Abstract
The first total synthesis of the furanobutenolide-derived cembranoid diterpenoid havellockate is disclosed. Our convergent strategy employs a Julia–Kocienski olefination to join two enantioenriched fragments to produce a diene that is subsequently used in a propiolic acid esterification/Diels–Alder cascade. This sequence generates the fused carbocyclic core of the natural product in short order. A challenging Zn-mediated Barbier allylation then forges the final C–C bond as well as establishes two vicinal stereogenic centers. Finally, a Cu-catalyzed aerobic oxidation facilitates the formation of the β-hydroxybutanolide to complete the total synthesis.
Graphical Abstract

Marine organisms provide a seemingly endless supply of complex natural products, which have captivated synthetic chemists for decades.1 Soft corals of the genus Sinularia are no exception, as dozens of macrocyclic and polycyclic molecules have been isolated from their extracts.2 The polycyclic furanobutenolide-derived cembranoid and norcembranoid classes of natural products have long served as challenging and elusive synthetic targets, with only four members having succumbed to total synthesis efforts to date.3,4
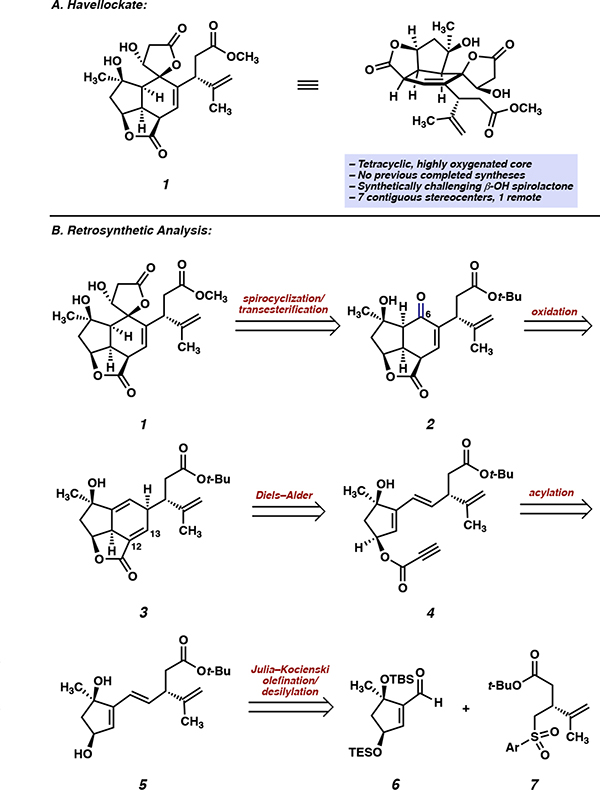
Havellockate (1), a C20-cembranoid first isolated in 1998 from Sinularia granosa, is a prime example of the complexity and synthetic challenge posed by the polycyclic furanobutenolide-derived cembranoid family (Figure 1A).5 Havellockate (1) is characterized by a highly oxygenated cis-fused tricyclic core common to other related natural products. However, it is adorned with a spiro-fused β-hydroxybutanolide ring, a unique feature not found in other members of the polycyclic furanobutenolide-derived cembranoid family. In addition to these topological features, havellockate (1) possesses eight stereogenic centers, seven of which are contiguous around the highly substituted and densely functionalized core. Although no completed syntheses of havellockate (1) have been reported, previous efforts by Mehta6 and Barriault7 highlight the difficulty of accessing this target via total synthesis.
Figure 1.
A) Structural features of havellockate (1). B) Retrosynthetic analysis of havellockate (1).
When devising a retrosynthetic strategy targeting havellockate (1), we first opted to disconnect the spirolactone to reveal enone 2 (Figure 1B). In the forward direction, it was envisioned that the β-hydroxybutanolide could be installed via 1,2-addition of a suitable nucleophile into the carbonyl at C(6) (havellockate numbering throughout), with subsequent elaboration to the requisite lactone. Enone 2, in turn, could be accessed from tricycle 3 after installation of the C(6) carbonyl and migration of the Δ12,13 olefin. Tricycle 3 was envisaged as arising from the intramolecular Diels–Alder reaction of propiolic ester 4, which would be formed by esterification of diol 5 with propiolic acid. This approach is analogous to the strategy we employed in our successful synthesis of scabrolide A.4d Key intermediate 5 could be forged via a convergent Julia–Kocienski olefination8 between aldehyde 6 and sulfone 7, which we imagined could be accessed from commercially available starting materials in enantioenriched form.
The synthesis of aldehyde 6 commences with known enone 8,9 which undergoes bromination followed by conjugate addition/elimination to afford β-cyanoenone 10 (Scheme 1A).10 Luche reduction and subsequent alcohol protection furnishes nitrile 11, which is converted to aldehyde 6 by reduction with DIBAL. The sulfone coupling partner is accessed from known acyl oxazolidinone 1211 by γ-deprotonation followed by α-alkylation with t-butyl bromoacetate, which proceeds in >20:1 dr. Reductive auxiliary removal then affords enantioenriched alcohol 13 (Scheme 1B). A Mitsunobu reaction with 1-phenyltetrazole thiol (PTSH, 14) and oxidation of the resultant sulfide (15) yields the desired sulfone (16).12
Scheme 1.
A) Synthesis of aldehyde 6. B) Synthesis of sulfone 16.
With both coupling partners in hand, we next investigated the key convergent olefination reaction to unite them (Scheme 2). Pleasingly, addition of KHMDS to a mixture of aldehyde 6 and sulfone 16 effects the desired Julia–Kocienski olefination and,13 following desilylation of the product, diol 5 is obtained in 57% yield over the two steps. This key olefination forges the requisite C=C bond with high (>20:1) selectivity for the E-isomer. With diol 5 in hand, the stage is set for the key acylation/Diels–Alder sequence.
Scheme 2.
Julia–Kocienski olefination uniting aldehyde 6 and sulfone 16, acylation and Diels–Alder of diol 5, oxidation of tricycle 3 to enone 2, and failed Grignard addition to enone 2.
Based upon our experience with similar esterification reactions,4d we did not anticipate difficulty in preparing Diels–Alder substrate 4. Upon treatment of diol 5 with propiolic acid under Steglich conditions,14 conversion to ester 4 occurs. However, attempting to isolate and purify ester 4 results in decomposition of this sensitive intermediate (we have observed that ester 4 decomposes after prolonged exposure to silica as well as upon concentration to the neat compound, which we attribute to the lack of substitution of the propiolate alkyne). Consequently, the reaction mixture is rapidly passed through a plug of silica, then the partially purified product is used immediately in the subsequent step. Heating ester 4 to 110 °C in xylenes triggers the [4+2] cycloaddition to afford Diels–Alder adduct 3 as a single diastereomer. Despite the modest yield of these two steps, this key sequence serves to construct the characteristic [5–5–6] framework of havellockate (1). 15
To access enone 2, Diels–Alder adduct 3 is subjected to a three-step oxidation protocol. First, a V-catalyzed directed epoxidation of the Δ6,7 olefin furnishes epoxide 17 as a single diastereomer. A Ti-catalyzed reductive epoxide opening16 provides diol 18, which then undergoes oxidation with IBX. Fortuitously, the oxidation of the C(6) alcohol proceeds with the concomitant migration of the Δ12,13 olefin, generating the desired enone (2) directly.
With enone 2 in hand, we set out to explore the installation of the final three carbon atoms of the natural product and the construction of the spiro-fused butanolide ring. We initially envisioned that a diastereoselective 1,2-addition of a Grignard reagent into enone 2 could forge the final C–C bond required and install the three carbon atoms of the butanolide ring. However, to our surprise, no trace of addition product 20 is observed upon addition of vinyl magnesium bromide (as a model nucleophile) to a solution of enone 2 in THF. We attribute this to the basicity of the Grignard reagent, which likely deprotonates the vinylogous β-ketoester (2, proton highlighted in red) forming enolate 19 and rendering the desired 1,2-addition unfeasible.
After surveying several classes of nucleophiles,17 we discovered that exposure of enone 2 to the allylzinc reagent generated from 3-bromo-1-acetoxypropene (22)18 results in productive reaction, giving rise to an inseparable mixture of isomeric addition products.19 Treatment of this mixture with methanolic ammonia deacetylates the products, and renders them separable by flash chromatography (Scheme 3). After this two-step sequence, triol 23 is isolated in modest yield, and as a single diastereomer. Gratifyingly, an X-ray of triol 23 reveals that it possesses the correct stereochemistry at both C(5) and C(6) corresponding to the β-hydroxybutanolide ring of havellockate (1). We attribute this high degree of diastereoselectivity to a closed transition state, which is commonly invoked for Zn-mediated allylation reactions, and approach of the nucleophile from the less hindered α-face of the enone (see SI for details).20
Scheme 3.
Top: Zn-mediated allylation of enone 2 to forge triol 23, and failed transesterification. Bottom: Transesterification of enone 2, allylation of enone 25, and completion of the total synthesis of havellockate 1.
Now in possession of an intermediate containing all of the carbon atoms of the natural product and all of the stereogenic centers correctly established, we sought to perform a transesterification to replace the t-butyl ester of triol 23 with the requisite methyl ester (i.e. triol 24). Unfortunately, despite investigating a variety of conditions for the selective removal of the t-butyl ester, we were unable to effect this transformation, and our efforts resulted only in decomposition or undesired side reactivity of this intermediate. After extensive experimentation, we found that enone 2 is a suitable intermediate upon which to carry out the transesterification sequence. This is achieved by cleavage of the t-butyl ester with formic acid,21 followed by formation of the methyl ester (25) using diazomethane.
To our delight, methyl ester-bearing enone 25 also serves as a competent substrate for the key allylation reaction. Following deacetylation with NaOMe, triol 24 is furnished in 11:1 dr, and in similar yield to the t-butyl system. Elaboration of the newly installed allyl group is accomplished by employing an anti-Markovnikov Ir-catalyzed hydrosilylation of the D4,18 olefin, using conditions reported by Ding and coworkers.22 A subsequent Tamao–Fleming oxidation23 then furnishes tetrol 26. 24
At this stage, the remaining objectives were the oxidation of the C(18) alcohol to the carboxylic acid oxidation state, and the closure of the butanolide ring, which would furnish havellockate (1). Given the presence of other oxidation-prone functionality within tetrol 26 (i.e., 2° alcohol, allylic 3° alcohol) we recognized that a protocol for the selective oxidation of 1° alcohols would be required. Consequently, we turned to the Cu-catalyzed aerobic oxidation system reported by Stahl and coworkers.25 Upon exposure of tetrol 26 to the Stahl oxidation conditions, we were delighted to observe that tetrol 26 undergoes a two-stage oxidation/cyclization to close the butanolide ring and furnish havellockate directly. Presumably, this involves initial oxidation to generate aldehyde 27 which likely undergoes cyclization to lactol 28. This lactol can then undergo facile oxidation to the lactone yielding the natural product (1).26 All NMR and IR spectral data were in good accordance with the literature values and, additionally, we were able to unambiguously determine the structure of our synthetic material via X-ray crystallography (including absolute stereochemistry).
Unfortunately, the optical rotation of our synthetic sample of havellockate did not match that reported in the literature, either in sign or magnitude (αD25 − 56.7 ° for our synthetic material vs. αD25 + 23.7 ° for the natural material). To date, attempts to secure a sample of the natural product have been met with failure. With no authentic material to compare to we are left in the unenviable situation of not knowing, and potentially never knowing, whether there was an error in the original measurement or whether the absolute stereochemistry of the natural product could be enantiomeric to that depicted in 1. Should a new sample of natural havellockate become available, this lingering question could be further addressed.
In conclusion, we have completed the first total synthesis of the polycyclic furanobutenolide-derived cembranoid havellockate (1). The synthesis begins with the preparation of two enantioenriched fragments which are united via a Julia–Kocienski olefination. This is followed by an intramolecular Diels–Alder reaction that forges the central 6-membered ring of the natural product. Finally, a challenging diastereoselective enone 1,2-acetoxyallylation reaction is employed for the installation of the final carbon-carbon bond and remaining stereogenic centers. A late-stage hydrosilylation/Tamao–Fleming sequence is followed by a Cu-catalyzed oxidative cyclization which closes the butanolide ring and completes the synthesis.
Supplementary Material
ACKNOWLEDGMENT
This manuscript is dedicated to the memory of David A. Evans (1941–2022), a great scientist, educator, mentor, and friend. The authors wish to thank NSF (CHE1800511), NIH (R35GM145239), and the Heritage Medical Research Institute Investigator Program. The authors thank David VanderVelde for NMR assistance and maintenance of the Caltech NMR facility, Dr. Michael Takase and the Caltech XRD facility for XRD assistance, and Dr. Mona Shahgholi for mass spectrometry assistance. The authors thank the Beckman Institute for their support of the Caltech XRD facility, as well as the Dow Next Generation Instrument Grant.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, spectroscopic data (1H NMR, 13C NMR, IR, HRMS) (PDF)
Crystallographic data (CIF)
REFERENCES
- (1).Carroll AR; Copp BR; Davis RA; Keyzers RA; Prinsep MR Marine Natural Products. Nat. Prod. Rep. 2022, 39, 1122–1171. [DOI] [PubMed] [Google Scholar]
- (2).Li Y; Pattenden G Perspectives on the Structural and Biosynthetic Interrelationships Between Oxygenated Furanocembranoids and Their Polycyclic Congeners Found in Corals. Nat. Prod. Rep. 2011, 28, 1269–1310. [DOI] [PubMed] [Google Scholar]
- (3).Craig RA II; Stoltz BM Polycyclic Furanobutenolide-Derived Cembranoid and Norcembranoid Natural Products: Biosynthetic Connections and Synthetic Efforts. Chem. Rev. 2017, 117, 7878–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Tang B; Bray CD; Pattenden G A Biomimetic Total Synthesis of (+)-Intricarene. Tetrahedron Lett. 2006, 47, 6401–6404. [Google Scholar]; (b) Roethle PA; Hernandez PT; Trauner D Exploring Biosynthetic Relationships Among Furanocembranoids: Synthesis of (−)-Bipinnatin J, (+)-Intricarene, (+)-Rubifolide, and (+)-Isoepilophodione B. Org. Lett. 2006, 8, 5901–5904. [DOI] [PubMed] [Google Scholar]; (c) Stichnoth D; Kölle P; Kimbrough TJ; Riedle E; de Vivie-Riedle R; Trauner D Photochemical Formation of Intricarene. Nat. Commun. 2014, 5, 5597. For previous syntheses of the polycyclic furanobutenolide-derived norcembranoid scabrolide A, see: [DOI] [PubMed] [Google Scholar]; (d) Hafeman NJ; Loskot SA; Reimann CE; Pritchett BP; Virgil SC; Stoltz BM The Total Synthesis of (−)-Scabrolide A. J. Am. Chem. Soc. 2020, 142, 8585–8590 [DOI] [PubMed] [Google Scholar]; (e) Meng Z; Fürstner A Total Syntheses of Scabrolide A and Nominal Scabrolide B. J. Am. Chem. Soc. 2022, 144, 1528–1533. For the recent total synthesis of rameswaralide see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Truax NJ; Ayinde S; Liu JO; Romo D Total Synthesis of Rameswaralide Utilizing a Pharmacophore-Directed Retrosynthetic Strategy. J. Am. Chem. Soc. 2022, in press. https://pubs.acs.org/doi/10.1021/jacs.2c08245. The total synthesis of ineleganolide was also recently disclosed: [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Tuccinardi JP; Wood JL Total Synthesis of Complex Furanocembranoids. Presented at 47th National Organic Symposium, La Jolla, CA, June 26–June 30, 2022. [Google Scholar]
- (5).Anjaneyulu ASR; Venugopal MJRV; Sarada P; Clardy J; Lobkovsky E Havellockate, A Novel Seco and Spiro Lactone Diterpenoid from the Indian Ocean Soft Coral Sinularia granosa. Tetrahedron Lett. 1998, 39, 139–142. [Google Scholar]
- (6).Mehta G; Kumaran RS Studies Towards the Total Synthesis of Novel Marine Diterpene Havellockate. Construction of the Tetracyclic Core. Tetrahedron Lett. 2001, 42, 8097–8100. [Google Scholar]
- (7).Beingessner RL; Farand JA; Barriault L Progress toward the Total Synthesis of (±)-Havellockate. J. Org. Chem. 2010, 75, 6337–6346. [DOI] [PubMed] [Google Scholar]
- (8).Blakemore PR The Modified Julia Olefination: Alkene Synthesis via the Condensation of Metallated Heteroarylalkylsulfones with Carbonyl Compounds. J. Chem. Soc., Perkin Trans 1 2002, No. 23, 2563–2585. [Google Scholar]
- (9).Brill ZG; Grover HK; Maimone TJ Enantioselective Synthesis of an Ophiobolin Sesterterpene via a Programmed Radical Cascade. Science 2016, 352 (6289), 1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bauta W; Booth J; Bos ME; DeLuca M; Diorazio L; Donohoe T; Magnus N; Magnus P; Mendoza J; Pye P; Tarrant J; Thom S; Ujjainwalla F New Strategy for the Synthesis of the Taxane Diterpenes: Formation of the BC-Rings of Taxol via a [5+2]-Pyrylium Ylide-Alkene Cyclization, Ring Expansion Strategy. Tetrahedron Letters 1995, 36 (30), 5327–5330. [Google Scholar]
- (11).Evans DA; Ennis MD; Mathre DJ Asymmetric Alkylation Reactions of Chiral Imide Enolates. A Practical Approach to the Enantioselective Synthesis of α-Substituted Carboxylic Acid Derivatives. J. Am. Chem. Soc. 1982, 104 (6), 1737–1739. [Google Scholar]
- (12).Pospíšil J; Markó IE Efficient and Stereoselective Synthesis of Allylic Ethers and Alcohols. Org. Lett. 2006, 8 (26), 5983–5986. [DOI] [PubMed] [Google Scholar]
- (13).See SI for details of the optimization of the Julia–Kosienski olefination.
- (14).Neises B; Steglich W Simple Method for the Esterification of Carboxylic Acids. Angew. Chem., Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar]
- (15).(a) Corey EJ; Munroe JE Total Synthesis of Gibberellic Acid. A Simple Synthesis of a Key Intermediate. J. Am. Chem. Soc. 1982, 104 (22), 6129–6130. [Google Scholar]; (b) Corey EJ; Jardine P. da Silva.; Rohloff JC Total Synthesis of (+)-Forskolin. J. Am. Chem. Soc. 1988, 110 (11), 3672–3673. [Google Scholar]; (c) Nicolaou KC; Li WS An Intramolecular Diels–Alder Strategy to Forskolin. J. Chem. Soc., Chem. Commun. 1985, No. 7, 421–421. [Google Scholar]
- (16).Gansäuer A; Bluhm H; Pierobon M Emergence of a Novel Catalytic Radical Reaction: Titanocene-Catalyzed Reductive Opening of Epoxides. J. Am. Chem. Soc. 1998, 120, 12849–12859. [Google Scholar]
- (17). Additions with organocerium, chromium, indium, and alkyl and vinyl organozinc nucleophiles were attempted. In all cases no addition product was observed.
- (18).(a) Lombardo M; Morganti S; Trombini C 3-Bromopropenyl Esters in Organic Synthesis: Indium- and Zinc-Mediated Entries to Alk-1-ene-3,4-diols. J. Org. Chem. 2003, 68, 997–1006. [DOI] [PubMed] [Google Scholar]; (b) Lombardo M; Morganti S; d’Ambrosio F; Trombini C 3-Bromo-propenyl Acetate in Organic Synthesis. The Zinc-Promoted α-Hydroxyallylation of Ketones. Tetrahedron Lett. 2003, 2823–2826. [Google Scholar]
- (19). This mixture has been tentatively characterized as a 1:1 mixture of branched and linear allylation products. See SI for details.
- (20).Mejuch T; Gilboa N; Gayon E; Wang H; Houk KN; Marek I Axial Preferences in Allylation Reactions via the Zimmerman–Traxler Transition State. Acc. Chem. Res. 2013, 46, 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Wuts PGM; Greene TW Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons, Ltd: Hoboken, NJ, 2014; pp 752. [Google Scholar]; (b) Chandrasekaran S; Kluge AF; Edwards JA Studies in β-Lactams. 6. Synthesis of Substituted β-Lactams by Addition of Nitromethane to 6-Oxopenicillanates and 7-Oxocephalosporanates. J. Org. Chem. 1977, 42 (24), 3972–3974. [Google Scholar]
- (22).Xie X; Zhang X; Yang H; Ji X; Li J; Ding S Iridium-Catalyzed Hydrosilylation of Unactivated Alkenes: Scope and Application to Late-Stage Functionalization. J. Org. Chem. 2019, 84, 1085–1093. [DOI] [PubMed] [Google Scholar]
- (23).Jones GR; Landais Y The Oxidation of the Carbon–Silicon Bond. Tetrahedron 1996, 52, 7599–7662. [Google Scholar]
- (24). Triol 26 and tetrol 27 were each isolated with an impurity that were evident in their NMR spectra. See SI for details.
- (25).Hoover JM; Stahl SS Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133 (42), 16901–16910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Xie X; Stahl SS Efficient and Selective Cu/Nitroxyl-Catalyzed Methods for Aerobic Oxidative Lactonization of Diols. J. Am. Chem. Soc. 2015, 137 (11), 3767–3770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.