Abstract
Interleukin-6 (IL-6) induces the activation of the Src family kinase Hck, which is associated with the IL-6 receptor β-chain, gp130. Here we describe the identification of an “acidic” domain comprising amino acids 771 to 811 of gp130 as a binding region for Hck, which mediates proliferative signaling. The deletion of this region of gp130 (i.e., in deletion mutant d771-811) resulted in a significant reduction of Hck kinase activity and cell proliferation upon stimulation of gp130 compared to wild-type gp130. In addition, d771-811 disrupted the growth factor-stimulated activation of Erk and the dephosphorylation of Pyk2. Based on these findings, we propose a novel, acidic domain of gp130, which is responsible for the activation of Hck, Erk, and Pyk2 and signals cell proliferation upon growth factor stimulation.
To exert its biological effects, interleukin-6 (IL-6) must bind to the IL-6 receptor (IL-6R), composed of two α-chains (IL-6Rα, 80 kDa) and two β-chains (IL-6Rβ or gp130, 130 kDa). Two moieties of IL-6 and two pairs of these receptor chains form a functional hexameric IL-6R complex (42, 43, 55). The subsequent intracellular signaling events are activated via gp130, which is the common β-chain of the receptors for cardiotrophin 1, ciliary neurotrophic factor, oncostatin M, leukemia inhibitory factor, IL-11, and IL-6 (24). Activation of the IL-6R stimulates at least two major signaling pathways, the Src homology 2 (SH2) domain containing protein tyrosine phosphatase 2 (Shp-2)/mitogen-activated protein kinase (MAPK) signaling cascade (8, 26, 31, 32, 41, 46) and the Janus kinase (Jak)/signal transducer and activator of transcription (STAT) pathway (6, 18, 25, 45). It was shown in recent in vivo studies that gp130-mediated signals were regulated by a balance between these two pathways (33). However, the signaling cascades mediating IL-6-induced cell growth are not fully defined. It was shown that Jak and STAT proteins are activated by IL-6 in multiple myeloma (MM) cells independently of the proliferative response. In contrast, MAPK was activated only in cells showing a proliferative response to IL-6 (32). Moreover, the physical separation of gp130 and Shp-2 reduced cell proliferation (26).
We have shown previously that at least three members of the Src family of tyrosine kinases, i.e., Fyn, Hck, and Lyn, coprecipitate with gp130 in lysates of MM cells (20). Stimulation of cells with IL-6 increased the activity of these kinases. The association of Hck kinase with gp130 appeared to be stronger than either of the other two kinases. Therefore, we decided to focus on the Hck kinase to elucidate the mechanism(s) and biological significance of the IL-6-mediated Src kinase activation. To identify the gp130 binding domain for Hck, several mutants of gp130 were constructed. These mutants were based on a chimeric receptor consisting of the extracellular part of the erythropoietin receptor (EPOR) and the intracellular part of human gp130 (23). These EPOR/gp130 receptor chimeras (Eg) allowed study of the activation of gp130 by erythropoietin (EPO) after transfection of a single molecule. By genetically modifying these chimeric receptor constructs, we identified a 41-amino-acid (aa) stretch (aa 771 to 811) located C terminal of the Box3 motif of gp130, which was necessary for Hck binding. This region was rich in negatively charged amino acids and therefore designated acidic domain in analogy to the Lck binding region of the IL-2R β-chain (22). Both C-terminal truncation up to aa 775 of gp130 and the deletion of its acidic domain significantly reduced the gp130-mediated cell proliferation upon growth factor stimulation.
MATERIALS AND METHODS
Reagents.
Purified recombinant murine EPO (rmEPO) was purchased from Boehringer (Mannheim, Germany) and purified murine IL-3 (rmIL-3) was obtained from Biosource International (Nivelles, Belgium). All reagents for cell lysis, protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting were purchased from Sigma (Munich, Germany) or Bio-Rad (Munich, Germany). Protein A-Sepharose was obtained from Pharmacia Biotech (Freiburg, Germany). The purified mouse monoclonal antibodies anti-His (C terminal) and anti-V5 were purchased from Invitrogen (Leek, The Netherlands). The specific antibody against serine-phosphorylated STAT3 was purchased from New England Biolabs (Schwalbach, Germany). The antibodies against phosphorylated Pyk2 were purchased from Biosource (Solingen, Germany). All other antibodies and the specific blocking peptide representing aa 8 to 37 of Hck were ordered from Santa Cruz Biotechnology (Santa Cruz, Calif.). [3H]thymidine and [33P]ATP were purchased from Amersham (Braunschweig, Germany). The Src kinase substrate Sam 68 was obtained as a 51-kDa tagged fusion protein from Santa Cruz Biotechnology. The tyrosine kinase inhibitor PP2 was purchased from Calbiochem (Bad Soden, Germany). Cell culture media and sera were obtained from BioWhittaker (Verviers, Belgium) and Gibco (Paisley, United Kingdom). The wild-type EPOR-gp130 (i.e., Eg) fusion protein was a kind gift from Friedemann Horn (Universität Leipzig, Leipzig, Germany). All enzymes for cloning procedures and the liposomal transfection reagent DOTAP were purchased from Boehringer. The mammalian expression vectors pcDNA3, pcDNA3.1(−)/myc-His, and pcDNA6/V5-His and the selection reagent blasticidin were purchased from Invitrogen. The expression vector pDpuro was a gift from Seth Corey, Pittsburgh, Pa. Puromycin was obtained from Sigma.
Cloning of Eg and mutants. (i) Cloning of Eg.
The chimeric receptor (Eg) was constructed by cloning the extracellular domain of the mouse EPOR to the cytoplasmatic domain of human gp130 by using an introduced EcoRI site as described elsewhere (23). This construct was then cloned into pcDNA3.1/myc-His by using introduced XbaI and BamHI sites and standard PCR methods.
(ii) Truncation mutations.
The C-terminal truncation (t) mutants t828, t770, t722, t702, t685, and t650 (with the numbers referring to the amino acid positions of wild-type human gp130) were constructed by using the full-length fusion protein (Eg) cloned into pcDNA3.1/myc-His as a template in a single-step PCR protocol. A universal sense primer, containing an integrated XbaI site and a Kozak signal sequence (5′-GGGCCCTCTAGACCAGCCATGGACAAACTC-3′) and the following truncation specific antisense primers containing an integrated BamHI site were used: t828a, ATCTGGGGATCCTTCATGCTGACTGCAGTTCTG; t770a, GACTTGGACTGAGGATCCTTGGTGTCTGTA; t722a, ACTGCTGGATCCTTCAGTATTAATTTTTTC; t702a, TTCTGGGGATCCCTTTTTGTCATTTGCTTCTAT; and t685a, GGCAATATGACTGGATCCAGGATCTGGAAC; and t650a, ATCTGGAACATTAGGGGATCCGTGTTTTTTAATTAG. By using the same strategy, the truncation mutants t775 and t710 were cloned into pcDNA6 by using BamHI and XbaI sites. The primers used were as follows: t775/t710s, GGGCCCGGATCCCCAGCCATGGACAAACTC; t710a, GAGCTCTCTAGACTGAGGCATGTAGCCGCC; and t775a, TCTTGATCTAGATTGGACTGACGGAACTTG.
(iii) Deletion mutations.
The deletion (d) mutants d681-721, d771-827, d771-811, d820-827, d812-827, and d812-819 were established by using Eg as a template in a two-step PCR protocol. In the first step, the deletions were performed by using internal, partially complementary primers (d681-721s, CACAATTTTAATTCAAAAGGACACAGCAGTGGTATT; d681-721a, AATACCACTGCTGTGTCCTTTTGAATTAAAATTGTG; d771-827s, AGTGGCTACAGACACCAAATTTCACATTTTGAAAGG; d771-827a, CCTTTCAAAATGTGAAATTTGGTGTCTGTAGCCACT; d771-811s, AGTGGCTACAGACACCAACAACAGTACTTCAAACAG; d771-811a, CTGTTTGAAGTACTGTTGTTGGTGTCTGTAGCCACT; d820-827s, TACTTCAAACAGAACTGCATTTCACATTTTGAAAGG; d820-827a, CCTTTCAAAATGTGAAATGCAGTTCTGTTTGAAGTA; d812-827s, GATGGTATTTTGCCCAGGAGTCAGCATGAATCCAGT; d812-827a, ACTGGATTCATGCTGACTCCTGGGCAAAATACCATC; d812-819s, GATGGTATTTTGCCCAGGAGTCAGCATGAATCCAGT; d812-819a, ACTGGATTCATGCTGACTCCTGGGCAAAATACCATC) and two end-standing primers binding 5′ (Ecosfp, CTGACCGCTAGCGAATTCACTTTTACTACC) and 3′ (Bamafp, GGTACCGAGCTCGGATCCCTGAGGCATGTAGCC) of gp130. The two corresponding PCR products were annealed in the second step by using Ecosfp and Bamafp for completion of the internal deleted EcoRI-BamHI fragments. These fragments were ligated to EcoRI-BamHI-cut Eg and inserted in pcDNA3.1(−)/myc-His.
(iv) Point mutations.
The point mutant Y814F was constructed by a two-step PCR protocol as described above by using specific internal oligonucleotides containing the desired mutation.
(v) Vectors for stable transfection in Baf-B03 cells.
For stable transfection of receptor mutants into Baf-B03 cells, the specific DNAs were cloned into the DNA6/V5-His vector by using XbaI and ApaI as restriction enzymes.
Construction of pDpuro-Hck.
The transfection vector pDpuro was constructed by fusion of the promoter region and the multiple cloning site of pcDNA3 into the backbone of pApuro. This was done in a two-step ligation protocol by using NcoI and PvuI restriction sites. Hck cDNA was obtained from the American Type Culture Collection and cloned into this vector by using the EcoRI site.
Cells, cell culture, and transfection.
Cos-7 cells were obtained from the German Collection of Microorganisms and Cell Culture (DSM) (Braunschweig, Germany). The IL-3-dependent murine pro-B-cell line Baf-B03 was a gift from Mark Showers (Dana-Farber Cancer Institute, Boston, Mass.). The IL-6-dependent murine plasmocytoma cell line 7TD-1 was obtained from the DSM. These cells were grown in RPMI 1640 supplemented with 5% fetal bovine serum (FCS), 5 pM recombinant IL-6, and 50 μM 2-mercaptoethanol. Cos-7 cells were routinely grown in Dulbecco modified Eagle medium supplemented with 10% FCS and l-glutamine. Baf-B03 cells were cultured in RPMI 1640 supplemented with 10% FCS and 10% WEHI-3B cell conditioned medium as a source for murine IL-3. Cos-7 cells were transiently transfected by using the liposomal transfection reagent DOTAP according to the manufacturer's protocol and as described previously (56). For transient cotransfection, 50 μg of fusion receptor DNA and 25 μg of Hck DNA (cloned into pcDNA3 expression vector) were used. Baf-B03 cells were stabily transfected by electroporation, by using 107 cells and 20 μg of DNA of the receptor constructs. Cells were resuspended in 800 μl of phosphate-buffered saline (PBS) without calcium and magnesium (BioWhittaker) and electroporated by using a pulse of 350 V and 950 μF. Selection of transfected cells was started 48 h later by using 8 μg of blasticidin/ml. After 10 days, single clones of positively transfected cells were established by limiting dilution. Highly expressing single clones were electroporated with Hck cDNA (cloned into pDpuro Hck expression vector) as described above. Selection of transfected cells was started 48 h later by the addition of 5 μg of puromycin/ml and 8 μg of blasticidin/ml. Generally, cells were cultured under selection until 3 days prior to stimulation and lysis. At least three similar expressing clones per mutant were cultivated for these experiments.
Preparation of cell lysates.
Prior to all experiments, cells were starved by serum deprivation for 16 to 20 h. Cos-7 cells and Baf-B03 cells were lysed with a lysis buffer containing 0.5% NP-40, 1 mM EDTA, 150 mM NaCl, 1 mM NaF, 50 mM Tris (pH 7.4), 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, and 2 mM sodium orthovanadate. In brief, 2 × 107 pelleted Baf-B03 cells were washed two times with PBS. For stimulation, the pellet was resuspended in 1 ml of PBS with 40 U of EPO/ml and incubated under light shaking for 15 min at 37°C. The reaction was stopped by adding 40 ml of ice-cold PBS. After an additional washing step, the pellet was resuspended in 300 μl of ice-cold lysis buffer per 2 × 107 cells. Cos-7 cells were scraped off the bottom of confluent 175-cm2 tissue flasks after starvation, washed once with PBS, and resuspended in 1.2 ml of ice-cold lysis buffer.
After rotation for 25 min on an overhead rotor at 4°C, the lysates were pelleted at 14,000 rpm and at 4°C for 15 min to remove insoluble material. The total protein concentration was measured by using a Bradford protein assay (Bio-Rad). Lysates were stored at −20°C or used immediately for experiments.
IP and Western blot.
For immunoprecipitation (IP), 500 μg of Cos-7 cell lysate or 500 μg of Baf-B03 cell lysate was incubated with 2 μg of the appropriate antibodies from 2 h up to 18 h at 4°C on an overhead rotator. A total of 100 μl of protein A beads were washed two times in IP washing buffer (0.1% NP-40, 1 mM EDTA, 150 mM NaCl, 1 mM NaF, 50 mM Tris [pH 7.4]) and then resuspended in 50 μl of IP washing buffer. Then, 100 μl of this mixture was added to each IP reaction. After an additional incubation for 2 h at 4°C, each precipitate was washed four times in 500 μl of IP washing buffer. After being boiled in 4× sample buffer, the precipitates were pelleted, and the supernatants were loaded onto 10% SDS gels. Peptide blocking experiments for Hck kinase and STAT3 were performed with the specific blocking peptides (Santa Cruz Biochemicals) according to the manufacturer's protocol. For normal expression controls of recombinant proteins, lysate containing 100 μg of proteins was subjected to electrophoresis.
After transfer of the proteins to Hybond-ECL nitrocellulose membranes (Amersham), membranes were blocked for 2 h in TBST (Tris-buffered saline with 0.05% Tween 20) containing 2% skim milk or 2% bovine serum albumin (BSA; Merck, Darmstadt, Germany). After a 1-min wash in TBST, the primary antibodies diluted 1:1,000 in TBST–1% BSA were incubated for 2 h or overnight. Membranes were washed four times with TBST, and then the appropriate peroxidase-linked secondary antibody diluted 1:3,000 in TBST–1% BSA was incubated for 35 min. After a final washing step, the proteins were detected by using the ECL System or ECL-Plus System from Amersham according to the manufacturer's guidelines.
In vitro kinase assay.
Immune complex tyrosine kinase assays were performed by using affinity-purified Sam 68 (Santa Cruz Biochemicals) as an external substrate for the measurement of Hck kinase activity. In brief, Hck kinase was precipitated from lysates of EPO-stimulated or unstimulated Baf-B03 transfectants as described above. The precipitates were washed two times with IP washing buffer and then one time with kinase buffer containing 25 mM HEPES (pH 7.3), 0.1% Triton X-100, 100 mM NaCl, 10 mM MgCl2, 3 mM MnCl2, and 200 μM Na3VO4. After the last wash 20 μl of kinase buffer was added to the precipitates, and the isotope-free tyrosine kinase assays were initiated by adding 5 μCi of [33P]ATP and 3 μg of Sam 68/μl. After incubation for 15 min at 30°C under constant shaking, the reaction was stopped by adding SDS sample buffer, followed by boiling for 5 min. The supernatant of the samples was then loaded onto an SDS gel, transferred to nitrocellulose, and finally tested for autoradiography.
Proliferation assay.
Proliferation assays were carried out in 96-well plates by using 5 × 103 Baf-B03 cells or 1 × 103 7TD-1 cells per well. Two days prior to these experiments, cells were seeded in equal concentrations into tissue culture flasks. All assays were performed with PBS-washed cells in RPMI medium supplemented with 10% FCS only. Wild-type cells or triplicate aliquots of monoclonal cells expressing recombinant proteins were stimulated with the indicated concentrations of EPO, IL-3, or IL-6. After incubation for 72 h at 37°C, the proliferation was assessed by microscopic cell counting after trypan blue staining.
RESULTS
Binding of Hck to gp130.
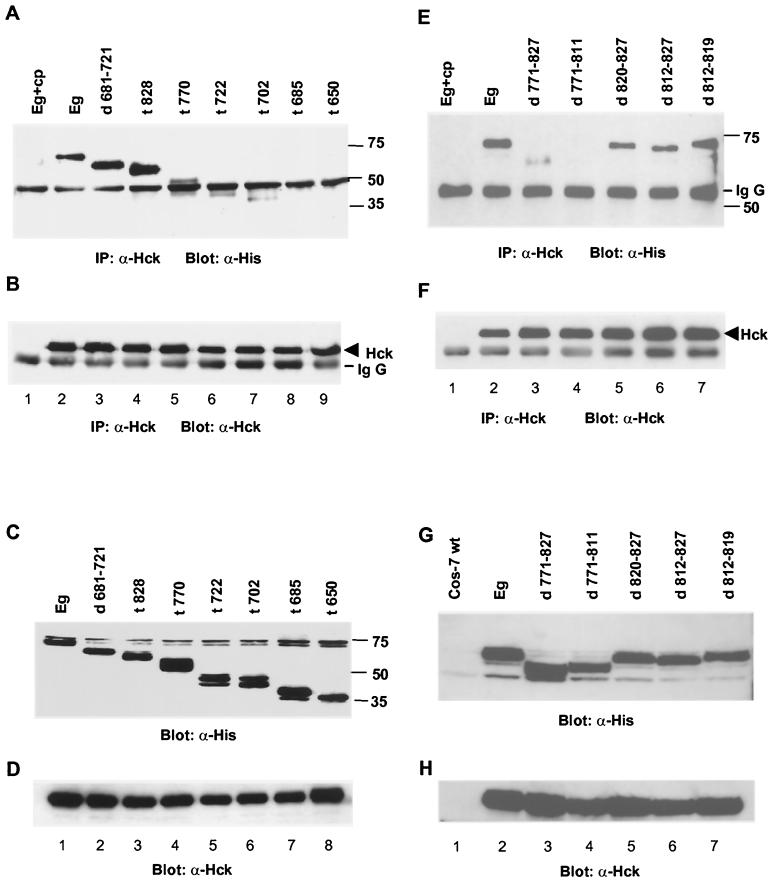
Src family kinases Fyn, Lyn, and Hck were physically associated with gp130 in MM and embryonic stem (ES) cells (16, 20). However, the structural requirements for this association remain unclear. In previous experiments, the interaction of gp130 with Hck was more prominent than with the other two kinases. Therefore, we focused on Hck in the studies presented here. In order to identify the Hck binding site of gp130, we generated several truncation and deletion mutants of gp130 (Fig. 1). For this purpose, we used a chimeric receptor comprising the extracellular domain of the EPOR and the transmembrane and intracellular parts of gp130 (Eg). This strategy allowed us to transfect only a single chimeric receptor molecule, since the EPOR is functional as a homodimer. Furthermore, when EPOR-negative cells were used, the effects of endogenously expressed IL-6R components could be excluded because activation of the chimeric receptor is achieved by stimulation with EPO. In order to facilitate the detection of the chimeric receptors, all receptor mutants were tagged with a poly-His peptide, allowing the detection by the antihistidine antibody, anti-His-C-term. These mutants were transfected together with a wild-type Hck expression plasmid, pcDNA3-Hck, into Cos-7 cells, which did not express EPOR endogenously. A similar protein expression of the various receptor mutants and of Hck was obtained (Fig. 2C, D, G, and H). The double or triple bands of the different receptor mutants (Fig. 2C and G) were most likely explained by differential glycosylation. However, only one of these forms seemed to coprecipitate with Hck (Fig. 2A and E). Lysates of doubly transfected Cos-7 cells were used for coprecipitation experiments, in which anti-Hck precipitates were resolved by SDS-PAGE and then immunoblotted with anti-His antibody to detect complexes of receptor mutants with Hck (Fig. 2A and E). Aliquots of the IP reaction mixtures were loaded on an additional gel and blotted with the Hck antibody (N-30) to verify that similar amounts of Hck were precipitated (Fig. 2B and F). To evaluate the specificity of the precipitating anti-Hck antibody, blocking experiments with a specific blocking peptide (see Materials and Methods) were also performed, showing that precipitation of Hck was completely disrupted by the peptide (Fig. 2A, B, E, and F, lanes 1). C-terminal truncation at aa 770 (mutant t770) led to a substantial loss of Hck binding (Fig. 2A, lane 5). In contrast, Hck binding remained unaffected when further C-terminal truncations of gp130 were used (mutant t828; Fig. 2A, lane 4). It was not possible to perform the experiment in the opposite direction, i.e., with an anti-His IP, followed by anti-Hck immunoblotting, because a significant fraction of Hck consistently bound unspecifically to protein A beads. Taken together, these results suggested that a putative Hck binding domain was located between aa 770 and 828 of gp130. This region did not include any of the homology boxes (Fig. 1A) shared among different growth factor receptors that were shown to be important docking regions for signaling proteins (2, 27, 29, 30, 37, 50). However, a striking feature of the gp130 region from aa 770 to 828 was a remarkably high content of negatively charged amino acids. It has been shown earlier that the Src family kinases Lck and Fyn were associated via domains with a high content of aspartic and glutamic acids with the IL-2R β-chain and IL-7R (22, 54). Therefore, we searched for potential Hck binding motifs in gp130 with a high fraction of negatively charged amino acids. Two such regions were identified: one between aa 771 and 827, matching the above mentioned putative Hck binding domain, and another between aa 681 and 721. Although a contribution of the region from aa 681 to 721 for Hck binding seemed very unlikely, considering the results obtained with gp130 truncation mutants (Fig. 2A, lanes 4 to 6), we constructed Eg mutants with internal deletions for both regions, d771-827 and d681-721, respectively (Fig. 1). When coexpressing these mutants with Hck in Cos-7 cells, the Hck-gp130 association remained unchanged when aa 681 to 721 were deleted (d681-721) (Fig. 2A, lane 3). In marked contrast, the internal deletion of residues 771 to 827 (d771-827) resulted in a >90% decrease of Hck coprecipitation with gp130 in Cos-7 cells (Fig. 2E, lane 3).
FIG. 1.
Schematic overview of gp130 truncation and deletion mutants. Several truncation (A) and deletion (B) mutants of gp130 were cloned as chimeric receptor molecules as described in Materials and Methods; the numbers of amino acids refer to wild-type gp130. The locations of tyrosine residues and the STAT3 binding region at tyrosine 814 are indicated. The homology boxes are shaded gray as boxes 1 to 3. All truncation (t) and deletion (d) mutants, as well as the wild-type gp130 (Eg) bearing C-terminal His and myc tags for expression in Cos-7 cells (or His and V5 tags for Baf-B03 cell experiments), are indicated.
FIG. 2.
Deletion of 41 aa inhibits Hck binding. Different gp130 mutants were expressed together with Hck cDNA in Cos7 cells. The expression of recombinant proteins was controlled by Western blotting with specific antibodies against the His tag (C and G) or Hck (D and H), respectively. The association of Hck to gp130 was tested by IP with Hck antibody and subsequent blotting with His-tag antibody for the detection of coprecipitated EPOR-gp130 fusion protein (A and E) Aliquots of the IP reaction mixtures were loaded on an additional gel and blotted with Hck antibody to demonstrate equal precipitation of Hck (B and F). In lanes 1 of panels A, B, E, and F, Hck interaction was blocked by preincubation with a specific blocking peptide. The molecular mass markers and the immunoglobulin G (Ig G) bands are indicated. α-Hck, anti-Hck antibody.
In order to define the Hck binding domain more precisely, we constructed additional mutants containing smaller deletions (Fig. 1B). It seemed possible that two well-characterized docking sites for STAT3, tyrosine residues 814 and 767 (Y814 and Y767), which lay within or near this putative Hck binding domain (18, 23), played a role in Hck binding. To address this problem, we created internal deletions, either encompassing Y814 (d812-827 and d812-819) or located N terminally (d771-811) or C terminally (d820-827) of it. The constructs were again coexpressed with Hck (pcDNA3 Hck) in Cos-7 cells (Fig. 2E to H). In addition, lysates of nontransfected Cos-7 cells were loaded (Fig. 2E and F, lanes 1) to control for the expression of Hck and receptor mutants. Cell lysates were immunoprecipitated with anti-Hck antibody and immunoblotted with anti-His antibody to detect Hck-gp130 complexes. As shown, a complete disruption of the Hck association with gp130 was observed with mutant d771-811 (Fig. 2E, lane 4). The faint signals (<10% of wild-type gp130) we obtained when the d771-827 mutant coprecipitated with Hck (Fig. 2E, lane 3) implied the existence of an additional Hck binding region in gp130. However, Hck binding was completely disrupted in the smaller deletion mutant d771-811 (Fig. 2E, lane 4), suggesting that structural changes of gp130 in the d771-827 mutant led to the exposure of an usually hidden binding motif in gp130. In marked contrast, Hck binding to mutants d812-827, d812-819, and d820-827 was similar to that with wild-type gp130 (Fig. 2E, lane 2 and lanes 5 to 7). These results suggested that the formation of the Hck-gp130 complex was independent of STAT3 association at Y814. In addition, we created mutants of gp130 in which the docking site for Shp-2, Y759, or the STAT3 docking sites, Y767 and Y814, were mutated to phenylalanine. All of these mutants coprecipitated with Hck to the same extent as wild-type gp130, indicating that Hck binding to gp130 occurred independently of these docking sites (data not shown). Therefore, we concluded that Hck bound to gp130 at a domain rich in negatively charged amino acids, spanning positions 771 to 811, and that this association was not mediated via tyrosine residues 759, 767, and 814. In analogy to the Lck kinase binding domain in the IL-2R β-chain (22), we termed this region the acidic domain of gp130.
Stimulation of gp130 but not d771-811 induces activation of Hck in the growth factor-dependent cell line Baf-B03.
To explore the functional significance of the association of Hck with gp130, we generated stable transfectants of the growth factor-dependent pro-B-cell line, Baf-B03 expressing the different gp130 mutants (see Materials and Methods). To investigate the activation state of Hck, we performed in vitro tyrosine kinase assays by using the affinity-purified Sam 68 as an Src kinase substrate. A stimulation-dependent increase in substrate phosphorylation occurred only in cells expressing Eg (Fig. 3, lane 6) but not in cells expressing d771-811 or Hck alone. Moreover, faint bands in lanes 6 and 7 showed that Hck was autophosphorylated upon stimulation in Eg-expressing cells only. Comparable precipitation of Hck was confirmed by immunoblotting with anti-Hck antibody. The similar expression of the receptor constructs Eg and d771-811 was confirmed by immunoblot with the anti-V5 antibody (data not shown). This result showed that the chimeric Epo/gp130 receptor was functional in activating Hck in response to EPO in Baf-B03 cells and that the disruption of the Hck binding domain lead to a decreased Hck tyrosine kinase activity after stimulation.
FIG. 3.
Stimulation of gp130 but not d771-811 induces activation of Hck in the growth factor-dependent cell line Baf-B03. Baf-B03 cells were transfected with expression vectors for Hck (pDpuro Hck) and for Eg and d771-811, respectively (pcDNA6/V5-His). These cells were then either stimulated with 40 U of EPO/ml (+) or with medium only (−). The activity of Hck was tested by performing in vitro kinase assays in the presence of radiolabeled ATP and the external Src kinase substrate Sam 68 as described in the text. After separation with SDS–10% PAGE the EPO-induced phosphorylation of Sam 68 was assessed by autoradiography (upper panel). Aliquots of the Hck immunoprecipitate were blotted with anti-Hck antibody (α-Hck) to control for comparable precipitation. The results were quantified by using phosphorimaging analysis software. Normalized induction levels of kinase activity were as indicated.
Deletion of the acidic domain of gp130 does not reduce the activation of STAT3.
Next, we investigated the phosphorylation status of STAT3 in d771-811-transfected Baf-B03 cells. Complete activation of STAT3 requires phosphorylation at tyrosine residue 705 and serine residue 727 (57, 59). To examine the activation status of STAT3, we precipitated endogenous STAT3 from normal and EPO-stimulated cells (Fig. 4D to F) and then blotted the immunoprecipitates with the anti-phosphotyrosine antibody PY99 (Fig. 4E) or an antibody specific for STAT3 phosphorylated at serine 727 (Fig. 4F). Baf-B03 transfectants expressed endogenous STAT3 (Fig. 4A), as well as the transfected receptor mutants (Fig. 4B) and Hck (Fig. 4 C), at similar levels. As shown in Fig. 4E and F, neither the deletion of residues 771 to 811 (lanes 3 and 4) nor the point mutation of tyrosine 814 (lanes 5 and 6) led to significant changes in STAT3 tyrosine or serine phosphorylation in comparison to cells transfected with wild-type Eg and Hck (lanes 1 and 2). Figure 4D shows that STAT3 was precipitated in comparable amounts and that a specific blocking peptide inhibited the precipitation of STAT3 (Fig. 4D, lane 7). These results demonstrated that the deletion of the Hck binding domain did not interfere with the overall activation of STAT3. The unchanged STAT3 phosphorylation observed with the point mutant Y814F can be explained by the fact that STAT3 binds to four phosphorylated tyrosine residues of gp130, namely, residues 767, 814, 905, and 915 (18). Therefore, the remaining three STAT3 docking motifs of gp130 were able to compensate for the Y814F mutation. Additionally, the results suggested that STAT3 activation was independent of the association of Hck with gp130, since the transfection of mutant d771-811 did not cause significant changes of EPO-induced STAT3 phosphorylation.
FIG. 4.
Deletion of the acidic domain of gp130 does not reduce the activation of STAT3. Baf-B03 cells were transfected with cDNAs for Hck and Eg, Y814F, or d771-811. These cells were either not treated (−) or stimulated with 40 U of EPO/ml (+). The lysates were tested for endogenous STAT3 expression by blotting with anti-STAT3 antibody (A), for the detection of the receptor constructs with V5 antibody (B), and for the detection of Hck with anti-Hck antibody (α-Hck) (C).Thereafter, lysates were used for precipitation experiments with anti-STAT3 antibody (right panel). (D) For control of STAT3 precipitation, aliquots of the IP reaction mixtures were incubated with anti-STAT3 antibody, and the specificity of STAT3 antiserum was tested by preincubation of anti-STAT3 with a specific blocking peptide (lane 7). (E and F) To check the STAT3 phosphorylation, separated lysates were blotted with the antiphosphotyrosine antibody PY99 (E) and then stripped and blotted with an antibody directed against serine 727-phosphorylated STAT3 (F).
The tyrosine kinase inhibitor PP2 reduces the gp130 mediated proliferation significantly.
To further explore the functional relevance of Hck for gp130-mediated proliferation, the selective Src kinase inhibitor PP2 was used (21). The IL-6-induced proliferation of the IL-6-dependent murine plasmocytoma cell line 7TD-1 was significantly reduced when the cells were incubated with PP2 (Fig. 5). This result supported our previous finding that Src kinases are involved in IL-6 signaling in MM cells (20). In an additional approach, we used Baf-B03 cells expressing either the wild-type chimeric receptor (Eg) or the mutant d771-811. Baf-B03 wild-type cells and cells coexpressing Eg or d771-811 and Hck stimulated with IL-3 showed a similar reduction of proliferation when PP2 was present (Fig. 5B, lower panel), indicating that the clones were comparable and confirming that Src kinases were also involved in IL-3-mediated signaling (3). However, EPO-induced proliferation of Baf-B03 cells expressing Eg was reduced five times when cells were cultivated in the presence of 10 μM PP2 (Fig. 5B, upper panel). In contrast, there was no additional decrease in cell proliferation of d771-811 transfectants, indicating that PP2 acted mainly on Hck kinase or its substrates in these cells. These results corroborated a function of Hck kinase in the proliferative signaling of gp130. To omit the potential lack of specificity of PP2 and to explore the biological relevance of the Hck-gp130 interaction in more detail, we used Baf-B03 cells expressing either the receptor constructs alone or together with Hck in an additional approach.
FIG. 5.
The tyrosine kinase inhibitor PP2 reduces gp130-mediated proliferation. (A) The IL-6-dependent murine plasmocytoma cell line 7TD-1 was kept in the absence (□) or presence (▪) of the tyrosine kinase inhibitor PP2 (10 μmol). After 3 days of IL-6 stimulation with the indicated amounts the cell proliferation was assessed by microscopic counting. (B) Baf-B03 cells expressing the indicated proteins were stimulated with 8 U of EPO/ml (solid bars, upper panel), 4 U of IL-3/ml (solid bars, lower panel), or medium alone (open bars, both upper and lower panels) for 72 h. Cells were kept in the absence (left) or presence (right) of 10 μmol of PP2. Proliferation was assessed by counting the cells under the microscope after trypan blue staining. Triplicate results from two different clones are shown, with the standard deviations indicated by the error bars.
The deletion of the Hck binding domain impairs gp130-mediated cell proliferation.
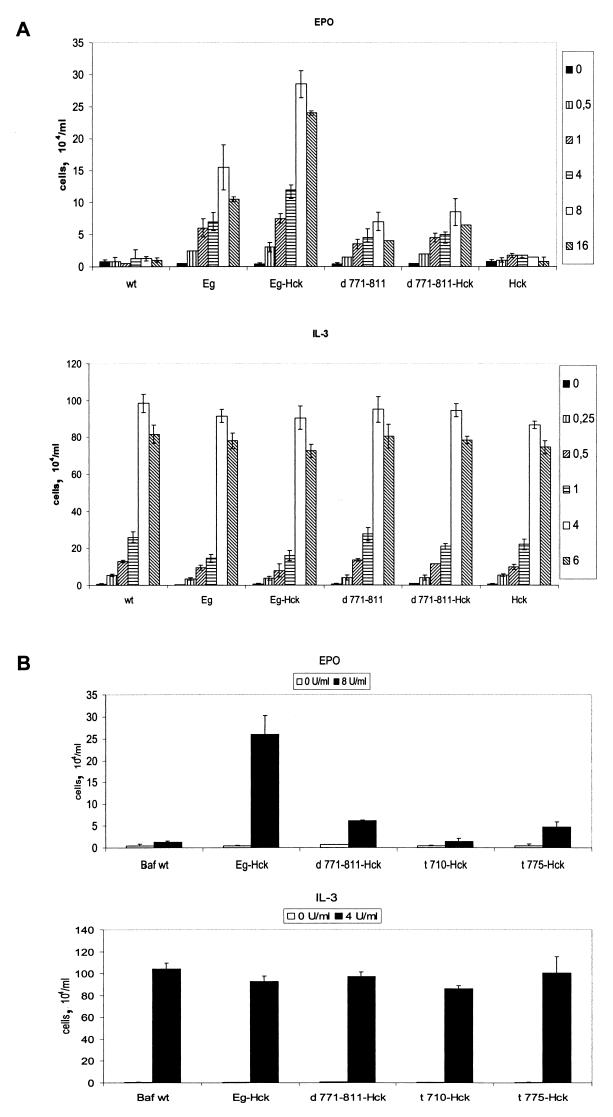
To investigate the biological function of the Hck-gp130 association, we used various Baf-B03 transfectants to perform proliferation assays. Similar expression of receptor mutants and Hck was controlled by immunoblotting lysates of the different clones with specific antibodies (data not shown). Since Baf-B03 cells depended on IL-3 for cell growth, maximal stimulation of cell proliferation was tested by stimulation with IL-3 (Fig. 6, lower panels). To evaluate the impact of an intact Hck-gp130 complex on proliferative signaling, we stimulated Baf-B03 cells expressing either Eg and d771-811 alone or together with Hck with various amounts of EPO or IL-3 (Fig. 6A). All clones and controls showed the same range of cell proliferation when stimulated with IL-3. Maximum growth was achieved with 4 U of IL-3/ml (lower panel). When the cells were stimulated with EPO, maximum growth was achieved with 8 U/ml but was about three times lower than with IL-3, a result most likely due to the difference between endogenous and transgenic effects. Wild-type cells and cells expressing Hck alone showed no proliferation in response to EPO. Not surprisingly, cells that expressed the receptor but lacking the Hck binding domain (d771-811) still grow in response to EPO, indicating that Hck-independent pathways were activated. Coexpression of Hck with d771-811 did not significantly change cell growth. Cells that expressed the full cytoplasmic domain of gp130 (Eg) proliferated about twofold more strongly in response to EPO than did the d771-811 transfectants. This effect was magnified when Hck was coexpressed. Taken together, these data supported the importance of an intact Hck-gp130 complex in these cells. In contrast to results published previously (17, 29), the membrane proximal region of gp130 was not sufficient for mediating proliferative effects, since cells transfected with the C-terminal truncation mutant t775 or t710 showed the same decrease in EPO-induced proliferation as did the cells bearing the d771-811 mutant (Fig. 6B, upper panel). Taken together, these findings suggested that the deletion of the acidic Hck binding domain significantly reduced the proliferative response to gp130 stimulation.
FIG. 6.
Deletion of the Hck binding domain impairs gp130-mediated cell proliferation. Clonally derived Baf-B03 cells expressing the indicated proteins were stimulated with the indicated amounts (in units per milliliter) of EPO (upper panels) or IL-3 (lower panels) for 72 h. Proliferation was assessed by microscopic counting after trypan blue staining. Triplicate results from two different clones are shown, with the standard deviations indicated by the error bars.
Pyk2 and Erk regulation is impaired by the disruption of the Hck binding site.
To investigate downstream signaling events mediated by the Hck-gp130 interaction, we first investigated the possible role of the focal adhesion-associated kinase RAFTK/Pyk2 (related adhesion focal tyrosine kinase), which was shown to be involved in Src kinase-mediated signaling events (7, 13). In addition, dexamethasone-induced phosphorylation of Pyk2 was strongly impaired in MM cells after stimulation with IL-6. Finally, the dephosphorylation of Pyk2 was leading to decreased apoptosis in these cells (10, 11). We used lysates of Baf-B03 cells cotransfected with Hck and Eg or d771-811, respectively. Control experiments showed that recombinant proteins and endogenous Pyk2 were expressed in similar amounts in all cells (Fig. 7A, lower panels). By blotting experiments with antibodies specific for Pyk2 phosphorylated at tyrosine residues Y402 or Y579 (Fig. 7A, upper panels), we could show that Pyk2 was strongly dephosphorylated at Y402 and, to a minor extent, at residue Y579 in cells expressing Eg and Hck, indicating the activation of a tyrosine phosphatase for Pyk2. In contrast, there was no significant decrease in Pyk2 phosphorylation in d771-811-expressing cells (Fig. 7A, lanes 5 and 6). Overexpression of Hck lead to a significant increase in basal phophorylation of Pyk2 (Fig. 7A, lanes 1 and 2), indicating that in these cells the endogenously expressed Src kinases, mainly Lyn, were not involved in Pyk2 phosphorylation. The double bands we could detect in these experiments were most likely due to a differential phosphorylation of Pyk2. Src and Pyk2 kinases both seemed to be implicated in MAPK signaling pathways (1, 7). Therefore, we investigated next whether we could detect a differential MAPK activation in cells expressing Eg or d771-811. After EPO stimulation of Baf-B03 cells expressing Eg, a time-dependent increase in Erk1/2 activation of about fivefold was detected by a phosphospecific Erk antibody. In contrast, only a twofold increase of Erk phosphorylation was observed after stimulation of d771-811-transfected cells (Fig. 7B, upper panels), suggesting that additional pathways are able to contribute to Erk activation after gp130 stimulation. Expression of Erk was controlled by blotting with a specific Erk antibody (lower panels). These findings suggest that the signaling pathway mediated by the Hck gp130 interaction strongly contributes to Erk activation.
FIG. 7.
Pyk2 and Erk regulation is impaired by the disruption of the Hck binding site. (A) Baf-B03 cells were cotransfected with the expression vectors for Eg (lanes 3 and 4) or d771-811 (lanes 5 and 6) and Hck. After stimulation with medium or EPO, cells were lysed as described in the text. Lysates were separated on 10% SDS gels. After blotting, the membranes were incubated with antibodies against phosphorylated Pyk2 (upper panels) or with several control antibodies as indicated (lower panels). (B) Baf-B03 cells expressing the indicated proteins were stimulated for the indicated times with 40 U of EPO/ml. Thereafter, membranes were subjected to Western blots by using antibodies specific for activated Erk1/2 (upper panels). Equal loading was confirmed by blotting with normal Erk antibody (lower panels).
DISCUSSION
We have shown previously that the Src kinase Hck is activated by stimulation of gp130 (20). The present study demonstrates that the Src family kinase Hck binds to gp130 via an acidic domain comprising aa 771 to 811. The internal deletion of this acidic domain resulted in a complete loss of Hck association and a severalfold reduction of growth factor-stimulated proliferation. The acidic domain of gp130 is directly C terminal to the box3 motif in gp130. In agreement with these findings, Ernst et al. (15) found that C-terminal gp130 deletions, including the box3 motif and all STAT3 binding motifs, impaired the activation of Hck by murine gp130 in ES cells. However, and in clear contrast to our results, the growth factor-induced proliferation was not reduced in comparison to wild-type gp130 (15). The acidic domains of murine and human gp130 are highly conserved (with only 1 aa being different in the acidic stretch). Therefore, the most likely explanation for the apparent difference in signaling is that ES cells represent a different cellular background for the effects of gp130 than the Baf-B03 cells we used and that Hck has various tasks in distinct cell types. Hence, Hck-dependent signaling pathways might support cell proliferation in Baf-B03 cells but suppress differentiation in ES cells (15).
The acidic domain of gp130 includes two motifs which play a role in receptor internalization, a serine residue at position 782 and a dileucine motif (L786 and L787) (14, 19). It was shown recently by mutational analysis that both signal generation and signal termination were independent of gp130 endocytosis (51, 52). It is intriguing to speculate about a role for Hck at this point, since it was shown that inhibition of CD4 endocytosis depended on interaction of CD4 with Src family kinase Lck in lymphocytes (35). The precise contribution of the gp130 internalization to the proliferative signaling remains to be identified.
The association of Hck kinase with gp130 will need require examination to identify the precise mechanism of interaction. The SH2 and SH3 domains of Src family kinases account for most interactions with other signaling molecules. In the case of the Src kinase SH2 domain, a phosphorylated tyrosine residue and the C-terminal flanking amino acids, which determine the specificity, serve as a binding motif. SH3 domains are able to recognize proline-rich sequences (34, 44, 58). For example, Src interacts with focal adhesion kinase (FAK) via its SH3 domain (53). The interaction of numerous signaling proteins with Src family kinases is mediated by SH2 domains. For instance, the association of Lyn and Blk with Syk kinase (4) and the binding of Hck to the β-subunit of the IL-3R (9) are mediated by the SH2 domain of the Src kinase(s). In marked contrast, the Hck binding region of gp130 identified in our studies did not contain any SH2 or SH3 consensus-binding motifs for Src kinases. The most striking feature of the Hck binding domain of gp130 was its relatively high content of negatively charged amino acids (9 of 41). A similar acidic domain has been shown to mediate the association of the Src family kinase Lck with the β-chain of the IL-2R. This interaction was mediated by the kinase domain (SH1) of Lck. Therefore, it should be investigated further whether the SH1 domain of Hck mediates the interaction with gp130. These experiments are currently under way.
The five C-terminal Y residues (Y759, Y767, Y814, Y905, and Y915) are docking sites for the association of downstream mediators of gp130 signaling. Y759 is involved in Shp-2 binding and IL-6-induced MAPK activation (32); Y767, Y814, Y905, and Y915 mediate STAT3 activation; and STAT1 binds toY905 and Y915 (18). Furthermore, it has been shown that the presence of the MAPK activation site alone is not sufficient for growth factor-mediated proliferation (J. D. French, R. C. Tschumper, J. A. Isaacson, E. Nygren, and D. F. Jelinek, unpublished data). Our findings add a new aspect to gp130-mediated effects on cell growth and survival in showing that activation of an Src family kinase, Hck, is critical for transmitting proliferative signals. Cells expressing receptors that lacked the Hck binding domain showed a fourfold-lower response to gp130 stimulation than cells expressing wild-type gp130. In addition, the proliferation of IL-6-dependent plasmocytoma cells (7TD-1) and Baf-B03 cells expressing wild-type gp130 was decreased to basal levels after treatment with the Src kinase inhibitor PP2, further supporting a role of Src kinases in proliferative signaling. However, because of the unspecificity of PP2, these results should be treated with care. The activation of STAT3 was not impaired by the deletion d771-811. It should be pointed out that the EPO-induced proliferation, as well as Erk activation, was not completely abrogated in cells expressing the d771-811 mutant receptor. This suggests that additional signaling events, such as the activation of Jaks and STATs, might also be involved in supporting the growth of Baf-B03 cells. Our studies could not confirm earlier findings that the region up to residue 775 of gp130 alone was necessary for mediating cell proliferation (17, 29). In our experiments, cells expressing the C-terminal truncation mutant t775 did not proliferate significantly upon gp130 stimulation (Fig. 6B). This difference might be explained by a severe disturbance of the protein integrity and/or loss of relevant docking sites at gp130 by large truncations such as t775, as recently suggested by others (40).
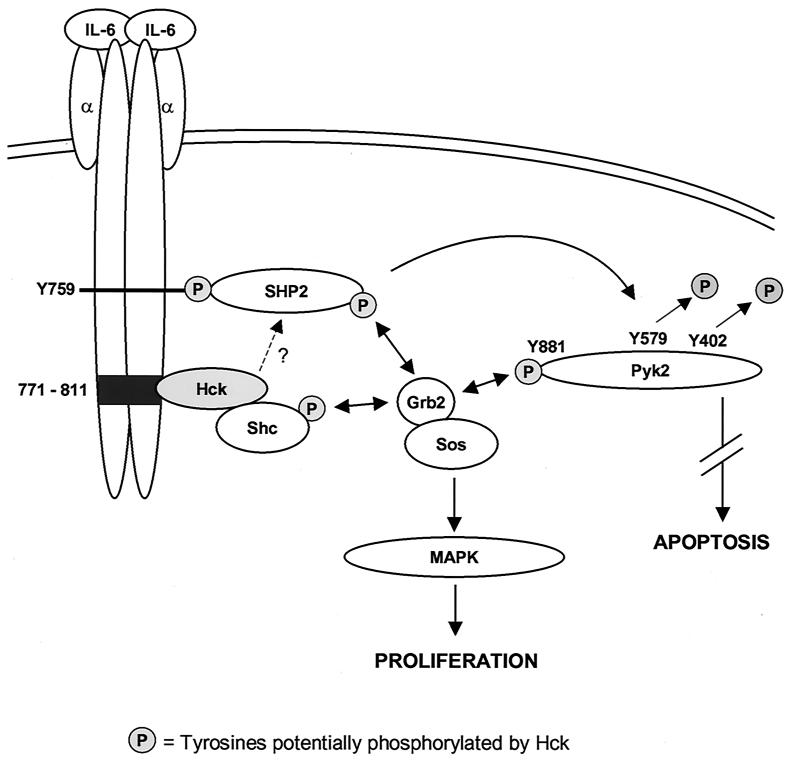
Our results suggest the activation of a tyrosine phosphatase downstream of Hck upon gp130 stimulation, since we could demonstrate a decrease in Pyk2 phosphorylation in Eg-expressing cells but not in d771-811-expressing cells (Fig. 7A). The dephosphorylation of Pyk2 blocks dexamethasone-induced apoptosis in MM cells (10). Our observation of a Hck-dependent dephosphorylation of Pyk2 would support the proposed role of Src kinases in blocking apoptotic signals (39). The tyrosine phosphatase Shp-2, whose docking site is located close to the Hck binding region of gp130, might be involved in Pyk2 dephosphorylation, since Pyk2 is a substrate for Shp-2 (48). Finally, the activation of Erk via the gp130-Hck interaction (Fig. 7B) confirms the proposed role for Src kinases by activating the Ras/MAPK signaling cascade (1, 31). In the context of a signaling complex involving gp130/Hck/Shp-2/Pyk2, it is possible that Hck phosphorylates docking sites for Grb2 in Shp-2 and/or Pyk2, thus leading to Erk activation (5, 38). This model and the finding that Shp-2 can be activated by Src kinases (47) suggest that Hck and Shp-2 might be members of the same signaling pathway. Moreover, it was shown recently that Pyk2 activation inhibits Erk activation, in contrast to the closely related FAK (60). Thus, the inactivation of Pyk2 by dephosphorylation of its major autophosphorylation site Y402 (38) in Eg-expressing Baf-B03 cells suggests that Pyk2 kinase is upstream of Erk in gp130 signaling. An alternative pathway, which might cause Erk activation, is a gp130/Hck/Shc/Grb2 complex, because we found Shc and Grb2 to be associated with Hck in LP-1 cells (unpublished data) and (31). Taken together, we suggest that Hck is involved in gp130 signaling by creating docking sites for signaling molecules such as Grb2 or Shp-2 and/or by activating adapter molecules such as Shc (Fig. 8).
FIG. 8.
Model of Hck involvement in gp130 signaling. Binding of IL-6 to the IL-6R results in the activation of Hck via gp130. Hck can then act on downstream signaling events by creating docking sites for signaling molecules such as Grb2 or Shp-2 and/or by activating adapter molecules such as Shc. The recruitment of Shp-2 to the Hck/gp130 complex might then lead to the dephosphorylation of Pyk2, resulting a block in apoptotic signaling in MM cells. Grb2 forms stable complexes with Sos, a GDP-GTP exchange factor for Ras. Thus, the binding of Grb2 to Shc, Pyk2, and/or Shp-2 via its SH2 domain can lead to the activation of Ras and Erk.
A role for Src family kinases has been described in the transmembrane signaling of many cytokines (1, 49). IL-2, IL-3, IL-6, IL-7, IL-12, granulocyte macrophage-colony stimulating factor (GM-CSF), and granulocyte-CSF were shown to activate Src kinases (3, 12, 16, 20, 28, 36, 54). Furthermore, Src kinases interact physically with the receptors for IL-2, IL-6, and IL-7 and the common β-chain of the receptors for IL-3, GM-CSF, and IL-5 (2, 20, 22, 54, 55). With the exception of the common β-chain of the receptors for IL-3, GM-CSF, and IL-5, where Lyn binds via its SH1 domain to a PXP motif in the receptor (2), the common denominator of the binding regions for Src kinases, such as Lck with the IL-2R and Fyn with the IL-7R, is their high content of negatively charged amino acids (22, 54). Our results suggest that the IL-6R β-chain, gp130, also uses an acidic domain for the interaction with Hck. Furthermore, we could show that the deletion of this Hck binding site in gp130 reduced cell proliferation and Erk activation in response to growth factor stimulation, suggesting that Hck contributed a mitogenic signal in addition to the previously described activation of Shp-2/Grb2/Erk (17).
In conclusion, our results suggest that the Src family kinase Hck mediates proliferative effects of gp130 by binding to a novel acidic domain thus far not described for gp130. This interaction seems to lead to the dephosphorylation of Pyk2 and to the activation of Erk. To further elucidate the mechanism of Hck activation, we are currently studying further downstream signaling events which depend on the interaction of this acidic domain of gp130 with Src kinases. Since IL-6 is important for the growth of MM cells and the expansion of early hematopoietic progenitor cells, these studies might enable us to identify some of the relevant signaling intermediates supporting the growth of these cells.
ACKNOWLEDGMENTS
We thank Susanne Anton for excellent technical assistance, Friedemann Horn (Leipzig, Germany) for providing the EpoR-gp130 chimera cDNA, and Mark Showers (Boston, Mass.) for providing Baf-B03 cells.
This work was supported by grants of the Deutsche Krebshilfe 10-1094-HA and 10-1678-HA2 (to M.H.).
REFERENCES
- 1.Abram C L, Courtneidge S A. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Pazdrak K, Stafford S, Alam R. The mapping of the Lyn kinase binding site of the common beta subunit of IL-3/granulocyte-macrophage colony-stimulating factor/IL-5 receptor. J Immunol. 1999;162:1496–1501. [PubMed] [Google Scholar]
- 3.Anderson S M, Jorgensen B. Activation of Src-related tyrosine kinases by IL-3. J Immunol. 1995;155:1660–1670. [PubMed] [Google Scholar]
- 4.Aoki Y, Kim Y T, Stillwell R, Kim T J, Pillai S. The SH2 domains of Src family kinases associate with Syk. J Biol Chem. 1995;270:15658–15663. doi: 10.1074/jbc.270.26.15658. [DOI] [PubMed] [Google Scholar]
- 5.Avraham H, Park S Y, Schinkmann K, Avraham S. Raftk/Pyk2-mediated cellular signaling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 6.Berger L C, Hawley T S, Lust J A, Goldman S J, Hawley R G. Tyrosine phosphorylation of Jak-Tyk kinases in malignant plasma cell lines growth-stimulated by interleukins 6 and 11. Biochem Biophys Res Commun. 1994;202:596–605. doi: 10.1006/bbrc.1994.1970. [DOI] [PubMed] [Google Scholar]
- 7.Blaukat I A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 8.Boulton T G, Stahl N, Yancopoulos G D. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–11655. [PubMed] [Google Scholar]
- 9.Burton E A, Hunter S, Wu S C, Anderson S M. Binding of Src-like kinases to the β-subunit of the interleukin-3 receptor. J Biol Chem. 1997;272:16189–16195. doi: 10.1074/jbc.272.26.16189. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan D, Hideshima T, Pandey P, Treon S, Teoh G, Raje N, Rosen S, Krett N, Husson H, Avraham S, Kharbanda S, Anderson K C. RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene. 1999;18:6733–6740. doi: 10.1038/sj.onc.1203082. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies F E, Shima Y, Tai Y T, Rosen S, Avraham S, Kharbanda S, Anderson K C. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 12.Corey S J, Burkhardt A L, Bolen J B, Geahlen R L, Tkatch L S, Tweardy D J. Granulocyte colony-stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc Natl Acad Sci USA. 1994;91:4683–4687. doi: 10.1073/pnas.91.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich E, Haft C R, Muys L, Heinrich P C, Graeve L. A dileucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and downregulation of the IL-6 receptor. J Biol Chem. 1996;271:5487–5494. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 15.Ernst M, Novak U, Nicholson S E, Layton J E, Dunn A R. The carboxyl-terminal domains of gp130-related cytokine receptors are necessary for suppressing embryonic stem cell differentiation: involvement of STAT3. J Biol Chem. 1999;274:9729–9737. doi: 10.1074/jbc.274.14.9729. [DOI] [PubMed] [Google Scholar]
- 16.Ernst M, Gearing D P, Dunn A R. Functional and biochemical association of Hck with the LIF/IL-6 receptor signal transducing subunit gp130 in embryonic stem cells. EMBO J. 1994;13:1574–1584. doi: 10.1002/j.1460-2075.1994.tb06420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 18.Gerhartz C, Heesel B, Sasse J, Hemmann U, Landgraf C, Schneider-Mergener J, Horn F, Heinrich P C, Graeve L. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin-6 signal transducer gp130. J Biol Chem. 1996;271:12991–12998. doi: 10.1074/jbc.271.22.12991. [DOI] [PubMed] [Google Scholar]
- 19.Gibson R M, Schiemann W P, Prichard L B, Reno J M, Ericsson L H, Nathanson N M. Phosphorylation of human gp130 at Ser-782 adjacent to the di-leucine internalization motif. J Biol Chem. 2000;275:22574–22582. doi: 10.1074/jbc.M907658199. [DOI] [PubMed] [Google Scholar]
- 20.Hallek M, Neumann C, Schäffer S, Danhauser-Riedl M, von Bubnoff N, de Vos G, Druker B J, Yasukawa K, Griffin J D, Emmerich B. Signal transduction of interleukin-6 involves tyrosine phosphorylation of multiple cytosolic proteins and activation of Src-family kinases Fyn, Hck, and Lyn in multiple myeloma cell lines. Exp Hematol. 1997;25:1367–1377. [PubMed] [Google Scholar]
- 21.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 22.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin S D, Perlmutter R M, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of a novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 23.Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grötzinger J, Wollmer A, Zhong Z, Darnell J E, Jr, Graeve L, Heinrich P C, Horn F. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin-6 signal transducer gp130. J Biol Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 24.Hibi M, Nakajima K, Hirano T. IL-6 cytokine family and signal transduction: a model of the cytokine system. J Mol Med. 1996;74:1–12. doi: 10.1007/BF00202068. [DOI] [PubMed] [Google Scholar]
- 25.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Thierfelder W E, Kreider B, Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Baumann H. Dual signaling role of the protein tyrosine phosphatase Shp-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999;19:5326–5338. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai C F, Ripperger J, Morella K K, Wang Y, Gearing D P, Fey G H, Baumann H. Separate signaling mechanisms are involved in the control of STAT protein activation and gene regulation via the interleukin 6 response element by the box 3 motif of gp130. J Biol Chem. 1995;270:14847–14850. doi: 10.1074/jbc.270.25.14847. [DOI] [PubMed] [Google Scholar]
- 28.Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter R M, Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993;12:759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami M, Narazaki M, Hibi M, Yawata H, Yasukawa K, Hamaguchi M, Taga T, Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci USA. 1991;88:11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narazaki M, Witthuhn B A, Yoshida K, Silvennoinen O, Yasukawa K, Ihle J N, Kishimoto T, Taga T. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci USA. 1994;91:2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann C, Zehentmaier S, Danhauser-Riedl G, Emmerich B, Hallek M. Interleukin-6 induces tyrosine phosphorylation of the Ras activating protein Shc, and its complex formation with Grb2 in the human multiple myeloma cell line LP-1. Eur J Immunol. 1996;26:379–384. doi: 10.1002/eji.1830260217. [DOI] [PubMed] [Google Scholar]
- 32.Ogata A, Chauhan D, Teoh G, Treon S P, Urashima M, Schlossman R L, Anderson K C. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 33.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, Itoh S, Narimatsu M, Maeda H, Fukada T, Itoh M, Okano H, Hibi M, Hirano T. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 34.Pawson T. Protein modules and signaling networks. Nature. 1995;373:573–579. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 35.Pelchen-Matthews A, Boulet I, Littman D R, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992;117:279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pignata C, Prasad K V, Hallek M, Druker B, Rudd C E, Robertson M J, Ritz J. Phosphorylation of Src family lck tyrosine kinase following interleukin-12 activation of human natural killer cells. Cell Immunol. 1995;165:211–216. doi: 10.1006/cimm.1995.1207. [DOI] [PubMed] [Google Scholar]
- 37.Rao P, Mufson R A. A membrane proximal domain of the human interleukin-3 receptor beta c subunit that signals DNA synthesis in NIH 3T3 cells specifically binds a complex of Src and Janus family tyrosine kinases and phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:6886–6893. doi: 10.1074/jbc.270.12.6886. [DOI] [PubMed] [Google Scholar]
- 38.Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. . (Erratum, 16:7182–7184.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz J, Dahmen H, Grimm C, Gendo G, Müller-Newen C, Heinrich P C, Schaper F. The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J Immunol. 2000;164:848–854. doi: 10.4049/jimmunol.164.2.848. [DOI] [PubMed] [Google Scholar]
- 41.Shi Z Q, Lu W, Feng G S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 42.Simpson R J, Hammacher A, Smith D K, Matthews J M, Ward L D. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somers W, Stahl M, Seehra J S. 1.9 Å crystal structure of interleukin 6: implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997;16:989–997. doi: 10.1093/emboj/16.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Songyang Z, Shoelson S E, Chauduri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider J R, Neel B G, Birge R B, Fajardo E J, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 45.Stahl N, Farugella T J, Boulton T G, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen O, Barberi G, Pellegrini S, Ihle J N, Yancopoulos G D. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 β receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi-Tezuka M, Yoshida Y, FukadA T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang H, Zhao Z J, Huang X Y, Landon E J, Inagami T. Fyn kinase-directed activation of SH2 domain-containing protein-tyrosine phosphatase SHP-2 by Gi protein-coupled receptors in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:12401–12407. doi: 10.1074/jbc.274.18.12401. [DOI] [PubMed] [Google Scholar]
- 48.Tang H, Zhao Z J, Landon E J, Inagami T. Regulation of calcium-sensitive tyrosine kinase Pyk2 by angiotensin II in endothelial cells: roles of Yes tyrosine kinase and tyrosine phosphatase SHP-2. J Biol Chem. 2000;275:8389–8396. doi: 10.1074/jbc.275.12.8389. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 50.Tanner J W, Chen W, Young R L, Longmore G D, Shaw A S. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J Biol Chem. 1995;270:6523–6530. doi: 10.1074/jbc.270.12.6523. [DOI] [PubMed] [Google Scholar]
- 51.Thiel S, Behrmann I, Dittrich E, Muys L, Tavernier J, Wijdenes J, Heinrich P C, Graeve L. Internalization of the interleukin 6 signal transducer gp130 does not require activation of the Jak/STAT pathway. Biochem J. 1998;330:47–54. doi: 10.1042/bj3300047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiel S, Sommer U, Kortylewski M, Haan C, Behrmann I, Heinrich P C, Graeve L. Termination of IL-6-induced STAT activation is independent of receptor internalization but requires de novo protein synthesis. FEBS Lett. 2000;470:15–19. doi: 10.1016/s0014-5793(00)01276-x. [DOI] [PubMed] [Google Scholar]
- 53.Thomas J W, Ellis B, Boerner R J, Knight W B, Withe I I G C, Schaller M D. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- 54.Venkitaraman A R, Cowling R J. Interleukin 7 receptor functions by recruiting the tyrosine kinase p59fyn through a segment of its cytoplasmic tail. Proc Natl Acad Sci USA. 1992;89:12083–12087. doi: 10.1073/pnas.89.24.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward L D, Hammacher A, Howlett G J, Matthews J M, Fabri L, Moritz R L, Nice E C, Weinstock J, Simpson R J. Influence of interleukin-6 (IL-6) dimerization on formation of the high-affinity hexameric IL-6 receptor complex. J Biol Chem. 1996;271:20138–20144. doi: 10.1074/jbc.271.33.20138. [DOI] [PubMed] [Google Scholar]
- 56.Warmuth M, Bergmann M, Priess A, Häuslmann K, Emmerich B, Hallek M. The Src kinase HCK interacts with Bcr-Abl by a kinase-independent mechanism and phosphorylates the Grb2-binding Site of Bcr. J Biol Chem. 1997;272:33260–33270. doi: 10.1074/jbc.272.52.33260. [DOI] [PubMed] [Google Scholar]
- 57.Wen Z, Thong Z, Darnell J E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Chen J K, Feng S, Dalgarno D C, Brauer A W, Schreiber S L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Blenis J C, Li H, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 60.Zhao J, Zheng C, Guan J. Pyk2 and FAK differentially regulate progression of the cell cycle. J Cell Sci. 2000;113:3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]