Abstract
Falls due to balance impairment are a major cause of injury and disability in the elderly. The study of neurophysiological correlates during static and dynamic balance tasks is an emerging area of research that could lead to novel rehabilitation strategies and reduce fall risk.
This review aims to highlight key concepts and identify gaps in the current knowledge of balance control in the elderly that could be addressed by relying on surface electromyographic (EMG) and electroencephalographic (EEG) recordings. The neurophysiological hypotheses underlying balance studies in the elderly as well as the methodologies, findings, and limitations of prior work are herein addressed.
The literature shows: 1) a wide heterogeneity in the experimental procedures, protocols, and analyses; 2) a paucity of studies involving the investigation of cortical activity; 3) aging-related alterations of cortical activation during balance tasks characterized by lower cortico-muscular coherence and increased allocation of attentional control to postural tasks in the elderly; and 4) EMG patterns characterized by delayed onset after perturbations, increased levels of activity, and greater levels of muscle co-activation in the elderly compared to younger adults.
EMG and EEG recordings are valuable tools to monitor muscular and cortical activity during the performance of balance tasks. However, standardized protocols and analysis techniques should be agreed upon and shared by the scientific community to provide reliable and reproducible results. This will allow researchers to gain a comprehensive knowledge on the neurophysiological changes affecting static and dynamic balance in the elderly and will inform the design of rehabilitative and preventive interventions.
Keywords: Electromyography, Electroencephalography, Balance, Older adults, Postural control
Introduction
Maintaining dynamic (as during walking) and static (e.g., standing posture) balance, which may seem effortless tasks, become demanding when diseases or physiological changes associated with aging take place [1]. In the elderly (age > 65 years), balance impairments are among the main causes of falls, resulting in both morbidity and mortality [2]. Due to the demographic transition phenomenon, the cumulative decline of several physiological systems associated with vulnerability and adverse consequences represents a major health issue leading to high health care costs [3].
Postural control involves an organized network of interacting systems. The activity of muscles is controlled by the central nervous system (CNS) to maintain balance via integration of musculoskeletal, visual and vestibular system inputs [4]. Proprioception, which originates from muscles and cutaneous receptors, provides information about body position in the environment, as well as the relative position of the body segments. The visual system provides information about the external environment. Cerebellar control provides a feedback-feedforward control of muscle activation. Lastly, the vestibular system generates information, using specialized organs situated in the inner ear, that enable tracking angular acceleration by relying on the semicircular canals and linear acceleration by relying on the saccule and the utricle [5]. The redundancy of afferent information from the musculoskeletal system and from the vestibular and vision systems is essential to enable the CNS to generate correct responses when these systems receive conflicting stimuli.
Although the relevance of this information is well known, how the brain controls balance by integrating these inputs is still poorly understood. Several protocols have been proposed to measure postural sway to evaluate balance in older adults and in people with neurological disorders [6]. Reliable and quantifiable clinical measures rely on computerized dynamic posturography, force plates, and wearable sensors [7]. These methods have the important shortcoming of capturing solely the final output of a complex process taking place at the cortico-subcortical, spinal, and muscle level.
In light of the increasing threat posed by falls to an aging society [8], researchers and clinicians have displayed a growing interest for the study of balance impairments in the elderly and the development of new assistive and rehabilitative interventions. Identifying cortical activity and muscle activity patterns associated with effective balance control strategies could provide a benchmark to assess the efficacy of rehabilitative interventions or to determine the targets of neurostimulation-based interventions. Being non-invasive and relatively low-cost, the study of surface electromyographic (EMG) and electroencephalographic (EEG) data could provide a useful tool to investigate the neurophysiology of balance [9]. Surface EMG data can be looked upon as the superposition of the action potential waveforms that travel along its muscle fibers during a muscle contraction. The resulting electrical signal can be processed to estimate the timing and magnitude of muscle activation [10]. Its clinical utility has been proven [11], [12], [13], [14].
EEG data enable the detection of motor planning and intention. EEG may also provide information on ongoing cognitive processes even in highly compromised people with weakness and abnormalities in muscle activation. EEG is a non-invasive low-cost imaging technique that allows clinicians and researchers to record the dynamics of brain activity (i.e., measures of voltage fluctuations collected on the scalp via non-invasive electrodes) with high temporal resolution (i.e., ms). Advances in hardware technology have allowed engineers to develop EEG wireless multi-channel systems [15]. Furthermore, advances in signal processing [16], [17] have allowed source-resolved EEG dynamics during walking, thus promoting the development of Mobile Brain/Body Imaging (i.e., MoBI) [18].
Until now, the relevance of the muscular and cortical systems involvement and their functional modifications in both dynamic and static balance control have been primarily studied in pathological conditions [19], [20], [21] and in robot-assisted rehabilitation [22], [23], [24].
To more clearly observe balance control impairments in experimental settings, dual-task paradigms are often implemented. They consist of combining a static or dynamic balance task with a cognitively-demanding task, such as mental calculations, visual or auditory disturbances (as in oddball-based experimental paradigms) [25], [26], [27]. The rationale underlying the choice of combining tasks is to challenge competing resources. This experimental approach aims to highlight the major role played by the brain cortex.
A detailed knowledge of the cortical activity and of how the CNS controls muscles during the performance of static and dynamic tasks is not currently available. In contrast, extensive literature describes the role of muscle activity during the performance of both static and dynamic balance control tasks [28].
A previous review about neuroimaging in human balance control [29] provided evidence of an increase in brain activation during the performance of balance control tasks by healthy adults, regardless of mechanical, cognitive, or sensory challenges they experienced. In fact, this review focused only on brain activity during balance control tasks and did not attempt to analyze muscle activity. Analyzing both muscle and brain activity may provide additional insights into the mechanisms involved in balance control.
The aim of this review is to summarize currently available data on the characteristics of muscular and cortical activation patterns during static and dynamic balance tasks in the elderly as derived by relying on EMG and EEG recordings. Furthermore, this review highlights gaps in the available knowledge of the neurophysiological mechanisms underlying the control of balance in the elderly, identifies potential biomarkers to be used in the design of intervention strategies, and suggests future research directions in this research field of growing interest.
Material and methods
Inclusion criteria
Only studies evaluating the following were considered in this review: healthy elderly (>60 y), or involving both elderly and young participants, without diagnosed neurological, cardiovascular, vestibular, musculoskeletal or any other systemic disorder; dynamic or static balance and EEG or EMG recordings.
Dynamic balance studies were considered if related to: 1) walking, either overground or on a treadmill; 2) any other dynamic action such as step-initiation, physical exercises/tests, slip-induced trials, etc. and 3) any physical functional balance assessment. Static balance studies were considered if assessing individuals during upright stance, with or without perturbations.
Case reports, clinical trials, clinical studies, comparative studies and methodological papers were included, whereas reviews, commentaries, editorials, conference abstracts or communication papers were excluded.
Database searches
The following databases were used: Scopus, Web of Science, PubMed, Cochrane library, IEEEXplore and Science Direct. Only search results written in English and published in the last ten years (from 2010 to April 2020) were considered. The search string was composed as follows: the search categories - i.e., motor task, balance condition, measurement and target population - were linked using the AND Boolean operator, which corresponds to the algebraic intersection, and the words describing each category were linked using the OR Boolean operator, which corresponds to the algebraic union:
(walk OR “upright stance”) AND (balance OR balanced OR balancing OR “dynamic balance” OR “static balance” OR stability OR “dynamic stability”) AND (aged OR elderly OR elderlies OR “older adults”) AND ((electroencephalography OR EEG) OR (electromyography OR EMG)).
The search string and databases used in the study were chosen with help from an expert librarian and agreed among the authors.
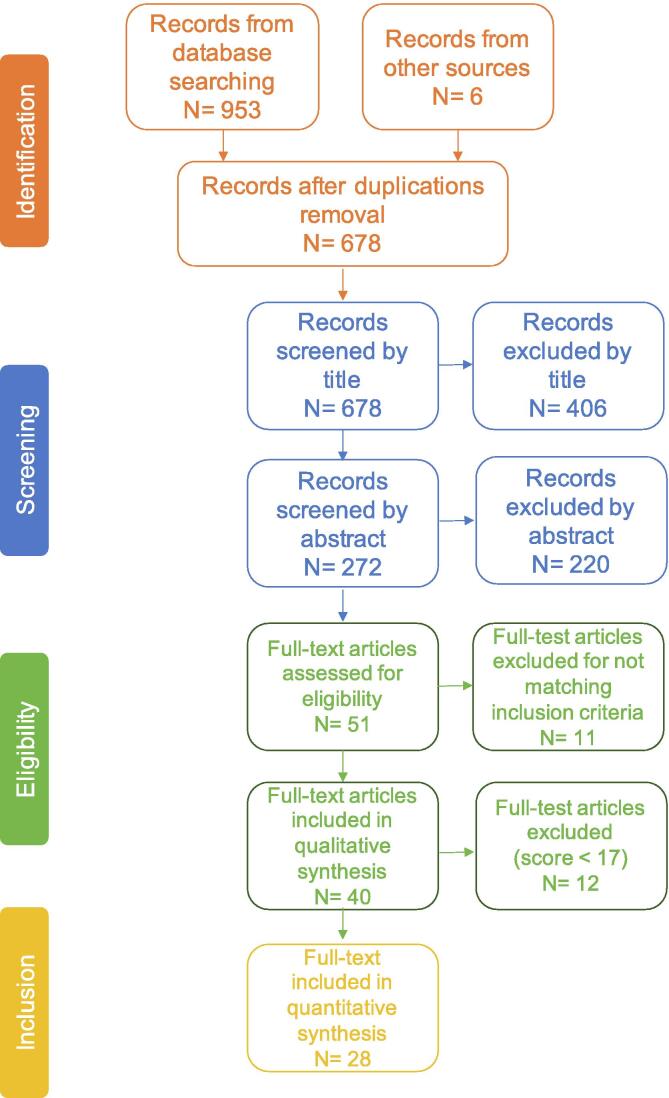
Fig. 1 shows a schematic representation of the database search. From a total of 953 papers published between 2010 and 2020, identified following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA statement) [30], 6 papers were added searching among the references of the selected studies. Duplicates were removed using the Mendeley software: 678 articles remained for further screening. Then a title screening was carried out, i.e., articles were excluded from further consideration if they revealed to be out of topic or to not meet the selection criteria. After title screening, 278 articles underwent an abstract screening. Fifty-two papers remained for full-text analysis. During both title and abstract screening, if all the investigators did not agree about the inclusion/exclusion decision, the record was moved to the next stage (Fig. 1: identification, screening, eligibility). This procedure was performed by one investigator (MZ) and cross-checked by two experienced researchers (MR, RDM). A fourth reviewer (EF) screened the papers in case of discordance among the others.
Fig. 1.
PRISMA-flow of the articles selection process.
In light of the high heterogeneity of the experimental setups and protocols, and of the analysis methods utilized to study the neurophysiological signals collected during the selected studies, we decided to carry out a scoping review [31].
Articles selection
Quality assessment
The quality of the papers was independently evaluated by three reviewers (MZ, MR, RDM). Fourteen criteria, adapted from two systematic reviews [32], [33], were assessed for each paper, belonging to the following seven sections: 1) aim of the work, 2) inclusion criteria (selection bias), 3) data collection and processing (performance bias), 4) data loss (attrition bias), 5) outcomes (detection bias), 6) presentation of the results, and 7) statistical approach.
Each item listed in Table 1, was scored from 0 to 2 by each reviewer, considering if the goals were not met (0), partially met (1), or fully met (2). Then the individual criteria scores were summed and averaged among reviewers. An article was included if the final score exceeded 60% of the maximum score (>17).
Table 1.
Quality check items.
| Aim of the work | |
|---|---|
| 1 | Description of a specific, clearly stated purpose |
| 2 | The research question is scientifically relevant |
| Inclusion criteria (selection bias) | |
| 3 | Description of inclusion and/or exclusion criteria |
| Data collection & processing (performance bias) | |
| 4 | Data collection is clearly described and reliable |
| 5 | Data processing is clearly described and reliable |
| 6 | Algorithms are clearly described and referenced |
| Data loss (attrition bias) | |
| 7 | Drop-outs < 20% |
| Outcomes (detection bias) | |
| 8 | Outcomes are topic relevant |
| 9 | The work answers the scientific question stated in the aim |
| Presentation of the results | |
| 10 | Presentation of the results is sufficient to assess the adequacy of the analysis |
| 11 | The main findings are clearly described |
| Statistical approach | |
| 12 | Appropriate statistical analysis techniques |
| 13 | Clearly states the statistical test used |
| 14 | Actual probability values reported for the main outcomes |
Data extraction form
A standardized form was created to extract data from the eligible papers. The following data were included in the extraction form: 1) first author and year of publication, 2) participant characteristics, 3) balance condition, 4) procedures, 5) signal analysis pipeline and 6) outcomes. EMG and EEG patterns reported by these studies were analyzed in a detailed manner for each manuscript and included in the final step of the literature review.
An example of the data extraction form is provided in Table 2.
Table 2.
Data extraction form.
| Organizational aspects | ||
|---|---|---|
| Reviewer | Date | Checked by |
| List of authors | Year of Publication | Journal/Source |
| Publication type: | Conference abstract/Conference paper/Full paper | Other |
| Fate: | Decision Pending | EX/IN-cluding |
| Notes/Short description | ||
| Aim | ||
| Results in brief | ||
| Reasons for Exclusion | ||
| Participants: | Not healthy (>60) and without control group | Healthy (<60) |
| Instrumentation: | Neither EEG nor EMG | |
| Task: | Imagined | Passive |
| Other: | Duplicate | Other: |
| Study description | ||
| Participants: | Number/Sex | Age interval |
| Disease: | None | Other |
| Balance condition: | Upright stance Static balance |
Walking Dynamic balance |
| Balance perturbation: | Expected | Unexpected |
| Visual | Cognitive | Other |
| Instrumentation: | EEG | List of electrodes/sample frequency |
| EMG Additional investigation |
List of muscles/sample frequency | |
| EEG analysis: | Protocol | |
| EMG analysis: | Protocol | |
| Other analysis: | Protocol | |
| Main outcomes | ||
| Confounders: | Same setting/operator | Statistic correction |
Results
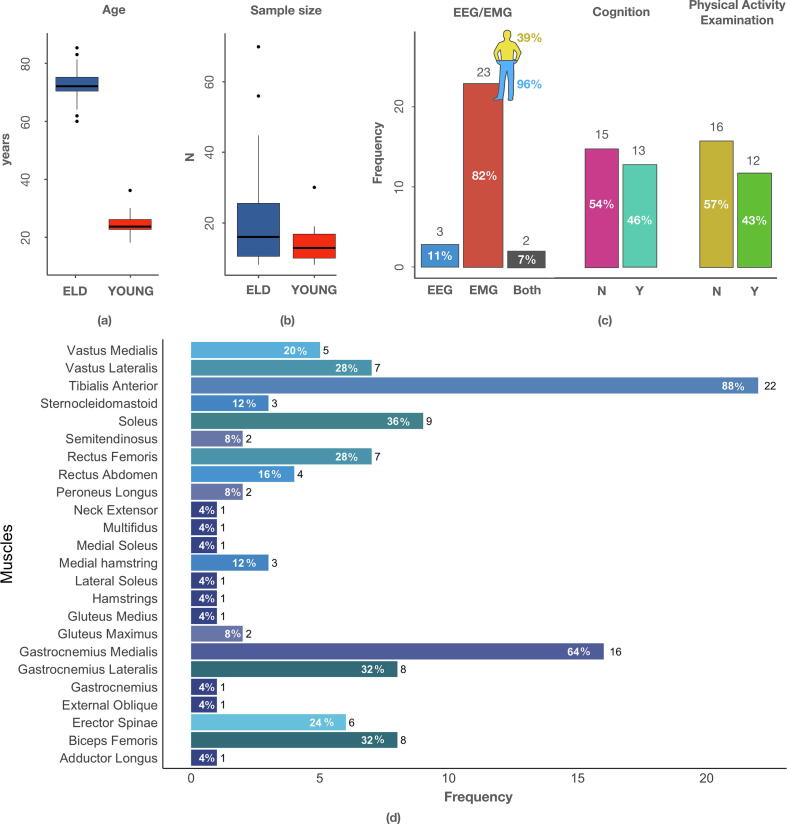
From the 51 papers selected for full-text analysis, 11 did not meet the inclusion criteria and were excluded. Among the remaining 40 full-papers, 28 (27 journal papers and 1 paper published in conference proceedings) received a score higher than 17. In scoring these papers, there was general agreement among the three raters as computed using the Fleiss' kappa [34], as shown in Table 3. Among those included, 23 papers were EMG-based studies, 3 were EEG-based studies, and in 2 papers both techniques were used. In these studies, participants were considered as older adults if their average age ranged between 65 and 83 y (with only one study including 60 year-old participants [26], and another one including participants with an average age of 61.9 y [35]). When a comparison with results obtained from a control population was carried out (i.e., 21 papers out of 28), young participants had an average age ranging between 18 and 36 y. It is worth considering that in some cases the small sample size considered for both elderly and younger participants might have negatively affected the relevance of the results. Indeed, the median sample size for the elderly population was 16 participants (ranging from 8 [35] to 70 [36]), and 10 for the younger controls (ranging from 8 [35] to 30 [37]). Fig. 2 provides additional details about the age and sample size of the elderly and younger adult groups recruited in the studies considered in this review.
Table 3.
Quality assessment: inter-rater reliability.
| Fleiss' kappa | Error | Confidence Interval | Agreement | z | p-value |
|---|---|---|---|---|---|
| 0.76893 | 0.091287 | 0.72238–0.81549 | Substantial | 8.4233 | <0.001 |
| Reject null hypothesis: Observed agreement is not accidental | |||||
Fig. 2.
Details about the included papers: (a) age of included participants for both elderly (ELD) and younger adult (YOUNG) groups; (b) sample size; (c) number of papers including EEG or EMG (for EMG, relative frequencies of papers investigating lower and upper body are also provided), number of papers investigating cognition and physical activity; (d) frequency bar plots of the muscles analyzed in the papers (relative frequencies are given close to each bar).
Different experimental protocols were utilized to evaluate balance performance in the elderly. Participants were asked to: 1) walk overground or on a treadmill (e.g., [38]); walk on a slippery surface (e.g., [39]), or step over an obstacle (e.g., [35]); 2) maintain the upright stance with either their eyes opened or their eyes closed (e.g., [40]), on a swaying platform (e.g., [41]), while performing the Functional Reach test (i.e., extending the arms as far forward as possible without moving the feet) (e.g., [42]), holding a load (e.g., [37]), performing a verbal or an arithmetic task (i.e., cognitive dual-task paradigm) (e.g., [43]).
The Supplementary Materials section includes a table showing the number and age of the participants in the 28 studies considered in this review, the experimental conditions in which the data were collected (i.e., balance task/s and perturbation conditions), the overall aim of each study, and their main outcomes.
Cognition, physical activity and executive functions
Cognitive and physical status were considered as covariates in the majority of the studies. In particular, to assess potential cognitive impairments, the Mini Mental State Examination test was used in [44], [45], [46], [47], [48], the Mental Status Questionnaire was used in [37], the Rapid Dementia Screening Test was used in [36], the Montreal Cognitive Assessment Questionnaire was used in [43], and the Cambridge Neuropsychological Test Automated Battery and Stanford Sleepiness Scale were used in [49].
The following tests were utilized to assess executive functions: the Trail Making Tests A and B were used in [25], [26], [27], the Stroop Interference Test and Digital Symbol Substitution test were used in [26], and the Binocular Visual Acuity was used in [49].
Many studies quantified also the physical performance using the International Physical Activity Questionnaire [49], [50], [51], the Berg Balance Scale [27], [46], [52], and the Timed Up-and-Go test [36], [37], [43]. Other assessment tests used in these studies were the Fried’s Criteria for frailty syndrome [50], the One Leg Standing test [37], the Chinese version of the Fall Efficacy Scale and of the Movement Specific Reinvestment Scale [45], the Four Square Step Test [53], and the Activities Specific Balance Confidence Scale [27], [43]. Test side dominance was also checked using the Edinburgh Handedness Inventory [43]; and Waterloo Footedness Questionnaire [49].
The following sections describe the protocols and data analysis pipelines used to extract the key features of balance control in the elderly, with specific reference to EMG and EEG recordings. The papers were then divided in two categories: those investigating static balance and those investigating dynamic balance. Studies were subsequently subdivided accordingly to the recorded neurophysiological signal: i.e., EMG-based studies, EEG-based studies and EEG-EMG-based studies.
Data acquisition and data analysis procedures
Notwithstanding the common aim of investigating how aging affects the mechanisms underlying static and dynamic balance control in different conditions of mechanical or cognitive load, the papers selected in this review displayed high heterogeneity in the methodologies utilized across studies. On average, people ranging between 65 and 83 y of age were considered in the group of older adults. Younger individuals were recruited in some studies. People 60 + years-old were considered eligible to participate in the study as older adults in [26] and an average age of 61.9 y (standard deviation of 2.9 y) marked the group of older adults in [35]. Results obtained in the elderly group were compared to the results in a control population of younger adults (age range of 18–36 y) in 21 of the 28 papers herein considered. In five papers, gender differences were detected as the studies focused on mechanisms hypothesized to affect in a distinct manner female [46], [50], [54], [55] or male subjects [51].
EMG-based studies
The majority of papers included EMG recordings (25 out of 28) as a tool to investigate balance control under different conditions. The EMG data were typically collected at a sampling rate of 1 kHz or higher (except for [56], in which a sampling rate of 600 Hz was used). Five studies collected data only from two muscles [27], [36], [37], [49], [57]. Electrode arrays were used in [51] to investigate the spatial distribution of the muscular activity in older women. Twenty-two studies investigated the activity of lower limb muscles and only 9 studies targeted trunk or neck muscles.
Despite the need to use standardized protocols for skin preparation, electrode placement, data collection, quality check, and signal processing [13], [58], only seven research articles included a statement concerning the standardized protocol utilized in the study. Five of these articles referred to the SENIAM protocol [25], [42], [50], [52], [59], and two of them to other protocols [38], [46].
Muscular activity was normalized using different methods: 1) using data collected during a control condition, 2) using the maximum signal amplitude, 3) by subtracting the mean and dividing by the standard deviation of the time series, 4) using data collected during a maximum voluntary contraction (MVC) (this was the case for 18 out of 28 papers), and 5) by normalizing the EMG time series using a reference value derived from data collected within the time interval corresponding to a cycle of the movement of interest, such as a gait cycle (this was the case for 7 out of 28 papers). Almost all the papers considered indices designed to compare the activity of agonist and antagonist muscles.
These indices were used as a measure of co-activation/co-contraction (e.g., ratio of signal amplitudes and ratio of the integrals of antagonist and total signals) [60], timing of activity onset and amplitude as derived from filtered EMG signals. Three papers suggested an alternative processing approach based on applying a data reduction algorithm to obtain the muscle synergies [35], [56], [61], which have been interpreted as spinal modules used by the nervous system to generate patterns of motion. The use of muscle synergies by the CNS is interpreted as a strategy to reduce the complexity of the neuromuscular control [56].
EEG-based studies
Five papers included the collection of EEG data. EEG data were collected at a sampling frequency ranging between 256 [49] and 1000 Hz [41], [48]. Data were gathered using 3 [45], 64 [41], [48], [49] or 72 electrodes [47]. The EEG data were filtered with a minimum high-pass cut-off frequency of 0.1 Hz [41], [48] and a maximum low-pass cut-off frequency of 80 Hz [49]. Despite the use of high-density electrode arrays, none of the studies herein reviewed relied on reconstructed EEG waveforms (estimated using EEG data recorded from the scalp) to determine cortical sources. All EEG channels from the peripheral and temporal sites were rejected a priori in [41]. The analysis of task-evoked N200/P300 components was limited to data collected from the fronto-central scalp sites (i.e., the three midline sites: FCz, Cz and CPz) in [47]. Also, the analysis was limited to data collected from Cz in [49] as the authors explored the coherence between EEG signals and EMG data collected from the tibialis anterior muscle. The coherence function can be looked upon as a measure of correlation between the frequency content of two signals. The authors used this function in an attempt to assess if the recruitment of muscle motor units and the modulation of their firing rate could be accounted for by the modulation of specific frequency components of the EEG data [49].
Static balance
EMG-based studies
Static balance was evaluated during upright stance and static conditions in 11 papers, with the aim of investigating the effect of aging on: 1) the activity of muscles controlling ankle movements [40], [50], [51] and potential differences in EMG activity in fallers vs. non-fallers [44], 2) the patterns of co-contraction of muscles controlling ankle movements [36], [57] in fallers vs. non-fallers [53], and 3) the control of balance during experiments carried out using a dual-task paradigm (either cognitive or manual extra load) [35], [37], [61]. Additionally, a new predictive data-driven model of the CNS mechanisms underlying postural control was developed and experimentally validated in [59].
Higher amplitude in the EMG activity of the muscles controlling ankle movements during quiet standing with eyes open and eyes closed was reported in the elderly compared to younger participants in [40], [50], [51]. The same findings marked the results in [44], where the authors investigated amplitude differences in plantar- vs. dorsi-flexor muscles in fallers vs. non-fallers, and observed a greater contribution to postural control of plantar-flexor muscles vs. dorsi-flexor muscles in the elderly compared to younger adults and in those with a history of falls compared to non-fallers.
Muscle co-contraction is the mechanism used to increase joint stiffness and is often associated with the performance of small postural adjustments during the performance of challenging motor tasks. Hence, the presence of co-contractions was looked upon by several researchers as a proxy for “postural instability”, as patterns of co-contraction lead to the loss of degrees of freedom that might be important in generating an appropriate response to an external perturbation. Indeed, higher co-contraction levels were observed in the elderly vs. younger adults when comparing the activity of individual muscles [50], [57] as well as when researchers investigated the characteristics of muscle synergies [35]. Moreover, in [53], the authors found a positive correlation between the level of co-contraction of the tibialis anterior and gastrocnemius muscles and fall reports, thus highlighting the potential use of metrics capturing the severity of co-contractions as proxy for fall risk. This finding is also supported by [44], where the authors observed an elevated level of muscle activity in the muscles controlling ankle movements in older adults with history of falls, which they suggested to be associated with greater postural sway. When studying postural control using a dual-task paradigm (i.e., holding a glass full of sand/water or performing a cognitive task) [37], a decrease in the activity of the tibialis anterior and gastrocnemius muscles was observed in older adults undergoing testing using a dual-task experimental paradigm (i.e., performing a cognitive task during quiet standing) when compared to the other conditions.
EEG- and EEG-EMG-based studies
In [45], the correlation between fear of falling - quantified using the movement specific reinvestment scale (MSRS) - and scalp EEG activity was investigated in the elderly. Results showed that higher MSRS scores (i.e., higher fear of falling) was associated with lower T3-Fz coherence.
In [41], EEG activity in the elderly and younger participants was recorded during postural tasks including quiet standing and standing on a swaying platform, as well as during dual-task experiments. Results showed: 1) an increase in delta activity (i.e., in the 1–4 Hz range) in both groups under challenging postural conditions, 2) a theta activity (i.e., in the 4–7 Hz range) response to an increase in cognitive requirements, and notably 3) an increase in gamma activity (i.e., in the 30–50 Hz range) in the elderly compared to younger participants.
In [48], not only EEG activity, but also EMG activity was recorded in the elderly and younger participants during quiet upright standing and after a perturbation induced by a platform translation or rotation. These experiments were carried out with eyes open and eyes closed. Gamma activity increased in the elderly in the eyes closed condition, whereas delta activity was higher in younger participants than in the elderly over the central and central parietal cortices. In the analysis of cortico-muscular coherence after balance perturbations, a higher coherence between C1 and the activity of the rectus femoris muscle was found in the elderly, whereas higher C1-tibialis anterior coherence was observed in younger participants. Post-perturbation potentials (PEP) were also analyzed showing that older participants displayed longer latency compared to younger adults. Interestingly, the delay in muscle activity onset in the elderly compared to younger adults ranged on average between 20 and 60 ms, the same delay observed between the groups for the N1 component of the PEP (i.e., the first EEG voltage deflection elicited by visual stimuli).
Dynamic balance
EMG-based studies
EMG recordings were used to investigate the effect of aging on muscle activity and postural control during dynamic balance tasks in 17 of the 28 papers considered in this review. This was sometimes done while asking subjects to simultaneously perform a cognitive task. In some studies, participants were asked to walk (either overground [27] or on a treadmill [25], [26], [46]) while doing arithmetic calculations [26], [27], [43]. In others, obstacles were positioned on the subjects’ walking path [46] or displayed in a virtual reality environment to challenge their balance [25]. The results of these studies demonstrated that an increase in cognitive load was significantly associated with an increase in the level of muscle co-contractions, as well as with motor and cognitive performance [27], with the latter not being affected by dual-tasks [26] and with no sizable effect on onset latencies and signal amplitude [43]. Apprehensive gait was marked by a greater level of activation of thigh muscles both in the elderly and in younger participants [46]. However, an increase in the level of co-contraction of agonist and antagonist muscles was observed only in the elderly population [27], [46]. An increase in muscle onset latency was observed in older adults in response to a perturbation when researchers analyzed the data of individual EMG channels [43] as well as when muscle synergies were examined [35], [56], [61] and when the activity of upper body muscles was studied [54]. Differences in motor performance were observed in terms of higher muscular activity for the elderly than their younger counterparts, especially when the activity of the hamstrings was examined [26] and the co-activation of agonist/antagonist muscles was considered [25]. Moreover, a greater level of co-contraction was observed in the elderly [27] when delivering visual stimuli [25].
In [36], [42], muscle activity in the elderly was monitored during a functional reaching test consisting of instructing subjects to maintain a fixed base of support while extending their arms as far forward as possible without moving their feet. In these conditions, the authors observed a temporal ordered muscle activation following a caudo-cranial pattern in the anterior muscles (i.e., tibialis anterior, rectus femoris, rectus abdominis, sternocleidomastoid), with subsequent activation of the posterior muscles (i.e., soleus, hamstring, erector spinae). The same activation pattern and two different balance strategies (hip and mixed) were observed during all the trials.
The impact of aging on muscle recruitment during the performance of demanding tasks, such as uphill and downhill walking, was investigated in [38]. Similar muscle activation patterns were observed when testing subjects while walking on a treadmill at different inclinations thus simulating uphill and downhill walking [38]. An increase in muscle activity level was observed in both the elderly and younger study participants with steeper inclinations, but a greater level of activity of the gluteus maximus and the gastrocnemius muscles was observed in the elderly group during uphill walking. Also, older adults exhibited greater muscle co-activation levels of leg muscles in all conditions. Higher muscle activation levels were generally observed in the elderly, with a significant correlation between age and level of muscle activation of trunk and lower limb muscles [52]. The same study highlighted a decrease in the activity of the rectus abdominis and gastrocnemius muscles during the stance phase of the gait cycle [52].
In [39], older adults with history of falls were trained on a slipping platform to identify the biomechanical and neuromuscular changes that such training induces, and to test for an improvement in balance control possibly associated with improvements in recovery reaction time. Results showed that the training group reduced the probability of falling by using a motor strategy marked by early activation of the hamstrings and tibialis anterior muscles and by a decrease in the level of muscle co-activation compared to the control group (which did not receive any training).
The effect of aging on muscle recruitment and activation patterns was also investigated and assessed via the analysis of muscle synergies based on data collected during step initiation and induced slip while walking [35], [61], and comparing the characteristics of muscle synergies in those who fell and those who recovered after the perturbation [56]. A less complex muscle response was observed in the group of those who maintained their balance, whereas a delayed activity onset (especially in the knee flexors/extensors of the slipping leg) and the recruitment of fewer muscle synergies was observed in participants who fell [56]. This finding was confirmed by [35], [61], which also highlighted that fallers displayed muscle synergies marked by more prominent levels of co-contraction of agonist/antagonist muscles, thus compromising their ability to respond to perturbations by exploiting “unused” degrees of freedom.
Despite the relevance of the vestibular system in maintaining balance control and the fact that it is part of the inner ear, the postural control of the head and upper body has not been extensively investigated. Specifically, the activity of muscles of the upper body was investigated to understand the mechanisms that support head stabilization during gait initiation [54] and during planned gait termination [55]. The activity of trunk muscles was analyzed during gait initiation, revealing a delayed onset of the activity of the sternocleidomastoid muscle paralleled by higher head angular displacement in the elderly. The delayed activation of muscles appeared to affect the ability to attenuate acceleration patterns originating from the trunk and transmitted to the head in the antero-posterior direction [54]. When examining gait termination instead, the sternocleidomastoid muscle activity appeared constant in both the elderly and younger adults in the first phase of gait termination, while it decreased during the end of the task only in the younger population, thus suggesting that a less stable mechanism of coordination between trunk and head movements would potentially lead to poor balance control [55].
EEG and EEG-EMG based studies
In [47], a cognitive dual-task (i.e., go-no-go task) during walking and a control task were performed by both the elderly and younger participants. Only the group of older adults showed a decrease in cognitive performance in the dual-task experimental condition compared to the control task. In addition, EEG activity was measured during both the dual-task and the control task conditions. Event related potentials (ERP) were calculated by identifying changes in N2 and P3 components. The N2 component, which displayed an earlier onset in younger participants compared to the elderly, showed a decrease in younger adults during the dual-task trials. However, no difference in this component was observed in older adults during the dual-task condition vs. the control condition. The group did not show any modulation in P3 latency, but exhibited a walking-related increase in P3 amplitude.
In [49], EEG and EMG activity was recorded during normal and visually-guided walking in the elderly and in younger participants to measure cortico-muscular and inter-muscular coherence. Results demonstrated that younger participants had higher cortico-muscular (considering EEG beta and gamma frequency bands) and inter-muscular coherence compared to the elderly. In fact, the magnitude of the coherence function increased in both groups during visually-guided walking.
Discussion
This review aimed to summarize current knowledge on the neuromuscular control of static and dynamic balance in the elderly. The paucity of studies investigating cortical activity showed that available knowledge concerning the CNS control of balance in healthy elderly is still insufficient [41], [45], [48]. This is not surprising, given that even in healthy young subjects the mechanisms underlying cortical control of postural stability are not fully understood [62], [63]. In light of the CNS changes induced by aging, the need for investigating its role in balance control in the elderly is of paramount importance.
Recent studies have demonstrated that EEG frequency rhythms, in particular in the delta and gamma EEG frequency bands, play a key role in maintaining posture in both static and dynamic tasks in the elderly (Fig. 3) [41], [45], [48]. Although the role of delta rhythm has not been fully understood from a neurophysiological point of view, an increase in the power spectral density function in the delta frequency range has been detected in data collected from older adults during dual-task experiments [41], [48]. It is possible that an elevated power in the delta frequency band might reflect an active cognitive task to help maintaining postural stability in the elderly. Beside the higher values in the delta frequency band, gamma rhythms in the frontal areas of the brain cortex appear to be involved in both cognitive processes and postural control, not only in the elderly but also in younger adults [64]. Perturbation-evoked cortical response in younger adults showed: 1) a scalp distribution of power in the delta, theta, alpha, and beta frequency range that increased at fronto-central midline electrode sites and 2) a significant synchronization of delta, theta, alpha, and beta activity [65]. To disentangle if cortical differences among healthy elderly and younger adults are due either to impairments in the balance control system (vestibular, musculoskeletal, visual) or to physiological changes that mark the aging brain, studies focusing on balance function in elderly fallers and non-fallers are needed to better understand postural control mechanisms in individuals prone to falls.
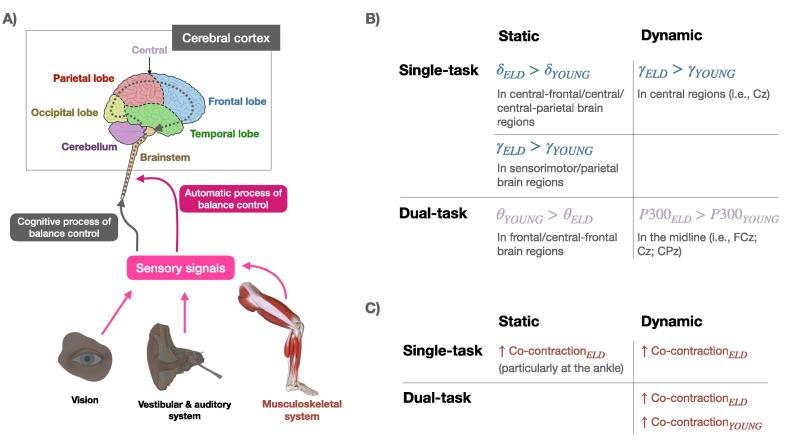
Fig. 3.
Summary: A) the network of balance control with and without the involvement of cognitive process of sensory signals; B) main observed differences in the cortical signals recorded from the elderly vs younger study participants – power bands are highlighted with the color of the relevant cortical area as in A); C) main observed differences on muscular activity between the elderly and younger study participants.
In healthy aging, structural [66] and functional [67] brain changes occur. As a result, the increased allocation of attentional resources to handle a challenging postural task may cause an increased recruitment of cortical areas. These different activation patterns highlight the increased cognitive load observed in older adults and point to the “frailty” of the system even in healthy aging. The interference of cognitive tasks with motor tasks is confirmed by data from the few EEG studies we identified in this review, which reported an increase during balance tasks in EEG band primarily associated with cognition (i.e., gamma) in the elderly [41], [48]. EEG findings are paralleled by EMG results showing delayed motor activations, a behavior consistent with the observation that cortical potentials in the elderly appear with a longer time lag compared to younger individuals. The identification of these EEG characteristics (e.g., power spectral changes in different frequency bands) in association with the performance of challenging postural control tasks suggests their potential use to achieve: 1) early detection of postural instability [63], 2) tracking functional recovery during rehabilitation, and 3) the development of new rehabilitative treatments tailored according to the physiological patterns observed on a subject-by-subject basis (Table 4). To accomplish these three goals, further interventional studies are needed to relate changes in postural behavior and cortical electrophysiology data recorded after the intervention.
Table 4.
Discussion Summary.
| Tool | Information provided | Possible translational implication |
|---|---|---|
| EEG | Balance cortical correlates | Early detectors of instability; Trackers of recovery; Drivers of new rehabilitative treatments |
| EMG | Balance control strategy Muscle synergies |
Targeted rehabilitation programs; Non-voluntary movement control restoration |
The analysis of muscle activity patterns has allowed researchers to study balance control strategies in healthy aging. Longer EMG latencies, higher amplitude levels, and more prominent muscle co-contractions were observed in the elderly compared to younger participants in the majority of the studies examined in this review [25], [35], [38], [46], [50], [57]. An increase in the activation of antagonist muscles (associated with an increase in the energy cost of maintaining balance) has been suggested to be part of physiological aging [68]. Of interest, challenging motor tasks in the elderly produces a further increase in the co-contraction levels [25], [38], which is most likely due to older adults perceiving static and dynamic tasks as more demanding than their younger counterparts. Conversely, during dual-task tests (either cognitive or based on motor tasks not strictly associated with balance - e.g., holding a glass of water [37]), younger adults increase the level of co-contraction, whereas the elderly display a deterioration in their motor performance, with what appears to be a decrease in muscle activity aimed to favor achieving a cognitive task [26], [41], [57]. Muscular activity in the elderly was higher than in younger adults, even under dual-task conditions [37]. Moreover, muscle synergies appear to be altered in the elderly and in a more prominent way in individuals reporting frequent falls. This knowledge based on EMG analyses may guide rehabilitative interventions, considering the age of the subject, both by: 1) addressing joint control and motor strategies that is important to focus on and 2) selecting the muscles to be targeted by strengthening interventions (Table 4).
When EEG and EMG data were simultaneously recorded, analyses focused on cortico-muscular coherence [48], which is generally reduced in the elderly, highlighted the decreased reactivity of the muscular and nervous systems with advancing age.
Overall, standardized protocols are needed to produce reliable and reproducible results that enable comparisons across studies. Future research would need to: 1) assess and include cognitive performance capacity of study participants as a covariate in the proposed statistical analyses, 2) use shared and validated protocols for muscle electrode positioning and analysis of EMG activity, 3) apply shared EEG signal analysis protocols, and 4) use high spatial resolution EEG systems to estimate EEG source waveform activity. Appendix A and B contains further details/discussion on papers limitations.
A potential limitation of our literature review is the lack of systematic sub-grouping of the elderly to account for some of the pathologies that affect this population. Our decision of including only studies on healthy participants and thus without neurological, cardiovascular, vestibular, musculoskeletal or any other systemic disorders was driven by our aim to study the effects of healthy aging on balance control and not the effects of specific pathologies.
Conclusions
The present review on muscular and cortical control during static and dynamic balance highlights the need to further investigate the CNS many contributions to assure postural stability. This holds particularly true when this topic is investigated in the elderly, a population at high risk of falls due to balance impairments. Despite being interesting and promising, the results of the studies herein reviewed leave significant gaps in our understanding of balance control in the elderly. This review highlights the urgent need to share protocols and data analysis pipelines to tackle a pressing demand in our ageing societies: to understand the neurophysiological basis of postural control and thus develop preventive and rehabilitative measures for the elderly that are consistent with the mechanisms underlying balance control impairments in this population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Italian Ministry for Foreign Affairs under the call “Progetti di Grande Rilevanza Internazionale” (PGR-01045 - SoftAct).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbas.2021.100013.
Contributor Information
Maria Rubega, Email: maria.rubega@unipd.it.
Roberto Di Marco, Email: roberto.dimarco@unipd.it.
Marianna Zampini, Email: marianna.zampini@studenti.unipd.it.
Emanuela Formaggio, Email: emanuela.formaggio@unipd.it.
Emanuele Menegatti, Email: emanuele.menegatti@unipd.it.
Paolo Bonato, Email: pbonato@mgh.harvard.edu.
Stefano Masiero, Email: stef.masiero@unipd.it.
Alessandra Del Felice, Email: alessandra.delfelice@unipd.it.
Appendix A. . Limitations of EMG-based studies
Only five of the studies herein reviewed [25], [42], [50], [52], [59], which reported EMG data, utilized the SENIAM guidelines to assure reproducibility of electrode placement [58]. Different studies showed significant heterogeneity in the selection of muscles to record EMG data during dynamic balance tests with no clear rationale supporting their choice (Fig. 2d), except for upright stance studies where authors recorded the activity of muscles controlling ankle movements based on the hypothesis of a prevalent ankle strategy to maintain balance, specifically, in response to perturbations.
Appendix B. . Limitations of EEG-based studies
In the five papers where EEG data were collected [41], [45], [47], [48], [49]; despite in four out of five studies, a high-density EEG set-up was exploited in data recordings, the processing was limited to a small sub-set of EEG channels. This limitation in the reported spatial resolution of the EEG results may be due to challenges encountered by researchers as they attempted to remove motion artifacts such as those associated with head movements during locomotion. In the last decades, many effective methods have been suggested, e.g., based on blind source separation [69] or adaptive filtering [70], but their computational complexity may not be suitable for online applications. To achieve real-time removal of non-cyclical movement artifacts, many trials are needed for the creation of an appropriate set of artifact templates and movement-related kinematic signals [16]. Contrary to prior claims [71], recent findings accurately quantify the extent to which head motion-related artifacts contaminate the EEG signal, showing that they are not as dominant relative to the actual cortical signal recorded by the EEG at slow to moderate walking speeds [72].
The work of Whitham et al. (2007) [73], showing that the main part of the EEG gamma signal disappears with temporary muscle paralysis, has challenged the assumption that EEG gamma bands could be recorded. A novel approach [74] has been proposed to reduce the effect of scalp and neck EMG, observing that scalp and neck muscle spikes had specific waveforms in the time domain which allowed researchers to separate them from the EEG gamma rhythms.
Appendix C. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brown RH, Ropper AH, Adams RD, Victor M. Adams and Victor’s principles of neurology, McGraw-Hill Companies, Inc, 2014.
- 2.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention, Age Ageing. 35 (2006) ii37--ii41. [DOI] [PubMed]
- 3.Mathers C.D., Stevens G.A., Boerma T., White R.A., Tobias M.I. Causes of international increases in older age life expectancy. The Lancet. 2015;385(9967):540–548. doi: 10.1016/S0140-6736(14)60569-9. [DOI] [PubMed] [Google Scholar]
- 4.Maurer C., Mergner T., Peterka R.J. Multisensory control of human upright stance. Exp Brain Res. 2006;171:231. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- 5.Winter D.A., Patla A.E., Frank J.S. Assessment of balance control in humans. Med Prog Technol. 1990;16:31–51. [PubMed] [Google Scholar]
- 6.Whitney S.L., Roche J.L., Marchetti G.F., Lin C.-C., Steed D.P., Furman G.R., et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait & Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siragy T., Nantel J. Quantifying dynamic balance in young, elderly and Parkinson’s individuals: a systematic review. Front Aging Neurosci. 2018;10:387. doi: 10.3389/fnagi.2018.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.W.H.O. (WHO), Falls, (n.d.). https://www.who.int/news-room/fact-sheets/detail/falls.
- 9.Annese VF, De Venuto D. Gait Analysis for Fall Prediction using EMG Triggered Movement Related Potentials, in: 2015 10TH IEEE Int. Conf. Des. Technol. Integr. Syst. NANOSCALE ERA, 2015.
- 10.Li L., Baum B.S. Electromechanical delay estimated by using electromyography during cycling at different pedaling frequencies. J Electromyogr Kinesiol. 2004;14(6):647–652. doi: 10.1016/j.jelekin.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.M.G. Benedetti, V. Agostini, M. Knaflitz, P. Bonato, Muscle activation patterns during level walking and stair ambulation, in: Appl. EMG Clin. Sport. Med., 2012: pp. 117–130.
- 12.Blanc Y., Dimanico U. Electrode placement in surface electromyography (sEMG) “Minimal Crosstalk Area” (MCA) Open Rehabil. 2010;3(1):110–126. [Google Scholar]
- 13.Merlo A., Campanini I. John Wiley & Sons; 2016. Surface electromyography: physiology, engineering, and applications. [Google Scholar]
- 14.Campanini I., Disselhorst-Klug C., Rymer W.Z., Merletti R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front Neurol. 2020;11:1–22. doi: 10.3389/fneur.2020.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratti E., Waninger S., Berka C., Ruffini G., Verma A. Comparison of medical and consumer wireless EEG systems for use in clinical trials. Front Hum Neurosci. 2017;11:1–7. doi: 10.3389/fnhum.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwin J.T., Gramann K., Makeig S., Ferris D.P. Removal of Movement Artifact From High-Density EEG Recorded During Walking and Running. J Neurophysiol. 2010;103(6):3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwin J.T., Gramann K., Makeig S., Ferris D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage. 2011;54(2):1289–1296. doi: 10.1016/j.neuroimage.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 18.Wagner J., Martinez-Cancino R., Delorme A., Makeig S., Solis-Escalante T., Neuper C., et al. High-density EEG mobile brain/body imaging data recorded during a challenging auditory gait pacing task. Sci Data. 2019;6(1) doi: 10.1038/s41597-019-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J.E. Apraxia: Review and Update. J Clin Neurol. 2017;13(4):317. doi: 10.3988/jcn.2017.13.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonnekes J, Giladi N, Guha A, Fietzek UM, Bloem BR, R\uužička E. Gait festination in parkinsonism: introduction of two phenotypes, J. Neurol. 266 (2019) 426–430. [DOI] [PMC free article] [PubMed]
- 21.Aprigliano F., Martelli D., Kang J., Kuo S.-H., Kang U.J., Monaco V., et al. Effects of repeated waist-pull perturbations on gait stability in subjects with cerebellar ataxia. J NeuroEng Rehabil. 2019;16:50. doi: 10.1186/s12984-019-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molteni F., Formaggio E., Bosco A., Guanziroli E., Piccione F., Masiero S., Del Felice A. Brain Connectivity Modulation After Exoskeleton-Assisted Gait in Chronic Hemiplegic Stroke Survivors: A Pilot Study. Am J Phys Med Rehabil. 2020;99(8):694–700. doi: 10.1097/PHM.0000000000001395. [DOI] [PubMed] [Google Scholar]
- 23.Lennon O., Tonellato M., Del Felice A., Di Marco R., Fingleton C., Korik A., et al. A Systematic Review Establishing the Current State-of-the-Art, the Limitations, and the DESIRED Checklist in Studies of Direct Neural Interfacing With Robotic Gait Devices in Stroke Rehabilitation. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formaggio E., Masiero S., Bosco A., Izzi F., Piccione F., Del Felice A. Quantitative EEG Evaluation During Robot-Assisted Foot Movement. IEEE Trans Neural Syst Rehabil Eng. 2017;25(9):1633–1640. doi: 10.1109/TNSRE.2016.2627058. [DOI] [PubMed] [Google Scholar]
- 25.Acuña S.A., Francis C.A., Franz J.R., Thelen D.G. The effects of cognitive load and optical flow on antagonist leg muscle coactivation during walking for young and older adults. J Electromyogr Kinesiol. 2019;44:8–14. doi: 10.1016/j.jelekin.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li K.Z.H., Abbud G.A., Fraser S.A., DeMont R.G. Successful adaptation of gait in healthy older adults during dual-task treadmill walking. Aging, Neuropsychol Cogni. 2012;19(1-2):150–167. doi: 10.1080/13825585.2011.628375. [DOI] [PubMed] [Google Scholar]
- 27.Lo J., Lo O.-Y., Olson E.A., Habtemariam D., Iloputaife I., Gagnon M.M., Manor B., Lipsitz L.A. Functional implications of muscle co-contraction during gait in advanced age. Gait & Posture. 2017;53:110–114. doi: 10.1016/j.gaitpost.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merletti R., Farina D. John Wiley & Sons; 2016. Surface electromyography: physiology, engineering, and applications. [Google Scholar]
- 29.Wittenberg E., Thompson J., Nam C.S., Franz J.R. Neuroimaging of human balance control: A systematic review. Front Hum Neurosci. 2017;11:1–25. doi: 10.3389/fnhum.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. others, Linee guida per il reporting di revisioni sistematiche e meta-analisi: il PRISMA Statement. PLoS Med. 2009;6 [Google Scholar]
- 31.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Method. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storm F.A., Cesareo A., Reni G., Biffi E. Wearable Inertial Sensors to Assess Gait during the 6-Minute Walk Test: A Systematic Review. Sensors. 2020;20:2660. doi: 10.3390/s20092660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taborri J., Agostini V., Artemiadis P.K., Ghislieri M., Jacobs D.A., Roh J., Rossi S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl Bionics Biomech. 2018;2018:1–19. doi: 10.1155/2018/3934698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardillo G. Compute the Fleiss’ kappa, (n.d.). https://github.com/dnafinder/Fleiss.
- 35.Wang Y., Watanabe K., Asaka T. Aging effect on muscle synergies in stepping forth during a forward perturbation. Eur J Appl Physiol. 2017;117(1):201–211. doi: 10.1007/s00421-016-3514-8. [DOI] [PubMed] [Google Scholar]
- 36.Nagai K., Yamada M., Uemura K., Tanaka B., Mori S., Yamada Y., et al. Effects of fear of falling on muscular coactivation during walking. Aging Clin. Exp. Res. 2012;24:157–161. doi: 10.3275/7716. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84864500317&partnerID=40&md5=41dabf4251de43b77b863d068ea216eb [DOI] [PubMed] [Google Scholar]
- 37.Makizako H., Furuna T., Ihira H., Shimada H. Age-related differences in the influence of cognitive task performance on postural control under unstable balance conditions. Int J Gerontol. 2013;7(4):199–204. [Google Scholar]
- 38.Franz J.R., Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture. 2013;37(3):378–384. doi: 10.1016/j.gaitpost.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parijat P., Lockhart T.E. Effects of moveable platform training in preventing slip-induced falls in older adults. Ann Biomed Eng. 2012;40(5):1111–1121. doi: 10.1007/s10439-011-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baudry S., Lecoeuvre G., Duchateau J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. J Appl Physiol. 2012;112(2):296–304. doi: 10.1152/japplphysiol.00913.2011. [DOI] [PubMed] [Google Scholar]
- 41.Ozdemir R.A., Contreras-Vidal J.L., Lee B.-C., Paloski W.H. Cortical activity modulations underlying age-related performance differences during posture–cognition dual tasking. Exp Brain Res. 2016;234(11):3321–3334. doi: 10.1007/s00221-016-4730-5. [DOI] [PubMed] [Google Scholar]
- 42.Maranesi E., Fioretti S., Ghetti G.G., Rabini R.A., Burattini L., Mercante O., Di Nardo F. The surface electromyographic evaluation of the Functional Reach in elderly subjects. J Electromyogr Kinesiol. 2016;26:102–110. doi: 10.1016/j.jelekin.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Laing J.M., Tokuno C.D. The effects of dual-tasking on arm muscle responses in young and older adults. Hum Mov Sci. 2016;46:159–166. doi: 10.1016/j.humov.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Cattagni T., Scaglioni G., Laroche D., Gremeaux V., Martin A. The involvement of ankle muscles in maintaining balance in the upright posture is higher in elderly fallers. Exp Gerontol. 2016;77:38–45. doi: 10.1016/j.exger.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Chan D.C.L., Wong T.W.L., Zhu F.F., Cheng Lam C., Young W.R., Capio C.M., Masters R.S.W. Investigating Changes in Real-time Conscious Postural Processing by Older Adults during Different Stance Positions Using Electroencephalography Coherence. Exp Aging Res. 2019;45(5):410–423. doi: 10.1080/0361073X.2019.1664450. [DOI] [PubMed] [Google Scholar]
- 46.Hallal C.Z., Marques N.R., Spinoso D.H., Vieira E.R., Gonçalves M. Electromyographic patterns of lower limb muscles during apprehensive gait in younger and older female adults. J Electromyogr Kinesiol. 2013;23(5):1145–1149. doi: 10.1016/j.jelekin.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Malcolm B.R., Foxe J.J., Butler J.S., De Sanctis P. The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: A mobile brain/body imaging (MoBI) study. NeuroImage. 2015;117:230–242. doi: 10.1016/j.neuroimage.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozdemir R.A., Contreras-Vidal J.L., Paloski W.H. Cortical control of upright stance in elderly. Mech Ageing Dev. 2018;169:19–31. doi: 10.1016/j.mad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Spedden M.E., Choi J.T., Nielsen J.B., Geertsen S.S. Corticospinal control of normal and visually guided gait in healthy older and younger adults. Neurobiol Aging. 2019;78:29–41. doi: 10.1016/j.neurobiolaging.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 50.M. Blaszczyszyn, A. Szczesna, K. Piechota, sEMG Activation of the Flexor Muscles in the Foot during Balance Tasks by Young and Older Women: A Pilot Study., Int. J. Environ. Res. Public Health. 16 (2019). 0-0. [DOI] [PMC free article] [PubMed]
- 51.dos Anjos F.V., Pinto T.P., Gazzoni M., Vieira T.M. The Spatial Distribution of Ankle Muscles Activity Discriminates Aged from Young Subjects during Standing. Front Hum Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.H.-J. Lee, W.H. Chang, S.H. Hwang, B.-O. Choi, G.-H. Ryu, Y.-H. Kim, Age-related locomotion characteristics in association with balance function in young, middle-aged, and older adults, J. Aging Phys. Act. 25 (2017) 247–253. [DOI] [PubMed]
- 53.Nelson-Wong E., Appell R., McKay M., Nawaz H., Roth J., Sigler R., Third J., Walker M. Increased fall risk is associated with elevated co-contraction about the ankle during static balance challenges in older adults. Eur J Appl Physiol. 2012;112(4):1379–1389. doi: 10.1007/s00421-011-2094-x. [DOI] [PubMed] [Google Scholar]
- 54.Maslivec A., Bampouras T.M., Dewhurst S., Vannozzi G., Macaluso A., Laudani L. Mechanisms of head stability during gait initiation in young and older women: A neuro-mechanical analysis. J Electromyogr Kinesiol. 2018;38:103–110. doi: 10.1016/j.jelekin.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Rum L., Laudani L., Vannozzi G., Macaluso A. Age-related changes in upper body contribution to braking forward locomotion in women. Gait & Posture. 2019;68:81–87. doi: 10.1016/j.gaitpost.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Sawers A., Pai Y.-C., Bhatt T., Ting L.H. Neuromuscular responses differ between slip-induced falls and recoveries in older adults. J Neurophysiol. 2017;117(2):509–522. doi: 10.1152/jn.00699.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwamoto Y., Takahashi M., Shinkoda K. Differences of muscle co-contraction of the ankle joint between young and elderly adults during dynamic postural control at different speeds. J Physiol Anthropol. 2017;36(1) doi: 10.1186/s40101-017-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merletti R. Surface electromyography: The SENIAM project. Eur. J. Phys. Rehabil. Med. 2000;36:167. [Google Scholar]
- 59.Jafari H, Pauelsen M, Röijezon U, Nyberg L, Nikolakopoulos G, Gustafsson T. On Internal Modeling of the Upright Postural Control in Elderly, in: 2018 IEEE Int. Conf. Robot. Biomimetics, 2018: pp. 231–236.
- 60.Falconer K., Winter D.A. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol. 1985;25:135–149. [PubMed] [Google Scholar]
- 61.Wang Y., Watanabe K., Asaka T. Muscle synergies in preparation to a step made with obstacle in elderly individuals. J NeuroEngineering Rehabil. 2015;12(1):10. doi: 10.1186/s12984-015-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson S.M., Ferris D.P. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. NeuroImage. 2019;198:93–103. doi: 10.1016/j.neuroimage.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slobounov S., Hallett M., Stanhope S., Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol. 2005;116(2):315–323. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Slobounov S., Cao C., Jaiswal N., Newell K.M. Neural basis of postural instability identified by VTC and EEG. Exp Brain Res. 2009;199(1):1–16. doi: 10.1007/s00221-009-1956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varghese J.P., Marlin A., B. Beyer K., Staines W.R., Mochizuki G., McIlroy W.E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci Lett. 2014;578:33–38. doi: 10.1016/j.neulet.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 66.Pettigrew C., Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep. 2019;19:1. doi: 10.1007/s11910-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boisgontier M.P., Nougier V. Ageing of internal models: from a continuous to an intermittent proprioceptive control of movement. Age (Omaha). 2013;35:1339–1355. doi: 10.1007/s11357-012-9436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hortobágyi T., Solnik S., Gruber A., Rider P., Steinweg K., Helseth J., DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait & Posture. 2009;29(4):558–564. doi: 10.1016/j.gaitpost.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 69.James C.J., Hesse C.W. Independent component analysis for biomedical signals. Physiol Meas. 2005;26(1):R15–R39. doi: 10.1088/0967-3334/26/1/r02. [DOI] [PubMed] [Google Scholar]
- 70.Sweeney K.T., Ward T.E., McLoone S.F. Artifact Removal in Physiological Signals—Practices and Possibilities. IEEE Trans. Inform. Technol. Biomed. 2012;16(3):488–500. doi: 10.1109/TITB.2012.2188536. [DOI] [PubMed] [Google Scholar]
- 71.Kline J.E., Huang H.J., Snyder K.L., Ferris D.P. Isolating gait-related movement artifacts in electroencephalography during human walking. J Neural Eng. 2015;12(4):046022. doi: 10.1088/1741-2560/12/4/046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nathan K., Contreras-Vidal J.L. Negligible motion artifacts in scalp electroencephalography (EEG) during treadmill walking. Front Hum Neurosci. 2016;9:1–12. doi: 10.3389/fnhum.2015.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitham E.M., Pope K.J., Fitzgibbon S.P., Lewis T., Clark C.R., Loveless S., Broberg M., Wallace A., DeLosAngeles D., Lillie P., Hardy A., Fronsko R., Pulbrook A., Willoughby J.O. Scalp electrical recording during paralysis: Quantitative evidence that EEG frequencies above 20Hz are contaminated by EMG. Clin Neurophysiol. 2007;118(8):1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 74.Nottage J.F., Morrison P.D., Williams S.C.R., ffytche D.H. A Novel Method for Reducing the Effect of Tonic Muscle Activity on the Gamma Band of the Scalp EEG. Brain Topogr. 2013;26(1):50–61. doi: 10.1007/s10548-012-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.