Abstract
This paper aims to provide guidance and a framework for commissioning tests and tolerances for the ExacTrac Dynamic image-guided and surface-guided radiotherapy (SGRT) system. ExacTrac Dynamic includes a stereoscopic X-ray system, a structured light projector, stereoscopic cameras, thermal camera for SGRT, and has the capability to track breath holds and internal markers. The system provides fast and accurate image guidance and intrafraction guidance for stereotactic radiosurgery and stereotactic ablative radiotherapy. ExacTrac Dynamic was commissioned on a recently installed Elekta Versa HD. Commissioning tests are described including safety, isocenter calibration, dosimetry, image quality, data transfer, SGRT stability, SGRT localization, gating, fusion, implanted markers, breath hold, and end-to-end testing. Custom phantom designs have been implemented for assessment of the deep inspiration breath-hold workflow, the implanted markers workflow, and for gating tests where remote-controlled movement of a phantom is required. Commissioning tests were all found to be in tolerance, with maximum translational and rotational deviations in SGRT of 0.3 mm and 0.4°, respectively, and X-ray image fusion reproducibility standard deviation of 0.08 mm. Tolerances were based on published documents and upon the performance characteristics of the system as specified by the vendor. The unique configuration of ExacTrac Dynamic requires the end user to design commissioning tests that validate the system for use in the clinical implementation adopted in the department. As there are multiple customizable workflows available, tests should be designed around these workflows, and can be ongoing as workflows are progressively introduced into departmental procedures.
Keywords: Deep inspiration breath hold, ExacTrac, stereotactic ablative radiotherapy, stereotactic radiosurgery
INTRODUCTION
Stereotactic radiosurgery (SRS) and stereotactic ablative radiotherapy (SABR) techniques involve delivery of an ablative dose to a target over a limited number of fractions. Patient setup accuracy must often be in the submillimeter range to ensure that a geometric miss does not occur during a treatment fraction. A geometric miss for a treatment with a high dose per fraction increases the risk of not achieving the desired dose to the target, and of normal tissue complications, particularly to organs at risk (OARs) such as the optic chiasm, optic nerves, lenses, brainstem, and inner ears. In addition, a geometric miss for a single fraction can potentially compromise target goals by underdosing the target. The American Association of Physicists in Medicine (AAPM) Task Group 142 Report recommends that medical linear accelerators use image guidance systems with a tolerance of 1 mm for deviations between imaging systems and treatment coordinate systems.[1]
To ensure submillimetric accuracy, stereotactic radiotherapy treatments combine patient immobilization (typically including a thermoplastic mask) with an image guidance system and a patient positioning system capable of patient shifts in a 6-degree of freedom (6DoF) coordinate system. Linac-based stereotactic radiotherapy treatments are often noncoplanar, using different couch rotations to enhance dose gradients around target volumes. Intrafraction imaging can be used to verify patient position and offer real-time monitoring of the patient, depending on the technology. On board, cone-beam computed tomography (CBCT) systems are widely available and are capable of high levels of geometric accuracy but are subject to several limitations. CBCTs are often unsuitable for noncoplanar treatment positions due to the geometry of the patient position system and the imaging system. They are time-consuming and can increase imaging dose significantly. Partial arc CBCTs may be used in certain situations, but their geometric accuracy and reduction in image quality should be assessed carefully.
An alternative image-guided radiotherapy (IGRT) system is ExacTrac® Dynamic (Brainlab AG, Munich, Germany). ExacTrac® Dynamic combines a fixed stereoscopic planar imaging system with surface-guided radiotherapy (SGRT). ExacTrac® Dynamic utilizes a structured light projector, a stereoscopic three-dimensional (3D) camera, and a thermal camera to generate a surface contour for patient tracking. The thermal data are correlated to the 3D camera data and used as an extra characteristic to aid in surface tracking. Stereoscopic planar X-ray images supply 3D internal information on patient setup rapidly, accurately, and with low patient dose.[2] After X-ray image acquisition, the surface reference image from the 3D camera and thermal camera is updated, providing information for any shifts in external position. Any movement of the patient surface beyond a set tolerance can be used to hold beam delivery on the linac and trigger a subsequent stereoscopic X-ray. For cranial treatments, patients can be immobilized with an open-face mask allowing the SGRT system to track the facial contour.
When integrated with an Elekta Versa HD linac and a Hexapod 6DoF patient positioning system, ExacTrac® Dynamic can be used in several ways to improve the treatment workflow. Patient prepositioning utilizes the stereoscopic and thermal camera system to match the patient surface to the surface as defined in the patient treatment plan. Prior to beam delivery, ExacTrac X-ray images can be used to perform a 6DoF shift of the patient positioning system. During beam delivery, ExacTrac Dynamic offers gated submillimetric patient monitoring using integrated surface and thermal imaging with X-ray verification available at appropriate gantry angles. In addition, ExacTrac® Dynamic can be used to monitor the position of implanted markers.
In a recent update, ExacTrac® Dynamic Version 1.1, a deep inspiration breath hold (DIBH) protocol, was implemented, coupling breath hold positioning with internal anatomy verification using stereoscopic X-rays. This workflow requires the acquisition of free breathing and DIBH planning computed tomography (CT) datasets and contours. The free-breathing external contour is used in conjunction with the SGRT system to position the patient. The user can then set a point on the surface to generate a breathing trace. Respiration and the patient surface can then be monitored tracked relative to the DIBH external contour. Beam gating is performed using information from the surface camera, with corrections performed using X-ray verification. The system can also supply visual feedback on the breath hold to the patient.
This paper describes the procedures undertaken to commission ExacTrac® Dynamic for use in stereotactic radiotherapy in our department and considerations and challenges encountered during the commissioning process, with a discussion of a suitable quality assurance program for ExacTrac® Dynamic.
MATERIALS AND METHODS
ExacTrac® Dynamic includes several subsystems that need to be commissioned, including the room-mounted pair of X-ray tubes (Varex G-892) and flat panel amorphous silicon detectors (Varian 3030DX), the camera system consisting of the light projector, stereoscopic cameras and thermal camera, the ExacTrac software, associated peripherals, and patient immobilization devices. In addition, ExacTrac® Dynamic's integration with other systems available on the linac needs to be assessed. The linac is an Elekta Versa HD with onboard CBCT imaging (XVI), an electronic portal imaging device (iView), and a Hexapod 6DoF couch. The Hexapod hardware includes an independent stereoscopic infrared camera and a reference frame with infrared markers that attaches to the couch. Associated software includes the Integrity linac control system, Mosaiq Oncology Information System (including record and verify capability), Pinnacle 16.2.1 treatment planning system, and iGuide Hexapod control software.
ExacTrac® Dynamic was commissioned for SRS and SABR treatments with tolerances suitable for stereotactic treatments. Published protocols were reviewed to aid in designing a commissioning plan and associated quality assurance procedures,[1,3,4,5,6,7,8] though they do not directly address a system with all of the functionality of ExacTrac® Dynamic. During acceptance testing, the interconnectivity and functionality of the ExacTrac® Dynamic workflow was assessed. In addition, a Function Checklist (as supplied by Brainlab) was completed. This checklist covers some of the functionality to be assessed in ExacTrac® Dynamic, and related tests can be expanded during commissioning. Calibrations were performed for the correlation between the thermal and 3D camera, X-ray correction images for both panels, and isocenter calibrations between the surface tracking system, the X-ray system, and the linac isocenter. Calibrations are performed with a thermal phantom and a system calibration phantom. The thermal phantom has features that are visible to both the 3D camera and thermal camera. The system calibration phantom has correlated surface and internal features and is also used as part of mandatory daily QA as designed by the vendor.
Commercial phantoms used as part of commissioning included anthropomorphic Brainlab head and pelvis phantoms, a uniform grid phantom for scaling, a Leeds phantom to assess image quality, a ball bearing phantom (Bill Ball Bearing, Elekta), the spherical Lucy® 3D QA Phantom (Standard Imaging) with a detector bore suitable for a diode, and CIRS plastic water block phantoms. Foundation makeup (BYS All Day Wear Foundation, Deep Tan and Ivory, BYS) was used to simulate skin tone on anthropomorphic phantoms, and was applied on top of micropore tape for ease of application and removal. Foam wedges were used for mounting and aligning phantoms with particular X-ray tubes/imagers, with ExacTrac X-ray images used for positioning. Detectors used include the RaySafe X2 system with sensors suitable for dosimetric measurements for the X-ray tubes, a survey meter for the radiation survey, and a PTW 60016 Diode P for megavoltage dose measurements.
ExacTrac® Dynamic includes workflows for implanted markers and DIBH. These workflows are intended to be used to compensate for different types of patient motion. The implanted marker workflow is used for treatments such as prostate, where the target volume can move internally with respect to the surrounding bony anatomy. The DIBH workflow is used for cases such as breast treatments where the target moves with the breathing cycle. Due to the dynamic nature of these treatments, the commissioning of these workflows requires the use of specialized phantoms that can accurately model patient motion. In particular, verification of the DIBH workflow requires a phantom that has an approximation of the patient surface and can simulate a breath cycle (in both free breathing and breath hold) that can be controlled remotely from the console room. Breath holds must be performed at a couch angle of 0°.
An in-house phantom was assembled for commissioning the DIBH workflow. The DIBH phantom used a thermoplastic sheet that was heat molded to approximate a human torso. Plastic ribs were attached to provide contrast details suitable for registration in ExacTrac. Two linear actuators were used to move the phantom vertically on the treatment couch, simulating the patient's motion during a breathing cycle. The linear actuators were constructed from 3D-printed plastic components and commercially available stepper motors driven by a Raspberry Pi single-board computer that could be controlled in the bunker room via Bluetooth or remotely in the console room via an Ethernet cable. The stepper motors in the DIBH phantom were controlled with a simple python script using the RpiMotorLib library (https://github.com/gavinlyonsrepo/RpiMotorLib). In the prototype version, only linear shifts were enabled. The interface can be easily extended to more complicated motions including arbitrary breathing waveforms. The script was deployed as a WebApp using the flask library (https://flask.palletsprojects.com/en/2.2.x/) which enabled the phantom to be controlled using a mobile phone from within the bunker or from the control room via a wifi extender. Scripting in Python was used to either simulate a full breathing cycle or to move the phantom-specific shifts in the vertical direction with submillimeter precision. The code for control of this phantom is found in Figure 1 and the phantom is illustrated in Figure 2.
Figure 1.
Python code to control the motors on the custom-made DIBH phantom. DIBH: Deep inspiration breath hold
Figure 2.
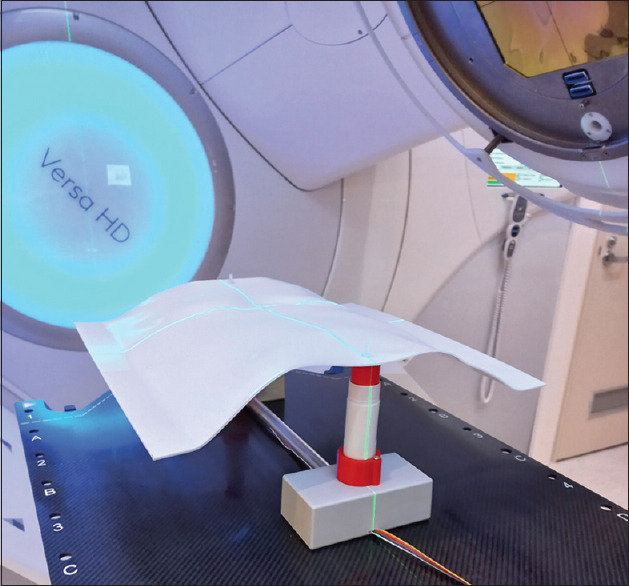
In-house phantom for SGRT molded to approximate the human torso. One of the programmable linear actuators is visible. SGRT: Surface-guided radiotherapy
For seed tracking, a wax block containing three seeds was positioned beneath the surface of the phantom. In addition to breath hold with X-ray monitoring and implanted markers, ExacTrac® Dynamic has implemented three other workflows. The standard X-ray workflow bases positioning on bony anatomy in ExacTrac X-ray images, with ExacTrac surface and X-ray available for monitoring during treatment. The external positioning with X-ray monitoring workflow uses an external system (such as CBCT) to position the patient with monitoring based on ExacTrac and reference acquisition based on X-ray bony anatomy. The external positioning with surface monitoring workflow uses an external system (such as CBCT) to position the patient with monitoring based on ExacTrac and reference acquisition based on surface position. Tests should be designed to test all functionality in each workflow to be commissioned for clinical use.
Commissioning tests can be grouped into several categories: Safety, isocenter calibration, dosimetry, image quality, data transfer, SGRT stability, SGRT localization, gating, fusion, implanted markers, breath hold, and end-to-end testing. A summary of the tests is found in Table 1.
Table 1.
Summary of tests performed as part of commissioning
| Category | Test | Method |
|---|---|---|
| Safety | Interlock functionality | Checked door, beam hold, beam inhibit functionality |
| Radiation warning lights | Checked radiation warning light functionality | |
| Radiation survey | Leakage survey for each tube | |
| Isoleft calibration | Repeatability | Checked that SGRT and X-ray calibration are consistent with MV isocenter through repeated hidden target type tests and tests using a ball bearing phantom |
| Reproducibility | Using independent setup, checked that SGRT and X-ray calibration were consistent with MV isocenter through repeated hidden target type tests and tests using a ball bearing phantom over multiple days | |
| Dosimetry | kVp accuracy | For each tube, with the detector in a perpendicular setup, measure kVp over a range of 50 kVp to 150 kVp |
| Timer accuracy | For each tube, measure the exposure time for five deliveries | |
| mAs linearity | For each tube, with the detector in a perpendicular setup, measure the dose for a range of mAs values from 1 to 65 | |
| HVL | For each tube, with the detector in a perpendicular setup, measure the HVL using the appropriate sensor | |
| Imaging dose | Position detector on the center of imaging panel. Affix the kV detector on the imaging panel surface and position at the center of the image. Record a series of exposure with kVp values from 40 kVp to 140 kVp for mAs settings of 12 and 32. Use a fit from a power law function to estimate dose area product | |
| Dose reproducibility | For each tube, with the detector in a perpendicular setup, measure a set of 20 mAs, 70 kVp exposures | |
| Couch transmission | Position detector on the center of imaging panel. Record imaging dose values for a series of kVp values with and without the couch positioned in the beam. Assess the potential effect of the couch on image quality | |
| Image quality | Geometric accuracy - scaling | Align the grid phantom to be parallel to the imaging plane. Measure from phantom features |
| Spatial resolution | Align the Leeds phantom to be parallel to the imaging plane for each panel. Measure from phantom features | |
| Contrast | As above | |
| Gantry angle limitations for imaging | Assess gantry angle ranges where stereoscopic and monoscopic X-ray images are available | |
| Data transfer | Patient orientation consistency | Export plans of anthropomorphic phantoms in each patient orientation and confirm consistency when using ExacTrac prepositioning and X-ray imaging |
| Data transfer integrity | Integrated into acceptance testing, transfer patient data from TPS including external contour information. Consistency of transferred data is checked along with the contour generated from the patient-external contour. Check that contoured OARs are correctly aligned with contours in the images. Process is checked with an anthropomorphic head phantom. Check that correct 6DoF shifts are sent to iGuide | |
| Coordinate system conventions | Confirmed that coordinate system follows manufacturer conventions and that coordinate transfers to iGuide and/or couch are consistent | |
| SGRT stability | SGRT stability with warm-ups | Use an anthropomorphic phantom to check the stability of SGRT as soon as possible after powering on system |
| SGRT stability with drift | Use anthropomorphic phantom to check the stability of SGRT after X-ray acquisition | |
| SGRT localization | SGRT field of view | Assess field of view limits with a phantom |
| SGRT translational and rotational accuracy | Use iGuide (Hexapod 6DoF couch) with an anthropomorphic phantom to induce translational and rotational shifts of known values and compare to the shift reported by SGRT | |
| Temporal accuracy (latency) | With a remote-controlled phantom, use motion with shifts with a set duration to trigger surface error detection. Check the duration of the motion is consistent, and that surface error is triggered as expected | |
| Effect of skin tone on surface tracking | Use an anthropomorphic phantom coated with foundation makeup representing various skin tones. Apply shifts of known magnitude and compare to the shift reported by the SGRT for each available skin tone preset | |
| Effect of the thermal camera on surface tracking | Using a phantom with a heating element, use the couch to move the phantom throughout the range of the camera system, looking for inconsistencies in surface tracking relative to couch position. Repeat this with and without heating | |
| Gating | Beam hold triggered by surface movement | With a remote-controlled phantom, use motion to trigger the surface error detection and check consistency with known motion |
| Beam hold triggered by internal anatomy movement | With a remote-controlled phantom, use motion and use planned stereoscopic checks to trigger the X-ray-based error detection and check consistency with known motion | |
| Beam hold at set MU/gantry angle | Check that the planned beam hold occurs at the set MU for static beams. Check that planned beam holds occur at the correct gantry angles for arcs | |
| Beam hold during image fusion calculation | Confirm that beam hold occurs during image fusion calculation if and only if this setting is selected | |
| Beam hold impact on dose | For each beam energy, deliver a conformal arc plan to the Lucy® phantom with a diode. Repeat the measurement with beam holds enabled | |
| Surface out-of-tolerance action delay | Check that the action delay set for automatic beam hold runs for the set time (0s to 5s) | |
| Implanted markers | Implanted marker definition | For a phantom with implanted markers, use the ExacTrac implanted marker definition page to define markers and check the functionality of tools, including automatic marker detection |
| Consistency of implanted marker position | Use ExacTrac to move the implanted marker phantom to a set position. Acquire a CBCT and check that the marker positions match those in the treatment plan after the shift. Repeat at two different couch angles | |
| Implanted marker workflow | Using a moveable phantom with implanted markers, use the implanted marker workflow to check the functionality and consistency of workflow features such as beam holds after the phantom offset | |
| Breath hold | Patient feedback system | Check that the patient feedback system can be mounted on the couch and adjusted |
| Respiratory point and breathing baseline | Using the custom respiratory phantom, define a respiratory point and check the quality and amplitude of the breath trace versus the set motion. Simulate free breathing to calculate a baseline | |
| DIBH gating window | Using the custom respiratory phantom, check that the beam activates when the phantom is within the gating window. Check that the beam is held if the patient leaves the gating window. Check for DIBH gating window settings between 0.5 mm and 3.0 mm | |
| Breath-hold workflow | Using the custom respiratory phantom, simulate all steps of the treatment workflow such as acquiring free breathing and breath hold planning CTs, entering breath hold and associated beam on, X-ray acquisition if surface out of tolerance, and patient shifts to correct movement | |
| Consistency of position | Check that the target position in respiratory phantom is in the expected location using CBCT | |
| Fusion | Fusion reproducibility | Set up anthropomorphic phantoms (head and pelvis) in various offset positions ranging around 5 mm translations and 2° rotations. Capture X-ray and use ExacTrac to complete bony fusion. Reset and repeat fusion 5 times. Repeat for 2 couch angles. Repeat test across multiple users to confirm that shifted phantom is in a reproducible position |
| Workflows | Workflow functionality | Check functionality of all settings available in workflows that will be used clinically. Many of these tests can be performed simultaneously with other tests in this table |
| X-ray verification triggers | Check that stereoscopic, and monoscopic X-ray verifications are acquired automatically if and only if consistent with settings in the workflow. Check that MU triggered X-ray verifications function as expected | |
| End-to-end | Test patient fraction | Set up an anatomical phantom with random offsets in all translational and rotational directions. Use stereoscopic imaging to acquire a bony fusion and then correct the phantom position. Verify position by identifying the location of a ball bearing in the phantom. This should be done for each workflow if possible |
CT: Computed tomography, SGRT: Surface-guided radiotherapy, DoF: Degree of freedom, CBCT: Cone-beam CT, DIBH: Deep inspiration breath hold, HVL: Half value layer, MV: Megavoltage, MU: Monitor units
Safety tests involve checking the functionality of interlocks and safety features of the unit as an integrated whole. Radiation leakage checks should be performed using a survey meter for each tube. Isocenter calibration checks confirm that the isocenter calibration of ExacTrac® Dynamic is stable for both repeated measurements and over longer periods of time. Dosimetry tests were performed independently for each tube using the RaySafe X2 and image quality tests were mostly performed with a Leeds phantom or grid phantom. Phantoms need to be aligned with the imaging plane, either by using foam holders or by setting them up in jigs on the surface of each panel.[9] For dosimetry tests, the R/F probe of the RaySafe X2 was positioned at the center of each imaging panel as determined in the images. Data transfer testing is important to confirm the integrity of data throughout the ExacTrac® Dynamic workflow. Coordinate system testing is particularly important as there are multiple coordinate systems interacting on this machine, including some with 6DoF. Coordinate systems include those from ExacTrac, the linac (including the couch), the record and verify system, XVI, and iGuide/Hexapod.
The stability of the SGRT system was assessed by placing an anthropomorphic phantom, acquiring an X-ray to generate a reference image for SGRT, and monitoring for any changes over time. This was done immediately after powering on the system to assess warm-up effects, and also during routine use to assess any drift over time. Translational and rotational accuracy of surface tracking was assessed by using SGRT to shift the anthropomorphic phantom from various start positions, imaging with stereoscopic X-rays, and fusing to a phantom reference image. To assess the reproducibility of the fusion, this was repeated 5 times for each position. This was also repeated for two different couch angles to assess any uncertainty introduced by noncoplanar orientations as reported in the literature.[10] Temporal accuracy was assessed by setting the phantom to move to an offset position for set time periods of 1 s, 2 s, and 5 s. The duration of the offset in ExacTrac was then compared to the set duration. In addition, the triggering of surface error tolerances on movement was checked.
Skin tone can impact the image and reconstruction quality of the SGRT 3D camera system as darker skin tones tend to absorb more light. The assessment was performed using the Brainlab head phantom with the region used for SGRT coated with foundation makeup representing various skin tones. Shifts of known magnitudes were applied to the phantom using the Hexapod couch, and the accuracy of the SGRT match was assessed. The phantom setup is shown in Figure 3.
Figure 3.
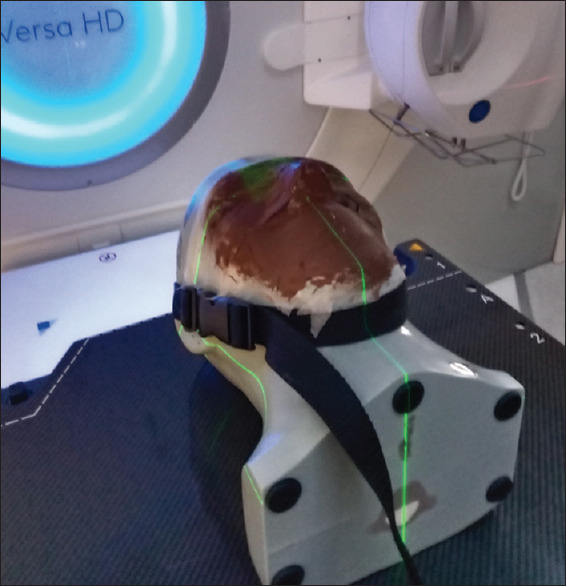
Brainlab head phantom with deep tan foundation makeup applied. The region used for SGRT is restricted to the area with foundation applied. SGRT: Surface-guided radiotherapy
To investigate the thermal camera, a simple rectangular plastic water phantom was set up with an edge aligned to the couch. An SGRT region of interest was selected that would allow tracking the motion of the phantom surface with minimal lateral sensitivity due to the uniform phantom surface. A heating element was set up under the top layer of the plastic water phantom so thermal information would be encoded in the image. After this was done, lateral shifts were applied to assess the accuracy of the SGRT system as indicated by thermal data alone. An illustration of this setup is found in Figure 4.
Figure 4.
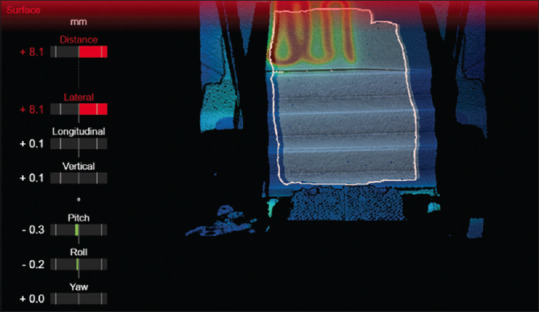
ExacTrac Dynamic surface guidance image of a simple plastic water phantom with a heating element
Gating tests used a Quasar Lung Motion Phantom and the drive system of one-dimensional water tank connected to a platform, allowing remote-controlled motion in 2 dimensions. This phantom is illustrated in Figure 5. A plastic water block phantom or pelvis anatomical phantom was set up on the platform, and treatment was delivered with intrafraction monitoring using ExacTrac® Dynamic. Motions were performed to check that SGRT deviations of more than the set tolerance would then trigger a beam hold. This was used to check that tolerances were applied successfully for each direction of motion and for distances not along each axis (root mean square). A similar test was performed to confirm that tolerances for motion in X-ray images would also trigger a beam hold. Triggering from SGRT was avoided by setting the SGRT tolerance to 5 mm with a tolerance of 1 mm used for the stereoscopic X-rays. In addition, beam holds for set MU for static beams, and beam holds for set gantry angles for arcs were tested. The impact of beam holds on dose delivery was assessed using the Lucy® phantom with a photon diode positioned at the center of the phantom. A conformal arc treatment was delivered to the phantom with intrafraction imaging (stereoscopic and monoscopic), and beam holds were enabled. The phantom was then irradiated using the same plan but with no intrafraction imaging or beam holds. The accumulated charge from the diode was then compared. This test was completed for each available beam energy.
Figure 5.

Phantom setup consisting of a Quasar lung motion phantom and a 1D water tank drive allowing remote movement of a phantom in two dimensions
The repeatability of fusions was checked using phantom studies. Brainlab head and pelvis phantoms placed at the isocenter were displaced with known shifts in all three directions. A stereoscopic image was captured, and automatic bony fusion was performed with shifts recorded. Fusions were repeated five times and at two different couch angles.
A test patient fraction was performed using the pelvis phantom setup with a random offset in all translations and rotations. Stereoscopic images were taken, and ExacTrac was used to perform an automatic bony fusion. Fusion results were recorded, the phantom was shifted, and an X-ray sphere detection was performed to check the consistency of the positioning of an internal ball bearing.
RESULTS
Table 2 summarizes the results from the commissioning tests. All values were found to be within set tolerances. Basic functional and imaging parameters for the ExacTrac® Dynamic system were verified using commercially available phantoms and software as part of the implementation checks. The ExacTrac system shows a consistent and stable dose, kVp, high contrast spatial resolution, contrast-to-noise ratio (CNR), and geometric accuracy for both the X-ray tubes. The mAs linearity test is calculated using all pairs of air kerma Kn and current time product measurements Qn where the condition 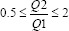 holds. Couch transmission was found to be at least 96% of the delivery with no couch present, which was deemed to have an acceptable impact on image quality. No significant difference was observed between the two tubes. The SGRT system was found to be stable and exhibit a minimal warm-up effect, with maximal translational and rotational differences of 0.3 mm and 0.4°, respectively. Tests including changes in skin tone and the thermal camera indicated similar stability. Data integrity was maintained throughout the workflow.
holds. Couch transmission was found to be at least 96% of the delivery with no couch present, which was deemed to have an acceptable impact on image quality. No significant difference was observed between the two tubes. The SGRT system was found to be stable and exhibit a minimal warm-up effect, with maximal translational and rotational differences of 0.3 mm and 0.4°, respectively. Tests including changes in skin tone and the thermal camera indicated similar stability. Data integrity was maintained throughout the workflow.
Table 2.
Summary of results tests performed as part of commissioning
| Category | Test | Result | Tolerance |
|---|---|---|---|
| Safety | Interlock functionality | Functional | Functionala |
| Radiation warning lights | Functional | Functionala | |
| Radiation survey | |||
| Isocenter calibration | Repeatability | ||
| Reproducibility | |||
| Dosimetry | kVp accuracy | Maximum deviation+2.1% | ±5% of nominal value |
| Timer accuracy | Maximum deviation−0.7% | ±5%a | |
| mAs linearity |
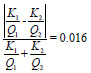
|
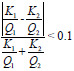
|
|
| HVL | 2.4mm Al @50 kVp 4.9 mm Al @100 kVp 6.6 mm Al @150 kVp | Legislated limitsa | |
| Imaging dose | Baseline | Baseline | |
| Dose reproducibility | Coefficient of variation=0.001 | Coefficient of variation <0.05a | |
| Couch transmission | >96% for 50 kVp and over | in house | |
| Image quality | Geometric accuracy - scaling | In tolerance | ≤1 mm2 |
| Spatial resolution | MTF (80)=0.819 lp/mm | Baselineb | |
| Contrast | Minimum CNR=8.916 | Baselineb | |
| Gantry angle limitations for imaging | As per specification | As per specification | |
| Data transfer | Patient orientation consistency | Consistent | Consistent |
| Data transfer integrity | Consistent | Consistent, organs at risk within 0.5 mm of expected positionc | |
| Coordinate system conventions | Consistent | Consistent | |
| SGRT stability | SGRT stability with warm-up | In tolerance | ≤1 mm after stabilizing, Stabilization time consistent with clinical used |
| SGRT stability with drift | In tolerance | ≤1 mm over 5 mind | |
| SGRT localization | SGRT field of view | Baseline | Baselinee |
| SGRT translational and rotational accuracy | Maximum translational deviation=0.3 mm Maximum rotational deviation=0.4° | Translational deviation ≤0.8 mm, rotational deviation ≤0.5°c | |
| Temporal accuracy (latency) | In tolerance | ≤100 mse | |
| Effect of skin tone on surface tracking | In tolerance | Translational deviation ≤0.8 mm, rotational deviation ≤0.5°c | |
| Effect of the thermal camera on surface tracking | In tolerance | Translational deviation ≤0.8 mm, rotational deviation ≤0.5°c | |
| Gating | Beam hold triggered by surface movement | Triggered within 0.1 mm of tolerance | As per specified tolerance |
| Beam hold triggered by internal anatomy movement | Triggered within 0.1 mm of tolerance | As per specified tolerance | |
| Beam hold at set MU/gantry angle | Maximum MU deviation of 21 MU for 6XFFF beam. Gantry angles within 0.5 | As per specification | |
| Beam hold impact on dose | Maximum deviation of 0.18% | Within 1%f | |
| Beam hold during image fusion calculation | Functional | Functional | |
| Surface out-of-tolerance action delay | |||
| Fusion | Fusion reproducibility | Maximum SD=0.08 mm | ≤0.5 mm3 |
| Implanted markers | Implanted marker definition | Functional | Functional |
| Consistency of implanted marker position | In tolerance | ≤0.5 mm and ≤0.5°c | |
| Implanted marker workflow | All functionality | All functionality | |
| Breath hold | Patient feedback system | Functional | Functional |
| Respiratory point and breathing baseline | In tolerance | ≤1 mm3 | |
| DIBH gating window | As per scale | As per scale | |
| Breath-hold workflow | All functionality | All functionality | |
| Consistency of position | In tolerance | ≤1 mm3 | |
| Workflows end-to-end | Workflow functionality Test patient fraction | All functionality Successful | All functionality Successful |
Key for tolerances: aLocal radiation safety legislation, aTG-142, cTolerances adapted from the essential performance characteristics supplied by the vendor. In general, tolerances are set to half of the values specified in the essential performance characteristics, dFrom TG-147, with modification due to SGRT being used in conjunction with stereoscopic X-rays, eTG-147, and ein-house tolerance. SD: Standard deviation, SGRT: Surface-guided radiotherapy, DIBH: Deep inspiration breath hold, HVL: Half value layer, MU: Monitor units, MTF: Modulation transfer function, CNR: Contrast-noise-ratio
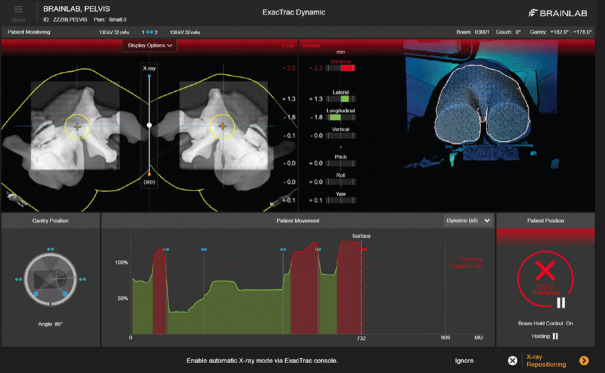
Figure 6 contains an example image of the ExacTrac workflow performed with the Brainlab pelvis phantom. The Brainlab pelvis phantom was moved remotely, triggering stereoscopic X-rays. This test allows investigation of the workflow while simultaneously confirming the coincidence between the SGRT, X-ray, and phantom motion.
Figure 6.
ExacTrac display while remotely moving the Brainlab pelvis phantom using the 2D motion platform
DISCUSSION
ExacTrac® Dynamic is a powerful tool for accurate and precise IGRT. As this system allows a variety of treatment workflows and techniques, it can be useful to limit the initial clinical implementation for a particular site and technique, with a gradual introduction of other treatment sites and workflows once the clinical department gains confidence and aptitude in the use of ExacTrac® Dynamic. The multidisciplinary team should be involved in decisions about the implementation of workflows taking into account clinical demand, departmental resources, and training. Training should cover all aspects of the workflows to be implemented for clinical use. Clear documentation on workflows, including staff responsibilities, should be maintained. It is important that the entire patient workflow is considered, as ExacTrac® Dynamic features require treatment plans to have specific features. Quality assurance should be adapted to the clinical implementation of the system.[11]
In order to maintain the submillimetric accuracy of IGRT using ExacTrac® Dynamic, quality assurance of the system needs to be more rigorous and have more restrictive tolerances when compared to onboard CBCT systems. ExacTrac® Dynamic implements a mandatory daily check sequence which involves positioning the system calibration phantom at the isocenter to check the consistency between the phantom position as indicated by the surface camera and the phantom position as indicated by ExacTrac X-ray. The system then uses Hexapod to move the phantom to the radiation isocenter, which needs to be confirmed by the user.
A more rigorous daily test can be designed using a commercial or custom-made phantom containing internal geometry and a ball bearing. An imaging sequence can be created to move the phantom to a known initial position, use IGRT to move the phantom, so the ball bearing is at the isocenter, and then confirm the coincidence of the radiation isocenter as determined by a Winston-Lutz type test with the isocenter as indicated by IGRT. This test can integrate tests of ExacTrac IGRT, Hexapod, and other IGRT systems such as CBCT.
It is mandatory to complete a thermal image to 3D camera calibration once a month using the ExacTrac calibration procedure. In addition, X-ray correction image calibration (flood fields), isocenter calibration (coincidence between X-ray and surface camera), and isocenter radiation calibrations (coincidence between X-ray and radiation isocenter) can be performed periodically. As a rigorous daily quality assurance procedure is used, any drifts can be monitored and calibrations arranged before there is a clinical issue. In addition, the validity of calibrations should be carefully assessed after upgrades, repairs, collisions, or any other changes or incidents that could affect the system setup.
For monthly quality assurance, a series of tests and inspections can be used based on vendor recommendations and clinical requirements. An anthropomorphic phantom such as the Brainlab head verification phantom can be prepositioned using the SGRT system, and an ExacTrac image can be acquired for fusion. The functionality of the fusion tools and the image quality can then be assessed. A visual inspection of all equipment, labeling, accessories, cables, and connectors should be performed. A dose area product test for a subset of tube potentials can be checked against baseline values. A test of the residual current circuit breaker should also be performed.
Annual quality assurance checks can be performed in conjunction with vendor-authorized support staff to check the functionality of the computer cabinet, emergency stops, interlocks, redundancy of signal cabling, X-ray on indication, and other scheduled maintenance. In addition, the accuracy of the SGRT system can be assessed by moving a suitable surface to a variety of known positions and comparing to the reported shifts. Similarly, the accuracy of the ExacTrac X-ray system can be assessed by moving a suitable phantom to various known offsets. Detailed testing should also be performed after repairs or upgrades, focusing on functionality that might be affected by the situation.
Limitations of ExacTrac® Dynamic should be considered carefully prior to clinical implementation. The 3D surface cameras and thermal cameras should be used on the patient's surface and the area to be monitored needs to have enough features to enable accurate tracking. Ideally, surface tracking should be in areas where motion is correlated well with target volumes and critical OAR. Open-faced mask designs can be helpful with this but must also be commissioned to ensure sufficient immobilization and suitable dosimetric properties. X-ray images are either monoscopic or stereoscopic planar images and do not contain as much information as CBCT images. Monoscopic images are particularly limited as no information in one dimension is available. Care should also be used with stereoscopic images, as any deformations in the patient's bony anatomy may affect the accuracy of the shift. The effect of placement uncertainty of bolus, particularly higher density bolus, should also be considered.
Recommendations for commissioning tests and tolerances were based on several documents, including AAPM Report No. 104: The role of in-room kV X-ray imaging for patient setup and target localization, Task Group 142 report: Quality assurance of medical accelerators, Report of Task Group 147: Quality assurance for nonradiographic radiotherapy localization and positioning systems, and Report of AAPM TG-179: Quality assurance for image-guided radiation therapy utilizing CT-based technologies.[1,3,4,5,6,7] Tests and tolerances needed to be modified to suit ExacTrac® Dynamic due to the features available in this system which were unavailable when these documents were published, such as surface tracking, intrafraction monitoring, and breath-hold workflows. Surface tracking tests in TG-147 were modified as ExacTrac is designed to take the surface at the time of X-ray imaging as a baseline, so tests of stability over extended timeframes are not required. Test tolerances were also designed around the essential performance characteristics of ExacTrac Dynamic as supplied by the vendor. In general, tolerances were set to values equating to half the values specified in the essential performance characteristics, allowing a margin when multiple independent sources of uncertainty are combined in quadrature. A recent paper by Bry et al. performed end-to-end tests on a similar system combining stereoscopic X-rays and SGRT and was able to obtain results within similar tolerances.[12] A paper by Swinnen et al. demonstrated a high degree of fidelity between SGRT and CBCT imaging systems.[13] There are several published quantitative analyses of patient setup uncertainty using the ExacTrac stereoscopic X-ray system.[14,15,16,17] The tests and tolerances in this report are intended to be a suggestion for departments commissioning ExacTrac® Dynamic for clinical use.
CONCLUSION
This paper summarizes recommended testing for commissioning of the ExacTrac® Dynamic system. As there are multiple customizable workflows available, it is recommended that commissioning be based on the workflows to be adopted into clinical practice. This may be an iterative process, as particular workflow settings may be more efficient or accurate in a particular clinical setting. Different workflows can be introduced over time as the multidisciplinary team gains confidence in the advantages and limitations of ExacTrac® Dynamic. It should be emphasized that commissioning involves the entirety of the multidisciplinary team, and that quality assurance, training, and documentation are vital to ensure quality and safety for clinical use of ExacTrac® Dynamic.
ExacTrac® Dynamic introduces a unique combination of surface guidance, thermal guidance, and stereoscopic X-ray image guidance that can be integrated into pretreatment and intrafraction imaging. As such, commissioning tests and tolerances should be designed to provide an adequate specification of this combination of system features.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Klein EE, Hanley J, Bayouth J, Yin FF, Simon W, Dresser S, et al. Task group 142 report: Quality assurance of medical accelerators. Med Phys. 2009;36:4197–212. doi: 10.1118/1.3190392. [DOI] [PubMed] [Google Scholar]
- 2.Graulieres E, Kubler S, Martin E, Ferrand R. Positioning accuracy of a single-isocenter multiple targets SRS treatment: A comparison between Varian Truebeam CBCT and Brainlab ExacTrac. Phys Med. 2020;80:267–73. doi: 10.1016/j.ejmp.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Yin FF, Wong J, Balter J, Benedict S, Bissonnette JP, Craig T, et al. Virginia, United States: Therapy Imaging Committee American Association of Physicists in Medicine; 2009. The role of in-Room KV Imaging for Patient Setup and Target Localisation: Report of Task Group 104; pp. 1–62. [Google Scholar]
- 4.Willoughby T, Lehmann J, Bencomo JA, Jani SK, Santanam L, Sethi A, et al. Quality assurance for nonradiographic radiotherapy localization and positioning systems: Report of task group 147. Med Phys. 2012;39:1728–47. doi: 10.1118/1.3681967. [DOI] [PubMed] [Google Scholar]
- 5.Bissonnette JP, Balter PA, Dong L, Langen KM, Lovelock DM, Miften M, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: A report of the AAPM TG-179. Med Phys. 2012;39:1946–63. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hallaq HA, Cerviño L, Gutierrez AN, Havnen-Smith A, Higgins SA, Kügele M, et al. AAPM task group report 302: Surface-guided radiotherapy. Med Phys. 2022;49:e82–112. doi: 10.1002/mp.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 8.Foote M, Bailey M, Smith L, Siva S, Hegi-Johnson F, Seeley A, et al. Guidelines for safe practice of stereotactic body (ablative) radiation therapy. J Med Imaging Radiat Oncol. 2015;59:646–53. doi: 10.1111/1754-9485.12336. [DOI] [PubMed] [Google Scholar]
- 9.Stanley DN, Papanikolaou N, Gutiérrez AN. Development of image quality assurance measures of the ExacTrac localization system using commercially available image evaluation software and hardware for image-guided radiotherapy. J Appl Clin Med Phys. 2014;15:4877. doi: 10.1120/jacmp.v15i6.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covington EL, Fiveash JB, Wu X, Brezovich I, Willey CD, Riley K, et al. Optical surface guidance for submillimeter monitoring of patient position during frameless stereotactic radiotherapy. J Appl Clin Med Phys. 2019;20:91–8. doi: 10.1002/acm2.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freislederer P, Kügele M, Öllers M, Swinnen A, Sauer TO, Bert C, et al. Correction to: Recent advances in surface guided radiation therapy. Radiat Oncol. 2020;15:244. doi: 10.1186/s13014-020-01661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bry V, Saenz D, Pappas E, Kalaitzakis G, Papanikolaou N, Rasmussen K. End to end comparison of surface-guided imaging versus stereoscopic X-rays for the SRS treatment of multiple metastases with a single isocenter using 3D anthropomorphic gel phantoms. J Appl Clin Med Phys. 2022;23:e13576. doi: 10.1002/acm2.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swinnen AC, Öllers MC, Loon Ong C, Verhaegen F. The potential of an optical surface tracking system in non-coplanar single isocenter treatments of multiple brain metastases. J Appl Clin Med Phys. 2020;21:63–72. doi: 10.1002/acm2.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling V, Hossain S, Jin H, Algan O, Ahmad S, Ali I. Quantitative evaluation of patient setup uncertainty of stereotactic radiotherapy with the frameless 6D ExacTrac system using statistical modeling. J Appl Clin Med Phys. 2016;17:111–27. doi: 10.1120/jacmp.v17i3.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SA, Yea JW, Kang MK, Park JW, Kim SK. Analysis of the setup uncertainty and margin of the daily ExacTrac 6D image guide system for patients with brain tumors. PLoS One. 2016;11:e0151709. doi: 10.1371/journal.pone.0151709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BC, Snyder WJ, Kim S, Kim T. Monitoring frequency of intra-fraction patient motion using the ExacTrac system for LINAC-based SRS treatments. J Appl Clin Med Phys. 2018;19:58–63. doi: 10.1002/acm2.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarnavski N, Engelholm SA, Af Rosenschold PM. Fast intra-fractional image-guidance with 6D positioning correction reduces delivery uncertainty for stereotactic radiosurgery and radiotherapy. J Radiosurg SBRT. 2016;4:15–20. [PMC free article] [PubMed] [Google Scholar]