Abstract
Theoretical work suggests that obesity is related to enhanced incentive salience of food cues. However, evidence from both behavioral and neuroimaging studies on the topic is mixed. In this work, we review the literature on cue reactivity in obesity and perform a preregistered meta-analysis of studies investigating effects of obesity on brain responses to passive food pictures viewing. Further, we examine whether age influences brain responses to food cues in obesity. In the meta-analysis, we included 13 studies of children and adults that investigated group differences (obese vs lean) in responses to food vs non-food pictures viewing. While we found no significant differences in the overall meta-analysis, we show that age significantly influences brain response differences to food cues in the left insula and the left fusiform gyrus. In the left insula, obese vs lean brain differences in response to food cues decreased with age, while in the left fusiform gyrus the pattern was opposite. Our results suggest that there is little evidence for obesity-related differences in responses to food cues and that such differences might be mediated by additional factors that are often not considered.
Keywords: obesity, cue reactivity, food cues, meta-analysis, fMRI
Introduction
Individuals vary in their susceptibility to obesity. An oft-proposed explanation is that an environment abundant in appetizing food cues triggers different levels of hunger and eating in different people. According to this explanation, individuals more susceptible to omnipresent food cues might overconsume palatable foods and have difficulty to restrict their caloric intake, which may lead to obesity (Polivy et al., 2008). However, there are few systematic reviews of cue-reactivity in obesity that unequivocally support the model.
The underlying theory is that foods and food-related cues can come to act as conditioned stimuli that predict the rewarding effects of ingestion. Food constituents, such as calories (Sclafani et al., 2011), are unconditioned stimuli that elicit unconditioned metabolic responses (Jansen, 1998; Hill, 2007). When reliably paired with caloric intake, food cues such as the sight or smell of food might become conditioned cues that ultimately invoke conditioned responses such as hunger, food craving, salivation or brain activity (Jansen, 1998; Hill, 2007; van den Akker et al., 2014). Several researchers have proposed that reactivity to food cues forms part of a trait that combines enhanced appetitive drive and reduced inhibitory control, which renders some individuals vulnerable to uncontrolled eating in an obesogenic environment (Vainik et al., 2019).
Some studies have related reactivity to food cues to high body mass index (BMI) and obesity (Stice et al., 2009 2010; Demos et al., 2012; van den Akker et al., 2014; Pursey et al., 2014). However, the literature contains a number of conflicting findings on the topic (see Boswell and Kober, 2016). This is largely due to the diversity of paradigms and outcome measures used. Classically, one of the main outcome measures in food cue reactivity paradigms is self-reported cue-induced food craving, which is a strong and conscious desire to eat (Jansen, 1998; Jansen et al., 2011; Boswell and Kober, 2016). It is different from trait craving, which is seen as a desire to eat arising independently of any external cues (Cepeda-Benito et al., 2000; Boswell and Kober, 2016). Both types of cravings can be measured with standardized questionnaires, such as the State and Trait Food-Cravings Questionnaire (Cepeda-Benito et al., 2000), or using visual analog scales (Nederkoorn et al., 2000). The second category of outcome measures in studies on food cue reactivity entails peripheral physiological measures, such as insulin levels changes (Jastreboff et al., 2013; Kroemer et al., 2013), vagal responses (Udo et al., 2014), heart-rate and heart-rate variability changes (Nederkoorn et al., 2000), or salivation (Boyland et al., 2017), among others. Finally, cue reactivity can be assessed with neurocognitive measures such as functional magnetic resonance imaging (fMRI) or electroencephalography (EEG) (van der Laan et al., 2011), eye-tracking (Doolan et al., 2014; Mehl et al., 2017), or cognitive paradigms (e.g. Morys et al., 2018; Oliva et al., 2019), as reviewed below.
A number of fMRI studies have been designed around the hypothesis that individuals with obesity show neurobehavioral alterations in response to food. Perhaps the simplest way of testing this hypothesis is via the presentation of passively viewed food images. These designs allow the comparison between fMRI activity in response to visual food stimuli vs non-food items (usually objects). Such paradigms have been used to investigate potential behavioral causes of unhealthy weight gain, such as making sub-optimal food choices, low cognitive control in response to food stimuli or dysregulation in emotional control.
Investigations of cue reactivity leave a large freedom to the researchers in terms of designing experiments and analyzing resulting datasets (Smeets et al., 2019). For example, while designing passive food picture viewing paradigms, one might consider contrasting reactivity to food pictures with non-food pictures (e.g. Davids et al., 2010), or high-calorie with low-calorie food pictures (e.g. Frank et al., 2014). Similarly, while investigating obesity-related changes in cue reactivity, experimenters tend to contrast obese groups with lean groups (e.g. Mehl et al., 2018), correlate cue reactivity with obesity measures (e.g. BMI; Boswell and Kober, 2016) or only investigate cue reactivity in obese individuals without a control group (e.g. Luo et al., 2013).
In the following sections of this article, we will focus on neurocognitive measures of cue reactivity in obesity, especially on neural correlates of passive food picture viewing. Specifically, we wish to test the hypothesis that there is either greater appetitive or reduced self-regulation response to food cues in obesity and that this is reflected in fMRI experiments. All the studies reviewed below meet a priori defined quality criteria. The most relevant criteria here are that all the studies examine fMRI differences between a condition of interest and a control condition and include obese and lean individuals.
Literature review
Passive image viewing in obesity
Studies investigating differences between obese and lean individuals in brain responses to food picture viewing have produced mixed findings. Bruce and colleagues showed that in obese children (n = 10), as opposed to healthy weight children (n = 10), food vs non-food pictures viewing elicits higher brain activations in the prefrontal cortex (PFC), both pre- and post-meal (Bruce et al., 2010). Lower post-meal reductions in brain activity in obese vs lean children were also observed in the limbic and reward processing regions, e.g. the nucleus accumbens. The authors concluded that this shows hyperreactivity of obese children to food cues and reduced satiety effects. Such interpretation is in line with a study by Rapuano and colleagues (n = 78) showing that children with a higher genetic risk for obesity have increased activation of the nucleus accumbens to food advertisement (Rapuano et al., 2017). Similarly, Davids and colleagues (n = 44) showed an increased activation in the dorsolateral prefrontal cortex (dlPFC) in response to food cues in obese vs lean children, but also increased caudate and hippocampal activations in lean vs obese children (Davids et al., 2010). Studies in adults showed increased brain response to food pictures in obese vs lean individuals in the insula (Stoeckel et al., 2008; Martin et al., 2010; Oltmanns et al., 2012; Scharmüller et al., 2012), caudate (Rothemund et al., 2007; Stoeckel et al., 2008; Nummenmaa et al., 2012), orbitofrontal cortex, amygdala, nucleus accumbens, anterior cingulate cortex, pallidum, putamen and hippocampus (Rothemund et al., 2007; Stoeckel et al., 2008; Martin et al., 2010; Oltmanns et al., 2012), or the PFC (Martin et al., 2010; Dimitropoulos et al., 2012). Generally, those regions are involved in processing of food cues (van der Laan et al., 2011), but also play a role in dietary self-control (Han et al., 2018; Neseliler et al., 2019), reward and emotional processing, working memory (Pursey et al., 2014), and interoception (Rahmani and Rahmani, 2019). Meta-analytical studies have shown that some of these brain regions, such as the nucleus accumbens, caudate, putamen or amygdala, are also activated in substance dependent individuals in response to drug cues (Tang et al., 2012; García-García et al., 2014), making a conceptual link between cue reactivity in addiction and obesity.
In contrast to these findings, decreased brain activation in obese vs lean individuals in response to food picture viewing has been shown in the anterior cingulate, lingual and superior occipital gyri (Heni et al., 2014), superior frontal gyrus (Nummenmaa et al., 2012), precentral gyrus, cingulate gyrus, dlPFC (Dimitropoulos et al., 2012) and the temporal lobe (Martin et al., 2010). Some of those regions overlap with those mentioned above and are also engaged in processing of food cues and dietary self-control (van der Laan et al., 2011; Han et al., 2018). Although reduced brain activation to food-cues may reflect impaired self-regulation, especially when it involves prefrontal areas, there remain inconsistencies in the neuroimaging findings. For instance, a number of studies did not show significant differences between lean and obese individuals for passive food picture viewing (Murdaugh et al., 2012; García-García et al., 2013b; Frank et al., 2014; Doornweerd et al., 2018; Morys et al., 2018). In their systematic review, Pursey and colleagues outline a large number of brain structures that show higher brain activity in obese vs lean participants in response to visual food cues (Pursey et al., 2014). This review, however, fails to differentiate between contrasts used in various studies (e.g. food > non-food cues, or high-calorie food > low-calorie food) and does not mention studies with no significant findings or findings where lean individuals had higher brain activity than obese individuals. A review by van der Akker and colleagues seems to further support the notion that obese individuals show higher responses to food cues than lean individuals (van den Akker et al., 2014). A contrasting view is presented in a behavioral meta-analysis by Boswell and Kober, who found no effect of BMI group on cue reactivity measures (Boswell and Kober, 2016). We believe that such large inconsistencies in the literature warrant a well-controlled meta-analysis on cue responsivity differences in obese and lean individuals.
Influence of food pictures on maladaptive behaviors in obesity
Reward sensitivity and decision-making.
Eating entails making food choices concerning what, how much, when or with whom to eat. These and other questions have been examined in the context of obesity. Specifically, studies have investigated whether obesity is associated with functional brain differences during food-related decision-making.
A study on children (n = 141), for instance, asked participants to perform food choices (either select the food displayed or reject it) under three conditions: the consideration of how healthy the food was, the consideration of tastiness, and a free (‘natural’) choice condition. Across all conditions, a higher BMI was associated with lower activity in the left dlPFC while selecting the food item displayed. This correlation was also found during the consideration of tastiness, which probably influenced the results most. The authors suggested that lower activity in the dlPFC might reflect lower cognitive control, which might jeopardize weight-loss interventions (van Meer et al., 2019). These findings, however, might be difficult to integrate with findings from another study on portion size choices. In this study, participants (n = 36) were asked to select how much food they wanted to eat for lunch that day from a series of food items displayed in the fMRI. The right inferior frontal operculum showed higher fMRI activity in overweight participants relative to lean individuals (Veit et al., 2019).
From a neuroeconomic perspective, food-related decision-making requires the integration of costs and benefits associated with each available choice (Essex and Zald, 2010). In this vein, auction tasks measure the willingness to pay for food and non-food items displayed during fMRI (Plassmann et al., 2007). These tasks provide a direct measurement of current subjective value. An fMRI study using an auction task (n = 81) showed that obese and overweight individuals paid more money for highly palatable foods than lean participants. Moreover, participants with obesity showed higher fMRI activity in the caudate, accumbens and anterior cingulate cortex relative to lean participants (Verdejo-Román et al., 2017). A similar auction task was used to show that these brain regions track the caloric density of food (Tang et al., 2014). Together, these studies raise the possibility that individuals with obesity might attribute a greater subjective value to palatable food.
Finally, food cues can affect decision-making not only in food domains, but, among others, in monetary paradigms. In the field of obesity, one fMRI study has examined monetary delay-discounting in obese and lean individuals (Morys et al., 2018, n = 36). Here, obese individuals showed less delay discounting (decreased impulsivity) when exposed to negative gustatory cues, which was related to decreased activity of the left dlPFC. This was, in turn, related to altered brain connectivity between the dlPFC and the ventromedial PFC, posterior cingulate gyrus and parietal cortex. Importantly, however, visual priming with food cues did not alter decision-making processes in obese or lean individuals. This suggests a lack of differences in visual food cue reactivity between obese and lean individuals, while pointing to the fact that more proximal cues (e.g. taste) might affect decision-making processes differently depending on weight status.
Inhibitory control paradigms.
In general, obese and lean individuals seem to differ in terms of their inhibitory control (Vainik et al., 2013). A question remains, however, whether such differences are specific to food cues or can be generalized to other domains. Two of the tasks measuring inhibitory control are the Stop-Signal Reaction Task and Go/No-Go task. These tasks have been adapted from their original designs to incorporate food stimuli (Loeber et al., 2012; Mühlberg et al., 2016). In general, behavioral studies using the food versions of these tasks have reported similar performance in obese and lean participants (Loeber et al., 2012; Mühlberg et al., 2016). In a similar vein, a neuroimaging study from Carbine et al. compared fMRI responses to high vs low calorie foods using a Go/No-Go task (n = 54). The authors did not find an effect of obesity on this type of inhibitory control (Carbine et al., 2018). Together, these findings suggest that obese and lean individuals have comparable inhibitory control responses towards food stimuli, both behaviorally and in terms of brain activity.
Another task, the approach/avoidance paradigm, allows testing food cue reactivity in the context of behavioral conflict (Mehl et al., 2018, 2019). A behavioral study by Mehl and colleagues (n = 60) showed increased approach for food cues in obese compared to lean individuals (Mehl et al., 2018). A follow-up fMRI study by the same group (n = 33) showed that approach bias for unhealthy food cues is related to increased activity in the right angular gyrus, while decreasing this bias by means of cognitive bias modification decreases the activity in the right angular gyrus and its connectivity to the right dorsal striatum (Mehl et al., 2019). Those findings, however, were identified in a group of obese individuals only and not contrasted with a group of lean individuals.
Influences on food cue reactivity independent of weight status
Factors beyond weight status might also influence food cue reactivity. Since the main focus of this paper is to investigate how weight status influences food cue reactivity, we will only briefly introduce other factors thought to influence neural responses to food cues. Lawrence and colleagues (n = 25) found that activity of the ventromedial PFC to food stimuli was positively related to self-reported hunger (Lawrence et al., 2012). In the same study, activity of the nucleus accumbens predicted BMI in individuals with low self-control. Cosme and colleagues corroborated these results and found evidence that neural food cue reactivity was related to self-control (n = 94, Cosme et al., 2019). Craving was also shown to be associated with neural food cue reactivity in the ventral striatum, anterior cingulate and orbitofrontal cortex (n = 60, Giuliani and Pfeifer, 2015). Interestingly, dietary restraint was associated with neural response to milkshake receipt in the orbitofrontal and dlPFC, but not to anticipated milkshake receipt or food pictures presentation (n = 39, Burger and Stice, 2011). Finally, and provided that emotions can affect eating behavior (Macht, 2008), the emotional context might also influence the neurobehavioral processing of food. In this vein, a study (n = 58) designed an emotional priming task to test whether the processing of food vs non-food pictures differed depending on the emotional context (i.e. negative, neutral or positive). The authors found that liking rates and amygdala activity differed according to emotional priming. Adiposity, however, measured using waist circumference, did not have an effect on the results (García-García et al., 2019). In line with this, a study by Lopez and colleagues found that the activity of the inferior frontal gyrus in response to passive food viewing was lower in individuals with low desire to eat and high positive mood (n = 75, Lopez et al., 2016).
Meta-analysis aims
Conflicting findings in available literature regarding neural correlates of cue reactivity in obesity might arise from a number of factors: small sample sizes, control conditions used (e.g. low-calorie foods or non-food objects), lack of control conditions, lack of a control group, region-of-interest (ROI) vs whole-brain fMRI analysis and, others. To overcome these limitations and provide an objective assessment of cue reactivity in obesity, we perform a meta-analysis with a focus on studies investigating obese vs lean group differences in passive food picture viewing paradigms. Such analysis enables us to provide evidence for and elucidate mechanisms of food cue reactivity differences between obese and lean individuals. Based on previous reviews on the topic of obesity and reward processing (Stice et al., 2009; García-García et al., 2013a; van den Akker et al., 2014; Pursey et al., 2014), we hypothesized that obese individuals would show higher fMRI activity in response to visual food stimuli than lean individuals in predominantly reward-related brain regions, such as the nucleus accumbens, caudate, pallidum, putamen, ventromedial and orbitofrontal cortex. In contrast, we also hypothesized that lower brain activity in obese vs lean individuals will be observed in the dlPFC and the temporal cortex, perhaps reflecting reduced inhibitory control. These hypotheses are an extended version of our preregistered hypotheses.
Materials and methods
Methods and analysis strategies used for the meta-analysis were preregistered prior to data collection. Protocols along with files used for the meta-analysis are available at https://osf.io/d53e6/.
Study selection
Morys and García-García independently performed a literature search in the following scientific databases: PubMed, Scopus and Google Scholar. Keywords included 1) obesity-related terms, such as ‘obesity’, or ‘obese’ or ‘overweight’, 2) ‘food’ and 3) ‘fMRI’, or ‘MRI’, or ‘brain’. The results were then cross-validated between the authors and fitting articles were selected for further analysis. Each included study had to meet all of the following criteria: 1) studies using fMRI measured whole-brain activity as outcome measures 2) studies investigating group differences in cue reactivity between obese and lean individuals, 3) studies using food vs non-food pictures contrast, 4) studies reporting cluster peak coordinates and t-statistics or z-statistics for each cluster, if significant results were found. The meta-analysis also included articles reporting non-significant findings. We included articles that investigated cue reactivity in children and in adult samples. Main exclusion criteria were: 1) studies on clinical populations (e.g. individuals with depression or type II diabetes), 2) lack of a (control) group of lean individuals, 3) studies using fMRI paradigms other than passive viewing (these studies, however, have been reviewed in the section ‘Influence of food pictures on maladaptive behaviors in obesity’). To the best of our knowledge, all the studies included were performed on independent (i.e. non-overlapping) samples of participants.
Seed-based d mapping meta-analysis
We conducted a meta-analysis using seed-based d-mapping (SDM, https://www.sdmproject.com/). This meta-analytic method allows the combination of fMRI studies using their cluster peak coordinates and effect sizes to find reliable, common patterns of activations in the brain for a specific effect of interest. It includes positive features from other meta-analytical methods, such as activation likelihood estimation or multi-kernel density analysis (e.g. weighting meta-analytic values by sample size of studies and using random-effects models), and extends those methods by adding certain improvements (Wager et al., 2007; Radua and Mataix-Cols, 2009; Radua et al., 2012). One of the improvements is the possibility of including both positive and negative effects in one analysis. Another one is including effect sizes from single studies to derive meta-analytic results.
Here, we investigated whether food cue reactivity differences between lean and obese individuals have consistent neural correlates. Additionally, because we recently found that some brain volume correlates of obesity might be age-dependent (García-García et al., 2019), we investigated whether age influences brain mechanisms of cue reactivity in obesity. To this end, we performed meta-regression analysis with SDM software using age as a covariate of interest. For this analysis, a study by Martin and colleagues (Berridge et al., 2010) was excluded because the authors did not report age of participants. We also investigated whether gender influenced weight group differences in neural responses to food vs non-food cues. Lastly, we performed a ROI analysis to investigate weight group differences in food cue reactivity in pre-defined ROIs derived from a previous meta-analysis investigating main effects of viewing food vs non-food stimuli (van der Laan et al., 2011). This analysis deviated from our preregistration protocol and is reported as an exploratory analysis. The ROIs from this study include the orbitofrontal cortex, inferior frontal gyrus, insula, amygdala and several visual areas. In assessment of the results, we used threshold-free cluster enhancement (TFCE) multiple comparison correction with a 0.05 threshold (‘threshold-free’ in TFCE refers to the cluster-forming threshold; Smith and Nichols, 2009) after 1000 permutations with a cluster extent > 100 voxels. For significant clusters, we performed Egger test for asymmetry of the funnel plot to investigate potential publication bias.
Results
Articles included in the meta-analysis
In our database search, we identified 13 studies that fulfilled our criteria. Two of the studies investigated cue reactivity in children, while 11 studies investigated adults aged 18 to 75 years. The meta-analysis included 407 individuals. Details can be found in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis: we searched for fMRI studies examining whole-brain differences between obese and lean individuals during viewing of food pictures
| Study | Year published | Sample size obese | Sample size lean | Mean age | BMI obese | BMI lean | Visual food stimuli | Control stimuli | Found significant clusters |
|---|---|---|---|---|---|---|---|---|---|
| Bruce et al. | 2010 | 10 | 10 | 13 | 31.3 | 18.8 | Appetizing low- and high-calorie food pictures | Pictures of animals | Yes |
| Davids et al. | 2010 | 22 | 22 | 14 | 29.4 | 19.7 | High-calorie food pictures | Neutral pictures of landscapes, buildings and work-related situations, pleasant pictures of babies, young animals, children playing. | Yes |
| Dimitropoulos et al. | 2012 | 22 | 16 | 25 | 31.6 | 22.7 | Low- and high-calorie food pictures | Furniture pictures | Yes |
| Doornweerd et al. | 2018 | 16 | 16 | 50 | 28.4 | 24.4 | Low- and high-calorie food pictures | Non-food items, such as trees, flowers, rocks, and bricks | No |
| Frank et al. | 2014 | 11 | 11 | 40 | 40.2 | 21.4 | Low- and high-calorie food pictures | Non-food pictures | No |
| Garcia-Garcia et al. | 2013 | 18 | 19 | 33 | 34.9 | 22.4 | Low- and high-calorie salty and sweet food pictures | Rewarding non-food stimuli | No |
| Heni et al. | 2013 | 12 | 12 | 24 | 30.5 | 21.2 | Low- and high-calorie food pictures | Pictures of objects with no association with eating | Yes |
| Martin et al. | 2010 | 10 | 10 | - | 34.0 | 22.1 | General food pictures | Gaussian blurred unrecognizable images of food and tools | Yes |
| Morys et al. | 2018 | 24 | 27 | 27 | 34.3 | 22.1 | Positive food pictures | Scrambled, unrecognizable versions of positive food pictures | No |
| Murdaugh et al. | 2012 | 25 | 13 | 47 | 32.9 | 22.6 | Low- and high-calorie food pictures | Pictures of cars | No |
| Nummenmaa et al. | 2012 | 19 | 16 | 47 | 43.9 | 24.1 | Appetizing and bland food pictures | Pictures of cars | Yes |
| Oltmanns et al. | 2012 | 10 | 10 | Age range: 20–45 | 35.1 | - | High-calorie sweet and savory food pictures | Non-food objects of use | Yes |
| Rothemund et al. | 2007 | 13 | 13 | 30 | 36.3 | 20.9 | Low- and high-calorie food pictures | Neutral non-food related stimuli | Yes |
Meta-analysis results
Contrary to our hypothesis, there were no significant differences in brain activity in obese vs lean participants for the contrasts of food > non-food pictures. However, meta-regression with age as a covariate revealed a significant cluster within the left posterior insula/Rolandic operculum, which was negatively related to age (peak MNI coordinates: −48, −8, 6; z-value: −1.416, size: 321 voxels; Figure 1, Table 2), and a cluster in the left fusiform gyrus which was positively related to age (peak MNI coordinates: −34, −54, −12; z-value: 3.681, size: 400 voxels; Figure 1, Table 2). Egger tests for funnel plot asymmetry performed for the peak voxel in those clusters did not show a significant publication bias (insula: t(10) = 0.02, P = 0.984; fusiform gyrus: t(10) = −0.02 P = 0.984). Upon further investigation, correlation analysis revealed that BMI in the lean groups was significantly related to mean age of samples included in the meta-analysis (r = 0.845, P = 0.001). This might mean that the clusters significantly related to age might in fact be related to the BMI of lean groups. We tested this in an additional meta-regression analysis where we included age as a predictor and regressed out effects of BMI of the lean group. In this analysis, we found that only the cluster in the left insula remained significantly related to age. This indicates that the cluster in the left fusiform gyrus was likely related to BMI of the lean group and not to the age of participants. We did not find an effect of gender on weight group food cue reactivity.
Fig. 1.
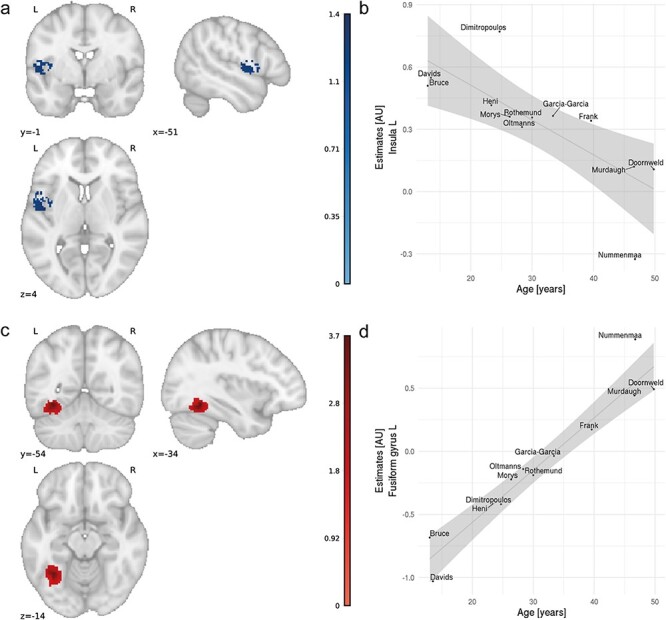
Results of meta-regression with age as a covariate of interest; a) cluster showing negative association with age for group differences between obese and lean groups in cue reactivity (z-scores); b) relationship between meta-analytic estimates from the cluster in the left insula and age; shaded area represents 95% confidence intervals; c) cluster showing positive association with age for group differences between obese and lean groups in cue reactivity (z-scores); d) relationship between meta-analytic estimates from the cluster in the left fusiform gyrus and age; shaded area represents 95% confidence intervals. AU, arbitrary units.
Table 2.
Local peaks for clusters found in the meta-regression analysis
| Peak voxel location | MNI coordinates | Z-value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Fusiform gyrus L | ||||
| Inferior longitudinal fasciculus | −34 | −54 | −12 | 3.681 |
| Fusiform gyrus L | −34 | −58 | −14 | 3.547 |
| Insula L | ||||
| Rolandic operculum L | −48 | −8 | 6 | −1.416 |
| Superior temporal gyrus L | −50 | 4 | 0 | −1.389 |
| Superior temporal gyrus L | −48 | 0 | 0 | −1.357 |
| Insula L | −46 | 6 | 2 | −1.336 |
| Frontal aslant tract L | −48 | 4 | 6 | −1.303 |
| Inferior frontal gyrus, opercular part L | −54 | 8 | 6 | −1.297 |
| Inferior frontal gyrus, opercular part L | −50 | 10 | 8 | −1.295 |
| Rolandic operculum L | −56 | 2 | 6 | −1.291 |
| Fronto-insular tract L | −48 | 0 | 8 | −1.286 |
| Inferior frontal gyrus, opercular part L | −56 | 6 | 10 | −1.278 |
| Insula L | −38 | 0 | 4 | −1.273 |
| Rolandic operculum L | −44 | −6 | 10 | −1.269 |
| Insula L | −44 | −2 | −2 | −1.212 |
L, left; MNI, Montreal Neurological Institute.
In order to further evaluate the possibility of weight group effects on cue reactivity, we performed an exploratory analysis using ROIs derived from a previous food cue meta-analysis (van der Laan et al., 2011). There were no significant differences in any ROIs, even with small-volume correction.
Discussion
In this article, we first reviewed the existing literature and then performed a pre-registered meta-analysis on food cue reactivity in obese individuals. Previous theoretical work suggests that obesity is related to an enhanced salience response towards visual food stimuli (Berridge et al., 2010). Moreover, greater brain responses to the sight of food have been proposed as a neural vulnerability factor for the development of obesity (Stice and Burger, 2019). However, although some exceptions exist (van Meer et al., 2019), most studies examining fMRI reactivity to food cues during decision-making, reward sensitivity or cognitive control tasks found no effects of BMI (Carbine et al., 2018; Morys et al., 2018; Adise et al., 2019). At the same time, results of neuroimaging studies investigating the influence of obesity on passive viewing of food cues are mixed. To investigate this issue further, we performed a meta-analysis of 13 studies with 407 participants investigating the influence of obesity on brain responses to food cues. We did not find evidence for overall altered cue processing in obese individuals. This is in line with a previous meta-analysis showing no influence of BMI on behavioral cue reactivity and craving (Boswell and Kober, 2016). However, we found two clusters in the brain that displayed an age-dependent relationship in this context: one in the left insula, which was negatively related to age, and one in the left fusiform gyrus, which was positively related to age. We did not find evidence for publication bias for either region. In our analyses, we only included studies that used appropriate control conditions (i.e. non-food cues) and control groups (lean individuals).
The first cluster was negatively related to age, which means that in children and young adults there was a weight group difference in left insula response to viewing food vs non-food pictures (Figure 1). This difference, however, decreased in older adults. Interestingly, a similar cluster in the left insula was previously observed to be activated for viewing food vs non-food pictures, independent of BMI (van der Laan et al., 2011; Huerta et al., 2014). Activation of the anterior and middle insula was also reported in studies investigating taste processing (Small, 2006), cephalic phase response (Tomasi et al., 2009) or food craving (Pelchat et al., 2004). Finally, insula was implicated in a meta-analysis of studies requiring subjects to intentionally regulate their level of craving in response to food (Han et al., 2018). The effect was BMI dependent.
The second cluster in which we found age-dependent effects of cue reactivity in obese vs lean individuals was located in the left fusiform gyrus, a structure also related to food picture viewing (van der Laan et al., 2011). The fusiform gyrus plays an important role in object recognition (Grill-Spector et al., 2001) and in the case of food vs neutral stimuli viewing is possibly related to increased attention for and visual processing of food images (Killgore and Yurgelun-Todd, 2007; van der Laan et al., 2011). Our age-dependent results are in line with a meta-analysis by van Meer and colleagues who showed that a similar brain region was activated to a higher degree in adults than in children/adolescents to food picture viewing (Van Meer et al., 2014). In our study, weight group difference in activation of this brain region as a response to food cues increased with age (Figure 1). Van Meer explains this finding as meaning that food cues gain salience in adults as compared to children, which is reflected in higher activity in the left fusiform gyrus. In the context of our study, however, such an interpretation should be considered carefully, since a follow-up analysis revealed that the cluster in the left fusiform gyrus is likely related to BMI in the lean group and not to age per se.
Our findings stand in contrast with the conclusions from previous reviews on the topic of cue reactivity in obesity (Stice et al., 2009; van den Akker et al., 2014; Pursey et al., 2014), which posit that there are indeed group differences in neural responses to food cues and that these differences present similarities with those found in substance addictions (García-García et al., 2014). Interestingly, these reviews did not consider age effects and concluded that obesity is related to altered neural processing of food cues independent of age. This is contrary to our meta-analytic findings. These reviews, however, included studies using other stimuli than food pictures (e.g. gustatory stimuli, such as milkshakes), neglected findings from studies reporting no group differences and might have overinterpreted findings that are not methodologically robust. We believe that failing to include studies with null findings, coupled with reviewing studies that used inadequate statistical thresholding and underpowered sample sizes (Eklund et al., 2016), might have led to incorrect conclusions. Such issues become evident when the neuroimaging literature is contrasted with the behavioral literature, as a meta-analysis performed by Boswell and Kober did not find evidence for the influence of BMI on food cue reactivity (Boswell and Kober, 2016). Here, the authors also showed that food cue reactivity predicts weight-gain. This is in line with other longitudinal findings in the neuroimaging domain showing that neural responses to food cues predict weight gain. For example, higher activity in the nucleus accumbens in response to food pictures tends to predict weight over a 6 months of follow-up (Demos et al., 2012). In their study in 2018, Stice and Yokum showed that activity in the motor processing areas, but not in the striatum, predicts BMI gain over 3 years (Stice and Yokum, 2018). Conversely, fMRI has also been used in individuals undergoing weight-loss programs: food cue reactivity in brain areas related to reward (Murdaugh et al., 2012), and self-regulation (Neseliler et al., 2019) both predicted successful outcomes. Therefore, a question remains how cue reactivity can be independent of BMI and obesity status but also predict future weight fluctuations (Boswell and Kober, 2016). Van der Akker and colleagues claim that cue reactivity might lead to increased food intake and weight gain only in more impulsive individuals (van den Akker et al., 2014). This suggests that there are multiple factors beyond BMI that need to be taken into account when investigating food cue reactivity. Some of those factors were reviewed in the Introduction to this article and include dietary restraint, self-control, hunger or food craving. Our meta-regression analysis shows that age might be another such factor. In addition, the nature of food cues (e.g. visual, olfactory or gustatory) might also differentially affect cue reactivity in obese individuals (Morys et al., 2018). Another possibility is that pathological eating (such as binge eating or food addiction patterns) might mediate the effects of obesity on fMRI responses to the sight of food. Unfortunately, findings from fMRI studies on compulsive overeating are notably inconsistent (García-García et al., 2020), and future research should consider both obesity and eating behavior to further test this possibility.
One limitation of the current meta-analysis is the use of BMI as a measurement of obesity. In some cases, individuals with high muscle mass who are not obese might be placed in the obese group based solely on BMI values. Further, only 3 studies included in the analysis reported the methods in which BMI was measured (self-reported vs measured), which might constitute an additional confound in the analysis as people tend to underreport their weight and overreport their height (Sherry et al., 2007; Merrill and Richardson, 2009). However, without the explicit knowledge of the methods used to measure BMI and body composition of individuals included in the studies we were not able to correct for this in our analyses.
Overall, our review and meta-analysis show that there is scant evidence for food cue reactivity differences between lean and obese individuals. Our findings show that only two brain areas were related to weight group differences in visual processing of food cues and that these effects were age-related. Hence, additional factors contributing to neural correlates of food picture viewing in lean and obese individuals, such as age, self-control, food craving, impulsivity, hunger or dietary restraint need to be investigated in future studies. We propose that studies of better quality—using large sample sizes, appropriate statistical thresholding and ideally preregistered designs and analyses plans—should be performed to investigate in depth how those additional factors influence cue reactivity in obesity. In addition, a meta-analysis of behavioral cue-reactivity studies investigating effects of age might shed more light on and replicate the current neuroimaging-based findings.
Contributor Information
Filip Morys, Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada.
Isabel García-García, Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada; Department of Clinical Psychology and Psychobiology, University of Barcelona, Barcelona, Spain.
Alain Dagher, Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada.
Funding
This work was supported by a Foundation Scheme award to A.D. from the Canadian Institutes of Health Research. I.G.G. is a recipient of a Postdoctoral Fellowship from the Canadian Institutes of Health Research.
Conflict of interest
The authors declare no conflict of interest.
References
- Adise, S., Geier, C.F., Roberts, N.J., White, C.N., Keller, K.L. (2019). Food or money? children’s brains respond differently to rewards regardless of weight status. Pediatric obesity, 14, e12469. doi: 10.1111/ijpo.12469 [DOI] [PubMed] [Google Scholar]
- van den Akker, K., Stewart, K., Antoniou, E.E., et al. (2014). Food cue reactivity, obesity, and impulsivity: are they associated? Current Addiction Reports, 1, 301–8. [Google Scholar]
- Berridge, K.C., Ho, C.Y., Richard, J.M., et al. (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research, 2, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell, R.G., Kober, H. (2016). Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obesity Reviews, 17, 159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland, E.J., Burgon, R.H., Hardman, C.A. (2017). Reactivity to television food commercials in overweight and lean adults: physiological, cognitive and behavioural responses. Physiology & Behavior, 177, 182–8. [DOI] [PubMed] [Google Scholar]
- Bruce, A.S., Holsen, L.M., Chambers, R.J., et al. (2010). Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity, 34, 1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, K.S., Stice, E. (2011). Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. NeuroImage, 55, 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbine, K.A., Duraccio, K.M., Kirwan, C.B., et al. (2018). A direct comparison between ERP and fMRI measurements of food-related inhibitory control: implications for BMI status and dietary intake. NeuroImage, 166, 335–48. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito, A., Gleaves, D.H., Williams, T.L., et al. (2000). The development and validation of the state and trait food-cravings questionnaires. Behavior Therapy, 31, 151–73. [DOI] [PubMed] [Google Scholar]
- Cosme, D., Ludwig, R.M., Berkman, E.T. (2019). Comparing two neurocognitive models of self-control during dietary decisions. Social Cognitive and Affective Neuroscience, 14, 957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids, S., Lauffer, H., Thoms, K., et al. (2010). Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity, 34, 94–104. [DOI] [PubMed] [Google Scholar]
- Demos, K.E., Heatherton, T.F., Kelley, W.M. (2012). Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32, 5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos, A., Tkach, J., Ho, A., et al. (2012). Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite, 58, 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan, K.J., Breslin, G., Hanna, D., Murphy, K., Gallagher, A.M. (2014). Visual attention to food cues in obesity: an eye-tracking study. Obesity (Silver Spring, Md.), 22, 2501–7. [DOI] [PubMed] [Google Scholar]
- Doornweerd, S., De Geus, E.J., Barkhof, F., et al. (2018). Brain reward responses to food stimuli among female monozygotic twins discordant for BMI. Brain Imaging and Behavior, 12, 718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A., Nichols, T.E., Knutsson, H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113, 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex, B.G., Zald, D.H. (2010). The neuroeconomics of food selection and purchase. In Dubé, L. et al. (eds). Obesity Prevention. San Diego: Academid Press, 89–104. [Google Scholar]
- Frank, S., Wilms, B., Veit, R., et al. (2014). Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. International Journal of Obesity, 38, 341–8. [DOI] [PubMed] [Google Scholar]
- García-García, I., Horstmann, A., Jurado, M.A., et al. (2014). Reward processing in obesity, substance addiction and non-substance addiction. Obesity Reviews, 15, 853–69. [DOI] [PubMed] [Google Scholar]
- García-García, I., Jurado, M.A., Garolera, M., et al. (2013a). Functional connectivity in obesity during reward processing. NeuroImage, 66, 232–9. [DOI] [PubMed] [Google Scholar]
- García-García, I., Kube, J., Morys, F., et al. (2020). Liking and left amygdala activity during food versus nonfood processing are modulated by emotional context. Cognitive, affective & behavioral neuroscience, 20, 91–102. doi: 10.3758/s13415-019-00754-8 [DOI] [PubMed] [Google Scholar]
- García-García, I., Morys, F., Dagher, A. (2019). Nucleus accumbens volume is related to obesity measures in an age-dependent fashion. Journal of Neuroendocrinology, e12812. doi: 10.1111/jne.12812 [DOI] [PubMed] [Google Scholar]
- García-García, I., Morys, F., Michaud, A., et al. (2020). Food addiction, skating on thin Ice: A critical overview of neuroimaging findings. Current Addiction Reports, 7, 20–29. [Google Scholar]
- García-García, I., Narberhaus, A., Marqués-Iturria, I., et al. (2013b). Neural responses to visual food cues: insights from functional magnetic resonance imaging. European Eating Disorders Review, 21, 89–98. [DOI] [PubMed] [Google Scholar]
- Giuliani, N.R., Pfeifer, J.H. (2015). Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage, 108, 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector, K., Kourtzi, Z., Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41, 1409–22. [DOI] [PubMed] [Google Scholar]
- Han, J.E., Boachie, N., Garcia-Garcia, I., et al. (2018). Neural correlates of dietary self-control in healthy adults: a meta-analysis of functional brain imaging studies. Physiology & Behavior, 192, 98–108. [DOI] [PubMed] [Google Scholar]
- Heni, M., Kullmann, S., Ketterer, C., et al. (2014). Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Human Brain Mapping, 35, 918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A.J. (2007). The psychology of food craving. Proceedings of the Nutrition Society, 66, 277–85. [DOI] [PubMed] [Google Scholar]
- Huerta, C.I., Sarkar, P.R., Duong, T.Q., et al. (2014). Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity, 22, 1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, A. (1998). A learning model of binge eating: cue reactivity and cue exposure. Behaviour Research and Therapy, 36, 257–72. [DOI] [PubMed] [Google Scholar]
- Jansen, A.T.M., Havermans, R.C., Nederkoorn, C. (2011). Cued overeating, In: Preedy, V.R., et al. (eds.). Handbook of Behavior, Food and Nutrition. Springer-Verlag, New York: 1431–43. [Google Scholar]
- Jastreboff, A.M., Sinha, R., Lacadie, C., et al. (2013). Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care, 36, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore, W.D.S., Yurgelun-Todd, D.A. (2007). Positive affect modulates activity in the visual cortex to images of high calorie foods. International Journal of Neuroscience, 117, 643–53. [DOI] [PubMed] [Google Scholar]
- Kroemer, N.B., Krebs, L., Kobiella, A., et al. (2013). (Still) longing for food: insulin reactivity modulates response to food pictures. Human Brain Mapping, 34, 2367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan, L.N., de Ridder, D.T.D., Viergever, M.A., et al. (2011). The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage, 55, 296–303. [DOI] [PubMed] [Google Scholar]
- Lawrence, N.S., Hinton, E.C., Parkinson, J.A., et al. (2012). Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage, 63, 415–22. [DOI] [PubMed] [Google Scholar]
- Loeber, S., Grosshans, M., Korucuoglu, O., et al. (2012). Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. International Journal of Obesity (2005), 36, 1334–9. [DOI] [PubMed] [Google Scholar]
- Lopez, R.B., Milyavskaya, M., Hofmann, W., et al. (2016). Motivational and neural correlates of self-control of eating: a combined neuroimaging and experience sampling study in dieting female college students. Appetite, 103, 192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, S., Romero, A., Adam, T.C., et al. (2013). Abdominal fat is associated with a greater brain reward response to high-calorie food cues in Hispanic women. Obesity (Silver Spring, Md.), 21, 2029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht, M. (2008). How emotions affect eating: a five-way model. Appetite, 50, 1–11. [DOI] [PubMed] [Google Scholar]
- Martin, L.E., Holsen, L.M., Chambers, R.J., et al. (2010). Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity, 18, 254–60. [DOI] [PubMed] [Google Scholar]
- van Meer, F., van der Laan, L.N., Adan, R.A., Viergever, M.A., Smeets, P.A. (2015). What you see is what you eat: An ALE meta-analysis of the neural correlates of food viewing in children and adolescents. NeuroImage, 104, 35–43. doi: 10.1016/j.neuroimage.2014.09.069 [DOI] [PubMed] [Google Scholar]
- van Meer, F., van der Laan, L.N., Eiben, G., et al. (2019). Development and body mass inversely affect children’s brain activation in dorsolateral prefrontal cortex during food choice. NeuroImage, 201, 116016. [DOI] [PubMed] [Google Scholar]
- Mehl, N., Bergmann, S., Klein, A.M., et al. (2017). Cause or consequence? Investigating attention bias and self-regulation skills in children at risk for obesity. Journal of Experimental Child Psychology, 155, 113–27. [DOI] [PubMed] [Google Scholar]
- Mehl, N., Mueller-Wieland, L., Mathar, D., Horstmann, A. (2018). Retraining automatic action tendencies in obesity. Physiology & Behavior, 192, 50–58. doi: 10.1016/j.physbeh.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Mehl, N., Morys, F., Villringer, A., et al. (2019). Unhealthy yet avoidable—how cognitive bias modification alters behavioral and brain responses to food cues in individuals with obesity. Nutrients, 11, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, R.M., Richardson, J.S. (2009). Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001–2006. Preventing Chronic Disease, 6, A121. [PMC free article] [PubMed] [Google Scholar]
- Morys, F., Bode, S., Horstmann, A. (2018). Dorsolateral and medial prefrontal cortex mediate the influence of incidental priming on economic decision making in obesity. Scientific Reports, 8, 17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberg, C., Mathar, D., Villringer, A., et al. (2016). Stopping at the sight of food – how gender and obesity impact on response inhibition. Appetite, 107, 663–76. [DOI] [PubMed] [Google Scholar]
- Murdaugh, D.L., Cox, J.E., Cook III, E.W., et al. (2012). fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage, 59, 2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn, C., Smulders, F.T.Y., Jansen, A. (2000). Cephalic phase responses, craving and food intake in normal subjects. Appetite, 35, 45–55. [DOI] [PubMed] [Google Scholar]
- Neseliler, S., Hu, W., Larcher, K., et al. (2019). Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metabolism, 29, 39–49.e4. [DOI] [PubMed] [Google Scholar]
- Nummenmaa, L., Hirvonen, J., Hannukainen, J.C., et al. (2012). Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One, 7, e31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, R., Morys, F., Horstmann, A., et al. (2019). The impulsive brain: neural underpinnings of binge eating behavior in normal-weight adults. Appetite, 136, 33–49. [DOI] [PubMed] [Google Scholar]
- Oltmanns, K.M., Heldmann, M., Daul, S., et al. (2012). Sibutramine promotes amygdala activity under fasting conditions in obese women. Psychopharmacology, 221, 693–700. [DOI] [PubMed] [Google Scholar]
- Pelchat, M.L., Johnson, A., Chan, R., et al. (2004). Images of desire: food-craving activation during fMRI. NeuroImage, 23, 1486–93. [DOI] [PubMed] [Google Scholar]
- Plassmann, H., O’Doherty, J., Rangel, A. (2007). Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 27, 9984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy, J., Herman, C.P., Coelho, J.S. (2008). Caloric restriction in the presence of attractive food cues: external cues, eating, and weight. Physiology & Behavior, 94, 729–33. [DOI] [PubMed] [Google Scholar]
- Pursey, K.M., Stanwell, P., Callister, R.J., et al. (2014). Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Frontiers in Nutrition, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J., Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder. British Journal of Psychiatry, 195, 393–402. [DOI] [PubMed] [Google Scholar]
- Radua, J., Mataix-Cols, D., Phillips, M.L., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27, 605–11. [DOI] [PubMed] [Google Scholar]
- Rahmani, M., Rahmani, F. (2019). Cortex, insula, and interoception. In: Rezaei, N., Saghazadeh, A. (eds.) Biophysics and Neurophysiology of the Sixth Sense, Cham: Springer International Publishing, 59–68 [Google Scholar]
- Rapuano, K.M., Zieselman, A.L., Kelley, W.M., et al. (2017). Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences of the United States of America, 114, 160–5. doi: 10.1073/pnas.1605548113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund, Y., Preuschhof, C., Bohner, G., et al. (2007). Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage, 37, 410–21. [DOI] [PubMed] [Google Scholar]
- Scharmüller, W., Übel, S., Ebner, F., et al. (2012). Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neuroscience Letters, 518, 106–10. [DOI] [PubMed] [Google Scholar]
- Sclafani, A., Touzani, K., Bodnar, R.J. (2011). Dopamine and learned food preferences. Physiology & Behavior, 104, 64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry, B. (2007). Accuracy of adolescent self-report of height and weight in assessing overweight status: a literature review. Archives of Pediatrics & Adolescent Medicine, 161, 1154–61. [DOI] [PubMed] [Google Scholar]
- Small, D.M. (2006). Central gustatory processing in humans. In: Hummel, T., Welge-Lussen, A. (eds). Taste and Smell, Basel: KARGER, 191–220. [DOI] [PubMed] [Google Scholar]
- Smeets, P.A.M., Dagher, A., Hare, T.A., et al. (2019). Good practice in food-related neuroimaging. American Journal of Clinical Nutrition, 109, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.M., Nichols, T.E. (2009). Threshold-free cluster enha- ncement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Stice, E., Burger, K. (2019). Neural vulnerability factors for obesity. Clinical Psychology Review, 68, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice, E., Spoor, S., Ng, J., et al. (2009). Relation of obesity to consummatory and anticipatory food reward. Physiology & Behavior, 97, 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice, E., Yokum, S. (2018). Relation of neural response to palatable food tastes and images to future weight gain: using bootstrap sampling to examine replicability of neuroimaging findings. NeuroImage, 183, 522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice, E., Yokum, S., Bohon, C., et al. (2010). Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage, 50, 1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel, L.E., Weller, R.E., Cook, E.W., et al. (2008). Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage, 41, 636–47. [DOI] [PubMed] [Google Scholar]
- Tang, D.W., Fellows, L.K., Dagher, A. (2014). Behavioral and neural valuation of foods is driven by implicit knowledge of caloric content. Psychological Science, 25, 2168–76. [DOI] [PubMed] [Google Scholar]
- Tang, D.W., Fellows, L.K., Small, D.M., et al. (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology & Behavior, 106, 317–24. [DOI] [PubMed] [Google Scholar]
- Tomasi, D., Wang, G.-J., Wang, R., et al. (2009). Association of body mass and brain activation during gastric distention: implications for obesity S. Gaetani (ed). PLoS One, 4, e6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo, T., Weinberger, A.H., Grilo, C.M., et al. (2014). Heightened vagal activity during high-calorie food presentation in obese compared with non-obese individuals—results of a pilot study. Obesity Research & Clinical Practice, 8, e258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik, U., Dagher, A., Dubé, L., et al. (2013). Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neuroscience and Biobehavioral Reviews, 37, 279–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik, U., García-García, I., Dagher, A., (2019). Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. The European Journal of Neuroscience, 50, 2430–45. doi: 10.1111/ejn.14352 [DOI] [PubMed] [Google Scholar]
- Veit, R., Horstman, L.I., Hege, M.A., Heni, M., Rogers, P.J., Brunstrom, J.M., Fritsche, A., Preissl, H., Kullmann, S. (2020). Health, pleasure, and fullness: changing mindset affects brain responses and portion size selection in adults with overweight and obesity. International Journal of Obesity (2005), 44, 428–37. doi: 10.1038/s41366-019-0400-6 [DOI] [PubMed] [Google Scholar]
- Verdejo-Román, J., Vilar-López, R., Navas, J.F., et al. (2017). Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Human Brain Mapping, 38, 666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T.D., Lindquist, M., Kaplan, L. (2007). Meta-analysis of functional neuroimaging data: current and future directions. Social Cognitive and Affective Neuroscience, 2, 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


