Abstract
Objectives
Pre-COVID-19 pandemic, patients who attended the emergency department (ED) for upper respiratory tract infection (URTI) were more likely to receive antibiotics if they expected them. These expectations could have changed with the change in health-seeking behaviour during the pandemic. We assessed the factors associated with antibiotics expectation and receipt for uncomplicated URTI patients in four Singapore EDs during the COVID-19 pandemic.
Methods
We conducted a cross-sectional study on adult patients with URTI from March 2021 to March 2022 in four Singapore EDs and assessed the determinants of antibiotics expectation and receipt using multivariable logistic regression models. We also assessed the reasons patients expect antibiotics during their ED visit.
Results
Among 681 patients, 31.0% expected antibiotics while 8.7% received antibiotics during their ED visit. Factors (adjusted odds ratio [95% confidence interval]) that significantly influenced expectation for antibiotics include: 1) prior consultation for current illness with (6.56 [3.30–13.11]) or without (1.50 [1.01–2.23]) antibiotics prescribed; 2) anticipation for COVID-19 test (1.56 [1.01–2.41]); and 3) poor (2.16 [1.26–3.68]) to moderate (2.26 [1.33–3.84]) knowledge on antibiotics use and resistance. Patients expecting antibiotics were 10.6 times (10.64 [5.34–21.17]) more likely to receive antibiotics. Those with tertiary education were twice (2.20 [1.09–4.43]) as likely to receive antibiotics.
Conclusion
In conclusion, patients with URTI who expected antibiotics to be prescribed remained more likely to receive it during the COVID-19 pandemic. This highlights the need for more public education on the non-necessity for antibiotics for URTI and COVID-19 to address the problem of antibiotic resistance.
Keywords: Antimicrobial resistance, Antibiotics expectation, Emergency department, COVID-19
1. Introduction
The rise of antimicrobial resistance (AMR) has been a long-standing threat to public health [1]. Bacteria that become antibiotic-resistant can cause human infections leading to higher medical costs, decreased work productivity, and increased mortality [1]. Antibiotic misuse, both from inappropriate prescribing by health care providers and overuse by the public, drives the development of AMR, necessitating an urgent need for behavioural change in antibiotic use to slow its progression [1]. The emergence of AMR, rendering the ineffectiveness of antibiotics, will outstrip the pace of development of new antibiotics and lead to a post-antibiotic era if no action is taken [2,3].
Some antimicrobial stewardship programs, such as delaying or shortening the duration of antibiotic prescription, have effectively reduced antibiotic use in the inpatient setting [4,5]. However, such programmes are under-established in ambulatory care where there is greater patient involvement in shared clinical decision-making. Interventions targeting patient education have shown only minor effects in reducing antibiotic prescribing [5]. One study found that providing patients with information on the efficacy and side-effects of antibiotics reduces but does not eliminate clinically inappropriate expectations and requests for antibiotics [6]. Despite the already lacklustre progress in tackling AMR pre-COVID-19 pandemic, the focus on pandemic response during the pandemic further disrupted actions against AMR [3]. Uncertainties surrounding the pandemic are changing patients’ health-seeking behaviour and may either shift their focus away from antibiotics or increase their expectations for receiving them.
Before the COVID-19 pandemic, patients’ expectations for antibiotics often contributed to physicians’ decisions to prescribe antibiotics. Experimental evidence from the United Kingdom showed that physicians were more willing to prescribe antibiotics if they believed that patients expected them, even if they thought the probability of a bacterial infection was low [7]. Patients’ expectations for antibiotics stems from their socio-cognitive knowledge, attitudes, and beliefs on the indications of antibiotics [6]. Studies have observed that lower education level, perceived severity of illness, previous positive experiences with antibiotics, history of antibiotics misuse, and the belief that antibiotics are effective are predictors of antibiotic expectation for upper respiratory tract infections (URTIs) [8], [9], [10].
During the COVID-19 pandemic, emergency departments (EDs) worldwide, including Singapore, experienced surges in attendance for acute respiratory illness, which accentuated the problem of overcrowding in EDs [11], [12], [13], [14], [15]. Although uncomplicated URTI should not be managed in EDs, the uncertainties surrounding COVID-19 management and the public's lack of understanding about COVID-19 could have changed health-seeking behaviour and influenced patients’ expectations for antibiotics when seeking care for URTI in the ED. Hence, we assessed the factors associated with the expectation for and receipt of antibiotics for uncomplicated URTI in four adult EDs in Singapore during the COVID-19 pandemic. We also assessed the reasons for their expectations and their behaviour surrounding the use of antibiotics.
2. Materials and methods
2.1. Study design and setting
We conducted a cross-sectional study on adults seeking medical care at the ED for uncomplicated URTI. Our study included EDs in four acute hospitals (Changi General Hospital, Khoo Teck Puat Hospital, National University Hospital, and Tan Tock Seng Hospital), covering all three healthcare clusters in Singapore.
2.2. Participants
We recruited 681 adults who attended the EDs with a diagnosis of URTI (ICD-10 J00-J06) between March 2021 and March 2022. Patients were asked to complete a survey questionnaire post-consultation; we excluded hospitalised patients and patients with multiple attendances to the ED within 30 days for the same illness to omit possible complicated URTI cases. These exclusion criteria were verified through review of electronic medical records prior to recruitment. We initially excluded COVID-19 suspects from the study because of a default hospital admission policy but included them after the national policy was revised in July 2021. Since then, the Singaporean government has advocated home recovery for COVID-19, as most of the population has been fully vaccinated against COVID-19 and the illness is predominantly mild. Study recruitment was suspended at one study site (from May 2021) because of operational restrictions in response to the ramp-up in COVID-19 response in the ED.
2.3. Questionnaire
We collected information on the patient's demographics (age, sex, race, nationality, education level), health status (vaccination status, illness symptoms, smoking status, Charlson's co-morbidity index), health-seeking behaviour (reasons for the ED visit, prior healthcare consultation for the same illness episode, payment method), and their expectations, knowledge, attitudes, and behaviour (KAB) on the use of antibiotics (Supplementary Table S1). The attitude and behaviour questions were measured on the five-point agreement Likert scale. We adapted the KAB questions on antibiotics from a literature review [16] and a priori knowledge of our previous research [10]. The questionnaire was interviewer-administered to enable interpretation consistency across all participants. All data collectors were independent of the patients’ care team and were trained to minimise bias in data collection.
2.4. Analysis
The outcome variables of interest are whether the patient 1) expected and 2) received an antibiotic prescription during the ED visit. We performed descriptive statistics to assess the differences between patients expecting antibiotics and patients prescribed antibiotics during the ED visit. We considered a positive vaccination status as follows: influenza vaccination within 12 months; ever had a pneumococcal vaccination; and at least a week after two doses of COVID-19 vaccination. The Charlson's co-morbidity index (CCI) was computed and classified into three categories (no co-morbidity, CCI 0; mild, CCI 1–2; moderate/severe, score CCI >2). We considered patients to have poor knowledge of antibiotics and AMR if they answered correctly ≤4 out of the 10 knowledge questions; moderate knowledge if they answered correctly 5 to 7 questions; and good knowledge if they answered correctly ≥8 questions.
We first performed univariate analyses to assess the differences between categories in the outcome variables to inform variable selection for the subsequent multivariable model. Next, we explored the independent factors associated with antibiotic expectation and the receipt of antibiotics using multivariable logistic regression by adding and dropping variables from an initial model. The best model was chosen based on the likelihood ratio tests of nested models and the lowest Akaike's Information Criteria (Supplementary Table S2A,S2B). We then present an anchored divergent graph on the reasons for expecting antibiotics and elaborate on other reasons that were not part of the Likert scale items.
In addition, we performed principal components analysis to classify the antibiotic use behaviours (Supplementary Table S1). Likert items with smaller coefficients were removed stepwise while optimising the total variance explained (the higher the better) and internal consistency (Cronbach's alpha) of each factor. Ungrouped behaviour statements were also dropped from the analysis. All analyses were performed with Stata version 15.0 (StataCorp LP, College Station, TX) and RStudio version 2022.02.3 (RStudio, PBC, Boston, MA).
3. Results and discussion
3.1. Baseline characteristics of respondents
Overall, 31.0% (211/681) of patients were expecting antibiotics, while 8.7% (59/681) received antibiotics during the ED visit. Of patients expecting antibiotics, 15.6% (33/211) received an antibiotic prescription. Table 1 shows the characteristics of patients expecting/not expecting antibiotics and patients who received/did not receive antibiotics.
Table 1.
Baseline characteristics of patients, by antibiotic expectation and antibiotic receipt.
| Antibiotic expectation |
Antibiotic receipt |
||||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics of respondents, n(%) | All patients (N=681) |
Expecting antibiotics (N=211) |
Did not expect antibiotics (N=470) |
P value | Prescribed antibiotics (N=59) |
Was not prescribed antibiotics (N=622) |
P value |
| Demographics | n(%) | n(%) | n(%) | n(%) | n(%) | ||
| Age, mean (SD) | 34.5 (12.7) | 33.6 (12.3) | 34.6 (12.9) | 0.228a | 34.2 (13.3) | 34.3 (12.7) | 0.113a |
| Male sex | 339 (49.8%) | 109 (51.7 %) | 230 (48.9%) | 0.566 | 25 (42.4%) | 314 (50.5%) | 0.292 |
| Race | |||||||
| Chinese | 314 (46.1%) | 92 (43.6 %) | 222 (47.2%) | 0.572 | 31 (52.5%) | 283 (45.5%) | 0.306 |
| Malay | 174 (25.6%) | 61 (28.9 %) | 113 (24.0%) | 9 (15.3%) | 165 (26.5%) | ||
| Indian | 114 (16.7%) | 33 (15.6 %) | 81 (17. %) | 11 (18.6%) | 103 (16.6 %) | ||
| Other races | 79 (11.6%) | 25 (11.9 %) | 54 (11.5%) | 8 (13.6%) | 71 (11.4%) | ||
| Nationality | |||||||
| Singaporean | 498 (73.1%) | 157 (74.4 %) | 341 (72.6%) | 0.550 | 40 (67.8%) | 458 (73.6%) | 0.550 |
| Permanent resident | 68 (10.0%) | 23 (10.9 %) | 45 (9.6%) | 8 (13.6%) | 60 (9.7 %) | ||
| Others | 115 (16.9%) | 31 (14.7 %) | 84 (17.9%) | 11 (18.6%) | 104 (16.7%) | ||
| Tertiary education | 224 (32.9%) | 56 (26.5 %) | 168 (35.7%) | 0.023b | 21 (35.6%) | 203 (32.6%) | 0.751 |
| Health status | |||||||
| Having a fever during the visit | 248 (36.4%) | 82 (38.9%) | 166 (35.3%) | 0.422 | 26 (44.1%) | 222 (35.7%) | 0.256 |
| Smoker | 149 (21.9%) | 49 (23.2%) | 100 (21.3%) | 0.640 | 12 (20.3%) | 137 (22.0%) | 0.893 |
| Charlson comorbidity severity | |||||||
| No comorbidity | 621 (91.2%) | 196 (92.9%) | 425 (90.4%) | 0.663c | 51 (86.4%) | 570 (91.6%) | 0.139c |
| Mild | 52 (7.6%) | 13 (6.2%) | 39 (8.3%) | 6 (10.2%) | 46 (7.4%) | ||
| Moderate/Severe | 8 (1.2%) | 2 (2.0%) | 6 (1.3%) | 2 (3.4%) | 6 (1.0%) | ||
| Influenza vaccinated (within 12 months) | 257 (37.7%) | 68 (32.2%) | 189 (40.2%) | 0.057 | 18 (30.5%) | 239 (38.4%) | 0.290 |
| Pneumonia vaccinated | 59 (8.7%) | 15 (7.1%) | 44 (9.4%) | 0.413 | 3 (5.1%) | 56 (9.0%) | 0.435c |
| COVID-19 vaccinated (2 doses) | 362 (53.2%) | 107 (50.7%) | 255 (54.3%) | 0.439 | 25 (42.4%) | 337 (54.2%) | 0.109 |
| Health seeking behaviour | |||||||
| Prior (non-ED) consult for same condition | |||||||
| No prior consult | 474 (69.6%) | 124 (58.8%) | 350 (74.5%) | <0.001d | 30 (50.9%) | 444 (71.4%) | <0.001d |
| Prior consult with antibiotics | 44 (6.5%) | 31 (14.7%) | 13 (2.8%) | 12 (20.3%) | 32 (5.14%) | ||
| Prior consult w/o antibiotics | 163 (23.9%) | 56 (26.5%) | 107 (22.8%) | 17 (28.8%) | 146 (23.5%) | ||
| Expects a COVID-19 test | 534 (78.4%) | 171 (81.0%) | 363 (77.2%) | 0.309 | 41 (69.5%) | 493 (79.3%) | 0.115 |
| Payment method | (n=676) | (n=208) | (n=468) | (n=57) | (n=619) | ||
| Employee benefits | 404 (59.8%) | 111 (53.4%) | 293 (62.6%) | 0.123 | 26 (45.6%) | 183 (29.6%) | 0.086c |
| Government/private insurance | 54 (8.0%) | 19 (9.1%) | 35 (7.5%) | 26 (45.6%) | 378 (61.1%) | ||
| Out-of-pocket | 209 (30.9%) | 74 (35.6%) | 135 (28.9%) | 4 (7.0%) | 50 (8.1%) | ||
| Social subsidies | 9 (1.3%) | 4 (1.9%) | 5 (1.1%) | 1 (1.8%) | 8 (1.3%) | ||
| Antibiotics use | |||||||
| Knowledge on antibiotics | |||||||
| Poor (Score ≤ 4) | 276 (40.5%) | 90 (42.7%) | 186 (39.57 %) | 0.002b | 20 (33.9%) | 256 (41.2%) | 0.261 |
| Moderate (Score 5-7) | 278 (40.8%) | 98 (46.5%) | 180 (38.3 %) | 30 (50.9%) | 248 (39.9%) | ||
| Good (Score ≥ 8) | 127 (18.6%) | 23 (10.9%) | 104 (22.13 %) | 9 (15.3%) | 118 (19.0%) | ||
| Expected antibiotics | 211 (30.1%) | - | - | 46 (78.0%) | 165 (26.5%) | <0.001d | |
Kruskal-Wallis test.
P < 0.05.
Fisher's exact test.
P < 0.001.
The mean age of participants was 34.5 (12.7) and between 21 and 88 years old. Half of the patients were male (49.8%), 46.1% were of the Chinese race, 73.1% were Singaporeans, and 32.9% had tertiary education. Approximately a third (36.4%) of patients had a fever during the visit, 91.2% had no comorbidities, 69.6% had not seen another healthcare provider for the same episode of illness, and 81.3% had poor to moderate knowledge of antibiotics (Scored <80% on the knowledge questionnaire).
3.2. Antibiotic expectation
There were no statistically significant differences between patients who expected antibiotics and those who did not expect antibiotics during their ED visit, except for prior health care consult for the same episode of illness and knowledge on antibiotics and AMR. A higher proportion of patients who were expecting antibiotics during the ED visit (14.7% vs. 2.8%, P < 0.001) received antibiotics from a prior consult (primary care or specialist outpatient clinic) for the same episode of illness. A higher proportion of patients who were expecting antibiotics during the ED visit also had poor to moderate knowledge (89.2% vs. 77.9%, P = 0.001) of antibiotics and AMR.
3.3. Antibiotic receipt
There were no statistically significant differences between patients who received antibiotics and those who did not receive antibiotics during their ED visit, except for prior health care consult for the same episode of illness and expectation for antibiotics. A higher proportion of patients who received antibiotics during their ED visit received antibiotics from prior consultations (primary care or specialist outpatient clinic) for the same episode of illness (20.3% vs. 5.1%, P < 0.001). A higher proportion of patients who received antibiotics expected antibiotics during the ED visit (78.0% vs. 26.5%, P < 0.001).
3.4. Determinants of expectation for antibiotics
Patients with a prior clinical consultation for the same illness were more likely to expect antibiotics during the ED visit. Compared with patients without prior consultation, patients who received antibiotics during a prior consultation were 6.5 times (adjusted odds ratio [aOR]: 6.56, 95% confidence interval [CI] 3.30–13.11, P < 0.001) more likely to expect antibiotics, while patients who did not receive antibiotics during their prior consultation were 1.5 times (aOR: 1.50, 95% CI 1.01–2.23, P = 0.046) more likely to expect antibiotics during the ED visit (Table 2 ).
Table 2.
Factors for antibiotics expectation.
| Model variables | Adjusted model |
||
|---|---|---|---|
| (Reference: Not expecting antibiotics) | Adjusted OR (95% CI) | P value | VIF |
| Expects a COVID-19 test | 1.56 (1.01, 2.41) | 0.045a | 1.07 |
| Prior (non-ED) consult for the same condition | |||
| No prior consult | Ref | ||
| Consult with antibiotics | 6.58 (3.30, 13.11) | <0.001b | 1.03 |
| Consult w/o antibiotics | 1.50 (1.01, 2.23) | 0.046a | 1.08 |
| Knowledge on antibiotics and antimicrobial resistance | |||
| Good | Ref | ||
| Moderate | 2.26 (1.33, 3.84) | 0.002a | 1.92 |
| Poor | 2.16 (1.26, 3.68) | 0.005a | 1.96 |
aP < 0.05.
bP < 0.001.
VIF, variance inflation factor.
Patients with poor (aOR: 2.16, 95% CI 1.26–3.68, P = 0.005) to moderate (aOR: 2.26, 95% CI 1.33–3.84, P = 0.002) knowledge of antibiotics and AMR were twice as likely to expect antibiotics compared with patients with good knowledge of antibiotics. In addition, patients expecting a COVID-19 test were 1.5 times (aOR: 1.56, 95% CI 1.01–2.41, P = 0.045) more likely to expect antibiotics (Table 2).
3.5. Determinants of antibiotics receipt
Patients expecting antibiotics during their ED visit were 10.6 times (aOR: 10.64, 95% CI 5.34–21.17, P < 0.001) more likely to receive antibiotics. Patients who received antibiotics during a prior consultation were thrice (aOR: 2.97, 95% CI 1.26–7.00, P = 0.013) as likely to receive antibiotics compared with patients with no prior consultation. Tertiary-educated patients were also twice (aOR: 2.20, 95% CI 1.09–4.43, P = 0.027) as likely to receive antibiotics. Although we did not observe statistical significance regarding severity of pre-existing comorbidity, the odds of patients receiving antibiotics increased with a higher severity of comorbidities compared with patients without any co-morbidity (Table 3 ).
Table 3.
Factors for receipt of antibiotics.
| Model variables | Adjusted model |
||
|---|---|---|---|
| (Reference: Did not receive antibiotics) | Adjusted OR (95% CI) | P value | VIF |
| Expects an antibiotic prescription | 10.64 (5.34, 21.17) | <0.001b | 1.08 |
| Expects a COVID-19 test | 0.52 (0.26, 1.03) | 0.061 | 1.08 |
| Age category | |||
| Above 50 years | Ref | ||
| 26–50 years | 0.60 (0.23, 1.55) | 0.290 | 2.78 |
| 25 years and below | 1.79 (0.63, 5.09) | 0.276 | 2.85 |
| Education level | |||
| Non-tertiary | Ref | ||
| Tertiary | 2.20 (1.09, 4.43) | 0.027a | 1.15 |
| Prior (non-ED) consult for the same condition | |||
| No prior consult | Ref | ||
| Consult with antibiotics | 2.97 (1.26, 7.00) | 0.013a | 1.09 |
| Consult w/o antibiotics | 1.29 (0.63, 2.65) | 0.484 | 1.1 |
| Pre-existing comorbidity | |||
| No comorbidity | Ref | ||
| Mild | 2.28 (0.75, 6.94) | 0.148 | 1.16 |
| Moderate/Severe | 6.17 (0.86, 44.24) | 0.070 | 1.08 |
aP < 0.05.
bP < 0.001.
VIF, variance inflation factor.
3.6. Reasons for expecting antibiotics
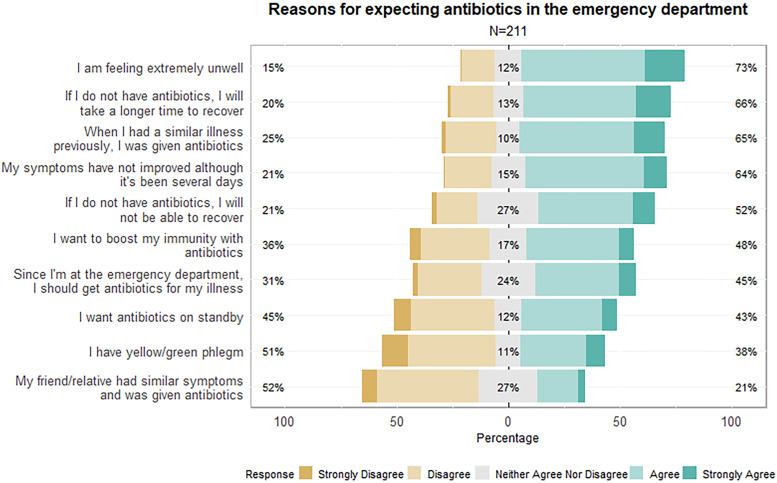
The top five reasons for patients expecting antibiotics in the emergency departments are: 1) feeling extremely unwell (73% agreement); 2) perception that the illness will take longer to recover without antibiotics (66% agreement); 3) having previous experiences of receiving antibiotics for similar illness (65% agreement); 4) prolonged symptoms without improvement (64% agreement); and 5) perception that recovery from the illness is only possible with antibiotics (52% agreement). In addition, 48% agreed that antibiotics could boost their immunity; 45% felt that they had to obtain antibiotics because they were at the ED; 43% wanted antibiotics for standby; 38% had yellow/green phlegm; and 21% were influenced by their friends and/or relatives (Fig. 1 ).
Fig. 1.
Reasons for expecting antibiotics in the emergency department, measured on a five-point Likert-scale.
In addition to the Likert scale statements, patients mentioned other reasons for expecting antibiotics during their ED visit. A few patients mistakenly thought that antibiotics were effective in treating viruses (including cough and flu), resolving inflammation, and improving their immunity. Some thought that antibiotics could generate antibodies and treat or prevent any infection. One patient mistakenly thought of antibiotics as a ‘cure-all’ medication. A few patients thought that the standard procedure for physicians was to prescribe antibiotics for their medical consultation, as they had prior experiences receiving antibiotics for similar illnesses. One patient wanted a stronger antibiotic, as the previous antibiotic received did not ‘cure his/her illness’, while one thought that antibiotics could substitute a sleeping pill. Another patient had concerns about developing URTI before his/her second dose of COVID-19 vaccination and was expecting antibiotics to speed up the recovery of URTI Figs. 2 and 3.
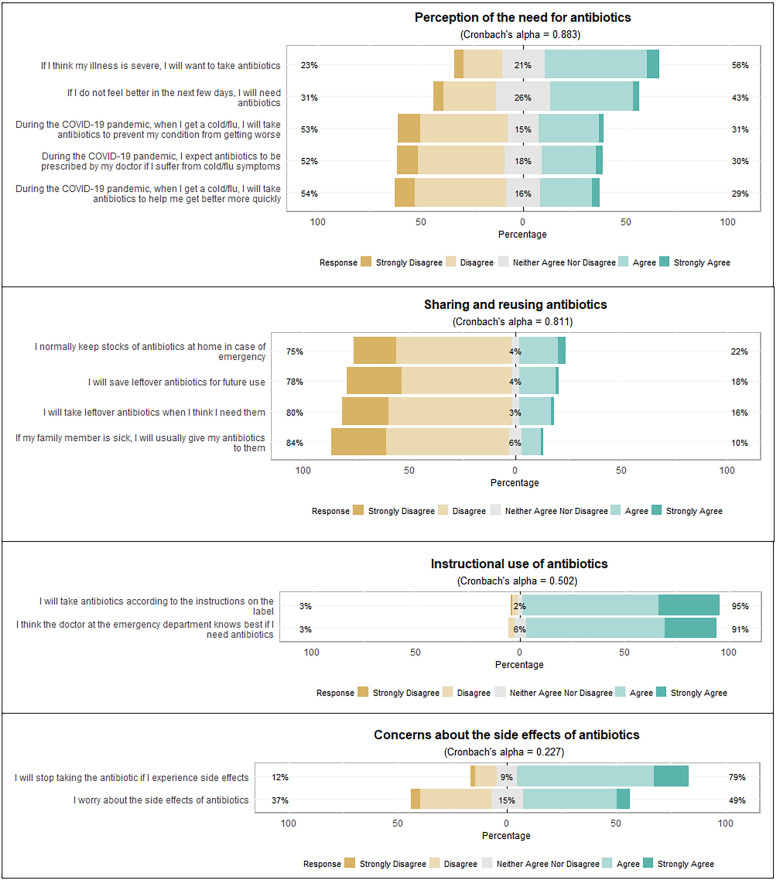
Fig. 2.
Statements on antibiotic use, measured on a five-point Likert-scale.
3.7. Antibiotic use behaviour
Four factors emerged from the factor analysis of antibiotics use behaviour. The first is the perception of the need for antibiotics. More than half of patients (56%) agreed that antibiotics are needed for a severe illness, while 46% agreed that antibiotics are needed if they do not feel better in the next few days. More than half of respondents disagreed that they would take or expect antibiotics to prevent/recover from the flu/cold during the COVID-19 pandemic (Fig. 2).
The second factor is sharing and reusing antibiotics. Three quarters (75%) of patients disagreed that they would keep stocks of antibiotics at home for an emergency, while about 80% of respondents disagreed that they would save and use leftover antibiotics or share antibiotics with their friends and family members.
The third and fourth factor had low internal consistency but shows interesting findings on antibiotic use behaviour. The third factor is the instructional use of antibiotics. More than 90% of patients agreed that they would take antibiotics according to instructions and would trust the ED physician on the need to use antibiotics. The last factor involves concerns about the side effects of antibiotics. Seventy-nine per cent of patients agreed that they would stop taking antibiotics if they experienced side effects, but a smaller proportion (49%) agreed that they worry about the side effects of antibiotics.
Our study explored patient-related factors associated with the expectation for and receipt of antibiotics for uncomplicated URTI in four EDs in Singapore during the COVID-19 pandemic. We also assessed the reasons patients expect antibiotics and their antibiotic use behaviours. Although these were extensively studied prior to the pandemic [7,[17], [18], [19], many studies were from western countries where different cultural settings and health systems can generate different patient expectations than those from Asian settings. We also took the perspective of patients seeking care for URTI in the EDs during surges of cases in the COVID-19 pandemic.
4. Discussion
The COVID-19 pandemic seemingly had a minor effect on the antibiotic expectation and prescribing rates for URTI despite changes in health-seeking behaviours. Approximately a third of patients (31%) expected antibiotics during their ED visit in our study, which is similar to a study (33%) conducted at one of our institutions pre-pandemic [10]. The antibiotic prescribing rate in our study (∼9%) was also similar to a pre-pandemic study which assessed antibiotic use for URTI in the ED in the United States [18]. We also found that patients expecting antibiotics were 10.6 times more likely to receive them, although the same US study reported that physicians were 5.3 times more likely to prescribe antibiotics if they believed that patients expected antibiotics [18]. The sheer number of COVID-19-related ED attendances should have brought the antibiotic prescribing rate down, but the uncertainties surrounding new variants of COVID-19 may have prompted physicians to loosen their prescribing criteria for anxious patients who perceived their illness as severe [20,21]. We found that the top reason for expecting antibiotics was the perceived severity of illness. This reason was also a predictor for antibiotic expectation in another study assessing the expectation for antibiotics in the Singapore primary care setting pre-pandemic [22].
Poor knowledge of the indications of antibiotics was a significant predictor of antibiotic expectation pre-COVID-19 and has remained a strong predictor of antibiotic expectation during the pandemic [22], [23], [24]. The misconceptions that antibiotics improve immunity, help one recover faster from an illness, and are a cure-all medication exist in our study and were deep-seated among the public [16,[25], [26], [27]. These misconceptions likely occurred because of ingrained myths surrounding the effectiveness of antibiotics [26] and patients’ past experiences with antibiotic use. We also found that patients with prior clinical consultation with an antibiotic prescription were 6.6 times more likely to expect antibiotics during their ED consultation, highlighting the importance of antimicrobial stewardship in primary care and the role of primary care physicians in promoting appropriate antibiotic use for URTI [28]. Highly educated patients were more likely to receive antibiotics as these patients may have appeared more confident about their needs and could have challenged the physician's decision in their care [29]. Given the diagnostic uncertainty of URTI and the time-strapped ED environment [30], physicians may compromise by prescribing antibiotics to patients, but further investigation is needed to support this hypothesis.
Antibiotic use behaviour was not substantially different pre- and during the COVID-19 pandemic, according to a community survey in Singapore [23]. The survey found that 24% (3% increment from pre-pandemic) of respondents would expect antibiotics and 21% (2% increment from pre-pandemic) would take antibiotics to prevent their condition from getting worse during the pandemic. Although a higher proportion of our study respondents (∼30%) agreed with the above two statements, ED respondents, who were unwell at the point of the survey, could have perceived a lower health status compared with community respondents who may not have been unwell at the point of the survey. One-fifth of respondents would keep stocks of antibiotics at home, and 10% to 16% would use or share them with their family without advice from a physician. Although most respondents did not agree to sharing or reusing antibiotics, these proportions have not improved over the years despite calls for actions to change the public's antibiotic use behaviour [22,31].
Our study had several limitations. The hospital and national protocols regarding the COVID-19 pandemic were evolving during our data collection. We initially excluded patients with COVID-19 infection from the study because of a default hospital admission policy. However, with mass vaccination and the transition to home recovery for COVID-19 infections due to milder illness, we subsequently included them in our study if they were medically diagnosed with URTI. Patients’ health-seeking behaviours could have varied at different periods of the pandemic. In addition, the antibiotic prescribing rate was self-reported by patients, which may differ from the rates prescribed by the physician. However, we expect the discrepancy between the self-reported and actual antibiotic prescribing rates to be low as we verified the antibiotic prescribing rates with the electronic medical records of one ED in this study and observed the discrepancy to be minimal (<5%).
The pandemic provides an invaluable opportunity for leveraging the mass communication channels to educate the public on uncomplicated URTI, as well as increase the public's knowledge of antibiotics and AMR [32,33]. Furthermore, since prior experiences with antibiotics likely occurred in primary care, future work can explore interventions in primary care to address patients’ expectations for antibiotics in the ED.
5. Conclusion
In conclusion, patients with URTI who expected antibiotics remained more likely than those who did not expect them to receive antibiotics during the COVID-19 pandemic. Perceived severity of illness and effectiveness of antibiotics in speeding up recovery were the top reasons for expecting antibiotics, while poor knowledge and prior experiences were strong predictors for expecting antibiotics. Our findings highlighted an opportunity for leveraging the COVID-19 mass communication channels to educate the public on the non-necessity of antibiotics for URTI to address the problem of antibiotic misuse and AMR.
Availability of data and material
The final dataset is partially available on request.
Funding
This project is supported by National Medical Research Council Clinician Scientist Award (award number: MOH-CSAINV18may-0002).
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Ethical approval
This study was approved by the National Healthcare Group Domain Specific Review Board in Singapore. NHG DSRB Ref: 2019/00174.
Acknowledgements
We would like to acknowledge Ho Kia Nam, Kirstie Neo, Nadiah Binte Abd Karim, Karthiga Natarajan, Chua Hoong Kai, Dillon Wee, Yvette Jee, and Khaing Nwe Win for data collection assistance for this study.
Editor: Wen-Chien Ko
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jgar.2023.02.025.
Appendix. Supplementary materials
References
- 1.World Health Organization. Antimicrobial resistance: global report on surveillance: World Health Organization. 2014.
- 2.Kåhrström CT. Entering a post-antibiotic era? Nat Rev Microbiol. 2013;11:146. doi: 10.1038/nrmicro2978. [DOI] [PubMed] [Google Scholar]
- 3.Kwon JH, Powderly WG. The post-antibiotic era is here. Science. 2021:471. doi: 10.1126/science.abl5997. [DOI] [PubMed] [Google Scholar]
- 4.Huang L-J, Chen S-J, Hu Y-W, Liu C-Y, Wu P-F, Sun S-M, et al. The impact of antimicrobial stewardship program designed to shorten antibiotics use on the incidence of resistant bacterial infections and mortality. Sci Rep. 2022;12:1–9. doi: 10.1038/s41598-022-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoolen B, de Ridder D, van Lensvelt-Mulders G. Patient-oriented interventions to improve antibiotic prescribing practices in respiratory tract infections: a meta-analysis. Health Psychol Rev. 2012;6:92–112. [Google Scholar]
- 6.Thorpe A, Sirota M, Orbell S, Juanchich M. Effect of information on reducing inappropriate expectations and requests for antibiotics. Br J Psychol. 2021;112:804–827. doi: 10.1111/bjop.12494. [DOI] [PubMed] [Google Scholar]
- 7.Sirota M, Round T, Samaranayaka S, Kostopoulou O. Expectations for antibiotics increase their prescribing: causal evidence about localized impact. Health Psychol. 2017;36:402. doi: 10.1037/hea0000456. [DOI] [PubMed] [Google Scholar]
- 8.Shlomo V, Adi R, Eliezer K. The knowledge and expectations of parents about the role of antibiotic treatment in upper respiratory tract infection--a survey among parents attending the primary physician with their sick child. BMC Fam Pract. 2003 Dec 30;4:20. doi: 10.1016/10.1186/1471-2296-4-20. [DOI] [PMC free article] [PubMed]
- 9.Dosh SA, Hickner JM, Mainous AG, Ebell MH. Predictors of antibiotic prescribing for nonspecific upper respiratory infections, acute bronchitis, and acute sinusitis. J Fam Pract. 2000;49:407–414. [PubMed] [Google Scholar]
- 10.Tan R, Huang Z, Guo H, Weng Y, Chow A. Antibiotic expectations of patients attending an emergency department with upper respiratory tract infections: clinical and behavioural determinants of antibiotic use. Int J Antimicrob Agents. 2022;59 doi: 10.1016/j.ijantimicag.2021.106511. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu P, Shah AB, Ahmad FB, Kerr J, Demeke HB, Graeden E, et al. Emergency department and intensive care unit overcrowding and ventilator shortages in US hospitals during the COVID-19 pandemic, 2020–2021. PHR. 2022 doi: 10.1177/00333549221091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savioli G, Ceresa IF, Gri N, Bavestrello Piccini G, Longhitano Y, Zanza C, et al. Emergency department overcrowding: understanding the factors to find corresponding solutions. J Pers Med. 2022;12:279. doi: 10.3390/jpm12020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouillon-Minois J-B, Raconnat J, Clinchamps M, Schmidt J, Dutheil F. Emergency department and overcrowding during COVID-19 outbreak; a letter to editor. Arch Acad Emerg Med. 2021;9:e28. doi: 10.22037/aaem.v9i1.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thangayah JR, Tan KBK, Lim CS, Fua T-P. Disease outbreak surge response: how a Singapore tertiary hospital converted a multi-story carpark into a flu screening area to respond to the COVID-19 pandemic. Disaster Med Public Health Prep. 2021;15:e37–e42. doi: 10.1017/dmp.2020.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L, Ng WM, Lin Z, Law LSC, Yong L, Liew YST, et al. Factors reducing inappropriate attendances to emergency departments before and during the COVID-19 pandemic: a multicentre study. Ann Acad Med Singap. 2021;50:818–826. [PubMed] [Google Scholar]
- 16.World Health Organization. Antibiotic resistance: multi-country public awareness survey. 2015.
- 17.Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:1–9. doi: 10.1186/s13756-016-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA, et al. Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Ann Emerg Med. 2007;50:213–220. doi: 10.1016/j.annemergmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Boiko O, Gulliford MC, Burgess C. Revisiting patient expectations and experiences of antibiotics in an era of antimicrobial resistance: qualitative study. Health Expect. 2020;23:1250–1258. doi: 10.1111/hex.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dozois DJ. Anxiety and depression in Canada during the COVID-19 pandemic: a national survey. Can Psychol. 2021;62:136. [Google Scholar]
- 21.Malesza M, Kaczmarek MC. Predictors of anxiety during the COVID-19 pandemic in Poland. Pers Individ Differ. 2021;170 doi: 10.1016/j.paid.2020.110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan DST, Huang JH, Lee MHM, Yu Y, Chen MI, Goh EH, et al. Knowledge, attitudes and practices towards antibiotic use in upper respiratory tract infections among patients seeking primary health care in Singapore. BMC Fam Pract. 2016;17:1–9. doi: 10.1186/s12875-016-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Hildon ZJ-L, Lye DCB, Straughan PT, Chow A. The associations between poor antibiotic and antimicrobial resistance knowledge and inappropriate antibiotic use in the general population are modified by age. Antibiotics. 2021;11:47. doi: 10.3390/antibiotics11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanichelli V, Tebano G, Gyssens IC, Vlahović-Palčevski V, Monnier AA, Stanic Benic M, et al. Patient-related determinants of antibiotic use: a systematic review. Clin Microbiol Infect. 2019;25:48–53. doi: 10.1016/j.cmi.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Thomas R, Greenwood H, Michaleff ZA, Abukmail E, Hoffmann TC, McCaffery K, et al. Examining Australian's beliefs, misconceptions and sources of information for COVID-19: a national online survey. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredericks I, Hollingworth S, Pudmenzky A, Rossato L, Syed S, Kairuz T. Consumer knowledge and perceptions about antibiotics and upper respiratory tract infections in a community pharmacy. Int J Clin Pharm. 2015;37:1213–1221. doi: 10.1007/s11096-015-0188-y. [DOI] [PubMed] [Google Scholar]
- 27.Shetty P. The antibiotic paradox. Lancet Infect Dis. 2002;2:704. [Google Scholar]
- 28.Kiel A, Catalano A, Clark CM, Wattengel BA, Mason J, Sellick J, et al. Antibiotic prescribing in the emergency department versus primary care: implications for stewardship. J Am Pharm Assoc. 2020;60:789–795. doi: 10.1016/j.japh.2020.03.016. (2003).e2. [DOI] [PubMed] [Google Scholar]
- 29.Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med. 2009;69:1805–1812. doi: 10.1016/j.socscimed.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Lim DW, Htun HL, Ong LS, Guo H, Chow A. Systematic review of determinants influencing antibiotic prescribing for uncomplicated acute respiratory tract infections in adult patients at the emergency department. Infect Control Hosp Epidemiol. 2022;43:366–375. doi: 10.1017/ice.2020.1245. [DOI] [PubMed] [Google Scholar]
- 31.Ceaser S, Wurtz R. ‘Leftover’ antibiotics in the medicine cabinet. Ann Intern Med. 2000;133:74. doi: 10.7326/0003-4819-133-1-200007040-00017. [DOI] [PubMed] [Google Scholar]
- 32.Anwar A, Malik M, Raees V, Anwar A. Role of mass media and public health communications in the COVID-19 pandemic. Cureus. 2020:12. doi: 10.7759/cureus.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thanh PT. Can risk communication in mass media improve compliance behavior in the COVID-19 pandemic? Evidence from Vietnam. Int J Sociol Soc Policy. 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The final dataset is partially available on request.