Abstract
Introduction
Use of online registries to efficiently identify older adults with cognitive decline and Alzheimer's disease (AD) is an approach with growing evidence for feasibility and validity. Linked biomarker and registry data can facilitate AD clinical research.
Methods
We collected blood for plasma biomarker and genetic analysis from older adult Brain Health Registry (BHR) participants, evaluated feasibility, and estimated associations between demographic variables and study participation.
Results
Of 7150 participants invited to the study, 864 (12%) enrolled and 629 (73%) completed remote blood draws. Participants reported high study acceptability. Those from underrepresented ethnocultural and educational groups were less likely to participate.
Discussion
This study demonstrates the challenges of remote blood collection from a large representative sample of older adults. Remote blood collection from > 600 participants within a short timeframe demonstrates the feasibility of our approach, which can be expanded for efficient collection of plasma AD biomarker and genetic data.
Keywords: acceptability, aging research, Alzheimer's disease, brain health registry, education, engagement, ethnicity, feasibility, genetics, internet, plasma biomarkers, race, research registry
1. BACKGROUND
Recent studies demonstrate the potential for plasma biomarkers of amyloid beta (Aβ), phosphorylated tau (p‐tau), and neurofilament light (NfL) to identify older adults with dementia due to Alzheimer's disease (AD), as well as those with preclinical (presymptomatic, biomarker positive) and prodromal (early symptomatic) AD. 1 Compared to traditional methods (positron emission tomography, magnetic resonance imaging [MRI], cerebrospinal fluid) to measure Aβ, tau, and neurodegeneration, blood samples for plasma biomarkers are easy to collect and non‐invasive, and therefore have the potential to be used broadly for community‐based phenotyping.
Online registries can efficiently identify those at increased risk for AD, including those with preclinical and prodromal AD, with the potential to minimize participant and staff burden and reduce cost compared to in‐clinic studies. 2 , 3 , 4 , 5 The Brain Health Registry (BHR) is an online registry to facilitate recruitment and longitudinally monitor participants for neuroscience research. BHR, with >90,000 participants, collects cognitive, everyday functioning, mood, health history, family history, and lifestyle data remotely using self‐ and study partner–report questionnaires and online neuropsychological tests. 6 , 7 , 8 However, online registries typically lack biomarker phenotyping. BHR, like many in‐clinic studies, trials, and other online registries, lacks diversity of ethnoracial, and socioeconomic groups, 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 which limits the impact and generalizability of research findings. 16 The overall goal of this work was to assess the feasibility of remote blood collection from a representative group of older adults in an online registry for plasma biomarker analysis.
Using the BHR platform, we developed a novel online infrastructure for blood collection from BHR participants through a collaboration with local phlebotomy centers and automated blood sample tracking and participant communication. We assessed the feasibility and acceptability of our approach, and addressed scalability by estimating the amount of individual support participants requested during the study. To identify selection biases, we tested the hypothesis that individuals from ethnocultural and education attainment groups that have historically been under‐included from AD and related dementias (ADRD) clinical research are less likely to enroll in and complete the study. 13 , 14 , 15
2. METHODS
2.1. Brain Health Registry
BHR is a public online research registry and cohort for recruitment, assessment, and longitudinal monitoring with a focus on cognitive aging. 6 , 7 , 8 Participants provide informed consent electronically and are invited to complete unsupervised online self‐report questionnaires and online neuropsychological tests every 6 months. Each participant can invite a study partner, who separately enrolls, consents, and answers questions about the participant's cognition and everyday functioning. 6 This study included a sample of BHR participants (n = 7150) who were invited to complete blood draws at local phlebotomy centers.
1. RESEARCH IN CONTEXT
Systematic review: Using PubMed, authors reviewed past literature on remote saliva and blood collection, and associations between demographic variables and Alzheimer's disease (AD) clinical study participation. Novel analyses included: (a) feasibility and acceptability of remote blood collection in older adults enrolled in an online study; and (b) participation of underrepresented ethnocultural and education groups in the study. We also describe novel, online infrastructure for collecting and tracking remote blood draw.
Interpretation: We demonstrate feasibility and acceptability of remote blood collection for AD plasma biomarker and genetic analysis from a large cohort of older adults with longitudinal online data. Similar to past studies in other settings, we failed to enroll underrepresented populations and engage them adequately for study completion.
Future directions: Future studies will determine the associations between AD plasma biomarkers and online cognitive and functional variables. We also plan to expand and deploy culturally tailored strategies to improve diversity.
HIGHLIGHTS
Six hundred twenty‐nine Brain Health Registry participants completed the study in 8 weeks.
Blood was obtained remotely using local phlebotomy centers.
We failed to adequately enroll underrepresented ethnocultural populations.
We failed to adequately enroll those with low education levels.
Future studies will process blood samples for Alzheimer's disease plasma biomarkers.
2.2. Enrollment
Inclusion criteria included existing BHR participants age 55 and over with no clinical or self‐reported diagnosis of dementia. Dementia diagnosis was assessed by: (1) self‐report of the question, “Please indicate whether you currently have or have had any of the following conditions in the past: Alzheimer's Disease, Dementia, Frontotemporal Dementia (FTD), or Lewy Body Disease (LBD)” or (2) had a clinically confirmed diagnosis of dementia from another study. 17 Participants were required to have completed two BHR online cognitive assessments: Cogstate Brief Battery (CBB) 18 , 19 , 20 , 21 , 22 and study partner‐report Everyday Cognition Scale (ECog). 23 Additionally, participants were required to reside in California around the metropolitan areas of San Francisco, Palo Alto, or Los Angeles. To maximize recruitment of individuals from underrepresented populations (URPs), which we define herein as those who did not self‐identify as White and those with less than a bachelor's degree educational attainment, the maximum geographical radius around sites was 100 miles for URPs and 50 miles for all others. Due to future goals of obtaining imaging data in the same cohort, participants deemed unsafe for MRI were also excluded. MRI safety was assessed with the questions, “Do you currently have: A cardiac pacemaker/defibrillator; Any surgical metal or any foreign objects in your body; Any stents, filter, or intravascular coils; Internal pacing wires; Sternum wires; or Claustrophobia?” and “Have you worked extensively with metal (grinding, welding, etc.)?”
Participants meeting inclusion criteria were sent an e‐mail invitation describing the study and providing instructions for enrollment. Participants were recruited between February 24 and March 10, 2020 and May 28 and June 30, 2020. (Due to the COVID‐19 pandemic, the University of California San Francisco [UCSF] stopped all non‐essential research, which resulted in a pause in recruitment and enrollment from March 10 to May 28.)
2.3. Electronic informed consent
This study was approved by the University of California, San Francisco Institutional Review Board and conducted in accordance with all regulations regarding the ethnical use of research participants. All participants completed an online, electronic informed consent document. 24 Potential participants received a study invitation e‐mail. Those who selected a “Tell me more” link were taken to a longer description of the study after they logged into the BHR participant portal. The potential participant was then given the option to select “I am interested” or “I am not interested.” If they selected “I am interested,” they were taken to the electronic consent. Of the 864 participants that enrolled, 12 (1.4%) ultimately withdrew consent.
2.4. Online measures
2.4.1. Sociodemographics
Participants self‐reported sociodemographic variables including: age, sex (male, female), race (Asian, Black/African American, Caucasian/White, Native American, Pacific Islander, Other, decline to state), ethnicity (Latino, non‐Latino, decline to state), and educational attainment. Throughout the article, we refer to those who selected “White/Caucasian” as “White,” following current guidance on the reporting of ethnocultural identity. 25 For our analyses, multiple categories of race and ethnicity were collapsed into two categories (White, non‐White) due to the low overall number of non‐White individuals. Further, the categorical education variable was converted into a continuous variable, years of education, ranging from 6 to 20 years. Throughout the article, we use the term URPs to define ethnocultural and educational attainment groups who ADRD clinical researchers have historically failed to adequately recruit, retain, and engage in their research. 25 Due to this failure, these groups are under‐included and underrepresented in the vast majority in ADRD clinical research studies, including BHR. For our analyses, URP specifically refers to those who self‐identify as non‐White and those with lower levels of educational attainment.
2.4.2. Self‐reported memory concerns and cognitive impairment
Subjective memory complaints (SMC) were assessed with the question, “Are you concerned that you have a memory problem?” Self‐reported mild cognitive impairment (MCI) was assessed with the question, “Please indicate whether you currently have or have had any of the following conditions in the past: Mild Cognitive Impairment.”
2.4.3. Post‐study questionnaire
After the blood sample was successfully collected, participants received an e‐mail asking them to complete an online questionnaire about their experience. The post‐study questionnaire included questions about difficulty of scheduling an appointment at a Quest Diagnostics phlebotomy center, expectation of time to complete the blood draw, and willingness to participate in a similar study in the future.
2.4.4. Decline survey
Participants who were invited to enroll could actively decline participation by marking “not interested” from their invitation e‐mail, clicking the “decline” button at the end of the consent form, or by e‐mailing the study team to withdraw consent after enrolling. Participants who actively declined participation could complete an optional Decline Survey, in which they could indicate their reason(s) for decline, including “This study takes too much time,” “This study does not interest me,” “I have concerns about privacy or sharing information,” and “Other [open text].”
Participants who consented but did not complete their blood draw were sent an automated e‐mail either 7 or 13 days after consent asking to provide a status update. Reminder e‐mails were sent if the participant did not respond. Participants could select multiple reasons: (1) “I already visited a Quest Diagnostics Center and completed my blood draw”; (2) “I have already scheduled an appointment at a Quest Diagnostics Center and will be completing my blood draw shortly”; (3) “I have not scheduled an appointment, but I still wish to participate in the study by completing a blood draw”; and (4) “I no longer want to complete a blood draw.” Participants who selected option 4 were asked to provide the reason for declined participation by selecting from a list that included: (1) “I am no longer interested in participating”; (2) “I am no longer interested in participating at this time, due to concerns about the coronavirus (COVID‐19) pandemic”; (3) “I have concerns about privacy or sharing information”; (4) “The blood draw was too time consuming to complete”; (5) “There is no Quest Diagnostics location near my home”; (6) “I tried to complete the blood draw but was unable to complete it for another reason”; and (7) “Other [open text].”
2.5. Participant communication and support
A study‐specific phone number and e‐mail address were provided for participants to contact BHR staff with questions about the study. Additionally, participants could generate inquiries to BHR via multiple channels including social media and web‐based forms on the BHR website. Participant communication is managed by BHR staff using Zendesk®, a third‐party support tool that uses an e‐mail/message ticketing system to support and communicate with participants. Each ticket is investigated, sorted, and solved by BHR staff.
2.6. Phlebotomy
Blood samples were collected remotely using an existing network of phlebotomy centers managed by Quest Diagnostics.
2.6.1. Phlebotomy scheduling
Participants who consented were assigned three unique identification codes for the sample collection (randomly generated dummy codes for the participant's first name, last name, and date of birth required to complete Quest Diagnostics online scheduling forms) and sent instructions on how to schedule a visit at a Quest Diagnostics Patient Service Center (PSC) of their choosing (Figure 1). Participants could select from any of the 440 PSCs in California. Participants scheduled an appointment via the Quest Diagnostics website by: (1) selecting a local PSC; (2) entering dummy codes in “First Name,” “Last Name,” and “Date of Birth” fields; and (3) completing any other required fields. Quest Diagnostics sent e‐mails with appointment details and confirmation numbers. Participants arrived at their chosen Quest Diagnostics PSC and signed in either using their dummy codes or confirmation number. Participants were called in by the PSC phlebotomist and asked to verify their codes.
FIGURE 1.
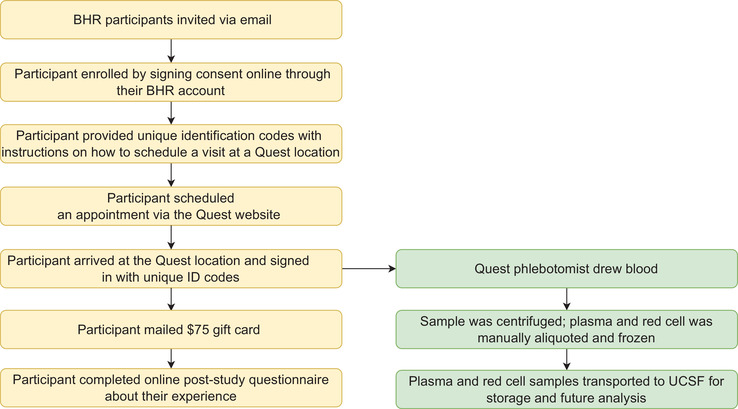
Participant flow. BHR, Brain Health Registry; UCSF, University of California, San Francisco
2.6.2. Blood sample handling
The PSC phlebotomist drew two 4 milliliter (mL) ethylenediaminetetraacetic acid tubes of blood labeled with a Quest Diagnostics test code and the participant's de‐identified codes. Immediately after collection, the sample was centrifuged at 1680± 90 g for 10 minutes. Plasma and blood cells were manually aliquoted. Aliquoted samples were stored at –20°C and transported on dry ice via courier to the Quest Diagnostics Core Lab. Specimens drawn that day were sent by late evening or early morning the next day and shipped overnight. Plasma and cell aliquots were stored at –80°C at the Quest Diagnostics Core Lab. Frozen samples were then shipped to the UCSF AIDS Specimen Bank (ASB), part of the Center for AIDS Research. Due to these constraints, participants were only allowed to schedule their appointments Monday through Wednesday before 1 pm. Participants that showed up outside of the time window were asked to reschedule. BHR was notified by ASB when sample collection was complete. Participants were then mailed a $75 gift card and asked to complete the online post‐study questionnaire about their experience.
FIGURE 2.
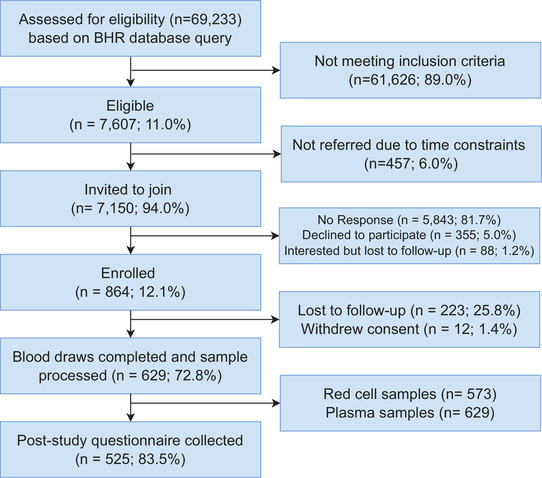
Study enrollment. BHR, Brain Health Registry
2.7. BHR biofluid collection management portal
Sample tracking and participant communication were automated using an adapted, expanded version of the BHR Biofluid Collection Management Portal, 26 which allows the study team to collect, store, maintain, and organize data related to remote blood and saliva collection. The application sent e‐mails automatically to provide participants with their unique codes and instructions on how to schedule an appointment, to alert participants when their sample was received by study staff, and to request a status update from participants if a sample was not collected after a specified time. The portal included a “code generator” that allowed study staff to specify criteria and generate unique participant identification codes. The portal also supported automated import of reports from ASB that automatically updated the database with collection date and status, and a participant payment management system.
2.8. Statistical analysis
Logistic regression was used to test for associations between demographic variables and four levels of study involvement: response to invitation, indication of interest, enrollment, and blood draw completion. As SMCs have previously been found to be associated with higher registry participation 27 and willingness with to participate in genetic studies, 26 we tested the hypotheses that SMC would be associated with increased odds of interest in the study and increased rate of enrollment and completion. We also hypothesized that ethnocultural and educational URP status is associated with lower odds of study participation. Predictors included: sex (male = 0, female = 1), age, education (years), ethnocultural status (non‐Latinx White = 0, URP = 1), and SMC (no = 0, yes = 1). The study involvement outcomes included the following categorical variables with “yes/no” responses: responded to invitation (among all invited), indicated interest (among total responded), enrolled (among total responded), and completed blood draw (among total enrolled). We included an additional, binary “yes/no” outcome variable of whether participants engaged in additional e‐mail communication with BHR (see section 2.5). Adjusted odds ratios (aOR), 95% confidence intervals (CI), and P‐values from the likelihood ratio test are reported for all logistic regression models. False discovery rate analyses were used to correct for multiple comparisons (four different outcome variables related to study participation and requested support). All analyses were done in R 28 and using four R packages (psych, 29 gmodels, 30 epiDisplay, 31 pROC 32 ).
3. RESULTS
3.1. Study participants
A total of 7150 BHR participants were invited to the study between February/March and May/June 2020 (Figure 2). Of invited participants, 864 (12.1%) enrolled, and 629 (72.8% of enrolled) completed a blood draw. Before joining the study, participants had been enrolled in BHR for an average of 4.8 years (±1.3 years; range: 53 days–6.9 years). Participants who completed a blood draw and had demographic information available (n = 624) had an average age of 67.1 (standard deviation [SD] ± 7.4) years, 438 (70.2%) were female, 547 (87.7%) identified as White, had an average of 16 (SD ± 2.3) years of education, and 12 (0.6%) self‐reported MCI (Table 1). All 629 samples were collected over 8 weeks, with 614 samples collected in the final 33 days.
TABLE 1.
Participant characteristics
| Invited (n = 7150) | Enrolled (n = 864) | Blood draw completed (n = 629) | Contacted BHR support (n = 264) | |
|---|---|---|---|---|
|
Age, mean ± SD (range) |
70 ± 8.5 (55.9, 110.3) |
68 ± 7.5 (55.9, 95.4) |
68 ± 7.5 (55.9, 95.4) |
71 ± 8.4) (56, 96.2) |
| Female, n (% of total) | 4967 (69%) | 606 (70%) | 438 (70%) | 188 (74%) |
| White, n (% of total) | 4926 (68%) | 739 (85%) | 552 (88%) | 222 (84%) |
|
Years education, mean ± SD (Range) |
16 ± 2.4 (6, 20) |
16 ± 2.4 (6, 20) |
16 ± 2.3 (6, 20) |
16 ± 2.5 (6, 20) |
| Subjective memory complaints, n (% of total) | 3273 (46%) | 364 (42%) | 244 (39%) | 114 (43%) |
| Self‐report MCI, n (% of total) | 231 (3%) | 35 (4%) | 22 (3%) | 12 (0.05%) |
Abbreviations: BHR, Brain Health Registry; MCI, mild cognitive impairment; SD, standard deviation.
3.2. Factors associated with study involvement
We considered whether sociodemographic factors or memory concerns were associated with the study involvement metrics—those who responded to the e‐mail invitation, indicated interest in the study, enrolled, and completed a blood draw (Table 2). Higher education levels were associated with higher probability of responding to the invitation, study enrollment, and completion. Identifying as non‐White was associated with lower probability of responding to the invitation and blood draw completion. Older participants had lower odds of responding to the invitation, indicating interest, and enrolling in this study. SMCs were associated with a lower probability of responding to the invitation and enrollment.
TABLE 2.
Associations between sociodemographic characteristics and study response, interest, enrollment, and completion
| Adjusted odds ratio | 95% confidence interval | p.fdr (LR‐test)* | |
|---|---|---|---|
| Responded to invitation (n = 7,150) | |||
| Female | 1.07 | 0.93, 1.23 | .581 |
| Years education | 1.09 | 1.06, 1.12 | .003 |
| Underrepresented race/ethnicity | 0.6 | 0.51, 0.72 | .005 |
| Age | 0.98 | 0.98, 0.99 | .002 |
| Reported subjective memory complaint | 0.82 | 0.72, 0.93 | .007 |
| Indicated interest in blood draw study (n = 1308) | |||
| Female | 0.82 | 0.61, 1.09 | .427 |
| Years education | 1.04 | 0.98, 1.09 | .173 |
| Underrepresented race/ethnicity | 0.9 | 0.62, 1.3 | .567 |
| Age | 0.94 | 0.92, 0.96 | .002 |
| Reported subjective memory complaint | 0.91 | 0.70, 1.18 | .657 |
| Enrolled in blood draw study (n = 940) | |||
| Female | 0.92 | 0.70, 1.2 | .672 |
| Years education | 1.07 | 1.01, 1.12 | 0.02 |
| Underrepresented race/ethnicity | 0.84 | 0.60, 1.19 | .426 |
| Age | 0.94 | 0.93, 0.96 | .002 |
| Reported subjective memory complaint | 1.0 | 0.78, 1.28 | .999 |
| Completed blood draw (n = 633) | |||
| Female | 1.01 | 0.71, 1.43 | .946 |
| Years education | 1.07 | 1.00, 1.14 | .055 |
| Underrepresented race/ethnicity | 0.57 | 0.37, 0.86 | .013 |
| Age | 1.01 | 0.99, 1.03 | .368 |
| Reported subjective memory complaint | 0.59 | 0.43, 0.82 | .005 |
| Contacted BHR Support (n = 263) | |||
| Female | 1.33 | 1.00, 1.78 | .24 |
| Years education | 1.09 | 1.04, 1.15 | .002 |
| Underrepresented race/ethnicity | 0.61 | 0.42, 0.89 | .013 |
| Age | 1.01 | 1.00, 1.03 | .192 |
| Reported subjective memory complaint | 0.92 | 0.71, 1.19 | .657 |
p.fdr = P‐value adjusted for multiple comparisons using false discovery rate analysis.
3.3. Acceptability
Of the 629 participants who completed a blood draw, 525 (83.5%) also completed a post‐study questionnaire. Of those, 486 (92.6%) rated blood draw scheduling as easy (1 or 2 based on a scale of 1 = least difficult to 5 = most difficult); 200 (38.1%) reported that it took “a lot less time” or “a little less time” than expected to complete the study; 238 (45.3%) reported that the time was “about what I expected”; and 510 (97.1%) reported that they would agree to participate in a similar, future study.
3.4. Reasons for declining participation
Of the 355 participants who actively declined participation and the 12 participants that withdrew consent, 18 (4.9%) completed a Decline Survey. The most common decline reason was concerns due to COVID‐19 (n = 6, 33.3%). Out of 327 consented participants who did not complete their blood draw and were asked by e‐mail about their blood draw status, 34 indicated that they no longer wanted to complete a blood draw. From the provided list of reasons participants most commonly selected “Other” (n = 12), describing issues with the phlebotomy center (n = 6), and “COVID‐19 concerns” (n = 9).
3.5. Volume and content of participant communication
Study staff received a total of 376 e‐mails from participants and potential participants. The five most common themes of the participant e‐mails were: confirming study interest (n = 61), participant payment (n = 47), phlebotomy center issues (n = 47), assistance with scheduling (n = 45), and residing outside of California (n = 37; Table 3). In addition, ≈176 participants called the study support telephone line. Of the participants who completed the blood draw (n = 629), 20% contacted BHR via e‐mail (n = 127). Of the total number of participants we identified as contacting BHR via Zendesk (n = 263), 48% completed the blood draw (n = 127). Those with higher education levels were more likely to request support, and URP individuals were less likely to request support (Table 2).
TABLE 3.
Participant e‐mail support themes
| Support themes | Number of e‐mails received (out of a total of 376 e‐mails) |
|---|---|
| Confirming interest | 61 |
| Participant payment | 47 |
| Phlebotomy center issues | 47 |
| Assistance with scheduling | 45 |
| Out of state (California) | 37 |
| Other | 29 |
| Access to results | 24 |
| COVID‐19 | 24 |
| Questions about study | 22 |
| BHR technical issue | 15 |
| No local Quest Diagnostics Center | 6 |
| Blood draw concerns | 4 |
| Privacy concerns | 2 |
| Time burden | 2 |
4. DISCUSSION
There were two major findings of this study. (1) Remote blood sample collection for AD plasma biomarker analysis from a large cohort of previously characterized older adults in an online registry is feasible and has high acceptability among participants. This was supported by enrollment of >600 participants (12% of those invited) within 8 weeks, a high study completion rate (73% of those enrolled), and positive participant feedback about their study experience. (2) Our study failed to refer and engage a representative number of individuals from underrepresented ethnocultural and educational groups. These results demonstrate that our approach has potential as a method to collect blood for plasma AD biomarker analysis in a large group of older adults. Major challenges of this approach include the need for effective, culturally tailored strategies to mitigate the selection bias for highly educated White individuals at multiple levels of study involvement, and to minimize the amount of one‐on‐one participant interaction needed to complete the study, for scalability.
To our knowledge, this study was the first to demonstrate feasibility and acceptability of remote blood sample collection in a large cohort of older adults engaged in longitudinal online evaluation. The high completion rate supports the feasibility, while the positive experience feedback shows participant acceptability. This adds to emerging evidence for the feasibility of adding biofluids collection to online studies, as was previously shown with remote saliva collection for apolipoprotein E (APOE) genotyping in BHR 26 and the Banner Alzheimer's Prevention Registry GeneMatch study. 33 Blood draws were collected in a relatively short time frame (> 600 blood draws within 8 weeks from February–June 2020), despite constraints on in‐person medical visits and research due to the COVID‐19 pandemic. Because blood draws can be performed locally (Quest Diagnostics phlebotomy centers can be found in most major metropolitan areas with ≈2000 patient locations in the United States), this approach has high potential for scalability. A number of factors likely contributed to the high study enrollment rate (12% of those invited). Those who enrolled had been BHR participants for an average of 4.8 years prior to being approached about this new study, and 78% had returned to BHR for longitudinal follow‐up prior to joining the study, suggesting a high level of engagement with BHR.
We prioritized recruitment of non‐White individuals and those with an education less than a bachelor's degree; 32% of those invited (n = 2224) were non‐White and 30% of those invited (n = 2197) had an education less than a bachelor's degree. Thus, the pool of eligible BHR participants falling into prioritized URP categories was adequate to oversample URP groups in the study. We also expanded the geographical radius from study sites allowed for individuals from URPs, resulting in an additional 544 individuals from URP groups who were eligible to enroll. However, neither “oversampling” at the recruitment stage nor expanded eligibility criteria were successful in recruiting and engaging a representative sample of ethnocultural URPs for remote blood collection. Although only 68% of those invited to the study were White, the final cohort of participants who completed the study included 88% White individuals. Our findings agree with past studies demonstrating failure to engage and retain URPs in clinical AD research, both in BHR, 27 and in clinic settings. 13 , 15 , 34 , 35 Our ability to test the hypotheses that individuals from ethnocultural and education attainment URPs are less likely to enroll in and complete the remote blood collection study is limited by the broader failure to adequately enroll and engage these same URPs in the larger (n > 90,000) pool of potential participants in BHR. This limitation warrants caution in interpretation of our results. Nonetheless, our findings suggest that the approach of establishing a large, registry‐based cohort to facilitate ADRD biomarker and genetic studies has the potential to amplify under‐inclusion of historically under‐included populations at each step of the enrollment “funnel.” Our results highlight the urgent need for development and evaluation of strategies that are effective at recruiting and engaging URPs at each step, including at the “top of the funnel” where participants join an online registry like BHR. Related to this, future analyses should include investigation of barriers and facilitators to participation, including the role of barriers (e.g., burden, competing demands, distance from blood draw sites), that are known to unduly burden URPs. 36 , 37 , 38 , 39 , 40
We have several new initiatives to address this limitation. These include deployment of surveys around barriers and motivators, and creation, evaluation, and optimization of culturally tailored recruitment and engagement materials and a Spanish‐language website, developed in collaboration with Community Science Partnership Boards within BHR. We hope that these initiatives will help us make evidence‐based decisions in redesigning our approach to recruitment, both in BHR and the additional studies to which BHR participants are referred. Further, our efforts failed to engage older participants and those with SMCs (Table 2), which is in agreement with previous studies. 26 , 27 Therefore, future recruitment and engagement strategies should be developed to appeal to these groups.
For our remote blood draw approach to be deployed for widespread screening in the community, it needs to be highly scalable. Our study identifies logistics that currently require a high level of one‐on‐one support: blood draw scheduling and instructions, as well as coordination between BHR and the phlebotomy centers. Overall, 20% of those who completed a blood draw contacted BHR via e‐mail. Interestingly, ethnocultural and educational URPs were less likely to communicate with and request support from BHR, which may contribute to their lower engagement and participation levels of these groups, because communicating with study staff was positively associated with successful blood draw completion. We plan to use this information to further automate the remote blood draw process and to optimize our novel Biofluids Management Portal, and to better engage URPs using the strategies described above. One limitation of using local phlebotomy centers was the variability of available cold storage (dry ice, etc.). As we learn more about plasma collection and storage requirements for AD biomarkers processing, the need for cold storage may be relaxed, allowing greater efficiency and scalability, and improved accessibility to participants in diverse locations.
The next step in this study is to process plasma to quantify Aβ42/40, p‐tau, and NfL, and to extract DNA for genome‐wide association studies. Plasma biomarker and genetic results will be used to investigate the relationship between these variables and online variables in BHR. One impactful line of research will be to identify combinations of online cognitive, everyday functioning, and health data (from questionnaires and cognitive tests) and plasma AD biomarkers (from remote blood collection) that best predict preclinical and prodromal AD, cognitive decline, and future disease progression. To accomplish this, we plan to acquire imaging and clinical data from participants in future studies. This novel approach could then be deployed more broadly in BHR, as well as in‐clinic observational studies and clinical trial screening, as an efficient way to identify older adults with preclinical and prodromal AD.
In conclusion, remote blood collection from a non‐representative group of older adults in the BHR online research registry is feasible. Major challenges include the need to expand efforts to effectively recruit and engage ethnocultural and educational URPs, older individuals, and those with SMCs; and to improve scalability, so that this approach can be used to facilitate future AD and aging observational research and clinical trials.
CONFLICTS OF INTEREST
Miriam Ashford, Joseph Eichenbaum, Aniekan Akenam, Derek Flenniken, and Alexander Happ have no conflicts of interest to declare. Juliet Focker has received support from the California Department of Public Health. Taylor Howell has received support for attendance to AAIC from UCSF and support for attendance to CTAD from NCIRE. Diana Truran serves as an NCIRE Audit Committee advisor (unpaid). Scott Mackin has received NIH grant funding for institutional support. Kaj Blennow has received support from Abcam, Axon, Biogen, JOMDD/Shimadzu, Lilly, MagQu, Prothena, GEECD/Roche Diagnostics, Siemens Healthineers, IFCC/SNIBE, Julius Clinical, and Novartis. He is the co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Eran Halperin has received support from the NIH, NSF, and consulted for KHealth and Ultima Genomics. Giovanni Coppola is a full‐time employee of Regeneron Pharmaceuticals. Michael Weiner receives support for his work from the following funding sources: NIH, Department of Defense, California Department of Public Health, University of Michigan, Siemens, Biogen, Larry L. Hillblom Foundation, Alzheimer's Association, and the State of California. He also receives support from Johnson & Johnson, Kevin and Connie Shanahan, VUmc, GE, Australian Catholic University, the Stroke Foundation, and the Veterans Administration. He has served on Advisory Boards for Cerecin/Accera, Roche, Alzheon, Inc., Merck Sharp & Dohme Corp., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Brain Health Registry, and ADNI. He serves on the editorial boards for Alzheimer's & Dementia, Topics in Magnetic Resonance Imaging, and Magnetic Resonance Imaging. He has provided consulting and/or acted as a speaker/lecturer to Cerecin/Accera, Inc., BioClinica, Nestle/Nestec, Roche, Genentech, NIH, The Buck Institute for Research on Aging, FUJIFILM‐Toyama Chemical (Japan), Garfield Weston, Baird Equity Capital, University of Southern California (USC), Cytox, and Japanese Organization for Medical Device Development, Inc. (JOMDD) and T3D Therapeutics. He holds stock options with Alzeca, Alzheon, Inc., and Anven. Rachel Nosheny receives support for her work from the NIH, the California Department of Public Health, and Genentech. This study and manuscript is produced by the researchers at the University of California, San Francisco and partner organizations, and partially supported by the California Department of Public Health Alzheimer's Disease Program funding from the 2019 California Budget Act (RFA18‐10612). The content may not reflect the official views or policies of the State of California.
ACKNOWLEDGMENTS
This work is supported by the National Institutes of Health, National Institute on Aging (K01AG055692 and R33AG062867). The authors gratefully acknowledge the entire Brain Health Registry staff and all BHR participants and study partners, as well as the past and current funding sources for the Brain Health Registry: National Institute on Aging, Alzheimer's Association, California Department of Public Health (18‐10929), Patient Centered Outcomes Research Institute, Alzheimer's Drug Discovery Foundation, Genentech Health Equity Innovations Fund, Larry L. Hillblom Foundation, the Rosenberg Alzheimer's Project, the Ray and Dagmar Dolby Family Fund, Connie and Kevin Shanahan, General Electric, and the Drew Foundation. The authors appreciate the contributions of Dr. Daniel Geschwind to this project.
Fockler J, Ashford MT, Eichenbaum J, et al. Remote blood collection from older adults in the Brain Health Registry for plasma biomarker and genetic analysis. Alzheimer's Dement. 2022;18:2627–2636. 10.1002/alz.12617
REFERENCES
- 1. Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood‐based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14(11):639‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langbaum JB, High N, Nichols J, Kettenhoven C, Reiman EM, Tariot PN. The Alzheimer's Prevention Registry: a Large Internet‐Based Participant Recruitment Registry to Accelerate Referrals to Alzheimer's‐Focused Studies. J Prev Alzheimers Dis. 2020;7:242‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong K, Cummings J. Healthybrains.org: from registry to randomization. J Prev Alzheimers Dis. 2016;3(3):123‐126. [DOI] [PubMed] [Google Scholar]
- 5. Grill JD, Hoang D, Gillen DL, et al. Constructing a local potential participant registry to improve Alzheimer's disease clinical research recruitment. J Alzheimers Dis. 2018;63(3):1055‐1063. [DOI] [PubMed] [Google Scholar]
- 6. Nosheny RL, Camacho MR, Insel PS, et al. Online study partner‐reported cognitive decline in the Brain Health Registry. Alzheimers Dement (N Y). 2018;4:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet‐based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14(8):1063‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mackin RS, Insel PS, Truran D, et al. Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: results from the Brain Health Registry. Alzheimers Dement (Amst). 2018;10:573‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grill JD, Hoang D, Gillen DL, et al. Constructing a local potential participant registry to improve Alzheimer's disease clinical research recruitment. J Alzheimers Dis. 2018;63(3):1055‐1063. [DOI] [PubMed] [Google Scholar]
- 10. Langbaum JB, Gordon D, Walsh TL, et al. The Alzheimer's Prevention Registry's Genematch program: update on progress and lessons learned in helping to accelerate enrollment into Alzheimer's prevention studies. Alzheimers Dement. 2018;14(7):P1073. [Google Scholar]
- 11. Canevelli M, Bruno G, Grande G, et al. Race reporting and disparities in clinical trials on Alzheimer's disease: a systematic review. Neurosci Biobehav Rev. 2019;101:122‐128. [DOI] [PubMed] [Google Scholar]
- 12. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moulder KL, Monsell SE, Beekly D, et al. Factors influencing lumbar puncture participation in Alzheimer's research. Alzheimers Dement. 2015;11(7):P780. [Google Scholar]
- 14. Bilbrey AC, Humber MB, Plowey ED, et al. The impact of latino values and cultural beliefs on brain donation: results of a pilot study to develop culturally appropriate materials and methods to increase rates of brain donation in this under‐studied patient group. Clin Gerontol. 2018;41(3):237‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bardach SH, Jicha GA, Karanth S, Zhang X, Abner EL. Genetic sample provision among National Alzheimer's Coordinating Center participants. J Alzheimers Dis. 2019;69:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weuve J, Sagiv SK, Fox MP. Quantitative bias analysis for collaborative science. Epidemiology. 2018;29(5):627‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nosheny RL, Camacho MR, Jin C, et al. Validation of online functional measures in cognitively impaired older adults. Alzheimers Dement. 2020;16(10):1426‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165‐178. [DOI] [PubMed] [Google Scholar]
- 19. Maruff P, Lim YY, Darby D, et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC Psychol. 2013;1(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuiper JS, Oude Voshaar RC, Verhoeven FEA, Zuidema SU, Smidt N. Comparison of cognitive functioning as measured by the Ruff Figural Fluency Test and the cogstate computerized battery within the lifelines cohort study. BMC Psychol. 2017;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cromer JA, Harel BT, Yu K, et al. Comparison of cognitive performance on the cogstate brief battery when taken in‐clinic, in‐group, and unsupervised. Clin Neuropsychol. 2015;29(4):542‐558. [DOI] [PubMed] [Google Scholar]
- 22. Sumner JA, Hagan K, Grodstein F, Roberts AL, Harel B, Koenen KC. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle‐aged women. Depress Anxiety. 2017;34(4):356‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet‐based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14(8):1063‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flanagin A, Frey T, Christiansen SL, Bauchner H. The reporting of race and ethnicity in medical and science journals: comments invited. JAMA. 2021;325(11):1049‐1052. [DOI] [PubMed] [Google Scholar]
- 26. Fockler J, Kwang W, Ashford MT, et al. Brain health registry GenePool study: a novel approach to online genetics research. Alzheimers Dement (N Y). 2021;7(1):e12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashford MT, Eichenbaum J, Williams T, et al. Effects of sex, race, ethnicity, and education on online aging research participation. Alzheimers Dement (N Y). 2020;6(1):e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Core Team. R: Language And Environment For Statistical Computing. R Foundation for Statistical Computing; 2017. [Google Scholar]
- 29. Revelle W. psych: Procedures for Personality and Psychological Research. Northwestern University:USA2916. [Google Scholar]
- 30. Warnes GR, Bolker B, Lumley T, Johnson RC, Various R programming tools for model fitting. In: 2018.
- 31. Chongsuvivatwong V epiDisplay: Epidemiological data display package. 2018.
- 32. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J‐C, Müller M. (2011) pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics, 12, (1), 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langbaum JB, Karlawish J, Roberts JS, et al. GeneMatch: a novel recruitment registry using at‐home APOE genotyping to enhance referrals to Alzheimer's prevention studies. Alzheimers Dement. 2019;15(4):515‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grill JD, Kwon J, Teylan MA, et al. Retention of Alzheimer disease research participants. Alzheimer Dis Assoc Disord. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blazel MM, Lazar KK, Van Hulle CA, et al. Factors associated with lumbar puncture participation in Alzheimer's disease research. J Alzheimers Dis. 2020(Preprint):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gelman CR. Learning from recruitment challenges: barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer's disease. J Gerontol Soc Work. 2010;53(1):94‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hughes TB, Varma VR, Pettigrew C, Albert MS. African Americans and clinical research: evidence concerning barriers and facilitators to participation and recruitment recommendations. Gerontologist. 2017;57(2):348‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams MM, Scharff DP, Mathews KJ, et al. Barriers and facilitators of African American participation in Alzheimer's disease biomarker research. Alzheimer Dis Assoc Disord. 2010;24 Suppl:S24‐S29. [DOI] [PMC free article] [PubMed] [Google Scholar]


