1. Introduction
In the quest to understand AD and find efficient therapeutic interventions, the dominant amyloid cascade hypothesis [1], [2] has been driven by several lines of evidence including: 1) genetic evidence from familial forms of AD (FAD), where fully penetrant mutations in the presenilins (PSEN1 and 2) and the amyloid β precursor protein (AβPP) genes are qualitative markers of disease [3]; 2) neuropathological deposition of Aβ in senile plaques (SP) and as cerebral amyloid angiopathy (CAA) is associated with dementia in both FAD and sporadic AD (SAD) [4], [5]; 3) evidence from animal, cell culture and molecular mechanistic models of AD mostly based on AD-associated genetic mutations and 4) amyloid-based biomarker evidence that has contributed to a biomedical/biological re-definition of AD in research contexts [6]. Taken together, the evidence is interpreted by the amyloid cascade hypothesis to give Aβ, whether as alternative amino acid sequence lengths or alternative aggregation states as monomers, dimers, oligomers or fibrils, a causal role in the initiation and progression of dementia. However, over the decades this interpretation has not been fully accepted by the AD research community [7], [8], [9] and the hypothesis has not yet delivered on its promise. The presenilin hypothesis [10], [11], an alternative hypothesis to understand the role of the genetic mutations in AD, suggests disease initiation and progression is related to the way mutations in PSENs affect APP cleavages via complex patterns of gain and loss of function. Both the amyloid cascade and presenilin hypotheses currently focus on measures of Aβ as markers of disease progression, in effect reducing this complex proteolytic system to measures of just a few components, (Aβ(1–40) and Aβ(1–42)).
Epidemiological population-representative, clinicopathological studies of brain ageing consistently find complex relationships between dementia and neuropathology that are further modulated by in-life factors such as age and education [12], [13], [14], [15] and consistently find cases with severe neuropathology with no dementia and cases with dementia and very little or no pathology. These complex relationships coupled with the lack of a qualitative diagnostic feature potentially undermine some experimental designs in dementia research [16] as neither cases nor controls can be selected with certainty leading to selection bias. Further, uncertainties in past and current AD research deriving from factors relating to basic science such as uncharacterised antibody reactivity profiles and cross-reactivities [17], [18], [19], [20], known biases in experimental design [16], [21], [22], and lack of clarity in definitions of Aβ [23] and AD [6], [16] suggest current research strategy could be improved. An alternative perspective, derived from systems biology and the complexity of dementia in the older population, is the amyloid precursor protein (APP) matrix approach [24], [25], [26].
The APP matrix approach [24], [25], [26] is the least acknowledged and least tested framework with which to understand the various roles of the amyloid beta protein (Aβ) in Alzheimer’s disease (AD) initiation and progression. Here we examine how the APP matrix approach provides an alternative framework to integrate and understand existing evidence and further we describe new ways of thinking required by this framework.
2. The amyloid precursor protein matrix approach
The APP matrix approach derives from a systems biology based approach to understanding flow through biomolecular networks where the APP proteolytic system can be understood as a hub [27], integrating wide ranging cellular systems via the regulation of APP cleavages and feeding this integration forward to wider cellular systems via cleavage products. There are several key concepts;
-
1)
The APP proteolytic system involves a dynamic balance between competing cleavages and is involved in cross talk between multiple cellular signalling systems where changes in this dynamic balance over physiological ranges can quickly respond to and integrate the demands of various homeostatic cellular systems within broader cellular functions e.g. synaptic plasticity. This perspective is supported by the observations that: i) APP levels may be rate limiting for cleavages [28] and APP695 expressed in neurons has a short half-life [29], both features required for fast organised cellular responses; ii) competing cleavage pathways with combined gains and losses of related functions e.g. loss of function associated with mutation in PSEN1 may be associated with gain of function for full length APP in neurite outgrowth [30] and iii) shared amino acid sequences with the potential for competitive binding of targets leading to different functional outcomes.
-
2)
Multiple disease associated drivers and pathways are possible through this complex proteolytic system and their relevance to both normal and disease states can be modulated by contributions from other cellular systems. Disease states may involve decoherence between APP processing and the physiological actions and demands of different signalling pathways in the wider cellular environment.
-
3)
To understand the role of the APP proteolytic system and to better place each fragment in its physiological context all fragments must be systematically considered and controlled for in experimental settings.
-
4)
Fundamental biochemical concepts such as dose–response relationships, compartmentation, competitive inhibition, competitive binding with agonistic or antagonistic actions, relative likelihood of interaction as described by dissociation constants, end product inhibition etc. should be considered for each proteolytic fragment.
The APP matrix approach aims to
-
1)
Create a descriptive molecular map of the cellular components and cellular functions associated with all the proteolytic fragments generated by the APP proteolytic system, representing each component as a single node.
-
2)
Characterise this map with reference to dynamic regulatory feedback loops, basic biochemical features such as cellular compartmentation, competitive inhibition, rates of reaction, dissociation constants etc. to predict how likely particular cleavages or interactions are to occur at any time, represented as weights on the connections between nodes.
-
3)
Describe and model differences in the behaviour of this system in different cell types and species represented as cell/species specific maps.
-
4)
Ultimately generate a dynamic functional model with predictive powers to characterise how specific molecular and neuropathological features relating to the APP proteolytic system are associated with clinical expression of dementia.
Aim 1. Creating a molecular map for the APP proteolytic system
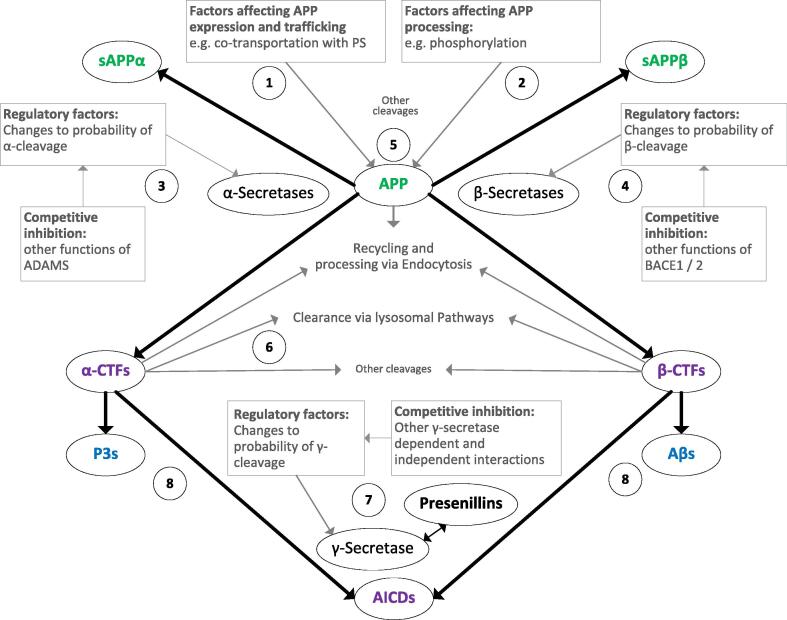
Mapping the APP proteolytic system, simplified for clarity in Fig. 1, is the first step in integrating the vast body of evidence. Nodes represent distinct molecular entities. Each amino acid sequence should be represented by a specific node with additional nodes depending on post-translational modifications and aggregation state such that the umbrella term “Aβ” could have as many as 100 individual nodes to capture differences in sequence length due to N and C terminal truncations, post translational modifications and aggregation states (monomer, homo- hetero- dimers, oligomers and fibrils). Each proteolytic fragment in a specific aggregation state is likely to have its own interaction profile and will likely be involved in different ranges of functional outputs. Although attempts have been made to describe and characterise proteolytic fragments derived from APP and their aggregation and solubility states e.g. [31], this list is currently incomplete, especially for the P3-type fragments derived via sequential α- and γ- cleavages.
Aim 2. Characterising the regulation and dynamic flow through the APP proteolytic system
Fig. 1.
Simplified map of APP proteolytic fragments and factors involved in regulating cleavages. Green: N-terminal fragments, blue: Aβ and P3 type fragments, purple: other fragments, grey: regulatory factors and processes. (1) Full length APP expression is likely rate limiting both for full length APP functional interactions and APP cleavages; factors that regulate APP expression and trafficking to particular cellular compartments require careful characterisation. (2) Factors that regulate the likelihood of the various APP cleavages, e.g. phosphorylation of threonine 668 in the intracellular domain [32] remain to be fully described and characterised. (3) α-cleavage, involving the A Disintegrin And Metalloproteinase (ADAM)s 10, 17 and 9 [33], generates sAPPα and the carboxy terminal fragment (CTF) C83 and is considered to be constitutive and in contrast to β-cleavage appears to include redundancy of multiple enzymes; competitive inhibition arising from other substrates of the enzymes involved [33], [34] remains to be fully described and characterised. (4) β-cleavage involving BACE1 generates sAPPβ and the CTF C99 [35]; competitive inhibition arising from other substrates of BACE1 [36] remains to be fully described and characterised. (5) Other cleavages, e.g. by BACE2 [37] and the N-terminal eta-cleavage [38], have been omitted here for clarity but must be included in the full APP matrix approach. (6) Factors regulating processing of particular CTFs in specific cellular compartments remain to be fully described; may involve processes such as other cleavages, endocytosis [39] or clearance via lysosomal pathways; contributions of CTFs to dementia [40] require further clarification. (7) Factors regulating the expression, trafficking and functions of PSs [41] either dependent or independent from the γ-secretase complex [42] and competitive inhibition between different γ-secretase substrates e.g. Notch 1 [43] require in depth investigation; while proteolysis of the CTFs C83 and C99 by γ-secretase may be differently affected by γ-secretase inhibitors [44] the relative affinities of γ-secretase with all CTFs that should be theoretically present have not been fully described, and this might be important with respect to mutations in the PSEN 1 and 2 genes. (8) Multiple molecular forms (amino acid sequences, post-translational modifications and aggregation states) have been collapsed into a single node for both Aβ and P3 for clarity. The different forms of both Aβ and P3 have not yet been fully described and characterised in brain donations from population representative clinicopathological studies of brain ageing. Further processing pathways e.g. via BACE2 [37] and catabolism of fragments e.g. Aβ [45] and CTFs [46] have also been omitted for clarity.
The weights associated with the connecting edges between components of the APP proteolytic system represent the likelihoods of interactions and can be understood as representing strength flow through a particular proteolytic pathway. In order to estimate the strengths of these connections, the effects of regulatory factors such as compartmentation, phosphorylation and catabolic cleavage etc. should be described and characterised for each connection with each change represented as an additional node e.g. APP phosphorylated at threonine 668 in the intracellular domain [32] has different behaviour to unphosphorylated APP. These connections can dynamically change as the different regulatory factors change in response to demands from the wider cellular environment.
To estimate the strength of the connecting edges, experimentally derived measures of dissociation constants, rates of reaction under different conditions, relative biding studies to estimate the agonistic or antagonistic behaviours of proteolytic fragments of similar sequence etc. are required and this will entail a matrix of experimental set ups so that the effects of each fragment alone and in combination with others can be investigated whilst also fully controlling for the other fragments. This evidence is completely absent from the current literature.
Aim 3. Maps for each cell type and species
It cannot be assumed that any one model of the APP proteolytic system applies across different cell types, e.g. the independence of Aβ deposition in the human brain as extracellular plaques or in vessel walls as CAA [47], or different species, e.g. relative abundances of specific Aβ-type peptides in brain tissues and cerebrospinal fluid are different in the APP23 transgenic mouse model compared to humans with the Swedish AβPP mutation [48]. Therefore multiple maps/models will be required and will be of vital importance to research strategy. Detailed characterisation for each cell type and species will allow the selection of the most relevant experimental mechanistic models. Without this basic characterisation, we have no way of knowing how features of any mechanistic model may support, misdirect or confound research strategy.
The detailed characterisation of the APP proteolytic system in human brain donations from population representative clinicopathological studies is essential for baseline estimates of the natural system in the human brain, against which all mechanistic experimental models should be compared. The same characterisations for each of the AD associated genetic mutations will add further mechanistic detail and can be potentially interpreted as partial knock-in or knock-out models. This “natural history” approach will greatly advance the translation of evidence relating to this proteolytic system between different research approaches and is absolutely fundamental to the selection of appropriate and efficient therapeutic targets that will impact on the initiation and development of dementia, especially in the older population amongst whom most dementia occurs.
Aim 4. Functional interpretations of the APP proteolytic system
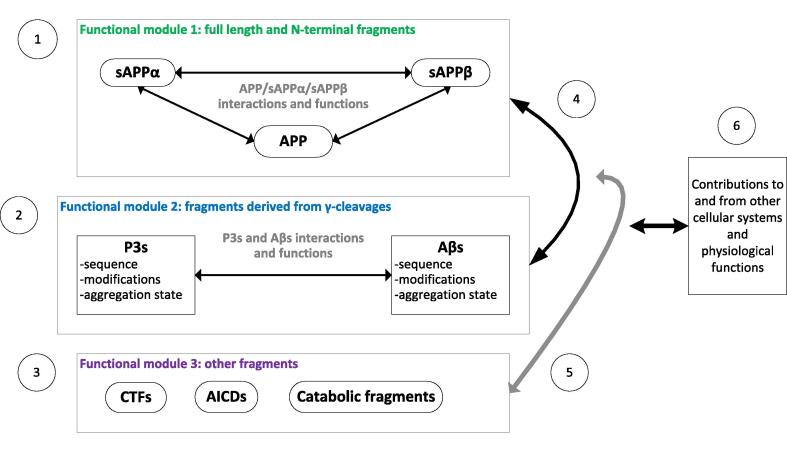
Although the APP matrix is in its infancy, it is possible to make predictions relating to the functions and roles of the APP proteolytic system in normal ageing and disease states with the scant and uncertain evidence we currently have. Predicted functional interactions are simplified in Fig. 2. The APP matrix approach has been used to highlight confounding and uncertainty in current approaches to measures of Aβ [17], [18], re-interpret the genetic evidence associated with mutations in the AβPP gene [49] and has been applied to synaptic plasticity [24], [25], [26] and ageing [50].
Fig. 2.
Simplified approach to investigating functional modules within the APP proteolytic system as guided by the APP matrix approach. Green: N-terminal fragments, blue: Aβ and P3 type fragments, purple: other fragments. (1) Functional block 1 derives from the synergistic actions of full length APP and the large N-terminal domains released following α-, β- and similar cleavages and may be involved in a variety of functions including neuroprotection [51], long term potentiation [53], [54] and neurite outgrowth [55]; Cleavage products from other cleavages including eta- and Aβ’ cleavages not shown for clarity. (2) Functional block 2 derives from the synergistic actions of Aβ-type, P3-type and other similar fragments released following γ-cleavage and may be involved in a variety of functions including inhibition of long term potentiation [56] and promotion of long term depression [57], multiple immune system functions [58] and may have complex interactions relating to metal ion homeostasis and oxidative stress [59], [60], [61]. No studies have investigated the synergistic functions of Aβ and P3 however different effects on synaptic function from different aggregations Aβ have been reported [62]. (3) Other proteolytic fragments derived from APP including the CTFs [40], APP intracellular domains (AICD)s [63]other cleavage products such as Aβ’ via BACE2 cleavage [64] and catabolic fragments, each of which may have functions that remain to be systematically investigated. (4) Beyond the synergistic interactions of those fragments sharing similar sequence homology, there are functional relationships between these functional blocks e.g. given that sAPPα is associated with long term potentiation and Aβ is associated with long term depression, the relative balance between both functional blocks will determine overall outcome in synaptic plasticity. (5) Functional effects deriving from other fragments have been described e.g. AICDs may be involved nuclear signalling [65]. Although catabolic fragments potentially act as small regulatory binding proteins and potentially feedback to APP processing pathways, this has not been systematically investigated. (6) The combined overall output from the APP proteolytic system can feed into other cellular and physiological systems via the complex ratios of all its fragments. Iteratively the wider cellular environment can also feedback via changes to the regulation of APP processing. This may involve multiple areas of cross talk between cellular signalling pathways that remain to be fully described and characterised.
If we consider the key concept of dynamic balance between the competing cleavages, we can predict that all the proteolytic fragments will be present at concentrations that can vary over time depending on wider regulatory factors. While the competing nature of these cleavages is well accepted, no study has systematically investigated this dynamic balance. Currently Aβ is measured too few times in experimental settings such that these measures represent cross-sections from a longitudinal sequence - other fragments are measured rarely or not at all. Current measures of Aβ-type fragments do not represent the behaviour of the APP proteolytic system over different time scales. An appropriate time series dataset with controls for all the fragments would be required to adequately describe the behaviours of all its constituents in relation to any biological process, data that is currently missing.
Given that the fragments share varying degrees of sequence homology, the APP matrix approach predicts that they can compete for binding sites, perhaps explaining the varying degrees of cross reactivity of APP proteolytic fragments with antibodies raised against a range fragments from the APP proteolytic system, previously discussed [17], [18]. Since cross reactivity for each antibody has not been systematically investigated to date, current interpretations of antibody reactivities remain uncertain and require clarification. The APP matrix approach requires a comprehensive list of potential binding targets for each fragment and their relative binding affinities over the possible range of concentrations. This information is currently completely missing so we cannot predict the likeliest physiological outcomes associated with specific changes in APP proteolysis.
Competitive binding may also modulate cellular processes. In terms of protection from excitotoxicity, the effects of Aβ and glucose deprivation, sAPPα was found to be ∼100× more neuroprotective than sAPPβ [51]. These effects, associated with amino acids in sAPPα C-terminus, were also modulated by heparinases, perhaps highlighting the importance of the role of the heparin binding domain affected differently by the α- and β-cleavages. The APP matrix approach suggests that since all fragments are present in varying concentrations and these fragments can compete for binding sites, sAPPβ can modulate the outcomes derived from the interactions of sAPPα and by extension perhaps also those associated with full length APP. Extending this prediction further, P3-type fragments potentially modulate the actions of Aβ-type fragments. These predictions have not been tested experimentally. A well-controlled matrix of experiments measuring relative binding affinities and functional outcomes would be required to investigate systematically how the effects of one APP proteolytic fragment are modulated, either agonistically or antagonistically, by varying concentrations of other APP proteolytic fragments and other cellular factors such as heparins.
The neurobiological substrates of cognitive function are not well understood. One widely accepted model of synaptic plasticity involves a wide range of organised and interdependent cellular processes so that dysfunction in any can lead to cognitive impairment [52]. Further, much of dementia research appears paradoxical, e.g. both over and under expression of Aβ-type fragments is associated with specific fully penetrant AβPP mutations associated with young onset AD [49] depending on mutation location. From the perspective of the APP matrix approach, the apparent paradoxes can be explained if we consider dose–response curves for each fragment in relation to wider physiological and cellular processes. It is likely that appropriate physiological processes associated with Aβ depend on appropriate modulation of its concentration within a narrow range, too little Aβ or too much, whether from genetic mutation or epigenetic change to gene expression in ageing, can lead to change in the dynamic balance between competing APP proteolytic pathways. These changes may be inappropriate in the context of wider cellular function and either aberrant loss or gain of Aβ may lead ultimately to cognitive impairments. Additionally, there may be situations where loss or gain of Aβ is appropriate, perhaps in response to wider physiological functions such as synaptic plasticity, injury or infection. From the perspective of the APP matrix approach, current cross sectional measures of the presence of Aβ-type fragments alone cannot represent the complexity of this proteolytic system and are confounded both by the neglect of other proteolytic fragments derived from APP and by neglect of factors deriving from the wider physiological context.
3. How this hypothesis relates to the other hypotheses
The APP matrix approach is a response to repeated calls over the decades to include considerations of the physiology of this proteolytic system in any theoretical mechanistic model [7], [8], [16]. It differs fundamentally from the amyloid cascade hypothesis in being iterative, dynamic and places all the proteolytic fragments derived from APP, not just Aβ, within physiological, regulatory and functional contexts in a way not biased by concepts of inherent neurotoxicity, relating to particular forms of Aβ, or neuroprotection, relating to soluble N-terminal product from α-cleavage (sAPPα), often used in the context of the amyloid cascade hypothesis. The APP matrix approach therefore avoids apparent paradoxes deriving from incomplete reductionist approaches seen in many areas of dementia research beyond amyloid by requiring systematic description of, and comprehensive controlling for, all factors involved.
The APP matrix approach is compatible with, and complementary to, the presenilin hypothesis when this hypothesis is adjusted to include consideration of all fragments derived from γ-cleavage. Analysis of PSENs and AβPP mutations located around the γ-cleavage site in humans suggests that these mutations generally share a reduction in total Aβ and increased or unchanged ratio of Aβ(1–42)/Aβ(1–40) [49]. This can be contrasted with the generally increased expression of Aβ seen for mutations around the α-cleavage sites suggesting that these mutations can be understood as loss of function mutations in terms of regulated cleavages that lead to gain of function in terms of the ratios between all the proteolytic fragments and that these perturbations lead to changes in the dynamic balance between competing cleavages and resultant ratios of fragments.
The APP matrix approach is compatible with a wide range of hypotheses relating to dementia initiation and progression and provides a framework with which contributions from different research areas, including the vascular system, metabolism, mitochondrial function, the immune system to the cell cycle etc., can be integrated via the regulation of APP proteolysis. There are multiple possible routes to disease through this complex proteolytic system, each depending on a dynamic balance between all factors involved. Disease can be initiated by any of these factors, either alone or in combination so that changes in APP proteolysis can both drive disease pathways and also be driven by wider physiological factors. The APP matrix approach allows partial contributions to disease progression from all systems involved and these contributions may vary between individuals. These contributions can also vary as disease progression leads to homeostatic responses that evolve over short and longer time scales. Evidence is accumulating that sporadic and familial AD may not share the same molecular [66] and neuropathological [67] profiles and therefore may not share the same therapeutic targets, supporting the multiple pathways suggested by the APP matrix approach. It remains to be investigated whether there are detailed molecular profiles that describe particular types of disease related pathways, each with their own specific therapeutic targets. Current AD biomarkers do not capture this level of detail and therefore their application to patient groups in clinical trials may be confounded by potentially different pathways.
4. Future directions
Investigations and tests based on the APP matrix approach involve major challenges that are shared by all other approaches to amyloid based research deriving from current uncertainties in basic science including uncertainties deriving from antibody cross reactivities and experimental designs [16] and further challenges deriving from its complex systems biology approach that range from the development of refined measurements to better designed and controlled mechanistic investigations. It is ambitious in its aims and will generate multiple avenues of investigation to characterise each factor associated with regulation of APP proteolysis and dementia. This will require the collaborative integration of evidence from multiple research groups and careful translation of evidence between different research approaches.
Perhaps the major challenge is to re-orientate thinking in the research community to minimise bias associated with ideas of direct causation via one or a few disease pathways, as illustrated by the amyloid cascade hypothesis. Considerations of dynamic complexity, fundamental to the APP matrix approach, may better represent the chaotic basis of cognitive function and allow a deeper understanding of the many possible ways dementia arises.
The search for therapeutic interventions for complex syndromes of ageing such as dementia presents great challenges across many research disciplines, from epidemiological characterisations of disease presentations in the population to detailed biomedical investigations of therapeutic targets at the molecular level. Each experimental approach is associated with particular limitations and uncertainties that impact on how we understand research progress to date. The integration and understanding of the vast and diverse dementia-related evidence requires individual researchers and research teams to develop broad research skill sets that, coupled with the use of appropriate theoretical frameworks, direct the design and interpretation of investigations. Theoretical frameworks fundamentally underpin research strategy and it is essential that these frameworks represent a useful approximation of the natural systems being investigated. We believe that the APP matrix approach will re-invigorate amyloid based dementia research and will generate data essential to understanding the role of the APP proteolytic system in AD and how this system interacts with wider cellular processes and physiological functions in the context of human cognitive function and disease.
Declarations of interest
None.
Acknowledgements
This work was supported by funding from the Addenbrooke’s Charitable Trust, the Paul G. Allen Family Foundation, Alzheimer’s Research, UK and the National Institute for Health Research, Senior Investigator Award, awarded to CB. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Biographies
Carol Brayne is a Professor of Public Health Medicine and Co-Chair of Cambridge Public Health Interdisciplinary Centre, University of Cambridge. Her research interests include Brain ageing, dementia, longitudinal and cohort studies and health inequalities.
Sally Hunter is a Research Associate with the Cambridge City over 75s Cohort. Her research interests focus on brain ageing in the population.
References
- 1.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Brouwers N., Sleegers K., Van Broeckhoven C. Molecular genetics of Alzheimer's disease: an update. Ann Med. 2008;40:562–583. doi: 10.1080/07853890802186905. [DOI] [PubMed] [Google Scholar]
- 4.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regland B., Gottfries C.G. The role of amyloid beta-protein in Alzheimer's disease. Lancet. 1992;340:467–469. doi: 10.1016/0140-6736(92)91780-c. [DOI] [PubMed] [Google Scholar]
- 8.Joseph J., Shukitt-Hale B., Denisova N.A., Martin A., Perry G., Smith M.A. Copernicus revisited: amyloid beta in Alzheimer's disease. Neurobiol Aging. 2001;22:131–146. doi: 10.1016/s0197-4580(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 9.Robinson S.R., Bishop G.M. Abeta as a bioflocculant: implications for the amyloid hypothesis of Alzheimer's disease. Neurobiol Aging. 2002;23:1051–1072. doi: 10.1016/s0197-4580(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 10.Shen J. Function and dysfunction of presenilin. Neurodegener Dis. 2014;13:61–63. doi: 10.1159/000354971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J., Kelleher R.J., 3rd. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brayne C., Richardson K., Matthews F.E., Fleming J., Hunter S., Xuereb J.H., et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–658. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 13.MRC-CFAS Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 14.Tanskanen M., Makela M., Notkola I.L., Myllykangas L., Rastas S., Oinas M., et al. Population-based analysis of pathological correlates of dementia in the oldest old. Ann Clin Transl Neurol. 2017;4:154–165. doi: 10.1002/acn3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savva G.M., Wharton S.B., Ince P.G., Forster G., Matthews F.E., Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 16.Hunter S., Smailagic N., Brayne C. Abeta and the dementia syndrome: simple versus complex perspectives. Eur J Clin Invest. 2018 doi: 10.1111/eci.13025. [DOI] [PubMed] [Google Scholar]
- 17.Hunter S., Brayne C. Do anti-amyloid beta protein antibody cross reactivities confound Alzheimer disease research? J Negat Results Biomed. 2017;16:1. doi: 10.1186/s12952-017-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter S., Brayne C. Erratum to: Do anti-amyloid beta protein antibody cross reactivities confound Alzheimer disease research? J Negat Results Biomed. 2017;16:8. doi: 10.1186/s12952-017-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouter Y., Lopez Noguerola J.S., Tucholla P., Crespi G.A., Parker M.W., Wiltfang J., et al. Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against Ntruncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol. 2015;130:713–729. doi: 10.1007/s00401-015-1489-x. [DOI] [PubMed] [Google Scholar]
- 20.Crespi G.A., Hermans S.J., Parker M.W., Miles L.A. Molecular basis for mid-region amyloid-beta capture by leading Alzheimer's disease immunotherapies. Sci Rep. 2015;5:9649. doi: 10.1038/srep09649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begley C.G., Ioannidis J.P. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res. 2015;116:116–126. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 22.Tsilidis K.K., Panagiotou O.A., Sena E.S., Aretouli E., Evangelou E., Howells D.W., et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter S. What is abeta? In: Perry G, editor. Journal of Alzheimer's Disease Editor's blog 2018.
- 24.Hunter S., Brayne C. Relationships between the amyloid precursor protein and its various proteolytic fragments and neuronal systems. Alzheimers Res Ther. 2012;4:10. doi: 10.1186/alzrt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter S., Brayne C. In: Springer handbook of bio-/neuro-informatics. Kasabov N., editor. Springer-Verlag; Berlin Heidelberg: 2014. Integrating data for modeling biological complexity; pp. 921–940. [Google Scholar]
- 26.Hunter S., Martin S., Brayne C. The APP proteolytic system and its interactions with dynamic networks in Alzheimer's disease. Methods Mol Biol. 2016;1303:71–99. doi: 10.1007/978-1-4939-2627-5_3. [DOI] [PubMed] [Google Scholar]
- 27.Turner P.R., O'Connor K., Tate W.P., Abraham W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 28.Moore S., Evans L.D., Andersson T., Portelius E., Smith J., Dias T.B., et al. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell reports. 2015;11:689–696. doi: 10.1016/j.celrep.2015.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyckman A.W., Confaloni A.M., Thinakaran G., Sisodia S.S., Moya K.L. Post-translational processing and turnover kinetics of presynaptically targeted amyloid precursor superfamily proteins in the central nervous system. J Biol Chem. 1998;273:11100–11106. doi: 10.1074/jbc.273.18.11100. [DOI] [PubMed] [Google Scholar]
- 30.Deyts C., Clutter M., Herrera S., Jovanovic N., Goddi A., Parent A.T. Loss of presenilin function is associated with a selective gain of APP function. eLife. 2016;5 doi: 10.7554/eLife.15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R., Sweeney D., Gandy S.E., Sisodia S.S. The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 32.Lee M.S., Kao S.C., Lemere C.A., Xia W., Tseng H.C., Zhou Y., et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deuss M., Reiss K., Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 34.Vincent B., Checler F. alpha-Secretase in Alzheimer's disease and beyond: mechanistic, regulation and function in the shedding of membrane proteins. Curr Alzheimer Res. 2012;9:140–156. doi: 10.2174/156720512799361646. [DOI] [PubMed] [Google Scholar]
- 35.Cole S.L., Vassar R. BACE1 structure and function in health and Alzheimer's disease. Curr Alzheimer Res. 2008;5:100–120. doi: 10.2174/156720508783954758. [DOI] [PubMed] [Google Scholar]
- 36.Cole S.L., Vassar R. The Alzheimer's disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun X., Wang Y., Qing H., Christensen M.A., Liu Y., Zhou W., et al. Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J. 2005;19:739–749. doi: 10.1096/fj.04-3426com. [DOI] [PubMed] [Google Scholar]
- 38.Willem M., Tahirovic S., Busche M.A., Ovsepian S.V., Chafai M., Kootar S., et al. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez R.G., Soriano S., Hayes J.D., Ostaszewski B., Xia W., Selkoe D.J., et al. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- 40.Chang K.A., Suh Y.H. Pathophysiological roles of amyloidogenic carboxy-terminal fragments of the beta-amyloid precursor protein in Alzheimer's disease. J Pharmacol Sci. 2005;97:461–471. doi: 10.1254/jphs.cr0050014. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Zhang Y.W., Wang X., Zhang H., You X., Liao F.F., et al. Intracellular trafficking of presenilin 1 is regulated by beta-amyloid precursor protein and phospholipase D1. J Biol Chem. 2009;284:12145–12152. doi: 10.1074/jbc.M808497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallhoff-Munoz V., Hu L., Chen X., Pautler R.G., Zheng H. Genetic dissection of gamma-secretase-dependent and -independent functions of presenilin in regulating neuronal cell cycle and cell death. J Neurosci. 2008;28:11421–11431. doi: 10.1523/JNEUROSCI.2873-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lleo A., Berezovska O., Ramdya P., Fukumoto H., Raju S., Shah T., et al. Notch1 competes with the amyloid precursor protein for gamma-secretase and down-regulates presenilin-1 gene expression. J Biol Chem. 2003;278:47370–47375. doi: 10.1074/jbc.M308480200. [DOI] [PubMed] [Google Scholar]
- 44.Hare J. Trafficking of amyloid beta-precursor protein products C83 and C99 on the endocytic pathway. Biochem Biophys Res Commun. 2010;401:219–224. doi: 10.1016/j.bbrc.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Baranello R.J., Bharani K.L., Padmaraju V., Chopra N., Lahiri D.K., Greig N.H., et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease. Curr Alzheimer Res. 2015;12:32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jager S., Leuchtenberger S., Martin A., Czirr E., Wesselowski J., Dieckmann M., et al. alpha-secretase mediated conversion of the amyloid precursor protein derived membrane stub C99 to C83 limits Abeta generation. J Neurochem. 2009;111:1369–1382. doi: 10.1111/j.1471-4159.2009.06420.x. [DOI] [PubMed] [Google Scholar]
- 47.Allen N., Robinson A.C., Snowden J., Davidson Y.S., Mann D.M. Patterns of cerebral amyloid angiopathy define histopathological phenotypes in Alzheimer's disease. Neuropathol Appl Neurobiol. 2014;40:136–148. doi: 10.1111/nan.12070. [DOI] [PubMed] [Google Scholar]
- 48.Schieb H., Kratzin H., Jahn O., Mobius W., Rabe S., Staufenbiel M., et al. Beta-amyloid peptide variants in brains and cerebrospinal fluid from amyloid precursor protein (APP) transgenic mice: comparison with human Alzheimer amyloid. J Biol Chem. 2011;286:33747–33758. doi: 10.1074/jbc.M111.246561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunter S., Brayne C. Understanding the roles of mutations in the amyloid precursor protein in Alzheimer disease. Mol Psychiatry. 2018;23:81–93. doi: 10.1038/mp.2017.218. [DOI] [PubMed] [Google Scholar]
- 50.Hunter S., Arendt T., Brayne C. The senescence hypothesis of disease progression in Alzheimer disease: an integrated matrix of disease pathways for FAD and SAD. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8445-3. [DOI] [PubMed] [Google Scholar]
- 51.Furukawa K., Sopher B.L., Rydel R.E., Begley J.G., Pham D.G., Martin G.M., et al. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 52.Peineau S., Rabiant K., Pierrefiche O., Potier B. Synaptic plasticity modulation by circulating peptides and metaplasticity: Involvement in Alzheimer's disease. Pharmacol Res. 2018 doi: 10.1016/j.phrs.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Ishida A., Furukawa K., Keller J.N., Mattson M.P. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. NeuroReport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 54.Taylor C.J., Ireland D.R., Ballagh I., Bourne K., Marechal N.M., Turner P.R., et al. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008;31:250–260. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Gakhar-Koppole N., Hundeshagen P., Mandl C., Weyer S.W., Allinquant B., Muller U., et al. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q., Rowan M.J., Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gosztyla M.L., Brothers H.M., Robinson S.R. Alzheimer's amyloid-beta is an antimicrobial peptide: a review of the evidence. J Alzheimers Dis. 2018;62:1495–1506. doi: 10.3233/JAD-171133. [DOI] [PubMed] [Google Scholar]
- 59.Zou K., Gong J.S., Yanagisawa K., Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atwood C.S., Obrenovich M.E., Liu T., Chan H., Perry G., Smith M.A., et al. Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 2003;43:1–16. doi: 10.1016/s0165-0173(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 61.Abramov A.Y., Canevari L., Duchen M.R. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742:81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Chiang H.C., Iijima K., Hakker I., Zhong Y. Distinctive roles of different beta-amyloid 42 aggregates in modulation of synaptic functions. FASEB J. 2009;23:1969–1977. doi: 10.1096/fj.08-121152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belyaev N.D., Kellett K.A., Beckett C., Makova N.Z., Revett T.J., Nalivaeva N.N., et al. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a {beta}-secretase-dependent pathway. J Biol Chem. 2010;285:41443–41454. doi: 10.1074/jbc.M110.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu K., Solano I., Mann D., Lemere C., Mercken M., Trojanowski J.Q., et al. Characterization of Abeta11-40/42 peptide deposition in Alzheimer's disease and young Down's syndrome brains: implication of N-terminally truncated Abeta species in the pathogenesis of Alzheimer's disease. Acta Neuropathol. 2006;112:163–174. doi: 10.1007/s00401-006-0077-5. [DOI] [PubMed] [Google Scholar]
- 65.Goodger Z.V., Rajendran L., Trutzel A., Kohli B.M., Nitsch R.M., Konietzko U. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci. 2009;122:3703–3714. doi: 10.1242/jcs.048090. [DOI] [PubMed] [Google Scholar]
- 66.Pera M., Alcolea D., Sanchez-Valle R., Guardia-Laguarta C., Colom-Cadena M., Badiola N., et al. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 2013;125:201–213. doi: 10.1007/s00401-012-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maarouf C.L., Daugs I.D., Spina S., Vidal R., Kokjohn T.A., Patton R.L., et al. Histopathological and molecular heterogeneity among individuals with dementia associated with Presenilin mutations. Mol Neurodegener. 2008;3:20. doi: 10.1186/1750-1326-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]