Abstract
The specificity and effectiveness of eye-movement training to remedy impaired visual exploration and reading with particular consideration of age and co-morbidity was tested in a group of 97 patients with unilateral homonymous hemianopia using a single subject /n-of-1 design. Two groups received either scanning training followed by reading training, or vice versa. The third group acted as a control group and received non-specific detailed advice, followed by training of scanning and reading. Scanning and reading performance was assessed before and after the waiting period, before and after scanning and reading training, and at short-term (11 weeks on average) and long-term follow-up (5 years on average). Improvements after training were practice-dependent and task-specific. Scanning performance improved by ∼40%, reading by ∼45%, and was paralleled by a reduction of subjective complaints. The advice (=control) condition was without effect. All improvements occurred selectively in the training period, not in treatment-free intervals, and persisted in the short- and long-term follow-up over several years. Age had only a minor, although significant effect on improvement in reading after training; co-morbidity had no significant impact on the outcome of training. In conclusion, visual impairments associated with homonymous hemianopia can be successfully and durably reduced by systematic and specific training of compensatory eye-movement strategies. The improvements in compensation strategies were independent of subjects’ age and of co-morbidity.
Keywords: Homonymous hemianopia, Rehabilitation, Ageing, Scanning, Visual search, Reading, Subjective midline, Short- and long-term follow up
Introduction
Homonymous visual field defects are a common consequence of injury to the postchiasmatic visual system and result in a severe disability, affecting patients’ daily activities including mobility, visuospatial orienting, and reading. About 30% of patients with acquired brain injury suffer from visual disorders (e.g., [40]). In their study of 850 cases, Zhang et al. [52] found homonymous visual field loss in ∼70% of posterior stroke survivors, 14% following traumatic injury and ∼12% following tumour; other causes were multiple sclerosis and brain surgery. Strokes mainly occur in older age, with a much higher prevalence of individuals > 65 years of age [48] and this is true for strokes chiefly affecting the occipital lobes [52].
Spontaneous recovery of vision has been reported in only ∼13% of cases, but is rarely complete, while<20% of patients develop effective compensatory behaviour spontaneously [55]. Thus, in the absence of an active intervention, some 70–80% of patients with homonymous visual field loss suffer from a severe and permanent visual disability. The disability is not merely the result of impaired global visual processing due to the restricted visual field but is exacerbated by a pronounced impairment in the guidance of scanning eye-movements, leading to laborious and highly time-consuming visual search strategies, which are partly characterized by piecemeal serial processing [55]. In addition, reading text is characteristically affected by homonymous visual field defects due to insufficient visual field sparing. Fluent reading requires asymmetric parafoveal visual input such that, in left-to-right readers, about 5° of visual angle to the left and 8° to the right of the fovea are crucial [53]. Interestingly, some 10% of patients with homonymous visual field loss do not show such visual impairments. In these cases, injury is restricted to the optic radiation and/or the striate cortex, and spares additional occipital fibre pathways and/or posterior thalamic or occipito-parietal structures [54], [55]. Thus, the extent of the visual field per se is not the sole determinant of efficient global visual processing. The integrity of the field of attention and sparing of visuospatial guidance of attention and eye-movements are likely to be as, or more, important for efficient global processing.
Visual rehabilitation in patients with homonymous visual field loss aims to restore the fullest possible degree of independence in professional and other daily activities, for example by devising means to compensate for the visual handicap. Because different saccadic strategies are involved in scanning and reading, visual scanning training does not lead to improved reading and practice with text does not result in improved visual exploration [45], indicating that specific practice with shifts of fixation in a task-specific manner is required [14]. Only one study systematically examined the influence of age on the effectiveness of treatment in subjects with homonymous visual field loss [43]. With the same amount of treatment older patients (70–85 yrs; mean age: 77 yrs; n = 19) showed the same rates of improvement as the younger group (20–35 yrs, mean age: 28 yrs; n = 19), indicating that age per se is not necessarily associated with a poorer treatment outcome.
Although several studies have suggested that saccadic compensation occurs as a result of systematic practice with visual scanning and reading (e.g., [8], [33], [41], [47]), it has been questioned whether improved performance is unequivocally due to specific practice because of the absence of a comprehensive design including a proper control group, as required by evidence-based medicine (EBM) criteria [34]. However, eye movement training seems more effective than control or placebo conditions [36]. The current comprehensive study is, therefore, designed to assess spontaneous adaptation, the extent to which training is practice-dependent and task specific, stability of training effects over time, and subjective reports on patients’ visual handicap. We used a multiple N-of-1 trials design in which the effects of interventions are assessed in each individual study participant over a series of periods during which different forms of training or practice were employed. In our design participants are randomly assigned to three groups which differed in the sequences in which they receive the types of training or practice. Furthermore, we considered especially the influence of age and co-morbidity on training effects and conducted short- (11 weeks on average) and long-term (5 years on average) follow-up examinations after the end of treatment to assess the persistence of the adopted compensatory strategies. For reasons of clarity, we report methodological details of the long-term follow up study separately.
Subjects and methods
Subjects
The study was carried out in a conventional setting of the day clinic at the Max Planck Institute of Psychiatry, Munich, Germany in accordance with the Declaration of Helsinki. Following detailed examination, consenting patients who were identified with homonymous visual field defects received treatment. The study commenced in January 2000 and was completed in October 2013. Patients gave their written informed consent for the use of data in anonymised form for scientific purposes. The majority of subjects (90%) were referred by neurologists or ophthalmologists; the remainder (10%) contacted the first author (JZ) directly for treatment options. Of the patients referred, 77 did not receive treatment for various reasons (field sparing > 30°); spontaneous adaptation to the visual field loss; severe cognitive impairments, transportation difficulties, or lack of interest in treatment). Of the 409 patients with unilateral visual field loss who received treatment, 264 patients were included in the study (see Fig. 1A). Inclusion criteria were: homonymous hemianopia, with visual field sparing ≤ 4° in left-sided and ≤ 6° in right-sided hemianopia, time elapsed since brain injury of at least 4 weeks, impaired scanning and reading performance (<90% of age-matched healthy subjects) at first and second assessments, and complete data sets. Because of incomplete data sets at short-term follow-up due to difficulties in transport or lack of interest of participants, the definitive group consisted of 97 subjects with either left- (n = 53) or right-sided (n = 44) hemianopia. In all subjects, aetiology of brain injury was verified by CT and/or MRT. Age of subjects varied between 21 and 84 years, with 25 subjects younger than 50 years (∼26%), and 44 subjects (∼45%) older than 65 years. None of the patients had received treatment for their visual field loss before. Patients showed no evidence of associated visual disorders, including reduced visual acuity (minimum Snellen acuity was 0.90 for near and far binocular vision), impaired spatial contrast sensitivity [49], visual adaptation or colour discrimination, visual disturbance due to disease or injury of the peripheral visual system, in particular macular disease, and oculomotor dysfunction (according to ophthalmic examination), aphasia, pure alexia or premorbid reading disorders. Furthermore, none of the patients suffered from visual or verbal memory impairments or visual neglect as assessed by the Behavioral Inattention Test [18] composed of line bisection, letter and star cancellation, figure copying and drawing from memory. All patients were native German speakers or possessed good comprehension and production performance in the German language and had at least five years of education. All patients complained of moderate to severe difficulties in overview/visual scanning and reading. When compared with age-matched normal subjects, all patients were above the cut-off value for speed in the paper-based visual scanning task (M + 1 SD: 14.5 s); normative values from 40 normal subjects; see [45]. In the reading task, 81 patients (83%) performed below the corresponding age-appropriate cut-off values of 80 control subjects [40 females, 40 males; n = 30 for ages 20–50, n = 25 for ages 51–65, and n = 25 for ages 66–80 years] which were 180 wpm (words per minute), 165 wpm, and 150 wpm, respectively, for the listed aged groups. The remaining 16 patients performed above their age-appropriate cut off values, but reported that their reading was much better before the brain injury.
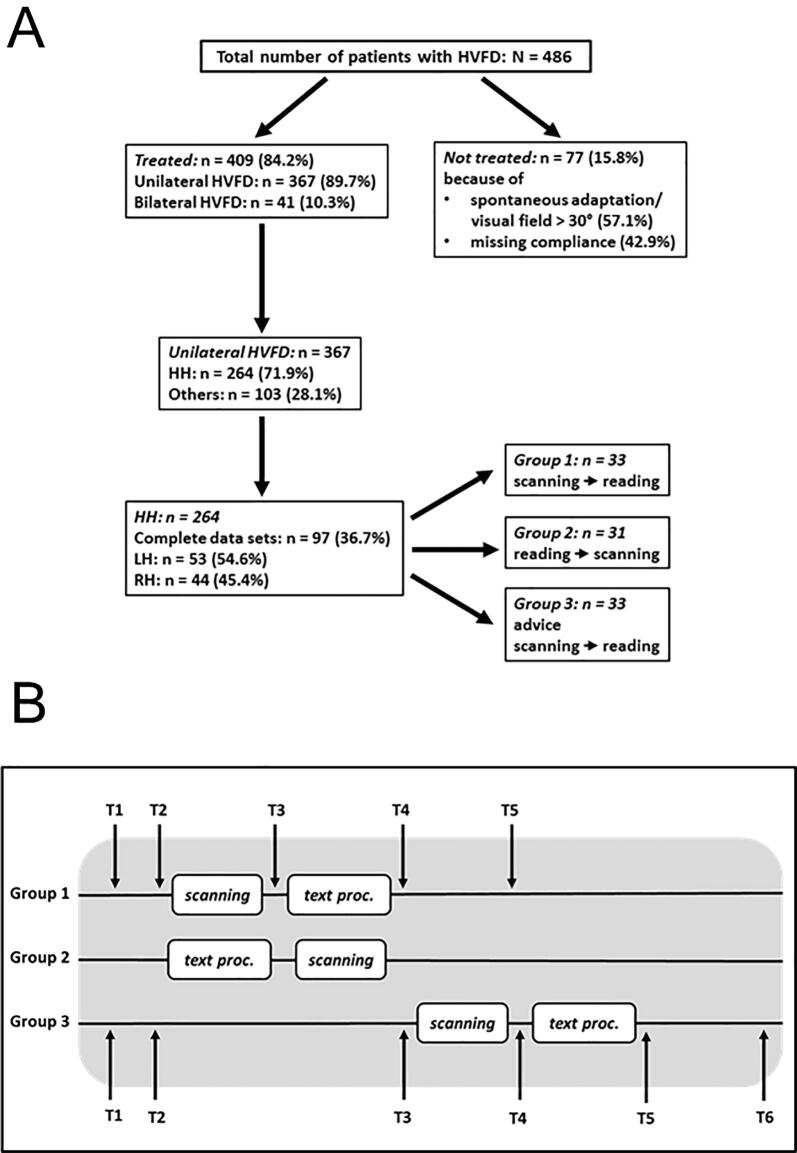
Fig. 1.
A: Diagram of selection of subjects with homonymous visual field defects (HVFD). Missing compliance (n = 33): too poor health (n = 24; 72.7%) or lack of interest in treatment (n = 9; 27.3%). B: Diagram of assessments and treatment periods. Mean time between onset of hemianopia (T1) and first assessment (T2) was 25 weeks, and between T2 and T3 (waiting period) 12 weeks. Follow-up assessment (T5 in groups 1 and 2, T6 in group 3) were performed approximately 11 weeks on average after the end of treatment. For further details, see text.
All patients were aware of their visual field loss at time of treatment, and no patient suffered from post-stroke depression, as assessed with the Geriatric Depression Scale (GDS; German version of short form [15]). Data for 27 subjects assigned to groups 1 and 2 have in part been published in earlier studies [45], [43].
Table 1A, Table 1B summarizes the demographical and clinical data and the statistical tests for group differences. Sixty-eight males and 29 females participated in the study, distributed evenly among groups. The majority of subjects (∼85%) suffered from cerebrovascular disease, mainly stroke; in the remaining subjects, homonymous visual field loss was caused either by traumatic brain injury or by surgical operation for an occipital tumour. Mean age at first assessment was ∼59 years; mean time since brain injury was 25 weeks. The mean interval between first and second assessment (beginning of intervention) was 12 weeks. Approximately equal numbers of subjects exhibited left- or right-sided hemianopia, with comparable mean visual field sparing of ∼2.4°, distributed evenly across groups. There was no significant difference between the groups with regard to sex, age, aetiology of and time since brain injury, side of hemianopia, visual field sparing, waiting interval between first assessment and training, and reading performance before training (see Table 1B). The only significant interaction was between side of hemianopia and performance in the paper-based visual scanning task, which just reached significance at the alpha level of 0.05 (p = 0.036). It is possible that this result may not reflect a true underlying effect given the number of statistical tests employed in assessing our baseline data.
Table 1A.
Demographical and clinical data of subjects. BI: brain injury (CV: cerebrovascular disease; CHT: closed head trauma; TU: tumour, operated); wks: weeks (mean, SD), range; Time s BI: time since brain injury; Interval: time between first assessment and intervention (groups 1 and 2) and systematic advice (group 3); VF-sp: visual field sparing (degrees visual angle; mean, SD, range); visual exploration performance (paper-based visual scanning task, in s, mean, SD, range) and reading performance (in words per minute, wpm; mean, SD, range) at baseline. LH, RH: left- and right-sided hemianopia, respectively.
| Variables | total group (n=97) | group 1 (n=33) | group 2 (n=31) | group 3 (n=33) | |
|---|---|---|---|---|---|
| Sex | female | 28 (28.9%) | 8 (24.2%) | 10 (32.3%) | 10 (30.3%) |
| male | 69 (71.1%) | 25 (75.8%) | 21 (67.7%) | 23 (69.7%) | |
| 58.9 (15.4; 21-84) | 59.8 (13.6; 25-84) | 59.9 (15.4; 24-83) | 57.1 (170; 21-81) | ||
| Aetiology of BI | |||||
| CV | 83 (85.5%) | 30 (90.9%) | 27 (87.1%) | 26 (78.8%) | |
| CHT | 05 (5.2%) | 00 (0.0%) | 03 (9.7%) | 02 (6.15) | |
| TU | 09 (9.3%) | 03 (9.1%) | 01 (3.2%) | 05 (15.1%) | |
| Time s BI (wks) | 25.0 (20; 4-107) | 24.8 (17.3; 4-84) | 23.3 (17.8; 4-85) | 27.4 (24.2; 4-107) | |
| Interval (wks) | 12.0 (5.6; 4-32) | 10.7 (3.7; 4-19) | 11.9 (6.2; 4-32) | 13.3 (6.5; 4-32) | |
| Side of hemianopia | |||||
| LH | 52 (53.6%) | 19 (57.6%) | 16 (51.6%) | 17 (51.5%) | |
| RH | 44 (46.4%) | 14 (42.4%) | 15 (48.4%) | 16 (48.5%) | |
| VF-sp (deg) | 2.3 (1.0; 1-5) | 2.1 (0.9; 1-4) | 2.5 (1.1; 1-5) | 2.4 (1.1; 1-5) | |
| LH | 2.4 (1.0; 1-4) | 2.2 (0.9; 1-4) | 2.5 (1.2; 1-4) | 2.5 (0.8; 1-4) | |
| RH | 2.3 (1.1; 1-5) | 2.1 (0.9; 1-4) | 2.4 (1.1; 1-5) | 2.2 (1.4; 1-5) | |
| Exploration (s) | 38.2 (14.4; 18-110) | 37.0 (13.8; 18-83) | 36.5 (16.9; 19-110) | 40.4 (12.4; 24-71) | |
| LH | 38.4 (12.6; 19-83) | 40.4 (14.5; 24-83) | 37.5 (11.9; 19-55) | 35.6 (10.1; 24-56) | |
| RH | 37.6 (16.4; 18-110) | 32.4 (11.6; 18-63) | 35.5 (21.5; 19-110) | 45.6 (12.8; 25-71) | |
| Reading (wpm) | 97.8 (36.7; 31-169) | 102.5 (36.6; 46-164) | 102.4 (34.6; 46-164) | 88.2 (38.0; 31-166) | |
| LH | 102.5 (32.9; 38-164) | 109.8 (32.3; 47-154) | 99.6 (33.07; 46-164) | 99.5 (33.5; 38-164) | |
| RH | 91.7 (40.4; 28-169) | 92.5 (40.1; 46-169) | 105.4 (37.1; 54-164) | 76.1 (39.7; 31-166) |
Table 1B.
Statistical tests used and results of the statistical analysis. Chi-squared tests were performed in Excel, Anovas in SPSS v27, and the Fisher Exact in R 3.6.1.
| Sex by group (2x3 Chi-squared) χ2 with 2df = 0.55, ns (p=0.759). |
| Age by group (3x1 Anova) F(2,94) = 0.33, ns (p=0.718) |
| Aetiology of Brain injury by group: Because expected frequencies are less than 5 in some cells, a Fisher exact test instead was used rather than a Chi-squared test. Deviations from random distribution are non-significant (p=0.321). |
| Time since brain injury by group (3x1 Anova) F(2,94) = 0.35, ns (p=0.709) |
| Interval by group (3x1 Anova) F(2,94) = 1.81, ns (p=0.169) |
| Side of Hemianopia by group (2x3 Chi-squared) χ2 with 2df = 0.32, ns (p=0.854). |
| Visual field sparing (before training) by side by group (3x2 Anova) |
| Group: F(2,91) = 0.67, ns (p=0.516) |
| Side: F(1,91) = 0.49, ns (p=0.484) |
| Group x Side: F(2,91) = 0.21, ns (p=0.812) |
| Exploration (before training) by side by group (3x2 Anova) |
| Group: F(2,91) = 0.93, ns (p=0.399) |
| Side: F(1,91) = 0.00, ns (p=0.993) |
| Group x Side: F(2,91) = 3.44, p<0.05 (p=0.812) |
| Reading (before training) by side by group (3x2 Anova) |
| Group: F(2,91) = 2.50, ns (p=0.117) |
| Side: F(1,91) = 1.65, ns (p=0.197) |
| Group x Side: F(2,91) = 1.45, ns (p=0.240) |
Based on the influence on cognitive performance and rehabilitation outcome reported in the literature (e.g., [11], [38]) selected co-morbidities were recorded. Patients’ co-morbidities were gleaned from available medical reports. Sixty-three patients (65%) had a diagnosis of co-morbidity, 30 patients of this group (48%) exhibited one co-morbidity, 22 patients (35%) two, and 11 (17%) three co-morbidities. The most frequent co-morbidity was hypertension (n = 46, or 73%), followed by hypercholesterolemia (n = 19, or 30%) and diabetes type 2 (n = 15, or 24%). Other co-morbidities included chronic obstructive pulmonary disease (COPD; n = 4) and hyperthyroidism (n = 2).
Methods
Monocular and binocular visual fields were measured using kinetic perimetry with a standard Tübingen perimeter [3]. Target diameter was 69 min of arc, its luminance was 102 cd/m2; background luminance was 3.2 cd/m2. The target was moved with a speed of ∼2°/s from the periphery towards the centre of the perimeter. Subjects were instructed to fixate a small red spot of light (diameter: 0.5°) in the centre of the sphere and to press a response button as soon as they detected the target. Fixation accuracy was monitored through a telescope. The visual field border was determined along 16 meridians. Perimetric resolution/measurement error was 0.5° within 15°, and 1° beyond 15° eccentricity.
We used the same standardised tasks as in our previous studies [45]. For scanning, patients were asked to perform a cancellation task by marking, with a pencil, all targets in an array as accurately and as quickly as possible; patients were not informed about the number of targets. The targets were 20 black diamonds among 22 non-targets consisting of black dots and crosses presented on a sheet of white paper (45° horizontally × 35° vertically), at a viewing distance of 30 cm. Measures of visual scanning performance were the time required and errors committed in the completion of the task. Normative data were available from 40 control subjects [45].
For the assessment of reading, patients were asked to read text aloud as accurately and as quickly as possible. Each of six versions of text consisted of 200 words (font: Arial, 14pt) arranged in 20 double-spaced, left-aligned lines printed on a white sheet of paper. The texts consisted of short sentences with simple syntactic structure and were standardized for content (taken from Gotthold E. Lessing’s animal fables, in German). The number of uncorrected errors and correctly read words per minute (wpm) were recorded. Normative data were available from a sample of 80 control subjects (cut-off values see above).
The cancellation and reading tests were administered under normal daylight conditions. Eye- and head-movements were unrestricted. We obtained informal but standardised subjective reports on the degree of visual impairment in everyday life affecting overview/visual exploration and reading, respectively, assigned to three categories: mild, moderate or severe. In addition, we asked in more detail about the type of visual impairment, i.e. slowness of vision, collision with obstacles or people, and getting lost. Furthermore, we asked patients for their visual difficulties in familiar (e.g. at home) and unfamiliar or complex surroundings (e.g., supermarkets, crowded places, new environments). This collection of individual visual difficulties has proved informative in previous studies [55] and p. 96]. All participants were asked the same questions in standardized form, and they were asked to assign the severity in each (for details, see Appendix).
Experimental design
With respect to time since onset of hemianopia, side of visual field loss and age, and baseline performance in the paper-based visual scanning and in the reading tasks, subjects were randomly allocated into three groups in our multiple N-of-1 design. As illustrated in Fig. 1B, group 1 (n = 33) first received training with visual scanning followed by training with reading; group 2 (n = 31) did the converse. The impact of scanning training on (subsequent) reading performance in group 1 was used as an indicator of the specificity of scanning training, and vice versa, the impact of reading training on (subsequent) scanning performance for the specificity of reading training in group 2. Group 3 (n = 33) did not receive systematic training within the treatment periods of groups 1 and 2. Instead subjects received detailed advice to use large gaze shifts to gain an overview of the visual scene to avoid obstacles in everyday life and, during reading, to ensure they produced gaze shifts to the beginning of each word/line (in the case of left-sided hemianopia) or to the end of a line/word (in the case of right-sided hemianopia). This was followed by training, as with Group 1.
A single subject baseline design was used, with a treatment-free-interval before and after training in groups 1 and 2 and before and after the advice period in group 3. Thus, every subject served as their own control. Scanning and reading performance were assessed at five time-points in groups 1 and 2. These were: T1, initial assessment; T2, before training; T3, after training with scanning (group 1) or reading (group 2); T4, after training with reading (group 1) or scanning (group 2); T5, at follow-up. In group 3, 6 time-points were used: T1, initial assessment, T2, before advice period, T3, after advice period, T4, before training with scanning, T5, after training with scanning, T5, after training with reading, T6, at follow up (see Fig. 1B and Table 1A, Table 1B). Assessments and treatments were carried out by different persons according to a predetermined protocol. Persons who carried out the assessments after treatment did not know the individual course of treatment.
Methods of treatment
For treatment, software-based reading and visual search training programs were used as developed by Zihl [55]. Training material was presented using a LCD monitor. The treatment was administered and supervised by the therapist, who also gave verbal feedback on reading or visual exploration performance (mainly omissions) during training (supervised learning). In addition, the therapist ensured that patients did not use head instead of eye movements or ‘guessing’, particularly in the training period with reading.
An individual training session lasted ∼45 min and consisted of 10 blocks of 30 trials, interspersed with short breaks. Training sessions were carried out at least once daily (in 29 subjects, 29.9%); the rest (68, or 70.1%) participated in 2 or 3 sessions per day, with at least a 45 min break between sessions. At each level of task difficulty, training was completed when subjects reached a pre-defined criterion (at least 90% correct responses).
Systematic training with visual scanning
For training in visual scanning, we used standardized versions of a visual search paradigm [45], [54], [55]. Subjects were instructed to always use large saccadic eye-movements towards the affected side and were systematically trained to gain an immediate complete overview and to use an efficient strategy to scan the scene. Training material consisted of computer-controlled visual search displays extending 50° horizontally and 42° vertically at a viewing distance of 120 cm. We used different target and distractor letters (size: 2.5°) of varying similarity on a black background. Subjects were instructed to fixate a cross in the centre of the display and to search, after its offset, for a single target letter among distractor letters. Targets were present on 60% of trials. Subjects were asked to respond as accurately and quickly as possible by pressing the left or right mouse buttons for the presence or absence of a target, respectively. Presentation and thus visual search time was unlimited. Subjects were given immediate feedback when they omitted targets. Training with visual scanning started with the easiest, i.e. parallel search condition (e.g. ‘T’ among ‘O’s) and progressed to a mixed condition (e.g. ‘S’ among ‘C’s) and eventually to a serial search condition (e.g. ‘O’ among ‘G’s). In addition to varying stimulus similarity, visual search difficulty was also systematically increased by increasing the number of stimuli (set size) from 15 to 20 letters. Training was terminated when the subject reached a defined performance level (at least 90% correct responses) for any level of difficulty used.
Systematic training with reading
For reading training a single-word reading task was used, as in earlier studies [45], [53], [54]. Single words of different lengths, ranging from three to 12 letters, were used. Letter size was 2.5°; spacing between letters was 0.4°. Text material was shown in yellow on a dark blue background to allow comfortable reading. Each training trial consisted of the time-limited presentation of a single word in the centre of the screen. Subjects were instructed to shift their gaze, as quickly as possible, from the centre of the screen to the beginning or end of each word (in cases with left- or right-sided hemianopia, respectively) before reading it aloud. Errors were corrected with immediate feedback. During the course of training, the length of the presented words was systematically increased. When a subject performed at > 90% correct, at a given word length, presentation time was reduced stepwise from 500 to 300 ms (for subjects < 60 years) or from 1000 to 500 ms (for subjects > 60 years). The final training stage involved the randomized presentation of words of different lengths. By adopting progressive time-limited word presentation, subjects were forced to make more rapid saccades to read the whole word before its disappearance.
Statistical analysis
Because the number of errors (omissions and commissions) in pre- and post-training cancellation tests was so few (<2), these data were excluded from the analysis; thus only cancellation time was considered for further statistical analysis. Analysis of reading performance was carried out on the number of correctly read words per minute (wpm). Cancellation times (s), reading performance (wpm) and the influence of age, extent of visual field sparing, side of hemianopia, time since brain injury and number of training sessions were analysed using analyses of variance with Bonferroni corrected post-hoc tests. Analyses of scanning and reading performance were performed both on raw scores and those expressed, for each subject, as a percentage of baseline performance at T1. Within group-comparisons for groups 1 and 2 were performed at T1 and T2 (“waiting period”), at T2, T3 and T4 (training periods), and at T5 (short-term follow-up). For group 3, within group comparisons were performed between T1 and T2 (“waiting” period), at T2 and T3 (“advice”), at T3, T4 and T5 (training periods), and at T6 (short-term follow-up). Between group comparisons were performed for all groups before and after the waiting period (T1, T2), after training with scanning and reading, respectively (groups 1 and 2: T3 and T4; Group 3: T4 and T5) and at short-term follow-up (groups 1 and 2: T5, group 3: T6). Patients’ subjective reports on the degree of visual impairment were analysed with chi-squared tests.
Results
Visual scanning and reading and short-term follow-up
The number of training sessions undertaken did not differ among groups. Group 1 participated on average in 11.2 scanning (range: 6–18) and 11.2 (range: 5–20) reading training sessions; Group 2 performed on average 10.7 (range: 4–20) scanning and 11.5 (range: 6–20) reading training sessions. In group 3, mean numbers of scanning and reading training sessions were 12.1 (range: 6–20) and 12.3 (range: 5–22), respectively.
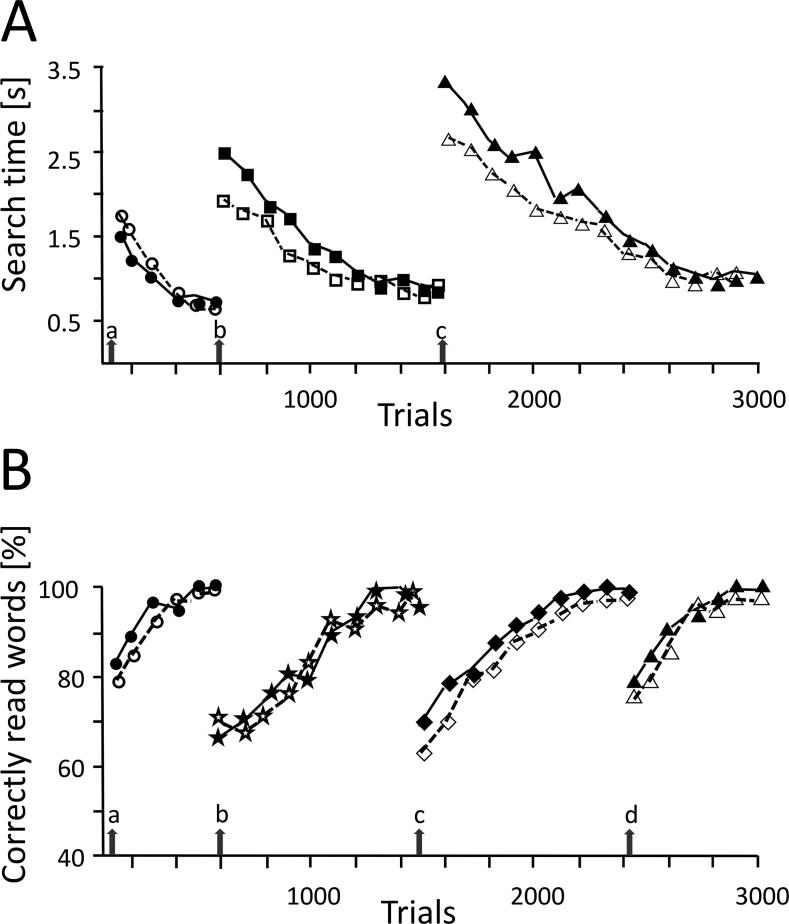
Fig. 2 shows typical examples for the outcome of systematic training with scanning and reading in a 32-year-old female patient (P1) with left- and a 29-year-old male patient (P2) with right-sided hemianopia. P1 had suffered an occipital stroke 9 weeks before the first assessment; training was started 9 weeks after the first assessment, i.e. 18 weeks after the onset of hemianopia. Visual field sparing in her left hemifield was 1°. Time to perform the cancellation task was 54 s at the first assessment, and 52 s at the second assessment, i.e. before training. Scanning training preceded reading training. After systematic training with visual search (12 sessions), she needed 32 s for the cancellation task (at follow up: 33 s). Reading performance was 77wpm in the first assessment, 81wpm before and 82wpm after scanning training. After reading training (13 sessions), her reading performance was 161wpm (follow up: 160wpm). Patient P2 was operated for an occipital tumour (meningioma) 14 weeks before the first assessment. Visual field sparing in his right hemifield was also 1°. Treatment was started 8 weeks after the first assessment, i.e., 22 weeks after the onset of hemianopia. Reading training was followed by scanning training. Reading performance was 75wpm at the first, and 82wpm at the second assessment. After reading training (15 sessions), his reading performance was 118wpm (at follow up: 135wpm). Time in the cancellation task was 52 s at the first, 51 s at the second assessment and 50 s after reading training. After training with visual search (14 sessions) cancellation time was 35 s (at follow up: 36 s). Both patients showed a remarkable increase in performance, in both scanning (P1: 33%, P2: 31%) and reading, (P1: 108%, P2: 80%), irrespective of the order of training.
Fig. 2.
Course of practice with visual search (A) and text processing (B) in a patient with left- (filled symbols; P1) and with right-sided hemianopia (open symbols; P2). P1 started with visual search practice followed by practice with text processing, while P2 started with practice with text processing followed by visual search practice. Arrows in A indicate the three levels of task difficulty (a: parallel, b: mixed, c: serial search modes). Set size was 30 in all conditions. Symbols indicate mean response in 30 trials at different stages of practice (number of trials). In B, arrows indicate the four levels of difficulty (a: 4–5 letter words; b: 8–9 letter words; c: 12–13 letter words; d: 15–16 letter words). Symbols indicate percentages of correct responses in 20 trials at different stages of practice (number of trials). Task difficulty was increased after the patient has reached either a stable search time (∼1 s) in three consecutive training blocks in A, or at least 90% correct responses in three consecutive training blocks in B. Note the increase in performance in both practice conditions in the course of practice in P1 and P2. For further details, see text.
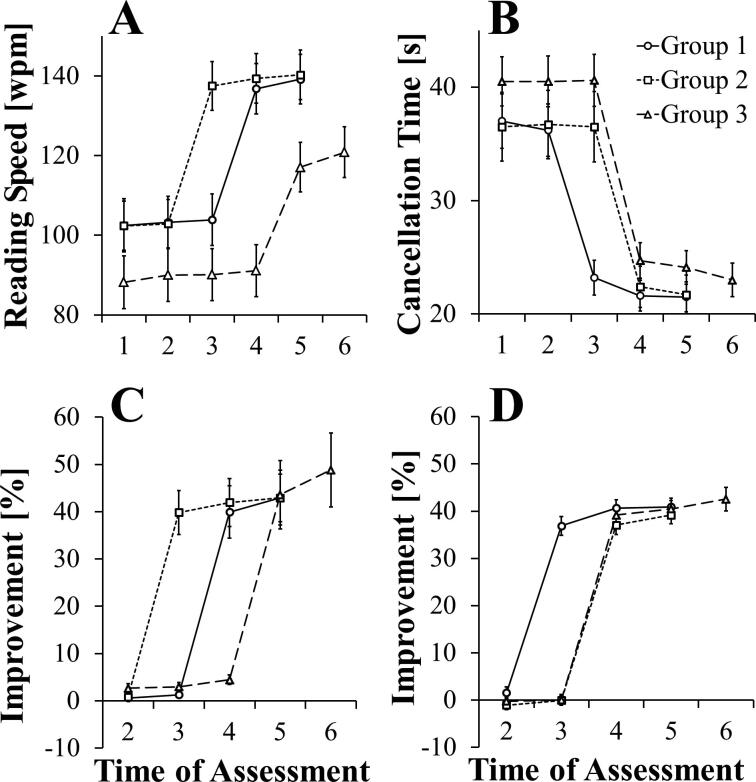
To compare the efficacy of the three different treatment conditions, analyses of variance were carried out using Groups (1–3) and Assessment Time (T1-T4 for groups 1 and 2; T1-T5 for group 3) as Factors. For Groups 1 and 2, cancellation times fell, on average, from ∼36 s to ∼22 s, and reading improved from ∼102 to ∼140 wpm, as a result of training. Inspection of Fig. 3a and 3b suggests that Group 3 was consistently ∼10–15% worse on measures of both scanning and reading across all assessment times compared with groups that had received equivalent histories of training on that activity, i.e. Groups 1 vs 3 for reading and group 2 vs 3 for scanning. However, although these differences were not significant at assessment times which preceded intervention, namely at T1 and T2 [Group F(2,94) = 1.54, ns], analyses of variance were carried out which take into account baseline performance, i.e. using measures expressed as percentage change in performance at T2 to T5 compared with performance at T1. These measures are presented in Fig. 3c and 3d, which show, for each group, percentage change in cancellation times and words per minute (wpm) from T2 to T6, respectively.
Fig. 3.
Performance in reading and exploration tasks for the three groups across assessment times, showing reading speed in word per minute for the reading task (A) and cancellation time for the exploration task (B). The improvements shown by each group relative to their performance at the first assessment time are shown for reading (C) and exploration (D). Error bars are standard errors of the means.
Separate analyses for scanning and reading measures were conducted, comparing groups across the assessment times T2 to T5. Highly significant main effects of Group and Assessment Time, and their interaction, for both measures indicate that each group showed a benefit from the training provided which was specific to the measure (Scanning: Group F(2,94) = 28.44, p < 0.001; Assessment Time F(3,282) = 798.05, p < 0.001; Interaction F(6,282) = 69.36, p < 0.001. Reading: Group F(2,94) = 8.75, p < 0.001; Assessment Time F(3,282) = 105.37, p < 0.001; Interaction F(6,282) = 21.47, p < 0.001.) At the assessment immediately following training, reading performance and cancellation times showed average improvements of 41.8% and 38.7%, respectively, which were comparable among groups (Scanning: Group 1: 40.7%, Group 2: 37.1%, Group 3:39.2%; Reading: Group 1: 39.9%, Group 2: 42.0%, Group 3: 43.6%). This was confirmed with tests of simple main effects, where the only differences among groups for each measure are between those that have received, and those that have yet to receive, training, i.e. the effects of training are apparent for Group 1 at T3, for scanning, and Group 2 and Group 3, for reading, at T3 and T4, respectively (Scanning: at T3, F(2,94) = 224.09, p < 0.001. Reading: at T3, F(2,94) = 66.50, p < 0.001; at T4, F(2,94) = 23.70, p < 0.001). Group differences at other assessment times did not approach significance (Scanning: highest F(2,94) = 1.603, p = 0.21 at phase 2. Reading: highest F(2,94) = 1.878, p = 0.16 at phase 2).
Post-hoc pairwise comparisons, with Bonferroni correction, of groups at T3 indicate that systematic training of scanning and reading substantially improves performance. Specific training in scanning clearly improves performance in the cancellation task in Group 1 compared with Group 2 who received reading [t(62) = 17.605, p < 0.001]. Similarly, reading training improved reading in Group 2, again compared with Group 1 who received scanning training [t(62) = 3.768, p < 0.001]. In short, scanning training accrued no benefit to reading and vice versa. Performance of Group 3 at T2 is indistinguishable from the group that receives no training both for scanning [t(62) = 0.07, n.s.] and for reading [t(64) = 0.13, n.s.]. Advice alone on strategies to cope with visual difficulties has no significant impact on reading and scanning performance at T3 which remained indistinguishable to performance achieved at T2 (Reading: Group 3: t(32) = 0.35, n.s. Scanning: Group 3: t(32) = 0.225, n.s.). Moreover, there was no change in performance between T2 and T3 when groups were again assessed on activities for which they had yet to receiving training (i.e. reading in Group 1 and scanning in Group 2) confirming that reading training had no effect on measures of visual scanning and vice versa (Reading: Group 1: t(32) = 1.16, n.s. Scanning: Group 2: t(30) = 0.946, n.s.). This is again confirmed by a similar analysis comparing performance between T3 and T4, again on the converse activity (i.e. scanning in Group 1 and reading in Group 2) where performance remained unchanged between assessments (Group 1 Scanning: t(32) = 1.94, n.s. Group 2 Reading: t(30) = 1.89, n.s.). The same picture emerges for Group 3, where scanning training had no effect on reading, i.e. reading performance did not differ for Group 3 between T3 and T4 [t(32) = 4.21, p < 0.001] and vice versa, i.e. scanning performance did not differ for Group 3 between T4 and T5 [t(32) = 1.93, n.s].
Individual patients received different numbers of training sessions in the course of treatment. Table 2 shows the number of sessions, the overall improvement rates and the rate of improvement per session for different levels of baseline performance in scanning and reading. For scanning, those with the more substantial impairment required more sessions and showed the greatest absolute improvement [F(5,90) = 4.95, p < 0.001]. This difference is not apparent for reading [F(5,90) = 0.48, p = 0.79]. Side of hemianopia had no effect on rate of improvement (Scanning: F(1,95) = 1.14, p = 0.29; Reading: F(1,95) = 0.00, p = 0.97).
Table 2.
Performances in visual exploration (search time, in s) and reading (words per minute, wpm) at baseline and after treatment, number of sessions, and improvement per session. n: number of subjects. Numbers indicate means (standard errors in brackets), and mean percentages (standard errors in brackets).
| Baseline | Sessions | Improvement | Improvement/session |
|---|---|---|---|
| Exploration | |||
| > 50 s (n = 18) | 14.0 (3.5) | 23.8 (9.7) [39.6% (11.2)] | 1.8 (0.8) [3.0% (1.4)] |
| 50–40 s (n = 17) | 12.3 (3.3) | 19.5 (4.7) [43.6% (8.6)] | 1.6 (0.4) [3.7% (1.0)] |
| 39–33 s (n = 17) | 12.8 (2.6) | 13.9 (3.3) [38.6% (9.0)] | 1.1 (0.3) [3.1% (0.9)] |
| 32–29 s (n = 20) | 10.6 (3.2) | 11.3 (3.0) [36.9% (9.6)] | 1.1 (0.4) [3.7% (1.2)] |
| 28–25 s (n = 13) | 08.8 (2.4) | 08.5 (3.1) [31.7% (11.5)] | 1.0 (0.4) [3.7% (1.4)] |
| 24–19 s (n = 12) | 08.3 (2.0) | 08.4 (2.0) [38.0% (6.9)] | 1.1 (0.4) [4.6% (1.6)] |
| Reading | |||
| 34–60 wpm (n = 18) | 12.8 (3.2) | 30.2 (15.4) [63.3% (40.7)] | 2.3 (0.9) [4.8% (2.1)] |
| 61–80 wpm (n = 17) | 13.7 (3.7) | 34.4 (22.3) [48.4% (31.4)] | 2.6 (1.7) [3.6% (2.3)] |
| 81–99 wpm (n = 15) | 14.1 (3.9) | 44.6 (19.3) [50.7% (23.0)] | 3.3 (1.7) [3.7% (1.9)] |
| 100–120 wpm (n = 18) | 11.2 (3.5) | 35.0 (13.4) [32.3% (12.7)] | 3.5 (2.2) [3.1% (2.2)] |
| 121–145 wpm (n = 15) | 09.2 (2.2) | 23.7 (11.4) [17.7% (8.6)] | 2.8 (1.4) [1.9% (0.7)] |
| 146–170 wpm (n = 14) | 08.4 (2.4) | 19.6 (8.1) [13.4% (6.2)] | 2.5 (1.3) [1.7% (0.8)] |
Short-term follow-up
Improvements as a result of intervention are maintained or, for reading, show mild improvement at follow-up (Scanning: Group 1 t(32) = 0.26, n.s.; Group 2 t(30) = 1.93, n.s.; Group 3 t(32) = 1.19, n.s.; Reading: Group1 t(32) = 3.25, p < 0.01; Group 2 t(30) = 1.19, n.s; Group 3 t(32) = 6.13, p < 0.001).
In summary, as a result of scanning training using a visual search task, all three groups showed a substantial improvement in scanning times in the cancellation task, with an average 39–41% reduction in latencies. Reading training resulted in an average 42–49% increase in wpm in the reading task. The order of the tasks was immaterial. When patients were offered training, in the absence of further structured advice about behavioural strategies to adopt, there was no evidence that scanning training influenced reading performance or vice versa, i.e. there was no transfer of training effects between the two tasks.
The role of age and co-morbidity
Analyses of age, the extent of visual field sparing, side of hemianopia, time since brain injury and number of training sessions indicate that, with the exception of age, these factors do not influence the outcomes described above. For reading there was an overall main effect of age on performance [F(1,54) = 6.71, p = 0.012] and this interacted with Assessment Time [F(3,162) = 3.59, p = 0.015] but not Group by Assessment Time. These statistics reflect a higher overall improvement in younger patients, but no differential effect on the three treatment groups. Table 3 illustrates the increase in visual exploration and reading performance for age subgroups (a) < 40 yrs, (b) 40–59 yrs, (c) 60–69 yrs and (d) > 70 years. As can be seen, the reduction in visual exploration time after training is in the same range in all subgroups (between ∼27% and 35%), while increase in reading performance after training was highest in the youngest group (∼55%), and lower in the other groups (b: ∼31%, c: ∼33%, and d: ∼30%). Interestingly, there are no substantial differences in the number of sessions required for the improvements, either for visual exploration or for reading.
Table 3.
A: Visual exploration (EX; time in s; M and SD) and reading performance (READ; words per minute, wpm; M and SD) before and after treatment for different age groups. n = number of subjects; yrs: years; diff: difference (in s or wpm, respectively). B: Mean number of sessions for visual exploration training (EX) and reading training (READ), respectively. SD and ranges in brackets.
| A | ||||||
|---|---|---|---|---|---|---|
| Age groups (n) | EX before | after | diff. | READ before | after | % |
| <40 yrs (n = 12) | 31.3 (13.4) | 20.7 (8.5) | −10.6 | 85.5 (28.7) | 132.4 (31.6) | +46.9 |
| 40–59 yrs (n = 30) | 30.1 (12.3) | 19.6 (4.6) | −10.7 | 104.4 (35.5) | 137.1 (31.6) | +32.7 |
| 60–69 yrs (n = 25) | 33.0 (11.1) | 24.0 (7.5) | −9.0 | 107.5 (36.0) | 143.2 (36.4) | +35.7 |
| 70–84 yrs (n = 30) | 37.5 (20.4) | 26.2 (12.1) | −11.3 | 90.8 (39.7) | 118.0 (39.8) | +27.2 |
| B | ||
|---|---|---|
| Age groups (n) | EX | READ |
| <40 yrs (n = 12) | 11.7 (4.7; 6–20) | 12.9 (5.4; 5–20) |
| 40–59 yrs (n = 30) | 10.8 (2.7; 7–17) | 11.7 (3.8; 6–22) |
| 60–69 yrs (n = 25) | 11.1 (3.4; 4–16) | 12.1 (3.6; 6–20) |
| 70–84 yrs (n = 30) | 11.9 (3.9; 5–20) | 10.8 (3.1; 6–18) |
The co-morbidities of our patients does not seem to have any particular impact on the performance at baseline and before and after treatment (see Table 4). The increase in scanning performance was similar in the group without and with co-morbidity (∼62%); the corresponding increase for reading performance is ∼38% and ∼30%. The number of training sessions is identical for both scanning and reading in both groups. Since quantitative values of the medical variables (blood pressure, cholesterol, blood sugar levels, thyroid hormone levels, etc.) were not available, we did not perform a statistical analysis of the influence of the individual co-morbidities. However, although patients with co-morbidity were, on average, 10 years older than those without, co-morbidity does not appear to have played an influential role, either in baseline performance, improvement rates or number of sessions required (maximum t for number of reading sessions t(95) = 0.649, p = 0.518).
Table 4.
Co-morbidity and demographic and treatment variables. Numbers indicate means, standard errors, and ranges (in brackets). A: Subjects without comorbidity (n = 34; 35.1%); B: Subjects with comorbidity (n = 63; 64.9%). EX1, EX2; visual scanning performance before and after training; Read1, Read2: reading performance before and after training.
| A (n = 34) | B (n = 63) | |
|---|---|---|
| Age (yrs) | 52.6 (17; 21–79) | 61.7 (13.6; 25–84) |
| Sex (f/m) | 11/23 (32.3%/67.7%) | 17/46 (27.0%/73.0%) |
| EX1 (s) | 34.1 (13.5) | 39.0 (15.8) |
| EX2 (s) | 21.3 (6.7) | 24.2 (10.4) |
| Improvement (%) | 62.4 | 62.1 |
| Sessions | 11 (3.2; 4–16) | 11 (3.7; 5–20) |
| READ1 (wpm) | 90.9 (31.4; 31–146) | 101.1 (38.7; 41–169) |
| READ2 (wpm) | 125.5 (31.4; 74–172) | 131.4 (37.9; 54–186) |
| Improvement (%) | 38.1 | 30.0 |
| Sessions | 12 (3.6; 5–20) | 12 (3.8; 6–22) |
Subjective reports before and after treatment
Patients’ difficulties were rated as mild, moderate or severe following each phase of testing; subjective reports of degree of visual impairment are summarised in Table 5, Table 6.
Table 5.
Patients’ subjective reports (response frequencies and respective percentages) on overall visual difficulties with overview/visual exploration (A), particular visual difficulties (B), and influence of familiar vs. unfamiliar surroundings (C) at the first assessment (T1), after the waiting period, i.e. before treatment (T2), after treatment (T3) and at follow- up (T4). LH: patients with left-, RH: patients with right-sided hemianopia. Response categories: mild difficulties, moderate difficulties, severe difficulties. T: assessment times 1–4, n: number of subjects.
| T/response categories | total group (n = 97) | LH (n = 53) | RH (n = 44) | |
|---|---|---|---|---|
| A) Overall visual difficulties | ||||
| T1 | Mild | 00 | 00 | 00 |
| Moderate | 24 (24.7%) | 13 (24.5%) | 11 (25.0%) | |
| Severe | 73 (75.3%) | 40 (75.5%) | 33 (75.0%) | |
| T2 | Mild | 00 | 00 | 00 |
| Moderate | 27 (27.8%) | 15 (28.3%) | 12 (27.3%) | |
| Severe | 70 (72.2%) | 38 (71.8%) | 32 (77.7%) | |
| T3 | Mild | 40 (41.2%) | 24 (45.3%) | 16 (36.4%) |
| Moderate | 55 (56.7%) | 29 (54.7%) | 26 (59.1%) | |
| Severe | 02 (02.1%) | 00 | 02 (04.5%) | |
| T4 | Mild | 51 (52.6%) | 30 (56.6%) | 21 (47.7%) |
| Moderate | 46 (47.4%) | 23 (43.3%) | 23 (52.3%) | |
| Severe | 00 | 00 | 00 | |
| B) Type of visual difficulties | ||||
| T1 | Vision too slow | 97 (100%) | 53 (100%) | 44 (100%) |
| Collisions | 38 (39.2%) | 24 (45.3%) | 14 (31.8%) | |
| Getting lost | 32 (33.0%) | 23 (43.4%) | 09 (20.5%) | |
| T2 | Vision too slow | 97 (100%) | 53 (100%) | 44 (100%) |
| Collisions | 34 (35.1%) | 21 (39.6%) | 13 (29.5%) | |
| Getting lost | 29 (29.9%) | 19 (35.8%) | 10 (22.7%) | |
| T3 | Vision too slow | 38 (39.2%) | 21 (39.6%) | 17 (38.6%) |
| Collisions | 13 (13.4%) | 08 (15.1%) | 05 (11.4%) | |
| Getting lost | 03 (03.1%) | 03 (05.7%) | 00 | |
| T4 | Vision too slow | 23 (23.7%) | 12 (22.6%) | 11 (25.0%) |
| Collisions | 04 (04.1%) | 02 (03.8%) | 02 (04.5%) | |
| Getting lost | 00 | 00 | 00 | |
| C) Surroundings | ||||
| T1 | Familiar | 38 (39.2%) | 24 (45.3%) | 14 (31.8%) |
| Unfamiliar | 97 (100%) | 53 (100%) | 44 (100%) | |
| T2 | Familiar | 28 (28.9%) | 17 (32.1%) | 11 (25.0%) |
| Unfamiliar | 92 (94.8%) | 51 (96.2%) | 41 (93.2%) | |
| T3 | Familiar | 09 (09.3%) | 06 (11.3%) | 03 (06.8%) |
| Unfamiliar | 17 (17.5%) | 11 (20.8%) | 06 (13.6%) | |
| T4 | Familiar | 00 | 00 | 00 |
| Unfamiliar | 08 (08.2%) | 07 (13.2%) | 01 (2.3%) | |
Table 6.
Patients’ subjective reports (response frequencies and respective percentages) on visual difficulties with reading at the first assessment (T1), after the waiting period, i.e. before treatment (T2), after treatment (T3) and at follow- up (T4). LH: patients with left-, RH: patients with right-sided hemianopia. Response categories: mild difficulties, moderate difficulties, severe difficulties. T: assessment times 1–4, n: number of subjects.
| T/response categories | Total group (n = 97) | LH (n = 53) | RH (n = 44) | |
|---|---|---|---|---|
| T1 | Mild | 00 | 00 | 00 |
| Moderate | 31 (32.0%) | 21 (39.6%) | 13 (29.5%) | |
| Severe | 66 (68.0%) | 32 (60.4%) | 31 (70.5%) | |
| T2 | Mild | 00 | 00 | 00 |
| Moderate | 32 (33.0%) | 20 (37.7%) | 14 (31.8%) | |
| Severe | 65 (67.0%) | 33 (62.3%) | 30 (68.2%) | |
| T3 | Mild | 34 (35.0%) | 24 (45.7%) | 15 (34.1%) |
| Moderate | 61 (62.9%) | 29 (54.7%) | 27 (61.4%) | |
| Severe | 02 (02.1%) | 00 | 02 (04.5%) | |
| T4 | Mild | 47 (48.5%) | 27 (50.9%) | 17 (38.6%) |
| Moderate | 50 (51.5%) | 26 (49.1%) | 27 (61.4%) | |
| Severe | 00 | 00 | 00 | |
All patients benefitted from training and, for both scanning and reading, the results were comparable. All patients reported moderate or severe visual impairments prior to treatment, irrespective of the side of the hemianopia (largest χ2(1) = 1.07, p = 0.30), with ∼55% reporting severe impairment in both scanning and reading and none a mild impairment. After training 72% of these described both impairments as moderate and 21% of the remainder described one or other impairment as mild. Of the 33% reporting severe difficulties with either scanning or reading, or those moderately impaired at both, 43% reported mild difficulties following training. At follow-up a further ∼10% of patients reported mild, rather than moderate, difficulties. In summary, significant benefits of training were evident such that, at follow-up, impairments in scanning originally described as severe by 43% of the cohort, were now deemed mild. For reading, the equivalent figure is 29%. At follow up, no patients described either impairment as severe.
While the training benefits were substantial, the effects of advice alone in group 3 failed to influence patients’ self-ratings of impaired scanning or reading. For scanning, prior to such advice, percentages for moderate and severe visual difficulties were 21.1% and 78.8%, respectively; after the period of advice, the corresponding percentages were 18.2% and 81.8%. For reading, percentages for moderate and severe difficulties were 27.3% and 72.7%, respectively; after the advice-period, the corresponding percentages were 30.3% and 69.7%. No meaningful changes in responses therefore occurred after the advice period.
The chief complaint prior to treatment was ‘slowed vision’ (100%), followed by reports on collisions (∼40%) and ‘getting lost’ (33%). Patients with left-sided hemianopia were particularly prone to reporting ‘getting lost’ than those with right hemianopia (43% vs. 20%; χ2(1) = 5.72, p = 0.02) but treatment was equally effective in both groups. After treatment, the rates for ‘slowed vision’ dropped down by about 60%, and for ‘collisions’ and ‘getting lost’ by about 20%. A further decrease of about 13–15% in the frequency of complaints was present at follow-up (T4); reports of collisions and getting lost were either negligible or absent.
Prior to treatment, all patients reported difficulties in unfamiliar surroundings and 40% reported similar problems in familiar surroundings. After training, only about 17% continued to report difficulties in unfamiliar surroundings, and 10% in familiar surroundings. Again, there was a tendency for patients with left hemianopia to report more difficulties than those with right hemianopia. At follow-up, no patient reported difficulties in familiar surroundings, and about 10% persisted in reporting difficulties in unfamiliar surroundings, again with left-sided hemianopic patients reporting more frequently difficulties (∼13%) compared with right-sided patients (∼2%).
In summary, patients’ subjective reports on their difficulties with overview/visual exploration and reading in everyday life paralleled the outcome of treatment. There were no substantial changes in reports of severity of impairment between assessments prior to training, but a marked shift to reported improvements after training. At follow-up, percentages of severe and moderate responses further decreased. Subjective complaints before treatment mainly concerned slowing of vision, followed by collisions and difficulties with visual-spatial orientation. Furthermore, substantially more difficulties were reported for unfamiliar compared with familiar surroundings, particularly by subjects with left-sided hemianopia. After treatment, self-ratings were considerably more favourable for all response categories and for unfamiliar and familiar surroundings. Advice for coping with everyday difficulties without training did not improve self-ratings for overview/visual exploration or reading. Aging effects showed only up for the speed of reading. There was no significant effect at all for the co-morbidity factor; this is all the more surprising since around 65% of the patients had co-morbidity.
Long-term follow-up
Short-term follow-up data have not revealed significant changes in scanning and reading performance at about 3 months after the end of treatment. However, no reports exist on the long-term outcome of compensation training in hemianopic subjects. For this study, we contacted all 97 subjects of the original study either by phone or by mail and invited them to participate in this study. We could not reach 22 subjects; nine subjects had died, 14 subjects could not participate due to illness or physical weakness, and six because of progressive macular disease and reduced visual acuity (<0.80 Snellen binocular near and far acuity acuity). Finally, we could recruit 46 subjects for the follow-up examination, 14 females and 32 males, with mean age of 67.2 years (SD: ±12.1, range: 42–87 years). More than half of subjects (26, or 56%) were older than 65 years. Data for the long-term follow-up study were collected between February 2014 and April 2015. Patients gave their written informed consent for the use of data in anonymised form for scientific purposes.
Demographic and clinical data are shown in Table 7. In the majority of subjects (n = 38; 82.6%), the homonymous visual field loss resulted from a stroke in the territory of the posterior artery; in the rest from occipital hemorrhage (n = 5), surgical removal of an occipital tumor (n = 2) or closed head trauma (n = 1). Subjects exhibited either a left- (n = 28) or right-sided hemianopia (n = 18), with mean visual field sparing of 3.5 deg (SD: ± 2.0, range: 1–8). Minimum Snellen acuity was 0.90 for near and far binocular vision and, according to ophthalmic examination, there were no signs of peripheral visual dysfunction or oculomotor dysfunction.
Table 7.
Summary of data of hemianopic subjects (n = 46) in the long-term follow-up (second follow-up) study. Interval: time between end of treatment and first follow-up (in weeks), and between first and second follow-up (in years). VF-sparing: visual field sparing (degrees visual angle; mean, SD, range); visual exploration performance (in s, mean, SD, range) and reading performance (in words per minute, wpm; mean, SD, range) at first and second follow-up. Line bisection: deviation to the left or right (in mm; mean, SD, range). GDS: geriatric depression scale (scores; mean, SD, range). MMSE: Mini-Mental-State-Examination (scores; mean, SD, range). Results of a two-sided paired t-Test for the mean difference between the second and first follow-up are presented in the last column.
| Variables | first follow-up | second follow-up | second follow-up – first follow-up |
|---|---|---|---|
| Age (yrs) | 61.9 (12.3; 32-81) | 67.2 (12.0; 43-87) | |
| Interval | 11.4 (3.3; 6-21) | 5 (2.9; 2-15) | |
| VF-sparing (deg) | 3.5 (2.0; 1-8) | 3.5 (2.0; 1-8) | ΔM=0.00, t(45)=0.00, p=1.00 |
| Exploration (s) | 22.9 (9.4; 12-66) | 30.6 (11.2; 8-65) | ΔM=7.70, t(45)=6.48, p<.001 |
| Reading (wpm) | 151.7 (34.6; 76-202) | 147.7 (37.6; 79-218) | ΔM = −4.04, t(45) = −1.67, p=.102 |
| Line bisection (mm) | 6.2 (1.4; 3-9) | 5.7 (1.4; 3-9) | ΔM = −0.43, t(45) = −2.76, p=.008 |
| GDS | 1.8 (1.5; 0-6) | 2.0 (2.0; 0-9) | ΔM=0.20, t(45)=0.92, p=.362 |
| MMSE | 28.7 (1.4; 25-30) |
Assessment procedures of visual field, of scanning and reading, and of subjective reports on visual difficulties in every-day life activities were the same as in the treatment study. In addition, we assessed mood with the GDS (short form; [15]) and global cognitive status with the MMSE [12], because both factors may affect cognitive and psychomotor speed, especially in older subjects [10], [39].
Because it has been assumed that shifts in midline towards the hemianopic side (so-called hemianopia measurement error) may indicate “strategic adaptation” to the visual field loss [4] or expansion of perceived space at the affected side [13], we also assessed line bisection performance. If this midline shift indicates successful functional adaptation to the hemianopia, then subjects with preserved or even further improved oculomotor compensation should show larger midline shifts towards their hemianopic side. We used the same procedure as described in an earlier study on line bisection in subjects with homonymous visual field defects [44]. We presented 20 cm long horizontal black lines (2 mm thick) on separate paper sheets five times in randomized order under normal daylight conditions. Each sheet was positioned in front of the subjects who used a pencil to mark the location on the line that appeared to be the line’s center. Viewing distance was 30 cm where the line subtended 33.7◦. All subjects were right-handed, and bisected the line using their right hand. To control for the effects of scanning direction and to ensure that subjects have perceived the entire line, we asked them to search for the left end of the line first, mark it with a pencil and then to search for the right end of the line and mark it as well. Subjects were allowed to repeat this procedure as often as they wished before bisecting the line. After marking the midpoint of the line with a short vertical line, we asked subjects to carefully check their bisection. They were allowed to correct their bisection once but never received any feedback on their performance. Eye and head movements were not restricted. The deviation to the left or right from the objective line centre (to 0.5 mm accuracy) in five consecutive trials served as bisection performance. In addition, we asked patients whether they still experienced a deviation to the left or right while going straight ahead.
Data was analysed with t-tests
Table 7 shows a summary of the data for the first and the second follow up. Performance in the paper-based visual scanning task and in the reading task of participants in the long-term follow-up showed a similar picture to that of the whole group if one compares the results between the different measurement times at the first follow-up. The only significant change in this subgroup in performance was, comparable to the whole group - between before and after treatment for both, visual exploration [t(45) = −11.93, p < 0.001] and reading performance [t(45) = −12.03, p < 0.001]. Visual exploration time at follow-up 2 was on average significantly increased by about 8 s on average, when compared to the corresponding data at follow-up 1 [t(45) = 6.48, p < 0.001]. Reading performance was also slightly diminished (4 wpm on average), but the difference was not significant [(t(45) = −1.67, n.s.)] In the line bisection task, all subjects with left-sided homonymous hemianopia shifted their subjective midline to the left (mean deviation: 5.8 mm; SD: 1.4, range: 3–8), while all subjects with right-sided hemianopia shifted midline to the right (mean deviation: 5.6 mm; SD: 1.5, range: 4–9). Overall deviation differed significantly between follow-ups 1 and 2 [t(45) = -2.76, p < 0.01], but the difference is rather marginal (M:0.83, SD:0.80, range: 0–3 mm). Mean variation between midline deviation at the first assessment and follow-up 2 is 1.37 mm (SD:0.71; range: 0–3 mm), which is in the range of normal subjects [58].
We found no significant differences in the GDS scores between the follow-up times [(t(45) = 0.92, n.s] and no subject scored above the cut-off value of 10, which would indicate depression [15]. In the MMSE, all subjects performed in the age-appropriate range (scores > 25; [12].
The vast majority of subjects did not report difficulties with either overview/visual exploration in their everyday-life activities (72%) or reading (85%); the rest reported minor difficulties (26%, and 15%, respectively). Subjects who reported difficulties with exploration/visual exploration also showed higher exploration time (n = 13; M: 41.8 s, SD: 10.8; range: 26–65 s) compared to subjects who did not report any difficulties (n = 33; M: 26.2 s, SD: 7.8; range: 8–44 s). A similar picture emerges for reading. Subjects who reported difficulties showed lower reading performance (n = 7; M: 95.7 wpm; range: 79–144) as compared with subjects without reported difficulties (n = 39; M:157.0 wpm, SD: 31.4; range: 89–218). Higher reading performance was linked to daily reading time; subjects with daily reading of less than three hours (n = 15, 32.6%) showed also lower reading performance (M:115 wpm, SD: 39.1; range: 79–183 wpm) compared with subjects with reading time of ≥ 3 h (n = 31; M: 163 wpm, SD: 24.9; range: 118–128) [t(44) = -5.09, p < 0.001].
Discussion
The aim of the present study was to test the specific effectiveness of eye-movement training for hemianopic deficits in reading and scanning when compared with a control intervention of advice. The question of the influence of age and possible co-morbidity at the time of treatment and in the follow-up examination was also a special research question.
The main outcome shows unequivocally that hemianopic patients benefit from systematic and specific practice to learn to make compensatory eye-movements. Neither spontaneous adaptation (waiting periods), nor advice for coping with visual difficulties in activities of daily living (ADL), nor visual training per se (control training conditions for scanning and reading, respectively) was associated with significant specific improvements in either scanning or reading performance. Improved scanning or reading performance was only found after the corresponding training. Furthermore, because visual field borders showed no significant changes after either type of training, improved visual ability can best be explained in terms of successfully learning compensatory strategies. The outcome of the study is consistent with earlier studies showing the importance of systematic and specific practice (e.g., [1], [23], [54], [32], [33], [42], [41], [45], [47], [53], [54], [55]), rather than nonspecific interventions (e.g., [29], [51]).
The use of a standardized, tailor-made training procedure improved scanning by ∼40%, and reading by ∼45%. Improvements were apparent early in training and reached a plateau after 10–12 sessions for both visual scanning and reading training (see Fig. 2), indicating that the improvement of the compensation strategies does not end after a few sessions [22]. Improvements were stable or, in single cases, enhanced at follow-up, particularly in reading. A similar continuing improvement in reading was also observed by Kerkhoff et al. [24] in their group of patients with hemianopic dyslexia.
The absence of significant transfer effects between scanning and reading training suggests that improvements are not based on a single compensatory mechanism. Of course, top-down influences play a crucial role in the acquisition of such successful compensatory visual behavior [21], [16], which eventually may become a routine [31], and thus can substitute effectively for the lost visual field in ADLs in both conditions, scanning and reading. Effective oculomotor guidance depends on the integrity of posterior and prefrontal structures, and their interplay [35]. Injury to these structures and/or their reciprocal fiber connections impair such guidance [56]and diminish successful compensation of the homonymous visual field loss by eye movements.
Speed of progress and degree of improvement differed substantially among individuals (see Table 2). Improvement rates in visual scanning varied between 30% and 44%, in reading rates varied between 13% and 63%; the number of sessions varied between 8 and 14. These differences may be explained by differences in individual cognitive capacities [7], differential ageing trajectories [37], but also by differences in the extent of posterior brain injury [53], [54]. Patients with lower baseline performance before treatment showed higher improvement rates per session in scanning but not in reading. This may be explained by different oculomotor adaptation processes involved in scanning and reading. In scanning, larger gaze shifts enable the subject to grasp the global aspects of a scene and code its spatial configuration transiently for guidance of subsequent fixation shifts required for local processing [2]. Reading, in contrast, requires a regular pattern of small saccades and fixations along the text. Readers rely on information from a ‘perceptual span‘, which includes information ahead of the eyes, to efficiently coordinate their eye-movements [27], [26], and to enable continuous text processing. The acquisition of this regular oculomotor pattern may require more practice to become a routine, compared with the more flexible oculomotor procedures involved in scanning. Surprisingly, but also fortunately, there were no significant effects of the factors age and co-morbidity on the treatment results.
In contrast to earlier studies (e.g., [9], [54]), we did not find significantly poorer reading performance in subjects with right-sided hemianopia before or after training. This may be the result of our selection criteria, which included similar baseline performance in all subgroups irrespective of the side of the visual field loss. It should be noted, however, that subjects in studies of hemianopic dyslexia are chiefly those with right-sided hemianopia (e.g., [28], [47]), possibly because this condition is of more interest for left-to-right reading and associated word-based analysis for semantic processing. However, subjects with left-sided hemianopia also suffer from impaired text processing. The degree of impairment may, however, be less pronounced and/or dyslexia may be less frequent, because less field sparing (∼5°) left of the fovea is required for fluent reading compared with the right (∼8°). Thus, intervention measures addressing subjects with right-sided hemianopia only (e.g., [32], [47] overlook subjects with dyslexia due to left-sided visual field loss. Furthermore, we found significant age effects, whereby younger patients showed a greater overall rate of improvement; this result contrasts with the outcome of an earlier study [43], but may be explained by the larger group size in this study. While subjects with co-morbidity were, on average, 10 years older than those without, and had a slightly lower (<10%) reading performance after training, they showed a similar rate of improvement suggesting that co-morbidity did not play a significant role. Of course, all subjects with co-morbidity were on appropriate efficient medication and regular medical control. It is known that hormonal and metabolic risk factors increase with age [6] and may have negative effects on cognition [11] and probably also on brain plasticity [38]. For a quantitative analysis of the influence of the co-morbidity, it would have been necessary to use the corresponding quantitative medical examination results at the various measurement times. Unfortunately, this data were not available to us, so our statement about the role of co-morbidity on the treatment outcomes is more of qualitative nature. In addition, reduced cognitive status at admission and psychopathological symptoms (e.g., affective disorders) can themselves also negatively affect the rehabilitation outcome [20], [50]. However, none of our subjects showed pronounced cognitive deficits or affective symptoms.
Patients’ reports of their visual difficulties paralleled the outcome at the various points of assessment. No changes in frequency of reported visual difficulties were found for the waiting period and the period of advice. After training with scanning, patients reported fewer difficulties with overview, avoiding of obstacles and spatial orientation, even in unfamiliar surroundings. Correspondingly, fewer difficulties with reading were reported after training with reading. The further decrease of percentages of reported difficulties at follow-up for reading, but not for scanning may be understood in terms of increasing routine-building for reading. Reading takes place under more or less similar conditions and, therefore, routine acquisition may progress even long-term. In contrast, scanning of the environment requires more diverse and more flexible patterns of oculomotor behavior.
The second follow-up testing, which was carried out about 5 years after treatment, did not reveal significant changes in visual exploration and reading performance, indicating that treatment effects persisted even over a protracted time. The slight worsening in performance, especially with respect to exploration (Md = 7.70 s), may be due to normal mental ageing, in particular processing speed and psychomotor ability, rather than global mental decline or depressed mood [19]. Considering that more than half of subjects (56%) were now over 70 years old (Md = 72 yrs), it seems rather surprising that performance levels were so high. A plausible explanation for the observation that visual exploration performance showed a larger decline than reading may be that reading typically takes place under the same condition, i.e. routine remains more or less the same. In contrast, the visual exploration must be able to be flexibly adapted to many different conditions, and the price of this flexibility may be an increased amount of time. Subjects’ subjective reports support this consideration. More subjects (28%) reported more (mild) difficulties with respect to visual exploration in their everyday-life activities as compared with difficulties with reading (15%). Interestingly, reading performance was linked to daily reading time. Subjects who spent more time reading each day also showed higher reading performance. While visual exploration is in use most of the day, and exploration and visual search activities require flexible adaptation to environmental changes, reading activities are limited in time and take place under more or less similar conditions. Thus, reading habits may also play a significant role for reading performance in our subjects [46]. Alternatively, subjects with lower reading performance may spent less time reading each day because they find reading difficult. However, some individuals with low reading performance reported that they had never spent much time reading before their stroke.
As already reported in earlier studies (e.g., [44]) we found no connection between the systematic midline deviation to the hemianopic side and the efficiency of oculomotor compensation of the visual field loss. Thus, this deviation represents a genuine visual spatial disorder, which is typically associated with a homonymous visual field defect [25], [58]. Over an expanded time span a significant, but small, reduction of the midline shift may occur. However, it seems unlikely that this change will have a significant impact on oculomotor compensation behavior (see also [58]).
Overall our findings show that visual impairment associated with homonymous hemianopia can be successfully and durably reduced by systematic and specific training of compensatory eye-movement strategies for both scene and text processing. Homonymous hemianopia is associated with a suboptimal bottom-up control of global visual processing because of a restriction of the field of view. The acquisition of effective compensatory eye-movement strategies after training enables the patient to regain a sufficiently large field of view via top-down guidance and control. Thus, the conventional vision-action relationship, whereby vision guides action [17], seems reversed. The use of appropriate, immediate feedback during the acquisition of efficient oculomotor compensation appears to be crucial [30]. For clinical purposes, the amount of training appears manageable (∼11 sessions for scanning, and ∼12 sessions for reading) and, since oculomotor learning is procedural learning and thus similar to skill learning, mass practice appears superior to distributed practice [5]. Furthermore, patients require systematic practice, until a stable plateau in improvement is reached, to achieve performance that serves as a reliable basis for coping with ADLs. Once achieved, the acquisition of efficient compensatory eye-movements have long-term beneficial effects.
Regarding age, this study offers positive news: no patient is too old to be treated for homonymous hemianopia. Of course, severe cognitive impairments can be a limiting factor; however, this rarely occurs in patients with occipital stroke. The same applies to co-morbidity: if accompanying systemic diseases are treated adequately, they do not appear to reduce the potential for rehabilitation. However, it only seems fair to respect the age-related increase in time spent on cognitive activities and to take it into account when evaluating test results, especially since not all older people show a significant cognitive slowing even in complex cognitive tasks [57].
Conclusions
Visual impairments associated with homonymous visual field loss can be successfully and durably reduced by systematic and specific training of compensatory eye-movement strategies for both scene and text processing. The duration of training is practicable and its effects mitigate the complaints of difficulties encountered in everyday life. In particular, older age and adequately treated co-morbidity were not found to reduce training effects, i.e. are not negative prognostic factors, neither concerning the outcome of training nor the number of sessions to achieve it. Short- and long-term follow-up data show successful transfer of learned compensation strategies to everyday life conditions, and thus underline the lasting effectiveness of systematic and specific visual training.
Funding
This study was supported by the Max Planck Institute of Psychiatry, Munich, Germany.
CRediT authorship contribution statement
J. Zihl: Conceptualization, Methodology, Data curation, Project administration, Writing - original draft. R.W. Kentridge: Formal analysis, Writing - original draft. F. Pargent: Formal analysis, Writing - original draft. C.A. Heywood: Conceptualization, Methodology, Data curation, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Christel Schmid, Jana Specht, Susanne Hörand, and Susanne Müller for their assistance with the recruitment, assessment and treatment of patients with homonymous visual field loss.
Appendix A.
Standardised subjective reports to assess visual difficulties in everyday life (A) and in reading (B) “Familiar surroundings” refer to difficulties at home, “unfamiliar surroundings” refer to difficulties in supermarkets, crowded places, complex or new environments. “Slower” reading refers to premorbid reading speed; difficulties were typically associated with finding the beginning of line/words (LH) or the end of line/words (RH), respectively. Patients rated their visual difficulties using three categories/scores: mild (1), moderate (2), and severe (3).
A difficulties in everyday life activities
-
(1)Vision “too slow”
-
•familiar surroundings
-
•unfamiliar surroundings
-
•
-
(2)Bumping against obstacles
-
•familiar surroundings
-
•unfamiliar surroundings
-
•
-
(3)“Getting lost”
-
•familiar surroundings
-
•unfamiliar surroundings
-
•
B difficulties with reading
-
•
difficult and/or slower reading
References
- 1.Aimola L., Lane A.R., Smith D.T., Kerkhoff G., Ford G.A., Schenk T.h. Efficacy and feasibility of home-based training for individuals with homonymous visual field defects. Neurorehabil Neural Repair. 2014;28:207–218. doi: 10.1177/1545968313503219. [DOI] [PubMed] [Google Scholar]
- 2.Antes J.R. The time course of picture viewing. J Exp Psychol. 1974;103:62–70. doi: 10.1037/h0036799. [DOI] [PubMed] [Google Scholar]
- 3.Aulhorn E, Harms H. Visual perimetry. In: Jameson, D Hurvich LM, editors. Handbook of sensory physiology: visual psychophysics Vol II/4. Berlin: Springer. 1972; p. 102-44. doi.org/10.1007/978-3-642-88658-4_5
- 4.Barton J., Black S. Line bisection in hemianopia. J Neurol Neurosurg Psychiatr. 1998;64:660–662. doi: 10.1136/jnnp.64.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaunieux H., Hubert V., Witkowski T., Pitel A.L., Rossi S., Danion J.M., et al. Which processes are involved in cognitive procedural learning? Memory. 2006;14:521–539. doi: 10.1080/09658210500477766. [DOI] [PubMed] [Google Scholar]
- 6.Carrera E., Maeder-Ingvar M., Rossetti A.O., Devuyst G., Bogousslavsky J. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007;24:97–103. doi: 10.1159/000103123. [DOI] [PubMed] [Google Scholar]
- 7.Carroll J.B., Maxwell S.E. Individual differences in cognitive abilities. Annu Rev Psychol. 1979;30:603–640. doi: 10.1146/annurev.ps.30.020179.003131. [DOI] [PubMed] [Google Scholar]
- 8.De Haan G.A., Melis-Danker B.J.M., Brouwer W.H., Tucha O., Heutink J. The effects of compensatory training on mobility in patients with homonymous visual field defects: a randomized controlled study. PLoS ONE. 2015;10(8) doi: 10.1371/journal.pone.0134459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca M., Spinelli D., Zoccolotti P. Eye movement patterns in reading as a function of visual field defects and contrast sensitivity loss. Cortex. 1996;32:491–502. doi: 10.1016/S0010-9452(96)80006-2. [DOI] [PubMed] [Google Scholar]
- 10.Ebaid D., Crewther S.G., MacCalman K., Brown A., Crewther D.P. Cognitive processing speed across the lifespan: beyond the influence of motor speed. Front Aging Neurosci. 2017;9:62. doi: 10.3389/fnagi.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etgen T., Bickel H., Förstl H. Metabolic and endocrine factors in mild cognitive impairment. Aging Res Rev. 2010;9:280–288. doi: 10.1016/j.arr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Fortenbaugh F.C., VanVleet T.M., Silver M.A., Robertson L.C. Spatial distortions in localization and midline estimation in hemianopia and normal vision. Vision Res. 2015;111:1–12. doi: 10.1016/j.visres.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gancarz G., Grossberg S. A neural model of saccadic eye movement control explains task-specific adaptation. Vision Res. 1999;39:3123–3143. doi: 10.1016/S0042-6989(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 15.Gauggel S., Birkner B. Validität, und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS) [Validity and reliability of a German version of the Geriatric Depression Scale (GDS)] Z Klin Psychol Psychother. 1999;28:18–27. doi: 10.1026//0084-5345.28.1.18. [DOI] [Google Scholar]
- 16.Gilbert C.D., Li W. Top-down influences on visual processes. Nature. 2013;14:350–361. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodale M.A. Transforming vision into action. Vision Res. 2011;51:1567–1587. doi: 10.1016/j.visres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Halligan P.W., Cockburn J., Wilson B.A. The behavioural assessment of visual neglect. Neuropsychol Rehabil. 1991;1:5–32. doi: 10.1080/09602019108401377. [DOI] [Google Scholar]
- 19.Harada C.N., Natelson Love M.C., Triebel K. Normal cognitive ageing. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heruti R.J., Lusky A., Dankner R., Ring H., Dolgopiat M., Barell V., et al. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch Phys Med Rehabil. 2002;83:742–749. doi: 10.1053/apmr.2002.32739. [DOI] [PubMed] [Google Scholar]
- 21.Hochstein S., Ahissar M. View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/S0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- 22.Jacquin-Courtois S., Bays P.M., Salemme R., Leff A.P., Husain M. Rapid compensation of visual search strategy in patients with chronic visual field defects. Cortex. 2013;49:994–1000. doi: 10.1016/j.cortex.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerkhoff G., Münssinger U., Haaf E., Eberle-Strauss G., Stögerer E. Rehabilitation of homonymous scotomata in patients with postgeniculate damage to the visual system: saccadic compensation training. Restorat Neurol Neurosci. 1992;4:245–254. doi: 10.3233/RNN-1992-4402. [DOI] [PubMed] [Google Scholar]
- 24.Kerkhoff G., Münssinger U., Eberle-Strauss G., Stögerer E. (1992b) Rehabilitation of hemianopic alexia in patients with postgeniculate visual field disorders. Neuropsychol Rehabil. 1992;2:21–42. doi: 10.1080/09602019208401393. [DOI] [Google Scholar]
- 25.Lanyon J., Barton J.J. Visual search and line bisection in hemianopia: computational modelling of cortical compensatory mechanisms and comparison with hemineglect. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0054919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConkie G.W., Rayner K. Asymmetry of the perceptual span in reading. Bull Psychon Soc. 1976;8:365–368. doi: 10.3758/BF03203972. [DOI] [Google Scholar]
- 27.McConkie G.W., Rayner K. The span of the effective stimulus during a fixation in reading. Percept Psychophys. 1975;17:578–586. doi: 10.3758/BF03198682. [DOI] [Google Scholar]
- 28.McDonald S.A., Spitzyna G., Shillcock R.C., Wise R.J.S., Leff A.P. Patients with hemianopic dyslexia adopt an inefficient eye movement strategy when reading text. Brain. 2006;129:158–167. doi: 10.1093/brain/awh678. [DOI] [PubMed] [Google Scholar]
- 29.Mödden C., Behrens M., Damke I., Eilers N., Kastrup A., Hildebrandt H. A randomized controlled trial comparing 2 interventions for visual field loss with standard occupational therapy during inpatient stroke rehabilitation. Neurorehabil Neural Repair. 2012;26:463–469. doi: 10.1177/1545968311425927. [DOI] [PubMed] [Google Scholar]
- 30.Mount J., Pierce S.R., Parker J., DiEgidio R., Woessner R., Spiegel L. Trial and error versus errorless learning of functional skills in patients with acute stroke. Neurorehabilitation. 2007;22:123–132. doi: 10.3233/NRE-2007-22208. [DOI] [PubMed] [Google Scholar]
- 31.Nelles G., Pscherer A., de Greiff A., Forsting M., Gerhard H., Esser J., et al. Eye-movement training-induced plasticity in patients with post-stroke hemianopia. J Neurol. 2009;256:726–733. doi: 10.1007/s00415-009-5005-x. [DOI] [PubMed] [Google Scholar]
- 32.Ong Y.-H., Brown M.M., Robinson P., Plant G.T., Husain M., Leff A.P. Read-Right: a “web app” that improves reading speeds in patients with hemianopia. J Neurol. 2012;259:2611–2615. doi: 10.1007/s00415-012-6549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong Y.-H., Jacquin-Courtois S., Gorgoraptis N., Bays P.M., Husain M., Leff A.P. Eye-search: a web-based therapy that improves visual search in hemianopia. Ann Clin Transl Neurol. 2015;2:74–78. doi: 10.1002/acn3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelak V.S., Dubin M., Whitney E. Homonymous hemianopia: a critical analysis of optical devices, compensatory training, and Nova Vision. Curr Treat Options Neurol. 2007;9:41–47. doi: 10.1007/s11940-007-0029-y. [DOI] [PubMed] [Google Scholar]
- 35.Pierrot-Deseilligny C., Milea D., Müri R.M. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Pollock A., Hazelton C., Rowe F.J., Jonuscheit S., Kernohan A., Angilley, et al. Interventions for visual field defects in people with stroke. Cochrane Database Syst rev. 2019;23:5(5):CD008388. doi: 10.1002/14651858.CD008388.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raz N., Rodriguez K.M. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuter-Lorenz P.A., Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Aranda C. Reduced writing and reading speed and age-related changes in verbal fluency tasks. Clin Neuropsychol. 2003;17:203–215. doi: 10.1076/clin.17.2.203.16508. [DOI] [PubMed] [Google Scholar]
- 40.Rowe FJ, Hepworth LR, Howard C, Hanna K, Cheyne CP, Currie J. High incidence and prevalence of visual problems after acute stroke: An epidemiology study with implications for service delivery. PLoS ONE 2019; 14(3): e0213035. doi: 10.1371/journal.pone.0213035 PMCID: PMC6402759. [DOI] [PMC free article] [PubMed]
- 41.Sahraie A., Cederblad A.M.H., Kenkel S., Romano J.G. Efficacy and predictors of recovery of function after eye movement training in 296 hemianopic patients. Cortex. 2020;125:149–160. doi: 10.1016/j.cortex.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Sahraie A, Smania N, Zihl J. Use of NeuroEyeCoachTM to Improve Eye Movement Efficacy in Patients with Homonymous Visual Field Loss. BioMed Research 2016; ID 5186461 9 pages. doi.org/10.1155/2016/5186461 [DOI] [PMC free article] [PubMed]
- 43.Schuett S., Zihl J. Does age matter? Age and rehabilitation of visual field disorders after brain injury. Cortex. 2013;49:1001–1012. doi: 10.1016/j.cortex.2012.04.00. [DOI] [PubMed] [Google Scholar]
- 44.Schuett S., Dauner R., Zihl J. Line bisection in unilateral homonymous visual field defects. Cortex. 2011;47:47–52. doi: 10.1016/j.cortex2010.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Schuett S., Heywood C.A., Kentridge R.W., Dauner R., Zihl J. Rehabilitation of reading and visual exploration in visual field disorders: transfer of specificity? Brain. 2012;135:912–922. doi: 10.1093/brain/awr356. [DOI] [PubMed] [Google Scholar]
- 46.Sörman D.E., Körning Ljungberg J., Rönnlund M. Reading habits among older adults in relation to level and 15-Year changes in verbal fluency and episodic recall. Front Psychol. 2018;9:1872. doi: 10.3389/fpsyg.2018.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitzyna G.A., Wise R.J.S., McDonald S.A., Plant G.T., Kidd D., Crewes H., et al. Optokinetic therapy improves text reading in patients with hemianopic alexia: a controlled trial. Neurology. 2007;68:1922–1930. doi: 10.1212/01.wnl.0000264002.30134.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truelsen T., Piechowski-Jóźwiak B., Bonita R., Mathers C., Bogousslasvky J., Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 49.Vistech Consultants, Inc. Vistech contrast sensitivity test. Daytona, Ohio: Vistech consultants, Inc; 1988.
- 50.West R., Hill K., Hewison J., Knapp P., House A. Psychological disorders after stroke are an important influence on functional outcomes. Stroke. 2010;41:1723–1727. doi: 10.1161/STROKEAHA.110.583351. [DOI] [PubMed] [Google Scholar]
- 51.Woodhead ZVJ, Ong Y-H, Leff P. Web-based therapy for hemianopic alexia is syndrome-specific BMJ Innov 2015; 1: 88–95. doi:10.1136/bmjinnov-2015-000041.
- 52.Zhang X., Kedar S., Lynn M., Newman N.J., Biousse V. Homonymous hemianopia in stroke. J Neuroophthalmol. 2006;26:180–183. doi: 10.1097/01.wno.0000235587.41040.39. [DOI] [PubMed] [Google Scholar]
- 53.Zihl J. Eye movement patterns in hemianopic dyslexia. Brain. 1995;118:891–912. doi: 10.1093/brain/118.4.891. [DOI] [PubMed] [Google Scholar]
- 54.Zihl J. Visual scanning behaviour in patients with homonymous hemianopia. Neuropsychologia. 1995;33:287–303. doi: 10.1016/0028-3932(94)00119-a. [DOI] [PubMed] [Google Scholar]
- 55.Zihl J. 2nd ed. Psychology Press; Hove (GB) and New York (USA): 2011. Rehabilitation of Visual Disorders after brain injury. [Google Scholar]
- 56.Zihl J., Hebel N. Patterns of oculomotor scanning in patients with unilateral posterior parietal or frontal lobe damage. Neuropsychologia. 1997;35:893–906. doi: 10.1016/S0028-3932(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 57.Zihl J., Fink Th., Pargent F., Ziegler M., Bühner M. Cognitive reserve in young and old healthy subjects: differences and similarities in a testing-the-limits paradigm using the DSST. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zihl J., Sämann Ph., Schenk ., Schuett S., Dauner R. On the origin of line bisection error in hemianopia. Neuropsychologia. 2009;47:2417–2426. doi: 10.1016/j.neuropsychologia.2009.04.009. [DOI] [PubMed] [Google Scholar]