Background:
Magnetic resonance imaging (MRI) is more accurate than mammography in screening for breast cancer. Exposure to ionizing radiation from repeated diagnostic X-rays may be a cause of breast cancer.
Methods:
We conducted systematic searches on PubMed, Cochrane and Embase to identify studies on women who underwent mammography or MRI screening. A meta-analysis was performed to compare the detection rate of breast cancer by mammography, MRI or both.
Results:
A total of 18 diagnostic publications were identified and included in the meta-analysis. Among the 1000 screened women, MRI alone increased the detection rate of breast cancer by 8 compared with mammography alone (RR 0.48, 95% CI 0.42–0.54), and MRI plus mammography increased the detection rate of breast cancer by 1 compared with MRI alone (RR 0.86, 95% CI 0.78–0.96). Subgroup analysis demonstrated that the diagnostic efficacy of MRI plus mammography in breast was obviously better than that of MRI alone or mammography alone.
Conclusions:
Screening with MRI alone might be the best choice for women at high risk of breast cancer.
Keywords: breast cancer, mammography, magnetic resonance imaging, meta-analysis
1. Introduction
Breast cancer is the most common cancer among women all over the world, and it is also the leading cause of cancer death among women in over 100 countries.[1] Women with a known family history of breast or mutations in breast cancer susceptibility gene 1/2 (BRCA1 and BRCA2) genes have higher lifetime risk of breast cancer than the general population.[2] Early diagnosis and correct treatment are the key to improve the prognosis of patients. Mammography is a common screening tool for breast cancer, which has been used in clinic for a long time. However, screening mammography associated with low-dose radiation to the breast may increase the incidence of breast cancer, especially in high-risk women.[3–5]
In addition, some studies have shown that the screening effect of mammography alone is not enough, especially in high-risk women, because the sensitivity of screening is relatively low and the incidence of interval cancers is relatively high in this population.[6–8] In contrast, magnetic resonance imaging (MRI) has no radiation risk and is sensitive enough to detect breast cancer at an early stage. Some experts insist that MRI can replace mammography as a routine screening tool for patients with high-risk breast cancer.[9–11] In the past decade, MRI has become a potential research tool for the detection and diagnosis of breast cancer. Several studies have demonstrated that in addition to mammography, high-risk women with breast cancer can also benefit from breast MRI.[12,13]
Several previous studies have compared the diagnostic performance of MRI with mammography in breast cancer screening. However, due to their small sample size and different research designs, the conclusions are not the same. To further evaluate the role of mammography, MRI, or both in the diagnosis of breast cancer, we conducted this meta-analysis to provide strong evidence to guide future decisions.
2. Methods
We conducted a systematic literature review and meta-analysis of publications on mammography and MRI diagnosis of breast cancer. This meta-analysis did not require any program or registration review.
2.1. Data sources and search strategy
The search was developed by W.D., Z.F., and Y.X. PubMed, Embase and Cochrane Library was used to identify all eligible trials between January 2000 and March 2021. Keywords used were: “breast cancer” or “breast carcinoma” or “breast mass”; “mammography” or “MG” or “MRI” or “Magnetic Resonance Imaging”; “high risk” or “high-risk” or “risk”; “screening.” Furthermore, all cross-referenced manuscripts and all review articles from retrieved articles were screened for related studies.
2.2. Inclusion and exclusion criteria
After screening the related studies in the databases, all included diagnostic trials needed to meet the following inclusion and exclusion criteria. Inclusion criteria were as follows: the study population were cases with a confirmed diagnosis of breast cancer; diagnostic methods used were mammography or MRI; the gold standard for diagnosis of breast cancer was pathological examination; Include high-risk factors: BRCA1 or BRCA2 mutation carriers, personal or family history of breast or ovarian cancer, history of prior chest radiation. Exclusion criteria were as follows: case report or review study type; patients had obvious symptoms of breast cancer during screening; and duplicated publication or data.
2.3. Data extraction
We used the keywords mentioned to retrieve qualified articles from the database. We selected the included articles in three steps. We used Endnote X7 Resources Management Software to organize, study titles and abstracts, and identify duplicates. After deleting duplicate articles, the titles of all articles would be reviewed and articles that did not match the inclusion criteria would be deleted. Next, the abstract and full text of the article were reviewed according to the inclusion criteria and research objectives. Two researchers independently completed the selection and quality evaluation of the study (Z.F. and Y.X.), and in case of disagreement, the study was submitted to a third person (C.W.). The information extracted from articles was summarized in a form of excerpt.
2.4. Quality assessment
We used the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) to evaluate the methodological quality of the included studies. QUADAS-2 mainly focuses on patient selection, index test reference standard, and flow and timing, which reflects the main quality of the diagnostic study. If the study meets the above criteria, the study belongs to the risk of low bias; otherwise it belongs to the risk of high bias.
2.5. Statistical analysis
The differences between the two groups were estimated by the pooled risk ratio (RR) along with 95% CIs. The summary RR estimates were estimated using a fixed-effect or random-effect model. Subgroup analyses were performed to detect the effects of stratification factors and other baseline characteristics. According to I2 statistics, statistical heterogeneity was estimated as follows: I2 < 30% meant “low heterogeneity”; I2 between 30% and 50% represented “moderate heterogeneity”; I2 > 50% represented “substantial heterogeneity.” if the heterogeneity was low or moderate, a fixed-effect model was used. Otherwise, the random-effect model was used after exploring the cause of heterogeneity. A 2-sided P value of < .05 indicated a significant difference. All calculations were performed, and figures were generated, using Review Manager Version 5.3 software (The Cochrane Collaboration, Oxford, UK).
3. Results
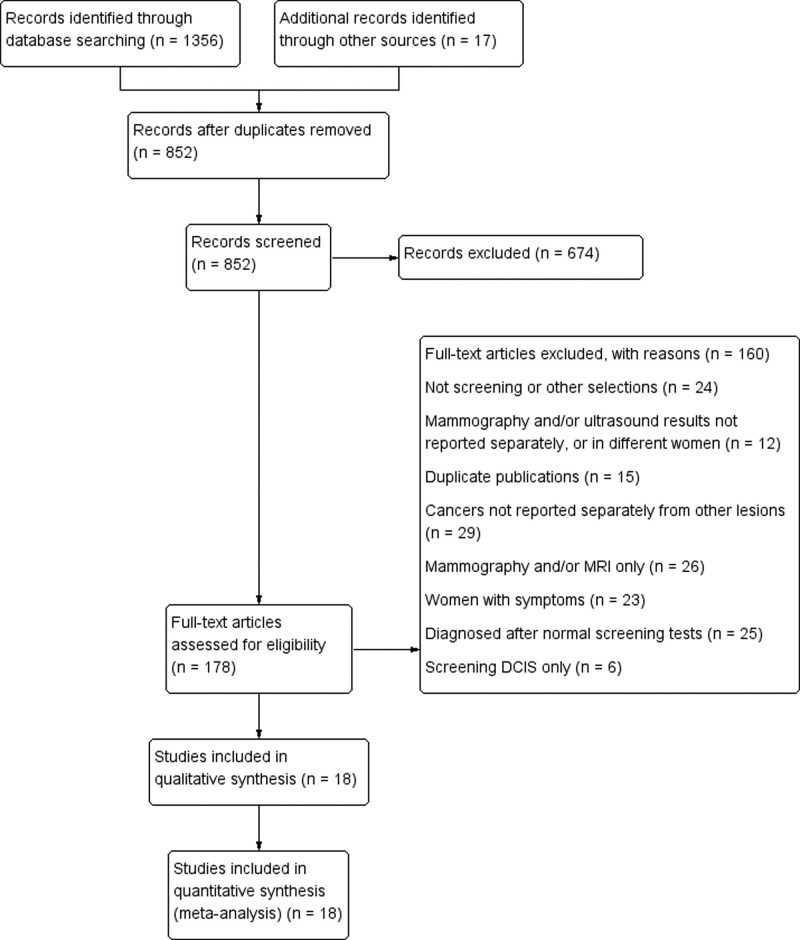
According to our search strategy, a total of 1373 records were retrieved from PubMed, Embase, and Cochrane Library. After removing the duplicate and irrelevant records, 178 full-text articles were available for the meta-analysis. However, after the full-text article evaluation, 160 studies were excluded. Finally, a total of 18 studies examined the benefits of screening with mammography and MRI in the same women at high risk of breast cancer (Fig. 1).
Figure 1.
Flow of selection of articles for the systematic review. DCIS = ductal carcinoma in situ, MRI = magnetic resonance imaging.
Among those included, 2 were retrospective studies and the other 16 were prospective work. A total of 21,157 women underwent 51,085 mammogram screenings, 45,060 MRI screenings, and 44,449 combined mammogram and MRI screenings. A total of 1799 cases of breast cancer were screened: 360 cases were detected by mammography only, 696 cases were detected by MRI only, 743 were detected by both. The general characteristics of the 18 studies included in the meta-analysis were demonstrated in Table 1.
Table 1.
General description and outcomes of the studies included in the meta-analysis.
| Authors, publication year | No of participants (studies) | Risk factors | Diagnostic criteria | Mammography | MRI | Mammography + MRI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N screens | Positive outcomes | Detected cancers | N screens | Positive outcomes | Detected cancers | N screens | Positive outcomes | Detected cancers | |||||||
| N | Per 1000 | N | Per 1000 | N | Per 1000 | ||||||||||
| Berg et al 2012[14] | 7473 (Prospective cohort) | BRCA mutation; personal history of breast cancer; history of prior chest irradiation | BI–RADS (3–5) + Biopsy | 7473 | 759 | 33 | 4.4 | 612 | 159 | 9 | 14.7 | 612 | 191 | 16 | 26.1 |
| Bigenwald et al 2008[15] | 505 (Prospective cohort) | BRCA mutation; family history of breast or ovarian cancer | Biopsy | 505 | NR | 11 | 21.8 | 505 | NR | 41 | 81.2 | NR | NR | NR | NR |
| Chiarelli et al 2020[12] | 20,053 (Prospective cohort) | BRCA mutation; family history of breast cancer; history of prior chest irradiation | Biopsy | 20,053 | 2003 | 109 | 5.4 | 20,053 | 3121 | 263 | 13.1 | 2,0053 | 4472 | 280 | 14.0 |
| Cho et al 2017[16] | 2065 (Prospective cohort) | Personal history of breast cancer | BI–RADS (3–5) + Biopsy | 2065 | 91 | 9 | 4.4 | 2065 | 221 | 15 | 7.3 | 2065 | 284 | 17 | 8.2 |
| Lehman et al 2007[17] | 171 (Prospective cohort) | BRCA mutation; family history of breast or ovarian cancer | BI–RADS (3–5) + Biopsy | 171 | 17 | 2 | 11.7 | 171 | 41 | 6 | 35.1 | 171 | 51 | 6 | 35.1 |
| Guindalini et al 2019[18] | 1223 (Prospective cohort) | BRCA mutation; personal history of breast cancer at age < 35 yr or family history of breast cancer; history of prior chest irradiation at age < 30 yr | Biopsy | 1223 | 34 | 7 | 5.7 | 2111 | 87 | 15 | 7.1 | 2209 | 106 | 16 | 7.2 |
| Kriege et al 2006[19] | 4134 (Prospective cohort) | Familial or genetic predisposition, cumulative lifetime risk of breast cancer > 15% | BI–RADS (3–5) + Biopsy | 4134 | 225 | 18 | 4.4 | 4134 | 452 | 32 | 7.7 | 4134 | 448 | 40 | 9.7 |
| Kuhl et al 2010[20] | 1679 (Prospective cohort) | BRCA mutation; familial and personal history of breast cancer | BI–RADS (4–5) + Biopsy | 1679 | 23 | 9 | 5.4 | 1679 | 52 | 25 | 14.9 | 1679 | 67 | 27 | 16.1 |
| Leach et al 2005[21] | 1881 (Prospective cohort) | BRCA or TP53 mutation; family history of breast or ovarian cancer; family history of classic Li-Fraumeni syndrome | BI–RADS (3–5) | 1881 | 135 | 14 | 7.4 | 1881 | 371 | 27 | 14.4 | 1881 | 371 | 33 | 17.5 |
| Lehman et al 2005[22] | 367 (Prospective cohort) | BRCA mutation; family history of breast cancer | BI–RADS (4–5) + Biopsy | 367 | 8 | 1 | 2.7 | 367 | 31 | 4 | 10.9 | 367 | 38 | 4 | 10.9 |
| Ng et al 2013[23] | 338 (Prospective cohort) | Chest irradiation at age ≤ 35 yr | BI–RADS (3–5) + Biopsy | 338 | NR | 13 | 38.5 | 338 | NR | 12 | 35.5 | 338 | NR | 18 | 53.3 |
| Podo et al 2002[24] | 781 (Prospective cohort) | BRCA mutation; family history of breast cancer | BI–RADS (4–5) + Biopsy | 781 | NR | 13 | 16.6 | 781 | NR | 24 | 30.7 | 781 | NR | 40 | 51.2 |
| Riedl et al 2015[25] | 1365 (Prospective cohort) | BRCA mutation; family history of breast cancer | BI–RADS (4–5) + Biopsy | 1365 | 53 | 15 | 11.0 | 1365 | 183 | 36 | 26.4 | 1365 | 204 | 38 | 27.8 |
| Sardanelli et al 2011[26] | 1095 (Prospective cohort) | BRCA mutation; family and personal history of breast or ovarian cancer | BI–RADS (4–5) + Biopsy | 1095 | 35 | 25 | 22.8 | 1045 | 75 | 42 | 40.2 | 1024 | 77 | 41 | 40.0 |
| Stoutjesdijk, et al 2001[27] | 262 (retrospective cohort) | BRCA mutation; family and personal history of breast or ovarian cancer | BI–RADS (3–5) + Biopsy | 262 | 15 | 5 | 19.1 | 258 | 30 | 13 | 50.4 | 75 | 21 | 12 | 160.0 |
| Vreemann et al 2018[28] | 6553 (retrospective cohort) | BRCA mutation | BI–RADS (4–5) + Biopsy | 6553 | 215 | 66 | 10.1 | 6553 | 399 | 112 | 17.1 | 6553 | 502 | 125 | 19.1 |
| Weinstein et al 2009[29] | 569 (Prospective cohort) | BRCA mutation; personal history of breast cancer, LCIS or atypical hyperplasia; history of prior chest irradiation | Biopsy | 569 | 72 | 7 | 12.3 | 571 | 129 | 12 | 571 | NR | 19 | 33.3 | |
| Weinstock et al 2015[30] | 571 (Prospective cohort) | Personal history of breast cancer | BI–RADS (4–5) + Biopsy | 571 | NR | 3 | 5.3 | 571 | NR | 8 | NR | 571 | NR | 11 | 19.3 |
BI–RADS = breast imaging reporting and data system, BRCA = breast cancer susceptibility gene, LCIS = lobular carcinoma in situ, NR = not report.
3.1. Meta-analysis mammography alone versus MRI alone
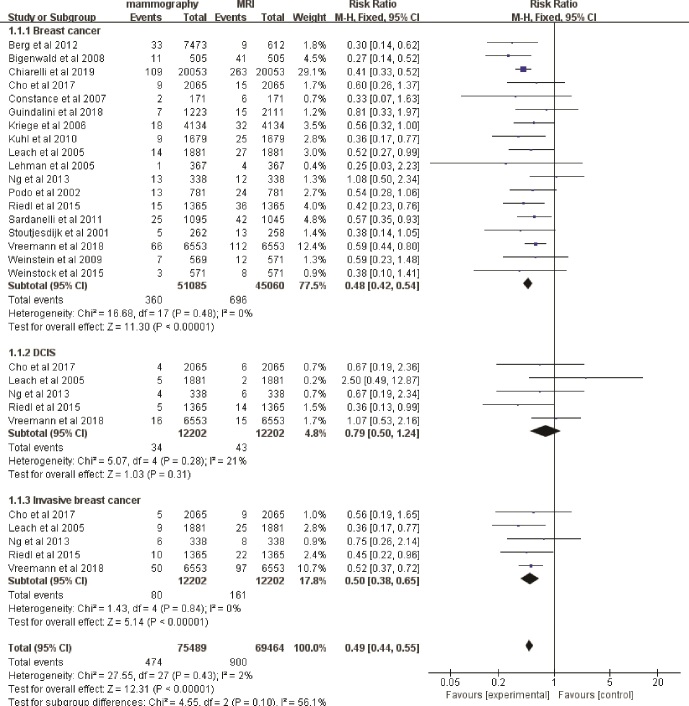
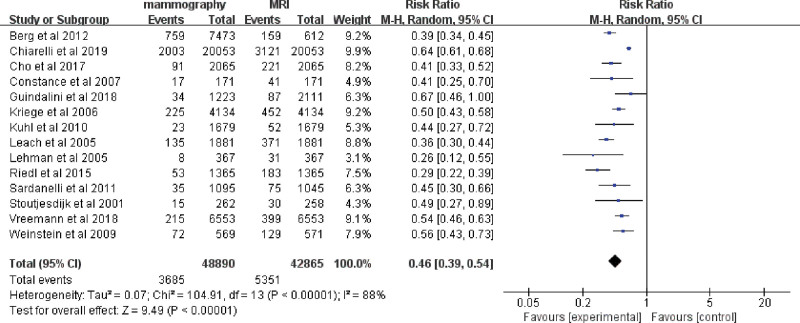
The mean cancer detection rate (CDR) of mammography was 7.0‰ (360/51,085), and the mean CDR of MRI was 15.4‰ (696/45,060). The fixed-effects model was used because there were no heterogeneities (I2 = 0%, P = .48) between these data. Among the 1000 screened women, MRI alone increased the detection rate of breast cancer by 8 compared with mammography alone (RR 0.48, 95% CI 0.42–0.54; Fig. 2), and the rate of invasive breast cancer detection was increased by 6 (RR 0.50, 95% CI 0.38–0.65; Fig. 2). Regarding the rate of ductal carcinoma in situ (DCIS), there was no clear evidence to support a difference between the two interventions (RR 0.79, 95% CI 0.50–1.24; Fig. 2). Accordingly, the recall rate of patients in MRI alone group was significantly higher than that in mammography alone group (RR 0.46, 95% CI 0.39–0.54; Fig. 3).
Figure 2.
Forest plot of detection rate of breast cancer, DCIS and invasive breast cancer screening by mammography alone versus MRI alone. DCIS = ductal carcinoma in situ, MRI = magnetic resonance imaging.
Figure 3.
Forest plot of recall rate of breast cancer screening by mammography alone versus MRI alone. MRI = magnetic resonance imaging.
Subgroups were analyzed to assess whether the variation between studies could be explained by patient’s age, research type, and BRCA1/2 mutation carriers. The results showed that the diagnostic efficacy of MRI alone on breast was significantly better than that of mammography alone in each subgroup (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I566).
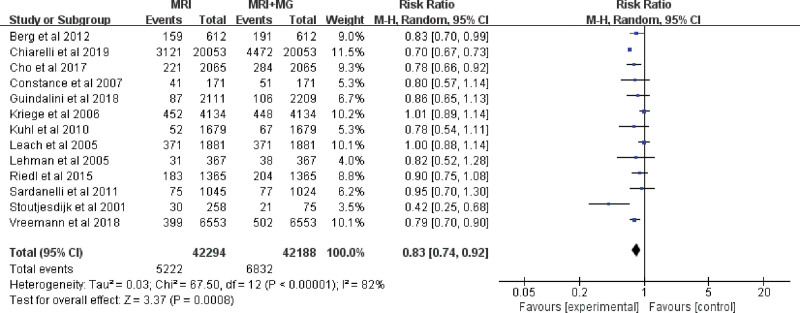
3.2. Meta-analysis MRI alone versus mammography plus MRI
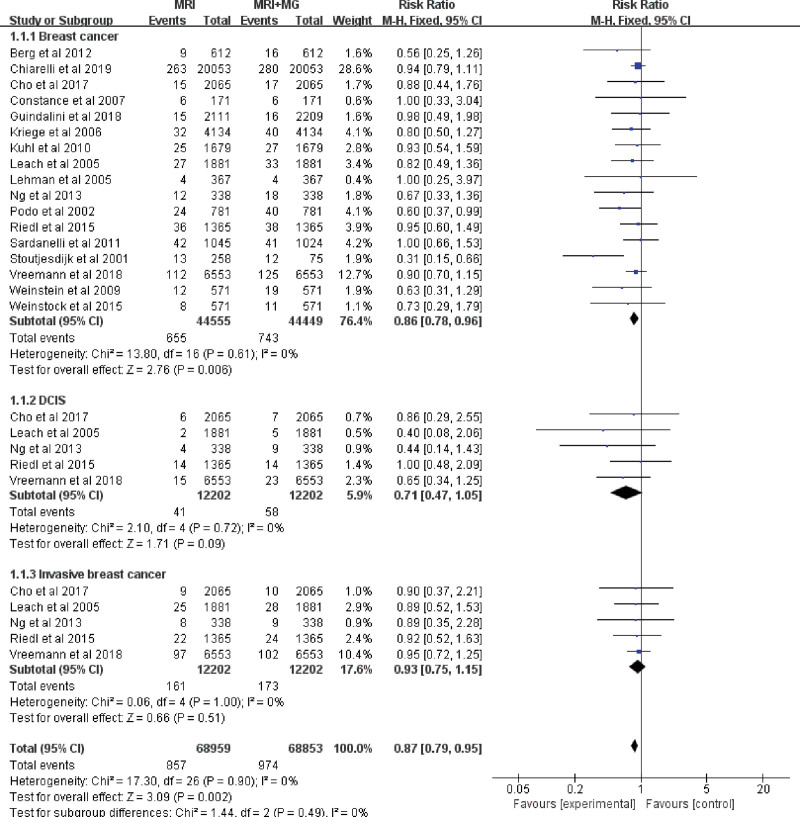
The mean CDR of MRI was 15.4‰ (696/45,060), and the mean CDR of MRI plus mammography was 16.7‰ (743/44,449). The fixed-effects model was used/conducted because there were no heterogeneities (I2 = 0%, P = .61) between these data. Among the 1000 screened women, MRI plus mammography increased the detection rate of breast cancer by 1 compared with MRI alone (RR 0.86, 95% CI 0.78–0.96; Fig. 4), while regarding the rate of DCIS and invasive breast cancer, there was no clear evidence to support the difference between the two interventions (RR 0.71, 95% CI 0.47–1.05 and RR 0.93, 95% CI 0.75–1.15; Fig. 4). Accordingly, the recall rate of patients in MRI plus mammography group was significantly higher than that in the MRI alone group (RR 0.83, 95% CI 0.74–0.92; Fig. 5). Subgroup analysis showed that the diagnostic efficacy of MRI plus mammography in prospective studies was obviously better than that of MRI alone. But in the subgroup less than 50 years old, the subgroup older than 50 years old, the subgroup of retrospective studies or the subgroup of BRCA mutation carriers, the detection rate of MRI plus mammography for cancer was not significantly better than that of MRI alone (Figure S2, Supplemental Digital Content, http://links.lww.com/MD/I567).
Figure 4.
Forest plot of detection rate of breast cancer, DCIS and invasive breast cancer screening by MRI alone versus mammography plus MRI. DCIS = ductal carcinoma in situ, MRI = magnetic resonance imaging.
Figure 5.
Forest plot of recall rate of breast cancer screening by MRI alone versus mammography plus MRI. MG = mammography, MRI = magnetic resonance imaging.
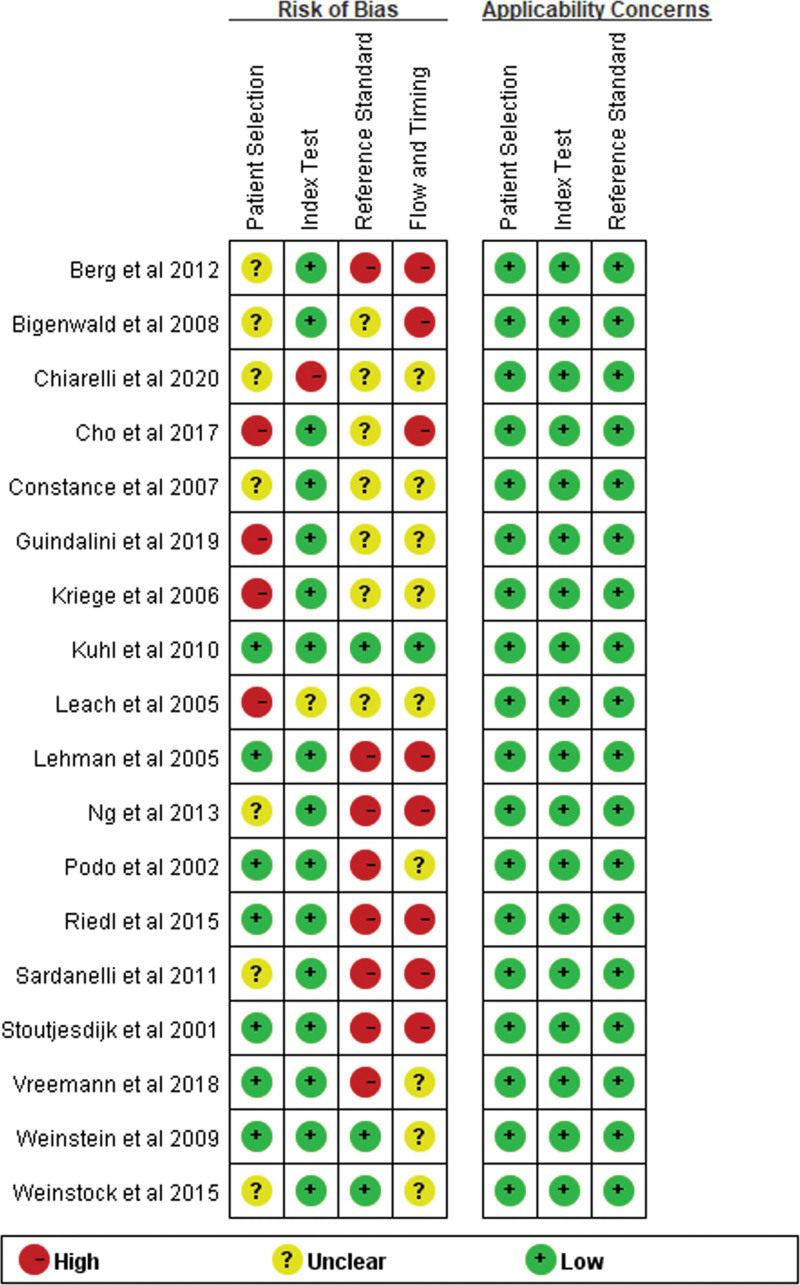
3.3. Risk of bias and applicability
Figure 6 showed the risk of bias corresponding to the inclusion of the study. Most studies reported the selection criteria and index test of the patients to be at low risk of bias in this respect. However, most studies were evaluated as having a high or unclear risk of bias in the reference test, since the pathologists who evaluated the pathological and biopsies results had prior knowledge of the screening tests. Follow-ups were also evaluated as a high or unclear risk of bias since patients who were not recalled missed the reference test. Therefore, the risk of this bias was high, which may reduce the quality of the publications.
Figure 6.
Risk of bias assessment according to QUADAS 2.
4. Discussion
In order to provide clinicians with convincing evidence to make decisions, we conducted a systematic review to examine the benefits and harms of screening with mammography, MRI or both for women at high risk of breast cancer. Our meta-analysis focused on the latest studies, which revealed that adding breast MRI to mammography could increase the detection rate of asymptomatic breast cancer. However, due to the potential corresponding risks of increased false positive findings, the degree of this trade-off was uncertain.
It has been recognized that large-scale screening and early treatment are extremely important to improve cancer prognosis and reduce the burden of medical treatment.[31] Currently, the common radiological tools of breast cancer are mammography, ultrasound and MRI. Ultrasound is a good way to evaluate palpable abnormalities, distinguish between cystic and solid lesions, and classifying solid masses. However, it also has limitations as a screening method because it is difficult to detect microcalcification in DCIS. In contrast, mammography is a simple and convenient method for diagnosis of DCIS, but its diagnostic sensitivity is affected by the radiation density of breast tissue, especially in oriental women.[32,33]
There is no doubt that mammography is the main imaging screening method for breast cancer screening. Nevertheless, screening mammography is associated with low-dose radiation to the breast, which can increase the genetic risk of breast cancer in high-risk women.[34–36] According to the results of in vitro studies, there is evidence that breast tissues with BRCA mutations may be more vulnerable to ionizing radiation than genetically intact breast tissues.[37–39] One recent study estimated that BRCA mutation carriers have a 1.5 times higher risk of radiation-induced breast cancer than non-carriers.[40] Therefore, considering the high risk and early onset of breast cancer, current recommendations for breast cancer screening may not be sufficient for high-risk patients.
Breast MRI not only has no electrical radiation, but also has higher diagnostic sensitivity than mammography. Meanwhile, the sensitivity of breast cancer diagnosis is not affected by dense tissue. Therefore, MRI has been recommended as supplemental screening for women at high risk of breast cancer who have a lifetime risk of 20% to 25% or greater based on family history, radiation history of anterior chest wall, or known or suspected BRCA or other high-risk genetic mutations.[41] The combination of MRI and mammography will have higher diagnostic sensitivity, because when two imaging methods were combined for diagnosis, the higher category was categorized as the final imaging diagnosis. However, it is also for this reason that the recall rate increased accordingly. The results of this study showed that combining MRI and mammography seemed to improve the positive rate of breast cancer screening, but it could also increase the recall rate of breast cancer screening. The mean cancer detection rate of combing MRI plus mammography was 16.7‰, and this strategy increased the cancer detection rate by 1/1000 compared with screening with MRI alone. However, the recall rate would also increase significantly. The recall rate of patients in MRI plus mammography group was 16.2%, which was 4 percentage points higher than that of screening with MRI alone.
High recall rates lead to high false positive diagnostic results which can lead to unnecessary and invasive diagnostic procedures, such as needle biopsy. Although there is evidence that women are more likely to be recalled frequently to investigate false positive results, since delays and misdiagnoses can lead to adverse evolution.[42] In order to save medical resources, it is best to carry out personalized screening according to the risk degree of patients. Women are likely to be divided to different screening imaging methods, which may increase the number of women and provide a more sensitive screening technique than mammography.[43]
Limitations of this study include the fact that the included studies lack complete follow-up data. Due to the lack of sufficient follow-up data, the absolute sensitivity of MRI and mammography is likely to be overestimated. Thus, although there is strong evidence that the addition of MRI contributes to the early detection of more breast cancer cases than traditional screening methods, the benefits of early detection in improving patient prognosis have not been quantified.
5. Conclusions
In conclusion, our findings support MRI as a screening tool for high-risk women. Although combined mammogram and MRI screening can increase the cancer detection rate slightly, it may also increase the potential corresponding risks of radiation and false positive findings. Therefore, screening with MRI alone might be the best choice for women at high risk of breast cancer.
Author contributions
Conceptualization: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei, Zhian Li.
Data curation: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei.
Formal analysis: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei, Guodong Ruan.
Funding acquisition: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei, Guodong Ruan.
Investigation: Wu Ding, Yingli Lin.
Methodology: Wu Ding, Guodong Ruan.
Project administration: Wu Ding, Zaiwei Fan, Guodong Ruan, Zhian Li.
Resources: Wu Ding, Yuehuai Xu, Guodong Ruan, Zhian Li, Yingli Lin, Jianming Zhu.
Software: Wu Ding, Yuehuai Xu, Guodong Ruan, Zhian Li, Jianming Zhu.
Supervision: Zaiwei Fan, Zhian Li.
Validation: Zaiwei Fan, Yuehuai Xu, Chunshou Wei, Guodong Ruan, Zhian Li, Yingli Lin.
Visualization: Yuehuai Xu, Chunshou Wei, Guodong Ruan, Jianming Zhu.
Writing – original draft: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei.
Writing – review & editing: Wu Ding, Zaiwei Fan, Yuehuai Xu, Chunshou Wei, Guodong Ruan, Yingli Lin, Jianming Zhu.
Supplementary Material
Abbreviations:
- BRCA1/2
- breast cancer susceptibility gene 1/2
- CDR
- cancer detection rate
- DCIS
- ductal carcinoma in situ
- MG
- mammography
- MRI
- magnetic resonance imaging
- RR
- risk ratio
WD, ZF, YX, and CW contributed equally to this work.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of meta-analysis, formal consent is not required.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Ding W, Fan Z, Xu Y, Wei C, Li Z, Lin Y, Zhu J, Ruan G. Magnetic resonance imaging in screening women at high risk of breast cancer: A meta-analysis. Medicine 2023;102:10(e33146).
Contributor Information
Wu Ding, Email: wuding0320@163.com.
Zaiwei Fan, Email: xjh13858415406@163.com.
Yuehuai Xu, Email: zhj13819508156@163.com.
Chunshou Wei, Email: xuping120120@163.com.
Zhian Li, Email: lza120120@163.com.
Yingli Lin, Email: qxy120120@163.com.
Jianming Zhu, Email: zjm120120120@163.com.
References
- [1].Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- [2].Daly MB, Pal T, Berry MP, et al.; CGC. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77–102. [DOI] [PubMed] [Google Scholar]
- [3].Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bernstein JL, Thomas DC, Shore RE, et al.; WECARE Study Collaborative Group. Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: a WECARE study report. Eur J Cancer. 2013;49:2979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pijpe A, Andrieu N, Easton DF, et al.; GENEPSO. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ. 2012;345:e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brekelmans CT, Seynaeve C, Bartels CC, et al.; Rotterdam Committee for Medical and Genetic Counseling. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol. 2001;19:924–30. [DOI] [PubMed] [Google Scholar]
- [7].Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–8. [DOI] [PubMed] [Google Scholar]
- [8].Komenaka IK, Ditkoff BA, Joseph KA, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100:2079–83. [DOI] [PubMed] [Google Scholar]
- [9].Gilbert FJ. Should we use MRI to screen women at high-risk of breast cancer? Cancer Imaging. 2005;5:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harkness EF, Astley SM, Evans DG. Risk-based breast cancer screening strategies in women. Best Pract Res Clin Obstet Gynaecol. 2020;65:3–17. [DOI] [PubMed] [Google Scholar]
- [11].Lam TH, Wong KH, Chan KK, et al. Recommendations on prevention and screening for breast cancer in Hong Kong. Hong Kong Med J. 2018;24:298–306. [DOI] [PubMed] [Google Scholar]
- [12].Chiarelli AM, Blackmore KM, Muradali D, et al. Performance measures of magnetic resonance imaging plus mammography in the high risk Ontario breast screening program. J Natl Cancer Inst. 2020;112:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Evans DG, Kesavan N, Lim Y, et al.; MARIBS Group. MRI breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat. 2014;145:663–72. [DOI] [PubMed] [Google Scholar]
- [14].Berg WA, Zhang Z, Lehrer D, et al.; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bigenwald RZ, Warner E, Gunasekara A, et al. Is mammography adequate for screening women with inherited BRCA mutations and low breast density? Cancer Epidemiol Biomarkers Prev. 2008;17:706–11. [DOI] [PubMed] [Google Scholar]
- [16].Cho N, Han W, Han BK, et al. Breast cancer screening with mammography plus ultrasonography or magnetic resonance imaging in women 50 years or younger at diagnosis and treated with breast conservation therapy. JAMA Oncol. 2017;3:1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–8. [DOI] [PubMed] [Google Scholar]
- [18].Guindalini RSC, Zheng Y, Abe H, et al. Intensive surveillance with biannual dynamic contrast-enhanced magnetic resonance imaging downstages breast cancer in BRCA1 mutation carriers. Clin Cancer Res. 2019;25:1786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kriege M, Brekelmans CT, Obdeijn IM, et al. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006;100:109–19. [DOI] [PubMed] [Google Scholar]
- [20].Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450–7. [DOI] [PubMed] [Google Scholar]
- [21].Leach MO, Boggis CR, Dixon AK, et al.; MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365:1769–78. [DOI] [PubMed] [Google Scholar]
- [22].Lehman CD, Blume JD, Weatherall P, et al.; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898–905. [DOI] [PubMed] [Google Scholar]
- [23].Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol. 2013;31:2282–8. [DOI] [PubMed] [Google Scholar]
- [24].Podo F, Sardanelli F, Canese R, et al. The Italian multi-centre project on evaluation of MRI and other imaging modalities in early detection of breast cancer in subjects at high genetic risk. J Exp Clin Cancer Res. 2002;21(3 suppl):115–24. [PubMed] [Google Scholar]
- [25].Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33:1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sardanelli F, Podo F, Santoro F, et al.; High Breast Cancer Risk Italian 1 (HIBCRIT-1) Study. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Invest Radiol. 2011;46:94–105. [DOI] [PubMed] [Google Scholar]
- [27].Stoutjesdijk MJ, Boetes C, Jager GJ, et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst. 2001;93:1095–102. [DOI] [PubMed] [Google Scholar]
- [28].Vreemann S, van Zelst JCM, Schlooz-Vries M, et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018;20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weinstein SP, Localio AR, Conant EF, et al. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27:6124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weinstock C, Campassi C, Goloubeva O, et al. Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. Springerplus. 2015;4:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zeng K, Fajardo LL, Kao S, et al. An in vitro evaluation of cone-beam breast CT methods. J X-Ray Sci Technol. 2008;16:171–87. [PMC free article] [PubMed] [Google Scholar]
- [32].Miller AB. The role of screening mammography in the era of modern breast cancer treatment. Climacteric. 2018;21:204–8. [DOI] [PubMed] [Google Scholar]
- [33].Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by Multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20:2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Friedenson B. Is mammography indicated for women with defective BRCA genes? Implications of recent scientific advances for the diagnosis, treatment, and prevention of hereditary breast cancer. MedGenMed. 2000;2:E9. [PubMed] [Google Scholar]
- [35].Goss PE, Sierra S. Current perspectives on radiation-induced breast cancer. J Clin Oncol. 1998;16:338–47. [DOI] [PubMed] [Google Scholar]
- [36].van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–80. [DOI] [PubMed] [Google Scholar]
- [37].Sharan SK, Morimatsu M, Albrecht U, et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature. 1997;386:804–10. [DOI] [PubMed] [Google Scholar]
- [38].Bebb G, Glickman B, Gelmon K, et al. “‘At risk’” for breast cancer. Lancet. 1997;349:1784–5. [DOI] [PubMed] [Google Scholar]
- [39].Vaidya JS, Baum M. Benefits and risks of screening mammography in women with BRCA1 and BRCA2 mutations (letter). JAMA. 1997;278:289–90. [PubMed] [Google Scholar]
- [40].Broeks A, Braaf LM, Huseinovic A, et al. Identification of women with an increased risk of developing radiation-induced breast cancer: a case only study. Breast Cancer Res. 2007;9:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saslow D, Boetes C, Burke W, et al.; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. [DOI] [PubMed] [Google Scholar]
- [42].Ganott MA, Sumkin JH, King JL, et al. Screening mammography: do women prefer a higher recall rate given the possibility of earlier detection of cancer? Radiology. 2006;238:793–800. [DOI] [PubMed] [Google Scholar]
- [43].Evans DG, Astley S, Stavrinos P, et al. Improvement in Risk Prediction, Early Detection and Prevention of Breast Cancer in the NHS Breast Screening Programme and Family History Clinics: A Dual Cohort Study. Programme Grants for Applied Research. Southampton (UK): NIHR Journals Library; 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.