Abstract
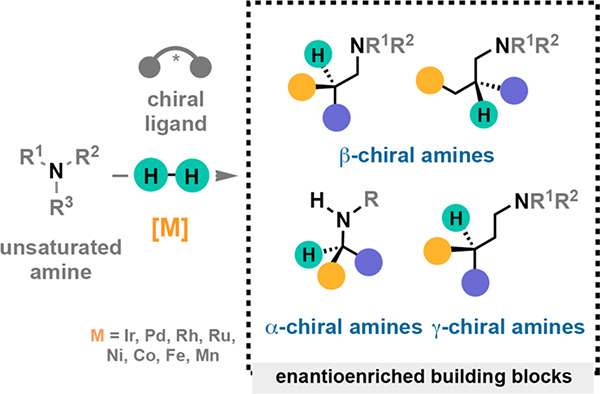
Chiral amines are key structural motifs present in a wide variety of natural products, drugs, and other biologically active compounds. During the past decade, significant advances have been made with respect to the enantioselective synthesis of chiral amines, many of them based on catalytic asymmetric hydrogenation (AH). The present review covers the use of AH in the synthesis of chiral amines bearing a stereogenic center either in the α, β, or γ position with respect to the nitrogen atom, reported from 2010 to 2020. Therefore, we provide an overview of the recent advances in the AH of imines, enamides, enamines, allyl amines, and N-heteroaromatic compounds.
1. Introduction
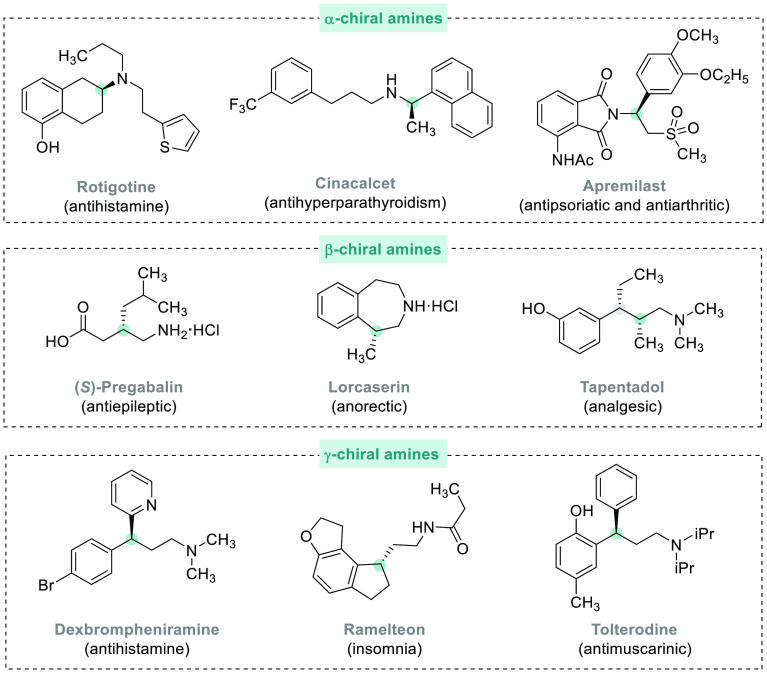
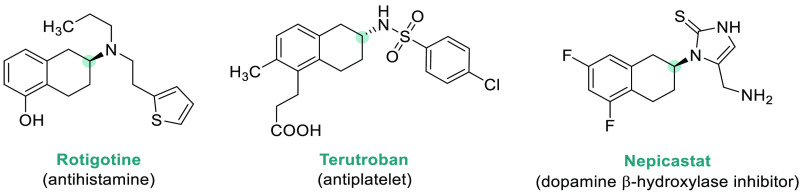
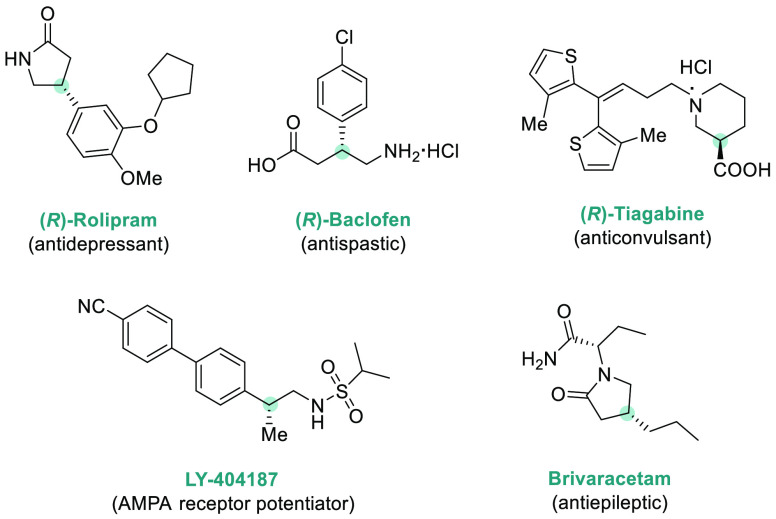
Chiral amines are key structural motifs present in a wide variety of natural products, drugs, and other biologically active compounds (Figure 1).1,2 Around 40–45% of the small molecule pharmaceuticals and many other industrially relevant fine chemicals and agrochemicals contain chiral amine fragments. Moreover, chiral amines can be used as resolving agents, chiral auxiliaries, or building blocks for the asymmetric synthesis of more complex molecules, including natural products. Therefore, the great demand for enantiomerically enriched amines in the life sciences has driven the development of innovative and sustainable synthetic routes toward their efficient preparation.3
Figure 1.
Selected pharmaceuticals with chiral amine fragments.
Despite the widespread relevance of chiral amines, traditional synthetic methods such as resolution are still being used. To overcome their intrinsic limitations, the use of catalytic methods has been widely investigated in recent decades, with asymmetric catalysis being a key research field in modern synthetic chemistry.4,5 Although biocatalytic6 and organocatalytic7 stategies have gained importance, the catalytic approach based on transition metals is still, arguably, the method most widely used.8 The design and synthesis of modular chiral ligands have allowed the preparation of novel metal complexes whose properties have been fine-tuned to afford highly active and efficient catalysts.
A relevant number of new metal-catalyzed transformations for the synthesis of chiral amines have been reported. Important achievements have been made in enantioselective methods involving, among others, reductive amination,9−12 hydroamination,13−15 allylic amination,16 or isomerization reactions.17,18 The metal-catalyzed enantioselective alkyl addition to imines has also been explored.19 Nonetheless, the asymmetric reduction of unsaturated compounds continues to be the most fundamental means of introducing chirality.20 In this regard, the asymmetric reduction of imines21,22 (via hydrosilylation or transfer hydrogenation, for example) provides an attractive route to chiral amines. However, direct asymmetric hydrogenation (AH) of unsaturated nitrogenated compounds is probably the most powerful and efficient strategy. AH offers excellent atom economy, with almost no waste or byproducts, and thus is a highly sustainable and “green” strategy for attaining optically active amines.23 Due to all these advantages, AH has become one of the major disciplines in homogeneous catalysis.24 Transition metal-catalyzed AH frequently shows excellent chemo-, regio-, and enantioselectivity, and it is considered a versatile and a reliable tool for the synthesis of chiral drugs.25 The AH of challenging organic substrates such as unfunctionalized olefins,26,27 nonaromatic cyclic alkenes,28 tetrasubstituted olefins,29 and (hetero)arenes30−34 has been extensively studied, reaching high levels of enantiocontrol. However, and in spite of the long-standing problems being partially solved, many challenges remain. Moreover, the environmental need to use more economical and accessible first-row transition metals (Mn, Fe, Co, and Ni) arises as a new complex task in a field dominated by Rh, Ir, and Ru since its origins.
Focusing on the enantioselective synthesis of chiral amines, important advances have been reported in the last ten years, many of them based on the AH of imines,35−39 enamines, and derivatives39−41 and N-heteroarenes.30−32 These advancements are largely driven by a plethora of new chiral phosphorus ligands,42 including phosphino-oxazolines43 and P-stereogenic phosphines.44,45 In addition, other chiral phosphine-free metal catalysts, bearing N-heterocyclic carbenes46 or C,N- and N,N-based ligands, have also shown outstanding catalytic activity.47 Thanks to these breakthroughs, a wide range of previously not easily accessible chiral amines have been obtained with excellent enantioselectivities.
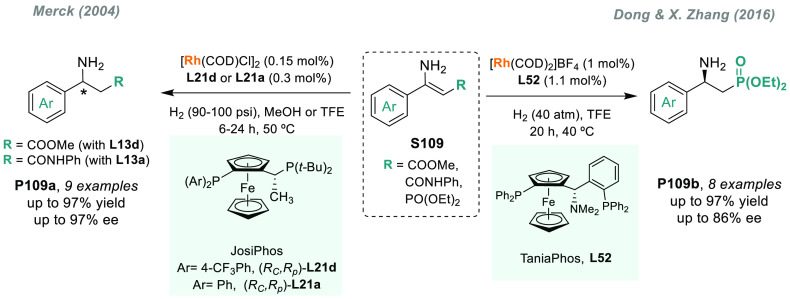
The development of new efficient routes for chiral amine synthesis has a strong and direct impact on medicinal chemistry and the pharmaceutical industry.48 Indeed, recent years have witnessed an increase in the synthesis of new chiral amino building blocks due to the great demand for expanding the chemical space in drug discovery platforms.49 AH has also found widespread use at the industrial level. The pioneering work of Knowles,50,51 Horner,52 and Kagan,53 followed by the great success of the Monsanto Company54 with the production of l-DOPA, opened up industrial-scale synthesis using AH.55−57 In 2009, Merck implemented a highly efficient and sustainable enantioselective synthesis of sitagliptin via rhodium-catalyzed AH on a manufacturing scale.58 In 2011, Pfizer developed the multikilogram synthesis of the amino acid imagabalin hydrochloride (PD-0332334), used for the treatment of generalized anxiety disorder (GAD), via AH.59 Another landmark in the field was the rhodium-catalyzed AH of β-cyanocynnamic esters60 to produce pregabalin (Lyrica), which is an important drug for the treatment of fibromyalgia and epilepsy.61
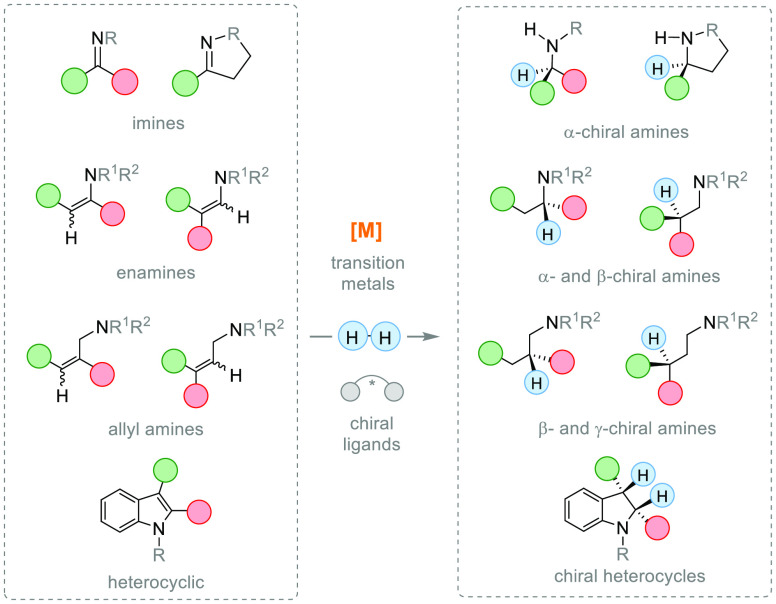
The present review focuses on the syntheses of chiral amines bearing a stereogenic center in either the α, β, or γ position with respect to the nitrogen atom reported between 2010 and 2020. Therefore, we provide an overview of the recent advances in the AH of the following substrates: (a) imines, (b) enamides, (c) enamines, (d) allyl amines, and (e) N-heteroaromatic compounds (Figure 2). Despite the fact that asymmetric reductive amination (ARA) is one of the most convenient methods for the prepration of chiral amines, this topic will not be covered specifically in this review since ARA has been extensively reviewed recently.10−12
Figure 2.
Synthesis of chiral amines via AH of unsaturated compounds using transition metal catalysis.
2. Asymmetric Hydrogenation of Imines
The asymmetric hydrogenation of prochiral imines35−40 is the most direct and efficient approach to prepare valuable α-chiral amines.62 It has been used at industrial scale, exemplified by the multiton-scale production of the herbicide (S)-metolachlor.63
Imines are more challenging substrates than their oxygenated analogs, namely ketones, due to the easy hydrolysis, the presence of E,Z stereoisomers, and nucleophilicity. Thus, extensive efforts have been devoted to the development of efficient synthetic procedures. In recent decades, considerable progress has been made in the AH of both N-protected and unprotected64 imines. While ruthenium has provided excellent results in asymmetric transfer-hydrogenation reactions, iridium has shown better performance for the direct hydrogenation of imines.65−67 In addition, catalytic systems based on earth-abundant metals such as iron or cobalt have started to give competitive results.68
2.1. N-Aryl Imines
2.1.1. N-Aryl Aryl Alkyl Imines
Several examples of the AH of N-aryl imines derived from acetophenones have been reported, reaching excellent levels of enantioselectivity. The reduction of acetophenone phenyl imine (S1, Scheme 1) is the standard substrate for this chemistry.
Scheme 1. Iridium- and Ruthenium-Catalyzed AH of N-Phenyl 1-Phenylethanimine.
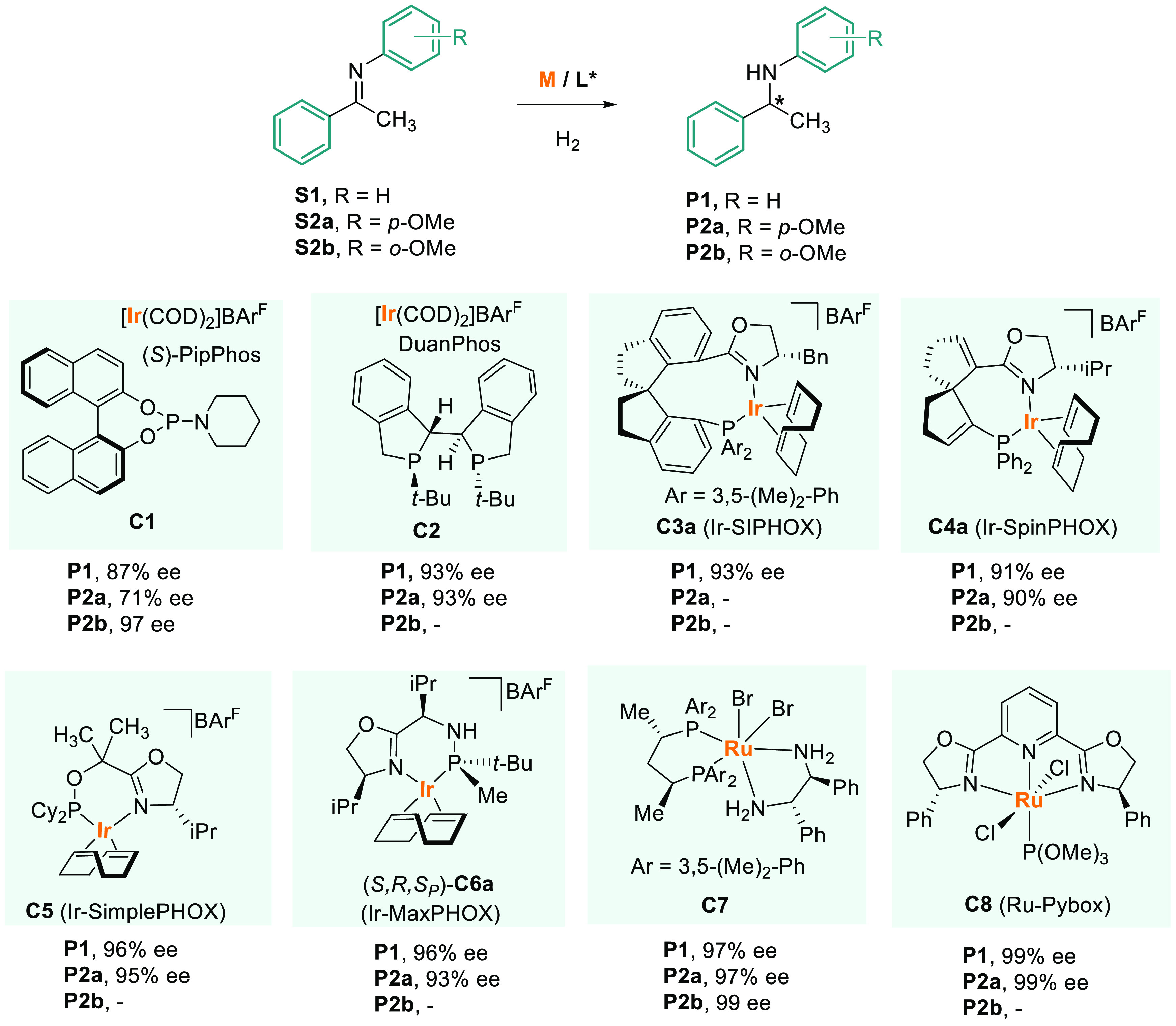
In 2009, de Vries, Feringa, and co-workers reported the iridium-catalyzed AH of N-aryl imines using readily available (S)-PipPhos as chiral monodentate phosphoramidite ligand (C1, Scheme 1).69 With the model substrate S1, they obtained a product of 87% ee, but the selectivity increased significantly using ortho-methoxyphenyl imines (S2b) as substrates. This work demonstrated that, although bidentate chiral ligands were considered a superior class in AH, modular monodentate ligands might also be highly efficient in certain cases.70
X. Zhang and co-workers used chiral diphosphine DuanPhos in the preparation of iridium catalysts C2.71 The AH of the standard substrate gave 93% ee. Similar substrates with substitutions in the aryl groups gave 90–93% ee.
Iridium complexes bearing phosphino-oxazoline chiral ligands have been widely used in the AH of N-aryl imines.72,73 Zhou’s74 and Ding’s75 groups, respectively, developed chiral complexes with a spiranic backbone C3a and C4a. Both reported high activity and achieved chiral amines in up to 97% ee. Pfaltz also showed that phosphino-oxazoline ligands provide an excellent platform for the iridium-catalyzed reduction of N-aryl imines. In 2010, he reported a range of Ir–P,N chiral complexes (SimplePHOX, C5) that were readily accessible by a short and convenient synthesis.76 The AH of S1 with C5 gave a product of 96% ee. In 2016, Riera and Verdaguer developed a novel family of chiral P-stereogenic phosphino-oxazoline ligands called MaxPHOX.77 These modular ligands are prepared from three different building blocks: an amino alcohol, an amino acid, and a P-stereogenic phosphinous acid.78 The key advantage of the Ir-MaxPHOX catalysts (C6a) resides in their structural diversity, which is conferred by four possible configurations and diverse substitution patterns. This feature allows fine-tuning of the catalyst for each specific reaction. Moreover, the absolute configuration of the P-center is crucial and has a great impact on catalytic activity. Using these Ir-MaxPHOX complexes, the AH of acyclic N-aryl ketimines smoothly proceeded with high enantiocontrol (up to 96% ee) at 1 bar of hydrogen.79
Ruthenium catalyst C7, first developed by Ohkuma and co-workers in 2012, afforded very high enantioselectivities on the model substrate S1 (97% ee) (Scheme 1).80 The Xyl-Skewphos/DPEN-Ru complex C7 was applied to the AH of a range of imines derived from aromatic and heteroaromatic ketones with a TON as high as 18,000 to afford chiral amines in up to 99% ee.
Another ruthenium complex, Ru-Pybox (C8), developed by Pizzano and Gamasa,81 afforded the corresponding amines with excellent enantioselectivities. C8 gave the best enantioselectivity for the model substrate S1 (99% ee).
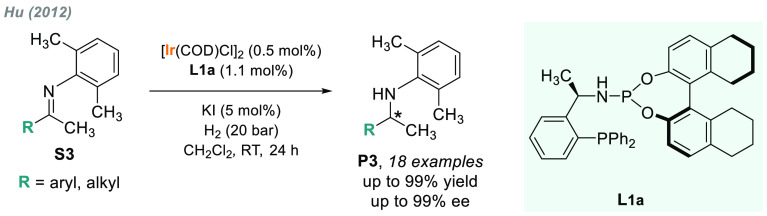
Sterically hindered N-aryl imines are difficult substrates. In 2001, X. Zhang and co-workers described Ir/f-binaphane as an excellent catalyst for the AH of sterically hindered N-aryl alkylarylamines.82 Later, in 2012, Hu reported an extended substrate scope by using the iridium complex derived from phosphine-phosphoramidite ligand L1a (Scheme 2).83,84 The corresponding chiral amines P3, which are important building blocks in organic synthesis and agrochemistry, were obtained in good to excellent enantioselectivities.
Scheme 2. Iridium-Catalyzed AH of Sterically Hindered N-Aryl Imines.
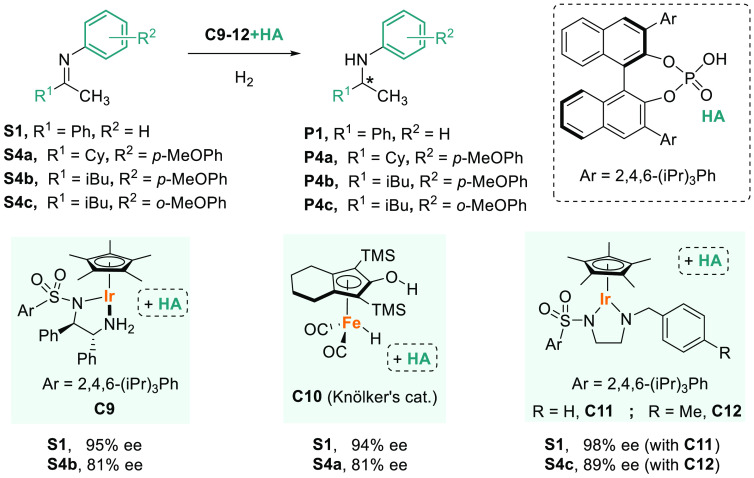
2.1.2. N-Aryl Dialkyl Imines
In contrast to aromatic imines, successful examples of the AH of imines derived from aliphatic ketones (S4) are rare and usually with low chiral induction. In 2008, Xiao pioneered the field with the highly efficient cooperative catalysis between the ruthenium complex C9 and achiral phosphoric acid (HA) (Scheme 3).85 In 2011, Beller and co-workers demonstrated that performing ligand-free AHs without the use of precious metal catalysts was possible.86 The combination of an achiral iron complex (Knölker’s catalyst, C10) with HAs enabled smooth hydrogenation for a wide range of N-aryl imines, including S1 and the dialkyl imine S4a. Similarly, in 2013, Xiao reported a family of achiral iridium-(Cp*) complexes containing diamine ligands that, in combination with a chiral HA, gave access to highly active catalysts (C11–C12) for the AH of N-aryl imines derived from both aryl and aliphatic ketones.87,88 While C11 was chosen as the best catalyst for S1 (98% ee, Scheme 3), for imine S4c the highest enantioselectivity was observed using C12, which is the best catalyst reported to date for these substrates.
Scheme 3. AH of N-Aryl Dialkyl Imines Using Binary Catalysts of a Metal Complex and a Chiral Phosphoric Acid (HA).
2.1.3. N-Aryl α-Imino Esters
The synthesis of enantiomerically pure α-amino acids and their derivatives is of great importance in pharmaceutical and synthetic chemistry.89 Chiral α-aryl glycines, in particular, have found wide applicability in the synthesis of significant antibacterial and cardiovascular drugs, such as amoxicillins and nocardicins.90 Several highly efficient asymmetric catalytic methods such as the asymmetric Strecker91 or Sharpless aminohydroxylation have been developed.92 Despite being a logical approach, the AH of the corresponding α-imino esters has scarcely been addressed, presumably because of the relatively poor reactivity of these types of imino substrates toward hydrogenation.
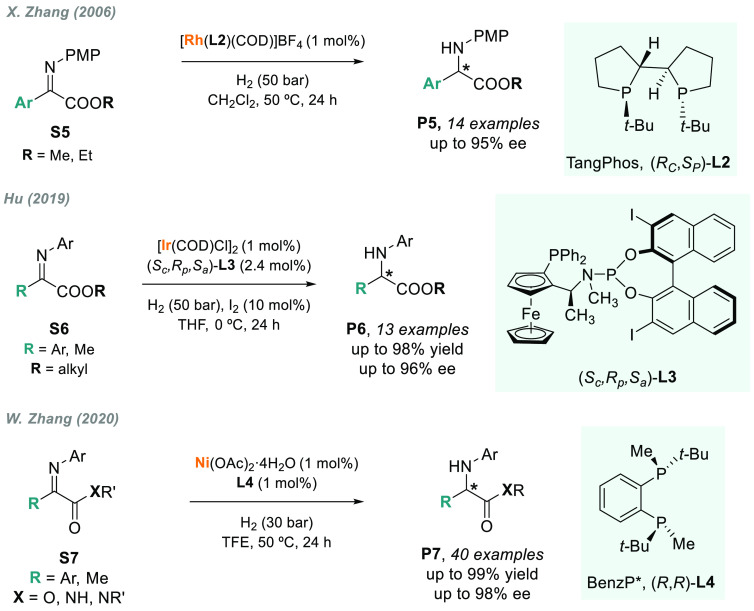
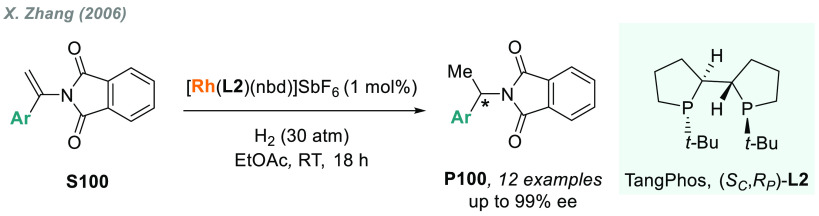
In 2006, X. Zhang and co-workers reported the first rhodium-catalyzed AH of S5 using a P-stereogenic diphosphine L2 (TangPhos), providing chiral glycines P5 with high yields and enantioselectivities (Scheme 4).93 However, the scope of this method was limited to p-methoxyphenyl (PMP)-protected α-imino esters. To overcome this constraint, Hu described the iridium-catalyzed AH of α-imino esters S6 with unsymmetrical hybrid chiral ferrocenylphosphine-phosphoramidite ligand L3 for the synthesis of optically active α-aryl glycines P6 (Scheme 4).94 The method features high asymmetric induction (up to 96% ee), with the iodo-substituent of the binaphthyl unit playing a fundamental role in the enantioselectivity.
Scheme 4. AH of N-Aryl α-Imino Esters.
To avoid the use of precious metals, in 2020, W. Zhang and co-workers reported an efficient nickel-catalyzed AH of N-aryl imino esters S7, affording chiral α-aryl glycines P7 in high yields and enantioselectivities (up to 98% ee) using a P-stereogenic dialkyl phosphine ligand, BenzP* L4 (Scheme 4).95 The reaction was performed on a gram scale at a low catalyst loading (S/C up to 2000). The preparation of two synthetic drug intermediates showcased the applicability of the method.
2.1.4. Exocyclic N-Aryl Imines
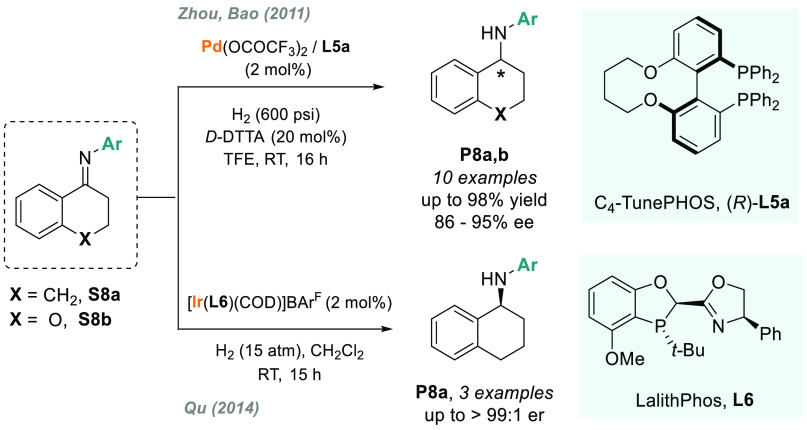
Typically, the AH of exocyclic ketimines derived from 1-tetralone or 4-chromanone exhibited low enantioselectivities, presumably due to the conformational strain upon metal coordination.96,97 In 2011, Zhou and Bao reported a highly enantioselective palladium-catalyzed hydrogenation using a catalytic amount of a Brønsted acid as activator (D-DTTA).98 By using C4-TunePhos L5a as a chiral ligand, this catalytic system provided straightforward access to enantioenriched cyclic amines P8a and P8b (86–95% ee, Scheme 5), which are privileged structural motifs present in a large number of drugs and natural compounds.99 Iridium-based catalytic systems were also used in this transformation. First, Bolm’s group made a significant advance in the iridium-catalyzed AH of exocylic imine S8a. They introduced a novel class of C1-symmetry sulfoximines as chiral ligands that, once coordinated, yielded the corresponding chiral amine adduct in 91% ee.100 Although it was a single example, the catalytic system also gave excellent results for acyclic N-aryl imines. Later, in 2014, Qu and co-workers reported a family of air-stable P-stereogenic dihydrobenzooxaphosphole oxazoline ligands (LalithPhos).101 In particular, Ir/L6 was chosen as the best catalyst for the AH of S8a, which afforded up to three examples of P8a in enantiopure form (Scheme 5).
Scheme 5. AH of Exocyclic N-Aryl Imines.
2.2. N-Alkyl Imines
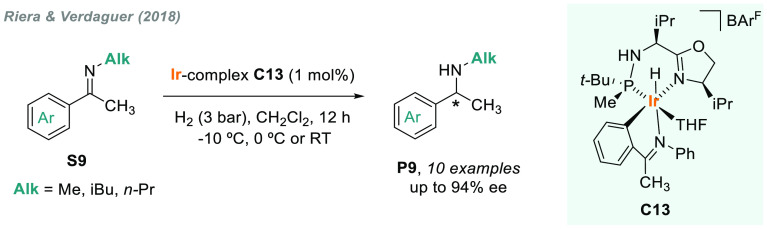
Chiral N-methyl or N-alkyl amine is a frequent pharmacophore in many pharmaceuticals and drugs, and despite other successful approaches,102,103 direct AH is the most convenient process. In sharp contrast with the good results obtained with N-aryl ketimines, the development of the AH of N-alkyl ketimines has been more difficult. The high basicity and nucleophilicity of the corresponding N-alkyl amines as reaction products often results in catalyst deactivation. Pfaltz pioneered the use of Ir-PHOX catalysts for the AH of the N-methyl imine of acetophenone, albeit with low enantioselectivity.73 Later, in 2013, he discovered that the catalyst in the hydrogenation of N-aryl imine is actually an iridacycle that forms upon reaction with the imine substrate.104 Prompted by this finding, and inspired by the excellent activity that Ir-MaxPHOX catalysts showed in the AH of N-aryl imines,79 Riera and Verdaguer’s laboratory recently reported a highly efficient AH of N-alkyl imines S9 using iridacycle C13 prepared fromMaxPHOX and the phenyl imine of benzophenone (Scheme 6).105 This catalyst allowed the first direct hydrogenation of methyl and alkyl imines derived from acetophenones in very mild conditions and in high enantioselectivity (up to 94% ee).
Scheme 6. AH of N-Methyl and N-Alkyl Imines Using Ir(III)-H Complex.
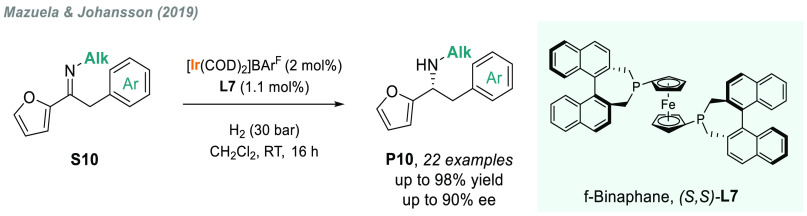
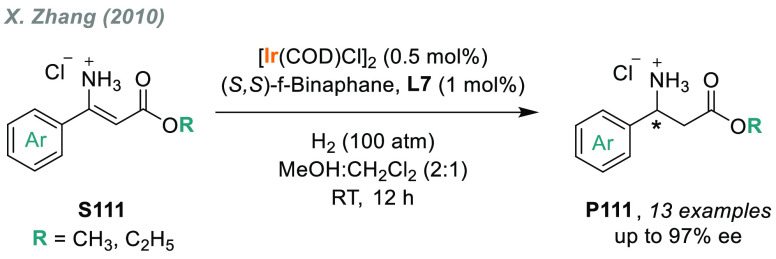
The AH of N-alkyl α-aryl furan-containing imines is a straightforward route to a wide range of unnatural N-alkyl aryl alanines. In this regard, Mazuela et al. reported that, using a Ir/(S,S)-f-Binaphane-L7 as catalyst, up to 22 N-alkyl imines were efficiently hydrogenated, providing chiral amines P10 (up to 90% ee), which can be further easily transformed into amino acids (Scheme 7).106 The effect of substituents on the nitrogen was remarkable, as the use of large alkyl substituents led to a significant decrease of enantioselectivity.
Scheme 7. Iridium-Catalyzed AH of N-Alkyl α-Aryl Furan-Containing Imines.
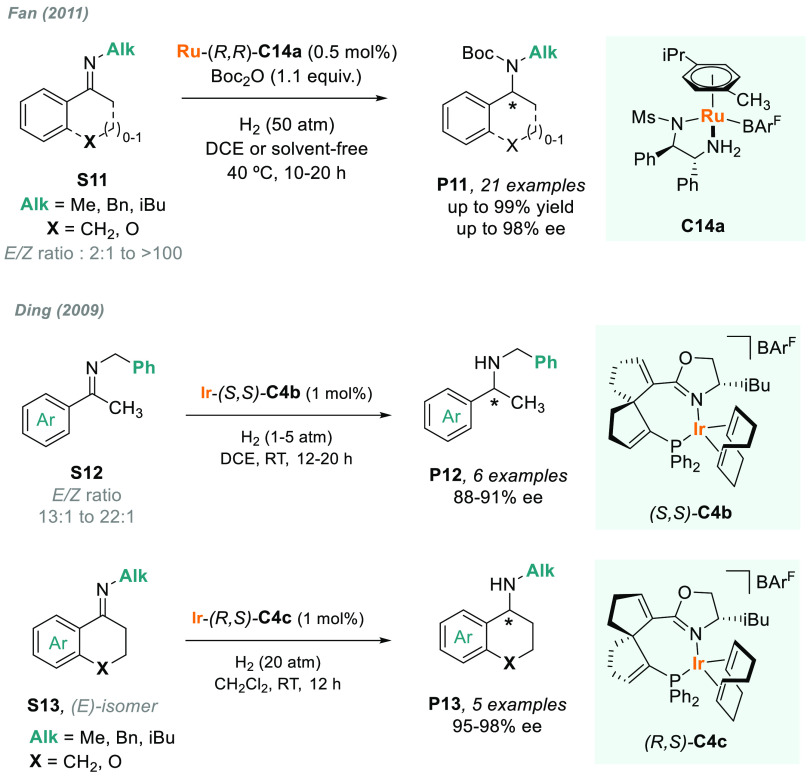
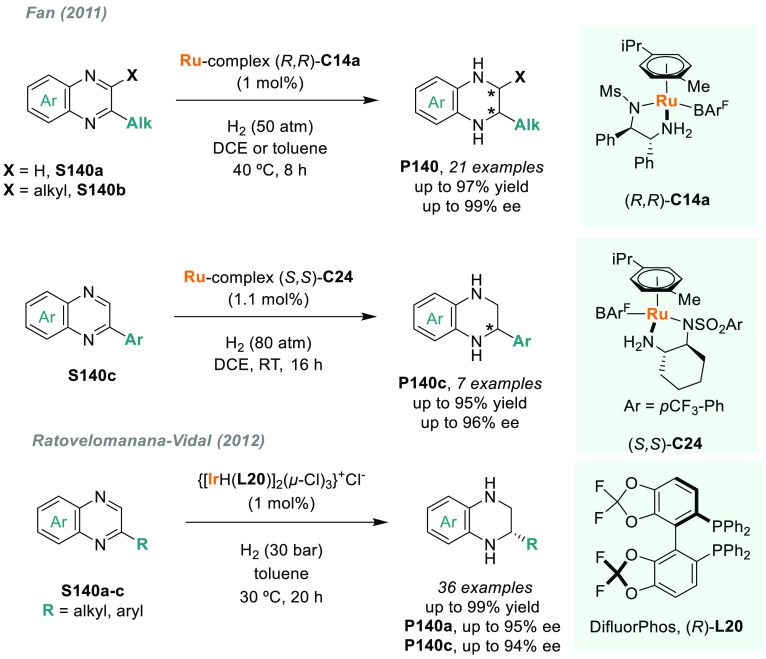
Fan described the phosphine-free, chiral, cationic Ru/MsDPEN complex C14a which was a highly active catalyst for the AH of a range of acyclic and exocyclic N-alkyl ketimines (Scheme 8).107 By using BArF as counterion, a broad range of often problematic substrates S11 were efficiently hydrogenated with low catalyst loadings, albeit with the use of Boc2O to avoid catalyst inhibition. Moreover, this system also operates under solvent-free conditions, thus providing a highly sustainable platform to optically active amines P11. The same group later reported a similar ruthenium complex that, together with a phosphoric acid as additive via cooperative catalysis, was also an efficient catalyst for the hydrogenation of N-alkyl ketimines S11 in the absence of Boc2O.108
Scheme 8. Metal-Catalyzed AH of N-Alkyl Imines.
Previously, in 2009, Ding and co-workers designed a new family of spiro phosphino-oxazoline chiral ligands that were successfully applied to the iridium-catalyzed AH of N-aryl imines.75 Of note, the catalytic system is also applicable for N-alkyl imines (Scheme 8). Actually, both acyclic (S12) and exocyclic (S13) imines were efficiently hydrogenated with high levels of enantioselectivity using two distinct precatalysts (diastereoisomers C4b and C4c, respectively) and without the need of additives.
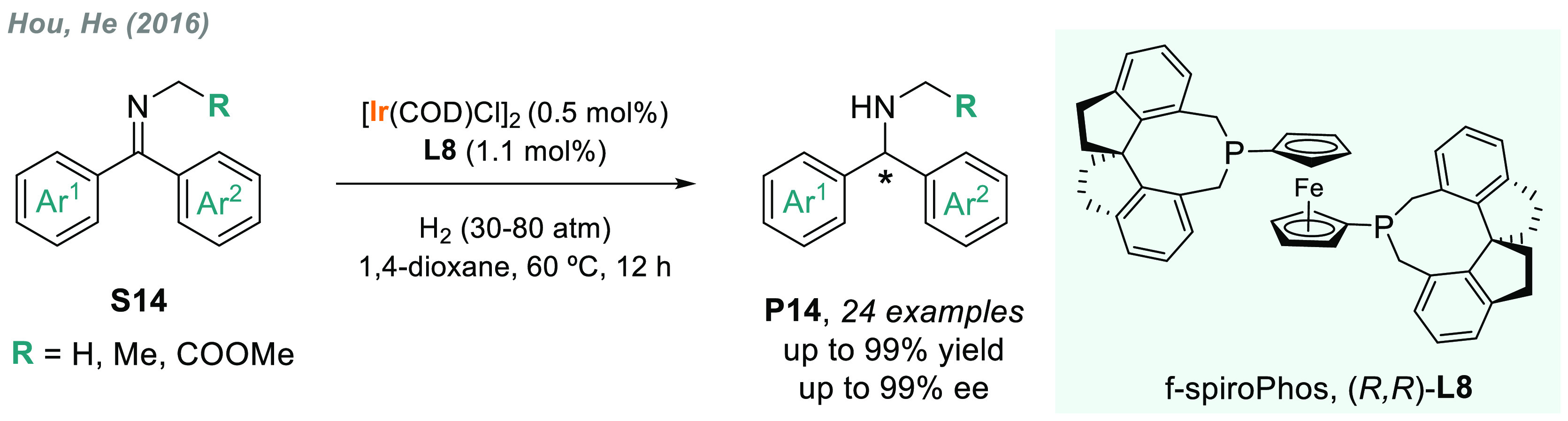
Phosphine ligands containing spiro scaffolds109 such as f-spiroPhos L8, first reported by Hou and co-workers,110 emerged as a new and powerful class of chiral ligands for asymmetric catalysis. In 2016, this group reported a highly efficient AH of diarylmethanimines, which are challenging substrates due to the difficulties to distinguish between two sterically similar aryl groups (Scheme 9).111 Hou detailed that, by using Ir/L8 as catalyst, a variety of chiral diarylmethylamines P14 were obtained with excellent enantioselectivities (up to 99% ee) and high TON. Previously, L8 had also been successfully applied to the rhodium-catalyzed AH of α,β-unsaturated nitriles,112 among other examples.
Scheme 9. Iridium-Catalyzed AH of Diaryl N-Alkyl Imines.
2.3. Cyclic N-Aryl Imines
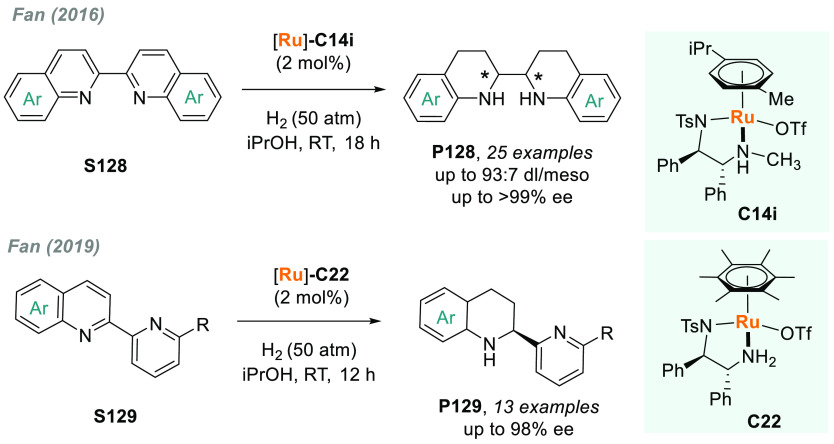
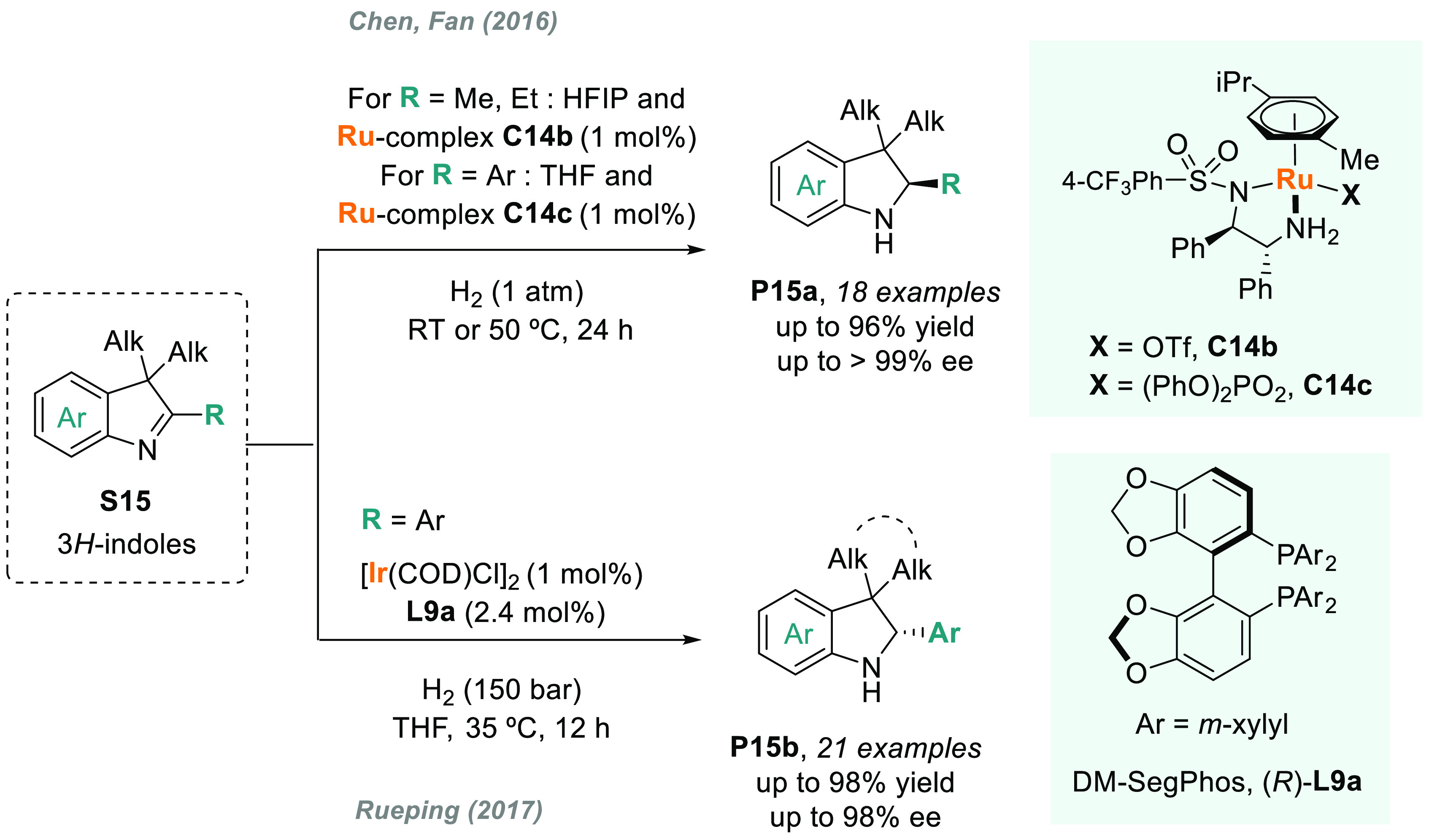
The AH of N-heteroarenes is one of the most important ways to access chiral N-heterocyclic compounds (see section 6). For instance, a direct strategy to obtain chiral indolines would be the direct AH of the corresponding indoles. However, indoles are a challenging class of substrates and their AH was unsuccessful for many years.113 In this field, Fan and Rueping’s groups simultaneously reported two independent catalytic systems that were highly efficient for the AH of 3H-indoles (Scheme 10). Fan and co-workers described a highly efficient enantioselective synthesis of 2-alkyl and 2-aryl indolines (P15a) via AH using Ru diamine catalysts C14b and C14c, respectively.114 The catalytic reaction proceeded smoothly at low H2 pressure and with a high enantioselectivity (>99% ee in the best cases). Both the counteranion and the solvent played a crucial role in catalytic performance. On the other hand, Rueping reported a highly enantioselective iridium-catalyzed AH of 3H-indoles S15 by using chiral diphosphine ligand L9a.115 A wide range of valuable disubstituted and spirocyclic 2-aryl indolines P15b were prepared in excellent results, albeit at elevated H2 pressure. Previously, the same group provided an operationally simple route to other biologically relevant heterocyclic compounds, such as dihydrobenzodiazepines, by AH.116
Scheme 10. Metal-Catalyzed AH of 3H-Indoles.
The enantioselective synthesis of seven-membered N-containing heterocycles has attracted considerable attention during recent decades, as they are versatile pharmacophores in medicinal chemistry. In 2012, Fan and co-workers reported that two Ru/diamine catalysts were highly efficient in the AH of benzodiazepines S16 (Scheme 11).117 Interestingly, an achiral anion influenced both the nature and the coordination effect and reversed the sense of the asymmetric induction. After an exhaustive catalyst screening, C14d was chosen for benzodiazepine-bearing aryl substituents, while C14e was used for alkyl groups. In the first case, both enantiomers were obtained using the same ligand but in the presence of different achiral counteranions. Recently, they also reported that the iridium complex C15 is a highly active catalyst for the AH of benzodiazepines S17 bearing aryl substituents (Scheme 11).118 For both catalytic systems, the corresponding optically active dihydrobenzodiazepines were obtained with good to excellent diastereoselectivity and excellent enantioselectivity. The same group reported other catalysts, including dendritic phosphinooxazoline iridium complexes, which proved highly efficient for both the partial and total AH of benzodiazepines.119−121
Scheme 11. Metal-Catalyzed AH of Benzodiazepines and Benzodiazepinones.
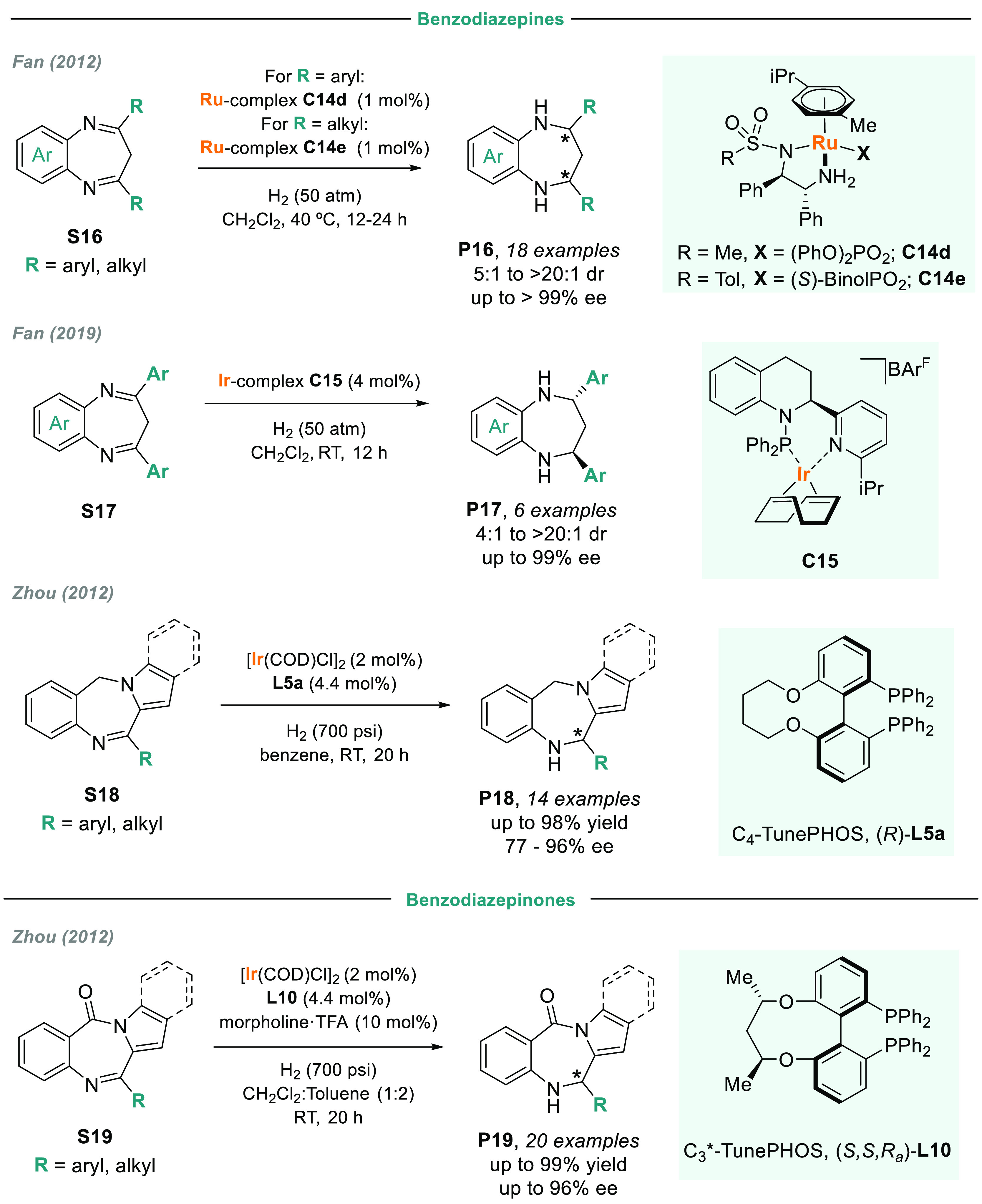
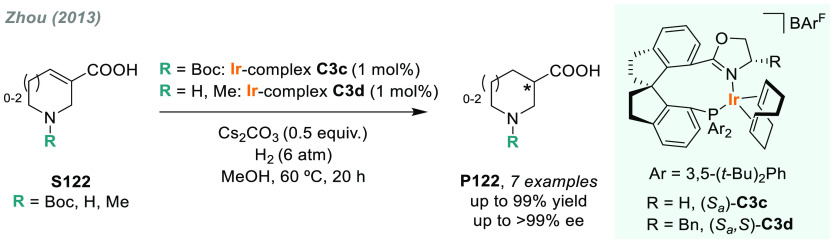
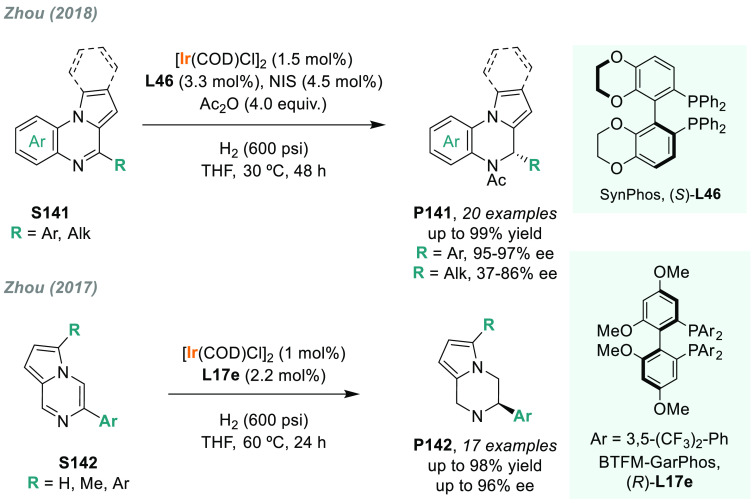
Zhou’s group used Ir chiral complexes also for the AH of benzodiazepines. They reported that Ir/C4-TunePhos-L5a is a highly active catalytic system for the AH of both pyrrole- and indole-fused benzodiazepines S18, reporting a moderate to excellent level of enantioselectivity (Scheme 11).122 Moreover, by switching to chiral ligand L10, outstanding results were also obtained for the AH of benzodiazepinones S19, thus offering a highly versatile catalytic approach for a range of chiral cyclic amines present in numerous important natural products and drugs. In addition, the same group later reported an iridium-catalyzed AH/oxidative fragmentation cascade for the synthesis of chiral dihydrobenzodiazepinones.
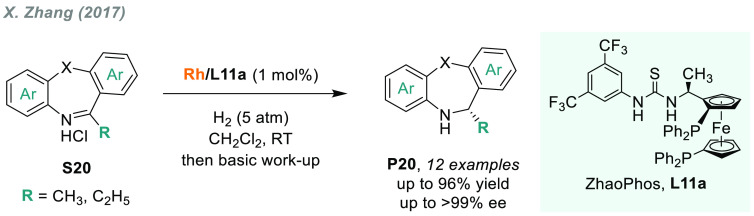
A number of successful examples of the AH of some benzo-fused seven-membered cyclic imines for the preparation of chiral benzazepines and benzodiazepines have recently been reported.123−126 X. Zhang and co-workers described a highly efficient AH of dibenzoazepine hydrochlorides S20 catalyzed by Rh/ZhaoPhos-L11a (Scheme 12).127 The corresponding chiral seven-member cyclic amines P20 were obtained in high yields and excellent enantioselectivities (>99% ee in the best cases). Interestingly, control experiments revealed that the anion-bonding interaction between the chloride ion of the substrate and the thiourea motif of L11a played a key role in enantioselectivity. The same reaction conditions were also useful for the AH of oxazepines.
Scheme 12. Access to Chiral Seven-Membered Cyclic Amines via Rhodium-Catalyzed AH.
Another important family of C=N-containing heterocycles are benzoxazines and derivatives (S21–S22). At the beginning of this decade, Beller,128 Ohkuma,129 and Zhou’s130 groups reported advances in the transition metal-catalyzed AH of this class of compounds. Later, in 2014, Fan expanded the catalytic application of Ru/MsDPEN complexes. In fact, C14f and C14g were excellent catalysts for the highly enantioselective AH of 3-aryl- and 3-styryl-substituted benzoxazines S21, respectively (up to 99% ee, Scheme 13).131 In contrast to previous work130 where 3-styryl-substituted benzoxazines were completely hydrogenated, this catalytic system showed an exquisite 1,2-selectivity with an appropriate counterion (C14g). On the other hand, the main drawback of this method is that ortho-substituted aryl substituents in benzoxazines S21 were not compatible. Unfortunately, when using these substrates, the reaction could not take place, probably due to undesired steric effects. In addition, the AH of 3-alkyl-substituted benzoxazines is underdeveloped.129
Scheme 13. Metal-Catalyzed AH of Benzoxazines and Benzoxazinones.
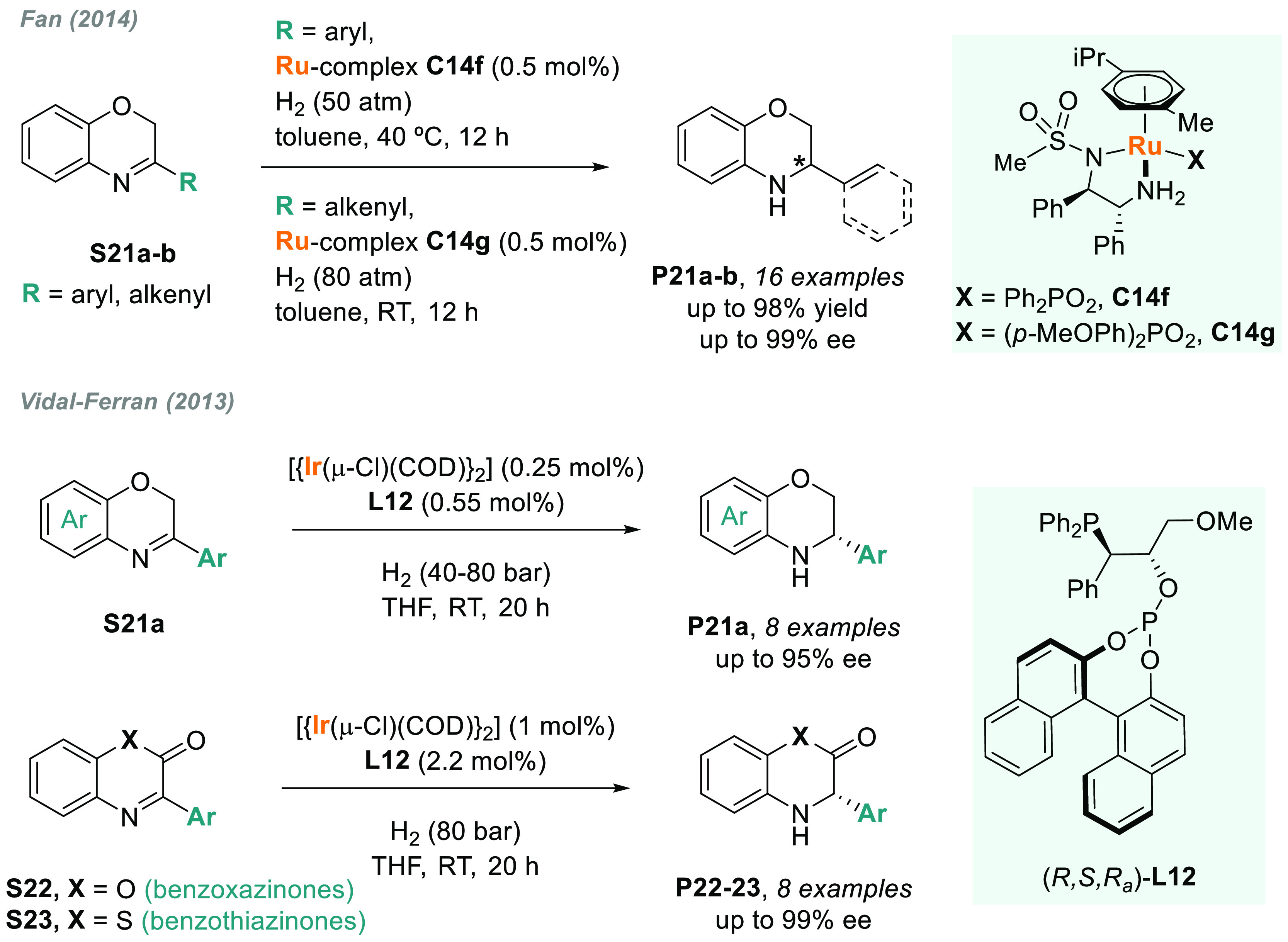
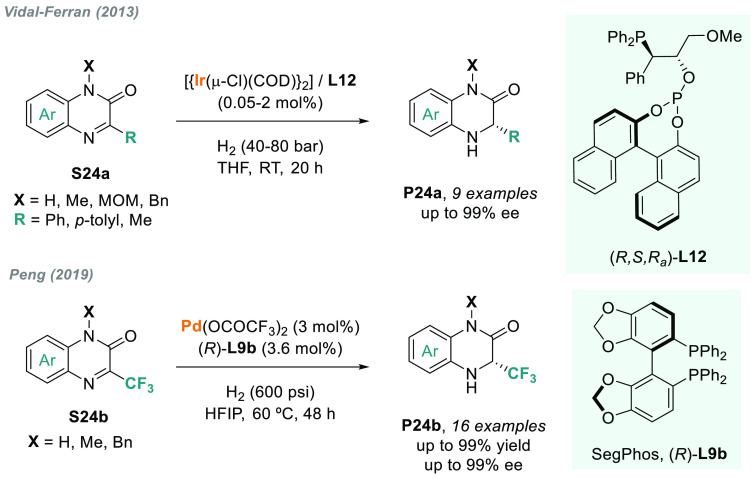
Moving to iridium catalysis, Vidal-Ferran designed a new phosphine-phosphite ligand L12, which, once coordinated to iridium, provided a highly active Ir(I) catalyst for the AH of 3-aryl-substituted benzoxazines (S21a), benzoxazinones (S22), and benzothiazinones (S23) (up to 99% ee, Scheme 13).132,133
The iridium catalyst with L12 was also the first-ever reported catalyst for the AH of quinoxalinones and N-substituted quinoxalinones S24a (Scheme 14). More recently, Peng and co-workers reported a highly enantioselective palladium-catalyzed AH of S24b.134 Using (R)-SegPhos L9b as the chiral ligand, and performing the reaction in HFIP, a wide array of optically active 3-trifluoromethylated dihydroquinoxalinones P24 were synthesized (>99% ee in the best cases, Scheme 14). However, the substituent on the aromatic ring impaired the reaction. In this regard, the introduction of a methyl group at the 5-position on the phenyl ring inhibited the reaction due to the steric effect.
Scheme 14. Metal-Catalyzed AH of Quinoxalinones.
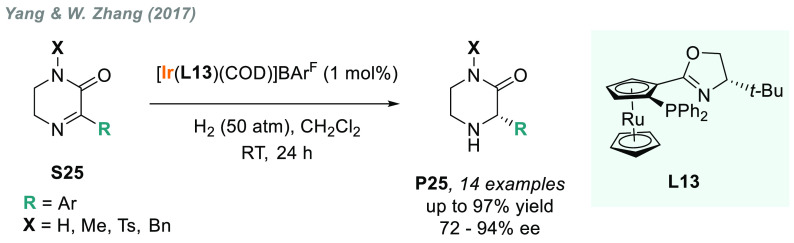
The AH of related nonaromatic systems such as 5,6-dihydropyrazin-2-ones S25 was recently reported by Yang, W. Zhang, and co-workers135 using a phosphine-oxazoline RuPHOX ligand (L13). The corresponding chiral piperazin-2-ones P25 were obtained in good yields and with moderate to good enantioselectivities (Scheme 15).
Scheme 15. Enantioselective Synthesis of Chiral Piperazin-2-ones via AH.
2.4. Cyclic N-Alkyl Imines
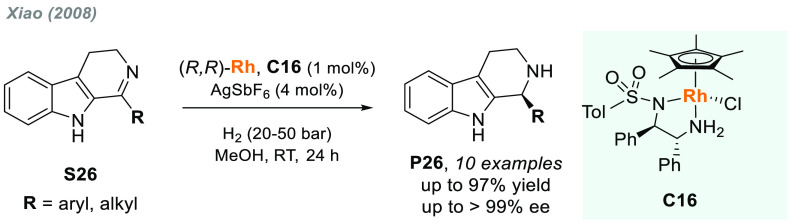
The great progress achieved in the AH of activated and N-aryl imines contrasts with the often problematic AH of N-alkyl imines. Buchwald’s group reported the titanocene-catalyzed AH of cyclic N-alkyl imines back in 1994.136 In 2008, Xiao and co-workers identified a Rh(III)-diamine complex (C16) as a highly active catalyst for the AH of cyclic N-alkyl imines S26 to give bioactive tetrahydro-β-carbolines137P26 in optically pure form (>99% ee in the best cases, Scheme 16).138 Remarkably, both aryl and alkyl substituents were well-tolerated, and mostly no differences in terms of enantioselectivities were observed.
Scheme 16. Rhodium-Catalyzed AH of Cyclic N-Alkyl Imines.
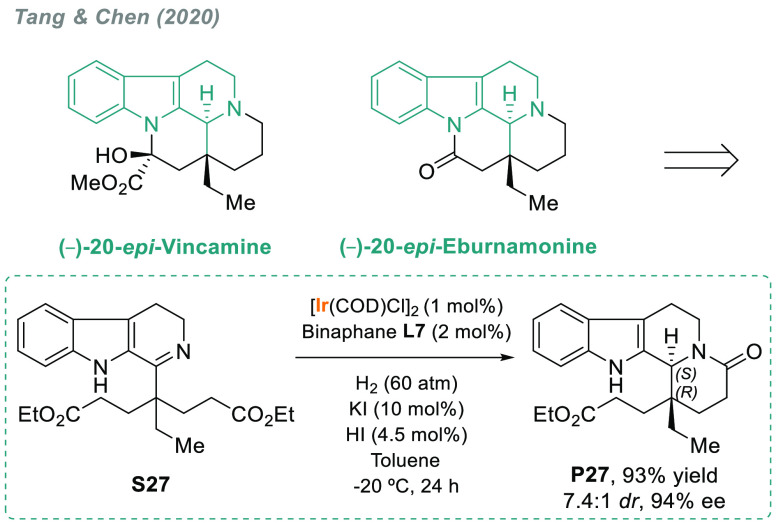
Dihydro-β-carbolines have been used to synthesize natural products. In 2020, Tang, Chen, and co-workers reported a concise asymmetric total synthesis of two examples of the Eburnamine–Vincamine alkaloids (Scheme 17).139 These syntheses featured a highly stereoselective iridium-catalyzed hydrogenation/lactamization cascade using f-binaphane L7 as a chiral ligand, thus allowing a stereocontrolled assembly of the C20/C21 adjacent chiral centers in P27.
Scheme 17. Iridium-Catalyzed Enantioselective Imine Hydrogenation/Lactamization Cascade.
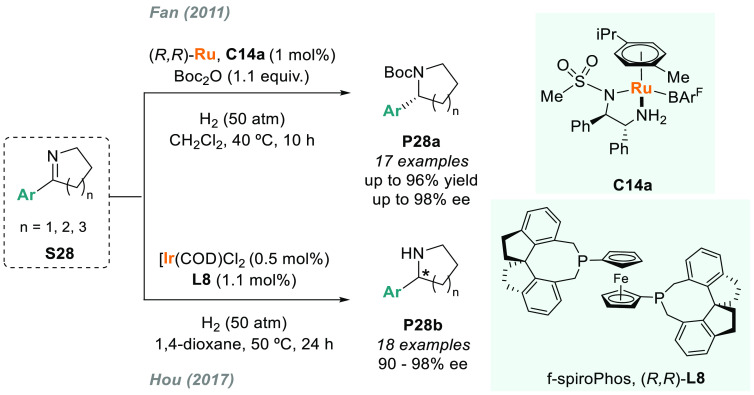
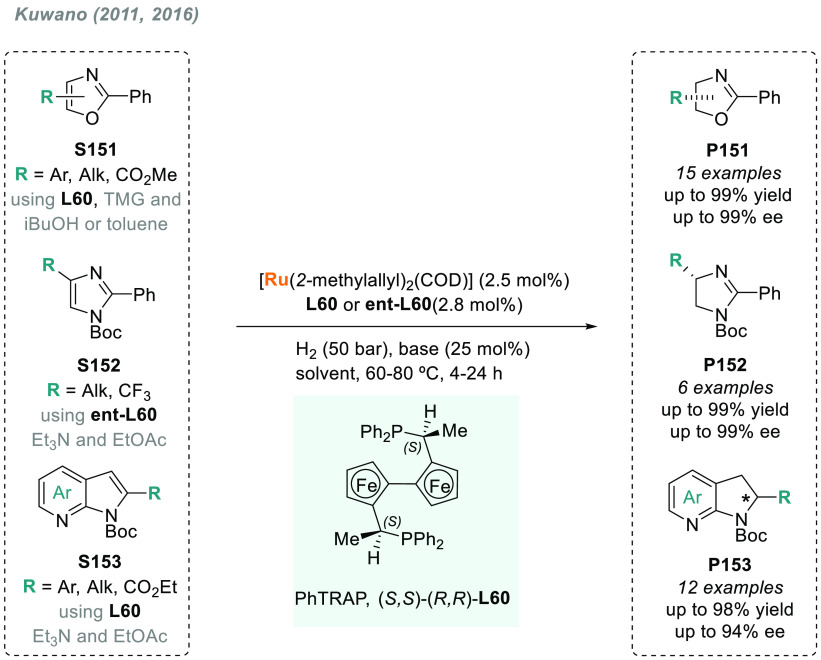
Chiral cationic Ru-MsDPEN complexes have also been employed in the AH of cyclic N-alkyl imines. In particular, Fan disclosed that C14a was an efficient catalyst for the AH of S28 to provide chiral cyclic amines P28a in excellent yields and enantioselectivities (Scheme 18).140 The same authors used a similar catalytic system for the AH of dibenzo[c,e]azepine derivatives to afford seven-membered cyclic amines with moderate to excellent enantioselectivities.141 However, in both cases, the use of Boc2O was required to prevent in situ catalyst deactivation. To circumvent this issue, Hou recently reported that the complex of iridium and (R,R)-f-spiroPhos L8 as the catalyst allowed the smooth hydrogenation of a range of 2-aryl cyclic imines S28 to P28b under mild conditions without any additive (Scheme 18).142 Hou also reported the synthesis of free cyclic amines via intramolecular reductive amination using a chiral iridium complex derived from L8.143 Previously, in 2010, X. Zhang reported an iridium-based catalytic system for the direct AH of S28 without in situ N-protection, albeit with lower enantioselectivities.144
Scheme 18. Synthesis of Chiral 2-Aryl Pyrrolidines and Piperidines via AH.
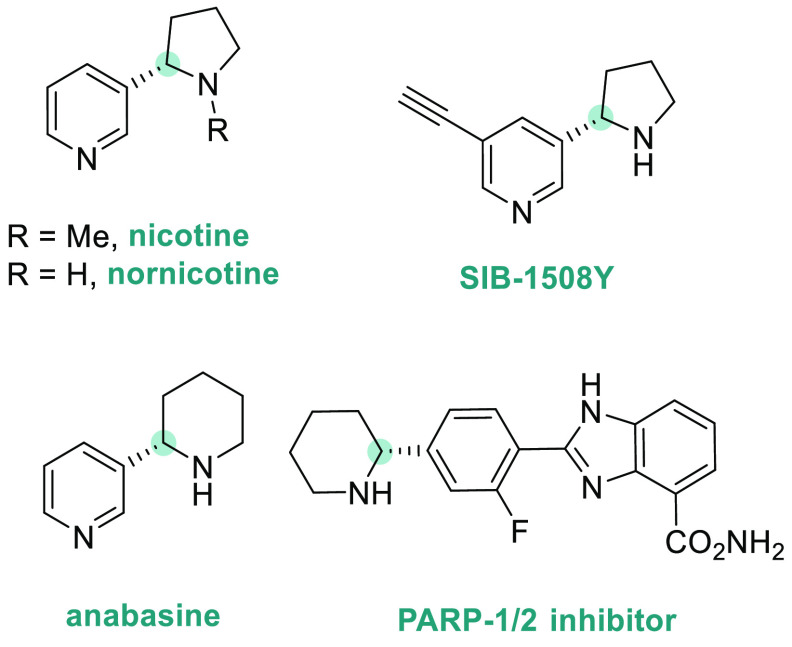
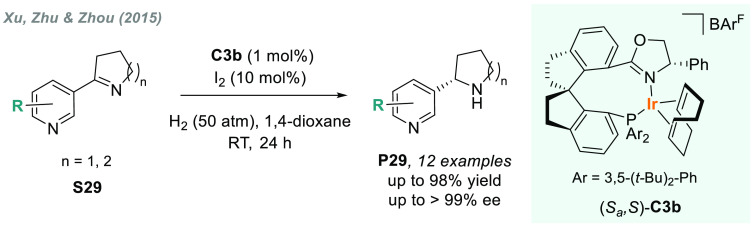
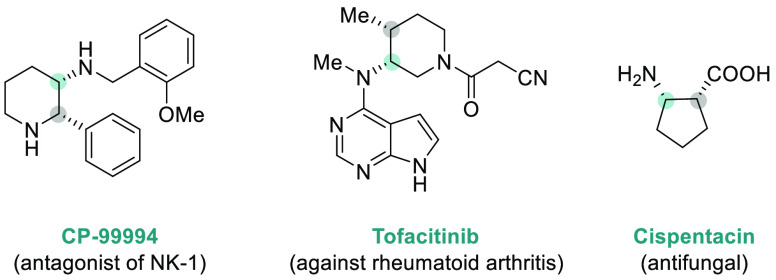
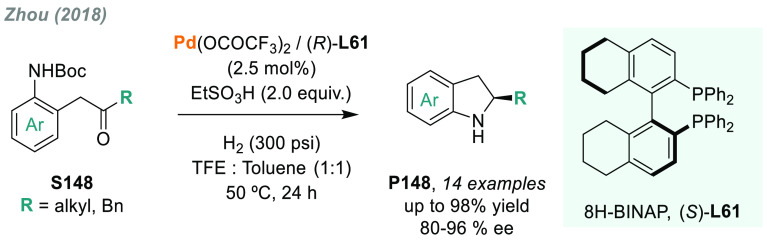
Optically active 2-aryl pyrrolidines and piperidines are an important class of structural units in many natural products and pharmaceuticals (Figure 3).145,146 In particular, chiral amines containing a pyridyl moiety, such as nicotine and its derivatives,147 are very common in alkaloid natural products and pharmaceuticals. However, the transition metal-catalyzed AH of pyridyl-containing unsaturated compounds remained a great challenge due to the strong coordinating ability of the pyridine moiety, which led to catalyst deactivation. To overcome this limitation, in 2015, Xu, Zhu, Zhou, and co-workers reported a highly efficient protocol to facilitate the exploration of nicotine-derived bioactive compounds.148 By using iridium catalyst C3b with a chiral spiro phosphine-oxazoline ligand (SIPHOX), a wide variety of chiral amines P29 were attained in excellent yields and enantioselectivities via direct catalytic AH of 2-pyridyl cyclic imines S29 (Scheme 19).
Figure 3.
Structures of biologically active compounds and pharmaceutical drugs containing a cyclic 2-aryl amine moiety.
Scheme 19. Iridium-Catalyzed AH of 2-Pyridyl Cyclic Imines.
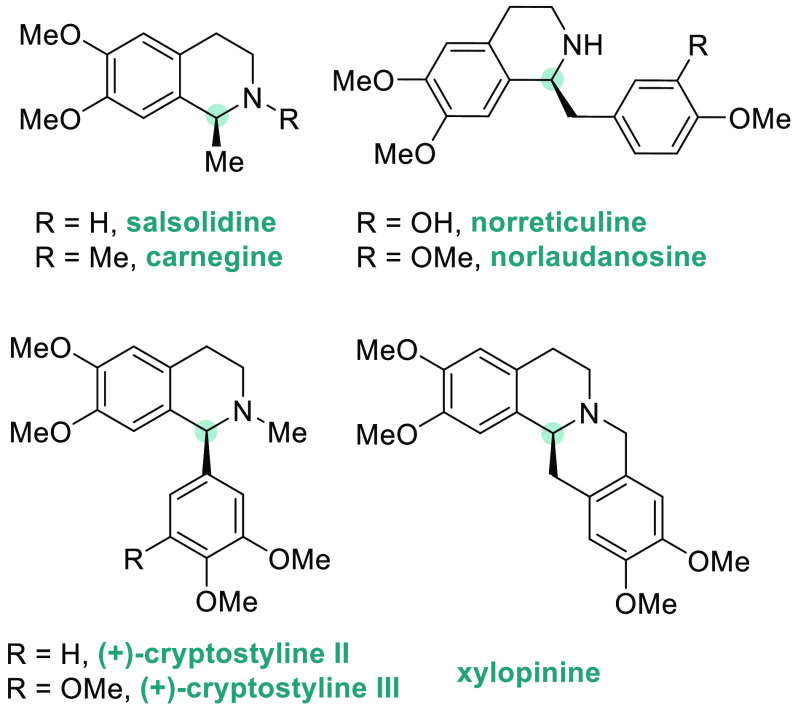
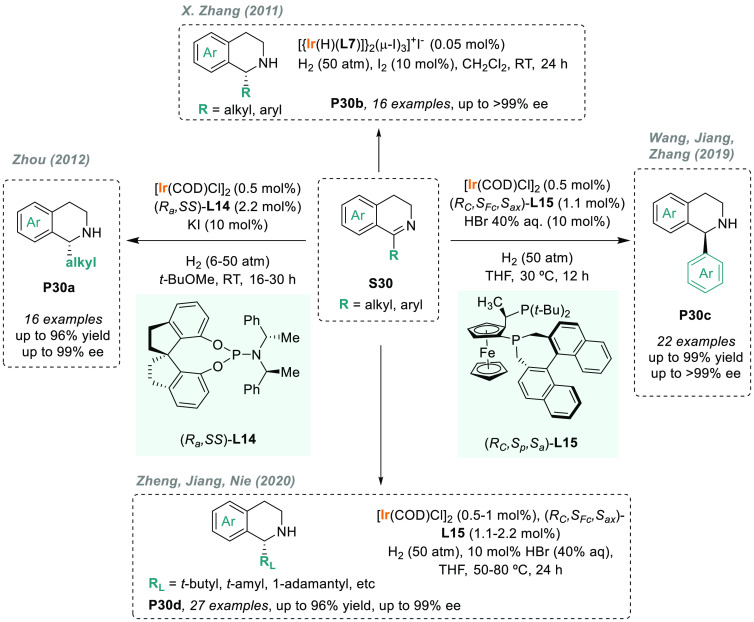
Tetrahydroisoquinolines (THIQs) are an important class of alkaloids present in many pharmaceutical drugs (Figure 4).149,150 Therefore, the development of new enantioselective methods for their synthesis is highly desired. To this end, Zhou’s group used ligand L14 from the family of spiro-ligands SIPHOS for the enantioselective synthesis of THIQs. In 2012, they developed a highly efficient iridium-catalyzed AH of 3,4-dihydroisoquinolines (DHIQs) S30 with good to excellent enantioselectivities (Scheme 20).151 The scope of the reaction was limited to alkyl substituents.138,152 X. Zhang’s laboratory developed an alternative catalytic system using the iodine-bridged dimeric iridium complex with (S,S)-f-Binaphane L7.153 This catalyst was applied to the AH of a wide range of 3,4-dihydroisoquinolines (S30) including, for the first time, those bearing aryl substituents. The corresponding THIQs P30b were afforded with excellent enantioselectivities and high TON (Scheme 20). Unfortunately, due to steric hindrance, the enantioselectivities varied dramatically with the substrates bearing a 1-ortho-substituted phenyl ring. To overcome this limitation, several catalytic systems were reported as alternatives.154−156 Of note, Wang, Jiang, S. Zhang, and co-workers reported a direct, simple, and efficient protocol toward enantioenriched chiral 1-aryl-substituted THIQs P30c.157 For this purpose, they applied novel JosiPhos-type binaphane ligand (t-Bu-ax-JosiPhos) L15 to the iridium-catalyzed AH of 1-aryl-substituted DHIQs S30 (Scheme 20). Interestingly, the new ligand adopted the privileged properties of both JosiPhos and f-binaphane in terms of rigidity and electron-donating ability. Moreover, the use of 40% HBr (aqueous solution) as an additive dramatically improved the asymmetric induction of the catalyst. In 2020, the same catalytic system was applied to the AH of sterically hindered cyclic imines P30d, achieved with good to excellent enantioselectivities (74–99% ee) (Scheme 20).158 This novel family of chiral ligands was also applied to the iridium-catalyzed AH of acyclic N-aryl imines.159
Figure 4.
Pharmaceuticals and alkaloids containing chiral 1-substituted THIQs.
Scheme 20. Enantioselective Synthesis of THIQs via Iridium-Catalyzed AH.
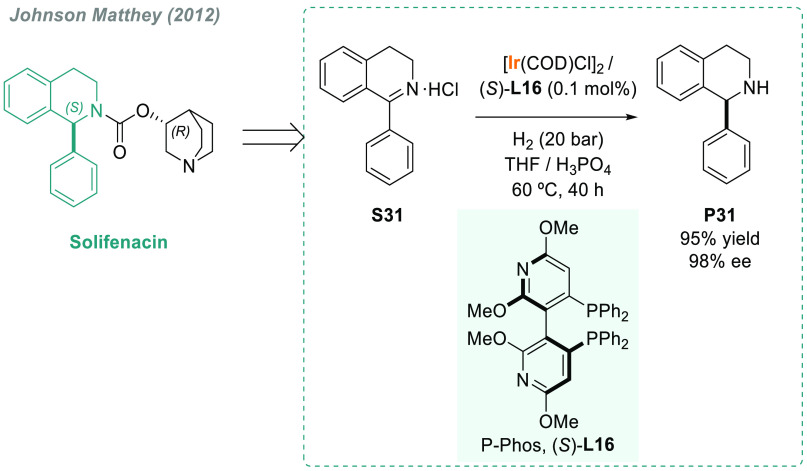
In 2013, Zanotti-Gerosa’s group (Johnson-Matthey) described a novel approach to the synthesis of the urinary antispasmodic drug solifenacin (Scheme 21).160 After an exhaustive optimization process, the group demonstrated the feasibility of the process for the AH of the hydrochloride salt S31. The use of this salt increased reactivity in the presence of the iridium catalyst with chiral ligand (S)-P-Phos (L16). The robustness of the protocol was proved by reproducing it on 200 g scale to give P31 in 95% yield and 98% ee.
Scheme 21. Asymmetric Synthesis of Solifenacin via Iridium-Catalyzed AH.
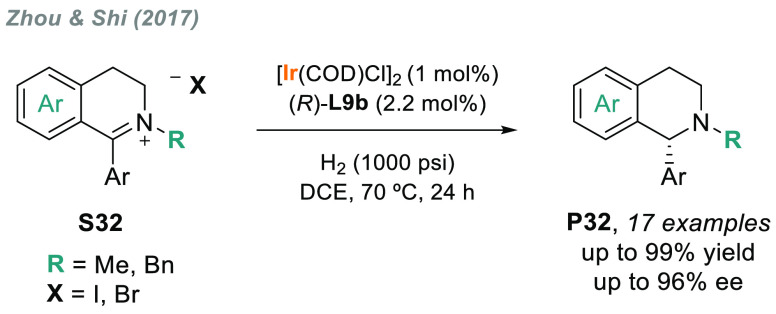
The AH of iminium salts is the method of choice for obtaining tertiary amines in terms of simplicity and atom economy. In this regard, Zhou’s group described an efficient and convenient method using Ir/(R)-SegPhos L9b for the AH of cyclic iminium salts bearing a dihydroisoquinoline moiety S32 (Scheme 22).161 The corresponding chiral tertiary amines P32 were afforded in good to excellent yields and with up to 96% ee.
Scheme 22. Iridium-Catalyzed AH of Cyclic Iminium Salts.
2.5. N-Sulfonyl Imines
2.5.1. Acyclic or exocyclic N-sulfonyl imines
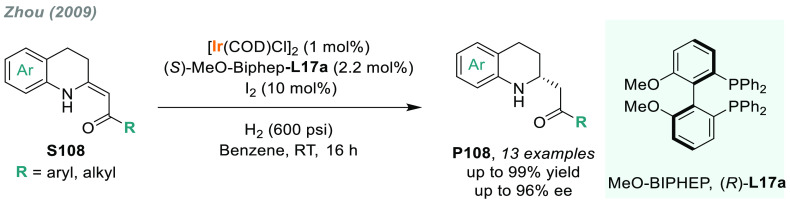
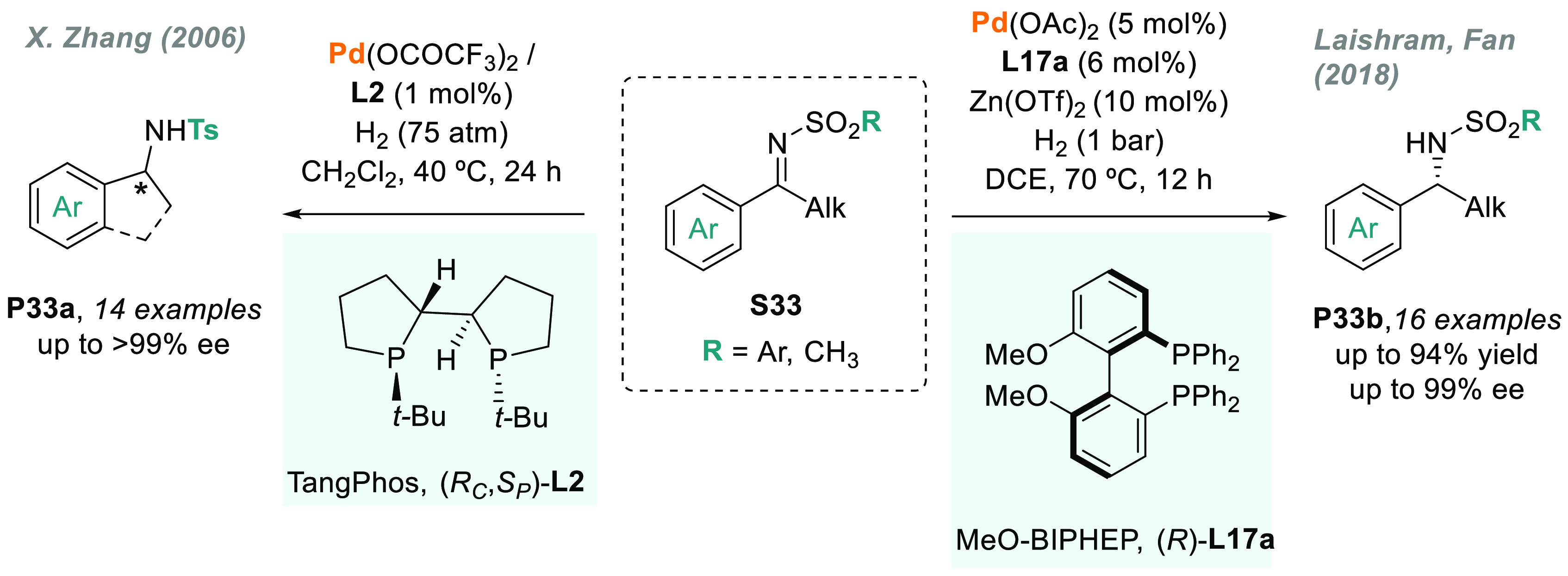
At the beginning of this decade, the instability of some imines prepared from ketones and the inhibitory effect of the amine products on the metal catalysts partially prevented their widespread use in AH. To overcome these limitations, N-sulfonyl imines, which are more stable than aryl or alkyl imines, emerged as a useful alternative. Moreover, the strong electron-withdrawing character of the sulfonyl group reduces the probability of eventual catalyst deactivation. In 2006, X. Zhang and co-workers reported an important breakthrough in the field: palladium-catalyzed AH using TangPhos (L2).162N-sulfonyl imines S33 (including exocyclic imines) were efficiently hydrogenated with high levels of enantioselectivity (>99% ee in the best cases, Scheme 23). However, high H2 pressure was required to hydrogenate the C=N bond with full conversion. Aiming to design a catalytic system able to work at low pressure, Laishram, Fan, and co-workers recently reported a cocatalytic system based on Pd and using Zn(OTf)2 as an essential additive.163 The combination of this Lewis acid, Pd(OAc)2, and the axially chiral diphosphine MeO-Biphep (L17a) furnished the corresponding N-sulfonyl amines P33b, which show high activity and optical purity working under 1 bar of H2 (Scheme 23).
Scheme 23. Palladium-Catalyzed AH of Aryl Alkyl N-Sulfonyl Imines.
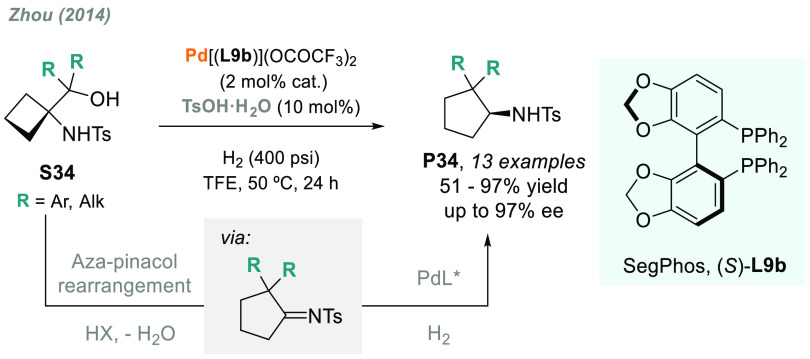
Palladium-based catalysts had a strong impact on AH.164 Furthermore, palladium-catalyzed processes involving tandem or cascade reactions are advantageous for the exploration of highly reactive intermediate species. In 2014, Zhou’s group reported an efficient palladium-catalyzed AH via hydrogenation of an intermediate generated from the acid-catalyzed aza-Pinacol rearrangement of S34 (Scheme 24).165 Using the axially chiral ligand (S)-SegPhos L9b, up to 13 examples of chiral five-membered exocyclic amines P34 were obtained in moderate to high yields and excellent enantioselectivities (up to 97% ee).
Scheme 24. Enantioselective Palladium-Catalyzed Hydrogenation of Cyclic N-Sulfonyl Amino Alcohols.
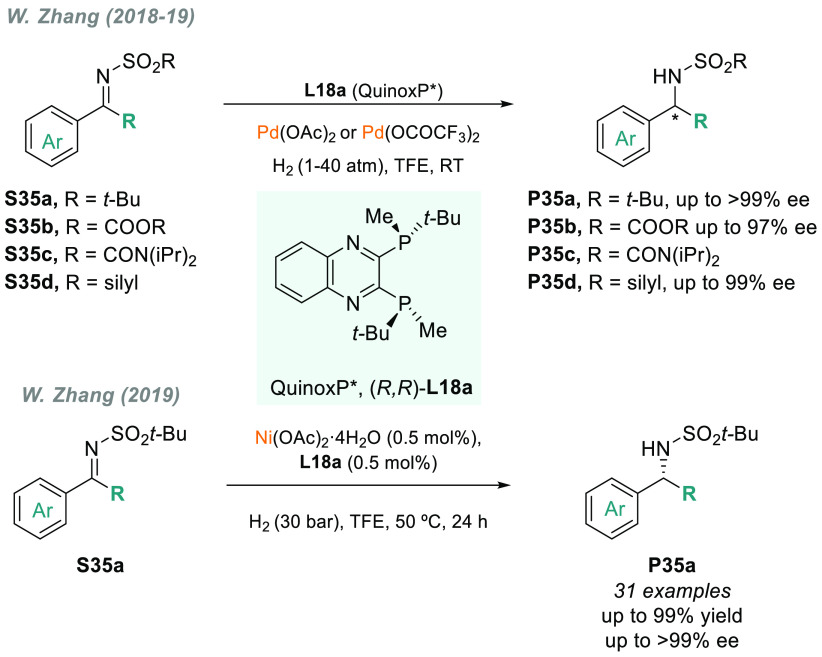
Imines bearing small substituents, such as methyl or ethyl groups connected to the carbon atom, have been widely used as substrates. In sharp contrast, the AH of sterically demanding imines (from ketones bearing bulky substituents) or other α-heteroatom-substituted imines is still rare. W. Zhang expanded the frontiers of the AH of N-sulfonyl imines by employing the P-stereogenic diphosphine Quinox-P* (L18a),166 previously designed by Imamoto (Scheme 25). In 2018, the group reported the palladium-catalyzed AH of sterically hindered N-tosylimines under 1 bar of H2 pressure with high catalytic activities (S/C up to 5000) and excellent enantioselectivities (up to 99% ee, Scheme 25, P35a).167 This methodology was also applied to dialkyl N-tosyl imines and N-sulfonyl α-iminoesters168 with the same level of enantiocontrol. W. Zhang and co-workers also described the AH of α-iminosilanes169 (up to 99% ee, Scheme 25, S35d), albeit using higher H2 pressures. The low activity of earth-abundant transition metal catalysts has prevented their broad adoption in AH.170 Undeterred by this challenge, W. Zhang’s group recently demonstrated that the combination of nickel complexes with QuinoxP* L18a allows the AH of N-sulfonyl imines S35a with high catalytic activity (S/C = 10500) and exquisite enantiocontrol (>99% ee in the best cases, Scheme 25).171
Scheme 25. Metal-Catalyzed AH of Different Acyclic α-Substituted N-Sulfonyl Imines.
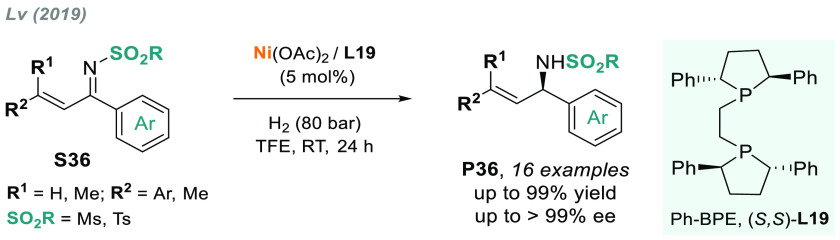
A similar catalytic system using Ph-PBE (L19) as a ligand was recently reported by Lv and co-workers (Scheme 26).172 The nickel-catalyzed chemoselective AH of α,β-unsaturated ketoimines S36 afforded chiral allylic amines P36 in excellent yields and enantioselectivities. The last two examples confirm that nickel can be an effective transition metal for AH—a concept also disclosed by Chirik173 and Hamada.174
Scheme 26. Nickel-Catalyzed Chemoselective AH of α,β-Unsaturated Ketoimines.
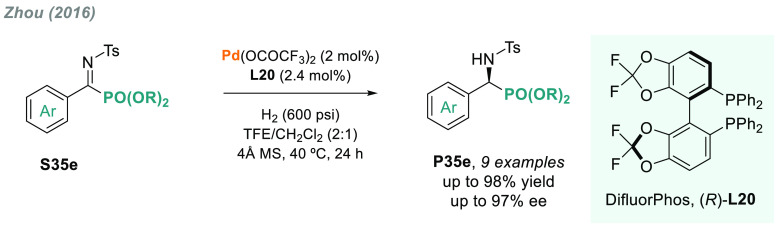
Other α-heteroatom N-sulfonyl imines have also been explored. Zhou and co-workers disclosed the palladium-catalyzed AH of a series of linear and cyclic α-iminophosphonates.175 The combination of Pd/(R)-DifluorPhos-L20 as catalyst provided an efficient route to obtain optically active α-amino phosphonates P35e with up to 97% ee (Scheme 27).
Scheme 27. Metal-Catalyzed AH of α-Substituted N-Sulfonyl Imines.
2.5.2. Cyclic N-Sulfonyl Imines
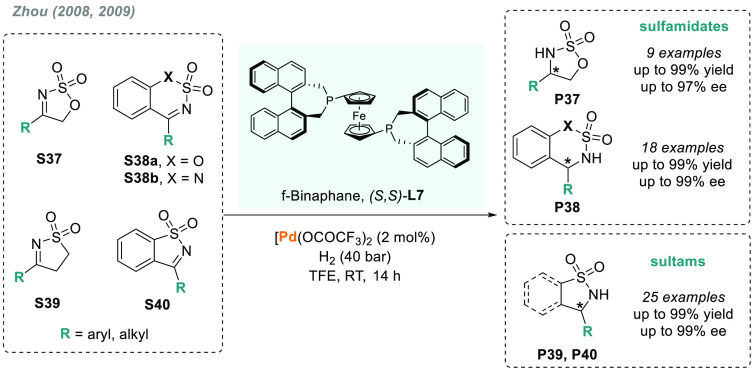
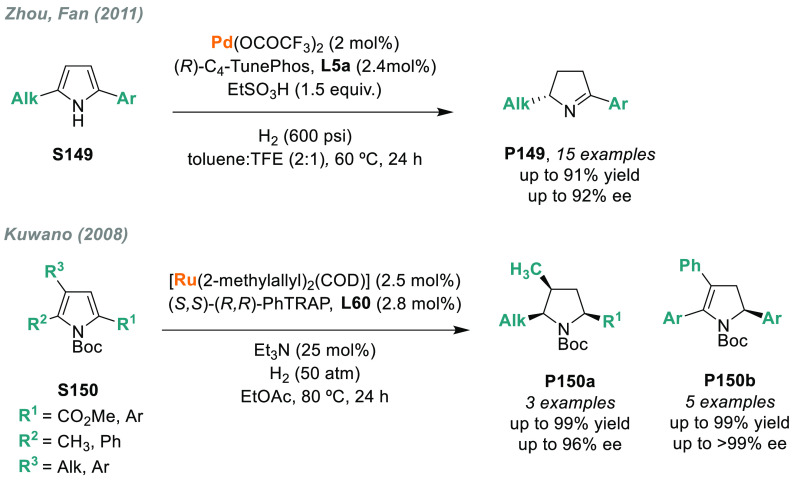
Sulfamidates (P37 and P38) and sultams (P39 and P40) are privileged building blocks in medicinal chemistry and useful chiral auxiliaries and ligands in asymmetric catalysis. They can be synthesized through the AH of the corresponding imines S37–S40 (Scheme 28). Zhou and co-workers reported an efficient AH of cyclic N-sulfonyl imines using Pd(CF3CO2)2/(S,S)-f-binaphane-L7 as catalyst, to afford the corresponding chiral amines in high enantioselectivity (up to 99% ee).176,177 The catalytic system was valid for both sulfamidates and sultams, and it was further extended to the AH of benzo-fused imines S38 and S40. Fan reported a previous version of this transformation using ruthenium catalysts, but with lower enantiomeric ratios.178
Scheme 28. Palladium-Catalyzed AH of Sulfamidites and Sultams Using (S,S)-f-Binaphane as a Chiral Ligand.
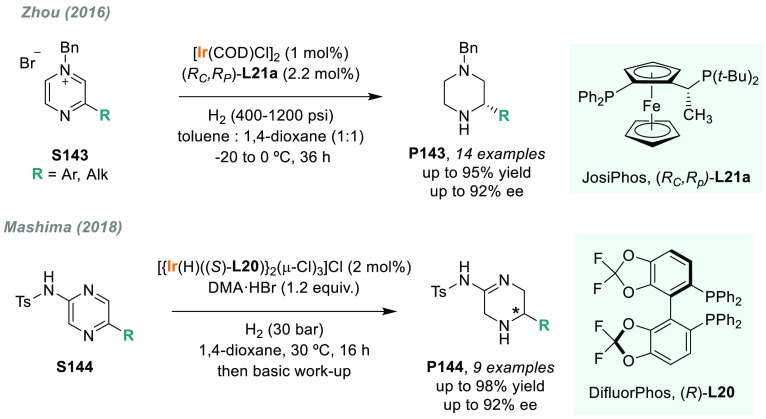
An example of the importance of chiral sulfamidates as drug building blocks is the Merck’s synthesis of MK-3207.179 The chirality of the benzylic stereocenter was introduced via the palladium-catalyzed AH of the cyclic sulfamidate imine S37a using either L7 or JosiPhos (L21a) as chiral ligands (Scheme 29).
Scheme 29. Synthesis of MK-3207 via Palladium-Catalyzed AH of Cyclic Sulfamidate Imine S37a.
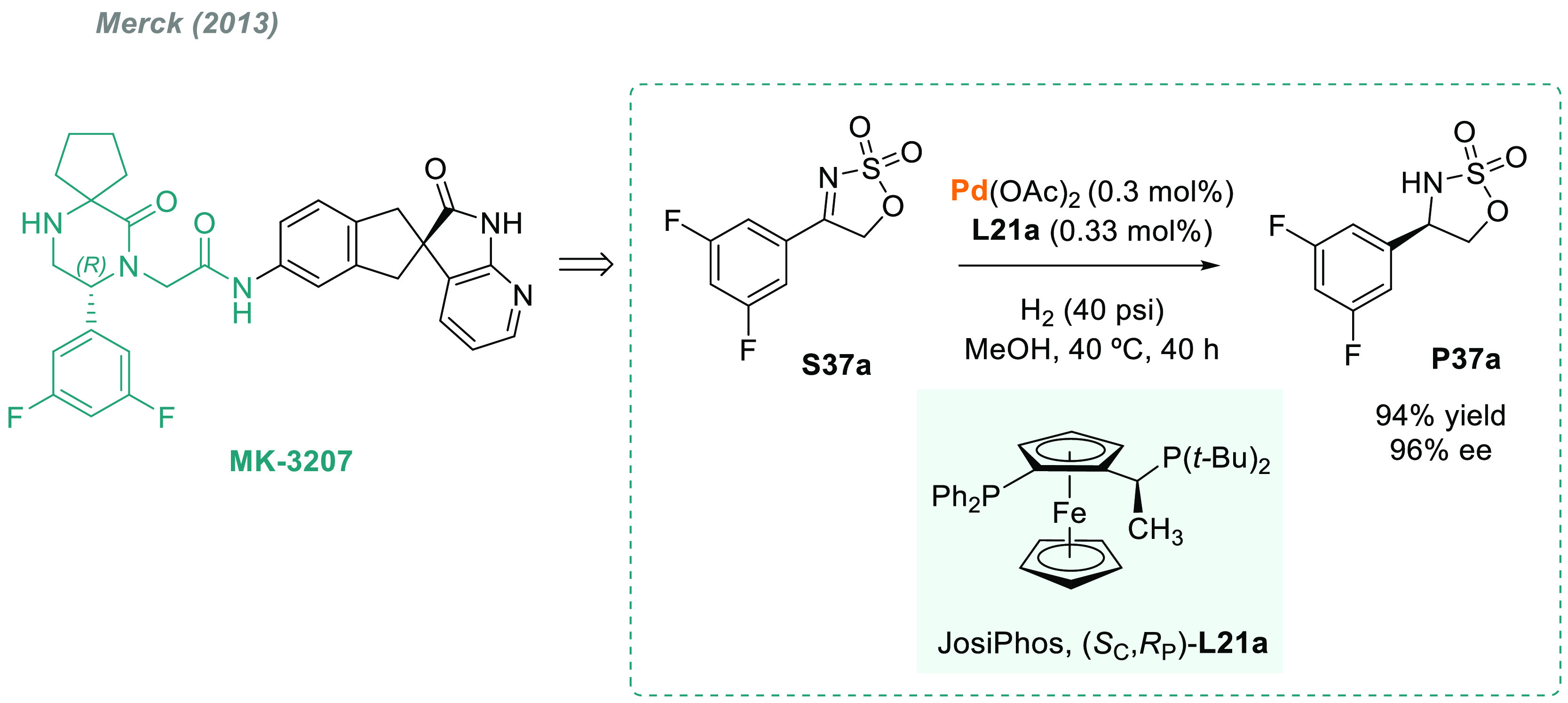
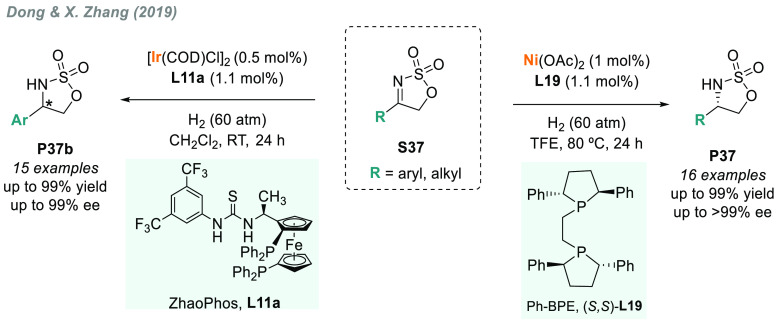
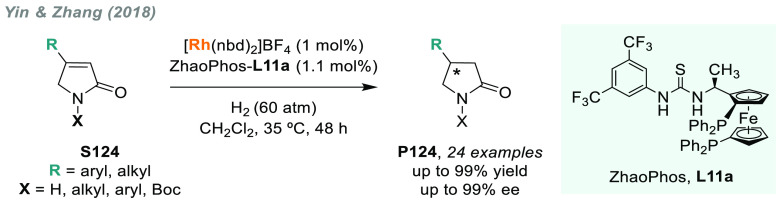
More recently, in 2019, Dong, X. Zhang, and co-workers performed the iridium-catalyzed AH of S37 using ZhaoPhos (L11a) to attain sulfamidates P37b with excellent activities and enantioselectivities (Scheme 30).180 ZhaoPhos is a family of chiral bifunctional diphosphine-thiourea ligands based on the synergistic cooperation between transition metal catalysis and organocatalysis.181 The substrate scope was limited to aryl or heteroaryl substituents. In contrast, the combination of Ni/L19, which gave excellent results for the AH of α,β-unsaturated ketoimines,172 is an alternative for the AH of S37 bearing both aryl and alkyl substituents.182 Several chiral cyclic sulfamidates P37 were prepared, even at gram scale, in high enantiomeric purity.
Scheme 30. Iridium- and Nickel-Catalyzed AH of Sulfamidate Imines S37.
The same catalytic system was used in the highly efficient AH of cyclic N-sulfonyl ketimino esters S38c, among other S38a-type substrates, which had not been disclosed before (Scheme 31).183 This transformation led to the facile synthesis of various chiral α-monosubstituted α-amino acid derivatives with excellent results.
Scheme 31. AH of Cyclic N-Sulfonyl Ketimino Esters S38 and Enesulfonamides S41.
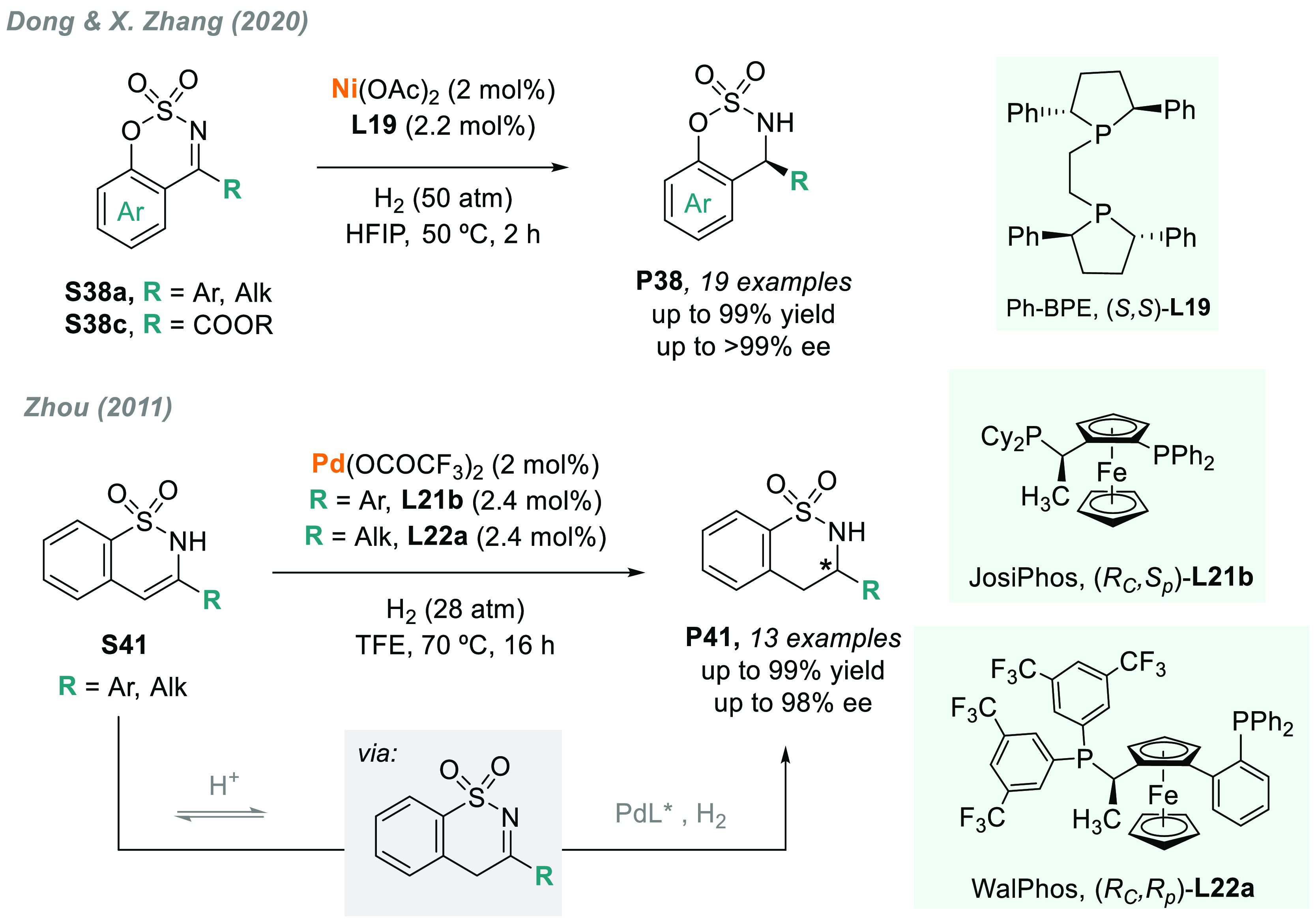
Another strategy for the synthesis of cyclic sultams is the AH of enesulfonamides S41. In 2011, Zhou’s group reported an innovative transformation, using a catalytic system based on Pd/JosiPhos-type ligands.184 JosiPhos-L21b and WalPhos-L22a, in particular, were excellent chiral ligands for enesulfonamides S41 bearing aryl and alkyl substituents, respectively (Scheme 31). Interestingly, labeling experiments confirmed that the hydrogenation was conducted via N-sulfonylimine intermediates. Later, in 2015, the same group reported the enantioselective synthesis of sultams by a palladium-catalyzed formal hydrogenolysis of racemic N-sulfonyloxaziridines with up to 99% ee.185
2.6. N-Phosphinyl Imines
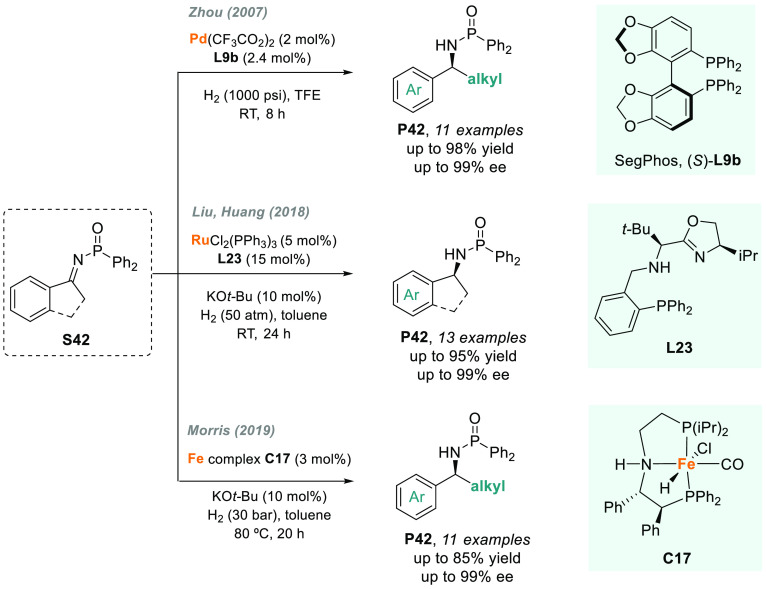
As described before, palladium complexes bearing diphosphine ligands are highly effective catalysts for the AH of N-sulfonyl imines in fluorinated solvents such as TFE (trifluoroethanol) or HFIP (hexafluoroisopropanol). Similarly, other activated imines, such as N-phosphinyl imines, are also suitable substrates for this catalytic system. After the pioneering work of Blaser,186 Zhou described the highly efficient palladium-catalyzed AH of activated imines, including N-diphenylphosphinyl ketimines.187 These ketimines (S42) were hydrogenated using L9b as a chiral ligand (Scheme 32), attaining excellent levels of enantioselectivity (up to 99% ee). The reaction showed a dramatic solvent effect, as only TFE led to high conversion toward P42.
Scheme 32. Metal-Catalyzed AH of N-Phosphinyl Imines.
Alternatively, Liu, Huang, and co-workers designed a phosphino-oxazoline ligand (L23) for the ruthenium-catalyzed AH of S42 (Scheme 32).188 The catalytic system exhibited good activity and excellent enantioselectivity, providing an efficient and mild approach to optically active secondary amines P42. Using iron as earth-abundant transition metal, Morris and co-workers reported that an unsymmetrical iron P-NH-P′ complex (C17, Scheme 32) gave excellent enantioselectivity for the AH of prochiral N-phosphinyl imines S42, but with poorer activity than the previous catalytic systems.189 The same group had previously foreseen that these iron-hydride catalytic species were highly active toward the AH of polar bonds.190 Nonetheless, the system failed when using dialkyl-substituted or exocyclic N-phosphinyl imines, which remains as a current challenge in the field.
2.7. N-Acyl Imines
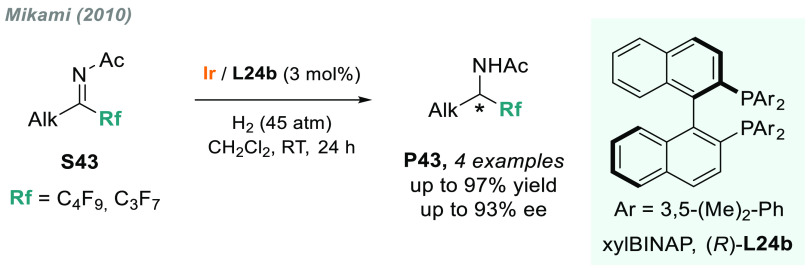
In 2010, Mikami and co-workers reported a catalytic AH of acyclic ketimines S43 bearing a perfluoroalkyl chain as substituent (Scheme 33).191 The introduction of fluorine into molecules enhances their lipophilicity, metabolic stability, and bioavailability, thus remarkably affecting the physicochemical properties.192−194 Using a Ir/L24b (a 3,5-dimethylphenyl analog of BINAP, L24a) as catalytic system, four examples of chiral perfluoroalkyl amines were obtained with excellent enantioselectivity. Moreover, this work established an important precedent in the field, as the direct AH of N-acyl imines is still rare.195
Scheme 33. Iridium-Catalyzed AH of N-Acyl Imines.
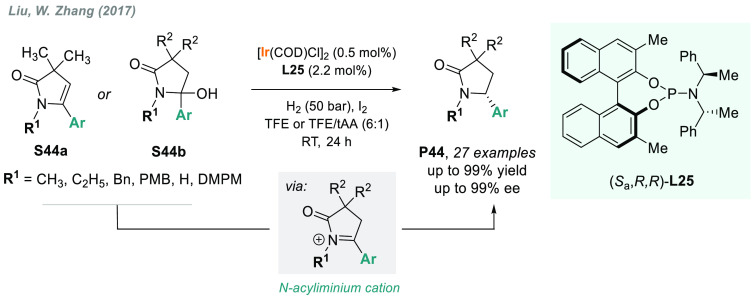
A novel strategy for the AH of β,γ-unsaturated γ-lactams S44a was described by Liu, W. Zhang, and co-workers using iridium catalysis in combination with a phosphoramidite ligand L25 and I2 (Scheme 34).196 The chiral γ-lactams P44 were obtained in excellent yields and enantioselectivities. Mechanistic studies detailed that the reduced products were obtained via the hydrogenation of N-acyliminium cations, rather than directly by the hydrogenation of S44a. Therefore, using the same catalytic system, these chiral γ-lactams were also prepared via in situ elimination/AH of racemic γ-hydroxy-γ-lactams S44b.197
Scheme 34. Synthesis of Chiral γ-Lactams via Iridium-Catalyzed AH of N-Acyliminium Cations.
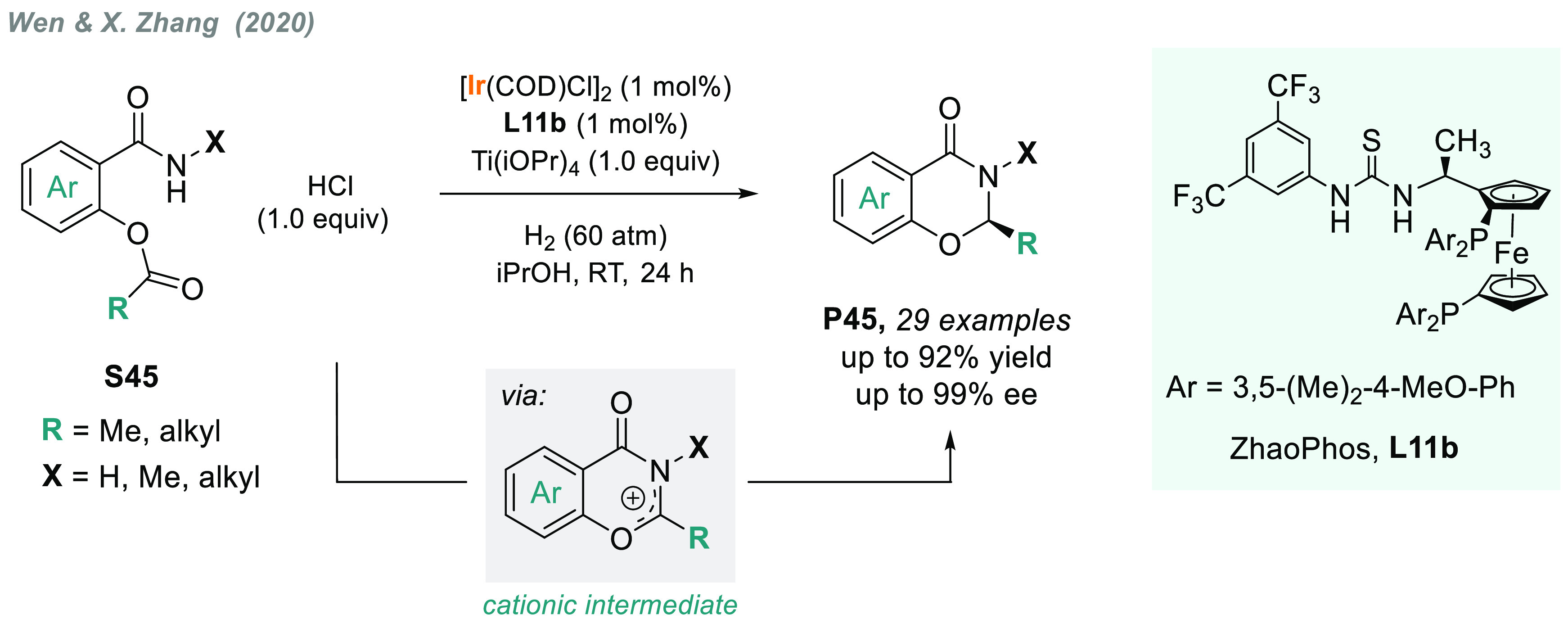
A related iridium-catalyzed AH of cationic species was recently reported by Wen, X. Zhang, and co-workers for the enantioselective synthesis of chiral N,O-acetals (Scheme 35).198 Under acidic conditions, O-acetylsalicylamides S45 underwent cyclization to generate cationic intermediates, which were subsequently hydrogenated by an iridium complex bearing a ZhaoPhos ligand (L11b), thus obtaining P45 in excellent yields and enantioselectivities.
Scheme 35. Synthesis of Chiral N,O-Acetals via Iridium-Catalyzed AH of Cationic Intermediates.
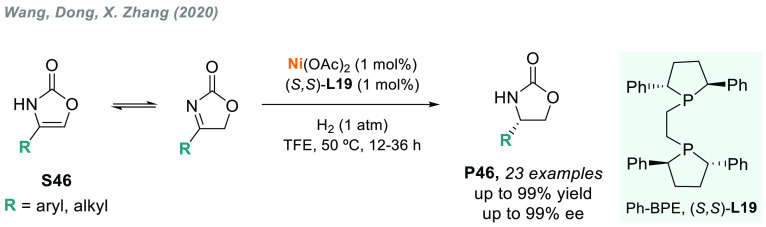
The same group recently reported the nickel-catalyzed AH of 2-oxazolones (S46) to afford 2-oxazolidinones in excellent yields and enantioselectivities (Scheme 36).199 Interestingly, deuterium labeling experiments and DFT calculations were conducted to reveal the catalytic mechanism for this hydrogenation, which indicated an equilibrium between the enamine and its imine isomer, with the latter being the substrate of choice for the asymmetric 1,2-addition of Ni(II)-H.
Scheme 36. Nickel-Catalyzed AH of 2-Oxazolones.
2.8. N-Heteroatom-Substituted Imines
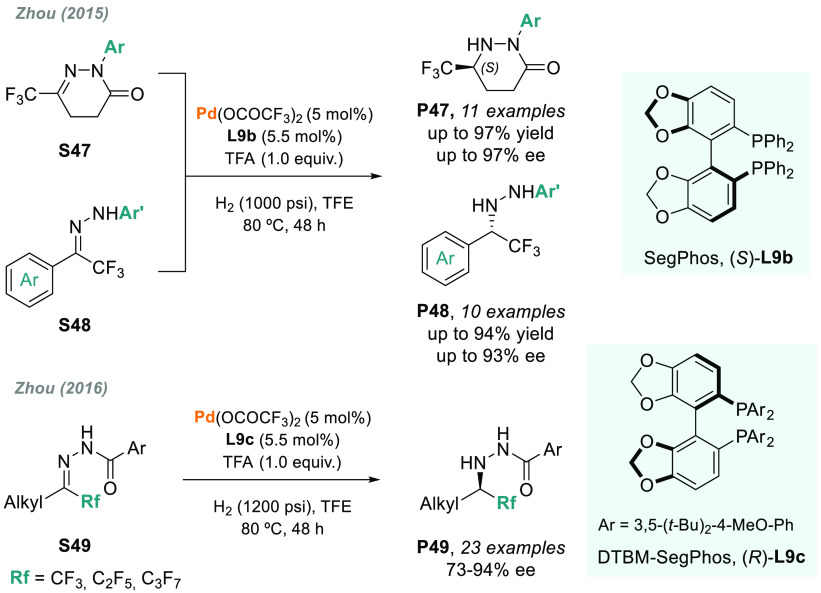
The hydrogenation of other N-heteroatom imines, such as hydrazones or oximes, remains a challenge. In 2015, Zhou and co-workers reported the enantioselective synthesis of cyclic and linear chiral trifluoromethyl-substituted hydrazines via the palladium-catalyzed AH of N-acyl and N-aryl hydrazones (S47 and S48, Scheme 37).200 Currently, many compounds bearing a hydrazine moiety, such as atazanavir or azacastanospermine, show pharmacological activity. By using Pd/(S)-SegPhos L9b as a catalyst and TFA as an essential additive, chiral hydrazines P47 and P48 were obtained in excellent yields and up to 97% ee. A year later, the same authors reported that, by using the bulkier DTBM-SegPhos (L9c) as a chiral ligand, the palladium-catalyzed AH of α-alkyl hydrazones S49 proceeded smoothly, thus affording the corresponding fluorinated hydrazines P49 in excellent enantioselectivities (Scheme 37).201
Scheme 37. Palladium-Catalyzed AH of α-Aryl Hydrazones and α-Alkyl Hydrazones.
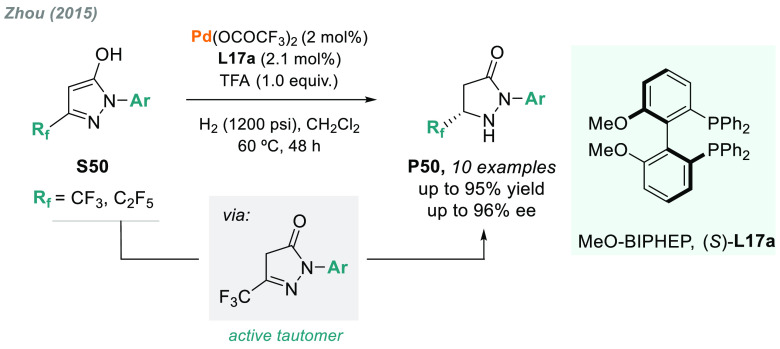
In addition, the same laboratory reported the palladium-catalyzed AH of fluorinated aromatic pyrazol-5-ols S50 (Scheme 38).202 The key for the success of this transformation is the Brønsted acid-promoted tautomerization, thus capturing the active form, followed by enantioselective hydrogenation. A wide variety of substituted pyrazolidinones P50 were synthesized with up to 95–96% ee using (S)-MeO-Biphep (L17a) as the chiral ligand.
Scheme 38. Palladium-Catalyzed AH of Fluorinated Aromatic Pyrazol-5-ols via the CH-Tautomer.
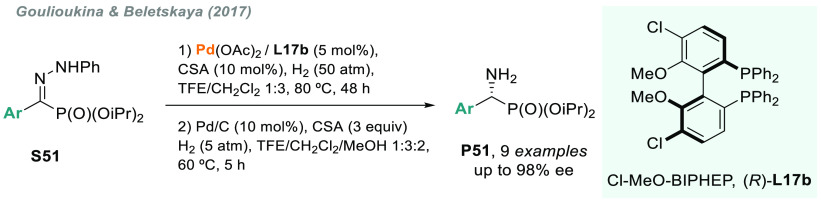
In 2017, Beletskaya and co-workers reported a convenient one-pot procedure for the asymmetric synthesis of α-amino phosphonates, which are also important structural motifs in many bioactive compounds. Using a combination of Pd and biaryl chiral ligand L17b, the AH of α-hydrazono phosphonates S51 proceeded with high enantiocontrol.203 Subsequent cleavage of the N–N bond after the addition of Pd/C and methanol into the crude reaction mixture afforded the optically active P51 (Scheme 39).
Scheme 39. Sequential Palladium-Catalyzed AH/Hydrogenolysis of α-Hydrazono Phosphonates.
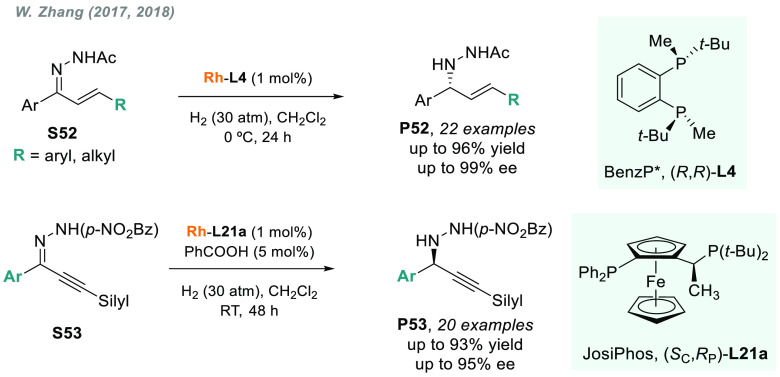
The laboratory of W. Zhang developed highly efficient protocols for the chemo- and enantioselective hydrogenation of allyl and alkynyl hydrazones using rhodium catalysts.204,205 When using BenzP* (L4) or JosiPhos (L21a) as a chiral ligand, allyl or alkynyl-aryl hydrazones (S52–S53) were hydrogenated with excellent results (Scheme 40).
Scheme 40. Rhodium-Catalyzed AH of Allyl and Alkynyl-aryl Hydrazones.
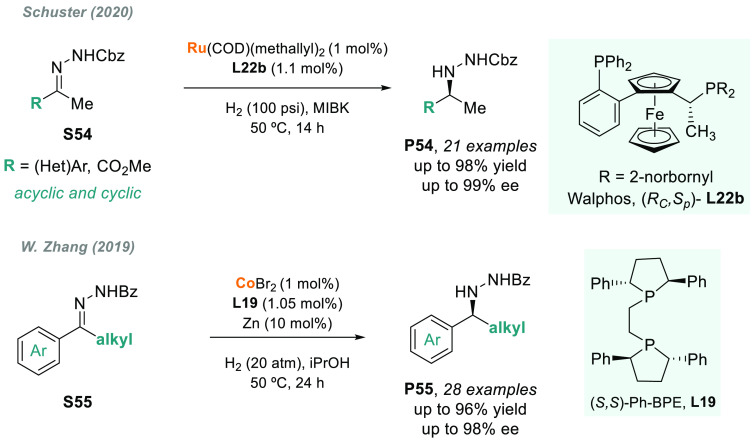
Alternatively, Schuster and co-workers described the ruthenium-catalyzed AH of hydrazones S54 using a Walphos-type ligand L22b (Scheme 41).206 The method allowed access to versatile chiral hydrazine building blocks P54 containing aryl, heteroaryl, cycloalkyl, and ester substituents, and the protocol was demonstrated on >150 g scale. The use of Rh complexes in the AH of hydrazones had been described early this decade, but with lower ee values.207,208
Scheme 41. AH of Hydrazones with Ruthenium and Cobalt Complexes.
The use of chiral Ir complexes in the AH of hydrazones was described by X. Zhang, using f-binaphane as the chiral ligand. Of note, they reported a direct catalytic asymmetric reductive amination of simple aromatic ketones with phenylhydrazide, thus offering an attractive route for the synthesis of chiral hydrazine-derived compounds.209
In 2019, W. Zhang and co-workers disclosed, for the first time, the efficient cobalt-catalyzed AH of C=N bonds.210 Although the use of cobalt as an earth-abundant transition metal in AHs was first pioneered by Chirik, the scope was limited to C=C or C=O bonds.211−213 Interestingly, the success of this reaction relies on the presence of an NHBz group (S55, Scheme 41), which acts as a directing group. The reactivity and enantioselectivity were further enhanced by assisted coordination to the cobalt atom and π–π nonbonding interactions between the phenyl groups on the substrates and the chiral diphosphine (S,S)-Ph-BPE L19. The resulting chiral nitrogen-containing compounds P55 were attained in high yields and excellent enantioselectivities (95–98% ee).
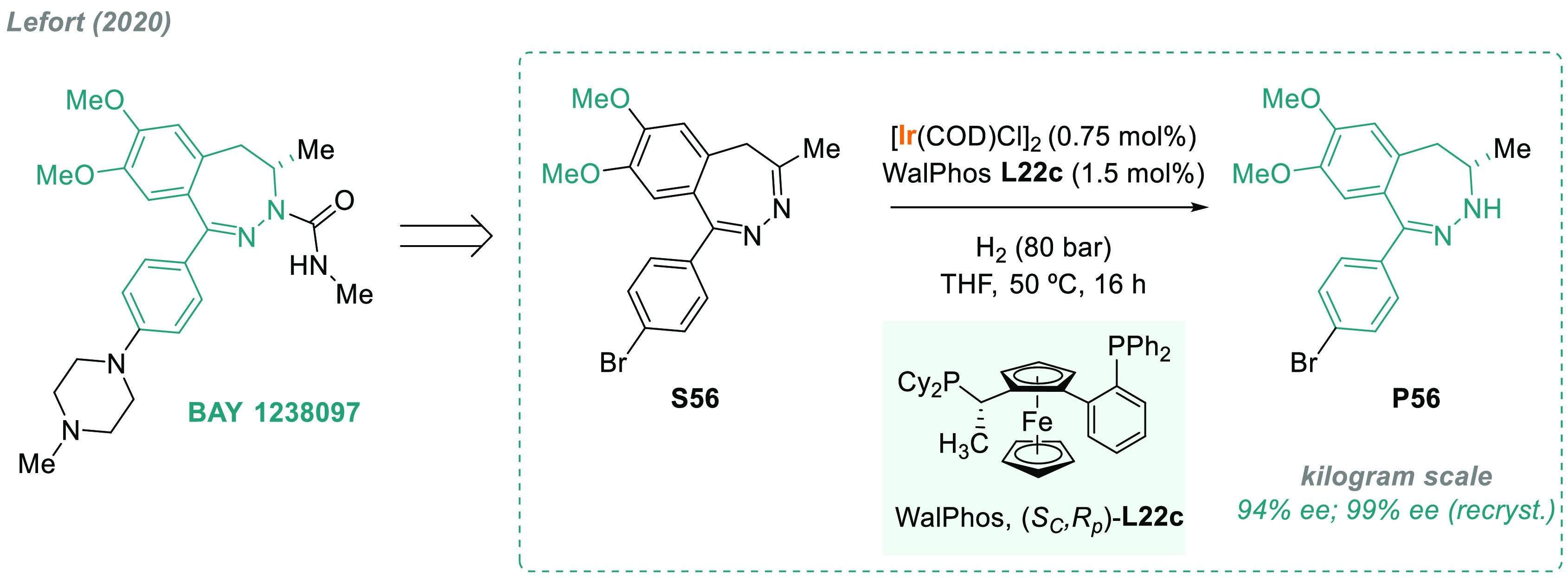
In 2020, Lefort and co-workers reported the first example of a regio- and enantioselective AH of a C=N–N=C motif.214 As shown in Scheme 42, the prochiral benzodiazepine S56 was efficiently hydrogenated using a chiral catalyst based on Ir and a Walphos bisphosphine L22c. No undesired hydrogenation of the C=N double bond in the 1,2-position was observed. Using the optimal conditions, the AH was performed on a kilogram scale leading to the production of P56, an intermediate of BET inhibitor BAY 1238097, in enantiopure form after crystallization.
Scheme 42. Enantioselective Synthesis of an Intermediate of BET Inhibitor BAY 1238097 via Iridium-Catalyzed AH.
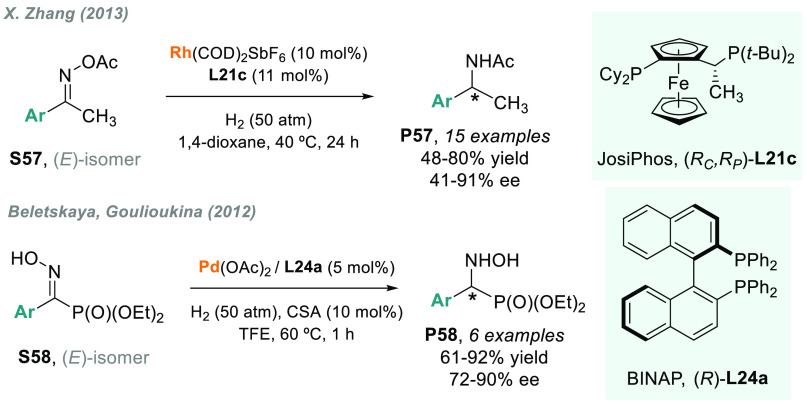
The AH of oximes and their derivatives remained a long-standing problem. To solve this gap in the field, X. Zhang and co-workers proposed the rhodium-catalyzed AH of oxime acetates S57 (Scheme 43).215 Unexpectedly, the reaction led to the formation of chiral acetamide P57 as the major product, thus affording a new strategy for the straightforward synthesis of chiral acetamides from oxime derivatives. After an exhaustive screening of phosphine ligands, JosiPhos L21c was found to give the highest enantioselectivity (up to 91% ee). The main limitations of this approach are the moderate activity, as well as the low enantiocontrol, in the case of ortho-substituted groups on the aromatic ring.
Scheme 43. Metal-Catalyzed AH of Ketoximes.
The AH of N-hydroxy-α-imino phosphonates S58 was studied by Goulioukina et al.216 using the Pd/BINAP (L24a) as catalytic system, first reported by Amii and co-workers.217 The synthesis of chiral P58 was achieved in up to 90% ee (Scheme 43). The catalytic reaction was performed using a Brønsted acid (CSA) as an activator and TFE as solvent. However, the scope was limited to phenyl and para-substituted aromatic rings.
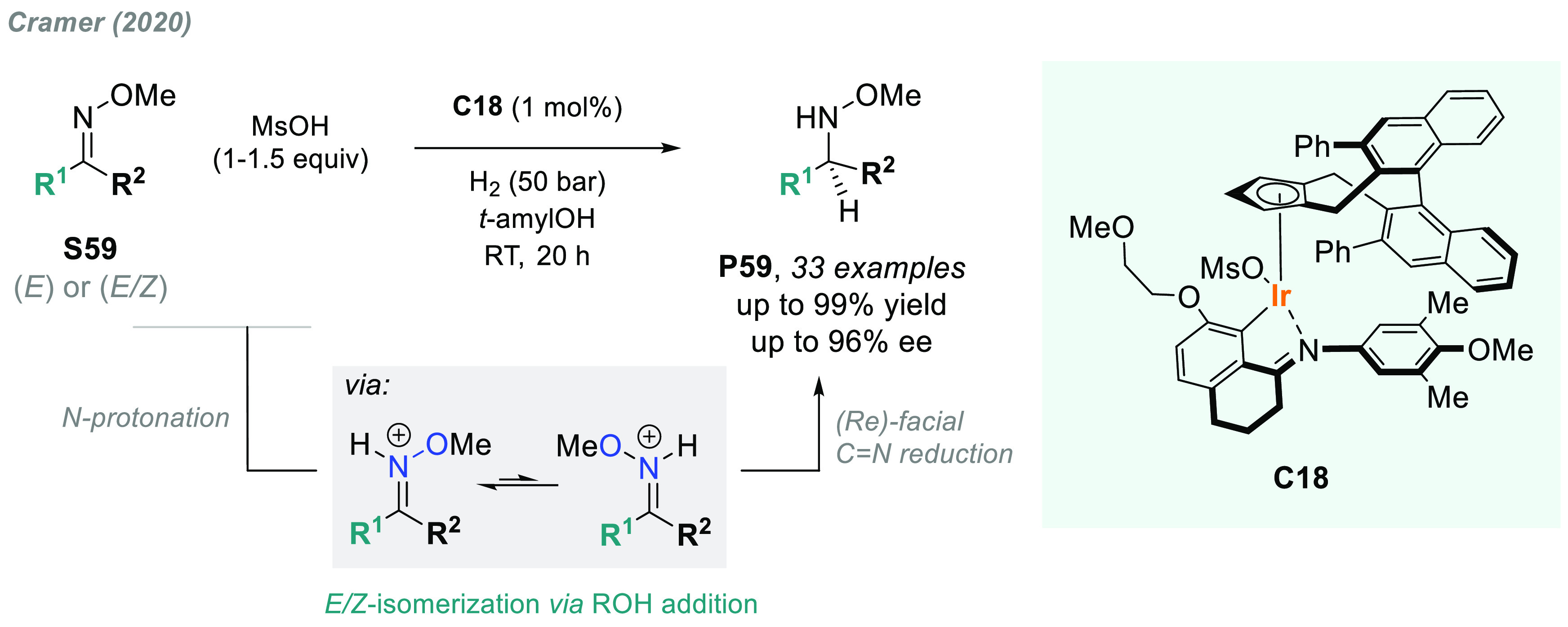
The selective reduction of an oxime to the corresponding chiral hydroxylamine derivative remains a challenge in this field because of undesired cleavage of the weak N–O bond. In this regard, in 2020, Cramer and co-workers described a methodology to overcome this limitation. He reported a robust cyclometalated Ir(III) complex C18 bearing a chiral cyclopentadienyl ligand as an efficient catalyst for this transformation (Scheme 44).218 Using MsOH as activator, this acid-assisted AH of oximes S59 avoids overreduction of the N–O bond via C=N reduction after substrate protonation, thus accessing valuable chiral N-alkoxy amines P59 in excellent yields and enantioselectivities.
Scheme 44. Iridium-Catalyzed Acid-Assisted AH of Oximes to Hydroxylamines.
2.9. Unprotected Imines
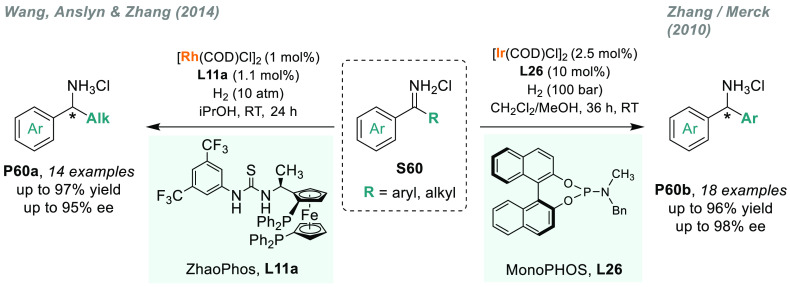
The transition metal-catalyzed AH of N-unprotected imines64 has been widely pursued. X. Zhang’s laboratory, in collaboration with Merck, developed the first efficient and atom-economic iridium-catalyzed AH of unprotected ketimines using HCl as Brønsted acid to activate the substrate.219 Ketimine hydrochlorides S60 were efficiently hydrogenated using Ir/(S,S)-f-Binaphane L7, although in high catalyst loading (5 mol %). Later, in 2014, Wang, Anslyn, X. Zhang, and co-workers improved this transformation in terms of TON using Rh/ZhaoPhos (L11a) as catalyst. Taking advantage of the anion binding interaction between the thiourea and chloride counterion, chiral amines P60a were afforded in high yields and enantioselectivities (Scheme 45).220 The iridium-catalyzed AH of substituted benzophenone imines S60 was also efficiently conducted in X. Zhang’s group.221 Enantioenriched diarylmethylamines P60b were obtained using a monodentate phosphoramidite L26 and rather harsh reaction conditions (100 atm H2, Scheme 45). Substitution at the 2-position on the aryl group in S60 is essential to achieve good enantiocontrol.
Scheme 45. Metal-Catalyzed AH of N-Unprotected Imines.
It is worth mentioning that direct asymmetric reductive amination (ARA) has become an important branch of asymmetric hydrogenation. However, as stated in the Introduction, this topic is not covered here since it has been comprehensively reviewed very recently.10−12
3. Asymmetric Hydrogenation of Enamides
In 1972, Kagan, Dang, and co-workers reported the first example of the AH of N-protected enamines, using the chiral ligand DIOP.53 Although the enantioselectivity was only moderate, this work opened a door toward the enantioselective synthesis of chiral amines. Knowles,222 Noyori,223 and Burk224 strongly contributed to the field by introducing DIPAMP, BINAP, and DuPhos ligands, respectively. Since then, many highly efficient Rh catalysts bearing chiral diphosphine ligands have been developed. In addition, at the beginning of the century, Reetz, Feringa, and Zhou’s groups independently demonstrated that Rh complexes bearing monodentate phosphorus chiral ligands were also highly efficient catalysts.225−227 The direct catalytic AH of enamides is, arguably, the method of choice for the synthesis of amino acids and chiral amines bearing a stereogenic center in the α or β position to the nitrogen atom.
3.1. Acyclic N-Acyl Enamines
3.1.1. Chiral Rh Catalysts
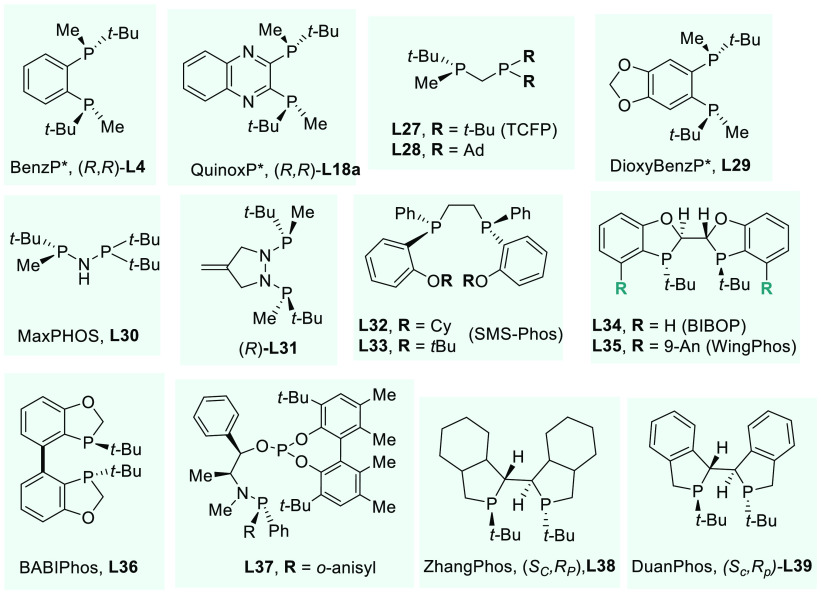
The presence of a coordinating group adjacent to the C=C makes N-acyl enamines ideal substrates for rhodium-catalyzed AH, which very often induces very high enantioselectivity.228 In contrast to imines, the use of iridium complexes in the AH of acyclic N-acyl enamines is uncommon.229 Nevertheless, the chiral ligand makes a critical contribution to the achievement of high activity and selectivity. Consequently, the development of more efficient ligands for a range of catalytic processes is still a vital research topic. During the past decade, new families of chiral phosphines,230 including monodentate phosphines,231−234 bis(aminophosphine)-type ligands,235−237 phosphino-phosphite (P-OP),238−244 phosphino-phosphoramidite,245−252 spiroketal253,254 or supramolecular-type255−259 biphosphines, and others,260,261 have found widespread use in the rhodium-catalyzed AH of N-acyl enamines.262 Among these, the past decade has witnessed the development of P-stereogenic electron-rich alkyl phosphines as highly proficient ligands.44,45,263−265Figure 5 shows the most relevant P-stereogenic ligands used in the rhodium-catalyzed AH of benchmark enamides (Table 1). These chiral ligands have stood out from others in the AH of standard N-acylenamines such as methyl α-acetamidoacrylate (MAA, S61), (Z)-methyl a-acetamido-3-phenyl acrylate (Z-MAC, S62), β-dehydroamino acids (S63),266 and N-(1-phenylvinyl)acetamide (PVA, S64).
Figure 5.
P-Stereogenic chiral ligands used in the metal-catalyzed AH of N-acyl enamines.
Table 1. Enantiomeric Excesses (%) in the Rhodium-Catalyzed AH of Benchmark N-Acyl Enamines Using the P-Stereogenic Ligands Shown in Figure 5.
| S61 | S62 | (E)- S63 | (Z)-S63 | S64 | S65 | |
|---|---|---|---|---|---|---|
| L27 | >99 | >99 | 99 | 96 | 98 | |
| L28 | >99 | >99 | >99 | 96 | 97 | 97 |
| L18a | >99 | >99 | >99 | 99 | >99 | 97 |
| L4 | >99 | >99 | >99 | 98 | 93 | 99 |
| L29 | >99 | >99 | >99 | 99 | 90 | 99 |
| L30 | 99 | 99 | 99 | 96 | 97 | 92 |
| L31 | 98 | 99 | 97 | |||
| L32 | >99 | >99 | 98 | 88 | >99 | |
| L33 | >99 | >99 | 97 | 80 | >99 | |
| L34 | >99 | 97 | 99 | 99 | 99 | |
| L36 | >99 | 90 | 96 | |||
| L37 | 99 | >99 | 96 | |||
| L38 | >99 | >99 | >99 | 97 | >99 | 96 |
| L39 | >99 | >99 | >99 | 97 | >99 | 96 |
In 2004, Hoge and co-workers established an important breakthrough in the field by preparing a C1-diphosphine with three-hindered quadrants (trichickenfootphos—TCFP, L27).267−269 This ligand showed very high enantioinduction for a wide variety of N-acyl enamines (S61–S64, Table 1). However, TCFP is difficult to handle in air, which explains why it has received little attention in asymmetric catalysis. To overcome this limitation, Imamoto recently prepared a crystalline, air-stable analog of TCFP by replacing the tert-butyl groups in the nonstereogenic phosphorus atom for the bulkier 1-adamantyl (L28).270 Its catalytic activity on the rhodium-catalyzed AH of enamides afforded excellent enantioselectivities for the substrates tested, including β-keto enamides (S65).271 In 2010, Riera and Verdaguer’s laboratory reported the first synthesis of optically pure, borane-protected primary and secondary aminophosphines.272 These compounds were found to be valuable P-stereogenic building blocks for the preparation of new chiral aminodiphosphine ligands. The synthesis and catalytic evaluation of small-bite angle MaxPHOS ligand (L30) was first described.273 Indeed, MaxPHOS is a nitrogen-containing analog of TCFP (L27). However, and in contrast to L27, the presence of an -NH- bridge between the two phosphine moieties allows the NH/PH tautomerism to take place. The protonation of MaxPHOS led to the stable PH form of the ligand, which turned into air-stable compounds both in the solid state and in solution. The complex Rh/L30 proved to be a highly enantioselective and robust system for the AH of a wide range of N-acyl enamines (Table 1). Later, a new class of P-stereogenic C2-symmetric ligands with a hydrazine backbone was also disclosed by Riera and Verdaguer.274L31, in particular, showed excellent catalytic performance in the rhodium-catalyzed AH of several benchmark substrates (Table 1).
C2-symmetric P-stereogenic ligands have been widely used in AH. Stephan’s laboratory performed the rhodium-catalyzed AH of a wide spectrum of representative enamides using L32 and L33 (SMS-Phos) as chiral ligands.275,276 Both catalytic systems showed excellent enantioselectivities (>99% ee for several model substrates; Table 1). The catalytic activity of the ligand was markedly affected by the nature of its aryl substituents in terms of both bulkiness and electronic properties. Of note, t-Bu-SMS-Phos L33 outperformed other reported ligands (Table 1), although the enantioselectivity dropped considerably when using tetrasubstituted vinyl acetamides.277
In 2010, Tang designed and synthesized a novel family of chiral bisdihydrobenzooxaphosphole ligands (BIBOP, L34).278,279 Their ease of preparation and excellent air stability make BIBOP a practical ligand. Moreover, it can also be highly modular by fine-tuning the substituents at the 4,4′-positions. The rhodium-catalyzed AH of various N-acyl enamines using BIBOP ligands was exploited, including in kilogram scale.280 When using Rh/L34 in the AH of benchmark substrates, the corresponding chiral amines were attained in excellent enantioselectivities (Table 1). The same group later developed a similar ligand named WingPhos (L35), and the introduction of 9-anthracenyl substituents conferred a deeper chiral pocket.281 Other ligands were efficiently applied to the rhodium-catalyzed AH of (E)-β-aryl enamides, which is a class of substrates that remained underdeveloped.282,283 More recently, a novel class of benzooxaphosphole ligands (BABIPhos, L36) has been reported.284 The high catalytic performance of these ligands was showcased in rhodium-catalyzed AH, although for S63 the enantioselectivity achieved was lower than with BIBOP.
The use of P-stereogenic N-phosphine-phosphinite ligands is still rare. Recently, Dieguez’s laboratory developed a family of these ligands (L37) that has been applied in rhodium-catalyzed AH.285 By choosing the appropriate ligand for each substrate family, benchmark enamides were hydrogenated, giving excellent results (Table 1).
Between 1998 and 1999, Imamoto pioneered the use of the tert-butylmethylphosphine synthon in C2 chiral diphosphines with the development of BisP* and MiniPHOS.286−288 Afterward, he improved the ligand design by introducing this P-stereogenic synthon into many other ligands such as QuinoxP* (L18a), BenzP* (L4), and DioxyBenzP* (L29).289−291 These conformationally rigid ligands are crystalline solids and, once coordinated to Rh, exhibited excellent enantioselectivities in the AH of a broad range of enamides and other functionalized alkenes (Table 1). L18a showed unbeatable enantioselectivities when acetamido acrylates and vinyl acetamides were used but gave poor conversion for the AH of β-keto enamide S65. In contrast, L4 and L29 gave the best results reported to date with S65.
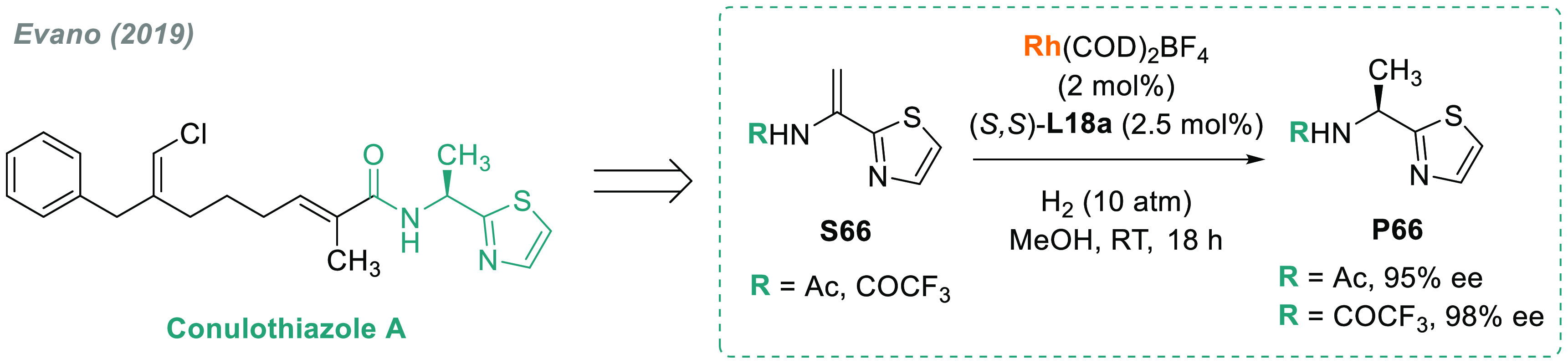
As an example of a synthetic application, Evano’s group recently developed a short and modular total synthesis of Conulothiazole A in 7 steps and 30% overall yield.292 One of the key steps was an efficient rhodium-catalyzed AH of a 2-enamido-thiazole S66 (Scheme 46) using (S,S)-QuinoxP* L18a. The catalytic system was extended to a variety of 2-enamido-heteroarenes with excellent results (up to 99% ee), thus providing efficient access to 2-aminoethyl-arenes, which are useful building blocks in medicinal chemistry. Of note, the rhodium-catalyzed AH of acetamidoacrylates or vinylacetamides has been widely used as a powerful tool in total synthesis of natural products293,294 and for the preparation of drugs and pharmacologically active compounds.295−302
Scheme 46. Total Synthesis of Conulothiazole A via Rhodium-Catalyzed AH.
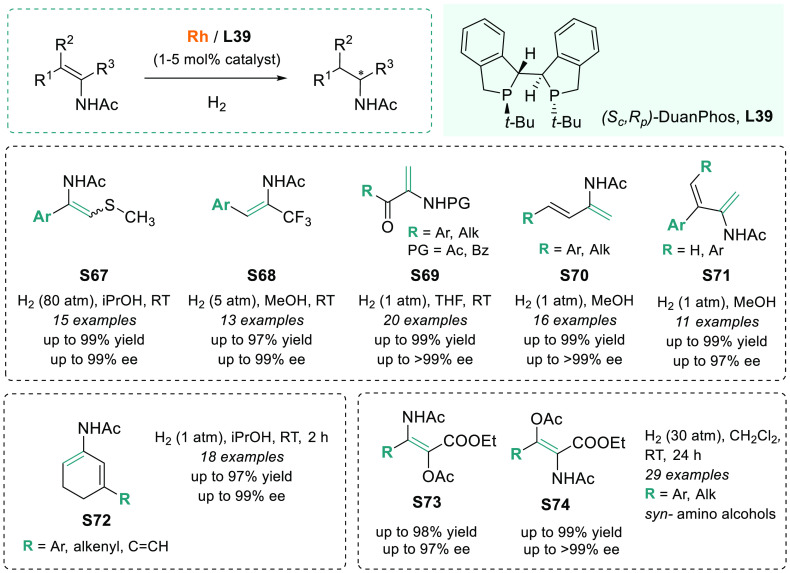
At the beginning of the decade, X. Zhang and co-workers reported the preparation of an electron-donating P-stereogenic biphospholane ligand (ZhangPhos, L38) for the rhodium-catalyzed AH.303,304 The group had also previously reported other P-stereogenic ligands with C2-symmetry, such as TangPhos (L2)305,306 or DuanPhos (L39),307,308 among others.309 Compared to those, ZhangPhos is conformationally more rigid, and it achieved better or similar enantioselectivities (up to 99% ee, Table 1). Moreover, L38 exhibited extremely high reactivity (up to 50 000 TON) in the rhodium-catalyzed AH of a wide range of N-acyl enamines and had the advantage that both enantiomers can be prepared by asymmetric synthesis.
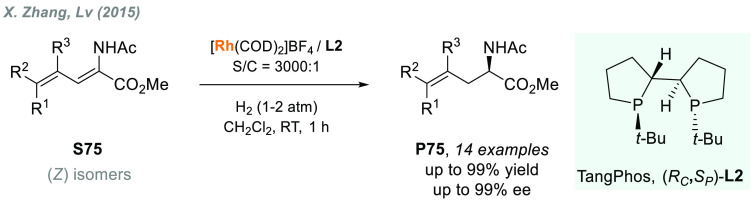
Nevertheless, Rh-DuanPhos is a highly versatile catalytic system that was used in many other functionalized substrates. Wiest, Dong, and co-workers recently applied this chiral catalyst in the cascade hydrogenation of cyclic dehydropeptides controlled by catalyst–substrate recognition.310 Previously, X. Zhang, Lv, and co-workers used this catalyst for the efficient AH of β-acetylamino vinylsulfides S67,311 α-CF3-enamides S68,312 α-dehydroamino ketones S69,313,314 aliphatic dienamides S70(315) and S71,316 and cyclic dienamides S72 (Scheme 47).317 The resulting chiral amines were afforded in excellent yields and enantioselectivities. Furthermore, other challenging functionalized substrates, such as tetrasubstituted enamides, were hydrogenated in a highly enantioselective manner. In particular, the AH of α-acetoxy β-enamido esters S73(318) and β-acetoxy α-enamido esters S74(319) for the preparation of syn amino alcohols was conducted using Rh/DuanPhos catalyst, achieving excellent results (Scheme 47). In 2015, the same group also reported the highly regio- and enantioselective synthesis of γ,δ-unsaturated amido esters P75 by AH of conjugated enamides using Rh/TangPhos-L2 (Scheme 48).320
Scheme 47. Scope of Substrates for Rhodium-Catalyzed AH Using DuanPhos.
Scheme 48. Rhodium-Catalyzed AH of Conjugated Enamides.
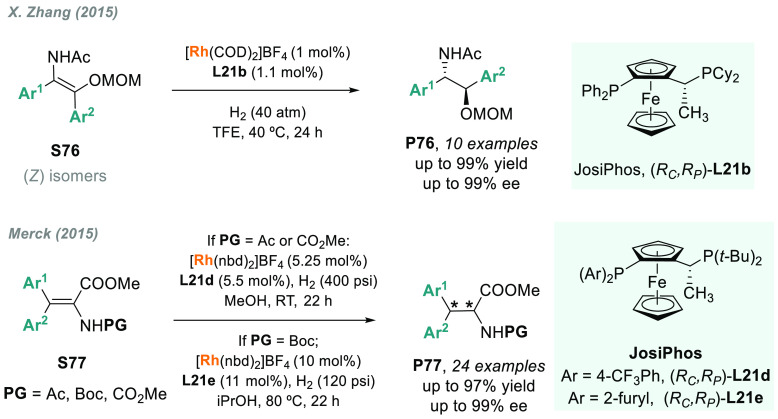
In addition, the AH of tetrasubstituted enamides in Z form was also accomplished by the same laboratory (Scheme 49).321 However, in this case, Rh-DuanPhos-L39 gave poor conversion for S76. In contrast, JosiPhos ligand L21b afforded a set of anti β-amino alcohol derivatives P76 in excellent yields and enantioselectivities. Simultaneously, scientists at Merck reported a concise, enantio- and diastereoselective route to novel nonsymmetrically substituted N-protected β,β-diaryl-α-amino acids and esters through the AH of tetrasubstituted enamides S77 (Scheme 49).322 Again, JosiPhos ligands (L21d and L21e) allowed complete stereocontrol over the two vicinal stereogenic centers. Remarkably, an example of S77 was previously hydrogenated by Ramsden and co-workers for the asymmetric synthesis of an intermediate of denagliptin.323 The rhodium-catalyzed AH of other tetrasubstituted enamides has also been investigated. A noteworthy example was the asymmetric synthesis of the cannabinoid-1 receptor inverse agonist taranabant, reported by a Merck team in 2009.324
Scheme 49. Rhodium-Catalyzed AH of (Z)- Tetrasubstituted Enamides.
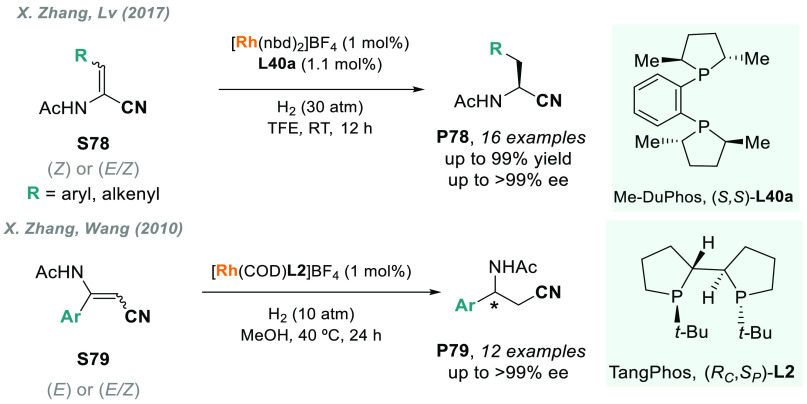
Another important transformation in this section is the rhodium-catalyzed AH of α-amino acrylonitriles S78, as it provides a concise route to the synthesis of chiral α-acylamino nitriles P78 (Scheme 50). These compounds are versatile synthetic intermediates, and they can be direct precursors of valuable α-amino acids. X. Zhang and co-workers recently reported that Rh-Me-DuPhos (L40a) is an efficient catalyst for this transformation, thus furnishing P78 in excellent yields and enantioselectivities.325 Previously, the same group described the highly enantioselective rhodium-catalyzed AH of β-acylamino acrylonitriles S79 using TangPhos (L2) or QuinoxP* (L18a) as chiral ligands (Scheme 50).326,327 Interestingly, in both cases, the hydrogenation of an E/Z mixture gave excellent enantioselectivities, thus making it unnecessary to isolate the substrate’s isomers.
Scheme 50. Rhodium-Catalyzed AH of α- and β-Amino Acrylonitriles.
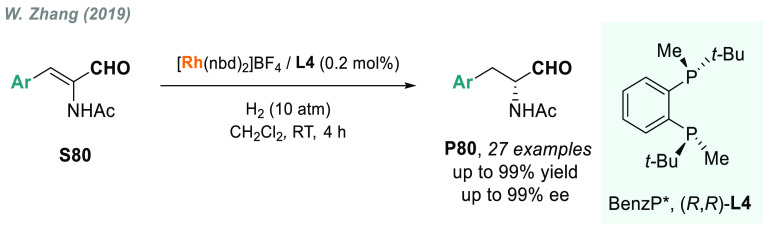
In 2019, W. Zhang and co-workers described a powerful strategy for the preparation of enantioenriched chiral α-amido aldehydes, which have many potential applications in organic synthesis and medicinal chemistry. Using a rhodium complex of a P-stereogenic biphosphine ligand ((R,R)-BenzP*, L4), α-formyl enamides S80 were hydrogenated in a highly chemo- and enantioselective manner (up to >99.9% ee, Scheme 51).328 Under different hydrogen pressures, the preparation of highly enantioenriched β-amido alcohols is also plausible. The method can be carried out on a gram scale, thus demonstrating its high efficiency and practicability.
Scheme 51. Rhodium-Catalyzed AH of α-Formyl Enamides.
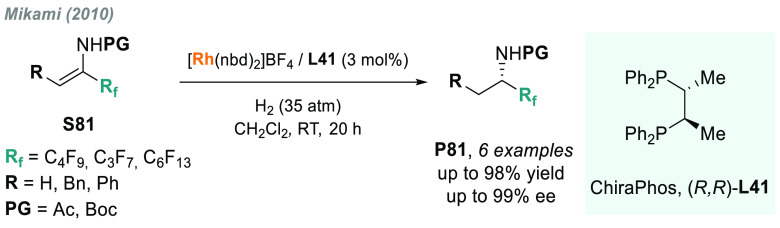
Although the AH of enamido esters, vinyl acetamides, or related compounds has received the most attention in the field, the AH of other α-and β-functionalized enamides constitutes a privileged methodology in the design of new pharmaceuticals and agrochemicals. In 2010, Mikami and co-workers described the enantioselective synthesis of α-(perfluoroalkyl)amines via the rhodium-catalyzed AH of enamides S81, which can be prepared by perfluoroalkylation of nitriles with Ti/Mg-reagents.329 By using ChiraPhos L41, acyclic perfluoroalkyl sec-amines were furnished with excellent enantioselectivities (Scheme 52). Also in 2010, Benhaim et al. reported the first enantioselective synthesis of β-trifluoromethyl α-amino acids using rhodium-catalyzed AH with TCFP (L27).330
Scheme 52. Enantioselective Synthesis of α-Perfluoroalkylated Chiral Amines.
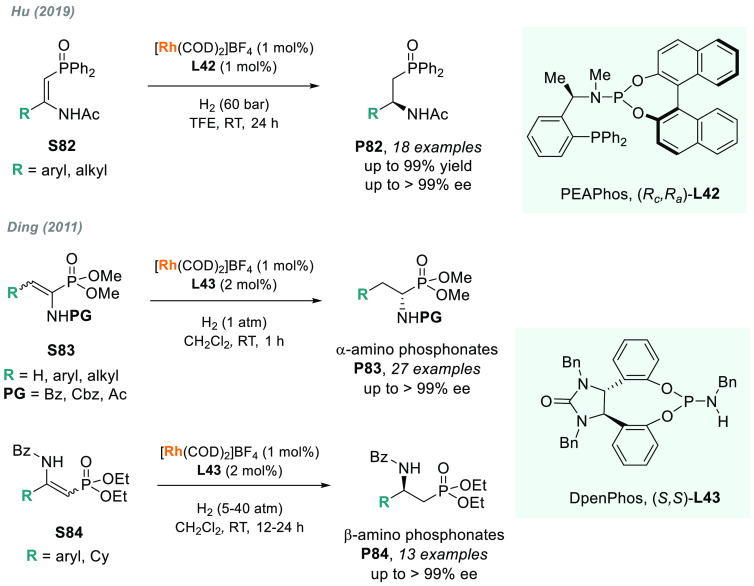
Another type of well-established chiral scaffold is β-amino phosphine derivatives. Hu and co-workers recently reported an unprecedented, catalytic AH of β-phosphorylated enamides S82 (Scheme 53).331 The method used rhodium catalysis derived from an unsymmetrical hybrid chiral phosphine-phosphoramidite ligand (L42). A wide range of aromatic and alkylic enantioenriched β-acetamidophosphine oxides P82 were efficiently prepared. These compounds could be readily hydrolyzed and reduced, thus providing an efficient route to important chiral β-aminophosphines.
Scheme 53. Rhodium-Catalyzed AH of Enamido Phosphonates and β-Phosphorylated Enamides.
Optically active α- and β-amino phosphonic acid derivatives can also be prepared by means of AH. In fact, in 2011, Ding designed a family of chiral monodentate phosphoramidite (DpenPhos) ligands that were found to be highly efficient in the rhodium-catalyzed AH of enamides S83 and S84 (Scheme 53).332 Of note, when L43 was used, a set of chiral amino phosphonates P83 and P84 were prepared with excellent results. In several cases, the enantioselectivity values obtained were higher than those reported previously.333,334
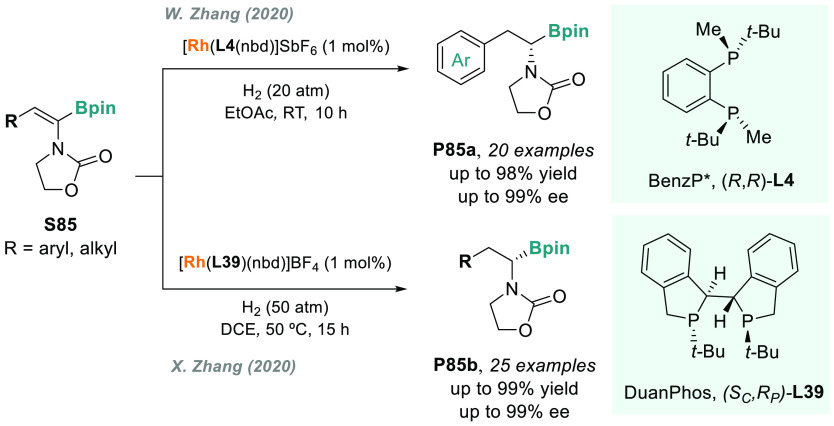
Organoboron compounds are also important due to their unique physical, chemical, and biological properties. However, the preparation of chiral α-aminoboronic acids, as mimics of chiral amino acids, is not trivial. In 2020, W. Zhang and X. Zhang independently pioneered this field describing the rhodium-catalyzed AH of α-boryl enamides (S85) using the P-stereogenic diphosphines L4 and L39, respectively (Scheme 54).335,336 Critical to the success of this method was the chelate coordination of the amido group to rhodium and the nonbonding interactions between the substrate and the ligand. Whereas by using L4 the method was limited to aryl substituents in the β position, the use of L39 allowed an expanded substrate scope, as alkyl substituents were also well tolerated. Chiral α-amidoboronic esters P85 were furnished in quantitative conversion and excellent enantioselectivity. Exquisite chemoselectivity was observed as no protodeboronation was detected.
Scheme 54. Rhodium-Catalyzed AH of α-Boryl Enamides.
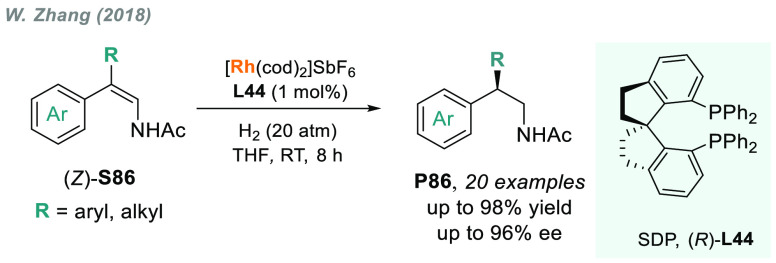
While the hydrogenative synthesis of chiral α-substituted amines has been widely addressed, synthetic methodologies for the preparation of β-chiral amines are rare. Only a few examples have been reported, mostly by AH of dehydroamino acids.337,338 The AH of β-branched simple enamines remained a long-standing challenge due to the difficulties related to the stereocontrol of the reaction. To overcome this issue, in 2018, W. Zhang and co-workers disclosed the first catalytic protocol using a Rh complex bearing a diphosphine ligand with a large bite angle (SDP, L44).339 β-Branched simple enamides with a (Z)-configuration (S86) were efficiently hydrogenated to optically pure β-chiral amines P86 in quantitative yields and with excellent enantioselectivities (Scheme 55).
Scheme 55. Enantioselective Synthesis of β-Stereogenic Amines via AH.
3.1.2. Ni and Co Catalysts
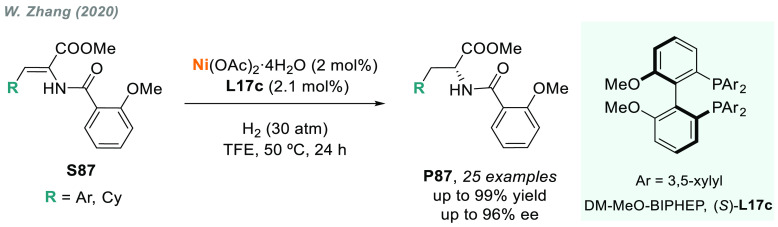
The limited availability, high cost, and toxicity of noble metals stimulated the research in their replacement with earth-abundant, inexpensive first-row transition metals. However, challenges such as different reaction mechanism and unexpected deactivation of the catalyst prevented their widespread use in asymmetric hydrogenation.340,341 While dozens of examples using Rh catalysis have been reported during the past decade, the use of earth-abundant transition metals has just started showing practical efficiency in AH. In 2020, W. Zhang and co-workers reported a highly efficient nickel-catalyzed AH of 2-amidoacrylates (Scheme 56).342 In contrast to the AH with Rh catalysts, where the amido-assisted activation strategy allowed attainment of high activity and enantioselectivity, Ni catalysts cannot utilize this approach as they have their own coordination modes. However, W. Zhang envisioned that other interactions between the substrate and catalyst would lead to high catalytic activity. Interestingly, when using S87 bearing an ortho-methoxy-substituted benzoyl group and Ni/BIPHEP-type ligand (L17c), the AH occurred smoothly and the corresponding chiral α-amino acid esters P87 were afforded in excellent enantioselectivities (up to 96% ee).
Scheme 56. Nickel-Catalyzed AH of 2-Amidoacrylates.
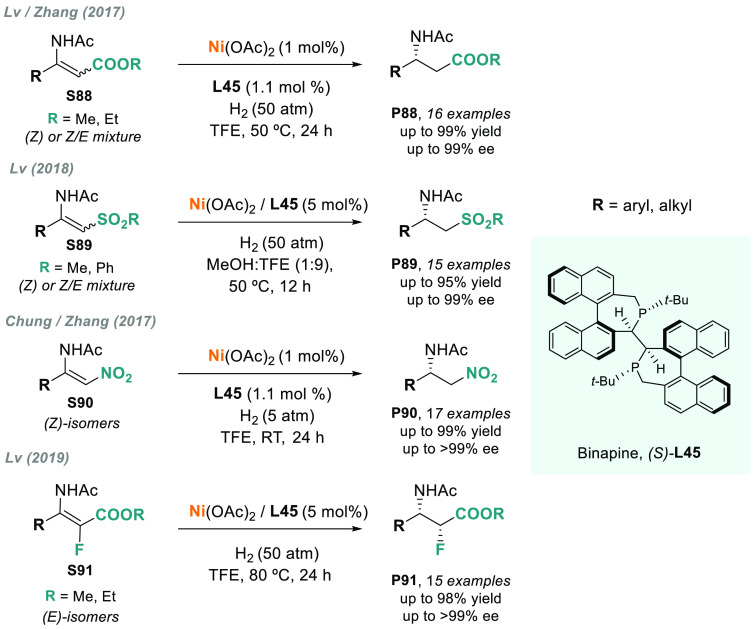
Nickel-catalyzed AH has also been used in the synthesis of chiral β-amino acid derivatives. Lv and X. Zhang and co-workers reported a highly enantioselective hydrogenation of (Z)-β-(acylamino)acrylates S88 to provide enantiomerically pure β-amino acid derivatives P88 using a commercially available binapine ligand (L45) (Scheme 57).343 High enantioselectivities were obtained even using Z/E isomeric mixtures. The same catalytic system proved to be fruitful for many other functionalized enamides, including benchmark substrates.344 In 2018, Lv and co-workers expanded its use for the Ni-catalyzed AH of β-acetylamino vinylsuflones S89 (Scheme 57).345 The methodology showed good compatibility with substituted (Z)-isomers and Z/E isomeric mixtures, thus being an alternative to the previously reported protocol using Rh/TangPHOS-L2.346 The resulting chiral sulfones P89 were obtained in high yields and excellent enantioselectivities, in gram scale in the presence of only 0.2 mol % of catalyst. This catalyst also showed high activity toward the AH of β-acylamino nitroolefins S90. These are usually challenging substrates for AH due to the weak binding affinity of the olefins with the electron-withdrawing nitro group, and in fact, only a few examples have been reported involving precious transition metal catalysts.347−349 Despite this, Chung, X. Zhang, and co-workers showed that Ni/Binapine could be used as catalyst to attain chiral β-amino nitroalkanes P90 with excellent enantioselectivity (>99% ee in the best cases) and high TONs using mild conditions (Scheme 57).350 Finally, Lv also reported the AH of tetrasubstituted β-enamino-α-fluoro esters S91 in high yields and excellent diastereo- and enantioselectivities using Ni/L45 (Scheme 57).351 Interestingly, key experiments revealed the critical role of acidic solvent in modulating the reaction pathway, as well as for the control of diastereoselectivity. This method provides a highly straightforward and concise route to α-fluoro-β-amino esters P91.352
Scheme 57. AH of β-Functionalized N-Acyl Enamines Using the Ni/Binapine System.
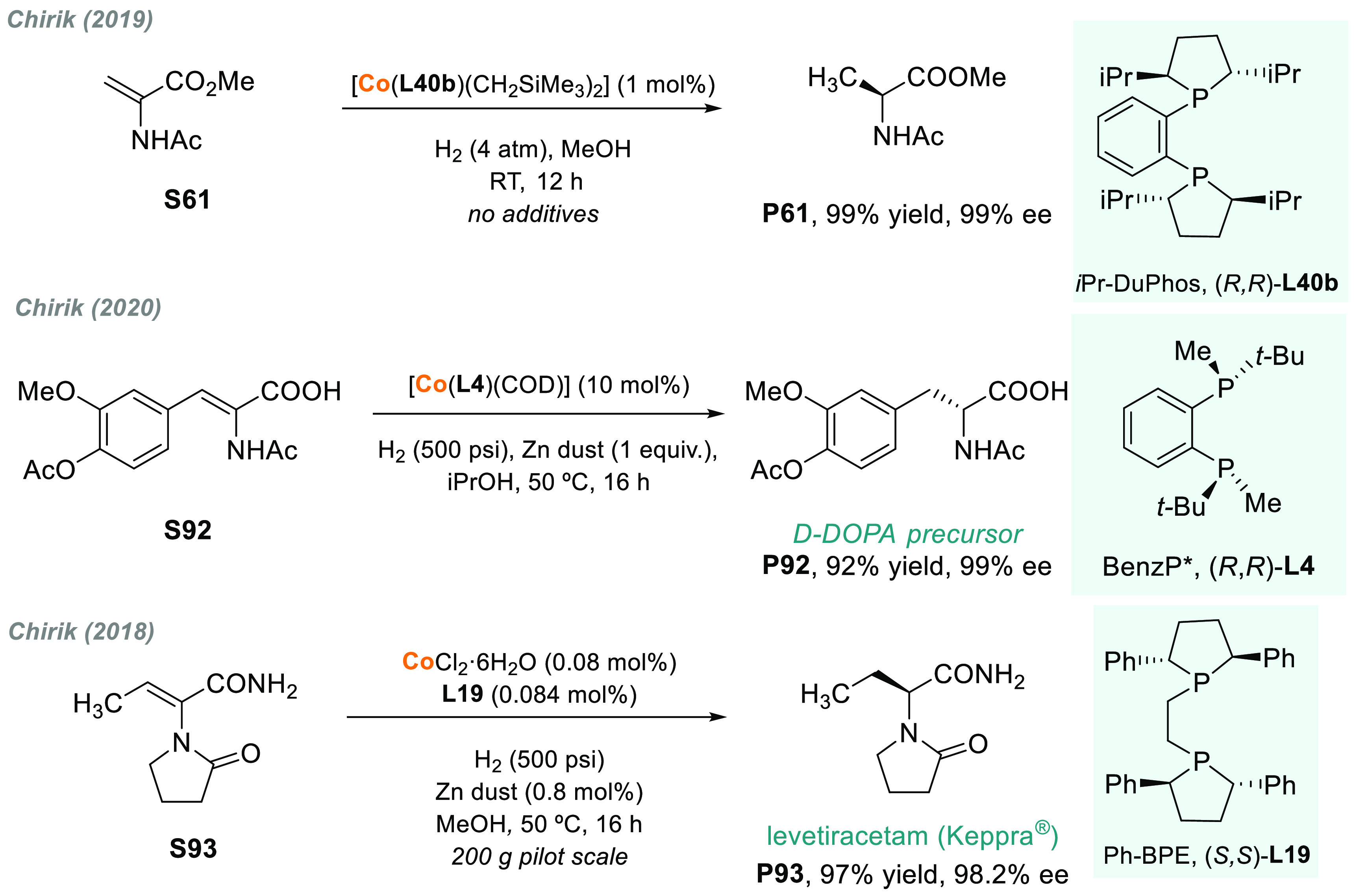
Cobalt has also gained great importance during the past decade in the field of AH. Chirik’s laboratory has pioneered the use of cobalt complexes bearing chiral diphosphines to attain hydrogenative processes with extraordinary activity and enantioselectivity.353,354 In 2019, the group demonstrated that cobalt complexes bearing DuPhos-type ligand (L40b) efficiently hydrogenated MAA S61 in excellent enantioselectivity (Scheme 58).355 More importantly, the reaction was carried out using MeOH, an industrially preferred green solvent which is often a poison for reduced earth-abundant metals, and without the use of additives. Other α,β-unsaturated carboxylic acids, including di-, tri-, and tetra-substituted acrylic acid derivatives, as well as dehydro-α-amino acid derivatives, were hydrogenated using Co/BenzP*-L4 (Scheme 58).356 Chiral carboxylic acids, including bioactive ones such as Naproxen, (S)-Flurbiprofen, and a D-DOPA precursor P92, were attained in high yields and enantioselectivities. Again, protic solvents such as MeOH were identified as optimal, and Zn dust was used stoichiometrically. The group had previously described the Co-catalyzed AH of enamides using zinc-activation, which promoted straightfroward single-electron reduction to enable the catalytic process (Scheme 58).357 The optimized protocol, using Co/L19, exhibited high activity and enantioselectivity and allowed the asymmetric synthesis of the epilepsy drug levetiracetam (P93) at 200-g scale with only 0.08 mol % of catalyst loading.
Scheme 58. Co-Catalyzed AH of 2-Functionalized N-Acyl Enamines.
3.2. Endocyclic N-Acyl Enamides
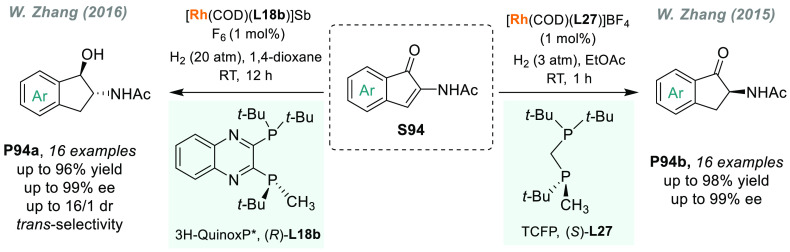
In contrast to acyclic enamides, which have been extensively studied, the AH of cyclic enamides remained a challenge before the past decade. Despite that, the resulting chiral cyclic amines are very useful structural motifs that can be found in a range of bioactive molecules.358 An example of this class of substrates are cyclic α-dehydro amino ketones (S94, Scheme 59). In 2016, W. Zhang and co-workers reported that P-stereogenic chiral ligand L18b, using Rh catalysis, efficiently hydrogenated S94 to chiral cyclic trans-β-amino alcohols P94a via a one-pot sequential AH with excellent enantioselectivities and diastereoselectivities.359 The same group achieved rhodium-catalyzed partial hydrogenation using small-bite angle ligand TFCP (L27) in a completely chemoselective manner (Scheme 59).360 Thus, chiral α-amino ketones P94b were exclusively obtained with excellent results, and both synthetic protocols were scaled up to gram scale. In contrast, the AH of cyclic β-keto enamides remains unexplored, with only one precedent in the literature and with very limited scope.361
Scheme 59. Partial and Total Rhodium-Catalyzed AH of Cyclic α-Dehydroamino Ketones.
Another family of long-standing challenging substrates are cyclic enamides derived from tetralones and chromanones. The resulting chiral amines are highly desirable as they are precursors of therapeutic drugs. In this regard, the AH of cyclic enamides has typically relied on the use of Rh and Ru catalysts.362−365 Among the most successful examples, Ratovelomanana-Vidal and co-workers reported up to 96% ee in the reduction of S95 (Scheme 60). The method employed Ru catalysis in combination with binap-type ligand SynPhos L46.366,367 Later, Tang and co-workers described the use of WingPHOS ligand (L35) in the rhodium-catalyzed AH of cylic enamides S95, which yielded the corresponding chiral amines P95 in up to 98% ee (Scheme 60).281
Scheme 60. Metal-Catalyzed AH of Cyclic Enamides Derived from α- and β-Tetralones.
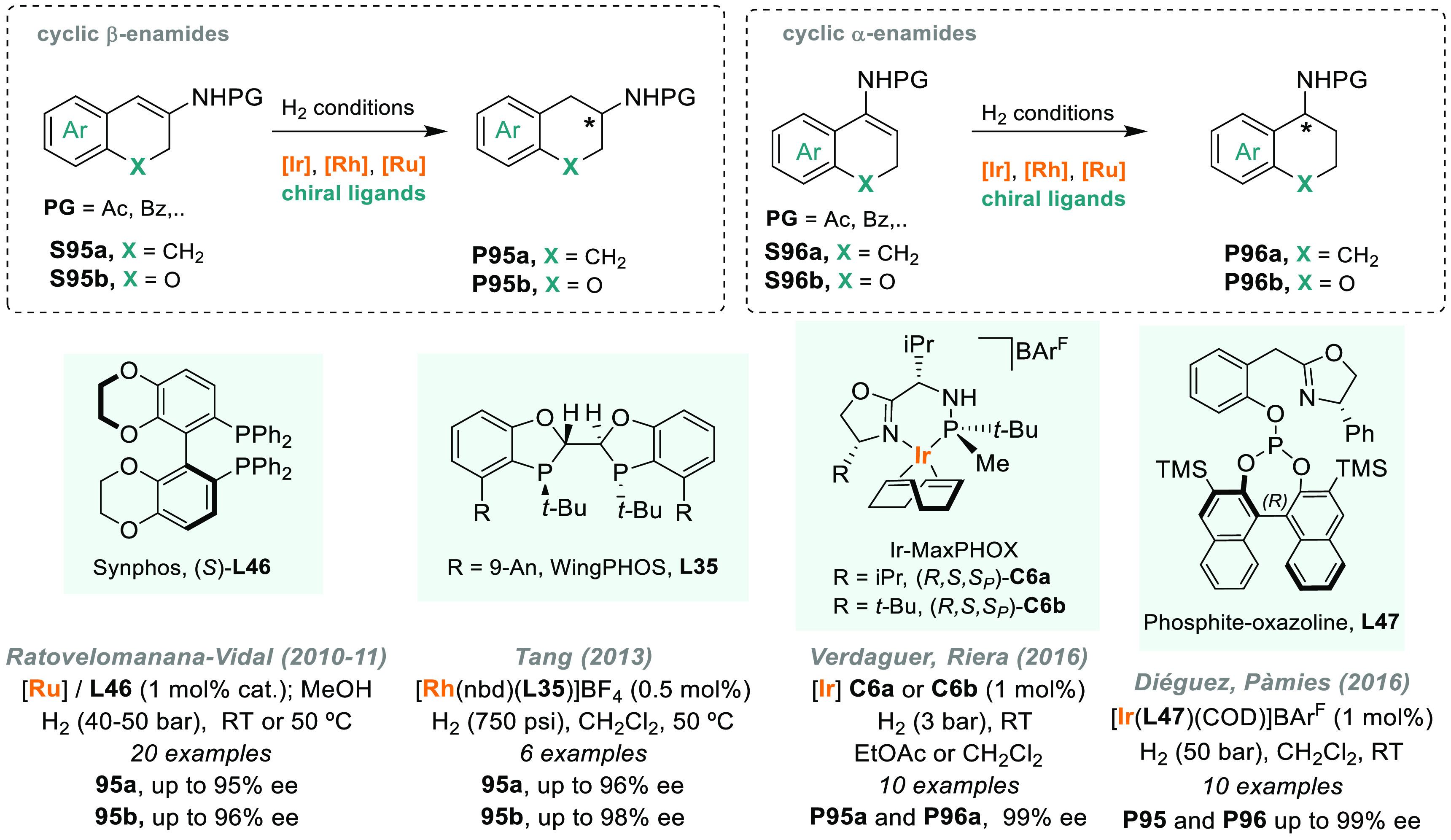
However, these methods suffer from harsh reactions conditions such as high H2 pressure or heating. In this regard, iridium catalysis, which has scarcely been used in the AH of N-acyl enamides and other alkenes bearing a metal-coordinating group, offered an excellent alternative. In 2016, Verdaguer, Riera, and co-workers reported the highly enantioselective iridium-catalyzed AH of cyclic enamides S95 and S96, derived from α- and β-tetralones (Scheme 60).77 They optimized the iridium complexes bearing P-stereogenic phosphino-oxazoline ligands (C6a or C6b). These catalytic systems provided the highest selectivity reported to date for the reduction of these substrates. The resulting chiral amines P95 and P96 were obtained in 99% ee. Moreover, the process was carried out in environmentally friendly solvents such as MeOH and EtOAc without loss of selectivity and under very mild conditions (3 bar of H2). When the ligand was replaced with a P-stereogenic phosphino-imidazole ligand, the enantioselectivity decreased considerably.368 Diéguez and co-workers also reported the iridium-catalyzed AH of cyclic enamides S95 and S96 in excellent enantioselectivities employing a phosphite-oxazoline ligand (L47, Scheme 60).369,370 The same group further extended this methodology using other modular ligands.371−374 Overall, these protocols allowed an efficient route to the asymmetric synthesis of 2-aminotetralines and 3-aminochromanes, key structural units in many biologically active agents such as rotigotine, terutroban, and nepicastat (Figure 6).375,376
Figure 6.
Pharmaceutical drugs containing the chiral 2-aminotetraline structure.
The AH of tetrasubstituted endocyclic enamides29 has been a focus of great attention over the last years. The resulting chiral cyclic amines with a substitution at the 2-position are important motifs in many bioactive molecules and drugs (Figure 7).
Figure 7.
Pharmaceutical drugs containing amines with vicinal chiral centers.
In 2017, Lv, X. Zhang, and co-workers developed a highly enantioselective hydrogenation of cyclic N-acyl enamines S97 to provide optically pure cycloalkyl amides P97a using Rh-Binapine (L45) as catalyst (Scheme 61).377 The resulting chiral amides had an aryl substituent in the vicinal position. The methodology could be applied to prepare biologically active compounds. More recently, in 2019, Tang, in collaboration with a team from Pfizer, demonstrated that an electron-rich P-stereogenic bisphosphorus ligand with deep chiral pockets (ArcPHOS, L48) could be applied to the rhodium-catalyzed AH of S97 bearing alkyl substituents at the 2-position (Scheme 61).378 Consequently, chiral amides P97b were attained in excellent yields and enantioselectivities. The methodology was showcased by a concise synthesis of Tofacitinib (Figure 7). Previously, Stumpf and co-workers reported a multikilogram scale asymmetric synthesis of the enantiomerically pure fluoropiperidine P97c via AH using a Ru/JosiPhos (L21a) catalyst with high enantiomeric excesses (Scheme 61).379 This fluorinated aminopiperidene is also present as a structural motif in the antibacterial clinical candidate AZD9742. In fact, researchers at AstraZeneca have recently reported its enantioselective synthesis by means of AH using [((S)-BINAP)RuCl2](p-cymene).380
Scheme 61. Metal-Catalyzed AH of Tetrasubstituted Cyclic Enamides.
Chiral cyclic β-amino acids,381 such as cispentacin (Figure 7), are important in the synthesis of β-peptides. In 2003, X. Zhang and co-workers pioneered the AH of tetrasubstituted cyclic β-(acylamino)acrylates using the chiral biaryl ligand C3-TunaPhos.382 Following this path, Zhou’s group hydrogenated tetrasubstituted cyclic β-(arylsulfonamido)acrylates. Using Pd/DuanPhos-L39 as catalyst, a range of five-membered chiral β-amino acid derivatives P97d were obtained in excellent yields and enantiomeric excesses (Scheme 61).383 More recently, the same group described the asymmetric hydrogenation of carbocyclic aromatic amines using a ruthenium-DuPhos (L40b) complex as catalyst.384
3.3. N-Sulfonyl Enamines
Little attention has been devoted to the AH of N-sulfonyl enamines, and only a few examples can be found in the literature.385,386 Following the latter example (P97d, Scheme 61), the enantioselective synthesis of chiral amino acids via AH of noncyclic α- and β-(arylsulfonamido)acrylates is of great importance. In 2005, a team from Merck published the synthesis of an anthrax lethal factor inhibitor via ruthenium-catalyzed AH of S98 (Scheme 62).387 Catalyst screening identified that JosiPhos L21d and the bis-thiophene atropoisomeric ligand L49 gave excellent enantioselectivities. Later, in 2016, Sato, Saito, and co-workers reported nickel-promoted regioselective carboxylation of internal enamides to afford a range of α-substituted β-aminoacrylates S99. These were then subjected to the rhodium-catalyzed AH using Walphos ligand L22d. Chiral amino acid derivatives P99 were furnished in a highly enantioselective manner (Scheme 62).388 On the other hand, the AH of cyclic N-sulfonyl enamines is rare. To the best of our knowledge, there is only one example in the literature, reported by Andersson’s group, using iridium complexes bearing chiral P,N ligands.389 However, the method was hampered by low conversions and moderate enantioselectivities with a very narrow scope.
Scheme 62. Metal-Catalyzed AH of α- and β-(Arylsulfonamido)acrylates.
3.4. Other Enamides
The AH of N-phthaloyl enamides is a promising method for the preparation of chiral amines. Apart from serving as a directing group, the phthalimido functionality can be easily removed under mild conditions. In 2006, X. Zhang and co-workers reported the highly enantioselective hydrogenation of α-aryl N-phthaloyl enamides using rhodium catalysts derived from TangPhos (L2) (Scheme 63).390 The resulting chiral α-methyl, aryl N-phthalimides P100 were generally obtained in excellent enantioselectivities, but these dramatically decreased for substrates bearing an ortho-substituent on the aromatic ring. In contrast, the preparation of enantioenriched α-methyl, alkyl N-phthalimides using AH has not yet been explored. Later, the authors reported the use of the same chiral ligand L2 in the rhodium-catalyzed AH of N-phthaloyl dehydroamino acid esters, thus affording highly valuable chiral α- or β-amino acid derivatives with good to excellent enantioselectivities.391
Scheme 63. Rhodium-Catalyzed AH of N-Phthaloyl Enamides.
The rhodium-catalyzed AH of heterocyclic β-aminoacrylates S101 was accomplished by Gallagher and co-workers in 2016 (Scheme 64).392 By using WalPhos L22d as a chiral ligand, several pyrrolidine and piperidone variants were efficiently hydrogenated, providing chiral heterocyclic amino acids P101 with high enantioselectivity. The use of the carboxylic acid was essential for the success of the reaction. Similarly, the AH of β,γ-unsaturated γ-lactams was described by Liu, W. Zhang, and co-workers, although the hydrogenation proceeded via N-acyliminium cations.196
Scheme 64. Metal-Catalyzed AH of Lactams.
On the other hand, Ding, Han, and co-workers recently described the double asymmetric hydrogenation of 3,4-dialkylidene-2,5-diketopiperazines using an iridium-SpinPhox (C4) complex as catalyst.393
The enantioselective synthesis of chiral 2-oxazolidinones, widely used as Evans’ chiral auxiliaries, has attracted considerable attention for the construction of new chiral building blocks and the development of new asymmetric transformations. An alternative to the conventional approach, limited to easily accessible chiral β-amino alcohols, is the direct AH of 2-oxazolones. In this regard, in 2018, Glorius and co-workers reported an innovative protocol for the ruthenium-catalyzed AH of S102 using L50 as precursor of the NHC ligand (Scheme 65).394 A variety of chiral 2-oxazolidinones P102 were obtained in excellent enantioselectivities (up to 96% ee). The formal synthesis of (−)-aurantioclavine was demonstrated as a synthetic application. X. Zhang’s group previously reported the rhodium-catalyzed AH version of this transformation using TangPhos L2, albeit with moderate enantioselectivities and restricted mainly to substrates bearing electron-donating groups on the aryl ring.395
Scheme 65. Ruthenium-Catalyzed AH of 2-Oxazolones.
4. Asymmetric Hydrogenation of Enamines
Although great progress has been made in the transition metal-catalyzed asymmetric hydrogenation of N-protected enamides, the introduction and removal of the protecting group reduce the overall efficiency of the method and limit its applications in the synthesis of optically active amines. To overcome this drawback, significant efforts have been devoted to the development of new chiral catalysts for the direct AH of enamines, including N-alkyl, N-aryl, or unprotected amines.40
4.1. N-Alkyl Enamines
In 2006, Zhou pioneered the AH of cyclic enamines using rhodium catalysts bearing monophosphorus ligands. In particular, spiro-phosphinite ligand L51 showed excellent enantioselectivites for simple N,N-dialkyl enamines S103 (Scheme 66).396,397 The reaction rates were enhanced using I2/acetic acid as additives. Later, in 2009, the same group reported that other N-alkylated enamines could be efficiently hydrogenated using a similar catalytic system Ir/L14/I2 (Scheme 66). This protocol allowed the hydrogenation of both endocyclic398 (S104) and exocyclic399 (S105) enamines in excellent enantioselectivities under very mild conditions (1 bar of H2 and RT). Pfaltz also contributed to the field using phosphino-oxazoline ligands for the iridium-catalyzed AH of S103-type substrates, albeit with lower ee values.400
Scheme 66. Metal-Catalyzed AH of Endocyclic and Exocyclic N-Alkyl Enamines.
In 2009, a team from Merck applied the direct AH of alkylated enamines to the synthesis of an HIV integrase inhibitor (Scheme 67).401 Using Rh and a JosiPhos ligand (L21e), a mixture of imine/enamine S106 was efficiently hydrogenated, affording P106, a direct precursor of the target drug, in 90% ee. Interestingly, a deuterium labeling study suggested that the AH proceeds predominantly via the enamine tautomer.
Scheme 67. Catalytic Synthesis of an HIV Integrase Inhibitor.
4.2. N-Aryl Enamines
The enantioselective synthesis of N-aryl β-enamino esters was first studied in 2005, when X. Zhang and co-workers developed an enantioselective strategy based on the rhodium-catalyzed AH of N-aryl enamines S107 (Scheme 68).402 Chiral N-aryl-substituted β-amino acid derivatives P107a were obtained in moderate to excellent enantioselectivities (79–96% ee) using a P-stereogenic ligand (TangPhos, L2). However, the reaction was highly substrate-dependent, and S107 bearing a CF3 as high electron-withdrawing group showed poor conversion. To overcome this limitation, Peng recently reported an alternative approach using Pd/L19 as catalyst.403 Chiral β-fluoroalkyl β-amino acid derivatives P107b were obtained in good yields and excellent enantioselectivites (Scheme 68). The use of p-anisic acid, which can promote the tautomeric transformation between imines and enamines, enhanced both the activity and enantioselectivity. The authors speculated that the hydrogenation could occur through an asymmetric reduction of the iminium ion rather than the enamine form of the substrate.
Scheme 68. Metal-Catalyzed AH of N-Aryl Enamines.
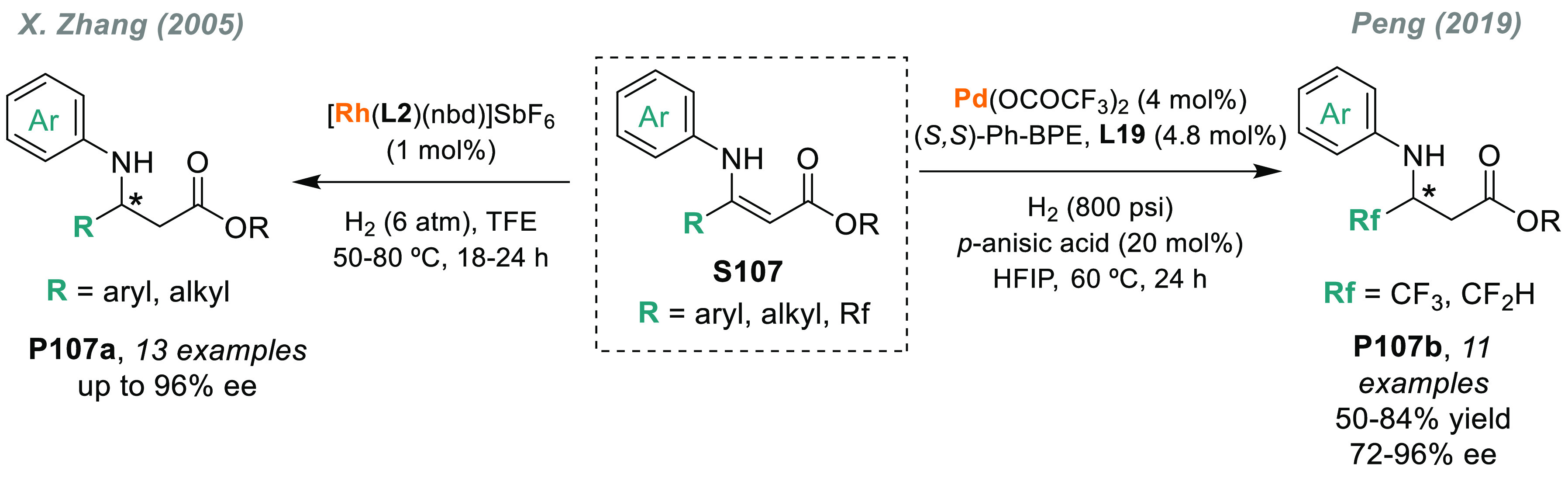
In 2009, Zhou and co-workers described the first highly enantioselective AH of exocyclic unprotected enamines S108 by using Ir/(S)-L17a/I2 as catalytic system (Scheme 69).404 The resulting chiral amines P108 were afforded in excellent yields and up to 96% ee.
Scheme 69. Iridium-Catalyzed AH of Exocyclic N-Aryl Enamines.
4.3. Unprotected Enamines
The AH of unprotected enamines has scarcely been studied because the transition metal catalyst is usually poisoned by the nucleophilic amino group. However, the direct AH of these substrates is highly desirable to avoid redundant introduction and subsequent removal of protecting groups and also for the preparation of pharmacologically relevant compounds. In 2004, a team from Merck reported the first example of catalytic AH of unprotected β-enamine esters and amides (S109), using Rh-JosiPhos complexes as catalysts (Scheme 70).405 Ligand L21d gave the best results in the AH of enamine esters, while L21a gave the highest rates and enantioselectivities for the AH of enamine amides. β-Amino acids derivatives P109a were attained in excellent yields and enantioselectivities, thus proving that the N-acyl group is not always a prerequisite for such transformations.
Scheme 70. Rhodium-Catalyzed AH of β-Functionalized Enamines.
The applicability of this protocol in late-stage functionalization was showcased by the asymmetric synthesis of Sitagliptin (P110, Scheme 71), which was implemented on a manufacturing scale.58P110 was obtained in 98% yield and 95% ee (improved to >99% ee by recrystallization) using (RC,Rp)-L21a. More recently, Chikkali and co-workers reported that Rh complexes bearing chiral FerroLANE ligands also catalyze the AH of S110 to yield sitagliptin with excellent enantioselectivity (98% ee).406 The asymmetric synthesis of P110 was also accomplished via direct asymmetric reductive amination (ARA) with unprecedented levels of asymmetric induction.407 In addition, Ru catalysis has been successfully applied in both ARA or direct AH of unprotected enamines for the preparation of other pharmacologically relevant compounds.408,409
Scheme 71. Asymmetric Synthesis of Sitagliptin via Rhodium-Catalyzed AH.
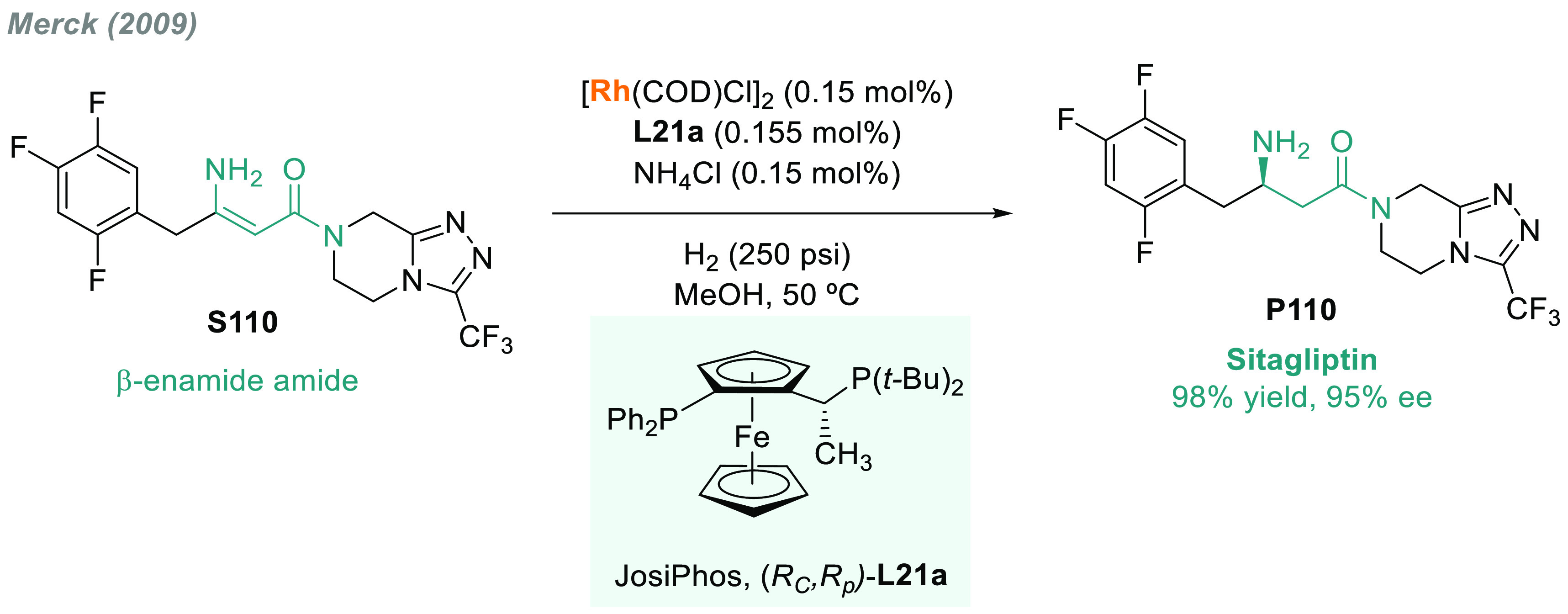
The rhodium-catalyzed AH of unprotected β-enamine phosphonates was described by Dong, X. Zhang, and co-workers (Scheme 70).410 By using Rh/TaniaPhos-L52, the method provided an efficient route to free β-amino phosphonates P109b, which are important intermediates in biochemistry and pharmaceuticals. This work provided an alternative to the protocol previously reported by Ding, in which the amine was necessarily protected by acyl groups (Scheme 53).332 Also, Ruchelman et al. reported the enantioselective hydrogenation of unprotected β-aminosulfones using Rh catalysis, which afforded a key intermediate of the phosphodiesterase 4 (PDE4) inhibitor Apremilast.411
Iridium catalysis has also been applied in the AH of unprotected enamines. X. Zhang demonstrated that β-enamine hydrochloride esters S111 can be suitable substrates for AH, despite the fact that primary amines might have a strong inhibitory effect on the iridium catalyst.412 The combination of Ir/f-binaphane (L7) and the use of hydrochlorides provided direct access to a range of enantiomerically enriched β-amino acids without use of an amino protecting group (Scheme 72).
Scheme 72. Iridium-Catalyzed AH of β-Enamine Hydrochloride Esters.
5. Asymmetric Hydrogenation of Allyl Amines
The AH of allyl amines remained relatively underdeveloped before the past decade. Allyl amines are usually considered minimally functionalized olefins as the unsaturated bond lacks close coordinating groups. For this reason, the AH of allyl amines is more challenging if compared to imines or enamines. In the early past decade, a range of new strategies were described for the metal-catalyzed AH of allyl amines, exhibiting high reactivity and enantiocontrol. Thus, the preparation of highly valuable β- and γ-substituted chiral amines is now more accessible. In this section, the most important precedents in the field will be described, along with pharmaceuticals and drugs that can be attained by means of the AH of allyl amines (Figure 8).
Figure 8.
Representative drugs that can be prepared via the AH of allyl amines.
5.1. N-Phthaloyl Allyl Amines
As previously stated, chiral β- and γ-amino acids and their derivatives are important building blocks in the synthesis of pharmaceuticals and other bioactive compounds. Zheng and co-workers reported the first highly enantioselective synthesis of chiral β-aryl-γ-amino acid ester derivatives P112 via rhodium-catalyzed AH of γ-phthalimido-substituted acrylates413 using the BoPhoz-type ligand414,415L53a (Scheme 73). The method showed high reactivity and enantioselectivity (up to 97% ee) for a range of (Z)-substrates S112. The method was successfully applied to the synthesis of several chiral pharmaceuticals including (R)-rolipram and (R)-baclofen (Figure 8) with high enantioselectivities. The same group later expanded the applicability of this approach to the asymmetric synthesis of β2-amino acids P113 via rhodium-catalyzed AH using another ligand of the BoPhoz family: L53b (Scheme 73).416 Interestingly, the presence of an N–H proton in the ligand significantly improved the enantioselectivity, whereas the introduction of a P-stereogenic center in the phosphino moiety proved unfruitful and displayed low conversion. The same catalytic system also exhibited excellent ee values for β-unsubstituted substrates S113 (99% ee). Other protocols for the stereoselective synthesis of chiral β2-amino acids include the rhodium-catalyzed AH of β-substituted α-aminomethyl acrylates that Börner and co-workers417,418 and Qiu and co-workers419,420 independently reported in this earlier past decade.
Scheme 73. Rhodium-Catalyzed AH of β- and γ-Phtalimido-Substituted Unsaturated Esters.
Chiral amines bearing a β-methyl stereogenic center are extremely interesting as they are present in numerous drugs and pharmaceuticals. The AH of 2-substituted allyl phthalimides is an efficient route toward their preparation. X. Zhang pioneered the field with the ruthenium-catalyzed AH of terminal disubtituted allylphthalimides S114 using C3-TunePhos ligand L5b (Scheme 74).421 Chiral β-alkyl-β-methyl amines P114a were attained in excellent yields and enantioselectivities. However, the scope of this reaction was limited to alkyl substituents, as the hydrogenation of an aromatic substrate gave moderate enantioselectivity. To overcome this limitation, Verdaguer and Riera’s laboratory recently reported the highly enantioselective hydrogenation of 2-aryl allyl phthalimides using iridium catalysis (Scheme 74).422 Ir-MaxPHOX C6b bearing a bulky substituent on the oxazoline ring gave the best enantioselectivities (>99% ee in the best cases for P114b), showing an exquisite functional group tolerance for a range of substrates. Several direct synthetic applications of this catalytic method were disclosed, such as the formal synthesis of (R)-Lorcaserin (Figure 1), which is a marketed anorectic drug, and also a novel approach to enantiomerically enriched 3-methyl indolines.
Scheme 74. Metal-Catalyzed AH of N-Allyl Phthalimides.
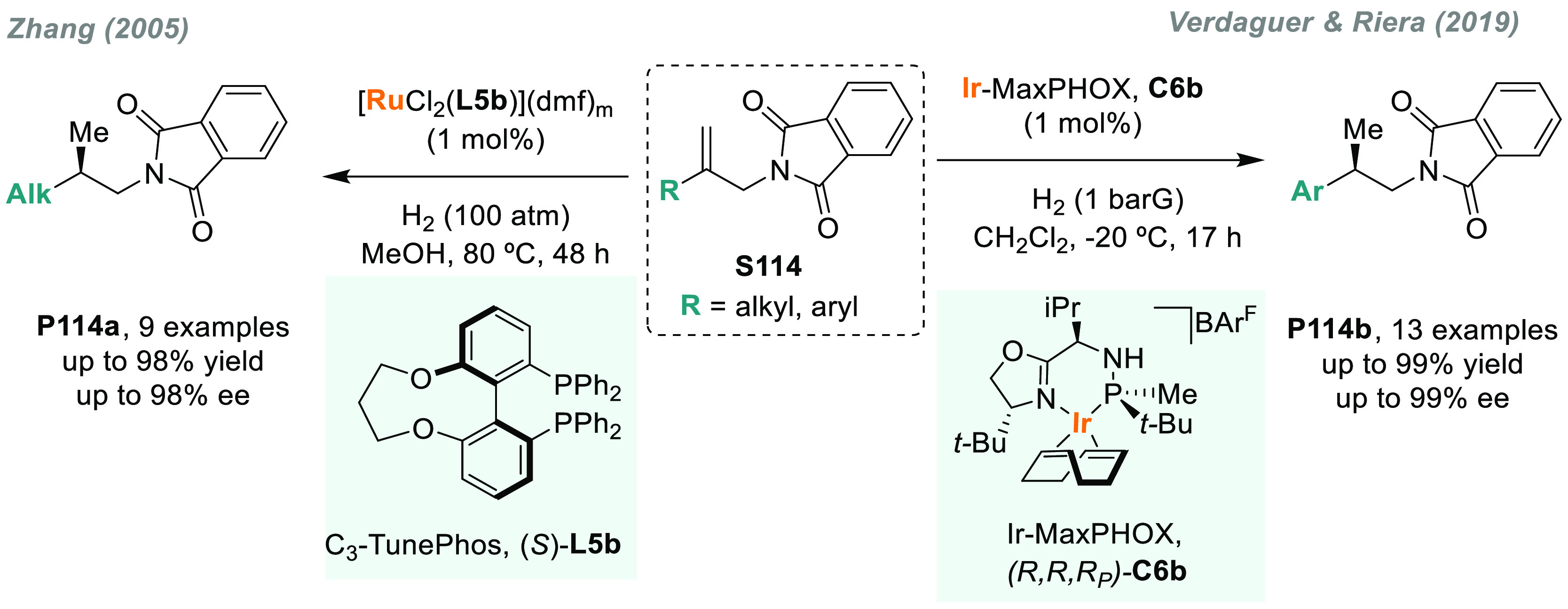
5.2. N-Sulfonyl Allyl Amines
Verdaguer and Riera’s group also reported the iridium-catalyzed AH of N-sulfonyl allyl amines S115,423 which can be easily prepared by the iridium-catalyzed isomerization of N-tosylaziridines.424 By using the commercially available iridium catalyst UbaPHOX (C19), first reported by Pfaltz,425,426 a wide range of chiral β-methyl amines were afforded with good to excellent enantioselectivities (Scheme 75). These compounds are also key intermediates for the preparation of allosteric modulators of AMPA receptor such as LY-404187 (Figure 8).427
Scheme 75. Iridium-Catalyzed AH of 2-Aryl N-Sulfonyl Allyl Amines.
Iridium complexes bearing chiral P,N ligands were also applied to the catalytic hydrogenation of cyclic N-sulfonyl allyl amines S116, as reported by Andersson and co-workers.428,389 The reaction was highly substrate-dependent, and an appropriate chiral ligand was used for each case. Substrates S116 bearing aliphatic substituents were efficiently hydrogenated using a phosphino-oxazoline ligand L54 whereas phosphino-imidazole L55 and phosphino-thiazole L56 gave excellent activities and selectivities for aromatic substrates, depending on their electronic properties (Scheme 76). In addition, the methodology was further expanded to the iridium-catalyzed AH of five- and seven-membered N-heterocyclic olefins. The chiral pyrrolidines, piperidines, and azepanes, which are highly valuable motifs for the synthesis of medicinal compounds and natural products, were attained in excellent enantioselectivities. Similarly, W. Zhang and co-workers recently reported the catalytic AH of 3-substituted 2,5-dihydropyrroles (S117) using an Ir-catalyst with an axially flexible chiral phosphine-oxazoline ligand named BiphPhox (L57).429 Chiral N-tosyl pyrrolidines P117 were efficiently prepared in good to excellent enantioselectivities (Scheme 76).
Scheme 76. Iridium-Catalyzed AH of Cyclic N-Sulfonyl Allyl Amines.
5.3. Other Allyl Amines
During the past decade, other remarkable examples have also been reported for the highly enantioselective hydrogenation of protected allyl amines. In 2010, a team from Merck described a general method for the ruthenium-catalyzed AH of trisubstituted N-acyl allyl amines S118 using the axially chiral ligand L49, affording chiral β-substituted amines P118 with excellent enantioselectivities (Scheme 77).430
Scheme 77. Ruthenium-Catalyzed AH of N-Acyl Allyl Amines.
Optically active amines with remote stereocenters, such as γ-substituted chiral amines, are often key contributors to the potent biological activity of many natural products and pharmaceuticals. Important examples of this class of compounds are dexbrompheniramine, which is an antihistaminic, and tolterodine, an anticholinergic (Figure 1). γ-Amino acids such as γ-aminobutyric acid (GABA) are also important in medicinal chemistry. Consequently, asymmetric synthesis of chiral derivatives of GABA with appropriate side chains is potentially important for the design of new drug-like molecules with enhanced pharmacological properties. Nevertheless, the direct preparation of γ-substituted chiral amines remains underdeveloped compared to the well-established methods for constructing α- and β-substituted chiral amines. In addition, most of the enantioselective syntheses of these compounds are indirect and often require multiple steps. Buchwald’s group first reported a direct approach to γ-chiral amines by enantioselective CuH-catalyzed reductive relay hydroamination.431 Hull and co-workers developed efficient conditions for the highly enantioselective synthesis of γ-branched amines via rhodium-catalyzed reductive amination.432 Alternatively, the redox neutral asymmetric isomerization of allylic amines is a method of choice with an excellent atom economy, although current methods are hampered by very limited substrate scope.17,433 In this scenario, the development of new transition metal-catalyzed AH processes to afford enantioenriched γ-substituted amines is extremely desirable. In 2011, Burgess and co-workers reported the asymmetric synthesis of α-methyl-γ-amino acid derivatives via catalytic AH using a carbene–oxazoline iridium complex C20 (Scheme 78).434 The method of optimization was based on varying peripheral aspects of the substrate rather than optimizing the catalyst via ligand modifications. Following this approach, O-TBDPS-protected allylic substrates gave the best results. Once hydrogenated under mild conditions, chiral γ-methyl amines were afforded in high stereocontrol. Anti-products P119 were formed from the Z-alkenes S119, while the E-isomers S120 gave syn-target compounds P120.
Scheme 78. Iridium-Catalyzed AH of N-Acyl Allyl Amines.
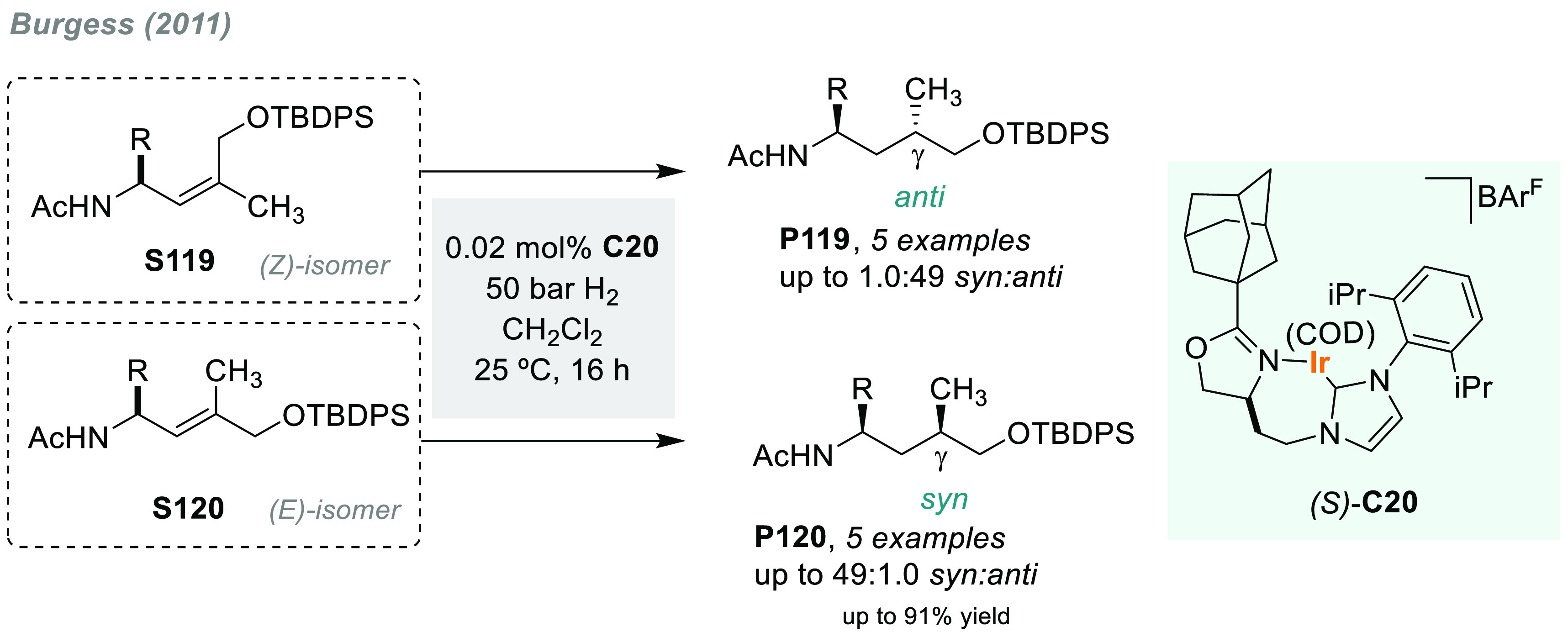
Similarly, Beller and co-workers recently used C21, an iridium-P,N-ligand complex, for the AH of N-acyl endocyclic allyl amines S121 (Scheme 79).435 Using HFIP as reaction solvent, the reaction proceeded efficiently to afford chiral γ-methyl amide P121, which is a key intermediate for the preparation of a common agrochemical building block.
Scheme 79. Synthesis of an Agrochemical Building Block via AH.
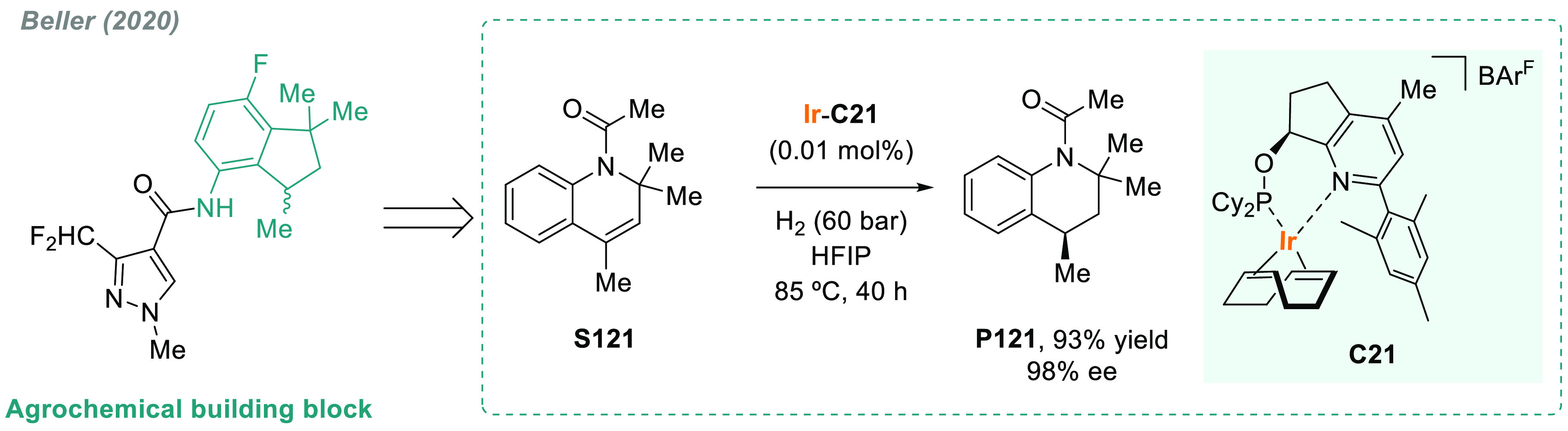
Another type of endocyclic allyl amines, amino acids S122, were hydrogenated by Zhou and co-workers using iridium complexes of P,N-ligands with a spiro backbone (C3c and C3d) (Scheme 80).436 In particular, C3c was chosen as the best catalyst for N-Boc allyl amines, while C3d was used for N-Me and unprotected allyl amines. The resulting chiral heterocyclic acids P122 were afforded in excellent enantioselectivities, and the potential utility was demonstrated by the concise asymmetric synthesis of (R)-nipecotic acid and (R)-tiagabine (Figure 8). In fact, chiral cyclic amines with a β-carboxylic acid or derivatives are common structural motifs in many bioactive compounds. For example, the key intermediate for the preparation of a histone deacetylase inhibitor (HDAC, Scheme 81) was obtained via the ruthenium-catalyzed AH of S123 using L17d.437 This achievement was reported by a team from Roche in 2014. They disclosed that the presence of a carboxylic acid was crucial for the rapid hydrogenation of the unsaturated bond.
Scheme 80. Iridium-Catalyzed AH of Endocyclic N-Allyl Amines.
Scheme 81. Asymmetric Synthesis of a HDAC Inhibitor via AH.
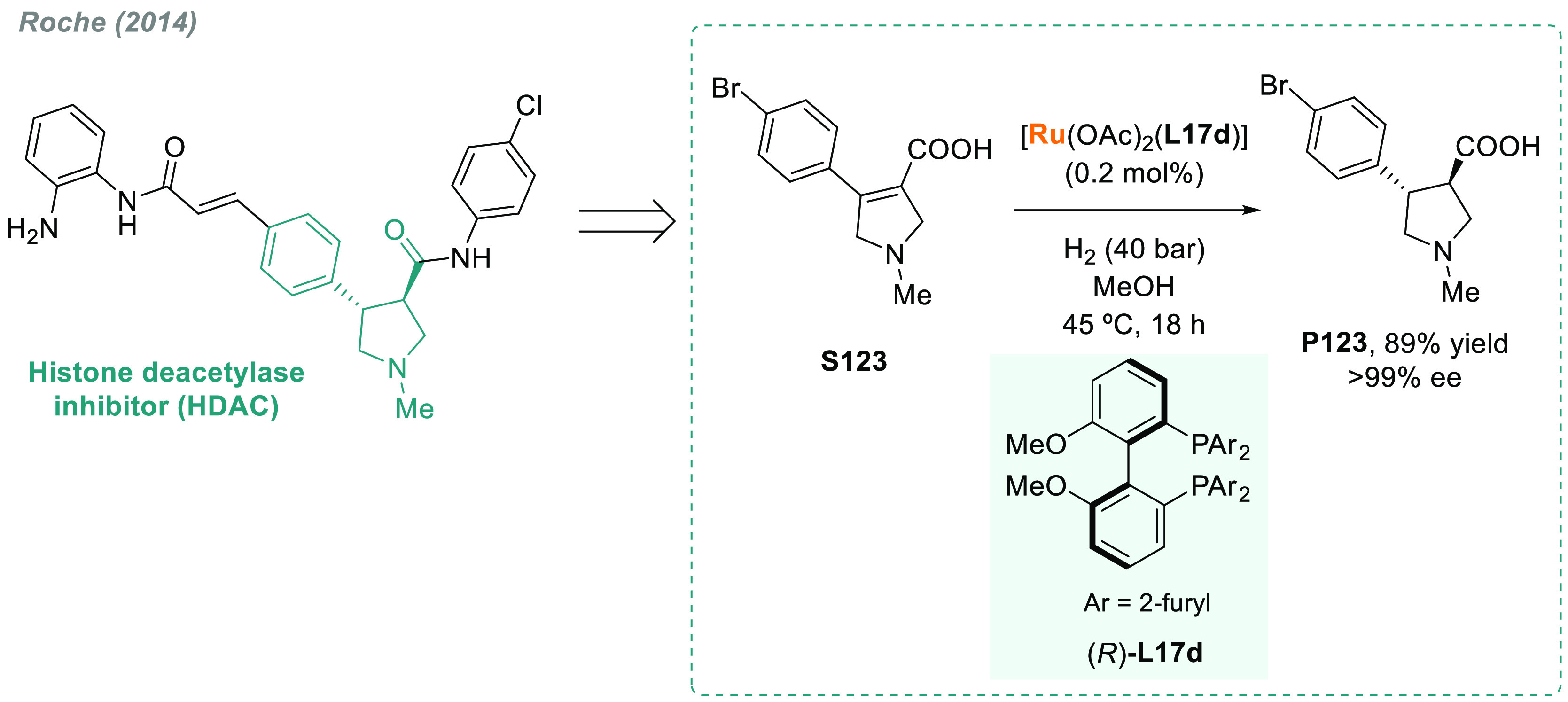
Chiral γ-lactams are ubiquitous in biological compounds. The antiepilepsy drug Brivaracetam and the PDE-4 inhibitor Rolipram are examples of γ-lactam clinical drugs (Figure 8). These lactams are key building blocks in medicinal chemistry as masked γ-amino acids. In their efforts to prepare γ-lactams in optically pure form, Yin, X. Zhang, and co-workers recently developed the AH of S124 using Rh/ZhaoPhos-L11a (Scheme 82).438 A wide range of enantioenriched γ-lactams P124 were furnished in excellent yields and enantioselectivities. Interestingly, the catalytic system successfully tolerated a free NH amide group which actually played a positive effect by hydrogen bonding with the thiourea motif of L11a.
Scheme 82. Rhodium-Catalyzed AH of β-Aryl-Substituted α,β-Unsaturated Lactams.
Another important drug with a chiral center in the γ-position of the amino group is ramelteon, a selective melatonin MT1/MT2 receptor agonist. Yamashita’s laboratory reported that Rh/JosiPhos-L21e was a highly effective catalyst for the AH of a key precursor, the unprotected allyl amine S125 (Scheme 83).439 Interestingly, the primary amino group might act as an anchoring group to the rhodium atom. Chiral amine P125 was smoothly prepared in 92% ee using very mild conditions.
Scheme 83. Rhodium-Catalyzed AH of Unprotected Primary Allyl Amine.
Finally, Huang, Geng, Chang, and co-workers recently reported a practical combination of AH and reductive amination in the enantioselective synthesis of the chiral β-aryl amines P126 (Scheme 84). Starting from readily available anilines and α,β-unsaturated aldehydes S126, they described a one-pot hydrogenation that involved DRA and AH, using Rh/L9b complex as catalyst.440 Control experiments revealed that the construction of the C–N bond beforehand helped to pave the way for the subsequent AH of the corresponding N-allyl amine.
Scheme 84. Combination of AH and Reductive Amination.
6. Asymmetric Hydrogenation of Heteroaromatic Compounds
The direct AH of heteroaromatic compounds provides straightforward access to the corresponding chiral saturated N-cyclic skeletons. This is a less explored area than the AH of prochiral unsaturated amines such as imines or enamides.30−32,441 This may be ascribed to several reasons: (a) the possible deactivation of the chiral catalysts due to the basicity of the nitrogen atom of the aromatic ring; (b) the lack of a coordinating group in simple aromatic compounds; and (c) the increased stability due to the aromaticity of these compounds, which might require harsher conditions. Despite all these issues, remarkable advances have been disclosed during the past decade. In this section, we will review these advances along with the pioneering works and research milestones in this field, focusing mainly on heteroarenes such as quinolines, pyridines, quinoxalines, and indoles.
6.1. Quinolines and Derivatives
Optically pure tetrahydroquinolines (THQs) and their derivatives are of great importance due to their pharmaceutical and agrochemical applications,442 as they are basic units in many natural products. Among the different strategies for their preparation, the AH of quinolines is the most effective.443
In 2011, Fan, Yu, Chan, and co-workers reported the AH of a wide range of quinoline derivatives (S127) catalyzed by chiral cationic η6-arene Ru(II)-diamine complexes (Scheme 85).444 Interestingly, for 2-alkylquinolines, C22 exhibited outstanding enantioselectivity as P127a (R = alkyl) were attained in excellent enantioselectivities (99% ee for most of the cases). To the best of our knowledge, this is the protocol with the highest level of enantioselectivity for 2-alkyl-substituted quinolines S127a. In contrast, the AH of 2,3-dialkyl quinolines S127b provided a very low ratio of diastereoselectivity. The same authors reported that similar Ru-diamine complexes were capable of efficiently hydrogenating S127a in solvent-free conditions,445 in neat water,446 in ionic liquids,447,448 and in oligo(ethylene glycol)s through host–guest interactions.449 On the other hand, the AH of 2-aryl-substituted quinolines was more challenging, and only a few examples have been reported. The same group extended the use of these Ru(II)–diamine complexes and found that C14h was a highly active catalyst for the AH of 2-aryl quinolines, affording the corresponding P127a (R = aryl) in excellent yields and enantioselectivities (Scheme 85).444 Previously, Fan, Xu, and co-workers had already disclosed that air-stable and phosphine-free iridium complexes were also efficient catalysts for the highly enantioselective hydrogenation of quinoline derivatives.450
Scheme 85. Metal-Catalyzed AH of Unfunctionalized 2-Quinolines and 2,3-Disubstituted Quinolines.
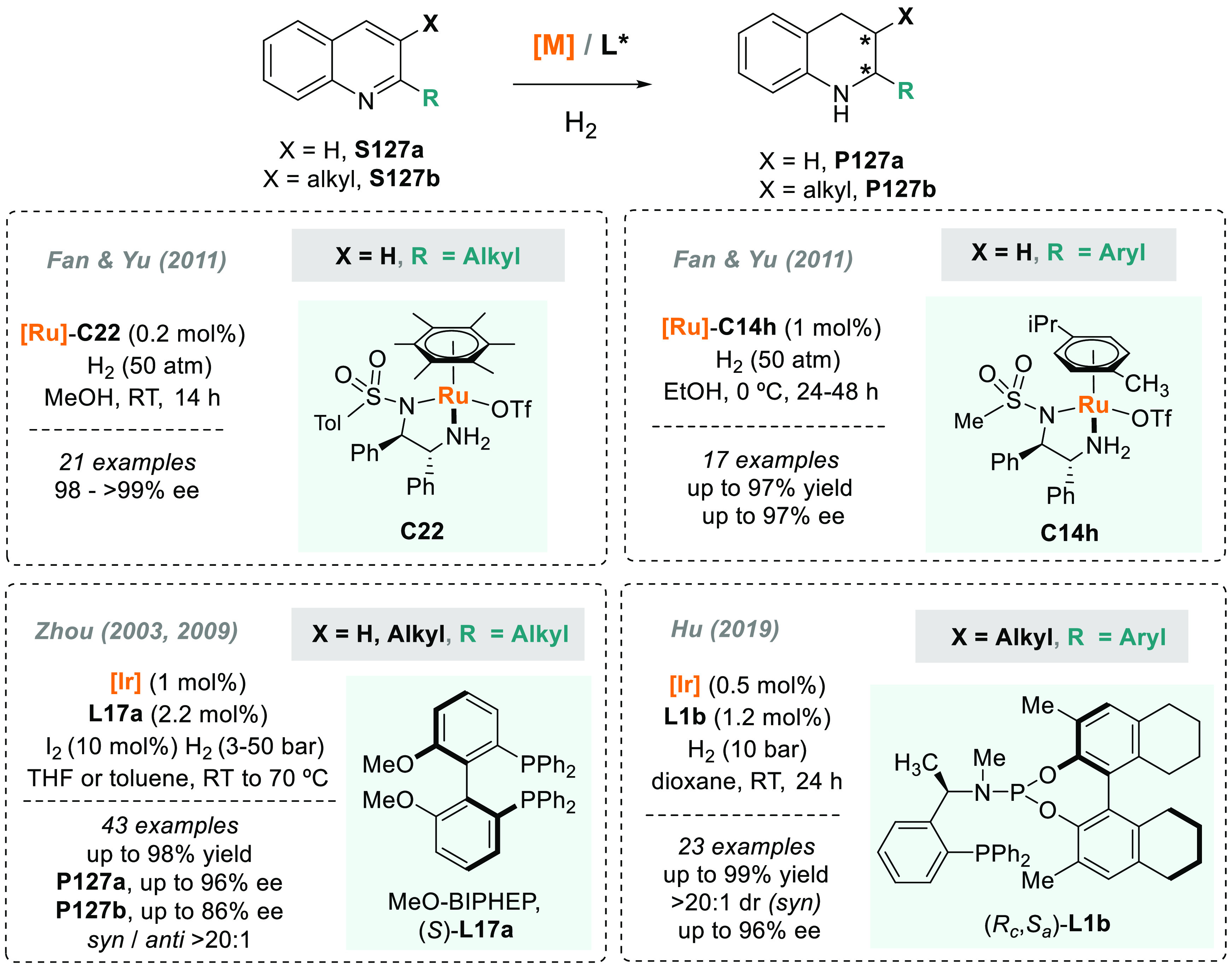
Y.-G. Zhou’s laboratory reported other important achievements in the field. In 2003, they pioneered the use of iridium complexes bearing axially chiral phosphines that, in combination with I2, were found to be highly active in the AH of 2-alkyl quinolines (Scheme 85).451 In particular, and when using L17a, P127 were obtained in excellent yields and enantioselectivities (up to 96% ee). Using the same catalytic system, the substrate scope was further expanded to a wide range of 2-functionalized quinoline derivatives.452−457 More importantly, 2,3-dialkylquinolines were also efficiently hydrogenated using the same catalyst, although P127b were furnished from moderate to good enantioselectivities (up to 86% ee). Zhou’s group later reported the iridium-catalyzed AH of quinolines activated by Brønsted acids, thus avoiding catalyst activation using I2.458
The highly enantioselective and catalytic hydrogenation of 3-alkyl-2-arylquinolines remained a challenging task until 2019, when Hu and co-workers examined the use of structurally fine-tuned phosphine-phosphoramidite L1-type ligands (Scheme 85).459 Using L1b as a chiral ligand, a highly diastereo- and enantioselective iridium-catalyzed AH of unfunctionalized 3-alkyl-2-arylquinolines was disclosed. The transformation displayed broad functional group tolerance, thus furnishing a wide range of 2,3-disubstituted tetrahydroquinolines P127b in up to 96% ee and with perfect cis-diastereoselectivity. In contrast, the AH of 2,3-diaryl quinolines remains unsolved. In general, the AH of 2,3-disubstituted quinolines represents a more difficult task due to the requirement of diastereocontrol in the construction of two vicinal stereogenic centers. Nevertheless, the AH of functionalized 2,3-disubstituted quinolines with a phthaloyl460,461 or an ester462 group at the 3-position and an alkyl group at the 2-position of quinoline has been accomplished.
The chemistry of chiral vicinal diamines and their derivatives has attracted a great deal of interest because they are key substructures in many biologically active compounds. In 2016, Fan and co-workers reported the highly enantioselective synthesis of vicinal diamines by direct AH of 2,2′-bisquinolines S128 (Scheme 86).463 Using the Ru/diamine complex C14i, the reaction proceeded with very good diastereoselectivity while reaching an unprecedented level of enantioselectivity (>99% ee in the best cases). The resulting chiral vicinal diamines P128 can be used as new chiral ligands. Similarly, the same group later described the use of Ru-complex C22 for the AH of 2-(pyridine-2-yl)quinoline derivatives S129 (Scheme 86).118 Based on the resulting chiral scaffolds P129, a small library of tunable chiral pyridine-aminophosphine ligands were readily prepared.
Scheme 86. Ruthenium-Catalyzed AH of 2,2′-Bisquinoline Derivatives.
In addition, the enantioselective hydrogenation of 2,6-bis(quinolinyl) pyridines (PyBQs) or terpyridine-type N-heteroarenes S130 was successfully developed in 2020 by Fan’s group using Ru(diamine) complex C14j as catalyst (Scheme 87).464 The method provided partially reduced chiral pyridine-amine-type products P130 in high yields with excellent diastereo- and enantioselectivity, which can serve as a new class of chiral nitrogen-donor ligands.
Scheme 87. Ruthenium-Catalyzed AH of 2,6-Bis(quinolinyl) Pyridines (PyBQs).
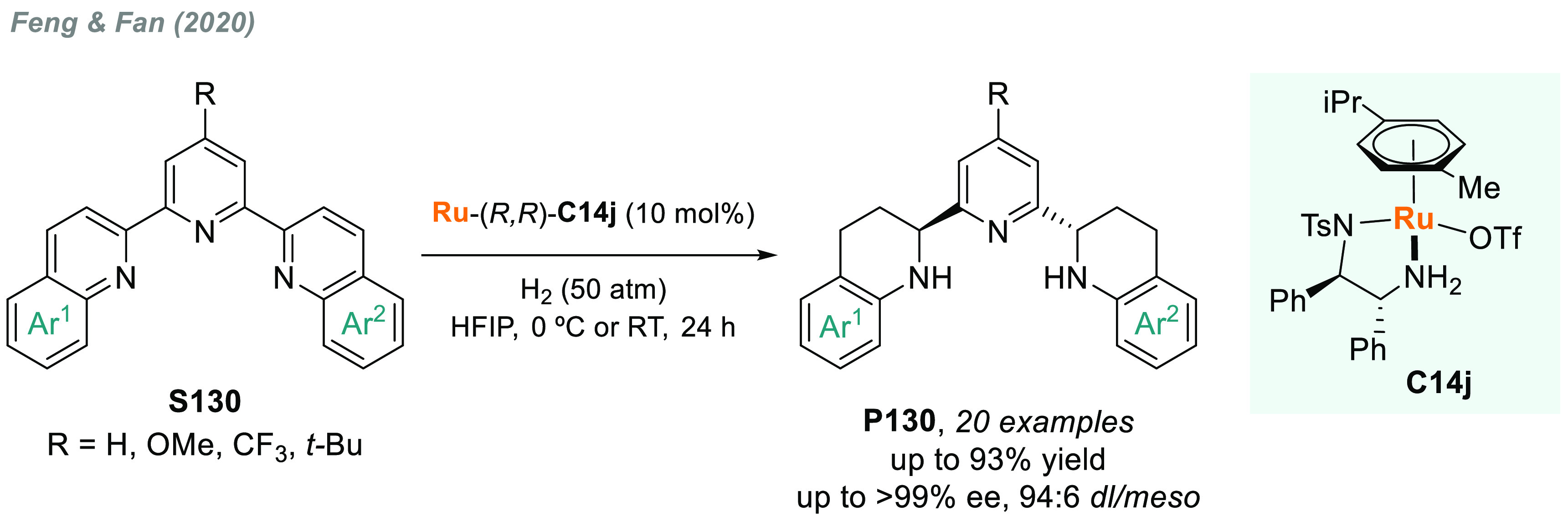
The AH of quinolines using a non-noble metal was also accomplished by Lan, Liu, and co-workers in 2020. Using a chiral pincer manganese catalyst, the AH of quinolines S127a was achieved in high yields and enantioselectivities (up to 97% ee, Scheme 88).465 Interestingly, an effective π–π interaction between the C=N double bond and the imidazole ring of the ligand L58 ensured precise regulation of the enantioselectivity. Undoubtedly, this represents an important precedent for the efficient AH of N-heteroaromatics without the need for precious metals.
Scheme 88. Mn-Catalyzed AH of Quinolines Enabled by π–π Interaction.
Although the catalytic AH of quinolines is the most direct and reliable approach, Mashima, Ratovelomanana-Vidal, Ohsima, and co-workers reported the AH of quinolinium salts using cationic Ir(III) halide complexes with difluorphos (L20).466 This catalyst system successfully converted both 2-aryl- and 2-alkyl-quinolinium salts to the corresponding THQs with excellent enantioselectivities (up to 95% ee). The AH of quinolinium salts has also been applied as the key step for the synthesis of vabicaserin.467
The asymmetric synthesis of THQs using tandem processes involving hydrogenation has been thoroughly explored in recent years. In 2019, Fan, He, and co-workers reported a novel strategy for the synthesis of chiral vicinal diamines based on a consecutive Ir- or Ru-catalyzed tandem intermolecular reductive amination/asymmetric hydrogenation.468 Using the appropriate catalyst (C14i or C23), 2-quinoline aldehydes (S131) and feedstock anilines were transformed into a broad range of sterically tunable chiral diamines P131, which were afforded in high yields with excellent enantioselectivity (Scheme 89). The usefulness and practicality of the method were exemplified by the transformation of P131 into sterically hindered chiral N-heterocyclic carbene precursors.
Scheme 89. Consecutive Intermolecular Reductive Amination/AH.
The same group developed a synthetic route to chiral THQs via sequential intramolecular hydroamination and ruthenium-catalyzed AH of anilino-alkynes S132 (Scheme 90).469 Alternatively, X. Zhang and co-workers reported a one-pot process involving N-Boc deprotection/intramolecular asymmetric reductive amination using S133 (Scheme 90).470 This latter methodology was also applied to the synthesis of tetrahydroisoquinolines471 as well as enantioenriched dibenz[c,e]azepines.472
Scheme 90. Asymmetric Synthesis of THQs through Sequential Processes.
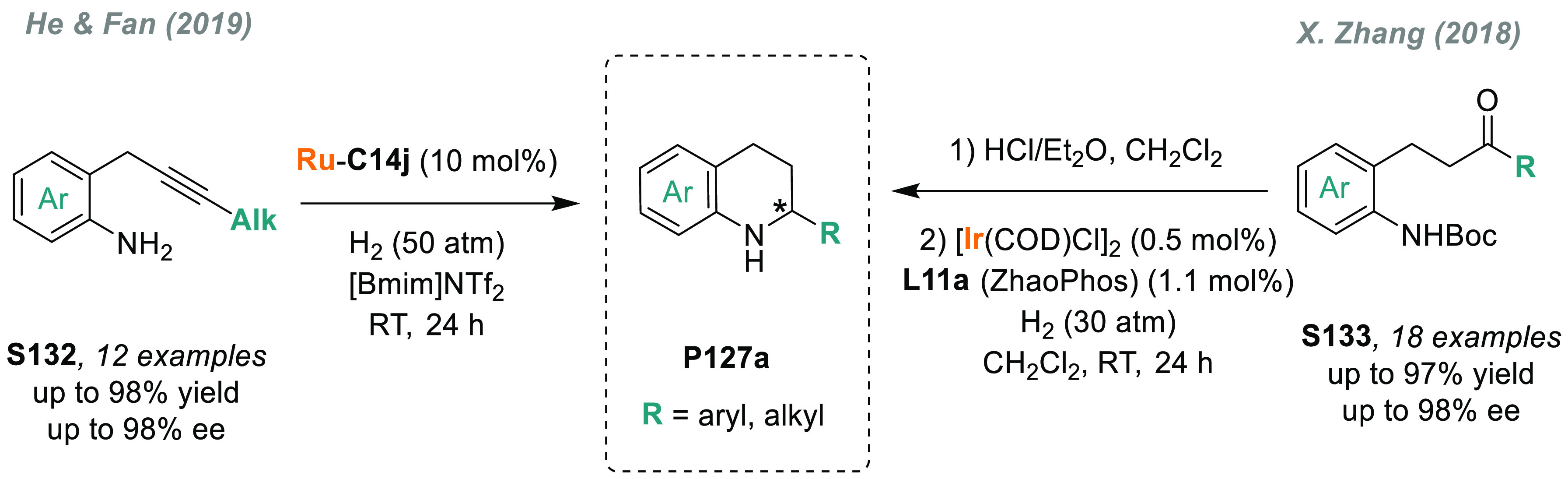
6.2. Isoquinolines, Pyridines, and Pyridinium Salts
In contrast to quinolines, the transition metal-catalyzed AH of isoquinolines has remained significantly underdeveloped. The resulting products (1,2,3,4-tetrahydroisoquinolines, THIQs) have a stronger basicity and coordination ability, which can lead to a more facile catalyst deactivation. Typically, the strategy most widely used for the AH of substituted isoquinolines was substrate activation via isoquinolinium salts.473−477 However, the requirement of prior formation of the salt for activation was a considerable limitation. Furthermore, in situ and transient activation using chloroformates478 or halogenides479,480 has also been reported. An example of this approach was provided by Zhou’s group in 2017 (Scheme 91).480 By using Ir/(R)-L9b as catalyst, a variety of both isoquinolines and pyridines (S134) were hydrogenated in excellent yields and enantioselectivities. The reaction was promoted using halogenide trichloroisocyanuric acid (TCCA) as traceless activation reagent. Mechanistic studies indicated that hydrogen halide generated in situ acted as the substrate activator. Nevertheless, and although this method avoided the tedious steps of the introduction and removal the activation groups, the reaction conditions were harsh as high temperatures and H2 pressure were required. To overcome these limitations, Stoltz and co-workers recently reported a highly straightforward method for the iridium-catalyzed AH of 1,3-disubstituted isoquinolines S135 under very mild conditions (Scheme 91).481 The reaction, which involves a commercially available ligand (L21f), proceeded in good yields with high levels of enantio- and diastereoselectivity. The key to the success of this approach was the introduction of a directing group at the C1 position that enabled hydrogenation to occur under mild reaction conditions. As a result, a wide variety of chiral THIQs P135 were prepared in optically pure form, in a broad scope and with a very high tolerance of Lewis basic functionalities. Using a similar catalytic system, the same authors applied this hydrogenation reaction as the key step to the concise total synthesis of (−)-jorunnamycin A and (−)-jorumycin (Scheme 92), which were attained with high efficiency after 15 and 16 steps, respectively.482
Scheme 91. Iridium-Catalyzed AH of Isoquinolines and Pyridines.
Scheme 92. Enantioselective Hydrogenation as the Key Step for the Total Synthesis of (−)-Jorunnamycin A and (−)-Jorumycin.
Pyridines are also challenging substrates. Xu and co-workers reported the iridium-catalyzed AH of trisubstituted pyridine derivatives. They described that the iridium complex generated in situ from [Ir(COD)Cl]2, difluorPhos-L20, and I2 was an excellent catalyst for the AH of S137, affording the corresponding chiral amines P137 in excellent yields and enantioselectivities (Scheme 93).483 The same group reported that P-Phos (L16) could also be used as a chiral ligand without diminishing the reactivity or enantioselectivity.484 These protocols, which could also be applied to the AH of quinolines, were more efficient than the previous one reported by Zhou’s group.485
Scheme 93. Iridium-Catalyzed AH of Trisubstituted Pyridines.
To facilitate the AH of pyridines, the activation of pyridine derivatives was first studied. In 2005, Legault and Charette demonstrated that chiral piperidine derivatives could be attained with high enantioselectivities (up to 90% ee) via the AH of N-acyliminopyridinium ylides using a Ir/P,N catalyst.486 Later, Andersson further developed this methodology with a screening of iridium catalysts with chiral P,N-ligands.487 In the search for an operationally simple protocol for large-scale synthesis, the N-benzylpyridinium salts emerged as the best method for pyridine activation. First described by Y.-G. Zhou’s group488 and applied by X. Zhang’s489 and Lefort’s laboratories,490 this method provided a better approach in terms of safety and applicability. Using their catalytic systems, the subsequent iridium-catalyzed AH of these N-benzylpyridinium salts furnished a wide variety of 2-arylpyridines in both high yields and enantioselectivities, but with limited application to 2-alkylpyridines. Qu et al. then reported the use of a new rigid P-stereogenic P,N-ligand L59 for the iridium-catalyzed AH of S138 (Scheme 94).491 Chiral α-(hetero)aryl piperidines P138a were afforded in high levels of enantioselectivity. Using the same ligand, chiral α-alkyl piperidines P138b were also prepared but with lower enantiocontrol.492 This catalytic system was also applied in the asymmetric construction of the indenopiperidine core of an 11β-HSD-1 inhibitor.493
Scheme 94. Iridium-Catalyzed AH of Pyridinium Salts.
In addition, Mashima and Zhou’s laboratories independently reported another strategy for the AH of Brønsted acid-activated multisubstituted pyridines using enantiopure binuclear iridium complexes, thus affording the corresponding chiral piperidines in high diastereo- and enantioselectivities (up to 90% ee).494,495 Later, Mashima expanded this methodology to the AH of 2-aryl-3-amidopyridinium salts, delivering the corresponding chiral piperidines with high diastereoselectivities and good enantioselectivities (up to 86% ee).496 These piperidines containing two contiguous centers as structural moieties are present in many neurokinin-1 (NK1) receptor antagonist derivatives, such as (+)-CP-99994 and Vofopitant. Due to the importance of these structural units, X. Zhang and co-workers recently described the AH of 2-aryl-3-phthalimidopyridinium salts S139 driven by the Ir/SegPhos (S)-L9b catalytic system (Scheme 95).497
Scheme 95. Iridium-Catalyzed AH of 2-Aryl-3-phthalimidopyridinium Salts.
Furthermore, the AH of 3-substituted pyridinium salts was developed by Lefort and co-workers using a Rh-JosiPhos catalyst with ee values up to 90%.498 Working with similar substrates, Y.-G. Zhou’s laboratory recently reported a highly enantioselective hydrogenation of 3-hydroxypyridinium salts using Ir/f-binaphane (L7) followed by sequential Swern oxidation, thus furnishing chiral 6-substituted piperidin-3-ones in excellent enantioselectivities (up to 95% ee).499 On the other hand, in 2018, Peters and co-workers reported an alternative synthesis of piperidines from isoxazolinones by Pd/Ir relay catalytic hydrogenation of the initially formed 3,4-dihydropyridines.500
6.3. Quinoxalines and Pyrazines
The 1,2,3,4-tetrahydroquinoxaline ring system is of great interest as a key structural unit in many therapeutically active compounds. In 2011, Fan’s group described that the family of half-sandwich Ru-diamine complexes, which had already been used for the AH of quinolines, were excellent catalysts for the AH of other heteroaromatic compounds such as quinoxalines. In particular, catalyst C14a was used for the enantioselective hydrogenation of 2-alkyl and 2,3-dialkyl quinoxalines S140 with up to 99% ee (Scheme 96).501 Of note, the bulky and weakly coordinating counteranion (BArF) was found to be critical for the high enantioselectivity and/or diastereoselectivity. On the other hand, and using C24, 2-aryl quinoxalines S140c were also hydrogenated in excellent ee values (up to 96% ee, Scheme 96). Later, in 2013, Ohkuma and co-workers reported an alternative protocol for the enantioselective hydrogenation of 2-alkyl quinoxalines using chiral ruthenabicylic complexes.129
Scheme 96. Ruthenium-Catalyzed AH of Quinoxalines.
Although this work is the most successful precedent in the field, iridium catalysts had also found use in the AH of quinoxalines. In this regard, Chan, Xu, Fan, and co-workers described a highly efficient AH of quinoxalines using Ir/H8-binapo at low catalyst loading.502 Later, Ratovelamana-Vidal and co-workers described a general AH of a wide range of 2-alkyl- and 2-aryl-substituted quinoxaline derivatives using a cationic dinuclear triply chloride-bridged Ir(III) complex bearing L20 as a chiral ligand.503,504 Of note, the efficiency of the catalytic system was demonstrated through a broad scope with excellent enantioselectivities (up to 95% ee, Scheme 96).
Mashima and co-workers showed that the use of amines as additives had a positive effect for the iridium-catalyzed AH of quinoxalines.505 In particular, they found that N-methyl-p-anisidine (MPA) was the best amine additive for achieving high enantioselectivity. More recently, Nagorny applied new chiral SPIROL-based phosphinite ligands for the iridium-catalyzed AH of heterocycles, including quinoxalines, in good to excellent enantioselectivities.506
While 2-alkyl- and 2-aryl-substituted quinoxalines have been hydrogenated at useful levels of enantioselectivity, the AH of other derivatives such as quinoxaline-2-carboxylates is still underdeveloped, although the resulting chiral cyclic amino acids are highly valuable. To the best of our knowledge, there is only one example in the literature, affording the corresponding chiral amino acid in moderate enantioselectivity (74% ee).507
Iridium catalysis was also applied to the AH of pyrrolo/indolo[1,2-a]quinoxalines (Scheme 97). In 2018, Zhou’s laboratory described the highly enantioselective hydrogenation of S141 with Ir/Synphos-L46, providing facile access to chiral P141 with up to 97% ee.508 However, the addition of acetic anhydride was pivotal for suppressing the rearomatization of hydrogenation products. The system was also applied to the AH of phenanthridines (see section 6.5). The same laboratory studied the iridium-catalyzed AH of pyrrolo[1,2-a]pyrazines S142 (Scheme 97).509 In this case, the preparation of the corresponding salt or the addition of a N-protecting group was not required. The reaction was performed under very mild conditions, and it exhibited excellent activity and enantioselectivity when using L17e as a chiral ligand. The group previously reported the enantioselective synthesis of P142 via the iridium-catalyzed AH of pyrrolo[1,2-a]pyrazinium salts, which were prepared after substrate activation using alkyl halides.510
Scheme 97. Iridium-Catalyzed AH of Pyrrolo/indolo[1,2-a]quinoxalines and of Pyrrolo[1,2-a]pyrazines.
In 2016, Zhou’s laboratory reported a facile method for the synthesis of chiral piperazines P143 through iridium-catalyzed AH of 3-substituted pyrazinium salts S143 using JosiPhos-type ligand L21a (Scheme 98).511 The system showed broad substrate scope with good to excellent selectivity (up to 92% ee). Furthermore, 2,3- and 3,5-disubstituted pyrazinium salts were also hydrogenated with high yields and enantioselectivities when using the appropriate chiral ligand. To demonstrate the applicability of the methodology, Vestipitant, a potent and selective NK1 receptor antagonist, was prepared in just two steps.
Scheme 98. Iridium-Catalyzed AH of Pyrazinium Salts.
In a similar approach, Mashima and co-workers reported the iridium-catalyzed enantioselective hydrogenation of tosylamido-substituted pyrazines S144. The addition of N,N-dimethylanilinium bromide (DMA·HBr) enhanced the catalytic activity of the iridium complexes, as well as the enantioselectivity (Scheme 98).512 Chiral tetrahydropyrazines with an amidine skeleton (P144) were obtained with good to excellent enantioselectivities (up to 92% ee) using dinuclear triply chloro-bridge Ir(III) complexes bearing chiral difluorPhos (L20). The resulting tetrahydropyrazines P144 are versatile precursors for the preparation of chiral piperazine derivatives without loss of enantioselectivity.
6.4. Indoles
Iridium chiral complexes were also applied to the AH of N-protected indoles to obtain chiral indolines, which are common structures occurring in alkaloids and other natural or synthetic products with biological activity.513 Pfaltz pioneered the use of cationic Ir complexes derived from PHOX or other chiral P,N-ligands in the AH of 2-substituted N-protected indoles S145a (Scheme 99).514 In particular, C25 gave the highest enantioselectivities, and the results obtained demonstrate that the protecting group (N-Boc, N-acetyl, or N-tosyl) influences both reactivity and enantiomeric excess. Moreover, various examples of 3-substituted indoles were also hydrogenated, using the right combination of catalyst and protecting group. On the other hand, Han, Ding, and co-workers recently reported that Ir/SpinPHOX C4a is an efficient catalyst for the AH of both 2- and 3-substituted N-protected indoles (Scheme 99).515 The corresponding chiral indolines were afforded in excellent yields and enantioselectivities (>99% ee in most cases).
Scheme 99. Iridium-Catalyzed AH of N-Protected Indoles.
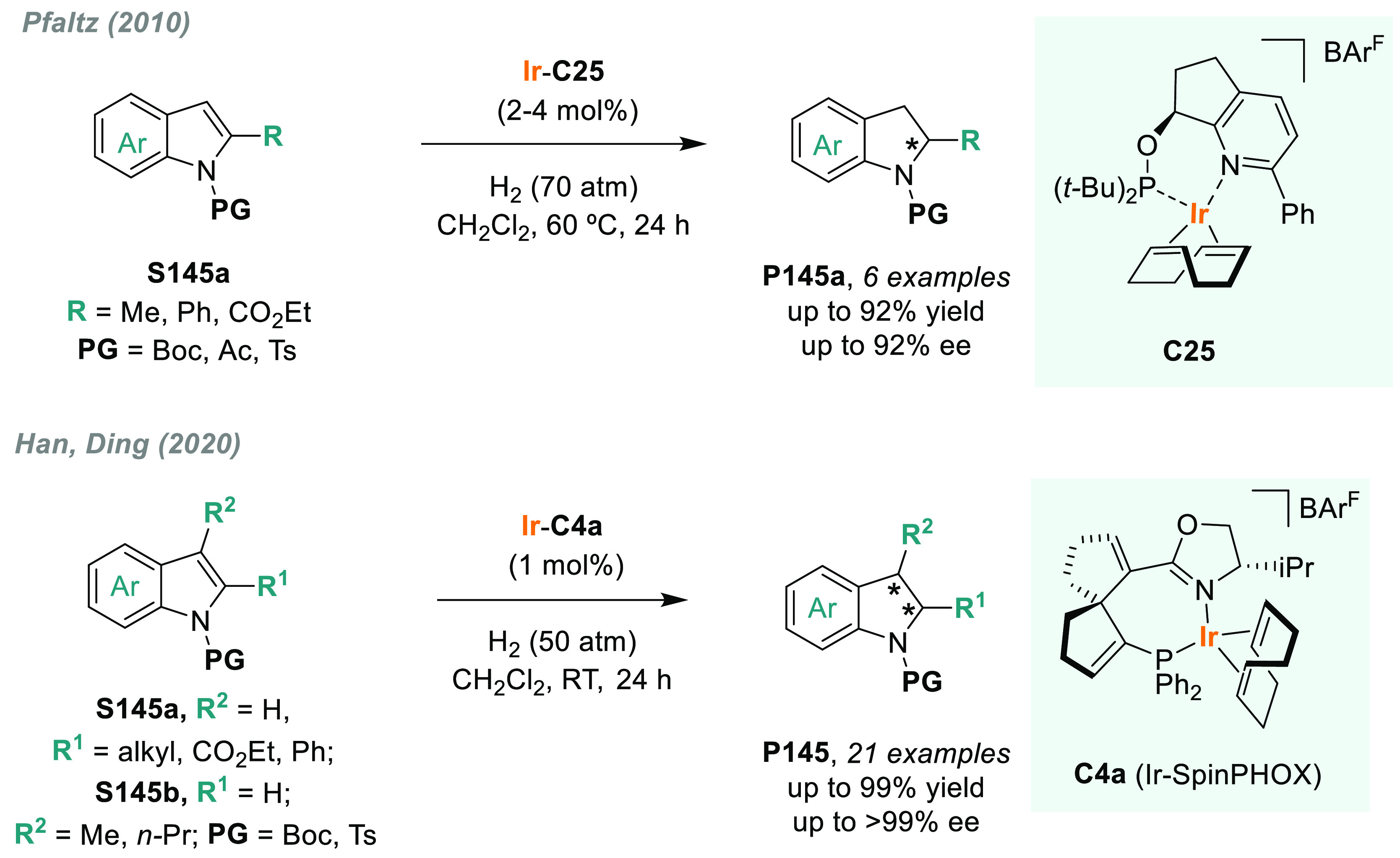
Prior to these iridium complexes, the only suitable catalytic systems known for the AH of N-protected indoles were Ru and Rh complexes bearing the trans-chelating chiral diphosphine (S,S)-(R,R)-PhTRAP ligand (L60), first reported by Kuwano’s group.516 In their work, a wide range of N-protected indoles were hydrogenated with high efficiency, thus providing the corresponding chiral indolines in excellent yields and enantioselectivities.517 For example, the ruthenium-catalyzed AH of N-Boc 2-substituted (S145a) and N-tosyl 3-substituted indoles (S145b) furnished the corresponding indolines (P145) in excellent yields using L60 (Scheme 100).518,519 2-Substituted indole esters were also hydrogenated by the groups of Minaard520 and Agbossou-Niedercorn521 using Rh complexes bearing PinPhos or WalPhos as catalysts, although only moderate enantioselectivities were achieved.
Scheme 100. Metal-Catalyzed AH of N-Protected Indoles Using PhTRAP.
The methodology developed by Kuwano and co-workers allows the preparation of a wide range of optically active indolines with a chiral center at the 3-position. A team at Bristol-Myers Squibb recently used it in the enantioselective total synthesis of (+)-Duocarmycin SA, which is a potent antitumor antibiotic (Scheme 101).522 The key tricyclic core was constructed through a highly enantioselective hydrogenation of indole S146 using L60.
Scheme 101. Synthesis of (+)-Duocarmycin SA via Rhodium-Catalyzed AH.
The progress achieved in the AH of N-protected indoles was in sharp contrast to the AH of simple unprotected indoles, which remain a challenge in organic synthesis. However, significant advances were made in this field in the last ten years.
In 2010, Y.-G. Zhou, X. Zhang, and co-workers developed the first highly enantioselective hydrogenation of simple indoles using Pd/(R)-H8-BINAP (L61) with a Brønsted acid (l-camphorsulfonic acid, l-CSA) as an activator and TFE as solvent (Scheme 102).523 This methodology provided an efficient route to make chiral indolines from unprotected indoles in excellent enantioselectivities (up to 96% ee). In 2014, Zhou’s laboratory reported an extensive substrate scope, including 2-substituted and 2,3-disubstituted indoles (S147).524 Using the same reaction conditions as the previous work, chiral indolines were prepared in up to 98% ee (Scheme 102). The main drawback of this approach was the low activity and/or enantioselectivity of the 2-aryl-substituted indoles. Interestingly, a mechanistic study using DFT calculations revealed that the hydrogenation occurs through a stepwise, ionic, and outer-sphere mechanism. As an alternative approach, W. Zhang reported that Pd/(S)-C10-BridgePhos is a highly efficient catalyst for the AH of 2-, 3-, and 2,3-substituted unprotected indoles.525
Scheme 102. Catalytic Strategies toward the AH of Unprotected Indoles.
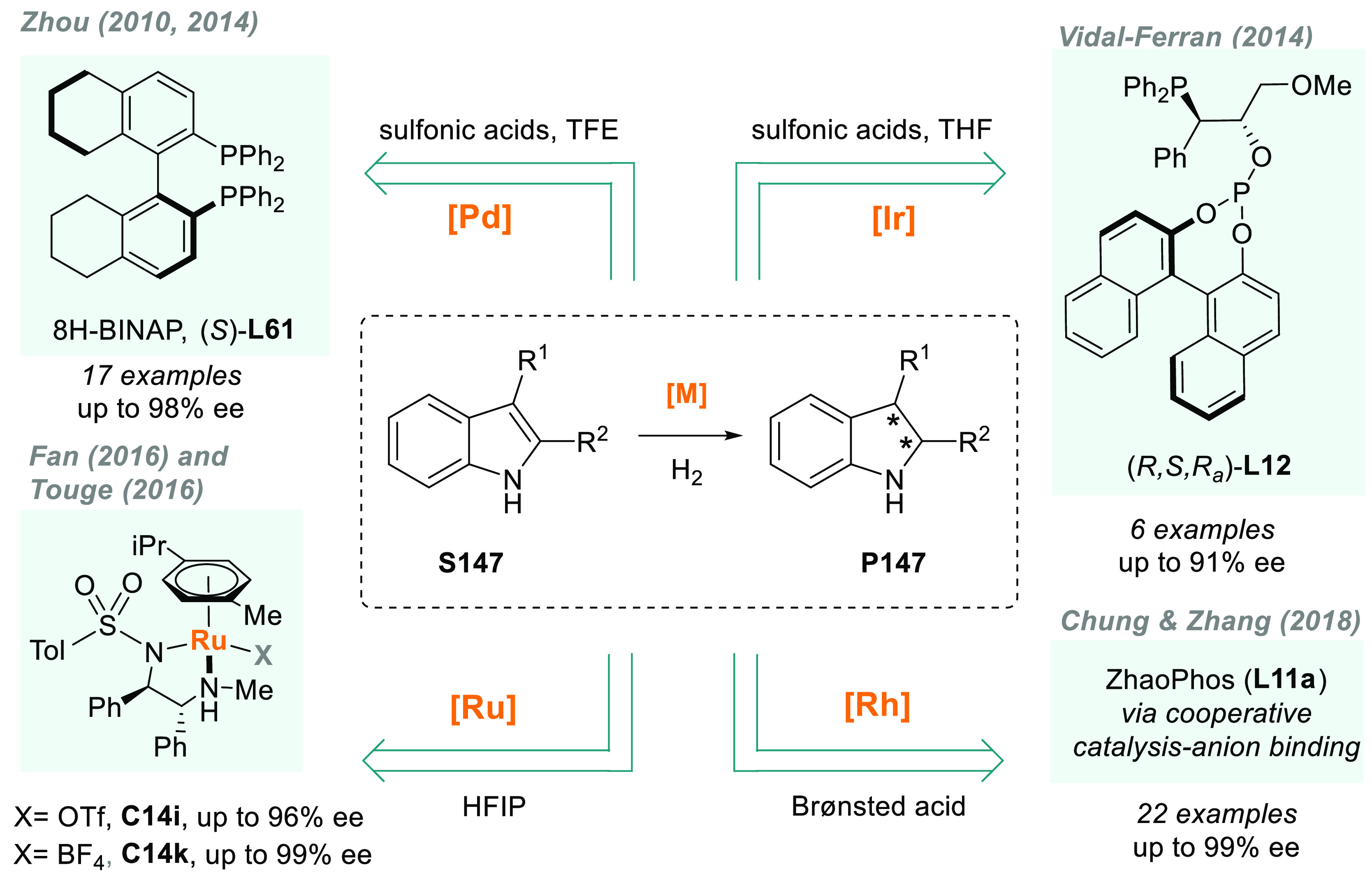
Sulfonic acids were also used as Brønsted acids in the iridium-catalyzed AH of S147, reported by Vidal-Ferran and co-workers, using a P-OP ligand L12 and THF as solvent (Scheme 102).526 Enantiomerically enriched indolines P147 were attained in up to 91% ee. The Brønsted acid (rac-CSA), which could be reused by addition of heterogeneous additives, activates the indole ring by breaking its aromaticity. Interestingly, the reaction proceeded by a stepwise process: Brønsted acid-mediated C=C isomerization, thus generating the corresponding iminium ion, followed by asymmetric hydrogenation.
Chung and X. Zhang also envisioned a similar strategy. They developed an efficient method to obtain optically pure indolines using a Rh/ZhaoPhos-L11a complex (Scheme 102).527 Various 2-substituted and 2,3-disubstituted indoles S147 were hydrogenated with high enantioselectivities. By employing HCl as Brønsted acid, an active iminium ion intermediate was formed and reduced. The thiourea anion binding of L11a proved crucial to achieve high enantioselectivity and reactivity.
Fan and co-workers also studied the AH of unprotected indoles using half-sandwich Ru–diamine complexes. In particular, when using C14i with a triflate as counteranion, indolines P147 were afforded in up to 96% ee (Scheme 102).114 Of note, the reaction was performed under very mild conditions at room temperature and using HFIP as solvent, which significantly influenced catalytic performance. Excellent enantio- and diastereoselectivities were obtained for a wide range of indole derivatives. Simultaneously, Touge and Arai and co-workers described that a similar Ru complex with a tetrafluoroborate as counteranion (C14k) also gave excellent yields and enantioselectivities in very mild conditions (Scheme 102).528
Subsequently, other related transformations using Pd catalysis emerged, including dehydration-triggered AH529 and consecutive Brønsted acid/Pd-complex-promoted tandem reactions.530 Moreover, 3-(toluenesulfonamidoalkyl)-indoles were synthesized and hydrogenated to form chiral indolines.531 Of note, Y.-G. Zhou’s group recently reported a facile synthesis of chiral indolines through the AH of in situ generated indoles.532 The reaction was developed through an intramolecular condensation, deprotection, and palladium-catalyzed AH using L61 in a one-pot process (Scheme 103). Chiral 2-alkyl-substituted indolines P148 were furnished in excellent yields and enantioselectivities (up to 96% ee).
Scheme 103. One-Pot Synthesis of Chiral Indolines via Palladium-Catalyzed AH.
6.5. Other Heteroaromatic Compounds
In 2011, Zhou, Fan, and co-workers disclosed the first AH of simple unprotected pyrroles. In their work, the Brønsted acid-activation mode was applied to the partial AH of pyrroles S149 (Scheme 104).533 Using sulfonic acid as activator, a highly enantioselective palladium-catalyzed partial hydrogenation using C4-TunePhos (L5a) was reported, providing chiral 2,5-disubstituted 1-pyrrolines P149 with up to 92% ee. Previously, Kuwano’s group applied the PhTRAP ligand L60 to the ruthenium-catalyzed AH of N-Boc protected pyrroles (Scheme 104).534 Using this catalytic system, pyrroles S150 were hydrogenated with high ee’s to give chiral pyrrolidines P150a or 4,5-dihydropyrroles P150b.
Scheme 104. Metal-Catalyzed AH of Pyrroles.
The Ru/L60 system was further exploited by Kuwano’s group for the AH of other single-ring heteroarenes. Using this catalytic system, oxazoles S151 and N-Boc imidazoles S152 were hydrogenated into the corresponding chiral oxazolines P151 and imidazolines P152, respectively (up to 99% ee, Scheme 105).535 These hydrogenation products are highly valuable synthetic intermediates, as they can be easily converted to 1,2-diamines or β-amido alcohols without loss of enantiopurity. On the other hand, fused aromatic rings consisting of two (or more) heteroarenes are challenging substrates due to the need to control chemoselectivity. Pyrido-fused pyrroles, namely azaindoles, are an important class of this type of substrate. In 2016, Kuwano and co-workers reported the chemo- and enantioselective reduction of 7-azaindoles S153 using Ru/L60 as catalyst (Scheme 105).536 The reaction occurred exclusively on the five-membered ring, thus furnishing the corresponding azaindolines P153 with up to 94% ee. Furthermore, the catalyst was also highly active for the AH of 6-, 5-, and 4-azaindoles.
Scheme 105. Ruthenium-Catalyzed AH of Oxazoles, Imidazoles, and Azaindoles Using PhTRAP.
Indolizines are another class of ring-fused heteroaromatic compounds. However, their selective reduction is a difficult task due to the nitrogen atom of the bridgehead position. In fact, the efficient AH of indolizines had not been accomplished until 2013, when Glorius and co-workers described the Ru-catalyzed AH of indolizines S154 using the chiral NHC-ligand L50 (Scheme 106).537 The corresponding hydrogenated products, indolizidines P154, were afforded in high yields and enantioselectivities, and the applicability of such a methodology was exemplified by the synthesis of unnatural (−)-monomorine in only two steps. In addition, the catalyst was also used for the high-yielding and completely regioselective AH of 1,2,3-triazolo-pyridines S155, albeit in low to moderate enantioselectivity (Scheme 106). Later, the same group used this catalytic system for the enantioselective hydrogenation of imidazo[1,2-a]pyridines, with enantiomeric ratios of up to 98:2.538 Very recently, Fan and co-workers have also reported the synthesis of benzo-fused indolizidines and quinolizidines via tandem AH/reductive amination using a Ru-DPEN catalyst (C14j).539
Scheme 106. Ruthenium-Catalyzed AH of Indolizines and 1,2,3-Triazolopyridines.
In 2015, Glorius and co-workers applied the same catalyst Ru/L50 for the AH of 2-pyridones S156 into the corresponding enantioenriched 2-piperidones P156 (Scheme 107).540 However, and even though this was the first related example using a homogeneous catalytic system, the method suffered from low enantioselectivities, and therefore there is still plenty of room for improvement.
Scheme 107. Ruthenium-Catalyzed AH of 2-Pyridones.
The enantioselective hydrogenation of quinozalinones was achieved by Zhou’s laboratory, using Pd or Ir catalysis (Scheme 108). First, the group disclosed that Pd/SynPhos [(S)-L46] was an excellent catalyst for the AH of fluorinated quinazolinones (94–98% ee).541 Later, the substrate scope was expanded to other substituted quinazolinones using iridium catalysis (with L9b), thus allowing the preparation of chiral dihydroquinazolinones P157 in excellent yields and enantioselectivities (Scheme 108).542
Scheme 108. Enantioselective Hydrogenation of Quinazolinones Using Pd or Ir Catalysts.
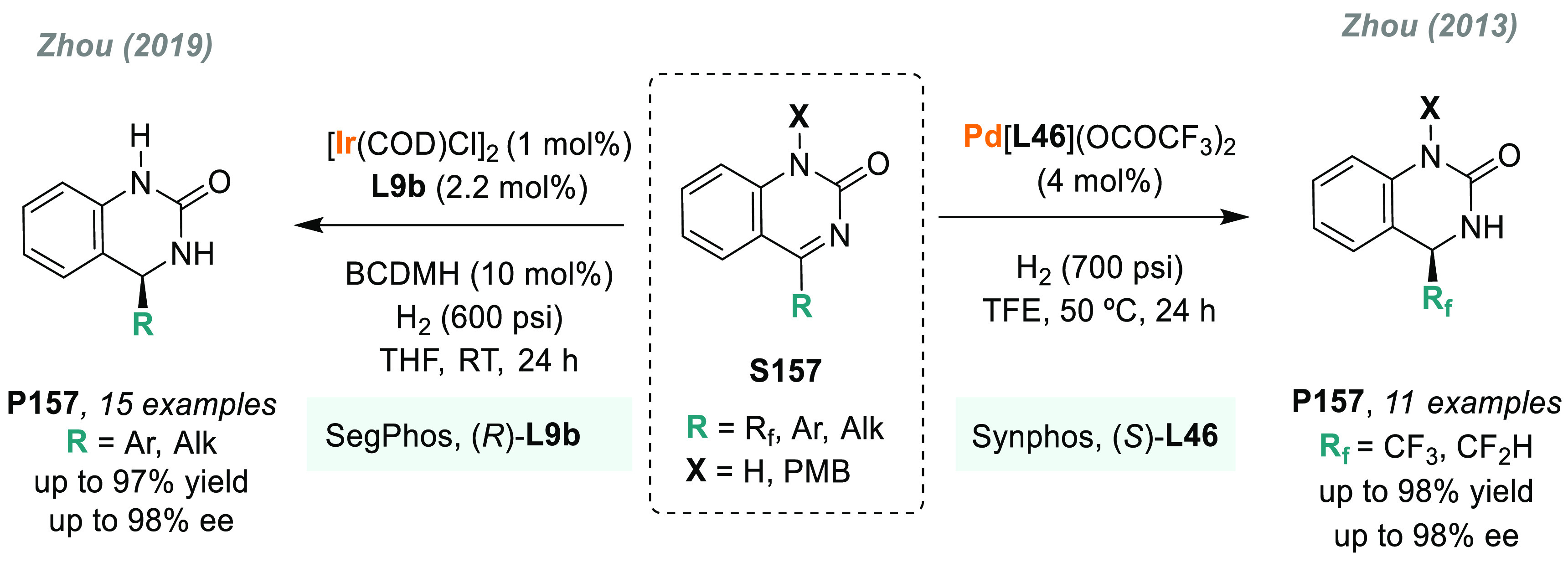
The AH of pyrimidines was first reported by Kuwano and co-workers in 2015.543 Using an Ir/L21a as catalyst, various 2,4-disubstituted pyrimidines S158 were converted into chiral 1,4,5,6-tetrahydropyrimidines P158 in high yield (Scheme 109). Interestingly, lanthanide triflate was used as additive for achieving high enantiocontrol, as well as for activating the heteroarene substrate. Similarly, the AH of 2-hydroxypyrimidines S159 was reported by Zhou’s group (Scheme 109).544 Taking advantage of the lactame-lactime tautomerism of the 2-hydroxypyrimidine, the authors developed two distinct catalytic systems for the efficient preparation of cyclic ureas P159, which are highly valuable structural motifs in many pharmacophores and other biologically active compounds. In particular, they reported an efficient Brønsted acid-activated palladium-catalyzed AH using chiral ligand L21a. By slightly modifying the catalytic system, di- and trisubstituted 2-hydroxypyrimidines were also hydrogenated in good yields. Alternatively, the same laboratory developed the same transformation using iridium catalysis with L7, in which the Brønsted acid was generated in situ.545 On the other hand, the AH of quinazolinium salts S160 was reported by Mashima and co-workers (Scheme 109).546 By using halide-bridged dinuclear iridium complexes bearing SegPhos ((S)-L9b) as ligand, the corresponding 1,2,3,4-tetrahydroquinazolines P160 were achieved in high enantiomeric excess along with some dihydroquinazolines.
Scheme 109. AH of Pyrimidines.
Iridium catalysis was also used for the efficient AH of isoxazolium salts (Scheme 110). Kuwano’s laboratory reported that iridium complexes bearing phosphino-oxazoline L62-type ligands were highly active catalysts for the AH of isoxazolium triflates S161.547 Interestingly, by fine-tuning the oxazoline substituent, isoxazolines P161a or isoxazolidines P161b were selectively obtained in good to high enantioselectivity.
Scheme 110. AH of Isoxazolium Salts.
Dihydrophenanthridines (DHPDs, P162) are important structural units in natural products and biologically active molecules. In addition, 9,10-dihydrophenanthridine proved to be a regenerable biomimetic hydrogen source, as it was designed as a NAD(P)H analog by Zhou.548
However, until 2017, the AH of substituted phenanthridines had not been documented. Phenanthridines are heteroarenes formed by three fused aromatic rings. Their hydrogenation is difficult due to the possible dehydrogenative aromatization of the reduced products. Moreover, the strong coordinative nitrogen atom can easily poison the metal catalyst. At that point, Zhou’s laboratory reported a highly enantioselective iridium-catalyzed AH of phenanthridines S162 through in situ protection of the reduced products with acetic anhydride to inhibit rearomatization (Scheme 111).508 This approach was also used for pyrrolo/indolo[1,2-a]quinoxalines, as previously stated (see Scheme 97). Aryl substituents were well-tolerated, and the corresponding DHPDs P162 were attained with excellent yields and enantioselectivities. For alkyl substituents, the level of enantioselectivity was only moderate. Nevertheless, alkyl-substituted phenanthridines S162 had previously been hydrogenated by Yang, Fan, and co-workers in a highly efficient manner (Scheme 111).549 By using a chiral cationic Ru-diamine complex C14l, the corresponding enantioenriched P162 were obtained. Interestingly, the counteranion was found to be critical for attaining high enantioselectivities (up to 92% ee).
Scheme 111. Metal-Catalyzed AH of Phenanthridines.
Fan and co-workers further exploited the use of chiral cationic Ru-diamine complexes to the AH of 1,5- and 1,8-naphthyridines (Scheme 112).550,551 By using the appropriate Ru catalyst, chiral amines containing the 1,2,3,4-tetrahydronaphthyridine ring were afforded in excellent yields and enantioselectivities. In fact, these chiral heterocycles have been used as rigid chelating diamine ligands for asymmetric synthesis and can be found in many biologically active compounds. Previously, in 2013, the same authors reported the highly enantioselective ruthenium-catalyzed AH of 1,10-phenanthroline and its derivatives using Ru-C14j.552
Scheme 112. Ruthenium-Catalyzed AH of 1,5- and 1,8-Naphthyridines.
7. Conclusions and Outlook
The metal-catalyzed asymmetric hydrogenation of prochiral unsaturated amines has been intensively studied in the last ten years, and it continues to be a growing field and a fundamental tool in synthetic organic chemistry. More than four hundred articles have been published since 2010. Although a huge number of catalysts have been described to date, there are some privileged ligands, such as DuanPhos, SegPhos, and ZhaoPhos, able to provide excellent results in a large variety of substrates. Moreover, some modular catalytic systems such as JosiPhos, WalPhos, and the ruthenium DPEN have also proved highly versatile, through fine-tuning the substituents and counterions. In the last ten years we have witnessed incredible advances and may conclude that asymmetric hydrogenation is, arguably, the cleanest and most convenient methodology for the synthesis of chiral amines. Nevertheless, to overcome the fierce competition of biocatalytic and resolution methodologies, further improvement is needed. In the years to come we might expect further development on the use of nonprecious metals to allow for greener and cheaper processes. On the other hand, catalytic systems that are active and resistant to basic and/or acidic conditions, catalyst deactivation, or poisoning by amines are much needed. These advances and others which we may not foresee now will for sure keep the metal-catalyzed synthesis of chiral amines as a thriving field in the next decade.
Acknowledgments
The authors thank IRB Barcelona and the Ministerio de Ciencia e Innovación (MICINN, PID2020-119535RB-I00) for financial support. IRB Barcelona is the recipient of institutional funding from MICINN through the Centres of Excellence Severo Ochoa award and from the CERCA Program of the Government of Catalonia. A.C. thanks the Ministerio de Ciencia e Innovación (MICINN) for a FPU fellowship.
Glossary
Abbreviations
- AH
asymmetric hydrogenation
- Alk
alkyl
- ARA
asymmetric reductive amination
- BArF
tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
- BCDMH
1-bromo-3-chloro-5,5-dimethylhydantoin
- BINAP
2,2′-bis(diphenylphosphino)-1,1′-binaphthyl
- Boc
tert-butoxycarbonyl
- Cbz
benzyloxycarbonyl
- COD
cyclooctadiene
- Cp*
pentamethylcyclopentadienyl
- CSA
camphor-10-sulfonic acid
- Cy
cyclohexyl
- d-DTTA
d-di-p-toluoyl-d-tartaric acid
- DCE
dichloroethane
- DHIQs
dihydroisoquinolines
- DHPDs
dihydrophenanthridines
- DFT
density functional theory
- DMF
dimethylformamide
- DPEN
trans-1,2-diphenylethylene diamine
- dr
diastereomeric ratio
- EDG
electron-donating group
- ee
enantiomeric excess
- EWG
electron-withdrawing group
- HA
chiral phosphoric acid
- HFIP
hexafluoroisopropanol
- MIBK
methyl isobutyl ketone
- MOM
methoxymethyl ether
- MS
molecular sieves
- Ms
methanesulfonyl (mesyl)
- nbd
norbornadiene
- NHC
N-heterocyclic carbene
- NIS
N-iodosuccinimide
- PG
protecting group
- PMB
p-methoxybenzyl
- PMP
p-methoxyphenyl
- Rf
perfluoroalkyl
- RT
room temperature
- S/C
substrate/catalyst ratio
- tAA
tert-amyl alcohol
- TBAI
tetrabutylammonium iodide
- TBDPS
tert-butyldiphenylsilyl
- TBS
tert-butyldimethylsilyl
- TCCA
trichloroisocyanuric acid
- TCFP
trichickenfootphos
- Tf
trifluoromethanesulfonyl (triflate)
- TFA
trifluoroacetic acid
- TFE
trifluoroethanol
- THF
tetrahydrofuran
- THIQs
tetrahydroisoquinolines
- THQs
tetrahydroquinolines
- TMS
trimethylsilyl
- TON
turnover number
- Ts
p-toluenesulfonyl (tosyl)
- Xyl
xylyl (dimethylphenyl)
Biographies
Albert Cabré studied chemistry at the Universitat Rovira i Virgili in Tarragona. In 2020, he received his Ph.D. from the Universitat de Barcelona under the supervision of Professor Antoni Riera. His doctoral studies focused on the development of new catalytic methods for Pauson-Khand reactions, isomerizations, and asymmetric hydrogenation processes. He performed a research stay in Professor David W. MacMillan’s laboratory at Princeton University (United States), where he worked on metallaphotoredox catalysis. He is the recipient of the 2020 Josep Castells Award for the best Doctoral Thesis from the Catalan section of the Spanish Royal Society of Chemistry. In 2020 he received a fellowship from the Fundación Ramon Areces to perform his postdoctoral studies at the Massachusetts Institute of Technology (United States), where he currently works under the guidance of Prof. Stephen L. Buchwald.
Xavier Verdaguer is full professor at the Inorganic and Organic Chemistry Department at the University of Barcelona. In 1994 he earned his Ph.D. degree from the University of Barcelona under the supervision of Professors Miquel A. Pericàs and Antoni Riera. He carried out a 2 year postdoctoral stay in the laboratories of Professor Stephen Buchwald at the Massachusetts Institute of Technology (MIT), where he worked on the titanocene-catalyzed asymmetric hydrosilylation of imines. In 2005 he was appointed research associate at the Asymmetric Synthesis Group of the Institute for Research in Biomedicine (IRB Barcelona). His research interests focus on the field of asymmetric synthesis and asymmetric catalysis. He is interested in the synthesis and application of P-stereogenic phosphine ligands and on the use of iridium cyclometalated catalysts for asymmetric hydrogenation and rearrangement processes.
Antoni Riera was born in Balsareny (Catalonia). He studied chemistry at the University of Barcelona, where he did his Doctoral thesis under the supervision of Professors Fèlix Serratosa and Miquel A. Pericàs. After a postdoctoral stage at the University of Pennsylvania (Philadelphia, USA) under the supervision of Professor Amos B. Smith III, in 1988 he returned to the Department of Organic Chemistry of the University of Barcelona as associate professor. In June 2003 he was promoted to full professor at the same university. Since 2005 he has served as group leader of the Asymmetric Synthesis Group of the Institute for Research in Biomedicine (IRB Barcelona). His main research area is organic synthesis. He works on synthetic methodology (Pauson-Khand reactions, asymmetric hydrogenation, new chiral ligands, etc.) and on the synthesis of biologically active compounds (amino acids, aza-sugars, peptides, protein inhibitors, PROTACs, and tetrazines).
The authors declare no competing financial interest.
References
- Nugent T. C., Ed. Chiral Amine Synthesis: Methods, Developments and Applications; Wiley-VCH: Weinheim, 2010; pp 1–479. [Google Scholar]
- Li W., Zhang X., Eds. Stereoselective Formation of Amines; Springer: Berlin, 2014; pp 1–282. [Google Scholar]
- Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Wood A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen E. N., Pfaltz A., Yamamoto H., Eds. Comprehensive Asymmetric Catalysis; Springer: Berlin, 2004; pp 1–1856. [Google Scholar]
- Caprio V., Williams J. M. J., Eds. Catalysis in Asymmetric Synthesis; Wiley VCH: Chichester, U.K., 2009; pp 1–408. [Google Scholar]
- Patil M. D.; Grogan G.; Bommarius A.; Yun H. Oxidoreductase-Catalyzed Synthesis of Chiral Amines. ACS Catal. 2018, 8, 10985–11015. 10.1021/acscatal.8b02924. [DOI] [Google Scholar]
- Rueping M.; Dufour J.; Schoepke F. R. Advances in Catalytic Metal-Free Reductions: From Bio-Inspired Concepts to Applications in the Organocatalytic Synthesis of Pharmaceuticals and Natural Products. Green Chem. 2011, 13, 1084–1105. 10.1039/c1gc15027h. [DOI] [Google Scholar]
- Trowbridge A.; Walton S. M.; Gaunt M. J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692. 10.1021/acs.chemrev.9b00462. [DOI] [PubMed] [Google Scholar]
- Nugent T. C.; El-Shazly M. Chiral Amine Synthesis - Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819. 10.1002/adsc.200900719. [DOI] [Google Scholar]
- Irrgang T.; Kempe R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. 10.1021/acs.chemrev.0c00248. [DOI] [PubMed] [Google Scholar]
- Murugesan K.; Senthamarai T.; Chandrashekhar V. G.; Natte K.; Kamer P. C. J.; Beller M.; Jagadeesh R. V. Catalytic Reductive Aminations Using Molecular Hydrogen for Synthesis of Different Kinds of Amines. Chem. Soc. Rev. 2020, 49, 6273–6328. 10.1039/C9CS00286C. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Hu L.; Wang Y. Z.; Zhang X.; Yin Q. Recent Advances on Transition-Metal-Catalysed Asymmetric Reductive Amination. Org. Chem. Front. 2021, 8, 2328–2342. 10.1039/D1QO00300C. [DOI] [Google Scholar]
- Pirnot M. T.; Wang Y. M.; Buchwald S. L. Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes. Angew. Chem., Int. Ed. 2016, 55, 48–57. 10.1002/anie.201507594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Shi S. L.; Niu D.; Liu P.; Buchwald S. L. Catalytic Asymmetric Hydroamination of Unactivated Internal Olefins to Aliphatic Amines. Science 2015, 349, 62–66. 10.1126/science.aab3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultzsch K. C. Transition Metal-Catalyzed Asymmetric Hydroamination of Alkenes (AHA). Adv. Synth. Catal. 2005, 347, 367–391. 10.1002/adsc.200404261. [DOI] [Google Scholar]
- Grange R. L.; Clizbe E. A.; Evans P. A. Recent Developments in Asymmetric Allylic Amination Reactions. Synthesis 2016, 48, 2911–2968. 10.1055/s-0035-1562090. [DOI] [Google Scholar]
- Tani K.; Yamagata T.; Otsuka S.; Akutagawa S.; Kumobayashi H.; Taketomi T.; Takaya H.; Miyashita A.; Noyori R.; et al. Highly Enantioselective Isomerization of Prochiral Allylamines Catalyzed by Chiral Diphosphine Rhodium(I) Complexes. Preparation of Optically Active Enamines. J. Am. Chem. Soc. 1984, 106, 5208–5217. 10.1021/ja00330a029. [DOI] [Google Scholar]
- Cabré A.; Khaizourane H.; Garçon M.; Verdaguer X.; Riera A. Total Synthesis of (R)-Sarkomycin Methyl Ester via Regioselective Intermolecular Pauson-Khand Reaction and Iridium-Catalyzed Asymmetric Isomerization. Org. Lett. 2018, 20, 3953–3957. 10.1021/acs.orglett.8b01525. [DOI] [PubMed] [Google Scholar]
- Kobayashi S.; Mori Y.; Fossey J. S.; Salter M. M. Catalytic Enantioselective Formation of C-C Bonds by Addition to Imines and Hydrazones: A Ten-Year Update. Chem. Rev. 2011, 111, 2626–2704. 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]
- Andersson P. G., Munslow I. J., Eds. Modern Reduction Methods; Wiley-VCH: Weinheim, 2008; pp 1–508. [Google Scholar]
- Foubelo F.; Yus M. Catalytic Asymmetric Transfer Hydrogenation of Imines: Recent Advances. Chem. Rec. 2015, 15, 907–924. 10.1002/tcr.201500203. [DOI] [PubMed] [Google Scholar]
- Echeverria P. G.; Ayad T.; Phansavath P.; Ratovelomanana-Vidal V. Recent Developments in Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones and Imines through Dynamic Kinetic Resolution. Synthesis 2016, 48, 2523–2539. 10.1055/s-0035-1561648. [DOI] [Google Scholar]
- Seo C. S. G.; Morris R. H. Catalytic Homogeneous Asymmetric Hydrogenation: Successes and Opportunities. Organometallics 2019, 38, 47–65. 10.1021/acs.organomet.8b00774. [DOI] [Google Scholar]
- de Vries J. G., Elsevier C. J., Eds. The Handbook of Homogeneous Hydrogenation; Wiley-VCH: Weinheim, Germany, 2007; Vol. 1–3; pp 1–1641. [Google Scholar]
- Etayo P.; Vidal-Ferran A. Rhodium-Catalysed Asymmetric Hydrogenation as a Valuable Synthetic Tool for the Preparation of Chiral Drugs. Chem. Soc. Rev. 2013, 42, 728–754. 10.1039/C2CS35410A. [DOI] [PubMed] [Google Scholar]
- Woodmansee D. H.; Pfaltz A. Asymmetric Hydrogenation of Alkenes Lacking Coordinating Groups. Chem. Commun. 2011, 47, 7912–7916. 10.1039/c1cc11430a. [DOI] [PubMed] [Google Scholar]
- Margarita C.; Andersson P. G. Evolution and Prospects of the Asymmetric Hydrogenation of Unfunctionalized Olefins. J. Am. Chem. Soc. 2017, 139, 1346–1356. 10.1021/jacs.6b10690. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Butt N. A.; Zhang W. Asymmetric Hydrogenation of Nonaromatic Cyclic Substrates. Chem. Rev. 2016, 116, 14769–14821. 10.1021/acs.chemrev.6b00564. [DOI] [PubMed] [Google Scholar]
- Kraft S.; Ryan K.; Kargbo R. B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641. 10.1021/jacs.7b07188. [DOI] [PubMed] [Google Scholar]
- Wang D. S.; Chen Q. A.; Lu S. M.; Zhou Y. G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557–2590. 10.1021/cr200328h. [DOI] [PubMed] [Google Scholar]
- Chen Z. P.; Zhou Y. G. Asymmetric Hydrogenation of Heteroarenes with Multiple Heteroatoms. Synthesis 2016, 48, 1769–1781. 10.1055/s-0035-1561622. [DOI] [Google Scholar]
- Kim A. N.; Stoltz B. M. Recent Advances in Homogeneous Catalysts for the Asymmetric Hydrogenation of Heteroarenes. ACS Catal. 2020, 10, 13834–13851. 10.1021/acscatal.0c03958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.-M.; Song F.-T.; Fan Q.-H.. Advances in Transition Metal-Catalyzed Asymmetric Hydrogenation of Heteroaromatic Compounds. In Stereoselective Formation of Amines; Li W., Zhang X., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; pp 145–190. [DOI] [PubMed] [Google Scholar]
- Wiesenfeldt M. P.; Nairoukh Z.; Dalton T.; Glorius F. Selective Arene Hydrogenation for Direct Access to Saturated Carbo- and Heterocycles. Angew. Chem., Int. Ed. 2019, 58, 10460–10476. 10.1002/anie.201814471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury-Brégeot N.; de la Fuente V.; Castillón S.; Claver C. Highlights of Transition Metal-Catalyzed Asymmetric Hydrogenation of Imines. ChemCatChem 2010, 2, 1346–1371. 10.1002/cctc.201000078. [DOI] [Google Scholar]
- Tang W.; Xiao J. Asymmetric Hydrogenation of Imines via Metal-Organo Cooperative Catalysis. Synthesis 2014, 46, 1297–1302. 10.1055/s-0033-1338603. [DOI] [Google Scholar]
- Abed R.; Abdine A.; Hedouin G.; Wencel-Delord J. Metal-Catalyzed Asymmetric Hydrogenation of C = N Bonds. ACS Catal. 2021, 11, 215–247. 10.1021/acscatal.0c03353. [DOI] [Google Scholar]
- Barrios-Rivera J.; Xu Y.; Wills M.; Vyas V. K. A Diversity of Recently Reported Methodology for Asymmetric Imine Reduction. Org. Chem. Front. 2020, 7, 3312–3342. 10.1039/D0QO00794C. [DOI] [Google Scholar]
- Xie J. H.; Zhu S. F.; Zhou Q. L. Transition Metal-Catalyzed Enantioselective Hydrogenation of Enamines and Imines. Chem. Rev. 2011, 111, 1713–1760. and references cited therein. 10.1021/cr100218m. [DOI] [PubMed] [Google Scholar]
- Xie J. H.; Zhu S. F.; Zhou Q. L. Recent Advances in Transition Metal-Catalyzed Enantioselective Hydrogenation of Unprotected Enamines. Chem. Soc. Rev. 2012, 41, 4126–4139. 10.1039/c2cs35007f. [DOI] [PubMed] [Google Scholar]
- Ponra S.; Boudet B.; Phansavath P.; Ratovelomanana-Vidal V. Recent Developments in Transition-Metal-Catalyzed Asymmetric Hydrogenation of Enamides. Synthesis 2021, 53, 193–214. 10.1055/s-0040-1705939. [DOI] [Google Scholar]
- Tang W.; Zhang X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3069. 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- Verendel J. J.; Pàmies O.; Diéguez M.; Andersson P. G. Asymmetric Hydrogenation of Olefins Using Chiral Crabtree-Type Catalysts: Scope and Limitations. Chem. Rev. 2014, 114, 2130–2169. 10.1021/cr400037u. [DOI] [PubMed] [Google Scholar]
- Cabré A.; Riera A.; Verdaguer X. P-Stereogenic Amino-Phosphines as Chiral Ligands: From Privileged Intermediates to Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 676–689. 10.1021/acs.accounts.9b00633. [DOI] [PubMed] [Google Scholar]
- Grabulosa A.; Granell J.; Muller G. Preparation of Optically Pure P-Stereogenic Trivalent Phosphorus Compounds. Coord. Chem. Rev. 2007, 251, 25–90. 10.1016/j.ccr.2006.05.009. [DOI] [Google Scholar]
- Zhao D.; Candish L.; Paul D.; Glorius F. N-Heterocyclic Carbenes in Asymmetric Hydrogenation. ACS Catal. 2016, 6, 5978–5988. 10.1021/acscatal.6b01736. [DOI] [Google Scholar]
- He Y. M.; Fan Q. H. Phosphine-Free Chiral Metal Catalysts for Highly Effective Asymmetric Catalytic Hydrogenation. Org. Biomol. Chem. 2010, 8, 2497–2504. 10.1039/b925199p. [DOI] [PubMed] [Google Scholar]
- Blaser H. U., Schmidt E., Eds. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; Wiley-VCH: Weinheim, Germany, 2004; pp 1–454. [Google Scholar]
- Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- Knowles W. S.; Sabacky M. J. Catalytic Asymmetric Hydrogenation Employing a Soluble, Optically Active Rhodium Complex. Chem. Commun. 1968, 1445. 10.1039/c19680001445. [DOI] [Google Scholar]
- Knowles W. S. Asymmetric Hydrogenation. Acc. Chem. Res. 1983, 16, 106–112. 10.1021/ar00087a006. [DOI] [PubMed] [Google Scholar]
- Horner L.; Siegel H.; Büthe H. Asymmetric Catalytic Hydrogenation with an Optically Active Phosphinerhodium Complex in Homogeneous Solution. Angew. Chem., Int. Ed. Engl. 1968, 7, 942. 10.1002/anie.196809422. [DOI] [Google Scholar]
- Kagan H. B.; Dang T. P. Asymmetric Catalytic Reduction with Transition-metal Complexes. 1. A Catalytic System of Rhodium(I) with (−) −2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane-New Chiral Diphosphine. J. Am. Chem. Soc. 1972, 94, 6429–6433. 10.1021/ja00773a028. [DOI] [Google Scholar]
- Knowles W. S.Asymmetric Hydrogenations-The Monsanto-Dopa Process. In Asymmetric Catalysis on Industrial Scale; Blaser H.-U., Schmidt E., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp 23–38. [Google Scholar]
- Johnson N. B.; Lennon I. C.; Moran P. H.; Ramsden J. A. Industrial-Scale Synthesis and Applications of Asymmetric Hydrogenation Catalysts. Acc. Chem. Res. 2007, 40, 1291–1299. 10.1021/ar700114k. [DOI] [PubMed] [Google Scholar]
- Ager D. J.; De Vries A. H. M.; De Vries J. G. Asymmetric Homogeneous Hydrogenations at Scale. Chem. Soc. Rev. 2012, 41, 3340–3380. 10.1039/c2cs15312b. [DOI] [PubMed] [Google Scholar]
- Lefort L.; Boogers J. A. F.; Kuilman T.; Vijn R. J.; Janssen J.; Straatman H.; De Vries J. G.; De Vries A. H. M. Rapid Identification of a Scalable Catalyst for the Asymmetric Hydrogenation of a Sterically Demanding Aryl Enamide. Org. Process Res. Dev. 2010, 14, 568–573. 10.1021/op100011y. [DOI] [Google Scholar]
- Hansen K. B.; Hsiao Y.; Xu F.; Rivera N.; Clausen A.; Kubryk M.; Krska S.; Rosner T.; Simmons B.; Balsells J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. 10.1021/ja902462q. [DOI] [PubMed] [Google Scholar]
- Birch M.; Challenger S.; Crochard J. P.; Fradet D.; Jackman H.; Luan A.; Madigan E.; McDowall N.; Meldrum K.; Gordon C. M.; et al. The Development of a Practical Multikilogram Synthesis of the Chiral β-Amino Acid Imagabalin Hydrochloride (PD-0332334) via Asymmetric Hydrogenation. Org. Process Res. Dev. 2011, 15, 1172–1177. 10.1021/op2001639. [DOI] [Google Scholar]
- Li X.; You C.; Yang Y.; Yang Y.; Li P.; Gu G.; Chung L. W.; Lv H.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of β-Cyanocinnamic Esters with the Assistance of a Single Hydrogen Bond in a Precise Position. Chem. Sci. 2018, 9, 1919–1924. 10.1039/C7SC04639A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Wu Z. M.; Liu S.; Tang X. L.; Zheng R. C.; Zheng Y. G. Efficient Chemoenzymatic Synthesis of Optically Active Pregabalin from Racemic Isobutylsuccinonitrile. Org. Process Res. Dev. 2019, 23, 2042–2049. 10.1021/acs.oprd.9b00285. [DOI] [Google Scholar]
- Yin Q.; Shi Y.; Wang J.; Zhang X. Direct Catalytic Asymmetric Synthesis of α-Chiral Primary Amines. Chem. Soc. Rev. 2020, 49, 6141–6153. 10.1039/C9CS00921C. [DOI] [PubMed] [Google Scholar]
- Blaser H. U.; Pugin B.; Spindler F.; Thommen M. From a Chiral Switch to a Ligand Portfolio for Asymmetric Catalysis. Acc. Chem. Res. 2007, 40, 1240–1250. 10.1021/ar7001057. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Jin W.; Jiang Q. Brønsted Acid Activation Strategy in Transition-Metal Catalyzed Asymmetric Hydrogenation of N-Unprotected Imines, Enamines, and N-Heteroaromatic Compounds. Angew. Chem., Int. Ed. 2012, 51, 6060–6072. 10.1002/anie.201200963. [DOI] [PubMed] [Google Scholar]
- Hopmann K. H.; Bayer A. Enantioselective Imine Hydrogenation with Iridium-Catalysts: Reactions, Mechanisms and Stereocontrol. Coord. Chem. Rev. 2014, 268, 59–82. 10.1016/j.ccr.2014.01.023. [DOI] [Google Scholar]
- Hopmann K. H.; Bayer A. On the Mechanism of Iridium-Catalyzed Asymmetric Hydrogenation of Imines and Alkenes: A Theoretical Study. Organometallics 2011, 30, 2483–2497. 10.1021/om1009507. [DOI] [Google Scholar]
- Tutkowski B.; Kerdphon S.; Limé E.; Helquist P.; Andersson P. G.; Wiest O.; Norrby P. O. Revisiting the Stereodetermining Step in Enantioselective Iridium-Catalyzed Imine Hydrogenation. ACS Catal. 2018, 8, 615–623. 10.1021/acscatal.7b02386. [DOI] [Google Scholar]
- Amézquita-Valencia M.; Cabrera A. The First Example of Asymmetric Hydrogenation of Imines with Co2(CO)8/(R)-BINAP as Catalytic Precursor. J. Mol. Catal. A: Chem. 2013, 366, 17–21. 10.1016/j.molcata.2012.08.019. [DOI] [Google Scholar]
- Mršić N.; Minnaard A. J.; Feringa B. L.; De Vries J. G. Iridium/Monodentate Phosphoramidite Catalyzed Asymmetric Hydrogenation of N-Aryl Imines. J. Am. Chem. Soc. 2009, 131, 8358–8359. 10.1021/ja901961y. [DOI] [PubMed] [Google Scholar]
- Minnaard A. J.; Feringa B. L.; Lefort L.; De Vries J. G. Asymmetric Hydrogenation Using Monodentate Phosphoramidite Ligands. Acc. Chem. Res. 2007, 40, 1267–1277. 10.1021/ar7001107. [DOI] [PubMed] [Google Scholar]
- Li W.; Hou G.; Chang M.; Zhang X. Highly Efficient and Enantioselective Iridium-Catalyzed Asymmetric Hydrogenation of N-Arylimines. Adv. Synth. Catal. 2009, 351, 3123–3127. 10.1002/adsc.200900692. [DOI] [Google Scholar]
- For a selected example of Asymmetric Hydrogenation of N-aryl imines using Rh catalysis, see:; Kutlescha K.; Irrgang T.; Kempe R. Novel Amido-Complexes for the Efficient Asymmetric Hydrogenation of Imines. Adv. Synth. Catal. 2010, 352, 3126–3130. 10.1002/adsc.201000733. [DOI] [Google Scholar]
- Schnider P.; Koch G.; Prétôt R.; Wang G.; Bohnen F. M.; Krüger C.; Pfaltz A. Enantioselective Hydrogenation of Imines with Chiral (Phosphanodihydrooxazole)Iridium Catalysts. Chem. - Eur. J. 1997, 3, 887–892. 10.1002/chem.19970030609. [DOI] [Google Scholar]
- Zhu S. F.; Xie J. B.; Zhang Y. Z.; Li S.; Zhou Q. L. Well-Defined Chiral Spiro Iridium/Phosphine-Oxazoline Cationic Complexes for Highly Enantioselective Hydrogenation of Imines at Ambient Pressure. J. Am. Chem. Soc. 2006, 128, 12886–12891. 10.1021/ja063444p. [DOI] [PubMed] [Google Scholar]
- Han Z.; Wang Z.; Zhang X.; Ding K. Spiro[4,4]-1,6-Nonadiene-Based Phosphine-Oxazoline Ligands for Iridium-Catalyzed Enantioselective Hydrogenation of Ketimines. Angew. Chem., Int. Ed. 2009, 48, 5345–5349. 10.1002/anie.200901630. [DOI] [PubMed] [Google Scholar]
- Baeza A.; Pfaltz A. Iridium-Catalyzed Asymmetric Hydrogenation of Imines. Chem. - Eur. J. 2010, 16, 4003–4009. 10.1002/chem.200903418. [DOI] [PubMed] [Google Scholar]
- Salomó E.; Orgué S.; Riera A.; Verdaguer X. Highly Enantioselective Iridium-Catalyzed Hydrogenation of Cyclic Enamides. Angew. Chem., Int. Ed. 2016, 55, 7988–7992. 10.1002/anie.201602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgué S.; Flores-Gaspar A.; Biosca M.; Pàmies O.; Diéguez M.; Riera A.; Verdaguer X. Stereospecific SN2@P Reactions: Novel Access to Bulky P-Stereogenic Ligands. Chem. Commun. 2015, 51, 17548–17551. 10.1039/C5CC07504A. [DOI] [PubMed] [Google Scholar]
- Salomó E.; Rojo P.; Hernández-Lladó P.; Riera A.; Verdaguer X. P-Stereogenic and Non-P-Stereogenic Ir-MaxPHOX in the Asymmetric Hydrogenation of N-Aryl Imines. Isolation and X-Ray Analysis of Imine Iridacycles. J. Org. Chem. 2018, 83, 4618–4627. 10.1021/acs.joc.8b00361. [DOI] [PubMed] [Google Scholar]
- Arai N.; Utsumi N.; Matsumoto Y.; Murata K.; Tsutsumi K.; Ohkuma T. Asymmetric Hydrogenation of N-Arylimines Catalyzed by the Xyl-Skewphos/DPEN-Ruthenium(II) Complex. Adv. Synth. Catal. 2012, 354, 2089–2095. 10.1002/adsc.201200464. [DOI] [Google Scholar]
- Menéndez-Pedregal E.; Vaquero M.; Lastra E.; Gamasa P.; Pizzano A. Highly Enantioselective Hydrogenation of N-Aryl Imines Derived from Acetophenones by Using Ru-Pybox Complexes under Hydrogenation or Transfer Hydrogenation Conditions in Isopropanol. Chem. - Eur. J. 2015, 21, 549–553. 10.1002/chem.201405276. [DOI] [PubMed] [Google Scholar]
- Xiao D.; Zhang X. Highly Enantioselective Hydrogenation of Acyclic Imines Catalyzed by Ir-f-Binaphane Complexes. Angew. Chem., Int. Ed. 2001, 40, 3425–3428. . [DOI] [PubMed] [Google Scholar]
- Hou C. J.; Wang Y. H.; Zheng Z.; Xu J.; Hu X. P. Chiral Phosphine-Phosphoramidite Ligands for Highly Efficient Ir-Catalyzed Asymmetric Hydrogenation of Sterically Hindered N-Arylimines. Org. Lett. 2012, 14, 3554–3557. 10.1021/ol301618r. [DOI] [PubMed] [Google Scholar]
- Li Q.; Hou C. J.; Liu X. N.; Huang D. Z.; Liu Y. J.; Yang R. F.; Hu X. P. Chiral Phosphine-Phosphoramidite Ligands for Highly Enantioselective Hydrogenation of N-Arylimines. RSC Adv. 2015, 5, 13702–13708. 10.1039/C4RA16062B. [DOI] [Google Scholar]
- Li C.; Wang C.; Villa-Marcos B.; Xiao J. Chiral Counteranion-Aided Asymmetric Hydrogenation of Acyclic Imines. J. Am. Chem. Soc. 2008, 130, 14450–14451. 10.1021/ja807188s. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Fleischer S.; Junge K.; Beller M. Cooperative Transition-Metal and Chiral Brønsted Acid Catalysis: Enantioselective Hydrogenation of Imines to Form Amines. Angew. Chem. 2011, 123, 5226–5230. 10.1002/ange.201100878. [DOI] [PubMed] [Google Scholar]
- Tang W.; Johnston S.; Iggo J. A.; Berry N. G.; Phelan M.; Lian L.; Bacsa J.; Xiao J. Cooperative Catalysis through Noncovalent Interactions. Angew. Chem., Int. Ed. 2013, 52, 1668–1672. 10.1002/anie.201208774. [DOI] [PubMed] [Google Scholar]
- Tang W.; Johnston S.; Li C.; Iggo J. A.; Bacsa J.; Xiao J. Cooperative Catalysis: Combining an Achiral Metal Catalyst with a Chiral Brønsted Acid Enables Highly Enantioselective Hydrogenation of Imines. Chem. - Eur. J. 2013, 19, 14187–14193. 10.1002/chem.201302437. [DOI] [PubMed] [Google Scholar]
- Ma J. A. Recent Developments in the Catalytic Asymmetric Synthesis of α- and β-Amino Acids. Angew. Chem., Int. Ed. 2003, 42, 4290–4299. 10.1002/anie.200301600. [DOI] [PubMed] [Google Scholar]
- Williams R. M.; Hendrix J. A. Asymmetric Synthesis of Arylglycines. Chem. Rev. 1992, 92, 889–917. 10.1021/cr00013a007. [DOI] [Google Scholar]
- Wang J.; Liu X.; Feng X. Asymmetric Strecker Reactions. Chem. Rev. 2011, 111, 6947–6983. 10.1021/cr200057t. [DOI] [PubMed] [Google Scholar]
- Reddy K. L.; Sharpless K. B. From Styrenes to Enantiopure α-Arylglycines in Two Steps. J. Am. Chem. Soc. 1998, 120, 1207–1217. 10.1021/ja9728177. [DOI] [Google Scholar]
- Shang G.; Yang Q.; Zhang X. Rh-Catalyzed Asymmetric Hydrogenation of α-Aryl Imino Esters: An Efficient Enantioselective Synthesis of Aryl Glycine Derivatives. Angew. Chem., Int. Ed. 2006, 45, 6360–6362. 10.1002/anie.200601540. [DOI] [PubMed] [Google Scholar]
- Hu X. H.; Hu X. P. Ir-Catalyzed Asymmetric Hydrogenation of α-Imino Esters with Chiral Ferrocenylphosphine-Phosphoramidite Ligands. Adv. Synth. Catal. 2019, 361, 5063–5068. 10.1002/adsc.201900888. [DOI] [Google Scholar]
- Liu D.; Li B.; Chen J.; Gridnev I. D.; Yan D.; Zhang W. Ni-Catalyzed Asymmetric Hydrogenation of N-Aryl Imino Esters for the Efficient Synthesis of Chiral α-Aryl Glycines. Nat. Commun. 2020, 11, 1–9. 10.1038/s41467-020-19807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonova A.; Diesen J. S.; Andersson P. G. Asymmetric Hydrogenation of Imines and Olefins Using Phosphine-Oxazoline Iridium Complexes as Catalysts. Chem. - Eur. J. 2006, 12, 2318–2328. 10.1002/chem.200500942. [DOI] [PubMed] [Google Scholar]
- Lu W. J.; Chen Y. W.; Hou X. L. Highly Enantioselective Iridium-Catalyzed Hydrogenation of Trisubstituted Olefins, α,β-Unsaturated Ketones and Imines with Chiral Benzylic Substituted P, N Ligands. Adv. Synth. Catal. 2010, 352, 103–107. 10.1002/adsc.200900618. [DOI] [Google Scholar]
- Zhou X. Y.; Bao M.; Zhou Y. G. Palladium-Catalyzed Asymmetric Hydrogenation of Simple Ketimines Using a Brønsted Acid as Activator. Adv. Synth. Catal. 2011, 353, 84–88. 10.1002/adsc.201000554. [DOI] [Google Scholar]
- Barluenga J.; Mendoza A.; Rodríguez F.; Fañanás F. J. A Palladium(II)-Catalyzed Synthesis of Spiroacetals through a One-Pot Multicomponent Cascade Reaction. Angew. Chem., Int. Ed. 2009, 48, 1644–1647. 10.1002/anie.200805519. [DOI] [PubMed] [Google Scholar]
- Moessner C.; Bolm C. Diphenylphosphanylsulfoximines as Ligands in Iridium-Catalyzed Asymmetric Imine Hydrogenations. Angew. Chem., Int. Ed. 2005, 44, 7564–7567. 10.1002/anie.200502482. [DOI] [PubMed] [Google Scholar]
- Qu B.; Samankumara L. P.; Ma S.; Frick K. R.; Desrosiers J. N.; Rodriguez S.; Li Z.; Haddad N.; Han Z. S.; McKellop K.; et al. A Mild Dihydrobenzooxaphosphole Oxazoline/Iridium Catalytic System for Asymmetric Hydrogenation of Unfunctionalized Dialins. Angew. Chem., Int. Ed. 2014, 53, 14428–14432. 10.1002/anie.201408929. [DOI] [PubMed] [Google Scholar]
- Verdaguer X.; Lange U. E. W.; Reding M. T.; Buchwald S. L. Highly Enantioselective Imine Hydrosilylation Using (S, S)-Ethylenebis(η5-Tetrahydroindenyl)Titanium Difluoride. J. Am. Chem. Soc. 1996, 118, 6784–6785. 10.1021/ja960808c. [DOI] [Google Scholar]
- Wakchaure V. N.; Kaib P. S. J.; Leutzsch M.; List B. Disulfonimide-Catalyzed Asymmetric Reduction of N-Alkyl Imines. Angew. Chem., Int. Ed. 2015, 54, 11852–11856. 10.1002/anie.201504052. [DOI] [PubMed] [Google Scholar]
- Schramm Y.; Barrios-Landeros F.; Pfaltz A. Discovery of an Iridacycle Catalyst with Improved Reactivity and Enantioselectivity in the Hydrogenation of Dialkyl Ketimines. Chem. Sci. 2013, 4, 2760–2766. 10.1039/c3sc50587a. [DOI] [Google Scholar]
- Salomó E.; Gallen A.; Sciortino G.; Ujaque G.; Grabulosa A.; Lledós A.; Riera A.; Verdaguer X. Direct Asymmetric Hydrogenation of N-Methyl and N-Alkyl Imines with an Ir(III)H Catalyst. J. Am. Chem. Soc. 2018, 140, 16967–16970. 10.1021/jacs.8b11547. [DOI] [PubMed] [Google Scholar]
- Mazuela J.; Antonsson T.; Knerr L.; Marsden S. P.; Munday R. H.; Johansson M. J. Iridium-Catalyzed Asymmetric Hydrogenation of N-Alkyl α-Aryl Furan-Containing Imines: An Efficient Route to Unnatural N-Alkyl Arylalanines and Related Derivatives. Adv. Synth. Catal. 2019, 361, 578–584. 10.1002/adsc.201801143. [DOI] [Google Scholar]
- Chen F.; Wang T.; He Y.; Ding Z.; Li Z.; Xu L.; Fan Q. H. Asymmetric Hydrogenation of N-Alkyl Ketimines with Phosphine-Free, Chiral, Cationic Ru-MsDPEN Catalysts. Chem. - Eur. J. 2011, 17, 1109–1113. 10.1002/chem.201002846. [DOI] [PubMed] [Google Scholar]
- Chen F.; Ding Z.; He Y.; Qin J.; Wang T.; Fan Q. H. Asymmetric Hydrogenation of N-Alkyl and N-Aryl Ketimines Using Chiral Cationic Ru(Diamine) Complexes as Catalysts: The Counteranion and Solvent Effects, and Substrate Scope. Tetrahedron 2012, 68, 5248–5257. 10.1016/j.tet.2012.03.019. [DOI] [Google Scholar]
- Xie J. H.; Zhou Q. L. Chiral Diphosphine and Monodentate Phosphorus Ligands on a Spiro Scaffold for Transition-Metal-Catalyzed Asymmetric Reactions. Acc. Chem. Res. 2008, 41, 581–593. 10.1021/ar700137z. [DOI] [PubMed] [Google Scholar]
- Yan Q.; Liu M.; Kong D.; Zi G.; Hou G. Highly Efficient Iridium-Catalyzed Asymmetric Hydrogenation of β-Acylamino Nitroolefins. Chem. Commun. 2014, 50, 12870–12872. 10.1039/C4CC05815A. [DOI] [PubMed] [Google Scholar]
- Kong D.; Li M.; Zi G.; Hou G.; He Y. Enantioselective Hydrogenation of Diarylmethanimines for Synthesis of Chiral Diarylmethylamines. J. Org. Chem. 2016, 81, 6640–6648. 10.1021/acs.joc.6b01273. [DOI] [PubMed] [Google Scholar]
- Yan Q.; Kong D.; Li M.; Hou G.; Zi G. Highly Efficient Rh-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Nitriles. J. Am. Chem. Soc. 2015, 137, 10177–10181. 10.1021/jacs.5b06418. [DOI] [PubMed] [Google Scholar]
- For another selected example, see:; Kluwer A. M.; Detz R. J.; Abiri Z.; Van Der Burg A. M.; Reek J. N. H. Evolutionary Catalyst Screening: Iridium-Catalyzed Imine Hydrogenation. Adv. Synth. Catal. 2012, 354, 89–95. 10.1002/adsc.201100422. [DOI] [Google Scholar]
- Yang Z.; Chen F.; He Y.; Yang N.; Fan Q. H. Highly Enantioselective Synthesis of Indolines: Asymmetric Hydrogenation at Ambient Temperature and Pressure with Cationic Ruthenium Diamine Catalysts. Angew. Chem., Int. Ed. 2016, 55, 13863–13866. 10.1002/anie.201607890. [DOI] [PubMed] [Google Scholar]
- Borrmann R.; Knop N.; Rueping M. Asymmetric Synthesis of Optically Active Spirocyclic Indoline Scaffolds through an Enantioselective Reduction of Indoles. Chem. - Eur. J. 2017, 23, 798–801. 10.1002/chem.201605450. [DOI] [PubMed] [Google Scholar]
- Borrmann R.; Koenigs R. M.; Zoller J.; Rueping M. Asymmetric Hydrogenation of Cyclic Imines and Enamines: Access to 1,5-Benzodiazepine Pharmacophores. Synthesis 2016, 49, 310–318. 10.1055/s-0036-1589401. [DOI] [Google Scholar]
- Ding Z. Y.; Chen F.; Qin J.; He Y. M.; Fan Q. H. Asymmetric Hydrogenation of 2,4-Disubstituted 1,5-Benzodiazepines Using Cationic Ruthenium Diamine Catalysts: An Unusual Achiral Counteranion Induced Reversal of Enantioselectivity. Angew. Chem., Int. Ed. 2012, 51, 5706–5710. 10.1002/anie.201200309. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Chen F.; He Y. M.; Li C.; Fan Q. H. Enantioselective Synthesis of Tunable Chiral Pyridine-Aminophosphine Ligands and Their Applications in Asymmetric Hydrogenation. Org. Biomol. Chem. 2019, 17, 5099–5105. 10.1039/C9OB00770A. [DOI] [PubMed] [Google Scholar]
- Ma B.; Ding Z.; Liu J.; He Y.; Fan Q. H. Highly Enantioselective Hydrogenation of 2,4-Diaryl-1,5-Benzodiazepines Catalyzed by Dendritic Phosphinooxazoline Iridium Complexes. Chem. - Asian J. 2013, 8, 1101–1104. 10.1002/asia.201300150. [DOI] [PubMed] [Google Scholar]
- Miao T.; Ma B.; Ding Z.; Liu Y.; He Y. M.; Fan Q. H. Chemoselective and Enantioselective Hydrogenation of 2,4-Diaryl-3H-Benzo[b]Azepines Catalyzed by Dendritic Phosphinooxazoline Iridium Complexes. Asian J. Org. Chem. 2017, 6, 1219–1221. 10.1002/ajoc.201700094. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Ding Z.; Chen F.; He Y. M.; Yang N.; Fan Q. H. Asymmetric Hydrogenation of Cyclic Imines of Benzoazepines and Benzodiazepines with Chiral, Cationic Ruthenium-Diamine Catalysts. Eur. J. Org. Chem. 2017, 2017, 1973–1977. 10.1002/ejoc.201700236. [DOI] [Google Scholar]
- Gao K.; Wu B.; Yu C. B.; Chen Q. A.; Ye Z. S.; Zhou Y. G. Iridium Catalyzed Asymmetric Hydrogenation of Cyclic Imines of Benzodiazepinones and Benzodiazepines. Org. Lett. 2012, 14, 3890–3893. 10.1021/ol301620z. [DOI] [PubMed] [Google Scholar]
- Gao K.; Yu C. B.; Li W.; Zhou Y. G.; Zhang X. Synthesis and Enantioselective Hydrogenation of Seven-Membered Cyclic Imines: Substituted Dibenzo[b, f][1,4]Oxazepines. Chem. Commun. 2011, 47, 7845–7847. 10.1039/c1cc12263k. [DOI] [PubMed] [Google Scholar]
- Guo R.; Gao K.; Ye Z.; Shi L.; Li Y. Iridium-Catalyzed Asymmetric Hydrogenation of Dibenzo [b, f][1,4] Thiazepines. Pure Appl. Chem. 2013, 85, 843–849. 10.1351/PAC-CON-12-07-02. [DOI] [Google Scholar]
- Wang J. Palladium-Catalyzed Asymmetric Hydrogenation of Dibenzo[b, f][1,4] Thiazepines Activated by Brønsted Acid. Tetrahedron Lett. 2013, 54, 5956–5959. 10.1016/j.tetlet.2013.08.068. [DOI] [Google Scholar]
- Balakrishna B.; Bauzá A.; Frontera A.; Vidal-Ferran A. Asymmetric Hydrogenation of Seven-Membered C = N-Containing Heterocycles and Rationalization of the Enantioselectivity. Chem. - Eur. J. 2016, 22, 10607–10613. 10.1002/chem.201601464. [DOI] [PubMed] [Google Scholar]
- Li P.; Huang Y.; Hu X.; Dong X. Q.; Zhang X. Access to Chiral Seven-Member Cyclic Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2017, 19, 3855–3858. 10.1021/acs.orglett.7b01726. [DOI] [PubMed] [Google Scholar]
- Fleischer S.; Zhou S.; Werkmeister S.; Junge K.; Beller M. Cooperative Iron-Bronsted Acid Catalysis: Enantioselective Hydrogenation of Quinoxalines and 2H-1,4-Benzoxazines. Chem. - Eur. J. 2013, 19, 4997–5003. 10.1002/chem.201204236. [DOI] [PubMed] [Google Scholar]
- Arai N.; Saruwatari Y.; Isobe K.; Ohkuma T. Asymmetric Hydrogenation of Quinoxalines, Benzoxazines, and a Benzothiazine Catalyzed by Chiral Ruthenabicyclic Complexes. Adv. Synth. Catal. 2013, 355, 2769–2774. 10.1002/adsc.201300604. [DOI] [Google Scholar]
- Gao K.; Yu C. B.; Wang D. S.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of 3-Substituted 2H-1,4-Benzoxazines. Adv. Synth. Catal. 2012, 354, 483–488. 10.1002/adsc.201100568. [DOI] [Google Scholar]
- Qin J.; Chen F.; He Y. M.; Fan Q. H. Asymmetric Hydrogenation of 3-Substituted 2H-1,4-Benzoxazines with Chiral Cationic Ru-MsDPEN Catalysts: A Remarkable Counteranion Effect. Org. Chem. Front. 2014, 1, 952–955. 10.1039/C4QO00188E. [DOI] [Google Scholar]
- For a selected publication for the enantioselective hydrogenation of benzoxazinones via a relay iron/chiral Brønsted acid catalysis, see:; Lu L. Q.; Li Y.; Junge K.; Beller M. Relay Iron/Chiral Brønsted Acid Catalysis: Enantioselective Hydrogenation of Benzoxazinones. J. Am. Chem. Soc. 2015, 137, 2763–2768. 10.1021/jacs.5b00085. [DOI] [PubMed] [Google Scholar]
- Núñez-Rico J. L.; Vidal-Ferran A. [Ir(P-OP)]-Catalyzed Asymmetric Hydrogenation of Diversely Substituted C = N-Containing Heterocycles. Org. Lett. 2013, 15, 2066–2069. 10.1021/ol400854a. [DOI] [PubMed] [Google Scholar]
- Chen M. W.; Deng Z.; Yang Q.; Huang J.; Peng Y. Enantioselective Synthesis of Trifluoromethylated Dihydroquinoxalinones: Via Palladium-Catalyzed Hydrogenation. Org. Chem. Front. 2019, 6, 746–750. 10.1039/C8QO01361F. [DOI] [Google Scholar]
- Wang Y.; Liu Y.; Li K.; Yang G.; Zhang W. Iridium-Catalyzed Asymmetric Hydrogenation of Unsaturated Piperazin-2-ones. Adv. Synth. Catal. 2017, 359, 1933–1941. 10.1002/adsc.201700175. [DOI] [Google Scholar]
- Willoughby C. A.; Buchwald S. L. Catalytic Asymmetric Hydrogenation of Imines with Titanocene Catalyst: Scope and Limitations. J. Am. Chem. Soc. 1994, 116, 8952–8965. 10.1021/ja00099a012. [DOI] [Google Scholar]
- For a selected review for their preparation, see:; Glinsky-Olivier N.; Guinchard X. Enantioselective Catalytic Methods for the Elaboration of Chiral Tetrahydro-β-Carbolines and Related Scaffolds. Synthesis 2017, 49, 2605–2620. 10.1055/s-0036-1589003. [DOI] [Google Scholar]
- Li C.; Xiao J. Asymmetric Hydrogenation of Cyclic Imines with an Ionic Cp*Rh(III) Catalyst. J. Am. Chem. Soc. 2008, 130, 13208–13209. 10.1021/ja8050958. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Chen X.; An Y.; Wang J.; Zhuang C.; Tang P.; Chen F. Enantioselective Total Syntheses of (−)-20-Epi-Vincamine and (−)-20-Epi-Eburnamonine by Ir-Catalyzed Asymmetric Imine Hydrogenation/Lactamization Cascade. Chem. - Eur. J. 2020, 26, 10439–10443. 10.1002/chem.202002404. [DOI] [PubMed] [Google Scholar]
- Chen F.; Ding Z.; Qin J.; Wang T.; He Y.; Fan Q. H. Highly Effective Asymmetric Hydrogenation of Cyclic N-Alkyl Imines with Chiral Cationic Ru-MsDPEN Catalysts. Org. Lett. 2011, 13, 4348–4351. 10.1021/ol201679f. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Chen F.; He Y. M.; Fan Q. H. Asymmetric Hydrogenation of Dibenzo[c, e]Azepine Derivatives with Chiral Cationic Ruthenium Diamine Catalysts. Org. Lett. 2019, 21, 5538–5541. 10.1021/acs.orglett.9b01859. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Kong D.; Wang R.; Hou G. Synthesis of Chiral Cyclic Amines via Ir-Catalyzed Enantioselective Hydrogenation of Cyclic Imines. Org. Biomol. Chem. 2017, 15, 3006–3012. 10.1039/C7OB00442G. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Yan Q.; Zi G.; Hou G. Enantioselective Direct Synthesis of Free Cyclic Amines via Intramolecular Reductive Amination. Org. Lett. 2017, 19, 4215–4218. 10.1021/acs.orglett.7b01828. [DOI] [PubMed] [Google Scholar]
- Chang M.; Li W.; Hou G.; Zhang X. Iridium-Catalyzed Enantioselective Hydrogenation of Cyclic Imines. Adv. Synth. Catal. 2010, 352, 3121–3125. 10.1002/adsc.201000473. [DOI] [Google Scholar]
- Escolano C.; Amat M.; Bosch J. Chiral Oxazolopiperidone Lactams: Versatile Intermediates for the Enantioselective Synthesis of Piperidine-Containing Natural Products. Chem. - Eur. J. 2006, 12, 8198–8207. 10.1002/chem.200600813. [DOI] [PubMed] [Google Scholar]
- Lebreton J. Recent Advances in the Total Synthesis of Piperidine and Pyrrolidine Natural Alkaloids with Ring-Closing Metathesis as a Key Step. Eur. J. Org. Chem. 2003, 2003, 3693–3712. 10.1002/ejoc.200300193. [DOI] [Google Scholar]
- Pogocki D.; Ruman T.; Danilczuk M.; Danilczuk M.; Celuch M. Application of Nicotine Enantiomers, Derivatives and Analogues in Therapy of Neurodegenerative Disorders. Eur. J. Pharmacol. 2007, 563, 18–39. 10.1016/j.ejphar.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Guo C.; Sun D. W.; Yang S.; Mao S. J.; Xu X. H.; Zhu S. F.; Zhou Q. L. Iridium-Catalyzed Asymmetric Hydrogenation of 2-Pyridyl Cyclic Imines: A Highly Enantioselective Approach to Nicotine Derivatives. J. Am. Chem. Soc. 2015, 137, 90–93. 10.1021/ja511422q. [DOI] [PubMed] [Google Scholar]
- Scott J. D.; Williams R. M. Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics. Chem. Rev. 2002, 102, 1669–1730. 10.1021/cr010212u. [DOI] [PubMed] [Google Scholar]
- Chrzanowska M.; Rozwadowska M. D. Asymmetric Synthesis of Isoquinoline Alkaloids. Chem. Rev. 2004, 104, 3341–3370. 10.1021/cr030692k. [DOI] [PubMed] [Google Scholar]
- Xie J. H.; Yan P. C.; Zhang Q. Q.; Yuan K. X.; Zhou Q. L. Asymmetric Hydrogenation of Cyclic Imines Catalyzed by Chiral Spiro Iridium Phosphoramidite Complexes for Enantioselective Synthesis of Tetrahydroisoquinolines. ACS Catal. 2012, 2, 561–564. 10.1021/cs300069g. [DOI] [Google Scholar]
- For another selected example, see:; Vilhanová B.; Václavík J.; Šot P.; Pecháček J.; Zápal J.; Pažout R.; Maixner J.; Kuzma M.; Kačer P. Enantioselective Hydrogenation of Cyclic Imines Catalysed by Noyori-Ikariya Half-Sandwich Complexes and Their Analogues. Chem. Commun. 2016, 52, 362–365. 10.1039/C5CC06712J. [DOI] [PubMed] [Google Scholar]
- Chang M.; Li W.; Zhang X. A Highly Efficient and Enantioselective Access to Tetrahydroisoquinoline Alkaloids: Asymmetric Hydrogenation with an Iridium Catalyst. Angew. Chem., Int. Ed. 2011, 50, 10679–10681. 10.1002/anie.201104476. [DOI] [PubMed] [Google Scholar]
- Berhal F.; Wu Z.; Zhang Z.; Ayad T.; Ratovelomanana-Vidal V. Enantioselective Synthesis of 1-Aryl-Tetrahydroisoquinolines through Iridium Catalyzed Asymmetric Hydrogenation. Org. Lett. 2012, 14, 3308–3311. 10.1021/ol301281s. [DOI] [PubMed] [Google Scholar]
- Ji Y.; Wang J.; Chen M.; Shi L.; Zhou Y. G. Dual Stereocontrol for Enantioselective Hydrogenation of Dihydroisoquinolines Induced by Tuning the Amount of N-Bromosuccinimide. Chin. J. Chem. 2018, 36, 139–142. 10.1002/cjoc.201700634. [DOI] [Google Scholar]
- Schwenk R.; Togni A. P-Trifluoromethyl Ligands Derived from JosiPhos in the Ir-Catalysed Hydrogenation of 3,4-Dihydroisoquinoline Hydrochlorides. Dalt. Trans. 2015, 44, 19566–19575. 10.1039/C5DT02019K. [DOI] [PubMed] [Google Scholar]
- Nie H.; Zhu Y.; Hu X.; Wei Z.; Yao L.; Zhou G.; Wang P.; Jiang R.; Zhang S. JosiPhos-Type Binaphane Ligands for Iridium-Catalyzed Enantioselective Hydrogenation of 1-Aryl-Substituted Dihydroisoquinolines. Org. Lett. 2019, 21, 8641–8645. 10.1021/acs.orglett.9b03251. [DOI] [PubMed] [Google Scholar]
- Li B.; Liu R.; Yang J.; Luo J.; Yao L.; Li M.; Zheng X.; Jiang R.; Nie H.; Zhang S. Iridium-Catalyzed Asymmetric Hydrogenation of Sterically Hindered Cyclic Imines for Enantioselective Synthesis of Tetrahydroisoquinolines. Org. Lett. 2021, 23, 140–144. 10.1021/acs.orglett.0c03858. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Zhou X.; Liu R.; Li M.; Li X.; Jiang R.; Nie H.; Zhang S. JosiPhos-Type Binaphane Ligands for the Asymmetric Ir-Catalyzed Hydrogenation of Acyclic Aromatic N-Aryl Imines. Catal. Commun. 2020, 136, 105906. 10.1016/j.catcom.2019.105906. [DOI] [Google Scholar]
- Ružič M.; Pečavar A.; Prudič D.; Kralj D.; Scriban C.; Zanotti-Gerosa A. The Development of an Asymmetric Hydrogenation Process for the Preparation of Solifenacin. Org. Process Res. Dev. 2012, 16, 1293–1300. 10.1021/op3000543. [DOI] [Google Scholar]
- Ji Y.; Feng G. S.; Chen M. W.; Shi L.; Du H.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Cyclic Iminium Salts. Org. Chem. Front. 2017, 4, 1125–1129. 10.1039/C7QO00060J. [DOI] [Google Scholar]
- Yang Q.; Shang G.; Gao W.; Deng J.; Zhang X. A Highly Enantioselective, Pd-TangPhos-Catalyzed Hydrogenation of N-Tosylimines. Angew. Chem., Int. Ed. 2006, 45, 3832–3835. 10.1002/anie.200600263. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Yang F.; Pu D.; Laishram R. D.; Fan R.; Shen G.; Zhang X.; Chen J.; Fan B. Pd/Zn(OTf) 2. Co-Catalyzed Asymmetric Hydrogenation of Imines under Normal Pressure of Hydrogen. Eur. J. Org. Chem. 2018, 2018, 6274–6279. 10.1002/ejoc.201801123. [DOI] [Google Scholar]
- Chen Q. A.; Ye Z. S.; Duan Y.; Zhou Y. G. Homogeneous Palladium-Catalyzed Asymmetric Hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511. 10.1039/C2CS35333D. [DOI] [PubMed] [Google Scholar]
- Yu C. B.; Huang W. X.; Shi L.; Chen M. W.; Wu B.; Zhou Y. G. Asymmetric Hydrogenation via Capture of Active Intermediates Generated from Aza-Pinacol Rearrangement. J. Am. Chem. Soc. 2014, 136, 15837–15840. 10.1021/ja5075745. [DOI] [PubMed] [Google Scholar]
- Imamoto T.; Sugita K.; Yoshida K. An Air-Stable P-Chiral Phosphine Ligand for Highly Enantioselective Transition-Metal-Catalyzed Reactions. J. Am. Chem. Soc. 2005, 127, 11934–11935. 10.1021/ja053458f. [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhang Z.; Li B.; Li F.; Wang Y.; Zhao M.; Gridnev I. D.; Imamoto T.; Zhang W. Pd(OAc)2-Catalyzed Asymmetric Hydrogenation of Sterically Hindered N-Tosylimines. Nat. Commun. 2018, 9, 1–10. 10.1038/s41467-018-07462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li F.; Wang F.; Hu Y.; Zhang Z.; Zhao M.; Zhang W. Pd(OAc)2-Catalyzed Asymmetric Hydrogenation of α-Iminoesters. Org. Lett. 2019, 21, 9060–9065. 10.1021/acs.orglett.9b03452. [DOI] [PubMed] [Google Scholar]
- Fan D.; Liu Y.; Jia J.; Zhang Z.; Liu Y.; Zhang W. Synthesis of Chiral α-Aminosilanes through Palladium-Catalyzed Asymmetric Hydrogenation of Silylimines. Org. Lett. 2019, 21, 1042–1045. 10.1021/acs.orglett.8b04073. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Butt N. A.; Zhou M.; Liu D.; Zhang W. Asymmetric Transfer and Pressure Hydrogenation with Earth-Abundant Transition Metal Catalysts. Chin. J. Chem. 2018, 36, 443–454. 10.1002/cjoc.201800053. [DOI] [Google Scholar]
- Li B.; Chen J.; Zhang Z.; Gridnev I. D.; Zhang W. Nickel-Catalyzed Asymmetric Hydrogenation of N-Sulfonyl Imines. Angew. Chem., Int. Ed. 2019, 58, 7329–7334. 10.1002/anie.201902576. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Zhang F.; Liu K.; Zhang X.; Lv H. Nickel-Catalyzed Chemoselective Asymmetric Hydrogenation of α,β -Unsaturated Ketoimines: An Efficient Approach to Chiral Allylic Amines. Org. Lett. 2019, 21, 8966–8969. 10.1021/acs.orglett.9b03365. [DOI] [PubMed] [Google Scholar]
- Shevlin M.; Friedfeld M. R.; Sheng H.; Pierson N. A.; Hoyt J. M.; Campeau L. C.; Chirik P. J. Nickel-Catalyzed Asymmetric Alkene Hydrogenation of α,β-Unsaturated Esters: High-Throughput Experimentation-Enabled Reaction Discovery, Optimization, and Mechanistic Elucidation. J. Am. Chem. Soc. 2016, 138, 3562–3569. 10.1021/jacs.6b00519. [DOI] [PubMed] [Google Scholar]
- Hibino T.; Makino K.; Sugiyama T.; Hamada Y. Homogeneous Chiral Nickel-Catalyzed Asymmetric Hydrogenation of Substituted Aromatic α-Aminoketone Hydrochlorides through Dynamic Kinetic Resolution. ChemCatChem 2009, 1, 237–240. 10.1002/cctc.200900084. [DOI] [Google Scholar]
- Yan Z.; Wu B.; Gao X.; Chen M. W.; Zhou Y. G. Enantioselective Synthesis of α-Amino Phosphonates via Pd-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 692–695. 10.1021/acs.orglett.5b03664. [DOI] [PubMed] [Google Scholar]
- Wang Y. Q.; Yu C. B.; Wang D. W.; Wang X. B.; Zhou Y. G. Enantioselective Synthesis of Cyclic Sulfamidates via Pd-Catalyzed Hydrogenation. Org. Lett. 2008, 10, 2071–2074. 10.1021/ol800591u. [DOI] [PubMed] [Google Scholar]
- Yu C. B.; Wang D. W.; Zhou Y. G. Highly Enantioselective Synthesis of Sultams via Pd-Catalyzed Hydrogenation. J. Org. Chem. 2009, 74, 5633–5635. 10.1021/jo900790k. [DOI] [PubMed] [Google Scholar]
- Chen F.; Li Z.; He Y.; Fan Q. H. Asymmetric Hydrogenation of Cyclic N-Sulfonylimines with Phosphine-Free Chiral Cationic Ru-MsDPEN Catalysts. Chin. J. Chem. 2010, 28, 1529–1532. 10.1002/cjoc.201090260. [DOI] [Google Scholar]
- McLaughlin M.; Belyk K.; Chen C. Y.; Linghu X.; Pan J.; Qian G.; Reamer R. A.; Xu Y. Practical Asymmetric Synthesis of a Chiral Piperazinone Derivative. Org. Process Res. Dev. 2013, 17, 1052–1060. 10.1021/op400150w. [DOI] [Google Scholar]
- Liu Y.; Huang Y.; Yi Z.; Liu G.; Dong X. Q.; Zhang X. Enantioselective Access to Chiral Cyclic Sulfamidates Through Iridium-Catalyzed Asymmetric Hydrogenation. Adv. Synth. Catal. 2019, 361, 1582–1586. 10.1002/adsc.201801566. [DOI] [Google Scholar]
- Zhao Q.; Chen C.; Wen J.; Dong X. Q.; Zhang X. Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 1905–1921. 10.1021/acs.accounts.0c00347. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yi Z.; Tan X.; Dong X. Q.; Zhang X. Nickel-Catalyzed Asymmetric Hydrogenation of Cyclic Sulfamidate Imines: Efficient Synthesis of Chiral Cyclic Sulfamidates. iScience 2019, 19, 63–73. 10.1016/j.isci.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Zhang X.; Wang H.; Cong H.; Zhang X.; Dong X.-Q. Synthesis of Chiral α-Substituted α-Amino Acid and Amine Derivatives Through Ni-Catalyzed Asymmetric Hydrogenation. Chem. Commun. 2020, 56, 4934–4937. 10.1039/D0CC01220C. [DOI] [PubMed] [Google Scholar]
- Yu C. B.; Gao K.; Wang D. S.; Shi L.; Zhou Y. G. Enantioselective Pd-Catalyzed Hydrogenation of Enesulfonamides. Chem. Commun. 2011, 47, 5052–5054. 10.1039/c1cc10313j. [DOI] [PubMed] [Google Scholar]
- Song B.; Yu C. B.; Huang W. X.; Chen M. W.; Zhou Y. G. Formal Palladium-Catalyzed Asymmetric Hydrogenolysis of Racemic N -Sulfonyloxaziridines. Org. Lett. 2015, 17, 190–193. 10.1021/ol503118v. [DOI] [PubMed] [Google Scholar]
- Spindler F.; Blaser H.-U. The Highly Enantioselective Hydrogenation of N-Diphenylphosphinylketimines with Cationic Rh Ferrocenyldiphosphine Catalysts. Adv. Synth. Catal. 2001, 343, 68–70. . [DOI] [Google Scholar]
- Wang Y. Q.; Lu S. M.; Zhou Y. G. Highly Enantioselective Pd-Catalyzed Asymmetric Hydrogenation of Activated Imines. J. Org. Chem. 2007, 72, 3729–3734. 10.1021/jo0700878. [DOI] [PubMed] [Google Scholar]
- Ma X.; Qiao L.; Liu G.; Huang Z. A New Phosphine-Amine-Oxazoline Ligand for Ru-Catalyzed Asymmetric Hydrogenation of N-Phosphinylimines. Chin. J. Chem. 2018, 36, 1151–1155. 10.1002/cjoc.201800343. [DOI] [Google Scholar]
- Seo C. S. G.; Tannoux T.; Smith S. A. M.; Lough A. J.; Morris R. H. Enantioselective Hydrogenation of Activated Aryl Imines Catalyzed by an Iron(II) P-NH-P′ Complex. J. Org. Chem. 2019, 84, 12040–12049. 10.1021/acs.joc.9b01964. [DOI] [PubMed] [Google Scholar]
- Lagaditis P. O.; Sues P. E.; Sonnenberg J. F.; Wan K. Y.; Lough A. J.; Morris R. H. Iron(II) Complexes Containing Unsymmetrical P-N-P′ Pincer Ligands for the Catalytic Asymmetric Hydrogenation of Ketones and Imines. J. Am. Chem. Soc. 2014, 136, 1367–1380. 10.1021/ja4082233. [DOI] [PubMed] [Google Scholar]
- Mikami K.; Murase T.; Zhai L.; Kawauchi S.; Itoh Y.; Ito S. Sequential Perfluoroalkylation and Asymmetric Reduction of Nitriles Triggered with Perfluoroalkyl Titanates: Catalytic Asymmetric Synthesis of Perfluoroalkyl Amines. Tetrahedron Lett. 2010, 51, 1371–1373. 10.1016/j.tetlet.2009.12.140. [DOI] [Google Scholar]
- Ojima I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; Blackwell: Oxford, 2009; pp 1–624. [Google Scholar]
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- For a recent work involving metal-free asymmetric hydrogenation of N-acyl imines, see:; Zhao B.; Shang R.; Wang G. Z.; Wang S.; Chen H.; Fu Y. Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. 10.1021/acscatal.9b04699. [DOI] [Google Scholar]
- Yuan Q.; Liu D.; Zhang W. Iridium-Catalyzed Asymmetric Hydrogenation of β,γ-Unsaturated γ-Lactams: Scope and Mechanistic Studies. Org. Lett. 2017, 19, 1144–1147. 10.1021/acs.orglett.7b00171. [DOI] [PubMed] [Google Scholar]
- Yuan Q.; Liu D.; Zhang W. Synthesis of Chiral γ-Lactams via in Situ Elimination/Iridium-Catalyzed Asymmetric Hydrogenation of Racemic γ-Hydroxy γ-Lactams. Org. Lett. 2017, 19, 1886–1889. 10.1021/acs.orglett.7b00651. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Zhao Q.; Wang H.; Yang T.; Wen J.; Zhang X. Asymmetric Hydrogenation of Cationic Intermediates for the Synthesis of Chiral N, O-Acetals. Chem. - Eur. J. 2020, 26, 11470–11477. 10.1002/chem.202002930. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yi Z.; Yang X.; Wang H.; Yin C.; Wang M.; Dong X. Q.; Zhang X. Efficient Access to Chiral 2-Oxazolidinones via Ni-Catalyzed Asymmetric Hydrogenation: Scope Study, Mechanistic Explanation, and Origin of Enantioselectivity. ACS Catal. 2020, 10, 11153–11161. 10.1021/acscatal.0c02569. [DOI] [Google Scholar]
- Chen Z. P.; Hu S. B.; Zhou J.; Zhou Y. G. Synthesis of Chiral Trifluoromethyl-Substituted Hydrazines via Pd-Catalyzed Asymmetric Hydrogenation and Reductive Amination. ACS Catal. 2015, 5, 6086–6089. 10.1021/acscatal.5b01641. [DOI] [Google Scholar]
- Chen Z. P.; Hu S. B.; Chen M. W.; Zhou Y. G. Synthesis of Chiral Fluorinated Hydrazines via Pd-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 2676–2679. 10.1021/acs.orglett.6b01118. [DOI] [PubMed] [Google Scholar]
- Chen Z. P.; Chen M. W.; Shi L.; Yu C. B.; Zhou Y. G. Pd-Catalyzed Asymmetric Hydrogenation of Fluorinated Aromatic Pyrazol-5-Ols via Capture of Active Tautomers. Chem. Sci. 2015, 6, 3415–3419. 10.1039/C5SC00835B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulioukina N. S.; Shergold I. A.; Rybakov V. B.; Beletskaya I. P. One-Pot Two-Step Synthesis of Optically Active α-Amino Phosphonates by Palladium-Catalyzed Hydrogenation/Hydrogenolysis of α-Hydrazono Phosphonates. Adv. Synth. Catal. 2017, 359, 153–162. 10.1002/adsc.201600945. [DOI] [Google Scholar]
- Hu Q.; Hu Y.; Liu Y.; Zhang Z.; Liu Y.; Zhang W. Rh-Catalyzed Chemo- and Enantioselective Hydrogenation of Allylic Hydrazones. Chem. - Eur. J. 2017, 23, 1040–1043. 10.1002/chem.201605579. [DOI] [PubMed] [Google Scholar]
- Fan D.; Hu Y.; Jiang F.; Zhang Z.; Zhang W. Rhodium-Catalyzed Chemo- and Enantioselective Hydrogenation of Alkynyl-Aryl Hydrazones. Adv. Synth. Catal. 2018, 360, 2228–2232. 10.1002/adsc.201800243. [DOI] [Google Scholar]
- Schuster C. H.; Dropinski J. F.; Shevlin M.; Li H.; Chen S. Ruthenium-Catalyzed Enantioselective Hydrogenation of Hydrazones. Org. Lett. 2020, 22, 7562–7566. 10.1021/acs.orglett.0c02756. [DOI] [PubMed] [Google Scholar]
- Yoshikawa N.; Tan L.; McWilliams J. C.; Ramasamy D.; Sheppard R. Catalytic Enantioselective Hydrogenation of N-Alkoxycarbonyl Hydrazones: A Practical Synthesis of Chiral Hydrazines. Org. Lett. 2010, 12, 276–279. 10.1021/ol902602c. [DOI] [PubMed] [Google Scholar]
- Haddad N.; Qu B.; Rodriguez S.; Van Der Veen L.; Reeves D. C.; Gonnella N. C.; Lee H.; Grinberg N.; Ma S.; Krishnamurthy D.; Wunberg T.; Senanayake C. H. Catalytic Asymmetric Hydrogenation of Heterocyclic Ketone-Derived Hydrazones, Pronounced Solvent Effect on the Inversion of Configuration. Tetrahedron Lett. 2011, 52, 3718–3722. 10.1016/j.tetlet.2011.05.017. [DOI] [Google Scholar]
- Chang M.; Liu S.; Huang K.; Zhang X. Direct Catalytic Asymmetric Reductive Amination of Simple Aromatic Ketones. Org. Lett. 2013, 15, 4354–4357. 10.1021/ol401851c. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Zhang Z.; Zhang J.; Liu Y.; Gridnev I. D.; Zhang W. Cobalt-Catalyzed Asymmetric Hydrogenation of C = N Bonds Enabled by Assisted Coordination and Nonbonding Interactions. Angew. Chem., Int. Ed. 2019, 58, 15767–15771. 10.1002/anie.201909928. [DOI] [PubMed] [Google Scholar]
- Co-catalyzed Asymmetric Hydrogenation of C=C bonds:; Monfette S.; Turner Z. R.; Semproni S. P.; Chirik P. J. Enantiopure C1-Symmetric Bis(Imino)Pyridine Cobalt Complexes for Asymmetric Alkene Hydrogenation. J. Am. Chem. Soc. 2012, 134, 4561–4564. 10.1021/ja300503k. [DOI] [PubMed] [Google Scholar]
- Co-catalyzed Asymmetric Hydrogenation of C=C bonds:; Chirik P. J. Iron- and Cobalt-Catalyzed Alkene Hydrogenation: Catalysis with Both Redox-Active and Strong Field Ligands. Acc. Chem. Res. 2015, 48, 1687–1695. 10.1021/acs.accounts.5b00134. [DOI] [PubMed] [Google Scholar]
- Co-catalyzed Asymmetric Hydrogenation of C=O bonds:; Zhang D.; Zhu E. Z.; Lin Z. W.; Wei Z. B.; Li Y. Y.; Gao J. X. Enantioselective Hydrogenation of Ketones Catalyzed by Chiral Cobalt Complexes Containing PNNP Ligand. Asian J. Org. Chem. 2016, 5, 1323–1326. 10.1002/ajoc.201600358. [DOI] [Google Scholar]
- Verzijl G. K. M.; Hassfeld J.; De Vries A. H. M.; Lefort L. Enantioselective Synthesis of a 2,3-Benzodiazepine Intermediate of BET Inhibitor BAY 1238097 via Catalytic Asymmetric Hydrogenation. Org. Process Res. Dev. 2020, 24, 255–260. 10.1021/acs.oprd.9b00519. [DOI] [Google Scholar]
- Huang K.; Li S.; Chang M.; Zhang X. Rhodium-Catalyzed Enantioselective Hydrogenation of Oxime Acetates. Org. Lett. 2013, 15, 484–487. 10.1021/ol303282u. [DOI] [PubMed] [Google Scholar]
- Goulioukina N. S.; Shergold I. A.; Bondarenko G. N.; Ilyin M. M.; Davankov V. A.; Beletskaya I. P. Palladium-Catalyzed Asymmetric Hydrogenation of N-Hydroxy-α-Imino Phosphonates Using Brønsted Acid as Activator: The First Catalytic Enantioselective Approach to Chiral N-Hydroxy-Aamino Phosphonates. Adv. Synth. Catal. 2012, 354, 2727–2733. 10.1002/adsc.201200170. [DOI] [Google Scholar]
- Abe H.; Amii H.; Uneyama K. Pd-Catalyzed Asymmetric Hydrogenation of α-Fluorinated Iminoesters in Fluorinated Alcohol: A New and Catalytic Enantioselective Synthesis of Fluoro α-Amino Acid Derivatives. Org. Lett. 2001, 3, 313–315. 10.1021/ol0002471. [DOI] [PubMed] [Google Scholar]
- Mas-Roselló J.; Smejkal T.; Cramer N. Iridium-Catalyzed Acid-Assisted Asymmetric Hydrogenation of Oximes to Hydroxylamines. Science 2020, 368, 1098–1102. 10.1126/science.abb2559. [DOI] [PubMed] [Google Scholar]
- Hou G.; Gosselin F.; Li W.; McWilliams J. C.; Sun Y.; Weisel M.; O’Shea P. D.; Chen C. Y.; Davies I. W.; Zhang X. Enantioselective Hydrogenation of N-H Imines. J. Am. Chem. Soc. 2009, 131, 9882–9883. 10.1021/ja903319r. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Wen J.; Tan R.; Huang K.; Metola P.; Wang R.; Anslyn E. V.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of Unprotected NH Imines Assisted by a Thiourea. Angew. Chem., Int. Ed. 2014, 53, 8467–8470. 10.1002/anie.201404570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G.; Tao R.; Sun Y.; Zhang X.; Gosselin F. Iridium-Monodentate Phosphoramidite-Catalyzed Asymmetric Hydrogenation of Substituted Benzophenone N-H Imines. J. Am. Chem. Soc. 2010, 132, 2124–2125. 10.1021/ja909583s. [DOI] [PubMed] [Google Scholar]
- Knowles W. S.; Sabacky M. J.; Vineyard B. D.; Weinkauff D. J. Asymmetric Hydrogenation with a Complex of Rhodium and a Chiral Bisphosphine. J. Am. Chem. Soc. 1975, 97, 2567–2568. 10.1021/ja00842a058. [DOI] [Google Scholar]
- Noyori R.; Ohta M.; Hsiao Y.; Kitamura M.; Ohta T.; Takaya H. Asymmetric Synthesis of Isoquinoline Alkaloids by Homogeneous Catalysis. J. Am. Chem. Soc. 1986, 108, 7117–7119. 10.1021/ja00282a054. [DOI] [Google Scholar]
- Burk M. J.; Wang Y. M.; Lee J. R. A Convenient Asymmetric Synthesis of α-1-Arylalkylamines through the Enantioselective Hydrogenation of Enamides. J. Am. Chem. Soc. 1996, 118, 5142–5143. 10.1021/ja953872n. [DOI] [Google Scholar]
- Reetz M. T.; Mehler G. Highly Enantioselective Rh-Catalyzed Hydrogenation Reactions Based on Chiral Monophosphite Ligands. Angew. Chem., Int. Ed. 2000, 39, 3889–3890. . [DOI] [PubMed] [Google Scholar]
- van den Berg M.; Minnaard A. J.; Schudde E. P.; van Esch J.; de Vries A. H. M.; de Vries J. G.; Feringa B. L. Highly Enantioselective Rhodium-Catalyzed Hydrogenation with Monodentate Ligands. J. Am. Chem. Soc. 2000, 122, 11539–11540. 10.1021/ja002507f. [DOI] [Google Scholar]
- Hu A. G.; Fu Y.; Xie J. H.; Zhou H.; Wang L. X.; Zhou Q. L. Monodentate Chiral Spiro Phosphoramidites: Efficient Ligands for Rhodium-Catalyzed Enantioselective Hydrogenation of Enamides. Angew. Chem., Int. Ed. 2002, 41, 2348–2350. . [DOI] [PubMed] [Google Scholar]
- Kleman P.; Pizzano A. Rh Catalyzed Asymmetric Olefin Hydrogenation: Enamides, Enol Esters and Beyond. Tetrahedron Lett. 2015, 56, 6944–6963. 10.1016/j.tetlet.2015.10.093. [DOI] [Google Scholar]
- Erre G.; Enthaler S.; Junge K.; Addis D.; Beller M. Iridium-Catalysed Asymmetric Hydrogenation of Enamides in the Presence of 3,3-Substituted H8-Phosphoramidites. Adv. Synth. Catal. 2009, 351, 1437–1441. 10.1002/adsc.200800799. [DOI] [Google Scholar]
- For a selected example using diamine ligands, see:; Opačak S.; Kokan Z.; Glasovac Z.; Perić B.; Kirin S. I. Backdoor Induction” of Chirality: Trans-1,2-Cyclohexanediamine as Key Building Block for Asymmetric Hydrogenation Catalysts. Eur. J. Org. Chem. 2019, 2019, 2115–2128. 10.1002/ejoc.201801647. [DOI] [Google Scholar]
- Lyubimov S. E.; Rastorguev E. A.; Verbitskaya T. A.; Petrovskii P. V.; Hey-Hawkins E.; Kalinin V. N.; Davankov V. A. The Use of a New Carboranylamidophosphite Ligand in the Asymmetric Rh-Catalyzed Hydrogenation of α- And β-Dehydroamino Acid Derivatives. Polyhedron 2011, 30, 1258–1261. 10.1016/j.poly.2011.02.003. [DOI] [Google Scholar]
- Frank D. J.; Franzke A.; Pfaltz A. Asymmetric Hydrogenation Using Rhodium Complexes Generated from Mixtures of Monodentate Neutral and Anionic Phosphorus Ligands. Chem. - Eur. J. 2013, 19, 2405–2415. 10.1002/chem.201202408. [DOI] [PubMed] [Google Scholar]
- Dobrota C.; Fiaud J. C.; Toffano M. P-Aryl-Diphenylphospholanes and Their Phospholanium Salts as Efficient Monodentate Ligands for Asymmetric Rhodium-Catalyzed Hydrogenation. ChemCatChem 2015, 7, 144–148. 10.1002/cctc.201402687. [DOI] [Google Scholar]
- Alegre S.; Alberico E.; Pàmies O.; Diéguez M. Rh-Catalyzed Asymmetric Hydrogenation Using a Furanoside Monophosphite Second-Generation Ligand Library: Scope and Limitations. Tetrahedron: Asymmetry 2014, 25, 258–262. 10.1016/j.tetasy.2013.12.010. [DOI] [Google Scholar]
- Sun X.; Li W.; Zhou L.; Zhang X. Enantioselective Hydrogenation of α-Dehydroamino Acid Esters Catalyzed by Rhodium Complexes with Chiral Bisaminophosphine Ligands. Adv. Synth. Catal. 2010, 352, 1150–1154. 10.1002/adsc.201000038. [DOI] [Google Scholar]
- Arribas I.; Álvarez E.; Pizzano A. Novel Bis(1,3,2-Diazaphospholidine) Ligands for Asymmetric Catalysis. Organometallics 2013, 32, 2497–2500. 10.1021/om400185q. [DOI] [Google Scholar]
- Bravo M. J.; Ceder R. M.; Muller G.; Rocamora M. New Enantiopure P, P-Bidentate Bis(Diamidophosphite) Ligands. Application in Asymmetric Rhodium-Catalyzed Hydrogenation. Organometallics 2013, 32, 2632–2642. 10.1021/om400119q. [DOI] [Google Scholar]
- Fernández-Pérez H.; Donald S. M. A.; Munslow I. J.; Benet-Buchholz J.; Maseras F.; Vidal-Ferran A. Highly Modular P-OP Ligands for Asymmetric Hydrogenation: Synthesis, Catalytic Activity, and Mechanism. Chem. - Eur. J. 2010, 16, 6495–6508. 10.1002/chem.200902915. [DOI] [PubMed] [Google Scholar]
- Farkas G.; Balogh S.; Szöllosy Á.; Ürge L.; Darvas F.; Bakos J. Novel Phosphine-Phosphites and Their Use in Asymmetric Hydrogenation. Tetrahedron: Asymmetry 2011, 22, 2104–2109. 10.1016/j.tetasy.2011.12.007. [DOI] [Google Scholar]
- Etayo P.; Núñez-Rico J. L.; Fernández-Pérez H.; Vidal-Ferran A. Enantioselective Access to Chiral Drugs by Using Asymmetric Hydrogenation Catalyzed by Rh(P-OP) Complexes. Chem. - Eur. J. 2011, 17, 13978–13982. 10.1002/chem.201103014. [DOI] [PubMed] [Google Scholar]
- Etayo P.; Núñez-Rico J. L.; Vidal-Ferran A. Chiral Rhodium Complexes Derived from Electron-Rich Phosphine-Phosphites as Asymmetric Hydrogenation Catalysts. Organometallics 2011, 30, 6718–6725. 10.1021/om200933b. [DOI] [Google Scholar]
- Fernández-Pérez H.; Benet-Buchholz J.; Vidal-Ferran A. Small Bite-Angle P-OP Ligands for Asymmetric Hydroformylation and Hydrogenation. Org. Lett. 2013, 15, 3634–3637. 10.1021/ol401494x. [DOI] [PubMed] [Google Scholar]
- Fernández-Pérez H.; Benet-Buchholz J.; Vidal-Ferran A. Enantiopure Narrow Bite-Angle P-OP Ligands: Synthesis and Catalytic Performance in Asymmetric Hydroformylations and Hydrogenations. Chem. - Eur. J. 2014, 20, 15375–15384. 10.1002/chem.201404731. [DOI] [PubMed] [Google Scholar]
- Song F. T.; Ouyang G. H.; Li Y.; He Y. M.; Fan Q. H. Metallacrown Ether Catalysts Containing Phosphine-Phosphite Polyether Ligands for Rh-Catalyzed Asymmetric Hydrogenation - Enhancements in Activity and Enantioselectivity. Eur. J. Org. Chem. 2014, 2014, 6713–6719. 10.1002/ejoc.201402735. [DOI] [Google Scholar]
- Pullmann T.; Engendahl B.; Zhang Z.; Hölscher M.; Zanotti-Gerosa A.; Dyke A.; Franciò G.; Leitner W. Quinaphos and Dihydro-Quinaphos Phosphine-Phosphoramidite Ligands for Asymmetric Hydrogenation. Chem. - Eur. J. 2010, 16, 7517–7526. 10.1002/chem.201000063. [DOI] [PubMed] [Google Scholar]
- Zhou X. M.; Huang J. Di; Luo L. B.; Zhang C. L.; Hu X. P.; Zheng Z. Chiral 1-Phenylethylamine-Derived Phosphine-Phosphoramidite Ligands for Highly Enantioselective Rh-Catalyzed Hydrogenation of β-(Acylamino) Acrylates: Significant Effect of Substituents on 3,3′-Positions of Binaphthyl Moiety. Org. Biomol. Chem. 2010, 8, 2320–2322. 10.1039/c000268b. [DOI] [PubMed] [Google Scholar]
- Balogh S.; Farkas G.; Szöllösy Á.; Darvas F.; Ürge L.; Bakos J. Fine Tuning of the Structure of Phosphine-Phosphoramidites: Application for Rhodium-Catalyzed Asymmetric Hydrogenations. Tetrahedron: Asymmetry 2013, 24, 66–74. 10.1016/j.tetasy.2012.11.013. [DOI] [Google Scholar]
- Pang Z. B.; Li H. F.; Tian M.; Wang L. L. Chiral Diphosphites Derived from (1R,2R)-Trans-1,2-Cyclohexanediol: A New Class of Ligands for Asymmetric Hydrogenations. Tetrahedron: Asymmetry 2015, 26, 1389–1393. 10.1016/j.tetasy.2015.10.020. [DOI] [Google Scholar]
- Balogh S.; Farkas G.; Tóth I.; Bakos J. Synthesis of New N-Substituted Chiral Phosphine-Phosphoramidite Ligands and Their Application in Asymmetric Hydrogenations and Allylic Alkylations. Tetrahedron: Asymmetry 2015, 26, 666–673. 10.1016/j.tetasy.2015.05.001. [DOI] [Google Scholar]
- Hammerer T.; Leitner W.; Franciò G. Synthesis of Phospholane-Phosphoramidite Ligands and Their Application in Asymmetric Catalysis. ChemCatChem 2015, 7, 1583–1592. 10.1002/cctc.201500070. [DOI] [Google Scholar]
- Schmitz C.; Holthusen K.; Leitner W.; Franciò G. Bidentate Phosphine-Phosphoramidite Ligands of the BettiPhos Family for Rh-Catalyzed Asymmetric Hydrogenation. Eur. J. Org. Chem. 2017, 2017, 4111–4116. 10.1002/ejoc.201700663. [DOI] [Google Scholar]
- Coll M.; Pàmies O.; Diéguez M. Asymmetric Rh-Catalyzed Hydrogenation Using a Furanoside Phosphite-Phosphoroamidite and Diphosphoroamidite Ligand Library. Dalt. Trans. 2012, 41, 3038–3045. 10.1039/c2dt11888b. [DOI] [PubMed] [Google Scholar]
- Argüelles A. J.; Sun S.; Budaitis B. G.; Nagorny P. Design, Synthesis, and Application of Chiral C2-Symmetric Spiroketal-Containing Ligands in Transition-Metal Catalysis. Angew. Chem., Int. Ed. 2018, 57, 5325–5329. 10.1002/anie.201713304. [DOI] [PubMed] [Google Scholar]
- Huang J.; Hong M.; Wang C. C.; Kramer S.; Lin G. Q.; Sun X. W. Asymmetric Synthesis of Chiral Spiroketal Bisphosphine Ligands and Their Application in Enantioselective Olefin Hydrogenation. J. Org. Chem. 2018, 83, 12838–12846. 10.1021/acs.joc.8b01693. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen P. W. N. M.; Rivillo D.; Raynal M.; Freixa Z. Enantioselective Supramolecular Catalysis Induced by Remote Chiral Diols. J. Am. Chem. Soc. 2011, 133, 18562–18565. 10.1021/ja207912d. [DOI] [PubMed] [Google Scholar]
- Pignataro L.; Bovio C.; Civera M.; Piarulli U.; Gennari C. A Library Approach to the Development of BenzaPhos: Highly Efficient Chiral Supramolecular Ligands for Asymmetric Hydrogenation. Chem. - Eur. J. 2012, 18, 10368–10381. 10.1002/chem.201201032. [DOI] [PubMed] [Google Scholar]
- Song F. T.; Ouyang G. H.; Li Y.; He Y. M.; Fan Q. H. Metallacrown Ether Catalysts Containing Phosphine-Phosphite Polyether Ligands for Rh-Catalyzed Asymmetric Hydrogenation - Enhancements in Activity and Enantioselectivity. Eur. J. Org. Chem. 2014, 2014, 6713–6719. 10.1002/ejoc.201402735. [DOI] [Google Scholar]
- Zhang X. C.; Hu Y. H.; Chen C. F.; Fang Q.; Yang L. Y.; Lu Y. B.; Xie L. J.; Wu J.; Li S.; Fang W. A Supramolecularly Tunable Chiral Diphosphine Ligand: Application to Rh and Ir-Catalyzed Enantioselective Hydrogenation. Chem. Sci. 2016, 7, 4594–4599. 10.1039/C6SC00589F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Ouyang G.; Tang Y.; He Y. M.; Fan Q. H. Diaza-Crown Ether-Bridged Chiral Diphosphoramidite Ligands: Synthesis and Applications in Asymmetric Catalysis. J. Org. Chem. 2020, 85, 8176–8184. 10.1021/acs.joc.0c00223. [DOI] [PubMed] [Google Scholar]
- For a selected example using chiral P, S-ligands, see:; Margalef J.; Borràs C.; Alegre S.; Alberico E.; Pàmies O.; Diéguez M. Phosphite-Thioether/Selenoether Ligands from Carbohydrates: An Easily Accessible Ligand Library for the Asymmetric Hydrogenation of Functionalized and Unfunctionalized Olefins. ChemCatChem 2019, 11, 2142–2168. 10.1002/cctc.201900132. [DOI] [Google Scholar]
- For a selected example using a chiral P, N-ligand, see:; Busacca C. A.; Lorenz J. C.; Saha A. K.; Cheekoori S.; Haddad N.; Reeves D.; Lee H.; Li Z.; Rodriguez S.; Senanayake C. H. Development of the BIPI Ligands for Asymmetric Hydrogenation. Catal. Sci. Technol. 2012, 2, 2083–2089. 10.1039/c2cy20337e. [DOI] [Google Scholar]
- For a selected review, see: Imamoto T. In Rhodium Catalysis in Organic Synthesis: Methods and Reactions; Tanaka K., Ed.; Wiley-VCH: Weinheim, 2019; Chapter 1, pp 3–37. [Google Scholar]
- Wang X. B.; Goto M.; Han L. B. Efficient Asymmetric Hydrogenation of α-Acetamidocinnamates through a Simple, Readily Available Monodentate Chiral H-Phosphinate. Chem. - Eur. J. 2014, 20, 3631–3635. 10.1002/chem.201304675. [DOI] [PubMed] [Google Scholar]
- Imamoto T.; Horiuchi Y.; Hamanishi E.; Takeshita S.; Tamura K.; Sugiya M.; Yoshida K. Utilization of Optically Active Secondary Phosphine-Boranes: Synthesis of P-Chiral Diphosphines and Their Enantioinduction Ability in Rhodium-Catalyzed Asymmetric Hydrogenation. Tetrahedron 2015, 71, 6471–6480. 10.1016/j.tet.2015.05.088. [DOI] [Google Scholar]
- Moritz J.-O.; Chakrabortty S.; Müller B. H.; Spannenberg A.; Kamer P. C. J. P-Chirogenic Diphosphazanes with Axially Chiral Substituents and Their Use in Rh-Catalyzed Asymmetric Hydrogenation. J. Org. Chem. 2020, 85, 14537–14544. 10.1021/acs.joc.0c01108. [DOI] [PubMed] [Google Scholar]
- For a selected article about the mechanism of their AH using Rh complexes, see:; Gridnev I. D.; Liu Y.; Imamoto T. Mechanism of Asymmetric Hydrogenation of β-Dehydroamino Acids Catalyzed by Rhodium Complexes: Large-Scale Experimental and Computational Study. ACS Catal. 2014, 4, 203–219. 10.1021/cs400767e. [DOI] [Google Scholar]
- Hoge G.; Wu H. P.; Kissel W. S.; Pflum D. A.; Greene D. J.; Bao J. Highly Selective Asymmetric Hydrogenation Using a Three Hindered Quadrant Bisphosphine Rhodium Catalyst. J. Am. Chem. Soc. 2004, 126, 5966–5967. 10.1021/ja048496y. [DOI] [PubMed] [Google Scholar]
- Wu H. P.; Hoge G. Highly Enantioselective Asymmetric Hydrogenation of β-Acetamido Dehydroamino Acid Derivatives Using a Three-Hindered Quadrant Rhodium Catalyst. Org. Lett. 2004, 6, 3645–3647. 10.1021/ol048386w. [DOI] [PubMed] [Google Scholar]
- Gridnev I. D.; Imamoto T.; Hoge G.; Kouchi M.; Takahashi H. Asymmetric Hydrogenation Catalyzed by a Rhodium Complex of (R)-(Tert-Butylmethylphosphino)(Di-Tert-Butylphosphino)-Methane: Scope of Enantioselectivity and Mechanistic Study. J. Am. Chem. Soc. 2008, 130, 2560–2572. 10.1021/ja076542z. [DOI] [PubMed] [Google Scholar]
- Sawatsugawa Y.; Tamura K.; Sano N.; Imamoto T. A Bulky Three-Hindered Quadrant Bisphosphine Ligand: Synthesis and Application in Rhodium-Catalyzed Asymmetric Hydrogenation of Functionalized Alkenes. Org. Lett. 2019, 21, 8874–8878. 10.1021/acs.orglett.9b02702. [DOI] [PubMed] [Google Scholar]
- For a selected publication in the AH of β-keto enamides, see:; Llopis Q.; Guillamot G.; Phansavath P.; Ratovelomanana-Vidal V. Enantioselective Synthesis of α-Acetal-B′-Amino Ketone Derivatives by Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2017, 19, 6428–6431. 10.1021/acs.orglett.7b03332. [DOI] [PubMed] [Google Scholar]
- Revés M.; Ferrer C.; León T.; Doran S.; Etayo P.; Vidal-Ferran A.; Riera A.; Verdaguer X. Primary and Secondary Aminophosphines as Novel P-Stereogenic Building Blocks for Ligand Synthesis. Angew. Chem., Int. Ed. 2010, 49, 9452–9455. 10.1002/anie.201004041. [DOI] [PubMed] [Google Scholar]; [erratum]
- Cristóbal-Lecina E.; Etayo P.; Doran S.; Revés M.; Martín-Gago P.; Grabulosa A.; Costantino A. R.; Vidal-Ferran A.; Riera A.; Verdaguer X. MaxPHOS Ligand: PH/NH Tautomerism and Rhodium-Catalyzed Asymmetric Hydrogenations. Adv. Synth. Catal. 2014, 356, 795–804. 10.1002/adsc.201300662. [DOI] [Google Scholar]
- Prades A.; Núñez-Pertíñez S.; Riera A.; Verdaguer X. P-Stereogenic Bisphosphines with a Hydrazine Backbone: From N-N Atropoisomerism to Double Nitrogen Inversion. Chem. Commun. 2017, 53, 4605–4608. 10.1039/C7CC01944K. [DOI] [PubMed] [Google Scholar]
- Zupančič B.; Mohar B.; Stephan M. Heavyweight “R-SMS-Phos” Ligands in the Olefins’ Hydrogenation Arena. Org. Lett. 2010, 12, 1296–1299. 10.1021/ol100184p. [DOI] [PubMed] [Google Scholar]
- Zupančič B.; Mohar B.; Stephan M. Impact on Hydrogenation Catalytic Cycle of the R Groups Cyclic Feature in “r-SMS-Phos.. Org. Lett. 2010, 12, 3022–3025. 10.1021/ol101029s. [DOI] [PubMed] [Google Scholar]
- Mohar B.; Stephan M. Practical Enantioselective Hydrogenation of α-Aryl- and α-Carboxyamidoethylenes by Rhodium(I)-{1,2-Bis[(o-Tert-Butoxyphenyl) (Phenyl)Phosphino]Ethane}. Adv. Synth. Catal. 2005, 355, 594–600. 10.1002/adsc.201200780. [DOI] [Google Scholar]
- Tang W.; Qu B.; Capacci A. G.; Rodriguez S.; Wei X.; Haddad N.; Narayanan B.; Ma S.; Grinberg N.; Yee N. K.; et al. Novel, Tunable, and Efficient Chiral Bisdihydrobenzooxaphosphole Ligands for Asymmetric Hydrogenation. Org. Lett. 2010, 12, 176–179. 10.1021/ol9025815. [DOI] [PubMed] [Google Scholar]
- For another class of chiral bisphosphorus ligands (POP), see:; Tang W.; Capacci A. G.; White A.; Ma S.; Rodriguez S.; Qu B.; Savoie J.; Patel N. D.; Wei X.; Haddad N.; et al. Novel and Efficient Chiral Bisphosphorus Ligands for Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2010, 12, 1104–1107. 10.1021/ol1000999. [DOI] [PubMed] [Google Scholar]
- Li W.; Rodriguez S.; Duran A.; Sun X.; Tang W.; Premasiri A.; Wang J.; Sidhu K.; Patel N. D.; Savoie J.; et al. The P-Chiral Phosphane Ligand (MeO-BIBOP) for Efficient and Practical Large-Scale Rh-Catalyzed Asymmetric Hydrogenation of N-Acetyl Enamides with High TONs. Org. Process Res. Dev. 2013, 17, 1061–1065. 10.1021/op400055z. [DOI] [Google Scholar]
- Liu G.; Liu X.; Cai Z.; Jiao G.; Xu G.; Tang W. Design of Phosphorus Ligands with Deep Chiral Pockets: Practical Synthesis of Chiral β-Arylamines by Asymmetric Hydrogenation. Angew. Chem., Int. Ed. 2013, 52, 4235–4238. 10.1002/anie.201300646. [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhang W.; Geng H.; Li W.; Hou G.; Lei A.; Zhang X. Efficient Synthesis of Chiral β-Arylisopropylamines by Using Catalytic Asymmetric Hydrogenation. Angew. Chem., Int. Ed. 2009, 48, 800–802. 10.1002/anie.200805058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. F.; Liu T.; Yang S.; Song S.; Zhou Q. L. Enantioselective Hydrogenation of (Z)- and (E)-β-Arylenamides Catalyzed by Rhodium Complexes of Monodentate Chiral Spiro Phosphorous Ligands: A New Access to Chiral β-Arylisopropylamines. Tetrahedron 2012, 68, 7685–7690. 10.1016/j.tet.2012.06.032. [DOI] [Google Scholar]
- Li G.; Zatolochnaya O. V.; Wang X. J.; Rodríguez S.; Qu B.; Desrosiers J. N.; Mangunuru H. P. R.; Biswas S.; Rivalti D.; Karyakarte S. D.; et al. BABIPhos Family of Biaryl Dihydrobenzooxaphosphole Ligands for Asymmetric Hydrogenation. Org. Lett. 2018, 20, 1725–1729. 10.1021/acs.orglett.8b00139. [DOI] [PubMed] [Google Scholar]
- Biosca M.; de la Cruz-Sánchez P.; Pàmies O.; Diéguez M. P-Stereogenic N-Phosphine-Phosphite Ligands for the Rh-Catalyzed Hydrogenation of Olefins. J. Org. Chem. 2020, 85, 4730–4739. 10.1021/acs.joc.9b03508. [DOI] [PubMed] [Google Scholar]
- Imamoto T.; Watanabe J.; Wada Y.; Masuda H.; Yamada H.; Tsuruta H.; Matsukawa S.; Yamaguchi K. P-Chiral Bis (Trialkylphosphine) Ligands and Their Use in Highly Enantioselective Hydrogenation Reactions. J. Am. Chem. Soc. 1998, 120, 1635–1636. 10.1021/ja973423i. [DOI] [Google Scholar]
- Yamanoi Y.; Imamoto T. Methylene-Bridged P-Chiral Diphosphines in Highly Enantioselective Reactions. J. Org. Chem. 1999, 64, 2988–2989. 10.1021/jo990131m. [DOI] [PubMed] [Google Scholar]
- Gridnev I. D.; Yasutake M.; Higashi N.; Imamoto T. Asymmetric Hydrogenation of Enamides with Rh-BisP* and Rh-MiniPHOs Catalysts. Scope, Limitations, and Mechanism. J. Am. Chem. Soc. 2001, 123, 5268–5276. 10.1021/ja010161i. [DOI] [PubMed] [Google Scholar]
- Imamoto T.; Tamura K.; Zhang Z.; Horiuchi Y.; Sugiya M.; Yoshida K.; Yanagisawa A.; Gridnev I. D. Rigid P-Chiral Phosphine Ligands with Tert-Butylmethylphosphino Groups for Rhodium-Catalyzed Asymmetric Hydrogenation of Functionalized Alkenes. J. Am. Chem. Soc. 2012, 134, 1754–1769. 10.1021/ja209700j. [DOI] [PubMed] [Google Scholar]
- Tamura K.; Sugiya M.; Yoshida K.; Yanagisawa A.; Imamoto T. Enantiopure 1,2-Bis(Tert-Butylmethylphosphino)Benzene as a Highly Efficient Ligand in Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2010, 12, 4400–4403. 10.1021/ol101936w. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Tamura K.; Mayama D.; Sugiya M.; Imamoto T. Three-Hindered Quadrant Phosphine Ligands with an Aromatic Ring Backbone for the Rhodium-Catalyzed Asymmetric Hydrogenation of Functionalized Alkenes. J. Org. Chem. 2012, 77, 4184–4188. 10.1021/jo300454n. [DOI] [PubMed] [Google Scholar]
- Nitelet A.; Gérard P.; Bouche J.; Evano G. Total Synthesis of Conulothiazole A. Org. Lett. 2019, 21, 4318–4321. 10.1021/acs.orglett.9b01490. [DOI] [PubMed] [Google Scholar]
- Corrêa B. K.; Silva T. R. C.; Raminelli C. Total Syntheses of (+)-Bernumidine and Its Unnatural Enantiomer. Tetrahedron Lett. 2018, 59, 3583–3585. 10.1016/j.tetlet.2018.08.046. [DOI] [Google Scholar]
- Barbie P.; Kazmaier U. Total Synthesis of Cyclomarin A, a Marine Cycloheptapeptide with Anti-Tuberculosis and Anti-Malaria Activity. Org. Lett. 2016, 18, 204–207. 10.1021/acs.orglett.5b03292. [DOI] [PubMed] [Google Scholar]
- Gracia S.; Marion C.; Rey J.; Popowycz F.; Pellet-Rostaing S.; Lemaire M. Enantioselective Straightforward Access to Benzo[b]Thiophene Analogs of Azatoxin. Tetrahedron Lett. 2012, 53, 3165–3168. 10.1016/j.tetlet.2012.04.055. [DOI] [Google Scholar]
- Lorenz J. C.; Busacca C. A.; Feng X. W.; Grinberg N.; Haddad N.; Johnson J.; Kapadia S.; Lee H.; Saha A.; Sarvestani M.; et al. Large-Scale Asymmetric Synthesis of a Cathepsin S Inhibitor. J. Org. Chem. 2010, 75, 1155–1161. 10.1021/jo9022809. [DOI] [PubMed] [Google Scholar]
- Reeves J. T.; Tan Z.; Reeves D. C.; Song J. J.; Han Z. S.; Xu Y.; Tang W.; Yang B. S.; Razavi H.; Harcken C.; et al. Development of an Enantioselective Hydrogenation Route to (S)-1-(2-(Methylsulfonyl)Pyridin-4-Yl)Propan-1-Amine. Org. Process Res. Dev. 2014, 18, 904–911. 10.1021/op5001513. [DOI] [Google Scholar]
- Ghosh A. K.; Rao K. V.; Akasapu S. An Enantioselective Synthesis of a MEM-Protected Aetheramide A Derivative. Tetrahedron Lett. 2014, 55, 5191–5194. 10.1016/j.tetlet.2014.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. E.; Jackson M.; Meek G.; Willets M. Large-Scale Synthesis of a Substituted d-Phenylalanine Using Asymmetric Hydrogenation. Org. Process Res. Dev. 2011, 15, 1163–1171. 10.1021/op200129m. [DOI] [Google Scholar]
- Cann R. O.; Chen C. P. H.; Gao Q.; Hanson R. L.; Hsieh D.; Li J.; Lin D.; Parsons R. L.; Pendri Y.; Nielsen R. B.; et al. Selection of an Enantioselective Process for the Preparation of a CGRP Receptor Inhibitor. Org. Process Res. Dev. 2012, 16, 1953–1966. 10.1021/op3003097. [DOI] [Google Scholar]
- Liu J.; Deng X.; Fitzgerald A. E.; Sales Z. S.; Venkatesan H.; Mani N. S. Protecting-Group-Free Synthesis of a Dual CCK1/CCK2 Receptor Antagonist. Org. Biomol. Chem. 2011, 9, 2654–2660. 10.1039/c0ob01004a. [DOI] [PubMed] [Google Scholar]
- Grayson J. I.; Roos J.; Osswald S. Development of a Commercial Process for (S)-β-Phenylalanine. Org. Process Res. Dev. 2011, 15, 1201–1206. 10.1021/op200084g. [DOI] [Google Scholar]
- Zhang X.; Huang K.; Hou G.; Cao B.; Zhang X. Electron-Donating and Rigid P-Stereogenic Bisphospholane Ligands for Highly Enantioselective Rhodium-Catalyzed Asymmetric Hydrogenations. Angew. Chem., Int. Ed. 2010, 49, 6421–6424. 10.1002/anie.201002990. [DOI] [PubMed] [Google Scholar]
- Huang K.; Zhang X.; Geng H.; Li S. K.; Zhang X. Highly Enantioselective Hydrogenation of β-Ketoenamides with the Rh-ZhangPhos Catalyst. ACS Catal. 2012, 2, 1343–1345. 10.1021/cs300037z. [DOI] [Google Scholar]
- Tang W.; Zhang X. A Chiral 1,2-Bisphospholane Ligand with a Novel Structural Motif: Applications in Highly Enantioselective Rh-Catalyzed Hydrogenations. Angew. Chem., Int. Ed. 2002, 41, 1612–1614. . [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhang W.; Geng H.; Li W.; Hou G.; Lei A.; Zhang X. Efficient Synthesis of Chiral β-Arylisopropylamines by Using Catalytic Asymmetric Hydrogenation. Angew. Chem., Int. Ed. 2009, 48, 800–802. 10.1002/anie.200805058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Zhang X. Practical P-Chiral Phosphane Ligand for Rh-Catalyzed Asymmetric Hydrogenation. Eur. J. Org. Chem. 2005, 2005, 646–649. 10.1002/ejoc.200400690. [DOI] [Google Scholar]
- Geng H.; Huang K.; Sun T.; Li W.; States U. Enantioselective Synthesis of Optically Pure β-Amino Ketones and γ-Aryl Amines by Rh-Catalyzed Asymmetric Hydrogenation. J. Org. Chem. 2011, 76, 332–334. 10.1021/jo102091f. [DOI] [PubMed] [Google Scholar]
- Huang K.; Zhang X.; Emge T. J.; Hou G.; Cao B.; Zhang X. Design and Synthesis of a Novel Three-Hindered Quadrant Bisphosphine Ligand and Its Application in Asymmetric Hydrogenation. Chem. Commun. 2010, 46, 8555–8557. 10.1039/c0cc02620d. [DOI] [PubMed] [Google Scholar]
- Le D. N.; Hansen E.; Khan H. A.; Kim B.; Wiest O.; Dong V. M. Hydrogenation Catalyst Generates Cyclic Peptide Stereocentres in Sequence. Nat. Chem. 2018, 10, 968–973. 10.1038/s41557-018-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W.; Lv H.; Zhang X. Rh/DuanPhos-Catalyzed Asymmetric Hydrogenation of β-Acetylamino Vinylsulfides: An Approach to Chiral β-Acetylamino Sulfides. Org. Lett. 2017, 19, 2877–2880. 10.1021/acs.orglett.7b01115. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Lu W.; Lv H.; Zhang X. Highly Efficient Synthesis of Chiral α-CF3 Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2015, 17, 1154–1156. 10.1021/acs.orglett.5b00087. [DOI] [PubMed] [Google Scholar]
- Sun T.; Hou G.; Ma M.; Zhang X. New Synthetic Strategy for High-Enantiopurity N-Protected α-Amino Ketones and Their Derivatives by Asymmetric Hydrogenation. Adv. Synth. Catal. 2011, 353, 253–256. 10.1002/adsc.201000680. [DOI] [Google Scholar]
- Gao W.; Wang Q.; Xie Y.; Lv H.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of α-Dehydroamino Ketones: A General Approach to Chiral α-Amino Ketones. Chem. - Asian J. 2016, 11, 231–233. 10.1002/asia.201500892. [DOI] [PubMed] [Google Scholar]
- Liu T. L.; Wang C. J.; Zhang X. Synthesis of Chiral Aliphatic Amines through Asymmetric Hydrogenation. Angew. Chem., Int. Ed. 2013, 52, 8416–8419. 10.1002/anie.201302943. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Gao W.; Lv H.; Zhang X. Enantioselective Synthesis of β-Substituted Chiral Allylic Amines: Via Rh-Catalyzed Asymmetric Hydrogenation. Chem. Commun. 2016, 52, 11850–11853. 10.1039/C6CC06047A. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Liu T. L.; Cao M.; Xue Z.; Lv H.; Zhang X. Highly Enantioselective Synthesis of Chiral Cyclic Allylic Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2014, 16, 3484–3487. 10.1021/ol501421g. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Huang W.; Yuan H.; Cai Q.; Chen L.; Lv H.; Zhang X. Rhodium-Catalyzed Enantioseletive Hydrogenation of Tetrasubstituted α-Acetoxy β-Enamido Esters: A New Approach to Chiral α-Hydroxyl-β-Amino Acid Derivatives. J. Am. Chem. Soc. 2014, 136, 16120–16123. 10.1021/ja509005e. [DOI] [PubMed] [Google Scholar]
- Guan Y. Q.; Gao M.; Deng X.; Lv H.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted β-Acetoxy-α-Enamido Esters and Efficient Synthesis of Droxidopa. Chem. Commun. 2017, 53, 8136–8139. 10.1039/C7CC03902F. [DOI] [PubMed] [Google Scholar]
- Gao M.; Meng J.-j.; Lv H.; Zhang X. Highly Regio- And Enantioselective Synthesis of γ,δ-Unsaturated Amido Esters by Catalytic Hydrogenation of Conjugated Enamides. Angew. Chem., Int. Ed. 2015, 54, 1885–1887. 10.1002/anie.201410213. [DOI] [PubMed] [Google Scholar]
- Meng J.; Gao M.; Lv H.; Zhang X. Highly Enantioselective Hydrogenation of O-Alkoxy Tetrasubstituted Enamides Catalyzed by a Rh/(R, S)-JosiPhos Catalyst. Org. Lett. 2015, 17, 1842–1845. 10.1021/acs.orglett.5b00401. [DOI] [PubMed] [Google Scholar]
- Molinaro C.; Scott J. P.; Shevlin M.; Wise C.; Ménard A.; Gibb A.; Junker E. M.; Lieberman D. Catalytic, Asymmetric, and Stereodivergent Synthesis of Non-Symmetric β,β-Diaryl-α-Amino Acids. J. Am. Chem. Soc. 2015, 137, 999–1006. 10.1021/ja511872a. [DOI] [PubMed] [Google Scholar]
- Appell R. B.; Boulton L. T.; Daugs E. D.; Hansen M.; Hanson C. H.; Heinrich J.; Kronig C.; Lloyd R. C.; Louks D.; Nitz M.; et al. The Large-Scale Synthesis of (S)-N-Boc-Bis(4-Fluorophenyl)Alanine. Org. Process Res. Dev. 2013, 17, 69–76. 10.1021/op3002855. [DOI] [Google Scholar]
- Wallace D. J.; Campos K. R.; Shultz C. S.; Klapars A.; Zewge D.; Crump B. R.; Phenix B. D.; McWilliams J. C.; Krska S.; Sun Y.; et al. New Efficient Asymmetric Synthesis of Taranabant, a CB1R Inverse Agonist for the Treatment of Obesity. Org. Process Res. Dev. 2009, 13, 84–90. 10.1021/op800270e. [DOI] [Google Scholar]
- Li X.; You C.; Yang Y.; Wang F.; Li S.; Lv H.; Zhang X. Rhodium-Catalyzed Enantioselective Hydrogenation of α-Amino Acrylonitriles: An Efficient Approach to Synthesizing Chiral α-Amino Nitriles. Chem. Commun. 2017, 53, 1313–1316. 10.1039/C6CC09662J. [DOI] [PubMed] [Google Scholar]
- Ma M.; Hou G.; Sun T.; Zhang X.; Li W.; Wang J.; Zhang X. Highly Efficient RhI-Catalyzed Asymmetric Hydrogenation of β-Amino Acrylonitriles. Chem. - Eur. J. 2010, 16, 5301–5304. 10.1002/chem.201000325. [DOI] [PubMed] [Google Scholar]
- Ma M.; Hou G.; Wang J.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of β-Acetylamino Acrylonitriles. Tetrahedron: Asymmetry 2011, 22, 506–511. 10.1016/j.tetasy.2011.01.023. [DOI] [Google Scholar]
- Zhang J.; Jia J.; Zeng X.; Wang Y.; Zhang Z.; Gridnev I. D.; Zhang W. Chemo- and Enantioselective Hydrogenation of α-Formyl Enamides: An Efficient Access to Chiral α-Amido Aldehydes. Angew. Chem., Int. Ed. 2019, 58, 11505–11512. 10.1002/anie.201905263. [DOI] [PubMed] [Google Scholar]
- Mikami K.; Murase T.; Zhai L.; Itoh Y.; Ito S. Highly Enantioselective Synthesis of α-(Perfluoroalkyl)Amines via Hydrogenation of Enamide Precursors in the Presence of Chiraphos-Rhodium Catalyst. Tetrahedron: Asymmetry 2010, 21, 1158–1161. 10.1016/j.tetasy.2010.05.021. [DOI] [Google Scholar]
- Benhaim C.; Bouchard L.; Pelletier G.; Sellstedt J.; Kristofova L.; Daigneault S. Enantioselective Synthesis of β-Trifluoromethyl α-Amino Acids. Org. Lett. 2010, 12, 2008–2011. 10.1021/ol100478d. [DOI] [PubMed] [Google Scholar]
- Du H. Q.; Hu X. P. Rh-Catalyzed Asymmetric Hydrogenation of (Z)-β-Phosphorylated Enamides: Highly Enantioselective Access to β-Aminophosphines. Org. Lett. 2019, 21, 8921–8924. 10.1021/acs.orglett.9b03174. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Li Y.; Wang Z.; Ding K. Asymmetric Hydrogenation of α- And β-Enamido Phosphonates: Rhodium(I)/Monodentate Phosphoramidite Catalyst. Angew. Chem., Int. Ed. 2011, 50, 11743–11747. 10.1002/anie.201104912. [DOI] [PubMed] [Google Scholar]
- Qiu M.; Hu X. P.; Huang J. Di; Wang D. Y.; Deng J.; Yu S. B.; Duan Z. C.; Zheng Z. Modular Phosphine-Aminophosphine Ligands Based on Chiral 1,2,3,4-Tetrahydro-1-Naphthylamine Backbone: A New Class of Practical Ligands for Enantioselective Hydrogenations. Adv. Synth. Catal. 2008, 350, 2683–2689. 10.1002/adsc.200800418. [DOI] [Google Scholar]
- Wang D. Y.; Huang J. Di; Hu X. P.; Deng J.; Yu S. B.; Duan Z. C.; Zheng Z. Readily Available Chiral Phosphine-Aminophosphine Ligands for Highly Efficient Rh-Catalyzed Asymmetric Hydrogenation of α-Enol Ester Phosphonates and α-Enamido Phosphonates. J. Org. Chem. 2008, 73, 2011–2014. 10.1021/jo702488j. [DOI] [PubMed] [Google Scholar]
- Fan D.; Zhang J.; Hu Y.; Zhang Z.; Gridnev I. D.; Zhang W. Asymmetric Hydrogenation of α-Boryl Enamides Enabled by Nonbonding Interactions. ACS Catal. 2020, 10, 3232–3240. 10.1021/acscatal.9b04543. [DOI] [Google Scholar]
- Lou Y.; Wang J.; Gong G.; Guan F.; Lu J.; Wen J.; Zhang X. Catalytic Asymmetric Hydrogenation of (Z)-α-Dehydroamido Boronate Esters: Direct Route to Alkyl-Substituted α-Amidoboronic Esters. Chem. Sci. 2020, 11, 851–855. 10.1039/C9SC04534A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarchuk T.; Babu S.; Stults J.; Zanotti-Gerosa A.; Roseblade S.; Yang S.; Huang P.; Sha C.; Wang Y. An Efficient Catalytic Asymmetric Synthesis of a β2-Amino Acid on Multikilogram Scale. Org. Process Res. Dev. 2014, 18, 135–141. 10.1021/op4002966. [DOI] [Google Scholar]
- Han C.; Savage S.; Al-Sayah M.; Yajima H.; Remarchuk T.; Reents R.; Wirz B.; Iding H.; Bachmann S.; Fantasia S. M.; et al. Asymmetric Synthesis of Akt Kinase Inhibitor Ipatasertib. Org. Lett. 2017, 19, 4806–4809. 10.1021/acs.orglett.7b02228. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu C.; Wang X.; Chen J.; Zhang Z.; Zhang W. Rhodium-Catalyzed Asymmetric Hydrogenation of β-Branched Enamides for the Synthesis of β-Stereogenic Amines. Chem. Commun. 2018, 54, 6024–6027. 10.1039/C8CC02798F. [DOI] [PubMed] [Google Scholar]
- Wen J.; Wang F.; Zhang X. Asymmetric Hydrogenation Catalyzed by First-Row Transition Metal Complexes. Chem. Soc. Rev. 2021, 50, 3211–3237. 10.1039/D0CS00082E. [DOI] [PubMed] [Google Scholar]
- Li Y. Y.; Yu S. L.; Shen W. Y.; Gao J. X. Iron-, Cobalt-, and Nickel-Catalyzed Asymmetric Transfer Hydrogenation and Asymmetric Hydrogenation of Ketones. Acc. Chem. Res. 2015, 48, 2587–2598. 10.1021/acs.accounts.5b00043. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Chen J.; Li B.; Zhang Z.; Gridnev I. D.; Zhang W. Nickel-Catalyzed Asymmetric Hydrogenation of 2-Amidoacrylates. Angew. Chem., Int. Ed. 2020, 59, 5371–5375. 10.1002/anie.201916534. [DOI] [PubMed] [Google Scholar]
- Li X.; You C.; Li S.; Lv H.; Zhang X. Nickel-Catalyzed Enantioselective Hydrogenation of β-(Acylamino)Acrylates: Synthesis of Chiral β-Amino Acid Derivatives. Org. Lett. 2017, 19, 5130–5133. 10.1021/acs.orglett.7b02417. [DOI] [PubMed] [Google Scholar]
- Tang W.; Wang W.; Chi Y.; Zhang X. A Bisphosphepine Ligand with Stereogenic Phosphorus Centers for the Practical Synthesis of β-Aryl-β-Amino Acids by Asymmetric Hydrogenation. Angew. Chem., Int. Ed. 2003, 42, 3509–3511. 10.1002/anie.200351465. [DOI] [PubMed] [Google Scholar]
- Long J.; Gao W.; Guan Y.; Lv H.; Zhang X. Nickel-Catalyzed Highly Enantioselective Hydrogenation of β-Acetylamino Vinylsulfones: Access to Chiral β-Amido Sulfones. Org. Lett. 2018, 20, 5914–5917. 10.1021/acs.orglett.8b02579. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Wang Y.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of β-Acetylamino Acrylosulfones: A Practical Approach to Chiral β-Amido Sulfones. ACS Catal. 2014, 4, 1570–1573. 10.1021/cs500261k. [DOI] [Google Scholar]
- Li P.; Zhou M.; Zhao Q.; Wu W.; Hu X.; Dong X. Q.; Zhang X. Synthesis of Chiral β-Amino Nitroalkanes via Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 40–43. 10.1021/acs.orglett.5b03158. [DOI] [PubMed] [Google Scholar]
- Yan Q.; Liu M.; Kong D.; Zi G.; Hou G. Highly Efficient Iridium-Catalyzed Asymmetric Hydrogenation of β-Acylamino Nitroolefins. Chem. Commun. 2014, 50, 12870–12872. 10.1039/C4CC05815A. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Dong D.; Zhu B.; Geng H.; Wang Y.; Zhang X. Rhodium-Catalyzed Enantioselective Hydrogenation of β-Acylamino Nitroolefins: A New Approach to Chiral β-Amino Nitroalkanes. Org. Lett. 2013, 15, 5524–5527. 10.1021/ol4026843. [DOI] [PubMed] [Google Scholar]
- Gao W.; Lv H.; Zhang T.; Yang Y.; Chung L. W.; Wu Y. D.; Zhang X. Nickel-Catalyzed Asymmetric Hydrogenation of β-Acylamino Nitroolefins: An Efficient Approach to Chiral Amines. Chem. Sci. 2017, 8, 6419–6422. 10.1039/C7SC02669B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y. Q.; Han Z.; Li X.; You C.; Tan X.; Lv H.; Zhang X. A Cheap Metal for a Challenging Task: Nickel-Catalyzed Highly Diastereo- and Enantioselective Hydrogenation of Tetrasubstituted Fluorinated Enamides. Chem. Sci. 2019, 10, 252–256. 10.1039/C8SC04002H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a selected publication using iridium catalysis, see:; Han Z.; Guan Y. Q.; Liu G.; Wang R.; Yin X.; Zhao Q.; Cong H.; Dong X. Q.; Zhang X. Iridium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted α-Fluoro-β-Enamino Esters: Efficient Access to Chiral α-Fluoro-β-Amino Esters with Two Adjacent Tertiary Stereocenters. Org. Lett. 2018, 20, 6349–6353. 10.1021/acs.orglett.8b02503. [DOI] [PubMed] [Google Scholar]
- Friedfeld M. R.; Shevlin M.; Hoyt J. M.; Krska S. W.; Tudge M. T.; Chirik P. J. Cobalt Precursors for High-Throughput Discovery of Base Metal Asymmetric Alkene Hydrogenation Catalysts. Science 2013, 342, 1076–1080. 10.1126/science.1243550. [DOI] [PubMed] [Google Scholar]
- Zhong H.; Friedfeld M. R.; Chirik P. J. Syntheses and Catalytic Hydrogenation Performance of Cationic Bis(Phosphine) Cobalt(I) Diene and Arene Compounds. Angew. Chem., Int. Ed. 2019, 58, 9194–9198. 10.1002/anie.201903766. [DOI] [PubMed] [Google Scholar]
- Zhong H.; Friedfeld M. R.; Camacho-Bunquin J.; Sohn H.; Yang C.; Delferro M.; Chirik P. J. Exploring the Alcohol Stability of Bis(Phosphine) Cobalt Dialkyl Precatalysts in Asymmetric Alkene Hydrogenation. Organometallics 2019, 38, 149–156. 10.1021/acs.organomet.8b00516. [DOI] [Google Scholar]
- Zhong H.; Shevlin M.; Chirik P. J. Cobalt-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carboxylic Acids by Homolytic H2 Cleavage. J. Am. Chem. Soc. 2020, 142, 5272–5281. 10.1021/jacs.9b13876. [DOI] [PubMed] [Google Scholar]
- Friedfeld M. R.; Zhong H.; Ruck R. T.; Shevlin M.; Chirik P. J. Cobalt-Catalyzed Asymmetric Hydrogenation of Enamides Enabled by Single-Electron Reduction. Science 2018, 360, 888–893. 10.1126/science.aar6117. [DOI] [PubMed] [Google Scholar]
- Royal T.; Dudognon Y.; Berhal F.; Bastard Y.; Boudet B.; Ayad T.; Ratovelomanana-Vidal V. Rhodium-Catalyzed Asymmetric Hydrogenation of N-(1-Benzylpiperidin-3-Yl)-Enamides: An Efficient Access to Valuable Enantioenriched 3-Aminopiperidine Derivatives. Synlett 2016, 27, 2009–2013. 10.1055/s-0035-1562235. [DOI] [Google Scholar]
- Hu Q.; Chen J.; Zhang Z.; Liu Y.; Zhang W. Rh-Catalyzed One-Pot Sequential Asymmetric Hydrogenation of α-Dehydroamino Ketones for the Synthesis of Chiral Cyclic Trans-β-Amino Alcohols. Org. Lett. 2016, 18, 1290–1293. 10.1021/acs.orglett.6b00212. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Hu Q.; Wang Y.; Chen J.; Zhang W. Rh-Catalyzed Asymmetric Hydrogenation of Cyclic α-Dehydroamino Ketones. Org. Lett. 2015, 17, 5380–5383. 10.1021/acs.orglett.5b02733. [DOI] [PubMed] [Google Scholar]
- Huang K.; Guan Z. H.; Zhang X. Synthesis of Chiral Cyclic β-Amino Ketones by Ru-Catalyzed Asymmetric Hydrogenation. Tetrahedron Lett. 2014, 55, 1686–1688. 10.1016/j.tetlet.2013.10.093. [DOI] [Google Scholar]
- Renaud J. L.; Dupau P.; Hay A.-E.; Guingouain M.; Dixneuf P. H.; Bruneau C. Ruthenium-Catalyzed Enantioselective Hydrogenation of Trisubstituted Enamides Derived from 2-Tetralone and 3-Chromanone: Influence of Substitution on the Amide Arm and the Aromatic Ring. Adv. Synth. Catal. 2003, 345, 230–238. 10.1002/adsc.200390017. [DOI] [Google Scholar]
- Arribas I.; Rubio M.; Kleman P.; Pizzano A. Rhodium Phosphine-Phosphite Catalysts in the Hydrogenation of Challenging N-(3,4-Dihydronaphthalen-2-yl) Amide Derivatives. J. Org. Chem. 2013, 78, 3997–4005. 10.1021/jo400345v. [DOI] [PubMed] [Google Scholar]
- Patureau F. W.; Worch C.; Siegler M. A.; Spek A. L.; Bolm C.; Reek J. N. H. SIAPhos: Phosphorylated Sulfonimidamides and Their Use in Iridium-Catalyzed Asymmetric Hydrogenations of Sterically Hindered Cyclic Enamides. Adv. Synth. Catal. 2012, 354, 59–64. 10.1002/adsc.201100692. [DOI] [Google Scholar]
- Jiang X. B.; Lefort L.; Goudriaan P. E.; De Vries A. H. M.; Van Leeuwen P. W. N. M.; De Vries J. G.; Reek J. N. H. Screening of a Supramolecular Catalyst Library in the Search for Selective Catalysts for the Asymmetric Hydrogenation of a Difficult Enamide Substrate. Angew. Chem., Int. Ed. 2006, 45, 1223–1227. 10.1002/anie.200503663. [DOI] [PubMed] [Google Scholar]
- Pautigny C.; Debouit C.; Vayron P.; Ayad T.; Ratovelomanana-Vidal V. Asymmetric Hydrogenation of Trisubstituted N-Acetyl Enamides Derived from 2-Tetralones Using Ruthenium-SYNPHOS Catalysts: A Practical Synthetic Approach to the Preparation of β3-Adrenergic Agonist SR58611A. Tetrahedron: Asymmetry 2010, 21, 1382–1388. 10.1016/j.tetasy.2010.03.024. [DOI] [Google Scholar]
- Wu Z.; Ayad T.; Ratovelomanana-Vidal V. Efficient Enantioselective Synthesis of 3-Aminochroman Derivatives through Ruthenium-Synphos Catalyzed Asymmetric Hydrogenation. Org. Lett. 2011, 13, 3782–3785. 10.1021/ol201786q. [DOI] [PubMed] [Google Scholar]
- Álvarez-Yebra R.; Rojo P.; Riera A.; Verdaguer X. Iridium Complexes with P-Stereogenic Phosphino Imidazole Ligands: Synthesis, Structure and Catalysis. Tetrahedron 2019, 75, 4358–4364. 10.1016/j.tet.2019.04.032. [DOI] [Google Scholar]
- Magre M.; Pàmies O.; Diéguez M. PHOX-Based Phosphite-Oxazoline Ligands for the Enantioselective Ir-Catalyzed Hydrogenation of Cyclic β-Enamides. ACS Catal. 2016, 6, 5186–5190. 10.1021/acscatal.6b01314. [DOI] [Google Scholar]
- Biosca M.; Magre M.; Pàmies O.; Diéguez M. Asymmetric Hydrogenation of Disubstituted, Trisubstituted, and Tetrasubstituted Minimally Functionalized Olefins and Cyclic β-Enamides with Easily Accessible Ir-P, Oxazoline Catalysts. ACS Catal. 2018, 8, 10316–10320. 10.1021/acscatal.8b03170. [DOI] [Google Scholar]
- Margalef J.; Pàmies O.; Diéguez M. Phosphite-Thiother Ligands Derived from Carbohydrates Allow the Enantioswitchable Hydrogenation of Cyclic β-Enamides by Using Either Rh or Ir Catalysts. Chem. - Eur. J. 2017, 23, 813–822. 10.1002/chem.201604483. [DOI] [PubMed] [Google Scholar]
- Biosca M.; Magre M.; Coll M.; Pàmies O.; Diéguez M. Alternatives to Phosphinooxazoline (t-BuPHOX) Ligands in the Metal-Catalyzed Hydrogenation of Minimally Functionalized Olefins and Cyclic β-Enamides. Adv. Synth. Catal. 2017, 359, 2801–2814. 10.1002/adsc.201700573. [DOI] [Google Scholar]
- Cruz-Sánchez P. D. La; Faiges J.; Mazloomi Z.; Borràs C.; Biosca M.; Pàmies O.; Diéguez M. Ir/Thioether-Carbene, -Phosphinite, and -Phosphite Complexes for Asymmetric Hydrogenation. A Case for Comparison. Organometallics 2019, 38, 4193–4205. 10.1021/acs.organomet.9b00514. [DOI] [Google Scholar]
- Biosca M.; Pàmies O.; Diéguez M. Ir-Biaryl Phosphite-Oxazoline Catalyst Libraries: A Breakthrough in the Asymmetric Hydrogenation of Challenging Olefins. Catal. Sci. Technol. 2020, 10, 613–624. 10.1039/C9CY02501D. [DOI] [Google Scholar]
- Beliaev A. Development of the Asymmetric Hydrogenation Step for Multikilogram Production of Etamicastat. Org. Process Res. Dev. 2016, 20, 724–732. 10.1021/acs.oprd.6b00041. [DOI] [Google Scholar]
- Cobley C. J.; Evans G.; Fanjul T.; Simmonds S.; Woods A. New Catalytic Route for the Synthesis of an Optically Active Tetralone-Derived Amine for Rotigotine. Tetrahedron Lett. 2016, 57, 986–989. 10.1016/j.tetlet.2016.01.060. [DOI] [Google Scholar]
- Li X.; You C.; Yang H.; Che J.; Chen P.; Yang Y.; Lv H.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted Cyclic Enamides: Efficient Access to Chiral Cycloalkylamine Derivatives. Adv. Synth. Catal. 2017, 359, 597–602. 10.1002/adsc.201601135. [DOI] [Google Scholar]
- Li C.; Wan F.; Chen Y.; Peng H.; Tang W.; Yu S.; McWilliams J. C.; Mustakis J.; Samp L.; Maguire R. J. Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib. Angew. Chem., Int. Ed. 2019, 58, 13573–13583. 10.1002/anie.201908089. [DOI] [PubMed] [Google Scholar]
- Stumpf A.; Reynolds M.; Sutherlin D.; Babu S.; Bappert E.; Spindler F.; Welch M.; Gaudino J. Kilogram-Scale Asymmetric Ruthenium-Catalyzed Hydrogenation of a Tetrasubstituted Fluoroenamide. Adv. Synth. Catal. 2011, 353, 3367–3372. 10.1002/adsc.201100195. [DOI] [Google Scholar]
- Benson H.; Bones K.; Churchill G.; Ford G.; Frodsham L.; Janbon S.; Millington F.; Powell L.; Raw S. A.; Reid J.; et al. Development of the Convergent, Kilogram-Scale Synthesis of an Antibacterial Clinical Candidate Using Enantioselective Hydrogenation. Org. Process Res. Dev. 2020, 24, 588–598. 10.1021/acs.oprd.0c00029. [DOI] [Google Scholar]
- For a selected review, see:; Weiner B.; Szymański W.; Janssen D. B.; Minnaard A. J.; Feringa B. L. Recent Advances in the Catalytic Asymmetric Synthesis of β-Amino Acids. Chem. Soc. Rev. 2010, 39, 1656–1691. 10.1039/b919599h. [DOI] [PubMed] [Google Scholar]
- Tang W.; Wu S.; Zhang X. Enantioselective Hydrogenation of Tetrasubstituted Olefins of Cyclic β-(Acylamino)Acrylates. J. Am. Chem. Soc. 2003, 125, 9570–9571. 10.1021/ja035777h. [DOI] [PubMed] [Google Scholar]
- Yu C. B.; Gao K.; Chen Q. A.; Chen M. W.; Zhou Y. G. Enantioselective Pd-Catalyzed Hydrogenation of Tetrasubstituted Olefins of Cyclic β-(Arylsulfonamido)Acrylates. Tetrahedron Lett. 2012, 53, 2560–2563. 10.1016/j.tetlet.2012.03.035. [DOI] [Google Scholar]
- Yan Z.; Xie H. P.; Shen H. Q.; Zhou Y. G. Ruthenium-Catalyzed Hydrogenation of Carbocyclic Aromatic Amines: Access to Chiral Exocyclic Amines. Org. Lett. 2018, 20, 1094–1097. 10.1021/acs.orglett.7b04060. [DOI] [PubMed] [Google Scholar]
- Kondoh A.; Yorimitsu H.; Oshima K. Regio- and Stereoselective Hydroamidation of 1-Alkynylphosphine Sulfides Catalyzed by Cesium Base. Org. Lett. 2008, 10, 3093–3095. 10.1021/ol8010979. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Lu X.; Lei A. Highly Enantioselective Hydrogenation of Exocyclic Double Bond of N-Tosyloxazolidinones Catalyzed by a Neutral Rhodium Complex and Its Synthetic Applications. Tetrahedron 2006, 62, 9237–9246. 10.1016/j.tet.2006.07.024. [DOI] [Google Scholar]
- Shultz C. S.; Dreher S. D.; Ikemoto N.; Williams J. M.; Grabowski E. J. J.; Krska S. W.; Sun Y.; Dormer P. G.; DiMichele L. Asymmetric Hydrogenation of N-Sulfonylated-α-Dehydroamino Acids: Toward the Synthesis of an Anthrax Lethal Factor Inhibitor. Org. Lett. 2005, 7, 3405–3408. 10.1021/ol050869s. [DOI] [PubMed] [Google Scholar]
- Saito N.; Abdullah I.; Hayashi K.; Hamada K.; Koyama M.; Sato Y. Enantioselective Synthesis of β-Amino Acid Derivatives: Via Nickel-Promoted Regioselective Carboxylation of Ynamides and Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Biomol. Chem. 2016, 14, 10080–10089. 10.1039/C6OB01234E. [DOI] [PubMed] [Google Scholar]
- Verendel J. J.; Li J. Q.; Quan X.; Peters B.; Zhou T.; Gautun O. R.; Govender T.; Andersson P. G. Chiral Hetero- and Carbocyclic Compounds from the Asymmetric Hydrogenation of Cyclic Alkenes. Chem. - Eur. J. 2012, 18, 6507–6513. 10.1002/chem.201104073. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Gao W.; Deng J.; Zhang X. Highly Enantioselective Hydrogenation of N-Phthaloyl Enamides. Tetrahedron Lett. 2006, 47, 821–823. 10.1016/j.tetlet.2005.11.064. [DOI] [Google Scholar]
- Chen J.; Liu Q.; Zhang W.; Spinella S.; Lei A.; Zhang X. A Convenient Synthesis and the Asymmetric Hydrogenation of N-Phthaloyl Dehydroamino Acid Esters. Org. Lett. 2008, 10, 3033–3036. 10.1021/ol800996j. [DOI] [PubMed] [Google Scholar]
- Campello H. R.; Parker J.; Perry M.; Ryberg P.; Gallagher T. Asymmetric Reduction of Lactam-Based β-Aminoacrylates. Synthesis of Heterocyclic B2-Amino Acids. Org. Lett. 2016, 18, 4124–4127. 10.1021/acs.orglett.6b02074. [DOI] [PubMed] [Google Scholar]
- Ge Y.; Han Z.; Wang Z.; Ding K. Ir-Catalyzed Double Asymmetric Hydrogenation of 3,6-Dialkylidene-2,5-Diketopiperazines for Enantioselective Synthesis of Cyclic Dipeptides. J. Am. Chem. Soc. 2019, 141, 8981–8988. 10.1021/jacs.9b02920. [DOI] [PubMed] [Google Scholar]
- Li W.; Wollenburg M.; Glorius F. Enantioselective Synthesis of 2-Oxazolidinones by Ruthenium(Ii)-NHC-Catalysed Asymmetric Hydrogenation of 2-Oxazolones. Chem. Sci. 2018, 9, 6260–6263. 10.1039/C8SC01869C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Tan X.; Zhu Z.; Dong X. Q.; Zhang X. New Synthetic Strategy for Chiral 2-Oxazolidinones Derivatives via Rhodium-Catalyzed Asymmetric Hydrogenation. Tetrahedron Lett. 2016, 57, 658–662. 10.1016/j.tetlet.2015.12.105. [DOI] [Google Scholar]
- Hou G. H.; Xie J. H.; Wang L. X.; Zhou Q. L. Highly Efficient Rh(I)-Catalyzed Asymmetric Hydrogenation of Enamines Using Monodente Spiro Phosphonite Ligands. J. Am. Chem. Soc. 2006, 128, 11774–11775. 10.1021/ja0644778. [DOI] [PubMed] [Google Scholar]
- For the Ir-catalyzed version, see:; Yan P.; Xie J.; Zhou Q. Asymmetric Hydrogenation of Unfunctionalized Enamines Catalyzed by Iridium Complexes of Chiral Spiro N, N-Diarylphosphoramidites. Chin. J. Chem. 2010, 28, 1736–1742. 10.1002/cjoc.201090293. [DOI] [Google Scholar]
- Hou G. H.; Xie J. H.; Yan P. C.; Zhou Q. L. Iridium-Catalyzed Asymmetric Hydrogenation of Cyclic Enamines. J. Am. Chem. Soc. 2009, 131, 1366–1367. 10.1021/ja808358r. [DOI] [PubMed] [Google Scholar]
- Yan P. C.; Xie J. H.; Hou G. H.; Wang L. X.; Zhou Q. L. Enantioselective Synthesis of Chiral Tetrahydroisoquinolines by Iridium-Catalyzed Asymmetric Hydrogenation of Enamines. Adv. Synth. Catal. 2009, 351, 3243–3250. 10.1002/adsc.200900602. [DOI] [Google Scholar]
- Baeza A.; Pfaltz A. Iridium-Catalyzed Asymmetric Hydrogenation of Unfunctionalized Enamines. Chem. - Eur. J. 2009, 15, 2266–2269. 10.1002/chem.200802576. [DOI] [PubMed] [Google Scholar]
- Zhong Y. L.; Krska S. W.; Zhou H.; Reamer R. A.; Lee J.; Sun Y.; Askin D. Catalytic Asymmetric Synthesis of an HIV Integrase Inhibitor. Org. Lett. 2009, 11, 369–372. 10.1021/ol802604v. [DOI] [PubMed] [Google Scholar]
- Dai Q.; Yang W.; Zhang X. Efficient Rhodium-Catalyzed Asymmetric Hydrogenation for the Synthesis of a New Class of N-Aryl β-Amino Acid Derivatives. Org. Lett. 2005, 7, 5343–5345. 10.1021/ol0523897. [DOI] [PubMed] [Google Scholar]
- Chen M. W.; Yang Q.; Deng Z.; Ding Q.; Peng Y. Synthesis of Chiral β-Fluoroalkyl β-Amino Acid Derivatives via Palladium-Catalyzed Hydrogenation. J. Org. Chem. 2019, 84, 10371–10379. 10.1021/acs.joc.9b01523. [DOI] [PubMed] [Google Scholar]
- Wang X. B.; Wang D. W.; Lu S. M.; Yu C. B.; Zhou Y. G. Highly Enantioselective Ir-Catalyzed Hydrogenation of Exocyclic Enamines. Tetrahedron: Asymmetry 2009, 20, 1040–1045. 10.1016/j.tetasy.2009.03.037. [DOI] [Google Scholar]
- Hsiao Y.; Rivera N. R.; Rosner T.; Krska S. W.; Njolito E.; Wang F.; Sun Y.; Armstrong J. D.; Grabowski E. J. J.; Tillyer R. D.; et al. Highly Efficient Synthesis of β-Amino Acid Derivatives via Asymmetric Hydrogenation of Unprotected Enamines. J. Am. Chem. Soc. 2004, 126, 9918–9919. 10.1021/ja047901i. [DOI] [PubMed] [Google Scholar]
- Khopade K. V.; Sen A.; Birajdar R. S.; Paulbudhe U. P.; Kavale D. S.; Shinde P. S.; Mhaske S. B.; Chikkali S. H. Highly Enantioselective Synthesis of Sitagliptin. Asian J. Org. Chem. 2020, 9, 189–191. 10.1002/ajoc.201900709. [DOI] [Google Scholar]
- Steinhuebel D.; Sun Y.; Matsumura K.; Sayo N.; Saito T. Direct Asymmetric Reductive Amination. J. Am. Chem. Soc. 2009, 131, 11316–11317. 10.1021/ja905143m. [DOI] [PubMed] [Google Scholar]
- Selected example:; Matsumura K.; Zhang X.; Hori K.; Murayama T.; Ohmiya T.; Shimizu H.; Saito T.; Sayo N. Practical, Catalytic Enantioselective Hydrogenation to Synthesize N-Unprotected β-Amino Esters. Org. Process Res. Dev. 2011, 15, 1130–1137. 10.1021/op2001035. [DOI] [Google Scholar]
- Selected example:; Mattei P.; Moine G.; Püntener K.; Schmid R. Asymmetric Synthesis of (S)-3-Amino-4-Methoxy-Butan-1-ol by Way of Reductive Amination. Org. Process Res. Dev. 2011, 15, 353–359. 10.1021/op1002775. [DOI] [Google Scholar]
- Zhou M.; Xue Z.; Cao M.; Dong X. Q.; Zhang X. Rhodium-Catalyzed Asymmetric Hydrogenation of Unprotected β-Enamine Phosphonates. Org. Biomol. Chem. 2016, 14, 4582–4584. 10.1039/C6OB00540C. [DOI] [PubMed] [Google Scholar]
- Ruchelman A. L.; Connolly T. J. Enantioselective Synthesis of the Apremilast Aminosulfone Using Catalytic Asymmetric Hydrogenation. Tetrahedron: Asymmetry 2015, 26, 553–559. 10.1016/j.tetasy.2015.03.010. [DOI] [Google Scholar]
- Hou G.; Zhang X.; Li W.; Ma M.; Zhang X. Highly Efficient Iridium-Catalyzed Asymmetric Hydrogenation of Unprotected β-Enamine Esters. J. Am. Chem. Soc. 2010, 132, 12844–12846. 10.1021/ja105674y. [DOI] [PubMed] [Google Scholar]
- Deng J.; Duan Z. C.; Huang J. Di; Hu X. P.; Wang D. Y.; Yu S. B.; Xu X. F.; Zheng Z. Rh-Catalyzed Asymmetric Hydrogenation of γ-Phthalimido-Substituted α,β-Unsaturated Carboxylic Acid Esters: An Efficient Enantioselective Synthesis of β-Aryl-γ-Amino Acids. Org. Lett. 2007, 9, 4825–4828. 10.1021/ol702193v. [DOI] [PubMed] [Google Scholar]
- Boaz N. W.; Debenham S. D.; Mackenzie E. B.; Large S. E. Phosphinoferrocenylaminophosphines as Novel and Practical Ligands for Asymmetric Catalysis. Org. Lett. 2002, 4, 2421–2424. 10.1021/ol0261736. [DOI] [PubMed] [Google Scholar]
- Boaz N. W.; Mackenzie E. B.; Debenham S. D.; Large S. E.; Ponasik J. A. Synthesis and Application of Phosphinoferrocenylaminophosphine Ligands for Asymmetric Catalysis. J. Org. Chem. 2005, 70, 1872–1880. 10.1021/jo048312y. [DOI] [PubMed] [Google Scholar]
- Deng J.; Hu X. P.; Huang J. Di; Yu S. B.; Wang D. Y.; Duan Z. C.; Zheng Z. Enantioselective Synthesis of β2-Amino Acids via Rh-Catalyzed Asymmetric Hydrogenation with BoPhoz-Type Ligands: Important Influence of an N-H Proton in the Ligand on the Enantioselectivity. J. Org. Chem. 2008, 73, 2015–2017. 10.1021/jo702510m. [DOI] [PubMed] [Google Scholar]
- Lühr S.; Holz J.; Zayas O.; Wendisch V.; Börner A. Synthesis of Chiral β2-Amino Acids by Asymmetric Hydrogenation. Tetrahedron: Asymmetry 2012, 23, 1301–1319. 10.1016/j.tetasy.2012.08.010. [DOI] [Google Scholar]
- Lühr S.; Holz J.; Zayas O.; Seidelmann O.; Domke L.; Börner A. Synthesis of Enantiopure β2-Homoalanine Derivatives via Rhodium Catalyzed Asymmetric Hydrogenation. Tetrahedron: Asymmetry 2013, 24, 395–401. 10.1016/j.tetasy.2013.02.011. [DOI] [Google Scholar]
- Guo Y.; Shao G.; Li L.; Wu W.; Li R.; Li J.; Song J.; Qiu L.; Prashad M.; Kwong F. Y. A General Approach to the Synthesis of β2-Amino Acid Derivatives via Highly Efficient Catalytic Asymmetric Hydrogenation of α-Aminomethylacrylates. Adv. Synth. Catal. 2010, 352, 1539–1553. 10.1002/adsc.201000122. [DOI] [Google Scholar]
- Li L.; Chen B.; Ke Y.; Li Q.; Zhuang Y.; Duan K.; Huang Y.; Pang J.; Qiu L. Highly Efficient Synthesis of Heterocyclic and Alicyclic β2-Amino Acid Derivatives by Catalytic Asymmetric Hydrogenation. Chem. - Asian J. 2013, 8, 2167–2174. 10.1002/asia.201300339. [DOI] [PubMed] [Google Scholar]
- Wang C.-J. J.; Sun X.; Zhang X. Enantioselective Hydrogenation of Allylphthalimides: An Efficient Method for the Synthesis of β-Methyl Chiral Amines. Angew. Chem., Int. Ed. 2005, 44, 4933–4935. 10.1002/anie.200501332. [DOI] [PubMed] [Google Scholar]
- Cabré A.; Romagnoli E.; Martínez-Balart P.; Verdaguer X.; Riera A. Highly Enantioselective Iridium-Catalyzed Hydrogenation of 2-Aryl Allyl Phthalimides. Org. Lett. 2019, 21, 9709–9713. 10.1021/acs.orglett.9b03865. [DOI] [PubMed] [Google Scholar]
- Cabré A.; Verdaguer X.; Riera A. Enantioselective Synthesis of β-Methyl Amines via Iridium-Catalyzed Asymmetric Hydrogenation of N-Sulfonyl Allyl Amines. Adv. Synth. Catal. 2019, 361, 4196–4200. 10.1002/adsc.201900748. [DOI] [Google Scholar]
- Cabré A.; Sciortino G.; Ujaque G.; Verdaguer X.; Lledós A.; Riera A. Iridium-Catalyzed Isomerization of N-Sulfonyl Aziridines to Allyl Amines. Org. Lett. 2018, 20, 5747–5751. 10.1021/acs.orglett.8b02450. [DOI] [PubMed] [Google Scholar]
- Blankenstein J.; Pfaltz A. A New Class of Modular Phosphinite - Oxazoline Ligands: Ir-Catalyzed Enantioselective Hydrogenation of Alkenes. Angew. Chem., Int. Ed. 2001, 40, 4445–4447. . [DOI] [PubMed] [Google Scholar]
- Pfaltz A.; Blankenstein J.; Hilgraf R.; Hörmann E.; Mcintyre S.; Menges F.; Schönleber M.; Smidt S. P.; Bettina W.; Zimmermann N. Iridium-Catalyzed Enantioselective Hydrogenation of Olefins. Adv. Synth. Catal. 2003, 345, 33–43. 10.1002/adsc.200390027. [DOI] [Google Scholar]
- Ornstein P. L.; Zimmerman D. M.; Arnold M. B.; Bleisch T. J.; Cantrell B.; Simon R.; Zarrinmayeh H.; Baker S. R.; Gates M.; Tizzano J. P.; et al. Biarylpropylsulfonamides as Novel, Potent Potentiators of 2-Amino-3- (5-methyl-3-hydroxyisoxazol-4-yl)- propanoic Acid (AMPA) Receptors. J. Med. Chem. 2000, 43, 4354–4358. 10.1021/jm0002836. [DOI] [PubMed] [Google Scholar]
- Verendel J. J.; Zhou T.; Li J. Q.; Paptchikhine A.; Lebedev O.; Andersson P. G. Highly Flexible Synthesis of Chiral Azacycles via Iridium-Catalyzed Hydrogenation. J. Am. Chem. Soc. 2010, 132, 8880–8881. 10.1021/ja103901e. [DOI] [PubMed] [Google Scholar]
- Meng K.; Xia J.; Wang Y.; Zhang X.; Yang G.; Zhang W. Ir/BiphPHOX-Catalyzed Asymmetric Hydrogenation of 3-Substituted 2,5-Dihydropyrroles and 2,5-Dihydrothiophene 1,1-Dioxides. Org. Chem. Front. 2017, 4, 1601–1605. 10.1039/C7QO00248C. [DOI] [Google Scholar]
- Steinhuebel D. P.; Krska S. W.; Alorati A.; Baxter J. M.; Belyk K.; Bishop B.; Palucki M.; Sun Y.; Davies I. W. Asymmetric Hydrogenation of Protected Allylic Amines. Org. Lett. 2010, 12, 4201–4203. 10.1021/ol101804e. [DOI] [PubMed] [Google Scholar]
- Zhu S.; Niljianskul N.; Buchwald S. L. A Direct Approach to Amines with Remote Stereocentres by Enantioselective CuH-Catalysed Reductive Relay Hydroamination. Nat. Chem. 2016, 8, 144–150. 10.1038/nchem.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Laffoon S. D.; Hull K. L. Asymmetric Synthesis of γ-Branched Amines via Rhodium-Catalyzed Reductive Amination. Nat. Commun. 2018, 9, 1185–1192. 10.1038/s41467-018-03535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S.; Takaya H.; Tani K.; Otsuka S.; Sato T.; Noyori R. Mechanism of the Asymmetric Isomerization of Allylamines to Enamines Catalyzed by 2,2’-Bis(Diphenylphosphino)-1,1’-Binaphthyl-Rhodium Complexes. J. Am. Chem. Soc. 1990, 112, 4897–4905. 10.1021/ja00168a040. [DOI] [Google Scholar]
- Zhu Y.; Khumsubdee S.; Schaefer A.; Burgess K. Asymmetric Syntheses of α-Methyl γ-Amino Acid Derivatives. J. Org. Chem. 2011, 76, 7449–7457. 10.1021/jo201215c. [DOI] [PubMed] [Google Scholar]
- Schneekönig J.; Liu W.; Leischner T.; Junge K.; Schotes C.; Beier C.; Beller M. Application of Crabtree/Pfaltz-Type Iridium Complexes for the Catalyzed Asymmetric Hydrogenation of an Agrochemical Building Block. Org. Process Res. Dev. 2020, 24, 443–447. 10.1021/acs.oprd.9b00466. [DOI] [Google Scholar]; Org. Process Res. Dev. 2020, 24, 1214–1214. 10.1021/acs.oprd.0c00206 [DOI] [Google Scholar]
- Song S.; Zhu S. F.; Pu L. Y.; Zhou Q. L. Iridium-Catalyzed Enantioselective Hydrogenation of Unsaturated Heterocyclic Acids. Angew. Chem., Int. Ed. 2013, 52, 6072–6075. 10.1002/anie.201301341. [DOI] [PubMed] [Google Scholar]
- Chen J.; Chen T.; Hu Q.; Puntener K.; Ren Y.; She J.; Du Z.; Scalone M. Practical Asymmetric Hydrogenation-Based Synthesis of a Class-Selective Histone Deacetylase Inhibitor. Org. Process Res. Dev. 2014, 18, 1702–1713. 10.1021/op500250b. [DOI] [Google Scholar]
- Lang Q.; Gu G.; Cheng Y.; Yin Q.; Zhang X. Highly Enantioselective Synthesis of Chiral γ-Lactams by Rh-Catalyzed Asymmetric Hydrogenation. ACS Catal. 2018, 8, 4824–4828. 10.1021/acscatal.8b00827. [DOI] [Google Scholar]
- Yamashita M.; Yamano T. Synthesis of Melatonin Receptor Agonist Ramelteon via Rh-Catalyzed Asymmetric Hydrogenation of an Allylamine. Chem. Lett. 2009, 38, 100–101. 10.1246/cl.2009.100. [DOI] [Google Scholar]
- Yuan S.; Gao G.; Wang L.; Liu C.; Wan L.; Huang H.; Geng H.; Chang M. The Combination of Asymmetric Hydrogenation of Olefins and Direct Reductive Amination. Nat. Commun. 2020, 11, 2–8. 10.1038/s41467-020-14475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. G. Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. 10.1021/ar700094b. [DOI] [PubMed] [Google Scholar]
- Sridharan V.; Suryavanshi P. A.; Menéndez J. C. Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- Luo Y. E.; He Y. M.; Fan Q. H. Asymmetric Hydrogenation of Quinoline Derivatives Catalyzed by Cationic Transition Metal Complexes of Chiral Diamine Ligands: Scope, Mechanism and Catalyst Recycling. Chem. Rec. 2016, 16, 2697–2711. 10.1002/tcr.201600095. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhuo L. G.; Li Z.; Chen F.; Ding Z.; He Y.; Fan Q. H.; Xiang J.; Yu Z. X.; Chan A. S. C. Highly Enantioselective Hydrogenation of Quinolines Using Phosphine-Free Chiral Cationic Ruthenium Catalysts: Scope, Mechanism, and Origin of Enantioselectivity. J. Am. Chem. Soc. 2011, 133, 9878–9891. 10.1021/ja2023042. [DOI] [PubMed] [Google Scholar]
- Wang Z. J.; Zhou H. F.; Wang T. L.; He Y. M.; Fan Q. H. Highly Enantioselective Hydrogenation of Quinolines under Solvent-Free or Highly Concentrated Conditions. Green Chem. 2009, 11, 767–776. 10.1039/b822822a. [DOI] [Google Scholar]
- Yang Z.; Chen F.; He Y. M.; Yang N.; Fan Q. H. Efficient Asymmetric Hydrogenation of Quinolines in Neat Water Catalyzed by Chiral Cationic Ru-Diamine Complexes. Catal. Sci. Technol. 2014, 4, 2887–2890. 10.1039/C4CY00418C. [DOI] [Google Scholar]
- Zhou H.; Li Z.; Wang Z.; Wang T.; Xu L.; He Y.; Fan Q. H.; Pan J.; Gu L.; Chan A. S. C. Hydrogenation of Quinolines Using a Recyclable Phosphine-Free Chiral Cationic Ruthenium Catalyst: Enhancement of Catalyst Stability and Selectivity in an Ionic Liquid. Angew. Chem., Int. Ed. 2008, 47, 8464–8467. 10.1002/anie.200802237. [DOI] [PubMed] [Google Scholar]
- Ding Z. Y.; Wang T.; He Y. M.; Chen F.; Zhou H. F.; Fan Q. H.; Guo Q.; Chan A. S. C. Highly Enantioselective Synthesis of Chiral Tetrahydroquinolines and Tetrahydroisoquinolines by Ruthenium-Catalyzed Asymmetric Hydrogenation in Ionic Liquid. Adv. Synth. Catal. 2013, 355, 3727–3735. 10.1002/adsc.201300698. [DOI] [Google Scholar]
- Wang T.; Chen Y.; Ouyang G.; He Y. M.; Li Z.; Fan Q. H. Solvent-Regulated Asymmetric Hydrogenation of Quinoline Derivatives in Oligo(Ethylene Glycol)s through Host-Guest Interactions. Chem. - Asian J. 2016, 11, 2773–2777. 10.1002/asia.201600445. [DOI] [PubMed] [Google Scholar]
- Li Z. W.; Wang T. L.; He Y. M.; Wang Z. J.; Fan Q. H.; Pan J.; Xu L. J. Air-Stable and Phosphine-Free Iridium Catalysts for Highly Enantioselective Hydrogenation of Quinoline Derivatives. Org. Lett. 2008, 10, 5265–5268. 10.1021/ol802016w. [DOI] [PubMed] [Google Scholar]
- Wang W. B.; Lu S. M.; Yang P. Y.; Han X. W.; Zhou Y. G. Highly Enantioselective Iridium-Catalyzed Hydrogenation of Heteroaromatic Compounds, Quinolines. J. Am. Chem. Soc. 2003, 125, 10536–10537. 10.1021/ja0353762. [DOI] [PubMed] [Google Scholar]
- Wang D. W.; Wang X. B.; Wang D. S.; Lu S. M.; Zhou Y. G.; Li Y. X. Highly Enantioselective Iridium-Catalyzed Hydrogenation of 2-Benzylquinolines and 2-Functionalized and 2,3-Disubstituted Quinolines. J. Org. Chem. 2009, 74, 2780–2787. 10.1021/jo900073z. [DOI] [PubMed] [Google Scholar]
- Maj A. M.; Suisse I.; Méliet C.; Hardouin C.; Agbossou-Niedercorn F. Highly Enantioselective Hydrogenation of New 2-Functionalized Quinoline Derivatives. Tetrahedron Lett. 2012, 53, 4747–4750. 10.1016/j.tetlet.2012.06.114. [DOI] [Google Scholar]
- Maj A. M.; Suisse I.; Hardouin C.; Agbossou-Niedercorn F. Synthesis of New Chiral 2-Functionalized-1,2,3,4-Tetrahydroquinoline Derivatives via Asymmetric Hydrogenation of Substituted Quinolines. Tetrahedron 2013, 69, 9322–9328. 10.1016/j.tet.2013.07.090. [DOI] [Google Scholar]
- Zhang D. Y.; Yu C. B.; Wang M. C.; Gao K.; Zhou Y. G. A New Electronically Deficient Atropisomeric Diphosphine Ligand (S)-CF3O-BiPhep and Its Application in Asymmetric Hydrogenation. Tetrahedron Lett. 2012, 53, 2556–2559. 10.1016/j.tetlet.2012.03.036. [DOI] [Google Scholar]
- Zhang D. Y.; Wang D. S.; Wang M. C.; Yu C. B.; Gao K.; Zhou Y. G. Synthesis of Electronically Deficient Atropisomeric Bisphosphine Ligands and Their Application in Asymmetric Hydrogenation of Quinolines. Synthesis 2011, 17, 2796–2802. 10.1055/s-0030-1260129. [DOI] [Google Scholar]
- Gou F. R.; Li W.; Zhang X.; Liang Y. M. Iridium-Catalyzed Asymmetric Hydrogenation of Quinoline Derivatives with C3*-Tunephos. Adv. Synth. Catal. 2010, 352, 2441–2444. 10.1002/adsc.201000485. [DOI] [Google Scholar]
- Wang D. S.; Zhou Y. G. Asymmetric Hydrogenation of Quinolines Activated by Brønsted Acids. Tetrahedron Lett. 2010, 51, 3014–3017. 10.1016/j.tetlet.2010.04.004. [DOI] [Google Scholar]
- Hu X. H.; Hu X. P. Highly Diastereo- And Enantioselective Ir-Catalyzed Hydrogenation of 2,3-Disubstituted Quinolines with Structurally Fine-Tuned Phosphine-Phosphoramidite Ligands. Org. Lett. 2019, 21, 10003–10006. 10.1021/acs.orglett.9b03925. [DOI] [PubMed] [Google Scholar]
- Cai X. F.; Guo R. N.; Chen M. W.; Shi L.; Zhou Y. G. Synthesis of Chiral Exocyclic Amines by Asymmetric Hydrogenation of Aromatic Quinolin-3-Amines. Chem. - Eur. J. 2014, 20, 7245–7248. 10.1002/chem.201402592. [DOI] [PubMed] [Google Scholar]
- Cai X. F.; Huang W. X.; Chen Z. P.; Zhou Y. G. Palladium-Catalyzed Asymmetric Hydrogenation of 3-Phthalimido Substituted Quinolines. Chem. Commun. 2014, 50, 9588–9590. 10.1039/C4CC04386C. [DOI] [PubMed] [Google Scholar]
- Chen Z. P.; Ye Z. S.; Chen M. W.; Zhou Y. G. Enantioselective Synthesis of Endocyclic β-Amino Acids with Two Contiguous Stereocenters via Hydrogenation of 3-Alkoxycarbonyl-2-Substituted Quinolines. Synthesis 2013, 45, 3239–3244. 10.1055/s-0033-1339849. [DOI] [Google Scholar]
- Ma W.; Zhang J.; Xu C.; Chen F.; He Y. M.; Fan Q. H. Highly Enantioselective Direct Synthesis of Endocyclic Vicinal Diamines through Chiral Ru(Diamine)-Catalyzed Hydrogenation of 2,2′-Bisquinoline Derivatives. Angew. Chem., Int. Ed. 2016, 55, 12891–12894. 10.1002/anie.201608181. [DOI] [PubMed] [Google Scholar]
- Li C.; Pan Y.; Feng Y.; He Y. M.; Liu Y.; Fan Q. H. Asymmetric Ruthenium-Catalyzed Hydrogenation of Terpyridine-Type N-Heteroarenes: Direct Access to Chiral Tridentate Nitrogen Ligands. Org. Lett. 2020, 22, 6452–6457. 10.1021/acs.orglett.0c02268. [DOI] [PubMed] [Google Scholar]
- Liu C.; Wang M.; Liu S.; Wang Y.; Peng Y.; Lan Y.; Liu Q. Manganese□Catalyzed Asymmetric Hydrogenation of Quinolines Enabled by π-π Interaction. Angew. Chem., Int. Ed. 2021, 60, 5108–5113. 10.1002/anie.202013540. [DOI] [PubMed] [Google Scholar]
- Tadaoka H.; Cartigny D.; Nagano T.; Gosavi T.; Ayad T.; Genêt J. P.; Ohshima T.; Ratovelomanana-Vidal V.; Mashima K. Unprecedented Halide Dependence on Catalytic Asymmetric Hydrogenation of 2-Aryl- and 2-Alkyl-Substituted Quinolinium Salts by Using Ir Complexes with Difluorphos and Halide Ligands. Chem. - Eur. J. 2009, 15, 9990–9994. 10.1002/chem.200901477. [DOI] [PubMed] [Google Scholar]
- Dragan V.; McWilliams J. C.; Miller R.; Sutherland K.; Dillon J. L.; O’Brien M. K. Asymmetric Synthesis of Vabicaserin via Oxidative Multicomponent Annulation and Asymmetric Hydrogenation of a 3,4-Substituted Quinolinium Salt. Org. Lett. 2013, 15, 2942–2945. 10.1021/ol401029k. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Pan Y.; He Y. M.; Fan Q. H. Consecutive Intermolecular Reductive Amination/Asymmetric Hydrogenation: Facile Access to Sterically Tunable Chiral Vicinal Diamines and N-Heterocyclic Carbenes. Angew. Chem., Int. Ed. 2019, 58, 16831–16834. 10.1002/anie.201909919. [DOI] [PubMed] [Google Scholar]
- Xu C.; Feng Y.; Li F.; Han J.; He Y. M.; Fan Q. H. A Synthetic Route to Chiral Benzo-Fused N-Heterocycles via Sequential Intramolecular Hydroamination and Asymmetric Hydrogenation of Anilino-Alkynes. Organometallics 2019, 38, 3979–3990. 10.1021/acs.organomet.9b00183. [DOI] [Google Scholar]
- Yang T.; Yin Q.; Gu G.; Zhang X. A One-Pot Process for the Enantioselective Synthesis of Tetrahydroquinolines and Tetrahydroisoquinolines: Via Asymmetric Reductive Amination (ARA). Chem. Commun. 2018, 54, 7247–7250. 10.1039/C8CC03586E. [DOI] [PubMed] [Google Scholar]
- For a selected publication for the synthesis of THIQs using ARA, see:; Zhou H.; Liu Y.; Yang S.; Zhou L.; Chang M. One-Pot N-Deprotection and Catalytic Intramolecular Asymmetric Reductive Amination for the Synthesis of Tetrahydroisoquinolines. Angew. Chem., Int. Ed. 2017, 56, 2725–2729. 10.1002/anie.201611181. [DOI] [PubMed] [Google Scholar]
- Yang T.; Guo X.; Yin Q.; Zhang X. Intramolecular Asymmetric Reductive Amination: Synthesis of Enantioenriched Dibenz[c, e]Azepines. Chem. Sci. 2019, 10, 2473–2477. 10.1039/C8SC04482A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Y.; Yamaji K.; Higashida K.; Sathaiah K.; Iimuro A.; Mashima K. Enhancing Effects of Salt Formation on Catalytic Activity and Enantioselectivity for Asymmetric Hydrogenation of Isoquinolinium Salts by Dinuclear Halide-Bridged Iridium Complexes Bearing Chiral Diphosphine Ligands. Chem. - Eur. J. 2015, 21, 1915–1927. 10.1002/chem.201405408. [DOI] [PubMed] [Google Scholar]
- Iimuro A.; Yamaji K.; Kandula S.; Nagano T.; Kita Y.; Mashima K. Asymmetric Hydrogenation of Isoquinolinium Salts Catalyzed by Chiral Iridium Complexes: Direct Synthesis for Optically Active 1,2,3,4- Tetrahydroisoquinolines. Angew. Chem., Int. Ed. 2013, 52, 2046–2050. 10.1002/anie.201207748. [DOI] [PubMed] [Google Scholar]
- Ye Z. S.; Guo R. N.; Cai X. F.; Chen M. W.; Shi L.; Zhou Y. G. Enantioselective Iridium-Catalyzed Hydrogenation of 1- and 3-Substituted Isoquinolinium Salts. Angew. Chem., Int. Ed. 2013, 52, 3685–3689. 10.1002/anie.201208300. [DOI] [PubMed] [Google Scholar]
- Guo R. N.; Cai X. F.; Shi L.; Ye Z. S.; Chen M. W.; Zhou Y. G. An Efficient Route to Chiral N-Heterocycles Bearing a C-F Stereogenic Center via Asymmetric Hydrogenation of Fluorinated Isoquinolines. Chem. Commun. 2013, 49, 8537–8539. 10.1039/c3cc45341c. [DOI] [PubMed] [Google Scholar]
- Wen J.; Tan R.; Liu S.; Zhao Q.; Zhang X. Strong Brønsted Acid Promoted Asymmetric Hydrogenation of Isoquinolines and Quinolines Catalyzed by a Rh-Thiourea Chiral Phosphine Complex: Via Anion Binding. Chem. Sci. 2016, 7, 3047–3051. 10.1039/C5SC04712A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. M.; Wang Y. Q.; Han X. W.; Zhou Y. G. Asymmetric Hydrogenation of Quinolines and Isoquinolines Activated by Chloroformates. Angew. Chem., Int. Ed. 2006, 45, 2260–2263. 10.1002/anie.200503073. [DOI] [PubMed] [Google Scholar]
- Shi L.; Ye Z. S.; Cao L. L.; Guo R. N.; Hu Y.; Zhou Y. G. Enantioselective Iridium-Catalyzed Hydrogenation of 3,4-Disubstituted Isoquinolines. Angew. Chem., Int. Ed. 2012, 51, 8286–8289. 10.1002/anie.201203647. [DOI] [PubMed] [Google Scholar]
- Chen M. W.; Ji Y.; Wang J.; Chen Q. A.; Shi L.; Zhou Y. G. Asymmetric Hydrogenation of Isoquinolines and Pyridines Using Hydrogen Halide Generated in Situ as Activator. Org. Lett. 2017, 19, 4988–4991. 10.1021/acs.orglett.7b02502. [DOI] [PubMed] [Google Scholar]
- Kim A. N.; Ngamnithiporn A.; Welin E. R.; Daiger M. T.; Grünanger C. U.; Bartberger M. D.; Virgil S. C.; Stoltz B. M. Iridium-Catalyzed Enantioselective and Diastereoselective Hydrogenation of 1,3-Disubstituted Isoquinolines. ACS Catal. 2020, 10, 3241–3248. 10.1021/acscatal.0c00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pototschnig G. M.; Mcdermott M. S. J.; Conklin D.; Gilmore C. D.; Tadross P. M.; Haley C. K.; Negoro K.; Glibstrup E.; Grünanger C. U.; Allan K. M.; et al. Concise Total Syntheses of (−)-Jorunnamycin A and (−)-Jorumycin Enabled by Asymmetric Catalysis. Science 2019, 363, 270–275. 10.1126/science.aav3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; Sun Y.; Xu L.; Wang T.; Fan Q. H.; Lam K. H.; Chan A. S. C. Highly Efficient and Enantioselective Hydrogenation of Quinolines and Pyridines with Ir-Difluorphos Catalyst. Org. Biomol. Chem. 2010, 8, 3464–3471. 10.1039/c002668a. [DOI] [PubMed] [Google Scholar]
- Tang W. J.; Tan J.; Xu L. J.; Lam K. H.; Fan Q. H.; Chan A. S. C. Highly Enantioselective Hydrogenation of Quinoline and Pyridine Derivatives with Iridium-(P-Phos) Catalyst. Adv. Synth. Catal. 2010, 352, 1055–1062. 10.1002/adsc.200900870. [DOI] [Google Scholar]
- Wang X. B.; Zeng W.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Pyridine Derivatives, 7,8-Dihydro-Quinolin-5(6H)-Ones. Tetrahedron Lett. 2008, 49, 4922–4924. 10.1016/j.tetlet.2008.05.138. [DOI] [Google Scholar]
- Legault C. Y.; Charette A. B. Catalytic Asymmetric Hydrogenation of N-Iminopyridinium Ylides: Expedient Approach to Enantioenriched Substituted Piperidine Derivatives. J. Am. Chem. Soc. 2005, 127, 8966–8967. 10.1021/ja0525298. [DOI] [PubMed] [Google Scholar]
- Cadu A.; Upadhyay P. K.; Andersson P. G. Iridium-Catalyzed Asymmetric Hydrogenation of Substituted Pyridines. Asian J. Org. Chem. 2013, 2, 1061–1065. 10.1002/ajoc.201300160. [DOI] [Google Scholar]
- Ye Z. S.; Chen M. W.; Chen Q. A.; Shi L.; Duan Y.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Pyridinium Salts. Angew. Chem., Int. Ed. 2012, 51, 10181–10184. 10.1002/anie.201205187. [DOI] [PubMed] [Google Scholar]
- Chang M.; Huang Y.; Liu S.; Chen Y.; Krska S. W.; Davies I. W.; Zhang X. Asymmetric Hydrogenation of Pyridinium Salts with an Iridium Phosphole Catalyst. Angew. Chem., Int. Ed. 2014, 53, 12761–12764. 10.1002/anie.201406762. [DOI] [PubMed] [Google Scholar]
- Renom-Carrasco M.; Gajewski P.; Pignataro L.; de Vries J. G.; Piarulli U.; Gennari C.; Lefort L. A Mixed Ligand Approach for the Asymmetric Hydrogenation of 2-Substituted Pyridinium Salts. Adv. Synth. Catal. 2016, 358, 2589–2593. 10.1002/adsc.201600348. [DOI] [PubMed] [Google Scholar]
- Qu B.; Mangunuru H. P. R.; Tcyrulnikov S.; Rivalti D.; Zatolochnaya O. V.; Kurouski D.; Radomkit S.; Biswas S.; Karyakarte S.; Fandrick K. R.; et al. Enantioselective Synthesis of α-(Hetero)Aryl Piperidines through Asymmetric Hydrogenation of Pyridinium Salts and Its Mechanistic Insights. Org. Lett. 2018, 20, 1333–1337. 10.1021/acs.orglett.8b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu B.; Mangunuru H. P. R.; Wei X.; Fandrick K. R.; Desrosiers J. N.; Sieber J. D.; Kurouski D.; Haddad N.; Samankumara L. P.; Lee H.; et al. Synthesis of Enantioenriched 2-Alkyl Piperidine Derivatives through Asymmetric Reduction of Pyridinium Salts. Org. Lett. 2016, 18, 4920–4923. 10.1021/acs.orglett.6b02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.; Qu B.; Zeng X.; Savoie J.; Fandrick K. R.; Desrosiers J. N.; Tcyrulnikov S.; Marsini M. A.; Buono F. G.; Li Z.; et al. Sequential C-H Arylation and Enantioselective Hydrogenation Enables Ideal Asymmetric Entry to the Indenopiperidine Core of an 11β-HSD-1 Inhibitor. J. Am. Chem. Soc. 2016, 138, 15473–15481. 10.1021/jacs.6b09764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. W.; Ye Z. S.; Chen Z. P.; Wu B.; Zhou Y. G. Enantioselective Synthesis of Trifluoromethyl Substituted Piperidines with Multiple Stereogenic Centers via Hydrogenation of Pyridinium Hydrochlorides. Org. Chem. Front. 2015, 2, 586–589. 10.1039/C5QO00069F. [DOI] [Google Scholar]
- Kita Y.; Iimuro A.; Hida S.; Mashima K. Iridium-Catalyzed Asymmetric Hydrogenation of Pyridinium Salts for Constructing Multiple Stereogenic Centers on Piperidines. Chem. Lett. 2014, 43, 284–286. 10.1246/cl.130943. [DOI] [Google Scholar]
- Iimuro A.; Higashida K.; Kita Y.; Mashima K. Asymmetric Hydrogenation of 3-Amido-2-Arylpyridinium Salts by Triply Chloride-Bridged Dinuclear Iridium Complexes Bearing Enantiopure Diphosphine Ligands: Synthesis of Neurokinin-1 Receptor Antagonist Derivatives. Adv. Synth. Catal. 2016, 358, 1929–1933. 10.1002/adsc.201600203. [DOI] [Google Scholar]
- Zheng L. S.; Wang F.; Ye X. Y.; Chen G. Q.; Zhang X. Asymmetric Hydrogenation of 2-Aryl-3-Phthalimidopyridinium Salts: Synthesis of Piperidine Derivatives with Two Contiguous Stereocenters. Org. Lett. 2020, 22, 8882–8887. 10.1021/acs.orglett.0c03261. [DOI] [PubMed] [Google Scholar]
- Renom-Carrasco M.; Gajewski P.; Pignataro L.; de Vries J. G.; Piarulli U.; Gennari C.; Lefort L. Asymmetric Hydrogenation of 3-Substituted Pyridinium Salts. Chem. - Eur. J. 2016, 22, 9528–9532. 10.1002/chem.201601501. [DOI] [PubMed] [Google Scholar]
- Huang W. X.; Yu C. B.; Ji Y.; Liu L. J.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Heteroaromatics Bearing a Hydroxyl Group, 3-Hydroxypyridinium Salts. ACS Catal. 2016, 6, 2368–2371. 10.1021/acscatal.5b02625. [DOI] [Google Scholar]
- Rieckhoff S.; Frey W.; Peters R. Regio-, Diastereo- and Enantioselective Synthesis of Piperidines with Three Stereogenic Centers from Isoxazolinones by Palladium/Iridium Relay Catalysis. Eur. J. Org. Chem. 2018, 2018, 1797–1805. 10.1002/ejoc.201800198. [DOI] [Google Scholar]
- Qin J.; Chen F.; Ding Z.; He Y. M.; Xu L.; Fan Q. H. Asymmetric Hydrogenation of 2-and 2,3-Substituted Ouinoxalines with Chiral Cationic Ruthenium Diamine Catalysts. Org. Lett. 2011, 13, 6568–6571. 10.1021/ol2029096. [DOI] [PubMed] [Google Scholar]
- Tang W.; Xu L.; Fan Q. H.; Wang J.; Fan B.; Zhou Z.; Lam K. H.; Chan A. S. C. Asymmetric Hydrogenation of Quinoxalines with Diphosphinite Ligands: A Practical Synthesis of Enantioenriched, Substituted Tetrahydroquinoxalines. Angew. Chem., Int. Ed. 2009, 48, 9135–9138. 10.1002/anie.200904518. [DOI] [PubMed] [Google Scholar]
- Cartigny D.; Berhal F.; Nagano T.; Phansavath P.; Ayad T.; Genêt J. P.; Ohshima T.; Mashima K.; Ratovelomanana-Vidal V. General Asymmetric Hydrogenation of 2-Alkyl- and 2-Aryl-Substituted Quinoxaline Derivatives Catalyzed by Iridium-Difluorphos: Unusual Halide Effect and Synthetic Application. J. Org. Chem. 2012, 77, 4544–4556. 10.1021/jo300455y. [DOI] [PubMed] [Google Scholar]
- Cartigny D.; Nagano T.; Ayad T.; Genêt J. P.; Ohshima T.; Mashima K.; Ratovelomanana-Vidal V. Iridium-Difluorphos-Catalyzed Asymmetric Hydrogenation of 2-Alkyl- and 2-Aryl-Substituted Quinoxalines: A General and Efficient Route into Tetrahydroquinoxalines. Adv. Synth. Catal. 2010, 352, 1886–1891. 10.1002/adsc.201000513. [DOI] [Google Scholar]
- Nagano T.; Iimuro A.; Schwenk R.; Ohshima T.; Kita Y.; Togni A.; Mashima K. Additive Effects of Amines on Asymmetric Hydrogenation of Quinoxalines Catalyzed by Chiral Iridium Complexes. Chem. - Eur. J. 2012, 18, 11578–11592. 10.1002/chem.201201366. [DOI] [PubMed] [Google Scholar]
- Sun S.; Nagorny P. Exploration of Chiral Diastereomeric Spiroketal (SPIROL)-Based Phosphinite Ligands in Asymmetric Hydrogenation of Heterocycles. Chem. Commun. 2020, 56, 8432–8435. 10.1039/D0CC03088K. [DOI] [PubMed] [Google Scholar]
- Maj A. M.; Heyte S.; Araque M.; Dumeignil F.; Paul S.; Suisse I.; Agbossou-Niedercorn F. First Catalytic Asymmetric Hydrogenation of Quinoxaline-2-Carboxylates. Tetrahedron 2016, 72, 1375–1380. 10.1016/j.tet.2016.01.033. [DOI] [Google Scholar]
- Hu S. B.; Zhai X. Y.; Shen H. Q.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Polycyclic Pyrrolo/Indolo[1,2-a]Quinoxalines and Phenanthridines. Adv. Synth. Catal. 2018, 360, 1334–1339. 10.1002/adsc.201701450. [DOI] [Google Scholar]
- Hu S. B.; Chen Z. P.; Song B.; Wang J.; Zhou Y. G. Enantioselective Hydrogenation of Pyrrolo[1,2-a]Pyrazines, Heteroaromatics Containing Two Nitrogen Atoms. Adv. Synth. Catal. 2017, 359, 2762–2767. 10.1002/adsc.201700431. [DOI] [Google Scholar]
- Huang W. X.; Yu C. B.; Shi L.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Pyrrolo[1,2- a]Pyrazinium Salts. Org. Lett. 2014, 16, 3324–3327. 10.1021/ol5013313. [DOI] [PubMed] [Google Scholar]
- Huang W. X.; Liu L. J.; Wu B.; Feng G. S.; Wang B.; Zhou Y. G. Synthesis of Chiral Piperazines via Hydrogenation of Pyrazines Activated by Alkyl Halides. Org. Lett. 2016, 18, 3082–3085. 10.1021/acs.orglett.6b01190. [DOI] [PubMed] [Google Scholar]
- Higashida K.; Nagae H.; Mashima K. Iridium-Catalyzed Asymmetric Hydrogenation of Tosylamido-Substituted Pyrazines for Constructing Chiral Tetrahydropyrazines with an Amidine Skelton. Adv. Synth. Catal. 2016, 358, 3949–3954. 10.1002/adsc.201600852. [DOI] [Google Scholar]
- Fattorusso E., Taglialatela-Scafati O., Eds. Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Wiley-VCH: Weinheim, 2008; pp 1–665. [Google Scholar]
- Baeza A.; Pfaltz A. Iridium-Catalyzed Asymmetric Hydrogenation of N-Protected Indoles. Chem. - Eur. J. 2010, 16, 2036–2039. 10.1002/chem.200903105. [DOI] [PubMed] [Google Scholar]
- Ge Y.; Wang Z.; Han Z.; Ding K. Iridium-Catalyzed Enantioselective Hydrogenation of Indole and Benzofuran Derivatives. Chem. - Eur. J. 2020, 26, 15482–15486. 10.1002/chem.202002532. [DOI] [PubMed] [Google Scholar]
- Kuwano R.; Sato K.; Kurokawa T.; Karube D.; Ito Y. Catalytic Asymmetric Hydrogenation of Heteroaromatic Compounds, Indoles. J. Am. Chem. Soc. 2000, 122, 7614–7615. 10.1021/ja001271c. [DOI] [Google Scholar]
- Kuwano R.; Kashiwabara M.; Sato K.; Ito T.; Kaneda K.; Ito Y. Catalytic Asymmetric Hydrogenation of Indoles Using a Rhodium Complex with a Chiral Bisphosphine Ligand PhTRAP. Tetrahedron: Asymmetry 2006, 17, 521–535. 10.1016/j.tetasy.2006.01.016. [DOI] [Google Scholar]
- Kuwano R.; Kashiwabara M. Ruthenium-Catalyzed Asymmetric Hydrogenation of N-Boc-Indoles. Org. Lett. 2006, 8, 2653–2655. 10.1021/ol061039x. [DOI] [PubMed] [Google Scholar]
- Kuwano R.; Kaneda K.; Ito T.; Sato K.; Kurokawa T.; Ito Y. Highly Enantioselective Synthesis of Chiral 3-Substituted Indolines by Catalytic Asymmetric Hydrogenation of Indoles. Org. Lett. 2004, 6, 2213–2215. 10.1021/ol049317k. [DOI] [PubMed] [Google Scholar]
- Mršić N.; Jerphagnon T.; Minnaard A. J.; Feringa B. L.; de Vries J. G. Asymmetric Hydrogenation of 2-Substituted N-Protected-Indoles Catalyzed by Rhodium Complexes of BINOL-Derived Phosphoramidites. Tetrahedron: Asymmetry 2010, 21, 7–10. 10.1016/j.tetasy.2009.11.017. [DOI] [Google Scholar]
- Maj A. M.; Suisse I.; Méliet C.; Agbossou-Niedercorn F. Enantioselective Hydrogenation of Indoles Derivatives Catalyzed by Walphos/Rhodium Complexes. Tetrahedron: Asymmetry 2010, 21, 2010–2014. 10.1016/j.tetasy.2010.06.030. [DOI] [Google Scholar]
- Schmidt M. A.; Simmons E. M.; Wei C. S.; Park H.; Eastgate M. D. An Enantioselective Total Synthesis of (+)-Duocarmycin SA. J. Org. Chem. 2018, 83, 3928–3940. 10.1021/acs.joc.8b00285. [DOI] [PubMed] [Google Scholar]
- Wang D. S.; Chen Q. A.; Li W.; Yu C. B.; Zhou Y. G.; Zhang X. Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles Activated by Brønsted Acids. J. Am. Chem. Soc. 2010, 132, 8909–8911. 10.1021/ja103668q. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Li L.; Chen M. W.; Yu C. B.; Fan H. J.; Zhou Y. G. Homogenous Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2014, 136, 7688–7700. 10.1021/ja502020b. [DOI] [PubMed] [Google Scholar]
- Li C.; Chen J.; Fu G.; Liu D.; Liu Y.; Zhang W. Highly Enantioselective Hydrogenation of N-Unprotected Indoles Using (S)-C10-BridgePHOS as the Chiral Ligand. Tetrahedron 2013, 69, 6839–6844. 10.1016/j.tet.2013.06.016. [DOI] [Google Scholar]
- Núñez-Rico J. L.; Fernández-Pérez H.; Vidal-Ferran A. Asymmetric Hydrogenation of Unprotected Indoles Using Iridium Complexes Derived from P-OP Ligands and (Reusable) Brønsted Acids. Green Chem. 2014, 16, 1153–1157. 10.1039/c3gc42132e. [DOI] [Google Scholar]
- Wen J.; Fan X.; Tan R.; Chien H. C.; Zhou Q.; Chung L. W.; Zhang X. Brønsted-Acid-Promoted Rh-Catalyzed Asymmetric Hydrogenation of N-Unprotected Indoles: A Cocatalysis of Transition Metal and Anion Binding. Org. Lett. 2018, 20, 2143–2147. 10.1021/acs.orglett.8b00312. [DOI] [PubMed] [Google Scholar]; Org. Lett. 2018, 20, 4390. 10.1021/acs.orglett.8b01912 [DOI] [PubMed] [Google Scholar]
- Touge T.; Arai T. Asymmetric Hydrogenation of Unprotected Indoles Catalyzed by η6-Arene/N-Me-Sulfonyldiamine-Ru(II) Complexes. J. Am. Chem. Soc. 2016, 138, 11299–11305. 10.1021/jacs.6b06295. [DOI] [PubMed] [Google Scholar]
- Wang D. S.; Tang J.; Zhou Y. G.; Chen M. W.; Yu C. B.; Duan Y.; Jiang G. F. Dehydration Triggered Asymmetric Hydrogenation of 3-(α-Hydroxyalkyl)Indoles. Chem. Sci. 2011, 2, 803–806. 10.1039/c0sc00614a. [DOI] [Google Scholar]
- Duan Y.; Chen M. W.; Ye Z. S.; Wang D. S.; Chen Q. A.; Zhou Y. G. An Enantioselective Approach to 2,3-Disubstituted Indolines through Consecutive Brønsted Acid/Pd-Complex-Promoted Tandem Reactions. Chem. - Eur. J. 2011, 17, 7193–7197. 10.1002/chem.201100576. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Chen M. W.; Chen Q. A.; Yu C. B.; Zhou Y. G. Pd-Catalyzed Asymmetric Hydrogenation of 3-(Toluenesulfonamidoalkyl)Indoles. Org. Biomol. Chem. 2012, 10, 1235–1238. 10.1039/C1OB06777J. [DOI] [PubMed] [Google Scholar]
- Yu C. B.; Wang J.; Zhou Y. G. Facile Synthesis of Chiral Indolines through Asymmetric Hydrogenation of: In Situ Generated Indoles. Org. Chem. Front. 2018, 5, 2805–2809. 10.1039/C8QO00710A. [DOI] [Google Scholar]
- Wang D. S.; Ye Z. S.; Chen Q. A.; Zhou Y. G.; Yu C. B.; Fan H. J.; Duan Y. Highly Enantioselective Partial Hydrogenation of Simple Pyrroles: A Facile Access to Chiral 1-Pyrrolines. J. Am. Chem. Soc. 2011, 133, 8866–8869. 10.1021/ja203190t. [DOI] [PubMed] [Google Scholar]
- Kuwano R.; Kashiwabara M.; Ohsumi M.; Kusano H. Catalytic Asymmetric Hydrogenation of 2,3,5-Trisubstituted Pyrroles. J. Am. Chem. Soc. 2008, 130, 808–809. 10.1021/ja7102422. [DOI] [PubMed] [Google Scholar]; J. Am. Chem. Soc. 2011, 133, 9136. 10.1021/ja2036332 [DOI] [Google Scholar]
- Kuwano R.; Kameyama N.; Ikeda R. Catalytic Asymmetric Hydrogenation of N-Boc-Imidazoles and Oxazoles. J. Am. Chem. Soc. 2011, 133, 7312–7315. 10.1021/ja201543h. [DOI] [PubMed] [Google Scholar]
- Makida Y.; Saita M.; Kuramoto T.; Ishizuka K.; Kuwano R. Asymmetric Hydrogenation of Azaindoles: Chemo- and Enantioselective Reduction of Fused Aromatic Ring Systems Consisting of Two Heteroarenes. Angew. Chem., Int. Ed. 2016, 55, 11859–11862. 10.1002/anie.201606083. [DOI] [PubMed] [Google Scholar]
- Ortega N.; Tang D. T. D.; Urban S.; Zhao D.; Glorius F. Ruthenium-NHC-Catalyzed Asymmetric Hydrogenation of Indolizines: Access to Indolizidine Alkaloids. Angew. Chem., Int. Ed. 2013, 52, 9500–9503. 10.1002/anie.201302218. [DOI] [PubMed] [Google Scholar]
- Schlepphorst C.; Wiesenfeldt M. P.; Glorius F. Enantioselective Hydrogenation of Imidazo[1,2-a]Pyridines. Chem. - Eur. J. 2018, 24, 356–359. 10.1002/chem.201705370. [DOI] [PubMed] [Google Scholar]
- Chen Y.; He Y. M.; Zhang S.; Miao T.; Fan Q. H. Rapid Construction of Structurally Diverse Quinolizidines, Indolizidines, and Their Analogues via Ruthenium-Catalyzed Asymmetric Cascade Hydrogenation/Reductive Amination. Angew. Chem., Int. Ed. 2019, 58, 3809–3813. and references cited therein. 10.1002/anie.201812647. [DOI] [PubMed] [Google Scholar]
- Wysocki J.; Schlepphorst C.; Glorius F. Asymmetric Homogeneous Hydrogenation of 2-Pyridones. Synlett 2015, 26, 1557–1562. 10.1055/s-0034-1378703. [DOI] [Google Scholar]
- Duan Y.; Zhu X. Y.; Ma J. A.; Zhou Y. G. Palladium-Catalyzed Asymmetric Hydrogenation of Fluorinated Quinazolinones. Tetrahedron Lett. 2013, 54, 6161–6163. 10.1016/j.tetlet.2013.08.078. [DOI] [Google Scholar]
- Feng G. S.; Zhao Z. B.; Shi L.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of Quinazolinones. Org. Chem. Front. 2019, 6, 2250–2253. 10.1039/C9QO00443B. [DOI] [Google Scholar]
- Kuwano R.; Hashiguchi Y.; Ikeda R.; Ishizuka K. Catalytic Asymmetric Hydrogenation of Pyrimidines. Angew. Chem., Int. Ed. 2015, 54, 2393–2396. 10.1002/anie.201410607. [DOI] [PubMed] [Google Scholar]
- Feng G. S.; Chen M. W.; Shi L.; Zhou Y. G. Facile Synthesis of Chiral Cyclic Ureas through Hydrogenation of 2-Hydroxypyrimidine/Pyrimidin-2(1H)-One Tautomers. Angew. Chem., Int. Ed. 2018, 57, 5853–5857. 10.1002/anie.201801485. [DOI] [PubMed] [Google Scholar]
- Feng G. S.; Shi L.; Meng F. J.; Chen M. W.; Zhou Y. G. Iridium-Catalyzed Asymmetric Hydrogenation of 4,6-Disubstituted 2-Hydroxypyrimidines. Org. Lett. 2018, 20, 6415–6419. 10.1021/acs.orglett.8b02723. [DOI] [PubMed] [Google Scholar]
- Kita Y.; Higashida K.; Yamaji K.; Iimuro A.; Mashima K. Asymmetric Hydrogenation of Quinazolinium Salts Catalysed by Halide-Bridged Dinuclear Iridium Complexes Bearing Chiral Diphosphine Ligands. Chem. Commun. 2015, 51, 4380–4382. 10.1039/C5CC00258C. [DOI] [PubMed] [Google Scholar]
- Ikeda R.; Kuwano R. Asymmetric Hydrogenation of Isoxazolium Triflates with a Chiral Iridium Catalyst. Chem. - Eur. J. 2016, 22, 8610–8618. 10.1002/chem.201600732. [DOI] [PubMed] [Google Scholar]
- Chen Q. A.; Gao K.; Duan Y.; Ye Z. S.; Shi L.; Yang Y.; Zhou Y. G. Dihydrophenanthridine: A New and Easily Regenerable NAD(P)H Model for Biomimetic Asymmetric Hydrogenation. J. Am. Chem. Soc. 2012, 134, 2442–2448. 10.1021/ja211684v. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Chen F.; Zhang S.; He Y.; Yang N.; Fan Q. H. Ruthenium-Catalyzed Enantioselective Hydrogenation of Phenanthridine Derivatives. Org. Lett. 2017, 19, 1458–1461. 10.1021/acs.orglett.7b00419. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Chen F.; He Y. M.; Fan Q. H. Asymmetric Ruthenium-Catalyzed Hydrogenation of 2,6-Disubstituted 1,5-Naphthyridines: Access to Chiral 1,5-Diaza-Cis-Decalins. Angew. Chem., Int. Ed. 2015, 54, 4622–4625. 10.1002/anie.201411105. [DOI] [PubMed] [Google Scholar]
- Ma W.; Chen F.; Liu Y.; He Y. M.; Fan Q. H. Ruthenium-Catalyzed Enantioselective Hydrogenation of 1,8-Naphthyridine Derivatives. Org. Lett. 2016, 18, 2730–2733. 10.1021/acs.orglett.6b01186. [DOI] [PubMed] [Google Scholar]
- Wang T.; Chen F.; Qin J.; He Y. M.; Fan Q. H. Asymmetric Ruthenium-Catalyzed Hydrogenation of 2- and 2,9-Substituted 1,10-Phenanthrolines. Angew. Chem., Int. Ed. 2013, 52, 7172–7176. 10.1002/anie.201301830. [DOI] [PubMed] [Google Scholar]