accurate than human DNA polymerases η, ι, and κ with respect to base substitutions during DNA synthesis (14, 15, 19, 23, 28, 38, 39). In contrast, the extraordinary ability of human Polμ to perform frameshift DNA synthesis is unmatched by any other DNA polymerases known. The hypermutation spectrum at the JH4-JH5 intron sequence shows base substitution as the vast majority of mutations (18). Among the 242 mutations observed, −1 deletion was scored only once, although more than one single-nucleotide repeat sequence is contained within every 10 bp of the JH4-JH5 intron (18). Since an intron sequence was analyzed, the hypermutation spectrum reported by Levy et al. (18) could not have been biased by selection. Clearly, the biochemical property of prevalent frameshift DNA synthesis has ruled out human Polμ as a significant somatic hypermutation DNA polymerase. DNA Polι appears to be a more likely candidate for somatic hypermutation, as originally proposed by us (38) and by Tissier et al. (28).
What, then, is the cellular function of human Polμ? Our biochemical studies may provide an important clue to the answer. Human Polμ is highly capable of realigning the primer and the template strands of DNA. Such realignment of two DNA strands is especially prominent at a mismatched primer end. Primer ends that contain one to four of the mismatches examined all promote Polμ-mediated DNA strand realignment. The result of the primer-template realignment is microhomology pairing between the primer end and the template strand downstream. Therefore, human Polμ is able to promote microhomology search and subsequent microhomology pairing between the primer strand and the template strand of DNA. Following microhomology pairing (1- to 3-base pairing), human Polμ can extend the primer end by 1 or a few nucleotides, consequently further stabilizing the DNA strand realignment and the paired microhomology region. The ability of human Polμ to promote microhomology search and microhomology pairing between the primer and the template strands of DNA strongly suggests a function for this polymerase in NHEJ during repair of double-strand DNA breaks. Several proteins have been identified for NHEJ, including Ku70, Ku80, DNA-PKcs, XRCC4, and DNA ligase IV (25). It is believed that the Ku70-Ku80 heterodimer binds to the DNA ends and holds the ends together (7, 25). The XRCC4-ligase IV complex is believed to be required to ligate DNA strands at the last step of NHEJ (7, 11, 34). A DNA polymerase has not been identified for NHEJ in higher eukaryotes, although NHEJ would conceptually require a DNA polymerase activity. We propose that human Polμ plays an important role in NHEJ. We hypothesize that human Polμ may function to promote microhomology search and microhomology pairing during NHEJ. Subsequent DNA polymerase activity of Polμ would stabilize the microhomology pairing to prepare the XRCC4-ligase IV complex for DNA ligation.
In addition to repairing damage-induced double-strand DNA breaks, NHEJ is also an essential mechanism of the V(D)J recombination. V(D)J recombination helps generate diversity of antigen-binding sites of antibodies and T-cell receptor proteins during lymphoid cell development. A role for Polμ in NHEJ would predict that this polymerase is important for V(D)J recombination in lymphoid cells. Ubiquitous expression of human Polμ in various tissues, including lymphoid tissues (1, 8), is consistent with a role of this polymerase in NHEJ and V(D)J recombination. Recently, Wilson and Lieber (35) reported evidence suggesting that yeast Pol4 (Polβ) is involved in NHEJ. Since yeast Pol4 appears to be more related to human Polλ and Polμ than to human Polβ (1), the results of Wilson and Lieber (35) support our model in which Polμ functions in NHEJ. As proposed most recently by Ruiz et al. (26), an 8-kDa domain with potential DNA-binding activity and an N-terminal BRCT domain similar to that of the TdT in human Polμ are consistent with a role of this polymerase in NHEJ and V(D)J recombination.
ACKNOWLEDGMENTS
This work was supported by a New Investigator Award in Toxicology from the Burroughs Wellcome Fund and research grant CA67978 from NIH.
REFERENCES
- 1.Aoufouchi S, Flatter E, Dahan A, Faili A, Bertocci B, Storck S, Delbos F, Cocea L, Gupta N, Weill J C, Reynaud C A. Two novel human and mouse DNA polymerases of the polX family. Nucleic Acids Res. 2000;28:3684–3693. doi: 10.1093/nar/28.18.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard W A, Wilson S H. Structural design of a eukaryotic DNA repair polymerase: DNA polymerase β. Mutat Res. 2000;460:231–244. doi: 10.1016/s0921-8777(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 3.Bentolila L A, Fanton d'Andon M, Nguyen Q T, Martinez O, Rougeon F, Doyen N. The two isoforms of mouse terminal deoxynucleotidyl transferase differ in both the ability to add N regions and subcellular localization. EMBO J. 1995;14:4221–4229. doi: 10.1002/j.1460-2075.1995.tb00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertocci B, Quint L, Delbos F, Garcia C, Reynaud C A, Weill J C. Probing immunoglobulin gene hypermutation with microsatellites suggests a nonreplicative short patch DNA synthesis process. Immunity. 1998;9:257–265. doi: 10.1016/s1074-7613(00)80608-1. [DOI] [PubMed] [Google Scholar]
- 5.Chang L M, Bollum F J. Molecular biology of terminal transferase. Crit Rev Biochem. 1986;21:27–52. doi: 10.3109/10409238609113608. [DOI] [PubMed] [Google Scholar]
- 6.Creighton S, Bloom L B, Goodman M F. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 7.Critchlow S E, Jackson S P. DNA end-joining: from yeast to man. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez O, Ruiz J F, Lain de Lera T, Garcia-Diaz M, Gonzalez M A, Kirchhoff T, Martinez A C, Bernad A, Blanco L. DNA polymerase mu (Pol μ), homologous to TdT, could act as a DNA mutator in eukaryotic cells. EMBO J. 2000;19:1731–1742. doi: 10.1093/emboj/19.7.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedberg E C, Feaver W J, Gerlach V L. The many faces of DNA polymerases: strategies for mutagenesis and for mutational avoidance. Proc Natl Acad Sci USA. 2000;97:5681–5683. doi: 10.1073/pnas.120152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Diaz M, Dominguez O, Lopez-Fernandez L A, de Lera L T, Saniger M L, Ruiz J F, Parraga M, Garcia-Ortiz M J, Kirchhoff T, del Mazo J, Bernad A, Blanco L. DNA polymerase lambda (Pol λ), a novel eukaryotic DNA polymerase with a potential role in meiosis. J Mol Biol. 2000;301:851–867. doi: 10.1006/jmbi.2000.4005. [DOI] [PubMed] [Google Scholar]
- 11.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber M R. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–484. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 12.Insel R A, Varade W S. Characteristics of somatic hypermutation of human immunoglobulin genes. Curr Top Microbiol Immunol. 1998;229:33–44. doi: 10.1007/978-3-642-71984-4_4. [DOI] [PubMed] [Google Scholar]
- 13.Ito J, Braithwaite D K. Compilation and alignment of DNA polymerase sequences. Nucleic Acids Res. 1991;19:4045–4057. doi: 10.1093/nar/19.15.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R E, Washington M T, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R E, Washington M T, Prakash S, Prakash L. Fidelity of human DNA polymerase η. J Biol Chem. 2000;275:7447–7450. doi: 10.1074/jbc.275.11.7447. [DOI] [PubMed] [Google Scholar]
- 16.Kornberg A, Baker T. DNA replication. 2nd ed. New York, N.Y: W. H. Freeman; 1991. [Google Scholar]
- 17.Kubota Y, Nash R A, Klungland A, Schar P, Barnes D E, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 18.Levy Y, Gupta N, Le Deist F, Garcia C, Fischer A, Weill J C, Reynaud C A. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc Natl Acad Sci USA. 1998;95:13135–13140. doi: 10.1073/pnas.95.22.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel T A. Low fidelity DNA synthesis by human DNA polymerase η. Nature. 2000;404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 20.McDonald J P, Rapic-Otrin V, Epstein J A, Broughton B C, Wang X, Lehmann A R, Wolgemuth D J, Woodgate R. Novel human and mouse homologs of Saccharomyces cerevisiae DNA polymerase η. Genomics. 1999;60:20–30. doi: 10.1006/geno.1999.5906. [DOI] [PubMed] [Google Scholar]
- 21.Morrison A, Christensen R B, Alley J, Beck A K, Bernstine E G, Lemontt J F, Lawrence C W. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson J R, Lawrence C W, Hinkle D C. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi E, Bebenek K, Matsuda T, Feaver W J, Gerlach V L, Friedberg E C, Ohmori H, Kunkel T A. Fidelity and processivity of DNA synthesis by DNA polymerase κ, the product of the human DINB1 gene. J Biol Chem. 2000;275:39678–39684. doi: 10.1074/jbc.M005309200. [DOI] [PubMed] [Google Scholar]
- 24.Ohmori H, Friedberg E C, Fuchs R P P, Goodman M F, Hanaoka F, Hinkle D, Kunkel T A, Lawrence C W, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker G C, Wang Z, Woodgate R. The Y-family of DNA polymerases. Mol Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 25.Rathmell W K, Chu G. Mechanisms for DNA double-strand break repair in eukaryotes. In: Nickoloff J A, Hoekstra M F, editors. DNA damage and repair. II. Totowa, N.J: Humana Press; 1998. pp. 299–316. [Google Scholar]
- 26.Ruiz J F, Dominguez O, Lain de Lera T, Garcia-Diaz M, Bernad A, Blanco L. DNA polymerase mu, a candidate hypermutase? Phil Trans R Soc Lond B. 2001;356:99–109. doi: 10.1098/rstb.2000.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharief F S, Vojta P J, Ropp P A, Copeland W C. Cloning and chromosomal mapping of the human DNA polymerase theta (POLθ), the eighth human DNA polymerase. Genomics. 1999;59:90–96. doi: 10.1006/geno.1999.5843. [DOI] [PubMed] [Google Scholar]
- 28.Tissier A, McDonald J P, Frank E G, Woodgate R. polι, a remarkably error-prone human DNA polymerase. Genes Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 29.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 30.Wagner S D, Neuberger M S. Somatic hypermutation of immunoglobulin genes. Annu Rev Immunol. 1996;14:441–457. doi: 10.1146/annurev.immunol.14.1.441. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z. Translesion synthesis by the UmuC family of DNA polymerases. Mutat Res. 2001;486:59–70. doi: 10.1016/s0921-8777(01)00089-1. [DOI] [PubMed] [Google Scholar]
- 32.Weaver D T. V(D)J recombination and double-strand break repair. Adv Immunol. 1995;58:29–85. doi: 10.1016/s0065-2776(08)60619-7. [DOI] [PubMed] [Google Scholar]
- 33.Wilson S H. Mammalian base excision repair and DNA polymerase β. Mutat Res. 1998;407:203–215. doi: 10.1016/s0921-8777(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 34.Wilson T E, Grawunder U, Lieber M R. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature. 1997;388:495–498. doi: 10.1038/41365. [DOI] [PubMed] [Google Scholar]
- 35.Wilson T E, Lieber M R. Efficient processing of DNA ends during yeast nonhomologous end joining. Evidence for a DNA polymerase β (Pol4)-dependent pathway. J Biol Chem. 1999;274:23599–23609. doi: 10.1074/jbc.274.33.23599. [DOI] [PubMed] [Google Scholar]
- 36.Wood R D, Shivji M K. Which DNA polymerases are used for DNA-repair in eukaryotes? Carcinogenesis. 1997;18:605–610. doi: 10.1093/carcin/18.4.605. [DOI] [PubMed] [Google Scholar]
- 37.Xin H, Lin W, Sumanasekera W, Zhang Y, Wu X, Wang Z. The human RAD18 gene product interacts with HHR6A and HHR6B. Nucleic Acids Res. 2000;28:2847–2854. doi: 10.1093/nar/28.14.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Yuan F, Wu X, Wang Z. Preferential incorporation of G opposite template T by the low-fidelity human DNA polymerase ι. Mol Cell Biol. 2000;20:7099–7108. doi: 10.1128/mcb.20.19.7099-7108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Yuan F, Xin H, Wu X, Rajpal D, Yang D, Wang Z. Human DNA polymerase κ synthesizes DNA with extraordinarily low fidelity. Nucleic Acids Res. 2000;28:4147–4156. doi: 10.1093/nar/28.21.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]