1. Introduction
In this commentary I argue that current evidence demonstrates a greater influence of the environment as opposed to genetic factors on human aging and longevity. Even though a few genetic factors have been highlighted as influencing the aging process, including APOE and FOXO3A, as yet, the list is sparse. Statistics on longevity and aging are based on life-expectancy and healthy-life expectancy. However, those born in the 1930s were not exposed to the massive increases in chemical pollution to which recent generations were exposed. A recent research focus is how epigenetic processes linking the environment to gene expression are implicated. Key discoveries are being made on how methylation patterns during development are linked to brain aging and cognitive processes. Similarly, life-style is increasingly recognized as a modulator of healthy longevity and cognitive agility or degeneration. Numerous environmental factors have been demonstrated to be involved, but diet, exercise and social context are currently seen to be of overriding importance.
2. Healthy life expectancy versus life expectancy
Statistics on longevity and aging are based on life expectancy and healthy life expectancy. The World Bank data base1 reports that the current life expectancy at birth in Japanese women is 87 and that for Japanese men 81 years. In the US it is for women 81 and only 76 for men, whereas in South Africa it is under 60 years. What accounts for the difference in countries and gender? Here I focus on environmental factors that modulate the aging process.
Of the many hypotheses that can be put forward I will focus on chemical pollution and particularly, air pollution and endocrine disrupting chemicals (EDCs). Many aspects of EDCs make them difficult to regulate as they display non-monotonic dose-responses and low dose responses [1]. That chemical contamination adversely affects healthy life expectancy as well as overall life expectancy is established, especially through increasing incidence of non-communicable disorders (NCD). As a result, even though life expectancy was still increasing at the end of the 20th century, the gap between healthy life expectancy was widening [2]. This is explained in large part as being due to an increase in non-infectious diseases that deteriorate healthy life expectancy. For instance, metabolic disorders such as obesity and diabetes [3], are linked to inflammatory disorders including arthritis [4]. All of these diseases have been shown to have environmental contributions involving EDCs [1].
By definition, the actual figures on which the current life expectancy statistics are based were those born at the beginning of the last century (1930s or before). As a result they were spared the massive increase in chemical production that was seen from the 1950s on. The UN produces data sets on chemical production. Their registers show that there has been a 300-fold increase in chemical production since 1970 [5].
Yet eight years previously, Rachel Carson had written Silent Spring [5]. The book galvanised the public’s attention on pesticides, but clearly did little to staunch the expansion of the chemical industries. Carson’s focus was on excessive use of dichlorodiphenyltrichloroethane (DDT), as a major cause of loss of bird and fish life. The population losses were not only due to reductions in food sources, insects, but also to accumulation in the bird’s organs, affecting reproductive capacity. Since then, the excessive use of DDT has been shown to reduce oestrogen levels in birds [6], so acting as an EDC. Carson also presciently realized that humans would be contaminated too and that ultimately effects would be seen on human health. This was unfortunately shown to be the case. Fifty years after widespread applications, Barbara Cohn reported that female foetuses that had been exposed to high levels of DDT in utero during the 1960s had an increased risk of breast cancer [7].
However, chemical production goes far beyond pesticides, now including molecules and mixtures that cover a multitude of consumer products such as plastics, food additives, food contact materials and packaging, building materials, personal care products and cosmetics. The result is that chemical pollution (both legacy products that are now banned and the new substitutes that replace them) are found not only in the serum and urine of pregnant women [8], but also in amniotic fluid [9] and the foetal/placental compartments [10].
3. The developmental origins of adult health and disease (DOHaD) as a driver of aging
The Developmental Origins of Adult Health and Disease (DOHaD) hypothesis is based on the concept that prenatal exposure to different stressors, whether chemical, psychological or infectious, determines later health and disease. Many examples have been described, from butterflies to mammals including humans.
In 1989, David Barker published in a commentary in Nature [11], correctly foreseeing, that in developed countries, death from infectious disease would decrease and that an increased incidence of non-infectious, NCDs would grow in industrialised and emerging economies. This rapid increase of NCDs exceeds all predictions that could have been made when Barker first published his thoughts. We know that industrialization is linked to the increased incidence of NCDs, such as metabolic disorders (including obesity and diabetes, cardiovascular disease), cancers of the reproductive system, reduced fertility and neurodevelopmental diseases Even though, they are all multifactorial, the incidence of these conditions is rising inexorably, with documented evidence that their origins can be traced to chemical pollution and EDCs [12]. An example is diabetes, with around 422 million people suffering from diabetes type 2 (T2D) that reduces life expectancy by 10 years or more, with incalculable costs in terms of suffering, care and medication as well as productivity. Various EDCs have been implicated in T2D risk, especially the ubiquitous plasticiser bisphenol-A and its substitutes [13]. The World Health Organisation (WHO) classed T2D as a pandemic in 2016.
Subsequent work by Barker and colleagues emphasized the first trimester of foetal life, the first 100 days, others have insisted that the window of time in which healthspan (and, ultimately lifespan) are determined should be widened to include the first 1000 days of life when nutrition in infant- and early childhood is a critical factor [14]. At the same time, we know that vulnerability to multiple stressors continues throughout life, i.e. from early gestation through to early childhood, adolescence and adulthood, although the adverse programming by the environment, including atmospheric pollution [15], seems to be most pronounced at younger life stages. Adverse programming by environmental factors involve a multiplicity of mechanisms (often cascading and amplifying), including inflammation, oxidative phosphorylation and epigenetic modulation of transcription at the levels of cellular and physiological metabolic responses.
4. Cognition and aging is linked to general health and metabolism
The concept of a healthy, active mind in a healthy body is even more apt today, particularly in the context of aging. In 2001, Whalley and Deary had concluded that ‘Childhood mental ability is a significant factor among the variables that predict age at death’ [16], at a time when cognitive epidemiology was just emerging. Later, in 2008, Ian Deary asked ‘Why do intelligent people live longer?’ [17]. Deary cites data from Sweden that shows that military conscripts with higher IQ scores had reduced risk of mortality and morbidity.
He puts forward a series of arguments and plausible mechanisms that could account for the differences in mortality. These range from the more obvious, such as better education and therefore better employment and, as a consequence, higher standards of living. For instance, those with more free-time could, for instance, take account of advice about healthy lifestyles and exercise. The final concept Deary refers to is ‘systems integrity’, where the basic idea is that, during prenatal life, if the mechanisms ensuring proper body and brain function proceed unperturbed by stress, then both will be more robust and resilient. Dreary has consolidated his theory with the idea of ‘optimal bodily functioning’. The result would be that those with higher intelligence and hence higher socio-economic conditions have reduced risk of disease such as cardiovascular disease, stroke, cancers (particularly, smoking related cancers), but also dementia. The principle argument has a number of parallels with the DOHaD theory and epigenetic changes during development (see previous section and the next).
However, given the increase in chemical and air pollution, even those that are gifted with greater intelligence might not continue to enjoy healthy lives, especially given the increase in neurodevelopmental disease that can be associated with decreased IQ [18]. The final point here is that all of these concepts linking aging of the brain and body are also intimately linked to questions of social justice. The important factors here include access to healthy diet, especially during pregnancy, education, medical care and where you live and how far you need to commute to work.
5. Air pollution and the epigenetic link: Programming through methylation
Numerous studies have linked epigenetic changes through methylation of DNA in blood samples as a predictive factor for all-cause mortality and hence the aging process, see for example, [19]. The authors examined the methylation status of the genome for the addition of methyl groups to the DNA. They then calculated the difference between DNA methylation-predicted age minus chronological age of the persons in the cohort. One of the cohorts showed a clear survival advantage for those with the lowest quartile of DNA methylation acceleration rates. Multiple other publications have confirmed these findings.
Most focus on the detrimental effects of air pollution in general have concentrated on the prenatal, perinatal and adolescent phases, with far less emphasis on the long-term consequences as these indivduals enter adulthood and old age. However, a number of publications provide links to DOHaD hypothesis and epigenetic changes. For instance, placental changes in leptin epigenetic markers were lower in those with higher particulate matter (PM) exposure [20]. As leptin is related to growth and development it will affect metabolic capacity not only in fetal life but also in later life.
That mortality is linked to air quality has been emphasized by the World Health Organisation (WHO) and the Organisation for Economic Cooperation and Development (OECD). Air and chemical pollution interact to affect not only one’s general health, notably the link to respiratory diseases such as asthma, but also, at all ages, behaviour and cognition. Many components of air pollution are detrimental to health including PM, heavy metals, Polycyclic Aromatic Hydrocarbons (PAHs), dioxins and volatile organic compounds (VOCs). The major sources of health-endangering air pollution are emissions from power stations (especially coal-powered stations), refineries, petrochemical industries including fertilizer production plants, municipal incinerators, domestic cleaning and traffic.
Two sources of degradation of air quality are well known: traffic-related pollution and agricultural sources. Air pollution is classed according to the size of the PM formed <10 µm and <2.5 µm. Traffic-related pollution is mostly associated with combustion products discharged through exhaust pipes. However, friction of tires on tarmac and brake linings contribute equally, even reaching 90% of vehicle emissions in Northern Europe, where more severe winters are found [21]. Air quality is known to be degraded by particulate matter from traffic, but less well known is the reaction between fertilisers applied in the surrounding countryside on air pollution in cities [22]. Here, the link is that the two interact to produce PM that contains ammonium sulphate and ammonium nitrate. To this one can add the VOCs that form aerosol pollution derived from a plethora of products including pesticides, printing inks in packaging, household cleaning agents and personal care products [23]. Ironically, the authors note that this form of air (and chemical) pollution has increased in the US simultaneously with decreased transport and traffic. More specifically, it has been demonstrated that the risk of developing dementia increases as a function of proximity to major roads [24]. Similarly, traffic-related pollution decreases cognitive function in older men [25], but less is known about agricultural fertilizer use and cognitive performance during aging.
So, here again we have a vicious circle: increased air pollution from whatever source whether traffic or agriculture, increases risk of brain aging. Air pollution also incites those working in offices in large cities to prefer prepackaged food deliveries. This situation is aggravated during COVID-19 lockdowns when restaurants are closed. In turn, increased food deliveries by motorized transport increases air pollution and the disposal of the packages also impacts air pollution whether they are disposed of by incineration or burying in landfills.
To date, most COVID-19 deaths were those with multiple risk factors including being overweight or diabetic, smoking, gender (men having higher death rates) and lower socio- economic status [26]. Those with lower economic classes have poorer nutrition and exposures to endocrine disruptors a fact that will exacerbate mortality if infected with COVID-19, again emphasising problems of social injustice.
6. Taking advantage of the natural environment improves brain health
There is a clear, positive, association between physical health during aging and natural environments. This holds true whether our horizons allow us to see blue seascapes, clear skies or green landscapes. The inverse is also true, with excess mortality being linked to environmental stressors that range from ambient air pollution, especially PAHs, to indoor air pollution, personal care products, VOCs and noise.
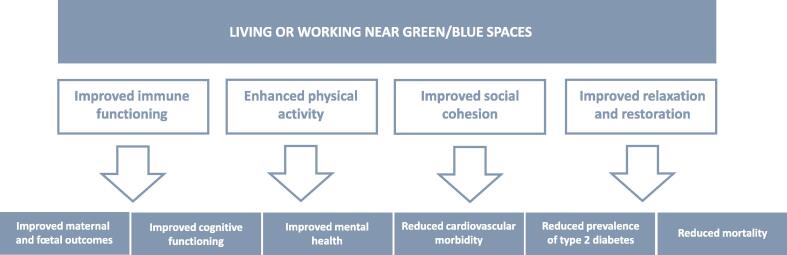
As per usual this will also depend on the individual’s socio-economic conditions, the quality of the environments that they experience on a daily basis and whether within them the individuals are able to maintain dynamic social relationships [27]. Oddly enough, urban living is associated with better cognitive health in aging [28]. Thus, there is a nexus between well-being, health and the environment that engenders resilience to the entropy that the aging process inevitably entails. Unfortunately, few today can achieve the optimal conditions required for health brain aging (Fig. 1).
Fig. 1.
Interactions of environmental factors with brain health. Schema of the numerous environmental factors that impinge on health and ultimately mortality placed in the context of the DOHaD hypothesis. Reducing environmental stressors during pregnancy and early childhood diminishes ill-health in childhood, adolescence and aging. At all stages taking advantage of green/blue environments improves cognitive function and mental health. Similarly, exercise improves immune function and thereby reduces incidence of non-communicable disease risk. Adapted from https://www.eea.europa.eu/publications/healthy-environment-healthy-lives [27].
7. Future directions and research
That the EU has invested €52 million in eight projects on adverse effects on human development caused by EDCs is certainly a step forward in understanding how environmental stressors affect health. The projects financed range from women’s reproductive health to developmental neurotoxicity and thyroid endpoints (including brain effects). However, the investment is somewhat limited when compared to the costs of neurodevelopmental and neurodegenerative diseases. In 2015 Bellinger et al. estimated the yearly costs of neurodevelopmental disease due to EDCs in the EU over €157 billion [29]. Global neurodegenerative disease costs are increasing exponentially too. In 2010 costs in Europe were at least €105 billion, [30]. The fear is that the neurodevelopmental costs could morph into neurodegenerative costs, with the increasingly top heavy age pyramid. Even though certain genetic factors have been identified as common to both forms of neuronal dysfunction, there is a need to invest greater research efforts into the how the environment modifies neuronal processes. A focus could be mechanistic studies on how epigenetic factors modulate physiological and cellular processes, both acutely as well as during development and aging.
Disclosure statement
Barbara Demeneix is a cofounder of WatchFrog.
Funding sources and acknowledgements
This work received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreements No 825161 (ATHENA), No 825759 (EndPoints), No 666869 (THYRAGE).
Biography
Barbara A. Demeneixis Emeritus Professor of Physiology at the National Natural History Museum in Paris, France. She previously led two large-scale EU-funded projects on development and aging, has advised the Organization for Economic Cooperation (OECD) and the European Parliament on the adverse impact of environmental chemicals on health and currently chairs the Endocrine Society European Task Force in Endocrine Disruption. Demeneix has been made a Chevalier (2004) and Officer (2014) of the French Légion d́ Honneur, and Commander (2018) of the French Order de Mérit for her scientific and technological contributions to society.
Footnotes
https://data.worldbank.org/indicator/SP.DYN.LE00.IN Consulted 1st November 2020
References
- 1.Gore A.C., et al. EDC-2: The endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parliament, U., Select Committe on Science and Technology First Report. 2003, https://publications.parliament.uk/pa/ld200506/ldselect/ldsctech/20/2004.htm: London UK.
- 3.Heindel J.J., et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace I.J., et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci USA. 2017;114(35):9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson R. Houghton Mifflin, Riverside Press; Boston, Cambridge, Mass: 1962. Silent spring; p. 368. [Google Scholar]
- 6.Peakall D.B. p,p'-DDT: effect on calcium metabolism and concentration of estradiol in the blood. Science. 1970;168(3931):592–594. doi: 10.1126/science.168.3931.592. [DOI] [PubMed] [Google Scholar]
- 7.Cohn B.A., et al. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. 2015;100(8):2865–2872. doi: 10.1210/jc.2015-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff T.J., Zota A.R., Schwartz J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fini J.B., et al. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci Rep. 2017;7:43786. doi: 10.1038/srep43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schonfelder G., et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):a703–a707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker D.J.P. Rise and fall of Western diseases. Nature. 1989;338(6214):371–372. doi: 10.1038/338371a0. [DOI] [PubMed] [Google Scholar]
- 12.Demeneix B., Slama R. European Parliament; Brussels, Belgium: 2019. Endocrine disruptors: from scientific evidence to human health protection. [Google Scholar]
- 13.Rancière F., et al. Exposure to bisphenol A and bisphenol S and incident type 2 diabetes: A case–cohort study in the French cohort D.E.S.I.R. Environ Health Perspect. 2019;127(10):107013. doi: 10.1289/EHP5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzenberg S.J., Georgieff M.K., Committee On N. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. 2018;141(2) doi: 10.1542/peds.2017-3716. [DOI] [PubMed] [Google Scholar]
- 15.Pope C A., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108(suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whalley L.J., Deary I.J. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322(7290):819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deary I. Why do intelligent people live longer? Nature. 2008;456(7219):175–176. doi: 10.1038/456175a. [DOI] [PubMed] [Google Scholar]
- 18.Bennett D., et al. Project TENDR: targeting environmental neuro-developmental risks the TENDR consensus statement. Environ Health Perspect. 2016;124(7):A118–A122. doi: 10.1289/EHP358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marioni R.E., et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saenen N.D., et al. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE cohort. Environ Health Perspect. 2017;125(2):262–268. doi: 10.1289/EHP38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keuken M., Denier van der Gon H., van der Valk K. Non-exhaust emissions of PM and the efficiency of emission reduction by road sweeping and washing in the Netherlands. Sci Total Environ. 2010;408(20):4591–4599. doi: 10.1016/j.scitotenv.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Lovarelli D., et al. Describing the trend of ammonia, particulate matter and nitrogen oxides: The role of livestock activities in northern Italy during Covid-19 quarantine. Environ Res. 2020;191 doi: 10.1016/j.envres.2020.110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald B.C., et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science. 2018;359(6377):760–764. doi: 10.1126/science.aaq0524. [DOI] [PubMed] [Google Scholar]
- 24.Chen H., et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017;389(10070):718–726. doi: 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- 25.Power M.C., et al. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119(5):682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman M., et al. A global analysis on the effect of temperature, socio-economic and environmental factors on the spread and mortality rate of the COVID-19 pandemic. Environ Dev Sustain. 2020:1–15. doi: 10.1007/s10668-020-01028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Environment Agency. Healthy environment, healthy lives: how the environment influences health and well-being in Europe. https://www.eea.europa.eu/publications/healthy-environment-healthy-lives, ed. E.E. Agency. 2019, Luxembourg: Publications of the European Union
- 28.Cassarino M., Setti A. Environment as ‘Brain Training’: A review of geographical and physical environmental influences on cognitive ageing. Ageing Res Rev. 2015;23:167–182. doi: 10.1016/j.arr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Bellanger M., et al. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100(4):1256–1266. doi: 10.1210/jc.2014-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustavsson A., et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]