Abstract
Obesity is a major public health problem that is associated with serious comorbidities and premature mortality. Cardiovascular disease (CVD) is the major cause of morbidity and mortality associated with obesity. Lifestyle modifications, pharmacological therapy, and weight reduction surgery are the major interventions to date available for obesity management. Bariatric surgery has been increasingly utilized as a therapeutic option for obesity. In this meta-analysis, we aim to assess the effects of bariatric surgery on CVD outcomes and cardiovascular mortality. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. PubMed, Embase, Cochrane Library, Google Scholar, and Web of Science were searched until 03/01/2022. Our search included three types of bariatric surgery: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, and gastric banding (GB). All were searched in conjunction with “coronary artery disease,” “ischemic heart disease,” “myocardial infarction,” “cerebrovascular accident,” “stroke,” “atrial fibrillation,” “heart failure,” “arrhythmias,” and “mortality.”
We included 49 studies meeting the study criteria. Bariatric surgery showed a beneficial effect on coronary artery disease (CAD) (hazard ratio (HR) of 0.68 {95% confidence interval (CI): 0.52-0.91}, p = 0.008), myocardial infarction (MI) (HR of 0.53 {95% CI: 0.44-0.64}, p < 0.01) heart failure (HF) (HR of 0.45 {95% CI: 0.37-0.55}, p < 0.01), cerebrovascular accident (CVA) (HR of 0.68 {95% CI: 0.59-0.78}, p < 0.01), and cardiovascular mortality (HR of 0.48 {95% CI: 0.40-0.57}, p < 0.01). The effect on atrial fibrillation (AF) did not reach statistical significance: HR of 0.81 (95% CI: 0.65-1.01), p = 0.07. Our study, that is, an updated meta-analysis, including the three types of procedure, confirms beneficial effects on the major CVD outcomes, including coronary artery disease, myocardial infarction, cerebrovascular accident, and heart failure, and on CVD mortality. This study provides updated insights into the long-term CV effects of bariatric surgery, an increasingly common intervention for obesity.
Keywords: stroke, cerebrovascular accident, myocardial infarction, coronary artery disease, cardiovascular disease, gastric banding, sleeve gastrectomy, roux-en-y gastric bypass, bariatric surgery, obesity
Introduction and background
Obesity is a multifactorial disorder associated with serious complications including diabetes, dyslipidemia, cancer, and cardiovascular disease (CVD) [1,2]. Its prevalence has been uptrending over the last few decades, and it has become a modern-day epidemic [3]. Per the 2013 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines, overweight is defined as a body mass index (BMI) of 25 to <30 kg/m2 and obesity as a BMI of 30 kg/m2 [4]. According to the 2017-2018 National Health and Nutrition Examination Survey (NHANES), at least two in five adults (42.4% prevalence) have obesity. This is an increase from the 1999-2000 data with a much lower prevalence of 30.5% [3]. The etiologies leading to obesity could be biological, psychosocial, socioeconomic, and environmental factors [2]. Although unhealthy dietary habits play a major role, racial differences [5] and socioeconomic factors play a major role in the high prevalence of obesity and its complications among minority populations [6]. A higher BMI was strongly associated with higher comorbid cardiovascular risk factors [1]. Of the BMI-related deaths, 41% were notably due to cardiovascular diseases [7].
Obesity is a major contributor to cardiovascular risk factors including hypertension, hyperlipidemia, coronary artery disease (CAD), heart failure (HF), stroke, sleep apnea, and arrhythmias [8]. Its pathogenesis is linked to proinflammatory factors and vessel wall remodeling, among others. Obesity accelerates atherosclerosis by promoting lipid deposition and atherothrombosis formation. It further activates the cytokines and interleukins causing endothelial dysfunction and vascular remodeling [2]. This translates into cardiovascular disease (CVD) events including CAD, myocardial infarction (MI), and stroke. Excess visceral adiposity leads to the activation of renin-angiotensin-aldosterone system, cytokine gene expression, and increased systemic circulation of proatherogenic factors [2,9]. This in turn leads to myocardial fat accumulation, increased stroke volume, cardiac wall remodeling, and fibrosis manifesting as heart failure [2,10]. Similar mechanisms lead to left atrial enlargement and fibrosis contributing to arrhythmogenesis [11].
Lifestyle modifications and increased physical activity are the initial modalities recommended in the management of obesity. Patients with a BMI of at least 40 or >35 kg/m2 with serious obesity-related comorbidities are considered eligible for bariatric surgery [12]. The commonly performed bariatric surgeries include sleeve gastrectomy, Roux-en-Y gastric bypass (RYGB), and gastric banding (GB) [12]. Sleeve gastrectomy is currently the most commonly performed owing to lower risk of complications. The benefits of bariatric surgery include greater long-term weight loss, reduction of major adverse cardiovascular events (MACE) [13], and cardiovascular mortality [14].
In this study, we aimed to perform an updated systematic review and a meta-analysis on bariatric surgery and major cardiovascular outcomes. The bariatric surgeries examined in our study include RYGB, sleeve gastrectomy, and gastric banding.
Review
Methods
Literature Search and Search Strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [15]. Articles were searched online by two investigators independently through five databases and additional online sources. PubMed, Embase, Cochrane Library, Google Scholar, and Web of Science were searched at the University Hospital of Brooklyn library. Articles were restricted to only English language and searched until 03/01/2022. The search included three common types of bariatric surgery: Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding. The search strategies included “Bariatric surgery” AND “Cardiovascular diseases,” “Roux en Y Gastric bypass” AND “Cardiovascular diseases,” “Sleeve gastrectomy” AND “Cardiovascular diseases,” and “Gastric banding” AND “Cardiovascular diseases.” Further, all three procedures were searched in conjunction with “coronary artery disease,” “ischemic heart disease,” “myocardial infarction,” “cerebrovascular accident,” “stroke,” “atrial fibrillation,” “heart failure,” and “arrhythmias.” We also reviewed prior meta-analysis articles to account for missing articles. The initial search included 3981 articles from all databases. After the removal of duplicates, 2515 articles were reviewed. A repeat search was done during manuscript writing, and additionally, one article was included in the analysis.
Study Selection and Quality Assessment
Articles were reviewed by assessing article titles and abstracts independently by two investigators (HC and TG). The intervention group included patients undergoing bariatric surgery (Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding). The control group included non-surgical obese patients.
Patients of age >18 years and BMI of >30 kg/m2 with a follow-up of at least 12 months were included. Further, these studies had to include a control group and should assess at least one of the outcomes. Exclusion criteria included the following: (i) patients with malignancy, (ii) case series and conference abstracts, and (iii) studies involving cardiovascular disease cohort at baseline. But studies noting incidental cardiovascular diseases among baseline comorbid characteristics were not excluded. The quality of the studies was evaluated by the Newcastle-Ottawa Scale (NOS). Studies with less than five points carry a high risk of bias, and those with more than seven points were deemed of good quality.
Outcomes Studied
Six outcomes were studied in total. This includes CAD, MI, HF, atrial fibrillation, cerebrovascular accident (CVA), and cardiovascular disease-specific mortality. Studies assessing all-cause mortality only were excluded.
Data Extraction
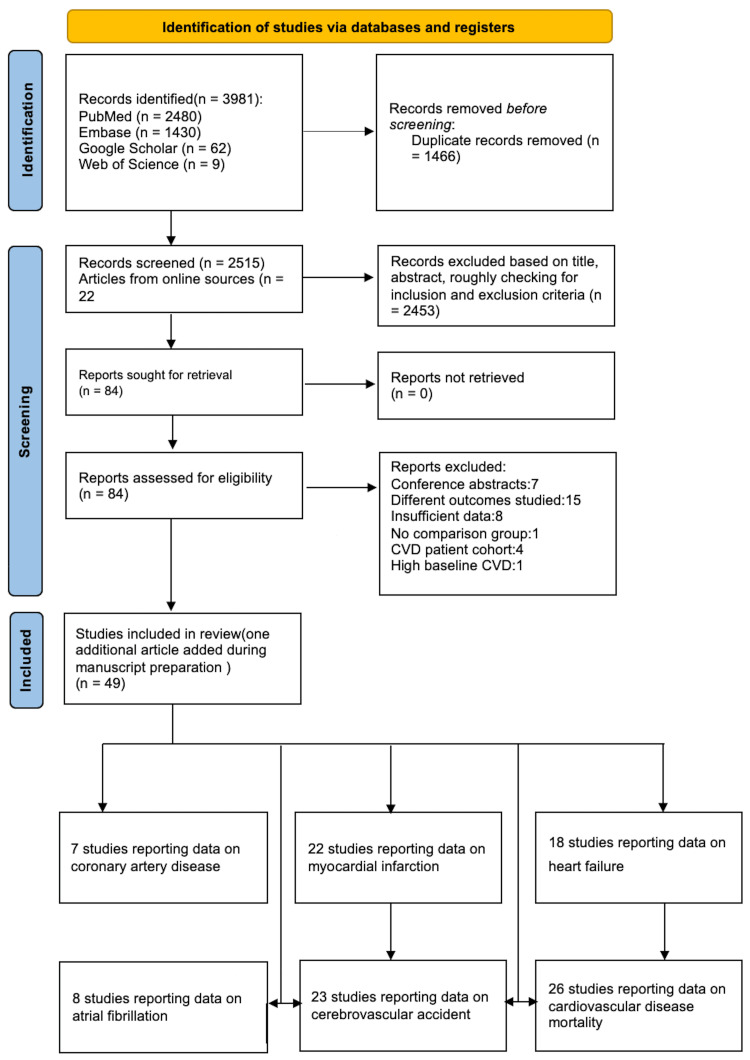
Eighty-five articles were reviewed in detail, of which 49 studies were included. The reasoning for study exclusion is elaborated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1). We extracted the following study details: sample size, gender, BMI, duration of follow-up, and end point data. The event data for intervention and control groups were obtained. Further, the adjusted and unadjusted hazard ratios (HR) with confidence intervals (CI) were extracted for the outcomes studied.
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart is shown elaborating the literature databases and the study selection.
CVD: cardiovascular disease
Statistical Analysis
The meta-analysis was performed with Cochrane’s Review Manager (RevMan) version 5.4. Adjusted hazard ratios were considered for the final analysis as the event rates were available for fewer studies. Hazard ratios (HR) were log transformed, and the confidence interval (CI) was used to measure standard error (SE). Genetic inverse variance and random effects model were used to obtain pooled HR and hence study the association between bariatric surgery and cardiovascular outcomes. Heterogeneity was assessed by Cochran’s Q statistic and quantified by I2 index. I2 values of <50%, 50%-75%, and >75% were considered to have low, moderate, and high heterogeneity, respectively. Publication bias was assessed using funnel plot analysis. A funnel plot was obtained for outcomes involving >10 studies.
Results
Out of the 3982 articles, 49 studies were included for data abstraction. All the included studies were cohort studies, both prospective and retrospective. Some of the studies excluded are the following: (i) studies involving malabsorptive surgery such as biliopancreatic diversion, (ii) studies that looked at outcomes in cohorts having preexisting MI and atrial fibrillation (since this would corroborate our outcome data, they were excluded), (iii) studies that had a high comorbid CVD at baseline, and (iv) studies assessing only all-cause mortality. The event rates and the hazard ratios for all the included studies are shown in Table 1.
Table 1. Included studies with the event rates and corresponding hazard ratios.
NA, not available; CI, confidence interval; HR, hazard ratio; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass
| Study name | Intervention group event rates | Control group event rates | Adjusted HR (CI) | Unadjusted HR (CI) |
| Coronary artery disease | ||||
| Bouchard et al., 2022 [13] | NA | NA | NA | NA |
| Fisher et al., 2018 [16] | NA | NA | 0.64 (0.42-0.99) | NA |
| Alkharaiji et al., 2019 [17] | 18/131 | 259/579 | 0.29 (0.16-0.52) | 0.31 (0.19-0.52) |
| Aminian et al., 2019 [18] | NA | NA | 0.69 (0.54-0.87) | NA |
| Singh et al., 2020 [19] | NA | NA | 0.85 (0.61-1.19) | NA |
| Ardissino et al., 2021 [20] | 15/593 | 17/593 | 0.6884 (0.3244-1.4610) | NA |
| Rassen et al., 2021 [21] | NA | NA | 1.10 (0.67-1.80) | NA |
| Myocardial infarction | ||||
| Bouchard et al., 2022 [13] | NA | NA | NA | NA |
| Alkharaiji et al., 2019 [17] | 13/131 | 95/579 | 0.98 (0.54-1.77) | 1.03 (0.57-1.86) |
| Ardissino et al., 2021 [20] | 6/593 | 6/593 | NA | NA |
| Sampalis et al., 2006 [22] | 35/1035 | 274/5746 | 0.71 (0.50-1.002) | NA |
| Sjöström et al., 2007 [23] | 13/2010 | 25/2037 | NA | NA |
| Romeo et al., 2012 [24] | NA | NA | 0.56 (0.34-0.93) | NA |
| Sjöström et al., 2012 [25] | 122/2010 | 136/2037 | NA | 0.71 (0.54-0.94) |
| Johnson et al., 2013 [26] | 8/2580 | 241/13371 | NA | NA |
| Douglas et al., 2015 [27] | 5/3618 | 18/3732 | 0.28 (0.10-0.74) | NA |
| Eliasson et al., 2015 [28] | 15/5694 | 39/5467 | 0.49 (0.24-1.01) | NA |
| Benotti et al., 2017 [29] | 12/1724 | 17/1724 | 0.89 (0.41-1.92) | 0.85 (0.41-1.79) |
| Brown et al., 2020 [30] | NA | NA | 0.39 (0.35-0.42) | NA |
| Michaels et al., 2020 [31] | 57/3242 | 323/3242 | NA | NA |
| Moussa et al., 2020 [32] | 37/3701 | 93/3701 | 0.41 (0.28-0.606) | NA |
| Stenberg et al., 2020 [33] | NA | NA | 0.53 (0.42-0.67) | 0.61 (0.50-0.75) |
| Wong et al., 2021 [34] | NA | NA | 0.534 (0.125-2.278) | NA |
| Höskuldsdóttir et al., 2020 [35] | NA | NA | 0.57 (0.24-1.35) | NA |
| Dash et al., 2021 [36] | NA | NA | 0.519 (0.301-0.894) | NA |
| Hung et al., 2021 [37] | 3/1436 | 15/1436 | 0.186 (0.054-0.643) | NA |
| Lundberg et al., 2021 [38] | 97/28204 | 518/40827 | 0.60 (0.41-0.88) | NA |
| Yuan et al., 2021 [39] | NA | NA | 0.24 (0.07-0.77) | 0.21 (0.07-0.69) |
| Mentias et al., 2022 [40] | NA | NA | 0.63 (0.59-0.68) | NA |
| Heart failure | ||||
| Bouchard et al., 2022 [13] | 182/3627 | 377/5420 | 0.80 (0.70-0.90) | NA |
| Alkharaiji et al., 2019 [17] | 13/131 | 91/579 | 0.89 (0.47-1.70) | 0.81 (0.44-1.49) |
| Aminian et al., 2019 [18] | NA | NA | 0.38 (0.30-0.49) | NA |
| Singh et al., 2020 [19] | NA | NA | 0.57 (0.34-0.96) | NA |
| Rassen et al., 2021 [21] | NA | NA | 0.82 (0.44-1.52) | NA |
| Sjöström et al., 2007 [23] | 2/2010 | 5/2037 | NA | NA |
| Johnson et al., 2013 [26] | 35/2580 | 1338/13371 | NA | NA |
| Benotti et al., 2017 [29] | 24/1724 | 55/1724 | 0.38 (0.22-0.64) | 0.53 (0.33-0.85) |
| Moussa et al., 2020 [32] | 22/3701 | 46/3701 | 0.403 (0.181-0.89) | NA |
| Wong et al., 2021 [34] | NA | NA | 0.283 (0.068-1.173) | NA |
| Höskuldsdóttir et al., 2020 [35] | NA | NA | 0.32 (0.15-0.67) | NA |
| Dash et al., 2021 [36] | NA | NA | 0.198 (0.109-0.36) | NA |
| Mentias et al., 2022 [40] | NA | NA | 0.46 (0.44-0.49) | NA |
| Persson et al., 2017 [41] | 89/22295 | 944/25564 | 0.37 (0.29-0.46) | NA |
| Sundström et al., 2017 [42] | 44/25804 | 29/13701 | NA | NA |
| Jamaly et al., 2019 [43] | 188/2003 | 266/2030 | 0.66 (0.51-0.81) | 0.65 (0.54-0.79) |
| Liakopoulos et al., 2020 [44] | 86/5321 | 233/5321 | 0.33 (0.24-0.46) | NA |
| Höskuldsdóttir et al., 2021 [45] | 47/5321 | 151/5321 | 0.27 (0.19-0.38) | NA |
| Atrial fibrillation | ||||
| Aminian et al., 2019 [18] | NA | NA | 0.78 (0.62-0.97) | NA |
| Singh et al., 2020 [19] | NA | NA | 0.93 (0.68-1.27) | 0.94 (0.60-1.28) |
| Rassen et al., 2021 [21] | NA | NA | 1.91 (1.10-3.33) | NA |
| Höskuldsdóttir et al., 2020 [35] | NA | NA | 0.69 (0.30-1.62) | NA |
| Yuan et al., 2021 [39] | NA | NA | 0.91 (0.43-1.90) | 0.64 (0.31-1.31) |
| Höskuldsdóttir et al., 2021 [45] | 104/5321 | 138/5321 | 0.59 (0.44-0.78) | NA |
| Jamaly et al., 2016 [46] | 247/2000 | 340/2021 | 0.69 (0.58-0.82) | NA |
| Lynch et al., 2019 [47] | 21/2522 | 73/2522 | NA | NA |
| Cerebrovascular accident | ||||
| Bouchard et al., 2022 [13] | 163/3627 | 233/5420 | 1.05 (0.74-1.12) | NA |
| Fisher et al., 2018 [16] | NA | NA | 0.69 (0.38-1.25) | NA |
| Alkharaiji et al., 2019 [17] | 8/131 | 40/579 | 0.87 (0.36-2.10) | 0.77 (0.34-1.72) |
| Aminian et al., 2019 [18] | NA | NA | 0.67 (0.48-0.94) | NA |
| Singh et al., 2020 [19] | NA | NA | 0.98 (0.66-1.45) | NA |
| Ardissino et al., 2021 [20] | 1/593 | 4/593 | 0.0227 (0.0009-5.45) | NA |
| Sjöström et al., 2007 [23] | 6/2010 | 6/2037 | NA | NA |
| Romeo et al., 2012 [24] | NA | NA | 0.73 (0.41-1.30) | NA |
| Sjöström et al., 2012 [25] | 93/2010 | 111/2037 | 0.66 (0.49-0.90) | NA |
| Johnson et al., 2013 [26] | 11/2580 | 214/13371 | NA | NA |
| Douglas et al., 2015 [27] | 17/3683 | 19/3748 | 0.91 (0.47-1.76) | NA |
| Benotti et al., 2017 [29] | 31/1724 | 49/1724 | 0.73 (0.45-1.17) | 0.77 (0.49-1.21) |
| Brown et al., 2020 [30] | NA | NA | 0.55 (0.51-0.59) | NA |
| Moussa et al., 2020 [32] | 4/3701 | 9/3701 | 0.536 (0.164-1.748) | NA |
| Stenberg et al., 2020 [33] | NA | NA | 0.81 (0.66-1.01) | 0.90 (0.75-1.09) |
| Wong et al., 2021 [34] | NA | NA | 0.811 (0.367-1.793) | NA |
| Höskuldsdóttir et al., 2020 [35] | NA | NA | 0.18 (0.04-0.82) | NA |
| Dash et al., 2021 [36] | NA | NA | 0.405 (0.169-0.971) | NA |
| Hung et al., 2021 [37] | 7/1436 | 42/1436 | 0.162 (0.073-0.360) | NA |
| Lundberg et al., 2021 [38] | 134/28204 | 486/40827 | 0.68 (0.48-0.96) | NA |
| Yuan et al., 2021 [39] | NA | NA | 1.23 (0.64-2.35) | 1 (0.53-1.91) |
| Mentias et al., 2022 [40] | NA | NA | 0.71 (0.65-0.79) | NA |
| Moussa et al., 2021 [48] | 19/4212 | 54/4212 | 0.352 (0.195-0.637) | NA |
| Cardiovascular mortality | ||||
| Carlsson et al., 2020 [14] | 167/2007 | 221/2040 | 0.70 (0.57-0.85) | NA |
| Sjöström et al., 2007 [23] | 20/2010 | 14/2037 | NA | NA |
| Sjöström et al., 2012 [25] | 28/2010 | 49/2037 | 0.47 (0.29-0.76) | 0.56 (0.35-0.88) |
| Johnson et al., 2013 [26] | 41/2580 | 985/13371 | NA | NA |
| Eliasson et al., 2015 [28] | 8/5694 | 33/5467 | 0.40 (0.15-1.05) | NA |
| Stenberg et al., 2020 [33] | NA | NA | NA | NA |
| Höskuldsdóttir et al., 2020 [35] | NA | NA | 0.15 (0.03-0.68) | NA |
| Hung et al., 2021 [37] | 0/1436 | 2/1436 | NA | NA |
| Lundberg et al., 2021 [38] | 196/28204 | 989/40827 | 0.78 (0.60-1.01) | NA |
| Liakopoulos et al., 2020 [44] | NA | NA | 0.36 (0.22-0.58) | NA |
| Höskuldsdóttir et al., 2021 [45] | 5/5321 | 31/5321 | NA | NA |
| MacDonald Jr et al., 1997 [49] | 2/154 | 12/78 | NA | NA |
| Christou et al., 2004 [50] | 49/1035 | 1530/5746 | NA | NA |
| Batsis et al., 2007 [51] | 4.5/173 | 6.8/139 | NA | NA |
| Adams et al., 2007 [52] | 55/7925 | 104/7925 | 0.51 (0.36-0.73) | 0.51 (0.36-0.73) |
| Pontiroli et al., 2016 [53] | 5/385 | 22/681 | NA | NA |
| Davidson et al., 2016 [54] | NA | NA | 0.51 (0.36-0.73) | NA |
| Lent et al., 2017 [55] | NA | NA | NA | NA |
| Pontiroli et al., 2018 [56] | 8/154 | 32/360 | NA | NA |
| Kauppila et al., 2019 [57] | 525/49977 | 30740/494842 | 0.57 (0.52-0.63) | NA |
| Doumouras et al., 2020 [58] (RYGB) | NA | NA | 0.58 (0.35-0.96) | NA |
| Doumouras et al., 2020 [58] (SG) | NA | NA | 0.39 (0.14-1.07) | NA |
| Sheetz et al., 2020 [59] | NA | NA | 0.47 (0.37-0.60) | NA |
| Courcoulas et al., 2021 [60] (RYGB) | NA | NA | 0.27 (0.20-0.37) | NA |
| Courcoulas et al., 2021 [60] (SG) | NA | NA | 0.57 (0.19-1.71) | NA |
| Doumouras et al., 2021 [61] | 9/3041 | 38/3041 | 0.32 (0.15-0.66) | NA |
Baseline study characteristics are shown in Table 2. The studies reported a mean age ranging from 32 to 62 and a mean BMI ranging from 37 to 50. All the studies were nonrandomized. Thirty-two of the studies were retrospective cohort studies, and the rest were either prospective or population-based studies.
Table 2. Baseline study characteristics of all included studies.
BMI, body mass index; CAD, coronary artery disease; ACS, acute coronary syndrome; HTN, hypertension; DM, diabetes mellitus; HbA1c, hemoglobin A1c; ESKD, end-stage kidney disease; CKD, chronic kidney disease; ASCVD, atherosclerotic cardiovascular disease; OSA, obstructive sleep apnea; MI, myocardial infarction; TIA, transient ischemic attack; IHD, ischemic heart disease; CHF, congestive heart failure; AF, atrial fibrillation; CV, cardiovascular; PAD, peripheral arterial disease; MSK, musculoskeletal; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; LAGB, laparoscopic adjustable gastric banding; CHD, coronary heart disease; ER, emergency room; ESRD, end-stage renal disease; HD, hemodialysis; HLD, hyperlipidemia; SCORS UB-04, South Carolina Office of Research and Statistics Uniform Billing-04; ICD9, International Classification of Diseases-9; Gen, general surgery; NA, not available
| Serial number | Study name | Design | Country | Type of intervention done | Study population | Inclusion criteria | Exclusion criteria | Sample size | Age (mean + SD) | BMI (mean + SD) | Follow-up duration | Primary outcome studied | Secondary outcome studied | |||
| Intervention | Control (con) | Intervention | Control | Intervention | Control | |||||||||||
| 1 | Bouchard et al., 2022 [13] | Population-based observational cohort study | Canada | Adjustable gastric banding (AGB: 42%), sleeve gastrectomy (SG: 23%), Roux-en-Y gastric bypass (RYGB: 11%), and duodenal switch (DS: 24%) | Two healthcare databases: 1) the Régie de l’Assurance Maladie du Québec (RAMQ) and 2) the Ministry of Health’s Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière (MED-ÉCHO), 2007-2012 | BMI of ≥35 with a comorbidity or BMI of ≥40, age of ≥18, and diagnosis of DM and/or HTN prior to the index date | Not specified | 3627 | 5420 | 48 ± 10 | 50 ± 10 | NA | NA | 7.05 years | Incident composite MACE (any coronary artery event, cerebrovascular event, heart failure (HF), or all-cause mortality) | Four individual components of the primary end point |
| 2 | Carlsson et al., 2020 [14] | Prospective matched cohort study | Sweden | Vertical banded gastroplasty (69%), AGB (18%), and RYGB (13%) | The Swedish Obese Subjects (SOS), 1987-2001 | Age of 37-60 years and BMI for males of ≥34 and females of ≥38 | Earlier gastric/duodenal surgery (surg), ongoing malignancy, MI of <6 months, and drug/alcohol | 2007 | 2040 | 47.2 ± 5.9 | 48.7 ± 6.3 | 42.4 ± 4.5 | 40.1 ± 4.7 | Surg: 24 years; con: 22 years | All-cause mortality | CV mortality |
| 3 | Fisher et al., 2018 [16] | Retrospective cohort | USA | RYGB (76%), SG (17%), and AGB (7%) | US health plan and care delivery systems, 2005-2011 | Age of 19-79 years, BMI of >35, and DM2 | <1 year of enrollment, cancer, pregnancy, gestational diabetes, CAD or cerebrovascular disease, and missing BMI | 5301 | 14934 | 49.5 ± 10 | 50.2 ± 10.1 | 44.7 ± 6.9 | 43.8 ± 6.7 | Surg: 4.7 years; con: 4.6 years | Macrovascular disease | CAD and stroke separately |
| 4 | Alkharaiji et al., 2019 [17] | Retrospective cohort | UK | RYGB or SG | The Health Improvement Network (THIN), 2017 | Age of >18 years and insulin-treated DM2 | DM1 or non-insulin-treated DM2 | 131 | 579 | 50.74 ± 11.0 | 51.96 ± 12.8 | 42.77 ± 9.6 | 40.6 ± 9.0 | 10 years | Patients’ (pt) survivability against nonfatal CV events: AMI, stroke, CHD, HF, and PAD | Health covariates such as body weight, calculated BMI, HbA1c, total cholesterol, systolic/diastolic blood pressure, and likelihood of insulin independency |
| 5 | Aminian et al., 2019 [18] | Retrospective cohort | USA | RYGB (63%), SG (32%), AGB (5%), and duodenal switch (0.002%) | Cleveland Clinical Health System, 2018 | Age of 18-80, BMI of ≥30, HbA1c of ≥6.5%, or ≥1 diabetic drug | Solid organ transplant, severe HF, active cancer, gastric cancer of <1 year, ER admission of <5 months, earlier gastric cancer surgery | 2287 | 39267 | 52.5 | 61.6 | 45.1 | 35.9 | 3.9 years | Six-point MACE | All-cause mortality, MI, CAD, HF, stroke, AF, and neuropathy |
| 6 | Singh et al., 2020 [19] | Retrospective cohort | UK | AGB, SG, RYGB, or duodenal switch (% NA) | The Health Improvement Network (THIN), 1990-2018 | >1 year registered in general practice | BMI of <30, age of >75 years, gastric cancer, gastric balloon, endo-barrier, or revisional bariatric surgery (BS) | 5170 | 9995 | 45.2 ± 10.6 | 45.3 ± 10.5 | NA | NA | 3.9 years | Cardiovascular disease (CVD) (IHD, HF, stroke, and TIA), all-cause mortality, incident hypertension, and AF | All-cause mortality, IHD, HF, stroke, TIA, and AF |
| 7 | Ardissino et al., 2021 [20] | Retrospective cohort | UK | Not specified | The Clinical Practice Research Datalink (CPRD) | Age of >18 years, BMI of ≥30, and DM2 | CKD of ≥3 and missing data: age, sex, BMI, and DM2 | 593 | 593 | 49.63 | 49.47 | 45.54 | 45.14 | 42.7 months | ASCVD | All-cause mortality, CAD, stroke, and PAD |
| 8 | Rassen et al., 2021 [21] | Retrospective cohort | USA | RYGB (50%), SG (44%), and gastric resection (8%) | Electronic health records licenced from Optum, 2007-2018 | Age of 18-80 years, DM2, and BMI of ≥30 | Solid organ transplant, severe HF, cancer in the past year, peptic ulcer disease on the index date, and ER admission of five prior to index date | 344 | 551 | 57.9 | 59 | 42.6 | 42.1 | Surg: 2.7 years; con: 2.4 years | Six-point MACE, B12 deficiency, anemia, and cholelithiasis | Not specified |
| 9 | Sampalis et al., 2006 [22] | Retrospective cohort | Canada | RYGB (81.3%) and vertical banded gastroplasty (18.7%) | McGill University Health Centre, 1986-2002 | Not specified other than BS | Cancer, hematological disease, CVD, digestive diseases, endocrinologic disease including diabetes and genitourinary, infectious, musculoskeletal, nervous system, psychiatric and mental, respiratory, and skin diseases | 1035 | 5746 | 45 ± 12 | 47 ± 13 | NA | NA | 2.5 years | Incidence of CV- and MSK-related conditions and treatments | Not specified |
| 10 | Sjöström et al., 2007 [23] | Prospective matched cohort | Sweden | Vertical banded gastroplasty (68%), AGB (19%), and RYGB (13%) | The Swedish Obese Subjects, 1987-2001 | Age of 37-60 years, BMI for males of ≥34 and females of ≥38 | Not specified | 2010 | 2037 | 46.1 ± 5.8 | 47.4 ± 6.1 | 41.8 ± 4.4 | 40.9 ± 4.3 | 10.9 ± 3.5 | All-cause mortality | Not specified |
| 11 | Romeo et al., 2012 [24] | Prospective, nonrandomized, controlled interventional trial | Sweden | RYGB (16%), gastric banding (18%), and vertical gastroplasty (66%) | The Swedish Obese Subjects (SOS), 1987-2001 | DM2, age of 37- 60, and BMI of ≥34 for males and ≥38 for females | Earlier gastric/duodenal ulcer surgery; earlier bariatric surgery; gastric ulcer/MI in the past six months; ongoing/active malignancy in the past five years; bulimic, drug/alcohol, psychiatric, or cooperative problems contraindicating bariatric surgery; and other contraindicating conditions, such as continuous glucocorticoid or anti-inflammatory treatment | 345 | 262 | 49 ± 6 | 50 ± 6 | 42 ± 5 | 40 ± 5 | 13.3 years | CV events (MI and stroke, whichever came first), as well as MI and stroke analyzed separately | Not specified |
| 12 | Sjöström et al., 2012 [25] | Prospective matched cohort | Sweden | Gastric bypass (13.2%), banding (18.7%), or vertical banded gastroplasty (68.1%) | The Swedish Obese Subjects (SOS), 1987-2001 | Age of 37-60 years and BMI for males of ≥34 and females of ≥38 | Earlier gastric/duodenal ulcer surgery; earlier bariatric surgery; gastric ulcer/MI in the past six months; ongoing/active malignancy in the past five years; bulimic, drug/alcohol, psychiatric, or cooperative problems contraindicating bariatric surgery; and other contraindicating conditions, such as continuous glucocorticoid or anti-inflammatory treatment | 2010 | 2037 | 46.1 | 47.8 | 42.4 | 40.1 | 14.7 years | Total mortality | MI and stroke |
| 13 | Johnson et al., 2013 [26] | Retrospective cohort | USA | Gastric bypass, adjustable gastric banding, vertical banded gastroplasty, or biliopancreatic diversion or sleeve gastrectomy | SCORS UB-04 | Moderate and severely obese patients with DM2, age of 18-77 years, and no documented history of (h/o) MI, angina, CHF, stroke, or advanced microvascular disease (previous nontraumatic amputation, laser eye/retinal surgery, blindness in at least one eye, ESRD, or creation of arteriovenous (AV) access for HD) | Type 1 diabetes, did not have diagnosis code specific to moderate or severe obesity, or had missing or incompatible data | 2580 | 13371 | 47.5 ± 10.6 | 52.1 ± 12.8 | NA | NA | Surg: 1.768 years; con: 1.58 years | Macrovascular (acute MI, stroke, or all-cause death) or microvascular (new diagnosis of blindness in at least one eye, laser eye or retinal surgery, nontraumatic amputation, or creation of permanent arteriovenous access for dialysis) | Macrovascular and microvascular complications considered separately, as well as other vascular complications, including revascularization of coronary, carotid, or lower extremity arteries or a new diagnosis of congestive heart failure or angina pectoris |
| 14 | Douglas et al., 2015 [27] | Retrospective cohort | UK | AGB (47.1%), RYGB (36.6%), SG (15.8%), and others (0.5%) | Clinical Practice Research Datalink, 2014 | >12 months of prior registration in database | Skin cancer and missing BMI data/BMI <35 | 3882 | 3882 | 45 ± 11 | 45 ± 11 | 44.7 ± 8.8 | 42.1 ± 6.5 | 3.4 years | Weight, BMI, DM2, HTN, angina, MI, stroke, fractures, OSA and cancer, mortality, and resolution of hypertension and DM2 | All-cause mortality and stroke |
| 15 | Eliasson et al., 2015 [28] | Retrospective cohort | Sweden | RYGB (100%) | National Diabetes Register and Scandinavian Obesity Surgery Registry, 2007-2014 | Age between 18 and 60 years | Not specified | 6132 | 6132 | 48.4 ± 9.8 | 50.5 ± 12.7 | 42 ± 5.7 | 41.4 ± 5.7 | 3.5 years | Total mortality, cardiovascular mortality, and fatal or nonfatal MI | MI and CV mortality |
| 16 | Benotti et al., 2017 [29] | Retrospective cohort | USA | RYGB (100%) | Geisinger Health Center, 2002-2012 | Age of 20-80 years, BMI of >35, and no preexisting CVD (ICD9 410-449) | Missing data to calculate Framingham Risk Score | 1724 | 1724 | 45.0 ± 10.6 | 45.1 ± 10.6 | 46.5 ± 6.0 | 46.5 ± 6.1 | 6.3 years | Combined MI/HF/stroke | Stroke, MI, and HF |
| 17 | Brown et al., 2020 [30] | Retrospective cohort | USA | RYGB (52.19%), SG (13.81%), and AGB (34%) | Statewide Planning and Research Cooperative System database, 2006-2012 | Age of ≥18 years | In-hospital death in earliest record, age of <18 years, duplicated records, and missing or unknown gender | 60445 | 268362 | 42.72 ± 11.55 | 43.28 ± 11.75 | NA | NA | Not specified | Any type of CV event, MI, and stroke | Cardiovascular events |
| 18 | Michaels et al., 2020 [31] | Retrospective cohort | USA | RYGB (78.9%), AGB (11.7%), SG (7.7%), and others (1.7%) | Single Virginia Academic Hospital, 1985-2015 | Not specified other than BS | Not specified | 3242 | 3242 | 43 | 43 | 47.7 | 48 | Surg: 6.1 years; con: 8.1 years | Incident MI, coronary catheterization, PCI, and CABG | Not specified |
| 19 | Moussa et al., 2020 [32] | Prospective cohort | UK | RYGB (38%), AGB (35%), SG (15%), others (1%), and undefined (11%) | UK Clinical Practice Research Datalink, 2020 | Not specified other than BS | BMI of <35, MACE before index date, lost to follow-up <12 months after index date, and missing data: age, BMI, and sex | 3701 | 3701 | 36 | 36 | 40.5 | 40.3 | 140.7 months | Combined MI/stroke | All-cause mortality, MI, stroke, and HF |
| 20 | Stenberg et al., 2020 [33] | Retrospective matched cohort | Sweden | RYGB (90.1%) and sleeve gastrectomy (9.9%) | Scandinavian Obesity Surgery Register (SOReg) and the Swedish National Patient Registers (NPR) | Not specified other than BS | <18 years, without HTN, those with antihypertensive therapy possibly for other reasons, and pt without at least one matched control with HTN | 11863 | 26199 | 52.1 ± 7.46 | 54.6 ± 7.12 | 41.9 ± 5.43 | NA | Surg: 61.1 ± 30.4 months; con: 60.7 ± 30.6 months | MACE | ACS, cerebrovascular event, all-cause mortality, CV mortality, and remission of HTN |
| 21 | Wong et al., 2021 [34] | Retrospective matched cohort study | China | Sleeve gastroplasty (80.5%), RYGB (16.2%), and revision procedure (3%) | Hospital Authority database in the Hong Kong adult diabetes population, 2006-2017 | DM2 | BMI of <27.5, non-DM2, history of CVD, and eGFR of <30 | 303 | 1399 | 51.35 ± 12.26 | 50.98 ± 13.44 | 37.44 ± 5.04 | 36.55 ± 6.49 | 32 months | All-cause mortality, composite CVD events (acute MI, other IHD, CHF, stroke, and PVD), ESKD, and severe hypoglycemia | NA |
| 22 | Höskuldsdóttir et al., 2020 [35] | Nationwide, matched, observational cohort study | Sweden | RYGB (100%) | National Diabetes Register and Scandinavian Obesity Surgery Registry, 2007-2013 | DM1 | Not specified | 387 | 387 | 41.7 ± 10.3 | 41.1 ± 14.5 | 40.8 ± 5.4 | 39.5 ± 7.0 | 9 years | All-cause mortality, CV disease, stroke, HF, and hospitalization for serious hypo- or hyperglycemic events, amputation, psychiatric disorders, changes in kidney function, and substance abuse | Not specified |
| 23 | Dash et al., 2021 [36] | Retrospective cohort study | Canada | RYGB (92.7%) and SG (7.3%) | University Health Network (UHN), 2008-2017 | BMI of ≥40 or ≥35 with comorbidities | Not Ontario residents, those who had surgery either before or >2.5 years after their referral date to the UHN bariatric program, and those who underwent procedures other than RYGB or SG, ineligible for surgery | 3098 | 5470 | 43.19 ± 10.36 | 46.15 ± 12.13 | 47.92 ± 8.07 | 47.37 ± 11.53 | NA | Stroke, MI, CHF hospitalization, or death | MI, stroke, HF, coronary revascularization, carotid revascularization, all-cause mortality, and hospitalization for chronic kidney disease, chronic liver disease, and psychiatric disease |
| 24 | Hung et al., 2021 [37] | Retrospective cohort | Taiwan | Gastric banding (13.87%), gastric bypass (52.68%), one-anastomosis gastric bypass (3.49%), and laparoscopic sleeve gastrectomy (29.87%) | Taiwan National Health Insurance Research Database (NHIRD), 2003-2008 | Age of 18-55 years and BMI of >35 kg/m2 with comorbidities or >40 kg/m2 | A primary diagnosis of any condition other than obesity, died during admission or within 30 days following the index admission, had a history of any CV disease, had undetermined sex, and were diagnosed with gastric malignancy | 1436 | 1436 | 32.39 ± 8.63 | 32.27 ± 9.25 | NA | NA | 89.65 months | Incidence of CV events | Not specified |
| 25 | Lundberg et al., 2021 [38] | Prospective cohort | Sweden | RYGB (100%) | Swedish National Patient Registry, 2001-2013 | Age of 20-65 years and BMI of ≥35 | Other bariatric surgery or died <2 years after obesity diagnosis | 28 204 | 40 827 | 40.8 ± 10.4 | 43.1 ± 11.8 | NA | NA | Surg: 4 years; con: 4.8 years | All-cause mortality, MI, ischemic stroke, and cardiovascular-related mortality | Not specified |
| 26 | Yuan et al., 2021 [39] | Retrospective cohort | USA | RYGB (100%) | Obesity clinic at Mayo Clinic, Rochester, MN, 1993-2012 | BMI of >35 | Pts underwent gastric banding and incomplete data | 308 | 701 | 44.2 ± 10.5 | 43.6 ± 12.6 | 46.4 ± 6.5 | 44.8 ± 6.9 | 1 year of index diagnosis | New-onset AF | MACE |
| 27 | Mentias et al., 2022 [40] | Prospective cohort | USA | SG (65.5%), gastric bypass (33.3%), and gastric banding (1.3%) | Medicare beneficiaries through 2013-2019 | Medicare beneficiaries enrolled in part A | >75 years, history of established HF, and enrolled in Medicare for <1 year before the study entry date. Patients that had an emergent/urgent admission, are admitted to a skilled nursing facility or long-term acute care, and are discharged to any destination other than home | 94885 | 94885 | 62.33 ± 10.62 | 62.33 ± 10.62 | 44.71 ± 7.3 | 44.71 ± 7.3 | 4 years | All-cause mortality | Time to admission with a diagnosis of new-onset HF, MI, and ischemic stroke. Secondary outcomes also included total rate of admissions with HF in follow-up |
| 28 | Persson et al., 2017 [41] | Retrospective cohort | Sweden | RYGB (92.8%), gastric banding (3.5%), vertical banded gastroplasty (3%), and gastroduodenal bypass (0.7%) | Swedish National Patient Registry, 2000-2011 | Age of 18-74 years with first recorded diagnosis of obesity | HF at or before obesity diagnosis and died on the same time of obesity diagnosis | 22295 | 25564 | 40.7 ± 10.7 | 44.3 ± 13.2 | NA | NA | 3.7 years | Incident HF and mortality | Not specified |
| 29 | Sundström et al., 2017 [42] | Prospective cohort | Sweden | RYGB (100%) | Scandinavian Obesity Surgery Registry (2007-2012) and Itrim health database (2006-2013) | BMI of 30-49.9 and age of ≥18 years | Crossover, HF at baseline, and missing data on education or marital status | 25804 | 13701 | 41.3 | 41.5 | 41.5 | 41.4 | 4.1 years | Incident HF | Nonischemic HF |
| 30 | Jamaly et al., 2019 [43] | Prospective matched cohort | Sweden | Vertical banded gastroplasty (68%), AGB (19%), and RYGB (13%) | The Swedish Obese Subjects, 1987-2001 | Age of 37-60 years and BMI for males of ≥34 and females of ≥38 | Earlier gastric/duodenal ulcer surgery; earlier bariatric surgery; gastric ulcer/MI in the past six months; ongoing/active malignancy in the past five years; bulimic, drug/alcohol, psychiatric, or cooperative problems contraindicating bariatric surgery; and other contraindicating conditions, such as continuous glucocorticoid or anti-inflammatory treatment | 2003 | 2030 | 47.2 ± 5.9 | 48.7 ± 6.3 | 42.4 ± 4.5 | 40.1 ± 4.7 | 22 years | Incident HF | Not specified |
| 31 | Liakopoulos et al., 2020 [44] | Retrospective observational cohort | Sweden | Gastric bypass | National Diabetes Register and the Scandinavian Obesity Surgery Register, 2007-2015 | Age of 18-75 years and DM2 | Not specified | 5321 | 5321 | 49 ± 9.5 | 47.1 ± 11.5 | 42 ± 5.7 | 40.9 ± 7.3 | Surg: 4.7 years; con: 4.6 years | Incident renal disease | CV diagnoses, heart failure, and mortality |
| 32 | Höskuldsdóttir et al., 2021 [45] | Nationwide, matched, observational cohort study | Sweden | RYGB (100%) | National Diabetes Register and Scandinavian Obesity Surgery Registry, 2007-2013 | Age of 18-65 years, BMI of >27.5, and DM2 | Procedures other than RYGB | 5321 | 5321 | 48.96 ± 9.50 | 47.14 ± 11.49 | 42.03 ± 5.65 | 40.95 ± 7.30 | 4.5 years | Hospitalization for HF and/or AF and mortality in patients with preexisting HF | Not specified |
| 33 | Jamaly et al., 2016 [46] | Prospective matched cohort | Sweden | Vertical banded gastroplasty (68%), AGB (19%), and RYGB (13%) | The Swedish Obese Subjects, 1987-2001 | Age of 37-60 years, BMI for males of ≥34 and females of ≥38 | H/o AF at baseline, gastric surgery, ongoing malignancy, recent myocardial infarction, a bulimic eating pattern, alcohol/drug abuse, or psychiatric problems likely to impair study compliance | 2000 | 2021 | 47.2 ± 5.9 | 48.6 ± 6.2 | 42.4 ± 4.5 | 40.1 ± 4.7 | 19 years | Incident AF | Not specified |
| 34 | Lynch et al., 2019 [47] | Retrospective cohort | USA | RYGB or SG (% NA) | Single Virginia Academic Hospital, 1985-2015 | Age of >18 years | Banded gastroplasty pts and preexisting AF | 2522 | 2522 | 42 | 42 | 47.1 | 47.7 | Surg: 6.2 years; con: 8.0 years | Incident AF | Not specified |
| 35 | Moussa et al., 2021 [48] | Retrospective cohort | UK | NA | UK Clinical Practice Research Datalink, 2021 | Not specified other than BS | Had primary event before enrollment | 4212 | 4212 | 50 | 51 | 40.4 | 40.5 | 11.4 years | Cerebrovascular event | Ischemic events, hemorrhagic events, individual components of the primary end point alone, and all-cause mortality |
| 36 | MacDonald Jr et al., 1997 [49] | Retrospective cohort | USA | RYGB (100%) | Obesity Research Program at East Carolina University, 1979-1994 | Non-insulin-dependent DM2 | No non-insulin-dependent DM2, no morbid obesity, and age of >64 years | 154 | 78 | 41.9 | 43.5 | 50.6 | 48.8 | Surg: 9 years; con: 6.2 years | All-cause mortality | Not specified |
| 37 | Christou et al., 2004 [50] | Observational two-cohort study | Canada | RYGB (79.2%), vertical banded gastroplasty (18.7%), and laparoscopic RY isolated gastric bypass (2.2%) | McGill University Health Centre between 1986 and 2002 | Not specified other than BS | Subjects with medical conditions (other than morbid obesity) at cohort inception into the study | 1035 | 5746 | 45.1 ± 11.6 | 46.7 ± 13.1 | NA | NA | Surg: 2.5 years; con: 2.6 years | Long-term mortality, morbidity, and healthcare use | Not specified |
| 38 | Batsis et al., 2007 [51] | Population-based, historical cohort | USA | RYGB (100%) | Mayo Clinic medical record, the Mayo Surgical Index, and the Rochester Epidemiology Project (REP), 1990-2003 | Not specified other than RYGB | Missing data and BMI of <35 | 197 | 163 | 44.0 ± 9.9 | 43.4 ± 11.2 | 49.5 ± 8.9 | 44 ± 5.7 | 3.3 years | All-cause mortality, cardiovascular mortality, cardiovascular events, and combined cardiovascular events/all-cause mortality | Not specified |
| 39 | Adams et al., 2007 [52] | Retrospective cohort | USA | RYGB (100%) | Single Utah surgical practice, 1984-2002 | Not specified | Not specified | 7925 | 7925 | 39.5 ± 10.5 | 39.3 ± 10.6 | 45.3 ± 7.4 | 46.7 ± 6.3 | 7.1 years | Death from any cause | Death from various specific causes: all deaths caused by disease: CV disease (HF, CAD, stroke, and other CV), diabetes, cancer, other diseases. All non-disease causes: accident unrelated to drugs, poisoning of undetermined intent, suicide, and others |
| 40 | Davidson et al., 2016 [54] | Retrospective cohort | USA | RYGB (100%) | Private surgical practice, Utah, 1984-2002 | Not specified other than BS | Not specified | 7925 | 7925 | 39.5 ± 10.5 | 39.5 ± 10.6 | 45.3 ± 7.4 | 46.7 ± 6.3 | 7.2 years | All-cause and cause-specific mortality | Not specified |
| 41 | Lent et al., 2017 [55] | Retrospective observational cohort | USA | RYGB (100%) | A large comprehensive medical center, 2004-2015 | Age of 18-70 years, BMI of >40 kg/m2 (or >35 kg/m2 with comorbidity of DM, HTN, HLD, or OSA), active in the primary care system for an extended period of time (three or more office visits over >2-year period), no prior h/o bariatric surgery, and no diagnosis of serious mental health disorders or illegal drug use | Surgery other than RYGB | DM: 625; no DM: 1803 | DM: 625, no DM: 1803 | DM: 52.5 ± 9.4; no DM: 48.3 ± 11 | DM: 52.5 ± 9.4; no DM: 43.9 ± 11 | DM: 44.9 ± 6.0; no DM: 47.4 ± 6.4 | DM: 44.9 ± 6.1; no DM: 47.3 ± 6.4 | 5.8 years | All-cause mortality, stratified by “baseline” diabetes status | Cause-specific mortality, stratified by “baseline” diabetes status |
| 42 | Pontiroli et al., 2018 [56] | Retrospective cohort | Italy | LAGB (100%) | Italian National Health System Lumbardy database (LAGB10 study group), 1995-2001 | BMI of ≥40 or ≥35 with comorbidities and age of 18-65 years | Not specified | 154 | 360 | 41.0 ± 10.13 | 42.2 ± 12.94 | 42.7 ± 4.62 | 39.1 ± 5.27 | 19.5 ± 1.87 years | All-cause mortality | Not specified |
| 43 | Kauppila et al., 2019 [57] | Population-based cohort | Denmark, Finland, Iceland, Norway, and Sweden | Gastric bypass (73.4%), vertical banded gastroplasty (11%), gastric banding (10.9%), other restrictive procedures (3.2%), or blocking procedures (1.5%) | Nordic Obesity Surgery Cohort (NordOSCO) | Not specified other than BS | Not specified | 49977 | 494842 | NA | NA | >15 years | All-cause mortality | Mortality, specifically in the obesity-related morbidities, cardiovascular disease, diabetes, cancer, and suicide | ||
| 44 | Doumouras et al., 2020 [58] (RYGB) | Population-based matched cohort | Canada | RYGB (87%) and sleeve gastrectomy (13%) | The Ontario Bariatric Network (OBN), 2010-2016 | Not specified other than BS | Non-Ontario residents, age of ≥70 years, BMI of 35 kg/m2 or less, h/o cancer within two years, active substance use disorder, accessed palliative care, pregnancy as of the index date, previous solid organ transplantation, active cardiac disease or major revascularization procedure within six months of the index date, or severe liver disease with ascites within one year of the index date | 13679 | 13679 | 45.23 ± 10.89 | 45.49 ± 11.63 | 47.21 ± 8.01 | 46.70 ± 8.44 | Gen: 4.89 years; con: 4.84 years | All-cause mortality | Cause-specific mortality |
| 45 | Doumouras et al., 2020 [58] (SG) | Population-based matched cohort | Canada | RYGB (87%) and sleeve gastrectomy (13%) | The Ontario Bariatric Network (OBN), 2010-2016 | Not specified other than BS | Non-Ontario residents, age of ≥70 years, BMI of 35 kg/m2 or less, h/o cancer within two years, active substance use disorder, accessed palliative care, pregnancy as of the index date, previous solid organ transplantation, active cardiac disease or major revascularization procedure within six months of the index date, or severe liver disease with ascites within one year of the index date | 13679 | 13679 | 45.23 ± 10.89 | 45.49 ± 11.63 | 47.21 ± 8.01 | 46.70 ± 8.44 | Gen: 4.89 years; con: 4.84 years | All-cause mortality | Cause-specific mortality |
| 46 | Sheetz et al., 2020 [59] | Retrospective cohort | USA | Sleeve gastrectomy (45.1%), Roux-en-Y gastric bypass (41.6%), gastric banding (12.8%), or duodenal switch (0.4%) | US Renal Data System registry, 2006-2015 | Not specified other than BS | <18 years, similarly coded surgery for a diagnosis of malignancy, BMI of <35, or without a recorded BMI | 1597 | 4750 | 49.8 ± 11.2 | 51.7 ± 11.1 | 45.6 ± 6.7 | 44.6 ± 6.8 | 3 years | All-cause mortality at five years | Disease-specific mortality and incidence of kidney transplant |
| 47 | Courcoulas et al., 2021 [60] (RYGB) | Retrospective matched cohort | USA | SG (45%) and RYGB (55%) | Kaiser Permanente regions Washington and California, 2005-2015 | Age of 19-79 years and BMI of ≥35 | <1 year of enrollment, pregnancy, h/o cancer (except non-melanoma skin cancer, and missing BMI data/BMI of <35 | 4.9 years | All-cause mortality | CV, cancer, and diabetes-related health | ||||||
| 48 | Courcoulas et al., 2021 [60] (SG) | Retrospective matched cohort | USA | SG (45%) and RYGB (55%) | Kaiser Permanente regions Washington and California, 2005-2015 | Age of 19-79 years and BMI of ≥35 | <1 year of enrollment, pregnancy, and h/o cancer (except non-melanoma | 4.9 years | All-cause mortality | CV, cancer, and diabetes-related health | ||||||
| 49 | Doumouras et al., 2021 [61] | Retrospective matched cohort | Canada | RYGB (86.7%) and sleeve gastrectomy (13.3%) | Ontario ICES database, 2010-2016 | DM2 and BMI of ≥35 | Non-Ontario pts, BMI of <35, age of ≥70 years, h/o cancer within two years, active substance abuse, had accessed palliative care, pregnant, had previous solid organ transplantation, had active cardiac disease or major revascularization procedure within six months of index date, or had severe liver disease with ascites within one year of the index date | 3455 | 3455 | 51.66 ± 9.20 | 52.41 ± 9.67 | 45.29 ± 7.55 | 44.06 ± 8.25 | 4.6 years | All-cause mortality | Cause-specific mortality and nonfatal morbidities |
Using the Newcastle-Ottawa Scale (NOS), studies were assessed for quality, of which all studies had at least a score of 7 and none were excluded. The quality assessment of the studies can be found in Table 3.
Table 3. Quality assessment of studies using the Newcastle-Ottawa Scale (NOS).
AHRQ: Agency for Healthcare Research and Quality
| Study name | Study type | Selection | Comparability | Exposure | Total score | AHRQ standards |
| Bouchard et al., 2022 [13] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Carlsson et al., 2020 [14] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Fisher et al., 2018 [16] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Alkharaiji et al., 2019 [17] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Aminian et al., 2019 [18] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Singh et al., 2020 [19] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Ardissino et al., 2021 [20] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Rassen et al., 2021 [21] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Sampalis et al., 2006 [22] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Sjöström et al., 2007 [23] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Romeo et al., 2012 [24] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Sjöström et al., 2012 [25] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Johnson et al., 2013 [26] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Douglas et al., 2015 [27] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Eliasson et al., 2015 [28] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Benotti et al., 2017 [29] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Brown et al., 2020 [30] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Michaels et al., 2020 [31] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Moussa et al., 2020 [32] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Stenberg et al., 2020 [33] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Wong et al., 2021 [34] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Höskuldsdóttir et al., 2020 [35] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Dash et al., 2021 [36] | Cohort | 2 | 2 | 3 | 7 | Good quality |
| Hung et al., 2021 [37] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Lundberg et al., 2021 [38] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Yuan et al., 2021 [39] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Mentias et al., 2022 [40] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Persson et al., 2017 [41] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Sundström et al., 2017 [42] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Jamaly et al., 2019 [43] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Liakopoulos et al., 2020 [44] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Höskuldsdóttir et al., 2021 [45] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Jamaly et al., 2016 [46] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Lynch et al., 2019 [47] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Moussa et al., 2021 [48] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Macdonald Jr et al., 1997 [49] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Christou et al., 2004 [50] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Batsis et al., 2007 [51] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Adams et al., 2007 [52] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Davidson et al., 2016 [54] | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Lent et al., 2017 [55] | Cohort | 4 | 2 | 3 | 9 | Good quality |
| Pontiroli et al., 2018 [56] | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Kauppila et al., 2019 [57] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Doumouras et al., 2020 [58] (RYGB) | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Doumouras et al., 2020 [58] (SG) | Cohort | 4 | 2 | 2 | 8 | Good quality |
| Sheetz et al., 2020 [59] | Cohort | 3 | 2 | 3 | 8 | Good quality |
| Courcoulas et al., 2021 [60] (RYGB) | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Courcoulas et al., 2021 [60] (SG) | Cohort | 3 | 2 | 2 | 7 | Good quality |
| Doumouras et al., 2021 [61] | Cohort | 4 | 2 | 3 | 9 | Good quality |
Effect on Coronary Artery Disease
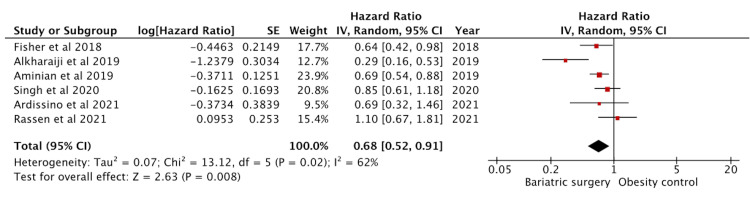
Seven studies reported effects on CAD, of which six had adjusted HR ratio data and were included in the analysis (Figure 2). One study by Bouchard et al. [13] reported a combined HR for both CAD and MI. Since individual data were not available, it was excluded from the analysis to avoid duplication of data and bias. Of the included studies, there were 17423 bariatric surgery patients and 43507 controls. The effect on CAD was significant with a pooled HR of 0.68 (95% CI: 0.52-0.91) (p = 0.008).
Figure 2. Forest plot with the included studies and the pooled hazard ratio for coronary artery disease.
CI: confidence interval
Effect on Myocardial Infarction
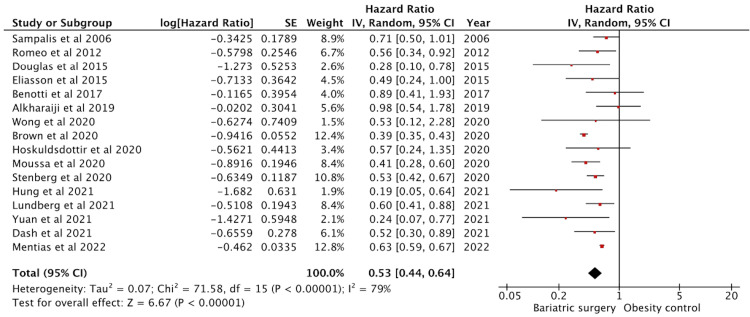
Twenty-two studies reported myocardial infarction outcomes. Sixteen studies had adjusted HR data and were included in the analysis (Figure 3). These studies had 231503 patients in the intervention group and 487727 in the control group. The effect on MI was significant with a pooled HR of 0.53 (95% CI: 0.44-0.64) (p < 0.01). The studies showed high heterogeneity with an I2 = 79%.
Figure 3. Forest plot with the included studies and the pooled hazard ratio for myocardial infarction.
CI: confidence interval
Like previously mentioned, Bouchard et al. [13] reported a combined incidence and hence was excluded. Johnson et al. [26], Sjöström et al. [23,25], Michaels et al. [31], and Ardissino et al. [20] provided only the incidence data and were not included in the analysis. Naslund et al. [62] studied the outcomes in patients with preexisting MI and was excluded.
Effect on Heart Failure
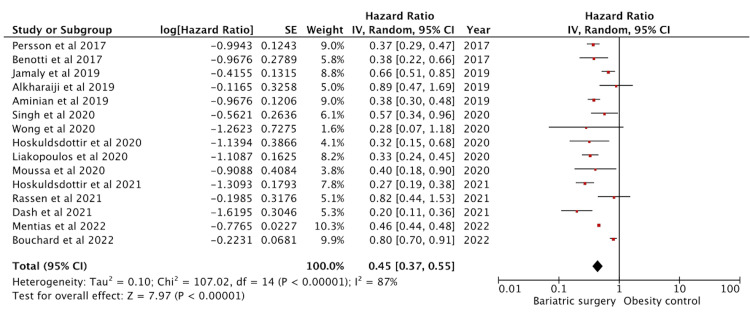
Eighteen studies reported heart failure outcomes. Fifteen studies had adjusted HR data and were included in the analysis (Figure 4). These studies amounted to a sample size of 180961 in the intervention group and 202891 in the control group. The effect on heart failure was significant with a pooled HR of 0.45 (95% CI: 0.37-0.55) (p < 0.01). The studies showed high heterogeneity with an I2 = 87%. Sundström et al. [42], Johnson et al. [26], and Sjöström et al. [23] had only provided relative risk data and were excluded from the analysis.
Figure 4. Forest plot with the included studies and the pooled hazard ratio for heart failure.
CI: confidence interval
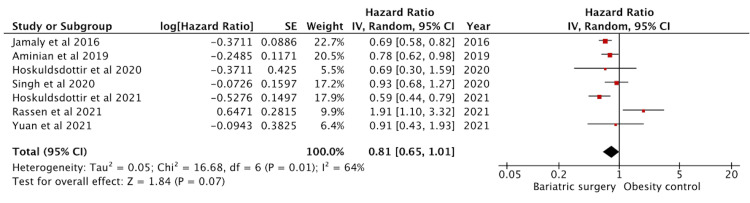
Effect on Atrial Fibrillation
Eight studies reported atrial fibrillation outcomes. Seven had adjusted HR data and were included in the analysis (Figure 5). These studies amounted to a sample size of 18309 in the intervention group and 32933 in the control group. The effect on atrial fibrillation was not significant with a pooled HR of 0.81 (95% CI: 0.65-1.01) (p = 0.07). Lynch et al. [47] provided relative risk data only and hence was excluded.
Figure 5. Forest plot with the included studies and the pooled hazard ratio for atrial fibrillation.
CI: confidence interval
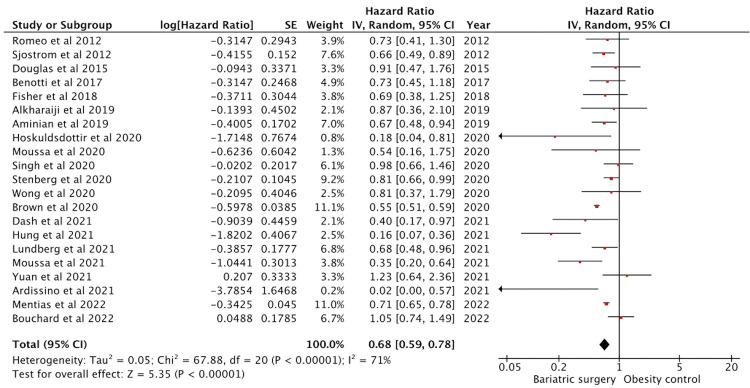
Effect on Cerebrovascular Accident
Twenty-three studies reported cerebrovascular accident (CVA) outcomes. Twenty-one studies had adjusted HR data and were included in the analysis (Figure 6). These studies amounted to a sample size of 238472 subjects and 513848 controls. The effect on CVA was significant with a pooled HR of 0.68 (95% CI: 0.59-0.78) (p < 0.01). The studies showed moderate heterogeneity with an I2 = 72%.
Figure 6. Forest plot with the included studies and the pooled hazard ratio for cerebrovascular accident.
CI: confidence interval
Johnson et al. [26] and Sjöström et al. [23] reported data for relative risks only and were excluded. Some studies for CVA reported ischemic outcomes (transient ischemic attack and ischemic stroke) and hemorrhagic outcomes (hemorrhagic stroke and intraparenchymal hemorrhage) separately. We have not made differentiation between the entities and have reported it as a composite CVA outcome.
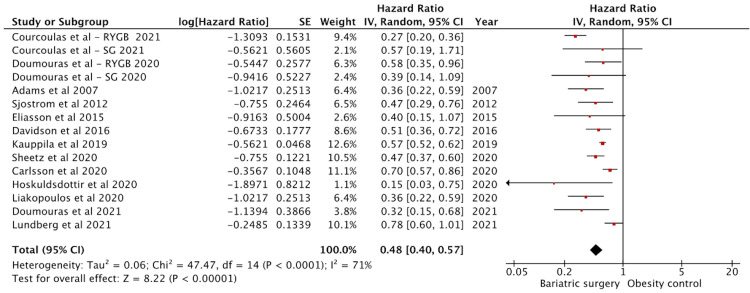
Effect on Cardiovascular Mortality
Twenty-six studies reported cardiovascular disease-specific mortality. Fifteen studies had adjusted HR data and were included in the analysis (Figure 7). There were 157750 in the surgery group and 643770 in the control groups. The effect on cardiovascular disease (CVD) mortality was significant with a pooled HR of 0.48 (95% CI: 0.40-0.57) (p < 0.01). The studies showed high heterogeneity with an I2 = 71%.
Figure 7. Forest plot with the included studies and the pooled hazard ratio for cardiovascular mortality.
CI: confidence interval
Pontiroli et al. [53,56], Höskuldsdóttir et al. [35,45], Stenberg et al. [33], Sjöström et al. [23], MacDonald Jr et al. [49], Lent et al. [55], Hung et al. [37], Batsis et al. [51], Johnson et al. [26], and Christou et al. [50] had insufficient data for hazard ratio and were excluded. Courcoulas et al. [60] and Doumouras et al. [58] studied CVD data separately on sleeve gastrectomy and RYGB. Hence, they were included as separate outcomes.
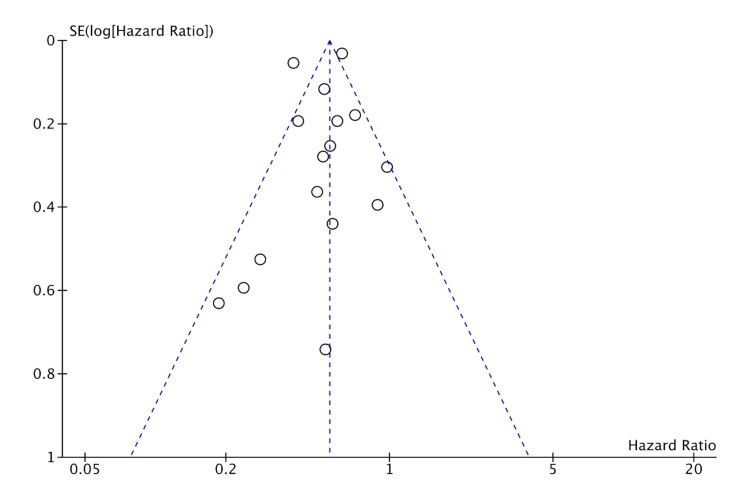
Publication bias
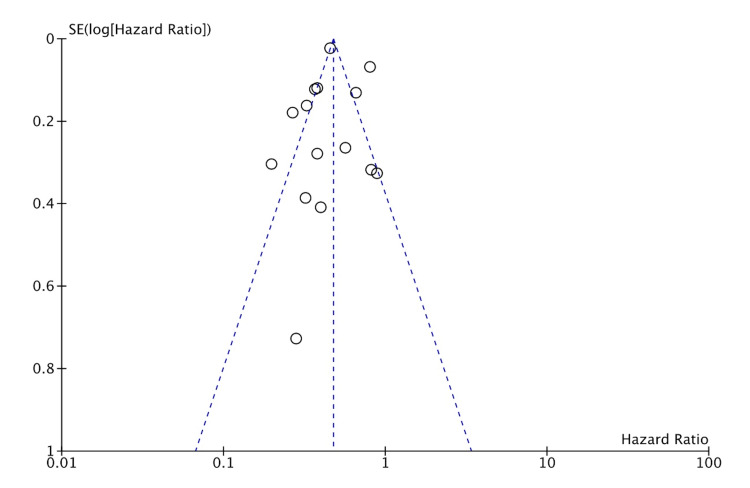
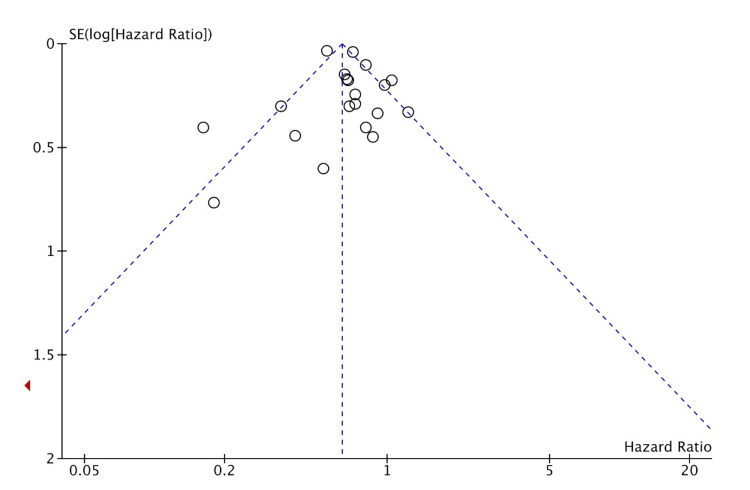
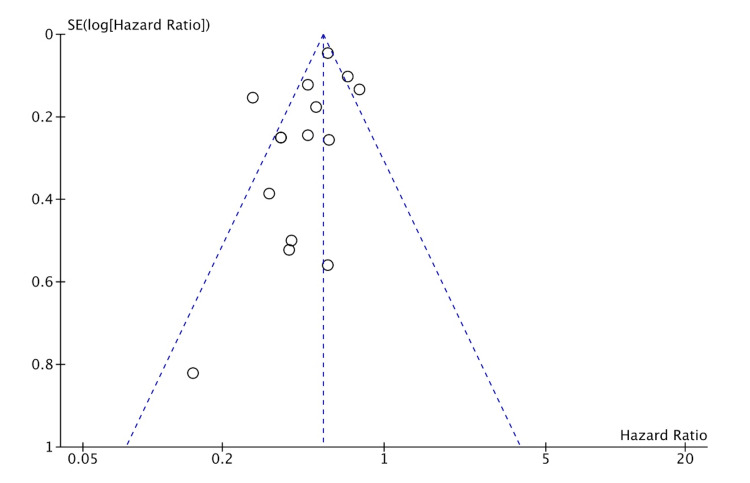
Publication bias was assessed for MI, HF, CVA, and CVD. The studies included had a moderate-to-high amount of heterogeneity. This is likely from many smaller studies included leading to effect size variation. This is suggestive of likely publication bias in favor of positive studies. But the funnel plots (shown in Figures 8-11) show the studies being symmetrically scattered around the midline. This is in concordance with the inverted funnel appearance reassuring that there is no publication bias [63].
Figure 8. Funnel plot depicting symmetrical distribution for myocardial infarction.
Figure 9. Funnel plot depicting symmetrical distribution for heart failure.
Figure 10. Funnel plot depicting symmetrical distribution for cerebrovascular accident.
Figure 11. Funnel plot depicting symmetrical distribution for cardiovascular mortality.
Discussion
In this updated meta-analysis, we analyzed six major long-term cardiovascular outcomes post-bariatric surgery. Five outcomes including CAD, MI, HF, CVA, and CVD mortality showed a significant risk reduction, whereas atrial fibrillation showed a non-significant risk reduction.
Bariatric Surgery and Atherosclerotic Disease
Obesity poses a high risk for atheroma formation [2]. Bariatric surgery provides a beneficial effect by altering molecular mechanisms involving inflammation. Bariatric surgery decreases the levels of oxidative stress and inflammatory markers [64]. It reduces circulating levels of adhesion molecules and improves endothelium-dependent vasodilatory response [65]. Objectively, several studies have shown that surgery reduces carotid intimal wall thickness in concordance with weight loss [66]. These processes in turn contribute to the risk reduction of atherosclerotic diseases such as CAD, MI, and CVA.
Although CAD and MI are atherosclerotic processes, they differ in their pathophysiology and clinical manifestations. CAD is defined as the presence of atherosclerotic plaque within the epicardial coronary arteries. Over time, risk factors potentiate plaque growth. During periods of myocardial oxygen demand, there is endothelial dysfunction causing plaque rupture. This in turn leads to atherothrombosis, vessel occlusion, and myocardial infarction [67]. Of significance, there was a 29.3% cumulative decrease in MI-related inpatient deaths and 3.6% cumulative increase in CAD-related inpatient deaths from 2001 to 2014 [68]. It is important to differentiate MI and CAD, as bariatric surgery is protective against both MI and CAD. Hence, we have studied the effects separately.
The pooled HR for CAD in our study was 0.68 (95% CI: 0.52-0.91). Currently, there are no prior meta-analysis exhibiting the association between bariatric surgery and CAD. The pooled HR for MI in our meta-analysis was 0.53 (95% CI: 0.44-0.64) from 16 studies. This is in concordance with previous studies. Kwok et al. reported a pooled OR of 0.46 (95% CI: 0.30-0.69) from four studies [69]. A more recent analysis by van Veldhuisen et al. reported a pooled HR of 0.58 (95% CI: 0.43-0.76) from seven studies [70]. The pooled HR for composite CVA in our meta-analysis was 0.68 (95% CI: 0.59-0.78) from 21 studies. Kwok et al. reported a similar pooled OR of 0.49 (95% CI: 0.32-0.75) from four studies [69].
Bariatric Surgery and Heart Failure
Bariatric surgery counteracts the effects of obesity on the heart, as described previously. Although there are no randomized controlled trials to show this effect on heart failure, few observational studies have been conducted. The mechanism by which this occurs could be multifactorial. Bariatric surgery reduces heart failure risk factors including hypertension, hyperlipidemia, and diabetes [51]. It also directly acts on the myocardium causing changes in the left ventricle (LV) wall and ejection fraction (EF) percentage. Vest et al. showed that bariatric surgery improved left ventricular systolic dysfunction and resulted in a statistically significant improvement in left ventricle ejection fraction (LVEF) [71]. Another study showed a 43% reduction in left ventricular mass with subsequent reduction in left atrial and right ventricular wall diameter and epicardial fat [72]. A meta-analysis done by Cuspidi et al. showed significant changes in LV thickness, improvement in LV diastolic function, and a decrease in left atrial diameter [73]. Cuspidi et al. also showed no significant improvement of EF percentage [73]. The pooled HR for HF in our study was 0.45 (95% CI: 0.37-0.55) from 15 studies. This is consistent with a prior similar meta-analysis [70,74].
Bariatric Surgery and Cardiovascular Mortality
Scandinavian countries have the most comprehensive obesity registries with a long-term follow-up [14,25,35,43,57]. The data from these have provided significant insight into the long-term outcomes after bariatric surgeries. Carlsson et al. followed 2007 patients over a mean of 24 years and found 457 deaths, of which 167 were from cardiovascular causes, the most common cardiovascular cause of death being myocardial infarction, heart failure, and sudden death [14]. Kauppila et al. reported from the Nordic population. Among 49977 patients that underwent bariatric surgery, there were 525 cardiovascular deaths with patients followed up to >15 years [57]. Sjöström et al. studied 2010 subjects with a mean follow-up of 14.7 years, encountering 28 cardiovascular deaths [25].
In our analysis, the pooled HR for CVD mortality was 0.48 (95% CI: 0.40-0.57) involving 15 studies. Our study has the largest pooled data with respect to cardiovascular mortality data to date. Wiggins et al. reported an OR of 0.50 (95% CI: 0.39-0.71) from three studies [75].
Given the significant cardiovascular benefits offered by bariatric surgery, the referral from primary care physicians has been lower. This could be attributed to knowledge gaps, hesitancy, or concerns regarding postoperative care. A recent Canadian survey showed that more than 50% of physician respondents did not feel equipped to counsel the patients on surgical options. And only 11.6% of the obese patients were being counselled [76]. In a Swedish survey, interestingly, 84% of respondents stated that the patients themselves initiated bariatric surgery referral [77]. Physician’s knowledge showed a positive correlation toward referral and management of postoperative issues [77]. This brings into perspective that education and awareness would lead to better patient sampling, thereby cumulatively improving cardiovascular outcomes.
Limitations
Firstly, the studies included are all nonrandomized cohort studies, which could involve selection and publication biases. Henceforth, longer randomized controlled trials are required. Secondly, most of the outcomes had high heterogeneity, which could be owed to the many smaller studies that were included. Thirdly, some studies had non-generalizable populations such as type 1 diabetes or type 2 diabetes specifically. However, we omitted populations that had cardiovascular diseases at baseline. Fourthly, only English studies were included owing to the ease of interpretation and analysis. Lastly, we failed to study the HR specific to each bariatric surgery, likely due to the scarcity of data for a pooled analysis.
Conclusions
Although the management of obesity requires a multimodal approach, recognizing the necessity for bariatric surgery early in the disease course is important. Both the physician and the patients should be aware of the treatment strategies to make a well-informed decision. Our study is an updated meta-analysis highlighting the consistency with the prior data. We included additional studies to provide more comprehensive data on six major cardiovascular outcomes. In conclusion, bariatric surgery showed a statistically significant risk reduction with CAD, MI, HF, CVA, and cardiovascular disease-specific mortality and a non-significant risk reduction of atrial fibrillation. However, these data are inclusive of RYGB, SG, and laparoscopic banding. Further research needs to be conducted to determine if these individual procedures have better overall outcomes than one another.
Acknowledgments
HC and SM formulated the idea and designed the research study. HC, TG, and NK collected the data and tabulated the findings. HC and AZ performed the statistical analysis. HC and SGM wrote the manuscript, and SGM assisted in editing the manuscript. All authors read and approved the final manuscript.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Comorbidity of overweight and obesity in a nationally representative sample of German adults aged 18-79 years. Schienkiewitz A, Mensink GB, Scheidt-Nave C. BMC Public Health. 2012;12:658. doi: 10.1186/1471-2458-12-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Powell-Wiley TM, Poirier P, Burke LE, et al. Circulation. 2021;143:0–1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevalence of obesity and severe obesity among adults: United States, 2017-2018. Hales CM, Carroll MD, Fryar CD, Ogden CL. https://pubmed.ncbi.nlm.nih.gov/32487284/ NCHS Data Brief. 2020:1–8. [PubMed] [Google Scholar]
- 4.2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Jensen MD, Ryan DH, Apovian CM, et al. Circulation. 2014;129:0–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Racism, segregation, and risk of obesity in the Black Women's Health Study. Cozier YC, Yu J, Coogan PF, Bethea TN, Rosenberg L, Palmer JR. Am J Epidemiol. 2014;179:875–883. doi: 10.1093/aje/kwu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Associations between obesity, obesogenic environments, and structural racism vary by county-level racial composition. Bell CN, Kerr J, Young JL. Int J Environ Res Public Health. 2019;16:861. doi: 10.3390/ijerph16050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health effects of overweight and obesity in 195 countries over 25 years. Afshin A, Forouzanfar MH, Reitsma MB, et al. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 9.The impact of obesity on the cardiovascular system. Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, Somodi S. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Relation of regional fat distribution to left ventricular structure and function. Neeland IJ, Gupta S, Ayers CR, et al. Circ Cardiovasc Imaging. 2013;6:800–807. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Benefits and risks of bariatric surgery in adults: a review. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. JAMA. 2020;324:879–887. doi: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 13.Long-term impact of bariatric surgery on major adverse cardiovascular events in patients with obesity, diabetes and hypertension: a population-level study. Bouchard P, Al-Masrouri S, Demyttenaere S, Court O, Andalib A. Obes Surg. 2022;32:771–778. doi: 10.1007/s11695-021-05849-1. [DOI] [PubMed] [Google Scholar]
- 14.Life expectancy after bariatric surgery in the Swedish obese subjects study. Carlsson LM, Sjöholm K, Jacobson P, et al. N Engl J Med. 2020;383:1535–1543. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. Rev Esp Cardiol (Engl Ed) 2021;74:790–799. doi: 10.1016/j.rec.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. Fisher DP, Johnson E, Haneuse S, et al. JAMA. 2018;320:1570–1582. doi: 10.1001/jama.2018.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effect of bariatric surgery on cardiovascular events and metabolic outcomes in obese patients with insulin-treated type 2 diabetes: a retrospective cohort study. Alkharaiji M, Anyanwagu U, Donnelly R, Idris I. Obes Surg. 2019;29:3154–3164. doi: 10.1007/s11695-019-03809-4. [DOI] [PubMed] [Google Scholar]
- 18.Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. Aminian A, Zajichek A, Arterburn DE, et al. JAMA. 2019;322:1271–1282. doi: 10.1001/jama.2019.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impact of bariatric surgery on cardiovascular outcomes and mortality: a population-based cohort study. Singh P, Subramanian A, Adderley N, et al. Br J Surg. 2020;107:432–442. doi: 10.1002/bjs.11433. [DOI] [PubMed] [Google Scholar]
- 20.Atherosclerotic disease burden after bariatric surgery in patients with obesity and type 2 diabetes. Ardissino M, Watson F, Amin R, Collins P, Moussa O, Purkayastha S. J Diabetes. 2021;13:640–647. doi: 10.1111/1753-0407.13151. [DOI] [PubMed] [Google Scholar]
- 21.Real-world evidence of bariatric surgery and cardiovascular benefits using electronic health records data: a lesson in bias. Rassen JA, Murk W, Schneeweiss S. Diabetes Obes Metab. 2021;23:1453–1462. doi: 10.1111/dom.14338. [DOI] [PubMed] [Google Scholar]
- 22.Impact of bariatric surgery on cardiovascular and musculoskeletal morbidity. Sampalis JS, Sampalis F, Christou N. Surg Obes Relat Dis. 2006;2:587–591. doi: 10.1016/j.soard.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Effects of bariatric surgery on mortality in Swedish obese subjects. Sjöström L, Narbro K, Sjöström CD, et al. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 24.Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Romeo S, Maglio C, Burza MA, et al. Diabetes Care. 2012;35:2613–2617. doi: 10.2337/dc12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bariatric surgery and long-term cardiovascular events. Sjöström L, Peltonen M, Jacobson P, et al. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 26.Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. Johnson BL, Blackhurst DW, Latham BB, et al. J Am Coll Surg. 2013;216:545–556. doi: 10.1016/j.jamcollsurg.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. PLoS Med. 2015;12:0. doi: 10.1371/journal.pmed.1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson AM, Ottosson J, Gudbjörnsdottir S. Lancet Diabetes Endocrinol. 2015;3:847–854. doi: 10.1016/S2213-8587(15)00334-4. [DOI] [PubMed] [Google Scholar]
- 29.Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. Benotti PN, Wood GC, Carey DJ, et al. J Am Heart Assoc. 2017;6:0. doi: 10.1161/JAHA.116.005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bariatric surgery lowers the risk of major cardiovascular events. Brown AM, Yang J, Zhang X, Docimo S, Pryo AD, Spaniolas K. Ann Surg. 2022;276:0–24. doi: 10.1097/SLA.0000000000004640. [DOI] [PubMed] [Google Scholar]
- 31.Bariatric surgery reduces long-term rates of cardiac events and need for coronary revascularization: a propensity-matched analysis. Michaels AD, Mehaffey JH, Hawkins RB, Kern JA, Schirmer BD, Hallowell PT. Surg Endosc. 2020;34:2638–2643. doi: 10.1007/s00464-019-07036-x. [DOI] [PubMed] [Google Scholar]
- 32.Effect of bariatric surgery on long-term cardiovascular outcomes: a nationwide nested cohort study. Moussa O, Ardissino M, Heaton T, et al. Eur Heart J. 2020;41:2660–2667. doi: 10.1093/eurheartj/ehaa069. [DOI] [PubMed] [Google Scholar]
- 33.Association between metabolic surgery and cardiovascular outcome in patients with hypertension: a nationwide matched cohort study. Stenberg E, Cao Y, Marsk R, Sundbom M, Jernberg T, Näslund E. PLoS Med. 2020;17:0. doi: 10.1371/journal.pmed.1003307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Effects of bariatric surgery on kidney diseases, cardiovascular diseases, mortality and severe hypoglycaemia among patients with type 2 diabetes mellitus. Wong CK, Wu T, Wong SK, et al. Nephrol Dial Transplant. 2021;36:1440–1451. doi: 10.1093/ndt/gfaa075. [DOI] [PubMed] [Google Scholar]
- 35.Potential benefits and harms of gastric bypass surgery in obese individuals with type 1 diabetes: a nationwide, matched, observational cohort study. Höskuldsdóttir G, Ekelund J, Miftaraj M, et al. Diabetes Care. 2020;43:3079–3085. doi: 10.2337/dc20-0388. [DOI] [PubMed] [Google Scholar]
- 36.Cardiorenal outcomes in eligible patients referred for bariatric surgery. Dash S, Everett K, Jackson T, et al. Obesity (Silver Spring) 2021;29:2035–2043. doi: 10.1002/oby.23294. [DOI] [PubMed] [Google Scholar]
- 37.The long-term risk of cardiovascular events in patients following bariatric surgery compared to a non-surgical population with obesity and the general population: a comprehensive national cohort study. Hung SL, Chen CY, Chin WL, Lee CH, Chen JH. Langenbecks Arch Surg. 2021;406:189–196. doi: 10.1007/s00423-020-02027-2. [DOI] [PubMed] [Google Scholar]
- 38.Risk of myocardial infarction, ischemic stroke, and mortality in patients who undergo gastric bypass for obesity compared with non-operated obese patients and population controls. Lundberg CE, Björck L, Adiels M, Lagergren J, Rosengren A. Ann Surg. 2021;277:275–283. doi: 10.1097/SLA.0000000000005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The long-term impact of bariatric surgery on development of atrial fibrillation and cardiovascular events in obese patients: an historical cohort study. Yuan H, Medina-Inojosa JR, Lopez-Jimenez F, et al. Front Cardiovasc Med. 2021;8:647118. doi: 10.3389/fcvm.2021.647118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long-term cardiovascular outcomes after bariatric surgery in the Medicare population. Mentias A, Aminian A, Youssef D, et al. J Am Coll Cardiol. 2022;79:1429–1437. doi: 10.1016/j.jacc.2022.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Risk of heart failure in obese patients with and without bariatric surgery in Sweden-a registry-based study. Persson CE, Björck L, Lagergren J, Lappas G, Giang KW, Rosengren A. J Card Fail. 2017;23:530–537. doi: 10.1016/j.cardfail.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Circulation. 2017;135:1577–1585. doi: 10.1161/CIRCULATIONAHA.116.025629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surgical obesity treatment and the risk of heart failure. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K. Eur Heart J. 2019;40:2131–2138. doi: 10.1093/eurheartj/ehz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renal and cardiovascular outcomes after weight loss from gastric bypass surgery in type 2 diabetes: cardiorenal risk reductions exceed atherosclerotic benefits. Liakopoulos V, Franzén S, Svensson AM, et al. Diabetes Care. 2020;43:1276–1284. doi: 10.2337/dc19-1703. [DOI] [PubMed] [Google Scholar]
- 45.Potential effects of bariatric surgery on the incidence of heart failure and atrial fibrillation in patients with type 2 diabetes mellitus and obesity and on mortality in patients with preexisting heart failure: a nationwide, matched, observational cohort study. Höskuldsdóttir G, Sattar N, Miftaraj M, et al. J Am Heart Assoc. 2021;10:0. doi: 10.1161/JAHA.120.019323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjöström L, Karason K. J Am Coll Cardiol. 2016;68:2497–2504. doi: 10.1016/j.jacc.2016.09.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bariatric surgery reduces incidence of atrial fibrillation: a propensity score-matched analysis. Lynch KT, Mehaffey JH, Hawkins RB, Hassinger TE, Hallowell PT, Kirby JL. Surg Obes Relat Dis. 2019;15:279–285. doi: 10.1016/j.soard.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Long-term cerebrovascular outcomes after bariatric surgery: a nationwide cohort study. Moussa O, Ardissino M, Tang A, et al. Clin Neurol Neurosurg. 2021;203:106560. doi: 10.1016/j.clineuro.2021.106560. [DOI] [PubMed] [Google Scholar]
- 49.The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. MacDonald KG Jr, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ. J Gastrointest Surg. 1997;1:213–220. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- 50.Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Brekke L, Lopez-Jimenez F. Obesity (Silver Spring) 2007;15:772–784. doi: 10.1038/oby.2007.589. [DOI] [PubMed] [Google Scholar]
- 52.Long-term mortality after gastric bypass surgery. Adams TD, Gress RE, Smith SC, et al. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 53.Long-term mortality and incidence of cardiovascular diseases and type 2 diabetes in diabetic and nondiabetic obese patients undergoing gastric banding: a controlled study. Pontiroli AE, Zakaria AS, Mantegazza E, Morabito A, Saibene A, Mozzi E, Micheletto G. Cardiovasc Diabetol. 2016;15:39. doi: 10.1186/s12933-016-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Association of patient age at gastric bypass surgery with long-term all-cause and cause-specific mortality. Davidson LE, Adams TD, Kim J, et al. JAMA Surg. 2016;151:631–637. doi: 10.1001/jamasurg.2015.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.All-cause and specific-cause mortality risk after Roux-en-Y gastric bypass in patients with and without diabetes. Lent MR, Benotti PN, Mirshahi T, et al. Diabetes Care. 2017;40:1379–1385. doi: 10.2337/dc17-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Pontiroli AE, Zakaria AS, Fanchini M, et al. Cardiovasc Diabetol. 2018;17:161. doi: 10.1186/s12933-018-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Effects of obesity surgery on overall and disease-specific mortality in a 5-country population-based study. Kauppila JH, Tao W, Santoni G, et al. Gastroenterology. 2019;157:119–127. doi: 10.1053/j.gastro.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 58.A longitudinal analysis of wait times for bariatric surgery in a publicly funded, regionalized bariatric care system. Doumouras AG, Albacete S, Mann A, Gmora S, Anvari M, Hong D. Obes Surg. 2020;30:961–968. doi: 10.1007/s11695-019-04259-8. [DOI] [PubMed] [Google Scholar]
- 59.Bariatric surgery and long-term survival in patients with obesity and end-stage kidney disease. Sheetz KH, Gerhardinger L, Dimick JB, Waits SA. JAMA Surg. 2020;155:581–588. doi: 10.1001/jamasurg.2020.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reduction in long-term mortality after sleeve gastrectomy and gastric bypass compared to non-surgical patients with severe obesity. Courcoulas AP, Johnson E, Arterburn DE, et al. Ann Surg. 2021 doi: 10.1097/SLA.0000000000005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Association between bariatric surgery and major adverse diabetes outcomes in patients with diabetes and obesity. Doumouras AG, Lee Y, Paterson JM, et al. JAMA Netw Open. 2021;4:0. doi: 10.1001/jamanetworkopen.2021.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Association of metabolic surgery with major adverse cardiovascular outcomes in patients with previous myocardial infarction and severe obesity: a nationwide cohort study. Näslund E, Stenberg E, Hofmann R, et al. Circulation. 2021;143:1458–1467. doi: 10.1161/CIRCULATIONAHA.120.048585. [DOI] [PubMed] [Google Scholar]
- 63.Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Sterne JA, Sutton AJ, Ioannidis JP, et al. BMJ. 2011;343:0. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 64.Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N. Obes Surg. 2004;14:659–665. doi: 10.1381/096089204323093453. [DOI] [PubMed] [Google Scholar]
- 65.Body weight and glucose metabolism have a different effect on circulating levels of ICAM-1, E-selectin, and endothelin-1 in humans. Pontiroli AE, Pizzocri P, Koprivec D, et al. Eur J Endocrinol. 2004;150:195–200. doi: 10.1530/eje.0.1500195. [DOI] [PubMed] [Google Scholar]
- 66.Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: a meta-analysis of literature studies. Lupoli R, Di Minno MN, Guidone C, Cefalo C, Capaldo B, Riccardi G, Mingrone G. Int J Obes (Lond) 2016;40:395–402. doi: 10.1038/ijo.2015.187. [DOI] [PubMed] [Google Scholar]
- 67.Current approach to the diagnosis of atherosclerotic coronary artery disease: more questions than answers. Nelson AJ, Ardissino M, Psaltis PJ. Ther Adv Chronic Dis. 2019;10:2040622319884819. doi: 10.1177/2040622319884819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Healthcare Cost and Utilization Project (HCUP) statistical briefs [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 69.Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Kwok CS, Pradhan A, Khan MA, et al. Int J Cardiol. 2014;173:20–28. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. van Veldhuisen SL, Gorter TM, van Woerden G, de Boer RA, Rienstra M, Hazebroek EJ, van Veldhuisen DJ. Eur Heart J. 2022;43:1955–1969. doi: 10.1093/eurheartj/ehac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clinical and echocardiographic outcomes after bariatric surgery in obese patients with left ventricular systolic dysfunction. Vest AR, Patel P, Schauer PR, Satava ME, Cavalcante JL, Brethauer S, Young JB. Circ Heart Fail. 2016;9:0. doi: 10.1161/CIRCHEARTFAILURE.115.002260. [DOI] [PubMed] [Google Scholar]
- 72.The early reduction of left ventricular mass after sleeve gastrectomy depends on the fall of branched-chain amino acid circulating levels. Castagneto-Gissey L, Angelini G, Mingrone G, et al. EBioMedicine. 2022;76:103864. doi: 10.1016/j.ebiom.2022.103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis. Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G. Am J Hypertens. 2014;27:146–156. doi: 10.1093/ajh/hpt215. [DOI] [PubMed] [Google Scholar]
- 74.Bariatric surgery among patients with heart failure: a systematic review and meta-analysis. Berger S, Meyre P, Blum S, Aeschbacher S, Ruegg M, Briel M, Conen D. Open Heart. 2018;5:0. doi: 10.1136/openhrt-2018-000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. PLoS Med. 2020;17:0. doi: 10.1371/journal.pmed.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.A survey of primary care physician referral to bariatric surgery in Manitoba: access, perceptions and barriers. El-Beheiry M, Vergis A, Choi JU, Clouston K, Hardy K. Ann Transl Med. 2020;8:0. doi: 10.21037/atm.2020.01.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Primary care physicians' knowledge, attitudes and concerns about bariatric surgery and the association with referral patterns: a Swedish survey study. Memarian E, Carrasco D, Thulesius H, Calling S. BMC Endocr Disord. 2021;21:62. doi: 10.1186/s12902-021-00723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]