Abstract
Background
Treatments for alopecia areata (AA) patients with extensive scalp hair loss are limited, and recent evidence supports a role for type 2 T-cell (Th2)-immune response in AA. Dupilumab, a monoclonal antibody inhibiting Th2 signaling, approved for type 2 diseases including atopic dermatitis, was evaluated in AA patients.
Methods
AA patients with and without concomitant atopic dermatitis were randomized 2:1 to receive weekly subcutaneous dupilumab (300 mg) or placebo for 24 weeks, followed by another 24-week dupilumab open-label phase. The primary outcome was change from baseline in the Severity of Alopecia Tool (SALT) score at week 24; secondary outcomes included a range of measures of hair regrowth.
Results
Forty and 20 patients were assigned to the dupilumab and placebo arms, respectively. At week 24, disease worsening was documented in the placebo arm, with a least-squares mean change in the SALT score of −6.5 (95% confidence-interval [CI], −10.4 to −2.6), versus a change of 2.2 (95% CI, −0.6 to 4.94) in the dupilumab arm (P<0.05). After 48 weeks of dupilumab treatment, 32.5%, 22.5% and 15% of patients achieved SALT30/SALT50/SALT75 improvement, respectively, while in patients with baseline IgE≥200IU/ml response rates increased to 53.8%, 46.2%, and 38.5%, respectively. Moreover, baseline IgE predicts treatment response with 83% accuracy. No new safety signals were detected.
Conclusions
This hypothesis-driven trial is the first to indicate the possible pathogenic role of the Th2 axis and Th2 targeting in AA patients. Patient selection based on baseline serum IgE levels may improve treatment results.
Keywords: Alopecia Areata, Atopic Dermatitis, Dupilumab, IgE, Th2
Graphical Abstract
• Recent evidence supports a role for Th2-immune response in AA. To test these observations, AA patients with and without concomitant atopic dermatitis were randomized to receive dupilumab or placebo.
• Patients with personal and/or family atopy and high baseline IgE (≥200IU/ml) exhibited greater efficacy of dupilumab.
• This hypothesis-driven trial supports the pathogenic role of Th2 activation in AA.
Abbreviations: AA, alopecia areata; IU/ml, international units per mililiter
INTRODUCTION
Alopecia areata (AA) is the most common cause of auto-inflammatory hair loss, with an estimated global lifetime risk of about 2% of the population.1 Disease severity ranges from patchy, limited hair loss to complete loss of scalp hair (alopecia totalis [AT]), or complete loss of scalp and body hair (alopecia universalis [AU]).2 Extensive AA patients often experience a chronic course of their disease; where there is ≥50% hair loss, only 8% of patients re-grow at least 25% of the hair on placebo treatment.2,3 In addition to devastating effects on patients’ quality of life and self-esteem,4 there is also emerging evidence on systemic inflammation in AA,5–7 further advocating to seek efficacious treatment options for these patients.
Common practices for extensive AA, where topical and intra-lesional treatments are often of limited applicability, include systemic immunosuppressors such as corticosteroids, cyclosporine, azathioprine and methotrexate, all associated with significant adverse events, particularly in long term use.8 While recent studies with janus kinase (JAK) inhibitors show promise in AA,9 patients lose their new hair regrowth within 3 months following drug discontinuation,10 requiring long-term use that may pose safety concerns.11 Thus, there is a high unmet need for new, specific treatments for long-term disease control in moderate-to-severe AA patients.
Development of novel, efficacious therapeutics is hindered by incomplete understanding of AA mechanisms, and immune modulators driving AA are still elusive. New data support that in addition to Th1/interferon (IFN)γ,12–14 atopic background and Th2-skewing may play a key pathogenic role in AA.6,7,15–17 Currently, there are no randomized, placebo-controlled trials evaluating narrow Th2 targeting agents in AA.
The objective of this randomized, placebo-controlled phase 2a study was to evaluate the efficacy and safety of dupilumab in AA. Dupilumab is a monoclonal antibody inhibiting Th2 signaling by blocking IL-4Rα, shared by IL-4 and IL-13, FDA-approved for the treatment of type 2 immune mediated diseases, including atopic dermatitis (AD).18–20
METHODS
Study Design and Patients
This phase 2a, randomized, double-blind, multicenter study was approved by the institutional review board at both study sites and registered on ClinicalTrials.gov (NCT03359356). Patients were randomized 2:1 to dupilumab or placebo arm, administered by identical syringes. Adults (age ≥18 years) diagnosed with AA (≥6 months and with ≥30% scalp hair loss) were enrolled.
Other inclusion criteria included: subjects who are able to understand and voluntarily sign an informed consent document, and adhere to the study visit schedule and other protocol requirements; approximately one-third of subjects will have active AD skin or a concomitant history of AD; negative pregnancy test; use of an approved contraceptive method; if subject is a female of non-childbearing potential, proper documentation should be provided; negative tuberculosis test prior to baseline visit (subjects with a positive or indeterminable test result must have a documented negative workup for tuberculosis and/or completed standard tuberculosis therapy); specific laboratory criteria, as detailed in the full protocol, should be met (including complete blood count parameters, serum creatinine, and liver function tests); subjects who are judged to be in otherwise good overall health.
Exclusion criteria included: subjects who are pregnant or breastfeeding; cause of hair loss is indeterminable and/or other concomitant causes of alopecia; a history of AA with no evidence of hair regrowth for ≥10 years; an active bacterial, viral, or helminth parasitic infections or a history of ongoing, recurrent severe infections requiring systemic antibiotics; subject with a known or suspected underlying immunodeficiency or immune-compromised state; a concurrent or recent history of severe, progressive, or uncontrolled renal, hepatic, hematological, intestinal, metabolic, endocrine, pulmonary, cardiovascular, or neurological disease; active hepatitis B, hepatitis C, human immunodeficiency virus (HIV), or positive HIV serology at the time of screening; a suspected or active lymphoproliferative disorder or malignancy or a history of malignancy within 5 years before the baseline assessment; subjects who received a live attenuated vaccine ≤30 days prior to study randomization; any uncertain or clinically significant laboratory abnormalities that may affect interpretation of study data or endpoints; any other medical or psychological condition that, in the opinion of the investigator, may present additional unreasonable risks as a result of study participation and/or interfere with clinic visits and necessary study assessments; history of adverse systemic or allergic reactions to any component of the study drug; severe, untreated asthma or a history of life-threatening asthma exacerbations while on appropriate anti-asthmatic mediations; use of systemic immunosuppressive medications, including, but not limited to, cyclosporine, systemic or intralesional corticosteroids, mycophenolate mofetil, azathioprine, methotrexate, tacrolimus, or ultraviolet (UV) within 4 weeks prior to randomization; use of an oral JAK inhibitor within 12 weeks prior to the baseline visit; use of topical corticosteroids, and/or tacrolimus, and/or pimecrolimus within 1 week prior to the baseline visit; previous treatment with dupilumab; current use or a plan to use anti-retroviral therapy at any time during the study.
For further details on inclusion/exclusion criteria, see full trial protocol in the Appendix.
Randomization and masking
The randomization for both sites was performed by a designated research personnel at the Icahn School of Medicine using an internet-based randomization system. The method uses random block sizes and randomly permuted blocks. Other research and clinical staff, study statisticians, and the participants were masked to treatment allocation during the study.
Procedures
An initial 24-week evaluation period (primary endpoint) in which patients were randomized to receive weekly, subcutaneous dupilumab or matching placebo (300 mg) was followed by another 24-week open-label phase in which all participants were treated with weekly dupilumab up to week 48 (secondary endpoint). Patients self-administrated dupilumab/placebo at their home after given instructions and guidance from the research team on baseline visit. From baseline visit to week 48 visit, participants were evaluated every four weeks. After week 48, follow-up continued at weeks 60 and 72.
Outcomes
The primary efficacy endpoint was change from baseline in the Severity of Alopecia Tool (SALT) score at week 24. The SALT score quantifies scalp hair loss, and ranges from 0 (no hair loss) to 100 (complete hair loss). The comparison versus placebo was chosen since there are no systemic FDA-approved treatments for AA.
Key secondary outcomes included change in SALT from week 48 to week 24 and to baseline and the proportion of patients achieving 30%/50%/75% SALT score improvement (SALT30/50/75) at week 24 and 48. Other outcomes included change in the Alopecia Areata Symptom Impact Scale (AASIS) and Alopecia Areata Quality of Life Index questionnaires (AA-QLI),21,22 proportion of patients with Alopecia Areata Physician’s Global Assessment (AA-PGA) improvement (Table S1), change in eyelash and eyebrow scores and in Eczema Area and Severity Index (EASI), and safety assessments. The full protocol and statistical analysis plan (SAP) can be found in the Appendix. Safety parameters included treatment-emergent adverse events (AEs) and clinical laboratory abnormalities. Safety labs and blood markers potentially related with AA/atopy (e.g., percentage of eosinophils, total serum IgE) were evaluated at baseline and weeks 12, 24, 36 and 48.
Statistical Methods
The sample size was based on the primary efficacy outcome, i.e., change in SALT score at week 24 in drug compared to placebo. For patients with similar disease severity, a change in SALT of less than 5 units was observed in placebo.23 Assuming that at week 24 dupilumab will induce a change in SALT of at least 25 with a standard deviation of 10, a total sample size of 54 patients randomized 2:1 dupilumab to placebo will provide 97% power to detect differences compared to placebo, based on a two-sided Student’s t-test with a type I error α=0.05. Assuming a 10% dropout rate, the planned sample size was a total of 60 patients.
All analysis was performed with R language (R-project.org, version 3.6.3). The primary outcome was analyzed by a linear mixed-effect model repeated measures with treatment and time point interaction as a fixed effect and a random effect for each subject. For continuous secondary outcomes we used a similar approach by linear mixed-effect models, stratified by variables such as IgE levels and atopy. Categorical outcomes were evaluated as two-sample proportions by Fisher’s exact test. Participants in analysis of clinical parameters by scoring tools (e.g., change in SALT/EASI/AASIS/AA-QLI) and safety were included per protocol. Proportion of patients achieving SALT30/50/75/90 improvement was analyzed including intention-to-treat populations and patients with missing values were considered non-responders. Confidence intervals have not been adjusted for multiplicity and cannot be used to infer definitive treatment effects for secondary efficacy endpoints. Correlation analysis was conducted using Pearson correlation coefficient, and P-values were adjusted by false discovery rate. To evaluate the utility of IgE as a possible predictor of dupilumab response in AA (for a SALT50 response at week 24), we used the receiver operating characteristic area under the curve (AUC).
Trial findings are described in accordance with CONSORT guidelines (Figure S1).24
RESULTS
Between December 13, 2017, and July 24, 2019, of 65 screened patients, 60 were randomized in a 2:1 ratio to receive dupilumab (n=40) or placebo (n=20). At week 24, 34 (85%) and 17 (85%) patients on dupilumab and placebo, respectively, completed treatment. In the open-label phase of this trial, from week 24 to week 48, 32 (94%) and 12 (70.6%) patients on dupilumab and placebo, respectively, completed treatment. The mean age was 44 years, 71.7% were women, 76.7% were white, the mean duration since last hair regrowth was 3.7 years, and 36.7% of patients had AT/AU (Table 1). Twenty-three patients (38.3%) had a history of AD, out of which seven (11.7%) had active AD at baseline, 27 (45%) participants had a family history of an atopic disease (e.g., AD, asthma), and 18 (30%) had high total serum IgE levels of ≥200 IU/ml. There were no significant differences in baseline patient characteristics between drug and placebo arms (Table 1).
Table 1.
Patient Baseline Characteristics.
| Placebo (N=20) | Dupilumab (N=40) | |
|---|---|---|
| Mean age, years (SD) | 46.5 (14.4) | 41.6 (13.8) |
| Female sex | 13 (65%) | 30 (75%) |
| Race | ||
| White | 15 (75%) | 31 (77.5%) |
| African American | 2 (10%) | 3 (7.5%) |
| Asian | 2 (10%) | 6 (15%) |
| Other | 1 (5%) | 0 (0%) |
| Mean duration since last hair regrowth, years (SD) | 3.5 (3) | 3.8 (2.9) |
| Mean SALT (SD) | 75.4 (26.1) | 70.5 (27.6) |
| Patients with SALT≥75 | 12 (60%) | 20 (50%) |
| Patients with SALT<75 | 8 (40%) | 20 (50%) |
| Patients with Alopecia Totalis/Universalis | 8 (40%) | 13 (32.5%) |
| Mean AASSIS score (SD) | 56.1 (33.8) | 48.4 (30.7) |
| Mean AA-QLI score (SD) | 51.8 (14.2) | 49.5 (12.7) |
| Mean eyelash assessment (0–4 scale) (SD) | 2 (1.6) | 2.07 (1.57) |
| Mean eyebrow assessment (0–4 scale) (SD) | 1.8 (1.54) | 1.9 (1.53) |
| Patients with AD history | 6 (30%) | 17 (42.5%) |
| Patients with active AD | 2 (10%) | 5 (12.5%) |
| Mean EASI score (SD) | 27.4 (15.6) | 13.6 (6.4) |
| Patients with family history of atopy | 9 (45%) | 18 (45%) |
| Mean IgE, IU/ml (SD) | 342.5 (826.7) | 525.8 (1211.3) |
| Patients with IgE≥200 IU/ml | 5 (25%) | 13 (32.5%) |
AASIS, denoted alopecia areata symptom impact scale, AA-QLI, alopecia areata quality of life index, AD, atopic dermatitis, EASI, eczema area and severity index, SALT, severity of alopecia tool, and SD, standard deviation.
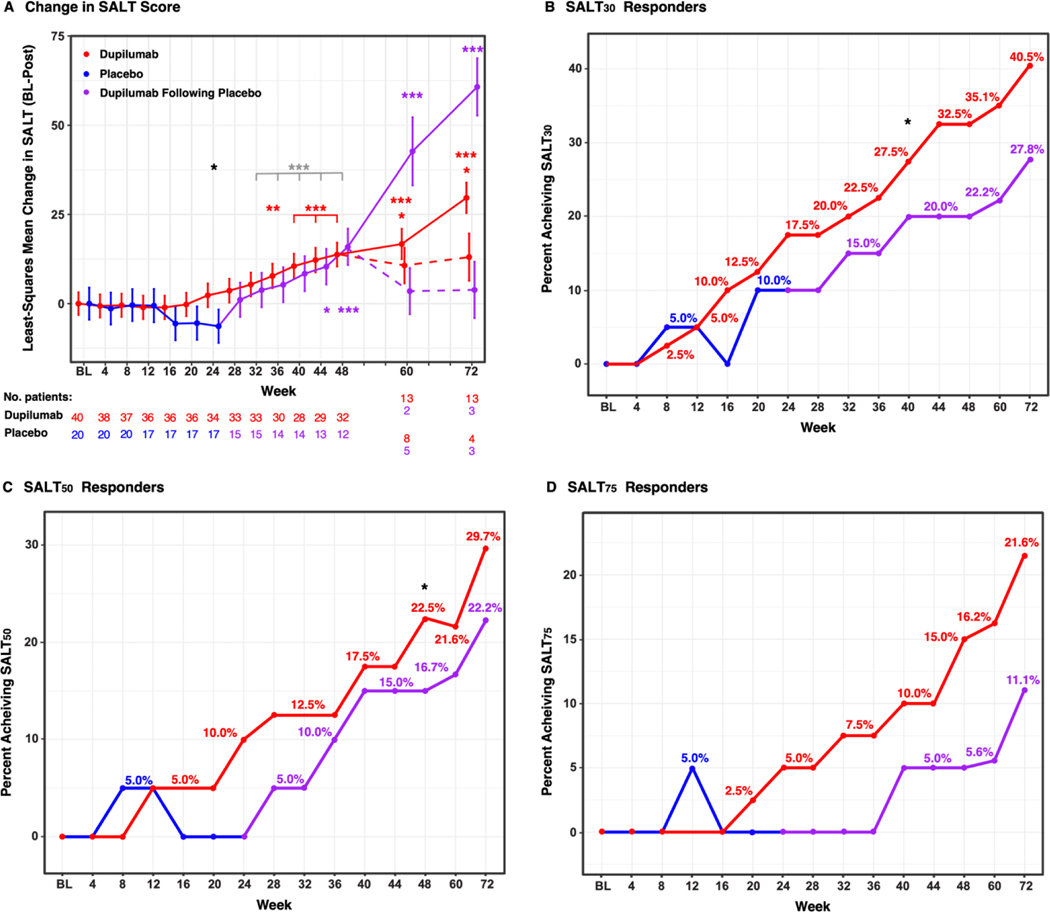
At week 24, worsening of AA was documented in the placebo group, with a least-squares mean change in the SALT score of −6.3 (95% CI, −14.6 to 1.8), while in the drug arm we observed a least-squares mean change of 2.3 (95% CI, −3.5 to 8.2) as compared to baseline (P<0.05). Change in least-squares mean SALT score from baseline to week 48 in the drug arm was 13.7 (95% CI, 7.8 to 19.7) compared to baseline (P<0.001). Change in least-squares mean SALT score from week 24 to week 48 in the placebo arm was 15.9 (95% CI 6.7 to 25.1) compared to placebo arm at week 24, when the open-label phase started (P<0.001). Change in least-squares mean SALT score from week 24 to week 48 in the drug arm versus the change in least-squares mean SALT score in the placebo arm from baseline to week 24 was 17.4 (95% CI, 2.84 to 31.93) (P=0.019). Change in least-squares mean SALT score from week 24 to week 48 in the placebo arm versus the change in least-squares mean SALT score in the placebo arm from baseline to week 24 was 22.3 (95% CI, 12.1 to 32.5) (P<0.001) (Table 2A and Figure 1A). When patients were stratified based on personal and/or family history of atopy, baseline SALT scores were not different between atopic and non-atopic participants. However, significant improvement in least-squares mean SALT score was detected much earlier in atopic as compared to non-atopic patients, that achieved significance at week 48 (Figure S2). Clinical pictures of participants are shown in Figure 2.
Table 2.
Efficacy Outcomes.
| A. | ||||
|---|---|---|---|---|
| Placebo (N=20) | Dupilumab (N=40) | P-value | ||
| Change in SALT from baseline to week 24 (SE) | −6.3 (4.2) | 2.3 (3) | 0.049 | |
| Key secondary endpoints: | ||||
| Change in SALT from baseline to week 48 in the drug-first arm (SE) | NA | 13.7 (3) | <0.0001 | |
| Change in SALT from week 24 to week 48 in the placebo-first arm (SE) | 15.9 (4.7) | NA | 0.0006 | |
| Change in SALT from week 24 to week 48 in the placebo-first arm versus change in SALT from baseline to week 24 in the placebo-first arm (SE) | 22.3 (5.2) | NA | <0.0001 | |
| Change in SALT from week 24 to week 48 in the drug-first arm versus change in SALT from baseline to week 24 in the placebo-first arm (SE) | NA | 17.4 (7.3) | 0.019 | |
| Proportion of patients achieving SALT30/50/75/90 at week 24 | ||||
| ≥30% (SALT30) | 2 (10%) | 7 (17.5%) | 0.66 | |
| ≥50% (SALT50) | 0 (0%) | 4 (10%) | 0.23 | |
| ≥75% (SALT75) | 0 (0%) | 2 (5%) | 0.55 | |
| ≥90% (SALT90)3 | 0 (0%) | 1 (2%) | 1 | |
| Proportion of patients achieving SALT30/50/75/90 at week 48 (compared to placebo-first arm at week 24) | ||||
| ≥30% (SALT30) | 4 (20%) | 13 (32.5%) | 0.067 | |
| ≥50% (SALT50) | 3 (15%) | 9 (22.5%) | 0.02 | |
| ≥75% (SALT75) | 1 (5%) | 6 (15%) | 0.17 | |
| ≥90% (SALT90) 3 | 1 (5%) | 4 (10%) | 0.29 | |
| Proportion of patients achieving SALT30/50/75/90 after 24 weeks of dupilumab treatment 1 | ||||
| Placebo arm after weeks 24–48 (open-label Dupilumab treatment) (N=20) | Drug arm after weeks 0–24 (N=40) | All patients after 24 weeks of dupilumab treatment (N=60) | ||
| ≥30% (SALT30) | 4 (20%) | 7 (17.5%) | 11 (18.3%) | 0.19 |
| ≥50% (SALT50) | 3 (15%) | 4 (10%) | 7 (11.7%) | 0.08 |
| ≥75% (SALT75) | 1 (5%) | 2 (5%) | 3 (5%) | 0.54 |
| ≥90% (SALT90)3 | 1 (5%) | 1 (2%) | 2 (3.3%) | 0.55 |
| B. | ||||
| Proportion of patients achieving SALT30/50/75/90 in the drug arm at week 24 based on baseline IgE level (threshold: 200IU/ml) 2 | ||||
| Low IgE (N=27) | High IgE (N=13) | Entire drug arm (N=40) | ||
| ≥30% (SALT30) | 2 (7.4%) | 5 (38.5%) | 7 (17.5%) | 0.03 |
| ≥50% (SALT50) | 0 (0%) | 4 (30.8%) | 4 (10%) | 0.008 |
| ≥75% (SALT75) | 0 (0%) | 2 (15.4%) | 2 (5%) | 0.1 |
| ≥90% (SALT90)3 | 0 (0%) | 1 (7.7%) | 1 (2%) | 0.32 |
| Proportion of patients achieving SALT30/50/75/90 in the drug arm at week 48 based on baseline IgE level (threshold: 200IU/ml) 2 | ||||
| Low IgE (N=27) | High IgE (N=13) | All drug arm (N=40) | ||
| ≥30% (SALT30) | 6 (22.2%) | 7 (53.8%) | 13 (32.5%) | 0.07 |
| ≥50% (SALT50) | 3 (11.1%) | 6 (46.2%) | 9 (22.5%) | 0.04 |
| ≥75% (SALT75) | 1 (3.7%) | 5 (38.5%) | 6 (15%) | 0.009 |
| ≥90% (SALT90)3 | 1 (3.7%) | 3 (23.1%) | 4 (10%) | 0.09 |
SALT denotes severity of alopecia tool, SE standard error. Bolded values show P<0.05.
P-values represent comparison between all patients completing 24 weeks of dupilumab treatment versus placebo arm at week 24.
P-values for comparisons by IgE levels are between high and low IgE subgroups.
All four patients achieving SALT90 at week 48 had complete hair regrowth, which means they could also be regarded as SALT100.
Figure 1. Least-squares Mean Change in the Severity of Alopecia Tool (SALT) Score (Baseline – Post Treatment) (A) and Responder Rates Over the Duration of the Study (Until Week 72) (B-D).
Red, blue and purple represent drug, placebo arms, and dupilumab after placebo (open-label), respectively. */**/*** denote significant (P<0.05/0.01/0.001 respectively) difference in the dupilumab arm as compared to placebo treatment (black), dupilumab open-label versus placebo treatment in the placebo arm (gray), and change from baseline at each time point in the dupilumab arm (red) and in the open-label phase of the placebo arm (purple). In (A), solid lines represent patients treated with dupilumab, dotted lines represent patients that were assessed on weeks 60 and 72 but were no longer treated with dupilumab after week 48.
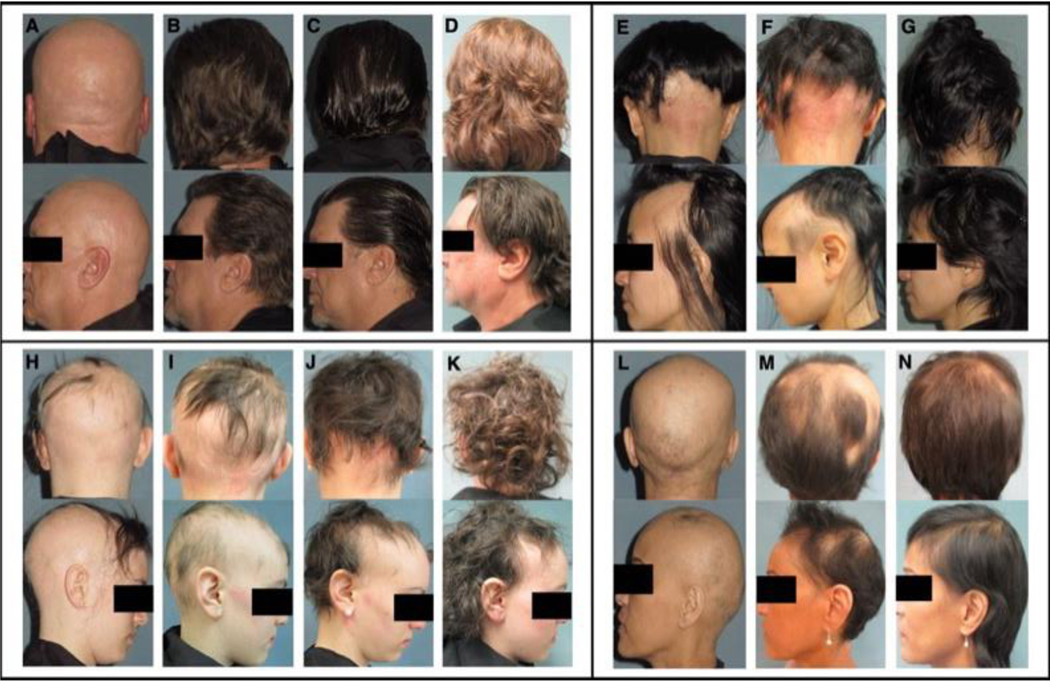
Figure 2. Scalp Pictures of Four Patients in the Drug Arm.
A-D and H-K show scalp hair at baseline, week 24, week 48, and week 72 visits in patients continuing treatment after week 48. E-G and L-N show scalp hair at baseline, week 24, and week 48 visits.
Upon grouping all patients treated with 24 weeks of dupilumab together (i.e., those completing week 24 in the drug arm with those completing weeks 24 to 48 in the placebo arm, after switching to dupilumab), SALT30 was achieved by 18.3% of patients, compared with 10% in the placebo group. The proportions of patients achieving SALT50 and SALT75 were 11.7% and 5%, respectively, versus 0% (for both SALT50 and SALT75) in the placebo group (Figure 1B–D) (P<0.05 for SALT50). By week 48, 32.5% of the patients achieved SALT30, 22.5% achieved SALT50, and 15% achieved SALT75 in the drug arm (Table 2A).
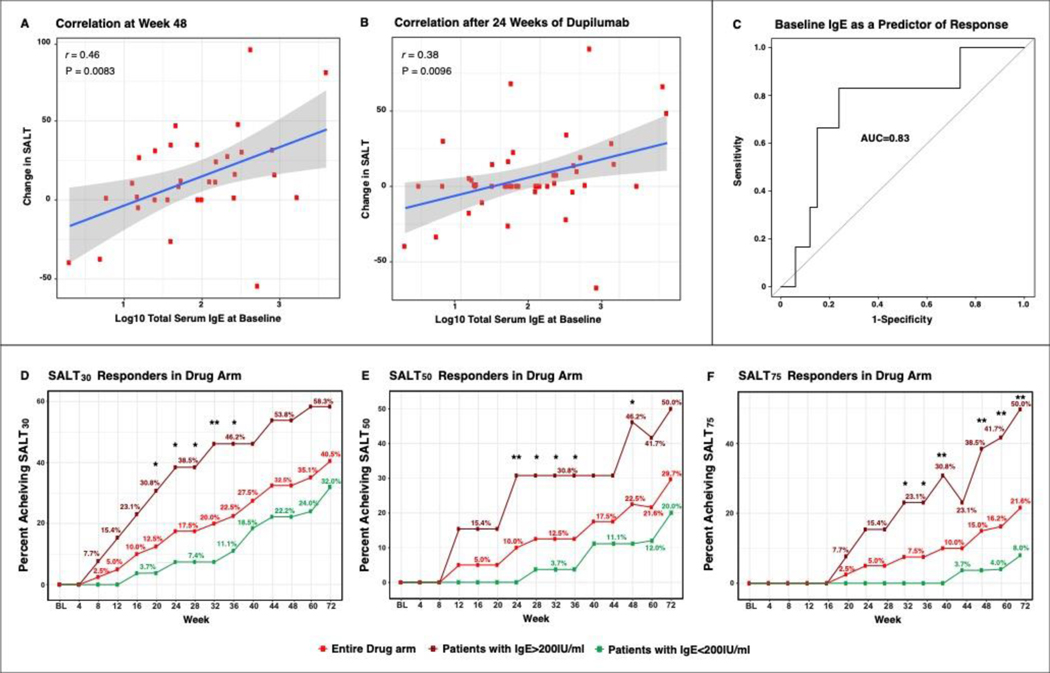
Baseline characteristics of patients achieving SALT30 were compared with patients that did not achieve SALT30 after 24 weeks of dupilumab treatment in both study arms (first 24 weeks for drug-first arm and weeks 24–48 in the placebo-first arm). Baseline SALT scores, IgE levels, and the proportion of patients with concomitant AD/AD history or with a family history of atopy were significantly different among SALT30-responders and non-responders (Tables S2 and S3) (P<0.05). Patients achieving SALT30 had significantly higher baseline IgE levels (946.2, 95% CI, 0 to 2,460.2) versus non-responders (338.8, 95% CI, 0 to 612.2). Moreover, change in SALT and IgE levels showed a robust and significant correlation both after 48 weeks in the drug arm (r=0.46, 95% CI, 0.13 to 0.7, P=0.0083), as well as after 24 weeks of dupilumab treatment in both study arms (r=0.38, 95% CI, 0.099 to 0.6, P=0.0096). Further, baseline serum IgE predicts dupilumab response with an AUC of 0.83 (Figure 3). To further clarify the possible utility of IgE to predict dupilumab response and potentially improve patient selection, we evaluated response in the entire dupilumab arm as compared to those patients with high and low baseline IgE (IgE≥/<200IU/ml) in this arm (Figure 3 and Table 2B). Patients with high IgE had the highest SALT30/50/75/90 response rates compared to the other groups (Figure 3). In fact, patients with high IgE had an odd ratio of 8.1 to achieve SALT30 response as compared with patients with low IgE at week 24. Concomitant AD/AD history or a family history of atopy were found in 90.9% of patients achieving SALT30 but only in 30.6% of non-responders (P<0.05). Consistent with previously published data,25 greater response to dupilumab treatment was observed in patients with shorter periods since last hair regrowth, as shown in Figure S3. Other baseline characteristics, including age, gender, race, mean duration since last hair regrowth, or other blood markers (i.e, CRP, LDH, eosinophil percentage) were not significantly different among SALT30 responders and non-responders.
Figure 3. Pearson Correlation of Changes in SALT Score with Baseline IgE Levels (A,B), Baseline Total Serum IgE as a Predictor of Response to Dupilumab (C), and Responder Rates Based on IgE Levels at Baseline (D-F).
Correlation of patients in the dupilumab arm after completing 48 weeks of treatment (A), correlation of all patients treated with 24 weeks of dupilumab together (i.e., those completing week 24 in the drug arm with those completing week 24 to week 48 in the placebo arm, after switching to dupilumab) (B). False discovery rate (FDR)<0.05 for both A and B. Receiver operating characteristic area under the curve (AUC) analysis utilizing IgE as a predictor of SALT50 response at week 24 (C), with IgE levels able to predict response in 83% of AA dupilumab-treated patients. Responder (SALT30/50/75) rates in the entire drug arm, along with lines depicting only patients with high or low IgE (threshold: 200IU/ml) in the drug arm (D-F). SALT, Severity of Alopecia Tool. */**/*** denote significant (P<0.05/0.01/0.001 respectively) difference in responder rates in patients with high versus low IgE at baseline.
Of patients with eyelash or eyebrow hair loss at baseline (a grade of ≤2, in a 0–4 scale), 18.2% and 27.3% achieved ≥1 grade improvement in the eyelash assessment and 7.1% and 20% achieved ≥1 grade improvement in the eyebrow assessment at week 24 compared to baseline in the placebo and drug arms, respectively. These proportions increased to 27.3% and 31.8% for eyelash assessment and 28.6% and 24% for eyebrow assessment at week 48 compared to baseline in the placebo and drug arms, respectively (Table S4).
Although changes in quality-of-life scores (both AA-QLI and AASIS) in drug vs placebo arm did not achieve significance, AASIS scores were significantly lower in responder versus non-responder participants at study endpoints (i.e., at both week 24 and week 48), reflecting less impact of AA on participants’ quality-of-life. For example, mean AASIS scores reported by responders at week 24 were 15.47 (95% CI, 1.06 to 30.08) for those achieving SALT30 and 4.75 (95% CI, 1.94 to 7.56) for those achieving SALT50, versus 54.59 (95% CI 41.68 to 67.50) in non-responders, i.e., participants with less than 30% SALT improvement (SALT0–30) (p<0.05) (Table S5).
Of the seven patients with active AD, AD severity (by EASI) improved in dupilumab-treated patients after both 24 and 48 weeks of treatment, with no improvement after placebo treatment (Table S5) (P<0.05).
AEs were reported in 20% and 25% of patients in the placebo and drug arms, respectively, during the first 24 weeks of this trial, and in 25% and 26.5%, respectively, during weeks 24 to 48 (Table S6). The most common AEs were mild upper-respiratory tract infection and mild-to-moderate conjunctivitis. Notably, all conjunctivitis cases were reported in participants with no personal history of AD. Less commonly, injection site reactions, gastrointestinal symptoms, and urinary tract infections were reported, all classified as mild. One patient receiving dupilumab experienced a serious AE (bowel obstruction) that was considered unrelated to study drug by study investigators. AE that led to discontinuation of the study drug occurred in one patient in the drug arm, during the first 24 weeks of the study (reversible drug eruption). In both study arms, there were no clinically relevant changes in hematology and chemistry blood tests or vital signs.
After completing week 48, patients were followed until week 72. Those interested in continuing dupilumab treatment received weekly dupilumab during this interval. Patients treated with dupilumab until week 72 showed continued improvement in SALT scores, with a least-squares mean change in the SALT score of 29.6 (95% CI, 21.8 to 37.5) in the drug-first arm compared to baseline, and of 60.7 (95% CI, 45.5 to 76) in the placebo-first arm compared to week 24. Patients discontinuing dupilumab experienced worsening after week 48, with a least-squares mean change in the SALT score of 13 (95% CI, 0.3 to 25.7) in the drug-first arm compared to baseline, and of 3.8 (95% CI −11.1 to 18.8) in the placebo-first arm compared to week 24 (Figure 1).
DISCUSSION
This is the first study showing the safety and efficacy of a Th2-specific immune antagonist in patients with moderate-to-severe AA. Recent evidence suggest that the Th2 immune axis may have a pathogenic role in AA. This is based upon epidemiological studies showing a strong association between AA and atopy,17,26 genetic associations between atopy-related genes and AA (e.g., the Th2 cytokine IL-13),27–29 significant up-regulation of Th2-related immune products in AA scalp and blood,6,7,15,16 elevated serum IgE levels in AA patients (including in non-atopic individuals),30–32 and case reports/series of hair regrowth with dupilumab treatment in AA patients.33–35
To explore a possible pathogenic role for the Th2 pathway in AA we conducted a randomized, placebo-controlled clinical trial, using the monoclonal antibody dupilumab, FDA-approved for type 2 diseases (AD, asthma, and nasal polyps).20 AA patients with and without AD/history of AD were enrolled, to evaluate whether Th2-targeting is beneficial for AA, both with and without concomitant AD. Due to higher systemic inflammation seen in patients with moderate-to-severe AA as compared to AD patients,5,15 we used a weekly dosing of dupilumab. The need for high dosing regimen is also supported by similar uses of different systemic immunosuppressants in extensive AA.36
There are currently no approved systemic treatments for moderate-to-severe AA, presenting a high unmet need for development of new treatments. While JAK inhibitors show promise in clinical trials for moderate-to-severe AA,9 hair regrowth is not maintained after discontinuation,10 necessitating long-term treatment for which safety data are still limited.11 In addition, as JAK inhibitors suppress multiple cytokines, including the Th1/IFN-γ but also the common γc cytokines,12 they cannot inform on the contribution of specific immune pathways to AA.
After 24 weeks, dupilumab-treated patients had responded significantly better to treatment as compared to placebo, largely due to the worsening in the placebo group, whereas the dupilumab-treated arm achieved disease stabilization. However, during the open-label phase of this trial, between weeks 24 to 48, continued improvement was seen in the mean SALT score in the drug arm with an abrupt positive change starting at week 24 in the placebo arm. These suggest controlled studies with longer treatment periods to primary endpoint are needed to improve the assessment of Th2 targeting versus placebo in AA.
We found that at week 48, 32.5% of the patients achieved SALT30, 22.5% achieved SALT50, and 15% achieved SALT75 in the drug arm. Further analysis revealed that responders were more likely to have personal or familial atopy (beyond AD) and/or high serum total IgE levels (≥200IU/ml) as compared to non-responders. Moreover, baseline IgE level were able to predict dupilumab response with 83% accuracy, and among patients with IgE≥200IU/ml, 53.8% achieved SALT30, 46.2% achieved SALT50, and 38.5% achieved SALT75 in the drug arm at week 48. Additionally, patients with IgE≥200IU/ml had an odd ratio of 8.1 to respond to dupilumab treatment as compared with patients with baseline IgE<200IU/ml, suggesting that IgE may potentially be a tool for patient selection to dupilumab treatment, a novel concept in AA management. A possible explanation to these observations may be that increased levels of serum IgE are indicative of Th2-associated inflammation in the hair follicle, which may respond better to Th2-targeting approaches.
This study has a few limitations. Our cohort was relatively small, and since this was a proof-of-concept, investigator-initiated study that enrolled 60 patients across two treatment arms, we only tested weekly dupilumab administration. While responder rates in our study may be lower than those seen with some JAK inhibitors,10 and dupilumab may require weekly dosing and longer treatment regimens for a meaningful response in AA, dupilumab use has accumulated a large bulk of multi-year longitudinal safety data across indications and does not necessitate blood monitoring,37 potentially making it a suitable treatment candidate for long-term use, as is needed in AA patients. Future, larger clinical trials are needed to determine the role of Th2 targeting in the therapeutic paradigm of AA patients. Additionally, the underlying mechanism linking increased serum IgE and therapeutic response to dupiluamb in AA needs to be further explored in future studies.
In conclusion, our hypothesis-driven trial is the first to indicate a possible pathogenic role of the Th2 axis and a potential for Th2 targeting in AA patients. It further suggests that this treatment approach may benefit from patient selection based on baseline serum IgE levels.
Supplementary Material
Funding
This publication was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University, Grant # UL1 TR001866. This investigator-initiated study was supported by a grant from Regeneron/Sanofi.
Role of the funding source
This investigator-initiated study was supported by a grant from Regeneron/Sanofi. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Declaration of interests
EGY has served as a consultant for AbbVie, Amgen, Allergan, Asana Bioscience, Celgene, Concert, Dermira, DS Bio- pharma, Escalier, Galderma, Glenmark, Kyowa Kirin, LEO Pharmaceuticals, Lilly, Mit- subishi Tanabe, Novartis, Pfizer, Regeneron, Sanofi, and Union Therapeutics; a member of advisory boards of Allergan, Asana Bioscience, Celgene, DBV, Dermavant, Dermira, Escalier, Galderma, Glenmark, Kyowa Kirin, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, and Sanofi; and a recipient of research grants from AbbVie, AnaptysBio, Anti- bioTx, Asana Bioscience, Boehringer-Ingelheim, Celgene, DBV, Dermavant, DS Biopharma, Galderma, Glenmark, Innovaderm, Janssen Biotech, Kiniska Pharma, LEO Pharmaceuticals, Lilly, Medimmune, Sienna Biopharmaceuticals, Novan, Novar- tis, Ralexar, Regeneron, Pfizer, UCB, and Union Therapeutics. MGL has received grant support from Ortho Dermatologics, UCB, AbbVie, Amgen, Eli Lilly, Incyte, and Janssen Research and Development, grant support and consulting fees from Pfizer, Dermavant Sciences, LEO Pharma, Boehringer Ingelheim, and Arcutis Biotherapeutics, and consulting fees from Allergan, Almirall, Avotres Therapeutics, BirchBioMed, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, EMD Serono, Evelo Biosciences, Inozyme Pharma, Meiji Seika Pharma, Menlo Therapeutics, Mitsubishi Pharma, NeuroDerm, Promius Pharma–Dr. Reddy’s Laboratories, Theravance Biopharma, Verrica Pharmaceuticals, Aditum Bio, BMD Skincare, and Kyowa Kirin. JGK has received grants paid to The Rockefeller University from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Innovaderm, Janssen, Kadmon, Kineta, Kyowa, LEO Pharma, Lilly, Novartis, Paraxel, Pfizer, Provectus, Regeneron, and Vitae and personal fees from AbbVie, Baxter, Biogen Idec, Boehringer Ingelheim, Bristol Myers Squibb, Delenex, Dermira, Janssen, Kadmon, Kineta, Lilly, Merck, Novartis, Pfizer, Sanofi, Serono, and XenoPort.
All other authors declare no competing interests.
(ClinicalTrials.gov number, NCT03359356)
REFERENCES
- 1.Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. The Journal of investigative dermatology. 2014;134(4):1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. Journal of the American Academy of Dermatology. 2006;55(3):438–441. [DOI] [PubMed] [Google Scholar]
- 3.Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. Journal of the American Academy of Dermatology. 2008;58(3):395–402. [DOI] [PubMed] [Google Scholar]
- 4.Okhovat JP, Marks DH, Manatis-Lornell A, Hagigeorges D, Locascio JJ, Senna MM. Association Between Alopecia Areata, Anxiety, and Depression: A Systematic Review and Meta-analysis. Journal of the American Academy of Dermatology. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Glickman JW, Dubin C, Renert-Yuval Y, et al. Cross-sectional study of blood biomarkers of patients with moderate to severe alopecia areata reveals systemic immune and cardiovascular biomarker dysregulation. Journal of the American Academy of Dermatology. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Song T, Pavel AB, Wen HC, et al. An integrated model of alopecia areata biomarkers highlights both TH1 and TH2 upregulation. The Journal of allergy and clinical immunology. 2018;142(5):1631–1634 e1613. [DOI] [PubMed] [Google Scholar]
- 7.Bain KA, McDonald E, Moffat F, et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. The British journal of dermatology. 2020;182(1):130–137. [DOI] [PubMed] [Google Scholar]
- 8.Cranwell WC, Lai VW, Photiou L, et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–170. [DOI] [PubMed] [Google Scholar]
- 9.Jabbari A, Sansaricq F, Cerise J, et al. An Open-Label Pilot Study to Evaluate the Efficacy of Tofacitinib in Moderate to Severe Patch-Type Alopecia Areata, Totalis, and Universalis. The Journal of investigative dermatology. 2018;138(7):1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2019;33(5):850–856. [DOI] [PubMed] [Google Scholar]
- 11.Gilhar A, Keren A, Paus R. JAK inhibitors and alopecia areata. Lancet. 2019;393(10169):318–319. [DOI] [PubMed] [Google Scholar]
- 12.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. The British journal of dermatology. 2006;155(3):515–521. [DOI] [PubMed] [Google Scholar]
- 14.Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. The Journal of investigative dermatology. 2005;124(1):288–289. [DOI] [PubMed] [Google Scholar]
- 15.Czarnowicki T, He HY, Wen HC, et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy. 2018;73(3):713–723. [DOI] [PubMed] [Google Scholar]
- 16.Suarez-Farinas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. The Journal of allergy and clinical immunology. 2015;136(5):1277–1287. [DOI] [PubMed] [Google Scholar]
- 17.Kridin K, Renert-Yuval Y, Guttman-Yassky E, Cohen AD. Alopecia Areata Is Associated with Atopic Diathesis: Results from a Population-Based Study of 51,561 Patients. The journal of allergy and clinical immunology In practice. 2020;8(4):1323–1328 e1321. [DOI] [PubMed] [Google Scholar]
- 18.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. The New England journal of medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. [DOI] [PubMed] [Google Scholar]
- 20.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. [DOI] [PubMed] [Google Scholar]
- 21.Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. Journal of the European Academy of Dermatology and Venereology : JEADV. 2013;27(3):e276–281. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza TR, Osei J, Duvic M. The Utility and Validity of the Alopecia Areata Symptom Impact Scale in Measuring Disease-Related Symptoms and their Effect on Functioning. J Investig Dermatol Symp Proc. 2018;19(1):S41–S46. [DOI] [PubMed] [Google Scholar]
- 23.Guttman-Yassky E PA, Page K, et al. Abstract #544: Alopecia areata lesions show significant changes in immune and keratin biomarkers that correlate with clinical improvement with oral Janus kinase inhibitors PF-06651600 (JAK3) and PF-06700841 (TYK2/JAK1). 2019. SID annual meeting; Chicago, IL, USA. [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. [DOI] [PubMed] [Google Scholar]
- 25.Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. Journal of the American Academy of Dermatology. 2017;76(1):22–28. [DOI] [PubMed] [Google Scholar]
- 26.Barahmani N, Schabath MB, Duvic M, National Alopecia Areata R. History of atopy or autoimmunity increases risk of alopecia areata. Journal of the American Academy of Dermatology. 2009;61(4):581–591. [DOI] [PubMed] [Google Scholar]
- 27.Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. The Journal of investigative dermatology. 2012;132(9):2192–2197. [DOI] [PubMed] [Google Scholar]
- 28.Betz RC, Pforr J, Flaquer A, et al. Loss-of-function mutations in the filaggrin gene and alopecia areata: strong risk factor for a severe course of disease in patients comorbid for atopic disease. The Journal of investigative dermatology. 2007;127(11):2539–2543. [DOI] [PubMed] [Google Scholar]
- 29.Petukhova L, Christiano AM. Functional Interpretation of Genome-Wide Association Study Evidence in Alopecia Areata. The Journal of investigative dermatology. 2016;136(1):314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasumagic-Halilovic E, Prohic A. Serum levels of total immunoglobulin e in patients with alopecia areata: relationship with clinical type of the disease. Acta Dermatovenerol Croat. 2006;14(3):149–152. [PubMed] [Google Scholar]
- 31.Zhao Y, Zhang B, Caulloo S, Chen X, Li Y, Zhang X. Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J Dermatol Venereol Leprol. 2012;78(6):709–714. [DOI] [PubMed] [Google Scholar]
- 32.Attia EA, El Shennawy D, Sefin A. Serum Interleukin-4 and Total Immunoglobulin E in Nonatopic Alopecia Areata Patients and HLA-DRB1 Typing. Dermatol Res Pract. 2010;2010:503587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada K, Irisawa R, Ito T, Uchiyama M, Tsuboi R. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: a case series of seven patients. The British journal of dermatology. 2020;183(2):396–397. [DOI] [PubMed] [Google Scholar]
- 34.Penzi LR, Yasuda M, Manatis-Lornell A, Hagigeorges D, Senna MM. Hair Regrowth in a Patient With Long-standing Alopecia Totalis and Atopic Dermatitis Treated With Dupilumab. JAMA Dermatol. 2018;154(11):1358–1360. [DOI] [PubMed] [Google Scholar]
- 35.Kageyama R, Ito T, Hanai S, et al. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. Int J Mol Sci. 2021;22(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meah N, Wall D, York K, et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. Journal of the American Academy of Dermatology. 2020;83(1):123–130. [DOI] [PubMed] [Google Scholar]
- 37.Beck LA, Thaci D, Deleuran M, et al. Dupilumab Provides Favorable Safety and Sustained Efficacy for up to 3 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. American journal of clinical dermatology. 2020;21(4):567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.