Abstract
Cancer cells strongly upregulate glucose uptake and glycolysis to produce vital biomolecules for cancer cell survival, proliferation, and metastasis as ATP, lipids, proteins, nucleotides, and lactate. The Warburg effect is tumours’ unique glucose oxidation to give lactate (not pyruvate) even in the presence of oxygen. Nicotinamide adenine dinucleotide (NAD/NADH.H) is used in glycolysis via glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and lactate dehydrogenase (LDH). Both catalyse reversible biochemical reactions to produce 1,3-diphosphoglycerate and lactate, respectively. In this expert opinion and based on published evidence, the author suggests that: “In transformed cells and hyperglycolytic cancer cells, the Warburg effect (permanent conversion of pyruvate to lactate) occurs secondary to a vicious cycle and a closed circuit between GAPDH and LDH (reaction of carcinogenesis) causing increased endogenous oxidative stress and subsequent carcinogenesis. Mitochondrial defects in cancer cells cause hyperglycolysis resulting in NADH.H accumulation (produced during GAPDH step) that obligatorily drives LDH to become an irreversible reaction in the direction of lactate formation (Warburg effect) but not pyruvate formation. Likewise, LDH oxidizes NADH.H producing excessive NAD+ that secondarily drives GAPDH reaction to be irreversible to produce NADH.H and so on. Pyruvate is an antioxidant while lactate is pro-oxidant, causing increased endogenous oxidative stress in cancer cells, tumour’s hypoxia and obligatory hyperglycolysis with NADH.H overproduction (GAPDH step) to be consumed in the LDH step for lactate production and NAD+ generation (utilized by GAPDH) and so on”. This confirms Warburg’s origin of cancer cells. Best anticancer applications based on this hypothesis are: breaking this closed vicious circle using siRNA to target GAPDH and LDH, avoiding strong oxidants (as many cancer chemotherapeutics), and using strong antioxidants for causing antioxidant–oxidant antagonism or antioxidant–lactate antagonism to inhibit the Warburg effect. Strong natural antioxidants of prophetic medicine (related to Prophet Muhammad peace be upon him) such as Zamzam water, Nigella sativa, costus, Ajwa date fruit, olive oil, Al-hijamah and natural honey are strongly recommended to prevent and antagonize the Warburg effect.
Keywords: Warburg effect, lactate, pyruvate, LDH, GAPDH, vicious cycle, closed circuit
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Nicotinamide adenine dinucleotide (NAD) is a coenzyme for many different enzymes (oxidoreductases) that catalyse the addition and removal of hydrogen atoms. When NAD acquires hydrogen atoms, it becomes reduced NAD (NADH.H). When reduced NAD (NADH.H) loses hydrogen, it gives oxidized NAD+. NADH.H plays an important role in complex I of electron transport chain (ETC) reactions. Herein, a possible partial role of NAD+/NADH.H in carcinogenesis is hypothesized and discussed. Continuous production of lactate (as an end product of glucose metabolism) is the unique characteristic metabolic criterion of highly aggressive cancer cells that differentiates them from the metabolic energy generating pathways in normal cells. NAD/NADH is a coenzyme that mediates redox reactions in a number of metabolic pathways, including glycolysis eg glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and lactate dehydrogenase (LDH) steps that are closely related to the Warburg effect. Increased NAD levels enhance glycolysis and fuel cancer cells. In fact, nicotinamide phosphoribosyltransferase (NPRT) is the rate-limiting enzyme for NAD synthesis in mammalian cells, and is frequently amplified in several cancer cells. NAD synthesis inhibitors significantly deplete NAD levels and subsequently suppress cancer cell proliferation through inhibition of energy production pathways eg glycolysis, tricarboxylic acid (TCA) cycle, and oxidative phosphorylation. NAD also serves as a substrate for poly(ADP-ribose) polymerase (PARP), sirtuin, and NAD glycohydrolase (CD38 and CD157) ie NAD regulates DNA repair, gene expression, and stress response through these enzymes.1
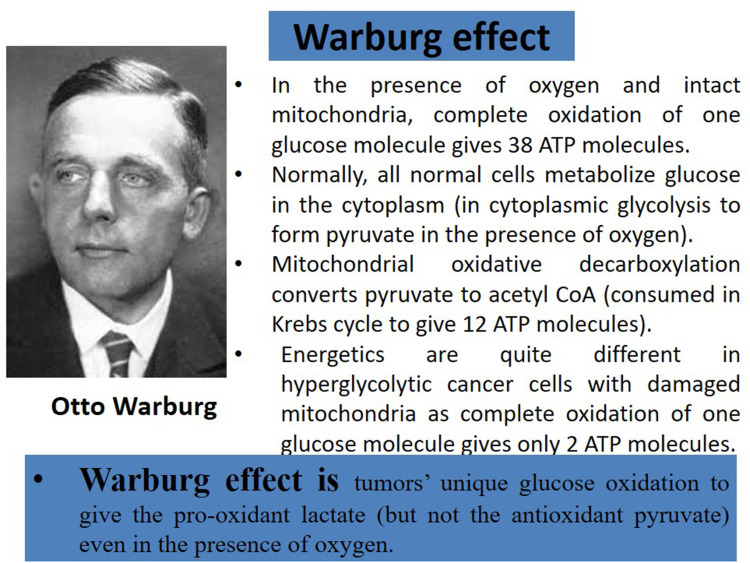
The Warburg effect is the hallmark of malignancy in hyperglycolytic cells that are driven by cytoplasmic glycolysis to convert glucose into lactic acid (even in the presence of oxygen) which is quite different from normal cells (Figure 1). However, it remains largely questionable to oncologists and cancer researchers: when the Warburg metabolic phenotype may play a role in tumour progression? And how that evolves? And more importantly, does the Warburg effect precede mitochondrial damage or result from it? And does carcinogenesis precede the Warburg effect or result from it?
Figure 1.
The Warburg effect is the hallmark of malignancy and differentiates energetics in hyperglycolytic cancer cells from normal cells.
For the author, mitochondrial damage precedes the occurrence of the Warburg effect and carcinogenesis ie a relatively significant degree of mitochondrial damage forces the energy metabolism of the transforming cells to rely on glycolysis resulting in overproduction of the end product pyruvate that is converted immediately into lactate via LDH (Box 1). A lot of details will be explored here in this article.
Box 1.
Which Comes First: Mitochondrial Damage, the Warburg Effect or Carcinogenesis?
For the author:
|
Importance of Glycolysis to Cancer Cells
Cancer cells strongly upregulate glucose uptake and cytoplasmic glucose oxidation (glycolysis) to produce ATP (to fuel malignancy), vital synthetic intermediates, glycolytic metabolites or pyruvate (the end product of glucose metabolism) and lactate. Glycolytic intermediates provide essential building biomolecules (lipids, proteins, nucleotides, and others) to the continuously and persistently dividing cancer cells and for tumour growth.2 Moreover, glycolysis is uncoupled from the mitochondrial tricarboxylic acid (TCA, Krebs) cycle and oxidative phosphorylation (OXPHOS) in cancer cells. However, the majority of glycolysis-derived pyruvate is shifted into lactate formation (instead of oxidation in the mitochondria to start the Krebs cycle) and becomes kept away from mitochondrial oxidative metabolism. This metabolic phenotype is known as the Warburg effect.2 The Warburg effect is anaerobic glycolysis (sequential glucose conversion into lactate but not pyruvate) even in the presence of oxygen in tumour cells.3
Benefits of the Warburg Effect to Cancer Cells
The Warburg effect is a unique trait of cancer metabolism. The Warburg effect is a dominant phenotype of most cancer cells particularly the most aggressive cancer cells. Under regular culture conditions, cancer cells exhibit the Warburg effect. Increased glycolysis in cancer cells (with lactate formation) carries a lot of benefits for these cells that suit malignancy.4 This includes the generation of nucleotides (to facilitate DNA replication), amino acids (cancer cells proteins), lipids (cell membranes and organelles membranes), folic acid (helps DNA synthesis), and nicotinamide adenine dinucleotide (NAD) that collectively unite to facilitate the biochemical and synthetic reactions for building blocks for cancer cell’s division.5 Under conditions of lactic acidosis, cancer cells did not exhibit the glycolytic phenotype but underwent a high ratio of oxygen consumption with minimal lactate production.6 Moreover, in co-cultures of human cervical carcinoma cells (HeLa cells) with human fibroblasts, there was a metabolic shift from oxidative phosphorylation towards glycolysis in cancer cells, and from glycolysis towards oxidative phosphorylation in fibroblasts. This metabolic shift was associated with hydrogen peroxide production.7
Expert Opinion
In transforming cells and hyperglycolytic cancer cells, the Warburg effect (permanent conversion of pyruvate to lactate) occurs secondary to a vicious cycle and a closed circuit between GAPDH and LDH (Biochemical reaction of carcinogenesis). Mitochondrial defects in cancer cells cause hyperglycolysis resulting in the accumulation of NADH.H produced during GAPDH step of cytoplasmic glycolysis. Excessively accumulating cytoplasmic NADH.H obligatorily drives LDH to become an irreversible reaction in the direction of lactate formation (Warburg effect) but not pyruvate formation. Likewise, LDH oxidizes NADH.H producing excessive NAD+ that secondarily drives GAPDH reaction to be irreversible to produce NADH.H and so on. This contributes to tumour hypoxia. Pyruvate is an antioxidant while lactate is not, hence endogenous oxidative stress in cancer cells increases and facilitates carcinogenesis. Strong antioxidants such as prophetic medicine remedies help combatting that.
Evidence in Favour of This Hypothesis
Both GAPDH and LDH are highly overexpressed in the primary culture of breast cancer cells.8 A strong link was reported between cancer progression and both GAPDH and LDH.9 GAPDH inhibition suppresses the Warburg effect in liver cancer cells. Moreover, selective inhibition of GADPH suppressed the growth and metastasis of hepatocellular carcinoma cells.10 In addition, LDH is a marker for tumour burden and response to treatment. LDH is a very valuable enzyme in the follow-up of cancer patients. Cancer cells release intracellular enzymes through damaged cell membranes to the serum which reflect the cellular metabolic state, presence of aerobic or anaerobic glycolysis, and the malignant activity.11 Significance of assaying the serum (LDH) level of 246 patients with Ewing’s sarcoma of bone (47 metastatic and 199 localized at presentation) was confirmed. Cancer patients with increased serum LDH levels had a higher metastatic potential and relapse rate after treatment than cancer patients with localized disease. Serum LDH was higher at the time of cancer recurrence confirming that serum LDH levels in Ewing’s sarcoma of bone can predict the prognosis and the response to therapy. Persistently high serum LDH, or an increasing value after a transient normalization, is usually followed by a relapse.12
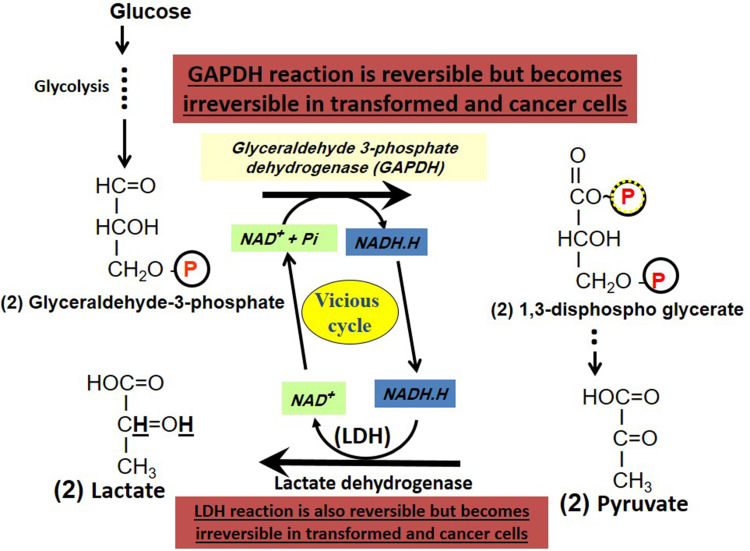
The above-mentioned hypothesis is illustrated in Figure 2. Comparison between lactate and pyruvate is given in Table 1 and Table 2. Also, the beneficial effects conferred by lactate (versus pyruvate) for cancer cells are also summarized (Table 1 and Table 2). Moreover, topics related to this hypothesis as importance of NAD/NADH.H, importance of oxidative stress, and importance of LDH and hypoxia are also discussed. Excess endogenous oxidative stress may induce and increase mitochondrial damage and increase hypoxia due to inability to make use of oxygen ie hypoxia.
Figure 2.
Origin of the Warburg effect. The Warburg effect originates from a vicious cycle and a closed circle existing between the two reversible glycolytic enzymes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and lactate dehydrogenase (LDH) where the former continuously produces NADH.H and the latter continuously utilizes it to form lactate from glucose even in the presence of oxygen (Warburg effect). Both enzymes act in harmony to supply the needed coenzymes (GAPDH utilizes NAD+ and LDH utilizes NADH.H) to each other.
Table 1.
Comparison Between Pyruvate and Lactate
| Pyruvate (Pyruvic Acid) | Lactate (Lactic Acid) | |
|---|---|---|
| Structure | 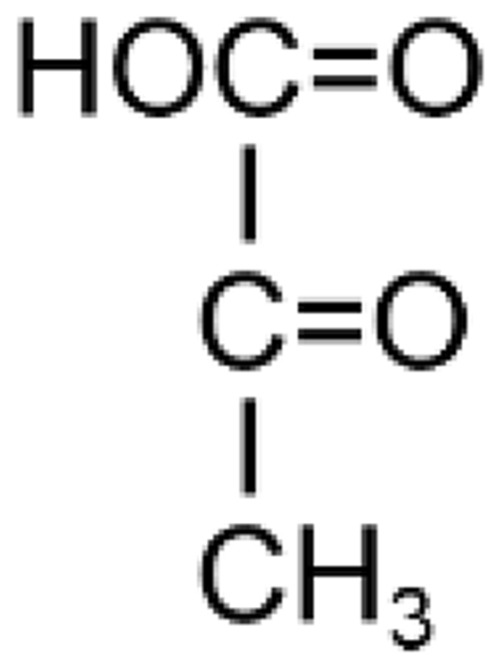 |
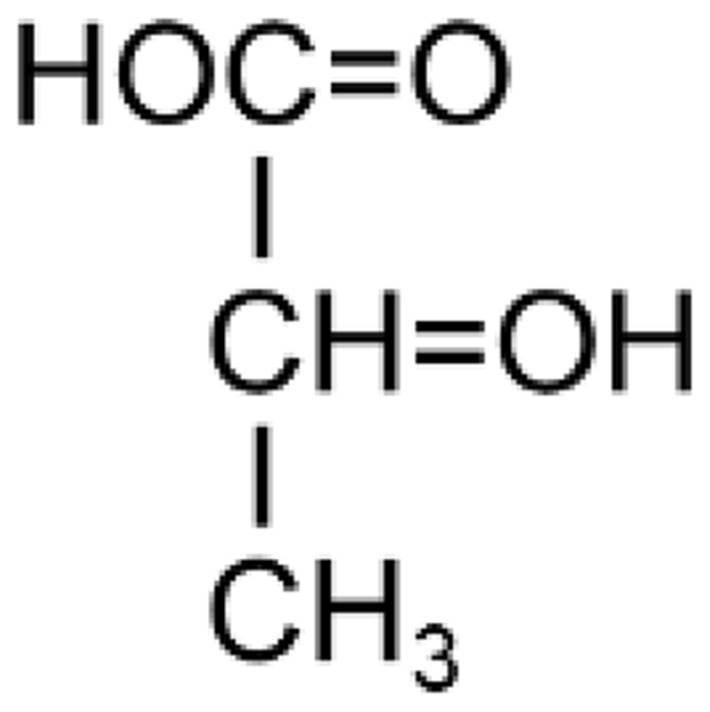 |
| Biochemical formula | 2 hydrogen atoms less than lactate | 2 hydrogen atoms more than pyruvate |
| Formation |
|
From (pyruvate + NADH.H) (using LDH) |
| Antioxidant function | Strong (so pyruvate participates in preventing an endogenous oxidative stress inside the normal cells). Adding pyruvate to H2O2 scavenged it13 | Absent. Lactate is pro-oxidant (so participates in increasing the endogenous oxidative stress in cancer cells). Adding lactate to exogenous H2O2 did not scavenge it13 |
| Normally formed as an end product of glucose oxidation | If oxygen is present (aerobic condition) to start the Krebs cycle | If oxygen is absent (anaerobic condition) |
| Formation (as an end product of glucose oxidation) in cancer cells form glucose | Transiently formed (as an end product) due to rapid conversion into lactate in hyperglycolytic cancer cells exhibiting the Warburg effect | Continuously formed inside cancer cells then becomes extruded to its microenvironment |
| Cytotoxicity to normal cells | Not cytotoxic. On the contrary, pyruvate is tissue-protective against oxidative stress | Excess lactate favours oxidant-induced cytotoxicity and acidic microenvironment |
Table 2.
Lactate is More Vital Than Pyruvate for Cancer Cells
| Pyruvate (Pyruvic Acid) | Lactate (Lactic Acid) | |
|---|---|---|
| Contribution to cancer cells’ extracellular acidity | No | Strong contribution |
| Conversion to acetyl CoA (active acetate) to start the cycle, ketogenesis, or lipogenesis | Directly via the enzyme pyruvate dehydrogenase. This occurs less in hyperglycolytic cancer cells due to consumption of pyruvate to form lactate and mitochondrial damage | None. Lactate must be converted first into pyruvate via LDH enzyme activity. Usually this does not occur in hyperglycolytic cancer cells due to consumption of pyruvate in forming lactate and the mitochondrial defects that impair the Krebs cycle |
| Fate in hyperglycolytic cancer cells exhibiting the Warburg effect | Formed from glucose then is rapidly converted to lactate (using LDH to produce the Warburg effect) | Formed from pyruvate, then becomes extruded outside cancer cells producing acidic microenvironment and does not become pyruvate again |
| Formation and transport | Mainly in normal cells' cytoplasm (from glucose oxidation), then enters the mitochondria using pyruvate translocase enzyme to give acetyl CoA to start the Krebs cycle that needs intact mitochondria and oxygen | In the cytoplasm (from pyruvate resulting from glucose oxidation). Then, lactate goes outside the cells. If lactate enters the mitochondria, it cannot be metabolized further due to mitochondrial lesions. Lactate formation is enhanced by mitochondrial lesions, and absent oxygen (in normal cells), tumour hypoxia or even in the presence of oxygen in cancer cells |
| Functions in hyperglycolytic cancer cells |
|
|
| Causing immunological evasion (escape) of tumours | Never | Very powerful. Lactate inhibits T lymphocytes and suppresses anticancer immunity |
A logic events cascade supporting this hypothesis (Figures 2 and 3) is also listed in the following:
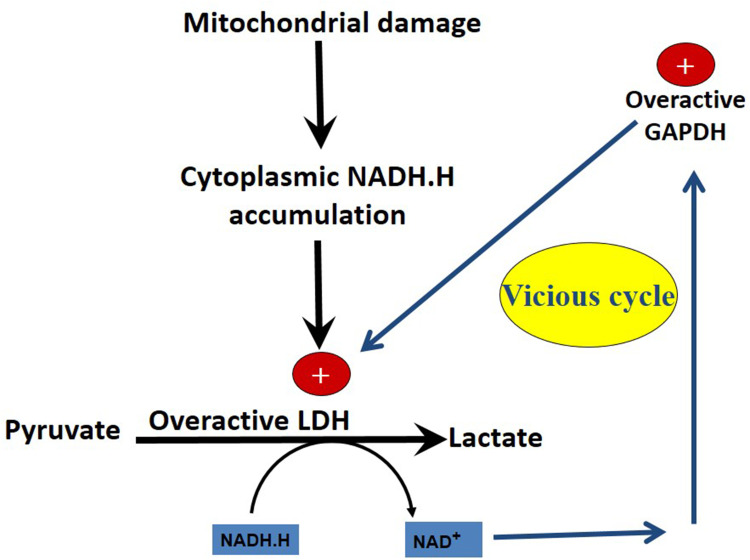
In transformed cells, significant damage in the mitochondria causes impaired mitochondrial energy-generating pathways and subsequent dependence on cytoplasmic glycolysis. The high energy demand in cancer cells causes hyperglycolysis and upregulation of glycolytic enzymes.
Hyperglycolysis in cancer cells empowers all enzymes of glycolysis including GAPDH.
GAPDH hyperactivity consumes excess NAD+ and produces excess NADH.H.
This causes excess cytoplasmic NADH.H accumulation and decreased cytoplasmic NAD+ availability.
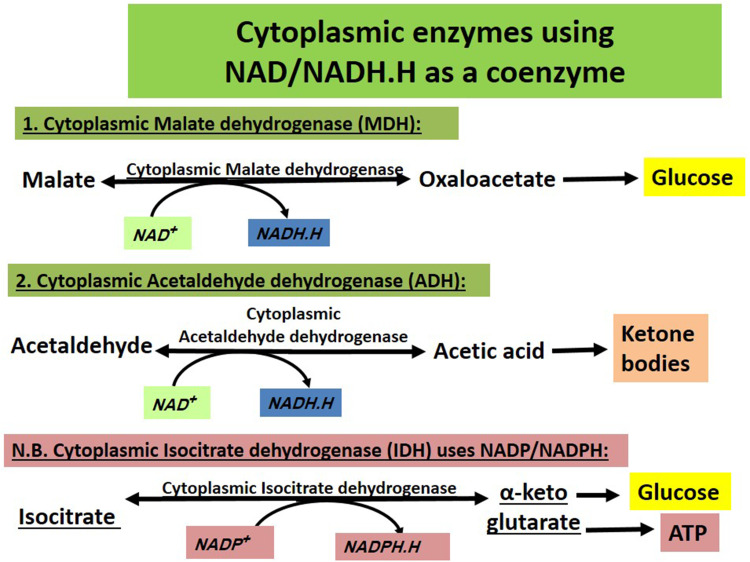
Other cytoplasmic enzymes using NAD+ are not upregulated in cancer cells or upregulated but not as glycolytic enzymes. Cytoplasmic malate dehydrogenase and acetaldehyde dehydrogenase produce NADH.H (ie facilitating lactate formation and the Warburg effect) when forming oxaloacetate and acetic acid, respectively ie when catalysing formation of glucose and ketone bodies. Cytoplasmic isocitrate dehydrogenase uses NADP/NADPH.H while malate dehydrogenase and acetaldehyde dehydrogenase use NAD+. So, NADH.H is not consumed (Figure 4).
NADH.H is not consumed by any of the glycolysis enzymes downstream to GAPDH till reaching the LDH step.
NADH.H cannot also be consumed in the mitochondria due to mitochondrial lesions.
All glycolytic enzymes downstream to GAPDH are also hyperactive to process their substrates into products.
At the LDH step, NADH.H is so profuse while NAD+ is scanty. So, the LDH step becomes irreversible and converts NADH.H into NAD+ with production of lactate (and no conversion back into pyruvate).
Resulting NAD+ is taken by GAPDH again. GAPDH becomes irreversible and produces NADH.H and so on.
A closed vicious cycle and a closed circuit soon arises and persists.
Overproduction of lactate (lacking antioxidant functions) at the expense of pyruvate (a strong antioxidant) persists causing increased endogenous oxidative stress in cancer cells (Table 1 and Table 2). This favours carcinogenesis.
Figure 3.
Mitochondrial damage precedes the development of the Warburg effect. The closed vicious cycle (GAPDH–LDH) maintains the Warburg effect and prevents pyruvate formation and deprive transforming cells and cancer cells from pyruvate-induced antioxidant effects.
Figure 4.
Cytoplasmic enzymes using NAD/NADH.H as a coenzyme. Those include malate dehydrogenase and acetaldehyde dehydrogenase but not isocitrate dehydrogenase (utilizes NADP/NADPH.H). Malate dehydrogenase and acetaldehyde dehydrogenase form NADH.H when they catalyse forming glucose and Ketone bodies, respectively and this adds to excess cytoplasmic NADH.H reserves ie stimulating formation of more lactate and more Warburg effect.
Best therapeutic applications for cancer treatment based on this hypothesis are: breaking this closed vicious circle between GAPDH and LDH, avoiding strong oxidants (including many cancer chemotherapeutics), adding strong antioxidants as adjuvant treatments, and using siRNA to target all the enzymes of the vicious cycle that are GAPDH and LDH and also the enzymes that catalyse the intermediate steps between them.
Endogenous Oxidative Stress in Cancer Cells: Pros versus Cons
Endogenous oxidative stress in cancer cells is a characteristic criterion of malignant cells in addition to tumour’s acidity and hypoxia. The author and Japanese co-researchers reported that pyruvate significantly scavenged oxidants in a cell-free medium while lactate did not scavenge the oxidants.13 Increasing such oxidative stress using a combination of 3-bromopyruvate and d-amino acid oxidase gene therapy (oxidative stress-energy depletion, OSED) helped reaching a lethal oxidative point to kill the malignant cells more selectively than normal cells based on the fact that most normal cells have an enough antioxidant reserve. That also helped combatting angiogenesis that is vital for tumours’ biology and survival.13–15 In addition, we previously reported that a malignant melanoma patient benefited also from 3-bromopyruvate therapy in significantly decreasing serum LDH, a vital enzyme for malignant cells.4 However, oxidative stress therapy is likely to produce oxidative stress-induced tissue harm also. So, including antioxidants as a partial or total component of the treatment protocols for cancer patients is quite vital to suppress the increased endogenous oxidative stress, spare the normal cells of chemotherapy-induced oxidative harm, minimize radiotherapy-induced oxidative tissue damage, and alleviate drug toxicities.
Why Glycolytic NADH (Produced During the GAPDH Step) is Not Consumed Before the LDH Step?
There is no biochemical glycolytic reactions needing NADH.H (produced during GAPDH step) as a hydrogen carrier till reaching the LDH step. Suppressed mitochondrial activity in cancer cells is multifactorial in origin eg decreased entry of reducing equivalents (as NADH) to the electron transport chain, impaired mitochondrial respiratory complexes, and mitochondrial DNA (mtDNA) mutations. That may cause increased generation of reactive oxygen species (ROS). Derangements of subunits of complex I also cause ROS generation with more subsequent mtDNA mutations ie a vicious circle arises that enhances mitochondrial dysfunction more.16
Importance of NAD/NADH for Cancer Cells
NAD+ is an essential coenzyme for various physiological processes eg cell growth, energy metabolism, DNA repair, and cell death that are disturbed in cancer cells. NAD+ is mainly synthesized by the NAD+ salvage pathway in cancer cells. NAD+ exhaustion inhibited energy metabolism in cancer cells via interfering with the Krebs cycle, oxidative phosphorylation, and glycolysis. This sensitized cancer cells to ROS-induced mitochondrial damage via depleting the antioxidant defence system, decreasing cancer cells’ proliferation, and causing cell death using cell signalling pathways (eg SIRT1 and p53).17 Cancer cells depend on NAD recycling. Inhibition of NAD salvage pathway caused metabolic collapse and cell death. Expression of NAD phosphoribosyltransferase (of the NAD salvage pathway) is increased significantly in pancreatic cancer cells and its suppression caused a drop in cellular NAD level and glycolytic activity. Expression of NAD phosphoribosyltransferase was independent of Kras and p16 status and its inhibition sensitized cancer cells to gemcitabine chemotherapy via decreasing the NAD+ level and suppressing the glycolytic activity.18
Interestingly, NAD+ regulates cancer cells’ energetics metabolism through different means via Sirtuins proteins that are a family of NAD+-dependent protein modifying enzymes. Sirtuins (SIRT1–SIRT7) have multiple catalytic functions such as deacetylase, desuccinylase, demalonylase, demyristoylase, depalmitoylase, and/or mono-ADP-ribosyltransferases. They play important roles in regulating cell’s metabolism, especially in glucose and lipid metabolism. Consequently, sirtuins play important roles in the biology of tumours.19 The sirtuins family of histone deacetylases are vital regulators of cancer metabolism. Sirtuins 1–7 (SIRT1–7) belong to class III of the histone deacetylase enzymes (dependent on NAD+ for activity). SIRT6 is a tumour suppressor that suppresses HIF1 transcription.20 Depleting NAD, lactate (Warburg effect), and inhibiting NAD phosphoribosyltransferase stopped the progression of glycolysis that is vital for cancer cells.21
Importance of GAPDH and LDH for Cancer Cells
GAPDH is vital for cellular metabolism and gene transcription. GAPDH expression is high in colon cancer and liver metastatic tissues ie GAPDH may promote cancer metastasis. Genetic silencing of GAPDH expression by short hairpin RNA (shRNA) caused a significant decrease of colon cancer cell glycolysis, decreased cancer cell proliferation, and changed cancer cell morphology. Transcriptional suppression of GAPDH downregulated the gene expression of cancer stem-like cells, inhibited epithelial-to-mesenchymal transition, and attenuated the migration and metastasis of colon cancer cells.22
Both GAPDH and LDH are the only enzymes in the glycolysis pathway that use the reducing equivalent (NAD/NADH). Both enzymes catalyse reversible pathways under physiological conditions. However, in hyperglycolytic cancer cells, both GAPDH and LDH work in a “forward direction” to catalyse the production of lactate and do not work in the “backward direction” to form pyruvate or (glucose from lactate or non-carbohydrate sources ie gluconeogenesis). With initiation of hyperglycolysis in cancer cells, more glucose is consumed and both GAPDH and LDH catalyse “irreversible steps” for lactate production. The reducing equivalent NADH is generated in the middle of glycolysis cascade of reactions through GAPDH. Under aerobic conditions in normal cells, NADH gives electrons to the ECT to renew NAD+. In hyperglycolytic cancer cells, mitochondrial defects inhibit such electron transport from NADH. This causes cytoplasmic accumulation of NADH accompanied by decreased NAD+ concentrations. The last step of glycolysis is a reversible step (catalysed by LDH). The accumulated coenzyme NADH will catalyse an irreversible “pyruvate to lactate” conversion using LDH and is consumed in the last step of glycolysis (Figure 2).
Lactate dehydrogenase-A (LDH-A) plays a key role in aerobic glycolysis (the Warburg effect) through regeneration of the electron acceptor NAD+ that is returned to GAPDH again to form NADH.H.23 LDHA has an aberrantly high expression in multiple cancers, which is associated with malignant progression. Cancer cells possess distinct metabolism from non‐transformed cells, providing sufficient biomaterials and energy. Lactate production in glycolysis contributes largely to malignant progression, like replenishing NAD+ for glycolysis, lowering pH for invasion, and triggering immune escape.24 LDHA is mainly located in the cytoplasm, but has also been found in the mitochondria and the nucleus.25–27
Due to the inhibition of pyruvate dehydrogenase in cancer cells, pyruvate accumulates and is directly converted into lactate, even in the presence of oxygen (Warburg effect). Subsequently, LDH reaction becomes unidirectional to form lactic acid and does not become reversible to form pyruvate (Figures 2 and 3). Lactate acidifies the cancer cells' microenvironment. That helps cancer cells growth and regenerates NAD+ that helps accomplishing different metabolic pathways eg glycolysis, DNA synthesis, DNA repair, and others. Aerobic respiring cancer cells stand in metabolic symbiosis (give and take) with Warburg glycolytic cancer cells. Aerobic respiring cancer cells consume a lot of glutamine to replenish Krebs cycle intermediates (α-ketoglutarate) coupled with ATP production and to replenish aspartate for nucleotide synthesis. Oncogene (Myc, AKT, etc.) activation and tumour suppressor inactivation (P53, PTEN, etc.) are involved in such metabolic effects.28 Tumours (and also melanoma patients) having low LDH-A expression exhibit resistance to MAPK signalling inhibitors, which target the Warburg effect. Interestingly, metastatic cancer cells accumulate more NADH (in favour of this hypothesis) and reactive oxygen species. In addition, the mitochondrial membrane potential of metastatic cells was lower than that of non-metastatic cells, indicating that the activity of NADH dehydrogenase and the mitochondrial oxidative chain were decreased in metastatic cells.29
HIF‐1 and LDH
Hypoxia‐inducible factor‐1 (HIF‐1) is a heterodimer containing α and β subunits. During hypoxia, stabilized HIF‐1α enters into the nucleus to combine HIF‐1β, and attach to hypoxia‐responsive elements to transactivate the targeted genes. In non-small‐cell lung cancer, LDH‐5 isozyme overexpression is linked to tumour hypoxia, angiogenic factor production, and poor prognosis. Koukourakis et al proved that LDHA was positively related to HIF‐1α.30 Later, Semenza et al found that hypoxia-responsive element existed in the promoter region of LDHA and demonstrated that HIF‐1 could occupy hypoxia-responsive element to transactivate LDHA expression.31 In addition, c‐Myc and HIF‐1 could collaborate to activate LDHA transcription in various cancer cells.32
Other Cytoplasmic Enzymes Utilizing NAD+/NADH.H (Figure 4)
Cytoplasmic malate dehydrogenase (reversibly generates NADH.H) is amplified in human tumours and correlates with poor prognosis. Proliferating cells rely on both cytoplasmic malate dehydrogenase and LDH to replenish cytosolic NAD+. To the author, cytoplasmic malate dehydrogenase-induced conversion of malate to oxaloacetate serves gluconeogenesis (formation of glucose from non-carbohydrate sources) and supplies cancer cells with endogenous glucose that suits best the hyperglycolytic phenotype in cancer cells. This also provides excess NADH.H that fuels LDH to form more lactate (Warburg effect) from pyruvate. Malate dehydrogenase working in the opposite direction for conversion of oxaloacetate to malate (to form other biochemical intermediates) seems to be less important for cancer cells as the need for energy and glucose is higher.
The enzyme acetaldehyde dehydrogenase helps the detoxification of ingested ethanol via converting the metabolite acetaldehyde (toxic) into acetic acid (non-toxic) with production of excess NADH.H (may increase LDH-induced lactate formation). Acetic acid is usually activated to acetyl CoA that favours ketone bodies formation in the liver cells' mitochondria ie helps supplying energetics that can be utilized by respiring cancer cell populations.
The enzyme cytoplasmic isocitrate dehydrogenase uses NADP/NADPH.H coenzymes and hence does not affect the NAD+/NADH.H ratio.
Otto Warburg Was Truthful (Figure 5)
Figure 5.
Otto Warburg was truthful. The best of the author’s knowledge and understanding agrees with Otto Warburg’s findings and reports: mitochondrial damage precedes the Warburg effect that precedes carcinogenesis.
This expert opinion agrees with Warburg’s report on the origin of cancer cells:
Cancer cells originate from normal body cells in two phases. The first phase is the irreversible injuring of respiration. The irreversible injuring of respiration is followed, as the second phase of cancer formation, by a long struggle for existence by the injured cells to maintain their structure, in which a part of the cells perish from lack of energy, while another part succeed in replacing their retrievably lost respiration energy by fermentation energy.33
This expert opinion explains how the Warburg effect occurs de novo based on a biochemical reaction and agrees with Warburg’s report in that mitochondrial injury precedes the Warburg effect. This expert opinion suggests that both mitochondrial injury and the Warburg effect facilitate causing carcinogenesis in transformed cells and maintain it in the highly aggressive (hyperglycolytic) cancer cells. However, it may be argued that some populations of cancer cells are not hyperglycolytic and still have intact mitochondria performing intact mitochondrial energy generating reactions eg Krebs cycle, oxidative phosphorylation, and ketolysis. For the author, such cells with intact mitochondria and mitochondrial pathways and respiration are driven by the aggressive hyperglycolytic cancer cells in a metabolic symbiosis where lactate produced and extruded by hyperglycolytic cancer cells is picked up by respiring cancer cells where it is converted back into pyruvate to start the Krebs cycle. Noteworthy, excessively produced NADH.H in respiring cancer cells is oxidized in oxidative phosphorylation to meet the high metabolic energy demands.
Natural Antioxidants for Combatting Lactate Effects
Natural antioxidants of prophetic medicine (related to Prophet Muhammad peace be upon him) are quite promising to combat the Warburg effect and lactate-induced augmentation of oxidant toxicity simply via an antioxidant–oxidant or antioxidant–lactate antagonism (Box 2). Zamzam water is a natural alkaline drinking water rich in many antioxidant minerals that catalyse major antioxidant enzymes in the human body. In addition, Zamzam water is rich in selenium and strontium that have antioxidant effects.34–37 Ajwa date fruit (Phoenix dactylifera) is a natural medicinal plant rich in both polyphenols and flavonoids and was reported to combat diclofenac-induced oxidative stress and cytotoxicity38 and was also reported to prevent and treat experimental liver carcinogenesis.41 Nigella sativa is a miracle herb and a natural pharmacy rich in so many antioxidant ingredients that combatted iron overload-induced oxidative stress in thalassaemic children40 and exerted potent antioxidant, antitoxic, and anticancer effects.41 Natural honey is also rich in antioxidants as flavonoids and polyphenols that strongly combat oxidant-induced harm.42,43 Costus speciosus (Saussurea lappa) is rich in antioxidant phytochemicals that exert potent antioxidant and anticancer effects. In addition, the prophetic medicine cocktail (TaibUVID nutritional supplements) including a combination of the above-mentioned natural products proved effective in combatting lethal viral infections as COVID-19 owing to its rich antioxidant contents.44–47 Moreover, Al-hijamah (wet cupping therapy of prophetic medicine) proved effective in decreasing iron overload-induced oxidative stress possibly due to excretion of excess oxidants outside the human body.48 Interestingly, Al-hijamah uses a physiological excretory mechanism ie percutaneous pressure-dependent filtration then excretion of disease-causing substances eg excess oxidants through the fenestrated dermal capillaries according to the evidence-based Taibah mechanism.48–57
Box 2.
Natural Antioxidants of Prophetic Medicine are Promising for Preventing and Treating the Warburg Effect
| *This occurs due to the so many natural antioxidant ingredients that may antagonize the Warburg effect (lactate-induced increased oxidative stress). *Natural and nutritional antioxidant ingredients may cause antioxidant–oxidant antagonism. *Natural and nutritional antioxidant ingredients may cause antioxidant–lactate (pro-oxidant) antagonism. *******Such promising natural antioxidants include:
|
Conclusion
Mitochondrial damage in cancer cells results in development of the Warburg effect. Pyruvate is an antioxidant while lactate is not, hence endogenous oxidative stress in cancer cells increases due to conversion of pyruvate into lactate. A vicious cycle arises between cytoplasmic GAPDH and LDH and is responsible for that. Excess NADH accumulation due to mitochondrial defects favours lactate formation. This contributes to tumour hypoxia and forces cancer cells to undergo further obligatory hyperglycolysis with overproduction of NADH (GAPDH step) to be consumed in the LDH step for lactate production and NAD+ generation (utilized by GAPDH) and so on. Best therapeutic applications for cancer treatment based on this hypothesis are: breaking this closed vicious circle, avoiding strong oxidants (including many cancer chemotherapeutics), adding strong antioxidants as adjuvant treatments, and using siRNA to target GAPDH and LDH. Natural antioxidants as Zamzam water are strongly recommended to prevent the Warburg effect.
Acknowledgments
The author is grateful to the Medical Research Centre and the Deanship of Scientific Research at Taibah University, Saudi Arabia for kindly supporting the library and research facilities for this article in the research project #8016.
Funding Statement
Funding was not received for this study.
Disclosure
The author reports no conflicts of interest in relation to this work.
References
- 1.Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622. doi: 10.3389/fonc.2018.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–164. doi: 10.1016/j.canlet.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto AM. Warburg effect (s)—a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016;4:1–8. doi: 10.1186/s40170-016-0145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Sayed SM, Mohamed WG, Seddik M-AH, et al. Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer. 2014;33:356. doi: 10.5732/cjc.013.10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Sayed SM, Mahmoud AA, El Sawy SA, et al. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med Hypotheses. 2013;81:866–870. doi: 10.1016/j.mehy.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 6.Xie J, Wu H, Dai C, et al. Beyond Warburg effect–dual metabolic nature of cancer cells. Sci Rep. 2014;4:1–12. doi: 10.1038/srep04927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druzhkova IN, Shirmanova MV, Lukina MM, Dudenkova VV, Mishina NM, Zagaynova EV. The metabolic interaction of cancer cells and fibroblasts–coupling between NAD (P) H and FAD, intracellular pH and hydrogen peroxide. Cell Cycle. 2016;15:1257–1266. doi: 10.1080/15384101.2016.1160974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa M, Inoue T, Shirai T, et al. Simultaneous expression of cancer stem cell-like properties and cancer-associated fibroblast-like properties in a primary culture of breast cancer cells. Cancers. 2014;6:1570–1578. doi: 10.3390/cancers6031570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. 2019;150:104511. doi: 10.1016/j.phrs.2019.104511 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Guo J, Kaboli PJ, et al. Analysis of key genes regulating the Warburg effect in patients with gastrointestinal cancers and selective inhibition of this metabolic pathway in liver cancer cells. Onco Targets Ther. 2020;13:7295. doi: 10.2147/OTT.S257944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Cancer Biomarkers. 2015;1:115–124. [DOI] [PubMed] [Google Scholar]
- 12.Bacci G, Avella M, McDonald D, Toni A, Orlandi M, Campanacci M. Serum lactate dehydrogenase (LDH) as a tumor marker in Ewing’s sarcoma. Tumori J. 1988;74:649–655. doi: 10.1177/030089168807400606 [DOI] [PubMed] [Google Scholar]
- 13.El Sayed S, Abou El-Magd R, Shishido Y, et al. 3-Bromopyruvate antagonizes effects of lactate and pyruvate, synergizes with citrate and exerts novel anti-glioma effects. J Bioenerg Biomembr. 2012;44:61–79. doi: 10.1007/s10863-012-9409-4 [DOI] [PubMed] [Google Scholar]
- 14.El Sayed S, Abou El-Magd R, Shishido Y, et al. D-Amino acid oxidase-induced oxidative stress, 3-bromopyruvate and citrate inhibit angiogenesis, exhibiting potent anticancer effects. J Bioenerg Biomembr. 2012;44:513–523. doi: 10.1007/s10863-012-9455-y [DOI] [PubMed] [Google Scholar]
- 15.El Sayed S, El-Magd A, Shishido Y, et al. D-amino acid oxidase gene therapy sensitizes glioma cells to the antiglycolytic effect of 3-bromopyruvate. Cancer Gene Ther. 2012;19:1–18. doi: 10.1038/cgt.2011.59 [DOI] [PubMed] [Google Scholar]
- 16.Gasparre G, Porcelli AM, Lenaz G, Romeo G. Relevance of mitochondrial genetics and metabolism in cancer development. Cold Spring Harb Perspect Biol. 2013;5:a011411. doi: 10.1101/cshperspect.a011411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy BE, Sharif T, Martell E, et al. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacol Res. 2016;114:274–283. doi: 10.1016/j.phrs.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 18.Ju H-Q, Zhuang Z-N, Li H, et al. Regulation of the Nampt-mediated NAD salvage pathway and its therapeutic implications in pancreatic cancer. Cancer Lett. 2016;379:1–11. doi: 10.1016/j.canlet.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Dong Z, Ke X. The roles of sirtuins family in cell metabolism during tumor development. Semin Cancer Biol. 2019;59:71. [DOI] [PubMed] [Google Scholar]
- 20.Kleszcz R, Paluszczak J, Baer-Dubowska W. Targeting aberrant cancer metabolism–The role of sirtuins. Pharmacol Rep. 2015;67:1068–1080. doi: 10.1016/j.pharep.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 21.Keshari KR, Wilson DM, Van Criekinge M, et al. Metabolic response of prostate cancer to nicotinamide phophoribosyltransferase inhibition in a hyperpolarized MR/PET compatible bioreactor. Prostate. 2015;75:1601–1609. doi: 10.1002/pros.23036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Tang Z, Huang A, et al. Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of SNAIL expression. Int J Oncol. 2017;50:252–262. doi: 10.3892/ijo.2016.3774 [DOI] [PubMed] [Google Scholar]
- 23.Pathria G, Scott DA, Feng Y, et al. Targeting the Warburg effect via LDHA inhibition engages ATF 4 signaling for cancer cell survival. EMBO J. 2018;37:e99735. doi: 10.15252/embj.201899735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y, Xiong Y, Qiao T, Li X, Jia L, Han Y. Lactate dehydrogenase A: a key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7:6124–6136. doi: 10.1002/cam4.1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy MA, Shukla SD. Nuclear activation and translocation of mitogen-activated protein kinases modulated by ethanol in embryonic liver cells. Mol Cell Res. 2000;1497:271–278. doi: 10.1016/S0167-4889(00)00058-6 [DOI] [PubMed] [Google Scholar]
- 26.Fiume L, Manerba M, Vettraino M, Di Stefano G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med Chem. 2014;6:429–445. doi: 10.4155/fmc.13.206 [DOI] [PubMed] [Google Scholar]
- 27.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc National Acad Sci. 1999;96:1129–1134. doi: 10.1073/pnas.96.3.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Icard P, Lincet H. The cancer tumor: a metabolic parasite? Bull Cancer. 2013;100:427–433. doi: 10.1684/bdc.2013.1742 [DOI] [PubMed] [Google Scholar]
- 29.Marquez J, Kratchmarova I, Akimov V, et al. NADH dehydrogenase complex I is overexpressed in incipient metastatic murine colon cancer cells. Oncol Rep. 2019;41:742–752. doi: 10.3892/or.2018.6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL, Jiang B-H, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529 [DOI] [PubMed] [Google Scholar]
- 32.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274 [DOI] [PubMed] [Google Scholar]
- 33.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 34.Abdullah A, Abdelsalam E, Abdullah B, Khaled A. Antioxidant effects of Zamzam water in normal rats and those under induced-oxidative stress. J Med Plants Res. 2012;6:5507–5512. [Google Scholar]
- 35.Mahmoud HS, Eltahlawi RA, Alhadramy O, et al. Zamzam water is pathogen-free, cardioprotective and tissue-protective: relieving the BBC concerns. Am J Clin Med Res. 2020;8:5–12. [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud HS, Eltahlawi RA, Jan AA, et al. Zamzam water is pathogen-free, uricosuric, hypolipidemic and exerts tissue-protective effects: relieving BBC concerns. Am J Blood Res. 2020;10:386. [PMC free article] [PubMed] [Google Scholar]
- 37.Zergui A, Aledeh M, Hamad S. Metallic profile of Zamzam water: determination of minerals, metals and metalloids by ICP-MS. J Trace Elements Minerals. 2022;2:100031. doi: 10.1016/j.jtemin.2022.100031 [DOI] [Google Scholar]
- 38.Hassan SMA, Aboonq MS, Albadawi EA, et al. The Preventive and Therapeutic Effects of Ajwa Date Fruit Extract Against Acute Diclofenac Toxicity-Induced Colopathy: an Experimental Study. Drug Des Devel Ther. 2022;16:2601. doi: 10.2147/DDDT.S344247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan F, Khan TJ, Kalamegam G, et al. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med. 2017;17:1–10. doi: 10.1186/s12906-017-1926-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Shanshory M, Hablas NM, Aboonq MS, et al. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J Herbal Med. 2019;16:100245. doi: 10.1016/j.hermed.2018.11.001 [DOI] [Google Scholar]
- 41.Ahmad A, Husain A, Mujeeb M, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Sayed SM, Baghdadi H, Abou-Taleb A, et al. Al-hijamah and oral honey for treating thalassemia, conditions of iron overload, and hyperferremia: toward improving the therapeutic outcomes. J Blood Med. 2014;5:219. doi: 10.2147/JBM.S65042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Al Jaouni SK. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid Med Cell Longev. 2016;2016:1–10. doi: 10.1155/2016/3643824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Sayed SM, Bahashwan S, Aboonq M, et al. Adjuvant TaibUVID nutritional supplements proved promising for novel safe COVID-19 public prophylaxis and treatment: enhancing immunity and decreasing morbidity period for better outcomes (A retrospective study). Int J Med Develop Countries. 2020;4:1375–1389. doi: 10.24911/IJMDC.51-1594011487 [DOI] [Google Scholar]
- 45.El Sayed SM, Almaramhy HH, Aljehani YT, et al. The evidence-based TaibUVID nutritional treatment for minimizing COVID-19 fatalities and morbidity and eradicating COVID-19 pandemic: a novel Approach for Better Outcomes (A Treatment Protocol). Am J Public Health Res. 2020;8:54–60. [Google Scholar]
- 46.El Sayed SM, Aboonq MS, El Rashedy AG, et al. Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID-19 pandemic: towards better public prophylaxis and treatment (A retrospective study). Am J Blood Res. 2020;10:266. [PMC free article] [PubMed] [Google Scholar]
- 47.El Sayed SM, Aboonq MS, Aljehani YT, et al. TaibUVID nutritional supplements help rapid cure of COVID-19 infection and rapid reversion to negative nasopharyngeal swab PCR: for better public prophylaxis and treatment of COVID-19 pandemic. Am J Blood Res. 2020;10:397. [PMC free article] [PubMed] [Google Scholar]
- 48.El-Shanshory M, Hablas NM, Shebl Y, et al. Al-hijamah (wet cupping therapy of prophetic medicine) significantly and safely reduces iron overload and oxidative stress in thalassemic children: a novel pilot study. J Blood Med. 2018;9:241. doi: 10.2147/JBM.S170523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Shanshory M, Hablas NM, Shebel Y, et al. Al-hijamah (the triple S treatment of prophetic medicine) exerts cardioprotective, tissue-protective and immune potentiating effects in thalassemic children: a pilot clinical trial. Am J Blood Res. 2020;10:447. [PMC free article] [PubMed] [Google Scholar]
- 50.El-Shanshory M, Hablas NM, El-Tahlawi R, et al. Al-hijamah (the triple S treatment of prophetic medicine) significantly increases CD4/CD8 ratio in thalassemic patients via increasing TAC/MDA ratio: a clinical trial. Am J Blood Res. 2022;12:125. [PMC free article] [PubMed] [Google Scholar]
- 51.Aboonq MS. Al-hijamah (wet cupping therapy of prophetic medicine) as a novel alternative to surgery for carpal tunnel syndrome. Neurosci J. 2019;24:137–141. doi: 10.17712/nsj.2019.2.20180036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baghdadi H, Abdel-Aziz N, Ahmed NS, et al. Ameliorating role exerted by Al-Hijamah in autoimmune diseases: effect on serum autoantibodies and inflammatory mediators. Int J Health Sci. 2015;9:207. doi: 10.12816/0024129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmoud HS, Abou-El-Naga M, Omar NAA, et al. Anatomical sites for practicing wet cupping therapy (Al-Hijamah): in light of modern medicine and prophetic medicine. Altern Integ Med. 2013;2:1–30. [Google Scholar]
- 54.El Sayed SM, Mahmoud H, Nabo M. Medical and scientific bases of wet cupping therapy (Al-hijamah): in light of modern medicine and prophetic medicine. Alternative Integrative Med. 2013;25:1–16. [Google Scholar]
- 55.El Sayed SM, Mahmoud HS, Nabo MMH. Methods of wet cupping therapy (Al-Hijamah): in light of modern medicine and prophetic medicine. Alternative Integrative Med. 2013;1–16. [Google Scholar]
- 56.El Sayed SM, Abou-Taleb A, Mahmoud HS, et al. Percutaneous excretion of iron and ferritin (through Al-hijamah) as a novel treatment for iron overload in beta-thalassemia major, hemochromatosis and sideroblastic anemia. Med Hypotheses. 2014;83:238–246. doi: 10.1016/j.mehy.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 57.El Sayed SM, Al-quliti A-S, Mahmoud HS, et al. Therapeutic benefits of Al-hijamah: in light of modern medicine and prophetic medicine. Am J Med Biol Res. 2014;2:46–71. doi: 10.12691/ajmbr-2-2-3 [DOI] [Google Scholar]