Abstract
We have previously demonstrated that overexpression of Cdc25B in transgenic mice resulted in mammary gland hyperplasia and increased steroid hormone responsiveness. To address how Cdc25B enhances the hormone responsiveness in mammary glands, we showed that Cdc25B stimulates steroid receptor-dependent transcription in transient transfection assays and in a cell-free assay with chromatin templates. Surprisingly, the effect of Cdc25B on steroid receptors is independent of its protein phosphatase activity in vitro. The direct interactions of Cdc25B with steroid receptors, on the other hand, were evidenced in in vivo and in vitro assays, suggesting the potential direct contribution of Cdc25B on the steroid receptor-mediated transcription. In addition, p300/CBP-associated factor and CREB binding protein were shown to interact and synergize with Cdc25B and further enhance its coactivation activity. Thus, we have uncovered a novel function of Cdc25B that serves as a steroid receptor coactivator in addition to its role as a regulator for cell cycle progression. This dual function might likely contribute to its oncogenic action in breast cancer.
Mammary gland development and tumorigenesis are tightly controlled by hormone stimuli. Through specific interaction with their cognate receptors, ovarian hormones (estrogen and progesterone) regulate the expression of a variety of genes that are involved in different stages of mammary gland development, including cell proliferation, differentiation, and apoptosis (13, 44, 54). It is well documented that estrogen receptor (ER) and progesterone receptor (PR) bind to palindromic response elements as homodimers and stimulate transcription of target genes by recruiting coactivators and general transcription factors (GTFs) to the promoters of hormone-responsive genes (4, 41, 63). In addition to specific hormone-receptor interactions, tissue responses to hormone stimuli are modulated by transcription cofactors. Recently, many transcription coactivators and corepressors have been identified. They regulate the magnitude of tissue responsiveness to hormone stimulation either by acting as a bridge between receptors and basal transcription factors and/or by changing the chromatin configuration of the promoter (14, 42, 58, 66).
In regards to the effect of ovarian hormones on mammary gland tumor development, it is known that prolonged exposure to estrogenic substances significantly increases the incidence of breast cancer (22, 23, 49, 50) and hormone ablative therapy has been successfully used to inhibit ER-dependent growth of breast cancer (27). It is thus hypothesized that any genetic alteration in favor of hormone activation could result in a growth advantage and contribute to breast cancer development. It is noted that many genes overexpressed and/or amplified in breast cancer can enhance the transcription activity of steroid receptors either through ligand-independent activation, e.g., Her2 (5, 60), or by acting as steroid receptor coactivators (SRCs) such as cyclin D1 (69, 70), AIB1 (2), SRA (33, 35), PBP/PPARBP (68), and ASC-2 (34).
Cdc25s (Cdc25A, -B, and -C) belong to a family of dual specificity proteins and activate cyclin–cyclin-dependent kinases (Cdks) by removal of inhibitory phosphates (11, 46). These phosphatases consist of a highly conserved catalytic domain containing an active site Cys-(X)5-Arg motif similar to the tyrosine phosphatase family and a variable N-terminal region implicated to serve a regulatory role through phosphorylation (11, 12, 37, 38, 52, 67). Ectopic expression of Cdc25A accelerates the G1/S transition through activation of cyclin E- and cyclin A-dependent kinases that have also been shown to be able to stimulate Cdc25A, constituting a similar feedback loop in the S-phase progression (7, 24, 26). Furthermore, Cdc25 is phosphorylated in response to DNA damage to create a binding site for 14-3-3 proteins, leading to the nuclear exclusion of Cdc25 and cell cycle arrest for DNA repair (10, 37, 57). In accordance with their critical roles in cell cycle regulation, Cdc25A and Cdc25B have been shown to be involved in cancer progression. Cdc25A and Cdc25B, but not Cdc25C, cooperate with activated ras to induce oncogenic focus formation of rat embryonic fibroblasts (18). Furthermore, Cdc25A and Cdc25B have been found to be overexpressed in many primary tumors, including breast cancer (18, 19, 23, 31, 65).
Cdc25B has been reported to have three isoform proteins, Cdc25B1, Cdc25B2 and Cdc25B3, by alternative mRNA splicing (3). Cdc25B2 appears to be most abundantly expressed in cancer cells. Previously, transgenic mice that overexpress Cdc25B (Cdc25B2) in the mammary gland under the control of the mouse mammary tumor virus (MMTV) promoter were generated to verify the oncogenic potential of Cdc25B in vivo (39). It was found that overexpression of Cdc25B leads to an increased rate of mammary epithelial cell proliferation, resulting in the formation of alveolar hyperplasia. In addition, cyclin D1 levels were elevated in these transgenic mammary glands. Cyclin D1 functions as a growth sensor, and its expression depends on extracellular signals. It has been shown that cyclin D1 expression is directly up regulated by ER in response to hormone stimulation and that the enhanced expression mediates estrogen-induced mitogenesis (1, 51, 55, 56). An increased level of cyclin D1 mRNA was also observed in breast cancer cells overexpressing the estrogen receptor (25, 28). Consistent with the above findings, genetic ablation of cyclin D1 dramatically affects mammary alveolar development associated with pregnancy (59). To examine how overexpression of Cdc25B augments the expression of cyclin D1 and alveolar hyperplasia, both of which are hormone regulable events, we investigated the potential effect of Cdc25B on steroid receptor-dependent transcription. In this report, we demonstrate that Cdc25B selectively enhances the transcription of steroid receptors, including estrogen, progesterone, glucocorticoid (GR), and androgen receptors. The effect of Cdc25B on receptor dependent transcription is further confirmed in vitro in a cell-free chromatin transcription assay. Cdc25B is able to physically interact with steroid receptors and functions synergistically with histone acetyltransferase (HAT)-containing coactivators, p300/CREB binding protein (CBP)-associated factor (PCAF) and CBP. In addition, the protein phosphatase activity of Cdc25B is not essential to enhance the steroid receptor-dependent transcription in in vitro assays.
MATERIALS AND METHODS
Plasmid construction.
Mammalian expression plasmids for ER, ER mutants (179C, N282g, and 3x), PRB, GR, AR, RAR (retinoid acid receptor), SRC-1, PCAF, CBP, reporter constructs p(ERE)3tata-Luc, and MMTV-Luc have been described previously (33, 40, 64). To construct the Cdc25B expression vector, the pBS-SK-Cdc25B vector was digested with BamHI, HindIII, and DraI, and then the BamHI-HindIII fragment containing Cdc25B cDNA was cloned into the corresponding sites of plasmid pCR3.1 (Invitrogen). For the Cdc25A expression vector, the pBS-SK-Cdc25A vector was digested with EcoRI, and Cdc25A cDNA was subcloned into the corresponding site of plasmid pCR3.1. To generate Cdc25B mutants, site-specific mutation was performed as described (32). Mutagenic oligonucleotide primers for Cdc25B mutants L29A, C446S, and R452A are as follows: L29A, 5′-CAG CGA GAG GCC TGG GGC GTG GCC CGG ACG-3′; C446S, 5′-CTC AGA TGA GAA CTC AGA GTG GAA AAT GAG-3′; and R452A, 5′-GCG GGG CCC AGC CTC AGA TGA GAA CTC ACA GTG-3′. Bold nucleotides indicate the silent mutations introduced to destroy the restriction site for the purpose of screening mutated clones. All the Cdc25B mutants were checked and confirmed by sequencing analysis. R452A actually carried a single nucleotide deletion that causes a frame shift and a 49-amino-acid (49-aa) C-terminal truncation. We named this mutant R452ΔC. To construct His-tagged Cdc25B, Cdc25B cDNA was PCR amplified with following primers: 5′ primer, 5′-CC GGA TCC ATG GAG GTG CCC CAG CCG GAG CC-3′, and 3′ primer, 5′-CCC AAG CTT TCA CTG GTC CTG CAG CCG GCT AC-3′. The PCR product was digested with BamHI-HindIII and cloned into the corresponding sites of plasmid pQE30 (Qiagen). Vectors expressing glutathione S-transferase (GST) fusion to various Cdc25B fragments were constructed by subcloning BamHI-XhoI-digested PCR fragments into plasmid pGEX4T (Pharmacia) in frame with GST. Primers used for these PCR amplifications are listed as follows: for full-length Cdc25B (539 aa), forward primer 5′-CCGGATCC ATG GAG GTG CCC CAG CCG GAG CC-3′ and reverse primer 5′-CCG CTC GAG TCA CTG GTC CTG CAG CCG GCT AC-3′; for aa 1 to 66, forward primer 5′-CCGGATCC ATG GAG GTG CCC CAG CCG GAG CC-3′ and reverse primer 5′-CCG CTC GAG GCC GAG CCC GGC GAG GTC-3′; for aa 81 to 273, forward primer 5′-CC GGA TCC AGC CGC AGC CGC CTG ACG CAC-3′ and reverse primer 5′-CCG CTC GAG CTT GCT GTA CAT GAC GAG GTC-3′; for aa 274 to 351, forward primer 5′-CCGGATCC ATG GAG GTG CCC AG CCG GAG CC-3′ and reverse primer 5′-CCG CTC GAG CTC TCG GTG GTC ACT GTC CAG-3′; and for aa 352 to 539, forward primer 5′-CC GGA TCC CTG ATT GGA GAT TAC TCT AAG G-3′ and reverse primer 5′-CCG CTC GAG TCA CTG GTC CTG CAG CCG GCT AC-3′. The VP16-Cdc25B expression plasmid was constructed by subcloning the BamHI-BSAAI fragment of pGEX4T-Cdc25B into the BamHI-HindIII (blunted) sites of pAB-VP16 plasmid.
Preparation and analyses of RNA.
Total RNA was isolated from mouse tissues using the Trizol reagent (Gibco) according to manufacturer's instructions. For RNase protection assays (RPAs), mouse cyclin D1 antisense riboprobe (PharMingen) or mouse lactoferrin antisense riboprobe was hybridized with 5 to 10 μg of total RNA together with a control mouse antisense cyclophilin (Ambion) or L32 riboprobe (PharMingen) and assayed according to the manufacturer's instructions using the RPA II kit (Ambion).
Histology and immunohistochemistry.
To prepare mammary gland sections, the left inguinal glands were fixed with 4% phosphate-buffered paraformaldehyde for 18 to 24 h, embedded in paraffin, and then sectioned at a 5-μm thickness. Tissue sections were deparaffinized and rehydrated according to standard protocols. The sections were then rinsed in phosphate-buffered saline (PBS) and quenched for endogenous peroxidases with 6% (vol/vol) H2O2 for 30 min. Following a 30-min blocking step with 10% (wt/vol) horse serum in PBS, the tissue sections were incubated with anti-cyclin D1 antibody (1:100 dilution; Oncogene) overnight at 4°C in a humidified chamber. After three washes with PBS, a biotin-conjugated secondary antibody was added. Biotin-avidin binding and detection were then carried out according to the manufacturer's protocols (Vector Lab). To enhance contrast, sections were counterstained with 0.1% methyl green for 1 min and mounted with aqueous mounting media.
Cell culture and transient transfection assays.
HeLa cells were routinely maintained in Dulbecco's modified medium (Gibco) supplemented with 10% fetal calf serum (FCS) (HyClone Laboratories). Cells were seeded 24 h before transfection in six-well tissue culture plates (2 × 105 cells/well) in phenol red-free Dulbecco's modified medium containing 10% charcoal–dextran-treated FCS. The DNA mixture was transiently transfected into cell with Lipofectin reagent (Gibco). Cells were transfected for 6 h and then washed with phosphate buffer to remove reagents. Cells were then incubated for an additional 24 h in phenol red-free medium containing 10% charcoal–dextran-treated FCS in the absence or presence of hormones. Cell extracts were prepared by adding 300 μl of lysis buffer (Promega) and assayed for luciferase activities (Monolight 2010 luminometer; Analytical Luminescence Laboratory). Values are corrected for protein concentration and presented as means of quadruplicate values obtained from representative experiments.
Chromatin assembly and in vitro transcription.
Chromatin assembly reactions were performed by incubation of DNA templates with Drosophila S190 extract, core histones, and an ATP-regenerating system as described previously (36). Typically, the components such as PRB, ligand, and/or Cdc25B were added after the DNA templates were assembled for 4 h at 27°C. Subsequently, the assembly reaction was carried out for an additional 30 min at 27°C and then subjected to in vitro transcription (36). In vitro transcription of chromatin with HeLa cell nuclear extracts was performed as previously described (36). Briefly, 100 ng of chromatin template was incubated at room temperature with HeLa cell nuclear extract (20 μg) and buffer in a 50-μl reaction volume (final volume) for 30 min. Subsequently, transcription was initiated by the addition of recombinant nucleoside triphosphates (0.5 mM final concentration), and the templates were transcribed for 1 h at 30°C. The resulting transcripts were detected by primer extension.
Immunoprecipitation.
Protein extracts from MCF-7 cells were prepared in cell lysis buffer (50 mM Tris HCl [pH 7.5], 250 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 1 mM dithiothreitol) with the following protease inhibitors (2 μg of leupeptin per ml, 2 μg of aprotinin per ml, 10 μg of trypsin inhibitor per ml, 10 μg of tosyl lysine chloromethyl ketone [TLCK] per ml, 10 μg of tosyl phenylalanine chloromethyl ketone per ml, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and phosphatase inhibitors (50 mM NaF, 0.1 mM Na3VO4, and 10 mM β-glycerophosphatases). Protein extracts (1.5 mg) were incubated with 2 μg of the rabbit polyclonal Cdc25B antibody (Santa Cruz Biotechnology) for 2 h at 4°C with shaking. Protein A-Sepharose beads (40 μl; Zymed) were then added for overnight incubation at 4°C with shaking. The beads were subsequently collected by microcentrifugation and washed three times with lysis buffer. The immunoprecipitates were denatured by boiling in 10 μl of Laemmli sample buffer and then separated in a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE) gel. ER protein in immunoprecipitates was detected by Western blotting using H222 antibody.
In vitro interaction assays.
GST-tagged ER and PRB were expressed in a baculovirus expression system in the absence of hormone. His-tagged Cdc25B was expressed in Escherichia coli and purified by using a nitrilotriacetate affinity column following the manufacturer's protocol (Qiagen). The purified and glutathione-bound ER or PRB was incubated with purified Cdc25B in binding buffer (20 mM Tris [pH 8.0], 60 mM KCl, 0.1% NP-40, 10% glycerol, 0.1 mM PMSF, and 0.5 μg of leupeptin per ml) for 2 h at 4°C. The beads were then washed five times with binding buffer. ER- or PR-bound Cdc25B was eluted and separated on an SDS–10% PAGE gel and then detected by Western blotting using Cdc25B antibody (Santa Cruz). For defining the interaction sites of Cdc25B with ER, GST-Cdc25B and its deletion mutant fusion proteins were expressed in E. coli and purified using glutathione-Sepharose beads (Pharmacia). ER was in vitro translated in the presence of [35S]methionine using the reticulocyte lysate system (Promega). The glutathione-bound full-length Cdc25B or its deletion mutants were incubated with 35S-labeled ER in binding buffer. The beads were then washed five times with binding buffer. The bound ER was eluted and separated in SDS–4 to 20% gradient PAGE and then detected by autoradiography. The direct interactions between Cdc25B and PCAF or SRC-1 were assessed by incubating GST-Cdc25B with either 35S-labeled PCAF or SRC-1 (TNT coupled reticulocyte lysate systems; Promega) in a binding buffer as described above. The bound PCAF or SRC-1 was eluted and separated in SDS–7.5% PAGE and detected by autoradiography.
HAT assay.
HAT assay procedures were adapted from previously described methods (61). In brief, reactions employed 100 ng of purified recombinant PCAF and increasing amounts of Cdc25B protein or nonspecific protein (vector control), in the presence of 1 μg of core histones, 50 mM HEPES (pH 8.0), 10% glycerol, 1 mM dithiothreitol, 1 mM PMSF, 10 mM sodium butyrate, and 10 μM [3H]acetyl coenzyme A (Amersham Pharmacia). Reaction mixtures were incubated at 30°C for 1 h and then subjected to SDS-PAGE analysis and autoradiography. All assays were performed at least three times, and nucleosome concentrations had approximately equal amounts of protein visualized by SDS-PAGE as did the mixed histone reactions.
RESULTS
Increased expression of ER responsive genes in mammary glands overexpressing Cdc25B.
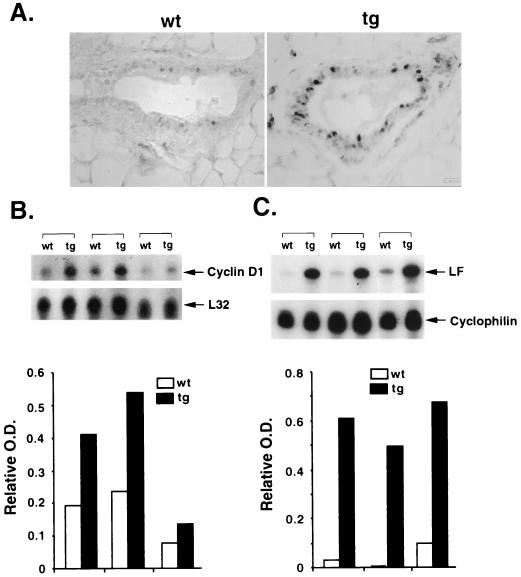
It has been shown that cyclin D1 is a potential ER target gene that mediates estrogen-dependent cell proliferation (1, 51, 55). Cyclin D1 expression is closely correlated with ER transcription activity in mammary cells. The potential role of Cdc25B in hormone responsiveness was first implied by an increased expression of cyclin D1 in mammary glands of multiple transgenic mouse lines ectopically overexpressing Cdc25B (39). As shown in Fig. 1A, mammary glands from 2-month-old virgin transgenic and nontransgenic mice were sectioned and processed for cyclin D1 immunostaining. The number of cyclin D1-positive cells and the level of cyclin D1 in transgenic glands were clearly greater than that in wild-type glands, suggesting an elevated ER transcriptional activity. Based on the notion that most of the estrogen response is mediated by direct binding of ER to an estrogen-responsive element to stimulate target gene expression (4, 41, 63), we further examined if the cyclin D1 expression is enhanced at the mRNA level by an RPA. RNA samples isolated from 3-to-4-month-old virgin mammary glands were hybridized with a mouse cyclin D1 antisense probe. Figure 1B shows that the cyclin D1 mRNA levels increased generally about twofold in virgin transgenic glands compared to their wild-type counterparts after normalized to the L32 internal control.
FIG. 1.
Marked increases in steroid receptor target gene expression in MMTV-Cdc25B transgenic mammary glands. (A) Immunohistochemistry of 2-month-old mammary gland sections from wild-type (wt) and transgenic (tg) mice for cyclin D1 expression. A significant number of epithelial cells from the tg section stained positive for cyclin D1 compared to those in the wt section. (B) RPA analysis of cyclin D1 transcripts. Five micrograms of RNA from mammary glands of different tg mice and their wt littermates was hybridized to cyclin D1-specific and control L32 antisense riboprobes. The protected fragments corresponding to cyclin D1 (202 bp) and L32 (112 bp) are indicated. (C) RPA analysis of lactoferrin (LF) transcripts. Ten micrograms of RNA from mammary glands was isolated and hybridized to LF and cyclophilin antisense probes as described above. Arrowheads indicate the protected fragments of LF and cyclophilin. RPA autoradiographies of cyclin D1 and LF were scanned and quantitated as relative optical density (O.D.) after being normalized to the internal control. Results are shown below their corresponding autoradiographies.
To examine whether other well-known estrogen-regulated genes are also affected, the expression of lactoferrin was also evaluated in transgenic glands by hybridizing RNA samples with the antisense mouse lactoferrin probe. The antisense cyclophilin probe was included in each sample as a loading control. Figure 1C shows that lactoferrin mRNA was barely detectable in normal virgin glands. A significant increase in lactoferrin mRNA was observed in transgenic virgin glands. Though varying from animal to animal, we found an average >20-fold induction of lactoferrin mRNA in Cdc25B transgenic virgin glands compared to the wild-type counterpart after normalizing the glands with cyclophilin mRNA. The observed greater response of lactoferrin to Cdc25B overexpression compared to that of cyclin D1 in transgenic mammary glands may be contributed in part by their different expression profiles in mammary glands. Cyclin D1 is broadly expressed in different cell types in mammary glands, whereas the Cdc25B transgene is only overexpressed in the epithelial cells of the mammary gland. Therefore, cyclin D1 expression in epithelial cells will be diluted by the contamination from other cell types in RPA analysis. This notion is further supported by the immunohistochemical analysis in which the number of cyclin D1-positive cells was scored directly (Fig. 1A). It is clear that cyclin D1 expression is greatly enhanced in the mammary epithelium of the transgenic mice compared to the nontransgenic mice. Taken together, these results suggest that overexpression of Cdc25B could enhance the responsiveness of the mammary gland to steroid stimulation.
Cdc25B is a coactivator for steroid receptors.
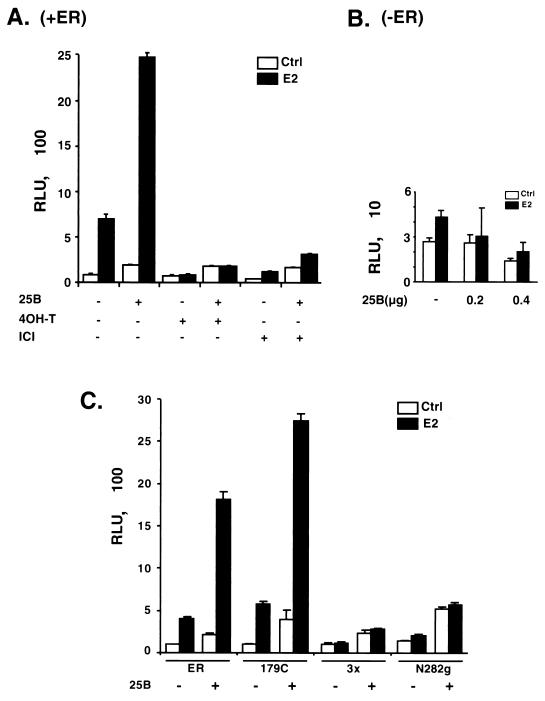
The increased expression of steroid receptor target genes in Cdc25B transgenic mammary glands prompted us to investigate the functional role of Cdc25B in the activation of steroid receptor-dependent transcription. We first examined whether Cdc25B could affect steroid receptor transactivation in mammalian cell lines by transient transfection assays. As shown in Fig. 2A, ER activation yielded about a 10-fold increase in the reporter gene expression after treatment with its ligand 17 β-estradiol. Coexpression of Cdc25B with ER consistently leads to a further ∼4-fold increase in hormone- and receptor-dependent transcriptional activity. A slight increase in hormone-independent ER transactivation was also observed, most likely due to the enhancement of ligand-independent activation of ER. Cdc25B has no effect on the reporter activity in the absence of the ER expression vector, indicating that the ability of Cdc25B to enhance gene expression requires the presence of ER (Fig. 2B). Similar results were observed when ER was coexpressed with another member of the Cdc25 proteins, Cdc25A (data not shown). In these experiments, ER levels were not increased by Cdc25B coexpression (data not shown). These results indicate that Cdc25B is able to enhance the hormone-dependent ER transactivation with a moderate effect on hormone-independent ER transcriptional activity.
FIG. 2.
(A and B) Cdc25B enhances transcription mediated by ER. HeLa cells were transiently transfected with 50 ng of ER expression plasmid and 100 ng of (ERE)3-tata-Luc reporter construct in the absence (−) or presence (+) of the Cdc25B expression plasmid, pCR3.1-Cdc25B (400 ng), using Lipofectin. After transfection, cells were washed and treated with 17 β-estradiol (E2) (10 −9 M) or its antagonists, 4OH-T (10−7 M) or ICI (10−6 M), as indicated for an additional 24 h. (C) Cdc25B acts mostly via the C-terminal AF2 domain of ER. One hundred nanograms of each ER mutant, helix 12 triple mutation (3x), AF1 deletion (179C), or AF2 deletion (N282g), was cotransfected with luciferase reporter and Cdc25B expression vector in HeLa cells and assayed as described in Materials and Methods. Luciferase activity was normalized per microgram of protein and expressed in relative luciferase activity units (RLUs). A single experiment representative of at least three independent experiments is detailed. The data shown indicate the means ± standard errors of the mean of quadruplicate estimations. 25B, Cdc25B.
We also investigated the effect of Cdc25B on transactivation of ER bound to the partial agonist 4-hydroxy-tamoxifen (4OH-T) and pure antagonist ICI 164384 (ICI). As expected, the addition of 4OH-T or ICI prevented the hormone-dependent transactivation of ER (Fig. 2A). Similarly, the addition of antagonist 4OH-T and ICI completely blocked the enhancement of Cdc25B on hormone-dependent ER activation while exerting no effect on hormone-independent ER transcription activity.
Two distinct activation functions, AF1 and AF2, have been identified in the amino-terminal AB region and in the carboxyl-terminal ligand binding EF region of ER, respectively. These activation functions determine the transcriptional activity of ER in target cells. In order to define the domain of ER that is responsible for the enhanced transcription activity, the effects of Cdc25B on the transcriptional activity of the AF1 (179C), AF2 (N282g), or helix 12 (3x) mutants of ER were examined in parallel with the wild-type ER. Figure 2C shows that the mutant 179C, which lacks the AF1 domain, induced the reporter gene expression in response to the hormone treatment due to the presence of AF2 function. Coexpression of Cdc25B with ER (179C) further enhanced the reporter activity to that of the extent similar to the wild-type ER. Deletion of AF2 (N282g) or mutation of helix 12 (3x) almost completely inactivated ER-dependent transcription activity. As expected, these receptor AF2 mutants failed to respond to the hormone treatment and exhibited constitutive transcriptional activity at 5 to 15% of the estrogen-dependent transcriptional activity of the wild-type ER. The addition of Cdc25B caused a slight increase in the hormone-independent transcription activity of these mutants. These results indicate that although Cdc25B augments both AF1- and AF2-dependent transcriptional activity, the enhancement of ER transactivation by Cdc25B mostly arises from the AF2 domain.
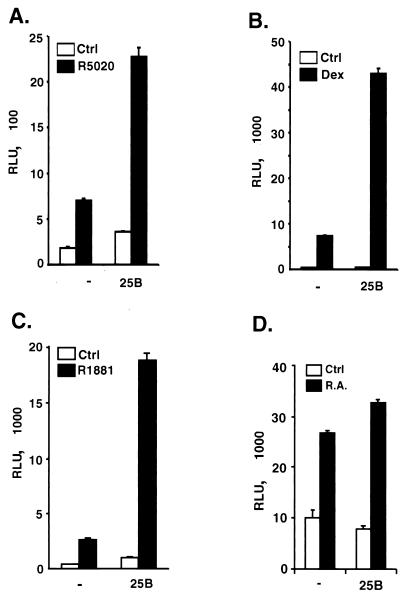
We next examined the effect of coexpression of Cdc25B on transcriptional activity of other intracellular nuclear receptors (NRs). As shown in Fig. 3, when Cdc25B was coexpressed with PR, the activity of PR was further stimulated ∼3.5-fold (Fig. 3A). Cdc25B also significantly enhanced hormone-dependent transcriptional activity of the GR receptor (Fig. 3B) and the androgen receptor (Fig. 3C), with an about sixfold further increase of reporter activity in both cases. In contrast, Cdc25B had a minimal effect on transcriptional activity of RAR under similar experimental conditions (Fig. 3D). These results suggest that Cdc25B appears to preferentially enhance the transcriptional activity of steroid receptors.
FIG. 3.
Effects of Cdc25B expression on transcriptional activities of other NRs. HeLa cells were transiently transfected with plasmids encoding the human receptors for progesterone (PRB) (100 ng) (A), GR (25 ng) (B), androgen (AR) (50 ng) (C), or RAR (50 ng) (D) and their cognate hormone-responsive reporter plasmids in the absence(−) or presence (+) of Cdc25B (400 ng). The cells were treated with the appropriate hormones as follows: PR, R5020 (10−7 M); GR, dexamethasone (Dex) (10−7 M); AR, R1881 (10−9 M); and RAR, retinoid acid (R.A.) (10−7 M). Luciferase activity was normalized per microgram of protein and expressed in relative luciferase activity units (RLUs). A single experiment representative of at least three independent experiments is shown. The data shown indicate the means ± standard errors of the mean of quadruplicate estimations.
Cdc25B-mediated coactivation of steroid receptors is independent of its cell cycle regulatory function.
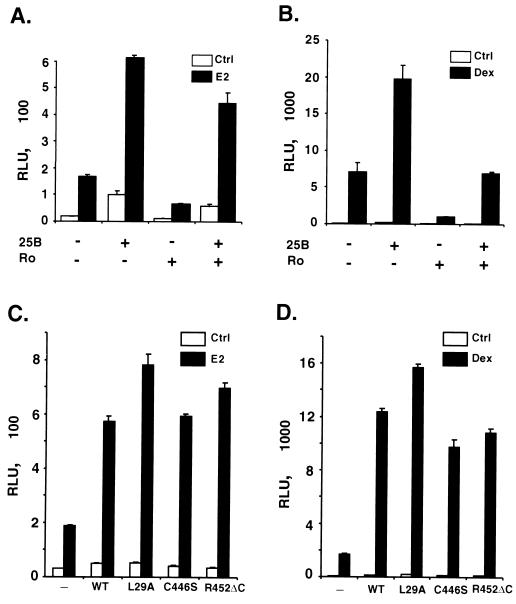
Cdc25B is able to dephosphorylate and activate Cdks to promote cell cycle progression. In addition, it has been shown that activation of Cdk2 is able to phosphorylate ER and enhance its transcriptional activity (53, 62). To determine if the effect of Cdc25B on steroid receptor transactivation is mediated by activation of Cdks, we used roscovitine to block the potential cyclin-dependent pathways in a transient transfection assay (45). Figure 4A illustrates that the addition of roscovitine leads to some general decrease in ER-dependent transcriptional activity. However, the enhancement of ER-mediated transcription by Cdc25B was not affected by the roscovitine treatment. Cdc25B still induced an ∼4-fold increase in ER-dependent transcription in the presence of ligand. A similar result was observed on GR-dependent transactivation (Fig. 4B), suggesting that Cdc25B can stimulate steroid receptor transcriptional activity independent of Cdk activity.
FIG. 4.
Cdc25B-mediated enhancement of steroid receptor transactivation is independent of its phosphatase activity. (A and B) HeLa cells were transfected with 50 ng of ER (A) or 25 ng of GR (B) expression plasmids, their appropriate reporter constructs, and 400 ng of Cdc25B expression vector as indicated. Transfected cells were treated with steroid receptor ligands for 24 h in the absence (−) or presence (+) of the cyclin E-Cdk2 inhibitor roscovitine (Ro, 60 μM). (C and D) HeLa cells were transfected with 50 ng of ER (C) or 25 ng of GR (D) expression plasmids and their appropriate reporter constructs, together with various Cdc25B mutants lacking the LXXLL motif (L29A) or phosphatase activity (C446S and R452ΔC). Luciferase activity was measured in the absence or presence of cognate receptor ligand and was normalized per microgram of protein and expressed in relative luciferase activity units (RLUs). A single experiment representative of at least three independent experiments is shown. The data shown indicate the means ± standard errors of the mean of quadruplicate estimations.
Cyclin D1 is another potential candidate that may be involved in Cdc25B's effect on steroid receptor activity. Cyclin D1 has been shown to stimulate the transcription of estrogen responsive genes independent of its cell cycle regulation function (47, 69, 70). It is reasonable to propose that increased cyclin D1 found in a Cdc25B-overexpressed mammary gland might contribute to the enhancement of Cdc25B on steroid receptor activity. To test this hypothesis, we performed a transient transfection assay in primary fibroblast cells isolated from cyclin D−/− mouse embryos (59). No significant changes were observed in the ability of Cdc25B to enhance the steroid receptor activity in cyclin D1 null fibroblast cells compared with the results from wild-type cells (data not shown), suggesting that cyclin D1 is not essential for the function of Cdc25B as an SRC.
To determine whether Cdc25B phosphatase activity is required for its effect on steroid receptor transactivation, we mutated the critical active sites from Cys446 and Arg452 to serine and alanine, respectively, to generate the phosphatase inactive protein C446S and R452ΔC as reported (67). These Cdc25B mutants were cotransfected into HeLa cells with the ER expression vector and the reporter plasmid. Figure 4C shows that C446S and R452ΔC retained their abilities to increase the hormone-dependent ER transactivation three- to fourfold, similar to the wild-type Cdc25B, indicating that phosphatase activity is not required for the Cdc25B-mediated increase in ER transactivation. The phosphatase-deficient mutants C446S and R452ΔC also stimulated the hormone-dependent GR transactivation (Fig. 4D), providing further evidence that the steroid receptor coactivation exerted by Cdc25B was unlikely to be mediated by its phosphatase activity.
Cdc25B enhances PRB-dependent cell-free transcription of chromatin.
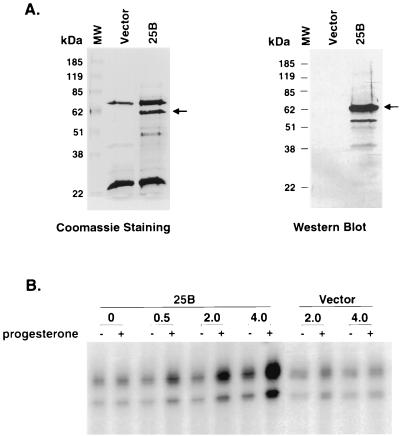
Several lines of evidence indicate that cell cycle related proteins are able to modulate steroid receptor transactivation either through Cdk-mediated phosphorylation or by direct protein-protein interaction (43, 47, 53, 62, 69, 70). To further substantiate our proposition that coactivation of steroid receptors by Cdc25B is achieved independent of these cell cycle-related events, we examined the ability of Cdc25B to enhance hormone-mediated PR transactivation in a cell-free system. His-tagged Cdc25B was bacterially expressed and purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography. An empty vector was included as a negative control in parallel with a Cdc25B expression vector throughout the protein purification procedures. The expected full-length Cdc25B was obtained after Ni-NTA purification as shown in Fig. 5A (left panel), and it was immunoreactive with anti-Cdc25B antibody (Fig. 5B, right panel). Two other major proteins were found to copurify with Cdc25B as shown with Coomassie staining. These proteins were nonspecific proteins because they were presented in the vector control fraction eluted from the Ni-NTA column and could not be recognized by anti-Cdc25B antibody. Other minor bands found in Coomassie staining likely represent the degradation of Cdc25B during protein purification procedures since they could be detected by anti-Cdc25B antibody in Western blot analysis. Purified Cdc25B protein and the vector control protein extracts were used in the subsequent in vitro transcription assays to ensure that the effect that we observed was due to the presence of Cdc25B protein rather than to copurified bacterial protein contaminants. As shown in Fig. 5B, when a low level of recombinant PRB was added to the preassembled chromatin, a slight activation of transcription was induced by the hormone treatment. A low level of PRB was used so that an optimal enhancement of Cdc25B could be achieved. The addition of exogenous Cdc25B further increased the hormone-dependent PR activation on chromatin, whereas it had a minimal effect on transcription in the absence of hormone. The effect of Cdc25B was dose dependent. A 10-fold increase in hormone-dependent PR transcription was observed when transcriptional activity of PR in the absence of Cdc25B was compared to that in the presence of the maximal dosage of Cdc25B tested. No significant change in PR transactivation was observed after the addition of the control vector protein extract. Thus, Cdc25B could enhance the PR-mediated transcription on chromatin in a ligand-dependent manner. This result suggested that Cdc25B could directly enhance the steroid receptor transactivation independent of the cell cycle.
FIG. 5.
(A) The full-length His6-tagged Cdc25B was expressed in E. coli and purified by Ni-NTA affinity chromatography. The recombinant protein was analyzed on an SDS–10% polyacrylamide gel and then subjected to staining with Coomassie brilliant blue (left) or Western blot analysis with polyclonal antibody against Cdc25B (right). Arrowheads indicate the full-length Cdc25B protein. It should be noted that two major nonspecific proteins copurified with Cdc25B. (B) Cdc25B enhances ligand-dependent transcription by purified PRB in vitro with a chromatin template. pPRE3-E4 was assembled into chromatin. Purified PRB (15 nM) and its ligand progesterone (10−7 M) were added to preassembled chromatin together with increasing amounts of Cdc25B protein or nonspecific protein (vector) as indicated, followed by a 30-min incubation at 27°C. The samples were then subjected to in vitro transcription analysis.
Cdc25B directly interacts with steroid receptors.
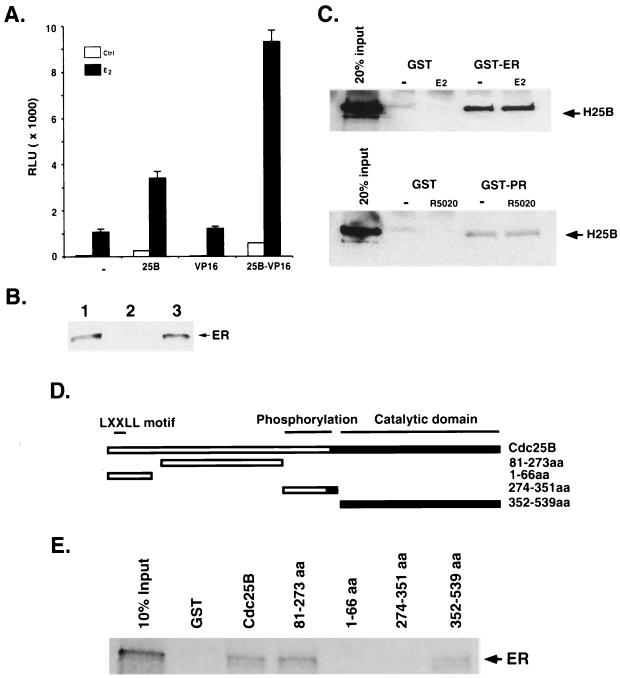
The finding that Cdc25B enhances steroid receptor transactivation independent of the cell cycle context suggests that Cdc25B may interact with steroid receptors to enhance transcription of hormone responsive genes. Therefore, we examined the interaction of Cdc25B with ER in vivo using a mammalian two-hybrid assay. Coexpression of VP16 did not significantly affect ER activity. In contrast, the chimeric Cdc25B-VP16 protein greatly increased the ligand- and ER-dependent transcription when compared to Cdc25B itself (Fig. 6A), suggesting that Cdc25B interacts with ER in vivo. The interaction of Cdc25B and ER was further substantiated by coimmunoprecipitation of ER with Cdc25B in a whole-cell extract of the MCF-7 cell line. As shown in Fig. 6B, an intense band of ER was detected in the immunoprecipitate using anti-Cdc25B antibody but not in the immunoprecipitate of negative control, whereby Cdc25B antibody was preabsorbed with excess Cdc25B peptide prior to immunoprecipitation. These results strongly suggested that Cdc25B interacts with ER in vivo.
FIG. 6.
Interaction of Cdc25B with steroid receptors in vivo and in vitro. (A) The expression vector (400 ng) for 25B-VP16, VP16, or Cdc25B was cotransfected with ER (50 ng) and (ERE)3-tata-Luc reporter construct (100 ng) into HeLa cells and assayed as described in Materials and Methods. (B) MCF-7 whole-cell extract (1.5 mg) was immunoprecipitated with peptide-absorbed Cdc25B antibody (lane 2) or Cdc25B antibody (lane 3). MCF-7 whole-cell extract (50 μg) (lane 1) was loaded as a control and probed with the ER antibody. ER was detected only in Cdc25B antibody immunoprecipitate, not in the immunoprecipitate using peptide-absorbed antibody, indicating that ER specifically coimmunoprecipitated with Cdc25B. (C) Purified full-length Cdc25B was incubated with baculovirus-expressed GST-ER (upper panel), GST-PR (low panel), or GST alone (control) bound to glutathione-Sepharose beads in the absence (−) or presence (+) of the appropriate ligand. ER- or PR-bound Cdc25B was analyzed on an SDS–10% polyacrylamide gel followed by Western blot analysis using an antibody specific to Cdc25B. (D) Schematic presentation of the GST fusion proteins of Cdc25B and its deletion mutants. Indicated are potential functional domains of Cdc25B and GST fusion proteins used in in vitro interactions. (E) The glutathione-bound full-length Cdc25B or its deletion mutants were incubated with 35S-labeled ER in binding buffer as described in Materials and Methods. The bound ER was eluted and separated in an SDS–4 to 20% gradient PAGE gel and then detected by autoradiography as indicated by the arrowhead.
To further confirm the direct interaction between Cdc25B and steroid receptors, we performed an in vitro GST pull-down assay. Figure 6C shows that a significant amount of Cdc25B is able to directly interact with ER in vitro, regardless of the absence or the presence of ligand. To examine whether Cdc25B also interacts with other members of the steroid receptor family, an analogous experiment was carried out using PR. Similarly, direct interaction between Cdc25B and PR was observed in vitro in both the absence and presence of ligand (Fig. 6C). Therefore, these results indicate that Cdc25B interacts directly with ER and PR in vitro. It should be emphasized that binding of receptor to the response element is a hormone-dependent event; thus, regardless of the interaction of Cdc25B with receptor is hormone dependent or not, the Cdc25B activated transcription is hormone dependent.
We next attempted to define regions of Cdc25B that are important for interaction with ER. A series of Cdc25B fragments was generated based on the potential functional domains of Cdc25B depicted in Fig. 6D and then fused to GST. As expected, full-length Cdc25B is able to interact with ER in this reciprocal GST pull-down assay (Fig. 6E). Two interaction domains are mapped within the Cdc25B. One domain is located within the C-terminal region from aa 352 to 539 that is relatively conserved among the Cdc25B family proteins (Fig. 6E). The second ER interaction domain is localized within the middle region of the molecule from aa 81 to 273 (Fig. 6E). Interestingly, the N-terminal fragment (aa 1 to 66) that contains an putative NR box (LXXLL motif) (21) failed to bind to ER. This is consistent with the result that Cdc25B with the NR box mutation (L29A) still could enhance ER and GR transactivation similar to wild-type Cdc25B (Fig. 4C and D). The fragment (aa 274 to 351) that comprises multiple phosphorylation sites critical for cell cycle regulatory function of Cdc25B is also unable to show steroid receptor interaction. Taken together, these results are consistent with our finding in the transactivation studies that Cdc25B physically interacts with steroid receptors to enhance their transcriptional activity.
Involvement of PCAF and CBP in Cdc25B coactivation.
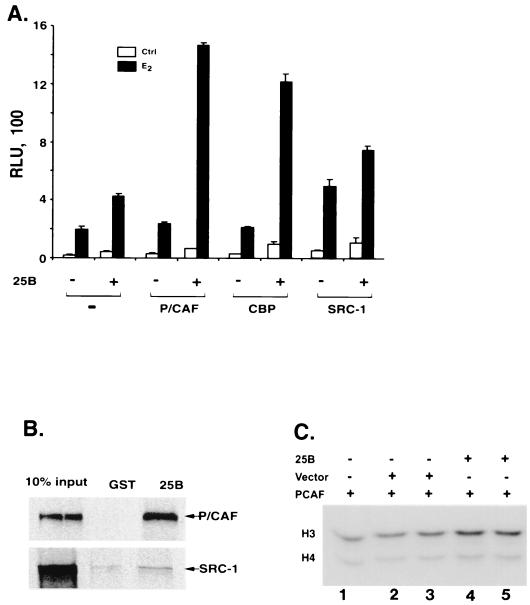
Activated NRs have been shown to bind to hormone response elements and to recruit transcription coactivators, such as the SRC-1 family of coactivators, PCAF, and CBP, to the target promoters (6, 9, 29). These coactivators, SRCs, PCAF, and CBP/p300, all contain HAT activity that can remodel chromatin structure and recruit basal transcription machinery to target promoters to enhance hormone-dependent transcription (6, 20, 29, 30, 36, 42). Similar to the SRC-1 family of coactivators, Cdc25B is able to interact with the steroid receptor and enhance the receptor transactivation in chromatin templates, suggesting that the coactivation function of Cdc25B may also involve the recruitment of HAT-containing factors to modify the chromatin structure. To test this hypothesis, HAT-containing proteins, PCAF and CBP, were included in transient transcription assays to examine their potential effects on Cdc25B-enhanced ER-dependent transcription. As shown in Fig. 7A, cotransfection of PCAF or CBP could synergize with Cdc25B to further increase the level of ER-mediated transcription. On the other hand, coexpression of SRC-1 only has a less than additive effect on the ER activity in presence of Cdc25B, suggesting preferential differences among the HAT proteins to Cdc25B.
FIG. 7.
Interaction of Cdc25B with PCAF. (A) Synergistic effect of PCAF and CBP on Cdc25B coactivation function. HeLa cells were transfected with (ERE)3-tata Luc reporter (100 ng) and ER expression plasmid together with (+) or without (−) expression vectors of Cdc25B (400 ng), PCAF (200 ng), CBP (200 ng), or SRC-1 (200 ng) or in combination as indicated. Luciferase activity was measured 24 h in the absence (−) or presence (+) of receptor ligand 17 β-estradiol. (B) The glutathione-bound full-length Cdc25B was incubated with 35S-labeled PCAF or SRC-1 as described in Materials and Methods. The bound PCAF or SRC-1 was eluted and separated in an SDS–7.5% PAGE gel and then detected by autoradiography as indicated by the arrowhead. (C) HAT assays were carried out using core histone as the substrate in the presence of purified PCAF together with increasing amounts of purified Cdc25B protein or nonspecific protein as indicated. Lane 1, PCAF; lanes 2 and 3, PCAF plus nonspecific protein (vector); and lanes 4 and 5, PCAF plus Cdc25B.
The ability of Cdc25B to recruit HAT proteins was further assessed through protein interaction assays. As shown in Fig. 7B, a significant amount of PCAF was coprecipitated with GST-Cdc25B but not with the GST control, indicating the direct interaction between Cdc25B and PCAF. Slightly weaker interactions were observed between Cdc25B-CBP (data not shown). However, the interaction of Cdc25B with SRC-1 is quite limited, and little SRC-1 was specifically pulled down by GST-Cdc25B. The weak interaction is consistent with the marginal increase of Cdc25B coactivation of ER-dependent transcription.
The direct interaction of Cdc25B with both ER and PCAF suggests that Cdc25B might act as a bridging factor to either increase the association of ER with PCAF or to remodel the chromatin in the promoter region of the target genes or both. The coimmunoprecipitation experiment indicated that there is no significant increase of PCAF coprecipitated with ER in the presence of Cdc25B (data not shown), suggesting that Cdc25B is unlikely to enhance the association of ER and PCAF. On the other hand, Fig. 7C shows that Cdc25B enhances HAT activity of PCAF in vitro. A preferential increase in H3 acetylation, but not H4 acetylation, is observed in the presence of Cdc25B compared to that in the nonspecific protein control, although PCAF acetylates both histone H3 and H4. Cdc25B itself has no HAT activity (data not shown). Taken together, Cdc25B interacts directly with both ER and PCAF. These direct interactions might enhance chromatin remodeling through acetylation of histones.
DISCUSSION
Cdc25 proteins are generally known as dual specificity protein phosphatases that dephosphorylate and activate Cdk activity critical for cell cycle progression (11, 46). Cdc25 proteins are involved in many biological processes. During DNA replication and in the presence of DNA damage, Cdc25 proteins are phosphorylated by activated Cdk-related kinases and bind to 14-3-3 proteins that exclude them from the nucleus, preventing Cdc25 from activating Cdc2 and delaying entry into mitosis (9, 15, 37). Cdc25 proteins are also downstream targets of proliferation signal pathways. Cdc25 phosphatase activity could be stimulated by Raf-dependent phosphorylation (17). Cdc25A and -B expression has been shown to be stimulated by the c-myc oncogene and might mediate c-myc-induced cell proliferation and apoptosis (16). Until now, the biological function of Cdc25 proteins has been ascribed to their phosphatase activity.
In the present study, we uncovered a novel function of Cdc25B. It serves as an SRC. Cdc25B was first shown to enhance ER-dependent transcription, mostly acting through the AF2 domain of ER. The ligand-dependent coactivation of Cdc25B could be blocked by partial ER agonist 4OH-T and pure antagonist ICI. Cdc25B also stimulates the transcriptional function of other steroid hormone receptors, including PR, GR, and AR. Another Cdc25 protein, Cdc25A, has a similar effect on steroid receptor-mediated transactivation (data not shown). These findings are of great interest since they demonstrate that protein phosphatases can also serve as coactivators to enhance hormone responsiveness. Thus, its coactivator activity may contribute to the observable elevated in vivo expression of hormone responsive genes, cyclin D1, and lactoferrin in mammary glands of transgenic mice overexpressing Cdc25B.
We next pursued the molecular mechanism underlying the Cdc25B coactivation function. We initially assumed that the Cdc25B coactivation function might be a secondary effect resulting from the cell cycle regulation and somehow linked to its phosphatase activity. In addition, the critical downstream targets of Cdc25B phosphatase, Cdk2 or Cdc2, have been shown to stimulate ER transactivation through ligand-independent receptor phosphorylation (53, 62). However, several pieces of evidence in our study indicate that this may not be the case. First, inhibition of active Cdks only resulted in a slight decrease in steroid receptor-mediated transcription. Furthermore, it did not alter the fold of induction of steroid receptor-mediated transcription enhanced by Cdc25B. Second, phosphatase inactive mutants of Cdc25B still retained their ability to enhance ER and GR transactivation to a level similar to that of the wild-type Cdc25B. Furthermore, Cdc25B is able to enhance PRB-dependent transcription in vitro in a cell-free chromatin template system. Thus, the coactivation function exerted by Cdc25B is most likely independent of its phosphatase activity.
Cdc25B was found to interact with ER in a mammalian two-hybrid assay and was coimmunoprecipitated with ER (Fig. 6A and B). The direct interaction between Cdc25B and steroid receptors was further substantiated by a GST pull-down assay. Though Cdc25B largely enhances hormone-dependent transcription of steroid receptors, the in vitro interaction of Cdc25B with steroid receptors is hormone independent. The discrepancy is likely due to the fact that only purified ER is present in an in vitro binding assay and that, therefore, Cdc25B is able to bind to ER in a hormone-independent manner. Since the binding of the ER complex to its response element in vivo depends on the hormone treatment, even though the binding of Cdc25B to ER and PR is hormone independent, Cdc25B-dependent activation of ER and PR would be hormone dependent. However, we cannot exclude the possibility that ER might be associated with many other proteins in the absence of ligand which could mask the site(s) for Cdc25B binding. Hormone treatment might dissociate these proteins from ER and allow Cdc25B to access and bind to ER. Thus, our results consistently show that Cdc25B could enhance steroid receptor transactivation through protein-protein interaction.
The ER interacting regions were mapped to the middle region of the molecule and also to the catalytic region that is relatively conserved in the Cdc25B family proteins. Surprisingly, the N-terminal fragment that contains a putative NR box (LXXLL motif) was not sufficient for its interaction with ER. Consistent with the above observation, an L29A mutant lacking the LXXLL motif retained its ability to coactivate the transcription activity of steroid receptors.
The SRCs are proposed to possess the ability to remodel the chromatin structure through its own HAT activity or through recruiting other factors that possess HAT activity to facilitate the access of GTFs to the target promoter (14, 42, 66). SRCs may also stabilize the preinitiation complex through direct or indirect interactions with GTFs. In a cell-free transcription system using a chromatin template, purified Cdc25B increased PR-dependent transcription on a chromatin template, clearly indicating its direct potential coactivator function. In addition, the involvement of HAT on Cdc25B coactivation is also implicated by the observation that Cdc25B-mediated ER transcription activity is markedly enhanced when coexpressed with PCAF or CBP. In fact, we showed that Cdc25B is able to interact directly with both ER and PCAF. These interactions do not seem to facilitate the recruitment of PCAF to the promoters of hormone-responsive genes, but they do modulate the HAT activity of PCAF in vitro to increase H3 acetylation. Thus, the alteration of the HAT activity of PCAF might result in the remodeling of the chromatin structure through histone modification. This might subsequently promote the formation and stabilization of the preinitiation complex in the promoter of ER target genes and contribute to the coactivation function of Cdc25B.The possibility that Cdc25B itself stabilizes the association of PCAF with ER to the hormone-responsive promoters could not be excluded at present.
Since proper function of steroid receptors is critical for normal mammary gland development, it is conceivable that cells with a genetic alteration in favor of hormone stimulation would gain a growth advantage and contribute to preneoplastic lesions. In fact, more than 70% of primary breast cancers are ER positive and exhibit hormone-dependent growth, although only 10 to 25% of mammary epithelial cells express ER in normal mammary glands (8, 48). Therefore, ER-positive epithelial cells appear to be more vulnerable for the oncogenic challenge than do ER-negative cells. With regard to the role of Cdc25B in breast cancer development, we speculate that Cdc25 proteins play dual functions. On one hand, Cdc25A and -B overexpression and amplification increase hormonal response and stimulate the expression of hormone target genes such as cyclin D1, lactoferrin, etc., resulting in abnormal epithelial cell proliferation. On the other hand, the Cdc25 proteins activate Cdk activity to deregulate DNA replication and the DNA damage checkpoint and promote cell cycle progression. The two functions of Cdc25 could complement or synergistically interact with each other to induce epithelial cell transformation and contribute to breast carcinogenesis. It will be of great interest to determine whether Cdc25B amplification and overexpression are involved in the transition of breast cancer from a hormone-dependent to a hormone-independent state.
In summary, we characterized a novel function of Cdc25B protein phosphatase as an SRC. Cdc25B directly interacts with ER and PR to enhance receptor transactivation independent of its well-known cell cycle regulatory function. Identification of this steroid receptor coactivation function may help us to understand the molecular mechanisms of Cdc25B in breast carcinogenesis and thereby design better therapeutic strategies for breast cancer therapy.
ACKNOWLEDGMENTS
We thank G. Greene for H222 antibody and M.-J. Tsai and Jiemin Wong for helpful discussions. We also thank B. W. O'Malley, D. Moore, S. Ellege, Z. Nawaz, and D. Bramblett for critical readings of the manuscript and L. Gong for excellent technical assistance.
This work was supported by an NIH grant and grant DAMD-17-94-J-4400 to S.Y.T. and a postdoctoral fellowship (DAMD-17-98-1-8025) to Z.-Q.M. Z.L. is a recipient of a National Research Service Award. E.S.W.N. is a recipient of the Croucher Foundation Fellowship, Hong Kong, SAR, China.
Z. Liu and E. S. W. Ngan contributed equally to this work.
REFERENCES
- 1.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 2.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 3.Baldin V, Cans C, Superti-Furga G, Ducommun B. Alternative splicing of the human CDC25B tyrosine phosphatase. Possible implications for growth control? Oncogene. 1997;14:2485–2495. doi: 10.1038/sj.onc.1201063. [DOI] [PubMed] [Google Scholar]
- 4.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 5.Benz C C, Scott G K, Sarup J C, Johnson R M, Tripathy D, Coronado E, Shepard H M, Osborne C K. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 6.Blanco J C, Minucci S, Lu J, Yang X J, Walker K K, Chen H, Evans R M, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castles C G, Fuqua S A W. Alterations within the estrogen receptor in breast cancer. In: Pasqualini J R, Katznellenbogen B S, editors. Hormone-dependent cancer. New York, N.Y: Marcel Dekker, Inc; 1996. pp. 81–105. [Google Scholar]
- 9.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Liu T H, Walworth N C. Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev. 1999;13:675–685. doi: 10.1101/gad.13.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta. 1997;1332:M53–M63. doi: 10.1016/s0304-419x(96)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Fauman E B, Cogswell J P, Lovejoy B, Rocque W J, Holmes W, Montana V G, Piwnica-Worms H, Rink M J, Saper M A. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. doi: 10.1016/s0092-8674(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 13.Fendrick J L, Raafat A M, Haslam S Z. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia. 1998;3:7–22. doi: 10.1023/a:1018766000275. [DOI] [PubMed] [Google Scholar]
- 14.Freedman L P. Increasing the complexity of coactivation in nuclear receptor signaling. Cell. 1999;97:5–8. doi: 10.1016/s0092-8674(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 15.Furnari B, Blasina A, Boddy M N, McGowan C H, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 17.Galaktionov K, Jessus C, Beach D. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9:1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 18.Galaktionov K, Lee A K, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 19.Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366–2368. [PubMed] [Google Scholar]
- 20.Glass C K, Rosenfeld M G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 21.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 22.Henderson B E, Ross R, Berstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 23.Hernandez S, Hernandez L, Bea S, Cazorla M, Fernandez P L, Nadal A, Muntane J, Mallofre C, Montserrat E, Cardesa A, Campo E. cdc25 cell cycle-activating phosphatases and c-myc expression in human non-Hodgkin's lymphomas. Cancer Res. 1998;58:1762–1767. [PubMed] [Google Scholar]
- 24.Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13:4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui R, Cornish A L, McClelland R A, Robertson J F, Blamey R W, Musgrove E A, Nicholson R I, Sutherland R L. Cyclin D1 and estrogen receptor messenger RNA levels are positively correlated in primary breast cancer. Clin Cancer Res. 1996;2:923–928. [PubMed] [Google Scholar]
- 26.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan V C. Overview from an international conference on long-term tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1992;84:231–234. doi: 10.1093/jnci/84.4.231. [DOI] [PubMed] [Google Scholar]
- 28.Kenny F S, Hui R, Musgrove E A, Gee J M, Blamey R W, Nicholson R I, Sutherland R L, Robertson J F. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5:2069–2076. [PubMed] [Google Scholar]
- 29.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 30.Kraus W L, Kadonaga J T. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo Y, Yasui W, Ue T, Yamamoto S, Yokozaki H, Nikai H, Tahara E. Overexpression of cyclin-dependent kinase-activating CDC25B phosphatase in human gastric carcinomas. Jpn J Cancer Res. 1997;88:947–952. doi: 10.1111/j.1349-7006.1997.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 33.Lanz R B, McKenna N J, Onate S A, Albrecht U, Wong J, Tsai S Y, Tsai M J, O'Malley B W. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee S K, Anzick S L, Choi J E, Bubendorf L, Guan X Y, Jung Y K, Kallioniemi O P, Kononen J, Trent J M, Azorsa D, Jhun B H, Cheong J H, Lee Y C, Meltzer P S, Lee J W. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem. 1999;274:34283–34293. doi: 10.1074/jbc.274.48.34283. [DOI] [PubMed] [Google Scholar]
- 35.Leygue E, Dotzlaw H, Watson P H, Murphy L C. Expression of the steroid receptor RNA activator in human breast tumors. Cancer Res. 1999;59:4190–4193. [PubMed] [Google Scholar]
- 36.Liu Z, Wong J, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc Natl Acad Sci USA. 1999;96:9485–9490. doi: 10.1073/pnas.96.17.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 38.Lu P J, Zhou X Z, Shen M, Lu K P. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- 39.Ma Z Q, Chua S S, DeMayo F J, Tsai S Y. Induction of mammary gland hyperplasia in transgenic mice over-expressing human Cdc25B. Oncogene. 1999;18:4564–4576. doi: 10.1038/sj.onc.1202809. [DOI] [PubMed] [Google Scholar]
- 40.Ma Z Q, Tsai M J, Tsai S Y. Suppression of gene expression by tethering KRAB domain to promoter of ER target genes. J Steroid Biochem Mol Biol. 1999;69:155–163. doi: 10.1016/s0960-0760(98)00154-x. [DOI] [PubMed] [Google Scholar]
- 41.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenna N J, Lanz R B, O'Malley B W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 43.McMahon C, Suthiphongchai T, DiRenzo J, Ewen M E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina D. The mammary gland: a unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996;1:5–18. doi: 10.1007/BF02096299. [DOI] [PubMed] [Google Scholar]
- 45.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 46.Millar J B, McGowan C H, Lenaers G, Jones R, Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuman E, Ladha M H, Lin N, Upton T M, Miller S J, DiRenzo J, Pestell R G, Hinds P W, Dowdy S F, Brown M, Ewen M E. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen O W, Hoyer P E, van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Res. 1987;47:5748–5751. [PubMed] [Google Scholar]
- 49.Pike M C, Krailo M D, Henderson B E, Casagrande J T, Hoel D G. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 50.Pike M C, Spicer D V, Dahmoush L, Press M F. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 51.Prall O W, Rogan E M, Musgrove E A, Watts C K, Sutherland R L. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds R A, Yem A W, Wolfe C L, Deibel M R, Jr, Chidester C G, Watenpaugh K D. Crystal structure of the catalytic subunit of Cdc25B required for G2/M phase transition of the cell cycle. J Mol Biol. 1999;293:559–568. doi: 10.1006/jmbi.1999.3168. [DOI] [PubMed] [Google Scholar]
- 53.Rogatsky I, Trowbridge J M, Garabedian M J. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–22302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- 54.Russo I H, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 55.Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci USA. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Said T K, Conneely O M, Medina D, O'Malley B W, Lydon J P. Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology. 1997;138:3933–3939. doi: 10.1210/endo.138.9.5436. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 58.Shibata H, Spencer T E, Onate S A, Jenster G, Tsai S Y, Tsai M J, O'Malley B W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 59.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 60.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 61.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 62.Trowbridge J M, Rogatsky I, Garabedian M J. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc Natl Acad Sci USA. 1997;94:10132–10137. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai M J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 64.Tzukerman M T, Esty A, Santiso-Mere D, Danielian P, Parker M G, Stein R B, Pike J W, McDonnell D P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 65.Wu W, Fan Y H, Kemp B L, Walsh G, Mao L. Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res. 1998;58:4082–4085. [PubMed] [Google Scholar]
- 66.Xu L, Glass C K, Rosenfeld M G. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 67.Xu X, Burke S P. Roles of active site residues and the NH2-terminal domain in the catalysis and substrate binding of human cdc25. J Biol Chem. 1996;271:5118–5124. doi: 10.1074/jbc.271.9.5118. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Qi C, Jain S, Le Beau M M, Espinosa III R, Atkins G B, Lazar M A, Yeldandi A V, Rao M S, Reddy J K. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc Natl Acad Sci USA. 1999;96:10848–10853. doi: 10.1073/pnas.96.19.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zwijsen R M, Buckle R S, Hijmans E M, Loomans C J, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zwijsen R M, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]