ABSTRACT
Purpose
This study aimed to assess the effects of 20 wk resistance exercise training with or without protein supplementation on body composition, muscle mass, muscle strength, physical performance, and aerobic capacity in prostate cancer patients receiving androgen deprivation therapy (ADT).
Methods
Sixty prostate cancer patients receiving ADT were randomly assigned to perform 20 wk of resistance exercise training with supplementation of 31 g whey protein (EX + PRO, n = 30) or placebo (EX + PLA, n = 30), consumed immediately after exercise and every night before sleep. A separate control group (CON, n = 36) only received usual care. At baseline and after 20 wk, body composition (dual-energy x-ray absorptiometry), muscle mass (computed tomography scan), muscle strength (1-repetition maximum strength tests), physical performance (Timed Up and Go Test, 30-Second Chair Stand Test, and Stair Climb Test), aerobic capacity (cardiopulmonary exercise test), and habitual dietary intake (food diary) were assessed. Data were analyzed using a two-factor repeated-measures ANOVA.
Results
Over time, muscle mass and strength increased in EX + PRO and EX + PLA and decreased in CON. Total fat mass and fat percentage increased in EX + PRO and CON, but not in EX + PLA. Physical performance did not significantly change over time in either group. Aerobic capacity was maintained in EX + PLA, but it decreased in EX + PRO and CON. Habitual protein intake (without supplements) averaged >1.0 g·kg body weight−1·d−1, with no differences over time or between groups.
Conclusions
In prostate cancer patients, resistance exercise training counteracts the adverse effects of ADT on body composition, muscle mass, muscle strength, and aerobic capacity, with no additional benefits of protein supplementation.
Key Words: HORMONE THERAPY, PROTEIN SUPPLEMENTATION, BODY COMPOSITION, PHYSICAL PERFORMANCE, AEROBIC CAPACITY
Androgen deprivation therapy (ADT) forms the cornerstone in the treatment of localized high-risk, locally advanced, and metastatic prostate cancer (PCa) (1,2). It is estimated that approximately 50% of all PCa patients will be treated with ADT in the course of their disease trajectory (3). The working mechanism of ADT is based on reducing androgen levels to castration level, because androgens are known to drive prostate tumor growth. Although ADT substantially improves survival, the decline in androgen levels has numerous adverse effects, including the loss of lean body mass and an increase in body fat mass (FM) (4–6).
It has been well established that resistance exercise training can be applied effectively to counteract the age-related loss of muscle mass and strength in healthy older adults (7–11). For PCa patients on ADT, randomized controlled trials (RCT) consistently show that resistance exercise training increases appendicular lean mass and muscle strength (12–17). The efficacy of resistance exercise training to reduce FM, increase physical performance, and improve aerobic capacity in PCa patients undergoing ADT is less evident (12–17). However, recent meta-analyses on the effect of prolonged resistance exercise training have reported positive results for these outcomes in the population of PCa patients, not restricted to ADT treatment only (18,19). We hypothesized that prolonged resistance exercise training represents an effective adjuvant treatment to attenuate the decline in muscle mass, reduce FM accrual, and increase strength and physical performance in PCa patients on ADT.
Protein supplementation during prolonged resistance exercise training has been shown to result in greater gains in muscle mass and strength in both young and older adults (10,20) compared with no protein supplementation. It could be speculated that dietary protein supplementation may also augment the anabolic effects of resistance exercise training in PCa patients on ADT. In support, protein ingestion during recovery from exercise has been shown to increase muscle protein synthesis rates in PCa patients on ADT (21). However, pilot work by Dawson et al. (17) failed to confirm the benefits of protein supplementation to further increase gains in muscle mass, strength, or physical performance after 12 wk of resistance exercise training in these patients. Clearly, more work is needed to determine the proposed benefits of protein supplementation on the prevention of muscle mass and strength loss in PCa patients on ADT. We hypothesized that protein supplementation augments the benefits of prolonged resistance exercise training to attenuate the decline in muscle mass, reduce FM accrual, and increase strength and physical performance in PCa patients on ADT.
To test our hypotheses, we enrolled PCa patients (starting) with ADT. Throughout their treatment, we assessed the effect of 20 wk of resistance exercise training with and without additional protein supplementation on body composition, muscle strength, physical performance, and aerobic capacity. To adequately assess the various changes in body composition and muscle mass, we applied both whole-body dual-energy x-ray absorptiometry (DXA) and leg computed tomography (CT), and quantified performance by both cardiopulmonary exercise testing and various strength and functional performance tests.
METHODS
Patients
Between September 2017 and February 2021, PCa patients starting or continuing treatment with gonadotrophin-releasing hormone agonist or antagonist, with or without anti-androgens, for a least 6 months were recruited. Patients were excluded if they were unable to participate in the exercise training regimen, had comorbidities severely compromising physical activity, exposed a high risk for pathological fractures due to bone metastases (as estimated by their treating urologist), had an estimated life expectancy <1 yr, were lactose intolerant or had a whey protein allergy, showed cognitive disorders or severe emotional instability, or were unable to speak, understand, or read the Dutch language. Potential participants were identified by their urologist or urology nurse and referred to the investigators who provided them with the full oral and written study information. Interested patients were invited for a screening visit to obtain informed consent and confirm eligibility. Their medical history and in- and exclusion criteria were evaluated and a cardiopulmonary exercise test was performed. All patients were cleared by a sports physician to perform the exercise training program.
The study was approved by the local Medical Ethical Committee of Maastricht University Medical Centre + (MUMC+) and confirmed to the principles outlined in the latest version of the Declaration of Helsinki for use of human subjects and tissue. This trial was registered at www.trialregister.nl as NL6258. The study was independently monitored by the Clinical Trial Center Maastricht.
Study design
This study was a multicenter partly RCT, comparing two intervention groups with a separately recruited control group. Patients were recruited in hospitals in the southern part of the Netherlands. For both intervention groups, patients were recruited in the MUMC+, the Zuyderland Medical Centre (Zuyderland MC), and the Máxima Medical Centre (MMC) between September 2017 and March 2020. Patients for the control group (CON) were recruited in the Jeroen Bosch Hospital (JBZ) and Canisius–Wilhelmina Hospital (CWZ) between June 2018 and February 2021, as well as in the MUMC+ and Zuyderland MC between October 2020 and February 2021. Patients for CON were asked to participate in a study about the side effects of ADT and were not informed about the exercise intervention.
At baseline and after 20 wk, an experimental test day was planned (in MUMC+ for patients of the MUMC+ and Zuyderland MC, MMC for patients of the MMC, and JBZ for patients of the JBZ and CWZ). Anthropometric measurements, whole-body DXA, and leg CT were performed. After providing a lunch, physical performance tests and maximal strength assessments were performed. The screening and baseline measurements were preferably separated by at least 7 d, in which patients wore an accelerometer and kept a food diary. At the end of the baseline test day, patients recruited for the intervention groups were randomly allocated in a double-blinded fashion to resistance exercise training with protein supplementation (EX + PRO) or with placebo supplementation (EX + PLA). An independent researcher performed the randomization by means of computer generated random numbers in stratified permuted blocks of 4.
After 20 wk of intervention or usual care, all procedures from the baseline test day and the cardiopulmonary exercise test were repeated. The cardiopulmonary exercise test was performed at least 48 h before or after the test day, to prevent any influence on the other outcome measurements. Furthermore, during the last week of the intervention or control period, patients again wore the accelerometer and filled in the food diary. Protein or placebo allocation was concealed from the research team and patients until all patients had performed their postmeasurements.
Exercise intervention program
Patients in the exercise groups performed a supervised progressive whole-body resistance exercise training program (60 min, twice a week) for 20 wk. Training started and ended with 5-min warm-up and cooling down on a cycle ergometer, interspersed by training on the leg press and leg extension separated by two upper body exercises. On the leg press and leg extension, 2 warm-up sets and 4 working sets were performed. Upper body exercises were paired (chest press with lateral pulldown and shoulder press with horizontal row) and were performed in an alternating manner between training sessions, with 1 warm-up set, followed by 3 working sets. All sets consisted of 10 repetitions with 1.5 and 3 min rest between sets and exercises, respectively. For lower body exercises, one-repetition maximum (1RM) was determined at the baseline test day; for upper body exercises, 1RM was determined during the first training week (as this was not an outcome measure). The training program was divided into cycles of 4 wk. During weeks 1–3 of the first cycle, the workload on each machine was gradually increased from 60% to 70% 1RM. In weeks 1–3 of the following cycles, the workload was increased from 65% to 70% 1RM. Every fourth week, workload was reduced to 60% 1RM to allow for proper recovery and to minimize the risk of injury. After 4, 8, 12, and 16 wk of training, 1RM was estimated using the multiple-repetition testing procedure (indirect 1RM) to progressively adjust the workload of the training sessions. For patients experiencing medical complications (e.g., treatment-related issues or injuries), training load was adjusted. Patients received a personalized training log for every training session, in which the actual training program/load was registered. Training adherence was calculated as the amount of performed exercise sets divided by the amount of prescribed sets.
Protein and placebo supplementation
Patients in the exercise groups ingested either a protein or a placebo supplement directly after every exercise session and each night before sleep. The protein supplement contained 31 g whey protein (Lacprodan® HYDRO.Rebuild, degree of hydrolysis 10%), 13 g carbohydrate, and 1.0 g fat, providing 774 kJ of energy (Arla Foods Ingredients Group P/S, Viby J, Denmark). The placebo supplement contained 1 g protein, 12 g carbohydrate, and 0.4 g fat, providing 234 kJ of energy (Arla Foods Ingredients Group P/S). Supplement provision was performed in a double-blinded fashion by an independent researcher. Patients received sachets containing dried contents, which needed to be dissolved in 250 mL water. All beverages were chocolate flavored to mask their contents. Adherence to the supplement ingestion was assessed by counting returned (full and empty) supplement sachets.
Habitual dietary intake and physical activity
Patients were instructed to refrain from exhaustive physical activity 48 h before the test days and to arrive at the study location by car or public transportation after an overnight fast. The week before both test days, patients recorded their dietary intake and their physical activity to gain insight into their habitual activity pattern and to identify potential changes during the intervention period. To assess dietary intake, patients kept a 3-d food dairy (on two weekdays and one weekend day). Average daily energy intake (MJ), energy percentages (En%) of protein, carbohydrate and fat, and protein intake relative to bodyweight (g·kg body weight−1·d−1) were calculated using Web-based software (Eetmeter; Voedingscentrum, Den Haag, The Netherlands). To assess physical activity, patients wore a small-sized triaxial accelerometer (ActiGraph wGT3X-BT; ActiGraph, Pensacola, FL) on the waist during wakefulness for 7 d. Accelerometer data were analyzed with ActiLife (version 6.13.4, ActiGraph), and average daily step count and percentage of time spent sedentary or in light, moderate, and (very) vigorous activity intensity were calculated. Data were included if the accelerometer was worn for ≥5 d and ≥10 h·d−1.
Body composition
Body weight was measured wearing underwear and directly after voiding using a digital scale to the nearest 0.1 kg. Height was measured by a fixed stadiometer to the nearest 0.5 cm. Body mass index (BMI) was calculated as kilograms per square meter. Waist circumference was measured twice at the midpoint between the top of the iliac crest and the lower margin of the lowest palpable rib at the end of a normal expiration using a stretch-resistant tape. A difference between both measurements of ≤1 cm was accepted and averaged to the nearest 0.5 cm. Whole-body and regional lean mass and FM were measured with whole-body DXA (Discovery A, Hologic, Marlborough, MA [MUMC+ and MMC]; or LUNAR iDXA, GE Healthcare, Chicago, IL [JBZ]).
Skeletal muscle mass
Skeletal muscle mass was assessed with a single-slice CT scan (SOMATOM Definition Flash, Siemens, München, Germany [MUMC+ and JBZ]; or Ingenuity CT, Philips Medical Systems, Eindhoven, The Netherlands [MMC]) to determine the anatomic cross-sectional area (CSA) of the quadriceps muscle, as described previously (22). A single-slice image was made 15 cm proximal to the top of the patella of both legs. Quadriceps muscle CSA of the dominant leg was calculated by manual tracing using ImageJ software (version 1.52p; National Institutes of Health, Bethesda, MD).
Muscle strength
Maximal strength was assessed by 1RM strength tests on the leg press and leg extension machines (Technogym, Milan, Italy). Patients started with a short warm-up on a cycle ergometer. Thereafter, proper lifting technique was demonstrated and practiced, and a specific warm-up of 10 and 5 repetitions on ~50% and 70% of the predicted 1RM was performed. This also served as practice session to familiarize the patients with the exercise. Subsequently, 1RM was determined by increasing the load after each successful single lift until failure. A 3-min rest between attempts was allowed. A repetition was valid if the entire lift was completed in a controlled manner without assistance.
Physical performance
Physical performance was assessed by three consecutive performance tests, always performed in the same order. The Timed Up and Go Test measures the time it takes to get up from a standard arm chair, walk 3 m on a comfortable pace, turn around, walk back, and sit down again (23). The 30-Second Chair Stand Test assesses how many times the patient can stand upright and sit down from a standard chair with his arms folded across the chest, over a 30-s period (24). The Stair Climb Test measures the time it takes to ascend and descend a flight of stairs as quickly as possible but in a safe manner (using the handrail was mandatory).
Aerobic capacity
Aerobic capacity was tested with a cardiopulmonary exercise test to exhaustion with continuous electrocardiography and respiratory gas analysis. Tests were performed on a cycle ergometer (Lode Corival, Groningen, the Netherlands [MUMC+]; or Ergoline, Bitz, Germany [MMC and JBZ]). Ventilatory parameters were measured breath by breath (Carefusion, San Diego, CA [MUMC+, MMC]; or Geratherm Respiratory, Bad Kissingen, Germany [JBZ]). After 3 min of unloaded cycling, the workload was increased according to an individualized ramp protocol aiming at a total test duration of 8–12 min (25). Patients were instructed to cycle with a pedaling rate of >60 rpm. The test was ended when the patient stopped cycling or was not able to maintain the required pedaling frequency. Maximal workload (Wmax), peak oxygen uptake (V̇O2peak), and peak respiratory exchange ratio (RERpeak) were recorded as the final 30-s averaged value of the test.
Sample size calculation and statistical analysis
Our initial sample size estimates of 72 patients in each exercise group and 64 in the control group were not feasible anymore because of the COVID-19-induced lockdown and its subsequent limitations. Therefore, we performed a new sample size calculation based on our already collected data. To detect a difference in quadriceps CSA (primary outcome) between groups over time, 15 patients per group were required (G-Power; power = 90%, alpha = 0.05, calculated Cohen’s f effect size = 0.24).
As already 30 patients in each exercise group had finished their 20 wk measurements, this number was enough to show the effect of the exercise intervention. Furthermore, including more patients would not result in identifying an effect of the protein supplementation, as the interim data showed absolutely no indication of any difference between both exercise groups (Cohen’s d = −0.035). Therefore, to end up with three groups of equal sizes, patient inclusion was continued in the control group only to reach a minimal inclusion of 36 patients (taking into account potential dropouts).
Data were expressed as mean ± SD, or as frequency and percentages. Baseline characteristics between groups were compared using one-way ANOVA with post hoc Bonferroni corrections (for continuous variables) or a chi-square test (for categorical variables). Data were analyzed on an intention-to-treat basis. A two-factor repeated-measures ANOVA time x treatment with time (baseline vs 20 wk) as a within-subjects factor and treatment group (EX + PLA vs EX + PRO vs CON) as a between-subjects factor was used. In case of a significant time–treatment interaction, paired-samples t-tests were performed to detect within-group changes over time. Additionally, univariate general linear models with post hoc Bonferroni corrections were used to detect between-group differences over time. For muscle strength, absolute 1RM values could not be compared due to slight differences in leg press and leg extension equipment at the different study locations. Therefore, percentage changes over time were calculated and compared between groups with univariate general linear models with post hoc Bonferroni corrections. Effect sizes were calculated using partial eta squared (ηpartial2). Significance was set at P < 0.05. All analyses were performed with the use of IBM SPSS Statistics (version 27.0; IBM Corp., Armonk, NY).
RESULTS
Patients
In total, 134 patients were screened to participate in the study, 126 patients were included, and 96 patients finished the study. Of the 30 patients that did not finish the study, 26 dropped out due to training and testing restrictions during the COVID-19-induced lockdown, 1 due to screening failure, and 3 for medical reasons (Fig. 1).
FIGURE 1.
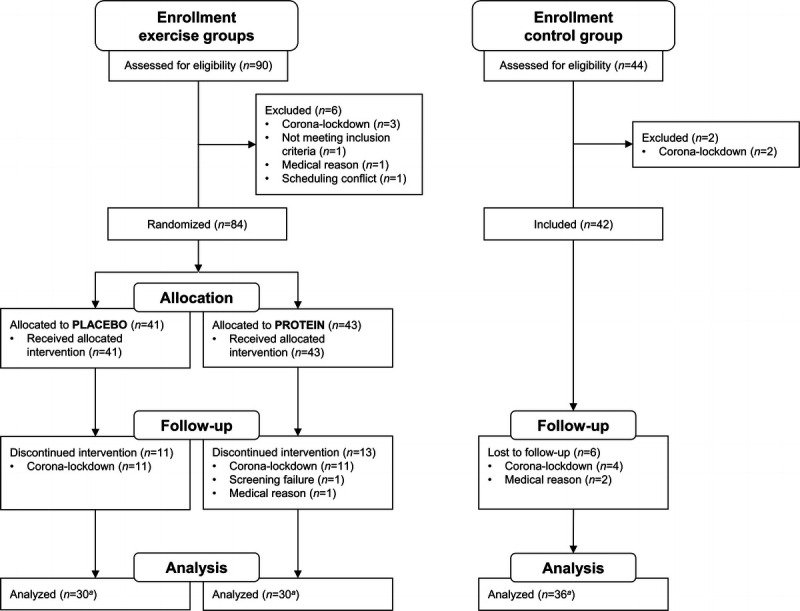
CONSORT flow diagram. CONSORT, Consolidated Standards of Reporting Trials. aNot all outcome measurements are available for all patients.
Patients’ baseline characteristics are presented in Table 1. Patients were 71 ± 7 y old and were slightly overweight (BMI = 26.9 ± 3.5 kg·m−2). Average time since PCa diagnosis was 24 months, 48% of all patients had bone metastases at study enrollment, and mean ADT treatment duration was 107 ± 206 d. Baseline characteristics did not differ between groups, except for ADT duration, which was significantly higher in EX + PLA compared with CON, and the number of patients receiving previous chemotherapy (n = 3 in EX + PLA, none in EX + PRO and CON). During the study period of 20 wk, 42 patients received additional chemotherapy (CTx) or radiation therapy (RTx) (EX + PLA: CTx n = 6, RTx n = 8; EX + PRO: CTx n = 2, RTx n = 7; CON: CTx n = 7, RTx n = 12), with no significant differences between groups (CTx, P = 0.264; RTx, P = 0.652). All statistical analyses were performed with and without “ADT duration at baseline” as covariate, and the unadjusted results are shown. In case of differences, both results are described.
TABLE 1.
Baseline patients’ characteristics.
| EX + PLA (n = 30) | EX + PRO (n = 30) | CON (n = 36) | |
|---|---|---|---|
| Age (yr) | 71 ± 7 | 73 ± 7 | 71 ± 7 |
| Body weight (kg) | 84.5 ± 11.1 | 82.2 ± 13.5 | 83.0 ± 12.8 |
| BMI (kg·m−2) | 27.5 ± 3.3 | 26.7 ± 3.4 | 26.7 ± 3.7 |
| Total body fat % (%) | 30 ± 4 | 30 ± 5 | 31 ± 6 |
| ADT duration (d)a | 190 ± 282b | 107 ± 208 | 37 ± 49 |
| Time since PCa diagnosis (months) | 12 ± 18 | 36 ± 52 | 23 ± 39 |
| Gleason score | 8.4 ± 1.0 | 7.9 ± 1.1 | 8.3 ± 1.0 |
| Bone metastases, n (%) | 14 (50.0) | 11 (39.3) | 19 (52.8) |
| Previous prostatectomy, n (%) | 7 (23.3) | 5 (16.7) | 10 (27.8) |
| Previous radiation, n (%) | 4 (13.3) | 9 (30.0) | 6 (16.7) |
| Previous chemotherapy, n (%)a | 3 (10) | 0 (0) | 0 (0) |
| No. of comorbidities, n (%)c | |||
| 0 | 5 (17.2) | 3 (10.0) | 10 (28.6) |
| 1 | 12 (41.4) | 11 (36.7) | 10 (28.6) |
| ≥2 | 12 (41.4) | 16 (53.3) | 15 (42.9) |
Values are presented as mean ± SD, or n (%) of participants. Not all data are available for all patients. Data available for time since PCa diagnosis: n = 35 (CON); for Gleason score: n = 35 (CON); for bone metastases: n = 28 (EX + PLA), n = 28 (EX + PRO); for number of comorbidities: n = 29 (EX + PLA), n = 35 (CON).
aSignificantly different between groups (P < 0.05).
bSignificantly different from CON (P < 0.05).
cComorbidity assessed by the adapted Self-administered Comorbidity Questionnaire.
EX + PLA, exercise training group with placebo supplementation; EX + PRO, exercise training group with protein supplementation; CON, control group.
Adherence and safety
Overall adherence to the training sessions was 84% ± 18% and 79% ± 23% in EX + PLA and EX + PRO, respectively, and did not differ between groups. Adherence to the intake of the supplements was 94% ± 8% and 86% ± 23% in EX + PLA and EX + PRO, respectively, with no differences between groups. During the exercise training, one patient experienced a collapse requiring admission to the emergency department for further cardiac examination. No abnormalities were found. No other serious adverse events during the exercise training or supplemental intake occurred.
Habitual dietary intake and physical activity
Habitual daily dietary intake and physical activity data are presented in Table 2. For total energy, protein, carbohydrate, and fat intake, no baseline differences between groups or changes over time were found. Overall habitual protein intake averaged 1.1 ± 0.3 g·kg body weight−1·d−1, with no differences between groups nor changes over time. Protein supplementation increased daily protein intake in EX-PRO by an average 34.5 ± 9.3 g·d−1, resulting in a daily protein intake of 1.4 ± 0.4 g·kg body weight−1·d−1. The total protein intake including placebo supplementation in EX-PLA averaged 1.1 ± 0.2 g·kg body weight−1·d−1.
TABLE 2.
Changes in habitual dietary intake and habitual physical activity over time.
| EX + PLA (n = 28a) | EX + PRO (n = 30a) | CON (n = 34a) | ||||
|---|---|---|---|---|---|---|
| Baseline | 20 wk | Baseline | 20 wk | Baseline | 20 wk | |
| Habitual dietary intake | ||||||
| Energy intake (MJ·d−1) | 9.2 ± 2.0 | 9.3 ± 2.2 | 9.0 ± 1.9 | 8.6 ± 1.8 | 9.4 ± 1.8 | 9.1 ± 1.7 |
| Protein intake (g·kg body weight−1·d−1) | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 |
| Protein intake (% of energy) | 16 ± 2 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 17 ± 3 |
| Carbohydrate intake (% of energy) | 38 ± 9 | 39 ± 8 | 42 ± 6 | 40 ± 7 | 40 ± 8 | 39 ± 6 |
| Fat intake (% of energy) | 38 ± 7 | 38 ± 7 | 36 ± 5 | 38 ± 6 | 36 ± 7 | 36 ± 6 |
| Habitual physical activity | ||||||
| Average steps per day (steps per day)b | 6212 ± 2901 | 5708 ± 2451 | 5586 ± 2774 | 5246 ± 2914 | 7008 ± 2216 | 5807 ± 1709 |
| % Time sedentary per day (%)c | 77 ± 7 | 77 ± 6 | 79 ± 7d | 78 ± 9 | 73 ± 7 | 74 ± 7 |
| % Time in light activity per day (%)c | 19 ± 6 | 19 ± 5 | 18 ± 6 | 18 ± 6 | 21 ± 6 | 21 ± 6 |
| % Time in moderate activity per day (%)b,c | 5 ± 3 | 4 ± 2 | 4 ± 3d | 4 ± 3 | 6 ± 3 | 5 ± 2 |
| % Time in vigorous and very vigorous activity per day (%) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Values are presented as mean ± SD. No time–treatment interaction was observed for any of the variables. The physical activity levels are assessed with the “Freedson Adult (1998)” filter, using the following cutoff points: sedentary, 0–99 counts per minute; light, 100–1951 counts per minute; moderate, 1952–5724 counts per minute; vigorous, 5725–9498 counts per minute; very vigorous, 9499 counts per minute and above.
aFor habitual physical activity data, n = 29 (EX + PLA), n = 26 (EX + PRO), n = 35 (CON).
bSignificant time effect (P < 0.05).
cSignificantly different between groups at baseline (P < 0.05).
dSignificantly different at baseline from CON (P < 0.05).
EX + PLA, exercise training group with placebo supplementation; EX + PRO, exercise training group with protein supplementation; CON, control group.
Body composition
Body composition parameters are presented in Table 3. No baseline differences between groups were observed. Over time, patients gained on average 1.5 ± 3.1 kg body weight (time effect, P < 0.001), resulting in 1.9% ± 3.9% increase in BMI (time effect, P < 0.001) and 1.0 ± 3.4 cm increase in waist circumference (time effect, P < 0.005), with no differences between groups. For DXA measurements, significant differences over time between groups were found for total lean mass, appendicular lean mass, total FM, and fat percentage (fat%). For total lean mass and appendicular lean mass, within-group analyses showed no significant changes over time in any of the three groups. However, between-group comparisons showed a significant difference (P = 0.039) in the change in appendicular lean mass over time between EX + PLA (+0.3 ± 1.0 kg) and CON (−0.4 ± 1.3 kg), which was no longer significant after adjusting for “ADT duration at baseline” (EX + PLA vs CON +0.7 ± 0.3 kg, P = 0.039 [unadjusted], and +0.7 ± 0.3 kg, P = 0.084 [adjusted]). For lean mass, between-group comparisons showed a strong trend (P = 0.053) toward a difference in the changes between EX + PLA (+0.6 ± 2.0 kg) and CON (−0.7 ± 2.4 kg). For FM and fat%, within-group analyses showed no significant changes over time in EX + PLA, whereas FM and fat% significantly increased over time in EX + PRO (FM = +1.1 ± 1.6 kg, fat% = +0.8 ± 1.6%, both P < 0.05) with even larger increases in CON (FM = +2.1 ± 1.7, fat% = +1.8 ± 1.8%, both P < 0.001). In accordance, between-group comparisons showed significant differences in the changes in FM and fat% between EX + PLA and CON, which became smaller after adjusting for “ADT duration at baseline” (FM: EX + PRO vs CON, −1.0 ± 0.5 kg, P = 0.138 [unadjusted], and −0.8 ± 0.5 kg, P = 0.328 [adjusted]; EX + PLA vs CON, −1.7 ± 0.5 kg, P = 0.002 [unadjusted], and −1.2 ± 0.5 kg, P = 0.034 [adjusted]; fat%: EX + PRO vs CON, −1.0 ± 0.4%, P = 0.064 [unadjusted], and −0.9 ± 0.4%, P = 0.155 [adjusted]; EX + PLA, −1.6 ± 0.4%, P < 0.001 [unadjusted], and −1.3 ± 0.4%, P = 0.014 [adjusted]).
TABLE 3.
Changes in body composition over time.
| Baseline | 20 wk | Time Effect | Time–Treatment Interaction | Within-Group Changes over 20 wk | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | P | P (ηpartial2) | Mean ± SD | P | |
| Body weight (kg) | <0.001 | 0.291 (0.026) | |||||
| EX + PLA | 30 | 84.5 ± 11.1 | 85.4 ± 10.4 | 0.8 ± 3.5 | |||
| EX + PRO | 30 | 82.2 ± 13.5 | 83.8 ± 13.9 | 1.6 ± 2.5 | |||
| CON | 36 | 83.0 ± 12.8 | 85.0 ± 13.3 | 2.1 ± 3.3 | |||
| BMI (kg·cm−2) | <0.001 | 0.327 (0.024) | |||||
| EX + PLA | 30 | 27.5 ± 3.3 | 27.8 ± 3.1 | 0.3 ± 1.1 | |||
| EX + PRO | 30 | 26.7 ± 3.4 | 27.2 ± 3.4 | 0.5 ± 0.8 | |||
| CON | 36 | 26.7 ± 3.7 | 27.3 ± 3.9 | 0.7 ± 1.1 | |||
| Waist circumference (cm) | 0.005 | 0.251 (0.029) | |||||
| EX + PLA | 30 | 104.6 ± 10.5 | 105.0 ± 9.6 | 0.4 ± 3.5 | |||
| EX + PRO | 30 | 103.5 ± 11.9 | 104.3 ± 11.4 | 0.8 ± 3.1 | |||
| CON | 36 | 102.3 ± 10.6 | 104.0 ± 10.3 | 1.7 ± 3.4 | |||
| Total lean mass (kg) | 0.466 | 0.032 (0.077) | |||||
| EX + PLA | 30 | 57.6 ± 6.9 | 58.3 ± 6.6 | 0.6 ± 2.0a | 0.088 | ||
| EX + PRO | 28 | 55.4 ± 7.0 | 55.9 ± 7.4 | 0.5 ± 1.9 | 0.161 | ||
| CON | 31 | 55.5 ± 6.6 | 54.8 ± 6.6 | −0.7 ± 2.4 | 0.125 | ||
| Appendicular lean mass (kg) | 0.913 | 0.028 (0.080) | |||||
| EX + PLA | 30 | 25.0 ± 3.3 | 25.3 ± 3.1 | 0.3 ± 1.0b | 0.104 | ||
| EX + PRO | 28 | 23.5 ± 3.2 | 23.7 ± 3.4 | 0.2 ± 1.1 | 0.378 | ||
| CON | 31 | 24.4 ± 3.0 | 23.9 ± 3.0 | −0.4 ± 1.3 | 0.072 | ||
| Total FM (kg) | <0.001 | 0.003 (0.127) | |||||
| EX + PLA | 30 | 25.6 ± 6.0 | 26.0 ± 5.4 | 0.4 ± 2.2b | 0.311 | ||
| EX + PRO | 28 | 25.6 ± 7.8 | 26.7 ± 7.6 | 1.1 ± 1.6 | 0.001 | ||
| CON | 31 | 26.9 ± 7.9 | 29.0 ± 8.4 | 2.1 ± 1.7 | <0.001 | ||
| Fat percentage | <0.001 | 0.001 (0.145) | |||||
| EX + PLA | 30 | 29.5 ± 4.5 | 29.7 ± 4.0 | 0.2 ± 1.7b | 0.544 | ||
| EX + PRO | 28 | 30.1 ± 5.1 | 30.9 ± 4.6 | 0.8 ± 1.6 | 0.011 | ||
| CON | 31 | 31.6 ± 5.3 | 33.4 ± 5.5 | 1.8 ± 1.8 | <0.001 | ||
Values are presented as mean ± SD. No significant differences were observed between groups at baseline.
aChanges over time are borderline significantly different from changes over time in CON (P = 0.053).
bChanges over time are significantly different from changes over time in CON (P < 0.05).
EX + PLA, exercise training group with placebo supplementation; EX + PRO, exercise training group with protein supplementation; CON, control group.
Skeletal muscle mass
At baseline, no significant differences in quadriceps CSA were observed between groups. However, changes over time were significantly different between groups (time–treatment P < 0.001). Within groups, quadriceps CSA increased over time in EX + PLA (+2.0 ± 3.0 cm2, P = 0.001) and EX + PRO (+1.9 ± 2.7 cm2, P < 0.001) and decreased in CON (−1.2 ± 2.5 cm2, P = 0.008). Between-group comparisons revealed significant differences in the changes over time between both EX + PLA (P < 0.001) and EX + PRO (P < 0.001), when compared with CON, with no differences between both exercise groups (Fig. 2).
FIGURE 2.
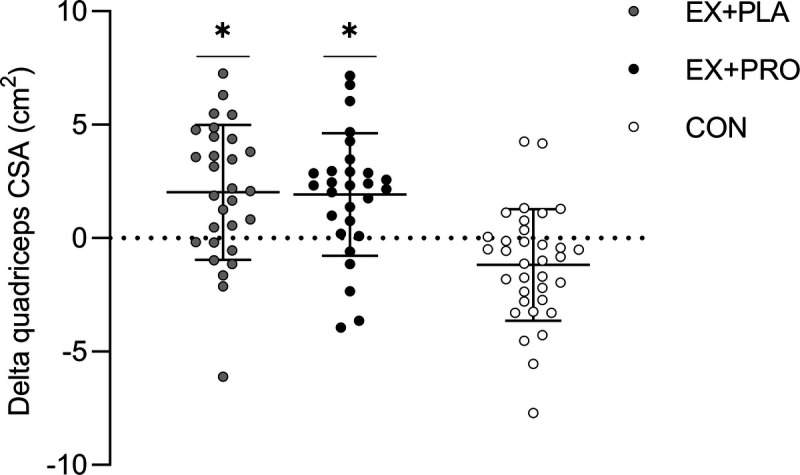
Changes in quadriceps CSA over the 20-wk intervention period. *Changes over time are significantly different from changes over time in CON (P < 0.001).
Leg strength
In both exercise groups, 1RM leg press and 1RM leg extension increased over time (EX + PLA, +12% ± 14% and +19% ± 15%, respectively; EX + PRO, +13% ± 11% and +15% ± 19%, respectively). In CON, 1RM leg press (−5% ± 11%) and leg extension (−6% ± 15%) decreased over time. For both exercise groups, changes over time in leg muscle strength were significantly different from the changes in CON (both P < 0.001), with no differences between both exercise groups (Fig. 3).
FIGURE 3.
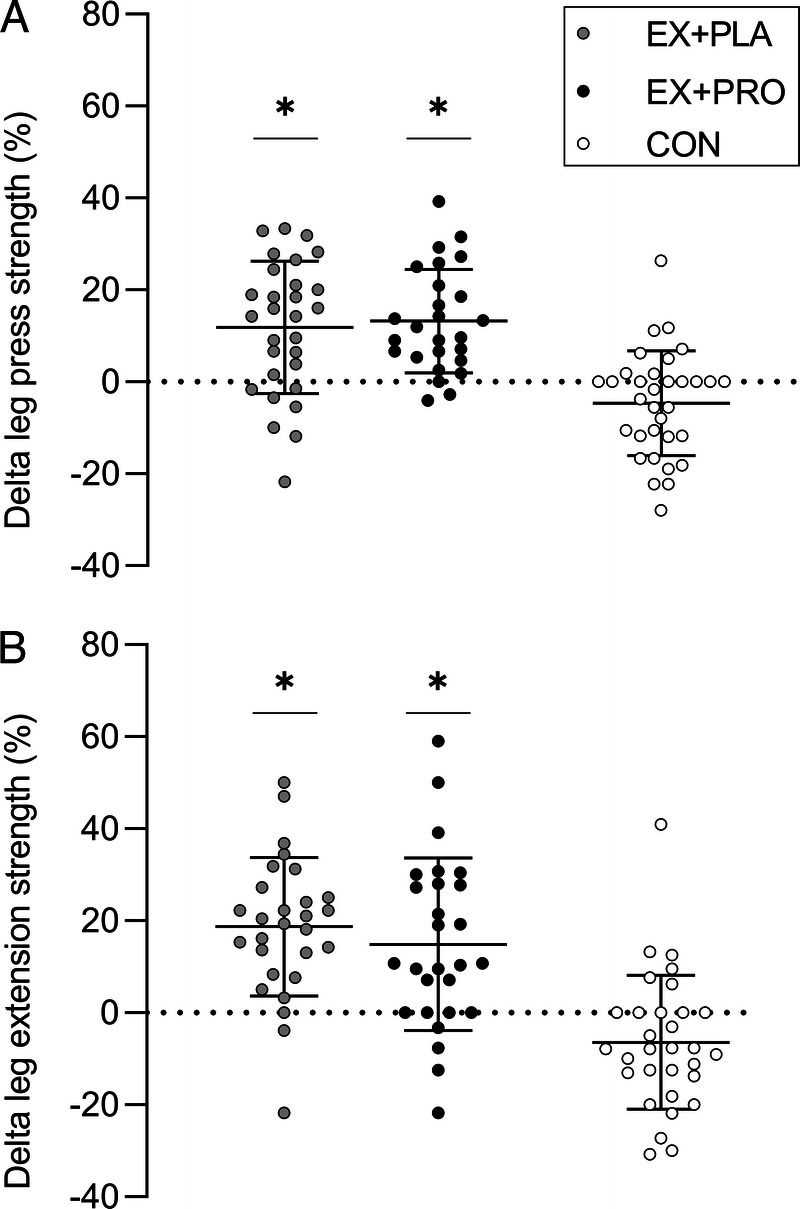
Percentage changes in 1RM leg press (A) and leg extension (B) strength over the 20-wk intervention period. *Changes over time are significantly different from changes over time in CON (P < 0.001).
Physical performance and aerobic capacity
Results are presented in Table 4. On the physical performance tests, no differences in baseline values or changes over time between groups were observed. For aerobic capacity, baseline differences between groups were found for Wmax, Wmax·kg body weight−1, and V̇O2peak (all P < 0.05). Changes over time were significantly different between groups for Wmax·kg body weight−1, V̇O2peak, and V̇O2peak·kg body weight−1 (all P < 0.05). Subsequent within-group analyses showed no changes over time in EX + PLA for all cardiopulmonary exercise test variables, whereas in EX + PRO V̇O2peak and V̇O2peak·kg body weight−1 decreased (both P < 0.05). Furthermore, in CON, V̇O2peak and V̇O2peak·kg body weight−1 declined even more, and Wmax·kg body weight−1 declined as well (all P < 0.001). As a result, changes over time were significantly different between EX + PLA and CON (all P < 0.05). Changes in RERpeak values did not differ between and within groups over time.
TABLE 4.
Changes in physical performance tests and cardiopulmonary exercise tests over time.
| Baseline | 20 wk | Time Effect | Time–Treatment Interaction | Within-Group Changes Over 20 wk | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | P | P (ηpartial2) | Mean ± SD | P | |
| Physical performance tests | |||||||
| Timed Up and Go Test (s) | 0.993 | 0.653 (0.009) | |||||
| EX + PLA | 30 | 9.6 ± 1.5 | 9.6 ± 1.5 | ||||
| EX + PRO | 28 | 9.6 ± 1.5 | 9.7 ± 1.7 | ||||
| CON | 36 | 9.3 ± 1.0 | 9.2 ± 1.3 | ||||
| 30-Second Chair Stand Test (times) | 0.011 | 0.092 (0.052) | |||||
| EX + PLA | 29 | 12.7 ± 2.9 | 13.9 ± 3.5 | 1.2 ± 2.6 | |||
| EX + PRO | 27 | 11.6 ± 2.3 | 12.5 ± 2.8 | 1.0 ± 2.2 | |||
| CON | 36 | 13.4 ± 3.3 | 13.3 ± 4.0 | −0.1 ± 2.8 | |||
| Stair Climb Test (s) | 0.024 | 0.862 (0.003) | |||||
| EX + PLA | 29 | 9.4 ± 3.2 | 9.8 ± 3.9 | 0.3 ± 2.3 | |||
| EX + PRO | 28 | 10.7 ± 3.3 | 11.1 ± 3.8 | 0.4 ± 1.4 | |||
| CON | 36 | 9.7 ± 2.2 | 10.3 ± 2.5 | 0.6 ± 1.6 | |||
| Cardiopulmonary exercise test | |||||||
| Wmax (W)a | <0.001 | 0.054 (0.070) | |||||
| EX + PLA | 28 | 156 ± 48 | 153 ± 43 | −4 ± 21 | |||
| EX + PRO | 24 | 130 ± 38b | 124 ± 41 | −6 ± 18 | |||
| CON | 32 | 164 ± 38 | 149 ± 34 | −15 ± 15 | |||
| Wmax (W·kg BW−1)a | <0.001 | 0.015 (0.100) | |||||
| EX + PLA | 28 | 1.84 ± 0.52 | 1.77 ± 0.47 | −0.07 ± 0.26c | 0.149 | ||
| EX + PRO | 24 | 1.58 ± 0.46b | 1.49 ± 0.52 | −0.09 ± 0.22 | 0.065 | ||
| CON | 31 | 2.01 ± 0.51 | 1.78 ± 0.46 | −0.23 ± 0.18 | <0.001 | ||
| V̇O2peak (mL·min−1)a | <0.001 | 0.008 (0.122) | |||||
| EX + PLA | 28 | 1832 ± 459 | 1811 ± 408 | −21 ± 239c | 0.653 | ||
| EX + PRO | 23 | 1615 ± 365 | 1510 ± 363 | −106 ± 199 | 0.018 | ||
| CON | 27 | 1886 ± 353 | 1672 ± 324 | −215 ± 225 | <0.001 | ||
| V̇O2peak (mL·min−1·kg BW−1) | <0.001 | 0.004 (0.139) | |||||
| EX + PLA | 28 | 21.5 ± 4.7 | 21.0 ± 4.1 | −0.6 ± 2.8c | 0.284 | ||
| EX + PRO | 23 | 19.5 ± 4.3 | 18.0 ± 4.6 | −1.5 ± 2.3 | 0.004 | ||
| CON | 26 | 23.1 ± 3.6 | 20.1 ± 3.6 | −3.0 ± 2.7 | <0.001 | ||
| RERpeak | 0.204 | 0.294 (0.032) | |||||
| EX + PLA | 28 | 1.18 ± 0.11 | 1.17 ± 0.08 | ||||
| EX + PRO | 23 | 1.18 ± 0.09 | 1.15 ± 0.08 | ||||
| CON | 27 | 1.19 ± 0.11 | 1.20 ± 0.10 | ||||
Values are presented as mean ± SD.
aSignificantly different between groups at baseline (P < 0.05).
bSignificantly different at baseline from CON (P < 0.01).
cChanges over time are significantly different from changes over time in CON (P < 0.05).
EX + PLA, exercise training group with placebo supplementation; EX + PRO, exercise training group with protein supplementation; CON, control group; Wmax, maximal workload; V̇O2peak, peak oxygen uptake; BW, body weight.
DISCUSSION
The present study showed that 20 wk of resistance exercise training was feasible, safe, and well tolerated, and effectively counteracted the negative effect of ADT treatment on body composition, muscle mass, leg strength, and aerobic capacity in men with advanced PCa. Protein supplementation did not further augment the benefits of resistance exercise training.
ADT is an important strategy in the treatment of advanced PCa. However, ADT is accompanied by detrimental side effects. In our control group in which patients only received usual care, total body weight and FM increased, whereas leg muscle mass, leg muscle strength, and aerobic capacity decreased. These findings are in line with previous studies on undesirable changes in body composition (4–6) and performance capacity (26,27) in men with PCa receiving ADT.
To combat these adverse effects, we subjected 60 PCa patients on ADT to 20 wk of supervised progressive resistance exercise training. The exercise training program was effective to counteract the most prominent ADT-induced side effects. In agreement with previous RCT on the effects of resistance exercise training in PCa patients on ADT (15–17), we showed improvements in appendicular lean mass. Furthermore, resistance exercise training effectively increased total lean mass and diminished FM gains during ADT treatment. The latter observations have not generally been reported in earlier RCT performing resistance training only during ADT (12–15,17). In addition to body composition measurements using DXA, we directly assessed quadriceps muscle mass by upper leg CT. We observed a significant ~3% increase in quadriceps muscle CSA in response to the resistance exercise training program. This was accompanied by ~10%–20% increase in leg press and leg extension strength. In comparison, in the control group, we observed ~2% decrease in quadriceps muscle CSA and ~5% decline in leg strength. Our data seem in line with previous findings reporting a 16% increase in quadriceps muscle thickness (28) and 6.4% increase in thigh muscle volume (29) as assessed by ultrasound and CT, respectively, after vigorous progressive resistance training interventions. A small pilot study applying a less conventional resistance exercise training program, however, failed to detect changes in quadriceps muscle volume with magnetic resonance imaging (30).
Despite the observed increases in leg muscle mass and strength, no significant improvements were observed on the physical performance tests. This seems to be in line with other (13,17) but certainly not all (15) studies on the effect of exercise training on physical performance in PCa patients on ADT. The absence of improvements in physical functioning is likely attributed to the limited sensitivity of the various tests used to assess physical function combined with insensitivity to detect changes in a well-performing population (ceiling effect). However, optimizing muscle mass and strength is unarguably important as strong associations between low muscle mass and strength to poorer clinical outcomes in cancer patients have been reported (31,32).
It has been well established that protein supplementation can further augment exercise-induced gains in muscle mass and strength (10,20,33). Previously, we observed that protein supplementation was required to allow measurable gains in fat-free mass during prolonged resistance exercise training in prefrail older adults (10). In the present study, we observed no surplus benefits of protein supplementation on any of the outcome parameters. A plausible reason could be the already sufficient habitual protein intake in our population (1.1 ± 0.3 g·kg body weight−1·d−1). This is in line with earlier studies in which we also reported no additional benefits of protein supplementation during prolonged exercise training in healthy, older populations habitually consuming ample protein (9,34,35). Furthermore, based on Dawson et al. (17), it could be speculated that the provided protein dose was insufficient to compensate for the anabolic resistance likely caused by inactivity and testosterone deprivation in this PCa patient population (21,36). Nonetheless, it seems safe to conclude that protein supplementation is not required to achieve gains in muscle mass and strength during a period of resistance exercise training in PCa patients. However, it should be evident that nutritional interventions to allow sufficient dietary protein intake should be considered in older PCa patients with a poor nutritional status.
Besides improvements in body composition, muscle mass, and strength, our intervention diminished the ADT-induced decrease in aerobic capacity. Similar findings have been reported in studies applying a combined endurance and resistance exercise training program (37–39). However, we only offered resistance exercise training, indicating that even resistance exercise training can effectively offset the negative effects of ADT on skeletal muscle oxidative capacity. This provides further support to advocate resistance exercise training as an adjuvant treatment strategy during and/or after ADT. In addition, the positive effects of resistance exercise training on oxidative capacity and body composition provide important health benefits, as both a decreased oxidative capacity as a higher fat% are associated with poorer oncologic prognosis (40–42) and a higher risk of comorbidities like cardiovascular disease (43–45).
In our study, all exercise sessions were supervised and group based. This enabled us to provide patients with optimal guidance and to tailor the program to variations in their daily condition (e.g., during chemotherapy), which is important for the prevention of injuries and optimizing the training load. Our exercise intervention was feasible, safe, and well tolerated, with an overall adherence score of 82% ± 21%. In agreement with previous studies (46), we experienced that the group-based setting of the exercise training enabled social interactions, provided unconstrained peer support, and increased pleasure. This all could have contributed to the excellent adherence and compliance to our exercise training program. However, our study also has limitations. The shorter ADT treatment duration in the control group could have potentially affected the outcome. Furthermore, because of the COVID-19 pandemic, the total group of included patients was 96 instead of the initially aimed 208 patients. As a consequence, we were underpowered to detect small but potentially clinically relevant differences between the protein supplemented and the placebo group.
Despite these limitations, our study clearly shows the many benefits of resistance exercise training that completely offset the negative side effects of ADT on muscle mass and function. Combined with a high compliance and adherence to the exercise program, we strongly advise the implementation of personalized and supervised resistance exercise training in PCa patients treated with ADT. Exercise should become part of the standard urological care for these patients. Therefore, specialists should actually prescribe exercise to all patients (starting) with ADT, including patients with bone metastases. Although it was no specific goal, our study adds evidence to earlier studies (47–49) that a well-designed resistance exercise program can be applied safely in patients with bone metastases, providing that there are no medical objections (e.g., high risk for pathological fractures). We advise an exercise program supervised by an exercise physiologist with PCa-specific expertise, delivered in small PCa-specific groups. Resistance exercise training should be a major component of this program, but the addition of an aerobic component might possibly have added value.
CONCLUSIONS
In conclusion, resistance exercise training is safe, feasible, and well tolerated in patients with advanced PCa on ADT. Resistance exercise training overcomes the negative side effects of ADT on body composition, muscle mass, muscle strength, and aerobic capacity in men with advanced PCa. Protein supplementation is not required to further augment gains in muscle mass and strength after resistance exercise training in PCa patients who habitually consume ample protein (>1.0 g·kg body weight−1·d−1).
Acknowledgments
The authors greatly appreciate the support and dedication of the patients who volunteered to participate in the study. They thank the staff of the urologic departments of the participating hospitals, the department of physiotherapy of MMC, the sport physicians of TopSupport Eindhoven, and the department of sports medicine of JBZ ‘s-Hertogenbosch for their collaboration and assistance. Many thanks also go to the colleagues of the M3 Research Unit Maastricht for their help and support, with special thanks to Joan Senden.
This project was organized by and executed under the auspices of TiFN (Wageningen, the Netherlands), a public–private partnership on precompetitive research in food and nutrition (Project code: 16NH03). Funding for this research was obtained from KWF Kankerbestrijding (Amsterdam, The Netherlands) and Arla Food Ingredients (Viby J, Denmark).
The researchers are responsible for the study design, data collection and analyses, decision to publish, and preparation of the manuscript. The industrial partner has contributed to the project through regular discussion and were involved in the study design. The protein and placebo supplements were produced by the industrial partner. The industrial partner had no role in data collection, analysis, interpretation of results, or decision to publish.
This trial was registered at www.trialregister.nl as NL6258.
U. R. M. is an employee of Arla Foods Ingredients. J. T. and L. B. V. have received research grants, consulting fees, speaking honoraria, or a combination of these from FrieslandCampina. L. J. Cv. L. has received research grants, consulting fees, speaking honoraria, or a combination of these for research on the effect of exercise and nutrition on muscle metabolism, which include funding from companies such as FrieslandCampina and Arla Foods Ingredients. A full overview on research funding is provided at https://www.maastrichtuniversity.nl/l.vanloon. S. B. has received a research grant, consulting fees, speaking honoraria, or a combination of these from Baxter, Nutricia, and Arla Food ingredients. M. B. has received a research grant from Arla Foods Ingredients. None of the other authors has any conflicts of interest to disclose. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
L. H. P. H., M. O., S. B., and M. B. contributed equally to this work.
Contributor Information
LISANNE H. P. HOUBEN, Email: l.houben@maastrichtuniversity.nl.
MAARTEN OVERKAMP, Email: m.overkamp@maastrichtuniversity.nl.
PUCK VAN KRAAIJ, Email: puckvankraaij@hotmail.com.
JORN TROMMELEN, Email: jorn.trommelen@maastrichtuniversity.nl.
JOEP G. H. VAN ROERMUND, Email: joep.van.roermund@mumc.nl.
PETER DE VRIES, Email: pe.devries@zuyderland.nl.
KEVIN DE LAET, Email: K.deLaet@mmc.nl.
SASKIA VAN DER MEER, Email: Sa.v.d.Meer@jbz.nl.
ULLA R. MIKKELSEN, Email: ulrmk@arlafoods.com.
LEX B. VERDIJK, Email: lex.verdijk@maastrichtuniversity.nl.
SANDRA BEIJER, Email: S.Beijer@iknl.nl.
MILOU BEELEN, Email: milou.beelen@maastrichtuniversity.nl.
REFERENCES
- 1.Mottet N van den Bergh RCN Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–62. [DOI] [PubMed] [Google Scholar]
- 2.Cornford P van den Bergh RCN Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II: 2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–82. [DOI] [PubMed] [Google Scholar]
- 3.Meng MV, Grossfeld GD, Sadetsky N, Mehta SS, Lubeck DP, Carroll PR. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology. 2002;60(3 Suppl 1):7–11. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63(4):742–5. [DOI] [PubMed] [Google Scholar]
- 5.Galvão DA Spry NA Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44–7. [DOI] [PubMed] [Google Scholar]
- 6.Berruti A Dogliotti L Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361–7. [PubMed] [Google Scholar]
- 7.Churchward-Venne TA Tieland M Verdijk LB, et al. There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc. 2015;16(5):400–11. [DOI] [PubMed] [Google Scholar]
- 8.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68(7):769–79. [DOI] [PubMed] [Google Scholar]
- 9.Leenders M Verdijk LB Van der Hoeven L, et al. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542–52. [DOI] [PubMed] [Google Scholar]
- 10.Tieland M Dirks ML van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–9. [DOI] [PubMed] [Google Scholar]
- 11.Beckwée D Delaere A Aelbrecht S, et al. Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J Nutr Health Aging. 2019;23(6):494–502. [DOI] [PubMed] [Google Scholar]
- 12.Segal RJ Reid RD Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–9. [DOI] [PubMed] [Google Scholar]
- 13.Winters-Stone KM Dobek JC Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winters-Stone KM, Dieckmann N, Maddalozzo GF, Bennett JA, Ryan CW, Beer TM. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol Nurs Forum. 2015;42(4):348–56. [DOI] [PubMed] [Google Scholar]
- 15.Nilsen TS Raastad T Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54(10):1805–13. [DOI] [PubMed] [Google Scholar]
- 16.Newton RU Galvão DA Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc. 2019;51(4):607–14. [DOI] [PubMed] [Google Scholar]
- 17.Dawson JK, Dorff TB, Todd Schroeder E, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. 2018;18(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez P Newton RU Taaffe DR, et al. Interventions for improving body composition in men with prostate cancer: a systematic review and network meta-analysis. Med Sci Sports Exerc. 2022;54(5):728–40. [DOI] [PubMed] [Google Scholar]
- 19.Lopez P, Taaffe DR, Newton RU, Galvão DA. Resistance exercise dosage in men with prostate cancer: systematic review, meta-analysis, and meta-regression. Med Sci Sports Exerc. 2021;53(3):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 21.Hanson ED Nelson AR West DW, et al. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J Clin Endocrinol Metab. 2017;102(3):1076–83. [DOI] [PubMed] [Google Scholar]
- 22.Backx EMP Hangelbroek R Snijders T, et al. Creatine loading does not preserve muscle mass or strength during leg immobilization in healthy, young males: a randomized controlled trial. Sports Med. 2017;47(8):1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. [DOI] [PubMed] [Google Scholar]
- 24.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–9. [DOI] [PubMed] [Google Scholar]
- 25.Mezzani A Agostoni P Cohen-Solal A, et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the exercise physiology section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16(3):249–67. [DOI] [PubMed] [Google Scholar]
- 26.Wall BA Galvão DA Fatehee N, et al. Reduced cardiovascular capacity and resting metabolic rate in men with prostate cancer undergoing androgen deprivation: a comprehensive cross-sectional investigation. Adv Urol. 2015;2015:976235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong J Payne D Caron J, et al. Reduced cardiorespiratory fitness and increased cardiovascular mortality after prolonged androgen deprivation therapy for prostate cancer. JACC CardioOncol. 2020;2(4):553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvão DA Nosaka K Taaffe DR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–52. [DOI] [PubMed] [Google Scholar]
- 29.Hanson ED Sheaff AK Sood S, et al. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. J Gerontol A Biol Sci Med Sci. 2013;68(4):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen PA, Dechet CB, Porucznik CA, LaStayo PC. Comparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: a pilot study. PM R. 2009;1(11):1019–24. [DOI] [PubMed] [Google Scholar]
- 31.Baracos V, Kazemi-Bajestani SM. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol. 2013;45(10):2302–8. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25(5):947–58. [DOI] [PubMed] [Google Scholar]
- 33.Snijders T Res PT Smeets JS, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145(6):1178–84. [DOI] [PubMed] [Google Scholar]
- 34.Verdijk LB Jonkers RA Gleeson BG, et al. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608–16. [DOI] [PubMed] [Google Scholar]
- 35.Holwerda AM Overkamp M Paulussen KJM, et al. Protein supplementation after exercise and before sleep does not further augment muscle mass and strength gains during resistance exercise training in active older men. J Nutr. 2018;148(11):1723–32. [DOI] [PubMed] [Google Scholar]
- 36.Hanson ED, Betik AC, Timpani CA, Tarle J, Zhang X, Hayes A. Testosterone suppression does not exacerbate disuse atrophy and impairs muscle recovery that is not rescued by high protein. J Appl Physiol (1985). 2020;129(1):5–16. [DOI] [PubMed] [Google Scholar]
- 37.Cormie P Galvão DA Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115(2):256–66. [DOI] [PubMed] [Google Scholar]
- 38.Wall BA GalvãO DA Fatehee N, et al. Exercise improves V˙O2max and body composition in androgen deprivation therapy-treated prostate cancer patients. Med Sci Sports Exerc. 2017;49(8):1503–10. [DOI] [PubMed] [Google Scholar]
- 39.Ndjavera W Orange ST O’Doherty AF, et al. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2020;125(1):28–37. [DOI] [PubMed] [Google Scholar]
- 40.Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26(2):272–8. [DOI] [PubMed] [Google Scholar]
- 41.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fosam A, Perry RJ. Current mechanisms in obesity and tumor progression. Curr Opin Clin Nutr Metab Care. 2020;23(6):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aspenes ST Nilsen TI Skaug EA, et al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43(8):1465–73. [DOI] [PubMed] [Google Scholar]
- 44.Field AE Coakley EH Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–6. [DOI] [PubMed] [Google Scholar]
- 45.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. [DOI] [PubMed] [Google Scholar]
- 46.Cormie P, Zopf EM. Exercise medicine for the management of androgen deprivation therapy-related side effects in prostate cancer. Urol Oncol. 2020;38(2):62–70. [DOI] [PubMed] [Google Scholar]
- 47.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–35. [DOI] [PubMed] [Google Scholar]
- 48.Galvão DA Taaffe DR Spry N, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. 2018;50(3):393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenfield SA Van Blarigan EL Panchal N, et al. Feasibility, safety, and acceptability of a remotely monitored exercise pilot CHAMP: a clinical trial of high-intensity aerobic and resistance exercise for metastatic castrate-resistant prostate cancer. Cancer Med. 2021;10(22):8058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


