Abstract
Nitric oxide (NO) is one of the most important molecules released by endothelial cells, and its antiatherogenic properties support cardiovascular homeostasis. Diminished NO bioavailability is a common hallmark of endothelial dysfunction underlying the pathogenesis of the cardiovascular disease. Vascular NO is synthesized by endothelial nitric oxide synthase (eNOS) from the substrate L-arginine (L-Arg), with tetrahydrobiopterin (BH4) as an essential cofactor. Cardiovascular risk factors such as diabetes, dyslipidemia, hypertension, aging, or smoking increase vascular oxidative stress that strongly affects eNOS activity and leads to eNOS uncoupling. Uncoupled eNOS produces superoxide anion (O2−) instead of NO, thus becoming a source of harmful free radicals exacerbating the oxidative stress further. eNOS uncoupling is thought to be one of the major underlying causes of endothelial dysfunction observed in the pathogenesis of vascular diseases. Here, we discuss the main mechanisms of eNOS uncoupling, including oxidative depletion of the critical eNOS cofactor BH4, deficiency of eNOS substrate L-Arg, or accumulation of its analog asymmetrical dimethylarginine (ADMA), and eNOS S-glutathionylation. Moreover, potential therapeutic approaches that prevent eNOS uncoupling by improving cofactor availability, restoration of L-Arg/ADMA ratio, or modulation of eNOS S-glutathionylation are briefly outlined.
Keywords: Cardiovascular disease, Endothelial dysfunction, eNOS uncoupling, Oxidative/nitroxidative stress, Peroxynitrite, Nitric oxide, ADMA, Tetrahydrobiopterin, BH4
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, highlighting the need to investigate its molecular mechanisms for effective treatment options. A common and early hallmark of CVD is endothelial dysfunction, i.e., a disturbance in the normal physiology of the endothelium that lines all blood vessels [1]. The endothelium is critical to cardiovascular homeostasis and plays a vital role in the pathophysiology of cardiovascular diseases associated with atherosclerosis, including hypertension, stroke, coronary artery disease, peripheral vascular disease, or heart failure [2, 3]. Endothelial cells produce and release a subset of diverse signaling molecules that orchestrate cardiovascular physiology by regulating hemostasis, vascular tone and permeability, inflammation, and angiogenesis [4]. Among these substances, nitric oxide (NO) is a key molecule that significantly influences the physiology of the endothelium and the cardiovascular system, and endothelial dysfunction is often simply defined as diminished NO bioavailability [5].
Nitric oxide was discovered as an endothelium-derived relaxing factor (EDRF), and research into its essential role in vascular tone regulation has been recognized by the Nobel Prize awarded to Furchgott, Ignarro, and Murad in 1998 [6]. NO, as a lipophilic molecule, easily diffuses from endothelium into adjacent vascular smooth muscle cells (VSMCs) and binds to the prosthetic haem group of soluble guanylate cyclase (sGC), thus activating the enzyme [7]. sGC catalyzes the dephosphorylation of guanosine triphosphate (GTP) to cyclic guanosine 3’,5’-monophosphate (cGMP), which acts as a second messenger and activates protein kinase G (PKG) [8]. As a result of PKG activity, cytoplasmic calcium (Ca2+) levels decrease, and the downstream signaling cascade leads to vascular smooth muscle relaxation and consequent vasodilation [9]. But regulating vascular tone is not the only role of NO as it also regulates vascular wall permeability, reduces proliferation and migration of VSMCs as well as platelet activation and aggregation [10]. Moreover, NO modulates the expression of endothelial adhesion molecules and thus prevents leukocyte recruitment and adhesion [11]. Therefore, besides being a potent vasodilator, NO generally has anti-atherosclerotic properties. Its pleiotropic effects are critical for vascular homeostasis, and dysregulation of NO signaling pathways is associated with the pathogenesis of CVD [12]. Endothelial dysfunction is characterized by impaired endothelium-dependent vasorelaxation due to diminished nitric oxide bioavailability resulting from an imbalance between its generation and degradation.
In endothelial cells, NO is produced by endothelial nitric oxide synthase (eNOS), one of three nitric oxide synthases (NOS) present in human tissues besides neuronal NOS (nNOS) expressed primarily in neurons and inducible NOS (iNOS) expressed in various cell types (especially in immune system cells) during infection or inflammation [13]. Thus, in addition to its essential role in the regulation of vascular physiology, NO also functions as a neurotransmitter in the nervous system and as a cytotoxic agent in the immune response [14, 15]. Given the very short NO half-life, its molecular effects are restricted to the site of its synthesis, hence eNOS expressed almost exclusively in endothelial cells is the major donor of vascular NO. Thus, diminished eNOS expression and activity are the major causes of reduced NO synthesis. On the other hand, NO scavenging by superoxide anion (O2−) is the main reason for decreased NO half-life in the vasculature [16]. Therefore it is not surprising that increased oxidative stress is involved in the pathogenesis of CVD [17].
A unique phenomenon called eNOS uncoupling combines oxidative NO scavenging with altered eNOS activity. Uncoupled eNOS generates highly reactive superoxide (O2−) instead of NO. eNOS uncoupling is often triggered by oxidative stress associated with cardiovascular risk factors, including diabetes, hypertension, dyslipidemia, smoking, and aging, via the mechanisms described below [18]. The presence of both functional and uncoupled eNOS in the cell results in the concomitant production of NO and O2− in the close vicinity. O2− reacts with NO, thus scavenging it and yielding harmful peroxynitrite radical (ONOO−), so a vicious cycle arises that potentiates oxidative stress and drives pathological changes [19]. Moreover, eNOS uncoupling occurs to some extent physiologically, as we have shown by using high-precision electrochemical microsensors able for concomitant real-time detection of NO, O2− and ONOO− generated in a single cell [20–22]. eNOS uncoupling is thought to be one of the major underlying causes of endothelial dysfunction observed in the pathogenesis of vascular diseases. The presence of uncoupled eNOS has been proven in patients with diabetes [23–25], hypertension [26], coronary artery disease [27], and congestive heart failure [28]. eNOS uncoupling was also demonstrated in animal experimental models of hypertension [29], diabetes [30], ischemia–reperfusion injury [31, 32], and ageing [33], and confirmed in vitro in human and animal endothelial cell cultures [34, 35].
This review discusses the molecular mechanisms leading to eNOS uncoupling and the proposed therapeutic approaches to prevent or reverse eNOS uncoupling and restore endothelial function as a possible strategy for CVD treatment.
eNOS regulation
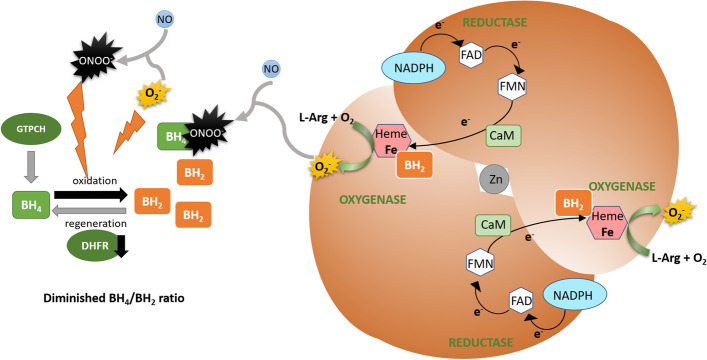
NO is produced by eNOS from L-Arg with molecular oxygen (O2) and nicotinamide adenine dinucleotide phosphate (NADPH) as co-substrates. The reaction requires several cofactors: heme, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and tetrahydrobiopterin (BH4), the latter being a critical determinant of eNOS coupling [13]. eNOS activity is also dependent on Ca2+/calmodulin (CaM) binding [36]. The enzyme exists as a homodimer, each monomer consisting of an N-terminal oxygenase domain with substrate L-Arg, heme, zinc, and BH4 cofactor binding sites, a central CaM-binding region, and a C-terminal reductase domain with NADPH, FAD, and FMN binding sites (Fig. 1) [37]. Functional eNOS dimer catalyzes the electron transfer from the C-terminal-bound NADPH through FAD and FMN of one monomer to the heme iron in the N-terminal oxygenase domain of the second monomer, and this interdomain electron transfer is facilitated by CaM. Heme iron is reduced, thus enabling the binding and reduction of O2. BH4 serves in this process as a one-electron donor for the heme-bound oxygen, which activates O2, enabling the following oxidation of L-Arg to L-citrulline (L-Cit) and NO (Fig. 1) [38–40]. The BH4 cofactor is thus essential for optimal eNOS activity [41].
Fig. 1.
Schematic eNOS homodimer structure with two monomers orientated "head-to-tail". Each monomer consists of a C-terminal reductase domain that binds NADPH, FAD, and FMN, a central calmodulin-binding region, and an N-terminal oxygenase domain that binds substrate L-Arg, oxygen, heme, and BH4. The formation of a homodimer enables the transfer of electrons from the reductase domain of one monomer to the oxygenase domain of the second monomer. The dimeric structure is stabilized by heme binding and by zinc ion in the zinc-thiolate cluster at the dimer interface. During catalysis, electrons from NADPH flow through the flavins FAD and FMN to the heme of the opposite monomer and CaM increases the rate of interdomain electron transfer. Heme reduction enables O2 binding, and BH4 can donate an electron to reduce and activate O2. When cellular redox balance is maintained, and the substrate L-Arg and the essential cofactor BH4 availabilities are optimal, O2 reduction is coupled to L-Arg oxidation and NO synthesis. L-Cit is formed as a byproduct
eNOS is expressed constitutively in endothelial cells and basal NO synthesis maintains resting vascular tone, however a number of factors dynamically influence the enzyme expression and activity [13, 42, 43]. eNOS is regulated by various stimuli through post-translational modifications: phosphorylation, acetylation, S-nitrosylation, S-glutathionylation, and protein–protein interactions [44, 45]. Palmitoylation and myristoylation of eNOS enable its localization to the plasmalemmal caveolae, where the enzyme is sequestered in its inactive state due to the interaction with caveolin-1 [46]. eNOS activity is dependent on intracellular Ca2+ concentration. In response to acetylcholine or bradykinin Ca2+ level increases, and Ca2+-activated CaM binds eNOS, disrupts its inhibitory interaction with caveolin, and stimulates NO synthesis [47]. Growth factors, hormones and shear stress affects the activity of kinases (PKA, Akt, and AMPK) and phosphatases, and modulate eNOS activity by altering the phosphorylation status of the enzyme [48]. Phosphorylation at Ser1177, Ser633 and Ser615 stimulates eNOS, whereas phosphorylation at Thr495 and Ser114 inhibits it [49]. Among the various post-translational modifications of eNOS, S-glutathionylation is of particular interest to this review as it may directly affect eNOS uncoupling [50], thus we will discuss this issue in more detail below.
Oxidative/nitroxidative stress in cardiovascular diseases
Reactive oxygen species (ROS) are produced as by-products of cellular metabolism and play an important role in physiological cell signaling [51, 52]. ROS can also contribute to the pathogenesis of various diseases, especially if their amount exceeds the capacity of the antioxidant defense system, causing oxidative stress and subsequent oxidative damage to lipids, proteins, and DNA [53]. Oxidative stress is a hallmark of CVD [54]. It is well documented that cardiovascular risk factors such as dyslipidemia, diabetes, hypertension, obesity, or smoking lead to increased production of ROS in the vascular wall, and the resulting oxidative stress promotes the development of endothelial dysfunction [55]. Oxidative stress is also a major cause of ischemia–reperfusion injury, observed after the restoration of blood flow to the ischemic tissue following myocardial infarction or stroke [56, 57].
Enzymes that produce free radicals in the vascular wall include NADPH oxidase, xanthine oxidase, the mitochondrial electron transport chain, and, importantly, uncoupled eNOS [58]. Superoxide generated by these enzymes can be reduced by superoxide dismutase (SOD) to H2O2 which is then eliminated by catalase and glutathione peroxidase (GPx). However, superoxide can also interact with NO and inactivate it, yielding toxic ONOO−, and the rate of this reaction is three times faster than the dismutation of O2− by SOD (6.7 × 109 mol/L−1 s−1) [59]. Thus the balance between antioxidant defense enzymes and the amount of ROS generated is key to proper NO bioavailability since excessive O2− scavenges NO. This balance is altered in various pathological conditions resulting in oxidative stress that plays an essential role in the development of CVD [60–62].
NADPH oxidases are considered as the major producers of O2− in the vasculature, and moreover, they are regarded the main source of “kindling radicals” that trigger the activation of additional ROS sources, e.g., via eNOS uncoupling [63]. The expression and activity of NADPH oxidases have been documented to increase in experimental models of diabetes [64, 65], hypertension [66, 67], smoking [68], obesity [69, 70], and with ageing [71]. Increased expression or activity of NADPH oxidase was reported in coronary and peripheral arteries of patients with coronary artery disease [72–74]. It is now well established that activation of NADPH oxidases contributes to cardiovascular pathogenesis [75].
NADPH oxidase- generated O2−, besides NO scavenging, triggers eNOS uncoupling (the mechanisms are discussed in the next section), and uncoupled eNOS becomes itself a source of superoxide [19]. The reaction of superoxide with NO yields peroxynitrite, ONOO−, a very potent oxidant that intensifies eNOS uncoupling by oxidizing its cofactor BH4 [76]. Moreover, ONOO− cause protein oxidation and nitration, leading to cellular injury [77]. Thus, uncoupled eNOS contributes significantly to vascular oxidative stress, and acting as a vicious cycle, it is considered to be one of the most important mechanisms leading to endothelial dysfunction. eNOS uncoupling has been reported in the vessels of patients with diabetes, hypertension, coronary artery disease [23–27] as well as in animal studies [29–31, 33].
Another source of free radicals that contributes to endothelial dysfunction is the electron transport chain and oxidative phosphorylation. Elevated levels of plasma glucose and free fatty acids increase mitochondrial superoxide production [78]. Excessive ROS production at the mitochondrial compartment is associated with cardiovascular diseases and has been observed in diabetes, aging, hypertension, and heart failure [79–82].
Also, xanthine oxidase-derived ROS contributes to endothelial dysfunction. The enzyme activation has been implicated in increased vascular O2− generation in patients with chronic heart failure [83], coronary artery disease [72, 84] and in the animal model of hypercholesterolemia [85]. It was reported that the expression and activity of xanthine oxidase in endothelial cells is upregulated by angiotensin II (Ang II) treatment [86]. Xanthine oxidase is also involved in endothelial dysfunction induced by smoking, since its inhibition restored endothelial function in heavy smokers [87].
According to the “kindling radical” hypothesis, individual sources of free radicals are interrelated and can stimulate each other, which is confirmed by the observations that inhibiting only one of them can restore the redox balance [88]. NADPH oxidase, xanthine oxidase and mitochondria-derived ROS, in addition to damaging cellular proteins, lipids, and DNA, can scavenge NO, and moreover, they can induce eNOS uncoupling [18]. Uncoupled eNOS not only does not produce NO but instead generates O2− exacerbating oxidative stress. In addition to NO scavenging and eNOS uncoupling, vascular oxidative stress induces oxidative damage of cellular macromolecules and the expression of proinflammatory genes, thus promoting atherogenesis [58, 89]. Oxidative stress is a major contributor to eNOS uncoupling and its mechanisms are described below.
Mechanisms of eNOS uncoupling
Under physiological conditions, i.e. in normal eNOS activity, the interdomain electron transfer and NADPH oxidation are coupled to NO synthesis. eNOS uncoupling refers to a situation in which eNOS produces superoxide instead of NO, thus becoming a source of harmful free radicals rather than antiatherosclerotic NO [18, 19].. Conditions implicated in eNOS uncoupling include oxidative depletion of the critical eNOS cofactor BH4, deficiency of eNOS substrate L-Arg, or accumulation of its analog asymmetrical dimethylarginine, and eNOS S-glutathionylation. These individual mechanisms are discussed below, but it is worth noting here that they are not mutually exclusive and may occur simultaneously.
Deficiency of BH4 cofactor
BH4 is an essential eNOS cofactor required for efficient electron transfer in the eNOS catalytic cycle that largely determines its activity [90]. Cellular production of BH4 is dependent on two alternative pathways: de novo synthesis or regeneration from its oxidized form dihydrobiopterin (BH2) through the salvage pathway [91]. BH4 is synthesized de novo from GTP by guanosine triphosphate cyclohydrolase I (GTPCH), 6-pyruvoyltetrahydropterin synthase (PTPS) and sepiapterin reductase (SR) and GTPCH is the rate-limiting enzyme in BH4 biosynthesis [91]. Importantly, under oxidative stress conditions, BH4 is rapidly oxidized to BH2 by superoxide anion or, especially strongly, by peroxynitrite derived from NO scavenging by O2− [76]. BH2 can be reduced back to BH4 via the salvage pathway by dihydrofolate reductase (DHFR) [92]. Thus, the cellular availability of BH4 is dependent on cellular redox status and the level of expression and activity of GTPCH and DHFR, the latter enzyme being particularly essential under oxidative stress conditions.
Cardiovascular risk factors are associated with oxidative stress, and excessive O2− oxidizes BH4 to BH2 [93]. BH2 can competitively replace BH4, but being catalytically incompetent as a cofactor, it promotes eNOS uncoupling, where electron transport is uncoupled from NO synthesis, and instead, O2− is generated (Fig. 2) [90, 94]. Moreover, the peroxynitrite formed from the reaction of O2− with NO very strongly oxidizes BH4 [76]. As a result, the cellular BH4/BH2 ratio drops, further increasing eNOS uncoupling and driving a vicious cycle of oxidative stress.
Fig. 2.
eNOS uncoupling due to BH4 deficiency. Under conditions of oxidative stress, O2− can combine with NO yielding ONOO−, which strongly oxidizes BH4 to BH2. Decreased DHFR expression or activity prevents effective regeneration of the cofactor. BH2 competes with BH4 at the heme oxygenase domain but is not catalitically active, thus disturbing the normal electron flow and promoting superoxide formation
The suboptimal concentration of BH4 and more importantly the resulting decrease in the BH4/BH2 ratio probably represent a major cause of eNOS uncoupling implicated in the pathophysiology of endothelial dysfunction [95, 96]. Oxidative depletion of BH4 as a cause of eNOS uncoupling and endothelial dysfunction has been described in vivo, in the aortas of mice with deoxycorticosterone acetate-salt (DOCA-salt) hypertension [29], spontaneously hypertensive mice and rats [97, 98], apolipoprotein E (poE)-deficient mice [99], or aged mice and rats [33, 100]. Human studies have also confirmed the relationship between BH4 depletion and endothelial dysfunction. Decreased vascular BH4 level, increased production of eNOS-dependent O2− proving eNOS uncoupling, and impaired vasorelaxations in response to acetylcholine were reported in patients with coronary artery disease [101]. Depletion of BH4 and reduced NO bioavailability were also shown in patients with peripheral arterial disease [102], diabetes [103], hypertension [26], and hypercholesterolemia [104]. Decreased BH4/BH2 ratio is also associated with endothelial dysfunction in heart failure with preserved ejection fraction (HFpEF) patients [105].
Importantly, the reduction of the BH4/BH2 ratio is not only a consequence of oxidative depletion of BH4, but may also a result from the reduced synthesis and regeneration of the cofactor due to decreased expression or activity of GTPCH and DHFR under oxidative stress conditions. Reduced GTPCH expression with a concomitant decrease in NO levels was observed in the aortas of aged mice, whereas GTPCH overexpression restored proper endothelial function [106]. Diabetes significantly affects the BH4/BH2 ratio in mouse aortas without changing the total biopterin level or GTPCH expression, which indicates that BH4 oxidation is the main cause of its deficiency [107]. Slightly different conclusions can be drawn from the studies on the diabetic rat model, where BH4 deficiency was shown to be due to decreased expression and activity of GTPCH [108]. Nevertheless, in both cases, the overproduction of GTPCH improved the BH4/BH2 ratio and restored endothelial function [107, 109]. Diabetes is also associated with impaired cofactor regeneration. Decreased DHFR expression and BH4 content along with increased eNOS-derived O2− were observed in aortas of streptozotocin (STZ)-induced diabetic mice model [110]. Accordingly, decreased DHFR expression, accumulation of BH2, decreased BH4/BH2 ratio, and eNOS uncoupling were observed in vitro, in hyperglycemic endothelial cells [96, 111]. Oxidative stress evoked by exposure of endothelial cells to Ang II in vitro resulted in downregulation of DHFR expression, decrease in BH4 levels, and eNOS uncoupling [112]. Decreased DHFR expression is also involved in eNOS uncoupling and vascular disorders in hypertensive rats [97] and in hypercholesterolemic mice [113]. The decreased BH4/BH2 ratio observed in various cardiovascular pathologies is therefore due to not only oxidative depletion of BH4, but also impaired synthesis and regeneration of this cofactor.
Deficiency of substrate L-Arg and accumulation of ADMA
L-Arg is the substrate for eNOS and the main precursor of NO, therefore, the availability of L-Arg is important for the activity of this enzyme, and the substrate insufficiency may lead to eNOS uncoupling (Fig. 3). The cellular content of this amino acid is dependent on dietary intake, whole-body protein turnover, endogenous synthesis, cellular uptake, and metabolism [114, 115]. Under physiological conditions, the intracellular concentration of L-Arg is saturating, as it significantly exceeds the Km of eNOS [116]. Nevertheless, exogenous L-Arg can still stimulate NO synthesis, which is a phenomenon known as the "L-arginine paradox" [117]. Therefore, the effective concentration of L-Arg, particularly the ratio of L-Arg to its methylated derivative, the asymmetric dimethylarginine (ADMA), an inhibitor of eNOS, is essential, and decreased L-Arg/ADMA ratio is associated with eNOS uncoupling [118]. The efficiency of cellular L-Arg uptake and the rate of its intracellular metabolism may also play a role and affect the final availability of L-Arg for eNOS. L-Arg is transported across the endothelial cell membrane mainly by cationic amino acid transporter (CAT) proteins belonging to the Na+-independent y + transport system (the letter y is for lysine, the first substrate described for this system, and the + denotes the positive charge of CAT substrates) [119]. The major endothelial L-Arg transporter, cationic amino acid transporter 1 (CAT-1), colocalizes with eNOS in plasma-membrane caveolae and could directly deliver L-Arg to eNOS or increase its local concentration in eNOS proximity [120, 121]. Moreover, the intracellular concentration of L-Arg is modulated by arginase- an enzyme that hydrolyzes L-Arg to ornithine and urea and competes with eNOS for a common substrate [122]. There are two isoforms of arginases in humans, with Arg-I being particularly important in the hepatic urea cycle and Arg-II being distributed throughout various tissues, especially kidneys [123]. Both isoforms have been reported to be expressed in endothelium, although their expression seems to be species and vascular bed–specific, e.g., both Arg-I and Arg-II are present in human aortic endothelial cells (HAECs), whereas in human umbilical vein endothelial cells (HUVECs), Arg-I is barely detectable [124, 125].
Fig. 3.
eNOS uncoupling due to diminished L-Arg/ADMA ratio. Under reduced L-Arg availability (resulting from excessive arginase activity) and/or accumulation of ADMA (due to decreased DDAH activity), the substrate concentration may not be sufficient to saturate eNOS and/or L-Arg is outcompeted by ADMA. As a result, molecular oxygen is a final electron acceptor, leading to superoxide formation
Cardiovascular risk factors are associated with diminished L-Arg availability [126]. The significance of L-Arg availability is emphasized by the beneficial effects of L-Arg supplementation on endothelial function and cardiovascular health, which will be discussed in more detail in the next section. Both L-Arg uptake and metabolism can be altered in CVD pathophysiology. It was shown that homocysteine-induced oxidative stress significantly decreased CAT-1 expression in endothelial cells, resulting in inhibition of L-Arg uptake, reduced NO production, and increased ONOO− formation, indicating eNOS uncoupling, which was abolished by L-Arg supplementation [127]. Still, the regulation of L-Arg transport by cardiovascular risk factors is not well understood. In turn, the role of arginases and its impact on L-Arg availability and CVD pathophysiology associated with oxidative stress is much better known and seems to be of great importance.
Arginases are involved in the pathogenesis of age-related diseases, including CVD, as aging affects their expression and activity [128]. In old rats and mice compared to young, arginase activity was significantly increased and accompanied by decreased NO production and increased O2− generation indicating eNOS uncoupling [129, 130]. Arginase inhibition or silencing significantly reduced eNOS-derived O2− level and restored eNOS coupling and endothelial function [129, 130]. In humans, the expression of Arg-I and Arg-II in the vascular wall was demonstrated to enhance with age and obesity, concomitantly with increased vascular superoxide and diminished NO levels [131]. Arginase is also involved in diabetes-induced vascular dysfunction. Diabetic patients showed increased expression of Arg-I and decreased NO production in coronary arterioles, resulting in reduced vasodilation that could be restored by arginase inhibition or L-Arg application [132]. Increased Arg-I expression and activity, and increased superoxide generation were also reported in aortas and liver of STZ-diabetic rats, which showed decreased NO-mediated vasodilation in coronary vessels, that could be restored by arginase inhibition [133]. Similarly, exposure of bovine coronary endothelial cells to high glucose concentrations resulted in increased Arg-I expression and activity and diminished NO levels, whereas silencing of Arg-I restored NO production [133]. Arginase plays also a crucial role in the pathophysiology of cholesterol-mediated endothelial dysfunction. Endothelial arginase is activated in atherogenic-prone apoE-deficient mice as well as in wild-type mice fed a high-cholesterol diet [134]. Inhibition or deletion of Arg-II prevents a diet-dependent decrease in NO production and increase in ROS production in the vessels, restores endothelial function, and prevents atherogenesis [134]. Similar results were obtained in vitro; oxidized low-density lipoprotein (LDL) activated Arg-II in HAECs, leading to impaired NO production [135, 136]. Arginase activation, decreased NO, increased ROS resulting in endothelial dysfunction and vascular stiffness were also observed in wild-type mice exposed to cigarette smoke, in contrast to Arg-II knockout mice, suggesting that Arg-II contributes to smoking-induced vascular dysfunction [137].
Most of the plasmatic and cellular L-Arg comes from physiological whole-body protein turnover [114]. However, L-Arg residues within proteins are commonly subjected to methylation carried-out post-translationally by a family of nine enzymes named protein arginine methyltransferases (PRMTs 1–9) [138]. Therefore, the subsequent breakdown of such proteins results in the release of methylated arginine derivatives: NG-monomethyl-L-arginine (L-NMMA), asymmetric dimethylarginine (ADMA), and symmetric dimethylarginine (SDMA) [139]. Methylarginines released from the protein breakdown into the cytosol pass into the bloodstream, and can be taken up by other cells via y + transporters, thus they can interfere with L-Arg uptake [140, 141]. Moreover, when taken up by endothelial cells, both ADMA and L-NMMA, but not SDMA, compete with L-Arg for eNOS binding but are not active as substrates, thus leading to eNOS uncoupling. ADMA is considered the most potent endogenous inhibitor of eNOS [142]. While under physiological conditions, ADMA plasma concentration fluctuates in the range of 1–2 µM, it increases significantly (up to tenfold) in the presence of oxidative stress associated with cardiovascular risk factors [143]. Increased ADMA levels in plasma have been correlated with endothelial dysfunction and are an independent risk factor for the development of systemic cardiovascular diseases [142].
The circulating ADMA is partially eliminated by the kidneys, but the most part is metabolized to L-Cit and dimethylamine by dimethylarginine dimethylaminohydrolases (DDAHs) that are expressed in two isoforms [144]. DDAH-1 is responsible for the systemic elimination of circulating ADMA, and its expression is most pronounced in the liver, kidneys, brain, and lungs, however it is also found in the endothelium. DDAH-2 role in ADMA metabolism seems to be more local, and DDAH-2 is expressed primarily in blood vessels, heart, placenta, and immune tissues [144]. Impaired DDAH activity is associated with ADMA accumulation observed in diverse clinical conditions [143]. Hence, plasma and intracellular ADMA levels result from its generation and metabolism regulated by PRMTs and DDAHs, respectively.
Oxidative stress affects the expression and activity of these enzymes, giving rise to ADMA accumulation, and a resulting decrease in L-Arg/ADMA ratio, i.e., diminished effective substrate availability, leads to eNOS uncoupling (Fig. 3). Elevated plasma ADMA levels and impaired endothelium-dependent vasodilation were observed in patients with hypercholesterolemia, hyperhomocysteinemia, diabetes, and hypertension [118, 145–148]. It was proved that oxidized LDL cholesterol enhances the expression of PRMTs, decreases DDAH activity, and increases the release of ADMA from human endothelial cells in vitro [149, 150]. Oxidized LDL was also demonstrated to decrease DDAH activity in hypercholesterolemic rabbits [150]. The activity of aortic DDAH was reduced, and plasma ADMA levels were increased in STZ-diabetic rats [151]. Consistently, in human endothelial cells exposed to high glucose, the activity of DDAH was significantly impaired with concomitant ADMA accumulation and reduction of cGMP level, indicating impaired eNOS activity that could be reversed by antioxidant treatment [151].
eNOS S-glutathionylation
As mentioned earlier, eNOS can be S-glutathionylated. In this type of post-translational modification, the tripeptide glutathione composed of glycine, cysteine, and glutamate is linked by a disulfide to specific cysteine residues of a protein [152]. Glutathione is produced ubiquitously in eukaryotes, and its intracellular concentration is in the millimolar range [153]. The reduced form of glutathione (GSH) is considered the essential non-enzymatic antioxidant in the body and the first line of defense against oxidants since it scavenges free radicals, undergoing oxidation to disulfide GSSG [154]. The ratio of reduced to oxidized glutathione (GSH/GSSG) is a marker of cellular health. Under physiological conditions, this ratio exceeds 100; thus, GSH constitutes over 99% of the cellular glutathione pool. Under pathological states associated with redox imbalance and oxidative stress, this ratio drops, and altered glutathione redox status (GSH/GSSG) increases protein S-glutathionylation by direct disulfide exchange between thiol protein and GSSG. On the other hand, ROS oxidizes protein thiols to sulfenic acid, which can be reduced by S-glutathionylation with GSH [155].
It is believed that S-glutathionylation is a regulatory mechanism that protects proteins from irreversible oxidation of sulfhydryl groups by oxidative stress to sulfinic and sulfonic acids resulting in protein degradation [155]. The consequence of such protection may be a change in the activity of the proteins, their oligomerization status, or the ability to interact with their ligands or protein partners, which is not always advantageous. Reversible protein S-glutathionylation can be thus considered as a redox switch that regulates cellular function under oxidative stress by modulating the activity of metabolic and signaling enzymes [156].
Importantly, eNOS activity can undergo such redox regulation. Chen et al. [50] reported that S-glutathionylation uncouples eNOS thus changing its activity and function. Oxidized glutathione was shown to dose-dependently induce S-glutathionylation of two conserved cysteine residues (Cys689 and Cys908) in the eNOS reductase domain, resulting in eNOS uncoupling characterized by a decreased NO production and an increased generation of O2− [50, 157, 158]. The proposed mechanism by which S-glutathionylation uncouples eNOS assumes that the glutathione binding alters protein structure. Both modified cysteine residues are located at the interface of the FAD- and FMN- binding sites, thus S-glutathionylation would disrupt FAD-FMN alignment and electron transfer between these flavins, resulting in the transfer of an electron to molecular oxygen and the production of a superoxide radical instead of NO (Fig. 4). S-glutathionylation-induced eNOS uncoupling mechanism is unique since O2− is generated in the reductase domain and is not inhibited by N(ω)-nitro-L-arginine methyl ester (L-NAME). In contrast, uncoupling mechanisms dependent on substrate and cofactor availability occur primarily at the heme of the oxygenase domain and can be blocked by L-NAME [50, 159].
Fig. 4.
eNOS uncoupling due to eNOS S-glutathionylation. Oxidative stress decreases the cellular GSH/GSSG ratio, leading to protein S-glutathionylation. Glutathionylated cysteine residues (Cys689 and Cys908) of eNOS are located at the interface of the FAD and FMN binding sites, thus disrupting FAD-FMN alignment and electron transfer between flavins, which causes the transfer of an electron to molecular oxygen and the production of a superoxide radical instead of NO. Prolonged retention of S-glutathionylated eNOS (SG-eNOS) in the cytoplasm can result in its degradation via chaperone-mediated autophagy (CMA), leading to irreversible loss of eNOS
The influence of S-glutathionylation-dependent eNOS uncoupling on endothelium function was tested on isolated rat vessels. Aortic segments exposed to glutathione reductase inhibitor, 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) showed markedly decreased endothelium-dependent vasodilation. Moreover, the involvement of this mechanism in the pathogenesis of cardiovascular diseases was confirmed in vivo. High levels of S-glutathionylated eNOS and impaired endothelium-dependent vasodilation were demonstrated in the vessels of spontaneously hypertensive rats; in contrast, control normotensive rats presented low eNOS S-glutathionylation levels and proper vasodilation response [50].
Ang II, the principal effector of the renin-angiotensin system linked to the pathogenesis of several CVD, was demonstrated to increase eNOS S-glutathionylation via NADPH oxidase activation in cultured endothelial cells, as well as in human arteries ex vivo [160]. Moreover, attenuation of Ang II signaling reduced the level of eNOS glutathionylation and improved endothelium-dependent vasorelaxation in vivo in rabbits [160]. S-glutathionylation-dependent eNOS uncoupling can also contribute to the pathophysiology of preeclampsia (PE) in humans since a high level of eNOS S-glutathionylation was detected in PE placentas in contrast to control placentas of healthy patients [161]. The significance of that mechanism was also confirmed in the pathophysiology of diabetes-related endothelial dysfunction by demonstrating the presence of S-glutathionylated eNOS in the aortas of STZ-induced diabetic rats [162]. Furthermore, eNOS uncoupling due to its S-glutathionylation was observed in endothelial cells in response to hypoxia-reoxygenation [35], and eNOS S-glutathionylation was confirmed in vivo in the coronary arteries of murine myocardial ischemia–reperfusion injury model [163]. Moreover, it was demonstrated that if S-glutathionylated eNOS is not deglutathionylated and persists in the cytosol, it is degraded via chaperone-mediated autophagy, resulting in irreversible loss of uncoupled eNOS, which protects cells from continuous production of O2− [164].
Interestingly, two mechanisms of eNOS uncoupling, i.e., the one dependent on BH4 depletion and that induced by the enzyme's S-glutathionylation, are interrelated. It was demonstrated that eNOS uncoupling induced by BH4 deficiency stimulates eNOS S-glutathionylation [165, 166]. O2− generated from uncoupled eNOS oxidizes eNOS cysteine 908 thiol residue, forming a protein thiyl radical susceptible to S-glutathionylation by GSH [166]. On the other hand, S-glutathionylation of eNOS induces BH4 deficiency. It was shown that inhibition of glutathione reductase in endothelial cells induces a fivefold increase in eNOS S-glutathionylation, and the resulting eNOS uncoupling (shown by superoxide generation) leads to BH4 oxidation, BH2 accumulation, and decreased BH4/BH2 ratio. The two mechanisms of eNOS uncoupling, S-glutathionylation-induced and BH4-dependent, are functionally related, and moreover, their effects are additive [165].
Pharmacological prevention of eNOS uncoupling
Understanding the mechanisms and the importance of eNOS uncoupling as a one of major causes of endothelial dysfunction has made reversing or preventing eNOS uncoupling an attractive therapeutic approach to prevent or treat cardiovascular complications. Since eNOS can be both an NO and an O2− producing enzyme, eNOS targeting may have a dual effect on vascular function, depending on its functional state. Thus, precise determination of eNOS activity and coupling state and the rate of eNOS-dependent NO and O2− generation are of particular importance when assessing the effects of drugs on endothelial function. For example, using electrochemical ultramicrosensors, we have demonstrated that antihypertensive drugs cicletanine, nifedipine, and third-generation β-blockers (nebivolol, carvedilol), Ang II AT1 receptor antagonists, as well as statins, concurrently stimulate NO release and scavenge O2− thus reducing the formation of ONOO− and preventing endothelial dysfunction [167–172]. Similarly, we have also demonstrated the potential of endogenous nicotinamide metabolite N1-methylnicotinamide (MNA+) in the prevention of eNOS uncoupling [173]. Since excessive vascular ROS generation is the key driver of endothelial dysfunction and the main trigger of eNOS uncoupling, attempts have been made to mitigate oxidative stress. However, antioxidant strategies will not be discussed here since this broad topic has been excellently reviewed elsewhere [174–177]. Instead, we will focus on strategies aiming to prevent eNOS uncoupling by targeting the exact mechanisms of this phenomenon such as restoration of BH4/BH2 ratio, L-Arg/ADMA ratio, and physiological eNOS glutathionylation level (Fig. 5). Below, they are briefly outlined.
Fig. 5.
Major causes of eNOS uncoupling as targets of potential therapeutic interventions. Oxidative stress associated with cardiovascular risk factors leads to eNOS uncoupling by: A decreased BH4/BH2 ratio due to oxidation of BH4 and impairment of DHFR expression/activity; B decreased L-Arg/ADMA ratio due to excessive arginase expression/activity and diminished DDAH expression/activity; C eNOS S-glutathionylation at Cys689 and Cys908
Restoration of the BH4/BH2 ratio
Given the critical role of BH4 in eNOS activity and endothelial health, numerous interventions involving BH4 supplementation have been attempted to improve vascular function. The efficacy of BH4 treatment in preventing eNOS uncoupling was demonstrated in animal models [29, 98, 178] as well as in human studies. Acute, intravenous BH4 administration augmented endothelium-dependent vasodilation in hypertensive individuals [26], in patients with hypercholesterolemia [104, 179], coronary artery disease [180], heart failure [181], as well as in chronic smokers [182], or in healthy subjects [183, 184]. However, chronic (several weeks), oral BH4 administration gave slightly discrepant results. BH4 supplementation improved endothelial function in patients with hypercholesterolemia [185], hypertension [186], and rheumatoid arthritis [187]. In contrast, BH4 administration for several weeks in patients with coronary artery disease did not improve endothelial function, but instead increased BH2 levels [188]. BH4 is very unstable and is easily oxidized pterin. Oxidative stress associated with cardiovascular disease oxidizes BH4, so the administration of additional BH4 under such conditions may further decrease the BH4/BH2 ratio. Therefore it was proposed that coadministration of BH4 with antioxidants could be a better strategy to restore the proper BH4/BH2 ratio [189, 190].
Alternatively, a BH4 precursor sepiapterin can be administrated that is converted to BH4 via the salvage pathway, and it was shown to restore tissue BH4 levels even more efficiently that BH4 supplementation in mice [191]. Sepiapterin was demonstrated to improve vascular reactivity in animal models of diabetes [192] and obesity [193]. Human studies revealed that sepiapterin administration is able to restore coronary flow mediated dilation in diabetic patients [24]. However, studies on isolated vessels of hyperlipidemic rabbits have shown that although sepiapterin restored vascular BH4 levels, it impaired NO-dependent vasodilation [194]. High concentrations of sepiapterin may compete with BH4 for binding to eNOS and thus promote eNOS uncoupling [195]. In addition, the conversion of sepiapterin to BH4 requires DHFR activity, therefore, in states in which the activity or expression of DHFR is reduced, sepiapterin supplementation may be ineffective [196].
Folic acid and its active circulating form 5-methyltetrahydrofolate (5-MTHF) have been also show to increase vascular BH4/BH2 ratio, reverse eNOS uncoupling and restore endothelial function [27, 197]. First, folic acid improves metabolic homocysteine clearance, and hyperhomocysteinemia is a CVD risk factor [198]. However, the effects of folate supplementation on endothelial function are also homocysteine-independent [199]. Folates can directly interact with eNOS and improve the binding affinity of BH4 to eNOS, chemically stabilize BH4 and enhance the regeneration of BH4 from BH2 [199]. Human experimental studies have proven the effectiveness of folates in improving eNOS-dependent endothelial function. Low-dose oral folic acid treatment was sufficient to improve vascular function in patients with coronary artery disease [200]. Infusion of 5-MTHF improved the impaired endothelium-dependent vasodilation in patients with familial hypercholesterolemia [201], diabetes [25], and coronary artery disease [27]. In contrast, in patients with chronic heart failure, 5-MTHF infusion did not improve endothelial function; however, it significantly reduced serum ADMA concentrations, suggesting a direct effect of 5-MTHF on ADMA metabolism [202]. Although the experimental clinical data were promising, clinical trials on the use of folic acid in CVD treatment have produced conflicting results that mostly failed to prove the beneficial effect of folic acid supplementation on cardiovascular health. Meta-analysis of several clinical trials data indicated that folic acid supplementation is not effective for cardiovascular events prevention in people with pre-existing vascular disease [203]. Other meta‐analyses indicated a modest but significant benefit of folic acid supplementation for stroke prevention with more significant benefit observed among participants without preexisting CVD or with lower plasma folate levels at baseline [204, 205].
Interestingly, BH4 levels can be increased by statins. Treatment of human endothelial cells in vitro with fluvastatin and cerivastatin augmented GTPCH expression and BH4 levels [206, 207]. These effects seem to be at least partly mediated by microRNA. Lovastatin has been shown to inhibit aberrant miR-133a expression that targets GTPCH, thereby restoring BH4/BH2 ratio, contributing to eNOS recoupling and preventing endothelial dysfunction [208]. Beneficial influence of statins on vascular BH4 content was also observed in animal and human studies. In STZ-diabetic rats, atorvastatin administration increased GTPCH expression, thus preventing eNOS uncoupling [209]. Simvastatin increased GTPCH activity and BH4 production in hypertensive rats [210]. In patients with coronary artery disease, atorvastatin upregulated GTPCH expression, increased BH4 levels and improved vascular NO bioavailability [211]. Patients with multiple coronary risk factors treated with atorvastatin were reported to have increased plasma BH4/BH2 ratio and showed improved flow-mediated dilation [212].
Restoration of L-Arg/ADMA ratio
L-Arg availability and its ratio to inhibitory ADMA is an important determinant of eNOS activity. Therefore, attempts have been made to restore L-Arg/ADMA ratio by direct L-Arg administration, inactivation of arginases or stimulation of ADMA elimination.
As mentioned earlier, many studies have reported a positive effect of L-Arg supplementation on eNOS activity (known as L-arginine paradox) in conditions associated with endothelial dysfunction such as dyslipidemia, diabetes, hypertension or coronary artery disease [213]. It is believed that L-Arg supply can prevent eNOS uncoupling through various mechanisms. Most of all, exogenous L-Arg supplementation can alter the L-Arg/ADMA ratio and thus overcome the inhibitory effects of ADMA on eNOS as well as on y + transporters [117]. Moreover, L-Arg itself acts as an antioxidant so that it can help to maintain a proper BH4/BH2 ratio [214]. Thus, L-Arg supplementation seemed to be an attractive therapeutic strategy for cardiovascular diseases, preventing eNOS uncoupling and increasing NO synthesis in the endothelium [215, 216]. However, the results of experimental clinical studies and clinical trials are contradictory. Most studies report a vasodilation effect induced by L-Arg administration. High doses of L-Arg administrated intravenously induced NO-dependent vasodilation in healthy subjects as well as in patients with peripheral arterial disease or coronary artery disease [217–220]. Oral L-Arg supplementation was shown to enhance endothelial function in patients with metabolic syndrome or with hyperhomocysteinemia [221, 222]. Meta-analysis of 11 randomized, double-blind, placebo-controlled trials involving 387 participants indicated that oral L-Arg administration significantly lowers blood pressure [223]. Since ADMA is endogenous inhibitor of eNOS and L-Arg/ADMA ratio determines eNOS activity [118], L-Arg supplementation is proposed to be particularly beneficial in patients with elevated ADMA levels, as supported by results of studies in animal models of hypercholesterolemia and arteriosclerosis [117]. Despite these promising results, optimism is not allowed due to studies that undermine the effectiveness of L-Arg supplementation and even show its harmfulness. Long-term administration of L-Arg in patients with peripheral arterial disease did not increased NO synthesis nor improved vascular reactivity [224]. In myocardial infarction therapy, L-Arg administration was not effective as it did not improve vascular stiffness measurements or ejection fraction and may be associated with higher post-infarction mortality [225]. The ineffectiveness of L-Arg supplementation, or even its harmfulness, may have several reasons. Cardiovascular diseases in which L-Arg supplementation therapy has been tested are associated with oxidative stress and oxidative depletion of the essential eNOS cofactor BH4, causing eNOS uncoupling. Thus, substrate supply without the additional amount of available cofactor may not be sufficient to reverse eNOS uncoupling. Moreover, high doses of L-Arg can induce the expression of arginases, which metabolizes L-Arg, and increase arginase activity is associated with eNOS uncoupling and endothelial dysfunction [122, 226, 227]. High levels of L-Arg have been also demonstrated to competitively inhibit DDAH activity, thus contributing to increased levels of ADMA which is endogenous inhibitor of eNOS [228].
Since elevated arginase expression and/or activity is associated with eNOS uncoupling, inhibition of arginase activity may be a good therapeutic strategy for endothelial dysfunction. To experimentally modulate arginase activity, specific inhibitors have been developed: N-hydroxy-L-arginine (NOHA) or N-hydroxy-nor-l-arginine (nor-NOHA), and boronic acid derivatives, such as 2(S)-amino-6-boronohexanoic acid (ABH), and S-(2-boronoethyl)-l-cysteine (BEC) s[229]. These inhibitors as well as genetically modified arginase knockout animals were tested in experimental studies which allowed to determine the effect of arginase on endothelial function and eNOS activity. Inhibition of arginase activity with BEC or deletion of Arg2 gene prevented eNOS uncoupling and atherogenesis in the vessels of hypercholesterolemic mice [134]. In mice with diet-induced obesity, deletion of Arg1 or Arg2 gene or inhibition of arginase activity with ABH prevented vascular dysfunction [230, 231]. Similarly, endothelial dysfunction in STZ-diabetic mice was reversed by ABH [232]. Also short-term clinical studies with local administration of arginase inhibitor have shown promising results. Intra-arterial infusion of the arginase inhibitor nor-NOHA for two hours significantly improved endothelium-dependent vasodilation in patients with familial hypercholesterolemia [233], coronary artery disease [234], diabetes [235, 236] and in elderly subjects [237]. However, the compounds used so far do not have specificity as to the enzyme isoform, and further studies are needed to search for novel and more specific arginase inhibitors [238]. It is also worth mentioning that increased arginase activity in diabetes is influenced by insulin administration, and insulin infusion has been shown to reduce arginase activity in patients with type 2 diabetes [239].
Moreover, excessive arginase activity can be mitigated with statins. Simvastatin and lovastatin blocked arginase activation by oxidized LDL in HAECs, and lovastatin prevented arginase activation in apoE-deficient mice fed a high-cholesterol diet [136]. In diabetic rats simvastatin diminished diabetes-induced arginase activity and Arg-I expression, reduced oxidative stress and restored proper vasorelaxation in response to acetylcholine [133]. Human studies demonstrated that atorvastatin decreased arginase activity in hypercholesterolemic patients [240].
Modulating the expression/activity of DDAH may also be a promising therapeutic strategy to restore the favorable L-Arg/ADMA ratio and several experimental studies have shown that the increase in DDAH expression reduces ADMA levels and stimulates NO synthesis. The use of purified recombinant DDAH-1 to lower ADMA levels was proved to be effective for the treatment of ischemia–reperfusion myocardial damage in isolated mouse hearts [241]. Some long-known medications can diminish ADMA levels by increasing DDAH expression. For example, nebivolol and telmisartan stimulated the expression of DDAH-2 in cultured endothelial cells, resulting in reduction of ADMA concentration. [242, 243]. Moreover, nebivolol treatment as well as telmisartan administration reduced serum ADMA levels and improved endothelial function in essential hypertensive patients [244, 245]. Also statins have been reported to increase ADMA metabolism by upregulation of DDAH. In cultured endothelial cells, simvastatin increased DDAH-1 expression and decreased ADMA content [246]. Rosuvastatin and atorvastatin increased DDAH expression and reduced serum ADMA levels in a rat model of pulmonary hypertension and in in high-fat diet-induced insulin-resistant rats with endothelial dysfunction, respectively [247, 248]. However, human studies have not produced conclusive results. Some clinical trials confirmed that statin treatment reduces circulating ADMA levels [249, 250], while others failed to prove such an effect [251, 252].
Modulating eNOS S-glutathionylation
S-glutathionylation is a reversible post-translational modification, and a mixed disulfide bond between a protein cysteine residue and glutathione can be reduced back in a process of deglutathionylation. The cellular antioxidant systems of glutaredoxin and thioredoxin are able to reduce thiol groups and restore protein function [253]. It was demonstrated that glutaredoxin (Grx1), a cytosolic oxidoreductase, can efficiently deglutathionylate eNOS in the presence of GSH, i.e., when the cellular redox status is favorable [254]. However, under oxidative stress conditions, when GSSG level is increased, Grx1 glutathionylate eNOS. Therefore, Grx1 activity is influenced by GSH/GSSG ratio [254]. Since lowered GSH/GSSG ratio promotes eNOS S-glutathionylation and uncoupling, supplementation of GSH may be beneficial for endothelial function. It was demonstrated that GSH administration reverses endothelial dysfunction and improves NO bioavailability in atherosclerotic patients, possibly due to its general anti-oxidant properties [255]. However, GSH could likely stimulate eNOS deglutathionylation by Grx1 and thus recouple the enzyme.
Another pathway that allows deglutathionylation is independent of GSH and involves thioredoxin (Trx), a small, ubiquitous redox protein. The primary function of Trx is the reduction of oxidized cysteine groups on proteins [253]. Trx possesses the disulfide reductase activity in its reduced state, which is maintained by the thioredoxin reductase in a NADPH-dependent reaction [256]. Interestingly, Trx can deglutathionylate eNOS even in the presence of high levels of GSSG under oxidative stress conditions [163]. Trx overexpression prevented eNOS glutathionylation and uncoupling in vivo in coronary arteries of ischemia/reperfusion treated mice and protected against myocardial infarction. Accordingly, in human coronary artery endothelial cells (HCAECs) in vitro, Trx overexpression protected against hypoxia/reoxygenation-induced eNOS glutathionylation and preserved eNOS activity, whereas Trx silencing resulted in increased eNOS S-glutathionylation and uncoupling [163].
Therapeutic potential of Trx has been also noted in studies on age-related hypertension in mice. It was demonstrated that overexpression of human Trx in mice protected against endothelial dysfunction and prevented the development of age-related hypertension [257]. Moreover, injection of recombinant human Trx via tail vein into aged wild-type mice reversed the existing hypertension. Both overexpression of Trx in transgenic mice and injection of Trx into old wild-type mice improved endothelial-dependent relaxation [257]. In aged mice eNOS S-glutathionylation was increased and eNOS was uncoupled, producing reduced amounts of NO and being a major source of vascular O2−. In contrast, in aged transgenic mice overproducing Trx the level of eNOS S-glutathionylation, NO and eNOS-derived O2− production were not significantly different from that of young mice [257]. These studies highlight the potential antihypertensive properties of Trx, which modulates the vascular redox state, prevents eNOS S-glutathionylation and preserves eNOS activity in the vessels of aged animals. Translational studies are needed to assess the potential of GSH, glutaredoxin and thioredoxin to modulate eNOS uncoupling and improve endothelial function in humans. Moreover, the efficiency of the eNOS deglutathionylation mechanisms affects the half-life of the eNOS protein, as it was demonstared in ischemia–reperfusion injury [164]. Persisting S-glutathionylation leads to the degradation of eNOS, thus protecting cells against oxidative damage, but on the other hand, it leads to irreversible loss of eNOS [164].
eNOS glutathionylation state could be also modified indirectly. Since Ang II increases eNOS S-glutathionylation and uncoupling, angiotensin-converting enzyme inhibition could reverse glutathionylation-dependent eNOS uncoupling [160]. Indeed, attenuation of Ang II signaling by captopril was demonstrated to reduce eNOS S-glutathionylation and endothelial O2− generation, simultaneously increasing NO production and improving vasorelaxation in rabbits [160].
Conclusions
It is now well recognized that eNOS uncoupling is associated with endothelial dysfunction and the pathophysiology of cardiovascular disease, thus, the mechanisms leading to eNOS uncoupling are considered a promising therapeutic target. However, direct interventions aimed at restoring eNOS cofactor and substrate availability, such as administration of BH4 analogs or supplementation of L-Arg, did not produce a clearly beneficial outcome. The results of arginase inhibition trials encourage further research to better understand the specificity and pharmacokinetics of new inhibitors. Further studies are also required to assess the therapeutic potential of GSH, Grx, and Trx in the treatment of endothelial dysfunction associated with eNOS S-glutathionylation. Moreover, already known drugs can modulate the eNOS coupling state. As mentioned above, statins were reported to restore favorable BH4/BH2 ratio and L-Arg/ADMA ratio through augmenting GTPCH expression, abolishing excessive arginase activity, or increasing DDAH activity. Thus it is worth emphasizing that the pleiotropic action of statins, among the many beneficial effects for endothelial physiology [258], also includes the prevention of eNOS uncoupling.
Acknowledgements
Not applicable.
Abbreviations
- 5-MTHF
5-Methyltetrahydrofolate
- ADMA
Asymmetrical dimethylarginine
- Ang II
Angiotensin II
- apoE
Apolipoprotein E
- BH2
Dihydrobiopterin
- BH4
Tetrahydrobiopterin
- CaM
Calmodulin
- cGMP
Cyclic guanosine monophosphate
- CVD
Cardiovascular disease
- DDAH
Dimethylaminohydrolase
- DHFR
Dihydrofolate reductase
- eNOS
Endothelial nitric oxide synthase
- FAD
Flavin adenine dinucleotide
- FMN
Flavin mononucleotide
- GPx
Glutathione peroxidase
- Grx1
Glutaredoxin
- GSH
Glutathione
- GSSG
Glutathione disulfide
- GTP
Guanosine triphosphate
- GTPCH
Guanosine triphosphate cyclohydrolase I
- L-Arg
L-arginine
- L-Cit
L-citrulline
- LDL
Low-density lipoprotein
- L-NAME
N(ω)-nitro-L-arginine methyl ester
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- O2−
Superoxide anion
- ONOO−
Peroxynitrite
- PRMT
Protein arginine methyltransferase
- ROS
Reactive oxygen species
- sGC
Soluble guanylate cyclase
- SOD
Superoxide dismutase
- STZ
Streptozotocin
- Trx
Thioredoxin
- VSMCs
Vascular smooth muscle cells
Author contributions
Conceptualization, AJJ, AP, JMW, LWD; writing—original draft preparation, AJJ, LK; writing—review and editing, AJJ, AP, LK; visualization AJJ; supervision, LK, JMW, LWD; funding acquisition, LK. All authors read and approved the final manuscript.
Funding
This study was supported by the Polish National Science Centre (NCN) OPUS Grant Nos. 2015/19/B/NZ7/03830 and 2019/33/B/NZ7/02699, and by the Ministry of Education and Science Poland Grant No. 10/E-389/SPUB/SP/2020.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 2.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. 2014;2014(3):291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 4.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196(3):430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 5.Farah C, Michel LYM, Balligand J-L. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 2018;15(5):292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- 6.SoRelle R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation. 1998;98:2365–2366. doi: 10.1161/01.CIR.98.22.2365. [DOI] [PubMed] [Google Scholar]
- 7.Montfort WR, Wales JA, Weichsel A. Structure and activation of soluble guanylyl cyclase, the nitric oxide sensor. Antioxid Redox Signal. 2017;26(3):107–121. doi: 10.1089/ars.2016.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1(10):2112–2118. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 10.Münzel T, Feil R, Mülsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by cyclic guanosine 3-cyclic monophosphate dependent protein kinase. Circulation. 2003;108(18):2172–2183. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 11.Carreau A, Kieda C, Grillon C. Nitric oxide modulates the expression of endothelial cell adhesion molecules involved in angiogenesis and leukocyte recruitment. Exp Cell Res. 2011;317(1):29–41. doi: 10.1016/j.yexcr.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26(1):33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esplugues JV. NO as a signalling molecule in the nervous system. Br J Pharmacol. 2002;135(5):1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 17.Cervantes Gracia K, Llanas-Cornejo D, Husi H. CVD and oxidative stress. J Clin Med. 2017;6(2):22. doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20(22):3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 19.Förstermann U. Endothelial NO synthase as a source of NO and superoxide. Eur J Clin Pharmacol. 2006;62(1):5–12. doi: 10.1007/s00228-005-0006-x. [DOI] [Google Scholar]
- 20.Kalinowski L, Malinski T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim Pol. 2004;51(2):459–469. doi: 10.18388/abp.2004_3584. [DOI] [PubMed] [Google Scholar]
- 21.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 22.Dobrucki LW, Marsh BJ, Kalinowski L. Elucidating structure-function relationships from molecule-to-cell-to-tissue: from research modalities to clinical realities. J Physiol Pharmacol an Off J Polish Physiol Soc. 2009;60(Suppl 4):83–93. [PubMed] [Google Scholar]
- 23.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 24.Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, et al. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63(4):1381–1393. doi: 10.2337/db13-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Etten RW, de Koning EJP, Verhaar MC, Gaillard CAJM, Rabelink TJ. Impaired NO-dependent vasodilation in patients with Type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45(7):1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 26.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals*. Am J Hypertens. 2002;15(4):326–332. doi: 10.1016/S0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 27.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels. Circulation. 2006;114(11):1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 28.Dixon LJ, Morgan DR, Hughes SM, McGrath LT, El-Sherbeeny NA, Plumb RD, et al. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107(13):1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88(2):e14–22. doi: 10.1161/01.RES.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 31.Moens AL, Champion HC, Claeys MJ, Tavazzi B, Kaminski PM, Wolin MS, et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117(14):1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janaszak-Jasiecka A, Siekierzycka A, Płoska A, Dobrucki IT, Kalinowski L. Endothelial dysfunction driven by hypoxia—the influence of oxygen deficiency on NO bioavailability. Biomolecules. 2021;11:34. doi: 10.3390/biom11070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y-M, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Circ Physiol. 2009;297(5):H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H-Y, Zeeshan HMA, Kim H-R, Chae H-J. Nox4 regulates the eNOS uncoupling process in aging endothelial cells. Free Radic Biol Med. 2017;113:26–35. doi: 10.1016/j.freeradbiomed.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 35.De Pascali F, Hemann C, Samons K, Chen C-A, Zweier JL. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry. 2014;53(22):3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoyagi M, Arvai AS, Tainer JA, Getzoff ED. Structural basis for endothelial nitric oxide synthase binding to calmodulin. EMBO J. 2003;22(4):766–775. doi: 10.1093/emboj/cdg078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol. 2011;210(3):271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Vanhoutte PM, Leung SWS. Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 40.Stuehr DJ, Haque MM. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br J Pharmacol. 2019;176(2):177–188. doi: 10.1111/bph.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J. 2011;438(3):397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 42.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol. 2006;291(5):C803–C816. doi: 10.1152/ajpcell.00457.2005. [DOI] [PubMed] [Google Scholar]
- 43.Kalinowski L, Janaszak-Jasiecka A, Siekierzycka A, Bartoszewska S, Woźniak M, Lejnowski D, et al. Posttranscriptional and transcriptional regulation of endothelial nitric-oxide synthase during hypoxia: the role of microRNAs. Cell Mol Biol Lett. 2016;21(1):16. doi: 10.1186/s11658-016-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulton D, Gratton J-P, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299(3):818–824. [PubMed] [Google Scholar]
- 45.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front Physiol. 2013;13(4):347. doi: 10.3389/fphys.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z, Oliveira S, Zimnicka AM, Jiang Y, Sharma T, Chen S, et al. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol Biol Cell. 2018;29(10):1190–1202. doi: 10.1091/mbc.E17-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43(3):532–541. doi: 10.1016/S0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 48.Kukreja RC, Xi L. eNOS phosphorylation: a pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol. 2007;42:280–282. doi: 10.1016/j.yjmcc.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. 2010;92(9):1186–1198. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Chen C-A, Wang T-Y, Varadharaj S, Reyes LA, Hemann C, Talukder MAH, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Cell Mol Physiol. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 52.Costa TJ, Barros PR, Arce C, Santos JD, da Silva-Neto J, Egea G, et al. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic Biol Med. 2021;162:615–635. doi: 10.1016/j.freeradbiomed.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020 doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013;34(6):313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 56.Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev. 2021;2021:6614009. doi: 10.1155/2021/6614009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jurcau A, Ardelean AI. Oxidative stress in ischemia/reperfusion injuries following acute ischemic stroke. Biomedicines. 2022;10:3. doi: 10.3390/biomedicines10030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Thomson L, Trujillo M, Telleri R, Radi R. Kinetics of cytochrome c2+ oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch Biochem Biophys. 1995;319(2):491–497. doi: 10.1006/abbi.1995.1321. [DOI] [PubMed] [Google Scholar]
- 60.Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15(26):2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 61.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7(4):257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 62.Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 63.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20(17):2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 65.Manea S-A, Antonescu M-L, Fenyo IM, Raicu M, Simionescu M, Manea A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018;16:332–343. doi: 10.1016/j.redox.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, et al. p22phox mRNA Expression and NADPH Oxidase Activity Are Increased in Aortas From Hypertensive Rats. Circ Res. 1997;80(1):45–51. doi: 10.1161/01.RES.80.1.45. [DOI] [PubMed] [Google Scholar]
- 67.Marchi KC, Ceron CS, Muniz JJ, De Martinis BS, Tanus-Santos JE, Tirapelli CR. NADPH oxidase plays a role on ethanol-induced hypertension and reactive oxygen species generation in the vasculature. Alcohol Alcohol. 2016;51(5):522–534. doi: 10.1093/alcalc/agw043. [DOI] [PubMed] [Google Scholar]
- 68.Kim M, Han C-H, Lee M-Y. NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol Res. 2014;30(3):149–157. doi: 10.5487/TR.2014.30.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, et al. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. 2011;16(6):223–229. doi: 10.1179/174329211X13049558293713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.La FJD, Dubis GS, Yan H, White JD, Nelson MAM, Anderson EJ, et al. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2016;36(12):2412–2420. doi: 10.1161/ATVBAHA.116.308339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40(12):2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26(2):333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 73.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429–1435. doi: 10.1161/01.CIR.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 74.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86(9):E85–90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. 2020;17(3):170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263(3):681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 77.Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618–625. doi: 10.1016/j.redox.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 79.Davidson SM, Duchen MR. Endothelial mitochondria. Circ Res. 2007;100(8):1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 80.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, et al. Nox2-Induced Production of Mitochondrial Superoxide in Angiotensin II-Mediated Endothelial Oxidative Stress and Hypertension. Antioxid Redox Signal. 2014;20(2):281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol. 2018;124(5):1194–1202. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkman DL, Robinson AT, Rossman MJ, Seals DR, Edwards DG. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am J Physiol Circ Physiol. 2021;320(5):H2080–H2100. doi: 10.1152/ajpheart.00917.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure. Circulation. 2002;106(24):3073–3078. doi: 10.1161/01.CIR.0000041431.57222.AF. [DOI] [PubMed] [Google Scholar]
- 84.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, et al. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107(10):1383–1389. doi: 10.1161/01.CIR.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 85.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, et al. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol. 2007;27(4):943–948. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 87.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107(3):416–421. doi: 10.1161/01.CIR.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 88.Daiber A, Oelze M, Daub S, Steven S, Schuff A, Kröller-Schön S, et al. Vascular Redox Signaling, Redox Switches in Endothelial Nitric Oxide Synthase (eNOS Uncoupling), and Endothelial Dysfunction. In: Laher I, et al., editors. Systems Biology of Free Radicals and Antioxidants. Berlin: Springer; 2014. pp. 1177–1211. [Google Scholar]
- 89.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–766. doi: 10.1161/01.RES.85.8.753. [DOI] [PubMed] [Google Scholar]
- 90.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 91.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(1):1–16. doi: 10.1042/bj3470001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284(41):28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20(18):3040–3077. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284(2):1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 95.Vásquez-Vivar J, Martásek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362(Pt 3):733–739. doi: 10.1042/bj3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294(4):H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee C-K, Han JS, Won K-J, Jung S-H, Park H-J, Lee HM, et al. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009;9(21):4851–4858. doi: 10.1002/pmic.200800973. [DOI] [PubMed] [Google Scholar]
- 98.Hong H-J, Hsiao G, Cheng T-H, Yen M-H. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38(5):1044–1048. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- 99.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in ApoE-Deficient Mice. Circulation. 2001;103(9):1282–1288. doi: 10.1161/01.CIR.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 100.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587(15):3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116(24):2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 102.Ismaeel A, Papoutsi E, Miserlis D, Lavado R, Haynatzki G, Casale GP, et al. The nitric oxide system in peripheral artery disease: connection with oxidative stress and biopterins. Antioxidants. 2020;9:7. doi: 10.3390/antiox9070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43(11):1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 104.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99(1):41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto E, Hirata Y, Tokitsu T, Kusaka H, Sakamoto K, Yamamuro M, et al. The pivotal role of eNOS uncoupling in vascular endothelial dysfunction in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2015;190:335–337. doi: 10.1016/j.ijcard.2015.04.162. [DOI] [PubMed] [Google Scholar]
- 106.Yan L, Zhang J, Dai X, Li J, Gao F, Zhang X, et al. Endothelium-Specific GTP Cyclohydrolase I Overexpression Restores Endothelial Function in Aged Mice. J Vasc Res. 2021;58(2):134–138. doi: 10.1159/000513464. [DOI] [PubMed] [Google Scholar]
- 107.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112(5):725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, et al. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349(Pt 1):353–356. doi: 10.1042/bj3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, et al. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J Off Publ Fed Am Soc Exp Biol. 2004;18(15):1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- 110.Oak J-H, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56(1):118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Z-W, Xie X-L, Zhou S-F, Li CG. Mechanism of reversal of high glucose-induced endothelial nitric oxide synthase uncoupling by tanshinone IIA in human endothelial cell line EA.hy926. Eur J Pharmacol. 2012;697(1–3):97–105. doi: 10.1016/j.ejphar.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 112.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102(25):9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller JD, Chu Y, Castaneda LE, Serrano KM, Brooks RM, Heistad DD. Vascular function during prolonged progression and regression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2013;33(3):459–465. doi: 10.1161/ATVBAHA.112.252700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36(3):249–255. doi: 10.1111/j.1440-1681.2008.05123.x. [DOI] [PubMed] [Google Scholar]
- 116.Hardy TA, May JM. Coordinate regulation of L-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med. 2002;32(2):122–131. doi: 10.1016/S0891-5849(01)00781-X. [DOI] [PubMed] [Google Scholar]
- 117.Bode-Böger SM, Scalera F, Ignarro LJ. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114(3):295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98(18):1842–1847. doi: 10.1161/01.CIR.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 119.Closs EI, Simon A, Vékony N, Rotmann A. Plasma Membrane Transporters for Arginine. J Nutr. 2004;134(10):2752S–2759S. doi: 10.1093/jn/134.10.2752S. [DOI] [PubMed] [Google Scholar]
- 120.Zharikov SI, Block ER. Characterization of L-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim Biophys Acta. 1998;1369(1):173–183. doi: 10.1016/S0005-2736(97)00191-0. [DOI] [PubMed] [Google Scholar]
- 121.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272(50):31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 122.Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB. Arginase: a multifaceted enzyme important in health and disease. Physiol Rev. 2018;98(2):641–665. doi: 10.1152/physrev.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab. 2004;81(Suppl 1):S38–44. doi: 10.1016/j.ymgme.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 124.Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98(3):334–343. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 125.Prieto CP, Krause BJ, Quezada C, San Martin R, Sobrevia L, Casanello P. Hypoxia-reduced nitric oxide synthase activity is partially explained by higher arginase-2 activity and cellular redistribution in human umbilical vein endothelium. Placenta. 2011;32(12):932–940. doi: 10.1016/j.placenta.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Tang WHW, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53(22):2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin L, Caldwell RB, Li-Masters T, Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol an Off J Polish Physiol Soc. 2007;58(2):191–206. [PubMed] [Google Scholar]
- 128.Moretto J, Girard C, Demougeot C. The role of arginase in aging: A systematic review. Exp Gerontol. 2019;116:54–73. doi: 10.1016/j.exger.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107(4):1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shin W, Berkowitz DE, Ryoo S. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp Mol Med. 2012;44(10):594–602. doi: 10.3858/emm.2012.44.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Masi S, Colucci R, Duranti E, Nannipieri M, Anselmino M, Ippolito C, et al. Aging modulates the influence of arginase on endothelial dysfunction in obesity. Arterioscler Thromb Vasc Biol. 2018;38(10):2474–2483. doi: 10.1161/ATVBAHA.118.311074. [DOI] [PubMed] [Google Scholar]
- 132.Beleznai T, Feher A, Spielvogel D, Lansman SL, Bagi Z. Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes. Am J Physiol Heart Circ Physiol. 2011;300(3):H777–H783. doi: 10.1152/ajpheart.00831.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102(1):95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102(8):923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 135.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99(9):951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 136.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214(2):279–287. doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sikka G, Pandey D, Bhuniya AK, Steppan J, Armstrong D, Santhanam L, et al. Contribution of arginase activation to vascular dysfunction in cigarette smoking. Atherosclerosis. 2013;231(1):91–94. doi: 10.1016/j.atherosclerosis.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 138.Blanc RS, Richard S. Arginine Methylation: The Coming of Age. Mol Cell. 2017;65(1):8–24. doi: 10.1016/j.molcel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 139.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43(3):542–548. doi: 10.1016/S0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 140.Strobel J, Mieth M, Endress B, Auge D, Koenig J, Fromm M, et al. Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1) J Mol Cell Cardiol. 2012;13(53):392–400. doi: 10.1016/j.yjmcc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Closs EI, Basha FZ, Habermeier A, Förstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric oxide Biol Chem. 1997;1(1):65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 142.Sibal L, Agarwal SC, Home PD, Boger RH. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr Cardiol Rev. 2010;6(2):82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sydow K, Münzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4(4):41–51. doi: 10.1016/S1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 144.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293(6):H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 145.Lajer M, Tarnow L, Jorsal A, Teerlink T, Parving H-H, Rossing P. Plasma Concentration of Asymmetric Dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2008;31(4):747–752. doi: 10.2337/dc07-1762. [DOI] [PubMed] [Google Scholar]
- 146.Yamagishi S, Ueda S, Nakamura K, Matsui T, Okuda S. Role of asymmetric dimethylarginine (ADMA) in diabetic vascular complications. Curr Pharm Des. 2008;14(25):2613–2618. doi: 10.2174/138161208786071326. [DOI] [PubMed] [Google Scholar]
- 147.Sydow K, Schwedhelm E, Arakawa N, Bode-Böger SM, Tsikas D, Hornig B, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57(1):244–252. doi: 10.1016/S0008-6363(02)00617-X. [DOI] [PubMed] [Google Scholar]
- 148.Gamil S, Erdmann J, Schwedhelm E, Bakheit KH, Abdalrahman IBB, Mohamed AO. Increased Serum Levels of Asymmetric Dimethylarginine and Symmetric Dimethylarginine and Decreased Levels of Arginine in Sudanese Patients with Essential Hypertension. Kidney Blood Press Res. 2020;45(5):727–736. doi: 10.1159/000508695. [DOI] [PubMed] [Google Scholar]
- 149.Böger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, et al. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87(2):99–105. doi: 10.1161/01.RES.87.2.99. [DOI] [PubMed] [Google Scholar]
- 150.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99(24):3092–3095. doi: 10.1161/01.CIR.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 151.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106(8):987–992. doi: 10.1161/01.CIR.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 152.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34(2):85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Lushchak VI. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gaucher C, Boudier A, Bonetti J, Clarot I, Leroy P, Parent M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants (Basel, Switzerland). 2018;7:5. doi: 10.3390/antiox7050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rashdan NA, Shrestha B, Pattillo CB. S-glutathionylation, friend or foe in cardiovascular health and disease. Redox Biol. 2020;37:101693. doi: 10.1016/j.redox.2020.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15(1):233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dulce RA, Schulman IH, Hare JM. S-Glutathionylation: A Redox-Sensitive Switch Participating in Nitroso-Redox Balance. Circ Res. 2011;108(5):531–533. doi: 10.1161/RES.0b013e3182147d74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duan DD, Kwan C. A molecular switch of “yin and yang”: S-glutathionylation of eNOS turns off NO synthesis and turns on superoxide generation. Acta Pharmacol Sin. 2011;32(4):415–416. doi: 10.1038/aps.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zweier JL, Chen C-A, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14:1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Galougahi KK, Liu C-C, Gentile C, Kok C, Nunez A, Garcia A, et al. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc. 2014;3(2):e000731. doi: 10.1161/JAHA.113.000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guerby P, Swiader A, Augé N, Parant O, Vayssière C, Uchida K, et al. High glutathionylation of placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2019;22:101126. doi: 10.1016/j.redox.2019.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schuhmacher S, Oelze M, Bollmann F, Kleinert H, Otto C, Heeren T, et al. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes. 2011;60(10):2608–2616. doi: 10.2337/db10-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Subramani J, Kundumani-Sridharan V, Hilgers RHP, Owens C, Das KC. Thioredoxin uses a GSH-independent route to deglutathionylate endothelial nitric-oxide synthase and protect against myocardial infarction. J Biol Chem. 2016;291(45):23374–23389. doi: 10.1074/jbc.M116.745034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Subramani J, Kundumani-Sridharan V, Das KC. Chaperone-mediated autophagy of eNOS in myocardial ischemia-reperfusion injury. Circ Res. 2021;129(10):930–945. doi: 10.1161/CIRCRESAHA.120.317921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling *. J Biol Chem. 2013;288(1):561–569. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen C-A, Lin C-H, Druhan LJ, Wang T-Y, Chen Y-R, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem. 2011;286(33):29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalinowski L, Dobrucki IT, Malinski T. Cicletanine stimulates nitric oxide release and scavenges superoxide in endothelial cells. J Cardiovasc Pharmacol. 2001;37(6):713–724. doi: 10.1097/00005344-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 168.Brovkovych V, Kalinowski L, Muller-Peddinghaus R, Malinski T. Synergistic antihypertensive effects of nifedipine on endothelium. Hypertension. 2001;37(1):34–39. doi: 10.1161/01.HYP.37.1.34. [DOI] [PubMed] [Google Scholar]
- 169.Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105(8):933–938. doi: 10.1161/hc0802.104283. [DOI] [PubMed] [Google Scholar]
- 170.Dobrucki LW, Kalinowski L, Dobrucki IT, Malinski T. Statin-stimulated nitric oxide release from endothelium. Med Sci Monit Int Med J Exp Clin Res. 2001;7(4):622–627. [PubMed] [Google Scholar]
- 171.Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertens. 2002;40(4):521–527. doi: 10.1161/01.HYP.0000034745.98129.EC. [DOI] [PubMed] [Google Scholar]
- 172.Kalinowski L, Dobrucki IT, Malinski T. Cerivastatin potentiates nitric oxide release and enos expression through inhibition of isoprenoids synthesis. J Physiol Pharmacol Off J Polish Physiol Soc. 2002;53(4 Pt 1):585–595. [PubMed] [Google Scholar]
- 173.Domagala TB, Szeffler A, Dobrucki LW, Dropinski J, Polanski S, Leszczynska-Wiloch M, et al. Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N1-methylnicotinamide in human blood vessels. Hypertens. 2012;59(4):825–832. doi: 10.1161/HYPERTENSIONAHA.111.183210. [DOI] [PubMed] [Google Scholar]
- 174.Nuttall SL, Kendall MJ, Martin U. Antioxidant therapy for the prevention of cardiovascular disease. QJM. 1999;92(5):239–244. doi: 10.1093/qjmed/92.5.239. [DOI] [PubMed] [Google Scholar]
- 175.Jain AK, Mehra NK, Swarnakar NK. Role of antioxidants for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Des. 2015;21(30):4441–4455. doi: 10.2174/1381612821666150803151758. [DOI] [PubMed] [Google Scholar]
- 176.Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, Megson IL. Antioxidants in cardiovascular therapy: panacea or false hope? Front Cardiovasc Med. 2015;2:29. doi: 10.3389/fcvm.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou D-D, Luo M, Shang A, Mao Q-Q, Li B-Y, Gan R-Y, et al. Antioxidant food components for the prevention and treatment of cardiovascular diseases: effects, mechanisms, and clinical studies. Oxid Med Cell Longev. 2021;2021:6627355. doi: 10.1155/2021/6627355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shinozaki K, Nishio Y, Okamura T, Yoshida Y, Maegawa H, Kojima H, et al. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res. 2000;87(7):566–573. doi: 10.1161/01.RES.87.7.566. [DOI] [PubMed] [Google Scholar]
- 179.Fukuda Y, Teragawa H, Matsuda K, Yamagata T, Matsuura H, Chayama K. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart. 2002;87(3):264–269. doi: 10.1136/heart.87.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maier W, Cosentino F, Lütolf RB, Fleisch M, Seiler C, Hess OM, et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35(2):173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 181.Setoguchi S, Hirooka Y, Eshima K, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol. 2002;39(3):363–368. doi: 10.1097/00005344-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 182.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers. Circ Res. 2000;86(2):e36–41. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 183.Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Køber L, et al. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol. 2003;285(2):H875–H882. doi: 10.1152/ajpheart.00008.2003. [DOI] [PubMed] [Google Scholar]
- 184.Walter R, Kaufmann PA, Buck A, Berthold T, Wyss C, von Schulthess GK, et al. Tetrahydrobiopterin increases myocardial blood flow in healthy volunteers: a double-blind, placebo-controlled study. Swiss Med Wkly. 2001;131(7–8):91–94. doi: 10.4414/smw.2001.06147. [DOI] [PubMed] [Google Scholar]
- 185.Cosentino F, Hürlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart. 2008;94(4):487–492. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 186.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008;22(6):401–407. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]
- 187.Mäki-Petäjä KM, Day L, Cheriyan J, Hall FC, Östör AJK, Shenker N, et al. Tetrahydrobiopterin supplementation improves endothelial function but does not alter aortic stiffness in patients with rheumatoid arthritis. J Am Heart Assoc. 2016;5(2):e002762. doi: 10.1161/JAHA.115.002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125(11):1356–1366. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mortensen A, Lykkesfeldt J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin-dependent manner? In vitro, in vivo and clinical studies. Nitric Oxide Biol Chem. 2014;36:51–57. doi: 10.1016/j.niox.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 190.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 191.Sawabe K, Yamamoto K, Kamata T, Wakasugi OK, Hasegawa H. Sepiapterin Administration Raises Tissue BH4 Levels More Efficiently Than BH4 Supplement in Normal Mice BT - Chemistry and Biology of Pteridines and Folates. In: Proceedings of the 12th International Symposium on Pteridines and Folates, National Institutes o. In: Milstien S, Kapatos G, Levine RA, Shane B, editors. Boston, MA: Springer US; 2002. p. 199–204. 10.1007/978-1-4615-0945-5_33
- 192.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140(4):701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295(4):H1514–H1521. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vásquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22(10):1655–1661. doi: 10.1161/01.ATV.0000029122.79665.D9. [DOI] [PubMed] [Google Scholar]
- 195.Tarpey MM. Sepiapterin treatment in atherosclerosis. Arteriosclerosis. 2002;22:1519–1521. doi: 10.1161/01.ATV.0000038144.37823.BF. [DOI] [PubMed] [Google Scholar]
- 196.Ionova IA, Vásquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, et al. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;295(5):H2178–H2187. doi: 10.1152/ajpheart.00748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, et al. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86(11):1129–1134. doi: 10.1161/01.RES.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 198.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moens AL, Vrints CJ, Claeys MJ, Timmermans J-P, Champion HC, Kass DA. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2008;294(5):H1971–H1977. doi: 10.1152/ajpheart.91503.2007. [DOI] [PubMed] [Google Scholar]
- 200.Shirodaria C, Antoniades C, Lee J, Jackson CE, Robson MD, Francis JM, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115(17):2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 201.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97(3):237–241. doi: 10.1161/01.CIR.97.3.237. [DOI] [PubMed] [Google Scholar]
- 202.Paul B, Whiting MJ, De Pasquale CG, Mangoni AA. Acute effects of 5-methyltetrahydrofolate on endothelial function and asymmetric dimethylarginine in patients with chronic heart failure. Nutr Metab Cardiovasc Dis. 2010;20(5):341–349. doi: 10.1016/j.numecd.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 203.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 204.Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:8. doi: 10.1161/JAHA.116.003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Y, Jin Y, Wang Y, Li L, Liao Y, Zhang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98(37):e17095. doi: 10.1097/MD.0000000000017095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hattori Y, Nakanishi N. Statin Increases GTP Cyclohydrolase I mRNA and 5,6,7,8-Tetrahydrobiopterin in Vascular Endothelial Cells. Pteridines. 2006;17(3):65–68. doi: 10.1515/pteridines.2006.17.3.65. [DOI] [Google Scholar]
- 207.Hattori Y, Nakanishi N, Akimoto K, Yoshida M, Kasai K. HMG-CoA Reductase Inhibitor Increases GTP Cyclohydrolase I mRNA and Tetrahydrobiopterin in Vascular Endothelial Cells. Arterioscler Thromb Vasc Biol. 2003;23(2):176–182. doi: 10.1161/01.ATV.0000054659.72231.A1. [DOI] [PubMed] [Google Scholar]
- 208.Li P, Yin Y-L, Guo T, Sun X-Y, Ma H, Zhu M-L, et al. Inhibition of Aberrant MicroRNA-133a expression in endothelial cells by statin prevents endothelial dysfunction by targeting GTP Cyclohydrolase 1 in Vivo. Circulation. 2016;134(22):1752–1765. doi: 10.1161/CIRCULATIONAHA.116.017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198(1):65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Z, Wang M, Xue S-J, Liu D-H, Tang Y-B. Simvastatin ameliorates angiotensin ii-induced endothelial dysfunction through restoration of Rho-BH4-eNOS-NO Pathway. Cardiovasc Drugs Ther. 2012;26(1):31–40. doi: 10.1007/s10557-011-6351-3. [DOI] [PubMed] [Google Scholar]
- 211.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang M-H, Tousoulis D, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124(3):335–345. doi: 10.1161/CIRCULATIONAHA.110.985150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takeda M, Yamashita T, Shinohara M, Sasaki N, Takaya T, Nakajima K, et al. Plasma tetrahydrobiopterin/dihydrobiopterin ratio. Circ J. 2009;73(5):955–962. doi: 10.1253/circj.CJ-08-0850. [DOI] [PubMed] [Google Scholar]
- 213.Rodrigues-Krause J, Krause M, Rocha IM, Umpierre D, Fayh AP. Association of l-arginine supplementation with markers of endothelial function in patients with cardiovascular or metabolic disorders: a systematic review and meta-analysis. Nutrients. 2019;11:67. doi: 10.3390/nu11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liang M, Wang Z, Li H, Cai L, Pan J, He H, et al. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018;115:315–328. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 215.Cylwik D, Mogielnicki A, Buczko W. L-arginine and cardiovascular system. Pharmacol Rep. 2005;57(1):14–22. [PubMed] [Google Scholar]
- 216.Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130(11):2626–2629. doi: 10.1093/jn/130.11.2626. [DOI] [PubMed] [Google Scholar]
- 217.Bode-Böger SM, Böger RH, Creutzig A, Tsikas D, Gutzki F-M, Alexander K, et al. l-Arginine infusion decreases peripheral arterial resistance and inhibits platelet aggregation in healthy subjects. Clin Sci. 1994;87(3):303–310. doi: 10.1042/cs0870303. [DOI] [PubMed] [Google Scholar]
- 218.Bode-Böger SM, Böger RH, Alfke H, Heinzel D, Tsikas D, Creutzig A, et al. L-arginine induces nitric oxide-dependent vasodilation in patients with critical limb ischemia. A randomized, controlled study. Circulation. 1996;93(1):85–90. doi: 10.1161/01.CIR.93.1.85. [DOI] [PubMed] [Google Scholar]
- 219.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46(5):489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dubois-Randé JL, Zelinsky R, Roudot F, Chabrier PE, Castaigne A, Geschwind H, et al. Effects of infusion of L-arginine into the left anterior descending coronary artery on acetylcholine-induced vasoconstriction of human atheromatous coronary arteries. Am J Cardiol. 1992;70(15):1269–1275. doi: 10.1016/0002-9149(92)90760-V. [DOI] [PubMed] [Google Scholar]
- 221.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, et al. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62(2):255–264. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 222.Reule CA, Goyvaerts B, Schoen C. Effects of an L-arginine-based multi ingredient product on endothelial function in subjects with mild to moderate hypertension and hyperhomocysteinemia - a randomized, double-blind, placebo-controlled, cross-over trial. BMC Complement Altern Med. 2017;17(1):92. doi: 10.1186/s12906-017-1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dong J-Y, Qin L-Q, Zhang Z, Zhao Y, Wang J, Arigoni F, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162(6):959–965. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 224.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 225.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 226.Scalera F, Closs EI, Flick E, Martens-Lobenhoffer J, Boissel JP, Lendeckel U, et al. Paradoxical effect of L-arginine: acceleration of endothelial cell senescence. Biochem Biophys Res Commun. 2009;386(4):650–655. doi: 10.1016/j.bbrc.2009.06.091. [DOI] [PubMed] [Google Scholar]
- 227.Xiong Y, Fru MF, Yu Y, Montani J-P, Ming X-F, Yang Z. Long term exposure to L-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling. Aging (Albany NY) 2014;6(5):369–379. doi: 10.18632/aging.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang J, Sim AS, Wang XL, Wilcken DEL. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell Mol Life Sci. 2006;63(23):2838–2846. doi: 10.1007/s00018-006-6271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Steppan J, Nyhan D, Berkowitz D. Development of Novel Arginase Inhibitors for Therapy of Endothelial Dysfunction. Front Immunol. 2013 doi: 10.3389/fimmu.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bhatta A, Yao L, Xu Z, Toque HA, Chen J, Atawia RT, et al. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc Res. 2017;113(13):1664–1676. doi: 10.1093/cvr/cvx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Atawia RT, Toque HA, Meghil MM, Benson TW, Yiew NKH, Cutler CW, et al. Role of arginase 2 in systemic metabolic activity and adipose tissue fatty acid metabolism in diet-induced obese mice. Int J Mol Sci. 2019;20:6. doi: 10.3390/ijms20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pernow J, Kiss A, Tratsiakovich Y, Climent B. Tissue-specific up-regulation of arginase I and II induced by p38 MAPK mediates endothelial dysfunction in type 1 diabetes mellitus. Br J Pharmacol. 2015;172(19):4684–4698. doi: 10.1111/bph.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kovamees O, Shemyakin A, Eriksson M, Angelin B, Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J Intern Med. 2016;279(5):477–484. doi: 10.1111/joim.12461. [DOI] [PubMed] [Google Scholar]
- 234.Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, et al. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation. 2012;126(25):2943–2950. doi: 10.1161/CIRCULATIONAHA.112.140335. [DOI] [PubMed] [Google Scholar]
- 235.Mahdi A, Kövamees O, Checa A, Wheelock CE, von Heijne M, Alvarsson M, et al. Arginase inhibition improves endothelial function in patients with type 2 diabetes mellitus despite intensive glucose-lowering therapy. J Intern Med. 2018;284(4):388–398. doi: 10.1111/joim.12785. [DOI] [PubMed] [Google Scholar]
- 236.Kövamees O, Shemyakin A, Checa A, Wheelock CE, Lundberg JO, Östenson C-G, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(11):3952–3958. doi: 10.1210/jc.2016-2007. [DOI] [PubMed] [Google Scholar]
- 237.Mahdi A, Pernow J, Kövamees O. Arginase inhibition improves endothelial function in an age-dependent manner in healthy elderly humans. Rejuvenation Res. 2019;22(5):385–389. doi: 10.1089/rej.2018.2135. [DOI] [PubMed] [Google Scholar]
- 238.Pudlo M, Demougeot C, Girard-Thernier C. Arginase inhibitors: a rational approach over one century. Med Res Rev. 2017;37(3):475–513. doi: 10.1002/med.21419. [DOI] [PubMed] [Google Scholar]
- 239.Kashyap SR, Lara A, Zhang R, Park YM, DeFronzo RA. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care. 2008;31(1):134–139. doi: 10.2337/dc07-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol. 2011;589(Pt 8):2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee Y, Singh J, Scott SR, Ellis B, Zorlutuna P, Wang M. A Recombinant dimethylarginine dimethylaminohydrolase-1-based biotherapeutics to pharmacologically lower asymmetric dimethyl arginine, thus improving postischemic cardiac function and cardiomyocyte mitochondrial activity. Mol Pharmacol. 2022;101(4):226–235. doi: 10.1124/molpharm.121.000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Garbin U, Pasini AF, Stranieri C, Manfro S, Boccioletti V, Cominacini L. Nebivolol reduces asymmetric dimethylarginine in endothelial cells by increasing dimethylarginine dimethylaminohydrolase 2 (DDAH2) expression and activity. Pharmacol Res. 2007;56(6):515–521. doi: 10.1016/j.phrs.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 243.Scalera F, Martens-Lobenhoffer J, Bukowska A, Lendeckel U, Täger M, Bode-Böger SM. Effect of telmisartan on nitric oxide asymmetrical dimethylarginine system. Hypertension. 2008;51(3):696–703. doi: 10.1161/HYPERTENSIONAHA.107.104570. [DOI] [PubMed] [Google Scholar]
- 244.Pasini AF, Garbin U, Stranieri C, Boccioletti V, Mozzini C, Manfro S, et al. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21(11):1251–1257. doi: 10.1038/ajh.2008.260. [DOI] [PubMed] [Google Scholar]
- 245.Ono Y, Nakaya Y, Bando S, Soeki T, Ito S, Sata M. Telmisartan decreases plasma levels of asymmetrical dimethyl-L-arginine and improves lipid and glucose metabolism and vascular function. Int Heart J. 2009;50(1):73–83. doi: 10.1536/ihj.50.73. [DOI] [PubMed] [Google Scholar]
- 246.Ivashchenko CY, Bradley BT, Ao Z, Leiper J, Vallance P, Johns DG. Regulation of the ADMA-DDAH system in endothelial cells: a novel mechanism for the sterol response element binding proteins, SREBP1c and -2. Am J Physiol Heart Circ Physiol. 2010;298(1):H251–H258. doi: 10.1152/ajpheart.00195.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pei Y, Ma P, Wang X, Zhang W, Zhang X, Zheng P, et al. Rosuvastatin attenuates monocrotaline-induced pulmonary hypertension via regulation of Akt/eNOS signaling and asymmetric dimethylarginine metabolism. Eur J Pharmacol. 2011;666(1–3):165–172. doi: 10.1016/j.ejphar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 248.Chen P, Xia K, Zhao Z, Deng X, Yang T. Atorvastatin modulates the DDAH1/ADMA system in high-fat diet-induced insulin-resistant rats with endothelial dysfunction. Vasc Med. 2012;17(6):416–423. doi: 10.1177/1358863X12467492. [DOI] [PubMed] [Google Scholar]
- 249.Lu TM, Ding YA, Leu HB, Yin WH, Sheu WHH, Chu KM. Effect of rosuvastatin on plasma levels of asymmetric dimethylarginine in patients with hypercholesterolemia. Am J Cardiol. 2004;94(2):157–161. doi: 10.1016/j.amjcard.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 250.Oguz A, Uzunlulu M. Short term fluvastatin treatment lowers serum asymmetric dimethylarginine levels in patients with metabolic syndrome. Int Heart J. 2008;49(3):303–311. doi: 10.1536/ihj.49.303. [DOI] [PubMed] [Google Scholar]
- 251.Eid HM, Eritsland J, Larsen J, Arnesen H, Seljeflot I. Increased levels of asymmetric dimethylarginine in populations at risk for atherosclerotic disease. Effects of pravastatin Atherosclerosis. 2003;166(2):279–284. doi: 10.1016/S0021-9150(02)00206-X. [DOI] [PubMed] [Google Scholar]
- 252.Valkonen V-P, Laakso J, Päivä H, Lehtimäki T, Lakka TA, Isomustajärvi M, et al. Asymmetrical dimethylarginine (ADMA) and risk of acute coronary events: Does statin treatment influence plasma ADMA levels? Atheroscler Suppl. 2003;4(4):19–22. doi: 10.1016/S1567-5688(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 253.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2(4):811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 254.Chen C-A, De Pascali F, Basye A, Hemann C, Zweier JL. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry. 2013;52(38):6712–6723. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999;34(2):507–514. doi: 10.1016/S0735-1097(99)00216-8. [DOI] [PubMed] [Google Scholar]
- 256.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 257.Hilgers RHP, Kundumani-Sridharan V, Subramani J, Chen LC, Cuello LG, Rusch NJ, et al. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med. 2017;9:376. doi: 10.1126/scitranslmed.aaf6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wolfrum S, Jensen KS, Liao JK. Endothelium-Dependent Effects of Statins. Arterioscler Thromb Vasc Biol. 2003;23(5):729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.