Abstract
Background:
The prevalence of type 2 diabetes mellitus (T2DM) has increased worldwide, including in Saudi Arabia.
Objective:
To systematically review the available literature and assess the pooled prevalence of T2DM in Saudi Arabia between 2000 and 2020.
Methods:
Observational studies that reported quantitative estimates of the prevalence of T2DM as their main outcome, included the general population of Saudi Arabia, and were published between 2000–2020 and in English were retrieved using three electronic databases (namely, CINAHL, Medline via PubMed, and Web of Science). Retrieved studies were screened, and relevant data were extracted. The Joanna Briggs Institute Critical Appraisal guideline was used to assess the methodological quality of included studies. A random-effects model was used to estimate the prevalence of T2DM.
Results:
Twenty-three studies were included in the systematic review, of which 19 were included in the meta-analysis (total pooled population: 258,283). The overall pooled prevalence of T2DM in Saudi Arabia was 16.4% (95% CI: 11.6–17.5). However, there was heterogeneity in the results of the studies [I2 = 99.31%, P < 0.0001] and the summary values varied from 3.18% (95% CI: 1.46–5.95) to 94.34% (95% CI: 89.53–97.38). Although the prevalence of T2DM by age varied across studies, in most studies, it was higher among the older age groups. In addition, the prevalence of diabetes widely varied across the different geographical regions of Saudi Arabia.
Conclusions:
This is the first meta-analysis that determined the pooled prevalence of T2DM in Saudi Arabia, and it revealed a high prevalence over the past two decades. However, owing to data collection inconsistencies in the identified studies, neither the modifiable (such as obesity, educational status, emotional support, etc.) nor the non-modifiable (such as gender and age) risk factors of T2DM could be determined, thereby indicating the need for a nationally collective effort in determining these factors.
Keywords: Meta-analysis, prevalence, random-effects model, Saudi Arabia, systematic review, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) is a major public health concern with increasing prevalence and long-term morbidity.[1] It is a debilitating and multifactorial disease that can be secondary to the existence of a genetic predisposition, is exacerbated by environmental factors, and can immensely the patients' quality of life.[2]
In 2019, the estimated prevalence of DM worldwide was 9.3% (463 million people), and this was expected to rise to 10.2% (578 million) by 2030 and to 10.9% (700 million) by 2045.[3] However, these estimates have already been revised: the International Diabetes Federation (IDF) estimated that in 2021, 10.5% (537 million) of all adults aged 20–79 worldwide had diabetes, and this would increase to 643 million adults by 2030, and 783 million adults by 2045. Consequently, whereas the global population is projected to expand by 20% in this period, the prevalence of DM is expected to increase by 46%.[4] Further, the estimated cost for diabetes care between 2011 and 2030 is projected to be approximately US$ 1.7 trillion, the burden of which is exacerbated by the fact that the majority of the increase in cases would occur in low-and middle-income countries.[2,4]
The Arab Gulf Cooperation Council (GCC), which includes Saudi Arabia, Kuwait, Bahrain, Oman, Qatar, and the United Arab Emirates, is one of the regions with the highest prevalence of DM.[5] Based on the IDF region-wise data, the prevalence of diabetes in Gulf countries among adults aged 20–79 years ranged from 8% to 22%. While the highest prevalence in GCC was in Kuwait (22%), the highest number of diabetes-related deaths were from Saudi Arabia.[6] The alarming increase in the number of patients with diabetes in Saudi Arabia has been attributed to the rapid epidemiological transition, urbanization, unhealthy diet, and reduced physical activity in the recent decades.[5] The increase in diabetes also places a considerable burden on the economy. In Saudi Arabia, the annual total direct expenditure of diabetes-related treatment in 2014 was estimated to be 17 billion Riyals, and the annual public medical healthcare expenditure on people with diabetes has been estimated to be tenfold that of those without diabetes.[7,8]
Several studies from Saudi Arabia have estimated the regional prevalence of DM; however, discrepancies between methods have even resulted in variation in the prevalence being reported from the same area. Therefore, the aim of this systematic review is to provide a pooled prevalence estimate for type 2 diabetes mellitus (T2DM) within the general population of Saudi Arabia, which can be useful for all the relevant stakeholders and policymakers.
METHODS
This study followed the PRISMA items for systematic review and meta-analysis guidelines.[9]
Data source and search strategy
A systematic literature search was performed to identify studies that reported the prevalence of T2DM in Saudi Arabia. A rigorous literature search was conducted using the following three academic electronic databases: CINAHL, Medline (via PubMed), and Web of Science. In addition, the reference lists of eligible studies were screened to identify relevant studies.
Studies that described the prevalence in relevance to either former diagnosis of diabetes or diagnostic blood glucose-level test were included. The search in PubMed was carried out using the following terms: (Diabetes) OR (Hyperglycemia) AND (Prevalence) OR (Trend) OR (Incidence) OR (Epidemiology) AND (Saudi) OR (KSA); these terms was similarly adapted for the other two databases. The publication date (range: 2000–2020) and language (only English) filters were used during the searches. All searches were independently carried out by two reviewers.
All references retrieved in the searches were uploaded to RAYYAN software, which was used for first removing duplicates and then for screening the titles and abstracts.[10] Studies were included for a full-text review if they met the following criteria:
Were observational (i.e., cross-sectional or cohort design);
Reported quantitative estimates of the prevalence of T2DM as the main outcome; and
Included the general population in Saudi Arabia.
Studies were excluded if they:
Were systematic reviews, meta-analysis, and discussion articles;
Full-text was not published in English, or were unpublished articles, conference proceedings, or thesis; and
Estimated the prevalence of type 1 diabetes mellitus and gestational diabetes.
Then, both the reviewers independently evaluated the full text of the eligible articles, and any disagreements were settled through discussion.
Data extraction method and data items
Using a set of factors, data from qualifying research were extracted into a predetermined data extraction file. Both the reviewers compared and extracted the following data: author details, publication year, study period, design, setting and region of Saudi Arabia, sampling and the number of participants, age range, type of diabetes, and prevalence with outcomes of interest.
Study quality assessment
Each paper was assessed for study quality by two assessors, and discrepancies were resolved through discussion. The included studies were critically appraised for methodological quality using the Joanna Briggs Institute (JBI) Critical Appraisal guideline.[11] All papers were assessed based on data relevance and methodological strength, and only articles that satisfied at least four of the nine criteria were included in the final analysis.
Effect measure and evidence synthesis
The prevalence of T2DM was determined as a ratio by dividing the number of people diagnosed with the disease by the total number of people in the study population. The Comprehensive Meta-Analysis (CMA) software version 2.0 was used to conduct the meta-analysis. Using the stated crude estimates and population denominators, standard errors and 95% confidence intervals (CI) were calculated, presuming exact binominal distribution, as defined by Clopper and Pearson.[12,13] In a meta-analysis of prevalence, when the estimate for a study approached either toward 0% or 100%, the variance for that study shifts toward zero, and as a result, its weight is exaggerated in the analysis. Accordingly, we carried out the meta-analysis with prevalence estimates and transformed it by using the logit transformation method. Back transformation was used to simplify the interpretation of the final pooled prevalence and the 95% CI. The prediction interval was calculated based on logit transformation using the CMA software and Microsoft Excel spreadsheet.[12] The prediction interval for the random-effects distribution was computed to acquire an understanding of the potential range of T2DM. The heterogeneity and publication bias, of the included studies in the meta-analysis were explored in accordance with the recommendations by Higgins and Thompson.[14]
A random-effects meta-analysis was used on individual study estimates to obtain a crude summary estimate for prevalence, where this model was used depending on the degree of the clinical and methodological heterogeneity between studies. Higgin's I2 and Cochran's Q-test were used to assess the heterogeneity.[15,16] I2 measures the proportion of the total variability that is due to between-study heterogeneity. The higher the I2 value, the greater the heterogeneity: I2 0% indicates no observed heterogeneity, while >50% indicates high heterogeneity.[17,18] Tau-squared statistics were performed to predict the variance between effects on test accuracy seen in different studies.[19] P values < 0.05 were considered significant.
Publication bias
The funnel plots and Egger's bias indicator test were used to detect publication bias. Funnel plots are scatter plots that indicate each study's influence concerning its sample size.[18] In the absence of skew or asymmetry, publication bias is ruled out. Egger's regression intercept is calculated when data from at least three trials are pooled.[19] In case of publishing bias, the results were adjusted using the Duval and Tweedie's trim and fill method.[19]
RESULTS
Search strategy and study selection
The searches resulted in the retrieval of 623 articles, of which 50 were duplicates. The remaining 573 articles were screened based on titles and abstracts, following which 544 records were excluded and the full text of 29 articles were assessed for eligibility. Six articles were excluded at this stage because either the prevalence of DM was not the main outcome (n = 3) or there was a significant risk for bias (n = 3), resulting in 23 studies being included in this systematic review [Figure 1].[20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]
Figure 1.
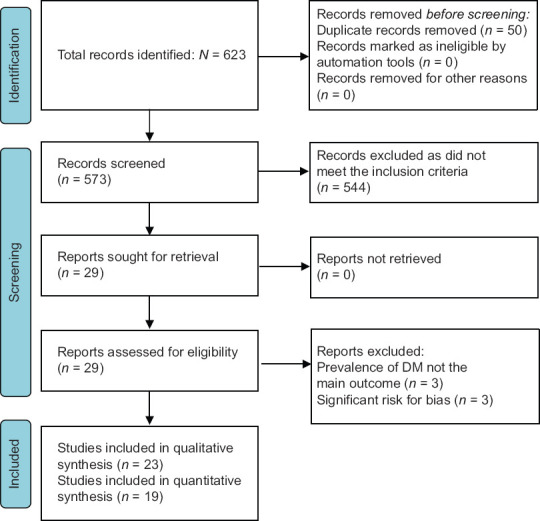
The PRISMA flowchart of study selection
Study quality assessment
The quality of all the 23 articles were appraised based on the relevance of data and methodology,[21] which led to the exclusion of the four articles [Supplementary Table 1].[20,21,22,23,24] Finally, 19 articles had suitable quantitative designs for the prevalence research and were used to conduct the meta-analysis [Table 1].
Supplementary Table 1.
JBI critical appraisal checklist applied for included articles reporting prevalence data
| Author Name/Year | Was the sample frame appropriate to address the target population? | Were the participants appropriately recruited? | Was the sample size adequate? | Were the study subjects and setting described in detail? | Was data analysis conducted with sufficient coverage of the identified sample? | Were valid methods used for the identification of the condition? | Was the condition measured in a standard, reliable way for all participants? | Was there appropriate statistical analysis? | Was the response rate adequate, and if not, was the low response rate managed appropriately? |
|---|---|---|---|---|---|---|---|---|---|
| Abou-Gamel et al.(2014) | F | F | F | UC | T | T | UC | UC | UC |
| Al-Orf (2012) | F | UC | F | UC | T | T | F | T | UC |
| Karim et al., (2000) | T | F | T | F | F | UC | UC | UC | UC |
| Al-Baghli et al. (2010) | T | T | T | T | T | T | T | T | T |
| Al-Daghri et al. (2011) | T | T | T | T | T | T | T | T | T |
| Al-Rubeaan et al. (2015) | T | T | T | T | T | T | T | T | T |
| Al-Qurashi et al. (2011) | T | F | T | T | T | UC | T | T | T |
| Al-Hanawi (2019) | T | T | T | T | T | F | UC | T | T |
| Al-Quwaidhi (2014) | *NA | *NA | *NA | T | UC | T | T | T | UC |
| Al-Zahrani et al. (2019) | T | T | T | T | UC | T | T | T | T |
| Alanazi et al. (2017) | T | UC | T | T | T | T | T | T | T |
| Aldossari et al.(2018) | T | T | T | T | T | T | T | T | T |
| Bahijri (2016) | T | T | T | T | UC | T | UC | T | T |
| Al Osaimi (2007) | T | T | UC | T | UC | T | T | T | T |
| Hadlaq (2017) | UC | UC | UC | T | T | T | T | T | T |
| El Bcheraoui (2013) | T | T | T | T | UC | F | T | T | T |
| A. Mohamed (2019) | T | T | T | T | UC | T | UC | T | T |
| Memish (2015) | T | T | UC | F | UC | T | T | T | T |
| Gutierre (2018) | T | F | T | F | T | T | T | T | T |
| Afifi (2015) | T | F | F | T | T | T | UC | T | T |
| Albakr 1 (2011) | F | F | F | T | F | T | T | T | T |
| Alhazmi (2017) | T | T | T | T | T | T | T | T | T |
| Albarakat (2019) | T | UC | T | T | T | T | T | T | T |
*NA, Not applicable; T, Yes; F, No; UC, Unclear.
Table 1.
Summary of the included studies
| Number | Author (s) (date of publication) | Study period | Setting (s) | Study type | Sample size | Age | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Karim et al., (2000)[24] | - | Al-Kharj Military Hospital | Retrospective cross-sectional study | 3747 case notes (1683 males and 2064 females) | All ages | ||
| 2 | Al Osaimi et al., (2007)[25] | March-April 2002 | East of Riyadh | Cross-sectional survey | 380 | Older than 18 | ||
| 3 | Al-Baghli et al., (2010)[40] | August 2004-February 2005 | Community screening campaign in Eastern province | - | 197,681 | Aged 30 years and above | ||
| 4 | Al-Quwaidhi et al., (2014)[21] | 2005 | Nationwide | Estimation study used discrete-state Markov model | - | Saudis aged >25 | ||
| 5 | Al-Rubeaan et al., (2014)[27] | 2007-2009 | Nationwide | Cross-sectional study | 18,034 | Aged 30 and above | ||
| 6 | Alqurashi, et al. (2011)[28] | June 2009 | Nationwide | Cross-sectional study | 6024: 2279 (37.8%) males and 3744 (62.2%) females | Above 12 | ||
| 7 | Memish et al., (2015)[43] | 2009 | Nationwide: one rural and two urban | Score | 1485 | >20 years of age | ||
| 8 | Albakr et al., (2013)[29] | 2010-2011 | Dhahran Mall and King Fahad University Hospital, Al-Khobar City | Cross-sectional community-based survey | 1552 subjects, 1206 Saudi’s, 346 non-Saudi | 15 years and above | ||
| 9 | Afifi, et al. (2014)[26] | 2010-2011 | The preventive medicine department at AFH, Taif city | Cross-section | 117 | 40 years and above | ||
| 10 | Al-Daghri et al., (2011)[30] | 2011 | PHCC across Riyadh | Cross-sectional study | 9149 | Ages 7-80 years | ||
| 11 | Abou-Gamel et al., (2014)[23] | November 2011 | The College of Science at Taibah University. 57.1% lived in urban regions | Cross-sectional study | 99 subjects | Above 30 years old | ||
| 12 | Al-Orf (2012)[22] | 2012 | Riyadh nursing home | Cross-sectional study | 31 elderly females | Ranged between 62 and 94 years | ||
| 13 | El Bcheraoui et al., (2014)[31] | April-June 2013 | Nationwide: Participants invited to local health clinics | Cross-sectional study | 10,735 | Aged 15 years or older | ||
| 14 | Al-Hanawi, et al. (2019)[41] | 2013 | Nationalwide | - | 8004 | Between 18 and 80 | ||
| 15 | Bahijri et al., (2016)[32] | 2015 | Jeddah | Cross-sectional study | 1420 comprising both Saudi and non-Saudi families | Aged >18 years | ||
| 16 | Hadlaq et al., (2017)[33] | December 2015-January 2016 | Riyadh | Cross-sectional study | 283, 8.5% male (n= 165) and 41.7% female (n=118) | The mean age was 36.6 years with SD of 13.5 years (18-65) | ||
| 17 | Gutierrez et al., (2018)[34] | October 2016 | Tabuk region | Nonexperimental, cross-sectional design | 432 | >18 years old; 89.4% were Saudi and 10.6% were non-Saudi | ||
| 18 | Aldossari et al., (2018)[35] | January-June 2016 | Al-Kharj (different governmental and private institutes) | Population-based cross-sectional study | 381 Saudi adult males | Aged 18 years of age or above | ||
| 19 | Alanazi et al., (2017)[36] | 2016-2017 | Turaif city, northern Saudi Arabia | Cross-sectional study | 1287 | 1 year to >65 years | ||
| 20 | Alhazmi et al., (2017)[37] | May 2017 | Turaif city, northern Saudi Arabia | Cross-sectional study | 402 | Aged between 6 and 63 years | ||
| 21 | Mohamed (2019)[38] | 2019 | Tabuk | Cross-sectional study | 120 (60 males and 60 females) | Age >20 | ||
| 22 | Albarakat & Guzu (2019)[42] | 2019 | Patients registered at home care center at family and community medicine department, Al-Kharj military industries corporation hospital | - | 159 | Older than 18 years | ||
| 23 | Al-Zahrani et al., (2019)[39] | 2019 | Alkharj public university, one governmental organization and one private institute | Exploratory cross-sectional survey | 638 Saudi females | 18 years of age and above | ||
|
| ||||||||
| Number | Type of diabetes | Sampling technique | Method |
Prevalence (%)
|
Overall prevalence (%) | |||
| Female | Male | |||||||
|
| ||||||||
| 1 | Not mentioned | Medical records were selected randomly | 5.32 | 2.55 | 4.08 | |||
| 2 | Not mentioned | Systematic random sampling | FPG | 24.2 | 11.3 | 15.8 | ||
| 3 | Not mentioned | Convenience sampling (approached participants in their workplaces, major public places, malls and other venues) | CFBG | 18.6 | 15.9 | 18.2 | ||
| FPG | ||||||||
| CCBG | ||||||||
| 4 | T2DM | - | OGTT | 2000 | 16.4 | 17.7 | 17.2 | |
| 2008 | 24.7 | 26.7 | 25.9 | |||||
| 2011 | 28.1 | 29.8 | 29.2 | |||||
| 2022 | 47.7 | 41.3 | 44.1 | |||||
| 5 | T2DM | Random sampling | FPG | 21.9 | 29.1 | 25.4 | ||
| 6 | Did not differentiate between type 1 and type 2 | Convenience sampling (patients were selected from those attending the department of primary care at King Fahad AFH) | Self-report | 27.6 | 34.1 | 30 | ||
| 7 | T2DM | Randomly chosen PHCCs | OGTT FPG | 18.3 | 13.9 | 15.6 | ||
| 8 | 4.6 type 1 and 14.3% type 2 | Not mentioned | Random capillary blood glucose | 18.7 | 20.6 | Self-reported diabetes 19.8, 17.6 Saudi, 27.5 non-Saudi Prevalence of high RBS was 21.5 | ||
| 9 | Both | Multi-phase screening plan | RBS >200 | - | - | 21.4 (25/117) of recruits with RBS ≥200 mg, 68 (17/25) of them are known diabetics | ||
| 10 | T2DM | Randomly selected using a cluster sampling strategy | FPG | 28.6 | 34.7 | 23.1 | ||
| 11 | Not specific | Not mentioned | RBG | - | - | 14.1 | ||
| 12 | T2DM | Not mentioned | FBG | 29 | - | 29 | ||
| 13 | 66.7% reported being diagnosed with T2DM and 19.9% did not know their type | Randomly selected from a national sampling frame | HbA1c | 11.70 | 14.84 | 13.4 | ||
| 14 | Not specific | Multi-stage stratified probability sample | Self-reported | 10.7 | 13.9 | 12 | ||
| 15 | T2DM | A 3-stage stratified cluster sampling technique | FBG, HbA1c | 11.4 | 12.9 | 12.1 | ||
| 16 | T2DM | Not mentioned | Fasting glucose test for 83 patients Random glucose test for 199 patients One refused | 3.4 | 3.0 | 3.2 | ||
| 17 | Not determined | The participants were voluntarily involved For a self-administered structured questionnaire | RBG | - | - | 5.6 | ||
| 18 | Not specific | Multi-stage sampling method | HbA1c | - | 9.2 | 9.2 | ||
| 19 | Not determined | Not mentioned | RBG | 4.3 | 1.6 | 5.8 | ||
| 20 | Not determined | Systematic random sampling technique | RBG | 7.8 | 2.2 | 4.5 | ||
| 21 | Not mentioned | Random sample | Fasting blood glucose | No significant differences between males and females Not mentioned | Not mentioned | 10 | ||
| 22 | 94.3% of participants had T2DM, whereas 3.8% had type 1 | Not mentioned Patients registered at home care center at family and community medicine department | HbA1c | - | - | 94.3 T2DM | ||
| 23 | Not mentioned | Multi-stage cluster Sampling | HbA1c | 3.8 | - | 3.8 | ||
AFH: Armed force hospital, SD: Standard deviation, T2D M: Type 2 diabetes mellitus, PHCCs: Primary health care centers, FPG: Fasting plasma glucose test, CFBG: Capillary fasting blood glucose, CCBG: Casual capillary blood glucose, OGTT: Oral glucose tolerance test, RBS: Random blood sugar, RBG: Random blood glucose, HbA1C: Flemoglobln A1C
Study characteristics
Of the 23 studies included in this systematic review, 18 were cross-sectional,[22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] one was an estimation study that used the discrete state Markov model,[20] and the remaining four did not clearly state the study design.[40,41,42,43] The sample sizes of DM patients among the included studies ranged from 31 to 197,681. In terms of geographical representation of the sampled population, six studies were conducted nationwide;[20,27,28,31,41,43] four studies each in Riyadh[22,25,30,33] and Al-Kharj city;[24,35,39,42] two studies each in the Eastern Province,[29,40] Turaif,[36,37] and Tabuk;[34,38] and one study each in Taif,[26] Jeddah,[32] and Madinah.[23] The prevalence of T2DM varied across regions.
Moreover, of the 23 included studies, five studies used the random sampling technique.[24,27,31,38,43] four used the multi-stage stratified sampling,[32,35,39,41] two each used systematic random sampling[25,37] and convenience sampling,[28,40] and one each used a cluster sampling strategy[30] and a multi-phase screening plan.[26] The remaining eight studies did not specify the sampling procedure.[20,21,22,23,29,33,34,36,42]
In terms of study settings, five studies were carried out in tertiary hospitals,[24,25,26,29,42] four in primary healthcare centers,[28,30,33,43] four in households,[27,31,32,41] two each in university hospitals[29,39] and shopping malls,[29,34] one in both governmental and private institutes,[35] and one in Riyadh Social Welfare Home;[22] one study was conducted across >300 primary health care centers and government hospitals, private hospitals, dispensaries, and public venues;[40] one study was conducted in a public academic institute.[23] Four studies did not mention their settings.[20,36,37,38] In terms of the prevalence of diabetes, it widely varied across various geographical regions [Table 1].
Meta-analysis
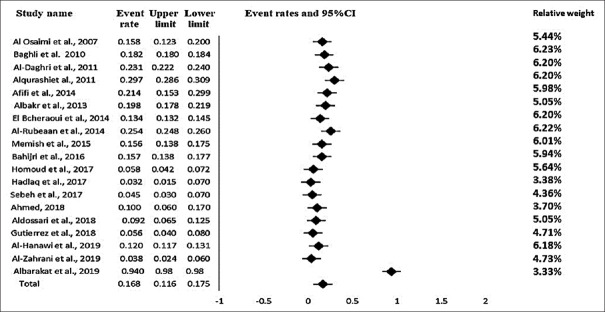
Nineteen studies were included in the meta-analysis, with a total pooled population of 258,283. The overall prevalence of T2DM in Saudi Arabia was estimated to be 16.4% (95% CI: 11.6–17.5) [Figure 2] and the prediction interval was (95% CI) 7.03–27.3, which presented the confidence interval of estimated prevalence for possible population-based observational research to be conducted on diabetes in the future. The true prevalence size varied across studies. The mean prevalence probability was 0.116–0.175. The true effect size for any single study ranged 0.0703–0.273. The test for consistency across studies revealed substantial heterogeneity [I2 = 99.31%, P < 0.0001] [Table 2]. Moreover, the Q-statistic was used to test the null hypothesis (i.e., all articles in the analysis have the same effect size). All studies with similar effect sizes would have an expected Q value equal to the degrees of freedom (df) (the number of articles minus 1), where the Q-value was 7145.293, and the P < 0.0001 [Table 2]. The variance of true effect sizes (T2, in log units) was 0.241. The standard deviation of the actual effects, which is represented by the letter “T = tau” (in log units), was 0.491.
Figure 2.
Forest Plot of the 19 studies showing the prevalence of T2DM among the Saudi population between 2000 and 2020. T2DM – Type 2 diabetes mellitus
Table 2.
The heterogeneity, publication bias and hypothesis testing of the included studies in the meta-analysis
| Variable | Publication bias |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity |
Random |
Hypothesis test |
||||||||
| Prevalence | 95% CI |
Random |
||||||||
| Q | P | df (Q) | I 2 | Lower limit | Higher limit | Z | P | Number of studies | ||
| T2DM | 2136.257 | <0.0001 | 18 | 99.31 | 0.164 | 0.116 | 0.175 | -19.442 | <0.0001 | 19 |
T2DM: Type 2 diabetes mellitus, CI: Confidence interval
The results of analyses by time showed variable prevalence of T2DM among the Saudi population between 2000 and 2020. A recent study found extremely high prevalence of T2DM: 94.3%.[42] The weights of the studies reported from the random effect model ranged from 3.33% to 6.22%, as shown in Figure 2.
Publication bias and sensitivity analysis
Funnel plot
The funnel plot was used to assess publication bias [Figure 3]. A mild asymmetric funnel plot of all studies across the prevalence of T2DM among the Saudi population was shown in Figure 3. In this instance, the mild asymmetry of the funnel plot suggests the presence of some publishing bias. It is plausible that the mild asymmetry is related to the effects of small and large studies (such as a sampling error).
Figure 3.
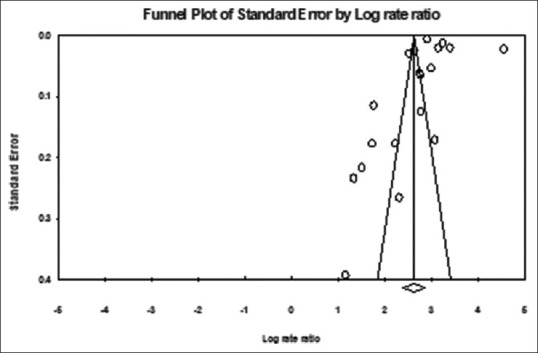
Funnel plot of standard error by log rate ratio associated with the prevalence of T2DM among the Saudi population. T2DM – Type 2 diabetes mellitus
Egger's bias indicator test
The Egger's bias test was used to assess the funnel plot asymmetry. In this analysis, the intercept (B0) was determined to be 0.48, 95% CI (−11.63–12.58), with t-value = 0.084 and df = 17.00. However, this test showed nonsignificant publication bias, where the suggested 1-tailed P value was 0.47 and the 2-tailed P value was 0.93.
Duval and Tweedie's trim and fill
Using these parameters, the method did not identify any missing study. The funnel plot for the trimmed and imputed study is shown in the Supplementary Figure 1 (198.5KB, tif) . According to the random-effects model, the point estimate (95% CI) for the included studies was 13.78 (10.96–17.34). The trim and fill method was used to obtain an adjusted point estimate of 13.78 (10.95–17.34).
DISCUSSIONS
This meta-analysis found that the pooled prevalence of T2DM in Saudi Arabia was 16.4%, with indications that that the prevalence of T2DM in Saudi Arabia is increasing. These findings are in line with the previous estimations by IDF: Saudi Arabia was identified as one of the top ten countries with the highest prevalence of T2DM, and the prevalence of diabetes in the Middle East and North Africa region was estimated as 18.1%, the highest in the world.[4,44] The current findings will help health policy planners in anticipating the potential increase in the prevalence of T2DM in Saudi Arabia over the next decade.[30,34,35,37,38]
In terms of non-modifiable risk factors of DM such as gender, there have been contrasting findings from Saudi Arabia. While several studies reported that the prevalence of DM was higher among females,[24,40,42,43] others have found the prevalence to be higher among males.[20,27,28,29,30,31,32,35,41] The possible explanation for discrepancies in the findings is that women tend to seek healthcare more frequently than men, thereby increasing the likeliness of them being diagnosed. On the other hand, several risk factors have been correlated with the high prevalence of DM among Saudi males, including tobacco smoking,[45] obesity,[46] and vitamin D deficiency.[30] Furthermore, in the Arab society, men tend be under a higher level of chronic psychological stress than women, which, over time, has a role in inflammatory and metabolic stress, eventually leading to DM.[47] In contrast to the above studies, Mohamed et al.[38] revealed no significant gender-wise difference in the prevalence of DM among Saudi patients. These discrepancies highlight need for a country-wide study to determine the prevalence of DM among males and females in Saudi Arabia.
In terms of age as a risk factor, DM among the elderly is common worldwide. We found that while the prevalence of T2DM by age varied across studies, in most studies, the prevalence was higher among the older age groups than the younger age groups.[29,31,32,35,36,39,42] In terms of proportion, in those aged ≥50 years, Albakr et al., Bahijri et al., and Albarakat and Guzu found that more than half of the people in this age group had DM, while another 10–15% had pre-diabetes.[29,32,42] One of the included studies found that the prevalence of DM was similar in the rural and urban areas.[43] However, the findings of this study cannot be generalized to Saudi Arabia, and thus there is need for additional studies to determine if region-wise factors contribute to DM in Saudi Arabia.
Comorbidities are common in patients with diabetes. A study from Al Kharj found that in addition to diabetes, 20.8% of the patients had ≥3 comorbidities and 74.2% had 1–2 comorbidities.[42] However, due to the lack of available comorbidity rates among T2DM patients in many of the included studies, subgroup analysis related to comorbidity rates was not possible. Therefore, additional research is needed to determine the comorbidity rates associated with T2DM in Saudi Arabia.
Obesity is a commonly known predictor of DM, regardless of the presence or absence of other factors.[35] In a country-wide study from Palau, both overall and central obesity were found to be significant predictors of prediabetes and/or diabetes; in fact, in obese individuals, diabetes occurred at a younger age than non-obese individuals.[48] In this meta-analysis, Alqurashi et al., in their logistic regression findings, showed that BMI >25 was significantly associated with diabetes,[28] a finding that was in accordance with those of several other included studies.[23,29,30,32,35,39]
Educational status is another factor that has been reported as a predictor of DM. Unsurprisingly, several studies included in this meta-analysis have found the prevalence of DM to be higher among socioeconomically disadvantaged groups with lower education levels and higher unemployment.[31,35,39,41,43] These finding are coherent with the findings of studies conducted in other countries.[49,50,51] Between these two variables, a recent study found that in Saudi Arabia, education inequality was higher than income inequality among patients with DM compared with those without DM. This study also found that rate of DM decreased with an increase in education level.[41]
In this meta-analysis, Al-Hanawi et al., Al-Zahrani et al., El Bcheraoui et al., and Aldossari et al.,[31,35,39,41] found that married individuals are more likely to develop DM than divorced, widowed, or single individuals. This may be explained by the fact that those married are likely to have greater responsibilities in their personal life and, as a result, are less physically active and more prone to obesity and an increased likelihood of developing DM. Among Greeks, married status has been found to be associated with obesity,[52] whereas among Americans,[53] marital status has been found to be associated with higher physical activity levels compared with those who are single, and among Malaysians,[54] physical activity did not differ according to marital statuses. These findings indicate that cultural factors may be associated with obesity, physical activity, and consequently, T2DM, and thus there is need for further studies from Saudi Arabia regarding this factor.
Some of the other risk factors of DM reported in the included studies were an increase in exposure to stress (which were indicators for elevating the prevalence of DM), family history, and the metabolic syndrome with its alarming manifestations among Saudi adults.[23,29,30,31,35,37]
Limitations and future directions
This meta-analysis has a few limitations that should be considered while interpreting its results. First, only studies that examined the prevalence of diabetes as the main outcome were included. Second, this review was limited to T2DM, and does not include the prevalence of T1DM and gestational diabetes; however, heterogeneous methods were used to measure glucose status and some of the included studies did not distinguish between T1DM and T2DM. Finally, we were unable to do any subgroup analysis of the prevalence for males/females and other associated factors of T2DM, as most of the included studies did not document gender or associated risk factor prevalence, thereby making it difficult to generate pooled gender or associated risk factor-specific prevalence figures for the region.
This systematic review found the pooled prevalence of T2DM in Saudi Arabia between 2000 to 2020 was 16.4%. The increasing trend in the prevalence of T2DM in Saudi Arabia indicates the need for urgent remedial actions by policymakers. This review also highlights the current deficiencies in the literature of T2DM from Saudi Arabia, and thus provides directions for future studies.
Peer review
This article was peer-reviewed by four independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Funnel plot for the trimmed and imputed study
REFERENCES
- 1.Roser M, Ritchie H. Burden of Disease. [Last accessed on 2022 Mar 05];2021 :1–25. Available from: https://ourworldindata.org/burden-of-disease . [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation . 10th. Brussels, Belgium: International Diabetes Federation; 2021. [Last accessed on 2022 Mar 08]. IDF Diabetes Atlas. Available from: https://www.diabetesatlas.org . [Google Scholar]
- 5.Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, et al. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr Diabetes Rev. 2016;12:359–68. doi: 10.2174/1573399811666150724095130. [DOI] [PubMed] [Google Scholar]
- 6.Aljulifi MZ. Prevalence and reasons of increased type 2 diabetes in Gulf cooperation council countries. Saudi Med J. 2021;42:481–90. doi: 10.15537/smj.2021.42.5.20200676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhowaish A. Economic costs of diabetes in Saudi Arabia. J Fam Community Med. 2013;20:1–7. doi: 10.4103/2230-8229.108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokdad AH, Tuffaha M. Cost of diabetes in the Kingdom of Saudi Arabia, 2014. J Diabetes Metab. 2015;06:6–11. [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelaide TU. JBI's Critical Appraisal Tools: The University of Adelaide. [Last accessed on 2021 Jul 20]. Available from: https://jbi.global/critical-appraisal-tools .
- 12.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–8. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 13.Clopper CJ, Pearson ES. the use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404. [Google Scholar]
- 14.Higgins JP, Thompson SG, Tierney J, Rydzewska L, Burdett S, Stewart L. 9th International Cochrane Colloquium. Lyon, France: 2001. Presenting Random Effects Meta-Analyses: Where are We Going Wrong. [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in knowledgebases. J Intell Inf Syst. 2006;27:159–84. [Google Scholar]
- 17.Sterne JA, Harbord RM. Funnel plots in meta-analysis. Stata J Promot Commun Stat Stata. 2004;4:127–41. [Google Scholar]
- 18.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraj R, Kumarasamy C. Systematic review and meta-analysis of cancer studies evaluating diagnostic test accuracy and prognostic values: Approaches to improve clinical interpretation of results. Cancer Manag Res. 2018;10:4669–70. doi: 10.2147/CMAR.S183181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Quwaidhi AJ, Pearce MS, Sobngwi E, Critchley JA, O'Flaherty M. Comparison of type 2 diabetes prevalence estimates in Saudi Arabia from a validated Markov model against the International Diabetes Federation and other modelling studies. Diabetes Res Clin Pract. 2014;103:496–503. doi: 10.1016/j.diabres.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 22.Al-Orf SM. The prevalence of diabetes mellitus in elderly females living in Riyadh Social Welfare Home. World Appl Sci J. 2012;17:1020–5. [Google Scholar]
- 23.Abou-Gamel M, Abdul-Nassir M, Rajeh A, Makhdoom A, Surrati A, Kateb A, et al. The prevalence of diabetes mellitus among working personnel in the faculty of science, Taibah University, Almadinah Almunawwarah, KSA. J Taibah Univ Med Sci. 2014;9:85–8. [Google Scholar]
- 24.Karim A, Ogbeide DO, Siddiqui S, Al-Khalifa IM. Prevalence of diabetes mellitus in a Saudi community. Saudi Med J. 2000;21:438–42. [PubMed] [Google Scholar]
- 25.Al Osaimi SM, AL-Gelban KS. Diabetes mellitus-prevalence and associated cardiovascular risk factors in a Saudi sub-urban community. Biomed Res. 2007;18:147–53. [Google Scholar]
- 26.Afifi RM, Omar SR, El Raggal AA. A community screening plan for the prevalence of some chronic diseases in specified adult populations in Saudi Arabia: 1- prediabetes and diabetes mellitus. Int J Diabetes Dev Ctries. 2015;35:149–56. [Google Scholar]
- 27.Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7:622–32. doi: 10.1111/1753-0407.12224. [DOI] [PubMed] [Google Scholar]
- 28.Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31:19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albakr W, Mohammad AS, Mohammed AM, Khamis AH. Prevalence and risk factors of diabetes mellitus (I & II) in a sample of adults population of Al-Khobar city, Saudi Arabia, within 2010-2011. Life Sci J. 2013;10:310–4. [Google Scholar]
- 30.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011;9:76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Bcheraoui C, Basulaiman M, Tuffaha M, Daoud F, Robinson M, Jaber S, et al. Status of the diabetes epidemic in the Kingdom of Saudi Arabia, 2013. Int J Public Health. 2014;59:1011–21. doi: 10.1007/s00038-014-0612-4. [DOI] [PubMed] [Google Scholar]
- 32.Bahijri SM, Jambi HA, Al Raddadi RM, Ferns G, Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia – A Community-Based survey. PLoS One. 2016;11:e0152559. doi: 10.1371/journal.pone.0152559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadlaq EM, Faraj ZT, Al Gamdi FM, Al Obathani FA, Abuabat MF, Awan KH. Early screening of diabetes and hypertension in primary care dental clinics at King Saud University in Riyadh, Kingdom of Saudi Arabia. J Contemp Dent Pract. 2017;18:652–9. doi: 10.5005/jp-journals-10024-2101. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez J, Alloubani A, Mari M, Alzaatreh M. Cardiovascular disease risk factors: Hypertension, diabetes mellitus and obesity among Tabuk Citizens in Saudi Arabia. Open Cardiovasc Med J. 2018;12:41–9. doi: 10.2174/1874192401812010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldossari KK, Aldiab A, Al-Zahrani JM, Al-Ghamdi SH, Abdelrazik M, Batais MA, et al. Prevalence of prediabetes, diabetes, and its associated risk factors among males in Saudi Arabia: A population-based survey. J Diabetes Res. 2018;2018:2194604. doi: 10.1155/2018/2194604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alanazi NH, Alsharif MM, Rasool G, Alruwaili AB, Alrowaili AM, Aldaghmi AS, et al. Prevalence of diabetes and its relation with age and sex in Turaif city, Northern Saudi Arabia in 2016-2017. Electron Physician. 2017;9:5294–7. doi: 10.19082/5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhazmi RS, Ahmed AA, Alshalan MH, Alfuhigi ZD, Alhazmi SF, Aldughmi AN, et al. Prevalence of diabetes mellitus and its relation with obesity in Turaif (Saudi Arabia) in 2017. Electron Physician. 2017;9:5531–5. doi: 10.19082/5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed NA. Prevalence of risk factors for diabetes mellitus and hypertension among adult in Tabuk – Kingdom of Saudi Arabia. Open Access Maced J Med Sci. 2019;7:831–7. doi: 10.3889/oamjms.2019.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Zahrani JM, Aldiab A, Aldossari KK, Al-Ghamdi S, Batais MA, Javad S, et al. Prevalence of prediabetes, diabetes and its predictors among females in Alkharj, Saudi Arabia: A cross-sectional study. Ann Glob Health. 2019;85:109. doi: 10.5334/aogh.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Baghli NA, Al-Ghamdi AJ, Al-Turki KA, Al Elq AH, El-Zubaier AG, Bahnassy A. Prevalence of diabetes mellitus and impaired fasting glucose levels in the Eastern Province of Saudi Arabia: Results of a screening campaign. Singapore Med J. 2010;51:923–30. [PubMed] [Google Scholar]
- 41.Al-Hanawi MK, Chirwa GC, Pulok MH. Socio-economic inequalities in diabetes prevalence in the Kingdom of Saudi Arabia. Int J Health Plann Manage. 2020;35:233–46. doi: 10.1002/hpm.2899. [DOI] [PubMed] [Google Scholar]
- 42.Albarakat M, Guzu A. Prevalence of type 2 diabetes and their complications among home health care patients at Al-Kharj Military Industries Corporation Hospital. J Family Med Prim Care. 2019;8:3303–12. doi: 10.4103/jfmpc.jfmpc_634_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Memish ZA, Chang JL, Saeedi MY, Al Hamid MA, Abid O, Ali MK. Screening for type 2 diabetes and dysglycemia in Saudi Arabia: Development and validation of risk scores. Diabetes Technol Ther. 2015;17:693–700. doi: 10.1089/dia.2014.0267. [DOI] [PubMed] [Google Scholar]
- 44.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Bassiony MM. Smoking in Saudi Arabia. Saudi Med J. 2009;30:876–81. [PubMed] [Google Scholar]
- 46.Al-Rethaiaa AS, Fahmy AE, Al-Shwaiyat NM. Obesity and eating habits among college students in Saudi Arabia: A cross sectional study. Nutr J. 2010;9:39. doi: 10.1186/1475-2891-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Attas OS, Al-Daghri NM, Alokail MS, Alfadda A, Bamakhramah A, Sabico S, et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: The influence of circulating adiponectin. Eur J Endocrinol. 2010;163:601–7. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilawe EH, Chiang C, Yatsuya H, Wang C, Ikerdeu E, Honjo K, et al. Prevalence and predictors of prediabetes and diabetes among adults in Palau: Population-based national STEPS survey. Nagoya J Med Sci. 2016;78:475–83. doi: 10.18999/nagjms.78.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele CJ, Schöttker B, Marshall AH, Kouvonen A, O'Doherty MG, Mons U, et al. Education achievement and type 2 diabetes-what mediates the relationship in older adults? Data from the ESTHER study: A population-based cohort study. BMJ Open. 2017;7:e013569. doi: 10.1136/bmjopen-2016-013569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen EM, Longo DR, Bardsley JK, Bridges JF. Education and patient preferences for treating type 2 diabetes: A stratified discrete-choice experiment. Patient Prefer Adherence. 2017;11:1729–36. doi: 10.2147/PPA.S139471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braverman-Bronstein A, Hessel P, González-Uribe C, Kroker MF, Diez-Canseco F, Langellier B, et al. Association of education level with diabetes prevalence in Latin American cities and its modification by city social environment. J Epidemiol Community Health. 2021;75:874–80. doi: 10.1136/jech-2020-216116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzotzas T, Vlahavas G, Papadopoulou SK, Kapantais E, Kaklamanou D, Hassapidou M. Marital status and educational level associated to obesity in Greek adults: Data from the National Epidemiological Survey. BMC Public Health. 2010;10:732. doi: 10.1186/1471-2458-10-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettee KK, Brach JS, Kriska AM, Boudreau R, Richardson CR, Colbert LH, et al. Influence of marital status on physical activity levels among older adults. Med Sci Sports Exerc. 2006;38:541–6. doi: 10.1249/01.mss.0000191346.95244.f7. [DOI] [PubMed] [Google Scholar]
- 54.Cai Lian T, Bonn G, Si Han Y, Chin Choo Y, Chee Piau W. Physical activity and its correlates among adults in Malaysia: A cross-sectional descriptive study. PLoS One. 2016;11:e0157730. doi: 10.1371/journal.pone.0157730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot for the trimmed and imputed study