Abstract
Background
Peripheral surgical trauma can trigger neuroinflammation and ensuing neurological complications, such as delirium. The mechanisms whereby surgery contributes to postoperative neuroinflammation remain unclear and without effective therapies. Here, we developed a microfluidic-assisted blood–brain barrier (BBB) device and tested the effects of omega-3 fatty acids on neuroimmune interactions after orthopaedic surgery.
Methods
A microfluidic-assisted BBB device was established using primary human cells. Tight junction proteins, vascular cell adhesion molecule 1 (VCAM-1), BBB permeability, and astrocytic networks were assessed after stimulation with interleukin (IL)-1β and in the presence or absence of a clinically available omega-3 fatty acid emulsion (Omegaven®; Fresenius Kabi, Bad Homburg, Germany). Mice were treated 1 h before orthopaedic surgery with 10 μl g−1 body weight of omega-3 fatty acid emulsion i.v. or equal volumes of saline. Changes in pericytes, perivascular macrophages, BBB opening, microglial activation, and inattention were evaluated.
Results
Omega-3 fatty acids protected barrier permeability, endothelial tight junctions, and VCAM-1 after exposure to IL-1β in the BBB model. In vivo studies confirmed that omega-3 fatty acid treatment inhibited surgery-induced BBB impairment, microglial activation, and delirium-like behaviour. We identified a novel role for pericyte loss and perivascular macrophage activation in mice after surgery, which were rescued by prophylaxis with i.v. omega-3 fatty acids.
Conclusions
We present a new approach to study neuroimmune interactions relevant to perioperative recovery using a microphysiological BBB platform. Changes in barrier function, including dysregulation of pericytes and perivascular macrophages, provide new targets to reduce postoperative delirium.
Keywords: blood–brain barrier, delirium, microglia, neurovascular unit, omega-3 fatty acid, organ-on-chip, pericyte, perivascular macrophage
Editor's key points.
-
•
Surgical trauma can trigger neuroinflammation and neurological complications, such as delirium.
-
•
A microfluidics-assisted blood–brain barrier (BBB) device was developed using primary human cells to test the effects of interleukin (IL)-1β-induced inflammation and a therapeutic omega-3 fatty acid emulsion.
-
•
Omega-3 fatty acids protected BBB permeability and endothelial tight junctions after exposure to IL-1β in the model and reduced surgery-induced barrier impairment, microglial activation, and delirium-like behaviour in vivo.
-
•
Systemic inflammation-induced changes in BBB function provide new targets to reduce postoperative delirium.
The blood–brain barrier (BBB) is a critical interface that maintains brain homeostasis and regulates neuroimmune interactions between the periphery and the CNS.1 It is part of the neurovascular unit (NVU) and plays key roles in protecting the brain from systemic factors, including pathogens, inflammatory molecules, and immune cells. Systemic inflammation, for example resulting from tissue injury or aseptic surgical trauma, can negatively impact the BBB and brain function, contributing to cognitive deficits.2 Delirium is a classical manifestation of ‘acute brain failure’ and a form of end-organ dysfunction resulting from systemic inflammation.3 Despite its acute course, delirium has devastating consequences, including significant morbidity, mortality, reduced quality of life, and increased healthcare costs.4,5 Neuroinflammation has been posited as a key driver of delirium onset; however, the specific mechanisms whereby systemic inflammation affects the brain are unknown.
We and others are interested in modelling features of delirium after orthopaedic surgery, a common procedure performed to repair traumatic fractures or joint and hip degenerations. Using this surgical model, we have described changes in BBB permeability followed by microglial activation in mice.6, 7, 8 Changes in barrier permeability are not limited to orthopaedic trauma, suggesting that neuroimmune crosstalk may be a critical driver of cognitive deficits, and evidence of BBB dysfunction in patients with delirium is emerging from neuroimaging studies after cardiac surgery.9,10 Biomarkers indicative of BBB breakdown were recently associated with delirium onset and severity, suggesting this barrier may be a critical interface in regulating neuroinflammation and cognitive decline after surgery.11
We have investigated the mechanisms of BBB opening after systemic inflammation by developing a human-derived BBB-on-chip model to characterise neuroimmune interactions. With this platform, we tested the protective effects of a clinically available fish oil emulsion (Omegaven®; Fresenius Kabi) as a prophylactic approach to protect the BBB and rescue delirium-like behaviour in mice. Fish oils have potent immunomodulatory effects via specialised pro-resolving mediators (SPMs) that are biosynthesised from omega-3 fatty acids.12 We previously showed that maresin-1, a macrophage-specific SPM, improved postoperative neurocognitive disorders by limiting monocytic infiltration into the brain after surgery.6 Here, we show that a clinically available fish oil emulsion rescues BBB-associated cellular damage and protects barrier function in a microfluidic-assisted BBB device with primary human cells after systemic inflammation. We also present in vivo evidence for involvement of a novel cell type, perivascular macrophages (PVMs) and pericytes, in the pathogenesis of postoperative neuroinflammation and a putative target to treat postoperative delirium.
Methods
See Supplementary materials for a detailed description of the methods.
Microphysiological BBB model
A microfluidic-assisted BBB device was designed with two layers of polydimethylsiloxane (SYLGARD™ 184; Dow Corning Corp., Midland, MI, USA) separated by polyester track etched membrane. The device was fabricated by soft lithography and assembled using plasma bonding of different layers.
Cell culture and experiment design
Human brain microvascular endothelial cells (hBMECs; ACBRI 376; Cell Systems, Kirkland, WA, USA), human brain vascular pericytes (hBVPs; 1200; ScienCell, Carlsbad, CA, USA), and human astrocytes (hAs; N7805100; Thermo Fisher, Waltham, MA, USA) were used to assemble a microphysiological BBB platform (see Supplementary materials). For omega-3 fatty acid experimental groups, the device was treated with 0.18% omega-3 fatty acids for 1 h before medium perfusion. For interleukin (IL)-1β experiment groups, IL-1β 10 ng ml−1 was added into the perfusion medium circulating the vascular compartment. Concentrations were determined by preliminary studies on the device.
Immunofluorescence and cell imaging
Cellular changes in the microphysiological BBB model were analysed by immunofluorescence (antibodies are listed in Supplementary Table 1) and high-speed confocal microscopy (Dragonfly Spinning Disk Confocal Microscope; Andor, Belfast, UK).
Trans-endothelial electrical resistance (TEER) measurements
Trans-endothelial electrical resistance (TEER) was measured using a custom-made Arduino-based device as a function of time before and after the treatments.
Animals
Inbred C57B/L6J and Cx3cr1GFP mice (males and females; 12–16 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were fed standard rodent chow (Prolab® RMH3500, Autoclavable; LabDiet, St Louis, MO, USA) and had ad libitum access to food and water. All experiments were conducted under an approved protocol from the Institutional Animal Care and Use Committee at Duke University Medical Center under guidelines described by the US National Science Foundation. Duke University is an AAALAC-certified institution.
Surgery and treatment
Mice were randomly assigned to three groups: control, surgery, and surgery with omega-3 fatty acid pretreatment. Omega-3 fatty acids (fish oil emulsion 10%, as Omegaven®; the qualitative and quantitative compositions of the emulsion are shown in Supplementary materials) or saline (as placebo) was administered 1 h before orthopaedic surgery at 10 μl g−1 (1 g kg−1) through tail vein injection. Orthopaedic surgery was performed on the left hind leg under aseptic conditions as described.6
Gene expression
At 24 h after surgery, the hippocampus was isolated from the rest of the brain to evaluate gene expression of neuroinflammatory markers with the NanoString nCounter®, Seattle, WA, USA.
Immunofluorescence
The mice were anaesthetised under deep isoflurane, and brains were harvested for immunofluorescence staining (antibodies are listed in Supplementary Table 1). Dextran diffusion through the BBB was evaluated by tetramethylrhodamine 70 kD dextran tracer (Thermo Fisher).
Five-choice serial reaction time task
The mice were tested for inattention in the Med Associates (St Albans, VT, USA) five-hole nose poke test chamber, as described.13 In brief, the mice were restricted to food to maintain 90% of free-feed weight and acclimatised to the chocolate sucrose pellets before the test. The mice were then trained to nose poke for food in the test chamber until reaching the criterion of receiving food rewards within a 0.5 s stimulus duration on at least 80% of daily trials for three consecutive days. After training, orthopaedic surgery was performed on the mice with omega-3 fatty acids or placebo treatment. The five-choice serial reaction time task (5-CSRTT) test was conducted daily for four consecutive postoperative days to determine the percentage of trials during each session where the mouse responded with a nose-poke, the daily percentage of correct nose-poke responses, and the daily average latency of the mouse to respond on test trials.
Statistical analyses
GraphPad version 9.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Multiple comparisons between groups were assessed by one-way analysis of variance followed by Bonferroni post hoc test, and data are expressed as mean (standard deviation). For biomarker endpoints, a power analysis was performed based on the current and previous findings of microglia activation. We achieved effect size f at 1.14; thus, n=12 for the three groups (n=4 per group) will yield power at 0.8. For the data presented as n=4 per group, the results were validated in two batches of experiments. Behaviour data were analysed with SPSS 28 (IBM SPSS Statistics, Chicago, IL, USA) using repeated measures analysis of variance. A posteriori tests to assess differences between treatment groups at different time points after the detection of significant effects of treatment or time by treatment interactions were assessed with Bonferroni corrected pairwise comparisons. Levene's test of equality of error variances was used to assess normality of distributions; unless noted, distributions were homoscedastic. The probability for significance was defined as P<0.05.
Results
Neuroinflammation in a human microphysiological BBB model
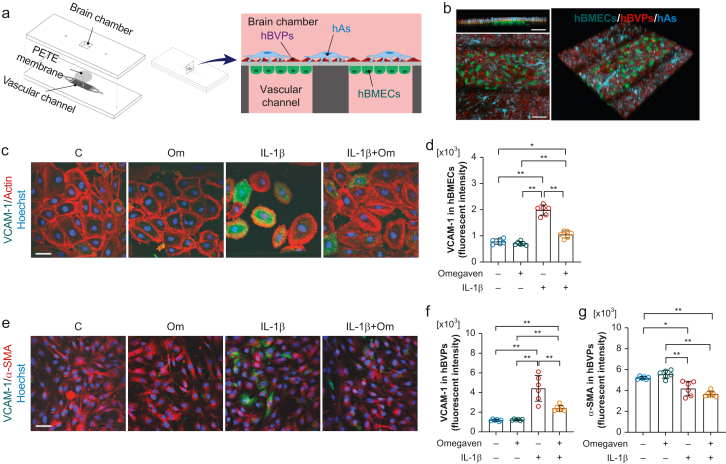
We established a BBB-on-chip model using primary human cells to characterise neuroimmune interactions after systemic inflammation (Fig 1a and b). Changes to key cellular targets that comprise the BBB were assessed after stimulation with IL-1β, a key circulating pro-inflammatory cytokine induced by surgical trauma.2,7 We applied a clinically relevant omega-3 fatty acid emulsion using the microfluidic-assisted device to test the efficacy in maintaining BBB integrity. IL-1β stimulation increased vascular cell adhesion molecule 1 (VCAM-1) expression both in hBMECs and hBVPs (P<0.001 vs control, respectively; n=6 per group; Fig. 1c–f). In addition, IL-1β reduced alpha-smooth muscle actin (α-SMA) in hBVPs (P=0.002 vs control; n=6 per group; Fig. 1e and g), suggesting impairment in pericytes, as described, after chronic inflammation.14 The omega-3 fatty acids eliminated the IL-1β-induced VCAM-1 overexpression both in hBMECs (P<0.001 vs IL-1β; Fig 1c and d) and hBVPs (P=0.0002 vs IL-1β; Fig 1e and f). Treatment did not affect the α-SMA reduction in pericytes (P=0.17 vs IL-1β; Fig. 1e and g).
Fig 1.
Blood–brain barrier (BBB)-on-chip model and interleukin (IL)-1β induced cell activation. (a) Illustration of the microphysiological platform for brain cells co-culture to generate model BBB tissue. The BBB-on-chip device consists of two layers of polydimethylsiloxane substrate separated by the polyester track etched (PETE) membrane, which acts as a basal membrane. The bottom layer has a vascular channel dividing into eight branches of 300 μm width each to culture human brain microvascular endothelial cells (hBMECs), and the top layer has a 5 mm × 5 mm square well that forms the brain chamber. The brain chamber is located on top of the vascular channels to co-culture brain cells (human brain vascular pericytes [hBVPs] and human astrocytes [hAs]) with a brain microvascular endothelial cells separated by the PETE membrane. (b) Representative fluorescence image of live brain microvascular endothelial cells, brain vascular pericytes, and human astrocytes in the BBB device. In counterclockwise order, each image represents a cross-sectional view, top view, 3D view, respectively (scale bar: 100 μm; green: hBMECs; red: hBVPs; cyan: hAs). Omega-3 fatty acids inhibit IL-1β-induced vascular cell adhesion molecule 1 (VCAM-1) overexpression in both (c and d) hBMECs (e and f) and hBVPs (e and g). IL-1β reduced alpha-smooth muscle actin (α-SMA) expression in hBVPs, which was not affected by omega-3 fatty acid treatment. Data expressed as mean (standard deviation); ∗P<0.01; ∗∗P<0.001; n=6 per group. All experiments were repeated three times. C, control; hAs, human astrocytes; hBMECs, human brain microvascular endothelial cells; hBVPs, human brain vascular pericytes; Om, omega-3 fatty acids.
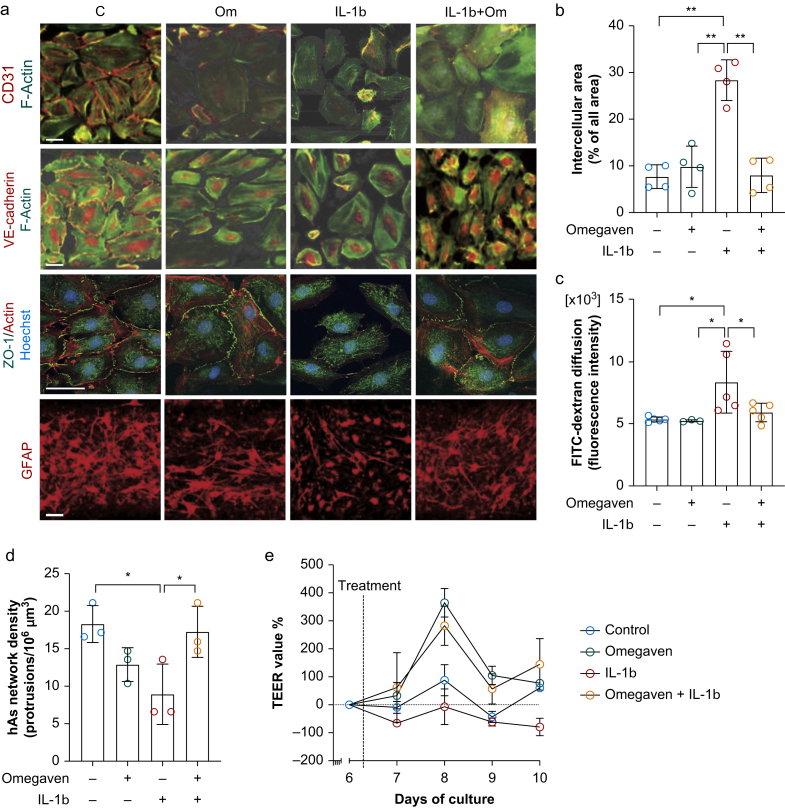
Next, we interrogated barrier integrity and found that cell–cell tightness between hBMECs was impaired by IL-1β exposure as evaluated by the percentage of intercellular areas in vascular endothelial cadherin-stained images (7.7 [2.5]% in control vs 28.4 [4.4]% in IL-1β; P<0.001; n=4 per group). Omega-3 fatty acids inhibited IL-1β-induced intercellular gaps (8.0 [3.7]%; P<0.001 vs IL-1β; n=4; Fig 2a and b). These findings were validated by the analysis of zonula occludens-1 in hBMECs (Fig 2a). Changes in BBB permeability were confirmed by assessing diffusion of fluorescein isothiocyanate (FITC)-labelled dextran (70 kDa) across the chambers. The fluorescence intensity of FITC in the brain chamber increased after IL-1β stimulation (5.3 [0.2] in control vs 8.3 [2.5] in IL-1β; P=0.004; n=5 per group). This effect was abolished by omega-3 fatty acid treatment (5.9 [0.7]; P=0.02 vs IL-1β; n=5; Fig 2c). BBB permeability was not affected by omega-3 fatty acid treatment alone (Fig. 2a–c). The network of hAs protrusion density, as visualised by glial fibrillary acidic protein (GFAP) staining, was reduced by IL-1β (18.3 [2.5] in control vs 8.9 [4] protrusions in 106 μm3 in IL-1β; P=0.03; n=3 per group; Fig. 2a and d). In contrast, pretreatment with omega-3 fatty acids rescued disruption of the hAs network density caused by IL-1β (17.3 [3.4] protrusions in 106 μm3; P=0.046 vs IL-1β; Fig. 2a and d). BBB integrity was also assessed by daily TEER values. The experimental groups treated with IL-1β showed consistently lower TEER values compared with the pretreatment level, whereas other groups had values that were unchanged or higher than pretreatment values over the course of the experiment (Fig 2e). Together, these findings in the BBB-on-chip model suggest a protective effect of omega-3 fatty acids in inhibiting inflammation in BBB-associated endothelial cells and pericytes, maintaining BBB homeostasis in response to an immune stressor.
Fig 2.
Omega-3 fatty acids inhibit interleukin (IL)-1β-induced blood–brain barrier (BBB) permeability dysfunction by increasing cell–cell tightness. (a) Representative images of immunofluorescence staining of CD31, vascular endothelial cadherin, ZO-1, and GFAP (scale bar: 100 μm). (b) Quantification of cell–cell tightness by measuring the percentage of intercellular area. Omega-3 fatty acids abolished IL-1β-induced intercellular area increase. (c) 70 kDa fluorescein isothiocyanate (FITC)–dextran diffusion across the BBB was significantly increased in response to IL-1β. This effect is prevented by omega-3 fatty acid pretreatment. (d) IL-1β induced significant reduction in the network density of astrocyte protrusions, which is rescued by omega-3 fatty acid pretreatment. (e) Relative changes in trans-endothelial electrical resistance (TEER) values compared with pretreatment values as a function of time for different experimental conditions. Data expressed as mean (standard deviation); ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001; n=4 per group for Panel B; n=3–5 per group for Panel C; n=3 per group for Panel D; n=2 per group for panel e. All experiments were repeated three times. C, control; GFAP, glial fibrillary acidic protein; hAs, human astrocytes; hBMECs, human brain microvascular endothelial cells; hBVPs, human brain vascular pericytes; Om, omega-3 fatty acids; ZO-1, zonula occludens-1.
Omega-3 fatty acids protect BBB integrity
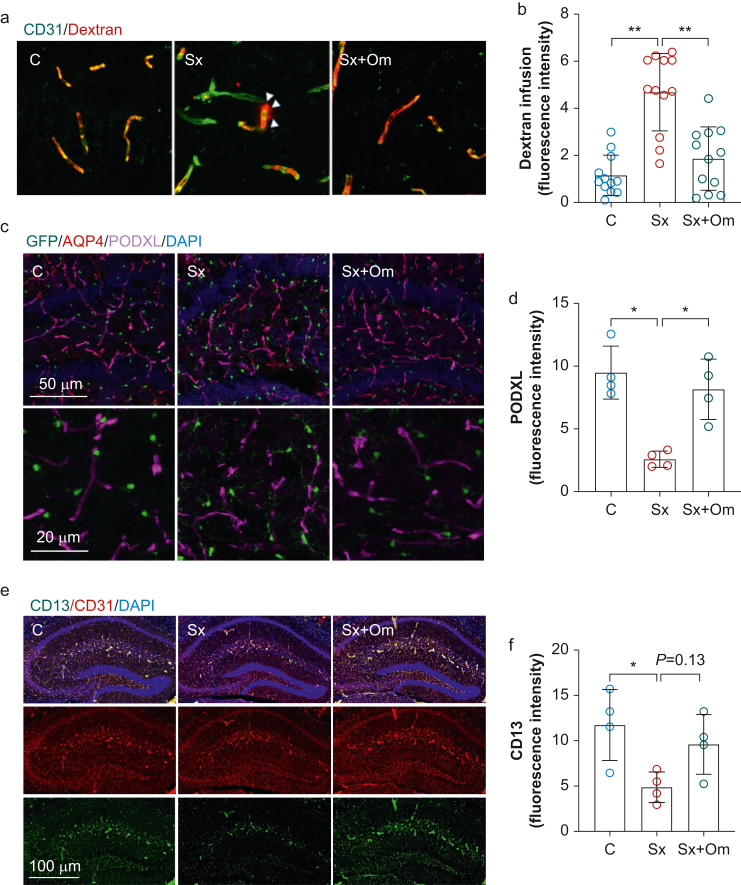
We evaluated changes in BBB opening using an established mouse model of postoperative neuroinflammation and delirium-like behaviour after orthopaedic trauma. At 24 h after surgery, we measured an increase in 70 kD dextran diffusion into the hippocampus (diffused dextran fluorescence intensity: 1.2 [0.9] in control vs 4.7 [1.6] in surgery [Sx]; P<0.001). This dextran diffusion was abolished by omega-3 fatty acid treatment (fluorescence intensity: 1.9 [1.3]; P<0.001 vs Sx; n=12 per group (three areas per mouse; four mice per group); Fig 3a and b). BBB permeability alterations after surgery and omega-3 fatty acid treatment were validated by peripheral myeloid cell infiltration in the hippocampus, as indicated by Iba1+TMEM119– cells (Supplementary Fig. S1). Recent work has shown a role for podocalyxin (PODXL) in maintaining endothelial barrier function, thus regulating BBB integrity during acute inflammation.15 We found reduced PODXL expression in the hippocampus of C57BL6 mice after surgery compared with controls at 24 h (fluorescence intensity in control: 9.5 [2.1]; Sx: 2.6 [0.6]; P=0.002; n=4 per group). Pretreating mice with omega-3 fatty acids (1 g kg−1) 1 h before surgery prevented the surgery-induced PODXL reduction (fluorescence intensity: 8.2 [2.4]; P=0.006 vs Sx; n=4; Fig 3c and d).
Fig 3.
Omega-3 fatty acids prevent surgery-induced blood–brain barrier disruption. Surgery induced significant dextran diffusion (a and b), a reduction of PODXL (c and d), and reduction of a pericyte marker (CD13) into the hippocampus (e and f). These changes were prevented by omega-3 fatty acid pretreatment. Data expressed as mean (standard deviation); ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001; n=4 per group (for panel b, the quantification was performed in three different regions in the hippocampus per mouse). AQP4, aquaporin 4; C, control; DAPI, 4′,6-diamidino-2-phenylindole; PODXL, podocalyxin; Sx, surgery; Sx+Om, surgery+omega-3 fatty acids.
In addition to endothelial cells, pericytes play a critical role in the regulation of the BBB function.16 To evaluate if orthopaedic surgery influenced pericytes, we assessed expression of CD13 in the hippocampus (Supplementary Fig. S2).17 Surgery significantly reduced hippocampal CD13 expression compared with control mice (fluorescence intensity in control: 11.7 [3.9]; Sx: 4.9 [1.7]; P=0.03; n=4 per group). Notably, pretreatment with omega-3 fatty acids effectively rescued CD13 expression at 24 h (fluorescence intensity: 9.6 [3.3]; P=0.13 vs Sx; n=4; Fig 3e and f). Taken together, these data suggest that omega-3 fatty acids contribute to maintenance of BBB integrity after surgery.
Orthopaedic surgery activates perivascular macrophages in the hippocampus
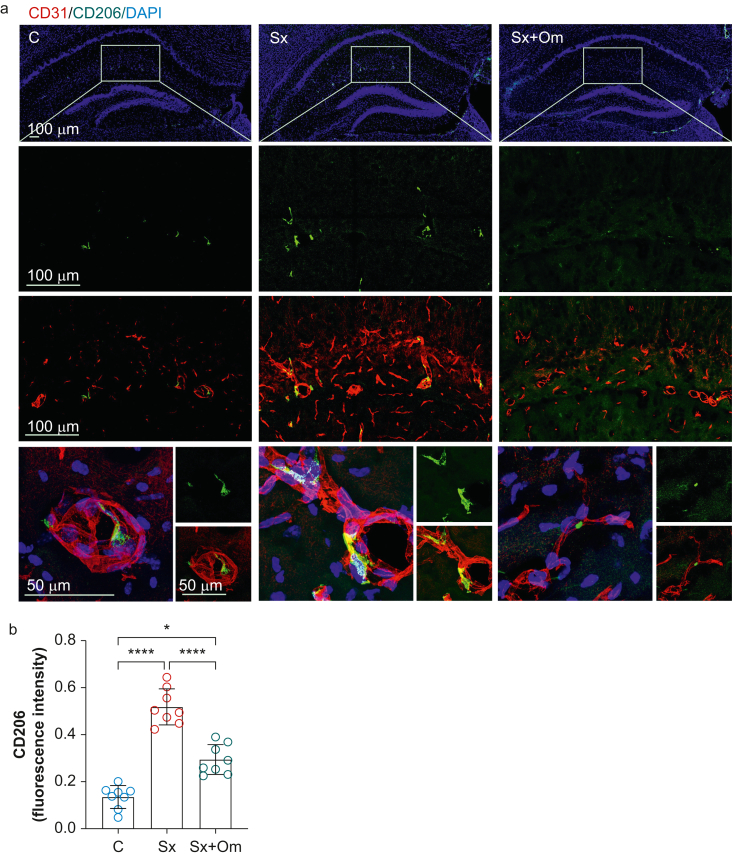
Perivascular macrophages are located inside the basement membrane and are a prominent component of the BBB and CNS microvasculature.18 We detected an increase in PVMs (CD206+ macrophages) in CD11b+ myeloid cells using flow cytometry in hippocampal homogenate (Supplementary Fig. S3). Although this technique provides a sensitive quantitative read-out, we performed immunohistochemistry to define PVM activation and the effects of omega-3 fatty acids. A similar induction of PVM intensity was observed in hippocampus 24 h after surgery, as measured by CD206 immunoreactivity (CD206 intensity: 0.14 [0.05] in control vs 0.52 [0.08] in Sx; P<0.0001; n=8 per group; Fig 4); this was rescued by omega-3 fatty acids pretreatment (0.29 [0.06]; P=0.0002 vs Sx; n=8; Fig 4). Together, these data highlight a new role for pericytes and PVM dysregulation after systemic trauma as putative targets for intervention in preventing BBB disruption.
Fig 4.
Omega-3 fatty acids inhibit surgery-induced perivascular macrophage activation. (a) Representative images of the immunofluorescence staining of a perivascular macrovascular marker, CD206, in the hippocampus. (b) Surgery enhanced expression of CD206 in the hippocampus, which was blocked by omega-3 fatty acid pretreatment. Data expressed as mean (standard deviation); ∗P<0.001; n=8 per group. C, control; DAPI, 4′,6-diamidino-2-phenylindole; Sx, surgery; Sx+Om, surgery+omega-3 fatty acids.
Modulation of microglial activation and delirium-like behaviour
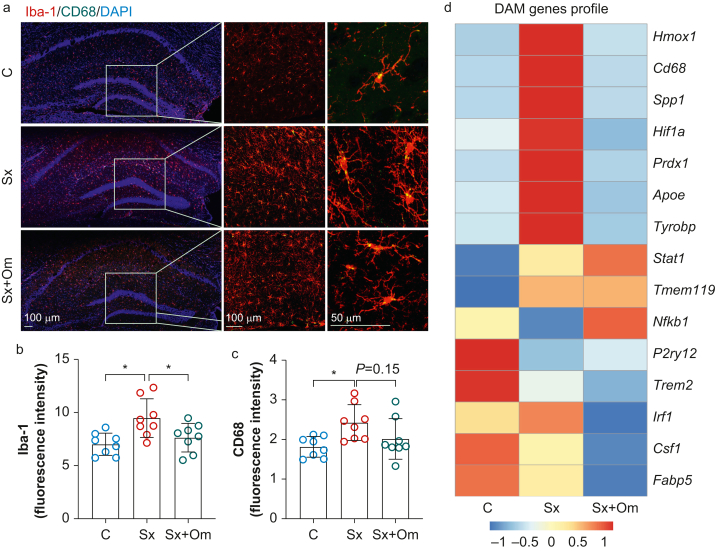
Consistent with prior work, surgery induced significant morphological changes in the expression of ionised calcium binding adaptor molecule-1 (Iba-1) (fluorescence intensity: 7 [1] vs 9.5 [1.8]; P=0.007; n=8 per group; Fig 5a and b) and CD68 (fluorescence intensity: 1.8 [0.3] vs 2.4 [0.5]; P=0.02; n=8 per group; Fig. 5a and c) in the hippocampus at 24 h, indicating activation of microglial cells. Pretreatment with omega-3 fatty acid (10 μl g−1) inhibited surgery-induced neuroinflammation (Iba-1 intensity: 7.6 [1.3]; P=0.046 vs Sx; CD68 intensity: 2 [0.5]; P=0.15 vs Sx; n=8 per group). We observed close contacts between microglia (Iba1+ cells) and microvasculature (labelled by CD31) after surgery (Supplementary Fig. S4). These contacts were less evident in mice treated with omega-3 fatty acids, suggesting that pre-emptive treatment can protect the CNS from surgery-induced BBB opening, although this could also be exerted by downregulating pro-inflammatory cytokines in the blood. This effect was reduced by omega-3 fatty acid pretreatment (2.6 [0.6]; P<0.001 vs Sx; n=4). To confirm functional changes in neuroinflammation after surgery, we performed NanoString gene expression analysis from hippocampal lysate. Multiple disease-associated microglia (DAM) signatures (Hmox1, CD68, Spp1, Hif1a, Prdx1, Apoe, and Tyrobp) that have been described in the context of neurodegenerative conditions19,20 were enhanced 24 h after surgery but had similar levels as controls in the omega-3 fatty acid treatment group (Fig 5d).
Fig 5.
Omega-3 fatty acids reduce surgery-induced microglia activation. (a) Representative images of immunofluorescence staining of microglia (Iba-1) and CD68 in the hippocampus. Surgery-induced microgliosis, with higher expression of Iba-1 (b) and CD68 (c) in the hippocampus. These effects were reduced by omega-3 fatty acids. (d) NanoString gene expression of hippocampal lysate shows changes in multiple microglia activation-associated genes, which were in part restored by omega-3 fatty acid treatment. Data expressed as mean (standard deviation); ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001; n=4 or 8 per group. C, control; DAM, disease-associated microglia; DAPI, 4′,6-diamidino-2-phenylindole; Iba-1, ionised calcium binding adaptor molecule-1; Sx, surgery; Sx+Om, surgery+omega-3 fatty acids.
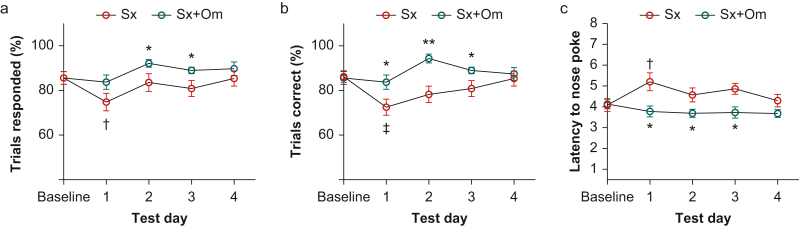
To evaluate how changes in neuroinflammation impact behaviour, we evaluated attention using the 5-CSRTT to model one feature for delirium diagnosis in humans. We found that mice treated with omega-3 fatty acids responded to more test trials on postoperative Days 2 and 3 (P=0.028–0.050) compared with placebo-treated mice (Fig 6a). Omega-3 fatty acid-treated mice showed higher accuracy on testing Days 1 (P<0.036), 2 (P<0.001), and 3 (P<0.050). This effect was primarily driven by a loss of accuracy in the first 24 h in placebo-treated animals after surgery relative to baseline (P<0.002; Fig 6b). As shown in Fig 6c, latency to nose poke increased from baseline to the first test day (P<0.018) in placebo-treated mice and remained prolonged compared with omega-3 fatty acid-treated mice on postoperative Days 1 (P<0.012), 2 (P<0.008), and 3 (P<0.038). These results indicate that omega-3 fatty acids protect against the surgery-induced deficit in attention, especially within the first 24 h of surgery.
Fig 6.
Postoperative inattention behaviour was prevented by omega-3 fatty acid pretreatment. Omega-3 fatty acid-treated mice showed better attention function after surgery in the five-choice serial reaction time task test as evaluated by a higher percentage of responded trials (a), more correct responses in all responded trials (b), and shorter response latency compared with placebo-treated mice (c). ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001 vs Sx on the same testing day; †P<0.05; ‡P<0.01 vs Sx baseline; n=9 per group. Sx, surgery; Sx+Om, surgery+omega-3 fatty acids.
Discussion
We developed a BBB-on-chip microphysiological model using primary human cells to interrogate immune-vascular changes of relevance to the pathogenesis of postoperative neurocognitive disorders. This platform can be broadly applied to screen for multiple perioperative stressors and therapeutics, as shown here using IL-1β as a candidate pro-inflammatory cytokine together with a clinically available therapeutic pro-resolving lipid emulsion. We also identified pericytes and PVMs as new putative cellular targets for postoperative inflammation and ensuing inattentive behaviour. This may have significant therapeutic implications for prevention of postoperative BBB opening, neuroinflammation, and behavioural deficits.
The BBB tightly regulates the neuronal microenvironment,1 but it is impaired in multiple neurological conditions ranging from stroke to dementia.21,22 BBB dysfunction is often identified by loss of tight junctions, alterations in transport properties, and changes in adhesion molecules that can lead to influx of systemic factors and immune cells into brain parenchyma.23,24 In this mouse model, we have shown that orthopaedic surgery leads to a loss of claudin-5 expression in the hippocampus6 and accumulation of systemic markers, such as fibrinogen and immunoglobulin G in the brain parenchyma.8 Whether postoperative neuroinflammation is solely or largely driven by systemic factors remains unclear. Using primary human cells to model neuroimmune interactions in the BBB-on-chip we found that systemic IL-1β induces VCAM-1 overexpression in both hBMECs and PVMs. IL-1β also reduced α-SMA in pericytes, another biomarker linked to chronic neuroinflammation,14 and impaired permeability of the microphysiological BBB as evaluated by increased inter-hBMEC area, FITC–dextran diffusion, and reduction of network density of hA protrusion. These findings provide new insights into the role of systemic inflammation on the BBB and how microphysiological devices can be applied to probe for perioperative stress responses and resiliency factors.
Cytokines can exert powerful effects on the brain, which can become maladaptive in the context of several neurological disorders.25 IL-1β is a prototypical cytokine that stimulates neuroinflammation in mice after orthopaedic surgery.7 It is upregulated in blood and CSF of patients with delirium, making it a predictive biomarker for neuroinflammation in the clinic.26 Excessive IL-1β levels in the CNS can directly contribute to cognitive decline and neuronal loss, which are reversable by IL-1 receptor antagonist.27,28 Future work will evaluate the impact of other cytokines and perioperative blood on the BBB. Indeed, blood-borne factors together with the pro-inflammatory milieu can negatively affect memory processes by impairing neurogenesis during ageing through affecting the BBB.29,30 Recent clinical studies in patients with delirium also suggest that BBB disruption may be a key contributor to postoperative neuroinflammation using both circulating biomarkers and neuroimaging approaches.10,11,31, 32, 33 Modulating the inflammatory response to surgery with omega-3 fatty acids may curtail the impact of soluble factors on the BBB and resolve delirium-like behaviour.
The BBB integrity is determined by multiple cell types in the NVU, including PVMs and pericytes. PVMs are key components of CNS microvasculature and integral to immune surveillance and BBB integrity.18 Brain PVMs are distinguished from other resident macrophages by their proximity to blood vessels and their expression of certain markers, including CD206.34 We showed that PVMs are critical in the response to surgery-induced systemic inflammation and may drive BBB breakdown in postoperative delirium. PVMs play a key role in cognitive decline after BBB breakdown in models of angiotensin II-induced hypertension,35,36 thus representing an attractive therapeutic targeted for a number of diseases.37 This study suggests a possible role for these cells in mediating vascular–immune interactions and onset of postoperative neuroinflammation. We also describe a novel protective effect of fish oil emulsion in preventing PVM activation and pericyte loss after surgery. The anti-inflammatory and anti-oxidant effects of omega-3 fatty acids have been demonstrated in peripheral inflammatory diseases, although their role in regulating CNS inflammation is poorly defined. It is possible that some of these effects are mediated in part by SPMs derived from omega-3 fatty acids.12 SPMs exert potent effects without evidence of immunosuppression or notable side-effects in preclinical models of postoperative neuroinflammation and pain.6,12 These markers are dynamically regulated in the CSF of older adults after surgery, including orthopaedic, and may have future implications for predicting vulnerable subjects prone to delirium or other complications.6,38 Further studies are warranted to characterise the role of lipid mediators on the BBB and their impact on barrier-associated immune cells.
There is a growing appreciation for vascular contributors to cognitive impairment, which may be relevant to the pathogenesis of delirium and postoperative neurocognitive disorders. Recently, we described a key role for the NVU in onset of delirium superimposed on dementia using an Alzheimer's disease-like mouse model. The vascular interface between the periphery and the CNS was negatively affected by surgery and involved in the onset of postoperative cognitive deficits.39 BBB opening and changes in pericytes and PVMs have been described in Alzheimer's disease.40,41 In fact, PVMs may also contribute to microglial activation as breaching of the BBB can trigger neuroinflammation and neurodegeneration. The response of microglia to surgery has been documented in several preclinical models and more recently by detecting soluble fragment of triggering receptor expressed on myeloid cells 2 (sTREM2) in the CSF of delirious subjects after hip fracture surgery.42 TREM2 is crucial in maintaining microglial homeostasis and progressing into DAM in response to stressors. Variation in TREM2 is a well-established risk factor in the development of late-onset Alzheimer's disease.43 We have found that surgery affects DAM genes, such as CD68, Spp1, Apoe, and Tyrobp. TREM2 expression was downregulated after surgery compared with naive controls, suggesting microglia can acquire a DAM-like profile after acute sterile trauma. The data presented here motivate future investigations into the mechanisms linking inflammation to changes in vascular and glia function after surgery, including the role of specific border-associated cell types. In this regard, our work provides new evidence for the implementation of organ-on-chip technologies and organoids to interrogate neuroimmune interactions and further elucidate these complex interactions in the perioperative setting.
There are limitations to our study that involve interrogation of barrier-specific protective properties of omega-3 fatty acids and reciprocal interactions with other cell types. Pharmacological or genetic targeting of barrier-associated immune cells, such as PVMs, has been challenging, as these cells express similar markers to meningeal macrophages and choroid plexus macrophages.34,44 Thus, it is not easy to selectively deplete PVMs using agents such as clodronate. We used IL-1β as a candidate inflammatory molecule, but the immune response to surgical trauma is complex and multifaced. Future work will expand the use of the BBB-on-chip model to adapt it to other perioperative stressors to test barrier function and the impact on microglial cells in organoids. A deeper characterisation of NVU changes during ageing and disease is also required, as these results will likely advance translation of new therapies to the clinic. Overall, this study provides new evidence for perioperative omega-3 fatty acid treatment in protecting the BBB from surgery-induced neuroinflammation and establishes a translatable microphysiological device with primary human cells to characterise neuroimmune interactions after systemic inflammation.
Authors' contributions
Conceptualisation: TY, SV, NT
Methodology: CK, RV, AIC, XZ, UK, HM, VK, NOF
Investigation: TY, CK, RV, UK, HM, VK, PB
Visualisation: CK, RV, RMR
Supervision: TY, RMR, WCW, SV, NT
Writing of original draft: TY, NT
Writing, review, and editing: RV, UK, WCW, SV
Acknowledgements
The authors thank the Duke University School of Medicine and the Duke Cancer Institute for the use of the Microbiome Core Facility, which provided assistance with the NanoString data. The authors are grateful to Benjamin Carlson from the Duke Light Microscopy Core Facility for his assistance and the Duke University Mouse Behavior and Neuroendocrine Core Facility, specifically the technical assistance provided to Nathan O. Franklin during the five-choice serial reaction time task by Abel Abadi, Christopher Means, and Crisanta Ritter.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: Microfluidics-assisted blood–brain barrier device: a powerful tool to study perioperative neurocognitive disorder by Z. Xie, Br J Anaesth 2023:130:e212–e214, doi: 10.1016/j.bja.2022.08.024
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.05.025.
Declarations of interest
An invention disclosure (SV, TY, and NT) on the blood–brain barrier-on-chip device is being evaluated by the Duke Office for Translation & Commercialization. The other authors declare that they have no conflicts of interest.
Funding
US National Institutes of Health (R01AG057525) to NT; US National Institutes of Health (R01AR071552) to SV; Alzheimer's Association Research Grant to NT; Fresenius Kabi Deutschland GmbH to NT; American Society of Nephrology and Kidney Cure Carl W. Gottschalk Research Scholar Award to TY.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Daneman R. The blood–brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 2.Yang T., Velagapudi R., Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21:1319–1326. doi: 10.1038/s41590-020-00812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson J.E., Mart M.F., Cunningham C., et al. Delirium Nat Rev Dis Primers. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie D.L., Marcantonio E.R., Zhang Y., Leo-Summers L., Inouye S.K. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Z., Hu J., Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 6.Yang T., Xu G., Newton P.T., et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122:350–360. doi: 10.1016/j.bja.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cibelli M., Fidalgo A.R., Terrando N., et al. Role of interleukin-1 beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrando N., Eriksson L.I., Ryu J.K., et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mietani K., Sumitani M., Ogata T., et al. Dysfunction of the blood-brain barrier in postoperative delirium patients, referring to the axonal damage biomarker phosphorylated neurofilament heavy subunit. PLoS One. 2019;14 doi: 10.1371/journal.pone.0222721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino J.G., Latour L.L., Tso A., et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol. 2013;34:518–523. doi: 10.3174/ajnr.A3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor J., Parker M., Casey C.P., et al. Postoperative delirium and changes in the blood–brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. 2022 doi: 10.1016/j.bja.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velagapudi R., Subramaniyan S., Xiong C., et al. Orthopedic surgery triggers attention deficits in a delirium-like mouse model. Front Immunol. 2019;10:2675. doi: 10.3389/fimmu.2019.02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persidsky Y., Hill J., Zhang M., et al. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab. 2016;36:794–807. doi: 10.1177/0271678X15606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cait J., Hughes M.R., Zeglinski M.R., et al. Podocalyxin is required for maintaining blood–brain barrier function during acute inflammation. Proc Natl Acad Sci U S A. 2019;116:4518–4527. doi: 10.1073/pnas.1814766116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armulik A., Genove G., Mae M., et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 17.Crouch E.E., Doetsch F. FACS isolation of endothelial cells and pericytes from mouse brain microregions. Nat Protoc. 2018;13:738–751. doi: 10.1038/nprot.2017.158. [DOI] [PubMed] [Google Scholar]
- 18.Lapenna A., De Palma M., Lewis C.E. Perivascular macrophages in health and disease. Nat Rev Immunol. 2018;18:689–702. doi: 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- 19.Deczkowska A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Keren-Shaul H., Spinrad A., Weiner A., et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. e17. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen M.A., Ryu J.K., Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19:283–301. doi: 10.1038/nrn.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S. doi: 10.1093/ajcn/83.2.447S. –55S. [DOI] [PubMed] [Google Scholar]
- 26.Cape E., Hall R.J., van Munster B.C., et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014;77:219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelly D.T., Griffin E.W., Murray C.L., et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. 2019;24:1533–1548. doi: 10.1038/s41380-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrientos R.M., Hein A.M., Frank M.G., Watkins L.R., Maier S.F. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang A.C., Stevens M.Y., Chen M.B., et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583:425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villeda S.A., Luo J., Mosher K.I., et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes C.G., Pandharipande P.P., Thompson J.L., et al. Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med. 2016;44:e809–e817. doi: 10.1097/CCM.0000000000001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey C.P., Lindroth H., Mohanty R., et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munster B.C., Aronica E., Zwinderman A.H., Eikelenboom P., Cunningham C., Rooij S.E. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraco G., Park L., Anrather J., Iadecola C. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl) 2017;95:1143–1152. doi: 10.1007/s00109-017-1573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iadecola C., Gottesman R.F. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124:1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santisteban M.M., Ahn S.J., Lane D., et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76:795–807. doi: 10.1161/HYPERTENSIONAHA.120.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang T., Guo R., Zhang F. Brain perivascular macrophages: recent advances and implications in health and diseases. CNS Neurosci Ther. 2019;25:1318–1328. doi: 10.1111/cns.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terrando N., Park J.J., Devinney M., et al. Immunomodulatory lipid mediator profiling of cerebrospinal fluid following surgery in older adults. Sci Rep. 2021;11:3047. doi: 10.1038/s41598-021-82606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P., Velagapudi R., Kong C., et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 2020;16:734–749. doi: 10.1002/alz.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park L., Uekawa K., Garcia-Bonilla L., et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121:258–269. doi: 10.1161/CIRCRESAHA.117.311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagare A.P., Bell R.D., Zhao Z., et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Henjum K., Quist-Paulsen E., Zetterberg H., Blennow K., Nilsson L.N.G., Watne L.O. CSF sTREM 2 in delirium—relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J Neuroinflammation. 2018;15:304. doi: 10.1186/s12974-018-1331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulland T.K., Colonna M. TREM2—a key player in microglial biology and Alzheimer disease. Nat Rev Neurol. 2018;14:667–675. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 44.Alves de Lima K., Rustenhoven J., Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.