Abstract
Cocaine Use Disorders (CUDs) are associated with an increased risk of human immunodeficiency virus (HIV) infection. Cocaine and the HIV envelope protein gp120 each induce distinct deficits to mesocorticolimbic circuit function and motivated behavior; however, little is known regarding how they interact to dysregulate these functions or how such interactions impact pharmacotherapeutic efficacy. We have previously shown that the selective, weak partial agonist of the dopamine D3 receptor (D3R), MC-25–41, attenuates cocaine-seeking behavior in male rats. Here, we sought to characterize changes in striatal neuroimmune function in gp120-exposed rats across abstinence from operant access to cocaine (0.75 mg/kg, i.v.) or sucrose (45 mg/pellet), and to examine the impact of gp120 exposure on MC-25–41-reduced cocaine seeking. After establishing a history of cocaine or sucrose self-administration, rats received intracerebroventricular gp120 infusions daily the first 5 days of abstinence and were sacrificed either on day 6 or after 21 days of forced abstinence and a cue-induced cocaine seeking test. We demonstrated that MC-25–41 treatment attenuated cue-induced cocaine seeking among control rats but not gp120-exposed rats. Moreover, postmortem analysis of nucleus accumbens (NAc) core neuroimmune function indicated cocaine abstinence- and gp120-induced impairments, and the expression of several immune factors within the NAc core significantly correlated with cocaine-seeking behavior. We conclude that cocaine abstinence dysregulates striatal neuroimmune function and interacts with gp120 to inhibit the effectiveness of a D3R partial agonist in reducing cocaine seeking. These findings highlight the need to consider comorbidities, such as immune status, when evaluating the efficacy of novel pharmacotherapeutics.
Keywords: Neuroinflammation, Addiction, CUDs, Abstinence, Cue reactivity, MC-25–41
1. Introduction
Human immunodeficiency virus (HIV) and cocaine use disorders (CUDs) remain pervasive global health concerns that disproportionately affect male minority populations as well as adolescent and young adult populations [12,48]. Combination antiretroviral therapy (cART) has dramatically reduced HIV-related mortality, and people living with HIV (PLWH) who achieve viral suppression generally live healthy, full lives [62,102]. However, cART has poor blood brain barrier (BBB) permeability [71], and PLWH who achieve viral suppression experience chronic neuroinflammation and often develop HIV-associated neurocognitive disorder (HAND; [40,41,90,112]). Substance misuse is often comorbid with HIV and is also a risk factor for HIV infection, particularly with opiates and psychostimulants [94]. Concurrent injection drug use and HIV infection may exacerbate neuroimmune dysfunction [27] and accelerate disease progression independent of adherence to cART [8,40,90]. Thus, developing improved therapeutics for HIV-associated neurocognitive dysfunction within the context of substance use disorders is essential.
HIV protein products such as the envelope glycoprotein gp120 and transactivator of transcription (Tat) are associated with dysregulation of neuroimmune signaling and disruptions to the BBB, which can increase viral invasion into the central nervous system (CNS) and subsequent neurotoxicity [94,95]. Both microglia and astrocytes generate a complex medley of immune responses under pathological conditions [20,25,44,54], and serve as viral reservoirs for HIV [23,38,56,98]. HIV induces persistent secretion of proinflammatory cytokines and chemokines [77,83], which impair cognitive function and, in severe cases, promote neurodegeneration throughout the CNS [26,41,81,97]. Within the rat striatum, acute exposure to gp120 or Tat causes dose-dependent increases in reactive astrogliosis [7], and adult gp120 transgenic mice exhibit increased striatal expression of glial fibrillary acidic protein (GFAP) and the chemokines CCL2 and CXCL10 [3]. An acute microinjection of a high dose of gp120 (500 ng) into the rat striatum induces significant upregulation of CCL3, an HIV-suppressive chemokine [1,21], as well as an increase in the expression of microglial and macrophage markers Iba1 and CD68 that persists for up to four weeks post-injection [57]. Subchronic, intracerebroventricular microinjections of gp120 (500 ng) within the CNS of rats increases striatal mRNA expression of interleukin-1β (IL-1β) and inducible nitric oxide synthase (iNOS), as well as protein expression of the active efflux transporter multidrug resistance-associated protein 1 (MRP1) [4]. Importantly, MRPs play a substantial role in the efflux of intracellular anti-HIV drugs, thus contributing to the poor BBB penetrance of cART [33,46,71,99]. While these studies implicate striatal neuroimmune signaling in the pathophysiology of HIV, it is still unclear how HIV and chronic drug use interact in this regard.
Like HIV, cocaine disrupts BBB integrity and induces immune responses within the brain through increased glial cell reactivity [27,94]. Cocaine also facilitates HIV invasion [2,27] and viral replication [88] in the brain even during the early asymptomatic stages of infection [81]. Furthermore, cocaine exposure enhances gp120 toxicity in rat primary astrocytes to a greater extent than that induced by either cocaine or gp120 separately [108]. Thus, combined HIV and cocaine use may further exacerbate immune dysregulation, aberrant dopaminergic signaling, and cognitive dysfunction [16]. Despite the clear and inextricable link between HIV and acute psychostimulant exposure and their impact on immune function, it is largely unknown how cocaine abstinence, which is associated with peripheral immune responses [5,111], alters CNS immune function within brain reward circuity and how this is modulated by HIV.
Motivation for cocaine progressively increases over protracted periods of abstinence, and several studies implicate the dopamine D3 receptor (D3R) in facilitating this process [69,80]. During the first three weeks of abstinence, D3Rs are up-regulated within the nucleus accumbens (NAc; [70]), a brain region associated with reward learning that is also critically involved in drug seeking. Interestingly, D3Rs may also contribute to the regulation of neuroimmune signaling through glial cell activation [63], suggesting a possible regulatory role in HIV- and cocaine-induced neuroimmune responses and drug relapse. Despite the known effects of cocaine and gp120 on striatal neuroimmune function, it remains unclear how gp120 interacts with cocaine abstinence to dysregulate mesolimbic neuroimmune function or whether this interaction impacts motivation to seek cocaine. We sought to address these gaps in the present study in male rats trained to self-administer cocaine that were then exposed to HIV gp120 for 5 days during a forced abstinence period. Exposure to gp120 after animals acquired cocaine self-administration is a novel design feature, which better models the acquisition of HIV as a collateral effect of chronic drug use in humans with CUDs. The present study also investigated inhibition of D3R signaling as a potential therapeutic strategy to ameliorate reward-seeking behavior in cases of comorbid HIV infection and CUDs. After 21 days of abstinence, cocaine-experienced rats were treated with the novel D3R weak partial agonist, MC-25–41, which effectively reduces both D3R signaling and cocaine motivation in rats without affecting spontaneous or cocaine-induced locomotion or sucrose motivation [17,80]. Given the paucity of information on cocaine-induced changes in neuroimmune signaling within mesolimbic reward circuitry, particularly regarding cocaine abstinence, we utilized a multiplex cytokine, chemokine, and growth factor array to explore changes in striatal neuroimmune function across abstinence and as a function of gp120 treatment history and MC-25–41 treatment.
2. Methods
2.1. Subjects
Adult male Sprague-Dawley rats (N = 50; 201–225 g upon arrival, Charles River Laboratories, Hollister, CA, USA) were housed individually in a temperature- and humidity-controlled vivarium on a 14:10 h reverse light:dark cycle. Animals had ad libitum access to water for the duration of the study but had restricted access to food, maintained at 85% of their free-feeding weight, during self-administration to facilitate cocaine acquisition. All experiments were conducted during the dark phase and were approved by and performed in accordance with the Institutional Animal Care and Use Committee of Arizona State University and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
2.2. Drugs and reagents
Cocaine hydrochloride (NIDA Drug Supply Program, RTI International, Research Triangle Park, NC, USA) was dissolved in sterile saline to a stock concentration of 10 mg/mL, which was further diluted with saline to achieve a dose of 0.75 mg/kg/0.10 mL and filtered through 0.2 μm syringe filters. HIV-1 IIIB gp120 was obtained from the NIH AIDS Reagent Program and was diluted in 1X phosphate buffered saline (PBS) to a final concentration of 45 ng/μL based on previous studies suggesting impaired cognition and a lack of neurodegeneration at this dose [6,31]. The N-(4-(4-(3-cyanophenyl)piperazin-1-yl)butyl)−4-(thiophen-3-yl)benzamide D3R partial agonist, referred to here as MC-25–41, was dissolved in 20% w/v cyclodextrin + 3% v/v 1 M HCl to achieve 10 mg/mL as we have previously described [17,80].
2.3. Surgical procedures
Guide cannulae were implanted intracranially and catheters were implanted into the jugular vein as previously described ([65], 2022). Briefly, animals were anesthetized using vaporized isoflurane (2–3%), and a sterile polyurethane catheter was inserted 2.5 mm into the right jugular vein of cocaine self-administering animals. The opposite end of the catheter was tunneled subcutaneously between the shoulder blades and attached to a cannula that was secured within a harness (Instech Laboratories, Plymouth Meeting, PA, USA). An intracranial guide cannula was stereotaxically implanted 1 mm dorsal to the right lateral ventricle (A/P: −0.8 mm, M/L: −1.6 mm, D/V: −2.6 mm) and secured to the skull using anchor screws and dental acrylic. All animals received buprenorphine (0.05 mg/kg/mL, s.c.) and meloxicam (1 mg/kg/mL, s.c.) at the end of surgery. Meloxicam was also administered 1 day post-operatively. Catheterized animals also received cefazolin (100 mg/mL, 0.1 mL, i.v.) and heparin (70 U/mL, 0.1 mL, i.v.), dissolved in saline, for 5 days post-operatively. Heparin alone was administered daily before and after each cocaine self-administration session to maintain catheter patency. Animals were given at least 5 days of recovery prior to beginning self-administration.
2.4. Cocaine and sucrose self-administration, forced abstinence, and cue-induced cocaine seeking
Animals were placed in individual operant conditioning chambers equipped with one active and one inactive lever, a cue light above each lever, a house light, a tone generator, and a food receptacle (30 × 24 × 21 cm; Med Associates Inc., St. Albans, VT, USA). Prior to self-administration, animals were habituated to their respective chambers for 1 hr with both levers retracted. Animals underwent 2 hr training sessions 6 days/week and were food-restricted as described above. Self-administration initially began on a fixed-ratio (FR) 1 schedule of reinforcement where one active lever response delivered a single infusion of cocaine (0.75 mg/kg/infusion, i.v., over 6 s) or a sucrose pellet (45 mg/pellet, Bio-Serv, Flemington, NJ, USA) paired with a compound stimulus light and auditory tone (500 Hz). Each reinforcer was followed by inactivation of the light and tone cues and illumination of the house light to signal a 20 s time-out period during which lever responses yielded no consequences. Inactive lever responses resulted in no reinforcer or associated cues but were still recorded. Within each session, the schedule of reinforcement advanced to a variable ratio (VR) 2, 3, and 5, sequentially, where a variable number of active lever responses averaging to the specified number was needed to achieve reinforcement. A minimum of 5 reinforcers earned was required within a reinforcement schedule within a given hour to advance to the next reinforcement schedule. Advancement to the next starting schedule between sessions required animals to end on a higher schedule than the starting one for 3 consecutive sessions. Stability criteria at the end of self-administration were defined as achieving at minimum 10 reinforcers on a VR5 starting schedule and ≤15% variability in reinforcers earned across 3 consecutive sessions with no upward or downward trends. Some animals (n = 17) underwent 5 days of forced abstinence and their brains were collected on day 6 for protein quantification. Other animals (n = 27) underwent 21 days of forced abstinence, followed by a 1 hr cue-induced, cocaine-seeking test where active lever responses resulted in presentation of the light/tone cues but no cocaine reinforcer. Immediately following cue testing, their brains were collected for protein quantification.
2.5. Microinjection and MC-25–41 treatment procedures
Beginning on day 1 of abstinence, all animals received a daily microinjection of gp120 (45 ng/μL, 1.0 μL, 0.5 μL/min, i.c.v.) or vehicle (1X PBS, 1.0 μL, 0.5 μL/min, i.c.v.) into the right lateral ventricle for 5 consecutive days. Some animals were then sacrificed the day after the fifth infusion (i.e., on day 6 of abstinence) to examine NAc cytokine, chemokine, and growth factor expression proximal to sub-chronic gp120 exposure. After 21 days of forced abstinence, remaining animals received a systemic injection of MC-25–41 (10 mg/kg/mL, i.p.) or vehicle (1 mL/kg of 20% cyclodextrin + 3% HCl in saline, i.p.) 10 min prior to undergoing the 1 hr cue-induced cocaine seeking test. This dosing regimen of MC-25–41 is based on results of our previous study showing a significant MC-25–41-induced reduction in cocaine self-administration on a progressive ratio schedule at this dose [80].
2.6. Tissue processing and measurement of cytokine, chemokine, and growth factor expression
On day 6 of abstinence or immediately after the cocaine seeking test, animals were deeply anesthetized with isoflurane until respiration ceased prior to rapid decapitation followed by brain removal. A 2 mm-thick coronal brain slice containing the NAc core was collected over ice and homogenized in an ice-cold RIPA lysis buffer solution containing protease and phosphatase inhibitors (Sana Cruz Biotechnology, Dallas, TX, USA). Tissue homogenates were centrifuged at 10,000 × g for 5 min and the supernatants were collected and stored at −80 °C. Samples were diluted 1:1 in 1X PBS and cytokine, chemokine, and growth factor expression levels were determined using the Rat Cytokine/Chemokine 27-Plex Discovery Assay® Array (Eve Technologies, Calgary, AB Canada). Each sample was analyzed in duplicate and the average of each pair of readings was used as the final measure for each sample across all targets.
2.7. Data analysis
Cocaine infusions and sucrose pellets earned across each of the qualifying sessions of self-administration were analyzed by repeated measures two-way ANOVAs, with self-administration session and treatment group as factors. Active and inactive lever presses during cue-induced cocaine seeking were analyzed with a three-way ANOVA, with treatment (vehicle vs. MC-25–41), microinjection (control vs. gp120), and lever (active vs. inactive) as factors. Cytokine, chemokine, and growth factor expression was analyzed via two-way ANOVAs, with reinforcer (or treatment) and microinjection as factors. For 21-day abstinence animals, cytokine, chemokine, and growth factor expression was further analyzed via simple linear regression to examine the correlation of NAc core neuroimmune signaling with active lever presses during cue-induced cocaine seeking. Tukey’s or Dunnett’s multiple comparisons tests were conducted to examine specific group differences when appropriate. Data from a total of 6 rats were excluded from analysis due to loss of catheter patency, failure to acquire cocaine self-administration, or clogged intracerebral guide cannula. All analyses were conducted at α = 0.05 significance level using GraphPad Prism 9.0 software.
3. Results
3.1. Cocaine and sucrose self-administration
Fig. 1A provides the procedural timeline from the experiment examining NAc core immune responses to 5-day gp120 exposure following cocaine or sucrose self-administration. A repeated-measures two-way ANOVA for cocaine infusions earned revealed a significant main effect of session (F(11,88) = 6.161, p < 0.0001) but no significant main effect of group or group-by-session interaction. Post hoc analysis of the session main effect revealed a significant increase in cocaine infusions earned on sessions 6–12 relative to session 1 (Dunnett’s test, ∗p < 0.05, Fig. 1B). For sucrose animals that underwent 5 days of abstinence, a repeated-measures two-way ANOVA for sucrose pellets earned revealed a significant main effect of session (F(11,110) = 23.35, p < 0.0001) but no significant main effect of group or group-by-session interaction. Post hoc analysis of the session main effect revealed a significant increase in sucrose pellets earned on sessions 2–12 relative to session 1 (Dunnett’s test, ∗p < 0.05, Fig. 1C). These results verify that the random assignment of animals to treatment conditions produced similar self-administration profiles.
Fig. 1. Cocaine and sucrose self-administration prior to gp120 exposure and 5-day abstinence.
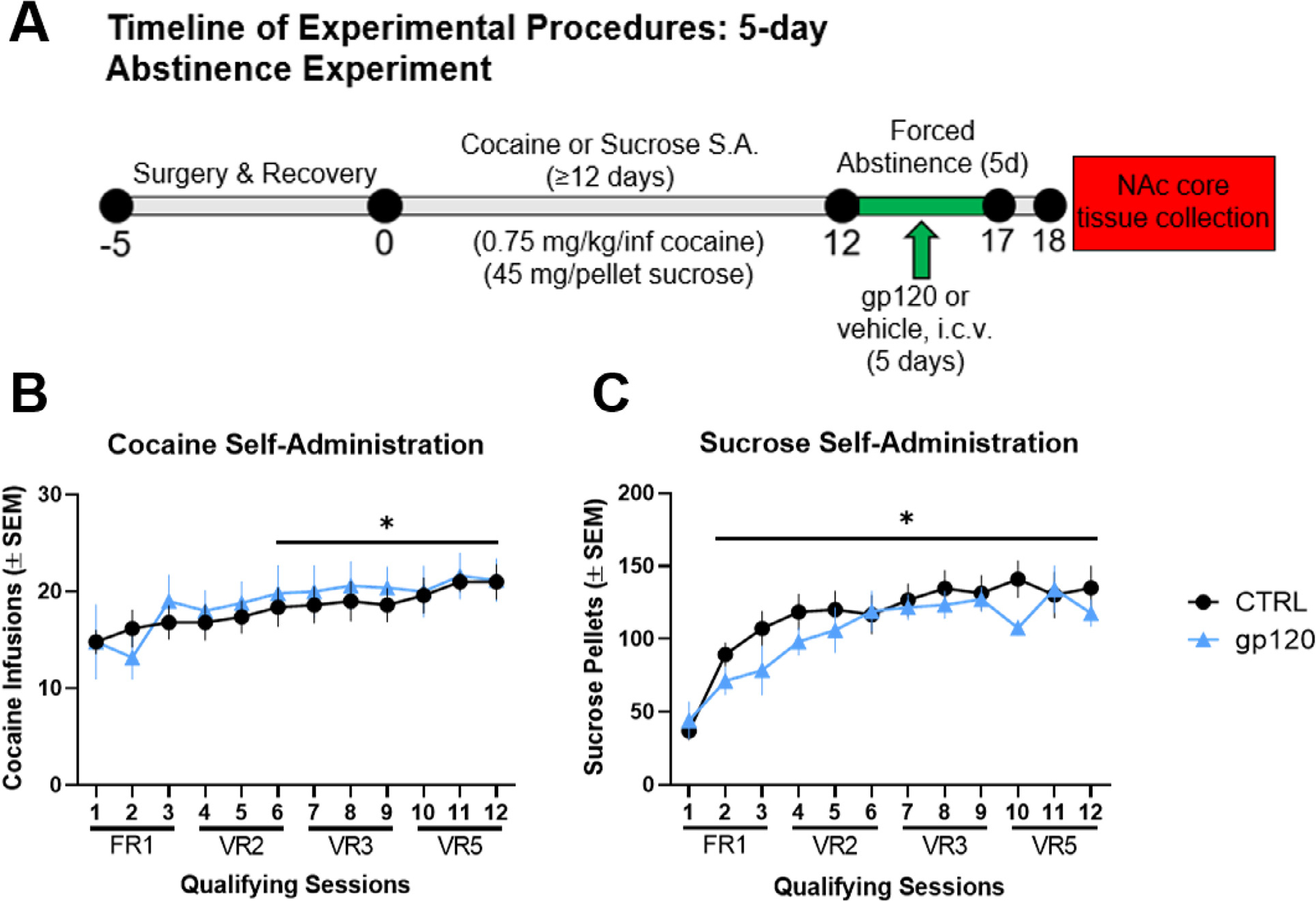
(A) Timeline of experimental procedures. (B) Mean (± SEM) cocaine infusions earned for each qualifying cocaine self-administration (S.A.) session and (C) mean (± SEM) sucrose pellets for each qualifying session of sucrose self-administration. There were no group differences during training (i.e., prior to gp120 treatment). ∗p < 0.05 relative to session 1, regardless of treatment group. n = 8–9/group.
3.2. Cue-induced cocaine seeking
The procedural timeline and results for the experiment investigating the effects of gp120 exposure on MC-25–41-induced attenuation of cue-induced cocaine seeking are shown in Fig. 2A. A repeated-measures, two-way ANOVA for cocaine infusions earned (Fig. 2B) revealed no significant main effect of session or group, and no significant group-by-session interaction. These results verify similar profiles of cocaine self-administration between groups prior to gp120 treatment and cue-induced cocaine seeking. A three-way ANOVA of lever presses during the cue test revealed significant main effects of microinjection type (F(1,23) = 5.153, p = 0.0329), drug treatment (F(1,23) = 14.77, p = 0.0008), and lever (F(1,23) = 97.76, p < 0.0001), as well as significant microinjection-by-treatment (F(1,23) = 5.195, p = 0.0322) and lever-by-treatment (F(1,23) = 10.03, p = 0.0043) interaction effects. Post hoc analysis revealed that MC-25–41 attenuated cue-induced cocaine seeking in unexposed rats but failed to do so in gp120-exposed rats [∗Tukey’s test, p < 0.05 comparing active lever presses (ALPs) and inactive lever presses (ILPs) for each group]. Indeed, the non-exposed, MC-25–41-treated rats had significantly lower ALPs relative to all other groups (#Tukey’s test, p < 0.05 relative to active lever presses of every other group, Fig. 2C). Taken together, these results suggest that MC-25–41 significantly decreases cue-induced cocaine seeking and that a history of gp120 exposure prevents this effect.
Fig. 2. HIV gp120 exposure during early cocaine abstinence prevents the attenuation of cue-induced cocaine seeking by the D3R partial agonist MC-25–41.
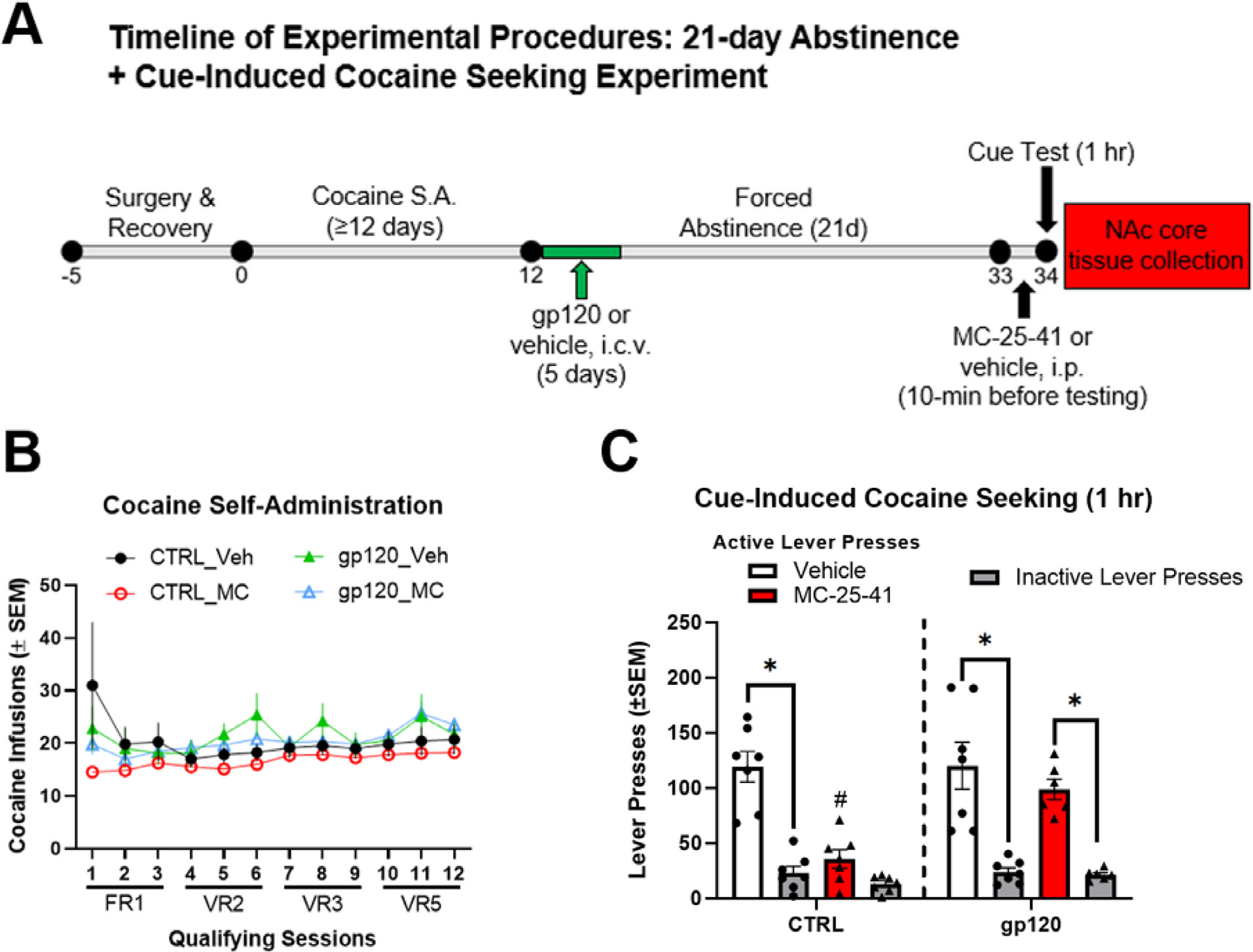
(A) Timeline of experimental procedures. (B) Mean (± SEM) cocaine infusions earned for each qualifying cocaine self-administration (S.A.) session prior to any treatments and (C) mean (± SEM) active lever presses (ALPs) and inactive lever presses (ILPs) during a 1-hr cue-induced cocaine seeking test. There were no group differences during training prior to abstinence and gp120 treatment. After gp120 treatment and 21 days abstinence, MC-25–41 significantly attenuated cue-induced cocaine seeking relative to vehicle treatment in control rats. However, MC-25–41 failed to attenuate cocaine seeking in rats treated with subchronic i.c.v. gp120 during the first 5 days of abstinence. ∗p < 0.05 between ALPs and ILPs for each group; #p < 0.05 relative to active lever presses of all other groups. Error bars = SEM; n = 6–7/group.
3.3. NAc core cytokine, chemokine, and growth factor expression
Two-way ANOVAs were used to examine the effect of early cocaine abstinence and gp120 exposure (5 days) on NAc cytokine, chemokine, and growth factor expression (Fig. 3A–J). The F-statistics, degrees of freedom, and p-values for significant main effects and interactions are provided in Table 1. A significant main effect of reinforcer was detected for fractalkine/CX3CL1, IL-4, IL-5, and IL-18, where early cocaine abstinence, regardless of gp120 exposure, resulted in downregulation of these targets compared to sucrose controls. Conversely, a significant main effect of gp120 exposure was detected for GM-CSF, where GM-CSF expression increased regardless of abstinence condition, although this effect was likely driven by the increase observed in the sucrose + gp120 rats. Significant main effects of both reinforcer and gp120 were detected for CCL5, where gp120 exposure increased CCL5 expression regardless of abstinence condition and cocaine abstinence decreased CCL5 expression regardless of gp120 exposure history. A significant gp120 X reinforcer interaction as well as significant main effects of gp120 and/or reinforcer were detected for eotaxin, IFNγ, MIP-2/CXCL2, and VEGF. Post hoc analyses of the interaction revealed that cocaine abstinence and gp120 exposure increased expression of these targets compared to the sucrose controls (∗∗Tukey’s test, p < 0.05), although for IFNγ, there was only a trend towards increased expression in the sucrose + gp120 group compared to sucrose controls (Tukey’s test, p = 0.0564). In summary, cocaine abstinence independently decreased NAc core expression of CX3CL1, IL-4, IL-5, IL-18, and CCL5, whereas gp120 independently increased GM-CSF and CCL5 expression. In addition to these independent effects, no synergistic or additive effects of cocaine abstinence and gp120 exposure were detected, as cocaine abstinence, gp120, and their combination produced similar increases in NAc core expression of IFNγ, eotaxin, MIP-2/CXCL2, and VEGF.
Fig. 3. Cocaine abstinence and i.c.v. gp120 exposure alter neuroimmune function in the NAc core.
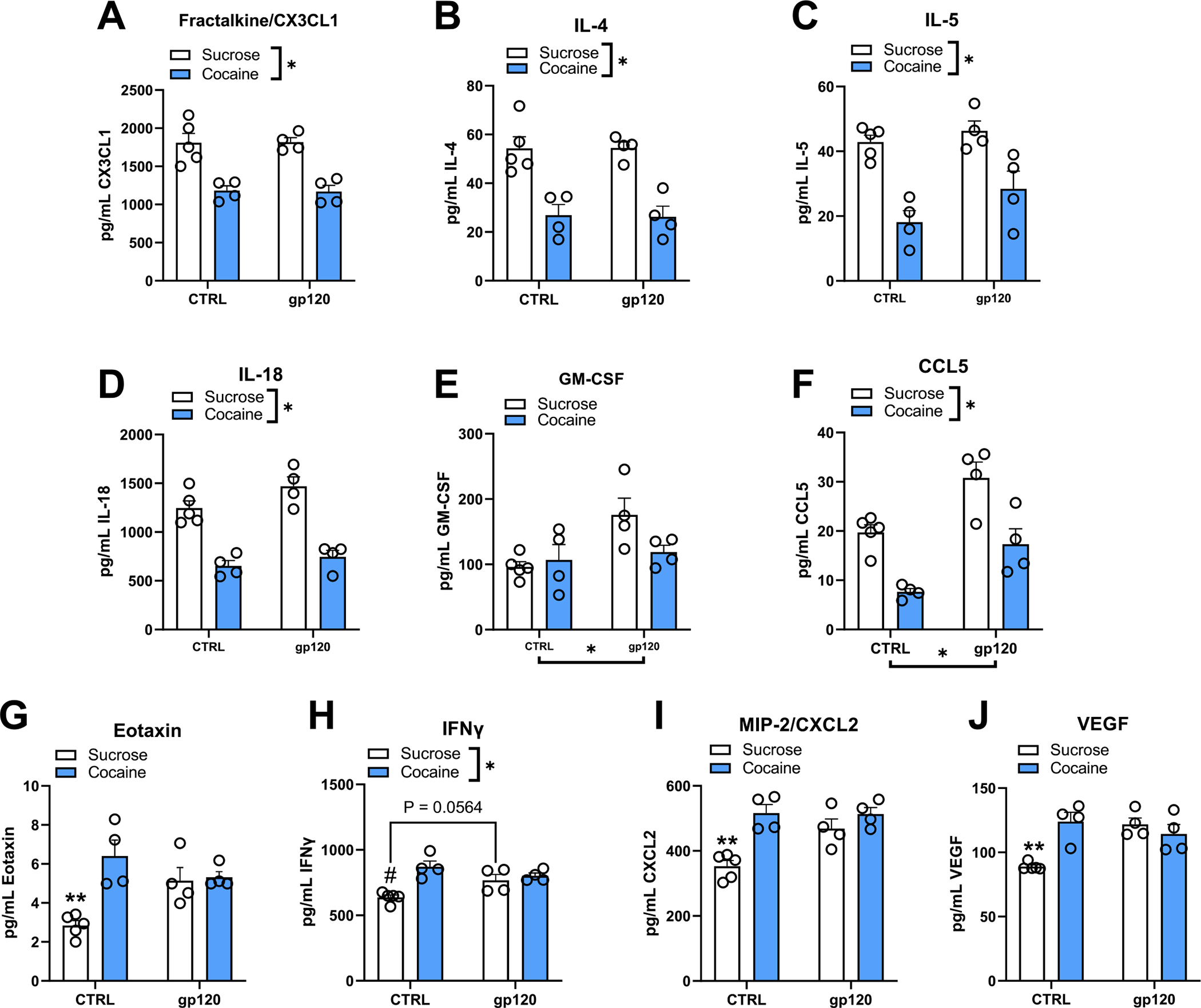
(A-J) Two-way ANOVAs revealed a significant main effect of early cocaine abstinence for fractalkine/CX3CL1, IL-4, IL-5, IL-18, and CCL5 where cocaine abstinence decreased the expression of these targets regardless of gp120. Conversely, a significant main effect of gp120 exposure was detected for CCL5 and GM-CSF, where gp120 increased the expression of these targets regardless of abstinence condition, although this is likely driven by the sucrose + gp120 group for GM-CSF. Significant interactions were detected between early cocaine abstinence and subchronic exposure to gp120 for eotaxin, IFNγ, MIP-2/CXCL2, and VEGF compared to unexposed, sucrose-abstinent rats (i.e., sucrose + CTRL group). Expression of these targets was increased to a similar degree due to cocaine abstinence, gp120 exposure, or their combination relative to sucrose controls. Overall, no additive or synergistic effects of combined gp120 and early cocaine abstinence were observed. ∗p < 0.05, ANOVA main effect (brackets indicate significant effect of the manipulation); ∗∗p < 0.05 relative to all other treatment groups; #p < 0.05 relative to CTRL-cocaine and gp120-cocaine groups. Error bars = SEM; n = 4–5/group.
Table 1.
Two-Way ANOVA Outputs for 5d Abstinence NAc core Cytokine, Chemokine, and Growth Factor Expression.
| Target | Reinforcer | p-value | Microinjection | p-value | Reinf.*Micro. | p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Eotaxin | F(1,13) = 12.29 | p = 0.0034 | F(1,13) = 1.27 | ns | F(1,13) = 10.01 | p = 0.008 |
| CX3CL1 | F(1,13) = 47.22 | p < 0.0001 | F(1,13) = 0.0002 | ns | F(1,13) = 0.013 | ns |
| GM-CSF | F(1,13) = 1.730 | ns | F(1,13) = 6.71 | p = 0.0224 | F(1,13) = 3.663 | ns |
| IFNγ | F(1,13) = 16.62 | p = 0.0013 | F(1,13) = 0.873 | ns | F(1,13) = 9.079 | p = 0.01 |
| IL-4 | F(1,13) = 42.42 | p < 0.0001 | F(1,13) = 0.003 | ns | F(1,13) = 0.011 | ns |
| IL-5 | F(1,13) = 34.94 | p < 0.0001 | F(1,13) = 3.636 | ns | F(1,13) = 0.907 | ns |
| IL-18 | F(1,13) = 75.45 | p < 0.0001 | F(1,13) = 4.386 | ns | F(1,13) = 0.727 | ns |
| CXCL2 | F(1,13) = 19.91 | p = 0.0006 | F(1,13) = 5.885 | p = 0.0306 | F(1,13) = 6.544 | p = 0.024 |
| CCL5 | F(1,13) = 30.22 | p = 0.0001 | F(1,13) = 19.84 | p = 0.0006 | F(1,13) = 0.089 | ns |
| VEGF | F(1,13) = 6.43 | p = 0.0248 | F(1,13) = 4.57 | ns | F(1,13) = 15.35 | p = 0.002 |
In addition to examining immune function during early cocaine abstinence, we examined whether NAc core neuroimmune signaling is altered by the 5-day exposure to gp120 after protracted abstinence from cocaine. Rats used for this assessment had also received treatment with vehicle or MC-25–41 prior to a cue-induced seeking test, and fresh NAc core tissue was harvested immediately after the test. Two-way ANOVAs were used to examine the effects of gp120 exposure history and MC-25–41 treatment on NAc core cytokine, chemokine, and growth factor expression (Fig. 4A–I). The F-statistics, degrees of freedom, and p-values for significant main effects and interactions are provided in Table 2. Only a significant main effect of gp120 was detected for eotaxin, IFNγ, and IL-13, where exposure history increased the expression of these targets regardless of MC-25–41 treatment. Conversely, only a significant main effect of treatment was detected for IL-4 and VEGF, where MC-25–41 reduced the expression of these targets regardless of gp120 exposure history. Similar main effects or trends toward them (p < 0.10) of both gp120 and treatment were found for GM-CSF, LIX/CXCL5, MIP-2/CXCL2, and IL-6, where gp120 exposure history independently increased, while MC-25–41 independently decreased, expression levels. Importantly, no significant interaction effects between gp120 and MC-25–41 treatment were detected for any of the measured analytes.
Fig. 4. MC-25–41 and gp120 independently alter NAc core cytokine, chemokine, and growth factor expression in cocaine-seeking rats.
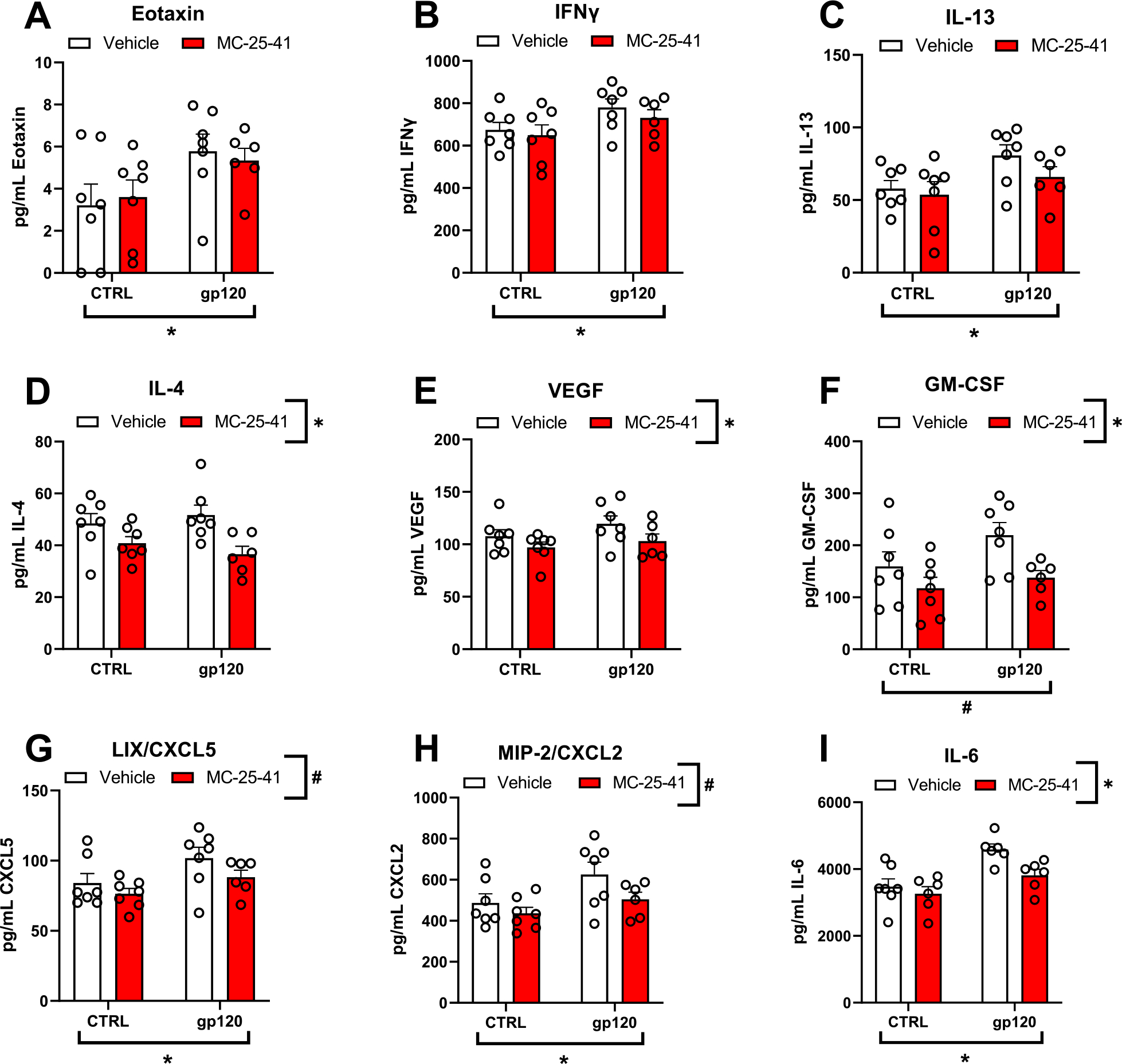
(A-I) Rats that had self-administered cocaine received daily i.c.v. microinfusions of gp120 for the first 5 (out of 21) days of abstinence prior to a cue-induced cocaine seeking test and were sacrificed for NAc core tissue collection immediately after testing. Two-way ANOVAs revealed a significant main effect of gp120 exposure history on eotaxin, IFNγ, IL-13, LIX/CXCL5, MIP-2/CXCL2, and IL-6 expression, where gp120 increased the overall expression of these markers regardless of MC-25–41 treatment (∗p < 0.05). GM-CSF also demonstrated a trend towards increased expression due of gp120 exposure history regardless of MC-25–41 treatment (#p < 0.10). There was also a significant main effect of MC-25–41 treatment on IL-4, VEGF, GM-CSF, and IL-6 expression (∗p < 0.05) and a trend toward an effect on LIX/CXCL5 and MIP-2/CXCL2 (#p < 0.10), where MC-25–41 treatment decreased the overall expression of these markers regardless of gp120 exposure history. Notably, no significant interactions were detected, indicating that MC-25–41 did not significantly attenuate any gp120-induced increases in immune factor expression back down to unexposed levels. Error bars = SEM; n = 6–7/group.
Table 2.
Two-Way ANOVA Outputs for 21d Abstinence + Cue-Induced Seeking NAc core Cytokine, Chemokine, and Growth Factor Expression.
| Target | Microinjection | p-value | Treatment | p-value | Micro.*Treat. | p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Eotaxin | F(1,23) = 6.522 | p = 0.0178 | F(1,23) = 0.0008 | ns | F(1,23) = 0.247 | ns |
| IFNγ | F(1,23) = 5.177 | p = 0.0325 | F(1,23) = 0.8085 | ns | F(1,23) = 0.0844 | ns |
| IL-13 | F(1,23) = 5.569 | p = 0.0271 | F(1,23) = 1.647 | ns | F(1,23) = 0.5017 | ns |
| IL-4 | F(1,23) = 0.0227 | ns | F(1,23) = 11.29 | p = 0.0027 | F(1,23) = 1.264 | ns |
| VEGF | ||||||
| GM-CSF | F(1,23) = 3.045 | p = 0.0943 | F(1,23) = 7.172 | p = 0.0134 | F(1,23) = 0.7248 | ns |
| CXCL5 | F(1,23) = 5.783 | p = 0.0246 | F(1,23) = 2.977 | p = 0.0979 | F(1,23) = 0.2292 | ns |
| CXCL2 | F(1,23) = 5.452 | p = 0.0286 | F(1,23) = 3.775 | p = 0.0644 | F(1,23) = 0.612 | ns |
| IL-6 | F(1,21) = 16.61 | p = 0.0005 | F(1,21) = 5.814 | p = 0.0251 | F(1,21) = 1.893 | ns |
To better understand the neuroimmune mechanisms that may underlie the failure of MC-25–41 to inhibit cocaine seeking in gp120-exposed rats, we examined whether neuroimmune-signaling molecules in the NAc core correlated with cue-induced cocaine seeking. Using simple linear regression analysis, we identified 5 neuroimmune factors that positively correlated with cue-induced cocaine seeking (IL-6, MIP-1α/CCL3, MIP-2/CXCL2, VEGF, and GM-CSF; Fig. 5A–E). In contrast, cocaine seeking correlated negatively with fractalkine/CX3CL1 expression (Fig. 5F). Given the failure of MC-25–41 to significantly attenuate the gp120-induced increase in MIP-2/CXCL2 (Fig. 4H) and the significant correlation of this factor with cue-induced cocaine seeking (Fig. 5C), it is possible that a gp120-induced increase in MIP-2/CXCL2 signaling may partially underlie the failure of MC-25–41 to inhibit cocaine seeking.
Fig. 5. Cue-induced cocaine seeking correlates with NAc core neuroimmune signaling.
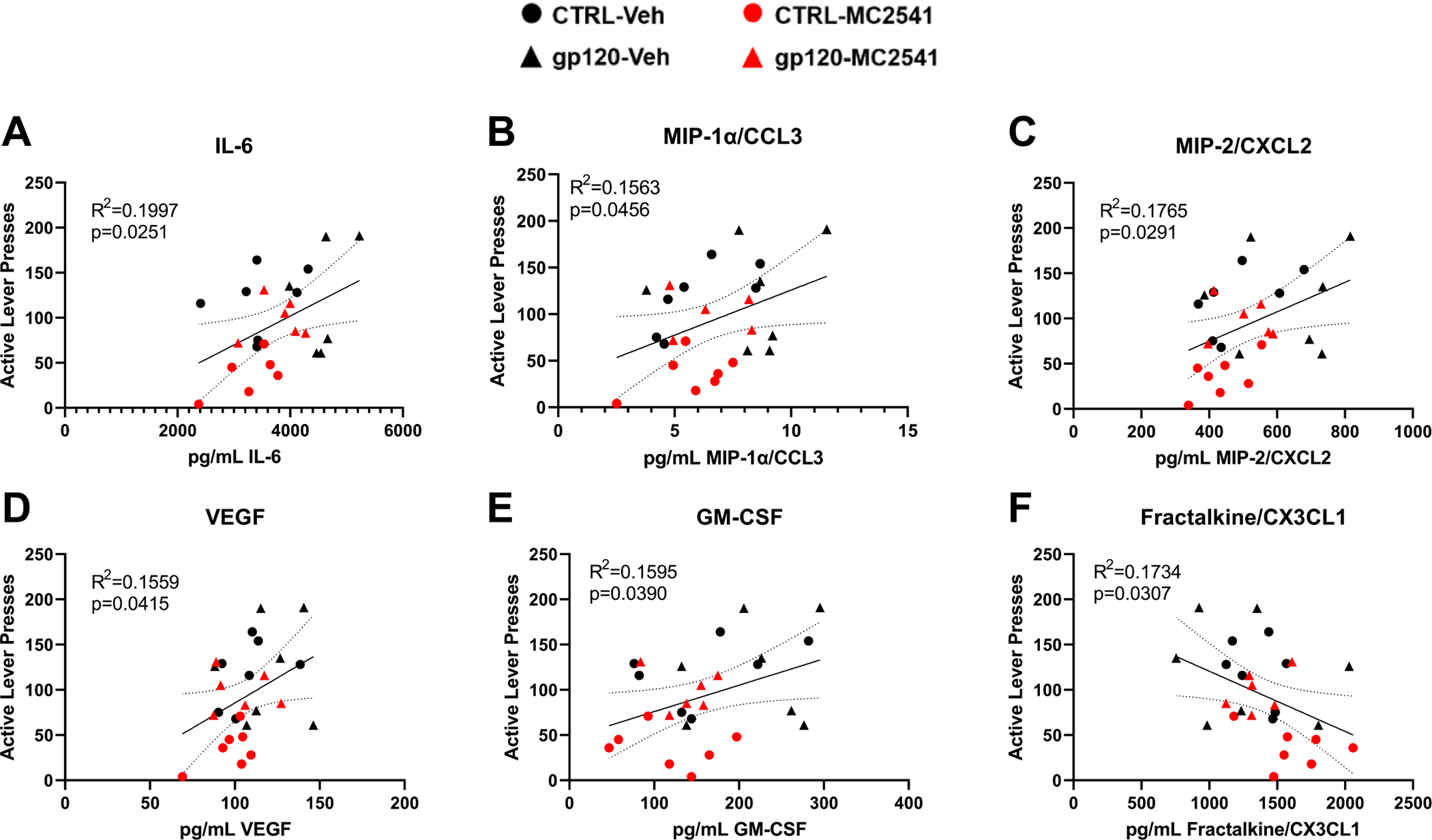
After gp120 exposure and protracted abstinence, rats were tested for cue-induced cocaine seeking, with some rats receiving an acute systemic injection of MC-25–41 (10 mg/kg, i.p.; red symbols) prior to testing. Active lever pressing during cue-induced cocaine seeking correlated positively with NAc core (A) IL-6, (B) MIP-1α/CCL3, (C) MIP-2/CXCL2, (D) VEGF, and (E) GM-CSF expression. In contrast, cocaine seeking correlated negatively with (F) fractalkine/CX3CL1 expression. Dashed lines = 95% confidence bands. n = 6–7/group.
4. Discussion
The present study demonstrates that a history of CNS exposure to the HIV protein gp120 during early abstinence impairs the therapeutic efficacy of a putative anti-craving medication in rodents [80]. Both early cocaine abstinence (5 days) and gp120 exposure, alone and in combination, were associated with immune dysfunction in the NAc core relative to sucrose controls. After a protracted period of cocaine abstinence, the dopamine D3R partial agonist MC-25–41 decreased cue-induced cocaine seeking in controls, but not in rats that received i.c.v. microinfusions of gp120 during the first 5 days of 21 days of cocaine abstinence (Fig. 2).
During early abstinence, cocaine decreased levels of fractalkine/CX3CL1, IL-4, IL-5, IL-18 and CCL5 regardless of whether rats were exposed to gp120, whereas gp120 exposure during early abstinence increased levels of CCL5 and GM-CSF regardless of reinforcement history (Fig. 3). Both gp120 exposure and cocaine history similarly increased levels of eotaxin, IFNγ, MIP-2/CXCL2, and VEGF during early abstinence. Among these immune factors altered by gp120, eotaxin, IFNγ, and MIP-2/CXCL2 were upregulated by gp120 exposure history relative to unexposed controls following late cocaine abstinence, which suggest a set of enduring neuroimmune adaptations that persist across abstinence (Fig. 4). Taken together, the findings suggest that exposure to HIV gp120 throughout the CNS may produce striatal neuroimmune-induced dysfunction that could impair the efficacy of medications intended to treat CUDs.
A history of gp120 exposure alone had no effect on cue-induced cocaine seeking; however, the expression of numerous immune factors within the NAc core positively correlated with this behavior and fractalkine/CX3CL1 negatively correlated with this behavior (Fig. 5). The finding that increasing levels of VEGF, GM-CSF, MIP-2/CXCL2, and IL-6 were associated with increased cue-induced cocaine seeking is interesting because MC-25–41 tended to decrease levels of these immune factors in the NAc core during late abstinence and attenuated cue-induced cocaine seeking in rats not exposed to gp120. However, gp120 significantly increased MIP-2/CXCL2 expression in the NAc core during late abstinence while MC-25–41 failed to significantly attenuate the expression of this immune factor (Fig. 4). Altogether, this suggests that gp120-induced increases in MIP-2/CXCL2 within the NAc core may underly the failure of MC-25–41 to inhibit cue-induced cocaine seeking in gp120-exposed rats (Fig. 2)
4.1. MC-25–41 reduces cue-motivated cocaine seeking
The D3R has long been implicated in cue-motivated drug seeking [15,36,47,69,76,101,107,109]. Both animal and human studies show up-regulation of D3Rs in response to cocaine experience [10,22,58,70,74]. Further evidence that D3R signaling plays a key role in motivation for cocaine is that antagonists or partial agonists attenuate cocaine self-administration under high- but not low-effort schedules of reinforcement [11,30,34,35,79,107]. We selected MC-25–41 for the present study among several compounds our group has developed [18,19] because it exhibits low efficacy (19.4% maximum activity in the forskolin-dependent adenylyl cyclase assay), high affinity (D3R Ki = 0.50 nM), and high selectivity for D3Rs (1486-fold selective over D2Rs; [80]). Importantly, MC-25–41 also has a longer half-life (>60 min in human and rat liver microsome assays) compared to other D3R compounds [17,80]. In line with our previous findings [80], MC-25–41 significantly attenuated cue-induced cocaine seeking after a period of protracted abstinence in unexposed rats. However, MC-25–41 failed to attenuate cocaine seeking in rats with a history of CNS gp120 exposure. The gp120 disruption of this MC-25–41 effect may be due in part to synaptic dysregulation caused by neuroimmune responses as discussed in more detail below.
4.2. Cocaine abstinence- and HIV gp120-induced immune responses in the NAc core
Both cocaine and HIV proteins such as gp120 can promote neuroimmune dysfunction and blood brain barrier (BBB) permeability, which may contribute to impaired synaptic homeostasis and subsequent drug seeking as well as increased HIV neuroinvasion [52,112,113]. Neuroinflammation induced by infection, psychostimulants, and other xenobiotics increases BBB permeability, which can facilitate neuroinvasion of peripheral immune cells that can impair synaptic homeostasis [29,37,52]. After 5 days of cocaine abstinence and concomitant gp120 exposure, cocaine alone upregulated MIP-2/CXCL2, eotaxin and VEGF to the same degree as cocaine + gp120 exposure, suggesting that gp120 exposure and early cocaine abstinence do not interact synergistically or additively in regulating these molecules. However, this upregulation of MIP-2/CXCL2 coincided with a positive correlation with cue-induced cocaine after a protracted abstinence period. Moreover, gp120 exposure history was associated with increased MIP-2/CXCL2 expression relative to unexposed rats after protracted abstinence, and MC-25–41 did not significantly attenuate MIP-2/CXCL2 expression. Among the other immune factors upregulated by gp120 exposure history at this timepoint, MC-25–41 decreased levels of IL-4, VEGF, GM-CSF, and IL-6 regardless of whether or not rats were previously exposed to gp120
Few studies have examined the causal role of cytokines and chemokines in SUDs-related behaviors. However, together with the extant literature, our findings suggest that the gp120-induced upregulation of MIP-2/CXCL2 in the NAc core is involved in the impaired efficacy of MC-25–41 to reduce cocaine seeking. MIP-2/CXCL2 critically mediates peripheral immune cell migration and subsequent release of proinflammatory factors into the brain parenchyma [51,92,106], and recruitment of these cells to the brain promotes depression- and anxiety-like behavior in rodents [61,105]. Studies in vitro demonstrate that gene expression of MIP-2/CXCL2 is significantly increased in human monocytes exposed to gp120 as well as in human neuronal progenitor cells exposed to cocaine [24,55]. Moreover, cultured human astrocytes repeatedly exposed to methamphetamine show marked upregulation of MIP-2/CXCL2 gene expression [45]. Nevertheless, there is a dearth of research on the impact of mesocorticolimbic MIP-2/CXCL2 signaling on drug-seeking behavior, although one study found a significant reduction in cocaine conditioned place preference after systemic inhibition of its receptor, CXCR2 [89]. Given the increase in NAc core MIP-2/CXCL2 by cocaine abstinence, gp120, and their combination at the early abstinence timepoint, this particular neuroimmune adaptation may represent a more enduring neuroimmune impairment that regulates cue-induced cocaine seeking. For example, this increase in MIP-2/CXCL2 could facilitate cueevoked excitatory neurotransmission at NAc core medium spiny neuron synapses that drive cocaine-seeking behavior [82]. Collectively, these results suggest that NAc neuroimmune dysfunction may regulate cue-motivated cocaine seeking, and immune signaling impairments induced by HIV may underlie treatment resistance among those living with comorbid HIV and CUDs.
HIV proteins impact mesocorticolimbic neuroimmune function in ways that may promote drug-seeking behavior, and in vivo transgenic animal models and in vitro studies have revealed important insights into this phenomenon. For instance, gp120 transgenic (Tg) mice exhibit increased translocator protein (TSPO; a microglial activation marker) binding within the striatum, hypothalamus, and hippocampus in response to a LPS challenge compared to wild type controls [110], suggesting an increase in microglial reactivity. These mice also exhibit increased sensitivity to methamphetamine-conditioned reward [50]. In rats, cocaine exposure upregulates NAc expression levels of CCR5 mRNA (which binds to gp120), and pharmacological inhibition of CCR5 attenuates cocaine conditioned place preference [68]. Within human mesencephalon/glia cell culture preparations that are rich in dopamine neurons, gp120 induces neurodegeneration and oxidative stress [43], implicating dopamine neurons as a susceptible cell population. Importantly, cocaine potentiates cellular toxicity, oxidative stress, and NF-κB pathway activation induced by gp120 exposure within rat primary astrocyte cell cultures [108]. We and others have demonstrated a critical role for NAc NF-κB pathway signaling in cue-motivated drug seeking ([65], 2022; [87]), and gp120 may induce neuroimmune dysfunction via stimulation of NF-κB pathway signaling [93]. In turn, this could drive drug-seeking behavior and impair the inhibitory effects of MC-25–41 on this behavior. Recently, de Guglielmo et al. [28] showed that HIV transgenic rats, which constitutively express 7 of the 9 HIV proteins, exhibit escalation of methamphetamine intake, increased responding on a progressive ratio schedule reinforcement, and enhanced neuroinflammation within the medial prefrontal cortex (mPFC) after a 4-week abstinence period. Paralleling these findings, we observed increased NAc core expression of proinflammatory factors such as eotaxin, IFNγ, IL-6, MIP-2/CXCL2, and LIX/CXCL5 in gp120-exposed rats regardless of MC-25–41 treatment after a similar length of abstinence. Nevertheless, the profile of neuroimmune changes observed here are distinct from this previous study, although this is likely due to model differences and/or brain region differences. Collectively, these studies highlight that HIV may modulate neuroimmune function to disrupt reward-seeking behavior, and that abstinence is a critical phase of the addiction cycle where individuals may be uniquely susceptible to HIV-induced dysregulation of drug-motivated behavior.
4.3. Limitations and future directions
One limitation of the present study is the lack of female subjects. We focused on males in this initial study because HIV still disproportionately impacts males. As of 2020, men accounted for 80% of new HIV cases within the United States, with over 70% of cases attributed to male-to-male sexual contact, with or without injection drug use, specifically [13]. However, it is important to note that women accounted for 18% of new HIV diagnoses within the United States [13]. Many preclinical studies in rodents indicate important sex differences in the effects of HIV on striatal synaptic morphology and physiology, dopamine signaling, and reward-seeking behavior [9,59,60,72,73]. Moreover, we have recently demonstrated that female rats fail to exhibit an attenuation of cue-induced cocaine seeking in response to NAc core NF-κB inhibition [66]. Thus, it is possible that females have a differential neuroimmune profile and behavioral response to MC-25–41 treatment following gp120 exposure and protracted abstinence compared to males. Future studies should probe this phenomenon.
Another potential limitation of the present study is the use of a single HIV protein (i.e., gp120) as opposed to a cocktail of multiple HIV proteins or the use of transgenic animals that express one or more of these proteins chronically. Indeed, gp120 and Tat can produce synergistic neurotoxicity that is greater than that produced by either protein alone [67], and chronic, low-level exposure within the CNS to several HIV proteins in transgenic rodents can recapitulate many aspects of HAND among virally-suppressed PLWH [64,100]. Nevertheless, one distinct advantage of the protein approach utilized in the present study is the temporal control over HIV protein exposure. Many PLWH who are diagnosed with a SUD acquired HIV collaterally through risky behaviors such as unsafe sexual practices, needle sharing, etc. In such cases, a chronic history of drug use, and the enduring impact of that drug history on mesocorticolimbic neurobiology, precedes HIV infection. Thus, the administration of HIV proteins into the CNS after a history of drug use is a distinct translational advantage over other preclinical models. Studies attempting to understand the neurobiological intersections of HIV and SUDs must carefully consider the chronological sequence in which substance use and HIV occur to parse their neurobehavioral interactions more accurately, particularly as it pertains to medications development efforts. Future studies could benefit from models such as the EcoHIV model, which provides temporal specificity over direct administration of a chimeric virus construct that closely mimics HIV and successfully infects murine immune cells [78]. Characterization of addiction-like behaviors and pharmacotherapeutic efficacy using such models would be a major advancement in the preclinical study of vulnerable subpopulations of people living with SUDs.
Collection of fresh NAc tissue for neuroimmune analyses precluded us from histologically verifying cannula placement for i.c.v. infusions, although cannula placement within the right lateral ventricle was visually inspected and confirmed during fresh tissue dissections. The present study focused on NAc core neuroimmune signaling given its critical role in abstinence-dependent cocaine seeking [75], however we realize that the behavioral effects observed involve corticolimbic neural circuitry. Given the systemic nature of the i.c.v. gp120 treatment, other brain regions that are known to be susceptible to HIV-induced neuropathology and are critically involved in cocaine-seeking behavior, such as the mPFC, may exhibit a differential profile of neuroimmune adaptations [28,85,103,104]. Such changes could also account for the disrupted MC-25–41 effects we observed on cue-induced cocaine seeking by gp120 exposure history. Future research should characterize the broader impact of HIV on neuroimmune function across brain regions, abstinence timepoints, and sex to better understand the mechanisms that underlie immunomodulation of drug-seeking behavior and associated synaptic plasticity.
5. Conclusions
We demonstrate here that sub-chronic CNS exposure to the HIV protein gp120 induces several neuroimmune adaptations within the NAc core of male rats and blocks the therapeutic efficacy of the novel D3R partial agonist MC-25–41. The latter finding highlights the importance of examining HIV and other comorbidities in medications development for SUDs. In further support, nearly 40% of young adults who have a serious mental illness also meet the diagnostic criteria for a substance use disorder (SUD), and over 60% of adolescents enrolled in community-based SUD treatment programs also met the DSM criteria for other mental disorders [42,49,86]. Furthermore, a large, multi-site study within the U.S. of over 10,000 PLWH found a SUD prevalence rate of 48%, with a 20% rate of polysubstance use and an 11% prevalence rate of CUDs, which is substantially higher than the national average [39]. SUDs/HIV comorbidity reduces cART adherence and healthcare utilization as well as increases difficulty in managing viral load [32]. Cocaine also impairs the efficacy of cART regardless of treatment adherence [84], possibly through direct drug-drug interactions [53]. Thus, with no FDA-approved medications that adequately treat CUDs, individuals with comorbid HIV and CUDs are particularly vulnerable to poorer health outcomes. Altogether, the present findings highlight potential neuroimmune mechanisms to explore with future research examining the modulatory impact of comorbidities such as HIV on the therapeutic efficacy of putative anti-craving medications.
Acknowledgments
We would like to thank Paula Overby & Erin Nagy for their technical assistance with this study. We also thank Dr. Robert R. Luedtke’s group for conducting the initial in vitro validation assays for MC-25–41 and for providing funding for the design and synthesis of MC-25–41 by BEB and PJC. Conceptualization, Methodology, Project administration: MDN, JLN, MFO; Funding Acquisition: MDN, JLN, MFO, & Dr. Robert R. Luedtke; Writing – original draft: MDN, JLN, MFO; Data curation, Investigation: MDN, MNP; Data analysis: MDN; Supervision, Resources: MDN, JLN, MFO; Writing – review and editing: MDN, MNP, JLN, MFO, PJC, BEB. This study was supported by National Institutes of Health grants F31 DA047072 (MDN), R21 DA048651 (JLN), and R01 DA043172 (MFO), R01 DA023957 (Dr. Robert R. Luedtke), as well as grants from the ARCS Foundation (MDN), Arizona State University School of Life Sciences (MDN), and Arizona State University Institute for Social Science Research (MDN, JLN).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
References
- [1].Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA, HIV-1 coreceptor activity of CCR5 and its Inhibition by Chemokines: independence from G protein signaling and importance of coreceptor downmodulation, Virology 234 (2) (1997) 340–348, doi: 10.1006/VIRO.1997.8673. [DOI] [PubMed] [Google Scholar]
- [2].An SF, Scaravilli F, Early HIV-1 infection of the central nervous system, Arch. anat. Cytol. Pathol 45 (2–3) (1997) 94–105 http://www.ncbi.nlm.nih.gov/pubmed/9382615. [PubMed] [Google Scholar]
- [3].Arabatzis TJ, Wakley AA, McLane VD, Canonico D, Cao L, Effects of HIV gp120 on neuroinflammation in immunodeficient vs. immunocompetent states, J. Neuroimmune Pharmacol 16 (2) (2021) 453, doi: 10.1007/S11481-020-09936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashraf T, Jiang W, Hoque MT, Henderson J, Wu C, Bendayan R, Role of anti-inflammatory compounds in human immunodeficiency virus-1 glycoprotein120-mediated brain inflammation, J Neuroinflammation 11 (1) (2014) 1–14, doi: 10.1186/1742-2094-11-91/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avila AH, Morgan CA, Bayer BM, Stress-induced suppression of the immune system following withdrawal from chronic cocaine, J. Pharmacol. Exp. Ther. 305 (1) (2003) 290–297, doi: 10.1124/JPET.102.045989. [DOI] [PubMed] [Google Scholar]
- [6].Bagetta G, Finazzi-Agro A, Palma E, Nistico G, Intracerebral injection of human immunodeficiency virus type 1 coat glycoprotein GP120 does not produce neurodegeneration in rats, Neurosci. Lett. 176 (1) (1994) 97–100, doi: 10.1016/0304-3940(94)90880-X. [DOI] [PubMed] [Google Scholar]
- [7].Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM, Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum, Brain Res. 879 (1–2) (2000) 42–49, doi: 10.1016/S0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- [8].Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A, Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users, J. Acquir. Immune Defic. Syndr 50 (1) (2009) 93–99, doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- [9].Bertrand SJ, Mactutus CF, Harrod SB, Moran LM, Booze RM, HIV-1 proteins dysregulate motivational processes and dopamine circuitry, Sci. Rep. 8 (1) (2018) 1–17, doi: 10.1038/s41598-018-25109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boileau I, Nakajima S, Payer D, Imaging the D3 dopamine receptor across behavioral and drug addictions: positron emission tomography studies with [11C]-(+)-PHNO, Eur. Neuropsychopharmacol 25 (9) (2015) 1410–1420, doi: 10.1016/j.euroneuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- [11].Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M, Cocaine self-administration in dopamine D3 receptor knockout mice, Exp. Clin. Psychopharmacol 20 (5) (2012) 352–363, doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Centers for Disease Control and Prevention. (2020). Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV Surveillance Supplemental Report, 25(1). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2020. [Google Scholar]
- [13].Centers for Disease Control and Prevention. (2021). HIV Surveillance Report, (32). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2021.
- [14].Cervo L, Carnovali F, Stark JA, Mennini T, Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of d3 and d2dopamine receptors, Neuropsychopharmacology 28 (6) (2003) 1150–1159, doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- [15].Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS, Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse, Neuroimage 42 (2) (2008) 869–878, doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen PJ, Taylor M, Griffin SA, Amani A, Hayatshahi H, Korzekwa K, Ye M, Mach RH, Liu J, Luedtke RR, Gordon JC, Blass BE, Design, synthesis, and evaluation of N-(4-(4-phenyl piperazin-1-yl)butyl)-4-(thiophen-3-yl)benzamides as selective dopamine D3 receptor ligands, Bioorg. Med. Chem. Lett. 29 (18) (2019) 2690–2694, doi: 10.1016/j.bmcl.2019.07.020. [DOI] [PubMed] [Google Scholar]
- [17].Cheung THC, Loriaux AL, Weber SM, Chandler KN, Lenz JD, Schaan RF, Mach RH, Luedtke RR, Neisewander JL, Reduction of cocaine self-administration and D3 receptor-mediated behavior by two novel dopamine D3 receptor-selective partial agonists, OS-3–106 and WW-III-55, J. Pharmacol. Exp. Ther. 347 (2) (2013) 410–423, doi: 10.1124/jpet.112.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheung THC, Nolan BC, Hammerslag LR, Weber SM, Durbin JP, Peartree NA, MacH RH, Luedtke RR, Neisewander JL, Phenylpiperazine derivatives with selectivity for dopamine D3 receptors modulate cocaine self-administration in rats, Neuropharmacology 63 (8) (2012) 1359, doi: 10.1016/J.NEUROPHARM.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clark KH, Wiley CA, Bradberry CW, Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection, Neurotox. Res 23 (2) (2013) 174–188, doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- [20].Cocchi F, DeVico AL, Garzino-Demo A, Cara A, Gallo RC, Lusso P, The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection, Nat. Med. 2 (11) (1996) 1244–1247, doi: 10.1038/NM1196-1244. [DOI] [PubMed] [Google Scholar]
- [21].Conrad KL, Ford K, Marinelli M, Wolf ME, Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration, Neuroscience 169 (1) (2010) 182–194, doi: 10.1016/J.NEUROSCIENCE.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cosenza MA, Zhao ML, Si Q, Lee SC, Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis, Brain Pathol. 13 (4) (2003) 442–455, doi: 10.1111/j.1750-3639.2003.tb00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crawford FC, Wood ML, Wilson SE, Mathura VS, Hollen TR, Geall F, Kolippakkam DN, Mullan MJ, Cocaine induced inflammatory response in human neuronal progenitor cells, J. Neurochem. 97 (3) (2006) 662–674, doi: 10.1111/J.1471-4159.2006.03760.X. [DOI] [PubMed] [Google Scholar]
- [24].Cui C, Shurtleff D, Harris RA, Neuroimmune mechanisms of alcohol and drug addiction, Int. Rev. Neurobiol 118 (2014) 1–12, doi: 10.1016/B978-0-12-801284-0.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dahal S, Chitti SVP, Nair MPN, Saxena SK, Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS, Front. Microbiol. 6 (2015) 931, doi: 10.3389/fmicb.2015.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dash S, Balasubramaniam M, Villalta F, Dash C, Pandhare J, Impact of cocaine abuse on HIV pathogenesis, Front. Microbiol 6 (OCT) (2015) 1–12, doi: 10.3389/fmicb.2015.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Guglielmo G, Fu Y, Chen J, Larrosa E, Hoang I, Kawamura T, Lorrai I, Zorman B, Bryant J, George O, Sumazin P, Lefebvre C, Repunte-Canonigo V, Paolo Sanna, Increases in compulsivity, inflammation, and neural injury in HIV transgenic rats with escalated methamphetamine self-administration under extended-access conditions, Brain Res. 1726 (2020) 146502, doi: 10.1016/j.brainres.2019.146502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Vries HE, Kuiper J, de Boer AG, Van Berkel TJC, Breimer DD, The blood-brain barrier in neuroinflammatory diseases, Pharmacol. Rev 49 (2) (1997) 143–156 https://pharmrev.aspetjournals.org/content/49/2/143.short. [PubMed] [Google Scholar]
- [29].Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ, Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-27701 1-A, Neuropsychopharmacology 28 (2) (2003) 329–338, doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- [30].Dong J, Lu DX, Pan R, Tang HM, Effect and mechanism of curcumin on learning and memory dysfunction induced by gp120 in rats, Chin. J. Cell. Mol. Immunol 24 (4) (2008) 328–331 http://www.ncbi.nlm.nih.gov/pubmed/18394334. [PubMed] [Google Scholar]
- [31].Durvasula R, Miller TR, Substance abuse treatment in persons with HIV/AIDS: challenges in managing triple diagnosis, Behav. Med 40 (2) (2014) 43–52, doi: 10.1080/08964289.2013.866540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eilers M, Roy U, Mondal D, MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells, Exp. Biol. Med. 233 (9) (2008) 1160, doi: 10.3181/0802-RM-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gál K, Gyertyân I, Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration i n rats, Brain Res. Bull. 61 (6) (2003) 595–601, doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- [34].Galaj E, Ananthan S, Saliba M, Ranaldi R, The effects of the novel da D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats, Psychopharmacology 231 (3) (2014) 501–510 (Berl.), doi: 10.1007/s00213-013-3254-y. [DOI] [PubMed] [Google Scholar]
- [35].Galaj E, Manuszak M, Babic S, Ananthan S, Ranaldi R, The selective dopamine D3 receptor antagonist, SR 21502, reduces cue-induced reinstatement of heroin seeking and heroin conditioned place preference in rats, Drug Alcohol Depend. 156 (2015) 228–233, doi: 10.1016/j.drugalcdep.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Galea I, The blood-brain barrier in systemic i nfection and i nflammation, Cell. Mol. Immunol. 18 (11) (2021) 2489–2501, doi: 10.1038/s41423-021-00757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gray LR, Turville SG, HItchen TL, Cheng WJ, Ellett AM, Salimi H, Roche MJ, Wesselingh SL, Gorry PR, Churchill MJ, HIV-1 entry and transinfection of astrocytes involves CD81 vesicles, PLoS ONE 9 (2) (2014) 1–8, doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, Mayer KH, Moore RD, Mugavero MJ, Napravnik S, Rodriguez B, Donovan DM, Prevalence and predictors of substance use disorders among HIV care enrollees in the United States, AIDS Behav. 21 (4) (2017) 1138, doi: 10.1007/S10461-016-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S,. . . Simpson DM, HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study, Neurology 75 (23) (2010) 2087–2096, doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hong S, Banks WA, Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications, Brain Behav. Immun. 45 (2015) 1–12, doi: 10.1016/J.BBI.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hser YI, Grella CE, Hubbard RL, Hsieh SC, Fletcher BW, Brown BS, Anglin MD, An evaluation of drug treatments for adolescents in 4 US cities, Arch. Gen. Psychiatry 58 (7) (2001) 689–695, doi: 10.1001/ARCHPSYC.58.7.689. [DOI] [PubMed] [Google Scholar]
- [42].Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB, Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage, J. Neurovirol. 15 (5–6) (2009) 401–410, doi: 10.3109/13550280903296346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Iovino L, Tremblay ME, Civiero L, Glutamate-induced excitotoxicity in Parkinson’s disease: the role of glial cells, J. Pharmacol. Sci 144 (3) (2020) 151–164, doi: 10.1016/J.JPHS.2020.07.011. [DOI] [PubMed] [Google Scholar]
- [44].Jackson AR, Shah A, Kumar A, Methamphetamine alters the normal progression by inducing cell cycle arrest in astrocytes, PLoS ONE 9 (10) (2014) e109603, doi: 10.1371/JOURNAL.PONE.0109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jaeger LB, Nath A, Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis, Dis. Models Mech 5 (3) (2012) 313–322, doi: 10.1242/dmm.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jordan CJ, Humburg B, Rice M, Bi GH, You ZB, Shaik AB, Cao J, Bonifazi A, Gadiano A, Rais R, Slusher B, Newman AH, Xi ZX, The highly selective dopamine D3R antagonist, R-VK4–40 attenuates oxycodone reward and augments analgesia in rodents, Neuropharmacology 158 (2019), doi: 10.1016/j.neuropharm.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kariisa M, Seth P, Scholl L, Wilson N, Davis NL, Drug overdose deaths involving cocaine and psychostimulants with abuse potential among racial and ethnic groups - United States, 2004–2019, Drug Alcohol Depend. 227 (2021) 109001, doi: 10.1016/J.DRUGALCDEP.2021.109001. [DOI] [PubMed] [Google Scholar]
- [48].Kelly TM, Daley DC, Integrated treatment of substance use and psychiatric disorders, Soc. Work Public Health 28 (2013) 3880, doi: 10.1080/19371918.2013.774673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kesby JP, Hubbard DT, Markou A, Semenova S, Expression of HIV gp120 protein increases sensitivity to the rewarding properties of methamphetamine in mice, Addict. Biol 19 (4) (2014) 593–605, doi: 10.1111/adb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kielian T, Barry B, Hickey WF, CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses1, J. Immunol. 166 (7) (2001) 4634–4643, doi: 10.4049/JIMMUNOL.166.7.4634. [DOI] [PubMed] [Google Scholar]
- [51].Kousik SM, Napier TC, Carvey PM, The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation, Front. Pharmacol. 3 (2012) 121, doi: 10.3389/FPHAR.2012.00121/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kumar S, Rao PSS, Earla R, Kumar A, Drug-drug interactions between antiretroviral therapies and drugs of abuse in HIV systems, Expert Opin. Drug Metab. Toxicol 11 (3) (2015) 343–355, doi: 10.1517/17425255.2015.996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kwon HS, Koh SH, Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes, Transl. Neurodegener 9 (1) (2020) 1–12, doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Levast B, Barblu L, Coutu M, Prévost J, Brassard N, Peres A, Stegen C, Madrenas J, Kaufmann DE, Finzi A, HIV-1 gp120 envelope glycoprotein determinants for cytokine burst in human monocytes, PLoS ONE 12 (3) (2017) e0174550, doi: 10.1371/JOURNAL.PONE.0174550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li H, McLaurin KA, Illenberger JM, Mactutus CF, Booze RM, Microglial HIV-1 expression: role in HIV-1 associated neurocognitive disorders, Viruses (5) (2021) 13, doi: 10.3390/v13050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Louboutin JP, Reyes BAS, Agrawal L, Van Bockstaele EJ, Strayer DS, HIV-1 gp120-induced neuroinflammation: relationship to neuron loss and protection by rSV40-delivered antioxidant enzymes, Exp. Neurol. 221 (1) (2010) 231–245, doi: 10.1016/J.EXPNEUROL.2009.11.004. [DOI] [PubMed] [Google Scholar]
- [57].fei D. Matuskey, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E, Lee DE, Hannestad J, Lim K, Zheng MQ, Lin S, Labaree D, Potenza MN, Carson RE, Malison RT, Ding YS, Dopamine D3 receptor alterations in cocaine-dependent humans imaged with [11C](+) PHNO, Drug Alcohol Depend. 139 (2014) 100–105, doi: 10.1016/j.drugalcdep.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE, Increased sensitivity to cocaine self-administration in HIV-1 transgenic rats is associated with changes in striatal dopamine transporter binding, J. Neuroimmune Pharmacol 10 (3) (2015) 493–505, doi: 10.1007/s11481-015-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McLaurin KA, Cook AK, Li H, League AF, Mactutus CF, Booze RM, Synaptic connectivity in medium spiny neurons of the nucleus accumbens: a sex-dependent mechanism underlying apathy in the HIV-1 transgenic Rat, Front. Behav. Neurosci 12 (2018), doi: 10.3389/FNBEH.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, Leclair KB, Janssen WG, Labonte B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, . . . Russo SJ, Social stress induces neurovascular pathology promoting depression, Nat. Neurosci. 20 (12) (2017) 1752–1760, doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Montaner JSG, Lima VD, Harrigan PR, Lourenqo L, Yip B, Nosyk B, Wood E, Kerr T, Shannon K, Moore D, Hogg RS, Barrios R, Gilbert M, Krajden M, Gustafson R, Daly P, Kendall P, Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV treatment as prevention” experience in a canadian setting, PLoS ONE 9 (2) (2014) e87872, doi: 10.1371/JOURNAL.PONE.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Montoya A, Elgueta D, Campos J, Chovar O, Falcon P, Matus S, Alfaro I, Bono MR, Pacheco R, Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation, J. Neuroinflammation 16 (1) (2019) 258, doi: 10.1186/s12974-019-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Moran LM, Booze RM, Mactutus CF, Modeling deficits in attention, inhibition, and flexibility in HAND, J. Neuroimmune Pharmacol 9 (4) (2014) 508–521, doi: 10.1007/s11481-014-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Namba MD, Kupchik YM, Spencer SM, Garcia-Keller C, Goenaga JG, Powell GL, Vicino IA, Hogue IB, Gipson CD, Accumbens neuroimmune signaling and dysregulation of astrocytic glutamate transport underlie conditioned nicotine-seeking behavior, Addict. Biol 25 (5) (2020) e12797, doi: 10.1111/adb.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Namba MD, Phillips MN, Neisewander JL, Olive MF, Nuclear factor kappa B signaling within the rat nucleus accumbens core sex-dependently regulates cue-induced cocaine seeking and matrix metalloproteinase-9 expression, Brain Behav. Immun 102 (2022) 252–265, doi: 10.1016/J.BBI.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD, Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine, Ann. Neurol. 47 (2) (2000) 186–194 . [DOI] [PubMed] [Google Scholar]
- [67].Nayak SU, Cicalese S, Tallarida C, Oliver CF, Rawls SM, Chemokine CCR5 and cocaine interactions in the brain: cocaine enhances mesolimbic CCR5 mRNA levels and produces place preference and locomotor activation that are reduced by a CCR5 antagonist, Brain Behav. Immun 83 (2020) 288–292, doi: 10.1016/j.bbi.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Neisewander JL, Cheung THC, Pentkowski NS, Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: implications for medications development, Neuropharmacology 76 (2014) 301–319, doi: 10.1016/j.neuropharm.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Neisewander JL, Fuchs RA, Tran-Nguyen LTL, Weber SM, Coffey GP, Joyce JN, Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior, Neuropsychopharmacology 29 (8) (2004) 1479–1487, doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- [70].Osborne O, Peyravian N, Nair M, Daunert S, Toborek M, The paradox of HIV blood-brain barrier penetrance and antiretroviral drug delivery deficiencies, Trends Neurosci. 43 (9) (2020) 695–708, doi: 10.1016/J.TINS.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP, Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice, Neuropsychopharmacology 39 (2) (2014) 380–388, doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Paris JJ, Fenwick J, McLaughlin JP, Estrous cycle and HIV-1 Tat protein influence cocaine-conditioned place preference and induced locomotion of female mice, Curr. HIV Res 12 (6) (2014) 388–396 http://www.ncbi.nlm.nih.gov/pubmed/25613137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I, Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral Phenotype: a positron emission tomography study with 11C-(+)-PHNO, Neuropsychopharmacology 39 (2) (2014) 311–318, doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y, Neurobiology of the incubation of drug craving, Trends Neurosci. 34 (8) (2011) 411–420, doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everittt BJ, Sokoloff P, Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist, Nature 400 (6742) (1999) 371–375, doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- [76].Polazzi E, Monti B, Microglia and neuroprotection: from in vitro studies to therapeutic applications, Prog. Neurobiol. 92 (3) (2010) 293–315, doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- [77].Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ, A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness, Proc. Natl Acad. Sci. 102 (10) (2005) 3765, doi: 10.1073/PNAS.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Powell GL, Bonadonna JP, Vannan A, Xu K, Mach RH, Luedtke RR, Neisewander JL, Dopamine D3 receptor partial agonist LS-3–134 attenuates cocaine-motivated behaviors, Pharmacol. Biochem. Behav. 175 (2018) 123–129, doi: 10.1016/J.PBB.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [79].Powell GL, Namba MD, Vannan A, Bonadonna JP, Carlson A, Mendoza R, Chen PJ, Luetdke RR, Blass BE, Neisewander JL, The long-acting D3 partial agonist MC-25–41 attenuates motivation for cocaine in sprague-dawley rats, Biomolecules 10 (7) (2020) 1–16, doi: 10.3390/BI0M10071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Purohit V, Rapaka R, Shurtleff D, Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia, Mol. Neurobiol. 44 (1) (2011) 102–110, doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- [81].Ragozzino D, Giovannelli A, Mileo AM, Limatola C, Santoni A, Eusebi F, Modulation of the neurotransmitter release in rat cerebellar neurons by GR0 beta, Neuroreport 9 (16) (1998) 3601–3606, doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- [82].Rao VR, Ruiz AP, Prasad VR, Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND), AIDS Res. Ther. 11 (1) (2014) 1–15, doi: 10.1186/1742-6405-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rasbach DA, Desruisseau AJ, Kipp AM, Stinnette S, Kheshti A, Shepherd BE, Sterling TR, Hulgan T, Mcgowan CC, Qian HZ, Active cocaine use is associated with lack of HIV-1 virologic suppression independent of nonadherence to antiretroviral therapy: use of a rapid screening tool during routine clinic visits, AIDS Care 25 (1) (2013) 109–117, doi: 10.1080/09540121.2012.687814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rocha A, Kalivas PW, Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking, Eur. J. Neurosci. 31 (5) (2010) 903–909, doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ross S, Peselow E, Co-occurring psychotic and addictive disorders: neurobiology and diagnosis, Clin. Neuropharmacol. 35 (5) (2012) 235–243, doi: 10.1097/WNF.0B013E318261E193. [DOI] [PubMed] [Google Scholar]
- [86].Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolaños CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, . . . Nestler EJ, Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward, J. Neurosci. 29 (11) (2009) 3529–3537, doi: 10.1523/JNEUR0SCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sahu G, Farley K, El-Hage N, Aiamkitsumrit B, Fassnacht R, Kashanchi F, 0chem A, Simon GL, Karn J, Hauser KF, Tyagi M, Cocaine promotes both initiation and elongation phase of HIV-1 transcription by activating NF-k-B and MSK1 and inducing selective epigenetic modifications at HIV-1 LTR, Virology 483 (2015) 185–202, doi: 10.1016/j.virol.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Saika F, Matsuzaki S, Kobayashi D, Kiguchi N, Kishioka S, Chemokine CXCL1 is responsible for cocaine-induced reward in mice, Neuropsychopharmacol. Rep 38 (3) (2018) 145–148, doi: 10.1002/NPR2.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sari H, Galbusera R, Bonnier G, Lin Y, Alshelh Z, Torrado-Carvajal A, Mukerji SS, Ratai EM, Gandhi RT, Chu JT, Akeju 0, 0rhurhu V, Salvatore AN, Sherman J, Kwon DS, Walker B, Rosen B, Price JC, Pollak LE, . . . Granziera C, Multimodal investigation of neuroinflammation in aviremic patients with HIV on antiretroviral therapy and HIV elite controllers, Neurol. Neuroimmunol. Neuroinflammation 9 (2) (2022), doi: 10.1212/NXI.0000000000001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC, Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury, Neurobiol. Dis. 40 (2) (2010) 394–403, doi: 10.1016/J.NBD.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [91].Shah A, Verma AS, Patel KH, Noel R, Rivera-Amill V, Silverstein PS, Chaudhary S, Bhat HK, Stamatatos L, Singh DP, Buch S, Kumar A, HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA, PLoS 0NE 6 (6) (2011) e21261, doi: 10.1371/J0URNAL.P0NE.0021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Silverstein PS, Shah A, Weemhoff J, Kumar S, Singh DP, Kumar A, HIV-1 gp120 and drugs of abuse: interactions in the central nervous system, Curr. HIV Res 10 (5) (2012) 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR, Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier, Brain Res. 1399 (2011) 96–115, doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Valcour V, Sithinamsuwan P, Letendre S, Ances B, Pathogenesis of HIV in the central nervous system, Curr HIV/AIDS Rep 8 (1) (2011) 54–61, doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Valdebenito S, Castellano P, Ajasin D, Eugenin EA, Astrocytes are HIV reservoirs in the brain: a cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer, J. Neurochem. 158 (2) (2021) 429–443, doi: 10.1111/JNC.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Varatharajan L, Thomas SA, The transport of anti-HIV drugs across blood-CNS i nterfaces: summary of current knowledge and recommendations for further research, Antiviral Res. 82 (2) (2009) A109, doi: 10.1016/J.ANTIVIRAL.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vigorito M, Connaghan KP, Chang SL, The HIV-1 transgenic rat model of neuroHIV, Brain Behav. Immun. 48 (2015) 336–349, doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vorel SR, Ashby CR, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL, Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats, J. Neurosci. 22 (21) (2002) 9595–9603, doi: 10.1523/jneurosci.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA, The survival benefits of AIDS treatment in the United States, J. Infect. Dis. 194 (1) (2006) 11–19. [DOI] [PubMed] [Google Scholar]
- [100].Wayman WN, Chen L, Hu XT, Napier TC, HIV-1 transgenic rat prefrontal cortex hyper-excitability is enhanced by Cocaine Self-Administration, Neuropsychopharmacology 41 (8) (2016) 1965–1973, doi: 10.1038/npp.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wayman WN, Chen L, Napier TC, Hu XT, Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to human immunodeficiency virus-1 Tat, Eur. J. Neurosci. 41 (9) (2015) 1195–1206, doi: 10.1111/EJN.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wohleb ES, Powell ND, Godbout JP, Sheridan JF, Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior, J. Neurosci. 33 (34) (2013) 13820–13833, doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wu F, Zhao Y, Jiao T, Shi D, Zhu X, Zhang M, Shi M, Zhou H, CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation, J. Neuroinflammation 12 (1) (2015) 1–15, doi: 10.1186/S12974-015-0316-6/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Gitajn L, Gardner EL, The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats, Neuropsychopharmacology 31 (7) (2006) 1393–1405, doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- [105].Yang Y, Yao H, Lu Y, Wang C, Buch S, Cocaine Potentiates Astrocyte Toxicity Mediated by Human Immunodeficiency Virus (HIV-1) Protein gp120, PLoS ONE 5 (10) (2010) e13427, doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, Newman AH, Dopamine D3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects, Neuropsychopharmacology 44 (8) (2019) 1415–1424, doi: 10.1038/s41386-018-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Young JW, Barback CV, Stolz LA, Groman SM, Vera DR, Hoh C, Kotta KK, Minassian A, Powell SB, Brody AL, MicroPET evidence for a hypersensitive neuroinflammatory profile of gp120 mouse model of HIV, Psychiatry Res. Neuroimaging 321 (2022) 111445, doi: 10.1016/J.PSCYCHRESNS.2022.111445. [DOI] [PubMed] [Google Scholar]
- [108].Zaparte A, Schuch JB, Viola TW, Baptista TAS, Beidacki AS, do Prado CH, Sanvicente-Vieira B, Bauer ME, Grassi-Oliveira R, Cocaine use disorder is associated with changes in Th1/Th2/Th17 cytokines and lymphocytes subsets, Front. Immunol. 10 (2019) 2435, doi: 10.3389/fimmu.2019.02435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zayyad Z, Spudich S, Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND), Curr. HIV/AIDS Rep. 12 (1) (2015) 16–24, doi: 10.1007/s11904-014-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhang L, Looney D, Taub D, Chang SL, Way D, Witte MH, Graves MC, Fiala M, Cocaine opens the blood-brain barrier to HIV-1 invasion, J. Neurovirol. 4 (6) (1998) 619–626, doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


