Abstract
Initiation of transcriptional silencing at mating type loci and telomeres in Saccharomyces cerevisiae requires the recruitment of a Sir2/3/4 (silent information regulator) protein complex to the chromosome, which occurs at least in part through its association with the silencer- and telomere-binding protein Rap1p. Sir3p and Sir4p are structural components of silent chromatin that can self-associate, interact with each other, and bind to the amino-terminal tails of histones H3 and H4. We have identified a small region of Sir3p between amino acids 455 and 481 that is necessary and sufficient for association with the carboxyl terminus of Rap1p but not required for Sir complex formation or histone binding. SIR3 mutations that delete this region cause a silencing defect at HMR and telomeres. However, this impairment of repression is considerably less than that displayed by Rap1p carboxy-terminal truncations that are defective in Sir3p binding. This difference may be explained by the ability of the Rap1p carboxyl terminus to interact independently with Sir4p, which we demonstrate by in vitro binding and two-hybrid assays. Significantly, the Rap1p-Sir4p two-hybrid interaction does not require Sir3p and is abolished by mutation of the carboxyl terminus of Rap1p. We propose that both Sir3p and Sir4p can directly and independently bind to Rap1p at mating type silencers and telomeres and suggest that Rap1p-mediated recruitment of Sir proteins operates through multiple cooperative interactions, at least some of which are redundant. The physical separation of the Rap1p interaction region of Sir3p from parts of the protein required for Sir complex formation and histone binding raises the possibility that Rap1p can participate directly in the maintenance of silent chromatin through the stabilization of Sir complex-nucleosome interactions.
Related forms of transcriptional silencing in the budding yeast Saccharomyces cerevisiae occur at silent mating type loci (HML and HMR) and immediately adjacent to the TG1–3 repeats at telomeres (reviewed in references 15, 21, and 36). Both mating type and telomeric silencing (referred to hereafter as telomere position effect [TPE]) require a common set of trans-acting factors that include a complex of silent information regulators (Sir2, Sir3, and Sir4 proteins) and the amino-terminal tails of the core histones H3 and H4. Although the precise molecular mechanisms underlying silencing are not known, genetic and biochemical evidence indicates that it results from the “spreading” of Sir protein complexes from sites of initiation (HM silencers or telomeres) to nearby chromatin.
Significantly, both Sir3p and Sir4p can interact directly with the histone H3 and H4 tails in vitro (23), pointing to a possible mechanism for the formation of a closed, or repressive, chromatin structure. Consistent with the idea that Sir2/3/4 protein complexes bring about repression through direct interactions with nucleosomes, silencing is neither promoter nor polymerase specific but instead appears to have a general effect either on chromatin accessibility (19, 34, 54) or on the subsequent action of chromatin-bound factors (51a).
A key question, whose answer is still not clearly understood, is how silencing is initiated only at specific chromosomal sites. None of the Sir proteins appear to recognize DNA directly, and the initiation of silencing thus requires a set of DNA-binding factors to recruit the Sir proteins to their sites of action on the chromosome. At telomeres this is accomplished in part by Rap1p, whose binding sites are contained within the TG1–3 repeat tracts that comprise the telomeric DNA (28, 32, 47). Rap1p also binds to three of the four mating type gene silencers, where it collaborates with the origin recognition complex (ORC) and/or Abf1p to initiate silencing (6, 52). Strikingly, none of these three DNA-binding factors is specific to silencers. Rap1p and Abf1p binding sites are found in numerous promoter regions, where they typically act to stimulate transcription (7). Likewise, ORC binding sites are found at all known origins of DNA replication, most of which are not silencers (the only known exception being the HMR-E silencer). Therefore, a full understanding of the role of these proteins in the initiation of silencing must also explain why they have quite different functions in other contexts.
An important clue to the molecular mechanism of Rap1p action at telomeres and silencers came from the identification of both Sir3p and Sir4p as Rap1p-interacting proteins in the two-hybrid system (47). Significantly, Rap1 and Sir3p can interact directly in vitro in the absence of other yeast proteins. These observations, together with other studies (11, 32), suggested that the role of Rap1p in the initiation of silencing is to recruit Sir proteins to the chromosome by direct protein-protein interactions with Sir3p and perhaps Sir4p. Strong support for this comes from the observation that direct targeting of Sir proteins to HMR, by the use of Gal4p DNA-binding domain (GBD)-Sir hybrids, bypasses the requirement for the normal silencer binding sites (10, 40). Similarly, GBD-Sir and LexA-Sir hybrids can restore silencing when targeted to a specific telomere in cells deleted for the carboxy-terminal Sir interaction domain of Rap1p (10, 37, 40).
To better understand the mechanisms underlying the recruitment of Sir3p and Sir4p to silencers and telomeres, we extended our analysis of the interactions of these two proteins with Rap1p. Using the two-hybrid system and a glutathione S-transferase (GST) hybrid pulldown assay, we have identified a Rap1p interaction domain of Sir3p that is both necessary and sufficient for the interaction with the carboxyl terminus of Rap1p. This short region of Sir3p, between amino acids 455 and 481, does not mediate Sir3p self-interaction or the Sir3p interaction with Sir4p, and it does not correspond to a previously characterized histone interaction domain (23). Deletion of this domain debilitates Rap1p-dependent targeted silencing but has much weaker effects on normal HM locus and telomeric silencing. In addition, the silencing defect caused by these Sir3p mutations is weaker than that obtained with Rap1p carboxy-terminal deletions, which themselves cause defects in Sir3p binding (47).
We provide evidence that this difference is due to the ability of Rap1p to interact independently with Sir4p. Significantly, overexpression of Sir4p suppresses the HMR silencing defect displayed by Sir3p mutants unable to bind Rap1p. These data support a model in which the establishment of silencing at HM loci and at telomeres involves the recruitment of the Sir complex to the chromosome via a set of cooperative interactions, involving direct binding of both Sir3p and Sir4p to the carboxyl terminus of Rap1p. Taken together with previous studies of Sir3p and Sir4p binding to histones (23), these new insights into Rap1p-Sir interactions also suggest a mechanism by which Rap1p may participate not only in initiation but also in the propagation and maintenance of silent chromatin.
MATERIALS AND METHODS
Yeast strains and silencing assays.
Growth and manipulation of yeast strains were done according to standard procedures (51). The yeast two-hybrid reporter strain CTY10-5D (MATa ade2-1 trp1-901 leu2-3,112 his3-200 gal4 gal80 URA3::lexAop-lacZ) was used in all studies involving LexA fusion proteins. This strain, and its derivatives with HIS3 disruptions of SIR1, SIR2, SIR3, SIR4, or RIF1, has been described elsewhere (47). All strains used for transcriptional silencing assays are isogenic to strain W303-1B (MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1) (59).
The two strains used for targeted silencing with GBD/Rap1p fusions are Leu− derivatives of two strains (relevant genotypes: ΔAΔE(UASG)3hmr::TRP1 gal4::LEU2 sir3::URA3 and ΔEΔB(UASG)3hmr::TRP1 gal4::LEU2 sir3::URA3) described previously (8). These strains were created as follows. First, the gal4::LEU2 gene was disrupted by gene replacement using a leu2::(UASG)::ADE2 construct in which the regulatory sequences of the ADE2 gene have been replaced with UASG, allowing Gal4p-dependent expression of the gene. The disruption was obtained by cotransformation with plasmid pMA210 (38), which expresses Gal4p, followed by selection on plates of synthetic complete (SC) medium lacking Ade and His (SC−Ade−His). Leu− transformants were identified by replica plating and then grown in liquid yeast extract-peptone-dextrose (YEPD) medium to allow loss of the pMA210 plasmid. The cultures were spread on YEPD plates, and cells with the desired phenotype (Leu− Ade− His−) were selected. These cells were then sequentially transformed, first with GBD/Rap1p fusions (8) in the HIS3 integrating vector pRS303 (53) and then with mutant sir3 alleles cloned into a low-copy-number CEN vector.
The strains used for the analysis of transcriptional silencing at the HMR locus and at telomeres are derived from a series of HMR::TRP1 and HMR::ADE2 strains or from a URA3-Tel VIIL strain, all of which have been described previously (57, 58). sir3 disruption derivatives of these strains were obtained by gene replacement using a sir3::HIS3 deletion/disruption construct (HMR::TRP1, HMR::ADE2, and URA3-Tel VIIL strains) or a sir3::URA3 deletion/disruption (HMR::ADE2) strain. The HIS3 disruption removes all Sir3p coding sequences between amino acids 108 and 945. The URA3 disruption deletes the entire Sir3p open reading frame (978 amino acids) up to position 972. Both gene disruptions cause a complete loss of repression of the reporter gene and can be complemented by a plasmid-borne copy of SIR3. For the experiments with mutant alleles of SIR3, the cells were transformed with SIR3 deletions cloned into plasmid pRS415 (LEU2 CEN) (53).
Assays for silencing using the HMR::TRP1 reporter were performed by spotting 10-fold serial dilutions of cultures grown in the appropriate synthetic selective medium as described previously (57). Repression was tested in HMR::ADE2 strains by examining colony color after 3 days of growth of the transformants at 30°C, followed by storage of the plates at 4°C for 1 or more days. Determination of the fraction of 5-fluoroorotic acid-resistant (5-FOAr) and Ura+ cells in URA3-Tel VIIL strains was done as follows. Independent colonies were grown in SC−Leu liquid medium overnight, diluted to an appropriate concentration, and then spread on SC−Leu, SC−Leu−Ura, and SC−Leu+5-FOA plates. Colonies were counted after 3 days at 30°C, and results from three or more independent cultures were used to calculate an average value.
Plasmids.
The LexA protein and all LexA fusion proteins were expressed from plasmid pBTM116 (2μm origin, TRP1 pADH1-lexA) (2). All LexA/Rap1p hybrids and the LexA/Sir4p(839–1358) and LexA/Sir3p(307–978) constructs have been described elsewhere (47). LexA/Sir1p, LexA/lamin, and LexA/Adh1p were gifts from Rolf Sternglanz (State University of New York at Stony Brook). All the Gal4p activation domain (GAD) hybrids described here were expressed from plasmid pACTII (2μm origin, LEU2, pADH1-GAD). The SIR3 fragment used to create GAD/Sir3p(307–978) was obtained from a LexA/Sir3p(307–978) fusion described previously (47). All the other GAD/Sir3p constructs used here were obtained by two- or three-way ligation of SIR3 fragments to the pACTII vector.
The construction of some of these constructs required intermediate cloning steps in pUC19 or pIC series plasmids. All the carboxy-terminal deletions of SIR3 in the pACTII vector have the same amino-terminal junction as GAD/Sir3p(307–978) and were created by cutting the SIR3 sequence with a suitable restriction enzyme followed by repair of the end with the Klenow fragment or T4 DNA polymerase and ligation to a flushed site in the polylinker of the vector. All amino-terminal GAD/Sir3p fusions other than GAD/Sir3p(307–978) were created by using a restriction sites in the SIR3 open reading frame or by creating a site at a suitable position by PCR. More detailed information on these constructs is available upon request.
GAD/Sir4p(839–1358) was obtained by ligating a BamHI-SalI fragment of SIR4 to the pACTII vector cut with BamHI and XhoI. GAD/Sir4p(839–1275) was obtained by removing SIR4 sequences beyond the EcoRV site. The internal deletions of SIR3 were created either by joining the flushed ends of DNA fragments cut at suitable restriction sites or by PCR cloning. All deletions cause the removal of SIR3 sequences without causing the insertion of amino acids that are not normally present in the protein. Allele Δ440–502 was created by joining an amino-terminal fragment cut with EcoRI followed by Klenow repair of the end to a carboxy-terminal fragment cut with HindIII and repaired with Klenow. Mutant Δ482–502 was created in the same manner by joining a BsrFI end repaired with Klenow to the same blunt HindIII end as above. All remaining SIR3 deletions were constructed using PCR by creating restriction sites at new positions and subsequently removing sequences between the novel site and an existing site in SIR3.
Alleles Δ440–480 and Δ440–454 were constructed by creating an EcoRI site at positions 481 and 455, respectively, followed by removal of SIR3 sequences between this novel site and the EcoRI site at amino acid position 439. Allele Δ456–479 was constructed by creating a BsrFI site at amino acid position 455 followed by ligation to the BsrFI site at position 480. Mutant Δ333–357 was constructed by creating a novel EagI site at position 333, and alleles Δ358–437 and Δ398–437 were constructed by creating new EcoRI sites at positions 357 and 397, respectively. Fragments from these deletion constructs of SIR3 were cloned in the pACTII vector for two-hybrid studies, in a pT7-Sir3p construct for in vitro studies, and in the pRS415 and pRS425 plasmids to test for transcriptional silencing in vivo. The GST/Rap1p and pT7-Sir3p(1–978) constructs used in this work have been described previously. pT7-Sir4p(839–1358) and pT7-Sir4p(839–1275) are derived from the corresponding GAD/Sir4p constructs. Constructs used for the overexpression of SIR1 and SIR4 were created by cloning a gene fragment in the 2μm URA3 vector pRS426 and in the CEN URA3 vector pRS316, respectively.
Transcriptional activation assays.
Transcriptional activation assays using LexA hybrids or LexA and GAD hybrid combinations were performed as previously described (47).
In vitro protein-binding assays.
Protein expression and purification and all in vitro binding studies were performed as described previously (47).
RESULTS
Amino acids 455 to 481 of Sir3p are sufficient to mediate a two-hybrid interaction with Rap1p but not Sir3p or Sir4p.
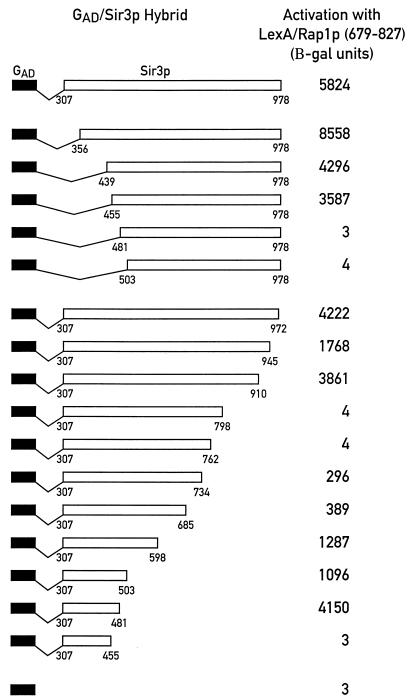
We showed previously that the carboxy-terminal two-thirds of Sir3p (amino acids 307 to 978) can interact with Rap1p, Sir4p, and itself in two-hybrid assays (47). Here we have extended this analysis to ask whether a defined region of Sir3p that is specifically involved in binding to Rap1p could be identified. Beginning with a construct expressing GAD/Sir3p(307–978), two sets of GAD/Sir3p hybrids with progressive carboxy- or amino-terminal deletions of Sir3p were created and tested for the ability to interact with a LexA/Rap1p(679–827) hybrid. As shown in the top panel of Fig. 1, GAD/Sir3p fusions with amino-terminal endpoints at positions 307, 356, 439, and 455 (and continuing to the carboxyl terminus at position 978) do not significantly differ in the strength of their interaction with LexA/Rap1p(679–827). However, two shorter fusions, GAD/Sir3p(481–978) and GAD/Sir3p(503–978), are completely defective in this interaction. The failure of these latter two GAD/Sir3p constructs to give a signal in the two-hybrid assay is not due to lack of expression of a functional protein, since both fusion proteins interact normally with LexA/Sir3p(307–978) (data not shown). From these results, we place the amino-terminal endpoint of the minimal region of Sir3p required for the two-hybrid interaction with Rap1p between amino acids 455 and 481.
FIG. 1.
Interaction of a series of GAD/Sir3p truncations with LexA/Rap1p(679–827) in the two-hybrid system. GAD/Sir3p fusions with progressive amino- and carboxy-terminal deletions of SIR3 sequences were assayed for their interaction with LexA/Rap1p(679–827) using the two-hybrid reporter strain CTY10-5D. Transcriptional activation, measured as units of β-galactosidase (B-gal) activity, was normalized to a value of 10,000 U for LexA/Gal4p(768–881) [(LexA/GAD)], which was included as a control in all experiments (not shown in the figure).
The analysis of a series of carboxy-terminal deletions gives a more complex picture than the amino-terminal set. Deletion of Sir3p sequences between amino acids 978 and 910 does not significantly affect the strength of the two-hybrid interaction with Rap1p, whereas two larger deletions (truncations at positions 798 and 762) completely abolish the two-hybrid signal (Fig. 1). Again, loss of the Rap1p interaction with these two latter constructs is not due to lack of expression of functional GAD/Sir3p hybrids, since both of these fusions can interact with LexA/Sir4p(839–1358) as strongly as GAD/Sir3p(307–978) (data not shown). Surprisingly, with Sir3p deletions beyond amino acid 762, the two-hybrid signal with LexA/Rap1p is restored, weakly in the case of GAD/Sir3p(307–734) and GAD/Sir3p(307–685), but to high levels with truncations at positions 598, 503, and 481 of Sir3p. The interaction is abolished again with further deletions to endpoints at amino acids 455 and 439. Taken together, these data suggest that the carboxy-terminal limit of the minimal Sir3p region required for the interaction with Rap1p may lie between amino acids 481 and 455. Although we do not know why the carboxy-terminal truncations ending between residues 685 and 798 of Sir3p weaken or abolish interactions with Rap1p in the two-hybrid assay, we note that this region of Sir3p has been linked to its binding to core histone N-terminal tail sequences (23). Perhaps, in the Sir3p truncations in question, histone interaction regions become exposed in such a way as to titrate out the two-hybrid interaction with Rap1p.
To rule out the possibility that the amino-terminal 307 amino acids of Sir3p that were not included in this analysis also contain sequences able to mediate an interaction with Rap1p, we tested a GAD/Sir3p(1–503) and a GAD/Sir3p(1–439) fusion. Only the first of these two constructs is able to give a signal in a two-hybrid assay with LexA/Rap1p(679–827) (data not shown), suggesting that the first 439 amino acids of Sir3p do not contain regions that are able to interact with Rap1p. This result, as well as all data shown in Fig. 1, were replicated with LexA/Rap1p(635–827) and LexA/Rap1p(653–827) hybrids.
We have shown previously that LexA/Rap1p carboxy-terminal fusions interact genetically with the endogenous SIR2, SIR3, SIR4, and RIF1 genes. Specifically, mutations in any one of these four genes enhance a cryptic activation potential of certain LexA/Rap1p hybrids. In addition, Rif1p and Sir3p appear to compete for binding to the carboxyl terminus of Rap1p in the two-hybrid assay (47). To ask if the analysis reported above was influenced by competition between the GAD/Sir3p fusions and endogenous Sir2, Sir3, Sir4, or Rif1 protein, we repeated these experiments in a set of reporter strains containing null mutations in each of the corresponding genes. Only minor quantitative differences between mutant and wild-type reporters were observed (data not shown), arguing against the possibility of significant perturbation by any of the endogenous factors tested.
The data described above suggest that amino acids 455 to 481 of Sir3p may contain all of the sequences required for a two-hybrid interaction with the carboxyl terminus of Rap1p. To test this hypothesis directly and to determine whether this small Sir3p fragment also mediates homodimerization or association with Sir4p, we constructed a GAD/Sir3p(455–481) fusion and tested it with LexA/Rap1p(679–827), LexA/Sir3p(307–978), and LexA/Sir4p(839–1358). As shown in Table 1, GAD/Sir3p(455–481) displays a significant association with LexA/Rap1p(679–827), demonstrating that a fragment of only 27 amino acids of Sir3p is sufficient to mediate a two-hybrid interaction with the carboxyl terminus of Rap1p. In contrast, this small Sir3p fragment does not give a signal with either LexA/Sir3p(307–978) or LexA/Sir4p(839–1358), suggesting that amino acids 455 to 481 of Sir3p are not sufficient to mediate a Sir3p self-interaction or binding to Sir4p.
TABLE 1.
Short stretch of 27 amino acids of Sir3p is sufficient to mediate a two-hybrid interaction with Rap1p but not Sir3p or Sir4p
| LexA hybrid | β-Galactosidase (U)
|
|
|---|---|---|
| GAD/Sir3p(455–481) | GAD | |
| LexA/Rap1p(679–827) | 80 | 3 |
| LexA/Sir3p(307–978) | 3 | 3 |
| LexA/Sir4p(839–1358) | 4 | 5 |
Deletion of amino acids 456 to 479 of Sir3p specifically abolishes the two-hybrid interaction with Rap1p.
To test the hypothesis that the region between amino acids 455 and 481 in Sir3p is necessary for the association with Rap1p but not required for either Sir3p self-interaction or binding to Sir4p, we constructed a series of GAD/Sir3p(307–978) fusions with small deletions spanning the region between amino acid positions 333 and 502. As shown in Table 2, the deletion of 24 amino acids of Sir3p between positions 456 and 479 completely abolishes the interaction with LexA/Rap1p(679–827), as do two larger deletions encompassing the same region (Δ440–480 and Δ440–502). None of these three mutations significantly impair the interaction with LexA/Sir4p(839–1358) or LexA/Sir3p(307–978) hybrids (Table 2), demonstrating the specificity of their effect on the interaction with Rap1p.
TABLE 2.
Deletion of as few as 24 amino acids of Sir3p abolishes the interaction with Rap1p but not Sir3p or Sir4p in the two-hybrid system
| GAD/Sir3p hybrid | β-Galactosidase (U)
|
||
|---|---|---|---|
| LexA/Rap1p (679–827) | LexA/Sir4p (839–1358) | LexA/Sir3p (307–978) | |
| GAD/Sir3p(307–978) | 5,824 | 3,238 | 3,597 |
| GAD/Sir3p(Δ456–479) | 3 | 4,297 | 1,384 |
| GAD/Sir3p(Δ440–480) | 4 | 3,354 | 1,201 |
| GAD/Sir3p(Δ440–502) | 4 | 3,331 | 1,704 |
| GAD/Sir3p(Δ440–454) | 5,337 | 3,555 | 3,292 |
| GAD/Sir3p(Δ398–437) | 5,709 | 2,944 | 3,136 |
| GAD/Sir3p(Δ358–437) | 6,587 | 2,501 | 3,333 |
| GAD/Sir3p(Δ333–357) | 6,445 | 2,845 | 3,378 |
| GAD/Sir3p(Δ482–502) | 7,052 | 2,329 | 2,694 |
| GAD | 3 | 5 | 2 |
To determine whether Sir3p sequences adjacent to the short region between amino acids 456 and 479 play a role in this interaction, constructs with deletions amino-terminal to position 456 (Δ440–454, Δ398–437, Δ358–437, and Δ333–357) and one deletion carboxy-terminal of position 479 (Δ482–502) were created. As shown in Table 2, none of these constructs show any defect in the interaction with LexA/Rap1p(679–827), LexA/Sir4p(839–1358), or LexA/Sir3p(307–978). The results of this analysis did not change when the interactions were tested in two-hybrid reporter strains carrying mutations in either SIR2, SIR3, SIR4, or RIF1 (data not shown). In summary, these data demonstrate that amino acids 456 to 479 of Sir3p are necessary for the interaction with the carboxyl terminus of Rap1p in the two-hybrid system but do not play any detectable role in binding of Sir3p to itself or Sir4p.
Amino acids 455 to 481 of Sir3p are required for binding to carboxyl terminus of Rap1p in vitro.
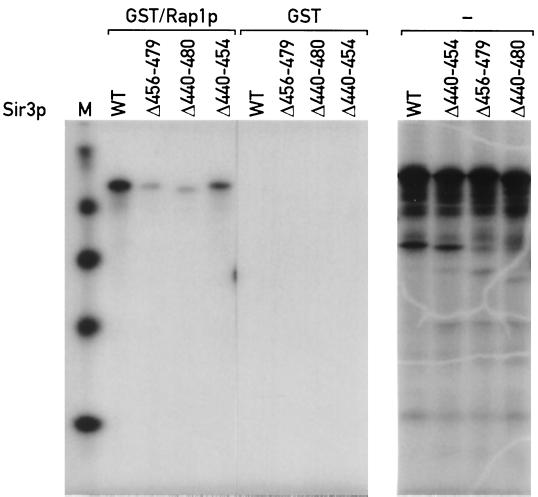
Using a GST pulldown assay, we showed previously that Sir3p can bind to the carboxyl terminus of Rap1p (47). The same approach was used to test whether Sir3p sequences required for Rap1p interaction in the two-hybrid assay are also necessary for binding in vitro (see Materials and Methods for details). Three 35S-labeled Sir3p mutant proteins were analyzed, two of which (Δ456–479 and Δ440–480) contain deletions that abolish the Rap1p-Sir3p two-hybrid interaction. The third mutant (Δ440–454) has a deletion of sequences that do not appear to play a role in the Rap1p interaction, as judged by the two-hybrid assay.
As shown in Fig. 2, wild-type Sir3p and Sir3p(Δ440–454) are both able to bind to the GST/Rap1p(562–827) fusion. In contrast, Sir3p(Δ456–479) and Sir3p(Δ440–480) show a strong impairment in binding to GST/Rap1p. These data confirm the results of the two-hybrid analysis of the interaction between Rap1p and Sir3p and indicate that amino acids 456 to 479 of Sir3p are required for strong binding to the carboxyl terminus of Rap1p in vitro. Previous studies have shown that this region of Sir3p does not appear to be required for in vitro binding to the amino termini of histones H3 and H4 (23). Taken together, the results described above identify amino acids 455 to 481 of Sir3p as a Rap1p interaction domain of this protein.
FIG. 2.
Sir3p mutants with a deletion of amino acids 456 to 479 are defective in binding to GST/Rap1p(562–827) in vitro. See Materials and Methods for details of the binding assay. Right, in vitro-produced labeled wild-type (WT) and mutant proteins (from a separate gel) before addition to GST/Rap1p(562–827) or GST-agarose beads. Left, material bound to the GST/Rap1p beads in each lane [Sir3p(1–978), Sir3p(Δ456–479), Sir3p(440–480), and Sir3p(440–454)] has the same mobility as the primary high-molecular-weight translation product in the right panel (note that the order of lanes in the two gels is not the same). Lane M, size standards.
Targeted silencing by GBD/Rap1p hybrids requires Rap1p interaction domain of Sir3p.
If the Rap1p interaction domain of Sir3p identified above plays any role in repression of transcription in vivo, its deletion should cause an impairment of silencing. In addition, mutations of Sir3p that do not affect the interaction with Rap1p, Sir4p, or Sir3p should not cause a silencing defect unless other functions required for silencing were affected. To test these ideas, we first used a targeted silencing assay (8) in which GBD/Rap1p hybrids are tethered to mutated HMR-E silencer elements containing Gal4p binding sites (UASG). In this assay, the restoration of silencing is critically dependent upon a small carboxy-terminal domain of Rap1p(667–827) with which Sir3p interacts.
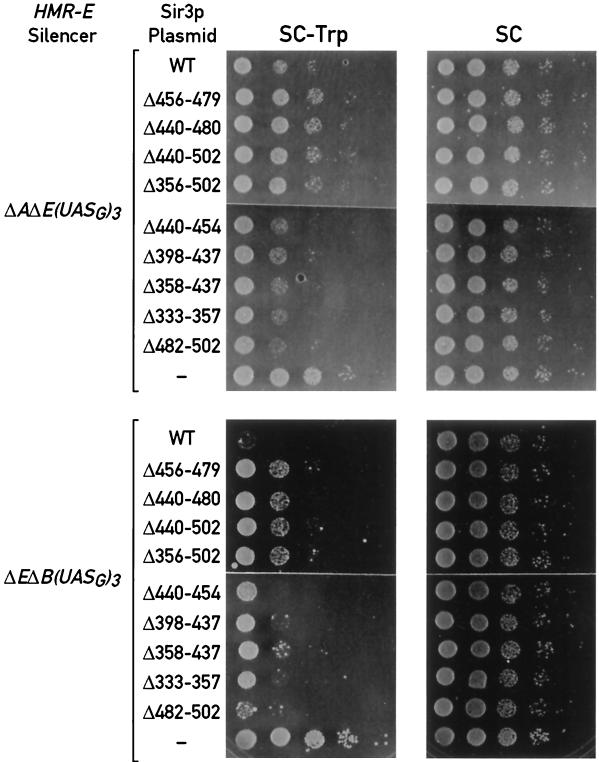
Two reporter strains with different mutant HMR-E silencers were used in these experiments. In the first strain, the HMR-E silencer is deleted for the ORC binding site (A) and the Rap1p-binding site (E), and the two elements are replaced by three UASG sites [ΔAΔE(UASG)3]. In the second strain, the Rap1p-binding site and the Abf1p-binding site (B) are removed and replaced by the same UASG trimer [ΔEΔB(UASG)3]. In both strains, the MATa1 gene at HMR is replaced by TRP1, and silencing is measured by the ability of the strains to grow on medium lacking tryptophan (57). To target the Rap1p carboxyl terminus to these altered silencers, a construct expressing a GBD/Rap1p(653–827) fusion protein is integrated at the HIS3 locus. To test for the SIR3 dependence of targeted silencing, the chromosomal copy of SIR3 is disrupted in these strains and either the wild-type or a series of mutant SIR3 alleles (the same mutations tested in the two-hybrid analysis introduced into the full-length SIR3 gene) are expressed from a low-copy-number centromere-containing (CEN) vector.
The top panel of Fig. 3 shows results obtained with the ΔAΔE(UASG)3::TRP1 strain. As expected, the TRP1 gene is efficiently repressed (as judged by an approximately 100-fold decrease in the ability to form colonies on medium lacking tryptophan) in the presence of wild-type SIR3 on a plasmid (top row) and is completely derepressed (full growth on −Trp medium) in cells transformed with the control vector alone (bottom row). Significantly, four SIR3 alleles with a deletion of the Rap1p interaction domain (Δ456–479, Δ440–480, Δ440–502, and Δ356–502) display an almost complete loss of repression (Fig. 3, rows 2 to 5). In contrast, Sir3p deletions that did not cause a defect in the two-hybrid interaction with Rap1p (Δ440–454, Δ398–437, Δ358–437, and Δ333–357) support silencing in this assay as well as wild-type Sir3p (Fig. 3, rows 6 to 9).
FIG. 3.
Targeted silencing of an hmr::TRP1 reporter by a GBD/Rap1p(653–827) hybrid with different HMR-E silencer deletions and a series of SIR3 alleles. Silencing is measured by comparing the ability of cells to grow in the absence (SC−Trp) and presence (SC) of tryptophan. Each row consists of spots representing 5-μl aliquots from a set of 10-fold serial dilutions of an overnight liquid culture. Photographs were taken after 2 to 3 days of growth at 30°C.
The results obtained with the same set of SIR3 alleles in the ΔEΔB(UASG)3::TRP1 strain (bottom panel of Fig. 3) are qualitatively similar. In this strain, however, targeted silencing in the SIR3 wild-type background appears much stronger, and the derepressing effect of the Rap1p interaction domain deletions (rows 2 to 5) is correspondingly weaker than in the ΔAΔE(UASG)3 silencer strain. Nonetheless, we again observe little or no silencing defect for the SIR3 alleles with deletions outside of the Rap1p interaction domain (rows 6 to 10). This difference between the two silencers suggests that the ORC plays a more important role than Abf1p in recruitment of the silencing complex to the chromosome in cooperation with Rap1p (60) (see Discussion). In both strains these results were replicated using GBD/Rap1p hybrids expressing either a longer or a shorter fragment of the carboxyl terminus of Rap1p (data not shown), suggesting that the different effect of the SIR3 mutations in the two strains is not the result of a peculiar feature of the GBD/Rap1p(653–827) hybrid.
The direct targeting of either Sir3p or Sir4p to the HMR locus in cells lacking a functional HMR-E silencer is also sufficient to restore repression (40). In order to determine whether the defect displayed by the SIR3 alleles tested above was specific to Rap1p-dependent silencing, we analyzed GBD-Sir3p(1–978)- and GBD-Sir4p(1–1358)-dependent repression in the presence of the same SIR3 mutants tested above. None of the SIR3 alleles displays a noticeable silencing defect with either GBD/Sir3p or GBD/Sir4p (data not shown). These data demonstrate that the defect caused by the SIR3 mutations is bypassed when either Sir3p or Sir4p is targeted to the silencer by a Rap1p-independent mechanism. Therefore, these small SIR3 deletions do not cause a general impairment of repression and are thus more likely to specifically affect a step in which Sir3p is recruited by Rap1p to the silencer.
Deletion of Rap1p interaction domain of Sir3p impairs silencing at HMR locus when the silencer ORC binding site is deleted.
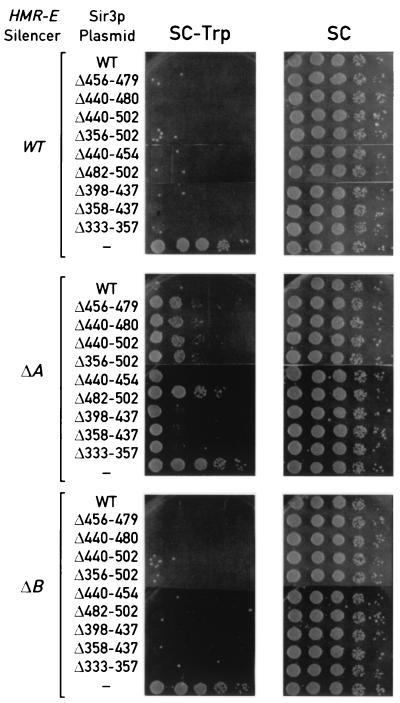
We next tested the effect of SIR3 mutations on silencing initiated by wild-type silencers at HMR as well as two mutated but fully functional silencers in which the redundant ORC (A element) or Abf1p (B element) binding sites at HMR-E were deleted (5, 26, 57). As shown in the top and bottom panels of Fig. 4, both wild-type and mutant alleles of SIR3 are able to restore full silencing in both HMR::TRP1 and hmrΔB::TRP1 cells. Only control cells lacking a functional copy of SIR3 are fully derepressed (last row of each panel in Fig. 4). In contrast, Sir3p mutants with a deletion of the Rap1p interaction domain (Δ456–479, Δ440–480, Δ440–502, and Δ356–502) display a partial silencing defect in hmrΔA::TRP1 cells (middle panels of Fig. 4). In these mutants, the ability to grow on medium lacking tryptophan is increased about 103-fold compared with cells expressing wild-type Sir3p (first rows of the panels), but is reduced 10−2- to 10−3-fold compared with cells transformed with the CEN plasmid control (last rows of the panels). Significantly, deletion of Sir3p sequences amino-terminal of the Rap1 interaction domain (Δ440–454, Δ398–437, Δ358–437, and Δ333–357) does not cause any impairment of repression in HMRΔA::TRP1 cells.
FIG. 4.
Transcriptional silencing at the HMR locus with different HMR-E silencer deletions and a series of SIR3 alleles. The assays were performed as described for Fig. 3.
The fact that Sir3p mutants defective in the Rap1p interaction have a partial silencing defect in hmrΔA::TRP1 cells but no impairment in HMR::TRP1 or hmrΔB::TRP1 cells differs from results obtained with Rap1p mutants defective in Sir3p interaction, in which there is a complete silencing defect in both hmrΔA::TRP1 and hmrΔB::TRP1 cells but no loss of repression in HMR::TRP1 cells (47). To test the possibility that these Sir3p mutants have a weak silencing defect that our assay was not sensitive enough to detect, we tested one of them (Δ456–479) in strains in which the ADE2 reporter is integrated at HMR in cells containing wild-type or mutant HMR-E silencer (ΔA and ΔB) (58). The ADE2 gene has been shown to be efficiently repressed at HMR and allows very sensitive detection of weak silencing defects by a simple nonselective colony color assay. Although two different SIR3 deletion-insertion mutations (removing amino acids 108 to 945 or nearly the entire open reading frame [ORF], amino acids 1 to 972) caused strong derepression of all three ADE2 reporters, the Sir3p(Δ456–479) deletion showed a strong silencing defect only in the hmrΔA::ADE2 strain (data not shown), consistent with the results obtained in the TRP1 reporter strains. Taken together, these data suggest that loss of Sir3p binding to the carboxyl terminus of Rap1p causes a partial silencing defect at HMR, but only when the HMR-E silencer lacks a functional ORC binding site.
Deletion of Rap1p interaction domain of Sir3p impairs telomeric silencing.
At telomeres, multiple Rap1p binding sites are found within the terminal TG1–3 repeats (16, 33), where the Rap1 protein is involved in regulation of both telomere structure and telomeric silencing (8, 27, 28, 32, 41, 47). We showed previously that mutations of the Rap1p carboxyl terminus that impair Sir3p binding also impair telomeric repression (47). If the Sir3p mutants described above were unable to bind to native Rap1p in vivo, one would predict that they should cause a telomeric silencing defect.
We tested this idea using a standard telomeric silencing assay in which the URA3 gene is placed immediately adjacent to a telomere created at the ADH4 locus (20). In these cells, the telomeric URA3 reporter gene is subject to a variegated form of silencing that results in the repression of the gene in ≈50% of the cells in a culture, which can be quantified by measuring the ability of the cells to grow in the presence of 5-FOA, which kills cells expressing URA3. We created a sir3Δ derivative of this strain and transformed the cells with plasmids carrying wild-type SIR3, two alleles encoding a deletion of the Rap1p interaction domain [Sir3p(Δ456–479) and Sir3p(Δ440–480)], and one allele with a mutation that does not cause an impairment of Rap1p interaction [Sir3p(Δ333–357)].
As shown in Table 3, growth in medium containing 5-FOA is impaired in cells expressing Sir3p(Δ456–479) and Sir3p(Δ440–480) compared with cells expressing wild-type Sir3p. About 45% of the cells expressing wild-type Sir3p are able to grow in the presence of 5-FOA. On the other hand, only 27 and 26% of the total cell population are able to grow in the same medium for cells expressing Sir3p(Δ456–479) and Sir3p(Δ440–480), respectively, resulting in a decrease of about 40 to 43% compared with the wild type. In contrast, the control mutant Sir3p(Δ333–357) gives wild-type levels of growth in 5-FOA of about 50%, demonstrating the specificity of the deletion of the Rap1p interaction domain in telomeric repression. No significant differences were measured between wild-type and mutant alleles of SIR3 in growth in the absence of uracil.
TABLE 3.
SIR3 alleles with a deletion of the Rap1p interaction domain have a defect in telomeric silencinga
| Sir3p construct | Growth (%) on medium:
|
|
|---|---|---|
| Containing 5-FOA | Lacking uracil | |
| Wild type | 45.3 | 68.5 |
| Sir3p(Δ456–479) | 27.2 | 69.9 |
| Sir3p(Δ440–480) | 25.6 | 66.0 |
| Sir3p(Δ333–357) | 49.9 | 75.6 |
A Δsir3 reporter strain containing the URA3 gene immediately adjacent to a telomere created at the ADH4 locus on chromosome VII-L was used in these experiments. The percentage of 5-FOAr and Ura+ cells was calculated by averaging the values obtained from three or more independent SIR3 transformants. These values reflect the number of cells carrying the plasmid-borne copy of SIR3 that are able to grow on the selective plates.
These data indicate that, as was the case for native silencing at HMR, loss of a single direct contact between Rap1p and Sir3p causes a relatively weak impairment of telomeric silencing compared to the effect of deleting the carboxy-terminal Sir3p-interacting domain of Rap1p (28, 47). One possible explanation for these results is that the Sir3p mutations that we created cause only a partial loss of Rap1p-Sir3p binding in vivo and thus do not significantly compromise the ability of Rap1p to recruit Sir3p to the chromosome. An alternative, though not mutually exclusive, possibility is that the carboxyl terminus of Rap1p possesses additional mechanisms to recruit a functional Sir complex. For example, Orc1p has been shown to play an important role in transcriptional silencing (3, 14, 44) and has extensive regions of similarity with Sir3p over the full length of the protein (4). We thus used the two-hybrid system to ask whether the Rap1p carboxyl terminus might also be able to interact with Orc1p, which might in turn recruit the Sir2/3/4 complex through its ability to interact with Sir1p (60). However, we found that a GAD/Orc1p(5–914) hybrid, capable of interacting with LexA/Sir1p, failed to interact with either LexA/Rap1p(635–827) or LexA/Rap1p(679–827) (data not shown). This result suggests that Orc1p may not interact with the carboxyl terminus of Rap1p and may be unable to substitute for Sir3p in Sir complex recruitment. Another possible explanation of the relatively weak silencing effect caused by a Rap1p/Sir3p interaction defect is that Rap1p interacts directly with Sir4p, which can itself associate with Sir3p. This possibility is addressed below.
Sir4p binds to Rap1p in vitro and interacts with Rap1p carboxyl terminus in vivo in the absence of endogenous Sir or Rif1 proteins.
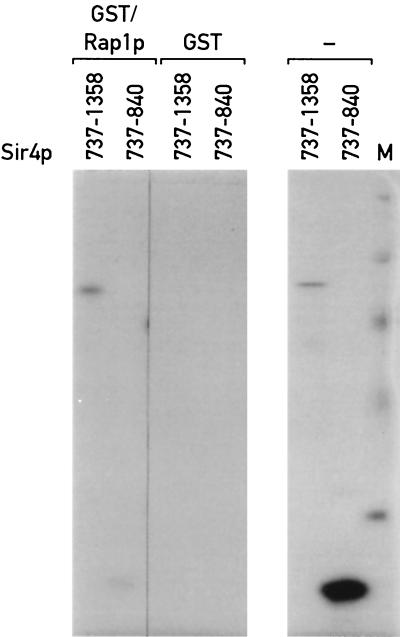
We had previously shown that the carboxyl terminus of Sir4p can interact with Rap1p in a two-hybrid assay, but were unable to determine whether or not this interaction is direct (47). To address this question, we first used a GST pulldown assay. The same GST/Rap1p(562–827) fusion and binding conditions used in the analysis of the Rap1p-Sir3p interaction were used with two different 35S-labeled fragments of Sir4p [Sir4p(737–1358) and Sir4p(737–840)]. As shown in Fig. 5, the Sir4p(737–1358) fragment can interact with GST/Rap1p(562–827), whereas the shorter Sir4p(737–840) fragment cannot bind specifically to Rap1p even when larger amounts of the 35S-labeled protein are used. These data indicate that functionally important parts of Sir4p and Rap1p can interact directly in vitro or at least without the assistance of other yeast proteins.
FIG. 5.
Sir4p carboxyl terminus binds to GST/Rap1p(562–827) in vitro. See Materials and Methods for details of the binding assay. Right, in vitro-produced labeled carboxy-terminal fragments of Sir4p(839–1358) and Sir4p(839–1275) (from a separate gel) before addition to GST/Rap1p(562–827) or GST-agarose beads. Left, material bound to the GST/Rap1p beads in the Sir4p(839–1358) lane has the same mobility as the higher-molecular-weight translation product in the right panel. Lane M, size standards.
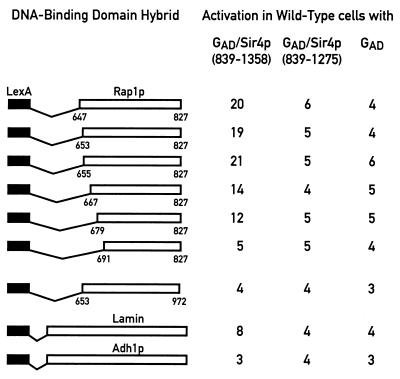
To determine whether Rap1p and Sir4p might associate directly in vivo, we extended our previous two-hybrid analysis in which an interaction between GAD/Sir4p(1205–1358) and LexA/Rap1p(635–827) but not shorter Rap1p fusions (amino-terminal endpoints at positions 647, 653, 655, 667, 679, and 691) was detected (47). Reasoning that a GAD/Sir4p construct expressing a larger fragment of the carboxyl terminus of Sir4p might be able to interact more strongly with Rap1p, we constructed and tested a GAD/Sir4p(839–1358) fusion with the same series of LexA/Rap1p constructs. As shown in Fig. 6 (first and third columns), LexA/Rap1p fusions with amino-terminal endpoints between positions 647 and 679 can weakly but specifically interact with GAD/Sir4p(839–1358). These five LexA/Rap1p fusions are also able to interact with GAD/Sir3p(307–978) and GAD/Rif1p(1614–1916) in two-hybrid assays (47). In contrast, LexA/Rap1p(691–827) does not show a significant difference in β-galactosidase signal with GAD/Sir4p(839–1358) compared to the GAD-alone control. This last LexA/Rap1p fusion is unable to interact with either GAD/Sir3p or GAD/Rif1p but expresses a protein of the expected size and in amounts comparable to those produced by the other LexA/Rap1p constructs (data not shown). As a control for the Rap1p-Sir4p interaction, we tested GAD/Sir4p(839–1358) with a LexA/Adh1p fusion. The last two rows of Fig. 6 show that GAD/Sir4p(839–1358) does not interact significantly above background with either LexA/Lamin or LexA/Adh1p, suggesting that the Rap1p-Sir4p interaction is specific.
FIG. 6.
Interaction of GAD/Sir4p(839–1358), GAD/Sir4p(839–1275), and GAD with a series of LexA/Rap1p hybrids using the two-hybrid system. LexA/Rap1p hybrids with different amino-terminal Rap1p fusion endpoints and either a wild-type Rap1p carboxyl terminus (647–827, 653–827, 655–827, 667–827, 679–827, and 691–827) or a linker insertion mutation at amino acid position 825 (653–825*) were assayed as before. The LexA/Lamin and LexA/Adh1p hybrids were included as negative controls.
Based on the results shown above, the possibility remains that the in vivo Sir4p-Rap1p interaction is indirect, mediated, for example, by an interaction between these two proteins and Sir3p (47) or another silencing protein(s). To determine whether the Rap1p-Sir4p two-hybrid interaction requires the function of the endogenous Sir proteins or Rif1p, we tested all of the interactions reported above in strains containing mutations in SIR1, SIR2, SIR3, SIR4, or RIF1. None of the interactions are abolished in the mutant strains (data not shown), supporting the idea that Rap1p and Sir4p interact directly in vivo and that this interaction does not require a functional Sir2/3/4 complex.
Silencing-defective mutations in Rap1p or Sir4p abolish the two-hybrid interaction between the two proteins.
Truncation of Sir4p at position 1237 in the sir4-42 mutant causes a silencing defect at HM loci and telomeres (25). To determine whether this phenotype might result from an inability of the truncated protein to interact with Rap1p, we created a GAD/Sir4p(839–1275) fusion and tested it for the ability to interact with the carboxyl terminus of Rap1p. As shown in the middle column of Fig. 6, deletion of the last 83 amino acids of Sir4p completely abolishes the two-hybrid interaction with all LexA/Rap1p fusions. This result demonstrates that the interaction of GAD/Sir4p with LexA/Rap1p is dependent on Sir4p sequences of the GAD hybrid and suggests that a short carboxy-terminal domain of Sir4p required for silencing may also be necessary for its physical association with Rap1p in vivo.
To ask whether the converse is also true, we analyzed a silencing-defective Rap1p mutation for its effect on the two-hybrid interaction with Sir4p. As shown in Fig. 6, the incorporation of a small linker insertion mutation at position 825 of Rap1p [Rap1p(825*)], which results in the addition of five amino acids at the carboxyl terminus of the protein, completely abolishes the ability of the LexA/Rap1p(653–825*) fusion to interact with GAD/Sir4p(839–1358). Since LexA/Rap1p(653–825*) is much less severely impaired in its interactions with both Sir3p and Rif1p compared to the wild type (1.6- and 3.8-fold decreases, respectively [47]), the complete absence of an interaction with GAD/Sir4p(839–1358) is unlikely to be due to decreased expression or stability of the LexA/Rap1p fusion protein. Taken together, these data are consistent with the idea that a direct Rap1p-Sir4p interaction plays an important role in silencing.
Increased gene dosage of SIR4 but not SIR1 improves silencing in sir3 mutants.
The gene dosage of both SIR1 and SIR4 can have profound effects on HMR silencing. For example, elevated gene dosage of SIR1 can suppress various silencing mutations (29, 55, 57), whereas increased SIR4 dosage can improve or disrupt silencing depending on the genetic background and the number of added copies of the gene (39, 42, 50, 57, 58). One possible mechanism for an improvement in silencing with increased SIR1 or SIR4 dosage is increased availability of these proteins at the silencers, which might act to strengthen the formation of a complex that nucleates heterochromatin assembly.
We reasoned that the decreased silencing seen at hmrΔA::TRP1 in SIR3 mutants defective in Rap1p binding might be explained by the combined loss of two independent interactions, one between the Sir2/3/4p complex and Rap1p and the other between the Sir2/3/4 complex and Sir1p/ORC (60). The loss of the first interaction would be the result of the SIR3 mutation, whereas the loss of the second would be explained by the deletion of the ORC binding site (the silencer A element). We therefore asked whether increased SIR1 or SIR4 dosage might improve silencing under these mutant conditions. As shown in Fig. 7, one or two extra copies of SIR4 can strongly suppress the silencing defect exhibited by SIR3 mutants that are defective in binding to Rap1p. The partial derepression of hmrΔA::TRP1 in the presence of Sir3p(Δ456–479) or Sir3p(Δ440–480) is completely reversed by the addition of one extra copy of SIR4 (Fig. 7, rows 2 and 3 of top panels). In contrast, much higher gene dosage of SIR1 (present on a 2μm plasmid) has no effect under these conditions (rows 2 and 3 of bottom panels). These results are consistent with the hypothesis that Sir4p interacts with the carboxyl terminus of Rap1p and contributes to the recruitment of the Sir complex to the HMR-E silencer. In addition, the data suggest that Sir1p may act at the HMR-E silencer only when a strong ORC binding site is present (10, 60).
FIG. 7.
Extra dosage of SIR4 suppresses the silencing defect caused by mutation of the ARS consensus sequence at the HMR-E silencer in combination with the deletion of the Rap1p interaction domain of Sir3p. Cells expressing different SIR3 alleles were transformed with a low-copy-number CEN plasmid containing the SIR4 gene (top panels) or a high-copy-number 2μm plasmid containing SIR1 (bottom panels). The assays were performed as described for Fig. 3.
DISCUSSION
Specific Rap1p interaction domain of Sir3p.
We have identified a minimal Rap1p interaction domain of Sir3p between amino acids 455 and 481 that, by several criteria, appears to play an important and specific role in the initiation of silencing. First, this short region is sufficient to mediate a two-hybrid interaction with the carboxyl terminus of Rap1p yet is incapable of interacting with either Sir3p or Sir4p. Second, deletion of amino acids 456 to 479 in Sir3p completely abolishes its association with Rap1p in the two-hybrid assay but does not affect its Sir3p or Sir4p interactions. Third, deletion of the 456 to 479 region impairs Sir3p binding to the carboxyl terminus of Rap1p in vitro. Fourth, deletion of this small region of Sir3p causes a complete loss of silencing at HMR when the initiation of repression is dependent on targeting of the carboxyl terminus of Rap1p, but not when it is initiated by either Sir3p or Sir4p targeting.
It is worth noting that the Rap1p interaction domain of Sir3p identified here maps well upstream on the linear protein sequence of regions required for self-association, Sir4p binding, or histone binding. A Sir3p interaction with the amino-terminal tails of histones H4 and H3 requires regions between amino acids 623 and 762 and 799 and 910 of Sir3p and is unaffected by deletion of the Rap1p interaction domain defined here (23). Similarly, we have also mapped Sir3p sequences required for self-association and Sir4p binding to two separate regions, both of which are carboxy-terminal to the Rap1p interaction domain (unpublished results), and within a broad region defined by Park and colleagues (49). The picture that emerges from these studies is that Sir3p may be able to interact simultaneously with several different proteins (Rap1p, Sir4p, and histones H3 and H4). The possible significance of this observation is discussed below.
Rap1p carboxy-terminal silencing domain can interact directly and independently with both Sir3p and Sir4p.
Previous studies showed that Rap1p and Sir4p coimmunoprecipitate from yeast extracts (11, 24, 56) and interact in the two-hybrid system (47). However, none of these studies were able to address the question of whether Rap1p and Sir4p interact directly and, if so, whether their association is important for silencing. Here we present the first demonstration that Rap1p and Sir4p can bind to each other directly in vitro in the absence of other yeast proteins and that their in vivo interaction in a two-hybrid assay is independent of SIR function. These findings suggest that the Rap1p-Sir4p association seen in large chromatin complexes is due, at least in part, to direct interactions that contribute to the stability of these complexes. As discussed in detail below, genetic data reported here indicate that direct binding between Rap1p and Sir4p is important in vivo for silencing.
On the basis of the results presented here, we propose that Sir3p and Sir4p interact directly and independently with partially overlapping regions of the carboxyl terminus of Rap1p. Although both Sir interactions require an intact Rap1p carboxyl terminus, the amino-terminal boundaries of the Rap1p sequences required for Sir3p and Sir4p association differ. A relatively small carboxy-terminal fragment (amino acids 679 to 827) interacts strongly with Sir3p but only weakly with Sir4p, which requires sequences upstream of position 679 for stronger Rap1p binding. This increase is specific to Sir4p and thus unlikely to be a trivial consequence of increased protein stability, because these larger Rap1p hybrids actually interact less well with Sir3p (47). The importance of Rap1p sequences upstream of 679 for silencing is underscored by the observation that GBD/Rap1p hybrids containing these additional sequences initiate repression much more efficiently than does GBD/Rap1(679–827) (8). It is worth pointing out that more amino-terminal sequences of Rap1p might also contribute to Sir protein binding, particularly for the case of Sir4p. However, LexA/Rap1p hybrids containing such sequences, which include the Rap1p DNA-binding domain, do not work in the two-hybrid system.
Given the above considerations, we favor the idea that Sir3p and Sir4p simultaneously contact the Rap1p carboxyl terminus in vivo. Although our experiments do not address this question directly, the fact that Sir3p and Sir4p can interact with each other (47) through regions not required for their interactions with Rap1p (our unpublished data) suggests that these two Sir proteins form a unique ternary complex with Rap1p that is stabilized by interactions among all three proteins. An alternative possibility that we cannot rule out at present is that Rap1p-Sir3p and Rap1p-Sir4p interactions are mutually exclusive, so that individual Rap1p molecules interact with one or the other Sir protein in forming a Rap1/Sir complex.
Cooperativity in Sir complex recruitment by Rap1p.
The apparent ability of Rap1p to interact independently with both Sir3p and Sir4p and the ability of these two proteins to interact with themselves and each other (9, 47) suggest a cooperative and redundant mechanism for recruitment of the Sir complex by Rap1p. This notion is strongly supported by the genetic studies described here. Thus, although a small region of Sir3p(455–481) is required (by both in vitro and in vivo criteria) for an interaction with Rap1p, SIR3 mutants lacking this specific domain display only a weak silencing defect even when the silencer being tested is totally dependent on Rap1p. Significantly, however, when such mutants are tested in a targeted silencing system (where Sir4p recruitment by GBD/Rap1p may be poor), they are severely silencing defective. The simplest interpretation of these results is that the Rap1p-Sir3p interaction that we have characterized is important but not essential for Rap1p's action at either HMR or a telomere. The same appears to hold for the Rap1p-Sir4p interaction, which is specifically abolished by a linker insertion mutation very near the carboxyl terminus of Rap1p with relatively little effect on TPE (47). In contrast, the loss of a Rap1p interaction with both Sir3p and Sir4p (due to Rap1p truncations at amino acids positions 716, 703, and 695) causes a complete TPE defect (28, 47).
The idea that Sir3p and Sir4p cooperate in recruitment to silencers and telomeres by Rap1p is further supported by the observation that increased SIR4 gene dosage significantly improves silencing in cells carrying a deletion of the Rap1p-interacting domain of Sir3p. Finally, the idea that Sir3p can interact independently with Rap1p in vivo is strongly supported by coimmunoprecipitation studies using an antigen-tagged version of Sir3p (24), which showed that mutation of SIR4 reduces but does not abolish Rap1p binding to Sir3p.
In addition to the Rap1p-Sir protein interactions described here, a large number of other protein-protein interactions have been implicated in either the establishment or maintenance of silencing at either HM mating type loci or telomeres (e.g., ORC-Sir1p, Yku70p-Sir4p, Sir1p-Sir4p, Sir3p-Sir3p, Sir4p-Sir4p, Sir4p-Sir2p, Sir3p-H3, Sir3p-H4, Sir4p-H3, and Sir4p-H4) (9, 11, 24, 31, 45–47, 60). Additional protein-protein interactions (e.g., Rap1-Rif1p, Rap1p-Rif2p, and Sif2p-Sir4p) appear to downregulate silencing at telomeres (12, 22, 28, 61). At present it is unclear why the recruitment and assembly of Sir proteins at HM loci and telomeres involve such a complex network of interactions. One possibility is that this complexity is necessitated by the tight regulation of Sir2/3/4-mediated silencing, which is particularly stable at HM mating type loci, less so at telomeres, and excluded from most other chromosomal sites (62). This “hierarchy” of silencing (1) has been linked to the different complexity of the silencers themselves, and the present work lends further support to this idea. For example, we found that mutation of the Rap1p interaction domain of Sir3p has a more severe effect at an HMR-E silencer lacking the ORC binding site (A element) than at a silencer lacking the Abf1 site (B element). These data indicate that the different silencer elements make independent and quantitatively different contributions to the strength of the silencer. This conclusion is supported by evidence that the silencer A element recruits Sir1p and, indirectly, Sir4p through the ORC (60), whereas Abf1p appears to act by recruiting Sir3p (P. Moretti and D. Shore, unpublished data).
Structural role for Rap1p in silent chromatin?
Initial molecular models suggested that silencer- or telomere-binding proteins (e.g., Rap1p, ORC, Abf1p, and Yku70/80) initiate silencing by recruiting a Sir2/3/4 complex to the chromosome, but do not participate directly in the subsequent “spreading” of this complex along adjacent nucleosomal DNA (21, 35, 36). However, several studies using chromatin immunoprecipitation (ChIP) have now shown that Rap1p and Yku80p are bound at distances of 2 to 4 kb from a telomere end, together with the Sir2/3/4 proteins (13, 24, 43, 56). These results are surprising in light of the fact that the TG1–3 repeat tracts (which constitute the telomeric Rap1p DNA-binding sites) extend only about 300 to 400 bp from the chromosome end and that Yku protein is generally thought to interact with the end of the TG1–3 repeat tract, based on its in vitro preference for DNA ends or duplex/single-strand junctions. The ChIP results have thus been interpreted to mean that the telomere repeat tract folds back on more internal nucleosomal regions through Sir-Sir interactions between these two domains (13, 17, 56), thus associating repeat tract-bound Rap1p indirectly with distal heterochromatin.
Our results suggest an additional explanation for this finding. Specifically, we propose that Rap1p might contribute directly to the stability of silent chromatin through simultaneous interactions with the Sir2/3/4 complex and (nonspecific) DNA sites. According to this model, Rap1p spreads together with the Sir2/3/4 complex by virtue of its ability to bind cooperatively to Sir3p and Sir4p and to nonspecific DNA sites. The finding that Sir3p may be able to simultaneously contact both Rap1p and histone tails raises the intriguing possibility that the Sir complex can promote the stable coassociation of Rap1p and nucleosomes on silent regions. This model might help to better explain two puzzling observations regarding telomeric heterochromatin. First, coimmunoprecipitation experiments with antibodies against Sir3p show that interactions with Rap1p and histone H4 are surprisingly interdependent, so that Sir3p-Rap1p binding appears to be lost in strains containing H4 amino-terminal tail mutations (23). These data are difficult to reconcile with a simple version of the telomere “fold-back” model but are consistent with the proposal that the bulk of Rap1p-Sir3p interactions at telomeres actually occurs within the silent chromatin itself through cooperative interactions between histones and Sir3/4 proteins on the one hand and DNA-bound Rap1p and Sir3/4 on the other. Second, indirect immunofluorescence experiments have revealed a significant loss of punctate Rap1p staining in strains carrying either SIR3 or SIR4 mutations (48), which cannot be explained by a loss of telomere clustering (18). This result would be predicted if one assumes again that a large pool of telomere-associated Rap1p is not bound directly to the TG1–3 repeat tracts but is instead held there by nonspecific binding and cooperative interactions with the Sir complex (17). It may prove difficult, however, to distinguish experimentally between different models to explain the spreading of Rap1p in telomeric heterochromatin. Furthermore, none of these models are mutually exclusive. Perhaps a good test of the idea that Rap1p is a structural component of the silent chromatin would be to determine if Rap1p also spreads together with the Sir complex at HM silent mating type loci, where a telomere fold-back model would not apply.
While our manuscript was under revision, a whole-genome analysis of both Rap1p and Sir protein binding in vivo was published (30). Data presented in that paper indicate considerable spreading of Rap1p (together with Sir2p, Sir3p, and Sir4p in most cases) to sequences outside of those bounded by the E and I silencers at both HML and HMR. As pointed out above, this observation is consistent with our model but difficult to accommodate in the context of a fold-back-type model for Rap1p spreading outside of its high-affinity binding sites.
ACKNOWLEDGMENTS
We thank members of the Shore laboratory, in particular Stephen Buck and Stéphane Marcand, for helpful comments throughout the course of this study. We are particularly grateful to Saul Silverstein for continuous support. We also thank Nicholas Roggli for help with the figures.
This work was supported by grants from the National Institutes of Health (GM-40094), the American Cancer Society (VM-62A), the Swiss National Science Foundation, and by funds from the Canton of Geneva.
REFERENCES
- 1.Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 2.Bartel P L, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 3.Bell S P, Kobayashi R, Stillman B. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 4.Bell S P, Mitchell J, Leber J, Kobayashi R, Stillman B. The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell. 1995;83:563–568. doi: 10.1016/0092-8674(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 5.Brand A H, Micklem G, Nasmyth K. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell. 1987;51:709–719. doi: 10.1016/0092-8674(87)90094-8. [DOI] [PubMed] [Google Scholar]
- 6.Buchman A R, Kimmerly W J, Rine J, Kornberg R D. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:210–225. doi: 10.1128/mcb.8.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchman A R, Lue N F, Kornberg R D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988;8:5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck S W, Shore D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 1995;9:370–384. doi: 10.1101/gad.9.3.370. [DOI] [PubMed] [Google Scholar]
- 9.Chien C-T, Bartel P L, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien C T, Buck S, Sternglanz R, Shore D. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell. 1993;75:531–541. doi: 10.1016/0092-8674(93)90387-6. [DOI] [PubMed] [Google Scholar]
- 11.Cockell M, Palladino F, Laroche T, Kyrion G, Liu C, Lustig A J, Gasser S M. The carboxy termini of Sir4 and Rap1 affect Sir3 localization: evidence for a multicomponent complex required for yeast telomeric silencing. J Cell Biol. 1995;129:909–924. doi: 10.1083/jcb.129.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockell M, Renauld H, Watt P, Gasser S M. Sif2p interacts with Sir4p amino-terminal domain and antagonizes telomeric silencing in yeast. Curr Biol. 1998;8:787–790. doi: 10.1016/s0960-9822(98)70304-5. [DOI] [PubMed] [Google Scholar]
- 13.de Bruin D, Kantrow S M, Liberatore R A, Zakian V A. Telomere folding is required for the stable maintenance of telomere position effects in yeast. Mol Cell Biol. 2000;20:7991–8000. doi: 10.1128/mcb.20.21.7991-8000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foss M, McNally F J, Laurenson P, Rine J. Origin recognition complex (ORC) in transcriptional silencing and DNA replication in S. cerevisiae. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 15.Gartenberg M R. The Sir proteins of Saccharomyces cerevisiae: mediators of transcriptional silencing and much more. Curr Opin Microbiol. 2000;3:132–137. doi: 10.1016/s1369-5274(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 16.Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser S M. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- 17.Gotta M, Cockell M. Telomeres, not the end of the story. Bioessays. 1997;19:367–370. doi: 10.1002/bies.950190503. [DOI] [PubMed] [Google Scholar]
- 18.Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser S M. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol. 1996;134:1349–1363. doi: 10.1083/jcb.134.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschling D E. Telomere-proximal DNA in Saccharomyces cerevisiae is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci USA. 1992;89:4062–4065. doi: 10.1073/pnas.89.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 21.Grunstein M. Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell. 1998;93:325–328. doi: 10.1016/s0092-8674(00)81160-5. [DOI] [PubMed] [Google Scholar]
- 22.Hardy C F J, Sussel L, Shore D. A RAP1-interacting protein involved in silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 23.Hecht A, Laroche T, Strahl-Bolsinger S, Gasser S M, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 24.Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy B K, Austriaco N R, Jr, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- 26.Kimmerly W, Buchman A, Kornberg R, Rine J. Roles of two DNA-binding factors in replication, segregation and transcriptional repression mediated by a yeast silencer. EMBO J. 1988;7:2241–2253. doi: 10.1002/j.1460-2075.1988.tb03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyrion G, Boakye K A, Lustig A J. C-terminal truncation of Rap1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyrion G, Liu K, Liu C, Lustig A J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 29.Laman H, Balderes D, Shore D. Disturbance of normal cell cycle progression enhances the establishment of transcriptional silencing in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:3608–3617. doi: 10.1128/mcb.15.7.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieb J D, Liu X, Botstein D, Brown P O. Promoter-specific binding of Rap1 revealed by genome-wide maps of protein-DNA association. Nat Genet. 2001;16:16. doi: 10.1038/ng569. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Lustig A J. Genetic analysis of Rap1p/Sir3p interactions in telomeric and HML silencing in Saccharomyces cerevisiae. Genetics. 1996;143:81–93. doi: 10.1093/genetics/143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Mao X, Lustig A J. Mutational analysis defines a C-terminal tail domain of RAP1 essential for telomeric silencing in Saccharomyces cerevisiae. Genetics. 1994;138:1025–1040. doi: 10.1093/genetics/138.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longtine M S, Wilson N M, Petracek M E, Berman J. A yeast telomere binding activity binds to two related telomere sequence motifs and is indistinguishable from RAP1. Curr Genet. 1989;16:225–239. doi: 10.1007/BF00422108. [DOI] [PubMed] [Google Scholar]
- 34.Loo S, Rine J. Silencers and domains of generalized repression. Science. 1994;264:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- 35.Lowell J E, Pillus L. Telomere tales: chromatin, telomerase and telomere function in Saccharomyces cerevisiae. Cell Mol Life Sci. 1998;54:32–49. doi: 10.1007/s000180050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustig A J. Mechanisms of silencing in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 37.Lustig A J, Liu C, Zhang C, Hanish J P. Tethered Sir3p nucleates silencing at telomeres and internal loci in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2483–2495. doi: 10.1128/mcb.16.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Ptashne M. A new class of yeast transcriptional activators. Cell. 1987;51:113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- 39.Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, Gasser S M. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 1996;10:1796–1811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- 40.Marcand S, Buck S W, Moretti P, Gilson E, Shore D. Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev. 1996;10:1297–1309. doi: 10.1101/gad.10.11.1297. [DOI] [PubMed] [Google Scholar]
- 41.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 42.Marshall M, Mahoney D, Rose A, Hicks J B, Broach J R. Functional domains of SIR4, a gene required for position effect regulation in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:4441–4452. doi: 10.1128/mcb.7.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin S G, Laroche T, Suka N, Grunstein M, Gasser S M. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 44.Micklem G, Rowley A, Harwood J, Nasmyth K, Diffley J F. Yeast origin recognition complex is involved in DNA replication and transcriptional silencing. Nature. 1993;366:87–89. doi: 10.1038/366087a0. [DOI] [PubMed] [Google Scholar]
- 45.Mishra K, Shore D. Yeast Ku protein plays a direct role in telomeric silencing and counteracts inhibition by Rif proteins. Curr Biol. 1999;9:1123–1126. doi: 10.1016/s0960-9822(99)80483-7. [DOI] [PubMed] [Google Scholar]
- 46.Moazed D, Kistler A, Axelrod A, Rine J, Johnson A D. Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci USA. 1997;94:2186–2191. doi: 10.1073/pnas.94.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 48.Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser S M. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75:543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- 49.Park Y, Hanish J, Lustig A J. Sir3p domains involved in the initiation of telomeric silencing in Saccharomyces cerevisiae. Genetics. 1998;150:977–986. doi: 10.1093/genetics/150.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renauld H, Aparicio O M, Zierath P D, Billington B L, Chhablani S K, Gottschling D E. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- 51.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Plainview, N.Y: Cold Spring Harbor Press; 1990. [Google Scholar]
- 51a.Sekinger E A, Gross D S. Silenced chromatin is permissive to activator binding and pic recruitment. Cell. 2001;105:403–414. doi: 10.1016/s0092-8674(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 52.Shore D, Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987;51:721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- 53.Sikorski R, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh J, Klar A J S. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 1992;6:186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- 55.Stone E M, Swanson M J, Romeo A M, Hicks J B, Sternglanz R. The SIR1 gene of Saccharomyces cerevisiae and its role as an extragenic suppressor of several mating-defective mutants. Mol Cell Biol. 1991;11:2253–2262. doi: 10.1128/mcb.11.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- 57.Sussel L, Shore D. Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1: isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci USA. 1991;88:7749–7753. doi: 10.1073/pnas.88.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sussel L, Vannier D, Shore D. Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3919–3928. doi: 10.1128/mcb.13.7.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 60.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 61.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 62.Wyrick J J, Holstege F C, Jennings E G, Causton H C, Shore D, Grunstein M, Lander E S, Young R A. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature. 1999;402:418–421. doi: 10.1038/46567. [DOI] [PubMed] [Google Scholar]