Abstract
Background:
Laparoscopic cholecystectomy (LC) is one of the most common abdominal operations. The difficult cases are still challenging for surgeons. There had been many studies providing several preoperative models to predict difficult LC or conversion. Randhawa's scoring system was a simple and practical predictive model for clinicians. The modification was reported to be more preferable for delayed LC. This study aimed to confirm the advantage of modified predictive model in larger sample size.
Materials and Methods:
This retrospective cohort study reviewed medical records of patients who underwent LC since January 2017 to December 2021. The difficulty of operation was categorized into three groups: easy, difficult, and very difficult. Multivariate analysis was performed to define significant factors of very difficult and converted cases. The predictive scores were calculated by using the original Randhawa's model and the modification, then compared with actual outcome.
Results:
There were 567 cases of delayed LC in this study, with 44 cases (7.8%) converted to open cholecystectomy. Four factors (previous cholecystitis, previous endoscopic retrograde cholangiopancreatography, higher ALP, and gallbladder wall thickening) for very difficult group and five factors (previous cholecystitis, previous cholangitis, higher white blood cell count, gallbladder wall thickening, and contracted gallbladder) for conversion were significant. The modification provided the better correlation and higher area of receiver operating characteristic (ROC) curve comparing with the original model.
Conclusion:
The modification of Randhawa's model was supposed to be more preferable for predicting the difficulty in elective LC.
Thai Clinical Trials Registry No. 20220712006.
Keywords: laparoscopic cholecystectomy, open cholecystectomy, conversion, prediction, scoring system
Introduction
Gallstone (GS) disease is one of the most common health problems causing abdominal pain and sometimes several serious complications. In patients with symptoms or complications of GS, laparoscopic cholecystectomy (LC) has become widely accepted as the procedure of choice instead of open cholecystectomy (OC). LC could be either performed as emergency operation at the episode of GS complications such as acute cholecystitis, acute cholangitis, and acute pancreatitis, or delayed to elective setting after those complications subsided. The timing of operation depends on several factors, for example, the clinical status of patients, the availability of operating room, and the timing of presentation that might be longer than 72 hours especially in referred cases.
Although laparoscopic technical skills have improved and instruments have been developed over decades, the difficult LC is still sometimes challenging for surgeons. The difficult cases usually result in longer operative time and higher complication rate such as intraoperative bleeding, bile leakage, or bile duct injury. Moreover, the conversion to OC might be inevitable in concern of patients' safety with varying range 1%–15% of all LC.1–4
In current literature, there had been many previous studies to identify significant factors affecting the difficulty and conversion of LC. Moreover, some of them had proposed systematic models to preoperatively predict the difficulty and conversion for individual patients who were undergoing LC. Almost all of these models were established for LC in emergency settings especially acute cholecystitis. Randhawa's model, as given in Table 1, was one of the preferable and practical scoring systems.5 It was simple using preoperative information, not including operative finding, then suitable to apply preoperatively.
Table 1.
| The original | The modification | Score |
|---|---|---|
| History | ||
| Age >50 years | Age >50 years | 1 |
| Male | Male | 1 |
| History of hospitalization for acute cholecystitis | History of previous biliary inflammation (cholecystitis, cholangitis) and procedure (ERCP) | 4 |
| Clinical parameters | ||
| BMI: 25–27.5 kg/m2 or | BMI: 25–27.5 kg/m2 or | 1 |
| >27.5 kg/m2 | >27.5 kg/m2 | 2 |
| Abdominal scar: infraumbilical or | Abdominal scar: infraumbilical or | 1 |
| supraumbilical | supraumbilical | 2 |
| Palpable gallbladder | Clinically palpable gallbladder or radiologically contracted gallbladder | 1 |
| Sonography | ||
| Wall thickness ≥4 mm | Wall thickness ≥4 mm | 2 |
| Pericholecystic collection | Pericholecystic collection | 1 |
| Impacted stone | Impacted stone | 1 |
Score 0–5 = easy; score 6–10 = difficult; score 11–15 = very difficult.
BMI, body mass index; ERCP, endoscopic retrograde cholangiopancreatography.
However, this model was established for LC at episode of acute cholecystitis. Tongyoo A et al had modified few terms in Randhawa's model to be eligible for delayed LC after any acute problems subsided.6 The condition “radiologically contracted gallbladder” was added into term of “palpable gallbladder,” and “history of hospitalization for acute cholecystitis” was replaced with “history of previous inflammation or procedure” including previous cholecystitis, cholangitis, and endoscopic retrograde cholangiopancreatography (ERCP). Our previous study had proved the modification was more preferable than the original scoring system in elective setting.
The aim of this study was to confirm the accuracy of the modified predictive model in larger scale of sample size. The classification of difficulty depended on operative time, intraoperative complications, and conversion to open surgery, then categorized into easy, difficult, and very difficult groups, as given in Table 2.
Table 2.
Operative Difficulty Grading5–8
| Grade | Parameters |
|---|---|
| Easy | Time taken <60 minutes No bile spillage No injury to duct, artery |
| Difficult | Time taken 60–120 minutes Bile/stone spillage Injury to bile duct |
| Very difficult | Time taken >120 minutes Conversion |
Materials and Methods
The study design was retrospective cohort study. The study proposal was approved by The Human Research Ethics Committee of Thammasat University (Medicine). The patients, who presented with symptomatic GS or complications of GS, then underwent LC since January 2017 to December 2021 in service of Hepato-Pancreato-Biliary and Transplantation unit in surgery department of Thammasat University Hospital, were considered to be enrolled into this study. The electronic medical record was thoroughly reviewed.
The important information including demographic data, clinical presentation, laboratory results, and radiological findings was collected. The operative time, intraoperative findings, perioperative complications, and conversion to open surgery were reviewed from operative notes. The laparoscopic procedure was carried out through three or four small incisions at umbilical and right upper quadrant areas. The operative time was counted from the opening of the first port-site incision to the closure of the last surgical wounds.
Some cases might be excluded because of the following reasons: (1) patients who underwent LC with other indication such as gallbladder polyp, (2) LC was performed in emergency setting for treatment of acute cholecystitis, and (3) there were any other procedures performed in the same setting of LC such as intraoperative ERCP. By the perioperative information, the patients were categorized into three groups by difficulty grading as given in Table 2.
The univariate analysis was performed using chi-square test for categorical data and Student's t-test for continuous data to define the significant factors affecting on very difficult LC and converted cases. Then multivariate analysis was carried out for both outcomes. Thereafter, the preoperative predictive scores of each patient were calculated using the original Randhawa scoring systems and also the modification of Tongyoo et al. The comparison between scores from both models was performed by many methods such as paired t-test, correlation coefficient, and area under receiver operating characteristic (ROC) curve. All of statistical analyses were performed by IBM SPSS® Statistics version 20 and their results were determined to be significant at P < .05.
Results
Between January 2017 and December 2021, 700 LC operations were performed by hepato-pancreato-biliary surgeons in our center. After some cases were excluded according to exclusion criteria, 567 patients were included in this study. The demographic data, clinical manifestation, previously performed procedure, and the findings of radiological studies are demonstrated in Table 3. More than half of our patients (58.2%) had previously encountered the episode of biliary tract inflammation, either cholecystitis or cholangitis, and the ERCP that might also induce inflammation of extrahepatic bile duct.
Table 3.
Clinical and Operative Information of Cholecystectomy Patients
| Age (years) | 57.8 ± 15.1 |
| Male (%) | 39.5 |
| BMI (kg/m2) | 25.89 ± 4.57 |
| Clinical history | |
| Previous cholecystitis (%) | 24.2 |
| Previous cholangitis (%) | 28.7 |
| Previously performed ERCP (%) | 42.3 |
| Previous pancreatitis (%) | 11.6 |
| Previous abdominal operation (%) | 15.2 |
| Radiological findings | |
| Gallbladder wall thickness ≥4 mm (%) | 31.6 |
| Impact gallstone (%) | 8.8 |
| Contracted gallbladder (%) | 17.3 |
| Pericholecystic fluid (%) | 8.8 |
| Large gallstone size ≥25 mm (%) | 5.5 |
| Operative procedure | |
| Operative time (minutes) | 78.0 ± 34.4 |
| Difficulty group (%): Easy | 36.0 |
| Difficult | 49.5 |
| Very difficult | 14.5 |
| Conversion (%) | 7.8 |
BMI, body mass index; ERCP, endoscopic retrograde cholangiopancreatography.
Among 567 cases, 44 cases (7.8%) were converted to OC due to severe adhesion in 72.7%. The other reasons were severely contracted gallbladder (GB), minor bile duct injury, small bowel injury, and arterial injury, 3 cases for each reason.
There were two pairs of comparison for statistical analysis. First of all, comparison between nonvery difficult and very difficult groups is shown in Table 4. There were nine factors potentially significant on relation with very difficult operation. However, after multivariate logistic regression analysis, only four revealed statistical significance.
Table 4.
Univariate and Multivariate Analysis for Risk Factors of Very Difficult Group
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Easy and difficult (485), n (%) | Very difficult (82), n (%) | P | Odds ratio (95% CI) | P | |
| Age | 57.51 ± 14.91 | 59.63 ± 15.96 | 0.24 | ||
| Age >50 years | 341 (70.3) | 62 (75.6) | 0.33 | ||
| Gender (male) | 187 (38.6) | 37 (45.1) | 0.26 | ||
| BMI | 25.82 ± 4.40 | 26.32 ± 5.49 | 0.44 | ||
| BMI 25–27.5 kg/m2 | 109 (22.5) | 18 (22.0) | 0.74 | ||
| >27.5 kg/m2 | 151 (31.1) | 29 (35.4) | |||
| Previous biliary inflammation/procedure | 254 (52.4) | 76 (92.7) | <0.01 | ||
| Acute cholecystitis | 93 (19.2) | 44 (53.7) | <0.01 | 2.94 (1.67–5.18) | <0.01 |
| Acute cholangitis | 125 (25.8) | 38 (46.3) | <0.01 | — | 0.18 |
| ERCP | 188 (38.8) | 52 (63.4) | <0.01 | 2.62 (1.53–4.48) | <0.01 |
| Acute pancreatitis | 59 (12.2) | 7 (8.5) | 0.34 | ||
| Abdominal scar | |||||
| Lower | 66 (13.6) | 14 (17.1) | 0.28 | ||
| Upper | 4 (0.8) | 2 (2.4) | |||
| WBC | 7004.3 ± 2108.9 | 7612.2 ± 2313.5 | 0.02 | — | 0.07 |
| TB | 0.59 ± 0.34 | 0.66 ± 0.45 | 0.15 | ||
| DB | 0.15 ± 0.08 | 0.18 ± 0.16 | 0.09 | — | 0.31 |
| ALP | 82.6 ± 38.8 | 99.6 ± 66.8 | 0.03 | 1.01 (1.001–1.01) | 0.03 |
| GB wall ≥4 mm | 123 (25.4) | 56 (68.3) | <0.01 | 4.14 (2.34–7.33) | <0.01 |
| Impacted stone | 39 (8.0) | 11 (13.4) | 0.11 | ||
| Contracted GB | 74 (15.3) | 24 (29.3) | <0.01 | — | 0.10 |
| Pericholecystic fluid | 27 (5.6) | 23 (28.0) | <0.01 | — | 0.20 |
| Large GS ≥25 mm | 26 (5.4) | 5 (6.1) | 0.79 | ||
ALP, alkaline phosphatase; BMI, body mass index; CI, confidence interval; DB, direct bilirubin; ERCP, endoscopic retrograde cholangiopancreatography; GB, gallbladder; GS, gallstone; TB, total bilirubin; WBC, white blood cell.
The latter comparison of successful LC and converted OC is given in Table 5. The operative time of successful LC and converted OC was 74.1 ± 31.7 and 124.8 ± 30.6 minutes, respectively, which was significantly different (P < .01). There were five variables with significant association with the conversion by multivariate analysis. Two significant factors, “previous episode of cholecystitis” and “GB wall >4 cm,” were associated with both very difficult operation and conversion of LC.
Table 5.
Univariate and Multivariate Analysis for Risk Factors of Converted Open Cholecystectomy
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Not converted (523), n (%) | Converted (44), n (%) | P | Odds ratio (95% CI) | P | |
| Age | 57.61 ± 15.19 | 60.30 ± 13.52 | .26 | ||
| Age >50 years | 369 (70.6) | 34 (77.3) | .35 | ||
| Gender (male) | 205 (39.2) | 19 (43.2) | .60 | ||
| BMI | 25.93 ± 4.64 | 25.38 ± 3.71 | .45 | ||
| BMI 25–27.5 kg/m2 | 115 (22.0) | 12 (27.3) | .67 | ||
| >27.5 kg/m2 | 168 (32.1) | 12 (27.3) | |||
| Previous biliary inflammation/procedure | 288 (55.1) | 42 (95.5) | <.01 | ||
| Acute cholecystitis | 108 (20.7) | 29 (65.9) | <.01 | 5.39 (2.47–11.77) | < .01 |
| Acute cholangitis | 140 (26.8) | 23 (52.3) | <.01 | 3.53 (1.75–7.09) | < .01 |
| ERCP | 212 (40.5) | 28 (63.6) | <.01 | — | .65 |
| Acute pancreatitis | 62 (11.9) | 4 (9.1) | .58 | ||
| Abdominal scar | |||||
| Lower | 74 (14.1) | 6 (13.6) | .71 | ||
| Upper | 5 (1.0) | 1 (2.3) | |||
| WBC | 7,001.7 ± 2,066.1 | 8,168.2 ± 2,763.2 | <.01 | 1.00 (1.00–1.00) | .03 |
| TB | 0.60 ± 0.36 | 0.62 ± 0.35 | .72 | ||
| DB | 0.15 ± 0.10 | 0.17 ± 0.11 | .28 | ||
| ALP | 84.2 ± 42.8 | 94.8 ± 59.1 | .13 | ||
| GB wall ≥4 mm | 147 (28.1) | 32 (72.7) | <.01 | 2.98 (1.37–6.71) | .01 |
| Impacted stone | 44 (8.4) | 6 (13.6) | .24 | ||
| Contracted GB | 84 (16.1) | 14 (31.8) | .01 | 2.54 (1.15–5.63) | .02 |
| Pericholecystic fluid | 35 (6.7) | 15 (34.1) | <.01 | — | .21 |
| Large GS ≥25 mm | 28 (5.4) | 3 (6.8) | .68 | ||
ALP, alkaline phosphatase; BMI, body mass index; CI, confidence interval; DB, direct bilirubin; ERCP, endoscopic retrograde cholangiopancreatography; GB, gallbladder; GS, gallstone; TB, total bilirubin; WBC, white blood cell.
For the next step of analysis, the original Randhawa's scoring system and Tongyoo modification were applied on the information of each patient. Table 6 reveals the significant difference between the scores from these two systems. The modification provided higher scores in every operative group and also in overall patients.
Table 6.
Comparison of Scores from Both Scoring Systems
| |
Mean ± SD |
P | ||
|---|---|---|---|---|
| Original Randhawa | Tongyoo modification | |||
| Operative difficulty group | 1 | 2.95 ± 2.19 | 4.18 ± 2.59 | <.01 |
| 2 | 3.89 ± 2.61 | 5.55 ± 2.79 | <.01 | |
| 3 | 6.27 ± 3.14 | 8.12 ± 2.10 | <.01 | |
| Overall | 3.89 ± 2.76 | 5.43 ± 2.92 | <.01 | |
SD, standard deviation.
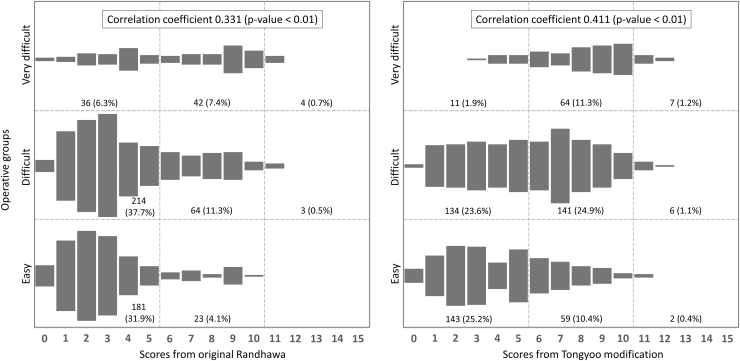
The correlation between the operative difficulty grades and the groups calculated by both scoring systems is shown in Figure 1. The distribution seemed to shift to the right side of chart. This finding was explained by the higher proportions of patients within difficult and very difficult groups predicted by the Tongyoo modification than by the original Randhawa model. And also, coefficient of correlation from the modification was higher than the original model.
FIG. 1.
Correlation between operative difficulty groups and predictive scores from the original and the modified models.
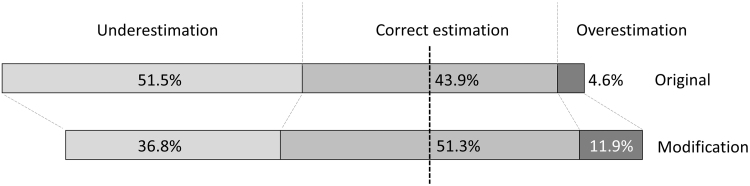
On summary of the accuracy of prediction, the distribution of correlation shifted from underestimation to make correct estimation and overestimation higher about 7% each, as shown in Figure 2.
FIG. 2.
The proportion of underestimation, correct estimation, and overestimation of the prediction comparing between the original and the modified models.
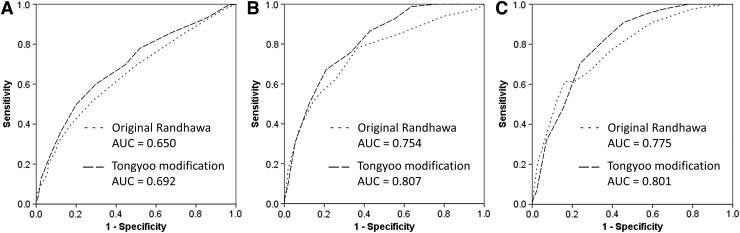
Moreover, the Tongyoo modification also provided the higher area under ROC curve for predicting three results: (1) difficult and very difficult operations, (2) very difficult operations, and (3) conversion to OC, as shown in Figure 3.
FIG. 3.
ROC curves of original Randhawa and Tongyoo modification to predict (A) difficult and very difficult groups, (B) very difficult group, and (C) conversion. ROC, receiver operating characteristic.
Discussion
Nowadays, LC is one of the most frequently performed intra-abdominal operations. In hands of experienced surgeons, this operation would spend a short period of procedural time and lead to good therapeutic result with low morbidity rate. However, the difficult cases are still challenging, and also, the conversion to OC is sometimes inevitable. The failure to clearly demonstrate “critical view of safety” due to the difficult dissection of Calot's triangle is one of the most common indications of conversion.2,3
These unexpected difficult operations and conversion are associated with the longer operative time, longer hospital stay, more expensive cost, and maybe higher morbidity. If there were any methods to distinguish the difficult cases from the easy cases effectively, it would be very helpful in clinical practice of surgeons to be able to plan for longer operative time in operative schedule, to prepare procedural instruments, and to preoperatively inform patients about probability of conversion to open surgery or even any complications.
There had been many published studies to identify several factors that were significant on the difficulty of LC. Male gender, increasing age, obesity, previous cholecystitis, thick gallbladder wall, pericholecystic collection, and contraction of gallbladder had been proven to be important factors.2,4–17 Several meta-analysis studies had also reported similar significant factors on conversion to open surgery.3,18,19 To predict difficult cases and apply to clinical practice easily, several preoperative predicting models had been generated using previously identified significant factors integrated into scoring systems.3,5,9,14,20,21 These models were beneficial in providing more objective and comparative parameters than considering each significant factor individually.
Among these predicting models, Randhawa's scoring system was the most preferable for our institute. The calculation was simple using only three parameters in each category of clinical history, clinical, and radiographic findings. Importantly, all of these factors were preoperative information. After the score was calculated, each patient could be categorized into predictive difficulty groups and could be informed before the operation. There have been several studies for external validation of Randhawa's scoring system with high sensitivity, positive predictive value, and area under ROC curve. However, this model was designed for setting of urgent LC during episode of acute cholecystitis, not for delayed operation after acute inflammation. Our previously published study proposed the modification by adjusting two parameters.6
We suggested to add “contacted gallbladder” into “palpable gallbladder” category, then changed to be “clinically palpable gallbladder or radiologically contracted gallbladder” for one score of the calculating system. And also, the condition of “History of acute cholecystitis” was replaced with “History of previous biliary inflammation and procedure,” which including acute cholecystitis, cholangitis, and ERCP. From the results of our previous study, the integration of these two adjusted parameters into the scoring system provided higher correlation coefficient and more area under ROC curve for very difficult cases and also conversion.
This study had confirmed the result of our previous report with the larger population. The modification generally made the scores higher than Randhawa's model, then some percentages of underestimation by Randhawa scores shifted to correct estimation or overestimation. Area under ROC curves from the new model was also larger for difficult, very difficult, and conversion. These results had supported that the modification was supposed to be more suitable for predicting the difficulty in delayed LC.
The retrospective design was the limitation of this study. However, there was very little information missing out of our electronic medical records. Most of demographic, laboratory, and radiological information was measurable objective data and almost completely recorded. And because the perioperative information was from the real situation of operative procedure, there had been some instrument errors that resulted in longer operative time for few minutes in some cases.
Conclusion
Difficult LC is one of the challenging situations for surgeons. There have been several predictive models proposed to preoperatively distinguish the difficult cases. Randhawa's scoring system was one of the published models that was preferable to be applied in emergent LC for acute cholecystitis. The modification was supposed to be more suitable for predicting the difficulty in elective LC and was still simple to apply for preoperative preparation.
Acknowledgments
The authors express gratitude to their colleagues, including interns, residents, and nurses, who were working at the Department of Surgery, Thammasat University Hospital, for help in clinical processes and operations.
Disclosure Statement
No competing financial interests exist.
Funding Information
This research was fully funded by Thammasat University, Thailand, with grant No. TUFT 18/2564. The publication of this study was supported by Research Group in Surgery, Faculty of Medicine, Thammasat University.
References
- 1. Goonawardena J, Gunnarsson R, de Costa A. Predicting conversion from laparoscopic to open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am J Surg 2015;210(3):492–500; doi: 10.1016/j.amjsurg.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 2. Hu ASY, Menon R, Gunnarsson R, de Costa A. Risk factors for conversion of laparoscopic cholecystectomy to open surgery: A systematic literature review of 30 studies. Am J Surg 2017;214(5):920–930; doi: 10.1016/j.amjsurg.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 3. Sutcliffe RP, Hollyman M, Hodson J, et al. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: A validated risk score derived from a prospective U.K. database of 8820 patients. HPB (Oxford) 2016;18(11):922–928; doi: 10.1016/j.hpb.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishizaki Y, Miwa K, Yoshimoto J, et al. Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br J Surg 2006;93(8):987–991; doi: 10.1002/bjs.5406 [DOI] [PubMed] [Google Scholar]
- 5. Randhawa JS, Pujahari AK. Preoperative prediction of difficult lap chole: A scoring method. Indian J Surg 2009;71(4):198–201; doi: 10.1007/s12262-009-0055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tongyoo A, Chotiyasilp P, Sriussadaporn E, et al. The pre-operative predictive model for difficult elective laparoscopic cholecystectomy: A modification. Asian J Surg 2021;44(4):656–661; doi: 10.1016/j.asjsur.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 7. Gupta N, Ranjan G, Arora MP, et al. Validation of a scoring system to predict difficult laparoscopic cholecystectomy. Int J Surg 2013;11(9):1002–1006.; doi: 10.1016/j.ijsu.2013.05.037 [DOI] [PubMed] [Google Scholar]
- 8. Husain A, Pathak S, Firdaus H. Assessment of operative predictors for difficulty in laproscopic cholecystectomy. Int J Contemp Med Res 2016;3(4):1232–1234. [Google Scholar]
- 9. Vivek MA, Augustine AJ, Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg 2014;10(2):62–67; doi: 10.4103/0972-9941.129947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramírez-Giraldo C, Alvarado-Valenzuela K, Isaza-Restrepo A, et al. Predicting the difficult laparoscopic cholecystectomy based on a preoperative scale. Updates Surg 2022;74(3):969–977; doi: 10.1007/s13304-021-01216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhandari TR, Khan SA, Jha JL. Prediction of difficult laparoscopic cholecystectomy: An observational study. Ann Med Surg (Lond) 2021;72:103060; doi: 10.1016/j.amsu.2021.103060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Buono G, Romano G, Galia M, et al. Difficult laparoscopic cholecystectomy and preoperative predictive factors. Sci Rep 2021;11(1):2559; doi: 10.1038/s41598-021-81938-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanisic V, Milicevic M, Kocev N, et al. A prospective cohort study for prediction of difficult laparoscopic cholecystectomy. Ann Med Surg (Lond) 2020;60:728–733; doi: 10.1016/j.amsu.2020.11.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqui MA, Rizvi SAA, Sartaj S, et al. A standardized ultrasound scoring system for preoperative prediction of difficult laparoscopic cholecystectomy. J Med Ultrasound 2017;25(4):227–231; doi: 10.1016/j.jmu.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucocq J, Scollay J, Patil P. Elective laparoscopic cholecystectomy: Recurrent biliary admissions predispose to difficult cholecystectomy. Surg Endosc 2022;36(9):6403–6409; doi: 10.1007/s00464-021-08986-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhardwaj R, Bali RS, Zahoor Y. Pre-operative factors for predicting a difficult laparoscopic cholecystectomy. Int Surg J 2018;5(9):4; doi: 10.18203/2349-2902.isj20183451 [DOI] [Google Scholar]
- 17. Yetkin G, Uludag M, Oba S, et al. Laparoscopic cholecystectomy in elderly patients. JSLS 2009;13(4):587–591; doi: 10.4293/108680809x1258998404604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang TF, Guo L, Wang Q. Evaluation of preoperative risk factor for converting laparoscopic to open cholecystectomy: A meta-analysis. Hepatogastroenterology 2014;61(132):958–965. [PubMed] [Google Scholar]
- 19. Philip Rothman J, Burcharth J, Pommergaard HC, et al. Preoperative risk factors for conversion of laparoscopic cholecystectomy to open surgery: A systematic review and meta-analysis of observational studies. Dig Surg 2016;33(5):414–423; doi: 10.1159/000445505 [DOI] [PubMed] [Google Scholar]
- 20. Nassar AHM, Hodson J, Ng HJ, et al. Predicting the difficult laparoscopic cholecystectomy: Development and validation of a pre-operative risk score using an objective operative difficulty grading system. Surg Endosc 2019;34(10):4549–4561; doi: 10.1007/s00464-019-07244-5 [DOI] [PubMed] [Google Scholar]
- 21. Chen G, Li M, Cao B, et al. Risk prediction models for difficult cholecystectomy. Wideochir Inne Tech Maloinwazyjne 2022;17(2):303–308; doi: 10.5114/wiitm.2022.114539 [DOI] [PMC free article] [PubMed] [Google Scholar]