Abstract
Large-scale study programs on CDK4/6 inhibitors, targeted therapies, and antibody–drug conjugates launched in recent years have yielded results from current studies which are now being published in journals and presented at international conferences. In this context, new results are available from the major CDK4/6 inhibitor studies. Also, an increasing amount of data is being published from large-scale genomic studies on efficacy and resistance mechanisms in patients treated with CDK4/6 inhibitors. These results now form the basis for further research plans to investigate combination therapies and treatment sequencing. Based on the latest published results, sacituzumab govitecan is now available as a second antibody–drug conjugate; this brings an advantage in terms of overall survival for patients with hormone receptor-positive (HRpos)/HER2-negative (HER2neg) breast cancer. In this review article, we summarize the latest developments and place them in context according to the current status of research.
Key words: advanced breast cancer, chemotherapy, therapy standard
Introduction
CDK4/6 inhibitors have become established as the new standard in first-line treatment for advanced or metastatic disease in patients with HRpos tumors. Data are being successively accumulated which help us to better understand the mechanisms of resistance and efficacy. Extensive genomic analysis gives new insights into the heterogeneity of the disease course in patients treated with CDK4/6 inhibitors. It is precisely these insights which may, in the coming years, determine the treatment sequences for patients with advanced-stage breast carcinoma. Moreover, the new antibody–drug conjugates represent the most cutting-edge innovations for patients with advanced-stage breast cancer. A whole series of new results have been published for both trastuzumab deruxtecan (T-DXd) and for sacituzumab govitecan.
Hormone Receptor-Positive Disease and CDK4/6 Inhibitors
Monarch 3
Among the large-scale, randomized phase III studies on palbociclib, ribociclib, and abemaciclib in patients with metastatic disease 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , the Monarch 3 study on first-line therapy with abemaciclib is the only one for which a final analysis of overall survival is not yet available. This situation did not change at this yearʼs ESMO 2022 Congress; however, an extensive, planned interim analysis of the Monarch 3 study was presented at this conference 17 , after parts of the data had already been published in the technical information for this product at the start of 2022 18 . The interim analysis presented in this context related to overall survival; the database used was closed in July 2021, and the median observation period was 5.8 years. With a p-value of 0.0301, the results are not yet statistically significant. Median overall survival was improved numerically, from 54.5 to 67.1 months. This corresponded to a hazard ratio (HR) of 0.754 (95% CI: 0.584 – 0.974) 17 . In the subgroup analysis, there was only one subgroup in which no consistent effect could be seen. The effect was most pronounced in the group of patients with negative progesterone receptor status (HR = 0.425; 95% CI: 0.368 – 0.702), while in the group with progesterone receptor-positive tumors, the effect achieved a hazard ratio of only 0.919 (95% CI: 0.682 – 1.238). These differences give rise to a number of hypotheses which may be investigated further in future studies. It remains to note that there is currently a discussion around the value of progesterone receptor determination, with some arguing that it should instead be used as a prognosis factor for patients with positive estrogen receptor status 19 . An update on progression-free survival, with the same database closure date, was also presented. In this case, the HR was 0.518 (95% CI: 0.415 – 0.648), which corresponds to an extension of the median progression-free survival (PFS) time from 14.8 months to 29.0 months. Furthermore, 26.7% of patients treated with abemaciclib were still progression-free after five years, while for patients receiving monotherapy with aromatase inhibitors, this figure was only 9.6% 17 . Another piece of clinically relevant information from this analysis of long-term follow-up data is that no additional safety signals could be seen, even with long-term exposure. The final analysis is expected to appear in 2023.
DAWNA-2
Compared to other CDK4/6 inhibitors, dalpiciclib, a CDK4/6 inhibitor which has not been approved for the European market, shows an inhibitory effect similarly strong to that of palbociclib, with a CDK4 : CDK6 IC50 ratio of 0.8. In preclinical trials, it showed potent inhibition of cell growth 20 , 21 . In previously published data from the DAWNA-1 study, patients showing a certain degree of endocrine resistance were treated with fulvestrant or fulvestrant + dalpiciclib 22 . The DAWNA-1 study was able to show an improvement in PFS. Now the results from the DAWNA-2 study have been presented; similar to the Monaleesa-2, Paloma-2 and Monarch 3 studies, this study focused on patients receiving first-line therapy for advanced stage disease 23 . The patients were randomized at a ratio of 1 : 2 to receive either letrozole or anastrozole, or therapy with an aromatase inhibitor plus dalpiciclib. The primary study goal was progression-free survival.
In this study, the median progression-free survival under endocrine monotherapy was increased from 18.2 months to 30.6 months. This corresponded to a hazard ratio of 0.51 (95% CI: 0.38 – 0.69) 23 . None of the sufficiently large subgroups showed an inconsistent effect in this regard. Just as in the DAWNA-1 study, both premenopausal and postmenopausal women were recruited. However, in the presentation of the DAWNA-2 study investigating aromatase inhibitors as endocrine therapy, it was not specified whether or not the premenopausal women received ovarian function suppression 22 . There was no difference in the reported therapeutic effects for premenopausal patients (HR = 0.53; 95% CI: 0.33 – 0.85) compared to postmenopausal patients (HR = 0.52; 95% CI: 0.36 – 0.75) 22 . In terms of side effects, hematological effects such as neutropenia (grade 4: 21.2%) were most prominent. As previously mentioned, dalpicilib has not yet been approved, either in Europe or the USA.
ELAINE 1
In the PADA-1 study, mutations in the estrogen receptor gene (ESR1) were associated with better efficacy when the treatment was switched to a combination with fulvestrant and palbociclib, compared to continued treatment with aromatase inhibitors 24 . In light of this, for patients with a somatic ESR1 mutation (sESR1) , the question arises as to what is the best combination partner for treatment with a CDK4/6 inhibitor. One drug currently in trial is lasofoxifene, which has been shown in preclinical trials to have better efficacy against tumors than fulvestrant 25 , 26 . This drug has now been trialed on a cohort in which an sESR1 mutation was detected during progression under treatment with aromatase inhibitors and CDK4/6 inhibitors. Given the small sample size of just 103 randomized patients, the hazard ratio at 0.699 (95% CI: 0.445 – 1.125; p = 0.138) was not statistically significant; nevertheless, it was highly promising. The median PFS in this therapy-resistant context was increased from 4.04 months to 6.04 months 27 .
MSK-Impact
Data from the MSK-IMPACT cohort relating to the prognosis for patients treated with CDK4/6 inhibitors have already been presented at the 2021 San Antonio Breast Cancer Symposium. At this conference, data were presented showing that a germline mutation in BRCA2 had an unfavorable effect on the prognosis for patients treated with a CDK4/6 inhibitor. Compared to patients with the wild-type genotype, patients with a mutation had a higher risk of progression (HR = 2.32; 95% CI: 1.38 – 3.91) 28 .
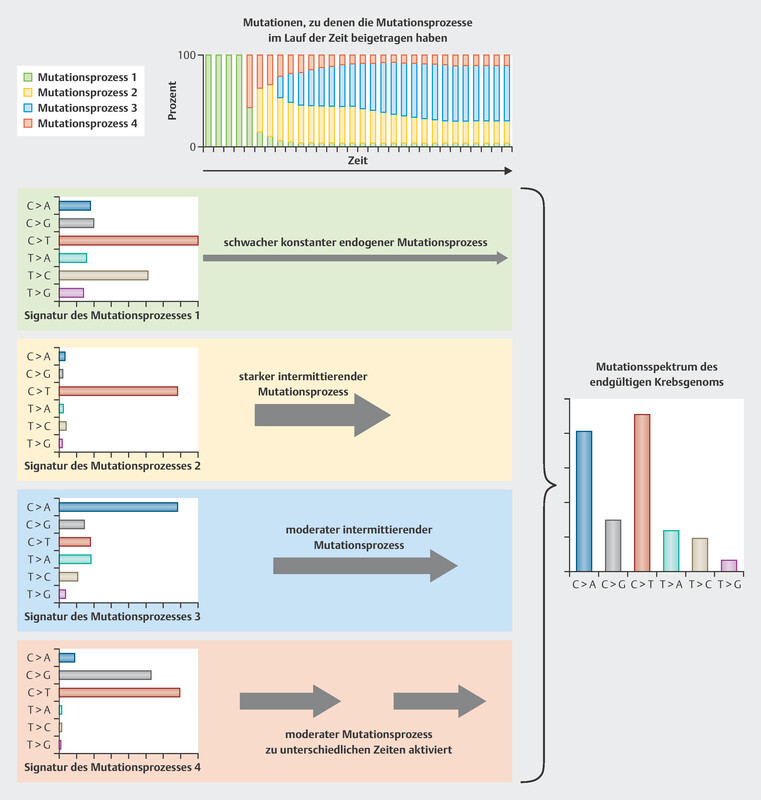
We are now being presented with new, extensive biomarker analyses for this cohort, again in relation to patients treated with CDK4/6 inhibitors 29 . For this purpose, the tumors of breast cancer patients were studied for complex mutational signatures. The classification of tumors according to complex mutational signatures is an attempt to categorize the tumors based on their somatic mutation patterns. In the pathogenesis of tumors, different stimuli and circumstances can lead to mutations, all of which have a characteristic mutational profile 30 . An example of the development of this kind of mutational profile is set out in Fig. 1 . These mutational profiles can be developed for different types of mutations (single base pair, doublet base pair, and INDELs). The study of the MSK-Impact cohort focused on single base substitution (SBS) mutations 29 , for which 96 different mutation classes have been described in a recently published article 31 . Current classifications of tumor genomes are available in COSMIC (Catalogue of Somatic Mutations in Cancer) 32 .
Fig. 1.
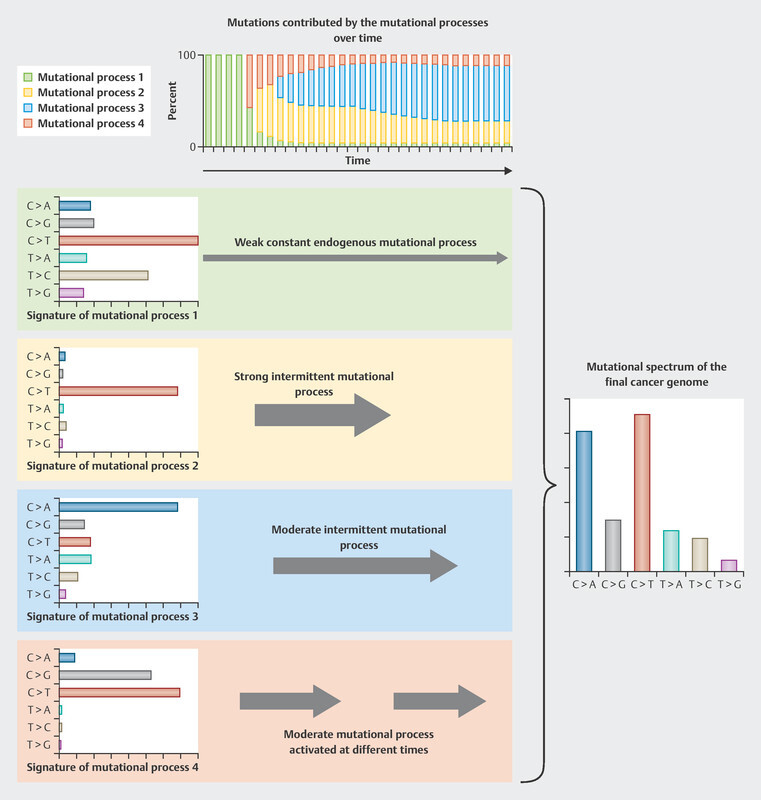
Model example of the development of mutational profiles (data from 30 , creative commons license CC BY, https://creativecommons.org/licenses/by/3.0/). Every stimulus leads to specific mutations of differing frequencies. Over a lifetime, different stimuli, either individually or in combination, give rise to a typical mutational profile. In the example given below, there are four mutational profiles which determine the pattern that can be found in tumors. Initially, the mutational patterns that arise through ongoing effects such as aging (mutation process 1) are predominant, then subsequently the effects of other mutation processes take over: first mutation process 4, then process 2, then process 3.
Some of these SBS signatures occur frequently in breast carcinomas, and can be classified according to the following etiological groups: clock-like, APOBEC, HRD, smoking, and mismatch repair deficiency. An overview is provided in Table 1 .
Table 1 Mutational profiles grouped according to etiology, based on single base substitutions (SBS) analyzed in the MSK-IMPACT cohort (data from 29 ).
| Etiological group | SBS groups | Description | Clinical implications |
|---|---|---|---|
| Clock-like | SBS1, SBS5 | With increasing age, these mutational profiles occur equally in both normal cells and neoplastic cells. | None yet |
| APOBEC | SBS2, SBS13 | Mutational patterns that can be induced by proteins of the AID/APOBEC family. AID/APOBEC proteins can cause mutations in DNA and RNA, and APOBEC3A is probably responsible for the majority of mutations in cancer cells. | None yet |
| Homologous recombination deficiency (HRD) | SBS3, SBS8 | This mutational pattern results from defects in the homologous recombination genes, primarily mutations in BRCA1 and BRCA2, or methylation of the BRCA1 promotor. | PARP inhibitors, platinum-based chemotherapy |
| Smoking | SBS4 | Mutational profile associated with smoking, e.g., as a consequence of exposure to benzopyrene. | None |
| Mismatch repair deficiency | SBS6, SBS15, SBS20, SBS26 | These mutational profiles are found in tumors with microsatellite instability. | Immune checkpoint inhibition |
The clinical data that have been presented relate firstly to a change in the mutational profile from primary tumor to metastasis, and secondly to the influence of the mutational profiles on the prognosis for patients receiving CDK4/6 inhibitors as first-line therapy. The two mutational profiles which increased the most during progression from early-stage HRpos/HER2neg disease to advanced-stage disease were APOBEC and HRD 29 . The research on mutational profiles in relation to their influence on the prognosis under first-line therapy with CDK4/6 inhibitors revealed clear differences. In patients with few mutations, the median progression-free survival was 17.8 months, compared to 12.3 months in patients with an APOBEC signature. In patients in whom an HRD signature could be identified, the median PFS was only 7.6 months ( Table 2 ) 29 .
Table 2 Progression-free survival in the MSK-Impact cohort receiving first-line therapy with a CDK4/6 inhibitor, grouped according to mutational profiles (data from 29 ).
| Group | Median PFS (95% CI) | HR (95% CI) | p-value |
|---|---|---|---|
| Less than five mutations | 17.8 (15.3 – 25.7) | 1 (reference) | |
| Clock-like and others | 14.5 (11.0 – 21.0) | 1.23 (0.9 – 1.7) | 0.185 |
| APOBEC | 12.3 (8.8 – 14.9) | 1.47 (1.1 – 1.9) | 0.012 |
| HRD | 7.6 (5.3 – 12.3) | 1.71 (1.2 – 2.5) | 0.006 |
This study also shed light on other relevant aspects of endocrine resistance. The extent to which these insights can be used to determine therapies or treatment sequences remains to be investigated in future studies. To date, treatment with a CDK4/6 inhibitor remains the standard first-line therapy for patients with advanced HRpos/HER2neg breast carcinoma. Given the short median PFS in patients with an HRD mutational profile, the question may arise as to whether this patient group would be better off treated with a PARP inhibitor. Making up 10.5% of CDK4/6 patients in the MSK-IMPACT cohort, this HRD group only represents a small proportion of patients; a specific study would therefore have to be conducted, or information gleaned from real world data, in order to gain further insights into this issue.
CAPTOR and MINERVA
Two studies that will help to contribute data in this context are the CAPTOR and MINERVA studies 33 , 34 .
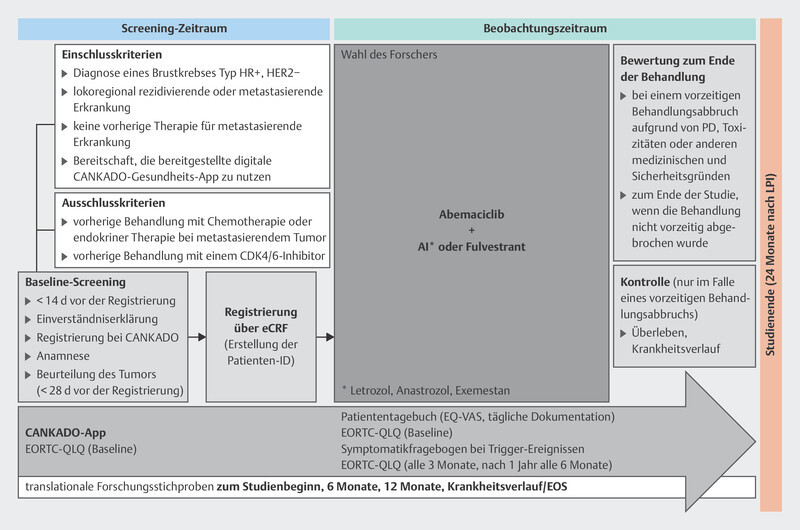
The MINERVA study 34 is investigating the efficacy of abemaciclib in patients with advanced HRpos/HER2neg breast carcinoma. As part of the study, biomaterial specimens may be collected for translational research programs, in particular circulating tumor DNA (ctDNA) and germline DNA. In addition, quality of life data are being recorded electronically using the health software application CANKADO. The study design is set out in Fig. 2 .
Fig. 2.
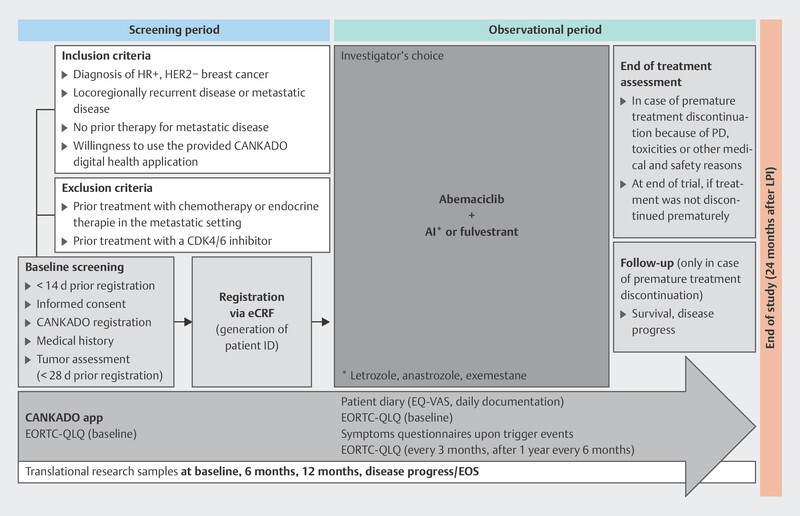
Design of the MINERVA study (AI: aromatase inhibitor, HR: hormone receptor, eCRF: electronic case report form, PD: progressive disease, LIP: last patient in, EORTC-QLQ and EQ-VAS: quality of life questionnaires, EOS: End of Study).
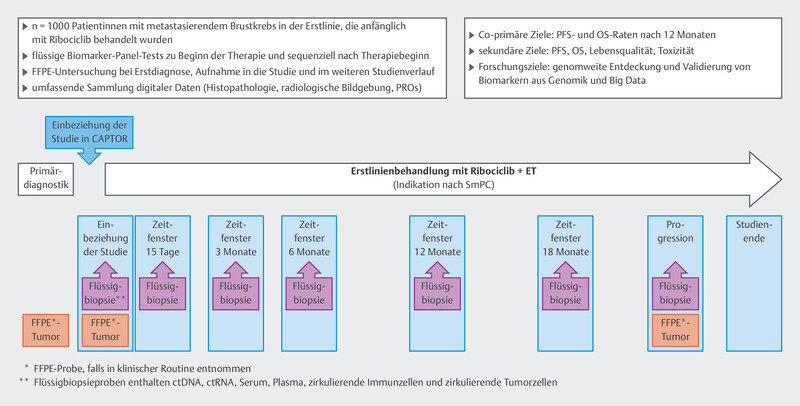
In the CAPTOR-BC study, the main focus is on investigating efficacy and resistance mechanisms using the latest methods. The visit timepoints have been optimized so as to gain extensive insights into the mechanism of action of ribociclib. For this purpose, efforts are being made to study different biomaterials: ctDNA, germline DNA, tumor tissue, serum, plasma, and leukocyte RNA. In addition, imaging data are to be associated with the efficacy of ribociclib. The study design is set out in Fig. 3 . As shown by the mutational profiles in the MSK-IMPACT cohort, it is often necessary to study thousands of genes and mutations. For this reason, a very large sample size of patients is needed in order to investigate aspects of resistance and efficacy. The CAPTOR-BC study network is specifically prepared to share algorithms and data with other research groups; this increases the chances of achieving substantial research.
Fig. 3.
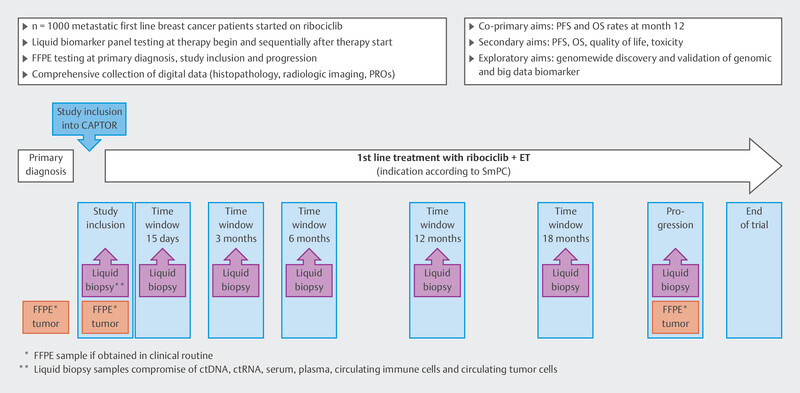
Design of the CAPTOR-BC study.
Hormone-Positive Disease and SERDs
The new oral selective estrogen degraders (SERDs) are currently under clinical development. They are being trialed in a large number of clinical studies, in a wide variety of clinical settings. Two randomized studies have now reached their primary study goal: the EMERALD study and the SERENA-2 study. While the EMERALD study has already been published in full 35 , so far only a press release is available for the SERENA-2 study 36 . Despite these two studies, the value of oral SERDS in treating HRpos/HER2neg breast cancer patients has yet to be established. The improvement to median PFS in the EMERALD study was only of marginal clinical significance. The median progression-free survival increased from 1.9 months to 2.8 months for the total cohort 35 . In addition, two similar studies which failed to reach their primary study goal have now been published: the acelERA study and the AMEERA-3 study 37 , 38 .
acelERA
The acelERA study recruited patients who had previously undergone either one or two systemic treatments for advanced-stage HRpos/HER2neg breast carcinoma. One of the treatments in this context had to be an endocrine therapy. The 303 patients were randomized to receive either monotherapy of the physicianʼs choice (aromatase inhibitor [IA] or fulvestrant [FUL]), or therapy with giredestrant). In the comparator arm, 75% of patients were treated with fulvestrant and 25% with aromatase inhibitors. An analysis of the total cohort did not reveal any difference in terms of progression-free survival. The median PFS in the group of patients receiving giredestrant was 5.6 months, compared to 5.4 months in the FUL/AI group. This corresponded to a hazard ratio of 0.81 (95% CI: 0.60 – 1.10) 38 . An ESR1 mutation was detected in a total of 90 patients. Among these patients, there was a greater difference in favor of giredestrant with an HR of 0.60 (95% CI: 0.35 – 1.03), with median PFS times of 5.3 months for giredestrant and 3.5 months for the patients treated with FUL/AI 38 .
Ameera-3
Patients in the Ameera-3 study had to have shown progression while receiving endocrine therapy in the adjuvant setting or as metastatic treatment, and were allowed to have received up to two endocrine therapies and up to one chemotherapy for the treatment of advanced-stage disease. The 290 patients in this study were randomized to receive either treatment with fulvestrant, aromatase inhibitors, or tamoxifen (TAM), or treatment with amcenestrant. In the comparator arm, 89.8% of the patients received fulvestrant, 6.8% received aromatase inhibitors, and 3.4% received tamoxifen. Again in this study, with an HR of 1.05 (95% CI: 0.79 – 1.4), no difference in progression-free survival could be seen for the total cohort. The median PFS times were 3.6 months for amcenestrant and 3.7 months for FUL/AI/TAM. Similarly in the Ameera-3 study, an analysis was performed of the subgroup containing 120 patients with an ESR1 mutation. In this case, the HR was in favor of amcenestrant at 0.9 (95% CI: 0.57 – 1.44).
Outlook for the SERD studies
Two positive and two negative studies have now been published, including the Serena-2 study. All of these studies were conducted in a therapeutic scenario with a large number of hormone therapy-resistant patients. In populations of this kind, it is generally difficult to find evidence of a therapeutic benefit. Also, the proportion of patients in the comparator arms who received the SERD fulvestrant was relatively high. In the Ameera-3 study, this proportion was 90%. Accordingly, it can be assumed that in this study, a SERD is in fact being compared to another SERD. In August, a press release was issued announcing that the Ameera-5 study had to be discontinued. In the Ameera-5 study, first-line therapy with palbociclib plus letrozole was compared to therapy with palbociclib plus amcenestrant. The study was discontinued following an assessment by the Data Safety Monitoring Board 39 . In light of the PARSIFAL study, these results did not come as a complete surprise 40 .
It is currently unclear whether the different results for these studies were due to efficacy or to the selection of the patient cohort. Even if oral SERDs can be presumed to have similar efficacy to that of fulvestrant, one of the major areas of potential is that these drugs could also be developed in the adjuvant setting. Some studies in this context have begun in the form of the lidERA study, the EMBER-4 study, and the CAMBRIA-1 study. Apart from one study, which was discontinued due to a lack of resources, fulvestrant has not yet been investigated in the adjuvant setting 41 .
Hormone-Positive Disease, ADCs and Chemotherapy
TROPiCs-02
Results of the TROPiCs-02 study on the anti-Trop2 antibody–drug conjugate sacituzumab govitecan have already been presented at the 2022 ASCO Annual Meeting. The TROPiCS-02 study included HR-positive, HER2-negative patients who had to have completed several preliminary therapies. These included at least endocrine therapy, taxane therapy, and therapy with a CDK4/6 inhibitor. Study participants had to have completed at least two and no more than four chemotherapy lines for metastatic disease. Thus, only HR-positive/HER2-negative patients who had clearly completed preliminary therapies were included in this study 42 .
Patients were randomized 1 : 1 to receive either treatment with sacituzumab govitecan or chemotherapy of the physicianʼs choice (capecitabine, vinorelbine, gemcitabine, eribulin). The aim of studies of this kind should be to improve efficacy while providing a more favorable side effect profile.
Initial results showed an improvement in PFS; however, the difference in overall survival was not statistically significant. Shortly after the publication of these results, another interim analysis has now been presented 43 .
While the first analysis of overall survival was based on 293 deaths, this second interim analysis was able to draw on a sample of 390 deaths 43 . Median overall survival was increased from 11.2 months (95% CI: 10.1 – 12.7) under chemotherapy to 14.4 months (95% CI: 13.0 – 15.7) under treatment with sacituzumab govitecan. This corresponded to an HR of 0.79 (95% CI: 0.65 – 0.96; p = 0.020). This improvement was statistically significant 43 .
As a result, just a short time after the advent of CDK4/6 inhibitors and T-DXd, another study of patients with advanced HRpos/HER2neg breast carcinoma was published which shows a benefit for overall survival in this patient group.
Meteora II
Even though chemotherapy is not the treatment of choice in patients with advanced HRpos/HER2neg breast carcinoma, it is often used as a treatment option in subsequent therapy lines 45 , especially following first-line therapy with CDK4/6 inhibitors 44 . However, many of these therapies are characterized by short median progression-free survival times 46 , 47 . It is suspected that some of the chemotherapy regimens, e.g., metronomic therapies, may also have immunomodulatory effects 48 , 49 . In light of this, studies comparing these kinds of chemotherapy regimens in HRpos/HER2neg patients are still useful and clinically relevant.
The recently presented METEORA-II study included patients who had received no more than one chemotherapy and no more than two endocrine therapies for the treatment of advanced HRpos/HER2neg disease 50 . The 140 patients in this study were randomized to receive either weekly paclitaxel, or a metronomic therapy with vinorelbine (days 1, 3, and 5), cyclophosphamide (oral, daily), and capecitabine (daily). Median progression-free survival in the paclitaxel arm was 6.9 months; in the arm receiving metronomic therapy, this was increased to 11.1 months (HR = 0.67; 95% CI: 0.46 – 0.96). There were no differences in terms of overall survival 50 .
Studies like the METEORA-II study show that the chemotherapy regimens which remain popular as subsequent therapy lines can yield clear differences in terms of efficacy. One study conducted by the AGO-B in this context is the AIRE study 51 , which is investigating the immunomodulatory effect of eribulin compared to chemotherapy of the physicianʼs choice in HER2-negative patients with advanced-stage breast carcinoma.
Interesting Research Data on Combination Therapies in HER2-Positive Patients
PHILA study
Some mechanisms involved in the treatment of patients with HER2-positive breast carcinoma have been described in connection with causes of resistance 52 . In some of these studies, the particular focus was on mutations in the PI3K signaling pathway 53 , 54 , 55 . The presence or accumulation of activating PI3K mutations was postulated to be a basis for some of the HER2 resistance. In light of this, the question arises as to whether the addition of a PI3K inhibitor could improve the prognosis for patients with HER2-positive disease. This question was pursued in the PHILA study 56 .
Patients with metastatic disease who had not yet received treatment in this context were randomized to receive either treatment with trastuzumab and docetaxel, or treatment with trastuzumab, docetaxel, and pyrotinib. Pyrotinib is an oral, bioavailable, irreversible pan-HER-receptor tyrosine kinase inhibitor. Median progression-free survival was increased from 10.4 months to 24.3 months. This corresponded to a hazard ratio of 0.41 (95% CI: 0.32 – 0.53) 56 .
Even though these results seem impressive, the standard first-line therapy is currently a combination of trastuzumab, pertuzumab, and chemotherapy, in accordance with data from the CLEOPATRA study 57 , 58 . In this study, the median progression-free survival was increased from 12.4 months to 18.5 months. Based on these data, in addition to therapy with monoclonal antibodies, the inhibition of the PI3K signaling pathway by a targeted molecule would certainly be an interesting approach to pursue in future studies, so as to test the ability of targeted therapies to overcome resistance.
MonarchHER
In recent years, there has been significant progress in the treatment of HER2-positive breast carcinoma, with the advent of new drugs such as pertuzumab, trastuzumab emtansine (T-DM1), margetuximab, neratinib, tucatinib, and T-DXd 16 , 59 , 60 , 61 , 62 . Except for neratinib, all of these therapies either contain or are combined with a chemotherapy. One study which gives insights into the chemotherapy-free treatment regimen is the MonarchHER study 63 . This study included patients with HRpos/HER2pos, advanced breast carcinoma, randomized into three therapy arms:
Abemaciclib + trastuzumab + fulvestrant,
Abemaciclib + trastuzumab,
Chemotherapy + trastuzumab.
The final overall survival data have now been published 63 . Out of a total of 237 randomized patients, a total of 157 deaths were recorded. No statistically significant differences were found, although the chemotherapy arm had the numerically lowest overall survival at 20.7 months, and the abemaciclib + trastuzumab + fulvestrant arm had the longest median overall survival at 31.1 months (HR = 0.71; 95% CI: 0.48 – 1.05; p = 0.086) 63 .
With these results, it has become clear that a confirmatory study should test the hypothesis of whether or not a therapy regimen in which chemotherapy is replaced by a CDK4/6 inhibitor results in an advantage for survival. In this context, the data from the DETECT-V study are of interest; this study pursued a similar line of inquiry, looking at the combination ribociclib/endocrine therapy/trastuzumab/pertuzumab. Initial data were presented at the 2022 San Antonio Breast Cancer Symposium.
Outlook
With the ongoing development of endocrine therapy options and insights into molecular mechanisms that are associated with efficacy and resistance in endocrine-based therapies, the focus is shifted increasingly towards the question of individual biomarkers for special therapy sequences. In this context, finding the best combination partner for the CDK4/6 inhibitors is just as important as the question of which subsequent therapies should be used, e.g., chemotherapy, T-DXd, or sacitzumab govitecan, and in which sequence. Especially given the insights into HRD mechanisms that can lead to endocrine resistance, PARP inhibitors have once again become a focus in the treatment of HRpos/HER2neg patients. For HER2-positive patients, there are numerous studies, either currently active or in the evaluation phase, investigating the value of the new antibody–drug conjugates. In this context, data from future studies investigating T-DXd in earlier therapy settings may once again change the therapeutic landscape.
Acknowledgements
This work was partly developed as a result of funding from the companies onkowissen.de, Gilead, Novartis, Pfizer, Roche, and MSD. None of the companies had any part in the preparation and recommendations of this manuscript. The authors are solely responsible for the content of the manuscript.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen onkowissen.de, Gilead, Novartis, Pfizer, Roche, und MSD. Keine der Firmen hatte einen Anteil an der Erstellung und den Empfehlungen dieses Manuskriptes. Für den Inhalt des Manuskriptes sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt B. A. received honoria and travel grants from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo and Pfizer. M. B. has no conflict of interest. M. B.-P. received honoraria for lectures and advisory role from Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, AstraZeneca, Amgen, Samsung, MSD, GSK, Daiichi-Sankyo, Gilead, Sirius Pintuition, Pierre Fabre, and study support from Mammotome, Endomag and Merit Medical. E. B. received honoraria from Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun and onkowissen.de for clinical research management and/or medical education activities. N. D. has received honoraria from MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen,Gilead, MCI Healthcare. P. A. F. reports personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi-Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from SeaGen, personal fees from Roche, personal fees from Hexal, personal fees from Agendia, personal fees from Gilead. T. N. F. has participated on advisory boards for Amgen, Daiichi-Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi-Sankyo, Roche, Novartis and Pfizer. A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo, Hexal and Pfizer. N. H. received honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi-Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz, Seagen. W. J. has received research grants and/or honoraria from Sanofi-Aventis, Daiichi-Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. H.-C. K. has received honoraria from Pfizer, Novartis, Seagen, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, SurgVision, Onkowissen, Gilead, Daiichi-Sankyo and MSD, travel support from Carl, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi-Sankyo, Tesaro and owns stock of Theraclion SA and Phaon Scientific GmbH. C. K.-L. reports stock by Theraklion and Phaon Scientific (self and family), honoraria by Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, SonoScape (self) and Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss MediTec, TEVA Pharmaceuticals Industries, Theraklion, Janssen-Cilag, GlaxoSmithKline, LIV Pharma (family), Consulting to Roche, Novartis, Pfizer, Celgene, Phaon Scientific (self) and Pfizer, Novartis, SurgVision, CarlZeissMeditec, Amgen, Onkowissen (family); research funding by Roche, Novartis, Pfizer (self) as well as Travel and Accomodation by Roche, Daiichi Sankyo, Novartis (self) and Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo (family). D. L. received honoraria from Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen, Teva. M. P. L. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi-Sankyo, PharmaMar, Roche, SamanTree, Sysmex and Hexal and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi-Sankyo, Grünenthal, pfm, Gilead, AstraZeneca, and Eisai. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead. Consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutional research support from Novartis, Roche, Seagen, Genentech. Travel grants: Roche, Pfizer, Daiichi-Sankyo. E. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, Pfizer, Seagen, Pierre Fabre MCI Deutschland GmbH, bsh medical communications GmbH, Onkowissen TV. F. S. participated on advisory boards for Novartis, Lilly, Amgen and Roche and received honoraria for lectures from Roche, AstraZeneca, MSD, Novartis and Pfizer. H. T. received honoraria from Novartis, Roche, Celgene, Teva, Pfizer, AstraZeneca and travel support from Roche, Celgene and Pfizer. C. T. received honoraria for advisory boards and lectures from Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen, Vifor. M. T. has participated on advisory boards for AstraZeneca, Celgene, Clovis, Daiichi-Sankyo, Eisai, Gilead Science, Grünenthal, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen and Roche and has received honoraria for lectures from Amgen, Aurikamed, Celgene, Clovis, Daiichi-Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor and AstraZeneca and has received trial funding by Exact Sciences and Endomag Manuscript support was done by Amgen, ClearCut, pfm medical, Roche, Servier, Vifor. M. U. all honoraria went to the institution/employer: Abbvie, Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen, Gilead. M. W. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer and Roche. I. W. has participated on advisory boards for Novartis, Daiichi-Sankyo, Lilly, Pfizer and received speaker honoraria from Astra Zeneca, Daiichi-Sankyo, MSD, Novartis, Pfizer, Roche. A. W. participated on advisory boards for Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro, Eisai and received honoraria for lectures from Novartis, Pfizer, Aurikamed, Roche, Celgene. The other authors have no conflict of interest to declare for this specific work./ B. A. hat von AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo und Pfizer Honorare und Reisekostenzuschüsse erhalten. M. B. hat keine Interessenkonflikte. M. B.-P. hat von Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, AstraZeneca, Amgen, Samsung, MSD, GSK, Daiichi-Sankyo, Gilead, Sirius Pintuition und Pierre Fabre Honorare für Vorträge und beratende Tätigkeiten sowie von Endomag, Mammotome und Merit Medical Studienunterstützung erhalten. E. B. hat von Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun und onkowissen.de Honorare für klinisches Forschungsmanagement und/oder medizinische Aus- und Weiterbildung erhalten. N. D. hat von MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead und MCI Healthcare Honorare erhalten. P. A. F. gibt an, persönliche Honorare von Novartis, Zuschüsse von Biontech, persönliche Honorare von Pfizer, persönliche Honorare von Daiichi-Sankyo, persönliche Honorare von AstraZeneca, persönliche Honorare von Eisai, persönliche Honorare von Merck Sharp & Dohme, Zuschüsse von Cepheid, persönliche Honorare von Lilly, persönliche Honorare von Pierre Fabre, persönliche Honorare von SeaGen, persönliche Honorare von Roche, persönliche Honorare von Hexal, persönliche Honorare von Agendia und persönliche Honorare von Gilead erhalten zu haben. T. N. F. hat in Beiräten bei Amgen, Daiichi-Sankyo, Novartis, Pfizer und Roche mitgewirkt und von Amgen, Celgene, Daiichi-Sankyo, Roche, Novartis und Pfizer Honorare für Vorträge erhalten. A. D. H. hat als Referent und Berater von AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo, Hexal und Pfizer Honorare erhalten. N. H. hat für Vorträge und/oder Beratung von Amgen, AstraZeneca, Daiichi-Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz und Seagen Honorare erhalten. W. J. hat von Sanofi-Aventis, Daiichi-Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Celgene und Johnson & Johnson Forschungsbeihilfen und/oder Honorare erhalten. H.-C. K. hat von Pfizer, Novartis, Seagen, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen, Gilead, Daiichi-Sankyo und MSD Honorare sowie von Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi-Sankyo und Tesaro Reisekostenzuschüsse erhalten und besitzt Aktien von Theraclion SA und Phaon Scientific GmbH. C. K.-L. hat Aktien von Theraklion und Phaon Scientific (selbst und Familie), Honorarien von Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, SonoScape (selbst) und Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss MediTec, TEVA Pharmaceuticals Industries, Theraklion, Janssen-Cilag, GlaxoSmithKline, LIV Pharma (Familie), Beratung von Roche, Novartis, Pfizer, Celgene, Phaon Scientific (selbst) und Pfizer, Novartis, SurgVision, CarlZeissMeditec, Amgen, Onkowissen (Familie); Forschungsförderung von Roche, Novartis, Pfizer (selbst) und Reiseunterstützung von Roche, Daiichi Sankyo, Novartis (selbst) und Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo (Familie). D. L. hat von Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen und Teva Honorare erhalten. M. P. L. hat in Beiräten bei AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi-Sankyo, PharmaMar, Roche, SamanTree, Sysmex und Hexal mitgewirkt und von MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi-Sankyo, Grünenthal, pfm, Gilead, AstraZeneca und Eisai Honorare für Vorträge erhalten. V. M. hat von Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape und Gilead Honorare als Referent erhalten. Beraterhonorare von Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutionelle Forschungsunterstützung von Novartis, Roche, Seagen, Genentech. Reisekostenzuschüsse von: Roche, Pfizer, Daiichi Sankyo. E. S. hat von Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, MCI Deutschland GmbH, Pfizer, Seagen, Pierre Fabre, MCI Deutschland GmbH, bsh medical communications GmbH und Onkowissen TV Honorare erhalten. F. S. hat in Beiräten bei Novartis, Lilly, Amgen und Roche mitgewirkt und von Roche, AstraZeneca, MSD, Novartis und Pfizer Honorare für Vorträge erhalten. H. T. hat von Novartis, Roche, Celgene, Teva, Pfizer und AstraZeneca Honorare sowie von Roche, Celgene und Pfizer Reisekostenzuschüsse erhalten. C. T. hat für die Mitwirkung in Beiräten und für Vorlesungen von Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen und Vifor Honorare erhalten. M. T. hat in Beiräten bei AstraZeneca, Celgene, Clovis, Daiichi-Sankyo, Eisai, Gilead Science, Grünenthal, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen und Roche mitgewirkt und von Amgen, Aurikamed, Celgene, Clovis, Daiichi-Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor und AstraZeneca Honorare für Vorträge sowie von Exact Sciences und Endomag finanzielle Mittel für Versuche erhalten. Manuskriptzuschüsse wurden von Amgen, ClearCut, pfm medical, Roche, Servier und Vifor geleistet. M. U. alle Honorare gingen an die Institution/den Arbeitgeber: Abbvie, Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen, Gilead. M. W. hat in Beiräten bei AstraZeneca, Lilly, MSD, Novartis, Pfizer und Roche mitgewirkt. I. W. hat in Beiräten bei Novartis, Daiichi-Sankyo, Lilly und Pfizer mitgewirkt und von AstraZeneca, Daiichi-Sankyo, MSD, Novartis, Pfizer und Roche Honorare als Referent erhalten. A. W. hat in Beiräten bei Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro und Eisai mitgewirkt und von Novartis, Pfizer, Aurikamed, Roche und Celgene Honorare für Vorträge erhalten. Bei den übrigen Autoren besteht kein für diese Arbeit anzugebender Interessenkonflikt.
References/Literatur
- 1.Slamon D J, Neven P, Chia S. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32:1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 2.Goetz M P, Toi M, Campone M. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 3.Slamon D J, Neven P, Chia S. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 4.Sledge G W, jr., Toi M, Neven P. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 5.Turner N C, OʼLeary B, Cutts R.Genetic landscape of resistance to CDK4/6 inhibition in circulating tumor DNA (ctDNA) analysis of the PALOMA3 trial of palbociclib and fulvestrant versus placebo and fulvestrant J Clin Oncol 201836(Suppl.)Abstr.. 1001 [Google Scholar]
- 6.Sledge G W, jr., Toi M, Neven P. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Turner N C, Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 8.Tripathy D, Im S A, Colleoni M. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 9.Finn R S, Martin M, Rugo H S. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi G N, Stemmer S M, Burris H A. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi G N, Stemmer S M, Burris H A. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 12.Im S A, Lu Y S, Bardia A. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 13.Slamon D J, Neven P, Chia S. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 14.Turner N C, Ro J, Andre F. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 15.Turner N C, Slamon D J, Ro J. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 16.Luftner D, Schutz F, Stickeler E. Update Breast Cancer 2021 Part 5 – Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2022;82:215–225. doi: 10.1055/a-1724-9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz M P, Toi M, Huober J. MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC) Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency Verzenios. 2022Accessed November 20, 2022 at:https://www.emaeuropa.eu/en/medicines/human/summaries-opinion/verzenios
- 19.Allison K H, Hammond M EH, Dowsett M. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Li Q, Yuan J. CDK4/6 inhibitor-SHR6390 exerts potent antitumor activity in esophageal squamous cell carcinoma by inhibiting phosphorylated Rb and inducing G1 cell cycle arrest. J Transl Med. 2017;15:127. doi: 10.1186/s12967-017-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long F, He Y, Fu H. Preclinical characterization of SHR6390, a novel CDK 4/6 inhibitor, in vitro and in human tumor xenograft models. Cancer Sci. 2019;110:1420–1430. doi: 10.1111/cas.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu B, Zhang Q, Zhang P. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021;27:1904–1909. doi: 10.1038/s41591-021-01562-9. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Zhang Q Y, Zhang P. Dalpiciclib plus letrozole or anastrozole as first-line treatment for HR+/HER2- advanced breast cancer (DAWNA-2): A phase III trial. Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 24.Bidard F C, Hardy-Bessard A C, Dalenc F. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367–1377. doi: 10.1016/S1470-2045(22)00555-1. [DOI] [PubMed] [Google Scholar]
- 25.Laine M, Fanning S W, Chang Y F. Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer. Breast Cancer Res. 2021;23:54. doi: 10.1186/s13058-021-01431-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreano K J, Baker J G, Park S. The Dysregulated Pharmacology of Clinically Relevant ESR1 Mutants is Normalized by Ligand-activated WT Receptor. Mol Cancer Ther. 2020;19:1395–1405. doi: 10.1158/1535-7163.MCT-19-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz M P, Plourde P, Stover D G. Open-label, randomized study of lasofoxifene (LAS) vs. fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2- breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 28.Safonov A, Bandlamudi C, Tallón de Lara P. Comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistanc. San Antonio Breast Cancer Symposium. 2021;2021:GS4-08. [Google Scholar]
- 29.Marra A, Gazzo A, Gupta A. Mutational signature analysis reveals patterns of genomic instability linked to resistance to endocrine therapy (ET) ± CDK 4/6 inhibition (CDK4/6i) in estrogen receptor-positive/HER2-negative (ER+/HER2-) metastatic breast cancer (MBC) Ann Oncol. 2022;33 07:S88–S121. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 30.Alexandrov L B, Stratton M R. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev. 2014;24:52–60. doi: 10.1016/j.gde.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandrov L B, Kim J, Haradhvala N J. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COSMIC Mutational Signatures (v3.3 – June 2022)- Single Base Substitution (SBS) Signatures. 2022Accessed November 12, 2022 at:https://cancer.sanger.ac.uk/signatures/sbs/
- 33.Comprehensive Analysis of Spatial, Temporal and Molecular Patters of Ribociclib Efficacy and Resistance in Advanced Breast Cancer Patients (CAPTOR-BC). 2022Accessed July, 16, 2022 at:https://clinicaltrials.gov/ct2/show/NCT05452213
- 34.Combination of Abemaciclib and Endocrine Therapy in Hormone Receptor Positive HER2 Negative Locally Advanced or Metastatic Breast Cancer With Focus on Digital Side Effect Management (MINERVA). 2022Accessed June 26, 2022 at:https://clinicaltrials.gov/ct2/show/NCT05362760
- 35.Bidard F C, Kaklamani V G, Neven P. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022;40:3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AstraZeneca Camizestrant significantly improved progression-free survival vs. Faslodex in SERENA-2 Phase II trial in advanced ER-positive breast cancer. 2022Accessed November 20, 2022 at:https://www.astrazeneca.com/media-centre/press-releases/2022/camizestrant-significantly-improved-progression-free-survival.html
- 37.Tolaney S M, Chan A, Petrakova K. AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physicianʼs choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2− advanced breast cancer (aBC) Ann Oncol. 2022;33 07:S80–S121. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 38.Jimenez M M, Lim E, Gregor M CM. Giredestrant (GDC-9545) vs. physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2– locally advanced/metastatic breast cancer (LA/mBC): Primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 39.Sanofi Sanofi provides update on amcenestrant clinical development program. 2022Accessed November 03, 2022 at:https://www.sanofi.com/en/media-room/press-releases/2022/2022-08-17-05-30-00-2499668
- 40.Di Cosimo S, Perez-Garcia J M, Bellet M. Palbociclib with Fulvestrant or Letrozole in Endocrine-Sensitive Patients with HR-Positive/HER2-Negative Advanced Breast Cancer: A Detailed Safety Analysis of the Randomized PARSIFAL Trial. Oncologist. 2023;28:23–32. doi: 10.1093/oncolo/oyac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Borrego M, Guerrero-Zotano A, Bermejo B. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2-) early breast cancer (EBC): results from the GEICAM/2006-10 study. Breast Cancer Res Treat. 2019;177:115–125. doi: 10.1007/s10549-019-05296-8. [DOI] [PubMed] [Google Scholar]
- 42.Rugo H S, Bardia A, Marmé F. Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physicianʼs choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. J Clin Oncol. 2022;40:LBA1001–LBA1001. doi: 10.1200/JCO.2022.40.17_suppl.LBA1001. [DOI] [Google Scholar]
- 43.Rugo H S, Bardia A, Marmé F. Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs. treatment of physicianʼs choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (mBC) Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 44.Engler T, Fasching P A, Luftner D. Implementation of CDK4/6 Inhibitors and its Influence on the Treatment Landscape of Advanced Breast Cancer Patients – Data from the Real-World Registry PRAEGNANT. Geburtshilfe Frauenheilkd. 2022;82:1055–1067. doi: 10.1055/a-1880-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartkopf A.Therapy landscapes and molecular markers, the German PRAEGNANT registry. ESMO Breast Cancer Conference 2022. 2022Accessed November 10, 2022 at:https://oncologypro.esmo.org/meeting-resources/esmo-breast-cancer-congress/therapy-landscapes-and-molecular-markers-the-german-praegnant-registry
- 46.Modi S, Jacot W, Yamashita T. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rugo H S, Bardia A, Marme F. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol. 2022;40:3365–3376. doi: 10.1200/JCO.22.01002. [DOI] [PubMed] [Google Scholar]
- 48.Emens L A, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opzoomer J W, Sosnowska D, Anstee J E. Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Front Immunol. 2019;10:1654. doi: 10.3389/fimmu.2019.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munzone E, Regan M M, Cinieri S. A randomized phase II trial of metronomic oral vinorelbine plus cyclophosphamide and capecitabine (VEX) vs. weekly paclitaxel (P) as first- or second-line treatment in patients (pts) with ER+/HER2- metastatic breast cancer (MBC): The METEORA-II trial (IBCSG 54-16) Ann Oncol. 2022;33 07:S88–S121. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 51.Assessing ImmunoResponse Post Eribulin: Eribulin and Immunogenicity in Advanced Breast Cancer (AIRE). NIH US National Library of Medicine 2022; NCT05033769. [Google Scholar]
- 52.Janni W, Schneeweiss A, Muller V. Update Breast Cancer 2019 Part 2 – Implementation of Novel Diagnostics and Therapeutics in Advanced Breast Cancer Patients in Clinical Practice. Geburtshilfe Frauenheilkd. 2019;79:268–280. doi: 10.1055/a-0842-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.OʼBrien N A, McDonald K, Tong L. Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res. 2014;20:3507–3520. doi: 10.1158/1078-0432.CCR-13-2769. [DOI] [PubMed] [Google Scholar]
- 54.Loibl S, von Minckwitz G, Schneeweiss A. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32:3212–3220. doi: 10.1200/JCO.2014.55.7876. [DOI] [PubMed] [Google Scholar]
- 55.Schlam I, Tarantino P, Tolaney S M. Overcoming Resistance to HER2-Directed Therapies in Breast Cancer. Cancers (Basel) 2022;14:3996. doi: 10.3390/cancers14163996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu B, Yan M, Ma F. Pyrotinib or placebo in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer (PHILA): A randomized phase III trial. Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 57.Swain S M, Baselga J, Kim S B. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baselga J, Cortes J, Kim S B. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller V, Welslau M, Luftner D. Update Breast Cancer 2022 Part 2 – Advanced Stage Breast Cancer. Geburtshilfe Frauenheilkd. 2022;82:590–600. doi: 10.1055/a-1811-6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aktas B, Fehm T N, Welslau M. Update Breast Cancer 2022 Part 4 – Advanced-Stage Breast Cancer. Geburtshilfe Frauenheilkd. 2022;82:922–931. doi: 10.1055/a-1912-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ditsch N, Stickeler E, Behrens A. Update Breast Cancer 2021 Part 2 – Advanced Stages, Long-Term Consequences and Biomarkers. Geburtshilfe Frauenheilkd. 2021;81:539–548. doi: 10.1055/a-1464-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tesch H, Muller V, Wockel A. Update Breast Cancer 2020 Part 4 – Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2020;80:1115–1122. doi: 10.1055/a-1270-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.André F, Nadal J C, Denys H. Final overall survival (OS) for abemaciclib plus trastuzumab ± fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): A randomized, open-label, phase II trial. Ann Oncol. 2022;33 07:S808–S869. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]