Abstract
Background
Blocking increased expression of nerve injury-specific long non-coding RNA (NIS-lncRNA) in injured dorsal root ganglia (DRG) through DRG microinjection of NIS-lncRNA small hairpin interfering RNA or generation of NIS-lncRNA knockdown mice mitigates neuropathic pain. However, these strategies are impractical in the clinic. This study employed a Food and Drug Administration (FDA)-approved antisense oligonucleotides strategy to examine the effect of NIS-lncRNA ASOs on neuropathic pain.
Methods
Effects of intrathecal injection of NIS-lncRNA antisense oligonucleotides on day 7 or 14 after chronic constriction injury (CCI) of the sciatic nerve, fourth lumbar (L4) spinal nerve ligation, or intraperitoneal injection of paclitaxel or streptozotocin on the expression of DRG NIS-lncRNA and C–C chemokine ligand 2 (CCL2, an NIS-lncRNA downstream target) and nociceptive hypersensitivity were examined. We also assessed whether NIS-lncRNA antisense oligonucleotides produced cellular toxicity.
Results
Intrathecal NIS-lncRNA antisense oligonucleotides attenuated CCI-induced mechanical allodynia, heat hyperalgesia, cold hyperalgesia, and ongoing nociceptive responses, without changing basal or acute nociceptive responses and locomotor function. Intrathecal NIS-lncRNA antisense oligonucleotides also blocked CCI-induced increases in NIS-lncRNA and CCL2 in the ipsilateral L3 and L4 DRG and hyperactivities of neurones and astrocytes in the ipsilateral L3 and L4 spinal cord dorsal horn. Similar results were found in antisense oligonucleotides-treated mice after spinal nerve ligation or intraperitoneal injection of paclitaxel or streptozotocin. Normal morphologic structure and no cell loss were observed in the DRG and spinal cord of antisense oligonucleotides-treated mice.
Conclusion
These findings further validate the role of NIS-lncRNA in trauma-, chemotherapy-, or diabetes-induced neuropathic pain and demonstrate potential clinical application of NIS-lncRNA antisense oligonucleotides for neuropathic pain management.
Keywords: antisense oligonucleotides, CCL2, chemotherapy, diabetes mellitus, dorsal root ganglion, nerve injury-specific long non-coding RNA, nerve trauma, neuropathic pain
Editor’s key points.
-
•
Genetic strategies such as siRNA injection and transgenetic mice are impractical in the clinic.
-
•
This study employed an FDA-approved antisense oligonucleotides strategy to examine the effect of intrathecal NIS-lncRNA antisense oligonucleotides on neuropathic pain.
-
•
Intrathecal NIS-lncRNA antisense oligonucleotides attenuated an increase in CCL2 in injured dorsal root ganglion and nociceptive hypersesnitivity caused by peripheral nerve trauma.
-
•
Intrathecal NIS-lncRNA antisense oligonucleotides also mitigated increases in CCL2 and nocieptive hypersensitivity caused by chemotherapetic neuropathy or diabetic neuropathy.
Neuropathic pain caused by peripheral nerve trauma, chemotherapy, diabetes mellitus, and neurological disease is a complex and debilitating major health problem. It impacts the quality of life for 6.9–10% of the population around the world.1 In the United States alone, over $600 billion dollars per year are spent on neuropathic pain-associated healthcare and productivity losses.1 Opioid analgesics are the most effective and widely used drugs for the management of this disorder; however, they are effective in <50% of neuropathic pain patients.2 Importantly, the physical dependence and abuse liability of opioids remain a serious public health concern,3 and the overreliance on opioids for neuropathic pain has contributed to an alarming epidemic of opioid misuse, abuse of prescription opioids, and opioid overdose mortality.4,5 The most commonly prescribed non-opioid medications for neuropathic pain are gabapentinoids (e.g. gabapentin and pregabalin), serotonin-norepinephrine reuptake inhibitors (e.g. duloxetine), and tricyclic antidepressants (e.g. amitriptyline).6 Although these medications overall have better safety and tolerance profile than opioids, their efficacy varies and is less than that of opioid analgesics.6 Moreover, some of their side-effects (including somnolence, dizziness, fatigue, and gastrointestinal symptoms) can be severe enough to significantly impact the long-term adherence.6,7 Therefore, there is clearly an urgent need for other safer non-opioid treatments of neuropathic pain.
Dysregulation of pain-associated genes at both transcriptional and translational levels in the dorsal root ganglia (DRG) under neuropathic pain conditions is considered to be a molecular basis for the pathogenesis of neuropathic pain. Long non-coding RNAs (lncRNAs) are longer than 200 nucleotides and play an important role in the development and maintenance of neuropathic pain via regulation of pain-associated gene expression.8 In addition to Kcna 1.2 antisense RNA,9 DS-lncRNA,10 and lncRNA H19,11 we recently identified nerve injury-specific lncRNA (NIS-lncRNA) in DRG neurones, which was dramatically upregulated in specific response to peripheral nerve trauma caused by chronic constriction injury (CCI) of unilateral sciatic nerve or unilateral fourth lumbar (L4) spinal nerve ligation (SNL) in injured DRG, but not in intact DRG and the ipsilateral lumbar spinal cord dorsal horn.12 Genetic knockdown of NIS-lncRNA in injured murine DRG through DRG microinjection of specific NIS-lncRNA small interfering RNA (siRNA) in CD1 mice or AAV5-Cre in NIS-lncRNAfl/fl mice attenuated CCI- or SNL-induced increase in the level of DRG C–C chemokine ligand 2 (CCL2), an endogenous initiator of neuropathic pain13 and nociceptive hypersensitivity during the development and maintenance periods.12 Similar results were also found in the SNL transgenic mice with sensory neurone-specific NIS-lncRNA knockdown.12 Conversely, overexpression of NIS-lncRNA in naive DRG elevated CCL2 expression and increased CCL2-mediated excitability in DRG neurones and led to enhanced responses to noxious stimuli.12 Thus, NIS-lncRNA contributes to neuropathic pain likely by promoting DRG CCL2 expression and may be a novel and potential target for therapeutic treatment of this disorder.
Given that the genetic knockdown strategies mentioned above are unfeasible in clinical practice, the present study made use of the chemically modified long-acting antisense oligonucleotides (ASOs), a strategy that has been approved by the US Food and Drug Administration (FDA) for clinical therapy.14 We first examined the effect of intrathecal (i.t.) administration of ASOs that specifically knocked down NIS-lncRNA (NIS-lncRNA ASOs) on the increased DRG CCL2 expression and established nociceptive hypersensitivity caused by CCI or SNL. We also validated its effect on the increased DRG CCL2 and established nociceptive hypersensitivity under the conditions of paclitaxel (PTX)-induced chemotherapy neuropathy or streptozotocin (STZ)-induced diabetic neuropathy. Finally, we determined whether i.t. NIS-lncRNA ASOs affected motor function or had toxic effects on DRG and spinal cord tissues.
Methods
Animal models
Adult male CD1 mice (about 7–8 weeks) were purchased from Charles River Laboratories (Wilmington, MA, USA) and were housed in the central housing facility under a standard 12-h light–dark cycle, with ad libitum food and water. The Institutional Animal Care and Use Committee of Rutgers New Jersey Medical School approved all procedures used in this study. Additionally, all animal experimental procedures were consistent with ethical guidelines issued by the National Institutes of Health and the International Association for the Study of Pain. All efforts were made to minimise the suffering of animals and reduce the number of animals used. To minimise intra- and inter-individual variability in behavioural outcome measurements, animals were acclimated for 1–2 days before behavioural testing. All the experimenters were blind to the treatment conditions.
Oligonucleotides
The synthesis and purification of chemically modified NIS-lncRNA ASOs and control missense oligonucleotides (MSOs) were carried out as described15 (ExonanoRNA LLC, Columbus, OH, USA). The sequences and chemistries of NIS-lncRNA ASOs and MSOs used are presented in Supplementary Table S1. The concentration was quantified by UV spectrometry. Lyophilised ASOs and MSOs were dissolved in 0.01 M sterile phosphate-buffered saline (PBS) without calcium or magnesium, sterilised through a 0.2-μm filter and then diluted to the desired concentration for dosing.
Experimental protocol
Mice were randomised to each model before experiments. The oligonucleotides or PBS were intrathecally injected on day 7 after surgery (for CCI, SNL, or the corresponding sham) or intraperitoneal (i.p.) injection of PTX (or vehicle 1), or on day 14 after i.p. injection of STZ (or vehicle 2). Behavioural nociceptive testing was carried out 1 day before surgery or i.p. injection and on days 7–35 after surgery or i.p. injection. Expression of NIS-lncRNA, Ccl2 mRNA and CCL2 protein in the L4 or L3/4 DRG and expression of extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2), and glial fibrillary acidic protein (GFAP) in the L4 or L3/4 spinal cord were assessed on day 35 after surgery or i.p. injection.
Neuropathic pain models
The CCI-induced neuropathic pain model was performed as described.16, 17, 18, 19 In brief, the mice were anaesthetised with isoflurane 2.5 vol% and maintained with isoflurane 2 vol%, the left sciatic nerve trunk was exposed above the hip, and three ligatures were tied loosely with 7–0 silk thread around the nerve ∼1 mm apart proximal to the trifurcation. Ligatures were loosely tied until a subtle flick of the ipsilateral hind limb was observed. The sham groups underwent identical procedures, but without the ligature of the sciatic nerve. We used 76 mice in total (seven groups, 8–12 mice/group) in this model.
The SNL-induced neuropathic pain model was generated as described.20, 21, 22 In brief, the animals were anaesthetised as described above. A dorsal incision was made at the level of the fifth lumbar transverse process. After the transverse process was removed, the unilateral fourth lumbar spinal nerve was exposed and tightly ligated with 7–0 silk thread. The ligated nerve was transected distal to the ligature. Skin and muscles were closed in layers. The sham groups underwent identical procedures, but without the ligature and transection of the respective nerve. We used 48 mice in total (four groups, 12 mice/group) in this model.
The PTX-induced chemotherapy neuropathic pain model was carried out as described.23, 24, 25 Briefly, PTX (6 mg ml−1 in Cremophor EL 50% (Sigma-Aldrich, St. Louis, MO, USA) and ethanol 50% (Sigma-Aldrich) was diluted in sterile saline to a final concentration of 0.4 mg ml−1 and administered i.p. at a dose of 4 mg kg−1 every other day for a total of four injections (days 0, 2, 4, and 6). Vehicle 1 (a mixture of ethanol and Cremophor EL (1:1) was prepared in the same manner as described above except for PTX. We used 32 mice in total (four groups, 8 mice/group) in this model.
The STZ-induced diabetic neuropathic pain model was conducted as described.26 Briefly, STZ (165 mg kg−1 in 10 mM citrate buffer, pH 4.4, Sigma-Aldrich) was injected intraperitoneally. Control mice received equal volume injection of sodium citrate buffer (vehicle 2). After 3 days of STZ injection, the mice were fasted for 12 h, and blood drawn from the tail vein was checked for blood glucose levels. Blood glucose levels exceeding 15 mmol L−1 indicated that the STZ model was successfully generated. We used 34 mice in total (four groups, 8–10 mice/group) in this model.
Behavioural testing
Behavioural testing including mechanical, thermal, and cold tests was carried out as described previously27, 28, 29 in order with 1-h intervals. Conditioned place preference (CPP) test was performed as reported previously27, 28, 29 at the third week after PBS or oligonucleotide injection. Locomotor functional testing was carried out as indicated16, 17, 18, 19 after all behavioural tests described above were completed.
Paw withdrawal frequencies (PWFs) in response to mechanical stimuli were measured with two calibrated von Frey filaments (0.07 g and 0.4 g; Stoelting Co., Wood Dale, IL, USA). In brief, each mouse was placed in a Plexiglas chamber on an elevated mesh screen. Each filament was applied to the plantar side of each hind paw for approximately 1 s and repeated 10 times at 5-min intervals. The occurrence of paw withdrawal in each of these 10 trials was expressed as a percent response frequency ([number of paw withdrawals/10 trials]×100=% response frequencies). This percentage was used as an indication of the amount of paw withdrawal.
Paw withdrawal latencies (PWLs) in response to noxious heat stimuli were examined with a Model 336 Analgesic Meter (IITC Inc./Life Science Instruments, Woodland Hills, CA, USA). Briefly, each mouse was placed in a Plexiglas chamber on a glass plate. Radiant heat from a light box was applied to the middle of the plantar surface of each hind paw through the glass plate. When the animal lifted its foot, the light beam was automatically turned off. The length of time between the start of the light beam and withdrawal of the hind paw was defined as the PWL. Each trial was repeated three times at 5-min intervals for each side. An automatic cut-off time of 20 s was used to avoid tissue damage to the hind paw.
PWLs to noxious cold (−1 to ∼0°C) were examined by placing the mouse in a Plexiglas chamber on the cold aluminium plate. The temperature was monitored continuously by digital thermometer. The length of time between the placement of the mouse and a positive nociceptive response (e.g. jumping or snapping at the relevant paw) was defined as the PWL. Each trial was repeated three times at 10-min intervals. A cut-off time of 20 s was used to avoid tissue damage.
The CPP test was conducted in an apparatus with two Plexiglas chambers connected with an internal door (Med Associates Inc., Fairfax, VT, USA). Each chamber had a unique floor texture and wall patterns. Movement of the mice and time spent in each chamber were monitored by photo beam detectors installed along the chamber walls and automatically recorded in MED-PC IV CPP software. Mice were first preconditioned for 30 min to habituate to the test environment with free access to both chambers. At the end of preconditioning, the duration time spent in each chamber was recorded within 15 min to check whether mice had a pre-existing chamber bias. Animals spending >80% or <20% of the total time in either chamber were excluded from further testing. The following conditioning protocol was performed each day for 3 days when the internal door was closed. The mice first received i.t. injection of saline (5 μl) specifically paired with one conditioning chamber in the morning for 15 min. Six hours later, lidocaine (0.8% in 5 μl saline) was given intrathecally paired with another opposite conditioning chamber in the afternoon for 15 min. The injection order of saline and lidocaine every day was alternated. On the test day, conditioned mice were randomly placed in one of the chambers with the interior door open. Movement and duration of time spent by each mouse in each chamber were recorded for 15 min for analysis of chamber preference. Difference scores were calculated as (test time-preconditioning time) spent in the drug chamber.
Locomotor functional tests including placing, grasping, and righting reflexes were carried out after the above-described behavioural tests. For the placing reflex, the hind limbs were placed slightly lower than the forelimbs and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. Then, whether the hind paws were placed on the table surface reflexively was recorded. For the grasping reflex, the mice were placed on a wire grid, and then whether the hind paws grasped the wire was recorded. For the righting reflex, the mice were placed on their back on a flat surface. Whether or not the mice could immediately right themselves was recorded. Each trial was repeated five times with 5-min intervals and the score for each test was recorded by counting the times of each normal reflex.
Intrathecal injection
Under isoflurane (2 vol%) anaesthesia, a dorsal midline incision was made in the lower lumbar region. The L4–5 intervertebral space was exposed by blunt dissection of the tissues. The mouse was fixed by attaching custom-made clamps to the vertebral column. The oligonucleotide solution (1 μL) or PBS (1 μL) was injected into the intrathecal space through a glass micropipette connected to a Hamilton syringe using a dissection microscope. The pipette was retained in the intrathecal space for 5 min after injection and before removal. Finally, the surgical field was irrigated with sterile saline, and the skin incision was closed with wound clips.
Quantitative real-time reverse transcription polymerase chain reaction
Total RNA extraction and quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) were carried out as described.16, 17, 18, 19 Briefly, unilateral L3/4 DRG from two CCI/sham mice, or unilateral L4 DRG from four SNL/sham mice were pooled together to obtain enough RNA. Total RNA was extracted by miRNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. After RNA was reverse transcribed with the ThermoScript reverse transcriptase (Invitrogen/ThermoFisher Scientific, Waltham, MA, USA) and oligo (dT) primers (Invitrogen/ThermoFisher Scientific), template (4 μL) was amplified in a Bio-Rad CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) by using the primers listed in Supplementary Table S2. Each sample was run in triplicate in a 20 μL reaction volume containing 250 nM forward and reverse primers, 10 μL of Advanced Universal SYBR Green Supermix (Bio-Rad Laboratories), and 20 ng of cDNA. PCR reaction mixtures were heated to 95°C for 3 min, and then subjected to 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, with a final incubation at 72°C for 7 min. All data were normalised to Gapdh, which has been demonstrated to be stable after peripheral nerve injury.9,30,31 The ΔCt method (2−ΔΔCt) was used to calculate the ratios of mRNA levels.
Western immunoblotting
Western immunoblotting was carried out as described previously.16, 17, 18, 19 In brief, after mice were euthanised under isoflurane, DRG or spinal cord was collected and flash frozen. To achieve sufficient protein, unilateral L3/4 DRG from two CCI/sham mice, or unilateral L4 DRG from four SNL/sham mice were pooled together. The tissues were homogenised in chilled lysis buffer (10 mM Tris, 5 mM MgCl2, 5 mM EGTA, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and 40 μM leupeptin). After the homogenates were centrifuged at 4°C for 15 min at 1000 g, the supernatant was collected. BSA assay was used to measure protein concentration. The samples were heated at 99°C for 5 min and loaded onto a precast polyacrylamide gel 4–20% (Bio-Rad Laboratories). The proteins were then electrophoretically transferred onto 0.2 μm pore size nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked with 3% non-fat milk in Tris-buffered saline containing Tween-20 0.1% for 1 h and then incubated with primary antibodies, including rabbit anti-p-ERK1/2 (1:1000, Cell Signaling, Danvers, MA, USA), rabbit anti- ERK1/2 (1:1,000, Cell Signaling), mouse anti-GFAP (1:1,000, Cell Signaling), mouse anti-CCL2 (1:500, Invitrogen), and rabbit anti-GAPDH (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight under gentle agitation. The proteins were detected by goat peroxidase-conjugated anti-mouse (1:3,000, Jackson ImmunoResearch, West Grove, PA, USA) or anti-rabbit secondary antibody (1:3,000, Jackson ImmunoResearch), developed by Western peroxide reagent and luminol/enhancer reagent (Clarity Western ECL Substrate, Bio-Rad) and were seen using the ChemiDoc XRS System with Image Lab software (Bio-Rad). The intensities of the blots were quantified with densitometry using Image Lab software (Bio-Rad).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) and cresyl violet histochemical staining
Terminal deoxynucleotidyl transferase-mediated (TdT) dUTP nick end labelling (TUNEL) and cresyl violet histochemical staining were performed as described.27,32, 33, 34 In brief, the mice were deeply anaesthetised and transcardially perfused with PBS 0.9%, 20 ml of 0.01 M (pH 7.4), followed by paraformaldehyde 4%, 100 ml in phosphate buffer 0.1 M (PB, pH 7.4). Lumbar DRG and lumbar spinal cord were harvested and post-fixed in the same fixative solution at 4°C for 4 h. The tissues were cryoprotected in sucrose 30% overnight. Two sets of the sections at thickness of 20 μm were collected from each tissue by grouping every second section. TUNEL histochemical staining was performed on one set of sections using an in situ cell death detection kit (Roche Molecular Biochemical, Pleasanton, CA, USA). Briefly, the sections were incubated with proteinase K solution (20 μg ml−1) for 20 min at room temperature, and then with a TUNEL reaction mixture composed of TdT at 37°C in a humidified chamber for 60 min. TdT enzyme-incorporated fluorescein was detected with converter-alkaline phosphatase (AP), consisting of sheep anti-fluorescein antibody conjugated with AP. The signal was detected using nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate as the colour substrate. Another set of sections was stained with cresyl violet. The sections were rinsed in distilled water and incubated for 30 min in a solution of cresyl violet 0.2% (cresyl violet acetate; Sigma, St. Louis, MO, USA) in acetate buffer, then washed in distilled water, dehydrated through a graded series of increasing ethanol concentrations, and cover-slipped. The images of whole DRG or spinal cord were taken using a Nikon microscope (Nikon Eclipse 80i, Melville, NY, USA). For DRG tissues, 15 sections (five sections/mouse) from three mice per group were examined under bright-field microscopy. The number of cells/section/group was counted and averaged. For spinal cord tissues, 12 sections (four sections/mouse) from three mice per group were examined. According to the anatomic structure, the spinal cord was divided into four regions: the superficial laminae (I–II), the nucleus proprius (III–IV), the neck of the dorsal horn (V–VI), and the ventral horn (VII–IX). The number of cells/section/group from these regions was counted and averaged.
Statistical analysis
The mice were assigned into various treatment groups randomly. All results were expressed as mean (standard error of the mean, sem). All of the data were statistically analysed using a one-way, two-way, or three-way analysis of variance (anova). When anova showed significant differences, pairwise comparisons between the means were analysed by the post hoc Tukey method (Sigma Plot 12.5, Inpixon, San Jose, CA). Significance was set at P<0.05.
Results
Effect of intrathecal administration of NIS-lncRNA antisense oligonucleotides on nerve trauma-induced neuropathic pain
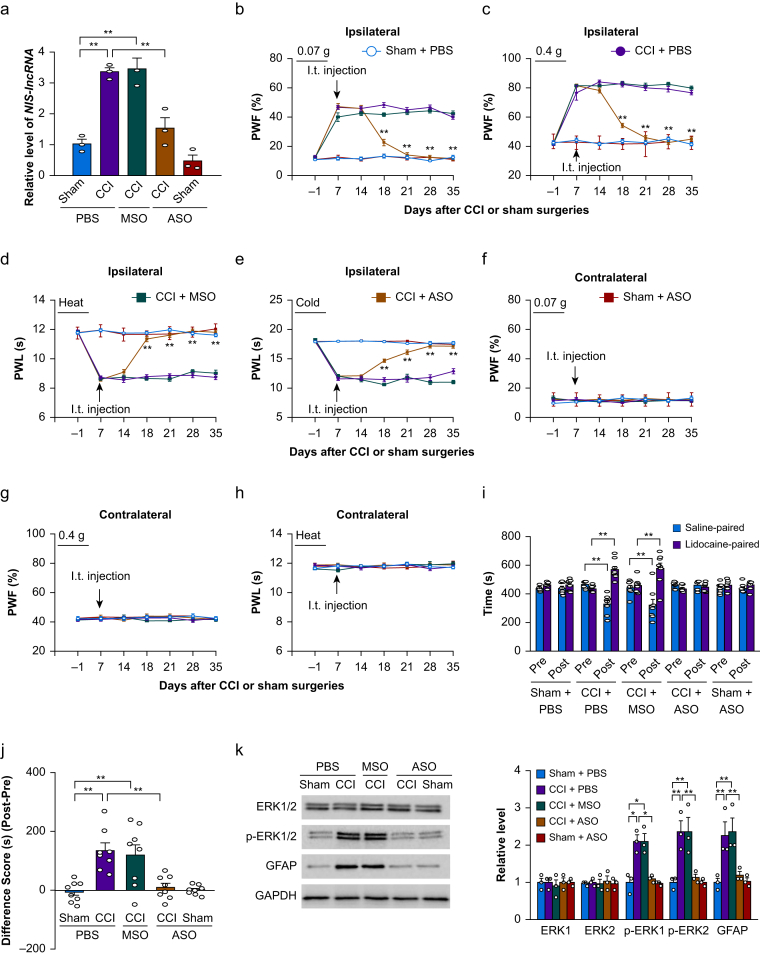
Our previous study showed that blocking the increased expression of DRG NIS-lncRNA alleviated CCI- or SNL-induced nociceptive hypersensitivities during the development and maintenance periods.12 Given that patients usually seek medical care after the onset of neuropathic pain, we intrathecally administered NIS-lncRNA ASOs (100 μg) on day 7 post-surgery. At this time point, CCI-induced nociceptive hypersensitivity was completely developed.16, 17, 18, 19 MSOs (100 μg) and PBS were used as the controls. As expected, the level of NIS-lncRNA was significantly increased by 3.3-fold in the ipsilateral L3/4 DRG of the PBS-treated CCI mice on day 35 after CCI, compared with the PBS-treated sham mice (Fig. 1a). This increase was not seen in the ASO-treated CCI mice (Fig. 1a). The MSO-treated CCI mice still displayed a marked elevation in the amount of NIS-lncRNA by 3.4-fold in the ipsilateral L3/4 DRG, compared with the PBS-treated sham mice (Fig. 1a). The basal level of NIS-lncRNA was not significantly changed in the ipsilateral L3/4 DRG of the ASO-treated sham mice (Fig. 1a). None of these treatments altered basal expression of NIS-lncRNA in the contralateral L3/4 DRG of the CCI or sham mice (data not shown).
Fig 1.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs) blocked the chronic constriction injury (CCI)-induced NIS-lncRNA increase in injured dorsal root ganglion (DRG) and nociceptive hypersensitivity. ASOs (100 μg), missense oligonucleotides (MSOs; 100 μg) or phosphate buffered saline (PBS) 0.01 M was injected intrathecally on day 7 after CCI or sham surgery. (a) Level of NIS-lncRNA in the ipsilateral lumbar 3/4 DRGs on day 35 after CCI or sham surgery in the mice with treatments as indicated. n=3 repeats (6 mice)/group. ∗∗P<0.01, by two-way analysis of variance (anova) followed by post hoc Tukey test. (b–h) Effect of i.t. administration of NIS-lncRNA ASOs, MSOs, or PBS on paw withdrawal frequency (PWF) to 0.07 g (b and f) and 0.4 g (c and g) von Frey filament stimuli and paw withdrawal latency (PWL) to heat (d and h) and cold (e) stimuli on the ipsilateral (b–e) and contralateral (f–h) sides on days as indicated after CCI or sham surgery. n=12 mice/group. ∗∗P<0.01 vs the PBS-treated CCI group at the corresponding days, by three-way anova with repeated measures followed by post hoc Tukey test. (i and j) Effect of i.t. administration of NIS-lncRNA ASOs, MSOs, or PBS on ongoing nociceptive responses as assessed by the conditioned place preference paradigm on day 28 post-CCI or sham surgery. Post, post-conditioning; Pre, preconditioning. n=8 mice/group. ∗∗P<0.01, by three-way anova with repeated measures followed by post hoc Tukey test (i) or two-way anova followed by post hoc Tukey test (j). (k) Effect of i.t. administration of NIS-lncRNA ASOs, MSOs, or PBS on the levels of phosphorylated extracellular signal-regulated kinase 1 and 2 (p-ERK1/2), ERK1/2, and glial fibrillary acidic protein (GFAP) in the ipsilateral lumbar 3/4 dorsal horn 35 days after CCI or sham surgery. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a loading control. n=3 repeats (6 mice)/group. ∗P<0.05, ∗∗P<0.01, by two-way anova followed by post hoc Tukey test.
Consistent with previous reports,16, 17, 18, 19 mechanical allodynia, as evidenced by the increases in PWFs to stimuli from 0.07 and 0.4 g von Frey filaments, and heat and cold hyperalgesia, as demonstrated by the reductions in PWLs to heat and cold stimuli, respectively, were detected on the ipsilateral side from days 7–35 after CCI in the PBS-treated CCI mice (Fig. 1b–e). I.t. injection of ASOs substantially blocked the CCI-induced mechanical allodynia and heat and cold hyperalgesia on the ipsilateral side from days 18–35 after surgery (Fig. 1b–e). In contrast, the MSO-injected CCI mice still exhibited mechanical allodynia and heat and cold hyperalgesia on the ipsilateral side, similar to the PBS-treated CCI mice, during the examination period (Fig. 1b–e). I.t. injection of neither ASOs nor MSOs altered basal responses on the contralateral side of CCI mice or on either side of sham mice during the observation period (Fig. 1b–h). All treated mice showed normal locomotor activity (Supplementary Table S3).
In addition to stimulation-induced evoked nociceptive hypersensitivity, we also observed the effect of i.t. ASOs on CCI-induced ongoing nociceptive responses using the CPP paradigm. Unsurprisingly, the PBS- or MSOs-treated CCI mice spent more time in the lidocaine-paired chamber on day 28 after surgery (Fig. 1i and j), indicating stimulation-independent spontaneous nociceptive responses to the nerve injury. In contrast, the ASO-treated CCI mice, like PBS- or ASOs-treated sham mice, did not exhibit significant preference toward either the saline- or lidocaine-paired chamber on day 28 after surgery (Fig. 1i and j), suggesting a lack of spontaneous nociceptive responses.
Whether i.t. ASOs affected the CCI-induced hyperactivities of dorsal horn neurones and astrocytes was also examined. In line with previous studies,16, 17, 18, 19 the levels of pERK1/2 (a marker for neuronal hyperactivation) and GFAP (a marker for astrocyte hyperactivation), but not total ERK1/2, were increased in the ipsilateral L3/4 dorsal horn on day 35 post-CCI in the PBS- or MSO-treated CCI mice (Fig. 1k). These increases were not found in the ASO-treated CCI mice (Fig. 1k). I.t. injection of ASOs did not alter basal levels of total ERK1/2, p-ERK1/2, and GFAP in the ipsilateral L3/4 dorsal horn of the ASOs-treated sham mice (Fig. 1k).
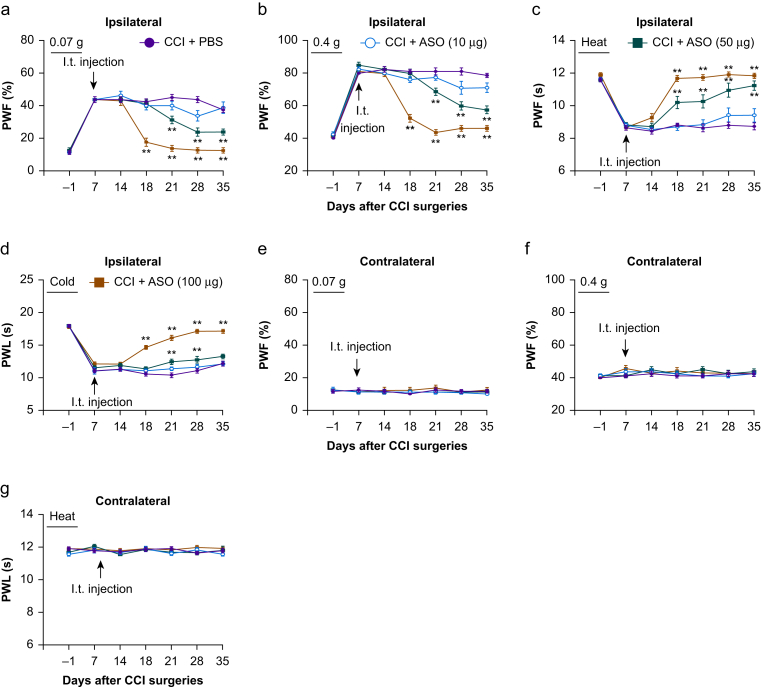
Finally, we examined whether the effect of ASOs on the CCI-induced nociceptive hypersensitivity was dose-dependent. On the ipsilateral side, the antinociceptive effect of ASOs at 100 μg on mechanical allodynia and heat and cold hyperalgesia occurred on day 18 post-CCI and persisted for at least 17 days (Fig. 2a–d), whereas the antinociceptive effect of ASOs at 50 μg on mechanical allodynia and cold hyperalgesia (except for heat hyperalgesia) was delayed (occurring at day 21 post-CCI, Fig. 2a–d). This dosage also produced a short-term antinociceptive effect (at two time points, day 21 and day 28) on cold hyperalgesia (Fig. 2d). I.t. injection of ASOs at 10 μg did not affect the CCI-induced mechanical allodynia and heat and cold hyperalgesia on the ipsilateral side during the observation period (Fig. 2a–d). As expected, i.t. injection of ASOs at these three doses did not alter basal paw withdrawal responses on the contralateral side in the ASO-treated CCI mice (Fig. 2e–g).
Fig 2.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs) dose-dependently attenuated the chronic constriction injury (CCI)-induced nociceptive hypersensitivity. Three doses of ASOs (10, 50, 100 μg) or phosphate buffered saline (PBS) 0.01 M was injected intrathecally on day 7 after CCI or sham surgery. Paw withdrawal frequency (PWF) to 0.07 g (a and e) and 0.4 g (b and f) von Frey filament stimuli and paw withdrawal latency (PWL) to heat (c and g) and cold (d) stimuli on the ipsilateral (a–d) and contralateral (e–g) sides were measured on days as indicated after CCI or sham surgery. n=8–12 mice/group. ∗∗P<0.01 vs the PBS-treated CCI group at the corresponding days, by three-way ANOVA with repeated measures followed by post hoc Tukey test.
The results were similar in the SNL/sham mice with i.t. administration of ASOs (100 μg) or PBS starting on day 7 after surgery (Supplementary Fig. S1 and Table S3).
Effect of intrathecal administration of NIS-lncRNA antisense oligonucleotides on chemotherapy-induced neuropathic pain
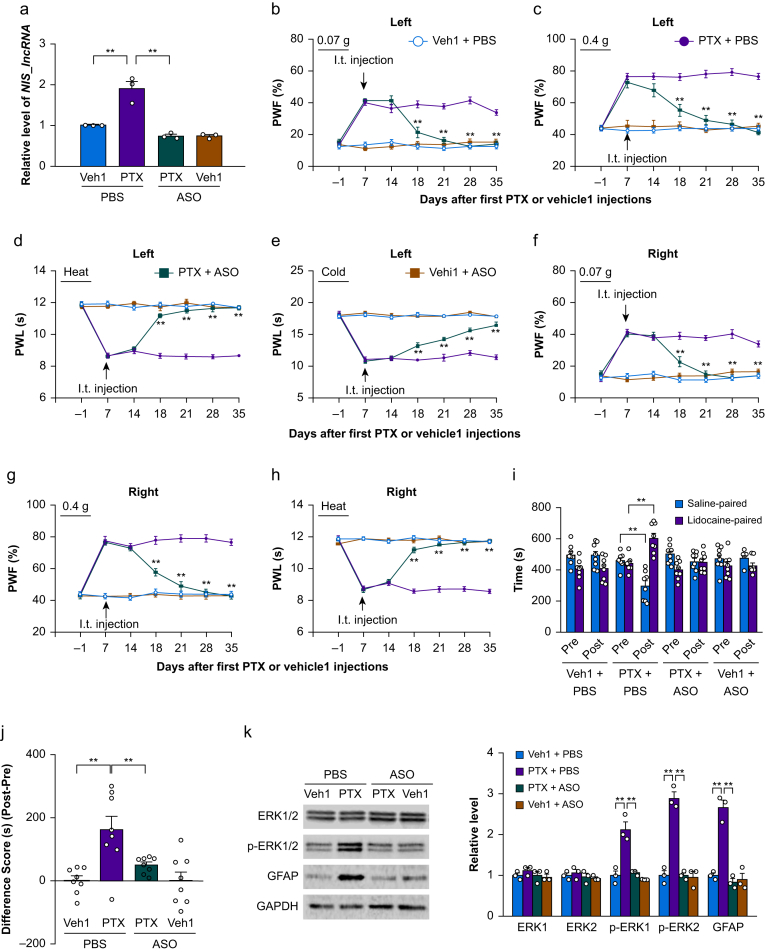
Besides peripheral nerve trauma, chemotherapeutic drugs can also lead to neuropathic pain.23, 24, 25 We examined the effect of i.t. administration of NIS-lncRNA ASOs on PTX-induced nociceptive hypersensitivity. ASOs (100 μg) or PBS was intrathecally administered on day 7 after the first i.p. injection of PTX or vehicle 1. At this time point, PTX-induced nociceptive hypersensitivity was markedly developed.23, 24, 25 Like after either CCI or SNL, the level of NIS-lncRNA was significantly increased by 1.9-fold in the unilateral L3/4 DRG of the PBS plus PTX-treated mice on day 35 after the first i.p. injection of PTX, compared with the PBS plus vehicle 1-treated mice (Fig. 3a). This increase was not found in the ASOs plus PTX-treated mice (Fig. 3a). As expected, the basal level of NIS-lncRNA was not altered in the L3/4 DRG of the ASOs plus vehicle 1-treated mice on day 35 after the first i.p. injection of vehicle 1 on either side (Fig. 3a).
Fig 3.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs) attenuated the paclitaxel (PTX)-induced NIS-lncRNA increase in dorsal root ganglion (DRG) and nociceptive hypersensitivity. ASOs (100 μg) or phosphate buffered saline (PBS) 0.01 M was injected intrathecally on day 7 after PTX or vehicle 1 (Veh1) injection. (a) Level of NIS-lncRNA in the unilateral lumbar 3/4 DRGs on day 35 after the first PTX or vehicle 1 injection in the mice with treatments as indicated. n=3 repeats (6 mice)/group. ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. (b–h) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on paw withdrawal frequency (PWF) to 0.07 g (b and f) and 0.4 g (c and g) von Frey filament stimuli and paw withdrawal latency (PWL) to heat (d and h) and cold (e) stimuli on the left (b–e) and right (f–h) sides on days as indicated after the first PTX or vehicle 1 injection. n=8 mice/group. ∗∗P<0.01 vs the PTX plus PBS-treated group at the corresponding days, by three-way anova with repeated measures followed by post hoc Tukey test. (i and j) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on ongoing nociceptive responses as assessed by the conditioned place preference paradigm on day 28 after the first PTX or vehicle 1 injection. Post, post-conditioning; Pre, preconditioning. n=8 mice/group. ∗∗P<0.01, by three-way anova with repeated measures followed by post hoc Tukey test (i) or two-way anova followed by post hoc Tukey test (j). (k) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on the levels of phosphorylated extracellular signal-regulated kinase 1 and 2 (p-ERK1/2), ERK1/2, and glial fibrillary acidic protein (GFAP) in the unilateral lumbar 3/4 dorsal horn 35 days after the first PTX or vehicle 1 injection. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control. n=3 repeats (6 mice)/group. ∗∗P<0.01, by two-way anova followed by post hoc Tukey test.
Consistent with previous reports,23, 24, 25 mechanical allodynia and heat and cold hyperalgesia were detected on both left and right sides of the PBS plus PTX-treated mice from days 7–35 after the initial i.p. injection of PTX (Fig. 3b–h). The antinociceptive effect of i.t. ASOs at 100 μg on the PTX-induced mechanical allodynia and heat and cold hyperalgesia on both left and right sides occurred on day 18 after the initial i.p. administration of PTX and lasted for at least 17 days (Fig. 3b–h). I.t. injection of neither ASOs nor PBS changed basal responses on either side of ASOs plus vehicle 1-treated mice during the observation period (Fig. 3b–h). All treated/injected mice showed normal locomotor activity (Supplementary Table S3). Moreover, on day 28 after the initial i.p. injection of PTX, i.t. administration of ASOs diminished the PTX-induced, stimulation-independent spontaneous nociceptive responses, as demonstrated by no significant preference for either the saline- or lidocaine-paired chamber in the ASOs plus PTX-treated mice (Fig. 3i and j). In addition, i.t. administration of ASOs abolished hyperactivities of dorsal horn neurones and astrocytes on both sides on day 35 after the initial i.p. injection of PTX, as evidenced by the absence of increases in the levels of p-ERK1/2 and GFAP in the L3/4 dorsal horns in the ASOs plus PTX-treated mice on both sides (Fig. 3k).
Effect of intrathecal administration of NIS-lncRNA antisense oligonucleotides on diabetic neuropathic pain
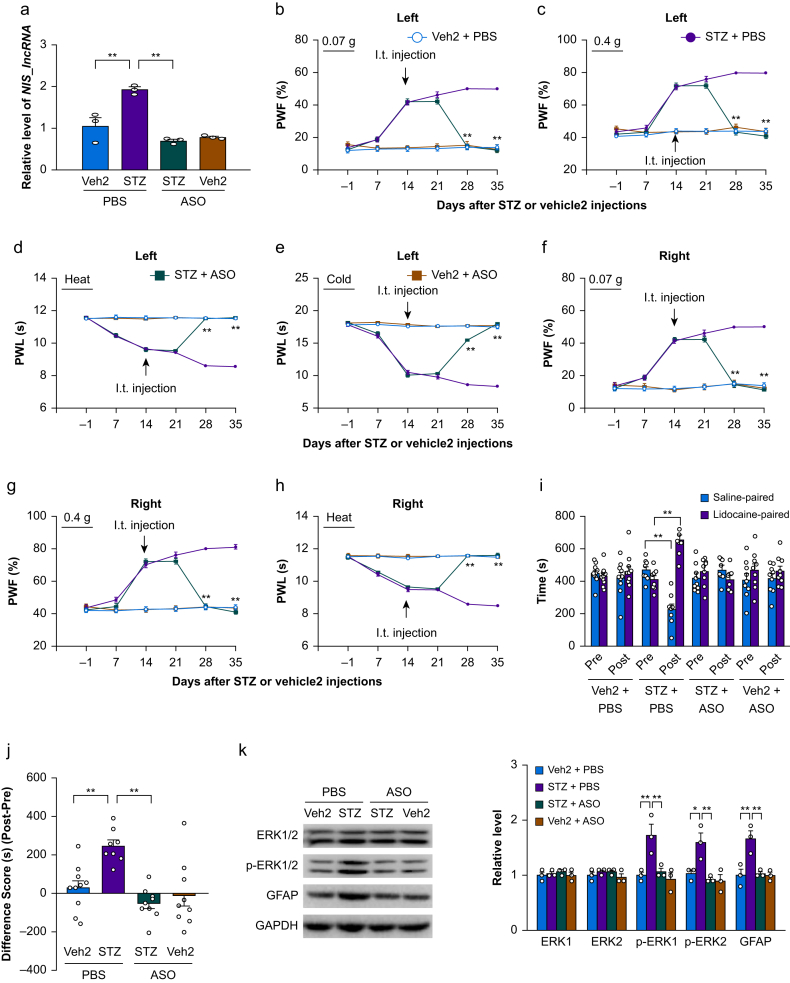
Diabetic neuropathic pain is often seen in clinical practice. Whether i.t. administration of NIS-lncRNA ASOs affected STZ-induced diabetic neuropathic pain was also examined. ASOs (100 μg) or PBS was intrathecally administered 14 days after i.p. injection of STZ or vehicle 2; at this time point, STZ produces significant nociceptive hypersensitivities.35 We found that the amount of NIS-lncRNA was markedly increased by 1.8-fold in the unilateral L3/4 DRG of the PBS plus STZ-treated mice on day 35 after STZ injection, compared with the PBS plus vehicle 2-treated mice (Fig. 4a). However, the ASOs plus STZ-treated mice did not display this increase (Fig. 4a). As expected, the basal level of NIS-lncRNA was not changed in the L3/4 DRG of the ASOs plus vehicle 2-treated mice on day 35 post-vehicle 2 injection on either side (Fig. 4a).
Fig 4.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs) attenuated the streptozotocin (STZ)-induced NIS-lncRNA increase in dorsal root ganglion (DRG) and nociceptive hypersensitivity. ASOs (100 μg) or phosphate buffered saline (PBS) 0.01 M was injected intrathecally on day 14 after STZ or vehicle 2 (Veh2) injection. (a) Level of NIS-lncRNA in the unilateral lumbar 3/4 DRGs on day 35 after STZ or vehicle 2 injection in the mice with treatments as indicated. n=3 repeats (6 mice)/group. ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. (b–h) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on paw withdrawal frequency (PWF) to 0.07 g (b and f) and 0.4 g (c and g) von Frey filament stimuli and paw withdrawal latency (PWL) to heat (d and h) and cold (e) stimuli on the left (b–e) and right (f–h) sides on days as indicated after STZ or vehicle 2 injection. n=8–10 mice/group. ∗∗P<0.01 vs the STZ plus PBS-treated group at the corresponding days, by three-way anova with repeated measures followed by post hoc Tukey test. (i and j) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on ongoing nociceptive responses as assessed by the conditioned place preference paradigm on day 28 after STZ or vehicle 2 injection. Post, post-conditioning; Pre: preconditioning. n=8–10 mice/group. ∗∗P<0.01, by three-way anova with repeated measures followed by post hoc Tukey test (i) or two-way anova followed by post hoc Tukey test (j). (k) Effect of i.t. administration of NIS-lncRNA ASOs or PBS on the levels of phosphorylated extracellular signal-regulated kinase 1 and 2 (p-ERK1/2), ERK1/2, and glial fibrillary acidic protein (GFAP) in the unilateral lumbar 3/4 dorsal horn 35 days after STZ or vehicle 2 injection. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control. n=3 repeats (6 mice)/group. ∗∗P<0.01, by two-way anova followed by post hoc Tukey test.
Similar to previous observations,35 i.p. injection of STZ led to mechanical allodynia and heat and cold hyperalgesia on both left and right sides of the PBS plus STZ-treated mice. These nociceptive hypersensitivities occurred 2 weeks post-STZ injection and persisted for at least 3 weeks (Fig. 4b–h). The 100 μg of ASOs given intrathecally 2 weeks post-STZ injection substantially reduced the mechanical allodynia and heat and cold hyperalgesia on both left and right sides (Fig. 4b–h). These antinociceptive effects occurred 4 weeks post-STZ injection and persisted for at least 1 week (Fig. 4b–h). I.t. injection of neither ASOs nor PBS altered basal responses on either side of vehicle 2 plus ASOs- or PBS-treated mice during the observation period (Fig. 4b–h). All treated/injected mice showed normal locomotor activity (Supplementary Table S3). We also found that i.t. administration of ASOs reduced the STZ-induced and stimulation-independent spontaneous nociceptive responses, as demonstrated by no significant preference for either the saline- or lidocaine-paired chamber in the ASOs plus STZ-treated mice 4 weeks post-STZ injection (Fig. 4i and j). Additionally, i.t. administration of ASOs completely blocked the STZ-induced hyperactivities of dorsal horn neurones and astrocytes, as evidenced by the absence of elevations in the levels of p-ERK1/2 and GFAP in the L3/4 dorsal horns of the ASOs plus STZ-treated mice 5 weeks after STZ injection on both sides (Fig. 4k).
Effect of intrathecal administration of NIS-lncRNA antisense oligonucleotides on CCL2 expression
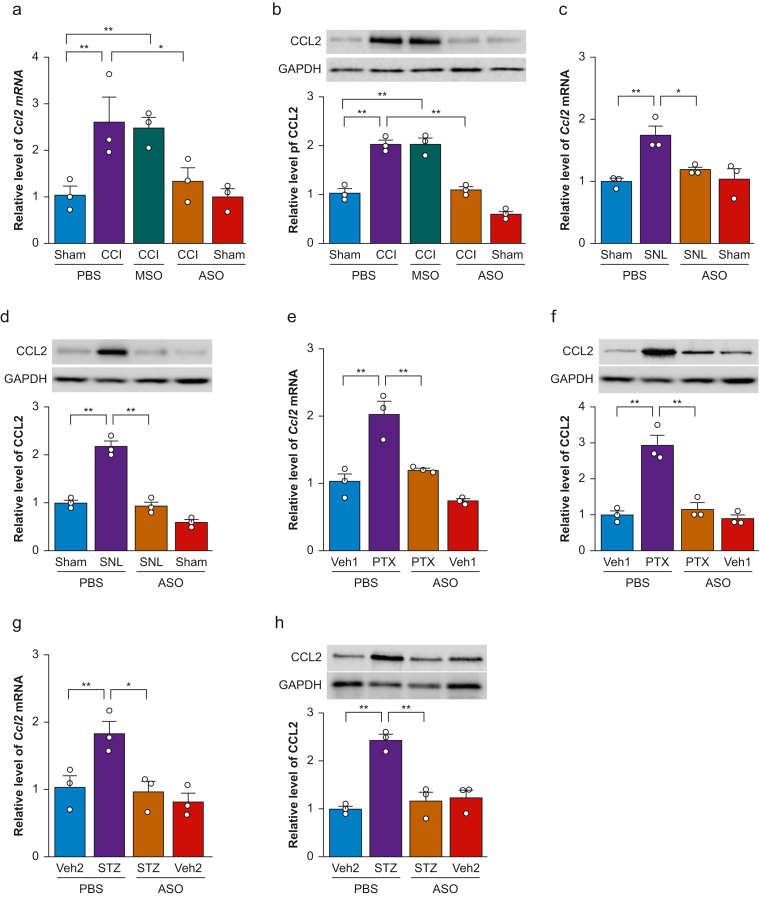
CCL2 is a downstream target of NIS-lncRNA in injured DRG under conditions of nerve trauma-induced neuropathic pain.12 We further examined whether i.t. administration of ASOs affected the CCI- or SNL-induced increases of CCL2 expression in injured DRG. Consistent with the previous study,13 CCI increased the levels of Ccl2 mRNA and CCL2 protein by 2.5-fold and 2.0-fold, respectively, in the ipsilateral L3/4 DRG of the PBS-treated CCI mice on day 35 after CCI, compared with the PBS-treated sham mice (Fig. 5a and b). Similarly, the amounts of Ccl2 mRNA and CCL2 protein were increased by 1.8-fold and 2.2-fold, respectively, in the ipsilateral L4 DRG of the PBS-treated SNL mice on day 35 after SNL, compared with those in the PBS-treated sham mice (Fig. 5c and d). These increases were absent in the ASOs-treated CCI or SNL mice (Fig. 5a–d). As expected, i.t. administration of MSOs did not affect CCI-induced increases in the levels of Ccl2 mRNA and CCL2 protein in the ipsilateral L3/4 DRG on day 35 post-CCI (Fig. 5a and b). Basal levels of Ccl2 mRNA and CCL2 protein were not significantly different in the ipsilateral L3/4 DRG or ipsilateral L4 DRG of the ASO-treated sham mice (Fig. 5a–d).
Fig 5.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs, 100 μg) blocked the increases of C–C chemokine ligand 2 (Ccl2) mRNA and CCL2 protein in dorsal root ganglion (DRG) under conditions of neuropathic pain caused by chronic constriction injury (CCI), spinal nerve ligation (SNL), paclitaxel (PTX) injection, or streptozotocin (STZ) injection. MSO, missense oligonucleotides (100 μg). PBS, phosphate buffered saline 0.01 M. (a and b) Levels of Ccl2 mRNA (a) and CCL2 protein (b) in the ipsilateral lumbar 3/4 DRGs on day 35 after CCI or sham surgery in mice with i.t. injection of ASOs, MSOs, or PBS on day 7 post-CCI or sham surgery. n=3 repeats (6 mice)/group/assay. ∗P<0.05, ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. (c and d) Levels of Ccl2 mRNA (c) and CCL2 protein (d) in the ipsilateral lumbar 4 DRG on day 35 after SNL or sham surgery in mice with i.t. injection of ASOs or PBS on day 7 post-SNL or sham surgery. n=3 repeats (12 mice)/group/assay. ∗P<0.05, ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. (e and f) Levels of Ccl2 mRNA (E) and CCL2 protein (f) in the unilateral lumbar 3/4 DRGs on day 35 after the first PTX or vehicle 1 (Veh1) injection in mice with i.t. injection of ASOs or PBS on day 7 after the first PTX or vehicle 1 injection. n=3 repeats (6 mice)/group/assay. ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. (g and h) Levels of Ccl2 mRNA (g) and CCL2 protein (h) in the unilateral lumbar 3/4 DRGs on day 35 after STZ or vehicle 2 (Veh2) injection in mice with i.t. injection of ASOs or PBS on day 14 after STZ or vehicle 2 injection. n=3 repeats (6 mice)/group/assay. ∗P<0.05, ∗∗P<0.01, by two-way anova followed by post hoc Tukey test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Like after CCI or SNL, i.p. injection of PTX markedly increased the levels of Ccl2 mRNA and CCL2 protein in the L3/4 DRG of the PBS plus PTX-treated mice on day 35 after the first PTX injection on both sides, compared with the PBS plus vehicle 1-treated mice (Fig. 5e and f). Furthermore, i.p. injection of STZ also substantially elevated the amounts of Ccl2 mRNA and CCL2 protein in the L3/4 DRG of the PBS plus STZ-treated mice on day 35 post-STZ on both sides, compared with the PBS plus vehicle 2-treated mice (Fig. 5g and h). These increases/elevations were not observed in the ASOs plus either PTX- or STZ-treated mice (Fig. 5e–h). Basal levels of Ccl2 mRNA and CCL2 protein were not altered in the L3/4 DRG of ASOs plus vehicle 1- or vehicle 2-treated mice on day 35 after the first vehicle 1 or vehicle 2 injection on either side (Fig. 5e–h).
Normal morphologic structure in the DRG and spinal cord after intrathecal administration of NIS-lncRNA antisense oligonucleotides
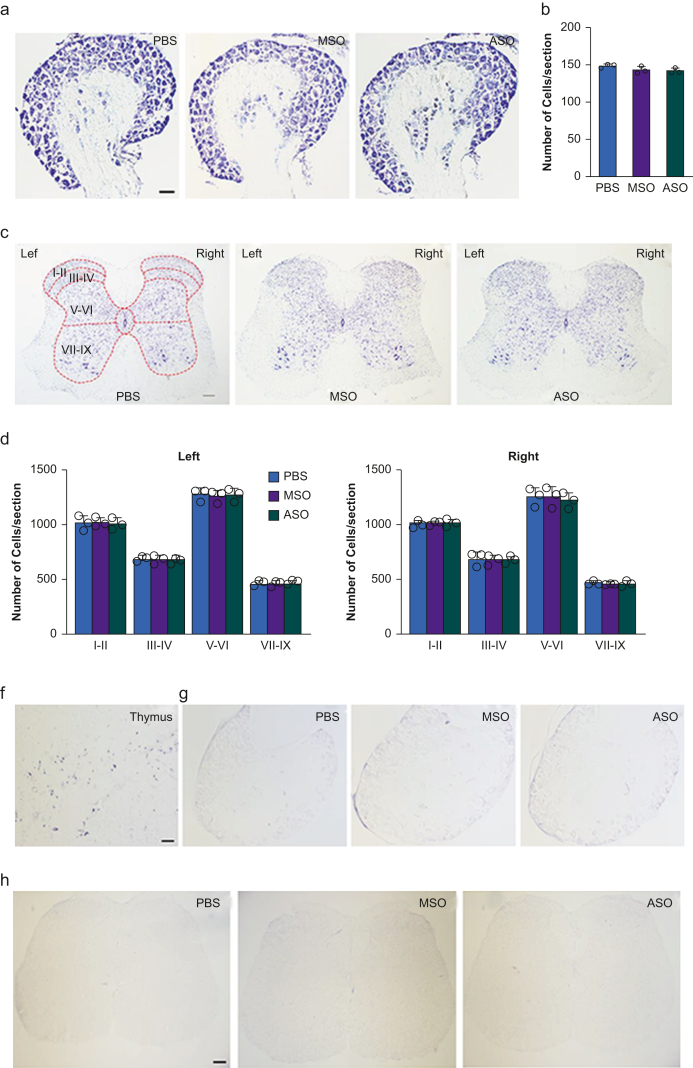
To examine whether i.t. administration of NIS-lncRNA ASOs produced cellular toxicity, we carried out cresyl violet staining32, 33, 34 and observed the morphology in the DRG and spinal cord 30 days after i.t. injection of ASOs, MSOs, or PBS. The neurones from the L3/4 DRG and L3/4 spinal cord did not have any pathologic changes in the ASO-treated mice, compared with PSB- or MSOs-treated mice (Fig. 6a–c). Neuronal cell architecture appeared normal in both regions (Fig. 6a–c). We counted the number of cresyl violet-stained cells in the L3/4 DRG and L3/4 spinal cord among these three treated groups. No significant differences were observed in the number of stained cells of the L3/4 DRG (Fig. 6b) and L3/4 spinal cord (Fig. 6d) among these groups. Furthermore, we examined whether i.t. administration of NIS-lncRNA ASOs led to neuronal damage in the DRG and spinal cord using TUNEL staining. Since, under normal conditions, apoptosis occurs in thymus,33,34 we used thymus as a positive control. As shown in Fig. 6f, many thymus cells were positive for TUNEL. However, no TUNEL-positive cells were detected in either DRG (Fig. 6g) or spinal cord (Fig. 6h) of mice with i.t. injection of PBS, MSOs, or ASOs.
Fig 6.
Intrathecal (i.t.) administration of nerve injury-specific long non-coding RNA (NIS-lncRNA) antisense oligonucleotides (ASOs, 100 μg) did not produce toxicity in dorsal root ganglion (DRG) and spinal cord. MSO, missense oligonucleotides (100 μg). PBS, phosphate buffered saline 0.01 M. (a–d) Cresyl violet staining in the L4 DRGs and L4 spinal cord of normal mice 30 days after i.t. injection of ASOs, MSOs, or PBS. Representative photograph showing cresyl violet stained cells in lumbar 4 DRG (a) and lumbar 4 spinal cord (c). Quantifying average number of cresyl violet-stained cells in lumbar 4 DRG (b) and in laminae I-II, III-IV, V-VI, and VII-IX of L4 spinal cord (d). n=3 mice/group. One-way anova followed by post hoc Tukey test. (f–h) Representative photograph showing TUNEL-positive cells in the thymus (f), lumbar 4 DRG (g), and lumbar 4 spinal cord (h) of naive mice 30 days after i.t. injection of ASOs, MSOs, or PBS. Thymus was used as a positive control. Scale bars: 100 μm for DRG and thymus and 200 μm for spinal cord.
Discussion
The present study demonstrated that i.t. administration of NIS-lncRNA ASOs dose-dependently attenuated CCI-induced evoked nociceptive hypersensitivities and ongoing nociceptive responses. Similar effects were observed in the ASO-treated mice after SNL or after systemic administration of PTX or STZ. I.t. administration of NIS-lncRNA ASOs also prevented the increases in the levels of NIS-lncRNA, Ccl2 mRNA, and CCL2 protein in the ipsilateral L3/4 DRG after CCI, in the ipsilateral L4 DRG after SNL, or in the bilateral L3/4 DRG after systemic injection of PTX or STZ. Finally, i.t. administration of NIS-lncRNA ASOs reduced neuronal and astrocyte hyperactivities in the ipsilateral L3/4 dorsal horn after CCI, in the ipsilateral L4 dorsal horn after SNL, or in the bilateral L3/4 dorsal horn after systemic injection of PTX or STZ. Taken together, these findings further validate a key mechanistic role for DRG NIS-lncRNA in the pathogenesis of peripheral neuropathic pain.
An ASO-based strategy has finally emerged as a viable therapeutic approach to the treatment of neurologic disorders after 30 yr of optimisation.14 ASOs are small, single-stranded synthetic nucleic acids between five and 50 nucleotides in length. ASOs bind to the targeted RNAs via Watson–Crick base paring and selectively suppress targeted RNA expression through RNase H (an endogenous enzyme)-mediated catalytic degradation.36 NIS-lncRNA ASOs and control MSOs designed and synthesised in the present study are 25 nucleotides with the phosphodiester backbone and locked nucleic acid modifications. Modification of the phosphodiester backbone may enable ASOs to be water soluble, resistant to exonucleases, enhanced stability, better cellular uptakes, and the ability to recruit RNase H enzyme for target degradation.36 A locked nucleic acid modification may enable ASOs to have greater potency and support RNase H activity.36 Indeed, our in vivo study showed that i.t. administration of NIS-lncRNA ASOs, but not control MSOs, significantly blocked the increase in DRG NIS-lncRNA and the concomitant nociceptive hypersensitivity caused by CCI, SNL, PTX injection or STZ injection. Given that NIS-lncRNA ASOs did not alter basal or acute pain and locomotor functions, this strongly supports the specificity and selectivity of NIS-lncRNA ASOs. Moreover, a single i.t. administration of NIS-lncRNA ASOs led to a long-lasting (at least 7 days) antinociceptive effect in four neuropathic pain models, which is consistent with other modified ASOs' analgesic effects reported previously.37 Interestingly, i.t. administration of ASOs failed to significantly suppress the basal level of DRG NIS-lncRNA in sham or vehicle-treated mice. The reason why ASOs did not significantly affect basal DRG NIS-lncRNA expression is unknown, but it is very likely that low or undetectable expression of DRG NIS-lncRNA under normal conditions12 cannot be further suppressed significantly by ASOs at the present dosages used. In addition, like DRG microinjection of NIS-lncRNA siRNA in CD1 mice or AAV5-Cre in NIS-lncRNAfl/fl mice,12 i.t. NIS-lncRNA ASOs possibly do not suppress NIS-lncRNA expression in the DRG under normal conditions. We also found that ASOs took about 11–14 days to produce an antinociceptive effect, which is similar to the 14-day latency of other modified ASOs' analgesic effects reported previously.37 Why the ASOs take these days to produce analgesic effects is unknown, but it is possibly that ASO-driven suppressing expression of targeted genes through the RNase H-mediated degradation may take the time. Further studies are required to reduce the latency of their analgesic effects.
Peripheral neuropathic pain can be the result from nerve trauma, chemotherapeutic agents, and some diseases (e.g. diabetes). The mechanisms underlying neuropathic pain may vary with these different aetiologies.38 In the present study, we carried out the CCI and SNL models to mimic the features of trauma-induced neuropathic pain, whereas we administered PTX and STZ intraperitoneally to induce neuropathic pain with chemotherapeutic and diabetic aetiologies, respectively. Consistent with our previous reports,12 the present study further confirmed the role of DRG NIS-lncRNA in nerve trauma-induced neuropathic pain, as evidenced by the blocking effect of i.t. NIS-lncRNA ASOs on CCI- or SNL-induced increases in DRG NIS-lncRNA/CCL2 and nociceptive hypersensitivity. Importantly, we found that i.p. administration of PTX or STZ, like CCI and SNL injury, also increased the expression of DRG NIS-lncRNA and CCL2 and that i.t. administration of NIS-lncRNA ASOs also attenuated this increase and reduced PTX- or STZ-induced nociceptive hypersensitivity. These findings indicate that the NIS-lncRNA-CCL2 signal pathway may be a common molecular mechanism underlying neuropathic pain with different aetiologies.
DRG CCL2 is considered as an endogenous initiator of neuropathic pain. Expression of Ccl2 mRNA and CCL2 protein was increased in injured DRG neurones in several models of neuropathic pain.13 CCL2 directly excited DRG neurones through activation of its preferred C–C motif receptor 2 (CCR2).39 I.t. injection of CCL2 led to mechanical and heat hypersensitivities.40,41 Conversely, i.t. injection of CCL2-neutralising antibodies or CCR2 blockers mitigated nociceptive hypersensitivity in various neuropathic pain models.13,40 CCR2 knockout mice also displayed reduced mechanical allodynia after partial ligation of the sciatic nerve.42 Our previous study demonstrated that nerve injury-induced CCL2 increase was determined at least in part by nerve injury-induced upregulation of NIS-lncRNA in injured DRG, as DRG microinjection of NIS-lncRNA siRNA in CD1 mice or AAV5-Cre in NIS-lncRNAfl/fl mice attenuated the nerve injury-induced CCL2 increase and CCL2-mediated hyperexcitability in injured DRG neurones.12 The present study further demonstrated that i.t. NIS-lncRNA ASOs produced similar effects. Thus, the antinociceptive effect of NIS-lncRNA ASOs is likely attributable to the consequent absence of increased CCL2 expression, the reductions in DRG neuronal hyperexcitability and primary afferent neurotransmitter release, and ultimately mediating decrease in dorsal horn central sensitisation. In line with this conclusion, the present study revealed that i.t. NIS-lncRNA ASOs reduced the hyperactivities of dorsal horn neurones and astrocytes on day 35 after CCI/SNL or PTX/STZ injection.
Conclusions
The present study demonstrated that intrathecal administration of NIS-lncRNA antisense oligonucleotides produced an antinociceptive effect on established neuropathic pain. Antisense oligonucleotides at the dose used did not affect basal/acute pain and locomotor function. Given that antisense oligonucleotides are an FDA-approved approach for the treatment of some neurological diseases,14 our study suggests that NIS-lncRNA antisense oligonucleotides might have utility in the treatment of neuropathic pain in a clinical setting. Nevertheless, attention should be paid to potential unwanted effects on renal or hepatic function, coagulation, and complement or antibody activation,14 although NIS-lncRNA antisense oligonucleotides may not produce the cellular toxicity reported here. Additionally, given that subcutaneous injection of antisense oligonucleotides has the effect on the targeted gene expression in DRG (not in spinal cord),37 further studies are needed to determine whether systemic administration of NIS-lncRNA antisense oligonucleotides can lead to antinociceptive effects on neuropathic pain.
Authors' contributions
Conceived the project and supervised all experiments: YXT.
Designed the project: CHW, TB, XL, SD, YXT.
Generated the animal models and performed behavioural experiments: CHW, TB.
Conducted Western blot experiments: CHW, XL.
Carried out TUNEL and cresyl violet staining: XL.
Did intrathecal injection and quantitative RT-PCR: SD.
Analysed the data: CHW, TB, XL, SD, HZ, AB, YXT.
Wrote the draft of manuscript; CHW, YXT.
Edited the manuscript: SD, YXT.
Read and discussed the manuscript: all authors.
Compliance with ethical standards
All procedures used were approved by the Animal Care and Use Committee at the Rutgers New Jersey Medical School (Newark, NJ).
Declaration of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by grants R01NS111553 to YXT and RF1NS113881 to SD and YXT from the US National Institutes of Health (Bethesda, MD, USA).
Handling editor: Nadine Attal
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.09.027.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gilron I., Baron R., Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor A.B. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Volkow N.D., McLellan A.T. Opioid abuse in chronic pain--misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 4.Brady K.T., McCauley J.L., Back S.E. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am J Psychiatry. 2016;173:18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd R.A., Seth P., David F., Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 6.Finnerup N.B., Attal N., Haroutounian S., et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Qian C., Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16:2075–2083. doi: 10.1111/pme.12845. [DOI] [PubMed] [Google Scholar]
- 8.Wu S., Bono J., Tao Y.X. Long noncoding RNA (lncRNA): a target in neuropathic pain. Expert Opin Ther Targets. 2019;23:15–20. doi: 10.1080/14728222.2019.1550075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X., Tang Z., Zhang H., et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16:1024–1031. doi: 10.1038/nn.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Z., Du S., Wang K., et al. Downregulation of a dorsal root ganglion-specifically enriched long noncoding RNA is required for neuropathic pain by negatively regulating RALY-triggered Ehmt2 expression. Adv Sci. 2021;8:e2004515. doi: 10.1002/advs.202004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen J., Yang Y., Wu S., et al. Long noncoding RNA H19 in the injured dorsal root ganglion contributes to peripheral nerve injury-induced pain hypersensitivity. Transl Perioper Pain Med. 2020;7:176–184. [PMC free article] [PubMed] [Google Scholar]
- 12.Du S., Wu S., Feng X., et al. A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J Clin Invest. 2022;132:e153563. doi: 10.1172/JCI153563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang B.C., Liu T., Gao Y.J. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212 doi: 10.1016/j.pharmthera.2020.107581. [DOI] [PubMed] [Google Scholar]
- 14.Wurster C.D., Ludolph A.C. Antisense oligonucleotides in neurological disorders. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756286418776932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swayze E.E., Siwkowski A.M., Wancewicz E.V., et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang L., Zhao J.Y., Kathryn T., Bekker A., Tao Y.X. BIX01294, a G9a inhibitor, alleviates nerve injury-induced pain hypersensitivities during both development and maintenance periods. Transl Perioper Pain Med. 2019;6:106–114. doi: 10.31480/2330-4871/097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z., Mao Y., Liang L., et al. The transcription factor C/EBPβ in the dorsal root ganglion contributes to peripheral nerve trauma-induced nociceptive hypersensitivity. Sci Signal. 2017;10(487) doi: 10.1126/scisignal.aam5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J., Wen J., Wu S., et al. Contribution of dorsal root ganglion octamer transcription factor 1 to neuropathic pain after peripheral nerve injury. Pain. 2019;160:375–384. doi: 10.1097/j.pain.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Li X., Feng X., et al. E74-like factor 1 contributes to nerve trauma-induced nociceptive hypersensitivity via transcriptionally activating matrix metalloprotein-9 in dorsal root ganglion neurons. Pain Adv Access published on. May 4, 2022 doi: 10.1097/j.pain.0000000000002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L., Han G., Wu S., et al. Toll-like receptor 7 contributes to neuropathic pain by activating NF-kappaB in primary sensory neurons. Brain Behav Immun. 2020;87:840–851. doi: 10.1016/j.bbi.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang L., Wu S., Lin C., Chang Y.J., Tao Y.X. Alternative splicing of Nrcam gene in dorsal root ganglion contributes to neuropathic pain. J Pain. 2020;21:892–904. doi: 10.1016/j.jpain.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Zheng B., Du S., et al. Eukaryotic initiation factor 4 gamma 2 contributes to neuropathic pain through downregulation of Kv1.2 and the mu opioid receptor in mouse primary sensory neurones. Br J Anaesth. 2020;126:706–719. doi: 10.1016/j.bja.2020.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Q., Wu S., Gu X., et al. DNMT3a-triggered downregulation of K2p 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int J Cancer. 2019;145:2122–2134. doi: 10.1002/ijc.32155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W., Liu W., Du S., et al. A Compound mitigates cancer pain and chemotherapy-induced neuropathic pain by dually targeting nNOS-PSD-95 interaction and GABAA receptor. Neurotherapeutics. 2021;18:2436–2448. doi: 10.1007/s13311-021-01158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Wen J., Zheng B., et al. CREB participates in paclitaxel-induced neuropathic pain genesis through transcriptional activation of Dnmt3a in primary sensory neurons. Neurotherapeutics. 2021;18:586–600. doi: 10.1007/s13311-020-00931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J.L., Lu J.H., Xie C.S., et al. Caveolin-1 in spinal cord modulates type-2 diabetic neuropathic pain through the Rac1/NOX2/NR2B signaling pathway. Am J Transl Res. 2020;12:1714–1727. [PMC free article] [PubMed] [Google Scholar]
- 27.Fu G., Du D., Huang T., et al. FTO (fat-mass and obesity-associated protein) participates in hemorrhage-induced thalamic pain by stabilizing toll-like receptor 4 expression in thalamic neurons. Stroke. 2021;52:2393–2403. doi: 10.1161/STROKEAHA.121.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang L.N., Zou Y., Wu S., et al. Fn14 Participates in neuropathic pain through NF-kappaB pathway in primary sensory neurons. Mol Neurobiol. 2019;56:7085–7096. doi: 10.1007/s12035-019-1545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz B.M., Wu S., Gu X., et al. Endothelin type A receptors mediate pain in a mouse model of sickle cell disease. Haematologica. 2018;103:1124–1135. doi: 10.3324/haematol.2017.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Guo X., Sun L., et al. N(6)-methyladenosine demethylase FTO contributes to neuropathic pain by stabilizing G9a expression in primary sensory neurons. Adv Sci (Weinh) 2020;7 doi: 10.1002/advs.201902402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng B.X., Malik A., Xiong M., Bekker A., Tao Y.X. Nerve trauma-caused downregulation of opioid receptors in primary afferent neurons: molecular mechanisms and potential managements. Exp Neurol. 2020;337 doi: 10.1016/j.expneurol.2020.113572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang T., Fu G., Gao J., et al. Fgr contributes to hemorrhage-induced thalamic pain by activating NF-kappaB/ERK1/2 pathways. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liaw W.J., Stephens R.L., Jr., Binns B.C., et al. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Tian X., Zhu H., Du S., et al. Injectable PLGA-coated ropivacaine produces a long-lasting analgesic effect on incisional pain and neuropathic pain. J Pain. 2021;22:180–195. doi: 10.1016/j.jpain.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J.H., Zhang M.B., Wang J.W., et al. Kalirin-7 contributes to type 2 diabetic neuropathic pain via the postsynaptic density-95/N-methyl-D-aspartate receptor 2B-dependent N-methyl-D-aspartate receptor 2B phosphorylation in the spinal cord in rats. Am J Transl Res. 2020;12:4819–4829. [PMC free article] [PubMed] [Google Scholar]
- 36.Schoch K.M., Miller T.M. Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron. 2017;94:1056–1070. doi: 10.1016/j.neuron.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohan A., Fitzsimmons B., Zhao H.T., et al. Antisense oligonucleotides selectively suppress target RNA in nociceptive neurons of the pain system and can ameliorate mechanical pain. Pain. 2018;159:139–149. doi: 10.1097/j.pain.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 38.Kankowski S., Grothe C., Haastert-Talini K. Neuropathic pain: spotlighting anatomy, experimental models, mechanisms, and therapeutic aspects. Eur J Neurosci. 2021;54:4475–4496. doi: 10.1111/ejn.15266. [DOI] [PubMed] [Google Scholar]
- 39.Jung H., Toth P.T., White F.A., Miller R.J. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y.J., Zhang L., Samad O.A., et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Steenwinckel J., Reaux-Le Goazigo A., Pommier B., et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31:5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbadie C., Lindia J.A., Cumiskey A.M., et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.