Abstract
We explored the effects of parietal damage on inhibitory effects of visuospatial attention, inhibition of return (IOR) and inhibitory tagging (IT), in the vertical meridian. We combined a vertical spatial cue paradigm with a Stroop task employing three different temporal intervals between the spatial cue and the target (700, 1200 and 2000 ms) in two groups of patients, one with damage to the parietal cortex and underlying white matter (the parietal patients group) and the other with damage in other brain areas not including the parietal lobe (the control patient group), and a healthy control group. Healthy controls showed the expected inhibitory effects, IOR at the 700 and 1200 intervals and IT at the 1200 interval (as evidenced in a reduction in the magnitude of Stroop interference at the cued location). On the other hand, only the group of parietal patients showed delayed onset of inhibitory effects, IOR and IT appeared at the 1200 ms and 2000 ms intervals, respectively. These findings provide evidence for a role of the parietal cortex, and the underlying fibre tracts, in inhibitory processing in the vertical meridian, with damage to the parietal cortex altering the time course of attention-dependent inhibition.
Keywords: Parietal lobe, Brain damage, Inhibition of return, Inhibitory tagging, Attention
1. Introduction
Inhibition of return (IOR) is the term used to refer to the slowing and/or reduced accuracy of responses to targets located at previously cued locations relative to targets presented at new (uncued) locations [[41], [42]]. IOR effects are usually observed in spatial orienting tasks where uninformative spatial cues are employed to attract attention automatically (exogenous orienting) to a location, and the cue-target intervals are greater than 250 ms [31]. Although this effect was first attributed to the inhibition of attentional (re)orienting to a previously attended location, in the last years alternative accounts have been put forward. These accounts include the idea that IOR reflects object-file integration [37], repetition suppression [51], or repetition habituation [18]. However, there is not a consensus as to which accounts are able to explain most empirical data (see [19], for a survey of IOR experts). Regarding the neural basis of the effect, IOR was first attributed to the integrity of the superior colliculus [[3], [42], [49]], but later studies have shown that collicular involvement might depend on the activation of higher brain regions [[16], [25]]. In addition, other cortical areas such as the intra-parietal sulcus [57], the temporoparietal junction of the right hemisphere [12], and the frontal eye fields [47], have also been associated with IOR in different tasks.
Previous studies with brain-damaged patients have provided evidence for the role of the parietal cortex in IOR in the horizontal meridian [[1], [54], [55]]. For instance, Vivas et al. [54] found typical IOR effects in parietal patients with unilateral damage either to the right or left hemisphere when targets were presented in the contralesional visual field. However, they did not find significant IOR effects in these patients when the target appeared in the ipsilesional visual field. The authors argued that unilateral parietal damage might result in an imbalance in the relative salience of locations in a spatial map implemented by the posterior parietal cortex, which signals salience of locations for attention. The relative increase in the salience of the ipsilesional signals in this spatial map may be sufficient to overrule any IOR applied to ipsilesional locations, in parietal patients [54]. Studies that have employed repetitive transcranial magnetic stimulation (rTMS) over the right or left parietal lobe support hemispheric differences in the control of visual attention in space. In line with the finding of higher frequency and greater severity of spatial neglect following right-hemisphere damage, right-parietal rTMS induces rightward, but left-parietal stimulation may fail to produce a similar leftward bias [[6], [7], [20]]. Although left-brain damage is less likely to produce severe neglect symptoms, it does result in inhibitory deficits of visuospatial attention [[54], [55], [36]].
This account of the effects of parietal damage stresses the role of the parietal lobe in lateralised shifting of attention. Consequently, we may expect effects of parietal damage on IOR to be reduced when stimuli are presented along the vertical meridian, where there is no need for lateralized shift of attention. In line with this hypothesis, Vivas et al. [55]; Experiment 1) found typical IOR effects in parietal patients (similar to those found in the healthy controls) with a vertical IOR procedure where cues and targets were presented in the top or bottom locations along a central vertical meridian. However, given that Vivas et al. [55] used a simple detection task and just one cue-target stimulus onset asynchrony (SOA) of 660 ms, the conclusion of spared IOR along the vertical meridian in parietal patients might be premature. Although the attentional representation of locations along the vertical axis has been less studied [27], it has been suggested that attention may be biased towards the upper visual field, and that the parietal lobe may play a role in directing attention to the lower visual field [44]. This asymmetry between the upper and lower visual fields has been documented mainly when directing attention has been manipulated both in an implicit way through peripheral cueing [[8], [56]] or saccades [44] and an explicit way through endogenous cueing [45]. In addition, deficits in inhibitory effects in the vertical line, as a result of parietal damage, may only be observed in more complex tasks (see [56], with moving objects).
Ongoing processing of stimuli presented at cued locations is also affected in an IOR paradigm. For instance, Fuentes et al. [23] reported inhibitory tagging (IT) following IOR. They combined a double-cue (cue-back) IOR procedure with semantic priming and flanker tasks in order to investigate how the processing of information was affected at the cued location. They found a striking reversal of both semantic priming and flanker interference at the location subject to IOR (see also [53]). For stimuli presented at the uncued location there were typical positive semantic and flanker interference effects; in contrast, for stimuli appearing at the cued location, semantically related stimuli yielded longer response times than semantically unrelated stimuli, while congruent flankers produced longer RTs than incongruent ones. In order to account for these findings, Fuentes and collaborators proposed that IOR generates an inhibitory tagging (IT) of stimuli presented at the inhibited location. The inhibitory tag disrupts responses to stimuli that are semantically related and/or congruent with the inhibited item (see [52], for evidence on Stroop interference). Fuentes and co-workers proposed that IOR and IT are two distinct, dissociable, inhibitory effects that cooperate to favour attentional allocation to novel unexplored locations [[21], [24]].
The hypothesis that the two inhibitory effects of IOR and IT are dissociable is supported by neuroimaging research [11] and neuropsychological studies with brain damaged [54] and psychiatric patients [22]. Specifically, Vivas and colleagues [[54], [55]] suggested that the parietal lobe, which is responsible for IOR, subsequently translates the attention/oculomotor bias into a signal to areas of the frontal cortex implicated in response selection. Research evidence suggests that frontal brain areas, and most likely the left dorsolateral prefrontal cortex (lDLPFC), generate IT. In agreement with this hypothesis, Vivas et al. [54] found IT, as evidenced by a reduced Stroop effect at the cued location, in unilateral parietal patients when stimuli where presented in the contralesional visual field but not in the ipsilesional visual field. The authors concluded that IOR is a necessary but not sufficient condition to observe IT. This conclusion was further supported by Chen et al. [11], who found activation in the lDLPFC related to IT in a Stroop-IOR task (see also [58], for similar evidence with a EEG source analysis methodology), and Martínez-Pérez et al. [39] with a transcranial direct current stimulation protocol in an affective priming paradigm.
In the present study we asked whether parietal damage may result in altered IOR and IT along the vertical axis in a complex discrimination task. Following Vivas et al. [55], we expect that IOR onset may be delayed rather than disrupted in patients with damage to the parietal cortex and underlying white matter. Furthermore, given the reliance of IT on IOR it may be that a delayed onset of IOR has a knock-on effect on the emergence of IT. That is, the expected reduction of Stroop interference at cued locations relative to uncued locations may be delayed (or not observed) in patients with parietal lesions.
2. Methods
2.1. Participants
Twenty-seven participants were recruited for the study. We examined ten patients with lesions affecting the parietal lobe (Mean age = 66.3 years). Seven of the parietal patients had unilateral lesions and three had bilateral lesions but they presented with behavioural deficits that were greater on one side of space (e.g., under conditions of visual extinction, with two stimuli on opposite sides of the vertical meridian). Six patients presented with some aspect of spatial neglect and four with extinction as clinically assessed using the BCoS battery [5]. The lesion analysis showed maximal overlap in the parietal white matter, superior longitudinal fasciculus, thalamic radiation, and in the inferior parietal lobe (see Fig. 1 for lesion overlap maps representing the spatial distribution of lesions across the parietal patients included in the current study). Please note that as good quality MRI scans were not available for one patient, his lesion map was not included in the overlap. Seven patients with lesions affecting several brain areas except the parietal lobe (ranged in age from 40 to 78 years; mean age = 63,7 years) served as the matched patient control group (hereafter PC). Four of the patients control group had unilateral lesions (three affecting the left hemisphere and one the right hemisphere) and three had bilateral lesions (see Table 1).
Fig. 1.
(a) Parietal Patients lesion overlap maps; (b) Control Patients lesion overlap maps.
Table 1.
Age, gender, aetiology, brain lesion location of the patients, Apple Cancelation and Visual Extinction Tests scores (from the Birmingham Cognitive Screen test battery) and Neglect/Extinction diagnosis. a Marked degeneration of the parietal lobes; b no MRI scan.
| Patient | Group | Age/Gender | Aetiology | Location | Apple Test Spatial/Object | Neglect diagnosis | Extinction Test | Extinction diagnosis |
|---|---|---|---|---|---|---|---|---|
| TM | Parietal | 73/male | Stroke | Right parietal | 12/4 | Left spatial and left object | 3 | Left visual extinction |
| RP | Parietal | 54/male | Stroke | Right parietal | 0/9 | Left object | 4 | Left visual extinction |
| MH | Parietal | 56/male | Anoxia | Left parietal | 0/-4 | Right object | 0 | No |
| PM | Parietal | 68/female | Stroke | Bilateral parietal | 0/0 | No | 0 | No |
| PJ | Parietal | 70/male | Stroke | Left parietal | 0/0 | No | 0 | No |
| PF | Parietal | 60/female | Stroke | Bilateral parietal | 0/7 | Left object | 0 | No |
| DB | Parietal | 74/male | Stroke | Left parietalb | 0/0 | No | 0 | No |
| JF | Parietal | 65/male | Dementia, Alzheimera | Bilateral parietal | 0/0 | No | 0 | No |
| RH | Parietal | 76/male | Stroke | Left parietal | 0/-8 | Right object | −4 | Right visual extinction |
| SB | Parietal | 67/male | Stroke | Right parietal | 15/3 | Left spatial and left object | 3 | Left visual extinction |
| GA | Control | 54/male | Herpes simplex encephalitis | Bilateral temporal and right frontal | 0/0 | No | 0 | No |
| PW | Control | 78/male | Stroke | Right fronto-temporal | 0/0 | No | 0 | No |
| AS | Control | 73/male | Stroke | Bilateral fronto-temporal | 2/0 | No | 0 | No |
| DS | Control | 76/male | Stroke | Left frontal |
0/0 | No | 0 | No |
| FK | Control | 40/male | Anoxia | Bilateral temporal | 0/0 | No | 0 | No |
| JoQ | Control | 63/male | Stroke | Right fronto-temporal | 0/0 | No | 0 | No |
| MalcH | Control | 62/male | Stroke | Left fronto-temporal | 0/0 | No | 0 | No |
Lesion maps for all patients were reconstructed using an outlier detection algorithm based on fuzzy clustering (for the full protocol and validation of the method see [[10], [50]]. Briefly, this procedure first identifies grey and white matter voxels that are different in the damaged brain as compared to a set of healthy controls (here we used for comparison a set of scans from 100 healthy controls, 55 males and 45 females, age range 20–87 years; see [10]. These outlier voxels are then combined into a single outlier image and thresholded to generate a binary map of the patient’s lesion [50]. To illustrate overlap within parietal regions in the group of patients studied here, the lesion maps from patients with unilateral left hemisphere lesions were flipped to the right side of the brain. The lesion overlap map is shown for nine axial slices in standard MNI space with the MNI Z-coordinates specified for the axial sections. The colour bar shows the number of patients with a lesion within a particular voxel (range 1–9). All patients were considered as chronic (more than nine months had lapsed after brain lesion).
Ten native English speakers served as the matched healthy control group (hereafter HC). These individuals ranged in age from 52 to 76 years, with a mean of 64.5 years. Both patient groups and the healthy control group had at least 12 years of education. They all had normal or corrected-to-normal vision in terms of acuity. All participants gave written informed consent for participation in the study. The ethic committee for Life Science at the University of Birmingham (UK) approved the study, which was carried out in accordance with the Declaration of Helsinki.
2.2. Stimuli
The stimuli were displayed on a 15.4-inch colour monitor (refresh rate of 60 Hz) controlled by a laptop computer. The experimental task was created using E-Prime, Version 1.1 (Psychology Software Tools, Pittsburgh, PA). The stimuli were presented against a black background in three white unfilled boxes arranged vertically in the centre of the screen. The stimuli consisted of a string of four Xs, and the colour words RED and GREEN. At a viewing distance of 60 cm each character was 0.48 high and 0.38 wide degrees of visual angle. The distance between the peripheral box and the fixation point was 4.30 degrees of visual angle. Responses were given through the computer’s keyboard.
2.3. Procedure
Each trial started with three boxes, one in the centre of the screen and one above and below (see Fig. 2). The three boxes remained on the screen for 1000 ms. Thereafter one of the peripheral boxes became thicker for 150 ms (the spatial cue). After this, the three boxes and the fixation remained on the screen for a further interval of 200 ms. Subsequently, the central box became thicker for 150 ms (the central cue), and after a further interval of 200, 700 or 1500 ms depending on the SOA condition (700, 1200, and 2000 ms), the target appeared inside the top or bottom box. The target consisted of a word in upper case (RED or GREEN) presented in one of two colours (red and green) for the congruent and incongruent conditions. For the neutral condition, the target consisted of a string of Xs presented in one of the above colours. Participants were instructed to press the colour patch with two fingers of their right hand (attached to the keys j and k) corresponding to the colour of the target as soon as possible, but not at the expense of accuracy. Stimuli were presented in binocular conditions.
Fig. 2.
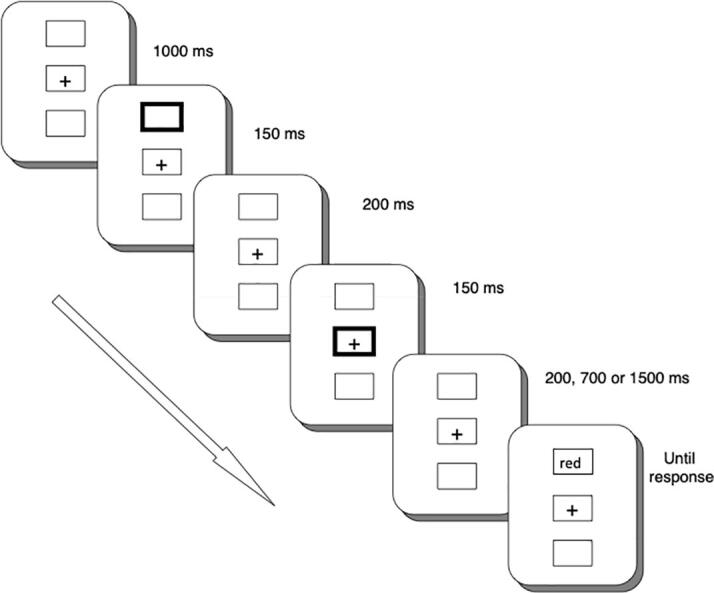
Sequence of stimuli in the IOR-Stroop procedure.
Participants received one practice block of 18 trials, and 6 experimental blocks of 72 trials each. In each experimental block, the target appeared at the cued location on half of the trials (36 trials), and it appeared at the uncued location on the remaining trials. Overall, there were 24 trials for each congruency-SOA condition.
3. Results
Mean correct response times and the percentages of errors for both groups are shown in Table 2. Correct RTs were submitted to a mixed design ANOVA with cue (cued, uncued), congruency (incongruent, congruent, neutral), and SOA (700, 1200, 2000 ms) as within-participants factors, and group (HC, PC, and parietal patients) as the between-participants factor.
Table 2.
Mean RTs (ms), Standard Deviation and percentage of errors (in parenthesis) as a function of group, SOA (700, 1200, and 2000 ms), congruency (neutral, congruent and incongruent) and cueing (cued, uncued).
| SOA | Congruency | Healthy controls |
Parietal patients |
Patients controls |
|||
|---|---|---|---|---|---|---|---|
| Cued | Uncued | Cued | Uncued | Cued | Uncued | ||
| 700 | Neutral | 733/93 (1.7 %) | 705/88 (0.0 %) | 1159/382 (2.1 %) | 1146/391 (1.2 %) | 1125/201 (0.6 %) |
1117/226 (4.8 %) |
| Congruent | 721/81 (0.8 %) |
711/107 (0.8 %) | 1138/397 (3.3 %) | 1138/374 (1.2 %) | 1123/197 (1.2 %) |
1165/271 (0.6 %) |
|
| Incongruent | 802/131 (1.7 %) | 785/139 (0.0 %) | 1240/416 (2.5 %) | 1287/440 (5.8 %) | 1306/301 (8.9 %) |
1293/243 (8.3 %) |
|
| 1200 | Neutral | 697/63 (1.2 %) | 701/128 (1.7 %) | 1118/376 (5.4 %) | 1122/372 (2.5 %) |
1114/215 (2.9 %) |
1135/223 (2.9 %) |
| Congruent | 709/83 (2.9 %) | 683/70 (1.7 %) | 1113/375 (1.7 %) | 1103/325 (2.1 %) | 1121/232 (1.19 %) |
1145/212 (1.18 %) |
|
| Incongruent | 758/86 (0.8 %) | 836/165 (0.0 %) | 1274/451 (2.1 %) |
1186/440 (4.2 %) | 1329/339 (6.5 %) |
1342/301 (3.5 %) |
|
| 2000 | Neutral | 687/83 (2.5 %) | 691/83 (0.8 %) | 1135/340 (4.2 %) | 1108/382 (3.7 %) | 1133/234 (1.4 %) |
1130/255 (2.3 %) |
| Congruent | 713/102 (1.7 %) | 689/85 (1.7 %) | 1138/395 (2.1 %) | 1113/375 (2.9 %) | 1146/251 (1.8 %) |
1178/282 (0.6 %) |
|
| Incongruent | 786/147 (2.1 %) | 797/147 (1.2 %) | 1193/400 (6.2 %) | 1308/444 (3.3 %) | 1325/315 (7.1 %) |
1381/348 (5.3 %) |
|
3.1. RT analysis
There were significant main effects of congruency [F(1, 24) = 28.35, p =.000, η2 = 0.54], and group [F(2, 24) = 8.75, p =.001, η2 = 0.42]. The congruency effect was due to slower RTs in the incongruent condition (1101 ms) compared with both the congruent condition (974 ms) [t(26) = 5.54, p =.000, r = 0.74], and the neutral condition (971 ms) [t(26) = 5.01, p =.000, r = 0.70]. The congruent condition and the neutral condition did not differ significantly [t(26) = 0.36, p =.72, r = 0.07]. Also, the HC group was overall faster (734 ms) than both parietal patients (1168 ms) and the PC group (1201 ms) [t(18) = 3.50, p =.003, r = 0.55, and t(15) = 5.60, p =.000, r = 0.82, respectively]. However parietal patients and PC did not show any significant difference in global RTs [t(15) = 0.20, p =.84, r = 0.021]. Furthermore, the four-way cueing × congruency × SOA × group interaction reached statistical significance [F(8, 96) = 2.09, p =.043, η2 = 0.15]. Further analysis of the four-way interaction showed that the cueing × congruency × SOA interaction was significant just for the HC group [F(4, 36) = 3.26, p =.022, η2 = 0.27] and for the parietal patients group [F(4, 36) = 2.93, p =.034, η2 = 0.27], but not for the PC group [F(4, 24) = 0.34, p =.85, η2 = 0.05]. For this control group, only the main effect of congruency was significant [F(2, 12) = 9.37, p =.004, η2 = 0.61]. Namely, RTs for the incongruent condition (1329 ms) were longer than for the congruent (1146 ms) [t(6) = 3.50, p =.014, r = 0.82] and neutral (1126 ms) conditions [t(6) = 3.15, p =.020, r = 0.79]. The congruent condition and the neutral condition did not differ significantly [t(6) = 0.63, p =.55, r = 0.025]. No other main effects or interactions reached statistical significance (all ps greater than 0.05).
3.2. IOR analysis for HC and parietal patients
For IOR, we compared RTs in the cued and the uncued locations (see Fig. 3). Because the three-way cueing × congruency × SOA interaction was significant only for HC and parietal patients, we further analysed IOR effects only in these two groups.
Fig. 3.

Cueing effect (RTuncued – RTcued) as a function of Stroop condition (incongruent, congruent and neutral) and SOA in Healthy Controls, Patients Control (*p <.05; ap =.06).
For the HC group, there were significant IOR effects at the 700 ms SOA (a 28 ms effect) for neutral trials [t(9) = 2.48, p =.035, r = 0.64], and at the 1200 ms SOA (a 26 ms effect) for congruent trials [t(9) = 2.74, p =.023, r = 0.67]. A facilitation effect (a reduction in RTs on cued trials relative to uncued trials) was observed at the 1200 ms SOA for incongruent trials (758 vs 836 ms, respectively) [t(9) = 2.79, p =.021, r = 0.68]. For the parietal patients, there was a significant IOR effect at the 1200 ms SOA (88 ms) for incongruent trials [t(9) = 2.40, p =.040, r = 0.61]. The facilitation effect at the 2000 ms SOA for incongruent trials was marginally significant [t(9) = 2.14, p =.06, r = 0.58]. Similar IOR results were found when we controlled for effects of group-related generalized slowing (see Supplementary material).
3.3. IT analysis for HC and parietal patients
For IT, we assessed the magnitude of the Stroop effect (incongruent minus congruent trials) across the cueing and SOA conditions for each group (see Fig. 4). For the HC group, there was evidence of IT at the 1200 ms SOA. At this SOA there was a reduction in the magnitude of the Stroop effect on cued trials relative to uncued trials (49 vs 153 ms, respectively) [t(9) = 3.27, p =.010, r = 0.74]. For the parietal patients, IT emerged later in time, at the longest 2000 ms SOA. The Stroop effect at the cued location was smaller (55 ms) than at the uncued location (195 ms) [t(9) = 2.73, p =.023, r = 0.67]. In fact, the Stroop effect was no longer significant at the cued location in the 2000 ms SOA for this group of participants [t(9) = 0.96, p =.36, r = 0.30]. There was no evidence for IT at earlier SOAs in the parietal patients.
Fig. 4.

Stroop effect (RTincongruent – RTcongruent) as a function of cue (cued, uncued) and SOA in Healthy Controls, Parietal Patients, and Patients Control (*p <.05).
The same pattern of results was found when the Stroop effect was computed as the difference between incongruent and neutral trials. For the HC group there was evidence of IT at the 1200 ms SOA. At this SOA there was a reduction in the magnitude of the Stroop effect on cued trials relative to uncued trials (61 vs 135 ms, respectively) [t(9) = 4.99, p =.001, r = 0.86]. For the parietal patients, IT emerged at the longest 2000 ms SOA. Stroop effects were 59 ms and 200 ms for the cued and uncued locations, respectively, and that difference was statistically significant [t(9) = 2.45, p =.037, r = 0.63].
Similar IT results were found when we controlled for effects of group-related generalized slowing (see Supplementary material).
3.4. Bilateral vs L/R lesions
The analyses were repeated to assess the effects of lesion location (left hemisphere, right hemisphere, or bilateral) in the parietal group. Correct RTs were submitted to a mixed ANOVA with cue (cued, uncued), congruency (incongruent, congruent, neutral), and SOA (700, 1200, 2000 ms) as within-participants factors, and lesion location (left, right, bilateral) as the between-participants factor. We replicated the previously reported main findings with the whole group of patients, but the factor lesion location did not interact with any of the other factors. Notably the cue × congruence × SOA × lesion location interaction did not approach significance [F(8, 28) = 1.10, p =.41, η2 = 0.24].
There was also no evidence that spatial neglect or extinction was a critical factor here. For example, separating the patients showing some aspect of neglect from the others failed also to reveal a significant cue × congruency × SOA × neglect interaction [F(4, 32) = 1.67, p =.18, η2 = 0.17]; the same held when we distinguished patients with and without extinction [F(4, 32) = 2.11, p =.10, η2 = 0.20]. For the PC group the cue × congruence × SOA × lesion location interaction was not significant either [F(8, 16) = 1.38, p =.28, η2 = 0.41].
3.5. Accuracy analysis
The percentage of errors was submitted to a mixed design ANOVA with cue (cued, uncued), congruency (incongruent, congruent, neutral), and SOA (700, 1200, 2000 ms) as within-participants factors, and group (HC, PC and parietal patients) as a between-participants factor. There was a significant main effect of congruency [F(1, 24) = 5.14, p =.033, η2 = 0.18]. The congruency effect was due to higher percentage of errors in the incongruent condition (3.7 %) compared with the congruent condition (1.7 %) [t(26) = 2.07, p =.048, r = 0.38]. The incongruent condition (3.7 %) and the neutral condition (2.3 %) did not differ significantly [t(26) = 1.6, p =.12, r = 0.30]. The different percentage of errors found in the congruent condition (1.7 %) compared with the neutral condition (2.3 %) was not significant [t(26) = 1.5, p =.16, r = 0.28]. Furthermore, the interaction SOA × congruency was also significant [F(4, 96) = 3.35, p =.013, η2 = 0.12]. This interaction was due to the presence of a lesser congruency effect (incongruent minus congruent) in the 1200 ms SOA (0.77 %) compared to the 700 ms SOA (3.16 %) [t(26) = 2.91, p =.007, r = 0.50] and the 2000 ms SOA (2.16 %) [t(26) = 2.6, p =.015, r = 0.45]. In the neutral condition the congruency effect (incongruent minus neutral) was higher in the 700 ms SOA (2.9 %) compared to the 1200 ms SOA (0.15 %) [t(26) = 2.92, p =.007, r = 0.50].
4. Discussion
The aim of this study was to investigate the contribution of the parietal lobe to attention-dependent inhibitory processes along the vertical meridian. In the HC group, we found significant IOR effects at the 700 and 1200 ms SOAs. At the longest SOA (2000 ms) IOR was no longer significant. Studies with healthy adults have shown that IOR lasts from 1.5 to 3 s [48]. However, it has also been shown that the time course of IOR is modulated by task difficulty, and with older participants [33]. Here, IOR decreased for the longer SOA (2000 ms). Interestingly, we also found an interaction between the time course of IOR and congruency. That is, at the 700 ms SOA, IOR was only significant for the neutral trials, whereas at the 1200 ms SOA IOR was only significant in the congruent condition. We believe that this finding may also be accounted for in terms of differential task demands for the congruent and neutral conditions. That is, in the congruent condition there are two dimensions (colour and word) and participants need to select and attend to one of these dimensions [38]. Thus, this condition may require a higher attentional control setting than the neutral condition (colour but not words), and this may extend the IOR effect. These results are in line with previous findings comparing IOR effects in younger and older adults in a variety of IOR tasks. Although previous findings support that location-based IOR is preserved with age [[26], [28], [33]], there are conditions that require higher attentional control settings that may affect how older adults deal with inhibitory processes in attentional orientation tasks. For instance, compared with younger adults, older adults show a delay onset of IOR when a single cue paradigm is used [[9], [35], [40]], and later resolution of IOR in both detection and discrimination tasks [[32], [33]].
Along with the IOR effect we found evidence for IT in the healthy control participants. Notably the HC group exhibited a reduced Stroop effect at the cued location relative to the uncued location when there was an intermediate SOA (1200 ms). This result contrasts with previous studies that failed to show a reduced Stroop effect at the cued location in older adults with similar characteristics to the healthy control participants that we tested in the present study [34]. However, the previous studies used a single 1400 ms SOA value, and therefore it is still possible that IT had already resolved at that cue-target interval. Note that one of the main features of IT is that it is rather short-lasting [[23], [39], [52]].
The group of parietal patients here differed from the healthy controls in several respects. First, the onset of IOR was delayed. The parietal patients showed IOR at the 1200 ms SOA (in contrast, IOR in the HC group was already evident at the 700 ms SOA). This result agrees with previous studies that suggest that delays in the time course of IOR can be of clinical interest as it permits to predict decline in general cognitive functioning not only in heathy adults [35] (although see [46]) but also in patients with a diagnosis of Alzheimer’s disease or in a high-risk (prodromal) stage [2].
The evidence for an emergence of IOR along the vertical meridian contrasts with the results from ipsilesional cueing, where IOR has been shown to be eliminated [[54], [55]]. One reason for the contrasting pattern could be that there is a gradient in resting activity across the visual field, which is highest in the ipsilesional field but then reduces towards the contralesional side [43]. This high baseline activation makes it difficult to suppress items on the ipsilesional side and so IOR is abolished. However, there may be intermediate activation in the vertical meridian. The consequence of this is that there can be some suppression associated to those locations, but this takes longer than normal – with the result that IOR emerges later. An opposite dissociation has been reported with patients affected by progressive supranuclear palsy (PSP; affecting the superior colliculus –SC–). While PSP patients exhibited typical IOR effects with lateralized stimuli in a horizontal display, they failed to show significant IOR effects in a vertical display [[42], [49]]. Posner et al. [42] argued that PSP patients had an impaired ability to make eye movements in the vertical direction, and thus only IOR in the vertical procedure was impaired in this group of patients.
In addition, the present group of parietal patients showed delayed onset of IT as compared to the HC group. In this case there was evidence that the Stroop effect was reduced at the cued location relative to the uncued location, but this only emerged at the longest SOA (2000 ms). That delay was not due to general slowing in attentional processes associated with the pathology because the same pattern of IOR and IT was observed with transformed scores. In contrast, we suggest that the delay in imposing inhibition at the central, vertical locations in turn led to a delay in IT. The results point to direct linkage between IOR and IT, with impairments in IOR after parietal damage being passed on through delays in IT. We propose that both the IOR and the IT effects can be linked to altered shifts in baseline neural activity across space, after parietal damage.
The argument for an altered shift in baseline activity fits with longstanding accounts of unilateral neglect (e.g., [30]. Here it has been argued that damage to attentional operations in one hemisphere leads to disinhibition of the ipsilesional hemisphere, which results in an attentional bias to the ipsilesional side. Consistent with this, suppressive TMS applied to the non-lesioned hemisphere of neglect patients can improve their attention to the contralesional side [20], while recovery of function in neglect is associated with decreased activity within the contralesional parietal cortex and increased activity in the lesioned hemisphere [13]. Here we argue that there remains some disinhibition even at the vertical meridian, though this is not so severe as when stimuli fall in the ipsilesional field. However, the extra activation of stimuli around the meridian means that IOR is delayed and there is then a consequent delay in activity in IT – though the basic mechanism of IT appears to be intact. This is consistent with IT being linked to frontal brain circuits not damaged in our parietal patients [[11], [39]]. Accordingly, our control patients, whose brain damage comprises mainly the frontal lobe (see Table 1), failed to show any evidence of either IOR or IT in the vertical meridian. This means that the pattern of results observed in the parietal patients are not due to brain lesion per se, but to lesions affecting the parietal lobe.
Interestingly, there is now increasing evidence to support the argument that attentional biases resulting from damage to the parietal lobe may not be symmetrical for both hemispheres [[14], [17]]. Against this, our analyses with lesion location did not yield differences between unilateral left and right hemisphere damage and bilateral patients. We also found that the effects did not differ between the parietal patients who did and did not manifest spatial neglect and those who did or did not show clinical extinction. However, any conclusion on the effects of lesion location and the effect of neglect should be cautious, given the small numbers of patients tested, which is a limitation of the present study. Nevertheless, the current results suggest important effects of lesion location over and above the clinical symptoms apparent in the parietal patients.
Finally, it should be noted that the present parietal patients not only incurred damage to the inferior parietal cortex but also damage to the underlying white matter, likely including the superior longitudinal fasciculus (SLF). There is increasing evidence that, in spatial disorders such as unilateral neglect, damage to the fibre tracts connecting the parietal and frontal cortices may be a critical factor (e.g., [15], with notably the SLF being implicated. Damage to these tracts may lead to a failure to pass inhibitory signals from frontal brain regions, so leading to a lack of modulation of spatial attention within the parietal cortex. A task for future work will be to assess this possibility in patients with finer-grained lesions.
Two limitations of the present study should be taken into consideration when interpreting the results. The first limitation concerns the rather small sample of patients. However, it is important to realise that the present study builds on the results of previous studies exploring the effects of IOR on the vertical meridian [55], where the sample size was half that of the present study. The second limitation concerns the inclusion of patients with lesions in the right and left hemisphere, some with additional pathologies such as Alzheimer's disease. However, although it would have been preferable to have a larger sample of patients so that they could be classified according to the location of the damage, note that in our previous work concerning the role of parietal lobe in IOR we had also patients with lesions in the right and left hemispheres and found no differences between them (see [[54], [55]]. This is further supported by some studies showing that although the effects of attentional orientation with peripheral cues are stronger when the damage affects the right parietal lobe, patients with damage to the left parietal lobe also showed such effects although the size was smaller (for a review, see [36]). For other pathologies such as Alzheimer’s disease, there is evidence that IOR-related brain areas such as the TPJ and inferior parietal cortex, are also affected in the progression of the disease [4] and, consequently, deficits in IOR has also been detected in patients with mild cognitive impairment and Alzheimer’s disease, even with a double-cue procedure similar to the one used here [29].
In conclusion, we provide evidence that there remain disturbances in IOR around the vertical meridian in patients with damage to the parietal cortex and underlying white matter, though here the result was that IOR was delayed in its emergence. In addition, we show that IT remained present in the parietal patients but was again delayed in time following the late importation of IOR.
Ethical standards
All participants gave written informed consent for participation in the study. The ethic committee for Life Science at the University of Birmingham (UK) approved the study, which was carried out in accordance with the Declaration of Helsinki.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors wish to acknowledge the contribution of Glyn W. Humphreys, who inspired our work on cognitive neuropsychology.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbas.2022.100043.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bartolomeo P., Siéroff E., Decaix C., Chokron S. Modulating the attentional bias in unilateral neglect: the effects of the strategic set. Exp Brain Res. 2001;137:432–444. doi: 10.1007/s002210000642. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A., Phillips M., Porter G., Leonards U., Bompas A., Tales A. Abnormal inhibition of return in mild cognitive impairment: is it specific to the presence of prodromal dementia? JAD. 2014;40:177–189. doi: 10.3233/JAD-131934. [DOI] [PubMed] [Google Scholar]
- 3.Berger A., Henik A. The endogenous modulation of IOR is nasal-temporal asymmetric. J Cogn Neurosci. 2000;12:421–428. doi: 10.1162/089892900562246. [DOI] [PubMed] [Google Scholar]
- 4.Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mezenge F., Egret S., et al. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci. 2015;35(29):10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickerton W.-L., Demeyere N., Francis D., Kumar V., Remoundou M., Balani A., et al. The BCoS cognitive profile screen: Utility and predictive value for stroke. Neuropsychology. 2015;29:638–648. doi: 10.1037/neu0000160. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois A., Chica A.B., Valero-Cabré A., Bartolomeo P. Cortical control of inhibition of return: Causal evidence for task-dependent modulations by dorsal and ventral parietal regions. Cortex. 2013;49:2229–2238. doi: 10.1016/j.cortex.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois A., Chica A.B., Valero-Cabré A., Bartolomeo P. Cortical control of Inhibition of Return: Exploring the causal contributions of the left parietal cortex. Cortex. 2013;49:2927–2934. doi: 10.1016/j.cortex.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco M., Williams P.E., Yeshurun Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision. 2002;2:4. doi: 10.1167/2.6.4. [DOI] [PubMed] [Google Scholar]
- 9.Castel A.D., Chasteen A.L., Scialfa C.T., Pratt J. Adult age differences in the time course of inhibition of return. J Gerontol Series B: Psychol Sci SocSci. 2003;58(5):P256–P259. doi: 10.1093/geronb/58.5.p256. [DOI] [PubMed] [Google Scholar]
- 10.Chechlacz M., Rotshtein P., Hansen P.C., Deb S., Riddoch M.J., Humphreys G.W. The central role of the temporo-parietal junction and the superior longitudinal fasciculus in supporting multi-item competition: Evidence from lesion-symptom mapping of extinction. Cortex. 2013;49:487–506. doi: 10.1016/j.cortex.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q., Wei P., Zhou X. Distinct neural correlates for resolving stroop conflict at inhibited and noninhibited locations in inhibition of return. J Cogn Neurosci. 2006;18:1937–1946. doi: 10.1162/jocn.2006.18.11.1937. [DOI] [PubMed] [Google Scholar]
- 12.Chica A.B., Bartolomeo P., Valero-Cabre A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J Neurosci. 2011;31(22):8143–8149. doi: 10.1523/JNEUROSCI.5463-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbetta M., Kincade M.J., Lewis C., Snyder A.Z., Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8(11):1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 14.Dietz M.J., Friston K.J., Mattingley J.B., Roepstorff A., Garrido M.I. Effective connectivity reveals right-hemisphere dominance in audiospatial perception: implications for models of spatial neglect. J Neurosci. 2014;34:5003–5011. doi: 10.1523/JNEUROSCI.3765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doricchi F., Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal–frontal disconnection? NeuroReport. 2003;14:2239–2243. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- 16.Dorris M.C., Klein R.M., Everling S., Munoz D.P. Contribution of the primate superior colliculus to inhibition of return. J Cognit Neurosci. 2002;14:1256–1263. doi: 10.1162/089892902760807249. [DOI] [PubMed] [Google Scholar]
- 17.Dragone A., Lasaponara S., Silvetti M., Macaluso E., Doricchi F. Selective reorienting response of the left hemisphere to invalid visual targets in the right side of space: Relevance for the spatial neglect syndrome. Cortex. 2015;65:31–35. doi: 10.1016/j.cortex.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Dukewich K.R. Reconceptualizing inhibition of return as. Psychon Bull Rev. 2009;16(2):238–251. doi: 10.3758/PBR.16.2.238. [DOI] [PubMed] [Google Scholar]
- 19.Dukewich K.R., Klein R.M. Inhibition of return: A phenomenon in search of a definition and a theoretical framework. Atten Percept Psychophys. 2015;77:1647–1658. doi: 10.3758/s13414-015-0835-3. [DOI] [PubMed] [Google Scholar]
- 20.Fierro B., Brighina F., Bisiach E. Improving neglect by TMS. Behav Neurol. 2006;17(3-4):169–176. doi: 10.1155/2006/465323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentes L.J. In: Cognitive neuroscience of attention. Posner M.I., editor. The Guilford Press; New York, NY, US: 2004. Inhibitory processing in the attentional networks; pp. 45–55. [Google Scholar]
- 22.Fuentes L.J., Boucart M., Vivas A.B., Alvarez R., Zimmerman M.A. Inhibitory tagging in inhibition of return is affected in schizophrenia: Evidence from the Stroop task. Neuropsychology. 2000;14:134–140. [PubMed] [Google Scholar]
- 23.Fuentes L.J., Vivas A.B., Humphreys G.W. Inhibitory tagging of stimulus properties in inhibition of return: effects on semantic priming and flanker interference. Quart J Experiment Psychol Sect A. 1999;52(1):149–164. [Google Scholar]
- 24.Fuentes L.J., Vivas A.B., Langley, Linda K., Chen Qi, González-Salina, Carmen . In: Cognitive neuroscience of attention. 2nd ed. Posner M.I., editor. Guilford Press; New York: 2012. Inhibitory mechanisms in the attentional networks: A multidisciplinary approach. [Google Scholar]
- 25.Gabay S., Behrmann M. Attentional dynamics mediated by subcortical mechanisms. Atten Percept Psychophys. 2014;76(8):2375–2388. doi: 10.3758/s13414-014-0725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartley A.A., Kieley J.M. Adult age differences in the inhibition of return of visual attention. Psychol Aging. 1995;10(4):670–683. doi: 10.1037//0882-7974.10.4.670. [DOI] [PubMed] [Google Scholar]
- 27.Heywood S., Churcher J. Structure of the visual array and saccadic latency: implications for oculomotor control. Quart J Experiment Psychol. 1980;32:335–341. doi: 10.1080/14640748008401169. [DOI] [PubMed] [Google Scholar]
- 28.Huether A.X.A., Langley L.K., Thomas L.E. Aging and inhibition of return to locations and objects. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.706549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X., Howard J.H., Rebeck G.W., Turner R.S., Forloni G. Spatial inhibition of return is impaired in mild cognitive impairment and mild Alzheimer’s disease. PLoS ONE. 2021;16(6):e0252958. doi: 10.1371/journal.pone.0252958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsbourne M. A model for the mechanism of unilateral neglect of space. Trans Am Neurol Assoc. 1970;95:143–146. [PubMed] [Google Scholar]
- 31.Klein R.M. Inhibition of return. Trends Cogn Sci. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- 32.Langley L.K., Fuentes L.J., Hochhalter A.K., Brandt J., Overmier J.B. Inhibition of return in aging and Alzheimers disease: performance as a function of task demands and stimulus timing. J Clin Exp Neuropsychol. 2001;23:431–446. doi: 10.1076/jcen.23.4.431.1235. [DOI] [PubMed] [Google Scholar]
- 33.Langley L.K., Fuentes L.J., Vivas A.B., Saville A.L. Aging and temporal patterns of inhibition of return. J Gerontol Series B: Psychol Sci Soc Sci. 2007;62(2):P71–P77. doi: 10.1093/geronb/62.2.p71. [DOI] [PubMed] [Google Scholar]
- 34.Langley L.K., Vivas A.B., Fuentes L.J., Bagne A.G. Differential age effects on attention-based inhibition: inhibitory tagging and inhibition of return. Psychol Aging. 2005;20:356–360. doi: 10.1037/0882-7974.20.2.356. [DOI] [PubMed] [Google Scholar]
- 35.Li T., Wang L., Huang W., Zhen Y., Zhong C., Qu Z., et al. Onset time of inhibition of return is a promising index for assessing cognitive functions in older adults. J Gerontol: Series B. 2020;75:753–761. doi: 10.1093/geronb/gby070. [DOI] [PubMed] [Google Scholar]
- 36.Losier B.J.W., Klein R.M. A review of the evidence for a disengage deficit following parietal lobe damage. Neurosci Biobehav Rev. 2001;25(1):1–13. doi: 10.1016/s0149-7634(00)00046-4. [DOI] [PubMed] [Google Scholar]
- 37.Lupiáñez J. In: Attention and time. Nobre A.C., Coull J.T., editors. Oxford University Press; 2010. Inhibition of return; pp. 17–34. [Google Scholar]
- 38.MacLeod C.M., MacDonald P.A. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4(10):383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Pérez V., Castillo A., Sánchez-Pérez N., Vivas A.B., Campoy G., Fuentes L.J. Time course of the inhibitory tagging effect in ongoing emotional processing. A HD-tDCS study. Neuropsychologia. 2019;135 doi: 10.1016/j.neuropsychologia.2019.107242. [DOI] [PubMed] [Google Scholar]
- 40.Muiños M., Palmero F., Ballesteros S. Peripheral vision, perceptual asymmetries and visuospatial attention in young, young-old and oldest-old adults. Exp Gerontol. 2016;75:30–36. doi: 10.1016/j.exger.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Posner M.I., Cohen Y. In: Attention and performance X. Bouma H., Bouwhuis D.G., editors. Erlbaum; Hillsdale, NJ: 1984. Components of visual orienting; pp. 531–556. [Google Scholar]
- 42.Posner M.I., Rafal R.D., Choate L.S., Vaughan J. Inhibition of return: Neural basis and function. Cogn Neuropsychol. 1985;2(3):211–228. [Google Scholar]
- 43.Pouget A., Driver J. Relating unilateral neglect to the neural coding of space. Curr Opin Neurobiol. 2000;10:242–249. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 44.Previc F.H. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behav Brain Sci. 1990;13:519–542. [Google Scholar]
- 45.Purokayastha S., Roberts M., Carrasco M. Voluntary attention improves performance similarly around the visual field. Atten Percept Psychophys. 2021;83(7):2784–2794. doi: 10.3758/s13414-021-02316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey-Mermet A., Gade M. Inhibition in aging: What is preserved? What declines? A meta-analysis, Psychon Bull Rev. 2018;25:1695–1716. doi: 10.3758/s13423-017-1384-7. [DOI] [PubMed] [Google Scholar]
- 47.Ro T., Farnè A., Chang E. Inhibition of return and the human frontal eye fields. Exp Brain Res. 2003;150(3):290–296. doi: 10.1007/s00221-003-1470-0. [DOI] [PubMed] [Google Scholar]
- 48.Samuel A.G., Kat D. Inhibition of return: A graphical meta-analysis of its time course and an empirical test of its temporal and spatial properties. Psychon Bull Rev. 2003;10:897–906. doi: 10.3758/bf03196550. [DOI] [PubMed] [Google Scholar]
- 49.Sapir A., Soroker N., Berger A., Henik A. Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci. 1999;2(12):1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- 50.Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.A.B. Sereno, S.R. Lehky, S. Patel, X. Peng, A neurophysiological correlate and model of reflexive spatial attention, in: advances in cognitive science, SAGE Publications India Pvt Ltd, B-42, Panchsheel Enclave, New Delhi 110 017 India, 2010: pp. 104–131.
- 52.Vivas A.B., Fuentes L.J. Stroop interference is affected in inhibition of return. Psychon Bull Rev. 2001;8(2):315–323. doi: 10.3758/bf03196167. [DOI] [PubMed] [Google Scholar]
- 53.Vivas A.B., Fuentes L.J., Estevez A.F., Humphreys G.W. Inhibitory tagging in inhibition of return: Evidence from flanker interference with multiple distractor features. Psychon Bull Rev. 2007;14:320–326. doi: 10.3758/bf03194071. [DOI] [PubMed] [Google Scholar]
- 54.Vivas A.B., Humphreys G.W., Fuentes L.J. Inhibitory processing following damage to the parietal lobe. Neuropsychologia. 2003;41(11):1531–1540. doi: 10.1016/s0028-3932(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 55.Vivas A.B., Humphreys G.W., Fuentes L.J. Abnormal inhibition of return: A review and new data on patients with parietal lobe damage. Cogn Neuropsychol. 2006;23:1049–1064. doi: 10.1080/02643290600588400. [DOI] [PubMed] [Google Scholar]
- 56.Vivas A.B., Humphreys G.W., Fuentes L.J. Object-based inhibition of return in patients with posterior parietal damage. Neuropsychology. 2008;22(2):169–176. doi: 10.1037/0894-4105.22.2.169. [DOI] [PubMed] [Google Scholar]
- 57.Vivas A.B., Paraskevopoulos E., Castillo A., Fuentes L.J. Neurophysiological activations of predictive and non-predictive exogenous cues: A cue-elicited EEG study on the generation of inhibition of return. Front Psychol. 2019;10:227. doi: 10.3389/fpsyg.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Zhou X., Zhang M. Temporary inhibitory tagging at previously attended locations: Evidence from event-related potentials: Temporary inhibitory tagging in IOR. Psychophysiol. 2012;49:1191–1199. doi: 10.1111/j.1469-8986.2012.01412.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



