Abstract
An 11–25% increase in total ventricular volume has been documented in astronauts following spaceflight on the ISS. Given the approximately 2-year time interval between pre- and post-flight MRI, it is unknown if ventricular enlargement simply reflects normal aging or is unique to spaceflight exposure. Therefore, we compared percent ventricular volume change per year (PVVC/yr) documented on pre- to post-flight MRI in a group of NASA ISS astronauts (n = 18, 16.7% women, mean age (SD) 48.43 (4.35) years) with two groups who underwent longitudinal MRI: (1.) healthy age- and sex-matched adults (n = 18, 16.7% women, mean age (SD) 51.26 (3.88) years), and (2.) healthy older adults (n = 79, 16.5% women, mean age (SD) 73.26 (5.34) years). The astronauts, who underwent a mean (SD) 173.4 (51.3) days in spaceflight, showed a greater increase in PVVC/yr than the control (6.86 vs 2.23%, respectively, p < .001) and older adult (4.18%, p = 0.04) groups. These results highlight that on top of physiologically ventricular volume changes due to normal aging, NASA astronauts undergoing ISS missions experience an additional 4.63% PVVC/yr and underscore the need to perform post-flight follow-up scans to determine the time course of PVVC in astronauts over time back on Earth along with monitoring to determine if the PVVC is ultimately clinically relevant.
One sentence summary
NASA astronauts who were exposed to prolonged spaceflight experienced an annual rate of ventricular expansion more than three times that expected from normal aging.
Abbreviations: PVVC, percent ventricular volume change; ISS, International Space Station
Keywords: Spaceflight, Cerebral ventricles, Normal aging
Introduction
We [1] and others [2], [3], [4] have reported mean increases in ventricular volumes of 11–25% in NASA astronauts following long-duration spaceflight on the International Space Station (ISS). A mean pre- to post-flight increase of 11.6% in total ventricular volume has also been reported in Russian cosmonauts compared with no change in ventricular volume for age- and sex-matched controls over the same inter-scan intervals [5], [6]. At follow-up MRI, performed on average 7 months after the cosmonauts return to Earth, ventricular volumes had decreased but had not yet returned to pre-flight baseline with a residual mean increase in total ventricular volume of 6.4% [5], [6]. Similarly, ventricular volumes remained enlarged above baseline values in astronauts 6 months to 1 year following return to Earth [3], [4].
Normal aging is associated with ventricular expansion of approximately 1.7–4.1% per year [7], [8], [9] for middle-age to older adults but is less than 1% in young adults [10]. Given the time interval between pre- and post-flight magnetic resonance imaging (MRI) of the brain, which is approximately 2 years including typically 6 months in spaceflight for most missions to the ISS, the ventricular enlargement documented in astronauts may reflect aging. To determine whether or not spaceflight is associated with ventricular expansion in NASA astronauts at post-flight beyond that which occurs during a similar time interval with normal aging, we compared the annualized percentage ventricular volume change (PVVC/yr) for ISS astronauts as measured by pre- and post-flight MRIs with a group of normal age- and sex-matched control participants. For comparison, we also included a third group composed of older adults.
Recently, ISS astronauts have reported visual changes during spaceflight and upon ophthalmologic exam, have been found to have optic disc edema, globe flattening, choroidal and retinal folds, cotton wool spots, and hyperopic refractive error shifts [11]. A few astronauts have undergone post-flight lumbar puncture with mildly elevated opening pressures of 21–28.5 cm H2O [11]. NASA has termed this constellation of findings the spaceflight associated neuro-ocular syndrome (SANS) and while the etiology of SANS is unknown, it has been likened to terrestrial idiopathic intracranial hypertension (IIH) [11]. To gain insight into the mechanism underlying the development of SANS, we also examined the PVVC/yr in astronauts who presented with clinical findings of SANS versus those astronauts who did not to determine if ventricular enlargement post-flight is a feature of SANS.
Methods
Experimental design
The study was approved by the institutional review boards of the NASA Johnson Spaceflight Center and the Medical University of South Carolina (MUSC). After the nature and possible consequences of the study, including potential loss of privacy, were explained to the astronauts, informed consent was obtained. All astronaut data was provided to the study team by the NASA Lifetime Surveillance of Astronaut Health Program. Normal control data was obtained through a data use agreement between MUSC and Washington University in St. Louis (WUSTL). This manuscript was reviewed by NASA to ensure astronaut anonymity.
Participants
Eighteen consecutive NASA astronauts who had undergone both long-term spaceflight on the ISS and high resolution, volumetric MRIs of the brain pre- and post-flight were included in the study (3 women, 15 men, mean spaceflight length (SD) 173.4 (51.3) days). Each astronaut had undergone MR imaging sometime before spaceflight and within two weeks upon return to Earth.
Normal control participants were obtained from the OASIS-3 (Open Access Series of Imaging Studies 3) database. OASIS-3 is a longitudinal neuroimaging, clinical, cognitive, and biomarker dataset for normal aging and Alzheimer’s Disease and represents a compilation of data that were collected across several ongoing projects at WUSTL. The database includes data from 613 well-characterized, cognitively normal adults ranging in age from 42 to 95 years without any documented medical diagnosis. Using the SPSS (IBM Corporation, SPSS®) case-control matching procedure, a group of 18 control participants from the OASIS-3 database were one-to-one matched to the ISS astronauts based on age, sex, and time between scans. Additionally, we screened the OASIS-3 database for a group of older adults aged 65–85 (n = 79) with similar profiles in sex and time between scans.
MR analysis
MRIs of the astronauts were performed on a 3 Tesla Siemens Verio system (n = 18, imaging parameters: TR = 1.9 s, TE = 2.3 ms, inversion time = 900 ms, flip angle of 9 degrees, NSA = 1, FOV = 250 × 250 mm, acquisition matrix = 256 × 256, and slice thickness = 1 mm) and MRIs of the controls were performed on a 3 Tesla Siemens Trim Trio (n = 81, imaging parameters: TR = 2.4 s, TE = 3.2 ms, inversion time = 1000 ms, flip angle of 8 degrees, NSA = 1, FOV = 250 × 250 mm, acquisition matrix = 256 × 256, and slice thickness = 1 mm) or a 1.5 Tesla Siemens Sonata (n = 16, imaging parameters: TR = 1.9 s, TE = 3.9 ms, inversion time = 1100 ms, flip angle of 15 degrees, NSA = 1, FOV = 250 × 240 mm, acquisition matrix = 256 × 240, and slice thickness = 1 mm) systems (Siemens, Erlangen, Germany). For each participant, the same scanner was used for both MRIs. The longitudinal design allowed for the study of within subject changes mitigating any potential errors associated with differences in scanner hardware between the groups.
The same fully automated software pipeline was used to calculate ventricular volumes for the astronaut and both control groups. For the control groups, ventricular volumes had been previously calculated and were available through the OASIS-3 database (FreeSurfer Software Suite; Version 6.0.0; http://surfer.nmr.mgh.harvard.edu). We applied the same automated pipeline (FreeSurfer Software Suite; Version 6.0.0) to the astronaut data set as was used for the control groups.
Percent ventricular volume change (PVVC) was calculated from the ventricular volume on the first and follow-up MRI scans according to:
The annual rate of change was obtained by dividing PVVC by the time interval between scans in years. PVVC/yr was calculated for the total ventricular volume (sum of the right and left lateral, third, and fourth ventricles) and for each ventricle separately.
Statistical methods
Kruskal-Wallis tests were used to evaluate group differences in time between scans, age, and MRI volume variables. Non-parametric approaches were used due to the presence of outliers and the violation of the normality assumption required for parametric approaches. Data are summarized by median (Mdn), Interquartile Range (IQR), and Median Absolute Deviation (MAD = median of (|Xi – median(Xi|)), except for age and flight duration, which are summarized with means and standard deviations (SD) to conform with NASA’s Astronaut Office privacy standards. Fisher’s Exact test was used to evaluate sex differences by group. Specific effects of SANS status versus matched controls were evaluated with Mann-Whitney U tests. Statistical significance was considered at the α = 0.05 threshold and 2-sided p-values are reported. All statistical analyses were performed using SPSS (IBM SPSS Statistics, Version 25).
Results
The ISS group consisted of 18 astronauts (16.7% women, mean age (SD) 48.43 (4.35) years; Table 1). There was no significant difference in age at first MRI scan or sex between the ISS and matched control (n = 18, 16.7% women, mean age (SD) 51.26 (3.88) years) groups (p = 1.00). The older adult group (n = 79, 16.5% women, mean age (SD) 73.26 (5.34) years) was significantly older than the ISS group and matched control groups (p < .001), however, there was no significant difference in the distribution of sex across the three groups (p = 1.00). Per our exclusion criteria, there were no significant differences in time between scans between ISS (Mdn = 2.06 years, IQR = 1.70 – 2.20, Range = 1.26 – 2.56, MAD = 0.15), matched control (Mdn = 2.50 years, IQR = 1.73 – 3.28, Range = 1.12 – 3.90, MAD = 0.75), and older adult groups (Mdn = 2.01 years, IQR = 1.68 – 2.30, Range = 1.00 – 2.59, MAD = 0.30; p = .17).
Table 1.
Participant demographics.
| Group | n (% female)* | Age at first MRI scan |
Time between MRI scans** |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | p-value | Median | IQR | Range | MAD | ||
| ISS | 18 (3F, 16.7%) | 48.43 | 4.35 | ISS vs controls, p = 1.00 ISS vs older adults, p < .001 |
2.06 | 1.70 – 2.20 | 1.26 – 2.56 | 0.15 |
| Controls | 18 (3F, 16.7%) | 51.26 | 3.88 | Controls vs older adults, p < .001 | 2.50 | 1.73 – 3.28 | 1.12 – 3.90 | 0.75 |
| Older Adults | 79 (13F, 16.5%) | 73.26 | 5.34 | 2.01 | 1.68 – 2.30 | 1.00 – 2.59 | 0.30 | |
*There was no significant difference in the distribution of sex across the three groups.
**There were no significant differences in time between scans between the three groups.
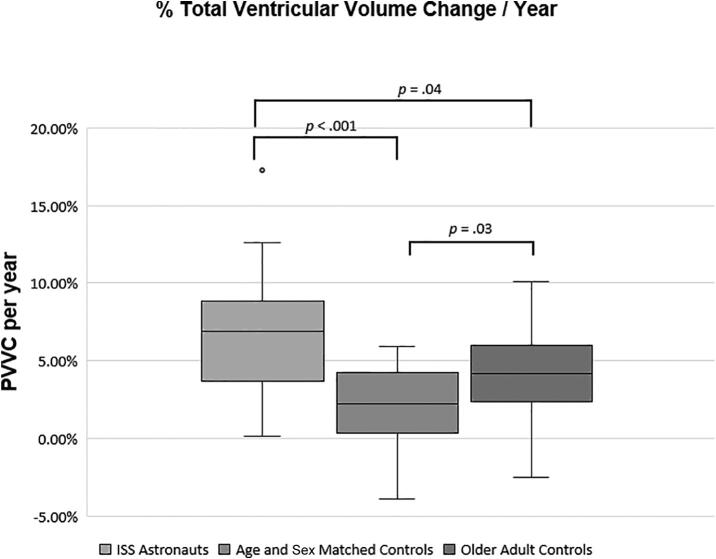
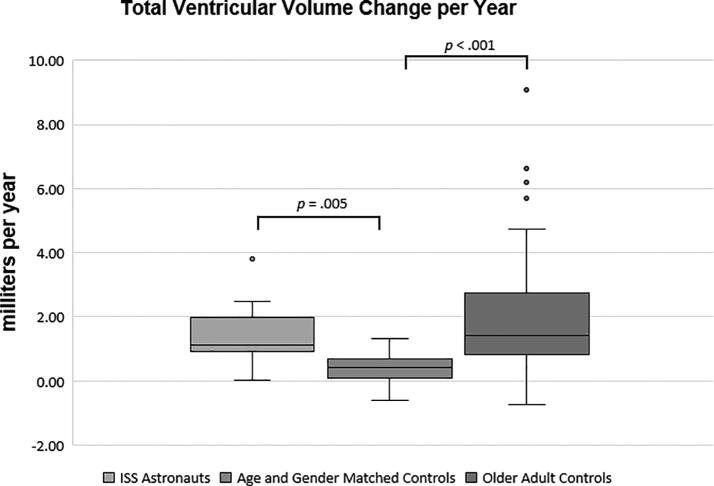
At the baseline MRI, there were no significant differences in total ventricular volumes between the ISS (Mdn = 18.59 ml, IQR = 15.46 – 26.32, Range = 10.97 – 36.49, MAD = 4.22) and matched control (Mdn = 19.40 ml, IQR = 14.60 – 24.72, Range = 10.53 – 37.30, MAD = 4.38) groups, p = .50. However, the older adult group had significantly greater total ventricular volumes (Mdn = 37.87 ml, IQR = 24.64 – 55.91, Range = 9.24 – 109.99, MAD = 13.63) than the ISS or matched control groups, p < .001. Concerning total ventricular volume change, the ISS group showed a significantly greater increase in PVVC/yr (Mdn = 6.86%, IQR = 3.67 – 8.86, Range = 0.13–17.30, MAD = 2.29) than the control group (Mdn = 2.23%, IQR = 0.32 – 4.26, Range = -3.90–5.93, MAD = 1.85; p < .001) and the older adults (Mdn = 4.18%, IQR = 2.37 – 6.01, Range = -2.48–10.12, MAD = 1.81; p = .04). (Table 2, Fig. 1). This was equivalent to median changes in total ventricular volume per year of 1.12 ml, 0.41 ml, and 1.41 ml respectively for the ISS, control, and older adult groups and these group differences were significant: the ISS group (p = .005) and the older adult group (p < .001) showed a significantly greater percent increase in volume than the matched control group (Fig. 2). As the older adults had larger total ventricular volumes at baseline than the other groups, with only a modest increase in volume at follow-up MRI compared to the ISS group, there was no significant difference in total ventricular volume change per year between the ISS and older adult groups, p = 1.00.
Table 2.
Annualized total ventricular volume change in PVVC/yr and in ml/yr.
| Group | n | Total Ventricular Volume (PVVC/yr) |
Total Ventricular Volume Change (ml/yr) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Range | MAD | p-value | Median | IQR | Range | MAD | p-value | ||
| ISS | 18 | 6.86 | 3.67–8.86 | 0.13–17.30 | 2.29 | ISS vs controls, p < .001 ISS vs older adults, p = .04 |
1.12 | 0.91–1.96 | 0.01–3.85 | 0.29 | ISS vs controls, p = .005 ISS vs older adults, p = 1.00 |
| Controls | 18 | 2.23 | 0.32–4.26 | −3.90–5.93 | 1.85 | Controls vs older adults, p = .03 | 0.41 | 0.10–0.67 | −0.62–1.31 | 0.27 | Controls vs older adults, p < .001 |
| Older Adults | 79 | 4.18 | 2.37–6.01 | −2.48–10.12 | 1.81 | 1.41 | 0.80–2.73 | −0.73–9.08 | 0.80 | ||
Fig. 1.
Annualized PVVC (total all ventricles). The boxplots show the median (horizontal line), IQR (the box), 1.5x the IQR (the whiskers), and outliers (dots).
Fig. 2.
Annualized total ventricular volume change (ml/yr). The boxplots show the median (horizontal line), IQR (the box), 1.5x the IQR (the whiskers), and outliers (dots).
Concerning ventricular volume change for the individual ventricles (Table 3), there were significant group differences in PVVC/yr for the left lateral, right lateral, and third ventricles. For the left lateral ventricle, the ISS group showed a significantly greater increase in PVVC/yr than the control group (p < .001) and the older adult group (p = .004). For the right lateral ventricle, the ISS group showed a significantly greater increase in PVVC/yr than the control group, p = .009, but not the older adult group (p = .29). For the third ventricle, the ISS group showed a significantly greater increase in PVVC/yr than the control group (p < .001) and the older adult group (p < .001). For the fourth ventricle, there were no significant group differences in the change in PVVC/yr (p = .19).
Table 3.
Annualized ventricular volume change for individual ventricles in PVVC/yr.
| Group |
n |
p-value vs ISS |
p-value vs Control |
||||
|---|---|---|---|---|---|---|---|
| Left Lateral Ventricle (PVVC/yr) |
|||||||
| Median | IQR | Range | MAD | ||||
| ISS | 18 | 7.95% | 4.16% – 11.31% | −0.02% – 18.51% | 3.43 | ||
| Control | 18 | 3.00% | −0.21% – 5.88% | −5.85% – 7.81% | 2.88 | p < .001 | |
| Older Adults | 79 | 4.77% | 2.34% – 6.26% | −2.36% – 11.87% | 1.79 | p = .004 | p = 0.27 |
| Right Lateral Ventricle (PVVC/yr) |
|||||||
| Median | IQR | Range | MAD | ||||
| ISS | 18 | 6.18% | 3.79% – 8.98% | −0.02% – 20.77% | 2.49 | ||
| Control | 18 | 2.56% | 0.80% – 4.43% | −1.30% − 7.47% | 1.68 | p = .009 | |
| Older Adults | 79 | 4.11% | 2.57% – 6.91% | −3.08% – 11.01% | 2.15 | p = 0.29 | p = 0.10 |
| 3rd Ventricle (PVVC/yr) |
|||||||
| Median | IQR | Range | MAD | ||||
| ISS | 18 | 12.56% | 8.26% − 15.84% | −1.19% − 20.42% | 3.52 | ||
| Control | 18 | −0.75% | −4.41% – 2.44% | −18.39% – 4.19% | 0.74 | p < .001 | |
| Older Adults | 79 | 2.97% | 0.65% – 4.62% | −4.51% – 81.11% | 1.92 | p < .001 | p = .005 |
| 4th Ventricle (PVVC/yr) |
|||||||
| Median | IQR | Range | MAD | ||||
| ISS | 18 | −0.98% | −4.14% – 2.04% | −5.83% – 3.62% | 3.02 | ||
| Control | 18 | 0.05% | −4.38% – 2.79% | −14.70% – 11.10% | 2.87 | *omnibus p = 0.19 | |
| Older Adults | 79 | 1.32% | −2.09% – 3.10% | −14.68% – 17.26% | 2.54 | ||
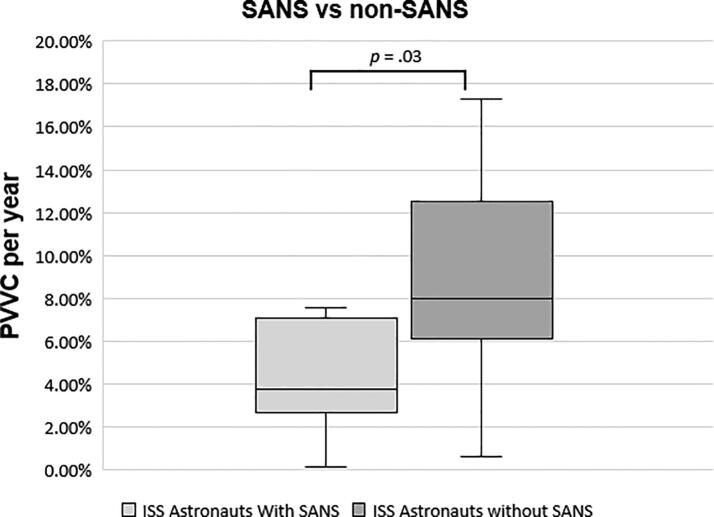
Out of the 18 ISS astronauts, 7 astronauts were found to have features of SANS including optic disc edema and choroidal folds following spaceflight. These 7 astronauts had significantly smaller total PVVC/yr than those astronauts who did not present with features of SANS (3.77% versus 8.02% respectively, p = 0.03, Fig. 3). The PVVC/yr in the 7 astronauts with SANS was not significantly different from their matched controls (3.77% vs 3.84%, p = 0.54), while the PVVC/yr in the astronauts without SANS was significantly greater than their matched controls (8.02% vs 1.64%, p > 0.001). That is, PVVC/yr appeared to be inversely associated with SANS.
Fig. 3.
Annualized PVVC (total all ventricles) in SANS vs non-SANS astronauts. The boxplots show the median (horizontal line), IQR (the box), 1.5x the IQR (the whiskers), and outliers (dots).
Discussion
By comparison with a normal, healthy age- and sex-matched control group, there is approximately 3 times the rate of enlargement of the ventricles in astronauts who participated in ISS missions beyond that expected over the same time interval in normal aging. The age- and sex-matched control group showed a natural physiological PVVC increase of 2.23%/yr over the approximately 2 years between the two MRI exams. On top of that, the astronaut group experienced an additional 4.63% increase in PVVC/yr attributable to the experience of spaceflight for approximately 6 months during the two-year time period. This finding in NASA astronauts is consistent with that previously reported in a group of Russian cosmonauts compared with age- and sex matched controls [5], [6]. Additionally, although ventricular expansion in normal subjects accelerates with age [12], [13], despite the younger age of the astronaut group, the annual % increase in ventricular size was over 1.5 times that of the older adult group (6.86% vs 4.18% per year, respectively).
One of the most important questions this study raises is whether or not after some time back on Earth, ventricular volumes in NASA astronauts return to baseline. Currently, follow-up MRI beyond the immediate post-flight time period (approximately 2 weeks) is not routinely performed in the astronaut population. However, Van Ombergen et al. [6] studied 7 cosmonauts who underwent imaging 7 months after return to Earth. They found ventricular volumes had decreased compared with post-flight values but had not yet returned to the pre-flight baseline [6]. Compared with pre-flight, the total ventricular volume had increased by 11.6% immediately post-flight and after 7 months post-flight, the total ventricular volume remained increased 6.4% over pre-flight values. Similarly, in 11 astronauts, Kramer et al. [4] found a 10.7% increase (equivalent to 2.8 ml) in total ventricular volume on MRI obtained within 24 h after return to Earth which remained increased by 4.2% above pre-flight values at MRI performed 1 year later. In 12 astronauts who spent 6–12 months in space and demonstrated ventricular enlargement immediately post-flight, Hupfeld et al. [3] found that ventricular volumes had decreased by 55 – 64% towards baseline at 6 months post-flight in some of the astronauts, but an astronaut who had spent 12 months in space experienced only a 2–8% return towards baseline ventricular volumes at 6 months. While this study also included a control group, they were not age- and sex matched to the astronaut cohort.
Terrestrial ventricular expansion greater than normal aging has been documented in a range of neurological disorders including declining cognitive status, reduced brain reserve, depression, and progression to mild cognitive impairment and Alzheimer’s disease [8], [14]. This study highlights the need to further investigate the etiology, time course, and any potential clinical consequences of ventricular enlargement in the astronaut population [15]. Importantly, routine serial follow-up MRI of NASA astronauts after spaceflight should be implemented to determine if ventricular volumes normalize after some time back on Earth [15], [16].
We also examined PVVC/yr in astronauts who presented with SANS, a constellation of clinical findings in astronauts that some have likened to IIH in terrestrial patients [11]. We found that in our cohort, the PVVC/yr in the astronauts with SANS was not significantly different from their matched controls while the greater PVVC/yr increase overall in the astronaut group appeared to be driven by the subset of astronauts without SANS. This finding suggest that some astronauts may not be susceptible to increased ventricular expansion with spaceflight but that in itself makes them vulnerable to SANS. This finding may contribute to hypotheses concerning the underlying etiology of SANS to be explored further in future studies. For example, it has been hypothesized that in astronauts the ventricles may act as buffer zones against the development of SANS based on the viscoelastic properties of the brain [6], [15], [17], [18].
The physiology underlying ventricular enlargement in astronauts and whether or not it represents a positive adaptive process to spaceflight are unknown. Post-flight MRI in space crews reveals no net brain tissue loss [19], [20], [21]. Additionally, as described above, the ventricular volume changes are partially reversible after some time back on Earth [3], [4], [6]. Therefore, we do not believe the ventricular enlargement in astronauts represents ex vacuo dilatation associated with brain atrophy, but instead is the result of altered CSF homeostasis from months-long exposure to the spaceflight environment.
Spaceflight-induced ventricular enlargement reveals an unsuspected gravitational dependency of CSF dynamics [15]. Mechanistically, under gravitational stress in the upright posture on Earth, intracranial pressure decreases significantly and perhaps diurnal shifts in posture may play an important role in maintaining normal CSF flow [22]. A gravitational dependency has also been demonstrated for proper functioning of the glymphatic system [23].
In this regard, spaceflight provides a unique insight into the physiology of CSF flow. Further study of the ventricular remodeling which occurs during spaceflight may help shed light on the pathophysiology of Earth-based CSF disorders of the elderly such as idiopathic normal pressure hydrocephalus [15], [21]. Additionally, our results have implications for the brain health of bed ridden patients, who are disproportionately elderly, and who, like astronauts, do not experience the daily shifts in gravitational gradients which occur when moving between the supine and upright standing positions.
Limitations of this study include the use of two different field strength Siemens MRI scanners. However, for each subject, the same scanner was used for both MRIs. Additionally, we believe the reported differences in ventricular volume are much larger than reproducibility errors introduced by differences in MRI scanners. A previous study [24] examined the agreement between ventricular volume estimates obtained on 1.5 and 3.0 Tesla Siemens scanners and analyzed using the FreeSurfer toolkit as in our study. They found mean volume differences of less than 1 ml in lateral ventricle measurements on the two MRI systems with a bias towards overestimation on the 1.5 Tesla system and close to zero for scans performed two times on the same system as in our study. The ventricular volume differences reported here were beyond the 95 percent limits of agreement. Another limitation is that during the 2.06 year median time interval between pre- and post-flight MRI scans, astronauts spent on average only 173.4 days in spaceflight on the ISS.
We chose to report both percentage changes in ventricular volumes (PVVC/yr) in addition to absolute values (ml/yr). The use of percentage change serves as an inter-individual control for differences in brain and head size.
Here we found that NASA astronauts exposed to prolonged spaceflight experience an annual rate of ventricular expansion which is more than three times that expected from normal aging. Importantly, our study points to the need for the inclusion of advanced MR protocols to further explore the mechanisms underlying both the ophthalmological changes of SANS, the structural changes to the brain experienced by astronauts on long-term missions to the International Space Station, whether or not these changes are clinically significant, and whether or not there is a return to baseline after some time back on Earth. This is particularly critical as NASA plans for future missions to the Moon and Mars. In addition, to supporting the health of NASA astronauts, these studies may contribute to a better of understanding of CSF homeostasis benefiting patients on Earth [15].
Funding
This work was supported by National Aeronautics and Space Administration (NASA), grant number NNX13AJ92G. Dr. Inglesby received support through the South Carolina NASA Established Program to Stimulate Competitive Research (EPSCoR) and the South Carolina Space Grant Consortium.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank Wafa Taiym and Sara Mason of the Lifetime Surveillance of Astronaut Health (LSAH) Program, NASA Johnson Space Center, who provided the astronaut imaging data. Control data were obtained from the OASIS-3 Database, Principal Investigators: T. Benzinger, D. Marcus, J. Morris; NIH P50AG00561, P30NS09857781, P01AG026276, P01AG003991, R01AG043434, UL1TR000448, R01EB009352.
Data and materials availability
All study data were provided by the NASA Lifetime Surveillance of Astronaut Health Office. The study was approved by the NASA Johnson Spaceflight Center and Medical University of South Carolina institutional review boards. All participants provided written informed consent for use and publication of their data. NASA has reviewed the manuscript and figures, which preserve astronaut anonymity and are compliant with NASA Astronaut Office privacy standards. To obtain access to the data, application should be made to the NASA Lifetime Surveillance of Astronaut Health Office. Any release of data must be approved by NASA.
References
- 1.Roberts D.R., Albrecht M.H., Collins H.R., Asemani D., Chatterjee A.R., Spampinato M.V., et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med. 2017;377(18):1746–1753. doi: 10.1056/NEJMoa1705129. [DOI] [PubMed] [Google Scholar]
- 2.Alperin N., Bagci A.M., Lee S.H. Spaceflight-induced changes in white matter hyperintensity burden in astronauts. Neurology. 2017;89(21):2187–2191. doi: 10.1212/WNL.0000000000004475. [DOI] [PubMed] [Google Scholar]
- 3.Hupfeld, K. E. et al. The Impact of 6 and 12 months in space on human brain structure and intracranial fluid shifts. Cereb Cortex Commun 1, tgaa023, doi: 10.1093/texcom/tgaa023 (2020). [DOI] [PMC free article] [PubMed]
- 4.Kramer L.A., Hasan K.M., Stenger M.B., Sargsyan A., Laurie S.S., Otto C., et al. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology. 2020;295(3):640–648. doi: 10.1148/radiol.2020191413. [DOI] [PubMed] [Google Scholar]
- 5.Van Ombergen A., Jillings S., Jeurissen B., Tomilovskaya E., Rühl R.M., Rumshiskaya A., et al. Brain tissue-volume changes in cosmonauts. N Engl J Med. 2018;379(17):1678–1680. doi: 10.1056/NEJMc1809011. [DOI] [PubMed] [Google Scholar]
- 6.Van Ombergen A., Jillings S., Jeurissen B., Tomilovskaya E., Rumshiskaya A., Litvinova L., et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc Natl Acad Sci U S A. 2019;116(21):10531–10536. doi: 10.1073/pnas.1820354116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack C.R., Weigand S.D., Shiung M.M., Przybelski S.A., O'Brien P.C., Gunter J.L., et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70:1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen S.K., Gutman B.A., Joshi S.H., Toga A.W., Jack C.R., Weiner M.W., et al. Mapping ventricular expansion onto cortical gray matter in older adults. Neurobiol Aging. 2015;36:S32–S41. doi: 10.1016/j.neurobiolaging.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fjell A.M., McEvoy L., Holland D., Dale A.M., Walhovd K.B. Brain changes in older adults at very low risk for Alzheimer's disease. J Neurosci. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horakova D., Cox J.L., Havrdova E., Hussein S., Dolezal O., Cookfair D., et al. Evolution of different MRI measures in patients with active relapsing-remitting multiple sclerosis over 2 and 5 years: a case-control study. J Neurol Neurosurg Psychiatry. 2008;79(4):407–414. doi: 10.1136/jnnp.2007.120378. [DOI] [PubMed] [Google Scholar]
- 11.Lee A.G., Mader T.H., Gibson C.R., Brunstetter T.J., Tarver W.J. Space flight-associated neuro-ocular syndrome (SANS) Eye (Lond) 2018;32(7):1164–1167. doi: 10.1038/s41433-018-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driscoll I., Davatzikos C., An Y., Wu X., Shen D., Kraut M., et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scahill R.I., Frost C., Jenkins R., Whitwell J.L., Rossor M.N., Fox N.C. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60(7):989. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 14.Erten-Lyons D., Dodge H.H., Woltjer R., Silbert L.C., Howieson D.B., Kramer P., et al. Neuropathologic basis of age-associated brain atrophy. JAMA Neurol. 2013;70(5):616. doi: 10.1001/jamaneurol.2013.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts D.R., Petersen L.G. Studies of hydrocephalus associated with long-term spaceflight may provide new insights into cerebrospinal fluid flow dynamics here on earth. JAMA Neurol. 2019;76:391–392. doi: 10.1001/jamaneurol.2018.4891. [DOI] [PubMed] [Google Scholar]
- 16.Roberts D.R., Stahn A.C., Seidler R.D., Wuyts F.L. Towards understanding the effects of spaceflight on the brain. The Lancet. Neurology. 2020;19(10):808. doi: 10.1016/S1474-4422(20)30304-5. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DR. et al. Reply. AJNR. Am J Neuroradiol 41, E16, doi:10.3174/ajnr.A6400 (2020). [DOI] [PMC free article] [PubMed]
- 18.Wostyn P., et al. The possible role of elastic properties of brain and optic nerve sheath in the development of spaceflight-associated neuro-ocular syndrome. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jillings S., Van Ombergen A., Tomilovskaya E., Rumshiskaya A., Litvinova L., Nosikova I., et al. Macro- and microstructural changes in cosmonauts' brains after long-duration spaceflight. Sci Adv. 2020;6(36):eaaz9488. doi: 10.1126/sciadv.aaz9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD. Brain structural plasticity with spaceflight. NPJ Microgravity 2, 2, doi:10.1038/s41526-016-0001-9 (2016). [DOI] [PMC free article] [PubMed]
- 21.Roberts DR. et al. Prolonged Microgravity Affects Human Brain Structure and Function. AJNR. Am J Neuroradiol 40, 1878-1885, doi: 10.3174/ajnr.A6249 (2019). [DOI] [PMC free article] [PubMed]
- 22.Lawley J.S., Petersen L.G., Howden E.J., Sarma S., Cornwell W.K., Zhang R., et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115–2127. doi: 10.1113/JP273557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H., Xie L., Yu M., Kang H., Feng T., Deane R., et al. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35(31):11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B., et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]