Abstract
Sepsis is a syndromic response to infection and is frequently a final common pathway to death from many infectious diseases worldwide. The complexity and high heterogeneity of sepsis hinder the possibility to treat all patients with the same protocol, requiring personalized management. The versatility of extracellular vesicles (EVs) and their contribution to sepsis progression bring along promises for one-to-one tailoring sepsis treatment and diagnosis. In this article, we critically review the endogenous role of EVs in sepsis progression and how current advancements have improved EVs-based therapies toward their translational future clinical application, with innovative strategies to enhance EVs effect. More complex approaches, including hybrid and fully synthetic nanocarriers that mimic EVs, are also discussed. Several pre-clinical and clinical studies are examined through the review to offer a general outlook of the current and future perspectives of EV-based sepsis diagnosis and treatment.
Introduction
Sepsis is an aberrant or dysregulated immune response to infection that leads to life-threatening organ dysfunction [1]. It is a highly prevalent condition that accounts for 18% of admissions to the intensive care unit (ICU) [2] and it is associated with a mortality rate higher than 25–30%, and even 40–50% when shock is present [3]. Although the incidence of sepsis is difficult to ascertain, conservative estimates indicate that sepsis is a leading cause of mortality and critical illness worldwide, accounted for almost 20% of all global deaths [4]. In addition, there is increasing awareness that patients who survive sepsis often have a reduced quality of life characterized by enduring cognitive and functional limitations [5, 6] turning survivorship into a public health problem with huge implications for patients, families, and the health care system.
The sepsis pathophysiology can be understood as a complex crosslinking of mechanisms, including inflammatory and anti-inflammatory responses, coagulopathies, systemic action of microorganisms, and multiple organ failure [7]. Such pathophysiology may substantially differ based on the underlying type of infection and individual host responses. In fact, a retrospective analysis using data from 63,858 patients allowed the identification and validation of four clinical sepsis phenotypes (α, β, γ, and δ) that were shown to correlate host-response patterns and clinical outcomes [8]. The high heterogeneity and the complex pathobiology of sepsis are the main reasons why pharmacological therapies are limited in preventing, managing, and diagnosing this syndrome, making it a “prototype” of a personalized treatment disease [9, 10].
Precision medicine aims to provide clinical treatments targeted to the needs of individual patients by considering their genetics, lifestyle and environment characteristics [11]. Regarding sepsis, precision medicine creates an individual approach on a case-by-case basis by identifying subgroups of patients with a high risk of adverse outcomes who may benefit from specific treatments or rescue therapies according to their particular phenotype [12]. In this regard, extracellular vesicles (EVs) bring along promises for tailoring sepsis treatment and diagnosis [13], since they possess several potential advantages compared with the cell therapies used for sepsis treatment, mainly addressing the inherent risks associated with live-cell transplants [14].
EVs, including exosomes, microvesicles and apoptotic bodies, are nanoscale (40–5000 nm size) phospholipid bilayer structures endogenously secreted from all cell types [15] and released in many body fluids such as urine, saliva, plasma, breast milk or cerebrospinal fluid [16]. Since EVs' discovery, research and methodological developments have led researchers to realize that they play crucial roles in cell-to-cell communications, the regulation of homeostatic and pathological processes, transferring proteins, bioactive lipid material, DNAs, RNA species as well as other cytoplasmic components [17]. The high biocompatibility and low immunogenicity, increased specificity to target cells or tissues, ability to cross biological barriers and use endogenous cellular machinery of loading, are features that make EVs an optimal candidate as drug delivery vehicles [18]. Moreover, EVs' composition depends on their generation pathway and the cells from which they originate. This confers them the capacity to provide evidence for early disease diagnosis and prognosis, disease severity evaluation, and treatment monitoring [19], making them a potential innovative approach for precision medicine.
The purpose of this article is to critically analyze the endogenous role of EVs in sepsis progression and highlight their use as diagnostic biomarkers and therapeutic agents for sepsis. We evaluate current progress and discuss future methods for EVs bioengineering to either boost the therapeutic effect that EVs show by themselves or how to use their characteristics as drug delivery vehicles. More complex approaches, including hybrid and fully synthetic nanocarriers that mimic natural EVs are also discussed. Throughout the review, relevant pre-clinical and clinical studies are presented, which are so far encouraging towards a near future use of EVs for a more accurate and precise treatment for sepsis.
Role of EVs in sepsis progression
Multiple pre-clinical and clinical studies of sepsis have reported increased numbers of circulating EVs in septic individuals [20, 21], and even higher levels in patients with septic shock, establishing a directly proportional relationship between the amount of EVs in plasma and the severity of the illness. Besides, circulating EVs have been associated with organ failure and mortality in critically ill sepsis patients [22]. Despite the EVs origin, whether from endothelial, epithelial, or immune cells, they all exhibit significant pro-inflammatory, pro-coagulant, and pro-permeability effects, influencing the behavior of EVs-targeting cells, which may contribute to the progression of the disease [23, 24]. Furthermore, circulating EVs populations and its content change during the several sepsis stages, which could be used to determine more precisely the severity and the pathology of each patient and, therefore, offer the possibility of a more precise intervention and therapy [21].
One of the most described actions of EVs during sepsis development is their pro-coagulant effect. Bacterial infections are associated with the release of EVs carrying tissue factor (TF, a significant initiator of the extrinsic coagulation cascade) by monocytes, which results in the activation of the coagulation pathway [24]. A recent study using a mouse model of pyroptosis showed that inflammasome activation leads to the release of TF-positive EVs into the blood, which in turn triggered blood coagulation, resulting in tissue perfusion deficit, organ dysfunction and lethality [27]. In addition, Wang et al. proposed TF-positive EVs as a biomarker for thrombosis risk in a mouse model of endotoxemia [28].
Another critical process during sepsis in which the EVs are also involved is the massive cytokine storm, which refers to multiple activated cascades that lead to an autoamplifying cytokine production, based on a profound increase in pro-inflammatory cytokines such as IL-1, IL-12, IL-18, tumor necrosis factor alpha (TNF-α), chemokines (IL8) and interferons into the circulation causing severe inflammation and tissue damage [29, 30]. Several studies have reported that some of the circulating cytokines are carried by EVs together with other chemokines and growth factors [31]. EVs from plasma of septic mice were shown to have the capability to enhance Th1/Th2 differentiation, promote T cells proliferation and augment T lymphocyte migration [31]. More specifically, EVs from pre-stimulated human neutrophils could act as attractors of monocytes via MCP-1 in vitro [32]. In addition, the kinetics of soluble or EV-associated cytokines and chemokines display different dynamics in the blood of LPS-injected mice. While the peak of soluble cytokines release is 2–12 h post-infection, the maximum of EVs carrying them is 12–24 h post-infection [31]. This, together with the fact that EVs exhibit an increased stability in the blood (allowing them to travel long distances within the body) [33], could suggest that EVs carrying pro-inflammatory cytokines is crucial for a sustained systemic inflammation over time.
EVs that are released during systemic inflammatory conditions can also contain DAMPs [34], including histones [35], heat shock proteins (HSPs) [36], and high-motility group box-1 (HMGB1) [37]. These proteins can interact with TLR4 or RAGE receptors, participating in the induction of different inflammatory pathways [34, 35]. C-reactive protein (CRP) is an acute phase protein that is part of the innate immune system and it is crucial for the activation of the adaptative immune response. It is predominantly secreted in response to tissue damage and systemic inflammatory conditions [38], and it is used for sepsis prognostic [39]. Indeed, Fendl et al. observed that the level of EVs containing CRP was significantly higher in septic patients than in healthy donors [40]. Similar results were observed in plasma EVs from moderate and severe acute pancreatic patients [41]. Both studies associated the presence of EVs carrying CRP with a major disease severity [40, 41].
Aside from proteins that directly trigger the transduction of signals, there are other EVs components like miRNAs capable of regulating gene expression [42]. Recent studies have evidenced the presence of a different plasma-circulating miRNA expression profile from EVs between septic and healthy patients [43, 44], which has been associated with sepsis severity. Interestingly, there are some specific miRNAs with a > 1.5-fold increase in EVs from septic mice compared to EVs from sham-operated control mice, such as miR-126-3p, miR-122-5p, miR-146a-5p, miR-145-5p, miR-26a-5p, miR-150-5p, miR-222-3p and miR-181a-5p which are closely related to inflammation and innate immune response, mediating the cytokine production via TLR7-MyD88 and NFκB signaling [20].
Severe sepsis is a stage of disease progression that eventually involves the alteration of vascular permeability [45, 46] triggering acute pulmonary edema, severe hypoxia, and consequently the development of acute respiratory distress syndrome (ARDS) [47]. Interestingly, EVs from septic patients' plasma have demonstrated to exert a detrimental role in microvascular permeability [48]. Specifically, EVs may cause direct injury to the endothelium modulating adherent junctions, tight junctions, caveolar and cytoskeletal proteins disturbing nitric oxide homeostasis [49].
Overall, the importance of endogenous EVs in sepsis was further highlighted in a study by Essandoh et al. in which GW4869, a neutral sphingomyelinase inhibitor that partially blocks the release of EVs, was used to successfully reduce the number of EVs and pro-inflammatory cytokines emitted from lipopolysaccharide-stimulated macrophages. This EV reduction was correlated with decreased systemic inflammation and mortality in a cecal ligation and puncture (CLP) mouse model[50].
EVs as diagnostic markers
In sepsis, early diagnosis is crucial, thus, one-quarter of septic patients receive inadequate treatment and a worse prognosis as a consequence of a delayed diagnosis [51]. The current diagnostic criteria for sepsis are based on non-specific clinical symptoms, which can also occur in a variety of other clinical conditions [52]. In addition, it is particularly challenging in high-risk groups, such as the elderly or infants, which often present with atypical symptoms and are at an increased risk for ARDS or other secondary complications [53]. At present, there are no valid and reliable biomarkers allowing an on-site diagnosis and the identification of high-risk septic patients [54]. Hence, the search for new diagnostic markers to accurately stratify the stages of sepsis and facilitate early diagnosis remains meaningful and needed [55].
Research of endogenous EVs and their specific roles in sepsis progression reveal numerous diagnostic capabilities in pre-clinical sepsis models, as well as in human patients [56]. As mentioned above, EVs plasma levels in general have been proposed as predictive biomarkers for organ failure and mortality rate of septic patients [22], as well as, specific miRNA expression profile in EVs from septic patients, that has been also associated with sepsis survival and disease stage [57]. More specifically, proteomic analyses of human blood EVs from septic patients have revealed the presence of SPTLC3 protein, which was negatively correlated with disease progression [58]. In addition, Dakhlallah et al. detected an increased number of plasma EVs containing an increased amount of DNA methyltransferases mRNAs in the septic shock cohort compared to critically ill, non-septic control and sepsis cohorts [21], offering an opportunity to more precisely and promptly intervene. Although these studies highlight the potential of EVs as a novel method to diagnose and monitor the progression of the disease, further studies are needed to implement these methods in the clinics.
EVs for sepsis treatment
Given the diversity of functions of EVs in the context of sepsis, they are currently being investigated as potential diagnostic biomarkers and therapeutic targets to suppress their detrimental role in sepsis progression [23]. Furthermore, the attributes associated with EVs, such as low immunogenicity and toxicity, excellent biocompatibility, and natural ability to cross biological barriers [19], have spurred pre-clinical and clinical investigations of EVs as a natural therapeutic strategy for many diseases [59–63]. Among all cell types, mesenchymal stem cells (MSCs) are major candidates for cell therapy over the last decades [64]. Several ongoing clinical trials use MSCs for sepsis and ARDS have provided promising results [65–67]. Hence, there has been a marked increase in published pre-clinical studies using MSC-EVs in animals with organ injury or immune dysfunction since 2013 until now [68]. All these studies have reinforced the crucial role of EVs derived from MSCs in reducing pathogen replication [69], phagocytosis [70], immunity regulation [71–73], and the regeneration of injured tissues, which are essential aspects to treat sepsis and its consequent organ dysfunction [66, 67] in vitro and in vivo.
Methods for the modification and enhancement of natural EVs effect
Despite all advantages and positive therapeutic outcomes that natural EVs have demonstrated to exert, their translation to the clinical field requires extensive multidisciplinary efforts. Yet, there are still many challenges to overcome, including a lack of scalable production methods, low reproducibility of isolation techniques, and high heterogeneity between batches [63]. However, research has shown that advancements in the EVs biomanufacturing process and bioengineering methods can potentially hurdle these obstacles [64], opening up new future opportunities for EVs-based precision nanomedicine [65].
EVs, as natural intracellular communicators, have been also proposed as vehicles for the delivery of both native and non-native molecules [74]. Compared with standard delivery methods, EVs have been shown to deliver functional cargo with decreased immune clearance [75], higher stability in circulation, enhanced drug efficacy while minimizing drug toxicity and off-target side effects [76]. In addition, their unique structure, made of a hydrophobic lipid bilayer and a hydrophilic core, allows for the loading of a multitude of different cargoes [15]. Two main approaches exist for EVs loading, which are described below at some length.
Non-cell-based methods
On the one hand, non-cell-based methods, also known as exogenous loading [77] (Fig. 1), involves direct filling of already isolated EVs with therapeutic agents. This process can be accomplished by means of a passive encapsulation without using any external stimuli [78]. By this technique, the cargo can diffuse into the EVs following the concentration gradient [79], causing a lipid rearrangement of the membrane [80] or using energy-dependent channels [81]. Besides, exogenous loading can also be performed by an active encapsulation in which the EVs are forced to capture the desired cargo. Different routes for the loading process can be used, including electroporation, sonication, extrusion, freeze–thaw cycles and transfection [82]. While the passive method allows the preservation of EVs morphology [83], the active encapsulation shows a better loading efficiency and fewer difficulties in assessing the purity of the final preparation [84]. Figure 1 schematically describes two such approaches. Several studies have already proven drug loading feasibility into EVs and a higher efficiency of sepsis drugs when encapsulated within EVs. For instance, Sun et al. provided evidence that curcumin delivered by exosomes was more stable and highly concentrated in the blood. They demonstrated that curcumin carried by exosomes showed an enhanced anti-inflammatory effect in a lipopolysaccharide (LPS)-induced septic shock mouse model [80]. Two other relevant studies in this field showed a less disruptive EVs loading method involving a transmembrane pH gradient. Jeyaram et al. were able to load thousands of copies of miR-146a per EV and maintain their immunomodulatory properties, suggesting their potential therapeutic use in inflammatory diseases such as sepsis [85]. Similarly, in order to silence chemokine receptor 2 (CCR2), Ding et al. loaded EVs from mouse-derived immortalized bone marrow-derived macrophages (iBMDM) with siCCR2 by electroporation [86]. After intravenous administration, siCCR2 was delivered to the spleen and inhibited the infiltration of some inflammatory monocytes or macrophages in the spleen, alleviating the subsequent sepsis symptoms in a CLP mouse model [86]. In 2018, Gao et al. studied the usefulness of neutrophil-derived EVs as a delivery platform for piceatannol [87], an anti-inflammatory drug, described for its protective effect against sepsis-induced acute lung injury [88] and myocardial dysfunction [89]. They observed that piceatannol-loaded EVs dramatically alleviated acute lung inflammation/injury and sepsis in mice administered with LPS [87], revealing their potential application for precise nanomedicine.
Fig. 1.
Non-cell-based methods for the modification of EVs cargo. EVs extracellular vesicles
Cell-based methods
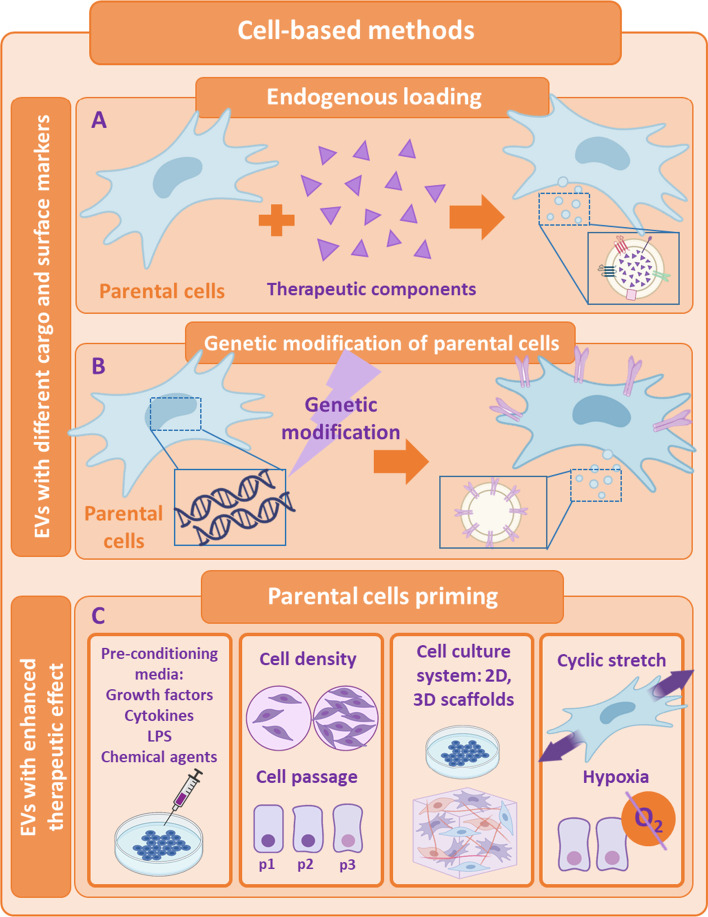
On the other hand, there are cell-based strategies for filling EVs, also known as endogenous loading (see Fig. 2, panel A). In this approach, parenteral cells can be incubated with drugs or drug-loaded nanoparticles (NPs) [90], allowing the secretion of natural EVs carrying a certain fraction of the therapeutic content of interest. For example, Perteghella et al. developed a new system to obtain curcumin-loaded EVs by previously treating MSCs with NPs carrying curcumin. In the end, MSCs were able to release EVs entrapping curcumin nanoparticles [91], enhancing the loading efficiency in comparison with non-cell-based methods. Furthermore, parental cells can also be genetically or metabolically modified in order to alter and thus enhance their targeting ability and biocompatibility [83]. A very recent study focused on COVID-19 treatment, allowed the obtaining of genetically modified CAR-T cells to secrete programmed nanovesicles (NVs) that expressed on their surface two antibody single-chain fragment variables, which are demonstrated to block spike protein of SARS-CoV-2 binding to ACE2 receptor in vivo. Moreover, obtained NVs were loaded with an anti-viral drug, remdesivir, by electroporation. These innovative NVs prevented SARS-CoV-2 from entering cells expressing ACE2 and also inhibited intracellular virus replication redirecting specifically the NVs to the major sites of viral infection [92].
Fig. 2.
Cell-based methods for the modification of EVs cargo or surface markers and for the enhancement of their natural effect. A Endogenous loading; B Genetic modification of parental cells; C Priming of parental cells. EVs extracellular vesicles
Enhancement of EVs' natural therapeutic effect
In addition to combating the obstacles that EVs as a therapy can present, there have also been described several strategies and progresses to improve the therapeutic natural effect exhibited by the EVs themselves [93]. EVs activity can be boosted by stress-induced adaptive responses of parental cells to the environment they are exposed to [94] (see Fig. 2, panel B). One of the most prominent strategy shown to significantly modify EVs intraluminal cargo, increase EVs secretion and enhance EVs potency is the alteration of parental cells cell-culture parameters [95, 96]. These modifications can be either biochemical (growth factors, cytokines, bacteria-derived molecules, pharmacological drugs or chemical agents) or biophysical (cell seeding density, cell-passages, 2D and 3D scaffolds, mechanical stimuli, etc.) [97, 98]. EVs secreted by primed MSCs have been extensively studied as a therapy for sepsis [99]. Increasing evidence indicate that EVs derived from IL-1β-primed MSCs induce more effectively M2-like polarization of macrophages, ameliorate the septic symptoms and increase the survival rate when administered in a CLP septic model. These findings are attributed to the appearance of exosomal miR-146a [100] and miR-21 [101], which are significantly upregulated in EVs from primed MSCs. Ti et al. reported similar results, although in their study, the MSCs-derived EVs with improved wound healing abilities and resolution of inflammation in a mouse model of diabetic cutaneous wound were obtained by pre-treating the MSCs with LPS, which, in this case, augmented the expression of exosomal let-7b [102]. Furthermore, inflammatory priming of MSCs with TNF-α or IFN-γ was also shown to trigger the secretion of EVs that were able to initiate the production of immunomodulatory factors, reducing Th1-cell proliferation [103] and inducing T-reg cell differentiation in vitro [104]. In this line, hypoxic preconditioning of MSCs seems to show akin effects since their secreted EVs also regulate inflammatory responses [105], inhibit apoptosis [106] and stimulate cellular proliferation [107] in different pre-clinical models by mainly modifying the miRNA expression profile from EVs, even though there are some studies that demonstrate the opposite [108].
The biophysical environment of MSCs can also modulate their secretory profile [109], and specifically, the EVs production [110]. MSCs that were cultured in a platform incorporating both physiomimetic lung extracellular matrix conditions and mechanical stimulation by cyclic stretch fostered their anti-inflammatory and immunosuppressive paracrine action in vitro [111] (see Fig. 2, panel C). Regarding the specific influence of physical atmosphere on EVs secretion and their immunomodulatory effect on sepsis, remains unclear. Even so, there are some studies that found a greater amount of protein, better outcomes in immunomodulation and vascularization in EVs isolated from MSCs seeded in 3D cultures [112], submitted to a cyclic mechanical stretch [113] or maintaining a low cell density and using only low passages for EVs collection [110] when administered in experimental models.
Bioinspired synthetic EVs
Bioinspired synthetic EVs are artificially produced EVs that mimic their natural counterparts. They are being extensively studied, since they can overcome the cumbersome production of natural EVs, their tedious isolation, and other already-mentioned barriers that hinder their fast clinical translation [114]. Bioinspired synthetic EVs can be bioengineered via different strategies. They can be produced by cell fragmentation, using supramolecular chemistry, or fusing exosomes with synthetic nanomaterials to produce biohybrid structures [115]. These different approaches and examples are described below with more detail and are summarized in Fig. 3 and Table 1.
Fig. 3.
Different approaches to obtain bioinspired synthetic EVs. Strategies based on cell fragmentation, supramolecular chemistry and biohybrid EVs
Table 1.
Summary of bioinspired synthetic EVs generated by cell fragmentation, supramolecular chemistry or biohybrid approaches, and inorganic NPs used in the context of sepsis
| Starting material | Preparation method | Cargo | Particle characterization | Biological effect | Refs. |
|---|---|---|---|---|---|
| Bioinspired synthetic EVs | |||||
| Human promyelocytic leukemia HL-60 cells | Cell fragmentation: nitrogen cavitation + differential UC | TPCA-1 |
Size: ~ 200 nm (cryo-TEM) ζ-potential: − 16 mV |
Reduction of lung inflammation | [122] |
| Human monocyte U397 cells | Cell fragmentation: alkaline solution + sonication + UC | Dexamethasone | Size: 130 nm (DLS) | Reduction of IL-8 levels and mitigation of SIRS | [121] |
| MSCs | Cell fragmentation: serial extrusion (10, 5 and 1 μm) + DGUC | – | Size: 50–150 nm (TEM) | Decrease of inflammatory cytokines levels and immunomodulatory effect | [124] |
| Polymers (HA, PEI) | Supramolecular chemistry: polymer coupling and self-assembly | miR-7651-5p, miR-615-5p, miR-6239, miR-690, miR-206-3p, miR-466i-5p and miR-146a-5p | Size: ~ 100 nm | Reduction of TNF-α and IL-6 levels and relief of LPS-induced sepsis | [127] |
| Lipids (DPPC, DOPC, cholesterol) and membrane proteins from leukocytes | Supramolecular chemistry: mixing and assembly | – |
Size: 94 nm (DLS) ζ-potential: − 27 mV |
Decrease of IL-6, IL-1b and TNF-α, rise of IL-10 and TGF-β, and prolongation of murine survival | [128] |
| Amphotericin B-loaded liposomes | Commercial product | Amphotericin B |
Size: 103 nm (DLS) ζ-potential: − 54 mV |
Identification of sepsis biomarkers for diagnosis purposes | [129] |
| Polymer (Biotin-PEG-b-PAE(-g-PEG-b-DSPE)-b-PEG-Biotin) | Supramolecular chemistry: polymerization and self-assembly | Ciprofloxacin and TPCA-1 | Size: 120 nm at pH = 7.4 (DLS) | Decrease in the number of leukocytes, bacteria and inflammatory cytokines, and mitigation of systemic inflammation | [130] |
| Macrophage membrane + lipids (PC and DSPE-PEG2000) | Biohybrid strategy: stirring + sonication + extrusion (800, 400 and 200 nm) | – |
Size: 203 nm (DLS) ζ-potential: − 23 mV |
Elimination of LPS and reduction of IL-1β, IL-6 and TNF-α levels | [134] |
| Inorganic NPs | |||||
| Fe3O4 NPs, chlorin e6 and aptamer | Fe3O4 NPs synthesis: thermal decomposition; EDC/NHS covalent coupling between Fe3O4 NPs, chlorin e6 and aptamer | – |
Size: 17 nm (TEM) ζ-potential: − 10 mV |
Detection, death and separation of bacteria from contaminated blood | [136] |
| Ceria NPs with mPEG-TK-PLGA coating | Ceria NPs synthesis: sol–gel; emulsion for polymer conjugation | Atorvastatin |
Size: 43 nm (DLS) ζ-potential: − 4 mV |
Suppression of excessive ROS levels in mitochondria and reduction of inflammation | [137] |
EDC 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, DLS dynamic light scattering, DGUC density gradient ultracentrifugation, DOPC 1,2-dioleoyl-sn-glycero-3-phosphocholine, DPPC 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DSPE 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine, HA hyaluronic acid, LPS lipopolysaccharide, MSCs mesenchymal stem cells, NHS N-hydroxysuccinimide, PC phosphatidylcholine, PEG polyethylene glycol, PEI polyethylenimine, ROS reactive oxygen species, SIRS systemic inflammatory response syndrome, TK thioketal, TPCA-1 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide, UC ultracentrifugation
Bioinspired synthetic EVs prepared by cell fragmentation
In the case of cell fragmentation, cells are forced to disintegrate to form nanosized vesicles. Their membranes contain natural lipids, proteins and nucleic acids from their parent cells, so they are very similar to natural EVs. Several fragmentation approaches have been reported to produce synthetic EVs starting from parental cells. Extrusion through nanosized polycarbonate membrane filters is widely used to turn cells into vesicles with reduced size, which preserve the topology of membrane proteins [116]. In addition, microfluidic systems have been developed to isolate, detect, analyze and engineer nanosized particles, such as exosomes [117, 118]. In these systems, living cells are forced to go through an array of parallel hydrophilic microchannels, in which they are broken into smaller fragments which then self-assemble forming artificial EVs [119]. Cells can also be disrupted by nitrogen cavitation to form exosome-like nanovesicles. This procedure relies on the dissolution of nitrogen in cells' cytoplasm under high pressure and a subsequent depressurization and release of this gas, causing the appearance of bubbles and the rupture of the cell membrane [120]. The exposure of cells to an alkaline solution forces the cells to break into membrane fragments, which can be re-assembled by sonication forming EVs.
One of the main advantages of cell fragmentation is the obtaining of homogeneous EVs populations. In addition, this approach is done in few steps, does not use organic solvents and can increase the production yield by more than 200-fold with respect to natural EVs [121], demonstrating the scalability of the method. Their physical and chemical compositions are not compromised, but the purification methods are still time-consuming.
Gao et al. successfully produced neutrophil-derived nanovesicles by two cycles of nitrogen cavitation under a pressure of 350–400 psi for 20 min, which triggered the physical disruption of neutrophils [122]. These neutrophil-derived EVs possessed integrin β2, a protein that binds to intercellular adhesion molecule 1 (ICAM-1), which is highly expressed in endothelial cells during inflammation [123]. These EVs were loaded with TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide) by incubation and were administered intravenously in an LPS model, reducing the lung inflammation and edema [122].
Furthermore, Go et al. developed EV-mimetic nanovesicles using human monocyte U397 cells, which were broken into membrane fragments by an alkaline solution and luminal cytosolic components were discarded by ultracentrifugation [121]. Sonication allowed the formation of EVs and their loading with dexamethasone, an anti-inflammatory agent. Using dexamethasone-loaded EVs, IL-8 levels in human umbilical vein endothelial cells (HUVECs) were reduced and systemic inflammatory response syndrome (SIRS) was mitigated in mice after intravenous administration of the nanovesicles loaded with the anti-inflammatory drug [121].
Park et al. produced EVs from MSCs by fragmenting cells through serial extrusions using different pore-sized polycarbonate membrane filters (10, 5 and 1 µm) [124]. The administration of MSC-derived EVs significantly decreased cytokine release into systemic circulation and monocyte and neutrophil infiltration in the peritoneum, demonstrating the immunomodulatory effect of MSC-derived EVs in a murine model of sepsis [124].
Bioinspired fully synthetic EVs prepared by supramolecular chemistry
Bioinspired artificial EVs can be produced by bottom-up approaches using individual components of the cellular membrane (lipids and proteins), which interact by supramolecular chemistry to form spherical structures that mimic EVs [115]. These nanocarriers show great potential as exosome-mimics when they are modified with chemical groups or conjugated with specific biomolecules, such as membrane proteins, peptides, or antibodies, to simulate the composition of natural EVs. These bottom-up approaches need a complete understanding of each component in the naturally derived exosomes to develop nanocarriers with superior characterization control and clean composition [116]. These entirely synthetic EVs may better satisfy the specifications of medical regulative agencies and have higher pharmaceutical acceptability than their natural counterparts because of their homogeneous and reproducible production, and besides, their cargoes can be loaded more efficiently. Nevertheless, further research is required to reproduce a complex lipid and protein natural structure starting from the building blocks. Liposomes, with their lipid double layer, and polymerosomes have emerged as strategic elements in cell mimicking [125]. Furthermore, biodegradable polymers, such as poly(lactic-co-glycolic acid) (PLGA), have also been used to develop nanocarriers that resemble EVs in size and morphology. PLGA nanocarriers have been proposed as a potential pulmonary drug delivery system, since they did not exhibit signs of cytotoxicity and showed and excellent lung biodistribution after intratracheal instillation, offering a suitable tool for the treatment of sepsis-induced acute lung injury [126]. Below, several studies are shown that demonstrate the efficacy of this type of nanocarriers for the treatment of sepsis-induced ARDS.
Li et al. synthesized hyaluronic acid-polyethylenimine (HA-PEI) NPs to mimic exosomes secreted by tumor cells that were shown to exert protective effects against sepsis [127]. This process included the chemical coupling of HA and PEI and the self-assembly of the conjugated polymer into nanosized particles, which were loaded with the seven miRNAs that were identified as the responsible for the therapeutic effect of tumoral exosomes. They observed that the obtained NPs did not alter either cell viability or permeability, and they were protective against inflammation [127]. Moreover, serum levels of cytokines TNF-α and IL-6 were decreased, and LPS-induced sepsis in mice and cynomolgus monkeys was relieved after the administration of these NPs, being promising candidates for the treatment of sepsis and cytokine-storm-related conditions [127].
Molinaro et al. engineered liposome-like nanocarriers, namely leukosomes, by blending synthetic lipids (1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and cholesterol at 4:3:3 molar ratio) with membrane proteins from leukocytes [128]. In vitro studies with macrophages treated with leukosomes demonstrated a decreased expression of pro-inflammatory genes (IL-6, IL-1b, and TNF-α) and a raised expression of anti-inflammatory ones (IL-10 and TGF-β), indirectly causing an anti-inflammatory response [128]. In vivo experiments in an LPS-induced model of sepsis elucidated that leukosomes allowed the targeting of inflamed tissues and significantly prolonged survival [128].
Papafilippou et al. used commercially available and clinically used amphotericin B-loaded liposomes (AmBisome®) to rapidly and accurately diagnose and differentiate between sepsis, triggered by an infection, and non-infectious acute systemic inflammation in humans [129]. After incubating these liposomes with plasma from two groups of patients, one suffering from sepsis and the other from a non-infectious acute systemic inflammation, a protein corona was formed through the spontaneous interaction of plasma proteins with liposomes in both cases [129]. The protein corona was deeply characterized and compared by mass spectrometry to demonstrate that the proposed synthetic nanosystem allowed the identification of 67 potential biomarker proteins for the reproducible distinction between non-infectious acute systemic inflammation and sepsis [129]. Therefore, the liposome-corona platform could fasten the clinical evaluation and precisely diagnose sepsis, avoiding unneeded antibiotic treatments.
Zhang et al. designed and synthesized polymeric micelles by self-assembly using an amphiphilic block copolymer (Biotin-PEG-b-PAE(-g-PEG-b-DSPE)-b-PEG-Biotin). The micelles were loaded with an antibiotic (ciprofloxacin) and an anti-inflammatory agent (TPCA-1) to prevent bacterial dissemination and mitigate inflammation [130]. In addition, the polymeric micelles were coated with ICAM-1 antibodies to target infected tissues, which enhanced drug delivery efficacy [130]. These nanosystems were responsive to the acidic pH and bacterial enzymes present in infectious microenvironments, triggering the release of the drugs [130]. The administration of the drug-loaded micelles in an acute peritonitis model significantly reduced the leukocytes, bacteria, and inflammatory cytokines, indicating a suppression of the peritoneal infection, leading to a mitigated systemic inflammation [130].
Biohybrid EVs
Natural EVs and synthetic nanostructures, such as liposomes or polymersomes, can be fused to form biohybrid vesicles that combine the advantages of both systems without altering their intrinsic properties [115]. Synthetic nanosystems contribute with stability, high and controlled production and the possibility of drug loading. There are different methods by which biohybrid EVs can be produced, such as the freeze–thaw technique, in which natural EVs and liposomes are mixed, frozen and thawed for several cycles [131]. Another option is by simply incubating natural EVs and liposomes, which may lead to the formation of biohybrid structures without external stimuli, since both have a lipid bilayer [132]. Finally, membranes of exosomes and liposomes break and merge to form hybrid EVs by the co-extrusion method, in which they undergo physical stress when going through membrane pores [133].
Regarding the utilization of biohybrid EVs for sepsis treatment, Jiang et al. used sonication to develop a macrophage-mimetic hybrid liposome by fusing macrophage membranes with artificial lipids (phosphatidylcholine and DSPE-PEG2000), which stabilized the natural membrane and prolonged the blood circulation time [134]. The resulting macrophage-mimetic hybrid liposome combined the advantages of both natural and artificial membranes. It reduced the toxicity of LPS in activated macrophages, protecting mice against septic shock, and reducing the levels of IL-1β, IL-6 and TNF-α [134].
Inorganic nanoparticles for sepsis
In addition to synthetic bioinspired EVs, several inorganic nanoparticles (NPs) have also been investigated for the diagnosis and treatment of sepsis to mimic the natural effect of EVs. The use of NPs for these purposes presents great potential due to the possibility of engineering NPs with different compositions, sizes, shapes, and surface charges and their capacity for surface functionalization, allowing targeting and selective binding [135]. Still, toxicity, rapid clearance, and difficulties crossing some biological barriers are these systems' main drawbacks [115]. However, magnetic NPs, metallic NPs or quantum dots, combined with lab-on-a-chip devices, point-of-care (POC) technologies or biosensors, have been examined for fast and sensitive sepsis detection and therapy [135].
As an example, Wang et al. developed a nanosystem for early sepsis diagnosis and extracorporeal blood disinfection as a treatment to eliminate the bacteria causing sepsis [136]. The platform was based on iron oxide (Fe3O4) NPs functionalized with chlorin e6 (Ce6) and bacterial species-identifiable aptamers (Apt) [136]. The aptamer helped to capture bacteria, while the Fe3O4 NPs allowed magnetic separation for the detection and enrichment of bacteria, and Ce6 acted as a photosensitizer that exhibited high photosensitizing efficacy. Based on this, the Fe3O4–Ce6–Apt nanosystem allowed a successful and rapid diagnosis of sepsis caused by single or multiple species of bacteria (S. aureus and E. coli) [136]. Due to the strong photodynamic effect and magnetic properties of the Fe3O4–Ce6–Apt nanosystem, it was also studied for the disinfection of extracted contaminated blood by first irradiating it with a NIR laser, causing the death of bacteria, and then magnetically removing the pathogens [136]. Disinfected blood could be reused for mice transfusion without adverse reactions, suggesting the successful potential of the Fe3O4–Ce6–Apt nanosystem for sepsis treatment [136]. In addition, Yu et al. designed and synthesized a reactive oxygen species (ROS)-responsive nanosystem which combined mitochondria-targeting ceria (CeO2) NPs with atorvastatin for acute kidney injury caused by sepsis [137]. CeO2 NPs were conjugated with triphenylphosphine, coated with mPEG-TK-PLGA, a ROS-responsive organic polymer that improved the biocompatibility of the nanosystem, and subsequently loaded with atorvastatin [137]. The NPs were accumulated in the kidneys and targeted specifically the mitochondria to suppress excessive ROS levels. They also efficiently reduced inflammation in vivo and exhibited antioxidant and antiapoptotic effects in vitro [137].
Conclusions
Sepsis is a complex syndrome characterized by its high heterogeneity between patients, which hinders its diagnosis and therefore, the administration of an efficient and definitive treatment. The need for a more personalized management of sepsis and the malleable characteristics of EVs, whether synthetic or natural, may be the focal point for the development of a therapy targeting all different pathways that confer its pathophysiology in the near future.
Here we have presented not only the role that EVs play in the progression of sepsis and how it could be used to develop more precise diagnosis and prognosis methods, but also highlighted the potential of natural EVs as a treatment for sepsis. In addition, the research discussed presently shows the wide range of emerging bioengineering strategies to enhance the beneficial effect of EVs or their use as cell-based delivery systems, which, in turn, leads to overcoming the inherent challenges that cellular therapy exerts. Furthermore, the recent research advancements in nanotechnology have opened up several exciting avenues to develop innovative approaches with a marked translational character, offering a potential precise therapy for sepsis.
Despite the promising prospects described in this review, future research is needed to further study the contribution of EVs in sepsis progression to provide a useful and prompt diagnostic method to determine more precisely the severity and the specific sepsis phenotype of each patient. Besides, additional investigation in regard to the septic pathological environment is required, as well as, the refinement of engineering processes and clinical development in order to obtain a definitive EVs-based sepsis treatment.
Acknowledgements
The authors thank Wid Mekseriwattana for helping with the preparation and revision of this manuscript.
Author contributions
AAB and ASP contributed equally in the design of the review, searched the literature, elaborated the figures and wrote the manuscript. MCR, AR, DC and AA critically revised the manuscript for important intellectual content and accurate English. All authors read and approved the last version of the manuscript.
Funding
This work was supported by the Fundación Ramón Areces (CIVP19S8207), the Ministerio de Economía y Competitividad-Instituto de Salud Carlos III (PI18/00677) and the Generalitat de Catalunya (2021SGR00446).
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aina Areny-Balagueró and Anna Solé-Porta contributed equally to this work
Contributor Information
Aina Areny-Balagueró, Email: aareny@tauli.cat.
Anna Solé-Porta, Email: asole@icmab.es.
Antonio Artigas, Email: aartigas@tauli.cat.
References
- 1.Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NKJ, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazar AE, Azamfirei L. Personalized medicine for the critically ill patient: a narrative. Rev Process. 2022 doi: 10.3390/pr10061200. [DOI] [Google Scholar]
- 6.Reinhart K, Daniels R, Kissoon N, et al. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med. 2017;377:411–414. doi: 10.1056/nejmp1704633. [DOI] [PubMed] [Google Scholar]
- 7.Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet. 2014;86:56–61. doi: 10.1111/cge.12368. [DOI] [PubMed] [Google Scholar]
- 8.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA J Am Med Assoc. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogos C, Kotsaki A, Pelekanou A, et al. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care. 2010;14:1–12. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Does Y, Limper M, Jie KE, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study) Clin Microbiol Infect. 2018;24:1282–1289. doi: 10.1016/j.cmi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Piffoux M, Nicolás-Boluda A, Mulens-Arias V, et al. Extracellular vesicles for personalized medicine: the input of physically triggered production, loading and theranostic properties. Adv Drug Deliv Rev. 2019;138:247–258. doi: 10.1016/j.addr.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-rodriguez JC, Plata-menchaca EP, Chiscano-camón L, et al. Precision medicine in sepsis and septic shock: from omics to clinical tools. World J Crit Care Med. 2022;11:1–22. doi: 10.5492/wjccm.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillalamarri N, Ren G, et al. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl Oncol. 2021;14:101095. doi: 10.1016/j.tranon.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res. 2018;19:1–14. doi: 10.1186/s12931-018-0921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra H, Drummen GPC, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igami K, Uchiumi T, Ueda S, et al. Characterization and function of medium and large extracellular vesicles from plasma and urine by surface antigens and Annexin V. PeerJ Anal Chem. 2020;2:e4. doi: 10.7717/peerj-achem.4. [DOI] [Google Scholar]
- 17.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Wu X, Chandra S, et al. Extracellular vesicles: emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Wang L, Zeng X, et al. Exosomes, a new star for targeted delivery. Front Cell Dev Biol. 2021 doi: 10.3389/fcell.2021.751079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Feng Y, Jeyaram A, et al. Circulating plasma extracellular vesicles from septic mice induce inflammation via MicroRNA- and TLR7-dependent mechanisms. J Immunol. 2018;201:3392–3400. doi: 10.4049/jimmunol.1801008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dakhlallah DA, Wisler J, Gencheva M, et al. Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. J Extracell Vesicles. 2019 doi: 10.1080/20013078.2019.1669881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im Y, Yoo H, Lee JY, et al. Association of plasma exosomes with severity of organ failure and mortality in patients with sepsis. J Cell Mol Med. 2020;24:9439–9445. doi: 10.1111/jcmm.15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang W, Shimizu M, Lee JW. Role of extracellular vesicles in severe pneumonia and sepsis. Expert Opin Biol Ther. 2022;22:747–762. doi: 10.1080/14712598.2022.2066470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuravi SJ, Yates CM, Foster M, et al. Changes in the pattern of plasma extracellular vesicles after severe trauma. PLoS ONE. 2017;12:1–17. doi: 10.1371/journal.pone.0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iba T, Ogura H. Role of extracellular vesicles in the development of sepsis-induced coagulopathy. J Intensive Care. 2018;6:1–12. doi: 10.1186/s40560-018-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalic-Cosic S, Dopsaj V, Kovac M, et al. Phosphatidylserine exposing extracellular vesicles in pre-eclamptic patients. Front Med. 2021;8:1–10. doi: 10.3389/fmed.2021.761453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Lu W, Zhang Y, et al. HHS Public Access. 2020;50:1401–1411. doi: 10.1016/j.immuni.2019.04.003.Inflammasome. [DOI] [Google Scholar]
- 28.Wang JG, Manly D, Kirchhofer D, et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–1098. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. 2020;89:107087. doi: 10.1016/j.intimp.2020.107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarczak D, Kluge S, Nierhaus A. Sepsis—pathophysiology and therapeutic concepts. Front Med. 2021;8:1–22. doi: 10.3389/fmed.2021.628302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao K, Jin J, Huang C, et al. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front Immunol. 2019;10:1–11. doi: 10.3389/fimmu.2019.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youn YJ, Shrestha S, Bin LY, et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics. 2021;11:2770–2787. doi: 10.7150/THNO.51756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarà M, Guidetti GF, Camera M, et al. Biology and role of extracellular vesicles (Evs) in the pathogenesis of thrombosis. Int J Mol Sci. 2019;20:2840. doi: 10.3390/ijms20112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair RR, Mazza D, Brambilla F, et al. LPS-challenged macrophages release microvesicles coated with histones. Front Immunol. 2018;9:1–15. doi: 10.3389/fimmu.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulapurkar ME, Ramarathnam A, Hasday JD, Singh IS. Bacterial Lipopolysaccharide augments febrile-range hyperthermia-induced heat shock protein 70 expression and extracellular release in human THP1 cells. PLoS ONE. 2015;10:1–15. doi: 10.1371/journal.pone.0118010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Li G, Liu Y, et al. Translocation of endogenous danger signal HMGB1 from nucleus to membrane microvesicles in macrophages. J Cell Physiol. 2016;231:2319–2326. doi: 10.1002/jcp.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13:301–315. doi: 10.1038/cmi.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anush MM, Ashok VK, Sarma RIN, Pillai SK. Role of c-reactive protein as an indicator for determining the outcome of sepsis. Indian J Crit Care Med. 2019;23:11–14. doi: 10.5005/jp-journals-10071-23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fendl B, Weiss R, Eichhorn T, et al. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-021-86489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrascal M, Areny-Balagueró A, de-Madaria E, et al. Inflammatory capacity of exosomes released in the early stages of acute pancreatitis predicts the severity of the disease. J Pathol. 2022;256:83–92. doi: 10.1002/path.5811. [DOI] [PubMed] [Google Scholar]
- 42.Tubita V, Callejas-Díaz B, Roca-Ferrer J, et al. Role of microRNAs in inflammatory upper airway diseases. Allergy Eur J Allergy Clin Immunol. 2021;76:1967–1980. doi: 10.1111/all.14706. [DOI] [PubMed] [Google Scholar]
- 43.Real JM, Ferreira LRP, Esteves GH, et al. Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit Care. 2018;22:1–11. doi: 10.1186/s13054-018-2003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu G, Fan J, Zheng G, et al. Diagnostic potential of plasma extracellular vesicle miR-483-3p and Let-7d-3p for sepsis. Front Mol Biosci. 2022;9:1–12. doi: 10.3389/fmolb.2022.814240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menezes SLS, Bozza PT, Castro Faria Neto HC, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 46.Herwig MC, Tsokos M, Hermanns MI, et al. Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiology. 2013;80:245–251. doi: 10.1159/000347062. [DOI] [PubMed] [Google Scholar]
- 47.Sharp C, Millar AB, Medford ARL. Advances in understanding of the pathogenesis of acute respiratory distress syndrome. Respiration. 2015;89:420–434. doi: 10.1159/000381102. [DOI] [PubMed] [Google Scholar]
- 48.Weber B, Franz N, Marzi I, et al. Extracellular vesicles as mediators and markers of acute organ injury: current concepts. Eur J Trauma Emerg Surg. 2022;48:1525–1544. doi: 10.1007/s00068-021-01607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatterjee V, Yang X, Ma Y, et al. Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Hear Circ Physiol. 2020;319:H1181. doi: 10.1152/AJPHEART.00579.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Essandoh K, Yang L, Wang X, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta Mol Basis Dis. 2015;1852:2362–2371. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coelho FR, Martins JO. Diagnostic methods in sepsis: the need of speed. Rev Assoc Méd Bras (English Ed) 2012;58:498–504. doi: 10.1016/s2255-4823(12)70236-9. [DOI] [PubMed] [Google Scholar]
- 52.Schenz J, Weigand MA, Uhle F. Molecular and biomarker-based diagnostics in early sepsis: current challenges and future perspectives. Expert Rev Mol Diagn. 2019;19:1069–1078. doi: 10.1080/14737159.2020.1680285. [DOI] [PubMed] [Google Scholar]
- 53.Hermann S, Brandes F, Kirchner B, et al. Diagnostic potential of circulating cell-free microRNAs for community-acquired pneumonia and pneumonia-related sepsis. J Cell Mol Med. 2020;24:12054–12064. doi: 10.1111/jcmm.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20:5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Liang G, Shen J, et al. Long non-coding RNAs as biomarkers and therapeutic targets in sepsis. Front Immunol. 2021;12:1–16. doi: 10.3389/fimmu.2021.722004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kronstadt SM, Pottash AE, Levy D, et al. Therapeutic potential of extracellular vesicles for sepsis treatment. Adv Ther. 2021;4:200259. doi: 10.1002/adtp.202000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashemian SMR, Pourhanifeh MH, Fadaei S, et al. Non-coding RNAs and exosomes: their role in the pathogenesis of sepsis. Mol Ther Nucleic Acids. 2020;21:51–74. doi: 10.1016/j.omtn.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Ku X, Wu C, et al. Exosomal proteome analysis of human plasma to monitor sepsis progression. Biochem Biophys Res Commun. 2018;499:856–861. doi: 10.1016/j.bbrc.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Tang TT, Lv LL, Lan HY, Liu BC. Extracellular vesicles: opportunities and challenges for the treatment of renal diseases. Front Physiol. 2019;10:1–12. doi: 10.3389/fphys.2019.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaimardanova A, Solovyeva V, Chulpanova D, et al. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res. 2020;15:586–596. doi: 10.4103/1673-5374.266908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Res Ther. 2019;10:1–18. doi: 10.1186/s13287-019-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian J, Casella G, Zhang Y, et al. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int J Biol Sci. 2020;16:620–632. doi: 10.7150/ijbs.39629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Abreu RC, Fernandes H, da Costa Martins PA, et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685–697. doi: 10.1038/s41569-020-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:1–15. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrnes D, Masterson CH, Artigas A, Laffey JG. Mesenchymal stem/stromal cells therapy for sepsis and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2021;42:20–39. doi: 10.1055/s-0040-1713422. [DOI] [PubMed] [Google Scholar]
- 66.Guillamat-Prats R, Camprubí-Rimblas M, Bringué J, et al. Cell therapy for the treatment of sepsis and acute respiratory distress syndrome. Ann Transl Med. 2017 doi: 10.21037/atm.2017.08.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Areny-balagueró A, Artigas A, Laffey JG, Closa D. Cellular therapies in ARDS. Signa Vitae. 2022 doi: 10.22514/sv.2022.059. [DOI] [Google Scholar]
- 68.Allan D, Tieu A, Lalu M, Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9:39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varkouhi AK, Mirjana J, Lindsay O, Stéphane G, Sakshi G, Razieh R, Claire M, Chris S, Paul C, Gu Z, Frank X, dos Santos CC, Curley GF, Laffey JG. Extracellular vesicles human umbilical cord coli-induced acute lung injury in rats. Anesthesiology. 2019;130:778–790. doi: 10.1097/ALN.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 71.Bin FS, Zhang HY, Meng XC, et al. Small extracellular vesicles derived from human MSCs prevent allergic airway inflammation via immunomodulation on pulmonary macrophages. Cell Death Dis. 2020 doi: 10.1038/s41419-020-2606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Li C, Liang Z, et al. Human mesenchymal stromal cells small extracellular vesicles attenuate sepsis-induced acute lung injury in a mouse model: the role of oxidative stress and the mitogen-activated protein kinase/nuclear factor kappa B pathway. Cytotherapy. 2021;23:918–930. doi: 10.1016/j.jcyt.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 76.Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Breyne K, Ughetto S, Rufino-Ramos D, et al. Exogenous loading of extracellular vesicles, virus-like particles, and lentiviral vectors with supercharged proteins. Commun Biol. 2022 doi: 10.1038/s42003-022-03440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villata S, Canta M, Cauda V. Evs and bioengineering: from cellular products to engineered nanomachines. Int J Mol Sci. 2020;21:1–32. doi: 10.3390/ijms21176048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Betzer O, Perets N, Angel A, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 82.Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 2019 doi: 10.1007/s11010-019-03545-4. [DOI] [PubMed] [Google Scholar]
- 83.Susa F, Limongi T, Dumontel B, et al. Engineered extracellular vesicles as a reliable tool in cancer nanomedicine. Cancers (Basel) 2019;11:1979. doi: 10.3390/cancers11121979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeyaram A, Lamichhane TN, Wang S, et al. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol Ther. 2020;28:975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding L, Zhou W, Zhang J, et al. Calming egress of inflammatory monocytes and related septic shock by therapeutic CCR2 silencing using macrophage-derived extracellular vesicles. Nanoscale. 2022;14:4935–4945. doi: 10.1039/D1NR06922E. [DOI] [PubMed] [Google Scholar]
- 87.Jin G, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil_derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2018;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng LY, Yuan M, Shi HT, et al. Protective effect of piceatannol against acute lung injury through protecting the integrity of air-blood barrier and modulating the TLR4/NFκB signaling pathway activation. Front Pharmacol. 2020;10:1–10. doi: 10.3389/fphar.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie L, Wu Y, Zhou C, et al. Piceatannol protects against sepsis-induced myocardial dysfunction via direct inhibition of JAK2. Int Immunopharmacol. 2021;96:107639. doi: 10.1016/j.intimp.2021.107639. [DOI] [PubMed] [Google Scholar]
- 90.Han Y, Jones TW, Dutta S, et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes. 2021;9:1–19. doi: 10.3390/pr9020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perteghella S, Crivelli B, Catenacci L, Sorrenti M, Bruni G, Necchi V, Vigani B, Sorlini M, Torre ML. Stem cell-extracellular vesicles as drug delivery systems: new frontiers for silk/curcumin nanoparticles. Int J Pharm. 2017;520:86–97. doi: 10.1016/j.ijpharm.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Zhu T, Xiao Y, Meng X, et al. Nanovesicles derived from bispecific CAR-T cells targeting the spike protein of SARS-CoV-2 for treating COVID-19. J Nanobiotechnol. 2021;19:1–17. doi: 10.1186/s12951-021-01148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brennan M, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30:1909125. doi: 10.1002/adfm.201909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31–39. doi: 10.1016/j.blre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Adlerz K, Patel D, Rowley J, et al. Strategies for scalable manufacturing and translation of MSC-derived extracellular vesicles. Stem Cell Res. 2020;48:101978. doi: 10.1016/j.scr.2020.101978. [DOI] [PubMed] [Google Scholar]
- 96.Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noronha NDC, Mizukami A, Caliári-Oliveira C, et al. Correction to: priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies (Stem Cell Research and Therapy (2019) 10 (131) DOI: 10.1186/s13287-019-1224-y) Stem Cell Res Ther. 2019;10:1–21. doi: 10.1186/s13287-019-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel DB, Santoro M, Born LJ, et al. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36:2051–2059. doi: 10.1016/j.biotechadv.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrzejewska A, Lukomska B, Janowski M. Mesenchymal stem cells: from roots to boost. Stem Cells. 2019;176:139–148. doi: 10.1002/stem.3016.Mesenchymal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 101.Yao M, Cui B, Zhang W, et al. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658. doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- 102.Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:1–14. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gieseke F, Kruchen A, Tzaribachev N, et al. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol. 2013;43:2741–2749. doi: 10.1002/eji.201343335. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Q, Fu L, Liang Y, et al. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J Cell Physiol. 2018;233:6832–6840. doi: 10.1002/jcp.26436. [DOI] [PubMed] [Google Scholar]
- 105.Jun EK, Zhang Q, Yoon BS, et al. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/AKT pathways. Int J Mol Sci. 2014;15:605–628. doi: 10.3390/ijms15010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng H, Chang S, Xu R, et al. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11:1–14. doi: 10.1186/s13287-020-01737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu W, Li L, Rong Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 108.Peltzer J, Lund K, Goriot ME, et al. Interferon-γ and hypoxia priming have limited effect on the miRNA landscape of human mesenchymal stromal cells-derived extracellular vesicles. Front Cell Dev Biol. 2020;8:1–11. doi: 10.3389/fcell.2020.581436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park KS, Bandeira E, Shelke GV, et al. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–15. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel DB, Gray KM, Santharam Y, et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2:170–179. doi: 10.1002/btm2.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Falcones B, Söderlund Z, Ibáñez-Fonseca A, et al. hLMSC secretome affects macrophage activity differentially depending on lung-mimetic environments. Cells. 2022;11:1–13. doi: 10.3390/cells11121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Putu D, Shoveller J, Montaner J, Feng C, Nicoletti R, Kate Shannon GO. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. doi: 10.1016/j.neuint.2016.08.003.Systemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao F, Zuo B, Tao B, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann Transl Med. 2021;9:798–798. doi: 10.21037/atm-21-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.García-Manrique P, Gutiérrez G, Blanco-López MC. Fully artificial exosomes: towards new theranostic biomaterials. Trends Biotechnol. 2018;36:10–14. doi: 10.1016/j.tibtech.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;191(19):1–20. doi: 10.1186/S12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/NN402232G. [DOI] [PubMed] [Google Scholar]
- 117.Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–3577. doi: 10.1039/C7LC00592J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao Z, McGill J, Gamero-Kubota P, He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip. 2019;19:1877–1886. doi: 10.1039/C8LC01279B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Q, Heon M, Zhao Z, He M. Microfluidic engineering of exosomes: editing cellular messages for precision therapeutics. Lab Chip. 2018;18:1690. doi: 10.1039/C8LC00246K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simpson RJ. Disruption of cultured cells by nitrogen cavitation. Cold Spring Harb Protoc. 2010;2010:pdb.proto5513. doi: 10.1101/PDB.PROT5513. [DOI] [PubMed] [Google Scholar]
- 121.Go G, Lee J, Choi DS, et al. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8:1801082. doi: 10.1002/ADHM.201801082. [DOI] [PubMed] [Google Scholar]
- 122.Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016;224:208–216. doi: 10.1016/J.JCONREL.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 124.Park KS, Svennerholm K, Shelke GV, et al. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019;10:1–14. doi: 10.1186/S13287-019-1352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rideau E, Dimova R, Schwille P, et al. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47:8572–8610. doi: 10.1039/C8CS00162F. [DOI] [PubMed] [Google Scholar]
- 126.Areny-Balagueró A, Mekseriwattana W, Camprubí-Rimblas M, et al. Fluorescent PLGA nanocarriers for pulmonary administration: influence of the surface charge. Pharmaceutics. 2022;14:1447. doi: 10.3390/PHARMACEUTICS14071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Zhang H, Chen C, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of sepsis. Adv Mater. 2022;34:2108476. doi: 10.1002/ADMA.202108476. [DOI] [PubMed] [Google Scholar]
- 128.Molinaro R, Pastò A, Corbo C, et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11:13576–13586. doi: 10.1039/C9NR04253A. [DOI] [PubMed] [Google Scholar]
- 129.Papafilippou L, Claxton A, Dark P, et al. Protein corona fingerprinting to differentiate sepsis from non-infectious systemic inflammation. Nanoscale. 2020;12:10240–10253. doi: 10.1039/D0NR02788J. [DOI] [PubMed] [Google Scholar]
- 130.Yang Zhang C, Gao J, Wang Z, et al. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30:1803618. doi: 10.1002/ADMA.201803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:1–11. doi: 10.1038/SREP21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin Y, Wu J, Gu W, et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5:1700611. doi: 10.1002/ADVS.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu A, Yang G, Liu Y, Liu T. Research progress in membrane fusion-based hybrid exosomes for drug delivery systems. Front Bioeng Biotechnol. 2022 doi: 10.3389/FBIOE.2022.939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang L, Li R, Xu J, et al. Endotoxin-adsorbing macrophage-mimetic hybrid liposome for sepsis treatment. Chem Eng J. 2019;371:15–25. doi: 10.1016/J.CEJ.2019.04.032. [DOI] [Google Scholar]
- 135.Lim J, Lee YY, Bin CY, et al. Sepsis diagnosis and treatment using nanomaterials. Biomed Eng Lett. 2021;11:197. doi: 10.1007/S13534-021-00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang J, Wu H, Yang Y, et al. Bacterial species-identifiable magnetic nanosystems for early sepsis diagnosis and extracorporeal photodynamic blood disinfection. Nanoscale. 2017;10:132–141. doi: 10.1039/C7NR06373C. [DOI] [PubMed] [Google Scholar]
- 137.Yu H, Jin F, Liu D, et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10:2342–2357. doi: 10.7150/THNO.40395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.