Abstract
Manganese (Mn) plays a complex role in the survival of pathogenic and symbiotic bacteria in eukaryotic hosts and is also important for free-living bacteria to thrive in stressful environments. This review summarizes new aspects of regulatory strategies to control intracellular Mn levels and gives an overview of several newly identified families of bacterial Mn transporters. Recent illustrative examples of advances in quantification of intracellular Mn pools and characterization of the effects of Mn perturbations are highlighted. These discoveries help define mechanisms of Mn selectivity and toxicity and could enable new strategies to combat pathogenic bacteria and promote growth of desirable bacteria.
Keywords: Manganese, Mn exporters, Mn importers, Metal homeostasis, Bacteria, MntR, yybP-ykoY riboswitch
Introduction
Transition metals (manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn)) are essential nutrients for bacteria, yet these metals can also be toxic in excess. The mammalian immune system takes advantage of this vulnerability and withholds critical metals, including Mn, Fe, and Zn, to starve invading bacteria in a process termed nutritional immunity [1–3]. In other situations, host defenses intoxicate bacteria with high concentrations of metals, notably Cu and Zn but also possibly Mn and Fe, during colonization of eukaryotic hosts [3–5].
Mn is required for the proper growth of many bacteria. Molecularly, Mn cofactors diverse enzymes involved in carbohydrate and nucleic acid metabolism, signaling, and oxidative stress resistance [6,7]. Mn also protects cells against oxidative stress, primarily by breaking down reactive oxygen species (ROS) (Box 1) [2,8]. However, despite these beneficial roles, excess Mn perturbs intracellular pools of other ions and causes mismetallation of important regulators and enzymes, sensitivity to ROS, and decreased virulence [5].
Box 1. Mn and oxidative stress.
Manganese (Mn) protects cells from oxidative stress via several distinct mechanisms [2,7,8]. Mn serves as a cofactor for enzymes that break down reactive oxygen species (ROS), mainly Mn superoxide dismutase (SOD) and Mn-cofactored catalases and peroxidases. In vitro, Mn can also nonenzymatically convert superoxide () to H2O2 and H2O2 to water when complexed with small molecule metabolites such as phosphate, bicarbonate, or certain amino acids [43–45]. Additionally, Mn has been shown to substitute for Fe in some mononuclear Fe enzymes in Escherichia coli under oxidative stress, protecting the enzymes from Fenton reaction–mediated oxidative damage while still retaining catalytic activity in the Mn-bound form [46,47]. The relative import of these different ROS protection mechanisms in different bacteria is an active area of research.
Alterations in total intracellular Mn levels can alter ROS sensitivity. For example, loss of Mn importers typically increases sensitivity of pathogenic and symbiotic bacteria to oxidative stress (as most recently shown in Refs. [42,48,49]). Interestingly, loss of Mn exporters, which leads to increased Mn levels, also affects ROS resistance, but has been shown to increase or decrease sensitivity to oxidative stress depending on the bacteria and the specific type of stress [6,20–23,25,27,28].
Therefore, to maintain an optimal Mn concentration, bacteria control intracellular Mn levels with Mn importers and exporters (Figure 1). Expression of these transporters is regulated by Mn-sensing transcription factors and riboswitches. Once inside the cell, Mn associates with various molecules, including proteins, nucleic acids, and small metabolites, in different intracellular pools [5].
Figure 1. Manganese homeostasis proteins across bacteria and their regulation by Mn.
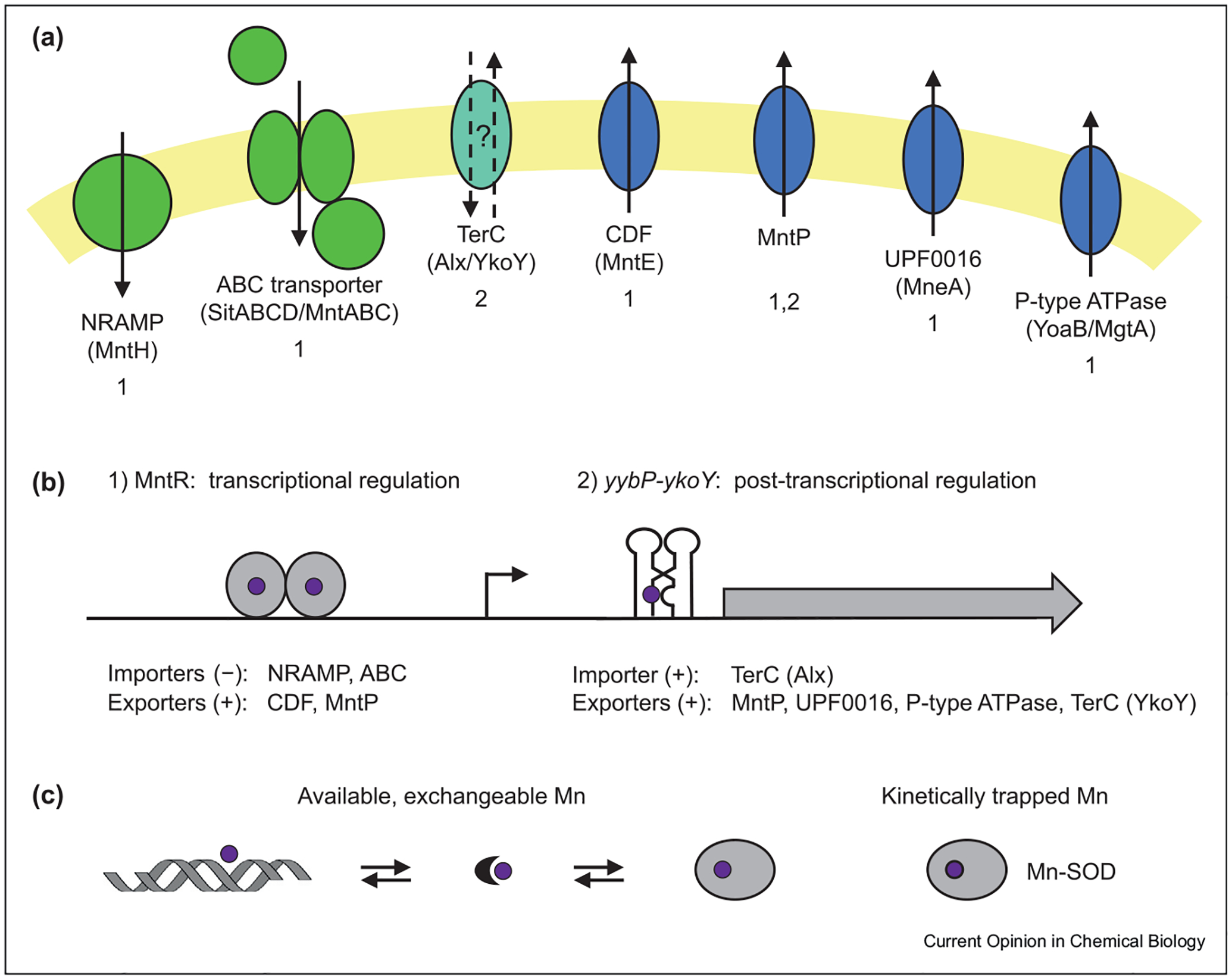
The complement of common Mn importer and exporter families in bacteria and their typical regulation by a transcription factor or a riboswitch. (a) Two major classes of Mn importers (green) are the NRAMP family, exemplified by MntH, and ABC transporters, the Mn-specific versions of which have various names in different species, commonly SitABCD in gram-negative bacteria and MntABC in gram-positive bacteria. Four major classes of exporters (blue) used to efflux Mn are the CDF, MntP, UPF0016, and P-type ATPase families, with specific protein examples as indicated. The TerC family proteins (blue-green) may function as a Mn importer or exporter depending on the organism [27,32]. Other rare Mn transporters are not shown, including the importers MntX (ArsP_2 type), MntA (P-type ATPase), and the exporter CtpC (P-type ATPase) [54–56]. The typical mechanism of regulation is indicated by a 1 or 2 below the protein names. (b) Two main mechanisms are employed to regulate gene expression of Mn transporters: (1) transcriptional regulation via MntR (or sometimes Mur [57], not shown) or (2) post-transcriptional regulation via the yybP-ykoY riboswitch. A + sign indicates upregulation, and a − sign indicates downregulation. Other metal homeostasis proteins impacted by these regulators are not shown, but often include Fe importers upregulated in various bacteria by MntR and several families of proteins of unknown function induced by the riboswitch. (c) Once inside cells, Mn (purple) is partitioned into different intracellular pools, including Mn bound to proteins, DNA and RNA, small molecule chelates (crescent), and possibly some fully hydrated ions. The total Mn quota consists of Mn ions that are weakly bound and exchangeable, as well as kinetically trapped Mn (e.g., buried in proteins such as Mn-SOD or MncA [2,52]).
This article summarizes recent findings regarding how Mn-binding regulators control gene expression, characterization of new classes of Mn exporters, the speciation and availability of intracellular Mn pools, and molecular mechanisms of Mn toxicity. Specifically, I review recent studies of (a) Mn regulators, including MntR oligomerization and the metal selectivity of the Mn-binding yybP-ykoY riboswitch; (b) newly identified Mn exporters; and (c) quantification and perturbation of intracellular Mn pools.
Regulators: Mn-binding transcription factors and riboswitches
Bacteria have two main mechanisms to regulate the expression of genes encoding Mn homeostasis proteins: Mn-binding transcription factors and a Mn-binding riboswitch (Figure 1).
The major transcriptional regulator controlling bacterial Mn homeostasis is the DtxR family protein, MntR [2,5]. In the absence of metal binding, the MntR apoprotein is found as a dimer in solution. Mn binding to MntR causes an allosteric change that allows it to bind DNA. Although noncognate metals bind MntR in vitro, they do not typically enable DNA binding or mediate MntR regulation of gene expression in vivo [2,5].
While protein-based sensors of Mn have been well characterized over the past 20 years, an RNA-based Mn sensor, the yybP-ykoY riboswitch, was only recently identified [9,10]. Found mainly in bacteria, riboswitches are RNA structures present in the 5’ untranslated regions of mRNAs. These riboregulators have two conformational states that switch upon binding a ligand to affect gene expression [11,12]. Metal-binding riboswitches have been found that respond to Mg, Ni, Co, and Mn to control expression of cognate metal transporters [13,14]. Recent work has expanded understanding of how both MntR and the yybP-ykoY riboswitch regulate expression of Mn homeostasis genes in different bacteria.
Mn-binding transcription factors
Long known as a repressor of Mn import genes, MntR has now been shown to directly activate transcription of Mn exporters in Bacillus subtilis [15]. Other recent studies explored two different aspects of MntR self-interaction that impact its regulation of gene expression. In one report, crystallization of the MntR homolog MtsR in Streptococcus pyogenes revealed contacts between dimers at the C-terminal FeoA domain [16]. The authors generated mutants that disrupted oligomerization without affecting dimerization. Using these mutants, they demonstrated that FeoA-dependent oligomerization can be important for proper regulation of gene expression in vitro and contributes to virulence in vivo [16].
Another form of MntR oligomerization was observed in the H2O2-resistant bacterium Streptococcus oligofermentans [17]. In this case, MntR monomers formed disulfide-linked dimers and higher order oligomers upon in vitro oxidation of two key Cys residues by H2O2. MntR mutants lacking the Cys residues showed fewer cross-linked complexes and stronger promoter binding in the presence of H2O2. In vivo, the mutants accumulated somewhat less Mn and survived less well upon H2O2 challenge. The authors propose that upon oxidative stress in S. oligofermentans, MntR is inactivated by oxidation and cross-linking of cysteines, allowing derepression of Mn importers and increased Mn uptake, which counteracts H2O2 toxicity [17].
It remains to be seen how widespread these oligomerization mechanisms are. The FeoA domain is not present in all MntR homologs, and the key cysteines mediating cross-linking are not conserved outside of Streptococci [16,17]. It will also be interesting to see whether these self-interactions affect all MntR-regulated genes similarly or whether they could contribute to a graded response to Mn and oxidative stress.
Mn-binding riboswitch
Initial experiments revealed that the yybP-ykoY riboswitch bound Mn at a specific site, causing a conformational change to induce gene expression [9,10]. Recent work investigated how the yybP-ykoY riboswitch binds Mn by comparing three versions of the riboswitch from the mntP, alx, and yoaB (denoted ykoY) genes [18]. The three homologs had a wide range of metal affinities. For instance, the mntP riboswitch bound Mn nearly 1000-fold more tightly than the alx riboswitch. To gain insight into the molecular mechanism of Mn selectivity, the authors crystallized three versions of the riboswitches with different Mn-binding affinities and reanalyzed the existing structure [10]. The plasticity of the metal-binding sites across the different structures suggested that ionic radius does not significantly contribute to the Mn specificity. Rather, by comparison with the structure of MntR, Mn selectivity was deduced to result from heptacoordination of the metal, which favors high-spin Mn over other metals, and the use of a softer nitrogen ligand [18].
Another study examined the yybP-ykoY riboswitch in Streptococcus pneumoniae regulating a P-type ATPase, MgtA, which was initially suggested to efflux calcium (Ca) [19]. Although the mgtA riboswitch was demonstrated to have a ~50-fold tighter binding affinity for Ca than Mn via isothermal titration calorimetry (ITC), it showed a significant preference for Mn over Ca for activation of gene expression using an in vitro transcriptional read-through assay. Supporting the functional preference for Mn, a mgtA-lacZ fusion showed strong induction by 20 μM Mn in vivo but not with 400 μM Ca or other metals [19].
Given the broad distribution of the riboswitch across bacteria in diverse ecologies, it will be important to further refine the relationship of Mn and other metals in the gene regulation imparted by the riboswitch, as well as in the activity of the gene products the riboswitch regulates, which so far have been limited to novel Mn exporters.
New families of Mn exporters
While the major classes of bacterial Mn importers have been known for some time [2,7], new families of Mn exporters are still emerging (Figure 1). The first discovered Mn exporter, the cation diffuser family (CDF) protein MntE, was identified a decade ago in S. pneumoniae [20]. Intriguingly, despite the observation that many pathogenic bacteria require Mn during infection and the fact that MntE reduces intracellular Mn levels, loss of MntE was shown to decrease virulence [20]. Subsequent studies confirmed this in other Streptococci [21,22], and recent work has shown that MntE is important for virulence in another genus, Staphylococcus aureus [23]. In addition, two other CDF family Mn exporters, MneP and MneS, have also been identified in B. subtilis by virtue of their upregulation by Mn via MntR [15].
Shortly after MntE, a second class of Mn exporter was identified, MntP [24–26]. Since the mntP gene was preceded by the yybP-ykoY riboswitch, which at the time had no known ligand, this discovery prompted the studies that demonstrated that the riboswitch bound and responded to Mn [9,10]. Like MntE, the loss of MntP reduces virulence [25,26]. More recent work has identified key amino acids in MntP that likely coordinate the Mn ion as it transits through the membrane [27].
The association of the Mn-responsive riboswitch with other membrane proteins next led to the identification of two additional classes of Mn exporter: a PII-type ATPase, called YoaB in Lactococcus lactis and MgtA in S. pneumoniae, and the UPF0016 family MneA in Vibrio species. Expression of these transporters protected against Mn toxicity and decreased intracellular Mn levels [10,19,27,28]. Mutational studies have elucidated key residues of MneA that mediate Mn efflux [27]. Both types of transporters appear to be specific for Mn export, as the YoaB/MgtA PII-type ATPase was induced specifically by Mn in vivo, and deletion of mneA in Vibrio caused sensitivity to Mn but not other ions [10,19,28]. However, a role in Ca efflux is also possible. Both exporters have eukaryotic homologs implicated in Ca homeostasis, and MgtA was shown to affect intracellular Ca levels [19,27]. Although Fe, and increasingly Zn, is known to affect Mn levels, there may also be a hitherto unappreciated interplay between Mn and Ca homeostasis in bacteria. As for the other Mn exporters, MgtA has been implicated in pathogenesis [29], and it will be important to determine whether MneA also contributes to virulence.
Interestingly, another protein upregulated by Mn via the yybP-ykoY riboswitch may be an Mn importer rather than an exporter. The TerC family protein Alx could not rescue the Mn sensitivity of cells lacking MntP in Escherichia coli. Moreover, expression of Alx led to increased intracellular Mn levels upon Mn stress [27]. Although it seems counterintuitive for cells to upregu-late Mn import when Mn levels are already high, the alx gene is also induced by high pH, which decreases Mn availability [30,31]. Since TerC is the most frequently associated protein family with the yybP-ykoY riboswitch, it will be interesting to establish the mechanism of Alx in Mn and pH homeostasis and determine if this role is typical of all TerC homologs [Note added in proof: A newly published study has demonstrated that two TerC homologs in B. subtilis not associated with pH regulation likely serve as Mn exporters [32].]
It is also noteworthy that while MntP is controlled by Mn both transcriptionally and post-transcriptionally through MntR and the yybP-ykoY riboswitch, this dual regulation is not found for the other Mn exporters (Figure 1). MneA, YoaB/MgtA, and TerC are not regulated by a MntR homolog, and the CDF family exporters are not associated with the yybP-ykoY riboswitch [9]. It is not yet clear why some Mn exporters are controlled by Mn-binding transcription factors and others by Mn-sensing riboswitches, but may relate to different physiological roles of the exporters, or ecological niches of the bacteria in which they are found [27].
Quantification of intracellular Mn pools and metal selectivity
Regulated expression of importers and exporters controls the total intracellular levels of metals, called the quota. Importantly, however, metals are partitioned into different pools once inside of the cell (Figure 1, Box 2). Methods to robustly measure these different Mn populations have been challenging to develop. Two main approaches used to quantify available metal pools have been electron paramagnetic resonance (EPR) spectroscopy and thermodynamic models to determine the sensitivities of regulatory proteins for different metals [5,33–35]. Recent work has continued to refine these approaches and provided insight into Mn speciation, availability, and toxicity within bacterial cells [6,36,37].
Box 2. Intracellular metal pools and metal selectivity.
The idea that different populations of metals exist within cells has been appreciated for quite some time; however, identifying and quantifying these pools has proved challenging [5,33,34,50]. The terms “available” or “buffered free” metal describe the labile pool kinetically accessible to proteins, while the total amount of metal in cells is called the “metal quota.” Metal quotas are typically measured with inductively coupled plasma mass spectrometry (ICP-MS); however, quantification of the available metal pools has been difficult to experimentally interrogate [50].
Besides direct detection techniques using spectroscopy (challenging for some metals, including Mn), thermodynamic models have been employed to determine available metal concentrations indirectly. This approach uses the affinity of metalloregulatory factors to infer the cytoplasmic set point for each metal [34]. The binding affinity (KA) of a regulator for its cognate metal can then be converted to the ΔG° of metallation. Since metalloregulators are the first responders in the battle to avoid excess metals, their free energy of metallation should represent the lower bound for a protein to obtain a given metal from cytoplasmic ligands [33,37]. Thus, if a protein has a less favorable free energy of metallation with that metal than this lower bound, it is not likely to obtain that metal from the available pool.
Another challenging topic to investigate continues to be how proteins and other molecules correctly select their cognate metal ion from among all other metals [50]. Differently from other biomolecules where intermolecular forces between complementary binding surfaces dictate strength of binding, divalent metals have been observed to show a stereotypical order of stability for complex formation with organic ligands, called the Irving–Williams series. Mg and Ca tend to bind most weakly, followed by Mn < Fe < Co < Ni < Cu > Zn [34,50–52]. Thus, among transition metals, Mn typically has the lowest intrinsic affinity for biomolecules. (A notable exception is unusually high stability complexes of Mn with nucleic acids [53].) This means that even for a bona fide Mn-binding protein, Mn will usually bind less tightly to the protein than Zn. (It should be noted, however, that kinetic trapping of a metal to a protein, for example, by protein folding, can increase the binding of a metal to low-affinity sites [37,52].) Thus, mismetallation of proteins with Zn and Cu is a constant problem for cells. Even Mn can become inappropriately associated with ligands, especially Fe proteins, if its levels increase and exceed the buffering capacity of cells [2,5,37,40]. Defining which proteins are affected by perturbations in metal pools to understand mechanisms of metal toxicity is an ongoing effort, and an important one. Emerging evidence suggests this “metal specificity problem” is an inherent aspect of metalloproteins and is exploited by eukaryotic hosts by subjecting bacteria to metal intoxication or starvation to kill invading pathogens [4,37].
Speciation of Mn inside cells
The Daly and Hoffman groups have defined a specific EPR signal that represents Mn bound to low molecular weight (LMW) compounds [36]. Such LMW compounds have been shown to help protect cells against oxidative stress and ionizing radiation (IR) (Box 1) [2,8,35,38]. While ROS- and IR-resistant bacteria accumulate higher quotas of Mn than ROS- and IR-sensitive bacteria [39], the amount of Mn present in the LMW ROS-scavenging complexes relative to the total intracellular Mn has not been clear. Using absorption-display EPR in live cells, the authors were able to distinguish two populations of intracellular Mn: (1) HeMn2+, high-symmetry LMW Mn complexes associated with resistance to IR and (2) L-Mn2+, low-symmetry Mn complexes. The ratio of H-Mn2+ to L-Mn2+ complexes in various organisms correlated well with the DNA repair efficiency of irradiated cells. The authors propose that this ratio in different cell types—including human cancers—can thus be used as a direct metric of radiation and ROS resistance [36].
Available metal pools predict metal selectivity in vivo
A recent comprehensive study inferred the available metal pools in Salmonella cells from measurements of binding affinities and concentrations of seven metal-sensing transcription factors and the metals Mn, Fe, Co, Ni, Zn, and Cu [37]. Using a thermodynamic model, they derived the available concentration of each metal and calculated the free energy of metallation (ΔG°) for the transcription factors with their cognate metal (Box 2). Importantly, the authors then showed that actual metal specificity of an example protein was predicted by the available amount of metals rather than the in vitro metal-binding affinities, as is often used. This and previous studies suggest that metal concentrations which exceed the buffering capacity of the cell could cause mismetallation [5,34,37]. Thus, this type of analysis could be used to analyze perturbations in metal pools on mismetallation. Although it would require significant effort, similar systematic quantification of available metal pools in different bacteria and under different growth conditions would be illuminating.
Toxicity due to perturbation of metal pools
The excess or deficiency of metals has long been known to inhibit bacterial growth. However, the specific cellular targets that are mismetallated or under-metallated and how concentrations of other metals are perturbed have been less clear [5]. Recently, a new target of Mn toxicity was identified in S. pneumoniae [6]. High Mn concentration led to hyperactivation of the protein phosphatase PhpP, which inhibited cell division. Interestingly, while high Mn concentrations have been associated with decreased Fe levels in E. coli and S. aureus, in this study, excess Mn led to increased Fe and Zn levels [6,23,40]. In vitro, Zn strongly inhibited activity of PhpP, suggesting that controlling the Mn:Zn ratio is important for proper cell functioning. Further studies are needed to define other cellular targets of Mn intoxication and deficiency across bacteria.
Perspective
In summary, significant progress has been made in understanding how Mn transporters and Mn-binding regulatory factors work, including recent work on Mn-dependent gene regulation, identifying new Mn homeostasis proteins, and characterizing Mn pools in cells. But by analogy with other metal homeostasis systems, dedicated factors that sequester excess Mn or that mobilize intracellular stores during Mn deficiency may also exist. Possible candidates include the MntR-regulated Dps protein and other genes associated with the yybP-ykoY riboswitch [7,9,41]. Additionally, while the importance of Mn for virulence of pathogenic bacteria is becoming even more apparent, similar work establishing the role of Mn during symbiotic interactions with eukaryotic hosts or within bacterial communities is mostly lacking. An exception is a recent study that provided mechanistic insight into Mn usage during colonization of Rhizobium leguminosarum with various plants [42]. Understanding the broader role of Mn in beneficial interactions of bacteria will also be an exciting area to follow in upcoming years.
Acknowledgements
The author wishes to acknowledge Julia Martin for critical discussion of this manuscript. Work in the Waters laboratory is supported by the University of Wisconsin Oshkosh Faculty Development program and Research Corporation for Science Advancement (Cottrell Scholars Award 23595 to LSW).
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Hood MI, Skaar EP: Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 2012, 10: 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisher JP, Giedroc DP: Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol 2013, 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer LD, Skaar EP: Transition metals and virulence in bacteria. Annu Rev Genet 2016, 50:67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheldon JR, Skaar EP: Metals as phagocyte antimicrobial effectors. Curr Opin Immunol 2019, 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.**.Chandrangsu P, Rensing C, Helmann JD: Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 2017, 15: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review that covers protein and RNA regulatory mechanisms of Zn, Fe, and Mn homeostasis, with an emphasis on metal selectivity and cellular responses to avoid metal limitation or toxicity.
- 6.*.Martin JE, Lisher JP, Winkler ME, Giedroc DP: Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol Microbiol 2017, 104:334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a cellular target of Mn toxicity in the pathogen S. pneumoniae, the PhpP phosphatase that controls cell division. The effect of Mn stress on intracellular metal pools was determined and the importance of identifying other molecular determinants underlying metal toxicity was highlighted.
- 7.Juttukonda LJ, Skaar EP: Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol 2015, 97:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culotta VC, Daly MJ: Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxidants Redox Signal 2013, 19:933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G: The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell 2015, 57:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price IR, Gaballa A, Ding F, Helmann JD, Ke A: Mn(2+)-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol Cell 2015, 57:1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breaker RR: Prospects for riboswitch discovery and analysis. Mol Cell 2011, 43:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serganov A, Nudler E: A decade of riboswitches. Cell 2013, 152:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedekind JE, Dutta D, Belashov IA, Jenkins JL: Metal-loriboswitches: RNA-based inorganic ion sensors that regulate genes. J Biol Chem 2017, 292:9441–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders AM, DeRose VJ: Beyond Mg(2+): functional interactions between RNA and transition metals. Curr Opin Chem Biol 2016, 31:153–159. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Shin JH, Pinochet-Barros A, Su TT, Helmann JD: Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol 2017, 103:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do H, Makthal N, Chandrangsu P, Olsen RJ, Helmann JD, Musser JM, Kumaraswami M: Metal sensing and regulation of adaptive responses to manganese limitation by MtsR is critical for group A streptococcus virulence. Nucleic Acids Res 2019, 14:7476–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Wang X, Yang F, Hu Q, Tong H, Dong X: Molecular insights into hydrogen peroxide-sensing mechanism of the metalloregulator MntR in controlling bacterial resistance to oxidative stresses. J Biol Chem 2017, 292:5519–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.*.Bachas ST, Ferre-D’Amare AR: Convergent use of heptacoordination for cation selectivity by RNA and protein metalloregulators. Cell Chem Biol 2018, 25:962–973 e965. [DOI] [PMC free article] [PubMed] [Google Scholar]; A structural analysis of different members of the yybP-ykoY family of riboswitches that provided insight into the molecular mechanism of Mn selectivity, including heptacoordination and the use of one soft ligand.
- 19.*.Martin JE, Le MT, Bhattarai N, Capdevila DA, Shen J, Winkler ME, Giedroc DP: A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res 2019, 13:6885–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration that the MgtA P-type ATPase preceded by the yybP-ykoY riboswitch serves as an exporter of Mn in S. pneumoniae. The authors examined metal binding and gene regulation of the riboswitch in vitro and in vivo.
- 20.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI: Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol 2009, 72:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ: Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Zheng C, Cao M, Zeng T, Zhao X, Shi G, Chen H, Bei W: The manganese efflux system MntE contributes to the virulence of Streptococcus suis serotype 2. Microb Pathog 2017, 110:23–30. [DOI] [PubMed] [Google Scholar]
- 23.Grunenwald CM, Choby JE, Juttukonda LJ, Beavers WN, Weiss A, Torres VJ, Skaar EP: Manganese detoxification by MntE is critical for resistance to oxidative stress and virulence of Staphylococcus aureus. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters LS, Sandoval M, Storz G: The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 2011, 193:5887–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Tao J, Mao D, He C: A novel manganese efflux system, YebN, is required for virulence by Xanthomonas oryzae pv. oryzae. PloS One 2011, 6, e21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veyrier FJ, Boneca IG, Cellier MF, Taha MK: A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog 2011, 7, e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*.Zeinert R, Martinez E, Schmitz J, Senn K, Usman B, Anantharaman V, Aravind L, Waters LS: Structure-function analysis of manganese exporter proteins across bacteria. J Biol Chem 2018, 293:5715–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]; This structure–function study provided bioinformatic and experimental evidence for critical amino acids mediating Mn efflux in the MntP and UPF0016 family of exporters. It also demonstrated that the riboswitch-associated TerC family protein Alx does not serve as a Mn exporter in E. coli.
- 28.Fisher CR, Wyckoff EE, Peng ED, Payne SM: Identification and characterization of a putative manganese export protein in Vibrio cholerae. J Bacteriol 2016, 198:2810–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosch JW, Sublett J, Gao G, Wang YD, Tuomanen EI: Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol Microbiol 2008, 70:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingham RJ, Hall KS, Slonczewski JL: Alkaline induction of a novel gene locus, alx, in Escherichia coli. J Bacteriol 1990, 172:2184–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S: A pH-responsive riboregulator. Genes Dev 2009, 23: 2650–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paruthiyil S, Pinochet-Barros A, Huang X, Helmann JD: Bacillus subtilis TerC family proteins help prevent manganese intoxication. J Bacteriol 2020, 202:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Outten CE, O’Halloran TV: Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292:2488–2492. [DOI] [PubMed] [Google Scholar]
- 34.Reyes-Caballero H, Campanello GC, Giedroc DP: Metalloregulatory proteins: metal selectivity and allosteric switching. Biophys Chem 2011, 156:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNaughton RL, Reddi AR, Clement MH, Sharma A, Barnese K, Rosenfeld L, Gralla EB, Valentine JS, Culotta VC, Hoffman BM: Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A 2010, 107: 15335–15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*.Sharma A, Gaidamakova EK, Grichenko O, Matrosova VY, Hoeke V, Klimenkova P, Conze IH, Volpe RP, Tkavc R, Gostincar C, et al. : Across the tree of life, radiation resistance is governed by antioxidant Mn(2+), gauged by paramagnetic resonance. Proc Natl Acad Sci U S A 2017, 114:E9253–E9260. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors developed an EPR technique that can distinguish Mn complexes associated with protection against ionizing radiation from other Mn species in living cells. This paper is an example of development of new spectroscopic techniques needed to probe transition metal ion speciation inside cells.
- 37.**.Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, et al. : Bacterial sensors define intracellular free energies for correct enzyme metalation. Nat Chem Biol 2019, 15:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive study quantifying metal and DNA binding affinities for several metalloregulators, allowing estimation of the intracellular buffered free metal pools for six transition metals. Methodology and sample calculations are provided to allow researchers to carry out similar analyses in other systems.
- 38.Daly MJ, Gaidamakova EK, Matrosova VY, Kiang JG, Fukumoto R, Lee DY, Wehr NB, Viteri GA, Berlett BS, Levine RL: Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PloS One 2010, 5, e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, et al. : Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 2004, 306:1025–1028. [DOI] [PubMed] [Google Scholar]
- 40.Martin JE, Waters LS, Storz G, Imlay JA: The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 2015, 11, e1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto K, Ishihama A, Busby SJ, Grainger DC: The Escherichia coli K-12 MntR miniregulon includes dps, which encodes the major stationary-phase DNA-binding protein. J Bacteriol 2011, 193:1477–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.*.Hood G, Ramachandran V, East AK, Downie JA, Poole PS: Manganese transport is essential for N2 -fixation by Rhizobium leguminosarum in bacteroids from galegoid but not phaseoloid nodules. Environ Microbiol 2017, 19:2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study investigating the role of Mn in bacterial symbiotic interactions with a eukaryotic host.
- 43.Archibald FS, Fridovich I: The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys 1982, 214:452–463. [DOI] [PubMed] [Google Scholar]
- 44.Barnese K, Gralla EB, Valentine JS, Cabelli DE: Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci U S A 2012, 109:6892–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadtman ER, Berlett BS, Chock PB: Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci U S A 1990, 87:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anjem A, Imlay JA: Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 2012, 287: 15544–15556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobota JM, Imlay JA: Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A 2011, 108:5402–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radin JN, Zhu J, Brazel EB, McDevitt CA, Kehl-Fie TE: Synergy between nutritional immunity and independent host defenses contributes to the importance of the MntABC manganese transporter during Staphylococcus aureus infection. Infect Immun 2019, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handke LD, Gribenko AV, Timofeyeva Y, Scully IL, Anderson AS: MntC-dependent manganese transport is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldron KJ, Robinson NJ: How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol 2009, 7:25–35. [DOI] [PubMed] [Google Scholar]
- 51.Irving H, Williams RJP: Order of stability of metal complexes. Nature 1948, 162:746–747. [Google Scholar]
- 52.Foster AW, Osman D, Robinson NJ: Metal preferences and metallation. J Biol Chem 2014, 289:28095–28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigel RK, Sigel H: A stability concept for metal ion coordination to single-stranded nucleic acids and affinities of individual sites. Acc Chem Res 2010, 43:974–984. [DOI] [PubMed] [Google Scholar]
- 54.Green RT, Todd JD, Johnston AW: Manganese uptake in marine bacteria; the novel MntX transporter is widespread in Roseobacters, Vibrios, Alteromonadales and the SAR11 and SAR116 clades. ISME J 2013, 7:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Z, Chen S, Wilson DB: Cloning, expression, and characterization of cadmium and manganese uptake genes from Lactobacillus plantarum. Appl Environ Microbiol 1999, 65: 4746–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padilla-Benavides T, Long JE, Raimunda D, Sassetti CM, Arguello JM: A novel P(1B)-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J Biol Chem 2013, 288:11334–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarvan S, Butcher J, Stintzi A, Couture JF: Variation on a theme: investigating the structural repertoires used by ferric uptake regulators to control gene expression. Biometals 2018, 31: 681–704. [DOI] [PubMed] [Google Scholar]


