Abstract
Specialized individual circuits in the brain are recruited for specific functions. Interestingly, multiple neural circuitries continuously compete with each other to acquire the specialized function. However, the dominant among them compete and become the central neural network for that particular function. For example, the hippocampal principal neural circuitries are the dominant networks among many which are involved in learning processes. But, in the event of damage to the principal circuitry, many times, less dominant networks compensate for the primary network. This review highlights the psychopathologies of functional loss and the aspects of functional recuperation in the absence of the hippocampus.
Keywords: Amnesia, Hippocampus, Learning & memory, Prefrontal cortex, Neuronal plasticity, Neurodegeneration, Aging
Introduction
One of the outstanding characteristics of the human brain is its ability to learn and adjust behavior based on experiences and memories. To produce such complex behaviors, the brain continuously undergoes structural as well as functional re-organization [177]. Brain plasticity is the ability of the neural network to rearrange its connections and functions, both at the system (circuit) and molecular (synaptic) levels in response to internal or external inputs [142]. For instance, learning-induced plasticity involves activation of postsynaptic NMDA glutamate receptor (NMDA-R) induced influx of Ca2+, followed by downstream signaling via secondary messengers, ultimately triggering long-term structural and functional changes in the neurons [109], [147], [230]. Extensive training or learning re-organizes the neural circuitries/network and helps the brain to store and retrieve the learned information for a longer period. If the affected circuitry is damaged, it results in a lifelong cognitive deficit. But interestingly, some of the lost abilities can be regained and compensated [206]. In this review, we are highlighting the events of structural and functional recovery and compensatory cognitive ability after the loss of the principal network.
Memories are encoded via a dedicated group of neuronal populations, which are initially active at the time of acquisition, and re-activated in their later phase at the time of retrieval [170]. According to the multiple memory system’s theory, distinct circuits are specialized to perform specific functions [160], [68]. These circuits do, however, engage in competition, with the dominant one performing the specific function. Studies suggest that synapses undergo synaptic scaling, which provides overall weight normalization at the neuronal level. As a result, competition is generated between different neuronal populations [234], [241], [230]. Different situations, such as perceptual categorization, action selection, decision-making, and attention, have all been thought to involve competitive selection [168]. Fear learning is the most well-known instance, which is mediated through multiple pathways (primary and alternative pathways) [68]. Fear-conditioned learning is dominated by the primary ‘dorsal hippocampus’ pathway. It normally sends inhibitory signals to the alternative prefrontal cortical pathways. These alternative pathways, however, are free from inhibitions in the absence of the primary pathway and hence mimic its compensatory functions, albeit less effectively.
The process of memory encompasses sequential events for acquisition, storage, and retrieval of new information. The physical and neural imprints of memories are encoded in the form of engrams [179], [212], [63], [115], [149], [95]. Conventionally, memory is broadly classified into long-term memory and short-term memory. Long-term memory is further divided into (A) declarative memory (i.e., explicit knowledge of facts and events), which is further sub-divided into (a) episodic memory (spatial and context-related memories) and (b) semantic memory (memories of facts and figures), and (B) Non-declarative memory (implicitly acquired, of unconscious knowledge) involves procedural learning, priming, etc. [20]. A broad classification of different memory systems is shown in Fig. 1. Short-term memory comprises working memory, which includes temporary storage and manipulation of sensory information for a very brief period [189], [10], [148]. The classical conditioning and non-associative forms of memory, including skill-based knowledge, develop gradually but with little ability to report what is being learned [68]. The consolidation of declarative memories involves the hippocampus and structures of the medial temporal lobe (MTL) for the processing of memory information during its acquisition and consolidation. On the contrary, non-declarative memories rely on structures like the neocortex, amygdala, and cerebellum. Further, within the neocortex, different sub-regions are thought to underlie different types of semantic knowledge [157], [156], [227]. For instance, knowledge of words and faces is mediated by the areas in the fusiform gyrus, whereas knowledge of the higher concepts is handled by anterior temporal regions [157], [27], [12]. The hippocampus is believed to be the central coordinator of memory processing as it combines an event’s spatial, temporal, and contextual attributes to form a conjunctive representation [62], [63], [41]. To perform this, it acquires the information either from a single network or multiple brain areas to stabilize a specific memory as a neuronal engram [67], [179], [212], [116]. The engram cells play an essential role in the stable representation of memory [231]. For instance, contextual fear conditioning is a learning paradigm used in animal models to study associative memory. When a neutral stimulus (context or tone) is paired with an aversive stimulus (e.g., electrical shock), the animal shows a conditioned response, i.e., freezing even in the absence of electrical shock. The attributes of the contextual and aversive stimuli are acquired individually by separate networks in different brain areas, the association is, however, formed in the hippocampal neurons [155], [154], [61]. The hippocampus acquires contextual representation, and the associative connections between the hippocampus and amygdala are strengthened in response to the electrical shock. Such processes suggest that the encoding simultaneously takes place in multiple memory-associated brain areas [171], [67], [169], [198], [13], [86]. Damage to these areas causes profound memory impairment leading to both retrograde as well as anterograde amnesia.
Fig. 1.
A broad classification of memory. Based on the attributes of memory retrieval patterns (short-lasting and long-tasting memories), memory is classified into two categories. Similarly, long term-memory is classified into two broad categories based on the active and passive nature of recollection.
There is very little possibility that cognitive function will improve following neuronal injury. In prodromal and early-stage neurodegenerative and neuropsychiatric illnesses, the patients can function at a level that is comparable to the general population while showing clear evidence of cortical and subcortical loss. This upkeep of typical performance is thought to result from the initiation of compensatory mechanisms, or adjustments in brain activation within a network specialized to a task, or in the recruitment of a region outside of the task network. The subject may improve their cognitive functions either (a) through behavioral adaptation and/or (b) by the development of compensatory neural pathways in the brain.
Behavioral compensation
Whether the behavioral alterations are merely the result of compensatory methods used in response to a damaged neurological system remains a crucial question. However, studies indicate that there might be a connection between subjective health and compensatory skills. [77], [129]. The compensatory strategies help the subjects to learn specific methods and skills to compensate for the cognitive deficiency and certain case reports highlight such compensatory strategies to improve cognitive functions. [78].
The patient JC: a curious case of behavioral compensation
The concept of memory compensation after the damage of the memory-associated areas dates back to a curious case of patient JC [247]. JC suffered from left cerebral artery aneurysm due to subarachnoid hemorrhage in an accident at the age of 20 years. After the accident, he was diagnosed with selective memory impairments (anterograde amnesia), along with severe epileptic seizures. During his clinical assessments, based on general IQ such as naming, reading, and visuospatial; perceptual & executive functions, he was able to score remarkably well. But, his scores were not comparable to those of normal healthy adults when assessed on memory tests/scales. Interestingly, he was able to remember events that preceded the accident during the retrograde amnesia tests. Also, his immediate memory seemed to be intact. JC’s initial treatment approaches to improve the condition included using mnemonics diaries, rehearsal strategies, and even splitting up a long task into small steps. These treatment strategies helped him to retain some information, but he still faced problems with routine memory tasks.
Gradually, he started following the habit of making notes and continued it for several years. For instance, he started making lists of his accounts and keeping dictaphone recordings for things he needed to remember in the near future. For example, telephone calls, things he had to buy, etc. This way, he was finally able to develop a compensatory system that could enable him to survive independently. Since his implicit memory was seemingly intact, he used it to develop skills that could help him compensate for other memory impairments.
JC developed ‘the system of compensatory memory’ into a foolproof system. He modified his system of compensatory memory many times. Even after so many modifications, in a common men’s view, it appeared to be a herculean task to make JC’s system of compensatory memory a foolproof system. However, it cannot be denied that JC had developed a very effective system to recall the partially consolidated/labile memory of the amnesic subjects. It is intriguing that JC was able to recall some of the memories, but under similar conditions, others could not. Later, Wilson and Watson [248] proposed that younger people (∼30 years of age) can develop compensatory memory relatively in better way than the aged subjects [248]. They also observed that the amnesic subjects without additional cognitive deficits developed memory compensation more adequately than the subjects having additional cognitive issues [248].
Neural compensation
Studies have demonstrated that in the events of brain damage, a neural compensation process also occurs. For example, the bilateral labyrinthectomy severely impairs postural stability but gradually with time the animal can stand unsupported, ataxia profoundly reduces, and locomotion speeds gradually increase [159]. Further, neuroimaging data show that the restructuring of cerebral resources in older persons may help them make up for cognitive and brain function deficits [114]. Similarly, in MRI findings on neurodegenerative disease, compensation is typically shown in the early stages of a variety of conditions, such as Alzheimer's and Parkinson's disease [88]. Compensatory mechanisms develop in older subjects and subjects suffering from neurodegenerative diseases, however, we have to have a clear understanding of how it can develop.
Development of compensatory pathways in neurodegenerative disorders
Alzheimer’s disease (AD)
Alzheimer's disease (AD) is the leading cause of dementia and cognitive decline in older populations. It is mainly characterized by the formation of amyloid β (Aβ) plaques and neurofibrillary tangles, exhibiting substantial loss of synapse and spine density [219]. Selective damage in brain areas involves the neocortex, hippocampus, amygdala, and limited regions of the medial nucleus of the thalamus, locus coeruleus, frontal & cingulate cortex, etc. [246], [214], [223]. Several studies have reported gradual and progressive deterioration of episodic memories [167], [210]. In the animal model of AD, various studies have demonstrated the compensation in terms of either cognitive performance or associated pathology. For example, Billings et al. have shown that training of spatial learning tasks such as longitudinal water maze produces a significant but transient improvement in learning other tasks, as well as a reduction in the deposition of amyloid-beta and tau proteins [19]. In the triple-transgenic mouse model of AD (3 × Tg-AD), the mice were trained and tested at 3-month intervals from 2 to 18 months. The performance improvement was observed after six months. Interestingly, it further delays the redistribution of amyloid-beta proteins to extracellular plaques and reduces the deposition of its oligomers associated with cognitive decline. Such a study demonstrates that repeated spatial training can significantly delay the development of neuropathology and a decline in spatial memory. Similarly, in another study, Jankowsky et al. reported the role of environmental enrichment in the alleviation of Alzheimer’s-associated learning and memory deficits. Intriguingly, AD mice showed a significant improvement in cognitive performances with environmental enrichment compared to the control group of animals [111]. Further, in another study, transgenic mice overexpressing Swedish doubly mutant amyloid precursor protein(APP), housed in the enriched environment were found to have significant global improvements in cognitive functions when compared with those put in standard housing for four months [7].
Mild cognitive impairment (MCI)
Studies on human subjects with mild cognitive impairment (MCI) have shown hyper-activation in the hippocampus, posterior medial temporal, and connected fusiform regions during visual memory tasks when compared to the older control group [57], [94], [125]. Another study using a similar methodology instead showed higher activation of the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC) in patients with MCI when compared with cognitively normal age-matched controls. In a SPECT study [30], voxel-based morphometric analysis was performed to obtain brain images of patients with incipient AD to investigate the relationship between grey matter (GM) atrophy and functional alteration (in terms of hypo-perfusion). On masking the atrophic area maps with those of the non-significantly hypo-perfused ones, they observed the atrophic areas (grey matter, white matter, or CSF) without significant functional deficits, which they referred to as functional compensatory areas, and the areas were posterior cingulate cortex, amygdala, the dorsal hippocampi, and the insular cortex.
Huntington’s disease (HD)
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease marked by chorea (involuntary, irregular unpredictable body movements), cognitive issues, and mood abnormalities, especially depression [203]. Initially, neurodegeneration begins in the dorsal striatum and later affects ventral striatal areas to some extent. Malejko et al. investigated the possibility of functional compensation of early neurodegeneration in prodromal Huntington's disease using structural and functional MRI [150]. They reported that patients with HD exhibited structural changes in the dorsal striatal area with marginal changes in the volume of the ventral striatal area. The patients showed prolonged reaction time in the motor response tasks, however, behavioral performance in the rewarding task was intact. Interestingly, the reward-associated fMRI signaling in the HD group was significantly different among the bilateral sides in the ventral striatum, orbitofrontal cortex/anterior insula, and other reward-associated areas [150]. Another PET study has demonstrated that the activation responses during learning significantly increased in the left mediodorsal thalamus and orbitofrontal cortex in pre-symptomatic HD patients [72]. However, Klerman et al observed that brain atrophy enhanced performance-related activity in the right parietal cortex along with the increased functional coupling between the right prefrontal cortex and a left hemisphere network [127]. This lateralized activation could be attributed to its pathophysiology where the left hemisphere is shown to be more susceptible to neurodegeneration [134], [251], [127], [165]. All these demonstrate the development of active compensatory processes in premanifest-HD, which could be attributed to their increased cognitive demands [196], [83], [87], [150], [127], [252].
Development of compensatory pathways in neuropsychiatric disorders
Obsessive-compulsive disorder (OCD)
Evidence suggests that patients with OCD may experience difficulties in the formation of working memory [54], [33] and some other cognitive functions [35], [201]. Working memory primarily depends on the frontoparietal network. However, de Vries et al. have reported hyperactivity in left dlPFC, left premotor/pre-supplementary motor area (SMA), and left precuneus areas after visuospatial working memory task when compared to healthy participants [52]. Another study also compared patterns of brain activity using fMRI in patients with OCD and healthy controls during spatial and verbal memory tasks [102]. Hyperactivity was observed in the regions of the left inferior frontal cortex, the middle part of the left inferior frontal sulcus, the left inferior frontal junctional (IFJ) area, and the intraparietal cortex. These brain areas are responsible for the articulatory rehearsal and phonetic maintenance of verbal information [90], [91], and the same areas have been reasoned as the compensatory areas to overcome working memory deficiency in patients with OCD.
Posttraumatic stress disorder
Post-traumatic stress disorder (PTSD) is characterized by recurrent memories from traumatic events in the past. It is a robust negative memory and, once acquired, remains for a lifetime. The life of an individual becomes miserable with time. Brain structural aberrations, such as a reduction in the hippocampal and ventromedial prefrontal cortex (vmPFC) volumes, have been found in PTSD patients [24], [92], [228]. A few studies have reported that PTSD is associated with selective volume loss of the CA3 and dentate gyrus subfields in the hippocampus [244]. It may be causing the inability to acquire context information, which is thought to be mediated through the dorsal hippocampus [120]. Nevertheless, studies have also reported the role of the basolateral amygdala (BLA) in the acquisition and expression of fear memory, which works in concert with the DH and vmPFC [11]. The dorsal hippocampal lesion causes a profound memory deficit [120], while the BLA seems to be playing an essential role in consolidating the fear memories in other brain areas [69], [153], [50], [186]. It is, therefore, likely that the hippocampus may encode the contextual information by forming an association with the aversive information, shock, for instance, by interacting with the BLA.
In rodents, lesions of the dorsal hippocampus ubiquitously extinguish context fear memories. However, animals whose hippocampus has been damaged before conditioning display the unique ability to overcome hippocampal loss, the mechanism of which remains unknown. Evidence for this neuronal compensation after the dorsal hippocampal loss was primarily reported by Wiltgen et al., wherein the formation of compensatory circuits after DH damage through training up to 16th-day post-lesioning was reported [249]. After the complete excitotoxic DH lesion, five training trials induced normal levels of contextual learning in rats. The study suggested the role of the neocortex in the acquisition of contextual fear memories in the absence of the dorsal hippocampus. However, the rate of learning is comparatively slower than the hippocampus [249]. The neocortex is thought to be the putative hippocampal independent learning area, albeit less efficient, since the neocortex, in concert with the hippocampus, drives the process of learning in intact mammals [179]. Similar results have also been observed in another study [137]. Although there is sufficient evidence for the compensation taking place for DH damage, the exact site and the nature of the compensation have not been explored yet. To determine its temporal aspects and whether the compensatory learning is as stable as that of the intact hippocampus, excitotoxic lesions of DH were performed in rats using a similar paradigm in another study. Rats were tested for contextual fear conditioning on the 1st, 3rd, 10th, and 30th-day post-training, to observe the temporal changes in fear expression. The results showed that the expression of contextual fear memories formed after the hippocampal damage diminished over time [254]. It is likely that the compensatory memories could be volatile in nature or a short-lived event. Interestingly, Fanselow has proposed an alternate hypothesis that the compensatory circuits form contextual memories only in the absence of the hippocampus but not in the presence of the hippocampus [68].
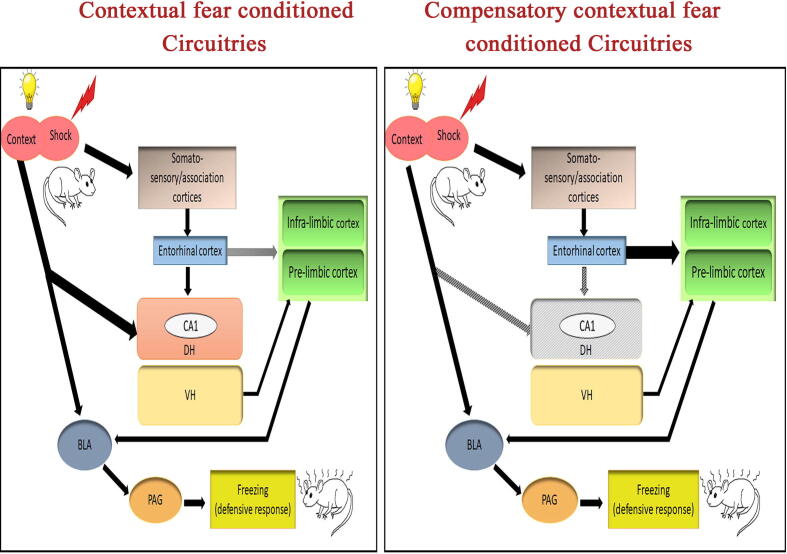
A recent study has hypothesized the recruitment of the infra-limbic (IL) and pre-limbic (PL) prefrontal cortices for compensatory memory following hippocampal damage. The PFC has a well-established role in the storage of long-term contextual fear memories. In this study, post-training lesions of the DH in rats showed a significant freezing response, which was no different from the sham group. In addition, the DH + IL or DH + PL lesioned rats were also examined to see the individual roles of these sub-regions, which were found to have completely lost the compensatory context fear in both conditions. This concluded that both IL and PL are individually necessary for the formation of compensatory circuitry after DH damage. In addition, the compensatory context memories also correspond to an increase and decrease in IL and PL activities, respectively [255]. The study was further extended to see whether a cross-talk between IL and PL results in the development of compensation for the DH. The DH + IL lesioned animals did not show any compensatory context-specific fear. Contralateral lesions of IL and PL and subsequent testing for contextual fear showed the progression of compensatory contextual fear conditioning [255]. The IL and PL have contrastingly different roles in fear expression [84], [239], and also send their connections to different sub-regions within the amygdala [238], [79], The medial prefrontal cortex may be a prospective site for the compensation of DH primarily because of its role in the long-term storage of remote contextual fear memories. Hence, it is likely that when the DH is intact, the prefrontal cortex and amygdala may be receiving the contextual fear memory along with the DH. Since the DH network could be acting as the primary and dominant network for the formation of an association of fear and context memories, the subsidiary network such as mPFC may not be that strong initially for the formation of the association in the presence of the DH. Nevertheless, in the absence of the DH, the mPFC network must have gained the property to compensate for the loss of DH. Very recently, it has been reported that memory deficits in hipocampus lesioned mice could be rescued using the cortical application of induced synchronized oscillatory signals (iSOS) in a contextual fear memory task. The freezing levels of control mice were found to be similar to those of the hippocampal-lesioned rescued mice [146]. The neural circuitry for contextual fear conditioning is thought to involve the dorsal hippocampus as the primary area. Individual contextual (neutral and aversive) inputs are separately acquired in different somatosensory/association cortices, from where these inputs are relayed onto the DH, as well as to the amygdala through the entorhinal cortex (EC) [211], [37], [34], [124]. The DH integrates these inputs to form a consolidated memory, which is later on transferred to higher cortical areas for long-term storage. In the absence of DH, the inputs coming from EC will no longer be able to relay the information to the DH. We reasoned that in such conditions, an existing connection between EC and mPFC will be strengthened and the CS-US association will be formed in the mPFC area in the absence of DH (Fig. 2).
Fig. 2.
Possible contextual and compensatory contextual fear conditioning circuitries in the brain. The sensory signals ‘conditioned stimulus’ (CS) and ‘unconditioned stimulus (US) are acquired individually in somatosensory cortices, dorsal hippocampus (DH), and basolateral amygdala (BLA). The BLA through the periaqueductal grey area helps in the induction of freezing behavior during memory retrieval. The somatosensory cortex project to the DH via the entorhinal cortex (EC). On the other hand, EC also projects to the medial prefrontal cortex (mPFC) which includes infralimbic (IL) and prelimbic (PL) areas. In the absence of DH, the CS and US signals will no more be able to integrate into the DH. Rather the EC may be able to send the CS & US signals to the IL & PL with multiple training trials. Hence, in the absence of DH, the development of EC-mPFC circuitry with time may help mPFC to take over the role of DH as a compensatory contextual fear conditioning center. Arrowheads denote neuronal projections from one area to another. The thickness of the arrows depicts the strength of connections between those areas. Black arrows show active projections. Grey arrows show damaged/inactive projections.
In parallel reports, the BLA lesions during the pre-training period abolish fear acquisition [191], [101], [80]. But, this loss of fear conditioning can be regained via excessive training [193], [196], [222]. This again supports the view of the existence of compensatory pathways, which take over the functions, albeit less efficiently. Another study has reported that the Bed nuclei of stria terminalis (BST) compensates for the excitotoxically damaged BLA. The freezing levels after training in BLA-damaged rats were similar to those of the intact rats [199]. Also, when BLA was lesioned along with BST, the freezing behavior was significantly reduced, suggesting that context fear acquisition was lost in the absence of BST [196].
Development of compensatory pathways in the aged brain
Apart from the causes due to underlying disease conditions, cognitive deficits have also been prevalent in the geriatric population. However, it is still uncertain whether cognitive functions necessarily decline with increasing age. The brain size, vasculature, and cognitive ability vary as we become older. With advancing age, the brain shrinks, and changes occur at every level from molecular (biochemical synthesis and release) to morphological as well as physiological levels.
Volumetric changes in the brain with aging
Aging induces structural, physiological, and behavioral changes in the brain. Aging commonly comes with cognitive decline [26], though it can’t be generalized [74]. The magnitude of cognitive decline varies for different cognitive processes. For example, the executive functions are typically the first to show impairment during normal aging, language abilities remain relatively intact [163], or even improve in terms of vocabulary, semantics, and speech processing [122], [237], [9].
Structural alterations have been universally reported in the form of gray and white matter atrophy [4], [40], [110], [97]. An fMRI study used voxel-based morphometric analysis to investigate differences in grey and white matter tissue volumes [70]. A significant reduction was found in the case of grey matter volume in the older population as compared to the younger ones, predominantly in the frontal, cingular and insular cortices. However, limbic and para-limbic regions were spared. Additionally, when compared between males and females separately, there was a steeper decline in grey matter volumes in females when compared with the males in both younger and older populations. On analyzing the white matter volumes, again, a negative correlation was found, mostly in the thalamic regions, in the older population as compared to the young ones. Apart from cortical areas, grey matter atrophy has also been reported in cerebellar regions [1], [2], [202], some of which are involved in cognition (language-specific and executive functions) rather than motor functions [25]. However, no significant gender differences were observed in this case. Similar patterns of decreased grey and white matter volumes with advancing age have been observed in other studies [89], [47], [119]. Although aging is known to be associated with grey matter atrophy, the degree of this atrophy is heterogeneous across the elderly population [73].
Alterations in neurotransmitter signaling with aging
Aberrant neuronal network activity in terms of altered neurotransmission is commonly reported in the aging brain. The excitatory neurotransmitter ‘Glutamate’ plays an essential role in memory consolidation through the process of long-term potentiation. Age-related decline in glutamate neurotransmission has been well reported [107], [130]. Aging-associated decreased glutamate levels have been observed in the hippocampus and areas of the cerebral cortex in rodents [218], [256], [93] and also in humans [216], [118], [253]. Moreover, it has been shown that this decline in glutamate levels is steeper in males than in females of the same age group [36]. Another study using proton MR spectroscopy investigated age-related changes in glutamate and glutamine levels in the brain between normal and aged populations. Reduced glutamate levels were observed in the grey matter motor cortex in the older population when compared with the younger subjects. At the same time, higher concentrations of glutamine were observed in the white matter corona radiata in the older population [118]. A recent study found decreased glutamate concentrations and significantly higher concentrations of glutamine in the older population compared with younger adults [209]. The higher concentrations of glutamine could imply impaired glutamate metabolism due to disequilibrium in the astroglial neurons since glial cells are the sources of glutamine. In addition, a decrease in the number of glutamatergic neurons has also been observed in aged rats in the cortical regions [104].
Another neurotransmitter, acetylcholine, is major neurotransmitter of the cholinergic system involved in the regulation of cortical activity [82]. Deterioration of cholinergic neurons with aging progressively leads to learning and memory deficits. Further, the enzymatic activity of Choline acetyltransferase (ChAT) (required for the synthesis of acetylcholine) in the hippocampus and cortex reduces progressively with age [189], [53]. In contrast, there are also reports which have found no significant difference in ChAT activity in the cortex as well as caudate-putamen region between younger and older populations [85], [44]. In addition, the muscarinic, as well as nicotinic receptor binding, has also been reported to be reduced in the hippocampus, entorhinal cortex, caudate, putamen, as well as other cortical areas [208], [45], [100].
Dopamine is essentially involved in the regulation of movement and control functions. It is also a major contributor to reward learning, motivation, and behavior. Age-related physiological alterations have been shown to reduce the number of dopaminergic neurons in the substantia nigra region, both in rodents [81], [174] and in humans [205], which is associated with motor impairments. In contrast, few reports demonstrate no significant differences in the number of dopaminergic neurons between control and aged subjects [71]. Deficits in dopaminergic signaling have also been associated with a decrease in several neurotrophic factors like brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and other neurotrophins [221]. Loss of these trophic factors has been shown to augment the onset of neurodegenerative disorders [184].
Several studies using MR spectroscopy revealed decreased GABAergic signaling in aging brains in both animal models [96]) and humans [143]; particularly the one mediated by GABA-A receptors [98], [207], [161], [195]. In addition, other neurotransmitter systems such as serotonergic, noradrenergic, etc. are also affected in the aged brain in terms of hyper-excitability and cytotoxicity [28]. An aging brain does not significantly lead to the deterioration of neurotransmitter signaling. Hence, not all functions are completely lost. However, a dysfunctional system of neurotransmission may augment the process of neurodegeneration and increase the vulnerability to age-related neurodegeneration [82].
Structural and morphometric neural alterations with aging
Several studies report a decrease in arborization of the dendritic trees of pyramidal neurons, mainly found in the prefrontal, superior temporal, and pre-central gyrus in humans [172], [51], primates [183], [58], [117] and mice [220], followed by loss of dendritic spines [185] and reduction in the number of oligodendrocytes [188], [65]. Disorganization of the myelin sheath along with axonal reduction has also been observed, which directly affects the conduction velocity. The altered action potential of neurons due to improper axonal insulation ultimately leads to cognitive and behavioral decline [190], [112], [22]. In addition, the role of glial cells in aging (other than their committed roles in maintaining synaptic homeostasis, glycogen storage, and synapse development) have also been emerging in reports [225].
Compensation for age-related neurodegeneration
Evidence of an age-related decrease in occipital activity with a substantial increase in frontal activity [also known as “posterior-anterior shift in aging” (PASA)], has provided models of compensatory cognition through various neuroimaging techniques. Few studies [140] investigated memory-dependent decision-making in younger and older populations using fMRI studies. Additional activation of the vmPFC apart from the regions of the frontoparietal network (dlPFC) was significantly higher in older than younger adults in a delayed vs intermediate memory choice task. Another fMRI study analyzed the encoding of face-name associations of healthy individuals. They found that the right hippocampus showed stronger neuronal activity during tasks of low as well as high encoding demands compared to baseline conditions in tasks. In contrast, the left hippocampus showed higher activities only in tasks requiring intermediate & high encoding demands. It was hypothesized that the left half might be an additionally recruited area to avoid a sharp decline in memory performance of the hippocampus of the right hemisphere. Some additional MRI studies have also reported an increase in regional cerebral blood flow in inferior frontal and anterior temporal cortices in early AD patients as compared to normal [145], [48], [140]. However, whether this increase correlates with the increase in cognitive functions is not known. While functional compensation is evident even after substantial damage to the memory systems, the reports are only limited to mild or no impairments. Nevertheless, the above reports are in line with the present hypothesis that in the absence of a brain circuit underlying a particular memory function, alternate neuronal circuities may be recruited to perform the functions.
Development of compensatory pathways and sleep
Role of sleep in memory consolidation and compensatory memory
Sleep is beneficial for the brain and body, and enhances memory consolidation and retention. Even a 6 min daytime nap helps facilitate memory [233], [128], [133]. It is known that during sleep, there is a replay of the firing pattern of the place cells as observed in the rats, similar to the one seen during active encoding. Also, studies have reported neuronal reactivation through the firing of similar patterns but with higher frequency during sleep [178]. These reactivations are generally observed in the hippocampus as sharp-wave ripples (having field potential oscillations of about 180 Hz) [56] during slow-wave sleep (SWS). SWS, in particular, is beneficial for the consolidation of declarative memories. In an fMRI study, it was found that hippocampal areas that were active during a spatial working memory task in subjects were reactivated during NREM sleep [187]. However, some studies have reported that this neuronal replay occurs during wakefulness as well. Such processes are often referred to as offline consolidation. [121], [162], [226]. Therefore, it is evident that sleep plays a permissive role in memory consolidation.
Sleep architecture may not necessarily change uniformly after learning different types of tasks. For example, cued fear conditioning is a sleep-dependent task but it may not require robust augmentation in sleep amount as such. Although, sleep architecture changes in fear-conditioned animals, NREM sleep amount increases while REM sleep amount decreases during 24 h of sleep-wake (S-W) recording after fear conditioning [113], [99], [131]. Nevertheless, such changes in sleep architecture did not occur in compensatory contextual memory (in the absence of DH). REM sleep remains suppressed after cued-fear conditioning [113], [131] and CxFC [200]. We have reported that REM sleep decreases after CxFC in DH-non-lesioned animals, even after multiple conditioning [120]. Moreover, we have recently reported that after cued-fear conditioning, REM sleep exclusively decreased in the consolidated memory group but not in the memory-impaired group [132]. We observed that DH-lesioned rats could express context fear after the second conditioning; nevertheless, it did not alter their REM sleep amount. No change in REM sleep amount in the DH-lesioned rats suggests that it might be related to the consolidation of fearful memories in some alternative brain circuits, such as the medial prefrontal cortex (mPFC), which may not influence sleep architecture.
Sleep disturbances and aging
It was observed that most of the age-related changes in sleep occur after the age of sixty [182]. With aging, the architecture of sleep shifts from the conventional characteristics in terms of prominent changes in the duration of sleep, as well as different vigilant sleep states, to micro-level changes like quantity/quality of sleep [152]. Amount of slow wave sleep (SWS) and K-complexes during NREM sleep stage, total sleep time, and sleep efficiency, as well as latency, reduced with aging [166], [138], [75], [158], [59]. Also, increased wakefulness during the nighttime has been prevalently reported [245], [236], [204], [126], [43], [240]. The medial prefrontal atrophy in the older age group has been correlated with disturbed sleep in terms of reduced SWS [151], which also led to the impairment in hippocampal-dependent memory [151]. Naps during the daytime have been considered a normal routine in most cases. However, this phenomenon is more prevalent in the older population as compared to the younger ones [76], [164], [66].
The putative neurobiological mechanisms underlying sleep alterations in aging involve neurodegenerative or neuropsychiatric disorders, sleep disorders, hormonal imbalances, or other abnormalities associated with aging [144]. It has been reported that a decrease in the release of galanin neuropeptide from the ventrolateral preoptic/intermediate nuclei has been correlated with advancing age [141]. In addition, a 40 % reduction in orexin-expressing neurons has been reported in older rats as compared to younger ones [123]. Sleep is predominantly regulated by circadian rhythmicity and homeostatic drive, which is further regulated by extracellular adenosine, a metabolic byproduct that accumulates while we are awake [60], [108]. Age-associated changes in adenosine production, as well as adenosine receptor levels, have been reported [38], [31], [213]. Hence, these as well as many other components could be attributed to the cause of sleep disturbances in the aging population.
Role of oscillatory waves of the brain in compensatory memory
Sleep-associated oscillatory waves such as hippocampal theta oscillations during REM sleep and gamma waves play a crucial role in the consolidation of memories. Polysomnographic studies demonstrate that theta activity remains elevated during consolidation as well as retrieval after context-dependent memory tasks [194], [5], [105], [23], [39], [46]. Overnight alterations in theta coherence between the hippocampus, medial prefrontal cortex, and amygdala during paradoxical sleep were specifically linked with changes in fear memory and thus it has been proposed that coherent theta activity in the limbic system promotes fear memory during paradoxical sleep [194]. Further, optogenetically induced theta rhythms (7 Hz) in the CA1 area rescued the consolidation of contextual fear memory in the sleep-deprived animals [180]. These studies suggest that REM sleep components such as theta waves during REM sleep plays an essential role in the consolidation of fear memories.
In addition to theta activity, gamma activity (25–100 Hz) has also been recognized for its role in cognitive function, including memory. It has been discovered that both human AD patients and rodent AD models exhibit aberrant gamma oscillations. Gamma entrainment has been demonstrated in recent studies to considerably lessen AD pathology and improve cognitive function in AD-prone mouse models through auditory and visual sensory stimulation. According to the initial evidence from AD patients, gamma entrainment therapy can alleviate several degenerative disease markers, reduce brain shrinkage and loss of functional connectivity, and enhance cognitive function [232].
Development of compensatory pathways and adult hippocampal neurogenesis
Increased neurogenesis from multipotent neural stem cells in the subgranular zone of the hippocampus promotes hippocampal-dependent learning [136], [15], [16]. Further, its suppression leads to memory impairment [55], [103], [224]. Additionally, impaired adult neurogenesis has been observed widely in various animal models of neurodegenerative disorders [250], [217], and in aged animals [8]. Few studies have proposed that environmental enrichment and exercise, both act as strong neurogenic stimuli, and may have therapeutic benefits to minimize the adverse effects of aging, and neurodegeneration [106], [243]. However, the role of adult neurogenesis in compensatory cognition of neurodegeneration is not known. Interestingly, in a recent study on rats, it was observed that one side hippocampectomy induced compensatory neurogenic response in the opposite hippocampal region. It was proposed that it may have been the reason for a considerable absence of a cognitive deficit in human patients who underwent unilateral hippocampectomy to treat refractory temporal lobe epilepsy [29]. The lack of cognitive deficiency in the patients of unilateral hippocampectomy could be attributed to the increased neurogenesis in the opposite hippocampus as a compensatory approach. Nevertheless, future studies await to provide in-depth knowledge of the role of adult neurogenesis and the development of compensatory processes.
Influence of brain and cognitive reserve on the development of compensatory pathways
JC’s case explained above was undoubtedly extraordinary, wherein he enabled himself to develop behavioral compensatory systems to live independently. Although, it is not known whether his compensatory behavior induced the neural circuit reorganization to allow neural compensation. However, numerous reports have shown that behavioral adjustments may induce cytoarchitectural changes including an increase in synaptic strength and synaptic turnover. A concept of cognitive reserve has been proposed which explains why there is a difference between a person's measurable level of brain pathology and expected cognitive performances. The brain reserve is a person's unique sensitivity to brain damage, which mainly depends on two factors: (a) the severity of the damage to the brain, and (b) quantitative measurement of brain reserve capacity (the brain size, number of neurons and projections, number of synapses, etc). Brain’s functional capacity decline when neurodegenerative processes diminish brain reserve capacity below a specific limit. In contrast to brain reserve, the cognitive reserve states that the threshold for the functional decline can be changed based on experience rather than being determined by quantitative brain measurements. As a result, people with the same brain reserve capacity can have varying degrees of cognitive reserve [17], [18], [3], [14], [197], [6]. The different cognitive exposures and activities throughout the lifespan can have an impact on current brain activity and cognitive reserve. The education level, heightened intellectual ability (vocabulary or knowledge), literacy level, degree of occupational complexity, and socioeconomic position contributes to reshape the neuronal circuitries and the compensatory cognitive processes [229], [173]. The ‘JC’ used his ultimate cognitive reserve which he developed by repeating intellectually stimulating leisure activities to recall some of the memories while under similar conditions, others could not.
There is evidence suggesting that memory functions can be regained even after damage through some unknown plastic mechanisms [249], [254], [120]. However, whether all forms of memories can be compensated is still not known. Available animal models, as well as human studies, suggest that some forms of memories may be compensated [235].
Summary
A wide variety of conditions, like normal aging, psychiatric conditions, and neurological disorders, can produce memory impairment. Also, anxiety disorders like depression, bipolar mania, and schizophrenia may cause memory impairment by affecting attention, concentration, and information processing [64]. Memory is reported to be severely affected by some neurological disorders like Alzheimer’s and Parkinson’s disease. Memory disorder in Alzheimer’s disease is complex and changes with the progression of the disease. Initial symptoms of memory impairment occur in recalling recent information. In Parkinson’s disease, memory impairment is strongly correlated with motor control. Older patients also show signs of dementia. Similarly, patients suffering from Huntington’s disease show deficits in the acquisition and retrieval of memory [64], [135].
Injury to the brain has been reported to induce signs of repair and formation of new connections in neurons proximal and distal to the damaged site [139], [181], [242], [215]. Some studies have proposed that the changes occurring in compensatory mechanisms are similar to that observed during normal brain development. These compensatory changes include circuit reorganization, neurogenesis, axonal growth, and dendritic plasticity [32], [176], [42], [175]. In a primate study, lesion of the primary motor cortex associated with hands led to compensatory growth of the ventral region of the premotor cortex (PMv) in the same hemisphere. This increased extension was mediated due to the modified axonal sprouting [192], [49]. Interestingly, some compensatory brain mechanisms have also been reported in neurodegenerative diseases like Alzheimer’s disease. Studies suggest that during the early and late periods of Alzheimer’s disease, the level of proteins inhibiting the amyloid beta increase in the brain. This compensatory process acts as a defense mechanism against the aggregation and spread of amyloid-beta, which is considered the hallmark pathology of Alzheimer’s disease [21]. Although there are many unanswered questions, for example, what molecular and cellular events drive the development of compensatory circuitries, whether a greater repetitive cognitive practice simply results in increased neurotrophic factors or rehabilitation intensity is directly related to the upregulation of greater synaptic number, at what point do these processes begin? The answers to these questions still await future studies. However, overall these findings elucidate the dynamic nature of the brain and its capability to adapt to new challenges and show the path of new research to understand the mystery of our brain.
Fundings
This work was supported by DBT India, DST (CSIR) and DST (PURSE) India, UPOE-II, UGC-CAS and, UGC-Resource Networking India, ICMR India funds.
Disclosure
We disclose no conflict of interest. The funding agencies have not played any role in research design, data collection, or data analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abe O., Yamasue H., Aoki S., Suga M., Yamada H., Kasai K., et al. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Alexander G.E., Ryan L., Bowers D., Foster T.C., Bizon J.L., Geldmacher D.S., et al. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alladi S., Bak T.H., Duggirala V., Surampudi B., Shailaja M., Shukla A.K., et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81:1938–1944. doi: 10.1212/01.wnl.0000436620.33155.a4. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J.M., Hubbard B.M., Coghill G.R., Slidders W. The effect of advanced old age on the neurone content of the cerebral cortex: Observations with an automatic image analyser point counting method. J Neurol Sci. 1983;58:235–246. doi: 10.1016/0022-510x(83)90220-4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson M.C., Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn Sci. 2014;18:279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arenaza-Urquijo E.M., Bejanin A., Gonneaud J., Wirth M., La Joie R., Mutlu J., et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017;59:72–79. doi: 10.1016/j.neurobiolaging.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Arendash G.W., Garcia M.F., Costa D.A., Cracchiolo J.R., Wefes I.M., Potter H. Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15:1751–1754. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- 8.Babcock K.R., Page J.S., Fallon J.R., Webb A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer's Disease. Stem Cell Rep. 2021;16:681–693. doi: 10.1016/j.stemcr.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baciu M., Boudiaf N., Cousin E., Perrone-Bertolotti M., Pichat C., Fournet N., et al. Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age (Dordr) 2016;38:3. doi: 10.1007/s11357-015-9857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 11.Balleine B.W., Killcross A.S., Dickinson A. The Effect of Lesions of the Basolateral Amygdala on Instrumental Conditioning. J Neurosci. 2003;23:666. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barense M.D., Groen I.I.A., Lee A.C.H., Yeung L.-K., Brady S.M., Gregori M., et al. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett AJ, Reilly W, Dimsdale-Zucker HR, Mizrak E, Reagh Z, Ranganath C (2020) Organization of cortico-hippocampal networks in the human brain. bioRxiv 2020.2006.2009.142166. [DOI] [PMC free article] [PubMed]
- 14.Barulli D., Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdugo-Vega G., Arias-Gil G., López-Fernández A., Artegiani B., Wasielewska J.M., Lee C.C., et al. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat Commun. 2020;11:135. doi: 10.1038/s41467-019-14026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besnard A., Sahay A. Enhancing adult neurogenesis promotes contextual fear memory discrimination and activation of hippocampal-dorsolateral septal circuits. Behav Brain Res. 2021;399 doi: 10.1016/j.bbr.2020.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialystok E., Craik F.I., Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45:459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Bialystok E., Craik F.I., Luk G. Bilingualism: consequences for mind and brain. Trends Cogn Sci. 2012;16:240–250. doi: 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billings L.M., Green K.N., McGaugh J.L., LaFerla F.M. Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci. 2007;27:751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird C.M., Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 21.Bobkova N., Vorobyov V. The brain compensatory mechanisms and Alzheimer's disease progression: a new protective strategy. Neural Regen Res. 2015;10:696–697. doi: 10.4103/1673-5374.156954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowley M.P., Cabral H., Rosene D.L., Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010;518:3046–3064. doi: 10.1002/cne.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyce R., Glasgow S.D., Williams S., Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science (New York, NY) 2016;352:812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 24.Bremner J.D., Randall P., Scott T.M., Bronen R.A., Seibyl J.P., Southwick S.M., et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Burke S.N., Barnes C.A. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 27.Bussey T.J., Saksida L.M. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- 28.Camandola S., Mattson M.P. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease. BBA. 2011;1813:965–973. doi: 10.1016/j.bbamcr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardoso G.T.M., Gomes-Leal W., Franco E.C.S., Pereira A., Jr., Gomes F.L., Brino A.L.F., et al. Compensatory Hippocampal Neurogenesis in the Absence of Cognitive Impairment Following Experimental Hippocampectomy in Adult Rats. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.709291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caroli A., Geroldi C., Nobili F., Barnden L.R., Guerra U.P., Bonetti M., et al. Functional compensation in incipient Alzheimer's disease. Neurobiol Aging. 2010;31:387–397. doi: 10.1016/j.neurobiolaging.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Castillo C.A., Albasanz J.L., León D., Jordán J., Pallàs M., Camins A., et al. Age-related expression of adenosine receptors in brain from the senescence-accelerated mouse. Exp Gerontol. 2009;44:453–461. doi: 10.1016/j.exger.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Castro-Alamancos M.A., Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 33.Cavedini P., Zorzi C., Piccinni M., Cavallini M.C., Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010;67:1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Chaaya N., Battle A.R., Johnson L.R. An update on contextual fear memory mechanisms: Transition between Amygdala and Hippocampus. Neurosci Biobehav Rev. 2018;92:43–54. doi: 10.1016/j.neubiorev.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain S.R., Fineberg N.A., Menzies L.A., Blackwell A.D., Bullmore E.T., Robbins T.W., et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang L., Jiang C.S., Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang S.D., Liang K.C. The hippocampus integrates context and shock into a configural memory in contextual fear conditioning. Hippocampus. 2017;27:145–155. doi: 10.1002/hipo.22679. [DOI] [PubMed] [Google Scholar]
- 38.Cheng J.T., Liu I.M., Juang S.W., Jou S.B. Decrease of adenosine A-1 receptor gene expression in cerebral cortex of aged rats. Neurosci Lett. 2000;283:227–229. doi: 10.1016/s0304-3940(00)00961-7. [DOI] [PubMed] [Google Scholar]
- 39.Clarke A., Roberts B.M., Ranganath C. Neural oscillations during conditional associative learning. Neuroimage. 2018;174:485–493. doi: 10.1016/j.neuroimage.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Coffey C.E., Wilkinson W.E., Parashos I.A., Soady S.A., Sullivan R.J., Patterson L.J., et al. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- 41.Collin S.H.P., Milivojevic B., Doeller C.F. Hippocampal hierarchical networks for space, time, and memory. Curr Opin Behav Sci. 2017;17:71–76. [Google Scholar]
- 42.Conner J.M., Chiba A.A., Tuszynski M.H. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Conte F., Arzilli C., Errico B.M., Giganti F., Iovino D., Ficca G. Sleep Measures Expressing ‘Functional Uncertainty' in Elderlies' Sleep. Gerontology. 2014;60:448–457. doi: 10.1159/000358083. [DOI] [PubMed] [Google Scholar]
- 44.Contestabile A., Ciani E., Contestabile A. The place of choline acetyltransferase activity measurement in the “cholinergic hypothesis” of neurodegenerative diseases. Neurochem Res. 2008;33:318–327. doi: 10.1007/s11064-007-9497-4. [DOI] [PubMed] [Google Scholar]
- 45.Court J.A., Lloyd S., Johnson M., Griffiths M., Birdsall N.J., Piggott M.A., et al. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Brain Res Dev Brain Res. 1997;101:93–105. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 46.Crivelli-Decker J., Hsieh L.-T., Clarke A., Ranganath C. Theta oscillations promote temporal sequence learning. Neurobiol Learn Mem. 2018;153:92–103. doi: 10.1016/j.nlm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Curiati P.K., Tamashiro J.H., Squarzoni P., Duran F.L.S., Santos L.C., Wajngarten M., et al. Brain structural variability due to aging and gender in cognitively healthy Elders: results from the Sao Paulo Ageing and Health study. AJNR Am J Neuroradiol. 2009;30:1850–1856. doi: 10.3174/ajnr.A1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai W., Lopez O.L., Carmichael O.T., Becker J.T., Kuller L.H., Gach H.M. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dancause N, Barbay S, Frost SB, Plautz EJ, Popescu M, Dixon PM, Stowe AM, Friel KM, Nudo RJ (2006) Topographically divergent and convergent connectivity between premotor and primary motor cortex. Cerebral cortex (New York, NY : 1991) 16:1057-1068. [DOI] [PubMed]
- 50.Davis M., Whalen P. Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatr 6: 13–34. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 51.de Brabander J.M., Kramers R.J., Uylings H.B. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 52.de Vries F.E., de Wit S.J., Cath D.C., van der Werf Y.D., van der Borden V., van Rossum T.B., et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76:878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Decker M.W. The effects of aging on hippocampal and cortical projections of the forebrain cholinergic system. Brain Res Rev. 1987;12:423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- 54.Delorme R., Goussé V., Roy I., Trandafir A., Mathieu F., Mouren-Siméoni M.C., et al. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur Psychiatry. 2007;22:32–38. doi: 10.1016/j.eurpsy.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng W., Saxe M.D., Gallina I.S., Gage F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diba K., Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickerson B.C., Salat D.H., Greve D.N., Chua E.F., Rand-Giovannetti E., Rentz D.M., et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR (2003) Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cerebral cortex (New York, NY : 1991) 13:950-961. [DOI] [PubMed]
- 59.Dubé J., Lafortune M., Bedetti C., Bouchard M., Gagnon J.F., Doyon J., et al. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35:7795–7807. doi: 10.1523/JNEUROSCI.3956-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dworak M., McCarley R.W., Kim T., Kalinchuk A.V., Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eichenbaum H. On the Integration of Space, Time, and Memory. Neuron. 2017;95:1007–1018. doi: 10.1016/j.neuron.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eichenbaum H., Cohen N.J. Oxford University Press; New York, NY, US: 2001. From conditioning to conscious recollection: Memory systems of the brain. [Google Scholar]
- 63.Eichenbaum H., Cohen N.J. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erickson K.R. Amnestic disorders. Pathophysiology and patterns of memory dysfunction. West J Med. 1990;152:159–166. [PMC free article] [PubMed] [Google Scholar]
- 65.Fabricius K., Jacobsen J.S., Pakkenberg B. Effect of age on neocortical brain cells in 90+ year old human females–a cell counting study. Neurobiol Aging. 2013;34:91–99. doi: 10.1016/j.neurobiolaging.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Fang W., Li Z., Wu L., Cao Z., Liang Y., Yang H., et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. 2013;14:950–954. doi: 10.1016/j.sleep.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Fanselow M.S. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 68.Fanselow M.S. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14:7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fanselow M.S., LeDoux J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 70.Farokhian F., Yang C., Beheshti I., Matsuda H., Wu S. Age-Related Gray and White Matter Changes in Normal Adult Brains. Aging Dis. 2017;8:899–909. doi: 10.14336/AD.2017.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fearnley J.M., Lees A.J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain J Neurol. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 72.Feigin A., Ghilardi M.F., Huang C., Ma Y., Carbon M., Guttman M., et al. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fjell A.M., McEvoy L., Holland D., Dale A.M., Walhovd K.B. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fletcher E., Gavett B., Harvey D., Farias S.T., Olichney J., Beckett L., et al. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018;32:436–449. doi: 10.1037/neu0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fogel S.M., Smith C.T. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Foley D.J., Vitiello M.V., Bliwise D.L., Ancoli-Israel S., Monjan A.A., Walsh J.K. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation '2003 Sleep in America' Poll. Am J Geriatr Psychiatry. 2007;15:344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 77.Freund A.M., Baltes P.B. Selection, optimization, and compensation as strategies of life management: correlations with subjective indicators of successful aging. Psychol Aging. 1998;13:531–543. doi: 10.1037//0882-7974.13.4.531. [DOI] [PubMed] [Google Scholar]
- 78.Freund A.M., Baltes P.B. Life-management strategies of selection, optimization and compensation: Measurement by self-report and construct validity. J Pers Soc Psychol. 2002;82:642–662. [PubMed] [Google Scholar]
- 79.Gabbott P.L., Warner T.A., Jays P.R., Salway P., Busby S.J. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 80.Gale G.D., Anagnostaras S.G., Godsil B.P., Mitchell S., Nozawa T., Sage J.R., et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao J., Miao H., Xiao C.H., Sun Y., Du X., Yuan H.H., et al. Influence of aging on the dopaminergic neurons in the substantia nigra pars compacta of rats. Curr Aging Sci. 2011;4:19–24. doi: 10.2174/1874609811104010019. [DOI] [PubMed] [Google Scholar]
- 82.Gasiorowska A, Wydrych M, Drapich P, Zadrozny M, Steczkowska M, Niewiadomski W, Niewiadomska G (2021) The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Frontiers in aging neuroscience 13. [DOI] [PMC free article] [PubMed]
- 83.Georgiou-Karistianis N., Gray M.A., Domínguez D.J., Dymowski A.R., Bohanna I., Johnston L.A., et al. Automated differentiation of pre-diagnosis Huntington's disease from healthy control individuals based on quadratic discriminant analysis of the basal ganglia: the IMAGE-HD study. Neurobiol Dis. 2013;51:82–92. doi: 10.1016/j.nbd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Gilmartin M.R., McEchron M.D. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- 85.Gilmor M.L., Erickson J.D., Varoqui H., Hersh L.B., Bennett D.A., Cochran E.J., et al. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- 86.Goto A. Synaptic plasticity during systems memory consolidation. Neurosci Res. 2022 doi: 10.1016/j.neures.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Gray M.A., Egan G.F., Ando A., Churchyard A., Chua P., Stout J.C., et al. Prefrontal activity in Huntington's disease reflects cognitive and neuropsychiatric disturbances: The IMAGE-HD study. Exp Neurol. 2013;239:218–228. doi: 10.1016/j.expneurol.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Gregory S., Long J.D., Tabrizi S.J., Rees G. Measuring compensation in neurodegeneration using MRI. Curr Opin Neurol. 2017;30:380–387. doi: 10.1097/WCO.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grieve S.M., Clark C.R., Williams L.M., Peduto A.J., Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gruber O., von Cramon D.Y. Domain-specific distribution of working memory processes along human prefrontal and parietal cortices: a functional magnetic resonance imaging study. Neurosci Lett. 2001;297:29–32. doi: 10.1016/s0304-3940(00)01665-7. [DOI] [PubMed] [Google Scholar]
- 91.Gruber O., von Cramon D.Y. The functional neuroanatomy of human working memory revisited. Evidence from 3-T fMRI studies using classical domain-specific interference tasks. Neuroimage. 2003;19:797–809. doi: 10.1016/s1053-8119(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 92.Gurvits T.V., Shenton M.E., Hokama H., Ohta H., Lasko N.B., Gilbertson M.W., et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hädel S., Wirth C., Rapp M., Gallinat J., Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imaging. 2013;38:1480–1487. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- 94.Hämäläinen A., Pihlajamäki M., Tanila H., Hänninen T., Niskanen E., Tervo S., et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Han D.H., Park P., Choi D.I., Bliss T.V.P., Kaang B.K. The essence of the engram: Cellular or synaptic? Semin Cell Dev Biol. 2022;125:122–135. doi: 10.1016/j.semcdb.2021.05.033. [DOI] [PubMed] [Google Scholar]
- 96.He X., Koo B.B., Killiany R.J. Edited Magnetic Resonance Spectroscopy Detects an Age-Related Decline in Nonhuman Primate Brain GABA Levels. Biomed Res Int. 2016;2016:6523909. doi: 10.1155/2016/6523909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hedden T., Gabrieli J.D. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 98.Heise K.-F., Zimerman M., Hoppe J., Gerloff C., Wegscheider K., Hummel F.C. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci. 2013;33:9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hellman K., Abel T. Fear conditioning increases NREM sleep. Behav Neurosci. 2007;121:310–323. doi: 10.1037/0735-7044.121.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hellström-Lindahl E., Court J.A. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behav Brain Res. 2000;113:159–168. doi: 10.1016/s0166-4328(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 101.Helmstetter F.J., Bellgowan P.S. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 102.Henseler I., Gruber O., Kraft S., Krick C., Reith W., Falkai P. Compensatory hyperactivations as markers of latent working memory dysfunctions in patients with obsessive-compulsive disorder: an fMRI study. J Psychiatry Neurosci. 2008;33:209–215. [PMC free article] [PubMed] [Google Scholar]
- 103.Hernández-Rabaza V., Llorens-Martín M., Velázquez-Sánchez C., Ferragud A., Arcusa A., Gumus H.G., et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 104.Heumann D., Leuba G. Neuronal death in the development and aging of the cerebral cortex of the mouse. Neuropathol Appl Neurobiol. 1983;9:297–311. doi: 10.1111/j.1365-2990.1983.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 105.Hsieh L.T., Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage. 2014;85(Pt 2):721–729. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu Y.S., Xu P., Pigino G., Brady S.T., Larson J., Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1DeltaE9 mice. Faseb j. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang D., Liu D., Yin J., Qian T., Shrestha S., Ni H. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27:2698–2705. doi: 10.1007/s00330-016-4669-8. [DOI] [PubMed] [Google Scholar]
- 108.Huang Z.L., Urade Y., Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem. 2011;11:1047–1057. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- 109.Hunt D.L., Castillo P.E. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jack C.R., Jr., Petersen R.C., Xu Y.C., Waring S.C., O'Brien P.C., Tangalos E.G., et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jankowsky J.L., Melnikova T., Fadale D.J., Xu G.M., Slunt H.H., Gonzales V., et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jermakowicz W.J., Casagrande V.A. Neural networks a century after Cajal. Brain Res Rev. 2007;55:264–284. doi: 10.1016/j.brainresrev.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jha S.K., Brennan F.X., Pawlyk A.C., Ross R.J., Morrison A.R. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–1080. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 114.Ji L., Pearlson G.D., Hawkins K.A., Steffens D.C., Guo H., Wang L. A New Measure for Neural Compensation Is Positively Correlated With Working Memory and Gait Speed. Front Aging Neurosci. 2018;10:71. doi: 10.3389/fnagi.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Josselyn S.A., Kohler S., Frankland P.W. Finding the engram. Nature reviews. Neuroscience. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- 116.Josselyn S.A., Tonegawa S. Science (New York; NY): 2020. Memory engrams: Recalling the past and imagining the future; p. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL (2009) The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cerebral cortex (New York, NY : 1991) 19:2248-2268. [DOI] [PMC free article] [PubMed]
- 118.Kaiser L.G., Schuff N., Cashdollar N., Weiner M.W. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalpouzos G., Chételat G., Baron J.C., Landeau B., Mevel K., Godeau C., et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 120.Kant D., Jha S.K. The formation of compensatory contextual fear memory in the absence of dorsal hippocampus does not change sleep architecture. Behav Brain Res. 2019;370 doi: 10.1016/j.bbr.2019.111944. [DOI] [PubMed] [Google Scholar]
- 121.Karlsson M.P., Frank L.M. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kavé G., Samuel-Enoch K., Adiv S. The association between age and the frequency of nouns selected for production. Psychol Aging. 2009;24:17–27. doi: 10.1037/a0014579. [DOI] [PubMed] [Google Scholar]
- 123.Kessler B.A., Stanley E.M., Frederick-Duus D., Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–88. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim W.B., Cho J.-H. Encoding of contextual fear memory in hippocampal–amygdala circuit. Nat Commun. 2020;11:1382. doi: 10.1038/s41467-020-15121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kircher T.T., Weis S., Freymann K., Erb M., Jessen F., Grodd W., et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klerman E.B., Dijk D.J. Age-related reduction in the maximal capacity for sleep–implications for insomnia. Curr Biol. 2008;18:1118–1123. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klöppel S., Gregory S., Scheller E., Minkova L., Razi A., Durr A., et al. Compensation in Preclinical Huntington's Disease: Evidence From the Track-On HD Study. EBioMedicine. 2015;2:1420–1429. doi: 10.1016/j.ebiom.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korman M., Doyon J., Doljansky J., Carrier J., Dagan Y., Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 129.Kraft P., Kraft B., Hagen T., Espeseth T. Subjective Socioeconomic Status, Cognitive Abilities, and Personal Control: Associations With Health Behaviours. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.784758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar A., Foster T.C. Alteration in NMDA Receptor Mediated Glutamatergic Neurotransmission in the Hippocampus During Senescence. Neurochem Res. 2019;44:38–48. doi: 10.1007/s11064-018-2634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kumar T., Jha S.K. Sleep deprivation impairs consolidation of cued fear memory in rats. PLoS One. 2012;7:e47042. doi: 10.1371/journal.pone.0047042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar T., Jha S.K. Influence of cued-fear conditioning and its impairment on NREM sleep. Neurobiol Learn Mem. 2017;144:155–165. doi: 10.1016/j.nlm.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Lahl O., Wispel C., Willigens B., Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 134.Lambrecq V., Langbour N., Guehl D., Bioulac B., Burbaud P., Rotge J.Y. Evolution of brain gray matter loss in Huntington's disease: a meta-analysis. Eur J Neurol. 2013;20:315–321. doi: 10.1111/j.1468-1331.2012.03854.x. [DOI] [PubMed] [Google Scholar]
- 135.Lang C.J.G., Majer M., Balan P., Reischies F.M. Recall and Recognition in Huntington's Disease. Arch Clin Neuropsychol. 2000;15:361–371. [PubMed] [Google Scholar]
- 136.Lazarov O., Hollands C. Hippocampal neurogenesis: Learning to remember. Prog Neurobiol. 2016;138–140:1–18. doi: 10.1016/j.pneurobio.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lehmann H., Sparks F.T., Spanswick S.C., Hadikin C., McDonald R.J., Sutherland R.J. Making context memories independent of the hippocampus. Learn Mem. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J., Vitiello M.V., Gooneratne N.S. Sleep in Normal Aging. Sleep Med Clin. 2018;13:1–11. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li S., Carmichael S.T. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 140.Lighthall N.R., Huettel S.A., Cabeza R. Functional Compensation in the Ventromedial Prefrontal Cortex Improves Memory-Dependent Decisions in Older Adults. J Neurosci. 2014;34:15648. doi: 10.1523/JNEUROSCI.2888-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lim A.S., Ellison B.A., Wang J.L., Yu L., Schneider J.A., Buchman A.S., et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain J Neurol. 2014;137:2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lledo P.M., Alonso M., Grubb M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 143.Long Z., Medlock C., Dzemidzic M., Shin Y.W., Goddard A.W., Dydak U. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:131–135. doi: 10.1016/j.pnpbp.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lord C., Sekerovic Z., Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol (Paris) 2014;62:302–310. doi: 10.1016/j.patbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 145.Luckhaus C., Flüß M.O., Wittsack H.J., Grass-Kapanke B., Jänner M., Khalili-Amiri R., et al. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer's dementia by perfusion-weighted magnetic resonance imaging. Neuroimage. 2008;40:495–503. doi: 10.1016/j.neuroimage.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 146.Luo W., Yun D., Hu Y., Tian M., Yang J., Xu Y., et al. Acquiring new memories in neocortex of hippocampal-lesioned mice. Nat Commun. 2022;13:1601. doi: 10.1038/s41467-022-29208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lüscher C., Malenka R.C. Cold Spring Harbor perspectives in biology 4. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ma W.J., Husain M., Bays P.M. Changing concepts of working memory. Nat Neurosci. 2014;17:347–356. doi: 10.1038/nn.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maddox S.A., Hartmann J., Ross R.A., Ressler K.J. Deconstructing the Gestalt: Mechanisms of Fear, Threat, and Trauma Memory Encoding. Neuron. 2019;102:60–74. doi: 10.1016/j.neuron.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malejko K., Weydt P., Süßmuth S.D., Grön G., Landwehrmeyer B.G., Abler B. Prodromal Huntington disease as a model for functional compensation of early neurodegeneration. PLoS One. 2014;9:e114569. doi: 10.1371/journal.pone.0114569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mander B.A., Rao V., Lu B., Saletin J.M., Lindquist J.R., Ancoli-Israel S., et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mander B.A., Winer J.R., Walker M.P. Sleep and Human Aging. Neuron. 2017;94:19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maren S. Neurotoxic Basolateral Amygdala Lesions Impair Learning and Memory But Not the Performance of Conditional Fear in Rats. J Neurosci. 1999;19:8696. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maren S., Phan K.L., Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 156.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- 157.Martin A., Chao L.L. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 158.Martin N., Lafortune M., Godbout J., Barakat M., Robillard R., Poirier G., et al. Topography of age-related changes in sleep spindles. Neurobiol Aging. 2013;34:468–476. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 159.McCall A.A., Yates B.J. Compensation following bilateral vestibular damage. Front Neurol. 2011;2:88. doi: 10.3389/fneur.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McDonald R.J., Devan B.D., Hong N.S. Multiple memory systems: the power of interactions. Neurobiol Learn Mem. 2004;82:333–346. doi: 10.1016/j.nlm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 161.McQuail J.A., Frazier C.J., Bizon J.L. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mednick S.C., McDevitt E.A., Walsh J.K., Wamsley E., Paulus M., Kanady J.C., et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meyer A.M., Federmeier K.D. Event-related potentials reveal the effects of aging on meaning selection and revision. Psychophysiology. 2010;47:673–686. doi: 10.1111/j.1469-8986.2010.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Milner C.E., Cote K.A. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 165.Minkova L., Gregory S., Scahill R.I., Abdulkadir A., Kaller C.P., Peter J., et al. Cross-sectional and longitudinal voxel-based grey matter asymmetries in Huntington's disease. NeuroImage Clinical. 2018;17:312–324. doi: 10.1016/j.nicl.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moraes W., Piovezan R., Poyares D., Bittencourt L.R., Santos-Silva R., Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15:401–409. doi: 10.1016/j.sleep.2013.11.791. [DOI] [PubMed] [Google Scholar]
- 167.Mucke L. Alzheimer's disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 168.Mysore SP, Kothari NB (2020) Mechanisms of competitive selection: A canonical neural circuit framework. eLife 9. [DOI] [PMC free article] [PubMed]
- 169.Nadel L., Hardt O. The spatial brain. Neuropsychology. 2004;18:473–476. doi: 10.1037/0894-4105.18.3.473. [DOI] [PubMed] [Google Scholar]
- 170.Nadel L., Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36:251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nadel L., Willner J. Context and conditioning: A place for space. Physiol Psychol. 1980;8:218–228. [Google Scholar]
- 172.Nakamura S., Akiguchi I., Kameyama M., Mizuno N. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol. 1985;65:281–284. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- 173.Nithianantharajah J., Hannan A.J. Mechanisms mediating brain and cognitive reserve: experience-dependent neuroprotection and functional compensation in animal models of neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:331–339. doi: 10.1016/j.pnpbp.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 174.Noda S., Sato S., Fukuda T., Tada N., Hattori N. Aging-related motor function and dopaminergic neuronal loss in C57BL/6 mice. Mol Brain. 2020;13:46. doi: 10.1186/s13041-020-00585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nudo R.J. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 176.Nudo R.J., Milliken G.W. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 177.O'Brien J. Design principles of electrical synaptic plasticity. Neurosci Lett. 2019;695:4–11. doi: 10.1016/j.neulet.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.O'Neill J., Pleydell-Bouverie B., Dupret D., Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 179.O'Reilly R.C., Rudy J.W. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 180.Ognjanovski N, Broussard C, Zochowski M, Aton SJ (2018) Hippocampal Network Oscillations Rescue Memory Consolidation Deficits Caused by Sleep Loss. Cerebral cortex (New York, NY : 1991) 28:3711-3723. [DOI] [PMC free article] [PubMed]
- 181.Ohab J.J., Fleming S., Blesch A., Carmichael S.T. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 183.Page T.L., Einstein M., Duan H., He Y., Flores T., Rolshud D., et al. Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett. 2002;317:37–41. doi: 10.1016/s0304-3940(01)02428-4. [DOI] [PubMed] [Google Scholar]
- 184.Palasz E., Niewiadomski W., Gasiorowska A., Wysocka A., Stepniewska A., Niewiadomska G. Exercise-Induced Neuroprotection and Recovery of Motor Function in Animal Models of Parkinson's Disease. Front Neurol. 2019;10:1143. doi: 10.3389/fneur.2019.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- 186.Paré D., Quirk G.J., Ledoux J.E. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 187.Peigneux P., Laureys S., Fuchs S., Collette F., Perrin F., Reggers J., et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 188.Pelvig D.P., Pakkenberg H., Stark A.K., Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 189.Perry E.K., Perry R.H., Gibson P.H., Blessed G., Tomlinson B.E. A cholinergic connection between normal aging and senile dementia in the human hippocampus. Neurosci Lett. 1977;6:85–89. doi: 10.1016/0304-3940(77)90070-2. [DOI] [PubMed] [Google Scholar]
- 190.Peters A., Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 191.Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 192.Plautz E.J., Barbay S., Frost S.B., Friel K.M., Dancause N., Zoubina E.V., et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 193.Ponnusamy R., Poulos A.M., Fanselow M.S. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Popa D, Duvarci S, Popescu AT, Léna C, Paré D (2010) Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proceedings of the National Academy of Sciences of the United States of America 107:6516-6519. [DOI] [PMC free article] [PubMed]
- 195.Porges E.C., Woods A.J., Edden R.A., Puts N.A., Harris A.D., Chen H., et al. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Poulos AM, Ponnusamy R, Dong HW, Fanselow MS (2010) Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proceedings of the National Academy of Sciences of the United States of America 107:14881-14886. [DOI] [PMC free article] [PubMed]
- 197.Prakash R.S., Voss M.W., Erickson K.I., Kramer A.F. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 198.Preston A.R., Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Quirk G.J., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qureshi M.F., Jha S.K. Short-Term Total Sleep-Deprivation Impairs Contextual Fear Memory, and Contextual Fear-Conditioning Reduces REM Sleep in Moderately Anxious Swiss Mice. Front Behav Neurosci. 2017;11:239. doi: 10.3389/fnbeh.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rajender G., Bhatia M.S., Kanwal K., Malhotra S., Singh T.B., Chaudhary D. Study of neurocognitive endophenotypes in drug-naïve obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr Scand. 2011;124:152–161. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 202.Ramanoël S, Hoyau E, Kauffmann L, Renard F, Pichat C, Boudiaf N, Krainik A, Jaillard A, Baciu M (2018) Gray Matter Volume and Cognitive Performance During Normal Aging. A Voxel-Based Morphometry Study. Frontiers in aging neuroscience 10. [DOI] [PMC free article] [PubMed]
- 203.Raymond L.A., André V.M., Cepeda C., Gladding C.M., Milnerwood A.J., Levine M.S. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Redline S., Kirchner H.L., Quan S.F., Gottlieb D.J., Kapur V., Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 205.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Reuter-Lorenz P.A., Park D.C. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol Rev. 2014;24:355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Richardson B.D., Ling L.L., Uteshev V.V., Caspary D.M. Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci. 2013;33:1218–1227a. doi: 10.1523/JNEUROSCI.3277-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rinne J.O., Paljärvi L., Rinne U.K. Neuronal size and density in the nucleus basalis of Meynert in Alzheimer's disease. J Neurol Sci. 1987;79:67–76. doi: 10.1016/0022-510x(87)90260-7. [DOI] [PubMed] [Google Scholar]
- 209.Roalf D.R., Sydnor V.J., Woods M., Wolk D.A., Scott J.C., Reddy R., et al. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol Aging. 2020;95:240–249. doi: 10.1016/j.neurobiolaging.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Roy D.S., Arons A., Mitchell T.I., Pignatelli M., Ryan T.J., Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rozeske R.R., Valerio S., Chaudun F., Herry C. Prefrontal neuronal circuits of contextual fear conditioning. Genes Brain Behav. 2015;14:22–36. doi: 10.1111/gbb.12181. [DOI] [PubMed] [Google Scholar]
- 212.Rudy J.W., O’Reilly R.C. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 213.Sánchez-Melgar A., Albasanz J.L., Palomera-Ávalos V., Pallàs M., Martín M. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol Neurobiol. 2019;56:2881–2895. doi: 10.1007/s12035-018-1281-8. [DOI] [PubMed] [Google Scholar]
- 214.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 215.Schoch K.M., Madathil S.K., Saatman K.E. Genetic manipulation of cell death and neuroplasticity pathways in traumatic brain injury. Neurotherapeutics. 2012;9:323–337. doi: 10.1007/s13311-012-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schubert F., Gallinat J., Seifert F., Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21:1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 217.Scopa C., Marrocco F., Latina V., Ruggeri F., Corvaglia V., La Regina F., et al. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ. 2020;27:934–948. doi: 10.1038/s41418-019-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Segovia G., Porras A., Del Arco A., Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 219.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science (New York, NY) 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 220.Shimada A., Tsuzuki M., Keino H., Satoh M., Chiba Y., Saitoh Y., et al. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol Appl Neurobiol. 2006;32:1–14. doi: 10.1111/j.1365-2990.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 221.Siegel G.J., Chauhan N.B. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 222.Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Simic G., Babic Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milosevic N., et al. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Snyder J.S., Choe J.S., Clifford M.A., Jeurling S.I., Hurley P., Brown A., et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Squire L.R., Genzel L., Wixted J.T., Morris R.G. Memory consolidation. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Squire L.R., Wixted J.T. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stein M.B., Koverola C., Hanna C., Torchia M.G., McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 229.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sweatt J.D. Neural plasticity and behavior – sixty years of conceptual advances. J Neurochem. 2016;139(Suppl 2):179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- 231.Tonegawa S., Morrissey M.D., Kitamura T. The role of engram cells in the systems consolidation of memory. Nat Rev Neurosci. 2018;19:485–498. doi: 10.1038/s41583-018-0031-2. [DOI] [PubMed] [Google Scholar]
- 232.Traikapi A., Konstantinou N. Gamma Oscillations in Alzheimer's Disease and Their Potential Therapeutic Role. Front Syst Neurosci. 2021;15 doi: 10.3389/fnsys.2021.782399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tucker M.A., Hirota Y., Wamsley E.J., Lau H., Chaklader A., Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 234.Turrigiano G.G., Leslie K.R., Desai N.S., Rutherford L.C., Nelson S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 235.Ullman M.T., Pullman M.Y. A compensatory role for declarative memory in neurodevelopmental disorders. Neurosci Biobehav Rev. 2015;51:205–222. doi: 10.1016/j.neubiorev.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Van Cauter E., Leproult R., Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 237.Verhaegen C., Poncelet M. Changes in naming and semantic abilities with aging from 50 to 90 years. J Int Neuropsychol Soc. 2013;19:119–126. doi: 10.1017/S1355617712001178. [DOI] [PubMed] [Google Scholar]
- 238.Vertes R.P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 239.Vidal-Gonzalez I., Vidal-Gonzalez B., Rauch S.L., Quirk G.J. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vienne J., Spann R., Guo F., Rosbash M. Age-Related Reduction of Recovery Sleep and Arousal Threshold in Drosophila. Sleep. 2016;39:1613–1624. doi: 10.5665/sleep.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vitureira N., Letellier M., Goda Y. Homeostatic synaptic plasticity: from single synapses to neural circuits. Curr Opin Neurobiol. 2012;22:516–521. doi: 10.1016/j.conb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang L., Yu C., Chen H., Qin W., He Y., Fan F., et al. Dynamic functional reorganization of the motor execution network after stroke. Brain J Neurol. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- 243.Wang X.-m., Zhang G.-f., Jia M., Xie Z.-m., Yang J.-j., Shen J.-c., et al. Environmental enrichment improves pain sensitivity, depression-like phenotype, and memory deficit in mice with neuropathic pain: role of NPAS4. Psychopharmacology (Berl) 2019;236:1999–2014. doi: 10.1007/s00213-019-5187-6. [DOI] [PubMed] [Google Scholar]
- 244.Wang Z., Neylan T.C., Mueller S.G., Lenoci M., Truran D., Marmar C.R., et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Webb W.B., Campbell S.S. The first night effect revisited with age as a variable. Waking Sleeping. 1979;3:319–324. [PubMed] [Google Scholar]
- 246.Wenk G.L. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 247.Wilson B.A., Hughes E. Coping with Amnesia: The Natural History of a Compensatory Memory System. Neuropsychol Rehabil. 1997;7:43–56. [Google Scholar]
- 248.Wilson B.A., Watson P.C. A Practical Framework for Understanding Compensatory Behaviour in People with Organic Memory Impairment. Memory. 1996;4:465–486. doi: 10.1080/741940776. [DOI] [PubMed] [Google Scholar]
- 249.Wiltgen B.J., Sanders M.J., Anagnostaras S.G., Sage J.R., Fanselow M.S. Context Fear Learning in the Absence of the Hippocampus. J Neurosci. 2006;26:5484. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Winner B., Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wolf R.C., Sambataro F., Vasic N., Baldas E.M., Ratheiser I., Bernhard Landwehrmeyer G., et al. Visual system integrity and cognition in early Huntington's disease. Eur J Neurosci. 2014;40:2417–2426. doi: 10.1111/ejn.12575. [DOI] [PubMed] [Google Scholar]
- 252.Wolff M.J., Jochim J., Akyürek E.G., Stokes M.G. Dynamic hidden states underlying working-memory-guided behavior. Nat Neurosci. 2017;20:864–871. doi: 10.1038/nn.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV (2008) Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cerebral cortex (New York, NY : 1991) 18:2241-2250. [DOI] [PMC free article] [PubMed]
- 254.Zelikowsky M., Bissiere S., Fanselow M.S. Contextual fear memories formed in the absence of the dorsal hippocampus decay across time. J Neurosci. 2012;32:3393–3397. doi: 10.1523/JNEUROSCI.4339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS (2013) Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proceedings of the National Academy of Sciences 110:9938. [DOI] [PMC free article] [PubMed]
- 256.Zhang X., Liu H., Wu J., Zhang X., Liu M., Wang Y. Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem Int. 2009;54:481–487. doi: 10.1016/j.neuint.2009.02.004. [DOI] [PubMed] [Google Scholar]