Abstract
Background:
Screening mammography guidelines do not explicitly consider racial differences in breast cancer epidemiology, treatment, and survival.
Objective:
To compare tradeoffs of screening strategies in Black women to those of White women screened under current guidelines.
Design:
An established Cancer Intervention and Surveillance Modeling Network model simulated screening outcomes using race-specific inputs for subtype distribution, breast density, mammogram performance, age-, stage-, and subtype-specific treatment effects, and non-breast cancer mortality.
Setting:
United States.
Participants:
1980 US birth cohort of Black and White women.
Intervention:
Screening strategies until age 74 with varying initiation ages and intervals.
Measurements:
Outcomes included benefits (life-years gained, breast cancer deaths averted, and mortality reduction), harms (mammograms, false positives, and overdiagnoses), and benefits-to-harm ratios (tradeoffs) by race. We evaluated efficiency (resources per unit benefit), mortality disparity reduction, and equity in tradeoffs. Equitable strategies for Black women were defined as those with tradeoffs closest to benchmark values for screening White women biennially from 50–74.
Results:
Biennial screening from 45–74 was the most efficient for Black women, while biennial screening from 40–74 was the most equitable. Initiating screening ten-years earlier in Black vs. White women reduced Black-White mortality disparities by 57% with comparable life-years gained/mammogram for both populations. Selection of the most equitable strategy was sensitive to assumptions about disparities in real world treatment effectiveness: the less effective treatment was for Black women, the more intensively Black women could be screened before tradeoffs fell short of those experienced by White women.
Limitations:
Single model.
Conclusion:
Initiating biennial screening in Black women at age 40 yields reduces breast cancer mortality disparities and yields benefit-to-harm ratios that are comparable to tradeoffs of White women screened biennially from 50–74.
Keywords: breast cancer, race, equity, disparities, screening mammography, decision analysis, simulation modeling, Black/African American
Introduction
Screening mammography guidelines provide recommendations for the overall US population(1, 2) but do not explicitly consider racial disparities in breast cancer epidemiology, screening, and treatment. Compared to White women, Black women in the US have a younger age at breast cancer diagnosis (58 v. 62 years),(3) are diagnosed more often with adverse features, including triple-negative(4) and advanced stage disease,(3) and have higher age-standardized breast cancer mortality rates (28.2 vs. 20.3 per 100,000).(3, 5)
These disparities are partially mediated through and further complicated by racism, particularly the institutionalized(6)/structural(7) and interpersonal(6) forms. Structural racism drives breast cancer disparities by influencing upstream healthcare factors (e.g. insurance access(8)) and broader societal constructs (e.g. poverty(9)), which influence stage and treatment receipt. Structural and interpersonal racism may also explain point-of-care disparities that drive screening and treatment differences.(10–13). Finally, all three forms of racism (institutionalized, interpersonal, and individualized(6)) racism influence competing mortality,(14, 15) which modifies screening outcomes. These complexities suggest that Black women may need different screening schedules to achieve similar screening outcomes as White women.
Unfortunately, no randomized trial data exist to optimize screening by race since few Black women were included in early trials(16, 17). Ideally, new trials would test screening schedules by race, but are not feasible due to the large sample sizes required. In these situations, simulation modeling can synthesize race-specific data and test a range of screening strategies. The Cancer Intervention and Surveillance Modeling Network (CISNET) models were previously used to inform breast cancer screening guidelines, but guideline-focused studies lacked race-specific modeling.(18, 19) Separate race-specific modeling studies lacked current knowledge about molecular subtypes and modern therapy. (20, 21)
In this study, we used an updated, race-specific CISNET model to identify equitable screening strategies, defined as strategies for Black women that yielded benefit-to-harm tradeoffs similar to those of White women screened according to the United States Preventive Services Task Force (USPSTF) guidelines.(1) The results are intended to inform discussions about health equity, given that race-neutral screening guidelines can do harm if they yield unequal outcomes and are applied instead of more equitable alternatives that retain acceptable tradeoffs.
Methods
We used CISNET Model GE (Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, NY) for this study. 12–14 The study was considered human subjects exempt by the Georgetown University Institutional Review Board due to public de-identified data use.
Screening Strategies and Population
We evaluated nine strategies that varied by starting age (40, 45, and 50 years) and interval (annual, biennial and hybrids: annual 40–49 and biennial thereafter; biennial 40–49 and annual thereafter; and the American Cancer Society recommendation: annual 45–54 and biennial thereafter(2)); with cessation at 74. The nine strategies were compared to biennial screening of White women from 50–74 since this is the implicit benchmark for outcomes and benefit-to-harm ratios based on US Preventive Service Task Force guidelines(1). In secondary analyses, we evaluated two additional strategies: initiation of annual screening at 30 or 35 through 39, followed by biennial screening from 40 to 74 (Appendix Figure 4a & 4b).
We modeled the cohort of US women born in 1980, who turn 40 in 2020, followed for their lifetimes starting from age 25 (because breast cancer is rare before then). As in prior modeling studies,(18) we assumed 100% of Black and White women used screening to focus on screening efficacy. This assumption was considered reasonable since contemporary studies show minimal to no difference in screening mammography use between Black and White women.(22)
Model Overview
The model has been described in detail elsewhere (schematic, Appendix Figure 1).(23–25) Additional information is available upon request. The model is available for use via collaboration. Briefly, model GE is a parallel-universe population simulation model that begins with estimates of breast cancer incidence and estrogen receptor /HER-2-specific survival trends in the absence of screening or adjuvant treatment.(24–27) Breast cancer is modeled to have a molecular sub-type specific distribution of preclinical screen-detectable periods (sojourn time) and clinical detection times. The model assumes one-third of ductal carcinoma in-situ cases do not progress to invasive cancer. Molecular subtype- and stage-specific treatment reduces the hazard of breast cancer death. Women can die of breast cancer or other causes.
Model Input Parameters
The model parameters(24, 27) were updated with race-specific inputs (Table 1). Race was typically defined by self-report. Breast cancer incidence was modeled based on an age-period-cohort model.(26) Race-specific rates were obtained by applying an age-specific relative risk of breast cancer for Black vs. White women using Surveillance, Epidemiology, and End Results (SEER) data.(28)
Table 1:
Model Input Parameters
| Parameter | Description and Race-Specificity | Source | Race Definition |
|---|---|---|---|
| Births | Birth Cohorts from 1890–2000 by race | Historical Statistics of the United States, Millennial Edition, Vol. 1. Cambridge University Press, 2006. (61) | Self-report |
| Incidence | Age-period-cohort model with age-specific relative risk of Black versus White Incidence | SEER (18, 26, 28) | Self-report prioritized if available, otherwise peer SEER standards, used data from medical records |
| Mammography Use | Assumed equal by race and 100% to isolate the impact of mammography under ideal screening conditions | - | - |
| Mammography Sensitivity | Age-specific rates for first and subsequent screening exams by race | Unpublished BCSC data, agreement DR285e(31) | Self-report |
| Breast Density | Prevalence by age and race | Unpublished BCSC data, agreement DR285e(31) | Self-report |
| ER/HER2 | Probability of ER/HER-2 conditional on age, stage, and race | Unpublished BCSC data, agreement DR285e(32) | Self-report |
| Sojourn time | Calibrated parameters; gamma distributions by age, ER and HER-2 status | Model GE calibration (24) | - |
| Unscreened Stage Distribution | Clinically-detected cases 2005–2017, by age and race | Unpublished BCSC data, agreement DR285e(32) | Self-report |
| Screened Stage Distribution | Digital screen and interval-detected cases 2005–2017, by age and race | Unpublished BCSC data, agreement DR285e(32) | Self-report |
| Survival without treatment | Survival by race from SEER 1975–1979, assumed equal by race | SEER(62) | Self-report prioritized if available, otherwise peer SEER standards, used data from medical records |
| Treatment efficacy | Reduction in hazard of breast cancer death, Meta-analyses of randomized trial results by ER/HER-2; assumed equal by race(34) | Clinical trial Meta-analyses (33, 34, 63) | - |
| Treatment dissemination | Assumed 100% for White women per previous modeling studies for USPSTF, Reduced for Black women to account for impact of disparities in treatment receipt; assumed 80% for Black women for base case with sensitivity analysis performed using range of 50%−100% | National Comprehensive Cancer Network data | Self-report |
| Non-breast cancer (other cause) mortality | Age-, race-, and cohort-specific other-cause mortality rates by year | Modeling performed by University of Wisconsin Breast CISNET group/CDC Wonder (39) | Self-report |
Table 1: Abbreviations: BCSC: Breast Cancer Screening Consortium; CISNET: Cancer Intervention and Surveillance Modeling Network; ER: estrogen receptor; SEER: Surveillance, Epidemiology and End Results;
Race-specific breast density was modeled using BIRADS categories(29) and assigned from ages 25–40. Density could decrease by one category or remain the same at age 50–64 and again at 65 based on prevalence observed in the Breast Cancer Surveillance Consortium database.(30, 31) We assumed density affected mammogram performance characteristics and incidence.
Screening sensitivity and specificity by age-, race- and density group were calibrated to Breast Cancer Surveillance Consortium data for invasive cancers and ductal carcinoma in-situ combined on initial vs. subsequent mammography.(31)
Stage was defined based on American Joint Committee on Cancer v.6, and dependent on age group (<50, 50+), density, molecular subtype and screen vs. clinical detection.(32) Stage- and molecular subtype-specific chemotherapy included anthracycline-based regimens with taxanes; estrogen receptor+ tumors included five years of endocrine therapy and HER-2/ErbB-2+ tumors included trastuzumab.
We model treatment effects by considering treatment efficacy and dissemination. Treatment efficacy was based on clinical trials(33) and was modeled as a reduction in the hazard of breast cancer death. We used data from pooled analyses of National Surgical Adjuvant Breast and Bowel Project (NSABP) trials to estimate race-specific treatment efficacy(34). That analysis demonstrated similar or slightly lower systemic therapy efficacy for Black relative to White women with treated on the same trials and considering age, stage, comorbidities, and estrogen receptor status.(34, 35) We therefore conservatively assumed equal efficacy by race.
However, outside of clinical trials, treatment effectiveness depends on differences in treatment dissemination, including access, delays, dose reductions, and discontinuation. Sub-optimal treatment dissemination occurs more often in Black than White women.(21, 36–38) In previous policy-oriented work,(18) we assumed full dissemination (i.e. all women receive the most effective therapy) to identify a pure effect of screening under optimal treatment conditions. However, given the differences in treatment dissemination by race, we used published data(36) to estimate the impact of disparities in dissemination. The best available evidence we identified showed that after accounting for mediators contained in our model, (e.g. stage and subtype), a residual Black-White disparity in breast cancer death remains (hazard ratio 1.24, Table 3, Model 3 from citation(36)). We converted this hazard ratio to a percentage (80.6%) and incorporated it into the dissemination parameter to account for decreased treatment effects in Black women.
We used existing US race- and age-specific non-breast cancer mortality rates.(26, 39) These mortality rates implicitly capture the net effect of racism, downstream disparities (e.g. comorbidities, social determinants of health, access to care) and other factors that differentially influence survival by race.
Analysis
We simulated 100 million life histories from birth to death, or age 120, to account for the entire potential life history in the absence of screening and treatment. Simulations strategies were repeated with screening and treatment effects for each strategy among Black women. We also simulated biennial screening of White women from 50 to 74 followed by optimal systemic therapy. The results for White women served as the benchmark for acceptable benefit-to-harm ratios. Benefits included percent reduction in breast cancer mortality, breast cancer deaths averted, and life-years gained (LYG). Harms included false positives, benign biopsies, and overdiagnoses, with the latter often leading to surgical treatment, e.g. lumpectomy or mastectomy. False positives were calculated using specificity estimates and defined as screens resulting in additional imaging that did not result in the diagnosis of breast cancer within 12 months(40). Overdiagnoses were defined as cases that would not have been clinically detected in the absence of screening because of lack of progressive potential or preceding death from competing non-breast cancer mortality. We calculated benefits, harms, and benefit-to-harm ratios for each combination of metrics. We chose LYG as our primary outcome metric given the differences in age-specific breast cancer incidence and non-breast cancer mortality by race. Number of mammograms and the ratio of LYG to mammograms were our primary harm and benefit-to-harm metrics for comparability to past guideline analyses(18, 19). Ratios of other metrics were secondary measures.
We used benchmarks for White women to identify the most equitable strategy for Black women, defined as strategies resulting in the most similar benefit-to-harm ratios (i.e. tradeoffs). We also quantified the change in the breast cancer mortality disparity compared to equivalent screening, defined as the difference between the Black-White mortality disparity under a) equivalent screening (i.e. B50–74 for both racial groups) and b) tailored screening (e.g. B50–74 in Whites and B45–74 in Blacks), divided by the disparity under equivalent screening.
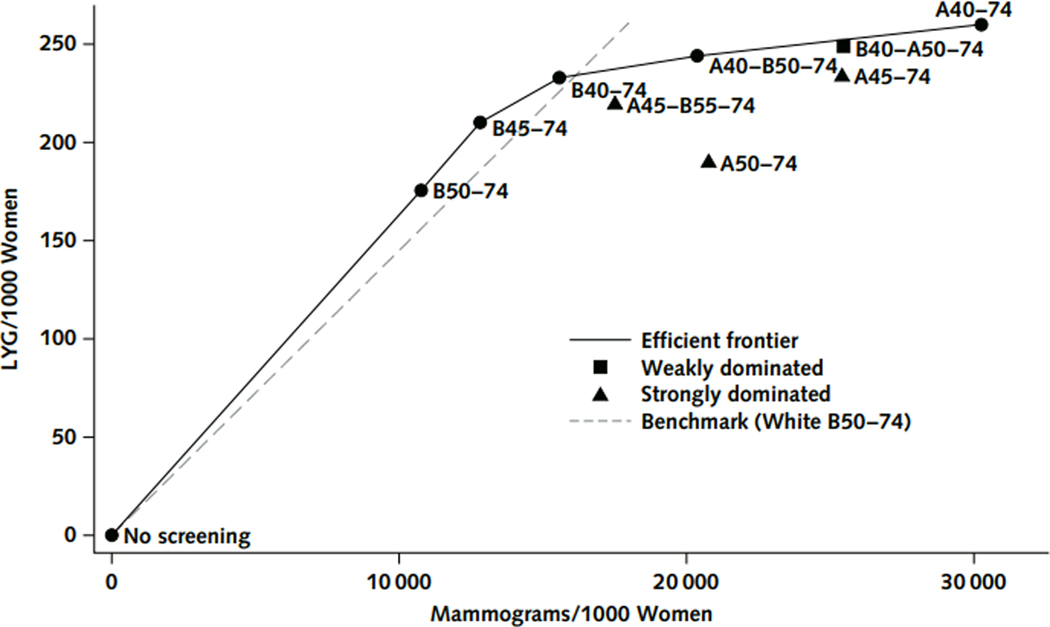
We displayed data for the screening scenarios among Black women on an efficiency frontier(41) by connecting the sequence of points representing the largest change in incremental benefits per harm. Strategies on the frontier were considered to be efficient. Strategies that caused more harms or required more mammograms but provided fewer benefits than any other strategy were considered to be strongly dominated. We also applied the concept of weak, or extended dominance. Weakly dominated strategies are strategies with an incremental harm-to-benefit ratio greater than that of a more beneficial strategy(42).
Sensitivity analyses tested the impact on results of a range of systemic therapy effects for Black vs. White women. We varied our base case estimate of 80% from 50–100% in sensitivity analysis, where 100% indicated that treatment effects were the same for Black and Whites.
Role of the Funding Source
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Results
Benefits
Among the strategies tested in Black women, benefits generally increased as the number of mammograms increased due to initiating screening earlier than age 50 and/or screening more frequently (Table 2). Efficient strategies for Black women always included the biennial strategies and the most intensive strategy, A40–74. Biennial screening from 45–74 was the most efficient for LYG/mammogram (Figure 1&2). Annual strategies starting at 45 or 50 and the American Cancer Society hybrid strategy were dominated (Figure 1 and Appendix Figure 3a–c). Efficient strategies were similar considering other metrics (Appendix Figure 3a–c). Marginal benefits for initiating biennial screening at age 40, 45, or 50 v. no screening and B50–74 are shown in Appendix Figure 5.
Table 2:
Benefits, Harms, and Benefit to Harm Ratios of Breast Cancer Screening Strategies for Black Women Compared to the Benchmark (B50–74) Strategy for White Women
| Per 1000 women screened (vs no screening) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Benefits | Harms | Benefit/Harm Ratio | Disparity Reduction | ||||||
| Strategy | Mammo-grams | Life-Years Gained | Breast cancer deaths averted* | Percent mortality reduction | False Positives | Over-diagnoses | Life-Years Gained Per Mammo-gram x 10−3 | Life-Years Gained Per Overdiagnosis | BC death disparity reduction (v. B50–74 for both races) |
| White women | |||||||||
| B50–74 | 11137 | 161 | 8.3 | 37% | 864 | 8.0 | 14.5 | 20.1 | - |
| Black women | |||||||||
| B50–74 | 10761 | 176 | 9.5 | 35% | 829 | 7.0 | 16.3 | 25.1 | 0% |
| B45–74 | 12826 | 210 | 10.5 | 39% | 1031 | 7.3 | 16.4 | 28.8 | 31.4% |
| B40–74 | 15576 | 233 | 11.3 | 42% | 1264 | 8.1 | 15.0 | 28.8 | 57.0% |
| A45-B55–74 | 17511 | 219 | 10.8 | 40% | 1399 | 7.4 | 12.5 | 29.6 | 42.2% |
| A40-B50–74 | 20370 | 244 | 11.7 | 43% | 1693 | 8.2 | 12.0 | 29.8 | 69.3% |
| A50–74 | 20660 | 192 | 10.4 | 38% | 1522 | 7.6 | 9.3 | 25.3 | 29.2% |
| A45–74 | 25411 | 234 | 11.9 | 44% | 1950 | 8.3 | 9.2 | 28.2 | 74.2% |
| B40-A50–74 | 25464 | 249 | 12.3 | 45% | 1957 | 8.7 | 9.8 | 28.6 | 86.0% |
| A40–74 | 30257 | 260 | 12.7 | 47% | 2385 | 8.8 | 8.6 | 29.5 | 97.7% |
Table 2: A indicates annual; B indicates biennial, numbers after “A” or “B” denote cessation and transition ages. Data shown represent the base case of 80% treatment effects/dissemination.
Breast cancer deaths per 1,000 women without screening: Black 27.07691 and White: 22.65354.
Figure 1:
Efficiency frontier for the base case (80% treatment effects for Black women) for life-years gained per mammogram (LGY/M). Treatment effects are described as “dissemination” here to clarify the assumptions made: efficacy was assumed to be equal for black and white women, but dissemination differed due to disparities in treatment receipt that impacted breast cancer survival. Efficient (blue circles and line), weakly dominated (green squares) and strongly dominated (red triangle) strategies are shown. The dashed line shows life-years gained per mammogram benchmark (B50–74 in White women). Strategies for Black women that fall above the line yield greater LYG/M than then benchmark and those that fall below the line yield fewer LYG/M than the benchmark.
Figure 2:
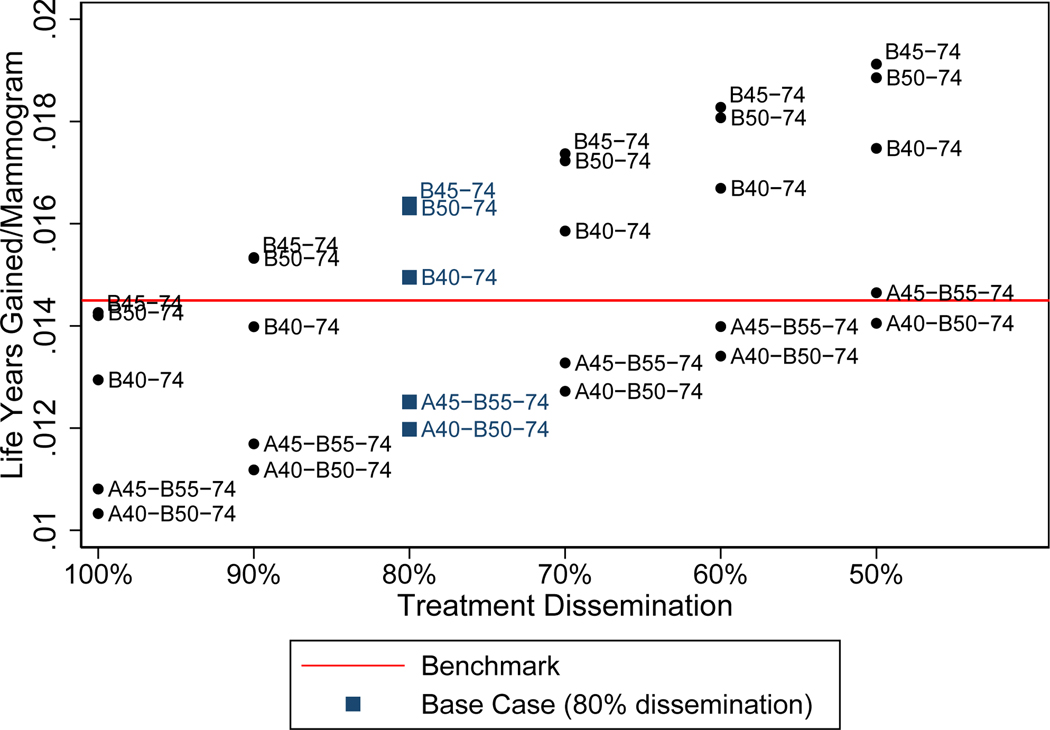
Life years gained/mammogram sensitivity analysis. The Black/White ratio of treatment effects is varied from 50–100%, with 80% representing the base case (blue squares). Treatment effects are described as “dissemination” here to clarify the assumptions made: efficacy was assumed to be equal for black and white women, but dissemination differed due to disparities in treatment receipt that impacted breast cancer survival. Strategies above and below the red line yield greater and lesser LYG/M than the benchmark (B50–74 in White women), respectively.
Equity in Benefit-to-Harm Ratios
The strategy that yielded the LYG per mammogram ratio closest to the benchmark (B50–74 for White women) was biennial 40–74, (15.0 vs. benchmark: 14.5 LYG per mammogram, respectively) (Table 2). Among the three strategies that yielded benefit-to-harm ratios that met or exceeded the benchmark, B40–74 resulted in the largest mortality reduction for black women (Figure 1 & Table 2). B40–74 resulted in 32% more LYG and 19% more breast cancer deaths averted than screening Black women biennially from ages 50–74 but required 45% more mammograms and resulted in 52% more false positives (calculated from Table 2). For secondary metrics, B40–74 remained the most equitable strategy (Appendix Figure 2a–c), with the exception that B45–74 was slightly more equitable when considering breast cancer deaths averted/false positive.
Impact on Mortality Disparities
If Black and White women were screened biennially from 50–74, there would be an excess of 3.29 deaths among Black women (17.62 vs. 14.33 per 1,000 for Black v. White women, respectively, calculated from Table 2). In contrast, if biennial screening was initiated in Black women beginning at 40, deaths would drop by 1.88 (from 17.62 to 15.74) per 1,000 women, removing 57% of the racial disparity (Table 2) in mortality expected under current guideline screening (1.88 of 3.29 excess deaths).
Sensitivity Analysis
The results were sensitive to assumptions about treatment disparities. As treatment dissemination decreased, relative benefits of screening increased, permitting use of progressively more intensive strategies before tradeoffs fell below benchmarks (Figure 2). If treatment were equally disseminated for Black and White women (but current levels of competing mortality disparities persisted), screening Black women biennially from 50–74 would yield similar benefit-to-harm ratios as the benchmark values for White women (Figure 2 and Appendix Table 1a–d). If disparities in treatment resulted in Black women experiencing 90% or less of treatment effectiveness experienced by Whites, biennial screening would need to start at 40 or 45 in Black women to achieve benefit-to-harm ratios comparable to benchmark values.
Discussion
To our knowledge, this is the first study to use simulation modeling to consider whether race-neutral breast cancer screening guidelines lead to unequal outcomes. Our results suggest that in self-identified Black women, initiation of earlier screening than is presently recommended for the overall US population by the US Preventive Services Task Force(1) or the American Cancer Society(2) can reduce mortality disparities and maintain acceptable benefit-to-harm tradeoffs. This highlights an important concept in health equity: equivalent interventions may yield inequitable outcomes(43).
Our results were highly sensitive to assumptions about disparities in treatment dissemination. Consistent with previous modeling studies(44), relative benefits of screening increased as treatment effectiveness decreased (i.e. Black-to-White disparities widened). This explains why more intensive screening strategies can be employed as disparities widen without compromising tradeoffs (relative to benchmark values). Although our previous policy-oriented studies(18) estimated screening benefits under ideal treatment conditions, this assumption ignores the impact of racism and other causes of disparities. Racism increases disparities in treatment and competing mortality, but these two inputs have opposing effects (i.e. competing mortality decreases relative screening benefits). Therefore, ignoring decades-old treatment disparities would have underestimated relative screening benefits for Black women.
Similar to conclusions from our past modeling analyses(18, 19), most annual screening strategies for Black women were inefficient; they had fewer benefits and more harms than biennial strategies. One explanation is that although higher age-specific breast cancer incidence in the 40s in Black vs. White women provides sufficient benefit to outweigh harms of starting screening at age 40, there may not be sufficient differences in the parameters we used to model tumor biology to warrant annual vs. biennial screening. We will reassess as knowledge about breast cancer biology evolves.
Comorbidities also vary in a complex, race-specific manner. For example, obesity decreases treatment effectiveness (due to suboptimal completion and other factors), but has mixed effects on breast cancer incidence. Obesity decreases breast cancer incidence pre-menopausally, but increases incidence post-menopausally. Unfortunately, the barriers that preclude equitable breast cancer treatment often prevent equitable treatment of comorbidities.(45) Our group previously modeled the impact of obesity on racial disparities in breast cancer and found that obesity had no net effect on disparities due to opposing pre- and post-menopausal effects(20). In the current study, the net impact of comorbidities on breast cancer incidence and treatment is already implicitly considered, given that our inputs are derived from real-world datasets that contain women with comorbidities. However, specific comorbidities may sufficiently alter screening outcomes for subsets of women. In future analyses, we will model screening recommendations for groups of women by race with specific comorbidities.
The role of screening in reducing disparities represents a dynamic interplay between tumor growth, early detection, and molecular-targeted therapy. This is illustrated by our finding that when disparities in treatment dissemination were eliminated, similar screening could yield similar outcomes for Black and White women, but if treatment disparities persist or widen, then Black women might benefit from more intensive screening than White women. Although earlier screening may partially mitigate the impact of treatment disparities, it should not supersede efforts to achieve treatment equity. Indeed, CISNET(21) and others(36) have shown that disparities in treatment represent one of the largest modifiable mediators of breast cancer survival disparities. Addressing treatment disparities therefore remains a high priority. However, aspects of treatment disparities are attributable to systemic racism, which is difficult to change and won’t be resolved in the near term. We therefore reduce harm by compensating for this with enhanced screening. Implementation of equitable screening represents a practical, sustainable, high-impact solution for reducing disparities that could be implemented in the short term.
However, elimination of breast cancer racial disparities goes beyond screening and treatment. Racial disparities in insurance and stage at diagnosis reflect the larger and longstanding issue of structural racism (employment, educational opportunity, etc.), (7, 8). Well-placed efforts within healthcare may, therefore, fall short of eliminating cancer inequity.
This study used a well-established CISNET model and followed best modeling practices. (18, 27) However, there are several caveats to consider. First, we used a single model. All models make structural assumptions about non-observable aspects of breast cancer, including the proportion of ductal carcinoma in-situ cases that progress to invasive cancer. We plan to expand these analyses with several CISNET models to test the impact of structural uncertainty on conclusions about race-specific screening schedules. There is also parameter uncertainty in any simulation model, but we used the model previously and calibrated to US trends using multiple real-world data sources.(27)
Second, our purpose was to establish whether there was a scientific rationale for recommending different screening strategies by race assuming full screening efficacy (i.e., 100% use). However, patterns of use may vary by age and race, affecting screening outcomes. For example, if younger Black women are less likely to complete biannual screening exams than older Black women, the benefits of starting screening at 40 vs. 50 would decrease. If there are differences in return to screening after a false positive by race(46) (or age(47)), then relative benefit-to-harm ratios for Black and white women might shift. We will address the age and race patterns in future analyses. We will account for the fact that tomosynthesis may decrease false positives(48) and that culturally competent coping strategies(49) and physician counseling(50) can reduce mammography avoidance after false positives. Our findings are likely to be relevant into the future until there are major changes in early detection technology or treatment paradigms. The models consider survival after local and systemic therapy, but do not model types of surgery. We did not model cost, but plan to in subsequent analyses. Earlier screening initiation may increase patient, payer, and societal costs, but earlier detection may reduce treatment costs and save more lives. Screening harms (e.g. false positives, benign biopsies, and overdiagnoses) can affect quality of life, but there are no current data to suggest that the quality of life effects differ by race. Additionally, our study is designed inform to population-level guidelines, and cannot fully capture nuances that may alter the risks and benefits for individual women whose characteristics differ substantially from those in our study.
Finally, race and racism (whether structural, interpersonal, or internalized (6)) are complex constructs. Many, including members of our own team, have published on race and recognize that associations between race and health or societal outcomes are often rooted in racism as opposed to biology. (51) (20, 21, 52–54) (55) (56, 57)
Our modeling used nationally representative data for US women that self-report as Black. Our choice of approach was guided by modeling best practices (23, 58), guidelines on presenting research on racial inequities(59), and consideration of the practicalities of making recommendations for screening in clinical practice. We use self-reported race because it is strongly associated with breast cancer mortality,(3) breast cancer molecular subtype distribution,(3) observed treatment effectiveness,(36) and competing mortality.(14) These associations persist even after socioeconomic status is considered(14, 36), suggesting that replacing socioeconomic status for race would not be methodologically appropriate in our study. These data also informed our modeling of treatment effects in Black women: Black women with different molecular sub-types of breast cancer derive equal benefits from equal treatment in clinical trials,(34) but treatment remains unequal in practice.(4, 57)
We acknowledge that racism and not race, islikely the primary driver of many of the disparities in inputs in our study. However, few datasets contain validated measures of racism, so self-reported race remains the best available variable at the present time. Racial disparities in breast cancer mortality are complex, and can persist after partial efforts to control for socioeconomic status. We are exploring data sources that could better capture the effects of lifetime socioeconomic status and racism in future studies. Until then, the majority of our model inputs are derived from U.S. population-based data. The results capture the heterogeneity in Black women and are generalizable to self-identify as such. Compared to screening guidelines for the overall US population, our results suggest that alternative screening guidelines provide an opportunity to reduce racial disparities in breast cancer mortality without increasing harms. Failing to consider race in this context may represent a missed opportunity to reduce breast cancer disparities while allowing Black women to derive the same screening tradeoffs as White women.
Overall, despite some improvements, (28, 60) Black-White breast cancer disparities persist. Our results suggest that Black women consider initiating biennial screening at age 40 instead of 50. Given that this screening strategy falls within the “individual decision making” category for the US Preventive Services Task Force, this represents a practical, evidence-based opportunity to advance equity.
Supplementary Material
Acknowledgment:
The authors acknowledge Ruth Etzioni Ph.D., Fred Hutchinson Cancer Research Center and University of Washington, Natasha Stout Ph.D., Harvard Pilgrim Healthcare Institute, Nicolien Van Ravesteyn Ph.D., Erasmus MC University Medical Center, for their insights on earlier versions of this work, as well as Mack Roach M.D., University of California, San Francisco, and James Dignam Ph.D., University of Chicago for their insights on investigating racial disparities in treatment efficacy. No compensation was received by Drs. Etzioni, Stout, Van Ravesteyn, Roach, or Dignam. Everyone who contributed significantly to the work is listed has been included as a co-author or is listed in the Acknowledgments.
Funding Source:
This research was funded by the National Institutes of Health at the National Cancer Institute Grant U01CA199218, U01C1199218-02, and P30CA046592. The research was also supported in part by the National Cancer Institute Grant R35CA197289 to JM and P30CA014520 to AT-D. Data collection and sharing were supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium. You can learn more about the Breast Cancer Surveillance Consortium at http://www.bcsc-research.org/.
Footnotes
Protocol: Not applicable
Computer code: Detailed information about the models is available at https://cisnet.cancer.gov/breast/profiles.html and in references(23, 40), but code or executables are not presently publicly available
Data: Output data from the models are available from Dr. Chapman (email: christinachap@gmail.com)
The views, statements, and opinions in this publication are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health, the Department of Veterans Affairs, or the Patient-Centered Outcomes Research Institute (PCORI), or its Board of Governors or Methodology Committee.
An earlier version of this work was presented in part at the National Medical Association Annual Convention, Honolulu, HI on July 29, 2019.
Professional Postal Addresses
Christina Hunter Chapman, University Hospital B2C490, SPC 5010, 1500 East Medical Center Drive, Ann Arbor, MI 48109–5010
Clyde Schechter, Department of Family & Social Medicine, 1300 Morris Park Avenue, Block Bldg, 4th Floor, Bronx, NY 10461
Christopher Cadham, Department of Oncology, Lombardi Cancer Center, Georgetown University School of Medicine, 3300 Whitehaven Boulevard, Suite 4100, Box 571469, Washington, DC 20057–1469
Amy Trentham-Dietz, PhD, Professor, Population Health Sciences, University of Wisconsin-Madison, 610 Walnut St., WARF Room 307, Madison, WI 53726
Ronald Gangnon, Department of Population Health Sciences, University of Wisconsin-Madison, 603 WARF, 610 Walnut St, Madison, WI 53726
Reshma Jagsi, University Hospital B2C490, SPC 5010, 1500 East Medical Center Drive, Ann Arbor, MI 48109–5010
Jeanne Mandelblatt, Department of Oncology, Lombardi Cancer Center, Georgetown University School of Medicine, 3300 Whitehaven Boulevard, Suite 4100, Box 571469, Washington, DC 20057–1469
Conflicts of Interest: The authors have no relevant conflicts to disclose.
Contributor Information
Christina Hunter Chapman, Center for Clinical Management Research, Veterans Administration Ann Arbor Healthcare System, Ann Arbor, Michigan, USA.; Department of Radiation Oncology at the University of Michigan School of Medicine in Ann Arbor, Michigan, USA.
Clyde B. Schechter, Departments of Family and Social Medicine and Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA..
Christopher J. Cadham, Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Amy Trentham-Dietz, Department of Population Health Sciences and the Carbone Cancer Center, University of Wisconsin-Madison, Madison, Wisconsin, USA..
Ronald E. Gangnon, Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison, Madison, Wisconsin, USA.; Department of Population Health Sciences and the Carbone Cancer Center, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Reshma Jagsi, Center for Bioethics and Social Sciences in Medicine at the University of Michigan, Ann Arbor, Michigan, USA
Jeanne S. Mandelblatt, Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
References
- 1.Siu AL, on behalf of the USPSTF. Screening for breast cancer: U.s. preventive services task force recommendation statement. Annals of internal medicine. 2016;164(4):279–96. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Fontham EH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the american cancer society. Jama. 2015;314(15):1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA surgery. 2017;152(5):485–93. Epub 2017/03/30. doi: 10.1001/jamasurg.2017.0005. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33. Epub 2019/02/15. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 5.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(7):1039–45. Epub 2015/05/15. doi: 10.1158/1055-9965.epi-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212–5. Epub 2000/08/11. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389(10077):1453–63. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 8.Ko NY, Hong S, Winn RA, Calip GS. Association of Insurance Status and Racial Disparities With the Detection of Early-Stage Breast Cancer. JAMA oncology. 2020;6(3):385–92. doi: 10.1001/jamaoncol.2019.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. JNCI: Journal of the National Cancer Institute. 2015;107(6). doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhargava A, Du XL. Racial and socioeconomic disparities in adjuvant chemotherapy for older women with lymph node-positive, operable breast cancer. Cancer. 2009;115(13):2999–3008. Epub 2009/05/20. doi: 10.1002/cncr.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38. Epub 2015/05/12. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 12.Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Racial Differences in Time to Breast Cancer Surgery and Overall Survival in the US Military Health System. JAMA surgery. 2019;154(3):e185113-e. doi: 10.1001/jamasurg.2018.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Medical care. 2009;47(7):765–73. Epub 2009/06/19. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- 14.Sautter JM, Thomas PA, Dupre ME, George LK. Socioeconomic Status and the Black–White Mortality Crossover. American Journal of Public Health. 2012;102(8):1566–71. doi: 10.2105/ajph.2011.300518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, et al. The Effect of Race and Sex on Physicians’ Recommendations for Cardiac Catheterization. New England Journal of Medicine. 1999;340(8):618–26. doi: 10.1056/nejm199902253400806. [DOI] [PubMed] [Google Scholar]
- 16.Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet. 2006;368(9552):2053–60. Epub 2006/12/13. doi: 10.1016/s0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro S. Periodic screening for breast cancer: the HIP randomized controlled trial. JNCI Monographs. 1997;1997(22):27–30. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Annals of internal medicine. 2016;164(4):215–25. Epub 2016/01/13. doi: 10.7326/m15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Annals of internal medicine. 2009;151(10):738–47. Epub 2009/11/19. doi: 10.7326/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Schechter CB, van Ravesteyn NT, Near AM, Heijnsdijk EA, Adams-Campbell L, et al. Collaborative modeling of the impact of obesity on race-specific breast cancer incidence and mortality. Breast cancer research and treatment. 2012;136(3):823–35. Epub 2012/10/30. doi: 10.1007/s10549-012-2274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ravesteyn NT, Schechter CB, Near AM, Heijnsdijk EA, Stoto MA, Draisma G, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(1):112–22. Epub 2010/12/02. doi: 10.1158/1055-9965.epi-10-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virk-Baker MK, Martin MY, Levine RS, Wang X, Nagy TR, Pisu M. Mammography utilization among Black and White Medicare beneficiaries in high breast cancer mortality US counties. Cancer causes & control : CCC. 2013;24(12):2187–96. Epub 2013/10/01. doi: 10.1007/s10552-013-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alagoz O, Berry DA, de Koning HJ, Feuer EJ, Lee SJ, Plevritis SK, et al. Introduction to the Cancer Intervention and Surveillance Modeling Network (CISNET) Breast Cancer Models. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(1_suppl):3s–8s. Epub 2018/03/20. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schechter CB, Near AM, Jayasekera J, Chandler Y, Mandelblatt JS. Structure, Function, and Applications of the Georgetown-Einstein (GE) Breast Cancer Simulation Model. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(1_suppl):66s–77s. Epub 2018/03/20. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, Cevik M, Schechter CB, Lee SJ, et al. Comparing CISNET Breast Cancer Models Using the Maximum Clinical Incidence Reduction Methodology. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(1_suppl):112s–25s. Epub 2018/03/20. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(6):905–12. Epub 2015/03/20. doi: 10.1158/1055-9965.epi-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. Jama. 2018;319(2):154–64. Epub 2018/01/11. doi: 10.1001/jama.2017.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surveillance E, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2018 Sub (1992–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. 2019. [Google Scholar]
- 29.Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS((R)) fifth edition: A summary of changes. Diagnostic and interventional imaging. 2017;98(3):179–90. Epub 2017/01/31. doi: 10.1016/j.diii.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Trentham-Dietz A, Kerlikowske K, Stout NK, Miglioretti DL, Schechter CB, Ergun MA, et al. Tailoring Breast Cancer Screening Intervals by Breast Density and Risk for Women Aged 50 Years or Older: Collaborative Modeling of Screening Outcomes. Annals of internal medicine. 2016;165(10):700–12. Epub 2016/08/23. doi: 10.7326/m16-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Meara ES. Sensitivity and Specificity by Race through October 2018 from the Breast Cancer Screening Consortium. Personal Communication, Received December 10, 2018. [Google Scholar]
- 32.O’Meara ES. Cancer Detection Rate, Breast Density, and Receptor Status by Race from the Breast Cancer Screening Consortium. Personal Communication, January 15 2019. [Google Scholar]
- 33.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. Epub 2011/12/14. doi: 10.1016/s0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. Journal of the National Cancer Institute Monographs. 2001(30):36–43. Epub 2002/01/05. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 35.Dignam JJ. Discussion about JNCI 2001. Personal Communication, May 11 2020. [Google Scholar]
- 36.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(20):2254–61. Epub 2015/05/13. doi: 10.1200/jco.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariotto AB, Feuer EJ, Harlan LC, Abrams J. Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975–1999. Journal of the National Cancer Institute Monographs. 2006(36):7–15. Epub 2006/10/13. doi: 10.1093/jncimonographs/lgj003. [DOI] [PubMed] [Google Scholar]
- 38.Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. Journal of the National Cancer Institute. 2002;94(21):1626–34. Epub 2002/11/07. doi: 10.1093/jnci/94.21.1626. [DOI] [PubMed] [Google Scholar]
- 39.Trentham-Dietz A, Gangnon R. Estimates of Non-Breast Cancer Mortality by Race. Personal Communication, May 17 2019. [Google Scholar]
- 40.Mandelblatt JS, Near AM, Miglioretti DL, Munoz D, Sprague BL, Trentham-Dietz A, et al. Common Model Inputs Used in CISNET Collaborative Breast Cancer Modeling. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(1_suppl):9s–23s. Epub 2018/03/20. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulden M. Calculating and Interpreting ICERs and Net Benefit. PharmacoEconomics. 2020;38(8):785–807. Epub 2020/05/12. doi: 10.1007/s40273-020-00914-6. [DOI] [PubMed] [Google Scholar]
- 42.HERC UDoVA. Cost-Effectiveness Analysis [cited 2021 February 3]. Available from: https://www.herc.research.va.gov/include/page.asp?id=cost-effectiveness-analysis.
- 43.Cookson R, Mirelman AJ, Griffin S, Asaria M, Dawkins B, Norheim OF, et al. Using Cost-Effectiveness Analysis to Address Health Equity Concerns. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2017;20(2):206–12. Epub 2017/02/27. doi: 10.1016/j.jval.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11). Epub 2014/09/27. doi: 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaVeist TA, Gaskin DJ, Richard P. Joint Center for Political and Economic Studies: The Economic Burden of Health Inequalities in the United States 2009 [cited 2020 June 10]. Available from: http://hsrc.himmelfarb.gwu.edu/cgi/viewcontent.cgi?article=1224&context=sphhs_policy_facpubs&sei-redir=1&referer=https%3A%2F%2Fscholar.google.com%2Fscholar%3Fq%3D%22darrell%2Bgaskin%22%2Beconomic%2Bburden%2Bof%2Bracial%2Bhealth%2Binequalities%26btnG%3D%26hl%3Den%26as_sdt%3D0%2C9.
- 46.Jafri NF, Ayyala RS, Ozonoff A, Jordan-Gray J, Slanetz PJ. Screening Mammography: Does Ethnicity Influence Patient Preferences for Higher Recall Rates Given the Potential for Earlier Detection of Breast Cancer? Radiology. 2008;249(3):785–91. doi: 10.1148/radiol.2493072176. [DOI] [PubMed] [Google Scholar]
- 47.Román R, Sala M, De La Vega M, Natal C, Galceran J, González-Román I, et al. Effect of false-positives and women’s characteristics on long-term adherence to breast cancer screening. Breast cancer research and treatment. 2011;130(2):543–52. Epub 2011/05/28. doi: 10.1007/s10549-011-1581-4. [DOI] [PubMed] [Google Scholar]
- 48.Lowry KP, Trentham-Dietz A, Schechter CB, Alagoz O, Barlow WE, Burnside ES, et al. Long-Term Outcomes and Cost-Effectiveness of Breast Cancer Screening With Digital Breast Tomosynthesis in the United States. JNCI: Journal of the National Cancer Institute. 2019;112(6):582–9. doi: 10.1093/jnci/djz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farr DE, Brandt HM, Friedman DB, Adams SA, Armstead CA, Fulton JK, et al. False-positive mammography and mammography screening intentions among black women: the influence of emotions and coping strategies. Ethnicity & Health. 2020;25(4):580–97. doi: 10.1080/13557858.2019.1571563. [DOI] [PubMed] [Google Scholar]
- 50.DeFrank JT, Rimer BK, Bowling JM, Earp JA, Breslau ES, Brewer NT. Influence of false-positive mammography results on subsequent screening: do physician recommendations buffer negative effects? Journal of medical screening. 2012;19(1):35–41. Epub 2012/03/23. doi: 10.1258/jms.2012.011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontanarosa PB, Bauchner H. Race, Ancestry, and Medical Research. Jama. 2018;320(15):1539–40. doi: 10.1001/jama.2018.14438. [DOI] [PubMed] [Google Scholar]
- 52.Deville C, Hwang WT, Burgos R, Chapman CH, Both S, Thomas CR Jr. Diversity in Graduate Medical Education in the United States by Race, Ethnicity, and Sex, 2012. JAMA internal medicine. 2015;175(10):1706–8. Epub 2015/08/25. doi: 10.1001/jamainternmed.2015.4324. [DOI] [PubMed] [Google Scholar]
- 53.Chapman CH, Hwang WT, Deville C. Diversity based on race, ethnicity, and sex, of the US radiation oncology physician workforce. International journal of radiation oncology, biology, physics. 2013;85(4):912–8. Epub 2012/11/06. doi: 10.1016/j.ijrobp.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Chapman CH, Gabeau D, Pinnix CC, Deville C Jr., Gibbs IC, Winkfield KM. Why Racial Justice Matters in Radiation Oncology. Adv Radiat Oncol. 2020;5(5):783–90. Epub 2020/08/25. doi: 10.1016/j.adro.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonham VL, Green ED, Pérez-Stable EJ. Examining How Race, Ethnicity, and Ancestry Data Are Used in Biomedical Research. Jama. 2018;320(15):1533–4. doi: 10.1001/jama.2018.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson RH. Otis Brawley’s ‘Skeptic’s View of Health Care Disparities & Health Care Reform’. Oncology Times. 2009;31(12):4, 6, 7. doi: 10.1097/01.COT.0000356948.37946.e2. [DOI] [Google Scholar]
- 57.Brawley OW. Is race really a negative prognostic factor for cancer? Journal of the National Cancer Institute. 2009;101(14):970–1. Epub 2009/07/02. doi: 10.1093/jnci/djp185. [DOI] [PubMed] [Google Scholar]
- 58.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Medical decision making : an international journal of the Society for Medical Decision Making. 2012;32(5):733–43. Epub 2012/09/20. doi: . [DOI] [PubMed] [Google Scholar]
- 59.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities 2020. [cited 2020 July 4]. Available from: https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/.
- 60.Takvorian SU, Oganisian A, Mamtani R, Mitra N, Shulman LN, Bekelman JE, et al. Association of Medicaid Expansion Under the Affordable Care Act With Insurance Status, Cancer Stage, and Timely Treatment Among Patients With Breast, Colon, and Lung Cancer. JAMA Network Open. 2020;3(2):e1921653-e. doi: 10.1001/jamanetworkopen.2019.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carter S, Gartner S, Haines M. Historical Statistics of the United States, Millenial Edition: Cambridge University Press; 2006. Ab11–30 p. [Google Scholar]
- 62.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W. SEER Cancer Statistics Review, 1975–2007 Bethesda, MD: National Cancer Institute; 2009. [cited 2011 January]. Available from: http://seer.cancer.gov/scr/1975-2007/. [Google Scholar]
- 63.Network NCC. Breast Cancer [cited 2015 April 27]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.