Abstract
To examine the relationships of retinal structural (optical coherence tomography) and visual functional (multifocal visual evoked potentials, mfVEP) indices with neuropsychological and brain structural measurements in healthy older subjects. 95 participants (mean (SD) age 68.1 (9.0)) years were recruited in the Optic Nerve Decline and Cognitive Change (ONDCC) study in this observational clinical investigation. OCT was conducted for retinal nerve fibre layer (RNFL) and mfVEP for amplitude and latency measurements. Participants undertook neuropsychological tests for cognitive performance and MRI for volumetric evaluation of various brain regions. Generalised estimating equation models were used for association analysis (p < 0.05). The brain volumetric measures including total grey matter (GM), cortex, thalamus, hippocampal and fourth ventricular volumes were significantly associated with global and sectoral RNFL. RNFL thickness correlated with delayed recalls of California verbal learning test (CVLT) and Rey complex figure test (RCFT). The mfVEP amplitudes associated with cerebral white matter (WM) and cingulate GM volumes in MRI and CVLT, RCFT and trail making test outcomes. A significant association of mfVEP latency with logical memory delayed recall and thalamus volume was also observed. Our results suggested significant association of specific RNFL and mfVEP measures with distinctive brain region volumes and cognitive tests reflecting performance in memory, visuospatial and executive functional domains. These findings indicate that the mfVEP and RNFL measurements may parallel brain structural and neuropsychological measures in the older population.
Keywords: Aging, MRI, Cognition, Memory, RNFL, mfVEP
Introduction
Aging is a complex and multifactorial physiological process that is influenced by a multitude of genetic, epigenetic, and environmental factors [45], [8], [55]. In recent years, there has been growing interest in elucidating the relationship between the visual system and brain changes in the healthy aging process as well as in age-related neurodegenerative disorders such as dementia [24]. Studies investigating human brain structures with magnetic resonance imaging (MRI) have demonstrated shrinkage of the adult brain with age, with approximately 5 % of the progressive brain weight reduction per decade after the age of 40 years [28]. However, this size diminution is variable, with certain brain regions showing a greater degree of change compared to the others. The structural matrix and interconnection of different brain regions have also been shown to alter with age with noticeable variations in several anatomically and functionally related areas such as the hippocampus, thalamus, posterior cingulate and parietal lobule [65], [46]. Along with the age-related reduction in the brain volume, expansion of the ventricular system due to cerebral atrophy has also been reported [19]. Further, both grey and white matter demonstrates progressive tissue shrinkage with age and grey matter atrophy may affect white matter and vice versa. [47]. Aging is also associated with declines in information processing speed as well as working memory capacity, which may reflect structural changes in specific regions of the brain [10]. Advances in PET imaging and cerebral spinal fluid (CSF) assays have provided critical insights into brain imaging and biochemical profiling. A major limitation of these approaches is that by the time alterations in the brain are diagnosed, irreversible degenerative changes have already occurred. Further, PET involves radiation exposure, and CSF sampling is invasive by nature which limits their widespread acceptability for screening purposes. Recent clinical studies suggest that any successful intervention to protect the neurons needs to be initiated prior to the damage, which makes it imperative to develop a sensitive and non-invasive biomarker that reflects or parallels the changes occurring in the brain [24].
Since the retina is an extension of the central nervous system (CNS), which is linked to the higher visual centres in the brain through the optic nerve, it provides a unique model to study age-related neurodegenerative changes. The axons of the retinal ganglion cells (RGCs) connect with the lateral geniculate nucleus (LGN) and superior colliculus (SC) and subsequently project to the visual cortex [15]. Retinal nerve fibre layer (RNFL) thickness as measured by optical coherence tomography (OCT) is commonly used as a structural marker to assess the level of axonal damage in the optic nerve [26], [11]. In a longitudinal study with 1251 healthy aging participants, the RNFL thinning as measured by OCT was shown to associate with worsening cognitive function as well as a higher risk of future cognitive decline [33]. The multifocal visual evoked potential (mfVEP) measures functional responses of the visual pathway and can demonstrate topographical mapping of visual field defects. The demyelinating changes in the visual system are primarily associated with latency disturbances in mfVEP while axonal loss manifests in the form of amplitude alterations [22], [54].
Retinal imaging methods are also non-invasive, widely acceptable, and cost-effective compared to CSF sampling and brain neuroimaging. While several studies suggest that visual pathway changes occur with normal ageing, it is not clear whether these changes parallel brain changes [62], [63]. Hence the aim of this study is to examine whether changes in the visual system occur concurrently to brain changes in aging. Brain regions that are recognised to have age-related changes in memory, visual spatial and executive functional domains etc. were selected for association with visual parameters [10], [30], [48]. The relationship is evaluated through the associations of visual structural (OCT) and functional (mfVEP) parameters with volumetric measures of brain regions (MRI) together with cognitive performance in a healthy aging cohort (Fig. 1).
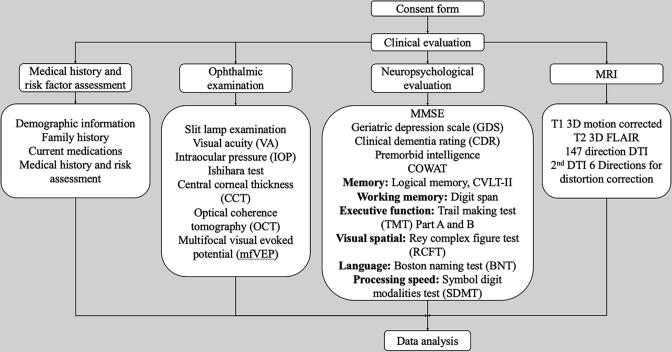
Fig. 1.
Flow chart describing the protocol for in-person clinical evaluation of study participants. The evaluation included 4 components: medical history and risk factor assessment, a neuropsychological evaluation, a detailed ophthalmic examination, and a brain magnetic resonance imaging (MRI). Specific measurements for each component are described. Abbreviations: MMSE = Mini-mental state examination; COWAT = Controlled Oral Word Association Test; CVLT-II = California verbal learning test second edition; FLAIR: fluid attenuated inversion recovery; DTI: diffusion tensor imaging.
Methods
Participant recruitment
Participants in the Optic Nerve Decline and Cognitive Change (ONDCC) study were recruited in response to recruitment flyers, study media releases and/or word of mouth in 2020–2021 in Sydney, NSW, Australia. The inclusion criteria comprised: (1) Male or Female, aged ≥50 years; (2) ability to provide consent for study either by themselves or through guardian; (3) ability to speak and write English sufficiently well to give written informed consent, complete self-reported questionnaires and psychometric assessment. Participants were excluded if they had (1) a significant history of any type of ocular diseases, including macular degeneration and glaucoma, that could affect the testing results; (2) ocular surgery within the last two months; (3) any evidence of dementia, depression, neurotrauma, malignancy, or any other major neurological disease; (4) intellectual disability; (5) did not adequately understand the study procedures or were unable to provide written informed consent at the time of the study entry. Of the 135 individuals who showed interest, 95 participants satisfied the criteria and were recruited in the study. Demographic information, including age, sex and education was collected at the time of recruitment. This study was conducted in accordance with the approval of the Human Research Ethics Committee (Medical Science) at Macquarie University and adhered to the tenets of the Declaration of Helsinki. All participants examined as part of the study were informed of the scope of the project and signed an informed consent form.
Clinical evaluation
The clinical evaluation was performed at the Ophthalmology clinic of Macquarie University Hospital (MUH), Sydney, NSW, Australia. Study participants underwent a medical history and risk factor documentation, comprehensive neuropsychological evaluation, and detailed ophthalmic assessment. MRI was carried out at the Macquarie Medical Imaging practice of MUH. All subjects completed a detailed questionnaire regarding the family history including a family history of Alzheimer’s disease or dementia, neuropsychiatric conditions and other systemic diseases, personal medical history, medication use and questions about smoking and alcohol use.
Ophthalmic examination
All study participants underwent complete ophthalmic examination including best-corrected visual acuity (BCVA), intraocular pressure (IOP) assessment, Ishihara test, central corneal thickness (CCT) measurements. Data obtained from the eyes that were observed normal upon fundus examination and slit lamp biomicroscope were considered for further analysis, [21], [53].
Optical coherence tomography (OCT)
All participants underwent spectral-domain optical coherence tomography (OCT) scans with Spectralis HRA + OCT; (Heidelberg Engineering, Germany). Retinal imaging was carried out on the same day as the cognitive evaluation. A peripapillary circular scan (diameter, 3.50 mm), a macula star-like radial scan and a macular posterior pole scan were performed to obtain the thickness profile of the retina. The global retinal nerve fibre layer (gRNFL), as well as sectoral RNFL thicknesses including nasal, temporal, temporal inferior, temporal superior, nasal inferior, nasal superior quadrants, were measured for analysis in this study. All tests were carried out by two operators utilising the same device under room light conditions. Only images with quality scores of higher than 25 and signal strength cut off and without segmentation failure, image artefacts or malposition were retained for subsequent analysis. Images obscured by voluntary or involuntary blinks were also excluded. All images were reviewed by two readers followed by automatic segmentation with Heidelberg software and manual correction before data extraction. Any participants with ocular abnormalities (including asymptomatic macular oedema, drusen etc. as confirmed by orthoptist or specialist) noticed during OCT scans were excluded from the study.
Multifocal visual evoked potential (mfVEP)
The mfVEP testing was performed utilising Vision Search 1 perimetry (VisionSearch, Sydney, Australia) controlled by Terra software (VisionSearch), and standard stimulus condition as described previously [54]. Briefly, 58 closely packed segments (eccentricity up to 24 degrees) in a cortically scaled dart-board configuration were used. Each segment contained 16 black-and-white checks, which reversed patterns according to a pseudorandom M sequence. Four gold-disc electrodes (Grass, West Warwick, RI) were used for bipolar recording with 2 electrodes positioned 4 cm apart on either side of the inion and 1 electrode placed 2.5 cm above and another 4.5 cm below the inion along the midline. Electrical signals were recorded by 2 channels, enabling monitoring the difference between superior and inferior, as well as between left and right electrodes. The largest peak-to-trough amplitude within the interval of 70 to 210 ms was ascertained for each channel. The locus of maximum amplitude among the channel trace was selected automatically by software to generate a combined topographic map for amplitude and latency analysis. Traces obtained from the combined channel were selected for all tests for every individual segment of the visual field using a specially designed algorithm embedded in the instrument [31]. All mfVEP test results were reviewed by a second reader before data extraction.
Neuropsychological evaluation
All study participants had a detailed neuropsychological evaluation using a battery of tests performed by trained personnel [50], [42]. The full battery comprised the MMSE [20], the national adult reading test (NART) [36], clinical dementia rating (CDR)[41], 15-item GDS [61], Controlled Oral Word Association Test (COWAT) [25], and other tests to assess cognitive domains including (1) memory (logical memory (LM) Wechsler Memory Scale (WMS), we used only story B (Wechsler, 1945), California verbal learning test second edition (CVLT-II) [14]; (2) working memory (digit span (Wechsler, 1997)); (3) executive function (trail making test (TMT) part A and B [60]; (4) visual-spatial (Rey complex figure test (RCFT)[40]; (5) language (30-item Boston naming test (BNT) [27]); (6) processing speed (symbol digit modalities test (SDMT) [59]. The premorbid Predicted Wechsler Adult Intelligence Scale, 4th edition (WAIS-IV) full-scale IQ was calculated using the following equation: 126.41–0.9775*NART errors [7].
Magnetic resonance imaging (MRI) brain imaging
All MRI scans were acquired on a 3.0 T GE Discovery™ MR750w Wide Bore MRI scanner (GE Healthcare, Milwaukee, WI, USA) with a 32-channel Nova Head Coil and a software version of DV26.0_R01_1725.a, located at Macquarie Medical Imaging, NSW, Australia. T1-weighted scans were acquired with a Magnetization-prepared Rapid Acquisition Gradient Echo (MPRAGE) pulse sequence with prospective motion correction (PROMO). The following scanning parameters were used: repetition time (TR) = 8.388 ms, echo time (TE) = 3.168 ms, inversion time (TI) = 900 ms, flip angle (FA) = 8 degrees, pixel bandwidth = 244.141 Hz, acquisition matrix = 256 × 256, 198 slices, yielding 1 mm isotropic voxels. Auto-calibrating Reconstruction for Cartesian imaging (ARC) was applied for parallel imaging (acceleration factor = 3 in the phase encoding direction).
Quantification of brain volumetric measures and quality control
We conducted whole brain volumetric analysis using T1-weighted MRAGE scans that were processed with standard FreeSurfer pipeline (version 7.1.0; [18]). The assessment included volumetric analyses of the following brain regions: ETIV (Estimated Total Intracranial Volume), Total GM (Gray matter), Cortex, Cerebral White Matter, Cingulate Gray matter, Parietal gray matter, Entorhinal cortex, Third Ventricle, Fourth Ventricle, Thalamus, Putamen and Hippocampus. Briefly, the processing included removal of non-brain tissue, transforming to standard space, segmentation of subcortical white matter and deep grey matter structures, intensity normalization, tessellation of the boundary between grey and white matter, automated topology correction, and reconstruction of grey-white and grey-cerebrospinal fluid surfaces. Volumes of cortical and subcortical structures were then quantified.
We then followed ENIGMA (Enhancing Neuro Imaging Genetics through meta-Analysis; (Medland et al.)) Cortical Quality Control Protocol 2.0 to assure quality for the FreeSurfer results. Both internal and external quality control images were visually checked. The unsatisfactorily segmented region volumes were excluded from further analyses. The number of removed values for each measure ranged from 0 to 15. (left banks of the superior temporal sulcus and right pericalcarine regions had 15 poor quality observations).
Statistical analysis
Statistical analysis was performed using SPSS software version 26 for Mac (SPSS, Inc., Chicago, IL, United States). The association between parameters and P-values were examined using the generalised estimating equation (GEE) models. We used the GEE models adjusting for sex as a cofactor, age at entry to study as continuous covariates, left or right eyes as within-subject covariates for all correlation analysis, premorbid Predicted WAIS-IV full-scale IQ as covariates for all neuropsychological test scores, and estimated total intracranial volume (eTIV) as covariates for all MRI parameters in statistical analysis [56]. OCT and mfVEP data were obtained from both eyes and included in the association analysis. Averaged amplitude and latency values from each segment were used for mfVEP analysis. Raw scores were used for all neuropsychological tests for statistical analysis. P-values were adjusted for multiple comparisons by the False Discovery Rate (FDR) method (Two-stage step-up procedure of Benjamini, Krieger and Yekutieli) and presented as adjusted P-values in the result section [4]. P < 0.05 was considered statistically significant.
Results
Participants
Ninety-five aging participants as defined by exclusion criteria listed in the methods section were enrolled in the ONDCC study. The detailed demographic data and clinical features are presented in Table 1. One-hundred and thirty-five persons showed their interest to participate, amongst these 116 (85.9 %) met the inclusion criteria and 95 (70.4 %) underwent the test sessions. 17 people who could not be either contacted to confirm eligibility, meet the age range for study or decided to withdraw from the study before the test day were excluded, and two more subjects were excluded because they were diagnosed with vision disorders. Amongst all the enrolled subjects, 81 (85.2 %) completed the brain MRI scan. Participants who suffered or suspected they suffered from claustrophobia, who needed sedation to complete the MRI scan or who had conditions that required participants to avoid MRI were recruited only for vision and cognitive assessments.
Table 1.
Demographic and baseline data of participants from Optic Nerve Decline and Cognitive Change (ONDCC) study.
| Demographic Data | No. | ||
|---|---|---|---|
| Total | 95 | ||
| No. with MRI | 81 | ||
| Mean | SD | ||
| Age at entry, yrs | 68.1 | 9.0 | |
| Female, No. (%) | 48.0 | 50.5 | |
| Global RNFL, μm | 96.3 | 9.1 | |
| Temporal RNFL, μm | 70.7 | 11.8 | |
| mfVEP | Amplitude, μV | 144.7 | 52.0 |
| Latency, ms | 142.3 | 11.3 | |
| MMSE | 28.4 | 2.1 | |
| GDS | 1.8 | 2.4 | |
| Premorbid Predicted WAIS-IV full-scale IQ | 112.5 | 8.9 | |
| eTIV, mm3 | 1564856.8 | 173619.7 | |
| Total GM Volume, mm3 | 600699.9 | 58976.4 | |
| Cortex Volume, mm3 | 438877.7 | 46761.3 | |
| Cerebral WM Volume, mm3 | 448426.2 | 66387.5 | |
Abbreviations: RNFL = Retinal nerve fiber layer; MMSE = Mini-mental state examination; GDS = Geriatric depression scale; NART = The national adult reading test; mfVEP = Multifocal visual evoked potential (mfVEP); eTIV = Estimated total intracranial volume; GM = Grey matter; WM = White matter.
Multifocal VEP parameters are associated with brain MRI
The amplitude of mfVEP was found to be significantly associated with both cerebral white matter (WM) volume (P = 0.02, adjusted P = 0.03) and grey matter (GM) cingulate volume (P = 0.02, adjusted P = 0.03) with greater volumes associated with higher amplitude. The thalamus volume also demonstrated a significant positive association with mfVEP latency (P = 0.04, adjusted P = 0.04). These associations between the two parameters were obtained after appropriate correction for eTIV and adjustment for age, sex, and inter-eye differences (Table. 2, data from all parameters including non-significant ones are presented in Supplementary Table 1).
Table 2.
Association between RNFL thickness, mfVEP and MRI measurements.
| Parameters | P-value | B-value |
95 % CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| VEPAmp | ||||
| Cerebral WM Vol | 0.02* | 143.354 | 25.025 | 261.682 |
| GM Cingulate Vol | 0.02* | 7.548 | 1.385 | 13.71 |
| VEPLat | ||||
| Thalamus Vol | 0.04* | 25.924 | 1.672 | 50.175 |
| RNFL_G | ||||
| Total GM Vol | 0.003* | 696.944 | 236.075 | 1157.813 |
| Cortex Vol | 0.03* | 439.532 | 34.111 | 844.953 |
| GM Cingulate Vol | 0.04* | 26.522 | 0.803 | 52.242 |
| Thalamus Vol | 0.02* | 20.404 | 2.986 | 37.822 |
| RNFL_T | ||||
| Forth Ventricle Vol | 0.01* | −7.650 | −13.535 | −1.765 |
| RNFL_TS | ||||
| Forth Ventricle Vol | 0.002* | −5.648 | −9.23 | −2.065 |
| RNFL_NS | ||||
| Hippocampus Vol | 0.02* | 7.625 | 1.148 | 14.102 |
Abbreviations: RNFL = Retinal nerve fiber layer; mfVEP = Multifocal visual evoked potential (mfVEP); Amp = Amplitude; Lat = Latency; G = Global; T = Temporal; S = Superior; Vol = Volume; GM = Grey matter; WM = White matter. P-values were examined using the generalised estimating equation (GEE) models. * P < 0.05.
RNFL thickness association with brain MRI volumes
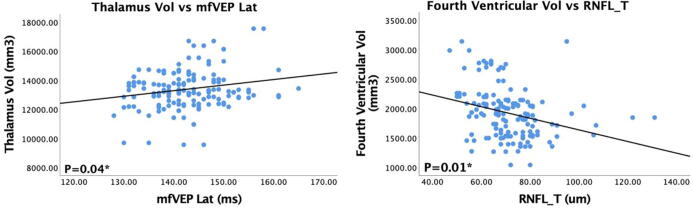
RNFL thickness measurement following OCT analysis revealed positive and statistically significant association of global RNFL thickness with the total GM (P = 0.003, adjusted P = 0.01), cortex (P = 0.03, adjusted P = 0.04), GM cingulate (P = 0.04, adjusted P = 0.04) and thalamus volumes (P = 0.02, adjusted P = 0.03) as demonstrated by MRI brain imaging. RNFL subsector analysis further revealed a statistically significant negative association specifically for the temporal (P = 0.01, adjusted P = 0.03) and temporal superior region (P = 0.002, adjusted P = 0.01) of RNFL with fourth ventricular volume. There was also a positive correlation between nasal superior RNFL thickness and hippocampus volume (P = 0.02, adjusted P = 0.03) (Table 2). The correlation between thalamus volume and mfVEP latency (A, P = 0.04) as well as forth ventricular volume and temporal RNFL thickness (B, P = 0.01) is plotted and shown in Fig. 2.
Fig. 2.
Scatterplots showing positive association between thalamus volume and multifocal visual evoked potentials (mfVEP) latency (left) and negative association between fourth ventricular volume and temporal retinal nerve fibre layer (RNFL) thickness (right). P-values were examined using the generalised estimating equation (GEE) models.
Association between mfVEP measures and neuropsychological tests
As MRI analysis demonstrated significant associations between mfVEP parameters and structural volumetric changes in the brain, after adjusting for age, sex and eTIV, we sought to determine whether mfVEP indices are also associated with the performance of participants in various neuropsychological tests. Table 3 presents the neuropsychological assessment scores against mfVEP measures for the study participants. Significant positive associations were observed between mfVEP amplitude measurements and the total learning scores (P = 0.03, adjusted P = 0.03), short-delay free recall (P = 0.02, adjusted P = 0.03) and long delay free recall scores (P = 0.008, adjusted P = 0.02) of the CVLT. The greater mfVEP amplitudes were observed to be associated with higher scores for all three sub-tests. Similarly, RCFT delayed recall scores (P = 0.03, adjusted P = 0.03) were identified to be positively associated with mfVEP amplitudes. Data analysis also revealed a negative association between the TMT part A scores and mfVEP amplitude with shorter reaction time for TMT part A associated with higher mfVEP amplitudes (P = 0.01, adjusted P = 0.03). The study also revealed that lower LM delayed recall scores were associated with prolonged mfVEP latency (P = 0.03, adjusted P = 0.03) (Table. 3, data from all parameters including non-significant ones are presented in Supplementary Table 2).
Table 3.
Association between mfVEP measures and Neuropsychological test score.
| Parameters | P-value | B-value |
95 % CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| VEPAmp | ||||
| CVLT-TL | 0.03* | 0.054 | 0.005 | 0.104 |
| CVLT-sdfr | 0.02* | 0.018 | 0.003 | 0.034 |
| CVLT-ldfr | 0.008* | 0.020 | 0.005 | 0.035 |
| TMT-Part A | 0.01* | −0.046 | −0.082 | −0.010 |
| RCFT-Immediate recall | 0.004* | 0.037 | 0.012 | 0.063 |
| RCFT-Delayed recall | 0.03* | 0.031 | 0.003 | 0.059 |
| VEPLat | ||||
| LM-Delay | 0.03* | −0.151 | −0.287 | −0.015 |
Abbreviations: mfVEP = Multifocal visual evoked potential (mfVEP); Amp = Amplitude; Lat = Latency; CVLT = California verbal learning test; TL = Total learning; sdfr = short-delay free recall; ldfr = long-delay free recall; TMT = Trail making test; RCFT = Rey complex figure test, LM = Logical memory. P-values were examined using the generalised estimating equation (GEE) models. *P < 0.05.
Association between RNFL thickness and neuropsychological test scores
There were significant positive associations of various sectoral RNFL thicknesses with CVLT total learning scores, short-delay free recall and long-delay free recall scores (P from 0.005 to 0.04, adjusted P-value 0.02 to 0.05). A statistically significant positive association was also observed between the immediate recall and delayed recall scores of RCFT and sectoral RNFL thickness (P ranging from 0.007 to 0.05, adjusted P-value from 0.01 to 0.05). In addition, MMSE scores also revealed significant positive associations with various sectoral RNFL thicknesses (Table 4).
Table 4.
Association between RNFL thickness and Neuropsychological test score.
| Parameters | P-value | B-value |
95 % CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| RNFL_G | ||||
| CVLT-TL | 0.04* | −4.92 | −9.708 | −0.137 |
| CVLT-ldfr | 0.02* | −2.08 | −3.766 | −0.401 |
| RCFT-Immediate recall | 0.03* | −3.74 | −7.037 | −0.451 |
| RCFT-Delayed recall | 0.03* | −3.63 | −6.842 | −0.407 |
| MMSE | 0.03* | −0.76 | −1.439 | −0.080 |
| RNFL_T | ||||
| CVLT-TL | 0.04* | 1.25 | 0.035 | 2.461 |
| CVLT-ldfr | 0.02* | 0.50 | 0.085 | 0.922 |
| RCFT-Immediate recall | 0.03* | 0.90 | 0.101 | 1.699 |
| RCFT-Delayed recall | 0.03* | 0.90 | 0.115 | 1.682 |
| MMSE | 0.02* | 0.21 | 0.03 | 0.387 |
| RNFL_TS | ||||
| CVLT-TL | 0.05 | 0.54 | 0.007 | 1.066 |
| CVLT-ldfr | 0.02* | 0.23 | 0.038 | 0.417 |
| RCFT-Immediate recall | 0.03* | 0.43 | 0.052 | 0.807 |
| RCFT-Delayed recall | 0.03* | 0.40 | 0.034 | 0.772 |
| RNFL_TI | ||||
| CVLT-ldfr | 0.03* | 0.24 | 0.029 | 0.45 |
| RCFT-Immediate recall | 0.05 | 0.39 | 0.008 | 0.77 |
| RCFT-Delayed recall | 0.03* | 0.42 | 0.038 | 0.792 |
| MMSE | 0.04* | 0.08 | 0.003 | 0.163 |
| RNFL_N | ||||
| CVLT-ldfr | 0.02* | 0.59 | 0.082 | 1.097 |
| RCFT-Immediate recall | 0.04* | 1.08 | 0.055 | 2.1 |
| RCFT-Delayed recall | 0.04 | 1.05 | 0.05 | 2.041 |
| MMSE | 0.03* | 0.22 | 0.017 | 0.426 |
| RNFL_NS | ||||
| CVLT-TL | 0.04* | 0.59 | 0.043 | 1.134 |
| CVLT-ldfr | 0.02* | 0.22 | 0.036 | 0.412 |
| RCFT-Immediate recall | 0.04* | 0.41 | 0.025 | 0.794 |
| RCFT-Delayed recall | 0.03* | 0.41 | 0.035 | 0.777 |
| MMSE | 0.04* | 0.09 | 0.006 | 0.168 |
| RNFL_NI | ||||
| CVLT-TL | 0.02 | 0.61 | 0.103 | 1.117 |
| CVLT-sdfr | 0.04* | 0.18 | 0.005 | 0.354 |
| CVLT-ldfr | 0.005* | 0.26 | 0.078 | 0.449 |
| RCFT-Immediate recall | 0.007* | 0.49 | 0.133 | 0.847 |
| RCFT-Delayed recall | 0.009* | 0.46 | 0.114 | 0.811 |
| MMSE | 0.03* | 0.08 | 0.007 | 0.158 |
Abbreviations: RNFL = Retinal nerve fiber layer; G = Global; T = Temporal; S = Superior; I = Inferior; N = Nasal; CVLT = California verbal learning test; TL = Total learning; sdfr = short-delay free recall; ldfr = long-delay free recall; RCFT = Rey complex figure test; MMSE = Mini-mental state examination. P-values were examined using the generalised estimating equation (GEE) models. *P < 0.05.
Discussion
This study demonstrated significant associations of structural and functional parameters of the visual system with specific brain volumetric indices and cognitive test scores in a healthy aging cohort. The global and sectoral RNFL thicknesses measured by OCT were significantly associated with a decreased volume of total GM, cortex, GM cingulate, thalamus, hippocampal volumes and increase in fourth ventricular volume in MRI, which are known measures of age-related degeneration. There were also significant associations between mfVEP measurements particularly its amplitude and cerebral WM, GM cingulate and thalamus volumes. Further, RNFL and mfVEP parameters were found to be significantly associated with functional measures of the brain as demonstrated by the performance of the participants in neuropsychological tests including CVLT, RCFT, LM, TMT and MMSE. Most statistically significant associations were positive except for associations with forth ventricular volume in MRI and TMT in cognitive function. This was expected as the size of the ventricular system has been shown to increase with age and longer individual TMT test finishing times are demonstrated to represent relative decline in executive function with age [19], [29].
We selected RNFL in OCT to represent the structural aspect in the visual pathway as it has been previously reported to have association with cognitive functions and is one of the most widely used retinal parameter when comparing with brain changes and cognition [49], [17]. There is ample evidence suggesting that OCT can play a role in identification of biomarkers for AD, with studies also examining the potential utility of OCT measures as biomarkers of cognitive function. The studies performed in this field [3], [57], [39] whilst varying in design (prospective, longitudinal, and cross-sectional) have found similar results. Mendez Gomez et al. reported a thicker RNFL associated with greater MRI volumetric measurements and diffusion tensor imaging (DTI) measures in the brain regions involved in the neurodegenerative processes in AD in elderly adults without dementia. Similarly, Shi et al. and Barret-Young et al. found RNFL thinning associated with cortex atrophy and memory decline in older adults and RNFL and GCL thickness to be associated once again with cognitive performance. Whilst most of these studies conclude that further longitudinal studies are needed to determine if retinal thinning precedes cognitive decline, the findings of this cross-sectional study will add to the current interest surrounding the parallels between healthy aging and brain changes especially in the visual functional aspect.
The thalamus is also known to have age-associated regional volume shrinkage, microstructural degradation, connectivity decline and subsequent loss inattention, processing speed, working and episodic memory associated cognitive functions [16]. As RNFL thickness is recognised to have an age-related reduction, our result of reduced volume of total GM, cortex, cingulate, thalamus, hippocampus and fourth ventricular volume enlargement associated with sectoral RNFL thinning is consistent with existing literature. The associations between RNFL thickness and brain volumetric measures also remained after statistical correction for age, thus potentially reflecting biological variability apart from the effects of normal aging. Further long-term follow-up of the study cohort in regard to the potential development of MCI symptoms would elucidate this finding and reflect potential cognitive deterioration. RNFL measures may thus potentially be a marker for volumetric changes in the brain, and the two tissues may exhibit parallel age-related degenerative effects.
This study showed a mild but significant positive association between mfVEP latency and thalamus volume. It is to be noted that retinal signals are directly received by the lateral geniculate nucleus (LGN) region of the thalamus, which forms functional connections with the visual cortex. Recent studies have highlighted that anatomical changes in the thalamus demonstrate an association with MRI diffusion alterations within the optic radiation [44]. In multiple sclerosis (MS) subjects, for instance, a reduction in thalamic volume has been observed [43], which was shown to correlate with optic radiation damage and GCIPL thinning [44]. Any effect on GCIPL or the optic radiation is likely to have an impact on the VEP latency. VEP latency changes are contributed by the complex interplay of retinal and visual cortex processing of signals in addition to thalamic processing, which makes it difficult to determine the role of the thalamus alone in regulating VEP latency. It is therefore acknowledged that prolonged VEP latency in isolation may provide little information about its pathophysiological relationship with thalamus volume change in ageing.
The analysis also showed significant associations between OCT/RNFL thickness and neuropsychological assessment results. Specifically, various immediate and delayed recall measures of CVLT and RCFT demonstrated a correlation with mfVEP and RNFL measurements. Previous studies in an elderly cohort have shown that subjects with higher age perform poorly on RCFT evaluation for both copy and immediate recall as well as delayed recall [6]. A positive association between TMT (part A) time to completion and age of healthy control subjects has also been suggested [2]. As it has been reported that VEP amplitude and mfVEP latency show an age-related decline [52], [9], [35], our findings of increased RCFT immediate/delayed recall and CVLT short/ long delay free recall scores and reduced time for TMT part A associations with higher mfVEP amplitudes in the older healthy cohort is in accordance with previous observations. Similarly previous studies have shown P100 amplitude and mRNFL thickness correlations with brain changes in AD patients [64], with studies concluding that pattern-reversal VEP (pVEP) and spatial frequency sweep VEP can potentially be useful in diagnosing AD [51], [34]. Elderly subjects with thin underlying skull bones and reduced subcutaneous fat tissue may demonstrate higher amplitudes due to improved signal conduction through the electrodes [32]. This study also established that lower CVLT total learning and delayed recalls are associated with decreased mfVEP amplitude and that lower LM delay scores are associated with prolonged mfVEP latency. The total learning scores of CVLT represent attention and learning and have been suggested to differentiate between MCI, AD and normal aging similar to the delayed recalls in discriminant function analysis [23]. These results support previous reports that the performance of subjects on CVLT decreases with age [14].
Similar observations of mild to moderate VEP latency delays in AD subjects compared to controls have been observed [12]. The latency of pattern-reversal VEP measurements has been demonstrated to increase with age over the entire age range for the P60 and N75 components and particularly for the P100 component between the age of 60 to 95 [1]. Interestingly, compared to long-delayed recall scores of CVLT, the short delay free recall scores demonstrated moderately significant relationships across the mfVEP and RNFL measures. It appears that encoding information into memory is more strongly related to mfVEP and RNFL than retention, which is interesting because encoding is more frontal lobe/front subcortical white matter dependent, whereas retention is more hippocampus dependent. It may suggest that mfVEP and RNFL are associated with diffuse atrophy (i.e., aging in general) rather than hippocampus specific effects (as one would see in AD), however, future studies are warranted to validate this hypothesis.
The findings for RCFT parallel the CVLT-II results, suggesting there is evidence for a relationship with poorer encoding, which then has a secondary effect on delayed recall. The results of the present study are also supported by previously reported findings from a longitudinal study which showed that alterations in short delay recall were the first measurement to indicate early cognitive changes while delayed recall performances changed more rapidly further along the AD disease progression scale [5]. The LM delayed score only correlated with mfVEP latency but not with other measurements in our study. A possible explanation may be that unlike the other two memory tests this test involves the presentation of just one story which may not comprehensively reflect the overall cognitive performance of the individual. Using only the single-story paradigm is a modification from standard presentation, which was necessary for the current cohort because the stories are different for the under 65 and over 65 age groups, so we used the story B that is suitable across both groups, however, this may have reduced the reliability of the test. Interestingly, we observed a significant positive association of MMSE scores with sectoral RNFL thickness changes. As one of the most widely used cognitive screening tests, annualized MMSE change has also been shown to correlate with whole-brain atrophy rate in AD patients [58]. The study has also examined the association between brain structural indices and neuropsychological test scores and found significant association among regional volumes and several cognitive domains including memory, visual spatial function and language, which is consistent with previous literature [30], [48].
The current study aimed to comprehensively investigate mfVEP, RNFL thickness, MRI volumetric data and cognitive parameters concurrently. The inclusion of visual functional tests- mfVEP in particular is novel and complements the previous studies conducted by Mammadova and Meijia-Vergara et al who determined retinal thickness correlations with cognitive performance of individuals in healthy cohorts [37], [38].
The battery of neuropsychological assessments can be physically and mentally tiring for older participants, and this limits the translation of comprehensive test protocols for large scale diagnostic applications. Majority of the previous studies have relied on MMSE as a screening test to assess potential association of retinal structure with cognitive function in healthy subjects [13]. More recently Ko et al employed a range of general cognitive functional tests reflecting reaction time and processing speed, and demonstrated that thinner RNFL in OCT was associated with reduced cognitive function [33]. The current study utilised a more comprehensive battery of neuropsychological tests representing various cognitive domains coupled with MRI analysis.
In summary, our findings indicate for the first time that the functional (mfVEP) and structural (OCT) vision test measures may parallel silent and co-existing brain structural and functional changes in the aging population. The cross-sectional design of the study is a limitation of the study which prevents the analysis of causal inferences, and the sample size is not large enough for detailed sub-analyses. The medical conditions are self-reported but verified wherever possible with the patient’s general practitioner. The socioeconomic status and comorbidities such as vascular-risk profile was not controlled for which is a limitation of the study. The axial length and visual acuity are also potential confounding factors when analysing the RNFL thickness and were not adjusted due to lack of data. The study is also limited with the lack of assessment of white matter hyperintensities. However, a strength is that it represents a community-based sample of healthy older individuals, the use of a wide number of parameters in both visual pathway and brain structural aspects as well as detailed neuropsychological evaluation all measured at the same time point. While the visual system anatomical changes can be investigated for subtle functional alteration using mfVEP, similar assessment for various other brain regions may not be possible without conducting comprehensive functional MRI measures. Future studies could focus on functional alterations in the specific brain regions and determining their association with retinal thickness and mfVEP differences in healthy ageing participants, and longitudinal follow up of these subjects will provide a better understanding of the pathophysiology of aging.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Health and Medical Research Council (NHMRC) Australia, Macquarie University, Perpetual Hillcrest, Ophthalmic Research Institute of Australia (ORIA), and National Multiple Sclerosis Society (NMSS), USA. StepUp for Dementia Research is funded by the Australian Government Department of Health and implemented by a dedicated team at the University of Sydney.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbas.2022.100049.
Contributor Information
Ting Shen, Email: drshenting@126.com.
Vivek K. Gupta, Email: vivek.gupta@mq.edu.au.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Allison T., Hume A.L., Wood C.C., Goff W.R. Developmental and aging changes in somatosensory, auditory and visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1984;58:14–24. doi: 10.1016/0013-4694(84)90196-2. [DOI] [PubMed] [Google Scholar]
- 2.Ashendorf L., Jefferson A.L., O'Connor M.K., Chaisson C., Green R.C., Stern R.A. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol. 2008;23:129–137. doi: 10.1016/j.acn.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-Young A., Ambler A., Cheyne K., Guiney H., Kokaua J., Steptoe B., et al. Associations between retinal nerve fiber layer and ganglion cell layer in middle age and cognition from childhood to adulthood. JAMA Ophthalmol. 2022;140:262–268. doi: 10.1001/jamaophthalmol.2021.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamini Y., Krieger A.M., Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 5.Bilgel M., An Y., Lang A., Prince J., Ferrucci L., Jedynak B., et al. Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimer's & Dementia. 2014;10:735–742.e4. doi: 10.1016/j.jalz.2014.04.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone K.B., Lesser I.M., Hill-Gutierrez E., Berman N.G., D'Elia L.F. Rey-Osterrieth Complex Figure performance in healthy, older adults: Relationship to age, education, sex, and IQ. Clin Neuropsychol. 1993;7:22–28. doi: 10.1002/1097-4679(199301)49:1<54::aid-jclp2270490108>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Bright P., Hale E., Gooch V.J., Myhill T., van der Linde I. The National Adult Reading Test: restandardisation against the Wechsler adult intelligence scale—fourth edition. Neuropsychol Rehab. 2018;28:1019–1027. doi: 10.1080/09602011.2016.1231121. [DOI] [PubMed] [Google Scholar]
- 8.Brooks-Wilson A.R. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338. doi: 10.1007/s00439-013-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown A., Corner M., Crewther D., Crewther S. Age related decline in cortical multifocal flash VEP: Latency increases shown to be predominately magnocellular. Front Aging Neurosci. 2019;10:430. doi: 10.3389/fnagi.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner R.L. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bussel I.I., Wollstein G., Schuman J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15-ii19. doi: 10.1136/bjophthalmol-2013-304326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coburn K.L., Arruda J.E., Estes K.M., Amoss R.T. Diagnostic utility of visual evoked potential changes in Alzheimer's disease. J Neuropsych Clin Neurosci. 2003;15:175–179. doi: 10.1176/jnp.15.2.175. [DOI] [PubMed] [Google Scholar]
- 13.Cunha, L. P., Lopes, L. C., Costa-Cunha, L. V. F., Costa, C. F., Pires, L. A., Almeida, A. L. M. & Monteiro, M. L. R. 2016. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s Disease. PLOS ONE, 11, e0153830. [DOI] [PMC free article] [PubMed]
- 14.Delis D.C., Freeland J., Kramer J.H., Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 15.Erskine L., Herrera E. Connecting the retina to the brain. ASN Neuro. 2014;6 doi: 10.1177/1759091414562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fama R., Sullivan E.V. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci Biobehav Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang K., Muthy Z.A., John G., Cathie S., Geraint R., Yang Q., et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. Jama Neurology. 2018 doi: 10.1001/jamaneurol.2018.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fjell A.M., Westlye L.T., Amlien I., Espeseth T., Reinvang I., Raz N., et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Graham E.C., You Y., Yiannikas C., Garrick R., Parratt J., Barnett M.H., et al. Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016;57:2311–2317. doi: 10.1167/iovs.15-19047. [DOI] [PubMed] [Google Scholar]
- 22.Graham S.L., Fortune B. Glaucoma: Medical Diagnosis and Therapy. Elsevier; 2015. Electrophysiology in glaucoma assessment; pp. 149–168. [Google Scholar]
- 23.Greenaway M.C., Lacritz L.H., Binegar D., Weiner M.F., Lipton A., Cullum C.M. Patterns of verbal memory performance in mild cognitive impairment, Alzheimer disease, and normal aging. Cognit. Behav. Neurol. 2006;19:79–84. doi: 10.1097/01.wnn.0000208290.57370.a3. [DOI] [PubMed] [Google Scholar]
- 24.Gupta V.B., Chitranshi N., den Haan J., Mirzaei M., You Y., Lim J.K., et al. Retinal changes in Alzheimer's disease—integrated prospects of imaging, functional and molecular advances. Progr Retinal Eye Res. 2020:100899. doi: 10.1016/j.preteyeres.2020.100899. [DOI] [PubMed] [Google Scholar]
- 25.Harrison J.E., Buxton P., Husain M., Wise R. Short test of semantic and phonological fluency: Normal performance, validity and test-retest reliability. Br J Clin Psychol. 2000;39:181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- 26.Kanamori A., Escano M.F., Eno A., Nakamura M., Maeda H., Seya R., et al. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica. 2003;217:273–278. doi: 10.1159/000070634. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan E.F., Weintraub S. 1982. The Boston Naming Test, ed 2., Philadelphia, Lea&Febiger.
- 28.Kemper, T. L. 1994. Neuroanatomical and neuropathological changes during aging and dementia.
- 29.Kennedy K.J. Age effects on Trail Making Test performance. Percept Mot Skills. 1981;52:671–675. doi: 10.2466/pms.1981.52.2.671. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy K.M., Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klistorner A., Garrick R., Barnett M.H., Graham S.L., Arvind H., Sriram P., et al. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology. 2013;80:242–245. doi: 10.1212/WNL.0b013e31827deb39. [DOI] [PubMed] [Google Scholar]
- 32.Klistorner A.I., Graham S.L. Electroencephalogram-based scaling of multifocal visual evoked potentials: effect on intersubject amplitude variability. Invest Ophthalmol Vis Sci. 2001;42:2145–2152. [PubMed] [Google Scholar]
- 33.Ko F., Muthy Z.A., Gallacher J., Sudlow C., Rees G., Yang Q., et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline. JAMA Neurology. 2018;75:1198. doi: 10.1001/jamaneurol.2018.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kromer R., Serbecic N., Krastel H., Beutelspacher S.C. Comparison of VEP with contrast sensitivity and other measurements of central visual function. Acta Ophthalmol. 2014;92:e141–e146. doi: 10.1111/aos.12176. [DOI] [PubMed] [Google Scholar]
- 35.Kuba M., Kremláček J., Langrová J., Kubová Z., Szanyi J., Vít F. Aging effect in pattern, motion and cognitive visual evoked potentials. Vision Res. 2012;62:9–16. doi: 10.1016/j.visres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Law R., O'Carroll R.E. A comparison of three measures of estimating premorbid intellectual level in dementia of the Alzheimer type. Int J Geriatr Psychiatry. 1998;13:727–730. doi: 10.1002/(sici)1099-1166(1998100)13:10<727::aid-gps851>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Mammadova N., Neppl T.K., Denburg N.L., West Greenlee M.H. Reduced retinal thickness predicts age-related changes in cognitive function. Front Aging Neurosci. 2020;12:81. doi: 10.3389/fnagi.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mejia-Vergara A.J., Karanjia R., Sadun A.A. OCT parameters of the optic nerve head and the retina as surrogate markers of brain volume in a normal population, a pilot study. J Neurol Sci. 2021;420:117213. doi: 10.1016/j.jns.2020.117213. [DOI] [PubMed] [Google Scholar]
- 39.Mendez-Gomez, J. L., Pelletier, A., Rougier, M. B., Korobelnik, J. F., Schweitzer, C., Delyfer, M. N., Catheline, G., Monferme, S., DARTIGUES, J. F., Delcourt, C. & Helmer, C. 2018. Association of retinal nerve fiber layer thickness with brain alterations in the visual and limbic networks in elderly adults without dementia. JAMA Netw Open, 1, e184406. [DOI] [PMC free article] [PubMed]
- 40.Meyers J.E.A.M. Psychological Assessment Resource Inc; 1995. Rey Complex Figure Test and Recognition Trial, Professional Manual. [Google Scholar]
- 41.Morris J.C. The clinical dementia rating (Cdr) –- current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 42.Numbers K., Lam B.C.P., Crawford J.D., Kochan N.A., Sachdev P.S., Brodaty H. Increased reporting of subjective cognitive complaints over time predicts cognitive decline and incident dementia. Int J Geriatric Psychiatry. 2021;36(11):1739–1747. doi: 10.1002/gps.5594. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulou A., Gaetano L., Pfister A., Altermatt A., Tsagkas C., Morency F., et al. Damage of the lateral geniculate nucleus in MS: Assessing the missing node of the visual pathway. Neurology. 2019;92(19):e2240–e2249. doi: 10.1212/WNL.0000000000007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlitzki M., Horbrügger M., Loewe K., Kaufmann J., Opfer R., Wagner M., et al. MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol - Neuroimmunol Neuroinflamm. 2020;7:e665. doi: 10.1212/NXI.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peel N.M., McClure R.J., Bartlett H.P. Behavioral determinants of healthy aging. Am J Prev Med. 2005;28:298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Péran P., Cherubini A., Luccichenti G., Hagberg G., Démonet J.F., Rascol O., et al. Volume and iron content in basal ganglia and thalamus. Hum Brain Mapp. 2009;30:2667–2675. doi: 10.1002/hbm.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A., et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 48.Riekkinen P.J., Hallikainen M., Pitkänen A., Hänninen T., Koivisto K., Vainio P., et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 49.Rufa A., Pretegiani E., Frezzotti P., Stefano N.D., Cevenini G., Dotti M.T., et al. Retinal nerve fiber layer thinning in CADASIL: an optical coherence tomography and MRI study. Cerebrovasc Dis. 2010;31:77–82. doi: 10.1159/000321339. [DOI] [PubMed] [Google Scholar]
- 50.Sachdev P.S., Brodaty H., Reppermund S., Kochan N.A., Trollor J.N., Draper B., et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70–90 years. Int Psychogeriatr. 2010;22:1248–1264. doi: 10.1017/S1041610210001067. [DOI] [PubMed] [Google Scholar]
- 51.Sen S., Saxena R., Vibha D., Tripathi M., Sharma P., Phuljhele S., et al. Detection of structural and electrical disturbances in macula and optic nerve in Alzheimer's patients and their correlation with disease severity. Semin Ophthalmol. 2020;35:116–125. doi: 10.1080/08820538.2020.1748203. [DOI] [PubMed] [Google Scholar]
- 52.Shaw N.A., Cant B.R. Age-dependent changes in the amplitude of the pattern visual evoked potential. Electroencephalogr Clin Neurophysiol. 1981;51:671–673. doi: 10.1016/0013-4694(81)90212-1. [DOI] [PubMed] [Google Scholar]
- 53.Shen T., Gupta V.K., Klistorner A., Chitranshi N., Graham S.L., You Y. Sex-specific effect of BDNF Val66Met genotypes on the progression of open-angle glaucoma. Invest Ophthalmol Vis Sci. 2019;60:1069–1075. doi: 10.1167/iovs.18-26364. [DOI] [PubMed] [Google Scholar]
- 54.Shen T., You Y., Arunachalam S., Fontes A., Liu S., Gupta V., et al. Differing structural and functional patterns of optic nerve damage in multiple sclerosis and neuromyelitis optica spectrum disorder. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 55.Shen T., You Y., Gupta V.K., Graham S.L. Factors Affecting Neurological Aging. Elsevier; 2021. Aging, brain-derived neurotrophic factor (BDNF) and its Val66Met polymorphism: a focus on neuropsychology, the visual system, and brain structures. [Google Scholar]
- 56.Shi Z., Cao X., Hu J., Jiang L., Mei X., Zheng H., et al. Retinal nerve fiber layer thickness is associated with hippocampus and lingual gyrus volumes in nondemented older adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;99 doi: 10.1016/j.pnpbp.2019.109824. [DOI] [PubMed] [Google Scholar]
- 57.Shi Z., Zheng H., Hu J., Jiang L., Cao X., Chen Y., et al. Retinal nerve fiber layer thinning is associated with brain atrophy: a longitudinal study in nondemented older adults. Front Aging Neurosci. 2019;11:69. doi: 10.3389/fnagi.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluimer J.D., van der Flier W.M., Karas G.B., Fox N.C., Scheltens P., Barkhof F., et al. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248:590–598. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- 59.Smith A. Western Psychological Services; 1973. Symbol Digit Modalities Test. [Google Scholar]
- 60.Smith S.R., Servesco A.M., Edwards J.W., Rahban R., Barazani S., Nowinski L.A., et al. Exploring the validity of the comprehensive trail making test. Clin Neuropsychol. 2008;22:507–518. doi: 10.1080/13854040701399269. [DOI] [PubMed] [Google Scholar]
- 61.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 62.Zaletel M., Štrucl M., Rodi Z., Zvan B. The relationship between visually evoked cerebral blood flow velocity responses and visual-evoked potentials. Neuroimage. 2004;22:1784–1789. doi: 10.1016/j.neuroimage.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X., Francis B.A., Dastiridou A., Chopra V., Tan O., Varma R., Greenfield D.S., Schuman J.S., Huang D., Group A.I.F.G.S. Longitudinal and cross-sectional analyses of age effects on retinal nerve fiber layer and ganglion cell complex thickness by Fourier-domain OCT. Transl Vision Sci Technol. 2016;5:1. doi: 10.1167/tvst.5.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao A., Fang F., Li B., Chen Y., Qiu Y., Wu Y., et al. Visual abnormalities associate with hippocampus in mild cognitive impairment and early Alzheimer's disease. Front Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.597491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu W., Wen W., He Y., Xia A., Anstey K.J., Sachdev P. Changing topological patterns in normal aging using large-scale structural networks. Neurobiol Aging. 2012;33:899–913. doi: 10.1016/j.neurobiolaging.2010.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.