Macrophages activate a cascade to drive anti-androgen therapy resistance in the prostate bone metastasis microenvironment.
Abstract
In the prostate bone metastasis microenvironment, macrophages activate a cascade that involves Activin A, the extracellular matrix, and SRC kinase and drives resistance to anti-androgen therapy. These findings (Li et al., 2023. J. Exp. Med. https://doi.org/10.1084/jem.20221007) have broad implications, including metastasis diversity in different tissue milieus and the interplay between hormones and immunity.
Androgen dependency is a feature of prostate cancer (PC), and hormone deprivation is the mainstay therapy to treat advanced tumors. Anti-androgen therapies, which include surgical intervention and administration of gonadotropin inhibitory factor and antagonists of the androgen receptor, show a robust clinical efficacy in the initial phases of the disease. However, PC in a portion of patients develops in a castration-resistant tumor (CRPC) that is refractory to hormonal therapies and progresses into a metastatic disease in most cases. Multiple cell-intrinsic mechanisms of resistance have been described, including in situ androgen synthesis and constitutive activation of the androgen receptor following genetic alterations (Lorente et al., 2015). Notably, profiling of the immune infiltrate in PC unveiled a contribution of the tumor microenvironment (TME) to therapy resistance, and novel cell non-autonomous mechanisms that hinder therapy efficacy recently emerged (Guan et al., 2022).
Insights from Diletta Di Mitri, Fabio Conforti, and Alberto Mantovani.
Cancer cells need to adapt to the tissue that hosts them, and thus shape the tissue microenvironment to create a niche that supports survival, proliferation, and ultimately invasion. Androgen deprivation in a castration-resistant murine model influenced tumor infiltrating B cells that in turn activate an IKK-α and Stat3-dependent proliferation program, resulting in therapy failure (Ammirante et al., 2010). On a similar line, IL-23 released by granulocytic myeloid suppressor cells activated androgen receptor signaling in PC cells to promote androgen deprivation therapy (ADT) resistance (Calcinotto et al., 2018). Tumor-associated macrophages (TAMs) are an essential component of the TME (Mantovani et al., 2022) and contribute to PC tumor progression by paracrine signaling (Masetti et al., 2022). Indeed, TAMs transfer cholesterol to tumor cells, thus contributing to androgen synthesis. Accordingly, TAM depletion reduces androgen levels within prostate tumors, and combinatorial administration of TAM-depleting agents and androgen deprivation shows strong preclinical efficacy (Escamilla et al., 2015; El-Kenawi et al., 2021).
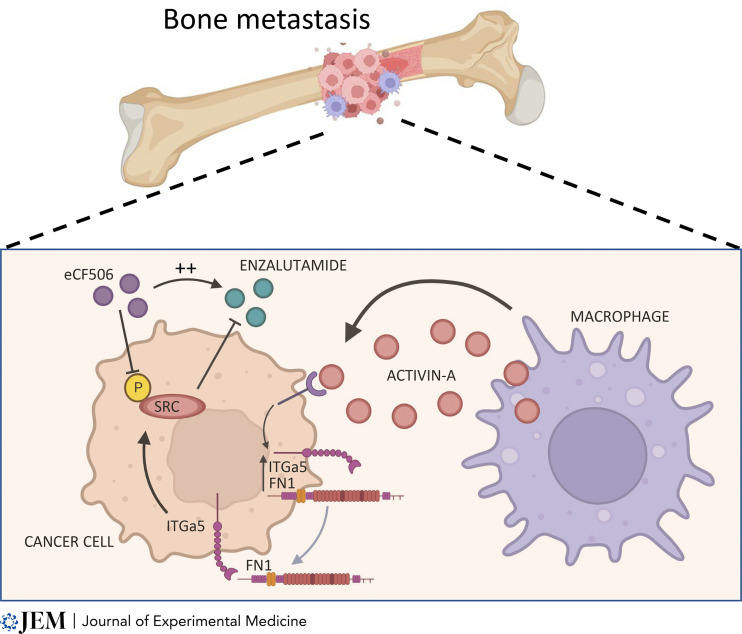
Bones are a major site of PC metastasis, which represent a formidable clinical challenge. In the present issue, Li et al. (2023) addressed the critical question of bone metastasis in PC, taking advantage of an androgen-sensitive metastatic model of PC that develops resistance in vivo. The authors describe a novel heterotypic interaction between cancer cells and macrophages that sustains resistance to androgen deprivation in the context of bone metastatic disease. The authors apply a deconvolution analysis to RNA datasets from PC patients and show that macrophages are enriched in bone metastasis when compared to other organs or primary tumors. Notably, TAM abundance in bone metastasis was associated with poor overall survival in patients with metastatic CRPC (mCRPC) that underwent anti-androgen therapies. To dissect the contribution of the TME to therapy resistance, the authors developed a novel model based on intra-cardiac injection of the MycCap-Bo cell line, derived from three rounds of in vivo selection of androgen sensitive bone homing cells. MycCap-Bo cells invade the bones and acquire resistance to enzalutamide 14 d after injection, representing a model allowing for the distinction between metastasis formation and resistance to therapy. Investigation of primary tumors and metastatic sites in this model revealed that TAM-derived Activin A induced an ECM-related transcription program in cancer cells, resulting in resistance to enzalutamide therapy (Fig. 1). The authors then show that Activin A induced Fibronectin 1 (FN1) that sustained the activation of an SRC-mediated signaling pathway in tumor cells, promoting cancer cell proliferation. Accordingly, SRC phosphorylation was reduced upon macrophage depletion. Finally, inhibition of SRC signaling by eCF506 administration in tumor-bearing mice significantly inhibited enzalutamide resistance.
Figure 1. TAMs and bone metastasis. Li et al. (2023) show that Activin A released by macrophages in the bone metastatic niche induces phosphorylation of SRC that activates an ECM-related transcription program in cancer cells, with production of FN1 resulting in resistance to enzalutamide therapy. Inhibition of SRC activation by eCF506 reinforces enzalutamide efficacy and overcomes resistance. Created with Biorender.com.
The study from Li et al. (2023) raises a number of critical questions and provides tools and indications for future investigations. The MycCap cancer cells colonize the bone when injected and are intrinsically sensitive to castration, acquiring resistance in vivo over time; these cells thus provide a useful model to investigate the microenvironment contribution to ADT failure specifically in metastasis. This metastatic androgen-dependent model of PC secondaries may be invaluable for further dissection of molecular pathways. Importantly, distinct tumor sites may respond differently to therapies, for reasons still to be elucidated. The results obtained by Li et al. (2023) corroborate this hypothesis and unveil dissimilarities in the composition of TME between primary tumors and bone metastasis that need to be further investigated. A more comprehensive profiling of the TME would be strongly needed to dissect the mechanisms that dictate the site dependency of tumor response to therapies.
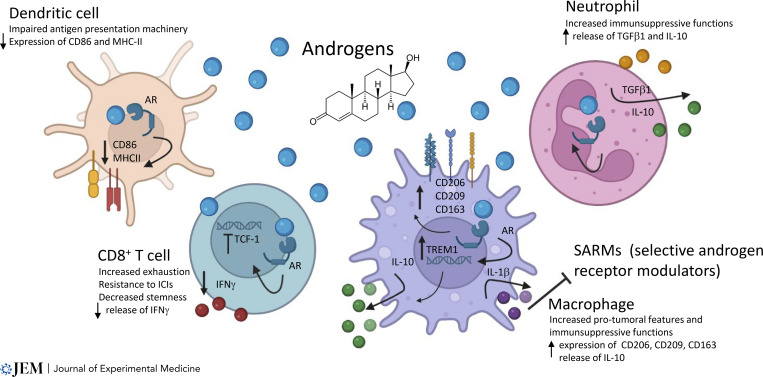
Interestingly, Li et al. (2023) ascribe resistance to ADT to the activation of an ECM–receptor process, the FN1-ITGa5 axis, and the downstream activation of the SRC pathway in cancer cells. However, the mechanisms by which the engagement of this ECM–receptor process lead to therapy resistance remain unclear. For example, does the activation of SRC flow into a reactivation of androgen signaling? Or, alternatively, do cancer cells become careless of androgen because of SRC-dependent sustained proliferation? These questions need to be addressed. The ECM is an underevaluated component of the TME that critically contributes to cancer progression and dissemination and recently emerged as predictive of tumor progression (Pearce et al., 2018). Importantly, the composition and architecture of the ECM may influence androgen dependency of PC cells, and mechanosensing pathways function as positive regulators of the androgen receptor in PC (Kuser-Abali et al., 2015). The possibility that the expression of FN1 downstream of the macrophage–cancer cell interaction can influence the composition of the ECM and can provoke androgen receptor activation is fascinating and should be investigated. Of note, the described axis results in ADT resistance, but whether the release of Activin A by macrophages and the consequent activation of ECM–receptor signaling in cancer cells is provoked or amplified by enzalutamide treatment remains uncovered. It has to be considered that in addition to its role in the sustenance of epithelial prostate cells, androgen signaling shapes the activation of the immune compartment (Fig. 2). For example, activation of the androgen receptor has been reported to impair T cell anti-cancer activity and to confer pro-tumoral functions to TAMs in preclinical settings (Pala et al., 2022b).
Figure 2. The complex interaction of androgens with the immune system. AR signaling regulates the activation of the immune compartment. Engagement of AR in dendritic cells causes a downregulation of CD86 and MHC-II, thus impairing antigen presentation. In CD8+ T cells, activation of the AR reduces the expression of TCF-1–dependent stemness and drives cells to exhaustion. In macrophages, AR signaling increases the transcription of TREM1 that in consequence sustains the upregulation of CD206, CD163, and CD209 and the release of IL-10. Additionally, exposure to androgens induces the release of IL-1β by TAMs that in turn hinders the efficacy of selective androgen receptor modulators (Mantovani et al., 2008). Activation of AR confers to neutrophils immunosuppressive capabilities mediated by TGFβ and IL-10 release. ICI, immune checkpoint inhibitor. Created with Biorender.com.
Finally, this paper reported intriguing new insights of SRC inhibition in PC. The SRC family of kinases (SFKs) mediates signaling pathways which have been implicated in PC cell growth, invasion, and metastasis in preclinical models (Fizazi, 2007). However, although this evidence points to SFKs as a promising therapeutic target, several prospective trials failed to show clinical benefit in patients with mCRPC from their blockade (Araujo et al., 2013). Li et al. (2023) provide evidence useful to understand the negative results obtained so far with SFKs inhibitors and to revisit their usage in mCRPC. First, in all trials conducted so far testing SFK inhibition, there was no patient selection according to metastatic sites of disease (Gao et al., 2022). The data reported by Li et al. (2023) suggest that the FN1-ITGA5-SRC pathway is significantly overactivated in bone metastases as compared with other metastatic sites. This raises the hypothesis that the specific patient subgroup with only bone metastases may be the one that most benefits from SFK inhibition.
Second, in all trials so far available, SFK inhibitors have been tested as monotherapy or in combination with chemotherapy (Gao et al., 2022). Li et al. show that the SRC pathway is involved in acquired resistance to next-generation androgen receptor (AR) inhibitors, and thus the therapeutic effect of its blockade should be specifically assessed in this context. Finally, another potential reason accounting for the failure of trials testing SFKs inhibitors in patients with mCRPC is their poor toxicity profile, partly due to their broad activity against multiple kinases and unwanted off-target effects (Kim et al., 2009). New compounds, such as eCF506, characterized by higher selectivity for SFKs and particularly SRC, while retaining potent antitumor activity, may address this limitation. Alternatively, the results reported here raise the option of targeting TAMs and their mediator(s) upstream, taking advantage of currently available strategies (Mantovani et al., 2022).
As is generally true for innovative research, the results reported by Li et al. (2023) open new vistas and raise questions. Further investigation is needed to shed light on the downstream consequences of the engagement of the FN1-ITGA5 axis on cancer cells and on the impact of such activation on androgen dependency. Importantly, a targeted investigation of the ECM and its components in resistant tumors may identify mechanisms that are behind the failure of androgen deprivation in CRPC. Bone metastasis represents a formidable clinical challenge in tumors other than PC, such as breast and colorectal. It will be important to assess whether similar pathways underlie resistance to chemo or immunotherapy in the bone TME of other tumors.
The report by Li et al. (2023) has broad implications that go beyond the specific issue of PC bone metastasis. The results reported here make the general point that TAMs and inflammatory mediators influence the action of hormones; in turn, there is evidence that hormones contribute to shaping the immune response also in non-sex-related tumors (Pala et al., 2022a). Disseminating cancer cells seed in organs with differences in the immunological contexture (e.g., bone versus liver), reflected in the metastasis TME. These components of metastasis responsiveness to hormonal and immunological therapies may pave the way to better therapeutic exploitation.
References
- Ammirante, M., et al. 2010. Nature. 10.1038/nature08782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo, J.C., et al. 2013. Lancet Oncol. 10.1016/S1470-2045(13)70479-0 [DOI] [Google Scholar]
- Calcinotto, A., et al. 2018. Nature. 10.1038/s41586-018-0266-0 [DOI] [Google Scholar]
- El-Kenawi, A., et al. 2021. Cancer Res. 10.1158/0008-5472.CAN-20-4028 [DOI] [Google Scholar]
- Escamilla, J., et al. 2015. Cancer Res. 10.1158/0008-5472.CAN-14-0992 [DOI] [Google Scholar]
- Fizazi, K. 2007. Ann. Oncol. 10.1093/annonc/mdm086 [DOI] [Google Scholar]
- Gao, L., et al. 2022. . Front. Oncol. 10.3389/fonc.2022.905398 [DOI] [Google Scholar]
- Guan, X., et al. 2022. Nature. 10.1038/s41586-022-04522-6 [DOI] [Google Scholar]
- Kim, L.C., et al. 2009. Nat. Rev. Clin. Oncol. 10.1038/nrclinonc.2009.129 [DOI] [PubMed] [Google Scholar]
- Kuser-Abali, G., et al. 2015. Nat. Commun. 10.1038/ncomms9126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.F., et al. 2023. J. Exp. Med. 10.1084/jem.20221007 [DOI] [Google Scholar]
- Lorente, D., et al. 2015. Nat. Rev. Urol. 10.1038/nrurol.2014.345 [DOI] [PubMed] [Google Scholar]
- Mantovani, A., et al. 2022. Nat. Rev. Drug Discov. 10.1038/s41573-022-00520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani, A., et al. 2008. Nature. 10.1038/nature07205 [DOI] [Google Scholar]
- Masetti, M., et al. 2022. J. Exp. Med. 10.1084/jem.20210564 [DOI] [Google Scholar]
- Pala, L., et al. 2022a. Sex and cancer immunotherapy: Current understanding and challenges. Cancer Cell. 40:695–700. 10.1016/j.ccell.2022.06.005 [DOI] [PubMed] [Google Scholar]
- Pala, L., et al. 2022b. Cancer Cell. 10.1016/j.ccell.2022.04.007 [DOI] [PubMed] [Google Scholar]
- Pearce, O.M.T., et al. 2018. Cancer Discov. 10.1158/2159-8290.CD-17-0284 [DOI] [Google Scholar]