Graphical abstract
Keywords: GSK-3β, Memory, Consolidation, Aging, Alzheimer's disease
Abstract
Glycogen synthase kinase 3β (GSK-3β) is a therapeutic target for various age-related neurodegenerative diseases. It is linked to the two main pathological features of Alzheimer’s disease (AD), tau and amyloid β (Aβ); GSK-3β is a major candidate to pathologically hyperphosphorylate tau and modulate Aβ production. However, inhibition of GSK-3β in clinical studies in humans has been found to not significantly improve cognitive function of AD patients, prompting us to study the physiological role of GSK-3β in old mice. Using a contextual fear-conditioning paradigm, we now report that old gsk-3β+/− mice are deficient in both short-term and long-term memory formation, suggesting that GSK-3β is required for memory formation at old age. Biochemical and immunohistochemical analyses showed that the number of synapses does not differ between gsk-3β+/− and age-matched wild-type (wt) littermate mice. Based on these observations, we propose that, GSK-3β may contribute to help maintain brain function during aging. Our results may explain the poor efficacy of GSK-3β inhibitors in preserving memory capacity in AD patients.
Introduction
Glycogen synthase kinase 3 (GSK-3) is a serine-threonine kinase encoded by two isoforms (α, β) in mammalian tissues [32]; GSK-3β has been isolated from the microtubule fraction of bovine brain as tau phosphorylation kinase I (TPK1) [11], which induces the hyperphosphorylated tau seen in Alzheimer’s disease (AD). GSK-3β is particularly abundant in the brain [32] where it phosphorylates a number of substrates other than tau [8], [12]. Results of studies involving GSK-3 inhibitors have suggested a role for GSK-3 in neurodegenerative disorders (e.g., Alzheimer’s disease), mental disorders and schizophrenia [5], [13], [17], [21], although a clear mechanistic explanation for these observations is still lacking.
Alzheimer’s disease is pathologically characterized by extracellular depositions of Aβ and intracellular accumulation of hyperphosphorylated tau fibril [2]. The number of neurofibrillary tangles (NFTs) and the extent of brain dysfunction are well correlated [6], [19]. It is now established that GSK-3β is a major tau kinase [11], [34] and that it is colocalized with NFTs which are comprised of hyperphosphorylated tau [33]. While GSK-3β is constitutively active, phosphorylation of the Ser9 residue in GSK-3β (pSer9-GSK3β) results in a conformation change that renders the kinase inactive [26]. Notably, the GSK-3β associated with NFT are unphosphorylated at Ser9 [22].
Evidence for a key role of GSK-3β in AD includes the demonstration that an antisense-oligonucleotide of GSK-3β inhibits Aβ-induced neuronal death and the formation of hyperphosphorylated tau [30], [28]. Since Aβ activates GSK-3β through inhibition of PI3K-Akt pathway [29], neuronal death seems to be triggered by hyperphosphorylated tau rather than Aβ itself. Other studies have shown that inhibition of GSK-3β attenuates NFT formation in P301L tau transgenic mouse [7], [20] and that NFT formation is potentiated when GSK-3β is co-expressed with FTDP-17 tau, accompanied by impairments of spatial memory [4], [9]. Moreover, the small molecule GSK-3β inhibitor SAR502250 was shown to have neuroprotective activity and the ability to alleviate behavioral impairments in app/tau double transgenic mice [7]. In addition, Tideglusib, a non-ATP competitive inhibitor of GSK-3β prevented tau phosphorylation, amyloid deposition, neuron loss and gliosis in the entorhinal cortex and hippocampus and also reversed a spatial memory deficit in app/tau double transgenic mouse line [24]. However, in a Phase 2a clinical study, Tidegulsib did not significantly improve cognitive performance in AD patients [16], although the drug tended to reduce brain atrophy in progressive supranuclear palsy (PSP) patients [10].
Given the inconsistencies between the results from preclinical and clinical studies, as well as our previous report on the crucial involvement of GSK-3β in memory reconsolidation in adult mice aged 7–14 months [14], and because AD is more frequent in older adults, we here investigated the role of GSK-3β on memory formation in 18–24 months old mice. Together with biochemical and immunohistochemical analyses of brains from mice aged up to 32 months, our results demonstrate an essential role for GSK-3β in both short-term and long-term memory formation at very advanced ages.
Materials and methods
Animals
Male gsk-3β+/− (C57/BL6J background) and wild-type (wt) littermate mice were used in these experiments. Animals were held under a 12 h light/12 h dark schedule, with free access to food and water at Gakushuin University. Behavioral assessments were carried out in mice aged 18–24 months; all animals were handled daily before behavioral testing. Biochemical and immunohistochemical analyses were performed on hippocampi and frontal cortices obtained from mice aged 19–32 months mice (old group) and mice aged 3 months (young adult group) after cervical dislocation. All experiments were approved by the institutional ethical committee for animal experiments at Gakushuin University.
Contextual fear-conditioning (CFC) test
Wild-type and GSK-3β heterozygous knockout mice were tested for contextual fear conditioning (CFC) in a plastic chamber (15 cm × 17 cm × 13 cm) with an electrified steel grid floor, within an outer (40 cm × 50 cm × 55 cm) chamber. The apparatus was outfitted with a CCD camera. Within the apparatus, mice were exposed to constant 55 dB white noise and 100 LUX illumination. Mice were allowed to acclimate to the novel environment of the inner box (5 min); this manipulation served as the conditioned stimulus (CS). They were subsequently given 6 consecutive electric shocks (shock intensity = 0.5 mA; inter-stimuli interval = 60 sec) which served as the unconditioned stimulus (US). One hour or 7 days after the initial conditioning, mice were re-exposed to CS for 5 min to assess short-term and long-term memory, respectively. All protocols were run using TimeFZ1 software (O'Hara & Co., Tokyo, Japan), which simultaneously analyzed the behavior of mice (rate = 2 frame/sec). The software detected “freezing” (i.e., the state of immobility) in mice: the percentage of freezing time in CS (5 min) was used as an index of memory formation.
Western blotting
Mouse tissues were homogenized in Tris-buffered saline (with 1 mM EDTA and 1 mM EGTA; [pH 7.4]) containing protease inhibitors (5 mg/ml Leupeptin, 2 mg/ml aprotinin, 5 mg/ml pepstatin) and phosphatase inhibitors (1 mM okadaic acid, 1 mM glycerophosphate, 1 mM Na3VO4, 1 mM NaF). After centrifugation at 24,000×g (15 min), the supernatant was solubilized in SDS sample buffer (with reducing agent) and subjected to SDS-PAGE, followed by Western blotting for detection of levels of GSK-3β or pSer9-GSK-3β levels, using mouse monoclonal anti-GSK-3β (Transduction Laboratories; 1:2000) and rabbit polyclonal anti-phospho-GSK-3β (Ser9) (Cell Signaling Technology; 1:1000) antibodies, respectively. Levels of GSK-3β and p-GSK-3β are shown after normalization against corresponding levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GAPDH was detected using a mouse monoclonal antibody (Sigma-Aldrich; 1:5000). In addition, the pellets from the initial centrifugation were homogenized in TBS with 1% Triton X-100 to yield a Triton X-100-soluble fraction; synaptophysin expression was measured by immunoblotting following SDS-PAGE using a mouse monoclonal anti-synaptophysin (Sigma-Aldrich; 1:2000) and normalized to those of β-tubulin, detected with mouse monoclonal anti-β-tubulin (Sigma-Aldrich; 1:3000). Finally, the Triton X-100-insoluble pellets were solubilized in SDS sample buffer (with reducing agent), electrophoresed (SDS-PAGE) and immunoblotted with mouse monoclonal anti-post-synaptic density protein 95 (PSD95) (Transduction Laboratories; 1:2000), the levels of which were normalized against those of β-actin (mouse monoclonal anti-β-actin; MP Biomedicals; 1:3000).
All homogenization steps were carried out at 4 °C. All separated proteins were blotted onto nitrocellulose membranes, treated with 5% skimmed milk (blocking reagent) for 1 h before incubation with the primary antibodies (16 h, 4° C). Antigen-antibody reactions were detected with respective HRP-conjugated secondary antibodies (for 2 h, RT) and visualized with chemiluminescent reagents (Chemi-Lumi One L, Nacalai Tesque, Kyoto, Japan). Semi-quantitative evaluation of signal strength was performed using an Amersham Imager 600 (GE Healthcare, Fujifilm).
Immunohistochemistry
Mice were anesthetized with isoflurane before perfusion with phospho-buffered saline (PBS, pH 7.4), followed by 10% neutral-buffered formalin. Brains were then removed and post-fixed in 10% neutral-buffered formalin (3 d, RT) before dehydration in xylene, paraffin embedding and sagittal sectioning (4 µm) using a microtome. After deparaffinization, sections were treated with citrate buffer (antigen retrieval), incubated in 8% skimmed milk (in TBS) to block non-specific binding, and sequentially incubated with mouse monoclonal antibodies against synaptophysin (Sigma-Aldrich; 1:200; 16 h, 4 °C) and rabbit polyclonal antibodies against PSD95 (Cell Signaling Technology; 1:100; 1 h, 37 °C), before incubation with corresponding secondary antibodies (1 h, 37 °C). Stained sections were mounted in Vectashield mounting medium (Vector Laboratories) before microscopic images were captured using a confocal microscope (FV1000, Olympus).
The relative level of fluorescence intensity was determined for each image. Next, to extract synaptic regions, the images were binarized with a specific value after adjusting for gaussian blur (σ = 0.7). Co-localized areas were then extracted by superimposing binarized synaptophysin- and PSD95-staining. Finally, we calculated the number of co-localized puncta in an area of 50 × 50 μm2 square. All images were processed using ImageJ [23].
Results
GSK-3β loss is accompanied by lower levels of pSer9-GSK-3β
GSK-3β is modified by kinase/phosphatase cascades to yield active GSK-3β and inactive pSer9-GSK-3β. An analysis of the phosphorylation state of GSK-3β in the hippocampus and frontal cortex of old gsk-3β+/− mice and littermates (wt) by Western blotting is shown in Fig. 1A.
Fig. 1.
Analysis of GSK-3β activity in gsk-3β+/− knockout mice. Western blots from TBS-soluble fractions of hippocampus and frontal cortex in wt mice (n = 3) and gsk-3β+/− mice (n = 3) (A, 21–32 months old; B, 3 months old) are shown. In both old and young mice, a significant reduction was found in GSK-3β expression levels (normalized to GAPDH) in hippocampus (C, *p < 0.05; E, **p < 0.01) and frontal cortex (D, *p < 0.05; F, **p < 0.01) respectively. No difference in the ratio of pSer9 GSK-3β to total GSK-3β was found in either hippocampus (G, p = 0.699; I, p = 0.145) or frontal cortex (H, p = 0.841; J, p = 0.289). Student’s t-test was applied in the statistical analyses. All data are plotted as mean ± SD; *p < 0.05. **p < 0.01.
As shown in Fig. 1C-D, normalizing GSK-3β levels to those of GAPDH revealed that the hippocampus and frontal cortex of gsk-3β+/− mice expressed only about half as much as that expressed by tissues from wt animals (p < 0.05). Importantly, the pSer9-GSK-3β to GSK-3β ratio did not differ between samples from old wt and age-matched gsk-3β+/− mice (Fig. 1G-H), indicating that the genetic manipulation (gsk-3β+/−) did not alter the relative activity of kinases and phosphatases. We further investigated whether young and old gsk-3β+/− mice showed a significant reduction in GSK-3β compared to wt and a conserved pGSK-3β/GSK-3β ratio (Fig. 1B, E-F, I-J). Compared to that in corresponding wt tissues, the ratio of pGSK-3β to GAPDH was 70 % in the hippocampus of young gsk-3β+/− mice, 42 % in the hippocampus of old gsk-3β+/− mice, 79 % in the frontal cortex of young gsk-3β+/− mice, and 51 % in the frontal cortex of old gsk-3β+/− mice. Taken together, this set of results shows that reduced GSK-3β activity is a phenotypic hallmark of gsk-3β+/− mice, and that reduced GSK-3β activity persists in gsk-3β+/− mice right through to old age.
GSK-3β is required for memory formation in old mice
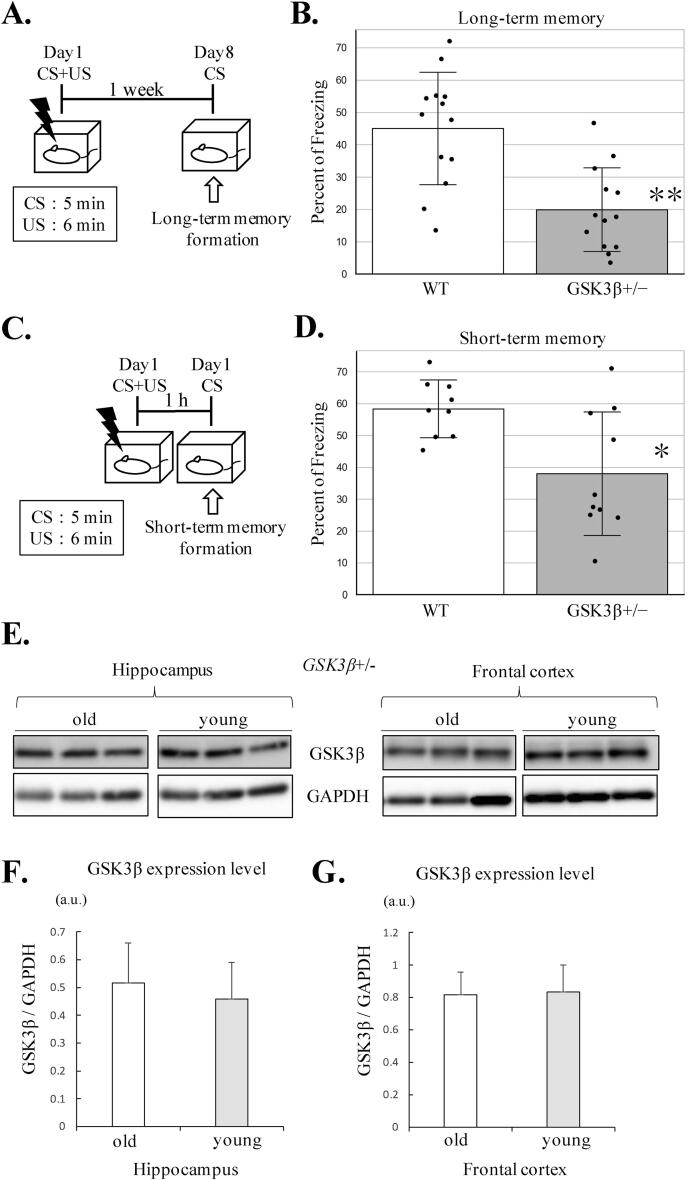
We previously reported that, as young adults (7–14 months old), gsk-3β+/− mice are not impaired in memory consolidation but that they display deficits in memory reconsolidation [14]. In the present study, we extended those earlier observations to include very old mice (18–24 months old) subjected to a test of contextual fear conditioning (CFC) (Fig. 2A, C) in which freezing time during CS served to judge contextual memory performance.
Fig. 2.
Old GSK-3β heterozygous mice show impairments in long-term and short-term memory formation. Contextual fear conditioning was used to assess long-term and short-term memory formation in mice aged 18–24 months, as depicted in (A, C). The conditioned stimulus (CS) consisted of placement in a plastic chamber for 5 min; the unconditioned stimulus (US) comprised exposure to 6 electrical footshocks (unconditioned stimulus, US) over 6 min. Seven days after conditioning (CS + US), gsk-3β+/− mice (n = 13) showed significant less freezing time than wt mice (n = 13) (B, **p < 0.01). A significant difference between wt (n = 9) and gsk-3β+/− (n = 10) (D, *p < 0.05) was also observed 1 h after conditioning. In (E), western blots from TBS-soluble fractions from old (n = 3) and young (n = 3) gsk-3β+/− mice (left, hippocampus; right, frontal cortex) are shown. There was no difference in the ratio of GSK-3β to GAPDH in either the hippocampus (F, p = 0.648) or frontal cortex (G, p = 0.909). Statistical analysis was performed using Mann-Whitney test in (B, D) and Student’s t-test in (F, G). All data are plotted as mean ± SD; data points are indicated as black dots; *p < 0.05; **p < 0.01.
On day 1 of the experiment, mobility did not differ between genotypes (wt and gsk-3β+/−; student’s t-test; p = 0.678) during exposure to the CS for 5 min, and mice were returned to their home cages after exposure to the US. One week later (day 8), both, wt and gsk-3β+/− mice were re-exposed to CS with the expectation that they would associate the CS with US through the process of memory consolidation. We observed that gsk-3β+/− mice were impaired at memory consolidation – they displayed shorter freezing times than wt mice during exposure to the CS (Fig. 2B). After a 1 h period of rest (home cage) from CS + US, both genotypes were exposed to the CS in order to evaluate their capacity for short-term memory formation. As compared to their wt counterparts, gsk-3β+/− mice froze for significantly shorter times when exposed to the CS, indicating that old gsk-3β+/− mice have a deficit in short-term memory formation (Fig. 2D).
Together with our earlier report [14], the above data show that GSK-3β deficiency impacts on different phases of memory formation in an age-related fashion; whereas GSK-3β only affects memory reconsolidation in young adults, it disrupts both short-term and long-term memory formation in old age.
In addition, we compared GSK-3β expression levels in old and young gsk-3β+/− mice (Fig. 2E). If aging gradually reduces GSK-3β expression in gsk-3β+/− mice, one could expect a dose-dependent behavioral abnormalities. However, GSK-3β expression levels in gsk-3β+/− mice did not show age-related declines (Fig. 2F–G).
GSK-3β deficiency does not influence synapse numbers in hippocampus and frontal cortex
The hippocampal CA1/CA3 layers are crucial to memory encoding and consolidation while the frontal cortex which plays a role in remote memory storage is innervated by the entorhinal cortex. Based on this, we investigated whether the memory impairments in old gsk-3β+/− mice might be underpinned by altered synapse numbers in the hippocampus and frontal cortex. Immunoblotting for PSD95 and synaptophysin were used for this purpose (Fig. 3A-B). Synapse numbers in the hippocampus and frontal cortex did not differ between wt and gsk-3β+/− mice, as assessed by western blotting (Fig. 3C-D).
Fig. 3.
Expression of synaptic markers in hippocampus and frontal cortex. Western blots from Triton X-100 soluble fractions (A) and from Triton X-100 insoluble fractions (B) in both wt mice (n = 3) and gsk-3β+/− mice (n = 3) (21–32 months old) are shown. There was no significant difference in the amount of Synaptophysin (SYN) in either hippocampus (p = 0.129) or frontal cortex (p = 0.370) (C), and also no significant difference in that of PSD95 in either hippocampus (p = 0.803) or frontal cortex (p = 0.271) (D). Student’s t-test was applied in the statistical analyses. All data are plotted as mean ± SD.
Furthermore, to perform a region-specific analysis, we quantified the number of synapses using immunohistochemistry on mouse brains utilizing primary antibodies against synaptophysin and PSD95 (Fig. 4A). Similar to the results from our biochemical analysis, significant differences in synapse number were not observed between old wt and gsk-3β+/− mice in either hippocampal substructures (CA1, CA3, dentate gyrus) or the frontal cortex (Fig. 4B-E, left). Moreover, as compared to their wt counterparts, gsk-3β+/− mice did not differ in terms of intensity of synaptophysin and PSD95 immunofluorescence (Fig. 4B-E, middle, right).
Fig. 4.
Analysis of immunofluorescence staining in hippocampal substructures and frontal cortex. Representative immunostaining images of PSD95 (red, as postsynaptic marker) and synaptophysin (green, as presynaptic marker) staining in hippocampal substructures (CA1, CA3, DG) and frontal cortex of old (19–25 months old) wt (n = 3) and GSK-3β+/− (n = 3) mice are shown (A). In merged images (A), the enlarged images shown in the insets are examples of colocalized areas (see Methods). No differences in the number of colocalized puncta in these regions (CA1, B left, p = 0.692; CA3, C left, p = 0.768; DG, D left, p = 0.494; FC, E left, p = 0.430) were found. Also, no differences in the normalized fluorescence intensity of PSD95 (CA1, B middle, p = 0.949; CA3, C middle, p = 0.743; DG, D middle, p = 0.606; FC, E middle, p = 0.555) and of SYN (CA1, B right, p = 0.305; CA3, C right, p = 0.332; DG, D right, p = 0.125; FC, E right, p = 0.651) were detected. Student’s t-test was applied in the statistical analyses. All data are plotted as mean ± SD. Scale bars in staining images, 10 μm; Scale bars in the enlarged images, 1 μm. DG, dentate gyrus; FC, frontal cortex; SYN, synaptophysin. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Together, the above results imply that alterations in synapse numbers do not account for the memory deficits observed in GSK-3β-deficient mice.
Discussion
In an extension of our previous report that GSK-3β plays a key role in memory reconsolidation in young adult mice [14], the present work demonstrates that old gsk-3β+/− mice are impaired at forming both, short-term and long-term memories. This observation bolsters our earlier evidence for the essentiality of GSK-3β in memory formation. Importantly, the results reported in this paper suggest that alterations in memory capacity during aging are subject to the influence of GSK-3β in manner that does not depend on age-related changes in synapse numbers.
Our earlier study [14] showed that GSK-3β is activated in the hippocampus during learning, consolidation and reconsolidation phases. In fact, levels of activated GSK-3β were highest during memory reconsolidation and, importantly, young adult gsk-3β heterozygosity only impaired memory reconsolidation [14]. Notably, conditional knockout of gsk-3β in young adult mice resulted in an inhibition of memory consolidation [15]. These results suggest that GSK-3β is involved in memory consolidation which becomes increasingly dependent upon activated GSK-3β as animals age. However, memory consolidation is not simply dependent on GSK-3β expression level, because GSK-3β expression levels did not differ in young and old gsk-3β+/− mice (Fig. 2F-G). This suggests that there is a mechanism of memory consolidation, in which the need for GSK-3β increases with age. Although the number of synapses did not differ significantly between old wt and gsk-3β+/− mice, the number of synapses in the medial prefrontal cortex of adult mice submitted to CFC is known to increase [1]. It therefore remains possible that we might have been able to observe some changes in synapse number and GSK-3β activity in the prefrontal cortex of old wt and gsk-3β+/− mice if analysis was performed immediately after CFC. Further studies are needed to clarify the mechanism of memory consolidation during old age. Reorganization of neural connectivity (re-connectivity) represents another mechanism that might underpin GSK-3β dependent memory consolidation during aging. Neural re-connectivity appears to be key to the ability to maintain brain functions during aging. For example, different place cells in the CA3 layer of the rat hippocampus become activated in response to different environments during early adulthood and become reactivated upon exposure to a new environment during old age [31]. Interestingly, whereas encoding of spatial memory in young humans involves medial temporal lobe structures such as the right hippocampus and left para-hippocampal gyrus, encoding of spatial memory in older subjects is accompanied by activation of the anterior-medial cingulate gyrus [18]. Based on the results of our experiments, we suggest that GSK-3β may play a crucial role in maintaining mnemonic functions during aging by facilitating neural connectivity.
Recently, GSK-3α, which is an isoform of GSK-3β, was reported to participate in synaptic plasticity [3], [25]. It is therefore important to note that the GSK-3β heterozygous mice used in this study showed decreases in GSK-3β levels without compensatory increases in GSK-3α [14]. It is important to note that activation of GSK-3β induces the generation of amyloid β (Aβ) [27] and the hyperphosphorylation of tau which lead to formation of neurofibrillary tangles (NFT) and eventually, neuronal death [7], [20], [29]. This means that the benefits of increasing GSK-3β activity to compensate for memory impairments during aging comes with the risk of initiating neurodegeneration. Therefore, although GSK-3β inhibitors may slow neurodegeneration, they do not alleviate memory deficits in patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Singularity Biology (No.8007)” (18H05414) of MEXT to AT.
Author contributions
RK, AT, YS and YT conceived and designed this article. RK conducted the experiments, analyzed the results and drafted the manuscript. AT, YS and YT conducted experiments and critically revised the manuscript. All authors approved the final version of the manuscript revision. The authors thank the anonymous reviewers for constructive remarks on previous versions of the manuscript.
References
- 1.Bero A.W., Meng J., Cho S., Shen A.H., Canter R.G., Ericsson M., et al. Early remodeling of the neocortex upon episodic memory encoding. PNAS. 2014;111(32):11852–11857. doi: 10.1073/pnas.1408378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Draffin J.E., Sánchez-Castillo C., Fernández-Rodrigo A., Sánchez-Sáez X., Ávila J., Wagner F.F., et al. GSK3α, not GSK3β, drives hippocampal NMDAR-dependent LTD via tau-mediated spine anchoring. EMBO J. 2021;40(2) doi: 10.15252/embj.2020105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel T., Lucas J.J., Gómez-Ramos P., Moran M.A., Avila J., Hernández F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27(9):1258–1268. doi: 10.1016/j.neurobiolaging.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Forlenza O.V., De-Paula V.J.R., Diniz B.S.O. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014;5(6):443–450. doi: 10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannakopoulos P., Gold G., Kövari E., von Gunten A., Imhof A., Bouras C., et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol. 2007;113(1):1–12. doi: 10.1007/s00401-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 7.Griebel G., Stemmelin J., Lopez-Grancha M., Boulay D., Boquet G., Slowinski F., et al. The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer’s disease in rodents. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-54557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimes C.A., Jope R.S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 9.Hernández F., Borrell J., Guaza C., Avila J., Lucas J.J. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83(6):1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- 10.Höglinger G.U., Huppertz H.-J., Wagenpfeil S., Andrés M.V., Belloch V., León T., et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Movem Disorders. 2014;29(4):479–487. doi: 10.1002/mds.25815. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro K., Shiratsuchi A., Sato S., Omori A., Arioka M., Kobayashi S., et al. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325(3):167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- 12.Jope R.S., Johnson G.V.W. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Jope R.S., Roh M.-S. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7(11):1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura T., Yamashita S., Nakao S., Park J.-M., Murayama M., Mizoroki T., et al. GSK-3beta is required for memory reconsolidation in adult brain. PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu E., Xie A.-J., Zhou Q., Li M., Zhang S., Li S., et al. GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-06173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovestone S., Boada M., Dubois B., Hüll M., Rinne J.O., Huppertz H.-J., et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimer’s Dis: JAD. 2015;45(1):75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 17.Lovestone S., Killick R., Di Forti M., Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30(4):142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Meulenbroek O., Petersson K.M., Voermans N., Weber B., Fernández G. Age differences in neural correlates of route encoding and route recognition. NeuroImage. 2004;22(4):1503–1514. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Nagy Z., Jobst K.A., Esiri M.M., Morris J.H., King E.-M.-F., MacDonald B., et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer’s disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia (Basel, Switzerland) 1996;7(2):76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- 20.Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F., et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. PNAS. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien W.T., Klein P.S. Regulation of glycogen synthase kinase-3 in patients with affective disorders. Biol Psychiatry. 2007;61(2):139–141. doi: 10.1016/j.biopsych.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei J.J., Braak E., Braak H., Grundke-Iqbal I., Iqbal K., Winblad B., et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58(9):1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serenó L., Coma M., Rodríguez M., Sánchez-Ferrer P., Sánchez M.B., Gich I., et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35(3):359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Shahab L., Plattner F., Irvine E.E., Cummings D.M., Edwards F.A. Dynamic range of GSK3α not GSK3β is essential for bidirectional synaptic plasticity at hippocampal CA3-CA1 synapses. Hippocampus. 2014;24(12):1413–1416. doi: 10.1002/hipo.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stambolic V., Woodgett J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X., Sato S., Murayama O., Murayama M., Park J.-M., Yamaguchi H., et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321(1-2):61–64. doi: 10.1016/S0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 28.Takashima A., Honda T., Yasutake K., Michel G., Murayama O., Murayama M., et al. Activation of tau protein kinase I/glycogen synthase kinase-3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neurosci Res. 1998;31(4):317–323. doi: 10.1016/s0168-0102(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 29.Takashima A., Noguchi K., Michel G., Mercken M., Hoshi M., Ishiguro K., et al. Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci Lett. 1996;203(1):33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]
- 30.Takashima A., Yamaguchi H., Noguchi K., Michel G., Ishiguro K., Sato K., et al. Amyloid beta peptide induces cytoplasmic accumulation of amyloid protein precursor via tau protein kinase I/glycogen synthase kinase-3 beta in rat hippocampal neurons. Neurosci Lett. 1995;198(2):83–86. doi: 10.1016/0304-3940(95)11964-x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson I.A., Ikonen S., Gallagher M., Eichenbaum H., Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25(29):6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodgett J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H., Ishiguro K., Uchida T., Takashima A., Lemere C.A., Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996;92(3):232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 34.Zheng-Fischhöfer Q., Biernat J., Mandelkow E.M., Illenberger S., Godemann R., Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252(3):542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]