Abstract
Background
Thymic stromal lymphopoietin (TSLP) is a key upstream regulator driving allergic inflammatory responses. We evaluated the efficacy and safety of ecleralimab, a potent inhaled neutralising antibody fragment against human TSLP, using allergen inhalation challenge (AIC) in subjects with mild atopic asthma.
Methods
This was a 12-week, randomised, double-blind, placebo-controlled, parallel-design, multicentre allergen bronchoprovocation study conducted at 10 centres across Canada and Germany. Subjects aged 18–60 years with stable mild atopic asthma were randomised (1:1) to receive 4 mg once-daily inhaled ecleralimab or placebo. Primary end-points were the allergen-induced change in forced expiratory volume in 1 s (FEV1) during the late asthmatic response (LAR) measured by area under the curve (AUC3–7h) and maximum percentage decrease (LAR%) on day 84, and the safety of ecleralimab. Allergen-induced early asthmatic response (EAR), sputum eosinophils and fractional exhaled nitric oxide (FENO) were secondary and exploratory end-points.
Results
28 subjects were randomised to ecleralimab (n=15) or placebo (n=13). On day 84, ecleralimab significantly attenuated LAR AUC3–7h by 64% (p=0.008), LAR% by 48% (p=0.029), and allergen-induced sputum eosinophils by 64% at 7 h (p=0.011) and by 52% at 24 h (p=0.047) post-challenge. Ecleralimab also numerically reduced EAR AUC0–2h (p=0.097) and EAR% (p=0.105). FENO levels were significantly reduced from baseline throughout the study (p<0.05), except at 24 h post-allergen (day 43 and day 85). Overall, ecleralimab was safe and well tolerated.
Conclusion
Ecleralimab significantly attenuated allergen-induced bronchoconstriction and airway inflammation, and was safe in subjects with mild atopic asthma.
Short abstract
Ecleralimab significantly attenuated allergen-induced airway responses and inflammation in subjects with mild asthma. It was generally safe and well tolerated, suggesting anti-TSLP may be a promising, new therapeutic class for inhaled asthma treatment. https://bit.ly/3U294EA
Introduction
Allergic asthma is characterised by eosinophilic inflammation and evidence of atopy, where T-helper type 2 immune pathway elements play an important role in the development and maintenance of airway inflammation and airway hyperresponsiveness (AHR). The majority of patients with asthma can be controlled by inhaled corticosteroids given alone or in combination with long-acting β2-agonists; however, ∼5% of patients still have uncontrolled severe disease [1]. The multiplicity of mechanisms and asthma phenotypes may necessitate different therapeutic approaches for successful management of disease, and many new therapeutic options are in clinical development [2].
A large body of evidence supports CD4+ T-cells and, more recently, type 2 innate lymphoid cells playing a critical role in type 2 inflammation and the development of asthma [3–5] by activating and/or recruiting B-cells, mast cells and eosinophils to the airways, which results in allergic responses and hallmark features of allergic asthma [1]. A key upstream regulator of the type 2 inflammatory response is thymic stromal lymphopoietin protein (TSLP) [6–8], which activates immune cells [9] and epithelial cells [10].
TSLP is associated with atopic diseases [11, 12] and TSLP gene promoter polymorphisms are associated with allergic inflammation, including asthma, increased IgE levels and eosinophilia [13]. Inhibition of TSLP via systemic administration (AMG 157 (tezepelumab)) attenuated allergen-induced airway responses and eosinophilia in mild atopic asthmatic subjects [14], and tezepelumab is now marketed for severe asthma following large clinical trials demonstrating efficacy [15, 16]. TSLP blockade may provide a potential advantage to current therapies by regulating several downstream pathways involved in asthma pathogenesis [15].
Ecleralimab is a potent neutralising antibody fragment (fragment antigen binding (Fab)) directed against human TSLP that has been developed for inhalation. This study used allergen inhalation challenge (AIC) as a model [17] to evaluate the efficacy and safety of ecleralimab on asthmatic airway responses to allergic triggers. Some results have been reported in the form of abstracts [18, 19].
Methods
Study design
A randomised, double-blind, placebo-controlled, parallel-design, multicentre AIC study was conducted at 10 centres across Canada and Germany from December 2017 to July 2019 (ClinicalTrials.gov: NCT03138811), consisting of a screening period, a baseline evaluation, a 12-week treatment period that included two AICs and a 4-week follow-up period.
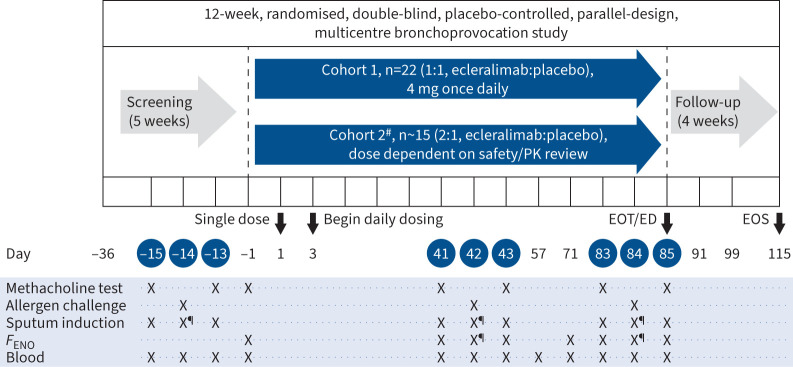
Two sequential cohorts were planned for the study. Ecleralimab at a dose of 4 mg was studied in Cohort 1 and subjects in Cohort 2 were to be given 16 mg once daily or less based on evolving safety and pharmacokinetics data from Cohort 1. Safety data and available pharmacokinetics data did not preclude dose escalation; however, it was decided to extend the dataset at a constant dose and Cohort 2 received 4 mg once daily (figure 1).
FIGURE 1.
Study design. The efficacy end-points were evaluated on days 42 and 84. #: efficacy interim analysis planned in the middle of Cohort 2 (∼15 subjects enrolled) with the potential to adjust the dose for an additional ∼18 subjects; ¶: 7 h post-challenge. PK: pharmacokinetics; EOT: end of treatment; ED: early discontinuation; EOS: end of study; FENO: fractional exhaled nitric oxide.
The study was approved by the independent ethics committees at participating centres in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All subjects provided written informed consent to participate in the study.
Subjects
Male and female subjects aged 18–60 years with stable mild atopic asthma requiring no asthma medications except short-acting β2-agonists less than twice weekly for treatment of symptoms were recruited. Subjects were required to have forced expiratory volume in 1 s (FEV1) ≥70% predicted [20], methacholine provocation concentration causing a 20% decrease in FEV1 (PC20) ≤16 mg·mL−1 during the screening methacholine inhalation challenge (MIC), positive skin prick test to common aeroallergens, and positive early asthmatic response (EAR; at least 20% decrease in FEV1 within 2 h of allergen inhalation) and positive late asthmatic response (LAR; at least 15% decrease in FEV1 between 3 and 7 h after inhalation) to a common inhaled allergen during the screening AIC.
Subjects were excluded if they required hospitalisation or emergency department treatment for asthma within the last 6 months, or if they had a history of clinically significant comorbidities or clinically significant chronic lung disease other than mild atopic asthma.
Randomisation and masking
Randomisation utilised interactive response technology (IRT) that assigned a randomisation number to the subject, which linked the subject to a treatment arm and specified a unique medication number to be dispensed. Randomisation codes, data on treatment and dosage allocation were tracked using IRT. Eligible subjects were randomly assigned to ecleralimab or placebo in Cohort 1 (1:1) and Cohort 2 (2:1).
Treatment allocations were concealed using identical packaging, labelling and schedule of administration and odour, and by the assignment of unblinded staff to assist subjects with investigational drug administration at study visits. No emergency unblinding took place during the study.
Procedures
Ecleralimab is formulated as a PulmoSol engineered powder in hard capsules and delivered to the lungs via a Breezhaler dry powder inhaler device, all provided by Novartis (Basel, Switzerland). Based on clinical and nonclinical safety data, a starting dose of 4 mg administered once daily for 12 weeks was expected to provide adequate pulmonary exposure to assess pharmacodynamic effects against allergen-induced airway responses. Each randomised subject received a single inhaled dose of ecleralimab or placebo on day 1 at the investigational site. Subsequent daily dosing began on day 3 provided no safety concerns were identified in the 48 h following the initial dose. Dosing was self-administered during the morning at home or at the study site during scheduled visits up to and including day 84. MIC and AIC testing commenced at least 1 h after dosing. Compliance to study treatment was recorded by the subject in a diary and assessed at each visit using blister pack counts and the diary.
13 visits were scheduled during the 12-week treatment period, including two allergen challenge triads (MIC–AIC–MIC) at days 41–43 and days 83–85. Safety, pharmacokinetics and pharmacodynamics assessments were also performed. To enter the treatment period, subjects needed to demonstrate return to baseline with FEV1 ≥70% predicted, and FEV1 and forced vital capacity were to be within 10% and the methacholine PC20 not more than 1 doubling concentration lower than the values measured at day −15 during screening.
MIC was conducted during screening (to qualify subjects for the study), pre-treatment on day 1, and 24 h pre-AIC and 24 h post-AIC, as previously described [21], until a 20% decrease in FEV1 occurred. The methacholine PC20 was calculated from the log concentration versus response curve.
AIC was conducted during screening and again at day 42 and day 84 as previously reported (see supplementary material) [22]. At screening, doubling concentrations of commercially available aero-allergen extracts (table 1) were inhaled at 12-min intervals until a ≥20% decrease in FEV1 was reached. FEV1 was then measured at regular intervals for 7 h to identify subjects with positive LAR. Allergen concentrations administered on days 42 and 84 were the same as those administered at screening. EAR and LAR were reported as time-adjusted area of percentage decrease in FEV1 (EAR AUC0–2h and LAR AUC3–7h), maximum percentage decrease in FEV1 (EAR% and LAR%) and minimum FEV1 (EARmin and LARmin).
TABLE 1.
Demographics and baseline characteristics (safety set)
| Ecleralimab 4 mg (n=15) | Placebo (n=13) | |
| Age (years) | 34.1±10.9 | 34.1±12.6 |
| Female | 8 (53) | 9 (69) |
| Body mass index (kg·m−2) | 26.8±3.9 | 23.7±2.3 |
| FEV1 (L) | 3.4±0.6 | 3.3±0.5 |
| FEV1 (% pred) | 91.0±10.6 | 92.1±15.5 |
| Methacholine PC20 (mg·mL−1)# | 1.41 (272) | 1.37 (136) |
| FENO (ppb)# | 44.02 (89) | 32.20 (72) |
| Blood eosinophils (×109 L−1)# | 0.23 (91) | 0.16 (80) |
| Sputum eosinophils (%)# | 1.97 (101) | 2.19 (135) |
| Inhaled allergen | ||
| Animal dander | 7 (46.6) | 7 (53.8) |
| House dust mite | 3 (20.0) | 0 (0) |
| Grass | 1 (6.7) | 1 (7.7) |
| Tree | 3 (20.0) | 5 (38.5) |
| Crops | 1 (6.7) | 0 (0) |
Data presented as mean±sd or n (%), unless otherwise stated. #: geometric mean (geometric coefficient of variation %). BMI: body mass index; FEV1: forced expiratory volume in 1 s; PC20: provocative concentration causing a 20% decrease in FEV1; FENO: fractional exhaled nitric oxide.
Sputum was induced before the start of treatment on day 1, and during each allergen challenge triad at 24 h pre-allergen and at 7 and 24 h post-allergen, and processed using a method modified from Pizzichini et al. [23].
Fractional exhaled nitric oxide (FENO) was sampled before each MIC, beginning on day −1 and also at 7 h post-allergen (prior to 7 h spirometry), using a Niox VERO (Aerocrine, Stockholm, Sweden) FENO testing device. Peripheral blood eosinophils were also assessed.
Safety assessments included physical examinations, systems review, open-ended health inquiry, ECGs, vital signs, haematology, blood chemistry, urinalysis, and monitoring of adverse events (AEs) and serious AEs (SAEs). Subjects completed an end-of-treatment period visit on day 85 and entered a 4-week follow-up period.
Outcomes
The primary end-points of this study included the allergen-induced LAR on day 84 assessed by LAR AUC3–7h and LAR%, and the safety and tolerability of daily ecleralimab administration assessed by AEs and SAEs.
Secondary end-points included day 42 and day 84 EAR AUC0–2h, EAR%, EARmin and LARmin, and day 42 LAR AUC3–7h and LAR%.
Exploratory end-points included sputum eosinophils, FENO, methacholine PC20 and blood eosinophil levels.
Statistical analysis
The sample size was calculated considering 80% power to detect an absolute difference either of 5% between ecleralimab and placebo in LAR AUC3–7h at day 84 (assuming that the standard deviation was 7.7) or to detect an absolute difference of 8% between ecleralimab and placebo in LAR% (assuming that the standard deviation was 12.7) using a Hochberg procedure with an overall family-wise controlled type 1 error rate of 10% and assuming a correlation coefficient of 0.8 between the two end-points.
Two unblinded interim analyses (IA) were conducted. IA1 included 20 subjects from Cohort 1 (n=10 in ecleralimab and n=10 in placebo) to confirm the sample size assumptions while assessing the variability in time-adjusted area of percentage decrease in FEV1 AUC3–7h and maximum percentage decrease in FEV1 following the AIC in this population. These data were not intended to change the conduct of this study and were not used to stop the study at this time, but could have informed a change in sample size. No pause in enrolment, pending the data from IA1, was required. IA2 included 28 subjects from Cohorts 1 and 2 (n=15 in ecleralimab and n=13 in placebo), and was conducted when 26 subjects had completed day 84. This analysis showed that there were no safety issues, and that the variability for AUC and maximum percentage decrease was slightly less than assumed (the correlation between these was 0.95). The study was terminated when 27 subjects completed the study. As the study met the primary end-point with the 4-mg dose, a decision was made to cancel the planned dose escalation phase.
The efficacy variables were analysed using the efficacy set, which included all subjects who received any study drug and experienced no protocol deviations with relevant impact on efficacy end-points. The two primary end-points, LAR AUC3–7h and LAR%, compared with placebo were analysed separately using an ANCOVA model for repeated measures. The model included treatment and visit as independent variables, treatment by visit interaction term, and baseline by visit interaction term where baseline values for time-adjusted area of percentage decrease in FEV1 AUC3–7h and maximum percentage decrease in in FEV1 are measured from the AIC at day −14 during the screening period. Model-based estimates of mean (least square estimates) were reported for ecleralimab and placebo groups on day 42 and day 84 along with the treatment difference, two-sided 90% confidence interval for treatment difference and one-sided p-value.
The secondary efficacy end-points were listed by treatment, subjects and visit/time.
EAR AUC0–2h, EAR%, EARmin and LARmin were also analysed using an ANCOVA model for repeated measures. Baseline values for all secondary efficacy end-points were measured from the screening AIC at day −14.
The exploratory efficacy end-points were listed by treatment, subjects and visit/time, and descriptive statistics were provided by treatment and visit/time, as needed. Methacholine PC20 and FENO data were log transformed prior to analysis. Comparisons of ecleralimab with placebo on change from baseline at day 41 and day 83 and allergen-induced shift (change from pre-challenge (day 42/43 to day 41 and day 84/85 to day 83) at 7 and 24 h post-challenge) were carried out using an ANCOVA model for repeated measures for sputum eosinophils, FENO and methacholine PC20 (24 h post-challenge). Blood eosinophil levels were compared on change from baseline at day 43 and day 71.
The safety analysis set included all subjects who received any study drug. The primary variable for the primary safety end-point was the occurrence of AEs/SAEs. The numbers and percentages of subjects with AEs by maximum severity of AEs were tabulated by body system and MedDRA preferred term with a breakdown by treatment. All the analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Subject disposition and demographics
A total of 28 subjects were enrolled in the study (15 received ecleralimab and 13 received placebo) and 27 subjects (96.4%) completed the study; one subject in the ecleralimab group was discontinued from the study due to unstable asthma prior to day 84 allergen challenge (supplementary figure S1).
The subject demographics and baseline characteristics were similar between the groups (table 1).
The two treatment groups developed similar EAR and LAR at the screening allergen challenge. Three subjects (n=2 in ecleralimab and n=1 in placebo) were excluded from the LAR and EAR analyses in the first challenge (day 42), with one ecleralimab subject also excluded in the second challenge (day 84) due to incorrect dosing of allergen; however, a sensitivity analyses including the data demonstrated no change from the reported results. These subjects were not excluded from the methacholine PC20 and FENO analyses.
Early and late asthmatic responses
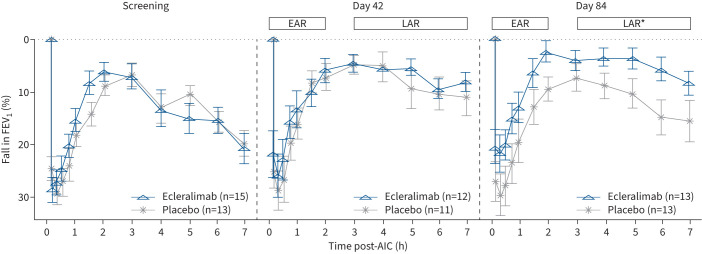
On day 84, ecleralimab significantly attenuated LAR AUC3–7h, being 4.2% in the ecleralimab group and 11.38% in the placebo group, representing a 64% reduction (treatment difference (Δ): −7.18% (90% CI −11.92– −2.44%); p=0.008). Similarly, ecleralimab significantly attenuated LAR%, being 9.3% in the ecleralimab group and 17.7% in the placebo group, demonstrating a 48% reduction (Δ: −8.42% (90% CI −15.66– −1.18%); p=0.029). In addition, LARmin was significantly higher in the ecleralimab group compared with the placebo group, being 2.96 versus 2.69 L, respectively, on day 84 (Δ: 0.27 L (90% CI −0.00–0.55 L); p=0.050). Small, nonsignificant reductions in LAR responses were found at day 42 for LAR AUC3–7h (Δ: −2.90% (90% CI −7.43–1.62%); p=0.141), LAR% (Δ: −2.64% (90% CI −9.02–3.74%); p=0.243) and LARmin (Δ: 0.09 L (90% CI −0.19–0.37 L); p=0.293) (figure 2).
FIGURE 2.
Effect of ecleralimab on allergen-induced early asthmatic response (EAR) and late asthmatic response (LAR) after days 42 and 84 of treatment. Data are presented as mean±se. FEV1: forced expiratory volume in 1 s; AIC: allergen inhalation challenge. *: p<0.05 significant difference between ecleralimab and placebo.
A numerical attenuation of the EAR was observed in subjects treated with ecleralimab at day 42 and day 84, but was not statistically different compared with placebo (supplementary table S1).
Allergen-induced airway inflammation
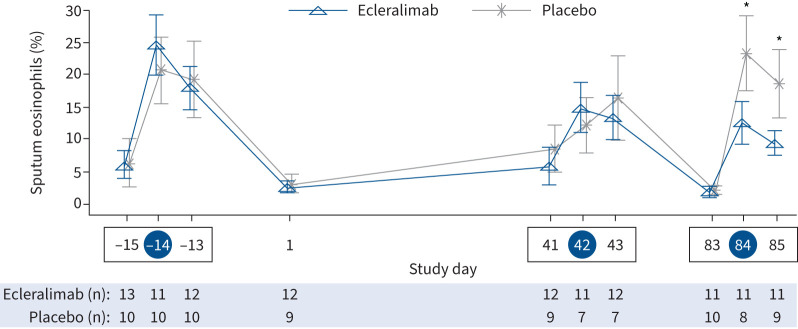
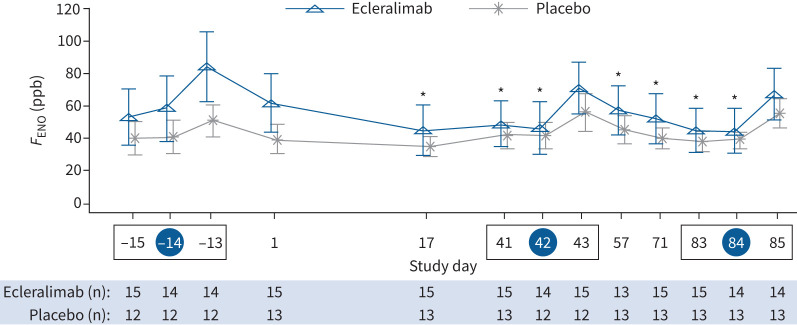
Ecleralimab significantly attenuated allergen-induced sputum eosinophils at 7 h (8.44% ecleralimab versus 23.19% placebo; Δ: −14.75% (90% CI −24.85– −4.65%); p=0.011) and 24 h (8.12% ecleralimab versus 16.84% placebo; Δ: −8.72% (90% CI −17.29– −0.15%); p=0.047) after day 84 AIC, corresponding to a 64% and 52% reduction, respectively, compared with placebo (figure 3). Compared with placebo there was no effect of ecleralimab on pre-allergen sputum eosinophil levels on day 41 (2.46% ecleralimab versus 6.14% placebo; Δ: −3.68% (90% CI −12.19–4.83%); p=0.231) or day 83 (−1.88% ecleralimab versus −1.32% placebo; Δ: −0.56% (90% CI −2.16–1.05%); p=0.27). There was a significant improvement from baseline in FENO by day 17 with ecleralimab compared with placebo (ratio to baseline: 0.73 versus 0.93) corresponding to a 27% versus 7% reduction (Δ: 0.78 (90% CI 0.63–0.97); p=0.031). Over the 12-week treatment period there were significant reductions in FENO (ppb) with ecleralimab (p<0.05), except at day 43 and day 85 at 24 h post-allergen (figure 4). There was no effect of ecleralimab on allergen-induced shift in FENO after either AIC.
FIGURE 3.
Effect of ecleralimab on sputum eosinophils before and after allergen challenge over 85 days. A blue circle around the study day indicates allergen inhalation challenge (AIC) and a box indicates an allergen challenge triad. Data are presented as mean±se. *: p<0.05 significant difference between ecleralimab and placebo.
FIGURE 4.
Effect of ecleralimab on fractional exhaled nitric oxide (FENO) change from baseline compared with placebo over 85 days. A blue circle around the study day indicates allergen inhalation challenge (AIC) and a box indicates an allergen challenge triad. Data are presented as mean±se. *: p<0.05 significant difference in change from baseline in ecleralimab versus change from baseline in placebo.
Airway hyperresponsiveness
There was a numerical increase in the methacholine PC20 on day 41 and day 83 in ecleralimab compared with placebo. Ecleralimab was better than placebo by 0.49 doubling doses on day 41 (Δ: 0.49 (90% CI −0.39–1.38) doubling doses; p=0.173) and by 0.18 doubling dose on day 83 (Δ: 0.18 (90% CI −0.54–0.90) doubling doses; p=0.335). There was no effect of ecleralimab on allergen-induced changes in methacholine PC20 measured on days 43 (day 43 to day 41) and 85 (day 85 to day 83). Moreover, there was no difference in peripheral blood eosinophil counts in subjects receiving ecleralimab compared with those receiving placebo.
Safety
A total of 22 subjects (78.6%) reported 93 treatment-emergent AEs: 51 AEs were reported in 10 subjects (66.7%) who received ecleralimab and 42 AEs were reported in 12 subjects (92.3%) who received placebo. The majority of AEs (75.0%) in both treatment groups were assessed as mild (table 2). The number, incidence and severity of treatment-emergent AEs were comparable between the treatment groups. The most frequent AEs were headache (25.0%), nasopharyngitis (17.9%) and oropharyngeal pain (17.9%) (supplementary table S2). No severe or serious AEs or deaths were reported. No subject discontinued study drug due to an AE. No clinically relevant changes were noted in the haematology, chemistry, urinalysis, ECG and spirometry results.
TABLE 2.
Overall incidence of adverse events (AEs)
| Ecleralimab 4 mg (n=15) | Placebo (n=13) | |||
| nE | nS (%) | nE | nS (%) | |
| Total AEs | 51 | 10 (66.7) | 42 | 12 (92.3) |
| AEs of mild intensity | 40 | 10 (66.7) | 30 | 11 (84.6) |
| AEs of moderate intensity | 11 | 6 (40.0) | 12 | 8 (61.5) |
| AEs of severe intensity | 0 | 0 | ||
| Study drug-related AEs | 4 | 4 (26.7) | 0 | |
| Serious AEs | 0 | 0 | ||
| AEs leading to discontinuation of study treatment | 0 | 0 | ||
| Study drug-related AEs leading to discontinuation of study treatment | 0 | 0 | ||
Percentage is based on the number of subjects. Only AEs occurring at or after first drug intake are included. nE: number of AEs in the category; nS: number of subjects with at least one AE in the category.
Discussion
Treatment with ecleralimab, the first inhaled anti-TSLP molecule, was shown to be safe and well tolerated in subjects with mild atopic asthma. Ecleralimab significantly attenuated the allergen-induced LAR on day 84, similar to that by i.v. anti-TSLP monoclonal antibody tezepelumab in a similar study [14]. Ecleralimab also reduced FENO after 17 days and allergen-induced sputum eosinophil levels after 3 months of dosing.
Emerging evidence suggests targeting TSLP may provide clinical benefit in asthma [15, 16] by reducing the inflammatory response to allergens [14]. In subjects with asthma, an increase in TSLP protein levels has been observed in both lung tissue and bronchoalveolar lavage fluid, and TSLP levels correlate with disease severity [11]. TSLP is thought to cause airway and blood eosinophilia among subjects with allergic asthma by activating airway dendritic cells and by polarising naïve T-cells to T-helper type 2 cells to produce pro-inflammatory cytokines, including interleukin (IL)-5 and IL-13 [24]. The significant inhibition of allergen-induced sputum eosinophils with ecleralimab on days 84 and 85 demonstrates that inhaled anti-TSLP Fab reduces allergen-induced airway inflammation in subjects with allergic asthma. There was no change in peripheral blood eosinophil counts in subjects receiving ecleralimab, which was expected due to the inhaled delivery.
The modest but statistically significant reduction of FENO levels suggests there is a constitutive release of TSLP from the airways of these subjects under baseline conditions [25, 26]. Furthermore, the reduction in allergen-induced eosinophils by ecleralimab indicates that TSLP, which is critical for maintaining eosinophilic airway inflammation, is effectively targeted with this inhaled formulation.
Methacholine challenge is a diagnostic test to evaluate the presence/severity of AHR consistent with a diagnosis of asthma including asthma control. While systemic anti-TSLP therapy has been reported to reduce baseline and allergen-induced eosinophilia in blood and airways in subjects, resulting in some improvements in AHR [14, 27], inhaled ecleralimab numerically improved baseline methacholine PC20 at days 41 and 83 by 0.49 and 0.18 doubling doses, respectively. In this same population, tezepelumab improved baseline methacholine PC20 at days 41 and 83 by 1.24 and 0.66 doubling doses, respectively [14]. Inhaled corticosteroid treatment over approximately the same period of time is reported to shift methacholine PC20 by 0.4–1.4 doubling doses [28]. Neither ecleralimab nor tezepelumab improved allergen-induced changes in methacholine PC20.
Ecleralimab inhaled at a dose of 4 mg once daily had no effect on EAR at day 42 and 84 allergen challenges, and no effect on LAR or sputum eosinophils at day 42 allergen challenge. The main reason for no effect on LAR at day 42 is due to higher than expected variability in two placebo outliers with significant improvement; when excluded, the LAR profiles look more similar to day 84. In contrast, in a separate study, treatment with systemic anti-TSLP therapy (700 mg i.v. every 4 weeks for 12 weeks) significantly inhibited EAR, LAR and sputum eosinophils at day 42 and 84 allergen challenges [14]. Collectively the results suggest at the doses studied there may be additional and/or more rapid blockade of cellular targets with systemic therapy. A higher dose of ecleralimab may have improved efficacy on these primary and secondary end-points; however, this was not studied because the dose was not escalated.
Ecleralimab was generally safe and well tolerated by the mild asthmatic subjects enrolled in this study. The study was terminated to proceed to development in severe asthmatic subjects, the intended population, rather than obtaining more data in mild asthmatic subjects and the decision for early termination was not based on any safety concerns regarding ecleralimab.
Limitations include the fact that the study was not powered to detect changes in secondary end-points such as methacholine PC20. We did observe a 0.49 doubling dose improvement in AHR; however, this change did not meet statistical significance. Although LAR at day 42 was numerically improved by ecleralimab, the lack of statistical significance is likely due to a lower sample size (only 12 received ecleralimab and 11 received placebo) and influenced by a marked placebo effect; the magnitude of LAR at day 42 AIC is considerably smaller than that at day −14 in the placebo group. No information on dose response could be obtained because of early termination of the study.
Ecleralimab, an inhaled Fab, reduced both allergen-induced late-phase bronchoconstriction and sputum eosinophilia, and was well tolerated. These findings demonstrate that ecleralimab is efficacious for attenuating allergen-induced responses in atopic asthmatic subjects.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01193-2022.supplement (210.6KB, pdf)
Shareable PDF
Acknowledgements
The study was funded by Novartis Pharma AG (Basel, Switzerland). We thank the investigators, study staff and subjects at the investigative sites for their support during the conduct of the study. The authors also thank Hiral Joshi, Phani Tejasvi and Chiranjit Ghosh of Novartis Medical and Knowledge Solutions (Hyderabad, India) for providing medical writing support in accordance with Good Publication Practice guidelines. This manuscript is dedicated to the memory of Dr J. Mark FitzGerald.
Footnotes
This study is registered at ClinicalTrials.gov with identifier number NCT03138811. Data sharing: Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymised to respect the privacy of subjects who have participated in the trial in line with applicable laws and regulations. Result summaries have been posted on the Novartis clinical trial database and other online public databases. More information on Novartis’ position on access to clinical trial results and patient-level data is available at: www.novartis.com/our-science/clinical-trials/clinical-trial-information-disclosure
Author contributions: All authors provided substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data for the work, and have been involved in drafting the work or revising it critically for important intellectual content. All authors have approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an editorial commentary: https://doi.org/10.1183/13993003.02389-2022
Conflict of interest: G.M. Gauvreau reports grants and personal fees from AstraZeneca, and grants from Biohaven, Genentech and BioGaia, outside of the submitted work. J.M. Hohlfeld reports grants from Novartis AG, during the conduct of the study; grants from AltamiraPharma GmbH, Astellas Pharma GmbH, AstraZeneca AB, Bayer AG, Beiersdorf AG, Boehringer Ingelheim Pharma GmbH & Co. KG, CSL Behring GmbH, Desitin Arzneimittel GmbH, F. Hoffmann-La Roche AG, Genentech, Inc., GlaxoSmithKline GmbH & Co. KG, Janssen Pharmaceuticals NV, M&P Pharma AG, Novartis AG, Sanofi-Aventis Deutschland GmbH and UCB Pharma GmbH and personal fees from Boehringer Ingelheim Pharma GmbH & Co. KG, CSL Behring GmbH, Merck & Co, Inc., Nocion Therapeutics, Inc., HAL Allergy Group and Novartis AG, outside of the submitted work. M.J. FitzGerald reported receipt of research funding from Novartis which was paid directly to UBC for the completion of this study and has also been in receipt of AllerGen CIHR and NIH grant funding unrelated to the current study. L-P. Boulet reports receiving grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron, personal fees from AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron, and honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events, outside of the submitted work. D.W. Cockcroft reports receiving grants from the Department of Medicine University of Saskatchewan, AstraZeneca, Novartis, CSACI and AllerGen NCE, outside of the submitted work. B.E. Davis has nothing to declare. S. Korn reports receiving personal fees from Novartis, outside of the submitted work. O. Kornmann reports receiving personal fees from Sanofi, Novartis, Boehringer Ingelheim, Adagio, AstraZeneca, Santhera and Chiesi, outside of the submitted work. R. Leigh reports receiving personal fees from Novartis, AstraZeneca, GlaxoSmithKline, Sanofi Genzyme and Valeo Pharma Inc., outside of the submitted work. I. Mayers has nothing to declare. H. Watz reports receiving grants and personal fees from Novartis, AstraZeneca, GlaxoSmithKline, Chiesi and Boehringer Ingelheim, outside of the submitted work. S.S. Grant, M. Jain and P.E. Pertel are employees of Novartis Institutes of Biomedical Research. M. Cabanski was an employee of Novartis during the time of the study. I. Jones and J.R. Lecot are employees of Novartis Pharma AG. H. Cao is an employee of Novartis Pharmaceuticals Corporation. P.M. O'Byrne has obtained grants in aid of research from Novartis for the conduct of the current study, as well as from AstraZeneca, Medimmune, Biohaven, Merck and Bayer for research outside the current study, and received personal fees for consulting or speaker fees from AstraZeneca, GSK, Medimmune, Chiesi, Menarini and Covis.
Support statement: The study was funded by Novartis Pharma AG (Basel, Switzerland) and AllerGen. The funders of the study participated in the study design and had access to the raw data. All authors, including those employed by the funders of the study, participated in the data collection, data analysis, data interpretation and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Backman H, Jansson SA, Stridsman C, et al. . Severe asthma – a population study perspective. Clin Exp Allergy 2019; 49: 819–828. doi: 10.1111/cea.13378 [DOI] [PubMed] [Google Scholar]
- 2.Gauvreau GM, Sehmi S, Ambrose CS, et al. . Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets 2020; 24: 777–792. doi: 10.1080/14728222.2020.1783242 [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, et al. . Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136: 2348–2357. [PubMed] [Google Scholar]
- 4.Al-Shami A, Spolski R, Kelly J, et al. . A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005; 202: 829–839. doi: 10.1084/jem.20050199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff PG, Modrek B, Choy DF, et al. . T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395. doi: 10.1164/rccm.200903-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soumelis V, Reche PA, Kanzler H, et al. . Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3: 673–680. doi: 10.1038/ni805 [DOI] [PubMed] [Google Scholar]
- 7.Kitajima M, Lee HC, Nakayama T, et al. . TSLP enhances the function of helper type 2 cells. Eur J Immunol 2011; 41: 1862–1871. doi: 10.1002/eji.201041195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park LS, Martin U, Garka K, et al. . Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000; 192: 659–670. doi: 10.1084/jem.192.5.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res 2012; 52: 211–223. doi: 10.1007/s12026-012-8264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semlali A, Jacques E, Koussih L, et al. . Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol 2010; 125: 844–850. doi: 10.1016/j.jaci.2010.01.044 [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Liu YJ, Arima K. Cellular and molecular mechanisms of TSLP function in human allergic disorders – TSLP programs the “Th2 code” in dendritic cells. Allergol Int 2012; 61: 35–43. doi: 10.2332/allergolint.11-RAI-0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying S, O'Connor B, Ratoff J, et al. . Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005; 174: 8183–8190. doi: 10.4049/jimmunol.174.12.8183 [DOI] [PubMed] [Google Scholar]
- 13.Harada M, Hirota T, Jodo AI, et al. . Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol 2011; 44: 787–793. doi: 10.1165/rcmb.2009-0418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauvreau GM, O'Byrne PM, Boulet LP, et al. . Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 15.Corren J, Parnes JR, Wang L, et al. . Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 16.Menzies-Gow A, Corren J, Bourdin A, et al. . Efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 17.Diamant Z, Gauvreau GM, Cockcroft DW, et al. . Inhaled allergen bronchoprovocation tests. J Allergy Clin Immunol 2013; 132: 1045–1055. doi: 10.1016/j.jaci.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 18.Gauvreau G, Hohlfeld J, Boulet LP, et al. . Efficacy of CSJ117 on allergen-induced asthmatic responses in mild atopic asthma patients. Eur Respir J 2020; 56: Suppl. 64, 3690. doi: 10.1183/13993003.congress-2020.3690 [DOI] [Google Scholar]
- 19.Gauvreau G, Hohlfeld J, Grant S, et al. . Efficacy and safety of an inhaled anti-TSLP antibody fragment in adults with mild atopic asthma. Am J Respir Crit Care Med 2020; 201: A4207. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A4207 [DOI] [Google Scholar]
- 20.Cooper BG, Stocks J, Hall GL, et al. . The Global Lung Function Initiative (GLI) Network: bringing the world's respiratory reference values together. Breathe 2017; 13: e56–e64. doi: 10.1183/20734735.012717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Killian DN, Mellon JJ, et al. . Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy 1977; 7: 235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x [DOI] [PubMed] [Google Scholar]
- 22.Boulet LP, Gauvreau G, Boulay ME, et al. . The allergen bronchoprovocation model: an important tool for the investigation of new asthma anti-inflammatory therapies. Allergy 2007; 62: 1101–1110. doi: 10.1111/j.1398-9995.2007.01499.x [DOI] [PubMed] [Google Scholar]
- 23.Pizzichini E, Pizzichini MM, Efthimiadis A, et al. . Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996; 154: 308–317. doi: 10.1164/ajrccm.154.2.8756799 [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Comeau MR, De Smedt T, et al. . Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005; 6: 1047–1053. doi: 10.1038/ni1247 [DOI] [PubMed] [Google Scholar]
- 25.Al-Sajee D, Sehmi R, Hawke TJ, et al. . Expression of IL-33 and TSLP and their receptors in asthmatic airways after inhaled allergen challenge. Am J Respir Crit Care Med 2018; 198: 805–807. doi: 10.1164/rccm.201712-2468LE [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Li Y, Lv Z, et al. . Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol 2018; 201: 2221–2231. doi: 10.4049/jimmunol.1800709 [DOI] [PubMed] [Google Scholar]
- 27.Sverrild A, Hansen S, Hvidtfeldt M, et al. . The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J 2022; 59: 2101296. doi: 10.1183/13993003.01296-2021 [DOI] [PubMed] [Google Scholar]
- 28.Davis BE, Blais CM, Cockcroft DW. Methacholine challenge testing: comparative pharmacology. J Asthma Allergy 2018; 11: 89–99. doi: 10.2147/JAA.S160607 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01193-2022.supplement (210.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01193-2022.Shareable (387.6KB, pdf)